- 1Department of Biology, University of Puerto Rico, Río Piedras, San Juan, Puerto Rico
- 2Department of Natural Sciences and Mathematics, Inter American University of Puerto Rico, Bayamón, Puerto Rico
Many species of social Hymenoptera demonstrate behavioral flexibility, where older workers that typically forage can revert to younger worker tasks, such as nursing, when these are absent. This flexibility is typical of the sterile worker class, yet rare in queens. In the little fire ant (Wasmannia auropunctata), queens have been reported to perform only egg laying. We examined behavior of queens of W. auropunctata after demographic manipulation. When half of the workers were removed from the colony, queens were observed caring for eggs, larvae and pupae as well as eating outside of the nest, like forager workers. We examined the relationship between these atypical queen behaviors and their juvenile hormone binding protein (JHbp) and vitellogenin (Vg) expression via QRT-PCR method. JHbp and Vg expression decreased when queens were performing worker tasks, resembling the expected expression pattern of typical sterile workers. Flexibility in queen behaviors in the little fire ant may be an important adaptation to changing environments. As a significant invasive species, such adaptation may increase the probability of colony survival during propagation. Our results not only present new insights in behavioral flexibility in social insects, but also increases our understanding of the success of this significant invasive species.
Introduction
Eusocial insects are characterized by having reproductive division of labor (Wilson, 1971). Within the colony one or more individuals carry out egg laying while sterile workers perform nest related tasks including queen and brood care (i.e., nursing), defense and foraging (Gordon, 1996). Division of labor in workers may be associated with age or morphological differences (Hölldobler and Wilson, 1990). Nevertheless, there are species where workers show behavioral flexibility, performing tasks that are not typical of their age or morphology. For example, in honey bees (Apis mellifera), nurses are known to forage precociously when foragers are absent, while forgers may revert to nursing according to the needs of the colony (Robinson, 1992). Similar behaviors has been observed in the eusocial wasp Polybia occidentalis (O’Donnell, 2001). Workers of the ant Pheidole dentata are found to increase their behavioral repertory as they age, where older workers perform tasks typical to younger individuals, yet the young workers are not proficient in older worker tasks (Calabi and Traniello, 1989; Seid and Traniello, 2006; Mertl and Traniello, 2009). Although behavioral flexibility in workers is an important adaptation that increases the chances of colony survival when the worker population decreases, it has not been reported in queens (Rüppell et al., 2002).
Queens produce eggs throughout most of their life. In species where the colony goes through a founding stage or independent colony foundation, queens perform worker type tasks until workers are reared. For example, in the red imported fire ant, Solenopsis invicta, the founding queen starts with nest construction, lays and tends the first batch of eggs until these emerge as adult workers, which take over worker duties. From this point on queens are known to only lay eggs (Tschinkel, 2006). Contrastingly, in species that reproduce through colony budding or dependent colony foundation, where one or more queens depart from the main colony with a group of workers, the queens do not experience a founding stage (Keller, 1991; Peeters and Ito, 2001). Since these queens keep a group of workers at all times it is not expected that they perform worker-like tasks.
We examined queen behavior in Wasmannia auropunctata, the little fire ant, a native of South America and an aggressive invasive species on all other continents except Antarctica (Le Breton et al., 2003; Wetterer and Porter, 2003; Mikheyev et al., 2008). Its colonies are composed of 200–500 monomorphic workers and one to twelve larger queens, and reproduce by colony fission (Wetterer and Porter, 2003; Foucaud et al., 2006; Mikheyev et al., 2009). Older workers, which typically carry out foraging duties, demonstrate behavioral flexibility by performing nursing duties when young workers (i.e., nurses) are absent (Rivera-Marchand and Fernández-Casas unpublished). The first objective of this study was to determine if queens of W. auropunctata demonstrate behavioral flexibility, performing worker tasks when necessary. Since colonies of the little fire ant do not experience a solitary founding stage (Wetterer and Porter, 2003; Foucaud et al., 2006; Mikheyev et al., 2009), queens typically have no need to perform worker tasks. We did not expect queens to perform worker duties until we had observed queens manipulating eggs. Based on these preliminary observations we expected that in the absence of workers, queens of W. auropunctata should have the behavioral flexibility to perform worker tasks.
Reproductive division of labor in eusocial Hymenoptera (ants, bees, and wasps), is under endocrine control (e.g., JH and Vg; Bloch et al., 2002; Amsalem et al., 2014). Juvenile hormone (JH) and vitellogenin (Vg) have important roles in regulating insect physiology (Dolezal et al., 2009, 2012) such as development, reproduction, and behavior (Robinson and Vargo, 1997; Dong et al., 2009; Azevedo et al., 2016). JH, considered a master hormone, has been found to control behavioral development in honey bees (Robinson and Vargo, 1997; Sullivan et al., 2000). It influences physiology in queens and guarding behavior in workers of primitive eusocial wasp Polistes canadensis (Giray et al., 2005). JH also affects queen maturation and reproduction in the invasive ant S. invicta where, high levels of JH induces alates to begin oogenesis (Vargo and Laurel, 1994; Brent and Vargo, 2003; Lu et al., 2009). Vg is a yolk precursor protein; its production is typically used to produce egg yolk by oviparous animals (Amdam et al., 2003), but it may also affect behavior (Nelson et al., 2007). In honey bees Vg has a role in the reproductive division of labor where concentrations are correlated with the hierarchy of the hive and reproductive division of labor (Corona et al., 2007; Nelson et al., 2007). Similar trends of Vg expression are seen in ants of S. invicta (Lewis et al., 2001; Lu et al., 2009) and Pogonomyrmex spp. (Corona et al., 2013; Libbrecht et al., 2013). Therefore, the second objective of this study was to measure gene expression of JH and Vg in relation to the tasks performed by queens. We expected egg laying queens to have higher levels of JH and Vg than worker-like queens.
Materials and Methods
Samples
Nests (N = 20) of the little fire ant W. auropunctata were collected from dry twigs and leaf litter in the northern region of the Caribbean island of Puerto Rico. They were housed in artificial nests which consisted of plastic boxes (25cm × 13cm × 7.5cm) coated with Fluon (Northern Products Inc., Alsip, IL, United States) on the sides. The boxes’ lids were perforated with a pin to allow air exchange. The nesting area within the nest box consisted of a 3 cm2 piece of thin (less than 1 cm thick) wood elevated 0.5 cm by a strip of clay placed along the edges of the wood. Each nest was kept at 25°C, a relative humidity (RH) between 80 and 85%, and 12 h light cycles. Nests were fed daily with 0.5 g of feeding mixture containing agar, eggs, honey, and vitamin supplements (Hölldobler and Wilson, 1994) placed in a feeding arena within the box at approximately 10 cm from the nesting area. Nests used in the study had multiple queens, eggs, larvae, and pupae. We marked 52 queens on the thorax or abdomen using unique color combinations of nail polish. Queens are easily distinguishable from workers by being 3 to 4 times larger (workers 1.2–1.5 mm long, queens 4.5–5 mm; Wetterer and Porter, 2003).
Behavioral Flexibility of Queens Assays
Six nests (N = 6) with a total of 19 queens were used for behavioral flexibility assays. Colonies were kept in the artificial nests 3 days prior to the start of experiment and fed daily. During the experiment observation period, queens were observed for 10 min daily for a total of 19 days; food was removed after each observation period. The 10 min observation period was determined after observing that the proportion of behaviors does not vary significantly in 10, 15, 20, and 30 min intervals. Observations were made for different tasks, including egg laying (a typical behavior), nursing (i.e., manipulating brood) and foraging (i.e., queens seen feeding in the designated arena), the latter two being non-typical behaviors. During the 10 min observational period, we tabulated by number of events, i.e., number of eggs laid, number of times brood was manipulated, and number of times queens walked to the feeding arena and was seen feeding. Control observation periods were performed for a period of 5 days. After the first 5 days of observations, worker population per nest was estimated via nest pictures. We then randomly culled approximately 50% of workers from the nest to simulate natural events in the wild. Preliminary observations (Rivera-Marchand and Fernández-Casas unpublished) indicated that the worker caste is equally divided between nurses and foragers. Daily 10 min observations continued for 14 days (Table 1). The remaining nests were used to measure JHbp and Vg gene expression.
Bioinformatic Analysis and Primer Design
Primers were designed for gene sequences related to JH and Vg expression. Since JH is a terpenoid, its gene expression levels were determined indirectly by measuring Juvenile hormone binding protein (JHbp), an associated protein. JHbp is directly correlated with the onset of JH production in the hemolymph (Kramer et al., 1976; Shemshedini and Wilson, 1990) because it prevents the absorption and enzymatic hydrolysis of JH, thereby maintaining a steady reservoir of the hormone in the hemolymph. As a consequence, free JH is virtually absent (Roe and Venkatesh, 1990; Tan, 2007). Also, JHbp/JH interaction is specific and of high affinity (KD = 10–9M), more than 99% of JH is bound to JHbp (De Kort and Granger, 1996; Tan, 2007). Other studies have further suggested this direct involvement as well (Prestwich et al., 1996; Hagai et al., 2007). Sequences for JHbp and Vg of W. auropunctata were obtained from NCBI Gene Bank. Vg sequences (XM_011697672.1, XM_011697673.1) were aligned using MAFFT (Multiple sequence alignment tool: Katoh et al., 2009). Primers (Table 2) were designed using primer3 from NCBI (Ye et al., 2012) with the obtained consensus sequence for Vg and the JHbp sequence (XM_011708554). Actin and GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) were used as housekeeping genes (Wong and Medrano, 2005; Scharlaken et al., 2008).
RNA Extraction, cDNA and qPCR of JHbp and Vg
Fourteen nests (N = 14), different from the ones used in the first behavioral assay, were used with a total of 33 queens. Nests were randomly assigned to control or experimental groups (nest with workers removed), behavioral assays were repeated. Seven days after worker removal, queens were collected by tasks; from control nests n = 15 and from experimental nests n = 18 and placed in a microtube with 20 μL of RNAlater reagent (Qiagen, Valencia, CA, United States) stored at −80°C for later RNA extraction. Afterward, samples were placed in a sterilized microtube and mechanically homogenized. RNA extraction was performed using the RNeasy Mini Kit (Qiagen). Extracted RNA was quantified for each sample in μg/μL units using a Nanophotometer (Implen, Westlake Village, CA, United States). RNA was normalized to a concentration (10 μg/μL) in a final volume of 20 μL and treated with DNase 1, following BioLabs (Ipswich, MA, United States) protocol to remove any DNA contamination. cDNA was synthesized from the normalized RNA using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad, Hercules, CA, United States) following the manufacturer’s protocol with 10 μL of RNA as a template. cDNA synthesis was verified in an electrophoresis 1% ETBR-gel.
qPCR was performed using the MJ Mini-Opticon Real-Time PCR (Bio-Rad) following the standard protocol of forty cycles; denature at 95°C for 10 s, annealing at 56°C for 30 s and elongation at 72°C for 15 s × 40, with post-amplification melt curve analysis. As a standard for quantification purposes, actin and GAPDH were used as reference genes (Wong and Medrano, 2005; Scharlaken et al., 2008). Primer efficiency was calculated using the standard curve analysis method where 1 μl of each cDNA sample were pooled and serial diluted in five points at 1:10. Reactions were prepared with 2 μL of first strand cDNA as a template in a master mix of 1 μL of forward and reverse primers per gene at [10 nM] and 5 μL of iTaq Universal SYBR Green Supermix (Bio-Rad) in a final volume of 10 μL. Relative gene expression was calculated using the geometric mean analysis method (Vandesompele et al., 2002), using the following equation:
E, primer efficiency; GOI, gene of interest; GeoMean, geometric mean; and REF, reference gene. ΔCt was calculated using the average Ct values of the control group for each gene (calibrator Ct). The relative expression values presented are relative to the control group.
Statistical Analysis
Behavior Analysis
For each task, relative probability was calculated by the number of queens performing a task with the total number of queens. Differences in relative probability between before and after worker removal were calculated with a Wilcoxon signed-rank test. To compare frequency of queen behaviors, frequency of typical and non-typical tasks were calculated by counting the number of events by queens before and after manipulation. Frequencies were compared using a Friedman test and Dunn’s test as a Post Hoc method.
Gene Expression
In order to compare relative expression between control and experimental samples, a Wilcoxon signed-rank test was used to measure differences in expression of JHbp and Vg. Here experimental samples were considered queens performing both nursing and/or foraging. To verify relative gene expression of JHbp and Vg differences among all tasks (egg laying, nursing, and foraging), a Kruskal-Wallis rank sum test was used with Dunn’s test as a Post Hoc method.
Data was analyzed using the statistical program R (R Core Team, 2014) v. 3.5.2 (2018-12-20) and the package agricolae (Statistical Procedures for Agricultural Research) v. 1.3-1. Graphs were done in Graph Pad Prism 6.0, (GraphPad Software, La Jolla, CA, United States). Data sets (Ortiz-Alvarado and Rivera-Marchand, 2020) can be found below https://datadryad.org/stash, https://doi.org/10.5061/dryad.j6q573nb2.
Results
Behavior Analysis
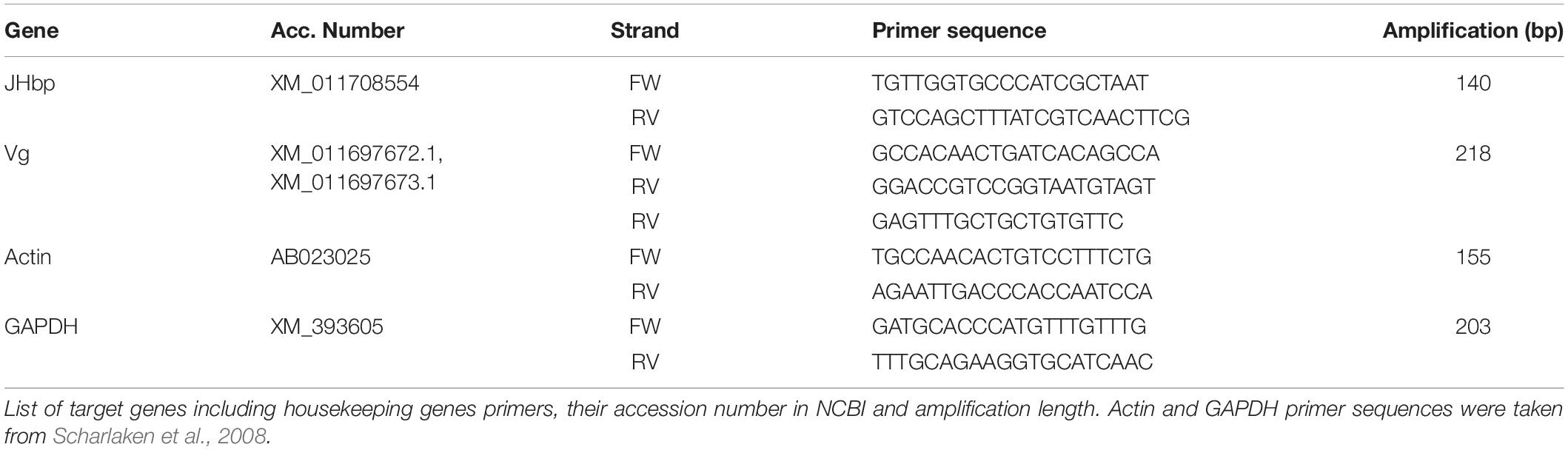
In the first 3 days of observations queens laid eggs while workers performed typical tasks. After worker removal queens performed worker tasks for nine consecutive days, which coincided with pupae emergence. During the period after worker removal in which queens behaved as workers, egg laying decreased significantly (Figure 1A: W = 62.5, p-value = 0.01) while nursing and foraging behaviors increased significantly (Figure 1B: W = 2.5, p-value = 0.002; Figure 1C: W = 15, p-value = 0.04). Of note, during the behavior observation period, some of the queens in the nests remained idle.
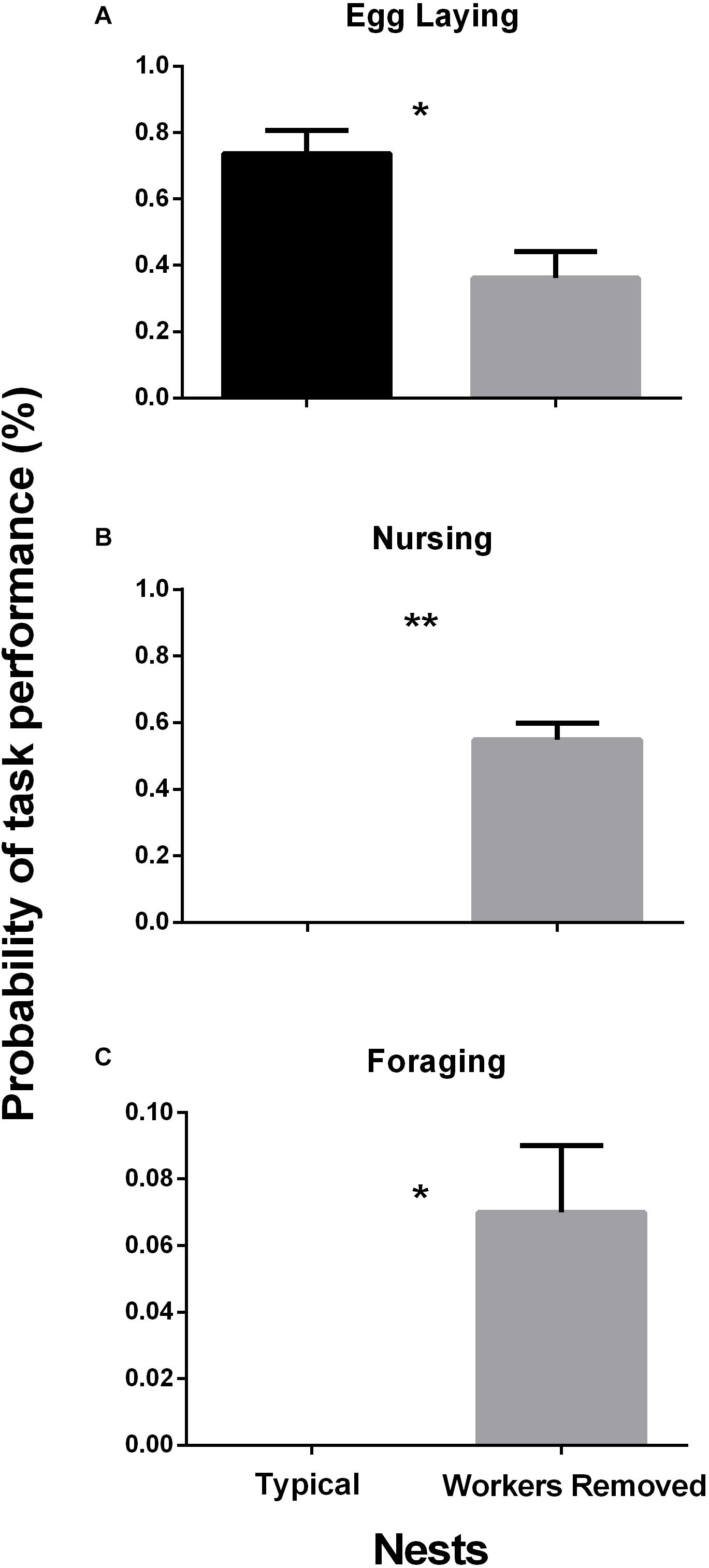
Figure 1. Relative probability of tasks in egg laying behavior (A), nursing behavior (B), and foraging behavior (C). (A) Egg Laying Behavior. Queen egg laying behaviors before and after workers were removed, task decreased significantly, W = 62.5, p-value = 0.01, Meantypical = 0.75, SEtypical = 0.07, MeanWR = 0.36, SEWR = 0.08. (B) Nursing Behavior. Queen nursing behaviors before and after workers were removed, task increased significantly, W = 2.5, p-value = 0.002, Meantypical = 0.00, SEtypical = 0.00, MeanWR = 0.55, SEWR = 0.05. (C) Foraging Behavior. Queen foraging behaviors before and after workers were removed, task increased significantly, W = 15, p-value = 0.04, Meantypical = 0.00, SEtypical = 0.00, MeanWR = 0.07, SEWR = 0.02. n = 19 queens. Behaviors observed tabulated as number of events occurred during the observation period. Asterisks (*) = p-value of ≤ 0.05, (**) = p-value of ≤ 0.001.
Throughout the experiment when queens performed non-typical tasks, egg laying decreased until new workers emerged (Figure 2). After new workers emerged, queens returned to egg laying and stopped performing worker tasks. Post hoc comparisons indicate that egg laying frequency from day 4 through 10 are significantly lower (p-value < 0.05) than egg laying before worker removal. As egg laying decreased, the frequency of nursing behavior by queens increased significantly (p-value < 0.05) from the fourth through tenth day of observations. While performing nursing, queens were seen manipulating eggs, larvae and pupae. Queens (N = 6) were also seen foraging during days 6 through 9. Only on day 7 was foraging frequency significantly higher than the rest of the experiment. On day 11 the queens returned to egg laying and by day fourteen queen behaviors resembled the behaviors during control period (p-value > 0.05), thus showing that the nests endured the experimental period and returned to a typical behavioral pattern.
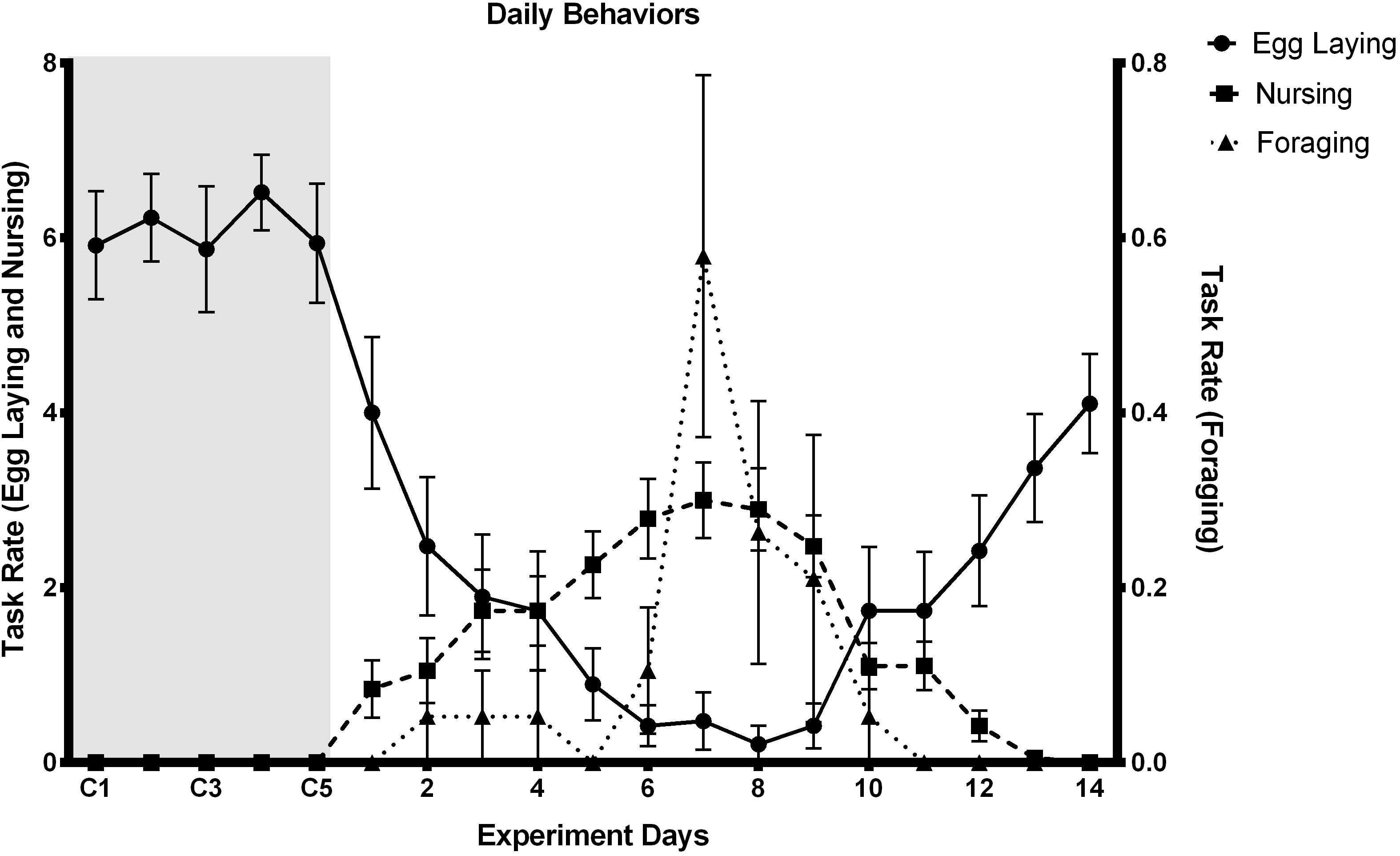
Figure 2. Daily behavior rate. Task frequency of Queens (n = 19) during the experiment. The shaded area represents tasks during the control period (5 days). Workers were removed on day 5 after final control observations (C5). Egg laying behavior decreased significantly at day 4; Chi-square = 118.524, df = 18, p-value ≤ 0.001, just as nursing behavior increased significantly; Chi-square = 14.58, df = 18, p-value ≤ 0.001, foraging increased significantly, at day 7; Chi-square = 37.71, df = 18, p-value ≤ 0.001. On day 12 the population began to grow as workers emerged. At day 13 queens increased egg laying and decreased nursing, completing a cycle. First 3 days show no significant difference with the last 3 days; Dunn p-value ≥ 0.05. Behaviors were observed tabulated as number of events occurred during the observation period.
Gene Expression
There’s a significant difference between the control and the experimental samples for JHbp and Vg expression. Queens from experimental nests performing worker related tasks have a lower relative gene expression of JHbp and Vg related to control nest queens (Figure 3A: W = 270, p-value ≤ 0.001; Figure 3C: W = 270, p-value ≤ 0.001). When relative gene expression of JHbp and Vg were compared between tasks, it shows a difference between egg laying compared to nursing and foraging for both JHbp and Vg; however, there is no difference in relative gene expression of JHbp and Vg in queens performing nursing or foraging tasks (Figure 3B:, Kruskal-Wallis chi-squared = 24.21, df = 2, p-value ≤ 0.001; Figure 3D; Kruskal-Wallis chi-squared = 25.19, df = 2, p-value ≤ 0.001).
Figure 3. Relative gene expression of JHbp (A,B) and Vg (C,D) in control and experimental nests. Results show differences in gene expression of JHbp and Vg between control or egg laying queens and experimental or nursing and foraging queens. Expression was measured in relative expression to endogenous genes Actin and GAPDH by geometric mean analysis method. n = 33 queens; control queens n = 15, experimental queens n = 18. (A) JHbp expression decreases from control to experimental queens, W = 270, p-value ≤ 0.001, Meancontrol = 1.30, SEexp = 0.26. (B) Comparing JHbp expression by tasks shows difference between egg laying compared to nursing and foraging, there’s no difference in expression between nursing or foraging queens Kruskal-Wallis chi-squared = 24.21, df = 2, p-value ≤ 0.001, Meanegg laying = 1.30, SEegg laying = 0.26, Meannursing = 0.20, SEnursing = 0.002, Meanforaging = 0.10, SEegg laying = 0.003. (C,D). Similar pattern as JHbp is seen with Vg expression, decreases in experimental queens (W = 270, p-value ≤ 0.001; Kruskal-Wallis chi-squared = 25.19, df = 2, p-value ≤ 0.001, Meancontrol = 1.22, SEexp = 0.22, Meanegg laying = 1.22, SEegg laying = 0.22, Meannursing = 0.10, SEnursing = 0.02, Meanforaging = 0.05, SEegg laying = 0.01; (C,D), respectively. Asterisks (**) = p-value of ≤ 0.001.
Discussion
Although behavioral flexibility is known to be a trait of workers in many species of social Hymenoptera (Gordon, 1991; Robinson, 1992; Giray et al., 2005; Seid and Traniello, 2006), queens of the little fire ant also demonstrate behavioral flexibility. When worker population decreases they perform worker tasks, principally nursing and, with less frequency, foraging (Figure 2). Although both behaviors were not initially expected, foraging in particular was a surprising outcome. Both egg laying and nursing are tasks done within the nest, so we hypothesize that the reason queens shift primarily to nursing is that it is less risky. Queens are risk aversive, and tend to perform nursing rather than foraging because the former is a less risky worker task. To our knowledge, this is the first time queens have been observed demonstrating behavioral flexibility after the founding stage. Given that W. auropunctata queens never experience a solitary founding stage (Foucaud et al., 2006; Mikheyev et al., 2009) and thus under typical conditions never perform worker-like duties, our results are even more remarkable.
During the experiment, there was an apparent transition phase, where queens gradually decreased their egg laying while increasing worker tasks. It is likely that queens may sense the shortage of workers due to a decrease in contact with workers. Studies have shown that ants typically communicate by cuticle hydrocarbons which are perceived by the olfactory organs (Vander Meer et al., 1989; Saïd et al., 2005; Ichinose and Lenoir, 2009; Bos et al., 2010), hence a decrease in the amount of cuticle hydrocarbons perceived might be an indicator to queens that the worker population has decreased. Maximum worker behavior frequencies were observed on the 8th day of the experiment, coinciding with minimum egg laying frequencies. We observed individual queens performing both nursing and foraging tasks. After the 9th day an increase in egg laying and a decrease in nursing were observed. From day 6 to 9, we observed queens eating at the feeding arena. Queens seem to decrease investments in reproduction in order to invest in brood care. As adult workers emerge and take over brood care tasks, queens begin investing in egg laying again. Since energy expenditures due to reproduction tend to be high in social insects (Oster and Wilson, 1978), queens of the little fire ant may not be able to invest in both egg laying and brood care. It is possible that these queens are diverting energy typically used for egg production to carry other nest duties. In our study queens performing worker tasks were not observed laying eggs. Moreover, studies on the reproductive biology of W. auropunctata have shown that all queens within nests produce viable eggs (de Ulloa, 2003). Therefore, we worked under the assumption that queens in the experiment were inseminated.
The changes in behaviors of queens were associated with changes in the expression of JHbp gene and Vg. When queens are performing typical tasks, the levels of JHbp and Vg tend to be higher, than when a queen displays worker behaviors. This suggests regulation by molecular and physiological mechanisms on behaviors, such as regulation by hormones/protein through gene expression. In honey bees, queens tend to have low levels of JH (Robinson et al., 1991; Fahrbach et al., 1995), the opposite is seen in the little fire ant queens, where queens performing egg laying had higher gene expression of JHbp gene than those performing worker related tasks (Figure 3A). When studying Vg, this protein has been found to have an effect on reproductive division of labor on honey bees, where queens have higher levels of Vg which decrease in nurses and foragers (Nelson et al., 2007; Page and Amdam, 2007). This is not an exception in other insects such as S. invicta and P. canadensis (Brent and Vargo, 2003; Sumner et al., 2006). The same pattern has been found in W. auropunctata queens, where queens have higher expression levels of Vg when laying eggs compared to the expression levels of Vg in the performance of worker related tasks (Figure 3B). Furthermore, studies have shown precocious foraging in workers induced by downregulation Vg using RNAi (Nelson et al., 2007; Antonio et al., 2008), suggesting changes in behavior are mediated by Vg.
The relationship between JH and Vg has been studied before (Robinson and Vargo, 1997; Lewis et al., 2001; Brent and Vargo, 2003; Barchuk et al., 2004). In most insects, there is a positive relationship between JH and Vg; increasing levels of JH cause an increase in Vg synthesis (Barchuk et al., 2004; Toth and Robinson, 2007). In S. invicta queens, high levels of JH and Vg are correlated. JH has been found to be an important promoter of vitellogenic oogenesis (Brent and Vargo, 2003; Lu et al., 2009) therefore, a decrease of JH seems to interrupt egg production. Our results show a similar relation with JH (JHbp) and Vg as queens’ reduction in Vg expression seems to correspond to a reduction in JHbp gene expression.
Behavioral flexibility in social insects ensures survival, particularly where environmental changes may affect colony demography. The adaptive value of behavioral flexibility in workers has been evidenced in honey bees (Robinson, 1992; Scheiner et al., 2004), different Pheidole species (Seid and Traniello, 2006; Mertl and Traniello, 2009) and the harvester ant (Pogonomyrmex barbatus; Gordon, 1991, 2002), where increased needs for one task due to environmental changes may lead to a behavioral response. Flexibility in queen behaviors in the little fire ant may be an important adaptation to changing environments. As a native to the tropics, this ant may face frequent disturbances that may deplete worker population. Moreover, the colonies of this ant tend to move their nests often (Wetterer and Porter, 2003). During the process of moving, workers such as foragers may be left behind as the nest is moved. The probability of colony survival may increase with queens performing worker duties as an adaptation for the population reduction that might occur during propagation. Evidence of this increased probability of survival can be seen in the final phase of the experiment where queens returned to their typical behaviors as the worker population increased. The results of this study not only present new insights in behavioral flexibility in social insects, but also increases our understanding of the success of this important invasive species. The little fire ant, is an important invasive species and agricultural pest that has colonized many areas around the world. Various ecological and reproductive adaptations are associated to its success and the unique queen behavior of this study adds to our knowledge of the suite of adaptations allowing this ant to be a successful invader.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://datadryad.org/stash and https://doi.org/10.5061/dryad.j6q573nb2.
Author Contributions
YO-A and BR-M conceived and designed the experiments presented in this manuscript, performed the writing of this manuscript, preparation of the figures, and editing. YO-A performed all of the experiments were in Puerto Rico and performed the data analysis on behavior and gene expression under the supervision of BR-M. All authors contributed to the article and approved the submitted version.
Funding
Financial support was provided by PR-LSAMP (grant no. 1026560) and the Agustin Stahl fellowships awarded to YO-A and the Inter American University of Puerto Rico.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Rafael Fernández-Casas, Carlos Ortiz-Alvarado, Emily Díaz-Iglesias, Luis Marrero-Ramos, Angel Rivera-Colón, and Yoselyn Rodríguez-Cruz aka the Wasmannia Team, for their effort in nest collection, establishment and extractions. We also thank Timothy Hendricks for the laboratory facilities and Rafael Canales-Pastrana and Bárbara González for their help in data analysis. Lastly we thank James D. Ackerman for helping with the revisions of previous versions of the manuscript.
References
Amdam, G. V., Norberg, K., Hagen, A., and Omholt, S. W. (2003). Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. U.S.A. 100, 1799–1802. doi: 10.1073/pnas.0333979100
Amsalem, E., Malka, O., Grozinger, C., and Hefetz, A. (2014). Exploring the role of juvenile hormone and vitellogenin in reproduction and social behavior in bumble bees. BMC Evol. Biol. 14:45. doi: 10.1186/1471-2148-14-45
Antonio, D. S. M., Guidugli-Lazzarini, K. R., Do Nascimento, A. M., Simões, Z. L. P., and Hartfelder, K. (2008). RNAi-mediated silencing of vitellogenin gene function turns honeybee (Apis mellifera) workers into extremely precocious foragers. Naturwissenschaften 95, 953–961. doi: 10.1007/s00114-008-0413-9
Azevedo, D. O., de Paula, S. O., Zanuncio, J. C., Martinez, L. C., and Serrão, J. E. (2016). Juvenile hormone downregulates vitellogenin production in Ectatomma tuberculatum (Hymenoptera: Formicidae) sterile workers. J. Exper. Biol. 219, 103–108. doi: 10.1242/jeb.127712
Barchuk, A. R., Maleszka, R., and Simões, Z. L. P. (2004). Apis mellifera ultraspiracle: cDNA sequence and rapid up-regulation by juvenile hormone. Insect Mol. Biol. 13, 459–467. doi: 10.1111/j.0962-1075.2004.00506.x
Bloch, G., Wheeler, D. E., and Robinson, G. E. (2002). Endocrine Influences on the Organization of Insect Societies in “Hormones, Brain, and Behavior”, Vol. 3, eds D. W. Pfaff, A. P. Arnold, A. M. Ettgen, S. E. Fahrbach, and R. T. Rubin (San Diego: Academic Press), 195–237.
Bos, N., Guerrieri, F. J., and d’Ettorre, P. (2010). Significance of chemical recognition cues is context dependent in ants. Anim. Behav. 80, 839–844. doi: 10.1016/j.anbehav.2010.08.001
Brent, C. S., and Vargo, E. L. (2003). Changes in juvenile hormone biosynthetic rate and whole body content in maturing virgin queens of Solenopsis invicta. J. Insect Physiol. 49, 967–974. doi: 10.1016/s0022-1910(03)00166-5
Calabi, P., and Traniello, J. F. (1989). Behavioral flexibility in age castes of the ant Pheidole dentata. J. Insect Behav. 2, 663–677. doi: 10.1007/bf01065785
Corona, M., Libbrecht, R., Wurm, Y., Riba-Grognuz, O., Studer, R. A., and Keller, L. (2013). Vitellogenin underwent subfunctionalization to acquire caste and behavioral specific expression in the harvester ant Pogonomyrmex barbatus. PLoS Genet. 9:1003730. doi: 10.1371/journal.pgen.1003730
Corona, M., Velarde, R. A., Remolina, S., Moran-Lauter, A., Wang, Y., Hughes, K. A., et al. (2007). Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. U.S.A. 104, 7128–7133. doi: 10.1073/pnas.0701909104
De Kort, C. A. D., and Granger, N. A. (1996). Regulation of JH titers: the relevance of degradative enzymes and binding proteins. Archiv. Insect Biochem. Physiol. 33, 1–26. doi: 10.1002/(sici)1520-6327(1996)33:1<1::aid-arch1>3.0.co;2-2
de Ulloa, P. C. (2003). Biologia reproductiva de Wasmannia auropunctata (R.)(H ymenoptera: formicidae). Rev. Acad. Colomb. Cienc. Exact. Físic. Nat. 27, 441–448.
Dolezal, A. G., Brent, C. S., Gadau, J., Hölldobler, B., and Amdam, G. V. (2009). Endocrine physiology of the division of labour in Pogonomyrmex californicus founding queens. Anim. Behav. 77, 1005–1010. doi: 10.1016/j.anbehav.2009.01.010
Dolezal, A. G., Brent, C. S., Hölldobler, B., and Amdam, G. V. (2012). Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J. Exper. Biol. 215, 454–460. doi: 10.1242/jeb.060822
Dong, S. Z., Ye, G. Y., Guo, J. Y., and Hu, C. (2009). Roles of ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae). Gen. Comparat. Endocrinol. 160, 102–108. doi: 10.1016/j.ygcen.2008.11.007
Fahrbach, S. E., Giray, T., and Robinson, G. E. (1995). Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol. Learn. Mem. 63, 181–191. doi: 10.1006/nlme.1995.1019
Foucaud, J., Jourdan, H., Breton, J. L., Loiseau, A., Konghouleux, D., and Estoup, A. (2006). Rare sexual reproduction events in the clonal reproduction system of introduced populations of the little fire ant. Evolution 60, 1646–1657. doi: 10.1111/j.0014-3820.2006.tb00509.x
Giray, T., Giovanetti, M., and West-Eberhard, M. J. (2005). Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proc. Natl. Acad. Sci. U.S.A. 102, 3330–3335. doi: 10.1073/pnas.0409560102
Gordon, D. M. (1991). Variation and change in behavioral ecology. Ecology 72, 1196–1203. doi: 10.2307/1941093
Gordon, D. M. (1996). The organization of work in social insect colonies. Nature 380, 121–124. doi: 10.1038/380121a0
Gordon, D. M. (2002). The regulation of foraging activity in red harvester ant colonies. Am. Nat. 159, 509–518. doi: 10.1086/339461
Hagai, T., Cohen, M., and Bloch, G. (2007). Genes encoding putative Takeout/juvenile hormone binding proteins in the honeybee (Apis mellifera) and modulation by age and juvenile hormone of the takeout-like gene GB19811. Insect Biochem. Mol. Biol. 37, 689–701. doi: 10.1016/j.ibmb.2007.04.002
Hölldobler, B., and Wilson, E. O. (1994). Journey to The Ants. Cambridge, MA: Harvard University Press.
Ichinose, K., and Lenoir, A. (2009). Ontogeny of hydrocarbon profiles in the ant Aphaenogaster senilis and effects of social isolation. Compt. Rend. Biol. 332, 697–703. doi: 10.1016/j.crvi.2009.04.002
Katoh, K., Asimenos, G., and Toh, H. (2009). Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537, 39–64. doi: 10.1007/978-1-59745-251-9_3
Keller, L. (1991). Queen number, mode of colony founding, and queen reproductive success in ants (Hymenoptera Formicidae). Ethol. Ecol. Evol. 3, 307–316. doi: 10.1080/08927014.1991.9525359
Kramer, K. J., Dunn, P. E., Peterson, R. C., and Law, J. H. (1976). “Interaction of juvenile hormone with binding proteins in insect hemolymph,” in The Juvenile Hormones, ed. L. I. Gilbert (Boston, MA: Springer), 327–341. doi: 10.1007/978-1-4684-7947-8_23
Le Breton, J., Chazeau, J., and Jourdan, H. (2003). Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on native litter ant fauna in a New caledonian rainforest. Austral Ecol. 28, 204–209. doi: 10.1046/j.1442-9993.2003.01266.x
Lewis, D. K., Campbell, J. Q., Sowa, S. M., Chen, M. E., Vinson, S. B., and Keeley, L. L. (2001). Characterization of vitellogenin in the red imported fire ant, Solenopsis invicta (Hymenoptera: Apocrita: Formicidae). J. Insect Physiol. 47, 543–551. doi: 10.1016/s0022-1910(00)00155-4
Libbrecht, R., Corona, M., Wende, F., Azevedo, D. O., Serrão, J. E., and Keller, L. (2013). Interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on polyphenism in ants. Proc. Natl. Acad. Sci. U.S.A. 110, 11050–11055. doi: 10.1073/pnas.1221781110
Lu, H. L., Vinson, S. B., and Pietrantonio, P. V. (2009). Oocyte membrane localization of vitellogenin receptor coincides with queen flying age, and receptor silencing by RNAi disrupts egg formation in fire ant virgin queens. FEBS J. 276, 3110–3123. doi: 10.1111/j.1742-4658.2009.07029.x
Mertl, A. L., and Traniello, J. F. (2009). Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): does morphological specialization influence task plasticity? Behav. Ecol. Sociobiol. 63, 1411–1426. doi: 10.1007/s00265-009-0797-3
Mikheyev, A. S., Bresson, S., and Conant, P. (2009). Single-queen introductions characterize regional and local invasions by the facultatively clonal little fire ant Wasmannia auropunctata. Mol. Ecol. 18, 2937–2944. doi: 10.1111/j.1365-294x.2009.04213.x
Mikheyev, A. S., Tchingnoumba, L., Henderson, A., and Alonso, A. (2008). Effect of propagule pressure on the establishment and spread of the little fire ant Wasmannia auropunctata in a Gabonese oilfield. Diver. Distribut. 14, 301–306. doi: 10.1111/j.1472-4642.2007.00463.x
Nelson, C. M., Ihle, K. E., Fondrk, M. K., Page, R. E., and Amdam, G. V. (2007). The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5:e62. doi: 10.1371/journal.pgen.100062
O’Donnell, S. (2001). Worker age, ovary development, and temporal polyethism in the swarm-founding wasp Polybia occidentalis (Hymenoptera: Vespidae). J. Insect Behav. 14, 201–213.
Ortiz-Alvarado, Y., and Rivera-Marchand, B. (2020). Data From: “Worker queens”? Behavioral Flexibility, Juvenile Hormone Binding Protein and Vitellogenin in Queens of the Little Fire Ant Wasmannia auropunctata. Dryad: Dataset Publishing. Available online at: https://doi.org/10.5061/dryad.j6q573nb2
Page, R. E. Jr., and Amdam, G. V. (2007). The making of a social insect: developmental architectures of social design. Bioessays 29, 334–343. doi: 10.1002/bies.20549
Peeters, C., and Ito, F. (2001). Colony dispersal and the evolution of queen morphology in social Hymenoptera. Annu. Rev. Entomol. 46, 601–630.
Prestwich, G. D., Wojtasek, H., Lentz, A. J., and Rabinovich, J. M. (1996). Biochemistry of proteins that bind and metabolize juvenile hormones. Archiv. Insect Biochem. Physiol. 32, 407–419. doi: 10.1002/(sici)1520-6327(1996)32:3/4<407::aid-arch13>3.0.co;2-g
R Core Team (2014). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robinson, G. E. (1992). Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665. doi: 10.1146/annurev.en.37.010192.003225
Robinson, G. E., Strambi, C., Strambi, A., and Feldlaufer, M. F. (1991). Comparison of juvenile hormone and ecdysteroid haemolymph titres in adult worker and queen honey bees (Apis mellifera). J. Insect Physiol. 37, 929–935. doi: 10.1016/0022-1910(91)90008-n
Robinson, G. E., and Vargo, E. L. (1997). Juvenile hormone in adult eusocial hymenoptera: gonadotropin and behavioral pacemaker. Archiv. Insect Biochem. Physiol. 35, 559–583. doi: 10.1002/(sici)1520-6327(1997)35:4<559::aid-arch13>3.0.co;2-9
Roe, R. M., and Venkatesh, K. (1990). Metabolism of juvenile hormones: degradation and titer regulation. Morphogenet. Hormon. Arthrop. 1, 126–179.
Rüppell, O., Schäffler, L., and Hölldobler, B. (2002). Lack of plasticity in the behavior of queens of the ant Leptothorax rugatulus emery (Formicidae: Hymenoptera). J. Insect Behav. 15, 447–454.
Saïd, I., Gaertner, C., Renou, M., and Rivault, C. (2005). Perception of cuticular hydrocarbons by the olfactory organs in Periplaneta americana (L.)(Insecta: Dictyoptera). J. Insect Physiol. 51, 1384–1389. doi: 10.1016/j.jinsphys.2005.09.001
Scharlaken, B., de Graaf, D. C., Goossens, K., Brunain, M., Peelman, L. J., and Jacobs, F. J. (2008). Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J. Insect Sci. 8:33.
Scheiner, R., Page, R. E., and Erber, J. (2004). Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35, 133–142. doi: 10.1051/apido:2004001
Seid, M. A., and Traniello, J. F. (2006). Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav. Ecol. Sociobiol. 60, 631–644. doi: 10.1007/s00265-006-0207-z
Shemshedini, L., and Wilson, T. G. (1990). Resistance to juvenile hormone and an insect growth regulator in Drosophila is associated with an altered cytosolic juvenile hormone-binding protein. Proc. Natl. Acad. Sci. U.S.A. 87, 2072–2076. doi: 10.1073/pnas.87.6.2072
Sullivan, J. P., Jassim, O., Fahrbach, S. E., and Robinson, G. E. (2000). Juvenile hormone paces behavioral development in the adult worker honey bee. Horm. Behav. 37, 1–14. doi: 10.1006/hbeh.1999.1552
Sumner, S., Pereboom, J. J., and Jordan, W. C. (2006). Differential gene expression and phenotypic plasticity in behavioural castes of the primitively eusocial wasp, Polistes canadensis. Proc. R. Soc. B Biol. Sci. 273, 19–26. doi: 10.1098/rspb.2005.3291
Tan, S. H. (2007). Stable Expression and Purification of Juvenile Hormone Binding Protein from Drosophila melanogaster Schneider line-2 cells. Doctoral Dissertation, University of Wisconsin, Madison. Available online at: http://digital.library.wisc.edu/1793/8134
Toth, A. L., and Robinson, G. E. (2007). Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341. doi: 10.1016/j.tig.2007.05.001
Vander Meer, R. K., Saliwanchik, D., and Lavine, B. (1989). Temporal changes in colony cuticular hydrocarbon patterns of Solenopsis invicta. J. Chem. Ecol. 15, 2115–2125. doi: 10.1007/bf01207442
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:research34.
Vargo, E. L., and Laurel, M. (1994). Studies on the mode of action of a queen primer pheromone of the fire ant Solenopsis invicta. J. Insect Physiol. 40, 601–610. doi: 10.1016/0022-1910(94)90147-3
Wetterer, J. K., and Porter, S. D. (2003). The Little Fire Ant, Wasmannia Auropunctata: Distribution, Impact And Control. Sociobiology 42, 1–41.
Wong, M. L., and Medrano, J. F. (2005). Real-time PCR for mRNA quantitation. Biotechniques 39, 75–85. doi: 10.2144/05391rv01
Keywords: behavior, flexibility, invasive, ant, queen, juvenile hormone, vitellogenin
Citation: Ortiz-Alvarado Y and Rivera-Marchand B (2020) Worker Queens? Behavioral Flexibility of Queens in the Little Fire Ant Wasmannia auropunctata. Front. Ecol. Evol. 8:241. doi: 10.3389/fevo.2020.00241
Received: 16 April 2020; Accepted: 02 July 2020;
Published: 04 August 2020.
Edited by:
Shu-Ching Chen, Florida International University, United StatesReviewed by:
Jose Eduardo Serrão, Universidade Federal de Viçosa, BrazilJocelyn G. Millar, University of California, Riverside, United States
Zachary Huang, Michigan State University, United States
Copyright © 2020 Ortiz-Alvarado and Rivera-Marchand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yarira Ortiz-Alvarado, eS5vcnRpemFsQGdtYWlsLmNvbQ==
 Yarira Ortiz-Alvarado
Yarira Ortiz-Alvarado Bert Rivera-Marchand
Bert Rivera-Marchand