
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 27 May 2020
Sec. Chemical Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00158
Mouse lemurs are small, nocturnal, arboreal solitary foragers and are endemic primates of Madagascar. This lifestyle and their high predation risk can explain why mouse lemurs rely heavily on olfaction for intraspecific communication. As they often use urine for this purpose, we investigated dichloromethane extracts of the urine of two mouse lemur species, the gray mouse lemur (Microcebus murinus) and the Godman’s mouse lemur (M. lehilahytsara), using gas-chromatography-mass spectrometry. We detected 977 different volatile compounds of different compound classes in 22 urine extracts obtained from nine M. murinus (four males, five females) and nine M. lehilahytsara (three males, six females) individuals. We compared the volatile profiles of the sexes and species using principal component analyses and discriminant function analyses and detected a significant difference in the urinary profiles of males and females and in the profiles of M. murinus and M. lehilahytsara. These very complex sex- and species-specific signatures could be used for distance communication in the context of species recognition, for mate search and in male-male competition. Our study provides important mechanistic insights into complex chemical signaling pathways in primates that are mirrored, in the case of mouse lemurs, by their extraordinarily rich repertoire of olfactory receptors. The production of highly informative olfactory signals may be complementing the complex acoustic signaling system of these solitary foragers suggesting the existence of a multimodal communication network that should be highly beneficial for any species living in dispersed social networks.
Olfactory cues play an important role in the intraspecific communication of many mammals (Müller-Schwarze, 2006; Apps et al., 2015). They constitute important chemical signals, often incorporated in urine, feces, or other scent marks. Such chemical cues can contain information about species identity, sex, group identity, kinship, individual identity, but also about their current social status, reproductive state, and health, among others (Brennan and Kendrick, 2006; Apps et al., 2015). In addition, these cues can be used to regulate space use within and among social groups or species, inform potential mates or competing conspecifics about the position of a sender and may therefore have considerable fitness consequences. Owl monkeys (Aotus nanymaae), for example, use odor signals expressed in their subcaudal scent secretions to communicate sex, age, and family (MacDonald et al., 2008). Some group-living diurnal lemur species such as Lemur catta and Propithecus coquereli deposit scent marks that allow them to identify species, sex and reproductive status (Hayes et al., 2004; Scordato and Drea, 2007; Scordato et al., 2007; Boulet et al., 2009). A first study on the chemical composition of the anogenital gland secretions of Lemur catta and Propithecus coquereli revealed that the two species are chemically distinguishable, as are the sexes in the case of Lemur catta (Hayes et al., 2004). The secretions mostly consisted of straight and branched long-chain alcohols, aldehydes, and esters. Scordato et al. (2007) analyzed scrotal, labial, and brachial gland secretions of Lemur catta and showed that labial and scrotal secretions were most similar in their composition, consisting of a series of organic acids and esters, squalene, and cholesterol derivatives. Male brachial secretions primarily contained squalene and appreciable amounts of cholesterol and derivatives, as well as lanosterol. They described seasonal differences in the composition of the secretions, supporting the observation that scent marking serves to advertise reproductive and physiological state and modulates intrasexual competition. In addition, labial and to some degree scrotal secretions showed stable individual profiles.
Very limited knowledge is so far available on the composition of olfactory urinary signals employed by nocturnal primates living in dispersed social systems. It is well-established, however, that mouse lemurs, Microcebus sp. (Primates, Strepsirrhini), rely heavily on olfactory communication (Perret, 1995). They have several apocrine scent glands that are used in different marking behaviors such as head rubbing or anogenital marking, and they perform urine washing (Glatston, 1983). The lemurs also show strong behavioral reactions to mammalian predator odors (Sündermann et al., 2008; Kappel et al., 2011). The main olfactory bulb and a functional vomeronasal organ, which are particularly well-developed, are used for the detection of chemical cues (Evans and Schilling, 1995; Hohenbrink et al., 2012, 2013, 2014). It was previously shown that chemical cues from their urine are involved in regulating sociosexual behavior. Dominant males release a pheromone with their urine that has an inhibitory effect on the reproductive function of conspecifics. This pheromone, possibly a steroid, leads to a decrease of plasma testosterone levels in subdominant males (Schilling et al., 1984). Furthermore, the testosterone level of males can also be significantly elevated by exposing them to prooestrous female urine (Perret, 1995; Hohenbrink et al., 2012, 2013, 2014). This pheromone-like effect is comparable to that described for mice (Vandenbergh, 1983).
Urinary chemical signals have been intensely studied in rodents. For example, it has been shown that the urinary pheromones of male mice (Mus musculus) can induce the estrous cycle in conspecific females (Whitten et al., 1968; Ma et al., 1999). In contrast, only one study reported the chemical composition of the urinary volatiles produced by lemurs previously. delBarco-Trillo et al. (2011) investigated 12 distantly related lemur species representing most families of the strepsirrhine primates and compared them to each other under the aspect of socioecological and phylogenetic patterns in their olfactory signals. As representative for the Cheirogaleidae to which the mouse lemurs belong, Cheirogaleus medius was investigated. The study showed that species that show urine marking express more volatile compounds in their urine than do non-markers. In addition, closely related species showed greater similarities in their volatiles profile than do more distantly related species. Dynamic headspace sampling was used to collect volatiles from urine.
In the present study, the chemical composition of urine volatiles was investigated in two closely related mouse lemurs, the gray mouse lemur, Microcebus murinus, and the Godman’s mouse lemur, Microcebus lehilahytsara (Cheirogaleidae). The two species live in the western dry deciduous forests and in the eastern montane evergreen forests of Madagascar, respectively. Up to two mouse lemur species can be found in various Malagasy forests (Radespiel, 2016), and we were therefore interested in the question whether the composition of the urine of any two mouse lemur species differ, so that these signatures could be potentially used for species discrimination. In addition, we addressed the question whether significant differences exist between female and male urine samples that could serve as a basis for sex discrimination. delBarco-Trillo et al. (2011) did not find any difference between male and female urine. We hypothesize that this might be due to their data collection method as they used dynamic headspace collection of volatiles resulting in a relatively low number of compounds identified. For example, heavier compounds are not readily detectable by the headspace method because of the minute amounts evaporating from the urine. Such compounds can, however, potentially act as signaling compounds, which has been shown for androstenone, a boar pheromone (Booth, 1987). Therefore, we decided to use solvent extraction to get a broad overview across compounds present in the urine that could potentially be perceived by the mouse lemurs when the animals sniff directly on the scent marks.
Urine samples were collected from nine (four males, five females) Microcebus murinus and from nine (three males, six females) M. lehilahytsara that were housed in the colony of the Institute of Zoology, University of Veterinary Medicine Hannover (Supplementary Table S1). Three males and one female were sampled twice. Samples were obtained ad libitum during the weekly handling routines when animals urinated spontaneously, or by collecting urine in modified sleeping boxes that contained a perforated metal floor that allowed collecting the urine with a metal funnel and test tube below. Although clean material was used, it was neither possible nor intended to collect sterile urine. We were looking for chemical signatures that potentially allow species or sex discrimination. Therefore, the chemical profiles carrying these information should be stable enough even during slight bacterial alteration potentially taking place during storage. These signatures should also be stable enough to be detected in urine marks in the environment with potentially much higher rates of bacterial contamination and therefore higher potential alteration of the urine marks chemical inventory. Sample collection for this study was non-invasive and was performed in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals, the European Directive 2010/63/EU on the protection of animals used for scientific purposes, and was approved by the relevant ethics committee of the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (reference number AZ 33.12-42502-04-14/1454). Both mouse lemur species were kept in the animal facility of the Institute of Zoology at the University of Veterinary Medicine Hannover, Germany (licensed by Ordnungsamt, Gewerbe- und Veterinärabteilung, Landeshauptstadt Hannover, AZ 42500/1H). All samples were frozen directly in inert sample tubes (KH-Flasche G 1, CS Chromatographie Service) at -20°C and stored until chemical analyses that took place shortly afterwards. Urine samples were collected between the 18th March and the 22nd April of the years 2013, 2014, or 2015, respectively, i.e., during the reproductive season of the colony (Wrogemann and Zimmermann, 2001; Wrogemann et al., 2001). All but one males (Guido) had fully developed testes on the day of sampling (Supplementary Table S1). All but two females were sampled during their interestrus, i.e., the time between two successive estrus periods (Wrogemann et al., 2001). Two females (Olympia, Irmi second sample), however, were sampled on the first day of their second estrus of the season.
Dichloromethane extracts of the mouse lemur urine were analyzed using gas chromatography coupled with mass spectrometry (GC/MS). Samples of 0.25–1.2 mL urine collected from each individual were extracted with 300 μL of dichloromethane by stirring at room temperature for 12 h. The two phases were separated and the organic phase was dried with NaCl. Extracts were analyzed by GC/MS (GC 7890B/MSD 5977A, Agilent Technologies, Santa Clara, CA, United States). The GC/MS system was equipped with a HP-5ms fused silica capillary column (30 m, 0.22 mm internal diameter, 0.25 μm film, Agilent Technologies). Conditions were as follows: inlet pressure: 67 kPa, He-flow: 1.2 ml/min, injector: 250°C, transfer line: 300°C, electron energy 70 eV. The GC oven temperature was kept at 50°C for 5 min, followed by a temperature gradient of 5°C/min to 320°C. Identification of compounds was performed by comparison of their mass spectra and retention indices (determined from a homologous series of n-alkanes, C8–C32) to those of commercial mass spectral libraries (Wiley 7, NIST 08) and an in-house data base. Water blank samples were prepared and extracted like the urine samples and analyzed by GC/MS. Compounds found in this sample were removed from the subsequent analyses. Only compounds eluting before retention index (RI) of <2000 (36 min) on the apolar column were included in the analysis, since volatile compounds elute before that time and later eluting compounds were overlaid by common fatty acid derivatives. In total, 22 urine samples of 18 different individuals were analyzed. A total of four individuals, two male M. murinus and one male and one female M. lehilahytsara, were sampled twice.
Presence or absence of all compounds was noted for each sample. Before statistical analyses, all compounds were removed that were present either in all samples or in one sample only. This approach yielded a total of 385 compounds for further analyses. The number of compounds in male and female samples of both species was first compared with a GLMM with the number of compounds as dependent variable, sex and species as fixed factors, and the individual identity as random factor. Since only four of 18 individuals were sampled twice, we used a Gauss-Hermite approximation (nAGQ = 10) to evaluate the log-likelihood and to reach model convergence. The model was calculated with the package lme4 in R (R Core Team, 2015).
The list with all presence and absence codes in all samples was subjected to a Principal Component Analysis in the program STATISTICA 12 (Statsoft, Inc.). A total of 21 principal components were derived with Eigenvalues ranging from 6.56 to 60.68, and single components explained between 1.71 and 15.76% of the variance contained in the dataset. The first 15 principal components (explaining 86.395% of the variance) were used in two discriminant function analyses (stepwise forward approach, p to enter = 0.05), one for the discrimination between the sexes and one for the discrimination between the species. All samples were classified by means of the calculated discriminant function according to sex (male, female) or species (M. murinus, M. lehilahytsara). The factor with the highest and significant contribution to the respective discriminant function was evaluated, and compounds with factor loadings of >0.6 or <−0.6 are identified and reported.
Pilot experiments showed that the highest number of compounds was observed by solvent extraction of urine with dichloromethane compared to solid phase microextraction (SPME) analysis. Therefore, the urine samples were extracted by the former procedure and analyzed by GC/MS. A total of 977 different compounds with a retention index of <2000 were detected in the 22 analyzed samples, of which 388 (40%) were identified (Supplementary Table S4). Despite this wealth of compounds, only 173 ± 49 (mean ± SD) compounds were present in a single urine extract. A wide range of different groups of compounds were present including alcohols, aldehydes, amides, ketones, (fatty) acids, esters, terpenes, steroids, and heterocycles including lactones, indoles, or quinolines. Of these, 42 components occurred in every sample or most samples (>82% occurrence) e.g., phenol, benzaldehyde, acetophenone, and nonanal, while 11 of them were unknown components (Table 1). The main compounds with the highest relative concentration in most samples were 2-methylpropyl acetate, dimethyl sulfone (1), 4-methylphenol (2), quinoline (3), nonanoic acid (4), 1-hexadecanol (5), hexadecenoic acid (6) and five unknown compounds (Figure 1).
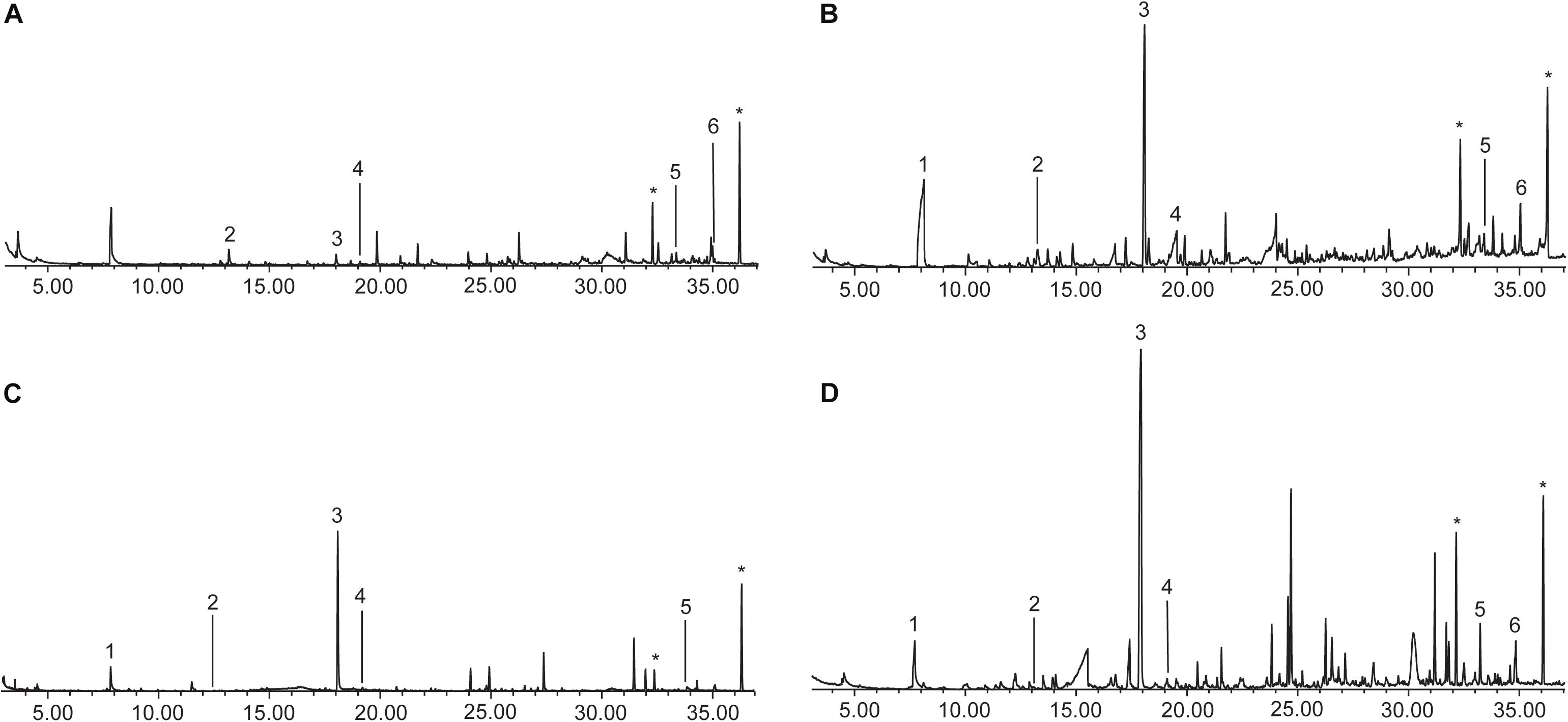
Figure 1. Typical total ion chromatograms (TIC) of a urine extract of a male (A) and a female (B) M. murinus and a male (C) and a female (D) M. lehilahytsara. Overall, females produce more compounds in higher quantities compared to males. 1 = dimethyl sulfone, 2 = 4-methylphenol, 3 = quinoline, 4 = nonanoic acid, 5 = 1-hexadecanol, 6 = hexadecanoic acid, *artifacts.
Ten male urine and twelve female urine extracts of both species were analyzed with regard to sex differences in volatile compounds. There were noticeable differences in the number of compounds found in the urine extracts of males and females of both species (Figure 2). The overall mean number of compounds was 145 compounds in male urine samples (132 in M. lehilahytsara, 154 in M. murinus), but 195 compounds in female urine samples (201 in M. lehilahytsara, 188 in M. murinus) (Figure 2). The GLMM revealed these differences to be significant in the overall analyses [Estimate(male) = −0.40388, SE = 0.10592, z-value = −3.813, p = 0.000137], while no influence of species on the numbers of compounds were detected [Estimate(murinus) = 0.05986, SE = 0.10264, z-value = 0.583, p = 0.559732]. The difference in compound numbers between the sexes was also significant when analyzing both species separately [Estimate(male, lehilahytsara) = −0.56280, p = 0.000233; Estimate(male, murinus) = −0.26074, p = 0.045].
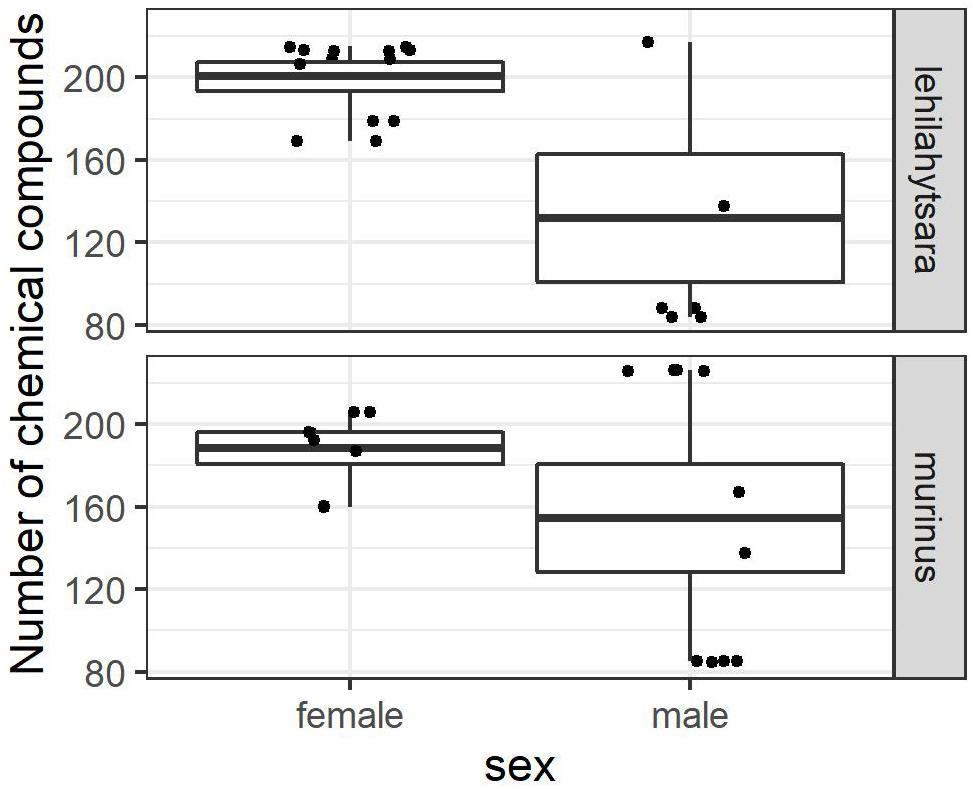
Figure 2. Number of volatile compounds per individual sample for male and female M. lehilahytsara (upper panel) and M. murinus (lower panel) (Mean, box: standard error, whiskers: minimum and maximum value). Individual values are displayed as jitter. Male samples contained significantly less compounds than female samples in both species (see text).
Generally, there was no single compound that was present in every sample of one sex and absent in the other one. However, the discriminant function analysis (DFA) based on the first 15 principal components provided a highly significant result [Wilks’ λ = 0.04290, F(10, 11) = 24.539, P < 0.000001]. Ten of the 15 factors (1, 3, 5, 10, 12, 2, 13, 14, 4, 8) were included in the discriminant function with six factors (1, 3, 5, 10, 12, 2) contributing significantly (Supplementary Table S2). This discriminant function classified all urine extracts of males and females correctly to their appropriate sex. The factor loadings on the first and most influential factor (factor 1, Eigenvalue 60.68, 15.8% variance explained) revealed five compounds to contribute importantly to the specificity of the male urine profile (Table 2). These were benzyl acetate and propionate, and three unknown compounds. Four of these compounds were never detected in female urine samples. In contrast, 42 compounds were identified as important volatile signatures in the female urine. We identified 22 of these compounds that belonged to various compound classes including short alcohols, fatty acids, esters or sulfur compounds. The remaining 20 compounds remained unknown. Despite being present in most female samples, however, the 42 compounds were not exclusively present in female urine.
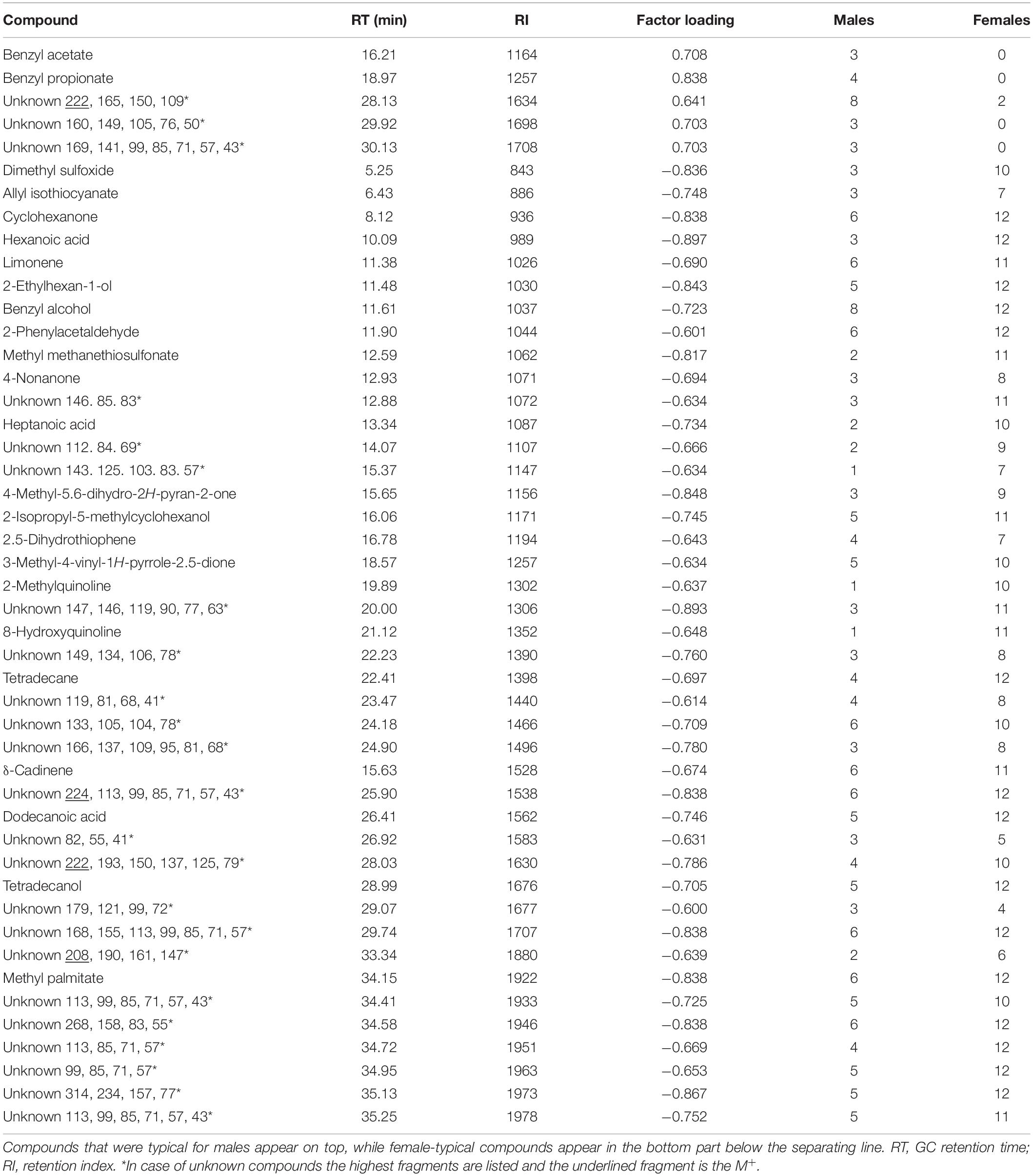
Table 2. Compounds of the urine profiles of male and female mouse lemurs that contributed substantially (factor loading <-0.6 or >+0.6 on the factor with the highest statistical contribution: Factor 1) to the discrimination of the sexes.
Eleven urine extracts of M. murinus and M. lehilahytsara, respectively, were analyzed to detect species-specific signatures in the urine constituents. Generally, there was no single compound that was systematically present in every sample of one species and absent in the other one. However, the DFA of the 15 first principal components provided a highly significant discriminant function [Wilks’ λ = 0.11607, F(7, 14) = 15.230, P < 0.000001]. Seven factors (2, 6, 5, 3, 10, 1, 15) were included in the function with four factors (2, 6, 5, 3) contributing significantly to the separation of the species (Supplementary Table S3). All urine extracts of M. murinus and M. lehilahytsara were assigned to the correct species by the DFA. The DFA revealed 15 compounds loading strongly onto the most influential factor 2 (Eigenvalue 43.52, 11.3% variance explained) which were characteristic for the M. murinus profile (Table 3). Ten of these compounds were specific for M. murinus, while the remainder occurred in M. lehilahytsara but in very low concentration. Nine of the fifteen compounds were identified mainly as ketones and aromatic amides, such as 3-octanone or 2-aminobenzamide. The DFA analysis further revealed seven compounds that were characteristic for the M. lehilahytsara profiles. However, all of these were unknown. Two of them were specific to M. lehilahytsara, while the remainder was only more abundant in this species than in M. murinus (Table 3).
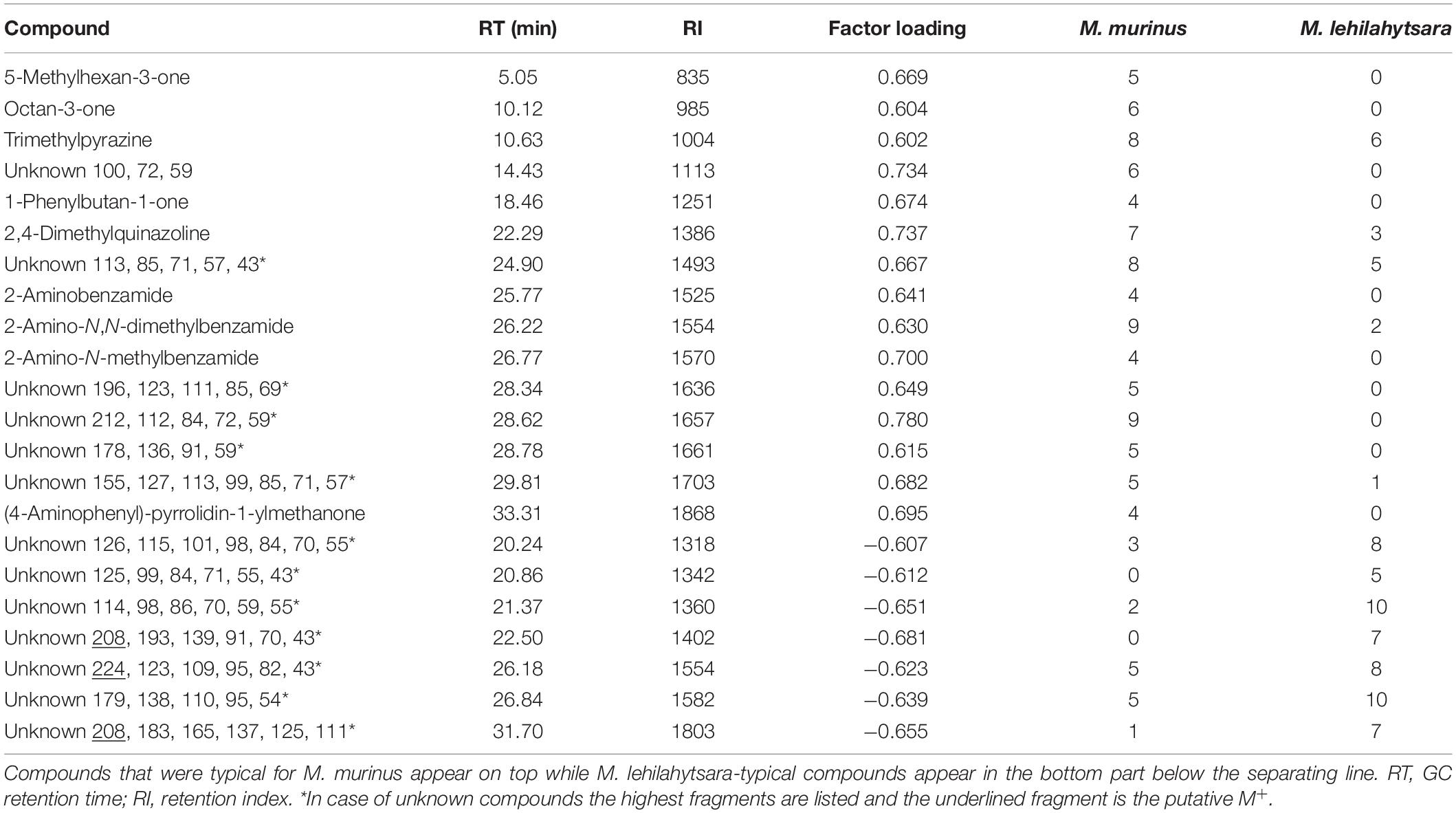
Table 3. Compounds of the urine profiles of M. murinus and M. lehilahytsara that contributed substantially (factor loading <-0.6 or >+0.6 on the factor with the highest statistical contribution: Factor 2) to the discrimination of the two species.
The large number of compounds detected by our method, over 900, suggests a very high potential of solvent extraction for compound identification and assessing signature mixtures. Even compounds not commonly detected by headspace methods, e.g., larger compounds with low vapor pressure such as cholesterol, or strongly H-bonding compounds such as amines or phenols could be successfully extracted. These compounds may potentially serve as olfactory signal components, and may be perceived e.g., by the vomeronasal organ or the main olfactory epithelium of the mouse lemurs. The effectiveness of solvent extraction is particularly evident when comparing with the previous study on urine volatiles of lemurs using solid phase dynamic extraction (SPDE) (delBarco-Trillo et al., 2011).
The large number of compounds present in most samples indicates the potential of urine to be used as scent mark by Microcebus spp. and might differentiate them from other genera, although there are so far no comparative results available from other lemur species obtained by the method used by us. In the study of delBarco-Trillo et al. (2011), SPDE-GC/MS was used to investigate the headspace volatiles of the urine of 12 lemur species. They reported 33 compounds as the highest number of urine compounds from one species. Benzaldehyde, nonanal, and decanal were present in almost every sample of the 12 species investigated.
These compounds were also present in almost all Microcebus samples investigated by us. Because of their common occurrence, it is not surprising that they were not diagnostic for sex or species in the DFA. In the headspace of the urine of Cheirogaleus medius, the only member of the Cheirogaleidae investigated so far, delBarco-Trillo et al. (2011) detected only 15 compounds. Some lemur-typical compounds like phenol, 4-methylphenol, or acetophenone were not detected. In contrast, these compounds were detected in every urine sample of the mouse lemurs investigated here. In general, the six compounds discussed in this paragraph are typical compounds of urine, not only in strepsirrhine primates but also in other mammals like rats, mice, cheetah or elephants (Burger et al., 2006; Röck et al., 2006; Osada et al., 2009). 4-Methylphenol and another widespread urinary compound also present in mouse lemur urine, cyclohexanone, are released from temporal gland secretions of elephants in larger amounts during musth, indicating a putative role as semiochemicals (Rasmussen and Perrin, 1999).
The comparison of the urine extracts of male and female mouse lemurs showed a significant difference between the sexes with females excreting significantly more compounds than males, although in males a wide variation in the number of compounds occurred. The total ion chromatograms (TIC) of males and females (Figure 1) also indicated that the concentration of the volatiles was higher in female samples. The statistical analyses further revealed that not only the number of compounds, but also the composition differed significantly between the sexes. The distinction of male and female urinary compounds is likely due to physiological and metabolic differences between the sexes, but it also suggests that females potentially convey more information via urine compared to males.
One of the very few compounds that were characteristic for males in our study were the two esters benzyl acetate and benzyl propionate (Table 2). Benzyl acetate is an attractant and pheromone for many Coleoptera and Hymenoptera, for example male euglossine bees (Willams and Whitten, 1983; Schiestl and Roubik, 2003). It seems not unlikely that these compounds may also play a role in the communication of mouse lemurs.
The analysis of the female mouse lemur urine profiles revealed a wide range of chemically different compounds to contribute to the specificity of their urine composition (Table 2). Some of these compounds are known from other mammals. Hexanoic acid was found in the gland secretions of L. catta (Knapp et al., 2006) and Old World monkeys (Mandrillus sphinx) (Setchell et al., 2010), in the urine of male rats (Osada et al., 2009) and male Bengal tigers (Burger et al., 2008; Osada et al., 2009), African wild dogs (Apps et al., 2012) and Iberian wolves (Martín et al., 2010; Apps et al., 2012). Heptanoic acid, dodecanoic acid, and 1-tetradecanol have also been identified in the urine of male Bengal tigers (Burger et al., 2008) and feces of the African Wild Dogs (Burger et al., 2008; Apps et al., 2012). Dodecanoic acid was reported from the labial glandular secretions of L. catta (Scordato et al., 2007) and the scent marks of female marmoset monkeys, Callithrix jacchus (Smith et al., 2001).
Our results are in contrast to those of delBarco-Trillo et al. (2011), who found no difference in the total number or relative abundance of compounds between male and female strepsirrhines. However, this may be due to the small number of samples they analyzed and the low overall number of compounds detected. In addition, the closest relative of the mouse lemurs, Cheirogaleus medius, was not analyzed under this aspect. Future comparative studies are needed to clarify if there is indeed a suite of compounds that is typical for all or most female lemur species or strepsirrhines and may then represent an ancestral communication signals, or whether they were largely derived during the rather recent radiation of the subclades, such as the genus Microcebus (Hotaling et al., 2016; Yoder et al., 2016). Future tests will be needed to evaluate the biological relevance of the discussed compounds for olfactory communication.
Microcebus murinus and Microcebus lehilahytsara can be clearly differentiated by the composition of the urine extracts. Both species showed a distinct urinary profile of compounds. Unfortunately, the structure of the compounds identified as being specific for M. lehilahytsara remain unknown (Table 2). Two of these compounds were specific for this species, while the other five compounds occurred occasionally in M. murinus as well (Table 3).
Fifteen compounds differentiated M. murinus from M. lehilahytsara. Within these two distinct compound classes prevailed, ketones and aromatic amides, and 10 compounds were specific for M. murinus (Table 3). A few of the identified compounds are known as potential semiochemicals in other mammals. For example, 3-octanone was also detected in the urine of the strepsirrhine Daubentonia madagascariensis (delBarco-Trillo et al., 2011) and the scent marks of Giant pandas, Ailuropoda melanoleuca (Hagey and MacDonald, 2003). 5-Methylhexan-3-one has been identified in the preorbital gland secretion of klipspringer, Oreotragus oreotragus (Burger et al., 1997) and the blue duiker, Cephalophus monicola (Burger and Pretorius, 1987; Burger et al., 1997). Trimethylpyrazine was detected in the scent marks of female marmoset monkeys, Callithrix jacchus (Smith et al., 2001) and in the urine of bobcats, Lynx rufus (Mattina et al., 1991) as well as maned wolves, Chrysocyon brachyurus (Mattina et al., 1991; Smith et al., 2001; Goodwin et al., 2013). 2,4-Dimethylquinazoline was reported from dorsal patches of males of the Curaçaoan long-nosed bat, Leptonycteris curasoae (Muñoz-Romo et al., 2012).
In conclusion, our findings show that urine of mouse lemurs contains many more volatile compounds than previously thought. Although samples were obtained from captive animals living under standardized diet and housing conditions (Wrogemann and Zimmermann, 2001), samples of the two species and the two sexes varied systematically in the composition of their compounds. However, not all variation must necessarily imply a communicative function. This is inherent in urine due to its function in the context as waste disposal. Moreover, the results indicate that potential signals or sex- and species-signatures hidden in the urine composition are very likely mixtures and do not consist of single or few compounds. The results of this study are complemented by a recent study on the protein content of the urine of both mouse lemur species (Unsworth et al., 2017). That study showed that some males of both species excrete high levels of WFDC12, an atypical member of the whey acidic protein family. This protein differs in one of 87 amino acids between the two mouse lemur species which may further contribute to olfactory species recognition in the context of sexual selection (Unsworth et al., 2017).
The number of samples in our study allowed only to investigate reliably whether differences in urine composition were detectable and differentiated between species or sex, but not both. However, it could be shown that male and female mouse lemurs and also both species have their own distinct chemical urine profile. Whether mouse lemurs actually use these signatures, however, can only be shown by extensive behavioral or physiological experiments with well-designed subsets of compounds. Olfactory discrimination between species has recently been shown with an operant conditioning paradigm in captive M. murinus and M. lehilahytsara (Kollikowski et al., 2019). The ability to discriminate species and sex based on urine signatures should be highly beneficial in the context of an efficient localization of potential mates, for mate choice, kin recognition and male-male-competition but also for other social behaviors like finding other members of the same sleeping group in the morning. It probably complements the acoustic signaling system in these species that has also been shown to contain individual-, group-, and species-specific signatures (Hafen et al., 1998; Braune et al., 2005, 2008; Leliveld et al., 2011). Future studies are needed to address the relative role of olfaction in multimodal signaling, the behavioral impact of urine and important compounds as well as the impact of seasonal changes and reproductive status on the urine composition of mouse lemurs.
All datasets generated for this study are included in the article/Supplementary Material.
All authors listed have made a substantial, direct and intellectual contribution to the work. JC, UR, and SS approved it for publication, in memory of EZ.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the Institute of Zoology and its animal facility for providing the urine samples for this study. They particularly acknowledge the technical help of Sarah Hohenbrink, Lisabelle Früh, Iris Grages, and Johanna Samtlebe during sample collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00158/full#supplementary-material
Apps, P., Mmualefe, L., and McNutt, J. W. (2012). Identification of volatiles from the secretions and excretions of African wild dogs (Lycaon pictus). J. Chem. Ecol. 38, 1450–1461. doi: 10.1007/s10886-012-0206-7
Apps, P. J., Weldon, P. J., and Kramer, M. (2015). Chemical signals in terrestrial vertebrates: search for design features. Nat. Prod. Rep. 32, 1131–1153. doi: 10.1039/C5NP00029G
Booth, W. D. (1987). Factors affecting the pheromone composition of voided boar saliva. J. Reprod. Fertil. 81, 427–431. doi: 10.1530/jrf.0.0810427
Boulet, M., Charpentier, M. J. E., and Drea, C. M. (2009). Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9:281. doi: 10.1186/1471-2148-9-281
Braune, P., Schmidt, S., and Zimmermann, E. (2005). Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): the role of olfactory and acoustic signals. Behav. Ecol. Sociobiol. 58, 587–596. doi: 10.1007/s00265-005-0944-4
Braune, P., Schmidt, S., and Zimmermann, E. (2008). Acoustic divergence in the communication of cryptic species of nocturnal primates (Microcebus ssp.). BMC Biol. 6:19. doi: 10.1186/1741-7007-6-19
Brennan, P. A., and Kendrick, K. M. (2006). Mammalian social odours: attraction and individual recognition. Philos. Trans. R. Soc. Lond. B 361, 2061–2078. doi: 10.1098/rstb.2006.1931
Burger, B. V., and Pretorius, P. J. (1987). Mammalian pheromone studies, VI. Compounds from the preorbital gland of the Blue Duiker, Cephalophus monticola. Z. Naturforsch. 42c, 1355–1357. doi: 10.1515/znc-1987-11-1238
Burger, B. V., Visser, R., Moses, A., and Le Roux, M. (2006). Elemental sulfur identified in urine of cheetah, Acinonyx jubatus. J. Chem. Ecol. 32, 1347–1352. doi: 10.1007/s10886-006-9056-5
Burger, B. V., Viviers, M. Z., Bekker, J. P. I., Le Roux, M., Fish, N., Fourie, W. B., et al. (2008). Chemical characterization of territorial marking fluid of male Bengal Tiger, Panthera tigris. J. Chem. Ecol. 34, 659–671. doi: 10.1007/s10886-008-9462-y
Burger, B. V., Yang, T.-P., Le Roux, M., Brandt, W. F., Cox, A. J., and Hart, P. F. (1997). Mammalian exocrine secretions XI. Constituents of the preorbital secretion of Klipspringer, Oreotragus oreotragus. J. Chem. Ecol. 23, 2383–2400. doi: 10.1023/B:JOEC.0000006681.33646.f4
delBarco-Trillo, J., Burkert, B. A., Goodwin, T. E., and Drea, C. M. (2011). Night and day: the comparative study of strepsirrhine primates reveals socioecological and phylogenetic patterns in olfactory signals. J. Evol. Biol. 24, 82–98. doi: 10.1111/j.1420-9101.2010.02145.x
Evans, C., and Schilling, A. (eds) (1995). The Accessory (Vomeronasal) Chemoreceptor System in Some Prosimians. New York, NY: Plenum Press.
Glatston, A. R. (ed.) (1983). Olfactory Communication in the Lesser Mouse Lemur (Microcebus murinus). New Delhi, IN: Today & Tomorrow’s printers & publishers.
Goodwin, T. E., Songsasen, N., Broederdorf, L. J., Burkert, B. A., Chen, C. J., Jackson, S. R., et al. (2013). “Hemiterpenoids and pyrazines in the odoriferous urine of the Maned Wolf (Chrysocyon brachyurus),” in Chemical Signals in Vertebrates 12, eds M. L. East and M. Dehnhard (New York, NY: Springer), 171–184. doi: 10.1007/978-1-4614-5927-9_13
Hafen, T., Neveu, H., Rumpler, Y., Wilden, I., and Zimmermann, E. (1998). Acoustically dimorphic advertisement calls separate morphologically and genetically homogenous populations of the grey mouse lemur (Microcebus murinus). Folia Primatol. 69(Suppl. 1), 342–356. doi: 10.1159/000052723
Hagey, L., and MacDonald, E. (2003). Chemical cues identify gender and individuality in Giant Pandas (Ailuropoda melanoleuca). J. Chem. Ecol. 29, 1479–1488. doi: 10.1023/A:1024225806263
Hayes, R. A., Morelli, T. L., and Wright, P. C. (2004). Anogenital gland secretions of Lemur catta and Propithecus verreauxi coquereli : a preliminary chemical examination. Am. J. Primatol. 63, 49–62. doi: 10.1002/ajp.20038
Hohenbrink, P., Dempewolf, S., Zimmermann, E., Mundy, N. I., and Radespiel, U. (2014). Functional promiscuity in a mammalian chemosensory system: extensive expression of vomeronasal receptors in the main olfactory epithelium of mouse lemurs. Front. Neuroanat. 8:102. doi: 10.3389/fnana.2014.00102
Hohenbrink, P., Mundy, N. I., Zimmermann, E., and Radespiel, U. (2013). First evidence for functional vomeronasal 2 receptor genes in primates. Biol. Lett. 9:20121006. doi: 10.1098/rsbl.2012.1006
Hohenbrink, P., Radespiel, U., and Mundy, N. I. (2012). Pervasive and ongoing positive selection in the vomeronasal-1 receptor (V1R) repertoire of mouse lemurs. Mol. Biol. Evol. 29, 3807–3816. doi: 10.1093/molbev/mss188
Hotaling, S., Foley, M. E., Lawrence, N. M., Bocanegra, J., Blanco, M. B., Rasoloarison, R., et al. (2016). Species discovery and validation in a cryptic radiation of endangered primates: coalescent-based species delimitation in Madagascar’s mouse lemurs. Mol. Ecol. 25, 2029–2045. doi: 10.1111/mec.13604
Kappel, P., Hohenbrink, S., and Radespiel, U. (2011). Experimental evidence for olfactory predator recognition in wild mouse lemurs. Am. J. Primatol. 73, 928–938. doi: 10.1002/ajp.20963
Knapp, L. A., Robson, J., and Waterhouse, J. S. (2006). Olfactory signals and the MHC: a review and a case study in Lemur catta. Am. J. Primatol. 68, 568–584. doi: 10.1002/ajp.20253
Kollikowski, A., Zimmermann, E., and Radespiel, U. (2019). First experimental evidence for olfactory species discrimination in two nocturnal primate species (Microcebus lehilahytsara and M. murinus). Sci. Rep. 9:20386. doi: 10.1038/s41598-019-56893-y
Leliveld, L. M. C., Scheumann, M., and Zimmermann, E. (2011). Acoustic correlates of individuality in the vocal repertoire of a nocturnal primate (Microcebus murinus). J. Acoust. Soc. Am. 129, 2278–2288. doi: 10.1121/1.3559680
Ma, W., Miao, Z., and Novotny, M. V. (1999). Induction of estrus in grouped female mice (Mus domesticus) by synthetic analogues of preputial gland constituents. Chem. Senses 24, 289–293. doi: 10.1093/chemse/24.3.289
MacDonald, E. A., Fernandez-duque, E., Evans, S., and Hagey, L. R. (2008). Sex, age, and family differences in the chemical composition of owl monkey (Aotus nancymaae) subcaudal scent secretions. Am. J. Primatol. 70, 12–18. doi: 10.1002/ajp.20450
Martín, J., Barja, I., and López, P. (2010). Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus). Biochem. Syst. Ecol. 38, 1096–1102. doi: 10.1016/j.bse.2010.10.014
Mattina, M. J. I., Pignatello, J. J., and Swihart, R. K. (1991). Identification of volatile components of bobcat (Lynx rufus) urine. J. Chem. Ecol. 17, 451–462. doi: 10.1007/BF00994344
Müller-Schwarze, D. (2006). Chemical Ecology of Vertebrates. Cambridge, MA: Cambridge University Press.
Muñoz-Romo, M., Nielsen, L. T., Nassar, J. M., and Kunz, T. H. (2012). Chemical composition of the substances from dorsal patches of males of the curacaoan long-nosed bat, Leptonycteris curasoae (Phyllostomidae: Glossophaginae). Acta Chiropt. 14, 213–224. doi: 10.3161/150811012X654411
Osada, K., Kashiwayanagi, M., and Izumi, H. (2009). Profiles of volatiles in male rat urine: the effect of puberty on the female attraction. Chem. Senses 34, 713–721. doi: 10.1093/chemse/bjp058
Perret, M. (ed.) (1995). Chemocommunication in the Reproductive Function of Mouse Lemurs. New York: Plenum Press.
R Core Team (2015). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radespiel, U. (2016). “Can behavioral ecology help to understand the divergent geographic range sizes of mouse lemurs?,” in The Dwarf and Mouse Lemurs of Madagascar, eds S. M. Lehman, U. Radespiel, and E. Zimmermann (Cambridge: Cambridge University Press), 498–519. doi: 10.1017/cbo9781139871822.027
Rasmussen, L. E. L., and Perrin, T. E. (1999). Physiological correlates of musth. Physiol. Behav. 67, 539–549. doi: 10.1016/S0031-9384(99)00114-6
Röck, F., Mueller, S., Weimar, U., Rammensee, H.-G., and Overath, P. (2006). Comparative analysis of volatile constituents from mice and their urine. J. Chem. Ecol. 32, 1333–1346. doi: 10.1007/s10886-006-9091-2
Schiestl, F. P., and Roubik, D. W. (2003). Odor compound detection in male euglossine bees. J. Chem. Ecol. 29, 253–257. doi: 10.1023/A:1021932131526
Schilling, A., Perret, M., and Predine, J. (1984). Sexual inhibition in a prosimian primate: a pheromone-like effect. J. Endocrinol. 102, 143–151. doi: 10.1677/joe.0.1020143
Scordato, E. S., and Drea, C. M. (2007). Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim. Behav. 73, 301–314. doi: 10.1016/j.anbehav.2006.08.006
Scordato, E. S., Dubay, G., and Drea, C. M. (2007). Chemical composition of scent marks in the Ringtailed Lemur (Lemur catta): Glandular differences, seasonal variation, and individual signatures. Chem. Senses 32, 493–504. doi: 10.1093/chemse/bjm018
Setchell, J. M., Vaglio, S., Moggi-Cecchi, J., Boscaro, F., Calamai, L., and Knapp, L. A. (2010). Chemical composition of scent-gland secretions in an old world monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses 35, 205–220. doi: 10.1093/chemse/bjp105
Smith, T. E., Tomlinson, A. J., Mlotkiewicz, J. A., and Abbott, D. H. (2001). Female marmoset monkeys (Callithrix jacchus) can be identified from the chemical composition of their scent marks. Chem. Senses 26, 449–458. doi: 10.1093/chemse/26.5.449
Sündermann, D., Scheumann, M., and Zimmermann, E. (2008). Olfactory predator recognition in predator-naïve gray mouse lemurs (Microcebus murinus). J. Comp. Psychol. 122, 146–155. doi: 10.1037/0735-7036.122.2.146
Unsworth, J., Loxley, G. M., Davidson, A., Hurst, J. L., Gómez-Baena, G., Mundy, N. I., et al. (2017). Characterisation of urinary WFDC12 in small nocturnal basal primates, mouse lemurs (Microcebus spp.). Sci. Rep. 7:42940. doi: 10.1038/srep42940
Whitten, W. K., Bronson, F. H., and Greenstein, J. A. (1968). Estrus-inducing pheromone of male mice: transport by movement of air. Science 161, 584–585. doi: 10.1126/science.161.3841.584
Willams, N. H., and Whitten, W. M. (1983). Orchid floral fragences and male euglossine bees: methods and advances in the last sesquidecade. Biol. Bull. 164, 355–395. doi: 10.2307/1541248
Wrogemann, D., and Zimmermann, E. (2001). Aspects of reproduction in the eastern rufous mouse lemur (Microcebus rufus) and their implications for captive management. Zoo Biol. 20, 157–167. doi: 10.1002/zoo.1017
Wrogemann, D., Radespiel, U., and Zimmermann, E. (2001). Comparison of reproductive characteristics and changes in body weight between captive populations of rufous and gray mouse lemurs. Internatl. J. Primatol. 22, 91–108. doi: 10.1023/A:1026418132281
Keywords: GC/MS, volatiles, pheromones, olfaction, mouse lemur, species differentiation, infochemicals
Citation: Caspers J, Radespiel U, Zimmermann E and Schulz S (2020) Volatile Urinary Signals of Two Nocturnal Primates, Microcebus murinus and M. lehilahytsara. Front. Ecol. Evol. 8:158. doi: 10.3389/fevo.2020.00158
Received: 15 November 2019; Accepted: 07 May 2020;
Published: 27 May 2020.
Edited by:
Stefano Colazza, University of Palermo, ItalyReviewed by:
Javier delBarco-Trillo, University College Cork, IrelandCopyright © 2020 Caspers, Radespiel, Zimmermann and Schulz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Schulz, U3RlZmFuLnNjaHVsekB0dS1icy5kZQ==
†Deceased
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.