- Station d’Ecologie Théorique et Expérimentale du CNRS (UMR5321), Moulis, France
Cognition has evolved to allow organisms to process, use and store information in their natural environment. Yet, cognitive abilities are traditionally measured in controlled laboratory conditions to obtain consistent and accurate measurements. Consequently, little is known about the actual effect of natural environmental variation on cognitive performances. Being able to modify cognitive performance according to environmental conditions (e.g., plasticity of attentional performances according to current predator densities) could provide evolutionary advantages. In this mini-review, we compile evidence for what we call “cognitive performance plasticity” (i.e., flexible adjustment of cognitive performance in response to the current environment). We then discuss methodological approaches associated with measurement of cognitive performance plasticity and cognition in general. Finally, we discuss the implications of acknowledging plasticity in cognitive performance, including a better understanding of the reproducibility crisis observed in cognitive sciences (Open Science Collaboration, 2015) and new lines of inquiry into the evolution of cognition and the adaptive value of cognitive performance plasticity.
Why Consider Context in Cognitive Sciences?
The recent growing interest of cognitive ecologists in an evolutionary ecology of cognition (Cauchoix and Chaine, 2016; Morand-Ferron et al., 2016; Boogert et al., 2018) and the emergence of environmental psychology (Stern, 2000; Sörqvist, 2016) raises the question of how current social or ecological conditions influence measures of cognitive performance. Whereas the impact of early environmental conditions on the development of cognitive abilities has recently been examined (Ebbesson and Braithwaite, 2012; Buchanan et al., 2013; Murphy et al., 2014; Ashton et al., 2018), we still know little about the effect of ongoing environmental variation on cognitive performance. Indeed, cognitive scientists traditionally conduct tests in controlled laboratory environments with homogenous social and physical contexts (Fize et al., 2011; Roitblat, 2014). By contrast, natural environments where cognitive traits have evolved are complex and dynamic such that cognitive performances recorded in one setting might not describe all possible expressions of the cognitive ability of interest but only its expression in that particular setting (Figure 1A).
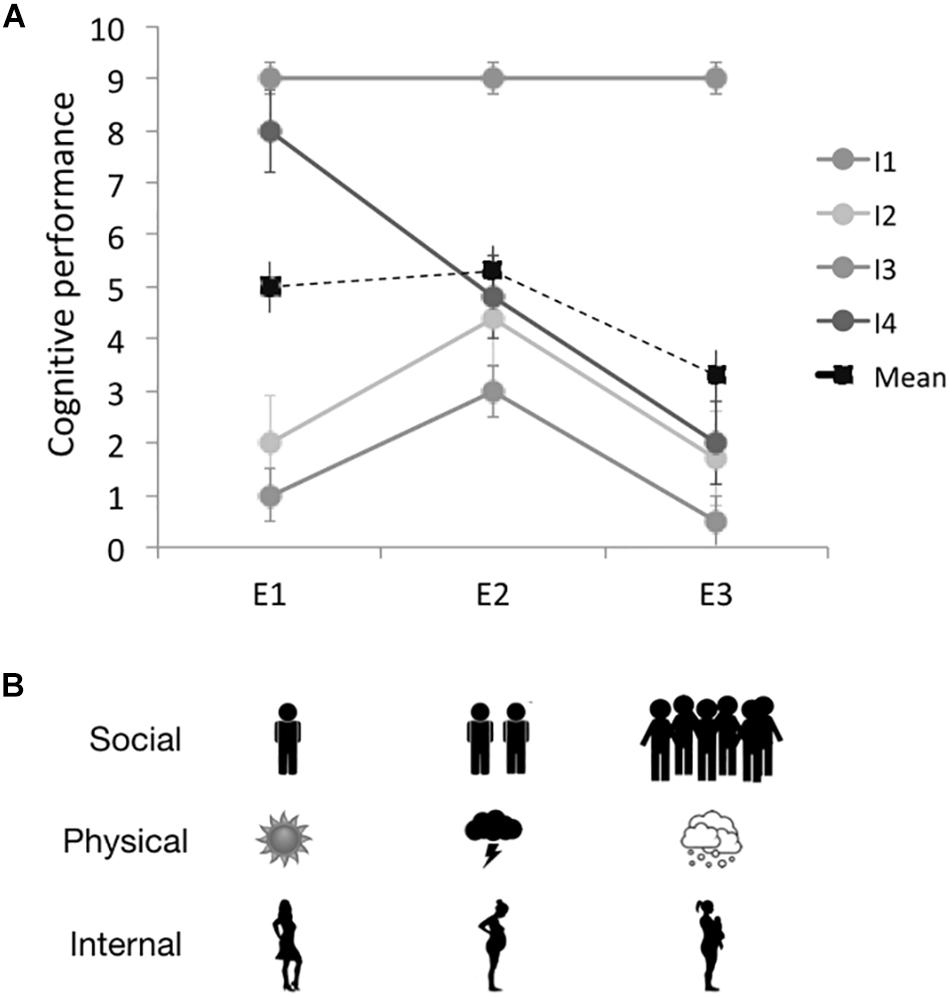
Figure 1. Examples of reaction norms for plasticity in cognitive performance elicited by current environmental factors. (A) Cognitive performance from each of four individuals (I1–4) and the population response (Mean) as a function of three different environmental conditions (E1, E2, and E3). Vertical lines represent standard errors across trials on the same individual in the same environment and highlights the need for repeated measurements of cognition for each individual in each environment. If individuals are tested in E2 only, little variation in cognitive performances would be detected among individuals. If a different lab tests individuals in a similar, but not identical environment (e.g., E3), there would be a clear lack of correspondence in results contributing to the reproducibility crisis at both the individual and population levels. (B) Examples of environmental factors that might influence cognitive performance including social factors (e.g., number of observers/group size), physical factors (e.g., weather/season) and internal factors (i.e., reproductive status/hormonal state).
Laboratory conditions during testing might thus only represent one of many possible environments, or worse, be out of the range of natural environments for a given species (Niemelä and Dingemanse, 2014) such as isolating gregarious species, increasing stress levels by taking individuals in captivity, or removing predation risk for prey species. Consequently, cognitive performance recorded under such conditions may not reflect cognitive performance expressed in nature (Niemelä and Dingemanse, 2014; McCune et al., 2019; but see Cauchoix et al., 2017). Furthermore, individuals might differ in their response to laboratory testing conditions if, for example, there are individual differences in response to stress (Ebner and Singewald, 2017).
An alternative to highly controlled laboratory settings is to acknowledge that cognitive performance might vary with environmental conditions and to measure and report those variations (i.e., cognitive performance plasticity). Therefore, instead of testing an individual in one standardized condition, we could measure an individual’s cognitive performance in different natural (or artificial) conditions (e.g., social vs. non-social, temperature gradient, etc.) to take into account plastic responses at the individual and population levels (Voelkl and Würbel, 2016; Barragan-Jason et al., 2018a; Caza and Atance, 2018). Such an approach would not only enable a more accurate measure of cognitive abilities, teasing out repeatable and plastic components (Dingemanse et al., 2010), but could also help us better understand the adaptive value of plastic cognitive responses. Plasticity in cognitive performance follows the concept of phenotypic plasticity in evolutionary biology which describes how a given genotype expresses different phenotypes under different environmental conditions (Bradshaw, 1965; Pigliucci, 2001; West-Eberhard, 2003). Plasticity in cognitive performance could borrow the conceptual framework from the field of “behavioral plasticity” which refers to the ability to flexibly adjust a behavior in response to environmental variations (e.g., temperature, elevation, etc; Dingemanse et al., 2010) including the notion of “social competence” which refers to the ability to flexibly adjust a behavior to best match a given social context (Taborsky and Oliveira, 2013). We believe that by focusing on plasticity in cognitive performance, rather than trying to limit variation during measurement of cognitive performance, we stand to gain a much richer understanding of cognition as a whole, how it is used in natural environments, and how it evolves under natural selection.
Evidence for Plasticity in Cognitive Performance
A number of different environmental factors could cause shifts in cognitive performance for the same individual or genotype. We provide examples below divided into three broad categories - social context, physical environment, and internal factors - as a first step in synthesizing and then better understanding plasticity in cognitive performance (Figure 1B).
The effect of the current social context on cognitive performance includes some of the best documented examples of plasticity in cognitive performance (Figure 1B). For instance, nine-spined sticklebacks engaged in a social learning task are more likely to copy the foraging choice of the larger of two groups they observe (Pike and Laland, 2010). Indeed, across a broad variety of species from insects to humans, the age, sex, size and social status of demonstrators also influence social learning (Rendell et al., 2011). Similarly, partner characteristics in chimpanzees (Suchak et al., 2018) and the social environment (presence or absence of female observers) in humans (Tognetti et al., 2016; Kelsey et al., 2018) greatly influences performance in cooperative and altruistic tasks. Less intuitively, social context can also modify performance in non-social cognitive tasks. For example, group size (number of individuals present on the site) seems to affect the efficiency of passerines engaged in a problem solving task in the wild (Chabaud et al., 2009; Morand-Ferron and Quinn, 2011) as well as success in a spatial discrimination task (Langley et al., 2018) in pheasants. In human children, the presence of an experimenter in a testing room modulates the expression of self-control (Barragan-Jason et al., 2018b). Similarly, the presence of conspecifics in baboons affects performance in a cognitive control task (Huguet et al., 2014). While most of the above studies contrast groups of individuals placed in each context, the last three (pheasants, children, baboons) specifically tested the same individuals in two different social contexts and clearly demonstrate how current/ongoing environmental variables can modify cognitive performance at the individual level (Huguet et al., 2014; Barragan-Jason et al., 2018b; Langley et al., 2018).
The current physical environment can also modulate cognitive performance (Figure 1B). Weather, pressure and temperature can affect working memory performance in humans (Keller et al., 2005) and mate choice copying in fruit flies (Dagaeff et al., 2016). Similarly, exposure to extreme environments (e.g., heat, hypoxia, and cold stress) impacts the expression of a number of cognitive functions in humans (Taylor et al., 2015). The emerging field of environmental psychology attests to growing interest in how the environment impacts cognitive performance (Stern, 2000; Sörqvist, 2016). One striking result from this new literature is that in humans, experiencing nature seems to almost instantaneously affect learning performances (Kuo et al., 2019). Changes in the physical environment of an area due to season can likewise influence cognitive performance. Seasonal plasticity in bird brains and in particular in the adult song system has been known for decades (Tramontin and Brenowitz, 2000). Increases in song rate during spring when males need to attract mates and defend territories is supported by important plasticity in neural nuclei involved in song production (Ball et al., 2004). New evidence suggests that brain activity related to executive function (i.e., working memory) tasks is also affected by season in humans (Meyer et al., 2016). Similarly, season modulates attention and spatial performance in african striped mouse (Maille et al., 2015). At a finer time scale, time of day can affect learning and memory performance in rats (Winocur and Hasher, 1999).
Internal factors, such as health, reproductive status, stress, motivation, mood, and hunger among other possibilities could also dramatically affect cognitive performance (Figure 1B). The impact of motivation on cognitive performance has been a preoccupation of cognitive scientists for decades (Padmala and Pessoa, 2010; Morand-Ferron et al., 2016;, Cauchard et al., 2017). For example, short-term fasting decreases psychomotor speed and executive functioning in humans (Sansone and Harackiewicz, 2000; Benau et al., 2014). On the contrary, short-term high fat food intake can deteriorate performance of rats in a maze test (Murray et al., 2009). Effects of stress on learning, memory, and cognitive flexibility is also well documented (Roozendaal et al., 2009; Schwabe et al., 2012; Seehagen et al., 2015; Goldfarb et al., 2017). Infection may also affect cognitive performance in humans (Boivin and Giordani, 1993; Kihara et al., 2006), mice (Desruisseaux et al., 2008), and birds (Dunn et al., 2011). Other internal factors such as reproductive status and hormonal levels modify both cognitive performance and neurophysiological activity (Figure 1B; Buckwalter et al., 1999; O’Reilly et al., 2004; Amin et al., 2006; Little, 2013; Sundström Poromaa and Gingnell, 2014). Finally, exposure to hazardous chemicals can also have important effects on cognition. For instance, acute exposure to even a low dose of pesticides directly impairs working memory in bees (Samuelson et al., 2016).
It is important to acknowledge that during cognitive testing all these environmental factors, grouped here in social, physical and internal categories, are likely to play a role in cognitive performance (Morand-Ferron et al., 2016). Furthermore, these different factors might also interact and produce different effects on cognition depending on their combination. For instance, the effect of weather on cognition is season dependent in humans (Keller et al., 2005). Positive relationships between higher temperature or barometric pressure (i.e., pleasant weather) on memory (i.e., digit span) modulated by the time spent outdoors only holds during the spring. In fact, the testing environment will always contain a specific value for each environmental category and most if not all combinations are possible. While understanding the simple effects of a given factor should be an initial goal in studies of plasticity in cognitive performance, we can already begin thinking about the more complex experimental designs needed to look for interactive effects of two or more environmental factors on cognitive performance.
Methods to Measure Plasticity in Cognitive Performance
In order to understand the causes and consequences of individual differences in cognitive abilities, cognitive ecologists are increasingly interested in measuring cognitive performance of animals directly in their natural environment (Figure 2A; Pritchard et al., 2016; Cauchoix et al., 2017; Boogert et al., 2018). Such an approach is ideal to evaluate how natural variation in the environment affects cognitive performance (Morand-Ferron and Quinn, 2011) but it requires a large number of repeated measurements on given individuals across contexts, which is challenging. The development of new technologies for automated and voluntary testing of cognitive abilities on free-ranging animals directly in their natural environment (Figure 2A) now allows us to record large numbers of trials for individuals in the wild (Fagot and Bonté, 2010; Gazes et al., 2013; Morand-Ferron et al., 2015; Cauchoix et al., 2017; Sonnenberg et al., 2019), although some bias may still exist in which individuals choose to participate. Moreover, the same kind of RFID (radio frequency identification) testing device (Figure 2A) which automatically identifies an individual during testing can be used to measure fine grained social interactions of free ranging birds and infer social structure or dominance hierarchy (Aplin et al., 2012; Evans et al., 2018). Such systems could be coupled with environmental sensors that constantly monitor physical environmental variables ranging from simple meteorological variables to air pollution (Ripoll et al., 2019) or even sensors to monitor individual weight as a proxy for condition (Larios et al., 2013; Hou et al., 2015). Together, these types of data provide us with repeated measures of cognitive performance for given individuals under a broad array of social and environmental settings which can then be used to understand plasticity in cognitive performance using the “behavioural reaction norm” approach (Dingemanse et al., 2010; Morand-Ferron et al., 2016). A similar approach in human cognitive psychology could use real-time tests and environmental sensors on smartphones (Dufau et al., 2011; Harari et al., 2017).
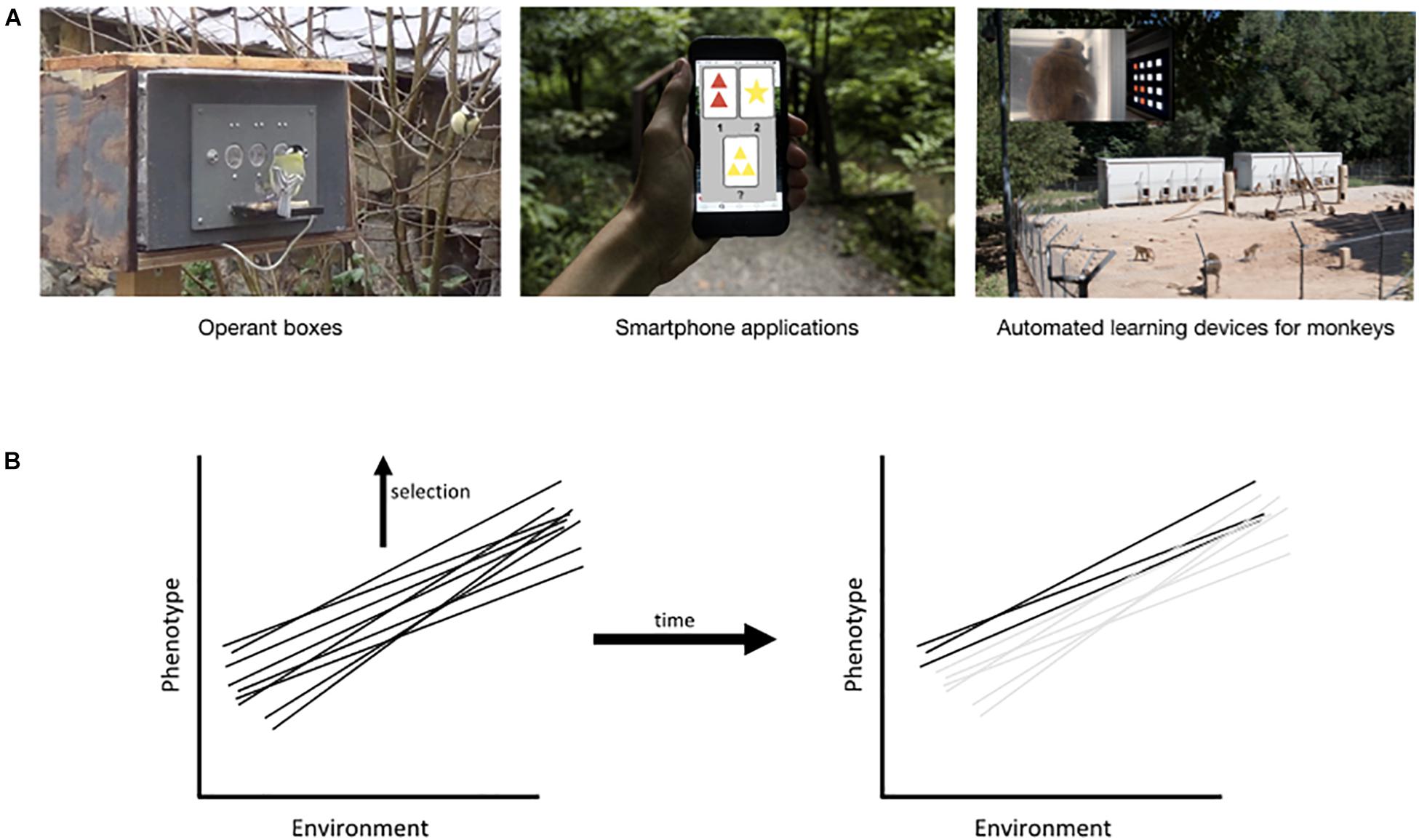
Figure 2. Methods to study plasticity in cognitive performance and its adaptive value (i.e., evolution of reaction norms). (A) Automated cognitive testing devices can gather repeated data from the same individual in natural contexts. Examples include operant boxes to study associative and reversal learning in wild birds (Morand-Ferron et al., 2015; Cauchoix et al., 2017), smartphone applications for cognitive tasks (e.g., executive function task), and automated learning devices for monkeys: ALDM (Fagot and Bonté, 2010; Claidière et al., 2017). (B) Plasticity can then be described by linear (or non-linear; e.g., Figure 1A) reaction norms. Selection can act on phenotypically plastic traits just as with normal traits except that selection will act on the reaction norm rather than a specific trait over the long term. Here we illustrate a population of genotypes that show a plastic expression of the phenotype across different environments (each line), and if selection favors increased values of the phenotype, the reaction norms that produce lower phenotypes will be eliminated over time. Note that selection can act primarily in one environment and therefore on the phenotype expressed in that environment, regardless of expression in other environments. Under such conditions, selection could be less efficient or slower in causing population change. In the case of cognition, the variety of expressions possible for an organism that is frequently in different environments could cause selection to act much more quickly on the shape of the reaction norm rather than exclusively on expression in one environment.
The correlational nature of such measurements in the wild do, however, have some limitations that could be complemented with experiments conducted in the laboratory or large semi-wild enclosures under controlled settings. Measurements of free ranging animals in the wild described above make it difficult to infer causality and may make it impossible to test independent effects of each environmental gradient. In such cases, controlled experiments that manipulate the social, physical or internal environment in the lab, as initially developed for rodent studies in biomedical research (Turner and Burne, 2013), could provide a complementary understanding. Virtual reality (Schoeller et al., 2018) or video playbacks (Snijders et al., 2016; Smit and van Oers, 2019) could provide an ideal tool to document responsiveness of cognitive performance to artificially controlled contexts in the lab. Other methods could include testing the same individual in a few different controlled environments by manipulating physical features (e.g., temperature), ecological features (e.g., predation risk), social context (e.g., group size), or internal state (e.g., hormonal manipulations) in the lab. Such experimental manipulation in the wild may also be possible. For instance, Cauchard et al. (2016) manipulated infection status of nesting great tits to evaluate the impact of parasitism on problem-solving performances. Finally, controlled experiments are the only way to understand how different contexts interact to influence cognitive performance through fully factorial designs crossing multiple levels of each environmental feature. Such experiments are ambitious in scale, but would provide us with a unique understanding of whether each environment has additive effects on cognition or complex non-linear impacts.
In practice, measuring plasticity in cognitive performance implies measuring one cognitive ability (e.g., attention, memory, etc.) in a few different contexts (Figures 1, 2B). Such contexts can be different categories (i.e., in presence or absence of a predator) or contexts that vary quantitatively in a specific dimension (e.g., temperature, group size; Figure 1B). Sample sizes depend on the goals of the project, but linear descriptions of plasticity, called reaction norms (Dingemanse et al., 2010), require multiple measures of each individual in each testing environment, which can be a challenge (Martin et al., 2011). For example, a study including 3 environments might ideally have 3 or more measures for each individual in each environment to control for the effects of noise or measurement error, implying more than 9 tests per individual. Such standards might be possible to reach for cognitive tasks in which individuals usually go through a high number of trials (Fagot and Bonté, 2010; Morand-Ferron et al., 2015) and often perform successive cognitive tasks (Cauchoix et al., 2017). Data from either observational or experimental approaches can then be analyzed much like behavioral plasticity using a mixed-model approach borrowed from quantitative genetics to partition phenotypic variation into its between-individual and within-individuals components (Dingemanse et al., 2010; Dingemanse and Dochtermann, 2013). This so called ‘behavioral reaction norm’ approach allows us to disentangle measures of individual consistency (personality or repeatability) and responsiveness to the environment (plasticity; Figures 1A, 2B). We recently used this approach to document the repeatability of individual differences in cognitive performances (Cauchoix et al., 2018) but it has not been applied yet to document plasticity in cognitive performance within a population. A powerful feature of this approach is that inclusion of a random slope in the mixed model allows individuals to vary differently according to environmental conditions which generates a description of plasticity for each individual (Figure 1A). The slope or shape of these individual reaction norms provides us with a trait - plasticity in cognitive performance - that can then be related to other features such as success later in life (Figure 2B; for a discussion of the benefits and challenges of this approach, see Houslay and Wilson, 2017).
Implications and Future Directions
If plasticity in cognitive performance is more than just noise as we have argued here, then directly studying it could provide new insight into past findings and would open whole new lines of research in cognitive sciences and cognitive ecology. For example, plasticity in cognitive performance could help resolve the reproducibility crisis in psychological sciences through an understanding of environmental variables that might generate differences between studies (Van Bavel et al., 2016; Voelkl and Würbel, 2016). Indeed, although recent studies report that some factors including contextual sensitivity (i.e., variations in time, culture and location) influence reproducibility, the effect of many other environmental factors on cognitive performance still need to be tested (Voelkl and Würbel, 2016). In addition, we still do not know how often cognitive performance is actually sensitive to the environment (i.e., plastic), if some cognitive abilities are more sensitive than others, and to what degree individuals vary in cognitive performance plasticity. While direct study of this phenomena would be ideal, systematically recording and reporting environmental variables in cognitives studies would enable future meta-analyses to answer such questions on a large scale.
If such plasticity is common, as we believe it is, there are important implications for the evolution of cognition. There is now a growing interest in linking individual variation in cognitive performance to fitness in wild non-human populations (Boogert et al., 2018), but plasticity in cognitive performance would modify our view of how selection acts on cognitive traits. Under what social and ecological conditions do we expect to see the evolution of plastic cognitive performance rather than fixed, invariant performance? The degree of plasticity in a population depends primarily on how stable the environment is and whether there are reliable cues to make plastic adjustment advantageous (Pigliucci, 2005). Variable environments with predictable cues to trigger plastic expression will favor high plasticity whereas stable environments or a lack of reliable cues will favor fixed phenotypic expression. Similarly, we might expect that the benefits of plastic modification of cognitive performance will depend on natural environmental variation which make it adaptive or maladaptive (Greggor et al., 2019). For instance, being able to modify attentional performance according to level of predation risk that can vary among seasons, time of day, and foraging areas, would enable an individual to allocate more time to feeding rather than vigilance (Lima and Bednekoff, 1999).
Furthermore, selection on plastic cognitive performance would act on reaction norms (Figure 2B) rather than the mean trait which can have important implications for evolutionary trajectories (Price et al., 2003; Duckworth, 2009). A stable environment will lead to low plasticity since selection will act on expression in one environment rather than the whole reaction norm which can lead to canalization of the trait in the long term. However, if the environment is naturally variable, then plasticity can be preserved as selection acts on different expressions of the phenotype in each environment and therefore on the shape of reaction norms rather than the mean. Likewise, plasticity will be preserved only if shifting the phenotypic expression provides a good match to the given environment and this match depends critically on reliable environmental cues to generate the correct phenotypic expression. These considerations will have important consequences for the role that cognition plays in adaptation to new environments including both colonization and climate change (Chevin et al., 2010; Chevin and Hoffmann, 2017). If the prior environment was variable, there might be sufficient plasticity to allow a decent fit to the new environment if the environmental cues triggering phenotypic expression are still appropriate (Lyon et al., 2008). In this case, plasticity could buffer against the negative effects of a new environment relative to a fixed phenotype, but will also mean the population takes longer to adapt to that new environment since selection on a reaction norm is generally thought to be weaker (Forsman, 2015).
Ignoring plasticity in cognition also carries costs such as concluding that there is low within population variation in cognitive performance (E2 in Figure 1A) or incorrectly describing evolutionary dynamics on plastic traits (Chaine and Lyon, 2008). In contrast, adopting a plasticity perspective adds complexities to experimental protocols, but has no influence on interpretation when traits are fixed. Given the potential for new insights into both cognition and ecology, we believe that a shift in perspective to plastic rather than fixed cognitive performance is critical.
Author Contributions
All authors have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the Agence Nationale pour la Recherche (ANR-18-CE02-0023 “SoCo”), and Human Frontiers Science Program (HFSP; RGP 0006/2015 “WildCog”) and is part of the Laboratoire d’Excellence (LABEX) entitled TULIP (ANR-10-LABX-41) and IAST through ANR grant ANR-17-EURE-0010 (Investissements d’Avenir program).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Nicolas Claidière and Joel Fagot for providing pictures of the automated learning devices for monkeys.
References
Amin, Z., Epperson, C. N., Constable, R. T., and Canli, T. (2006). Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage 32, 457–464. doi: 10.1016/j.neuroimage.2006.03.013
Aplin, L. M., Farine, D. R., Morand-Ferron, J., and Sheldon, B. C. (2012). Social networks predict patch discovery in a wild population of songbirds. Proc. Biol. Sci. 279, 4199–4205. doi: 10.1098/rspb.2012.1591
Ashton, B. J., Thornton, A., and Ridley, A. R. (2018). An intraspecific appraisal of the social intelligence hypothesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170288. doi: 10.1098/rstb.2017.0288
Ball, G. F., Auger, C. J., Bernard, D. J., Charlier, T. D., Sartor, J. J., Riters, L. V., et al. (2004). Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann. N. Y. Acad. Sci. 1016, 586–610. doi: 10.1196/annals.1298.043
Barragan-Jason, G., Atance, C. M., Hopfensitz, A., Stieglitz, J., and Cauchoix, M. (2018a). Commentary: revisiting the marshmallow test: a conceptual replication investigating links between early delay of gratification and later outcomes. Front. Psychol. 9:2719. doi: 10.3389/fpsyg.2018.02719
Barragan-Jason, G., Atance, C., Kopp, L., and Hopfensitz, A. (2018b). Two facets of patience in young children: waiting with and without an explicit reward. J. Exp. Child Psychol. 171, 14–30. doi: 10.1016/j.jecp.2018.01.018
Benau, E. M., Orloff, N. C., Janke, E. A., Serpell, L., and Timko, C. A. (2014). A systematic review of the effects of experimental fasting on cognition. Appetite 77, 52–61. doi: 10.1016/j.appet.2014.02.014
Boivin, M. J., and Giordani, B. (1993). Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. J. Pediatr. Psychol. 18, 249–264. doi: 10.1093/jpepsy/18.2.249
Boogert, N. J., Madden, J. R., Morand-Ferron, J., and Thornton, A. (2018). Measuring and understanding individual differences in cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170280. doi: 10.1098/rstb.2017.0280
Bradshaw, A. D. (1965). Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155. doi: 10.1016/S0065-2660(08)60048-6
Buchanan, K. L., Grindstaff, J. L., and Pravosudov, V. V. (2013). Condition dependence, developmental plasticity, and cognition: implications for ecology and evolution. Trends Ecol. Evol. 28, 290–296. doi: 10.1016/j.tree.2013.02.004
Buckwalter, J. G., Stanczyk, F. Z., McCleary, C. A., Bluestein, B. W., Buckwalter, D. K., Rankin, K. P., et al. (1999). Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology 24, 69–84. doi: 10.1016/S0306-4530(98)00044-4
Cauchard, L., Angers, B., Boogert, N. J., and Doligez, B. (2016). Effect of an anti-malaria drug on behavioural performance on a problem-solving task: an experiment in wild great tits. Behav. Process. 133, 24–30. doi: 10.1016/j.beproc.2016.10.012
Cauchard, L., Angers, B., Boogert, N. J., Lenarth, M., Bize, P., and Doligez, B. (2017). An experimental test of a causal link between problem-solving performance and reproductive success in wild great tits. Front. Ecol. Evol. 5:107. doi: 10.3389/fevo.2017.00107
Cauchoix, M., and Chaine, A. S. (2016). How can we study the evolution of animal minds? Front. Psychol. 7:358. doi: 10.3389/fpsyg.2016.00358
Cauchoix, M., Chow, P. K. Y., van Horik, J. O., Atance, C. M., Barbeau, E. J., Barragan-Jason, G., et al. (2018). The repeatability of cognitive performance: a meta-analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170281. doi: 10.1098/rstb.2017.0281
Cauchoix, M., Hermer, E., Chaine, A. S., and Morand-Ferron, J. (2017). Cognition in the field: comparison of reversal learning performance in captive and wild passerines. Sci. Rep. 7:12945. doi: 10.1038/s41598-017-13179-5
Caza, J. S., and Atance, C. M. (2018). Children’s behavior and spontaneous talk in a future thinking task. Psychol. Res. 83, 761–773. doi: 10.1007/s00426-018-1089-1
Chabaud, M.-A., Isabel, G., Kaiser, L., and Preat, T. (2009). Social facilitation of long-lasting memory retrieval in Drosophila. Curr. Biol. 19, 1654–1659. doi: 10.1016/j.cub.2009.08.017
Chaine, A. S., and Lyon, B. E. (2008). Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319, 459–462. doi: 10.1126/science.1149167
Chevin, L.-M., and Hoffmann, A. A. (2017). Evolution of phenotypic plasticity in extreme environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160138. doi: 10.1098/rstb.2016.0138
Chevin, L.-M., Lande, R., and Mace, G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8:e1000357. doi: 10.1371/journal.pbio.1000357
Claidière, N., Gullstrand, J., Latouche, A., and Fagot, J. (2017). Using automated learning devices for monkeys (ALDM) to study social networks. Behav. Res. Methods 49, 24–34. doi: 10.3758/s13428-015-0686-9
Dagaeff, A. C., Pocheville, A., Nöbel, S., Loyau, A., Isabel, G., and Danchin, E. (2016). Drosophila mate copying correlates with atmospheric pressure in a speed learning situation. Anim. Behav. 121, 163–174. doi: 10.1016/j.anbehav.2016.08.022
Desruisseaux, M. S., Gulinello, M., Smith, D. N., Lee, S. C., Tsuji, M., Weiss, L. M., et al. (2008). Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA. J. Infect. Dis. 197, 1621–1627. doi: 10.1086/587908
Dingemanse, N. J., and Dochtermann, N. A. (2013). Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. doi: 10.1111/1365-2656.12013
Dingemanse, N. J., Kazem, A. J. N., Réale, D., and Wright, J. (2010). Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Duckworth, R. A. (2009). The role of behavior in evolution: a search for mechanism. Evol. Ecol. 23, 513–531. doi: 10.1007/s10682-008-9252-6
Dufau, S., Duñabeitia, J. A., Moret-Tatay, C., McGonigal, A., Peeters, D., Alario, F.-X., et al. (2011). Smart phone, smart science: how the use of smartphones can revolutionize research in cognitive science. PLoS One 6:e24974. doi: 10.1371/journal.pone.0024974
Dunn, J. C., Cole, E. F., and Quinn, J. L. (2011). Personality and parasites: sex-dependent associations between avian malaria infection and multiple behavioural traits. Behav. Ecol. Sociobiol. 65, 1459–1471. doi: 10.1007/s00265-011-1156-8
Ebbesson, L. O. E., and Braithwaite, V. A. (2012). Environmental effects on fish neural plasticity and cognition. J. Fish Biol. 81, 2151–2174. doi: 10.1111/j.1095-8649.2012.03486.x
Ebner, K., and Singewald, N. (2017). Individual differences in stress susceptibility and stress inhibitory mechanisms. Curr. Opin. Behav. Sci. 14, 54–64. doi: 10.1016/j.cobeha.2016.11.016
Evans, J. C., Devost, I., Jones, T. B., and Morand-Ferron, J. (2018). Inferring dominance interactions from automatically recorded temporal data. Ethology 124, 188–195. doi: 10.1111/eth.12720
Fagot, J., and Bonté, E. (2010). Automated testing of cognitive performance in monkeys: use of a battery of computerized test systems by a troop of semi-free-ranging baboons (Papio papio). Behav. Res. Methods 42, 507–516. doi: 10.3758/BRM.42.2.507
Fize, D., Cauchoix, M., and Fabre-Thorpe, M. (2011). Humans and monkeys share visual representations. Proc. Natl. Acad. Sci. U.S.A. 108, 7635–7640. doi: 10.1073/pnas.1016213108
Forsman, A. (2015). Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–284. doi: 10.1038/hdy.2014.92
Gazes, R. P., Brown, E. K., Basile, B. M., and Hampton, R. R. (2013). Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory-based testing. Anim. Cogn. 16, 445–458. doi: 10.1007/s10071-012-0585-8
Goldfarb, E. V., Froböse, M. I., Cools, R., and Phelps, E. A. (2017). Stress and cognitive flexibility: cortisol increases are associated with enhanced updating but impaired switching. J. Cogn. Neurosci. 29, 14–24. doi: 10.1162/jocn_a_01029
Greggor, A. L., Trimmer, P. C., Barrett, B. J., and Sih, A. (2019). Challenges of learning to escape evolutionary traps. Front. Ecol. Evol. 7:408. doi: 10.3389/fevo.2019.00408
Harari, G. M., Müller, S. R., Aung, M. S., and Rentfrow, P. J. (2017). Smartphone sensing methods for studying behavior in everyday life. Curr. Opin. Behav. Sci. 18, 83–90. doi: 10.1016/j.cobeha.2017.07.018
Hou, L., Verdirame, M., and Welch, K. C. (2015). Automated tracking of wild hummingbird mass and energetics over multiple time scales using radio frequency identification (RFID) technology. J. Avian Biol. 46, 1–8. doi: 10.1111/jav.00478
Houslay, T. M., and Wilson, A. J. (2017). Avoiding the misuse of BLUP in behavioural ecology. Behav. Ecol. 28, 948–952. doi: 10.1093/beheco/arx023
Huguet, P., Barbet, I., Belletier, C., Monteil, J.-M., and Fagot, J. (2014). Cognitive control under social influence in baboons. J. Exp. Psychol. Gen. 143, 2067–2073. doi: 10.1037/xge0000026
Keller, M. C., Fredrickson, B. L., Ybarra, O., Côté, S., Johnson, K., Mikels, J., et al. (2005). A warm heart and a clear head. The contingent effects of weather on mood and cognition. Psychol. Sci. 16, 724–731. doi: 10.1111/j.1467-9280.2005.01602.x
Kelsey, C., Grossmann, T., and Vaish, A. (2018). Early reputation management: three-year-old children are more generous following exposure to eyes. Front. Psychol. 9:698. doi: 10.3389/fpsyg.2018.00698
Kihara, M., Carter, J. A., and Newton, C. R. J. C. (2006). The effect of Plasmodium falciparum on cognition: a systematic review. Trop. Med. Int. Health 11, 386–397. doi: 10.1111/j.1365-3156.2006.01579.x
Kuo, M., Barnes, M., and Jordan, C. (2019). Do experiences with nature promote learning? Converging evidence of a cause-and-effect relationship. Front. Psychol. 10:305. doi: 10.3389/fpsyg.2019.00305
Langley, E. J. G., van Horik, J. O., Whiteside, M. A., and Madden, J. R. (2018). Individuals in larger groups are more successful on spatial discrimination tasks. Anim. Behav. 142, 87–93. doi: 10.1016/j.anbehav.2018.05.020
Larios, D. F., Rodríguez, C., Barbancho, J., Baena, M., Angel, M. L., Marín, J., et al. (2013). An automatic weighting system for wild animals based in an artificial neural network: how to weigh wild animals without causing stress. Sens. Basel Sens. 13, 2862–2883. doi: 10.3390/s130302862
Lima, S. L., and Bednekoff, P. A. (1999). Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659. doi: 10.1086/303202
Little, A. C. (2013). The influence of steroid sex hormones on the cognitive and emotional processing of visual stimuli in humans. Front. Neuroendocrinol. 34, 315–328. doi: 10.1016/j.yfrne.2013.07.009
Lyon, B. E., Chaine, A. S., and Winkler, D. W. (2008). Ecology. A matter of timing. Science 321, 1051–1052. doi: 10.1126/science.1159822
Maille, A., Pillay, N., and Schradin, C. (2015). Seasonal variation in attention and spatial performance in a wild population of the African striped mouse (Rhabdomys pumilio). Anim. Cogn. 18, 1231–1242. doi: 10.1007/s10071-015-0892-y
Martin, J. G. A., Nussey, D. H., Wilson, A. J., and Réale, D. (2011). Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2, 362–374. doi: 10.1111/j.2041-210X.2010.00084.x
McCune, K. B., Jablonski, P., Lee, S.-I., and Ha, R. R. (2019). Captive jays exhibit reduced problem-solving performance compared to wild conspecifics. R. Soc. Open Sci. 6:181311. doi: 10.1098/rsos.181311
Meyer, C., Muto, V., Jaspar, M., Kussé, C., Lambot, E., Chellappa, S. L., et al. (2016). Seasonality in human cognitive brain responses. Proc. Natl. Acad. Sci. U.S.A. 113, 3066–3071. doi: 10.1073/pnas.1518129113
Morand-Ferron, J., Cole, E. F., and Quinn, J. L. (2016). Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. Camb. Philos. Soc. 91, 367–389. doi: 10.1111/brv.12174
Morand-Ferron, J., Hamblin, S., Cole, E. F., Aplin, L. M., and Quinn, J. L. (2015). Taking the operant paradigm into the field: associative learning in wild great tits. PLoS One 10:e0133821. doi: 10.1371/journal.pone.0133821
Morand-Ferron, J., and Quinn, J. L. (2011). Larger groups of passerines are more efficient problem solvers in the wild. Proc. Natl. Acad. Sci. U.S.A. 108, 15898–15903. doi: 10.1073/pnas.1111560108
Murphy, T., Dias, G. P., and Thuret, S. (2014). Effects of diet on brain plasticity in animal and human studies: mind the gap. Neural Plast. 2014:563160. doi: 10.1155/2014/563160
Murray, A. J., Knight, N. S., Cochlin, L. E., McAleese, S., Deacon, R. M. J., Rawlins, J. N. P., et al. (2009). Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 23, 4353–4360. doi: 10.1096/fj.09-139691
Niemelä, P. T., and Dingemanse, N. J. (2014). Artificial environments and the study of “adaptive” personalities. Trends Ecol. Evol. 29, 245–247. doi: 10.1016/j.tree.2014.02.007
Open Science Collaboration (2015). PSYCHOLOGY. Estimating the reproducibility of psychological science. Science 349:aac4716. doi: 10.1126/science.aac4716
O’Reilly, M. A., Cunningham, C. J., Lawlor, B. A., Walsh, C. D., and Rowan, M. J. (2004). The effect of the menstrual cycle on electrophysiological and behavioral measures of memory and mood. Psychophysiology 41, 592–603. doi: 10.1111/j.1469-8986.2004.00194.x
Padmala, S., and Pessoa, L. (2010). Interactions between cognition and motivation during response inhibition. Neuropsychologia 48, 558–565. doi: 10.1016/j.neuropsychologia.2009.10.017
Pigliucci, M. (2005). Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486. doi: 10.1016/j.tree.2005.06.001
Pike, T. W., and Laland, K. N. (2010). Conformist learning in nine-spined sticklebacks’ foraging decisions. Biol. Lett. 6, 466–468. doi: 10.1098/rsbl.2009.1014
Price, T. D., Qvarnström, A., and Irwin, D. E. (2003). The role of phenotypic plasticity in driving genetic evolution. Proc. Biol. Sci. 270, 1433–1440. doi: 10.1098/rspb.2003.2372
Pritchard, D. J., Hurly, T. A., Tello-Ramos, M. C., and Healy, S. D. (2016). Why study cognition in the wild (and how to test it)? J. Exp. Anal. Behav. 105, 41–55. doi: 10.1002/jeab.195
Rendell, L., Fogarty, L., Hoppitt, W. J. E., Morgan, T. J. H., Webster, M. M., and Laland, K. N. (2011). Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76. doi: 10.1016/j.tics.2010.12.002
Ripoll, A., Viana, M., Padrosa, M., Querol, X., Minutolo, A., Hou, K. M., et al. (2019). Testing the performance of sensors for ozone pollution monitoring in a citizen science approach. Sci. Total Environ. 651, 1166–1179. doi: 10.1016/j.scitotenv.2018.09.257
Roitblat, H. L. (2014). Animal Cognition. New York, NY: Psychology Press. doi: 10.4324/9781315802602
Roozendaal, B., McEwen, B. S., and Chattarji, S. (2009). Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433. doi: 10.1038/nrn2651
Samuelson, E. E. W., Chen-Wishart, Z. P., Gill, R. J., and Leadbeater, E. (2016). Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6:38957. doi: 10.1038/srep38957
Sansone, C., and Harackiewicz, J. M. (eds) (2000). “Intrinsic and extrinsic motivation: the search for optimal motivation and performance,” in Intrinsic and Extrinsic Motivation: The Search for Optimal Motivation and Performance, (San Diego, CA: Academic Press).
Schoeller, F., Bertrand, P., Gerry, L. J., Jain, A., Horowitz, A. H., and Zenasni, F. (2018). Combining virtual reality and biofeedback to foster empathic abilities in humans. Front. Psychol. 9:2741. doi: 10.3389/fpsyg.2018.02741
Schwabe, L., Joëls, M., Roozendaal, B., Wolf, O. T., and Oitzl, M. S. (2012). Stress effects on memory: an update and integration. Neurosci. Biobehav. Rev. 36, 1740–1749. doi: 10.1016/j.neubiorev.2011.07.002
Seehagen, S., Schneider, S., Rudolph, J., Ernst, S., and Zmyj, N. (2015). Stress impairs cognitive flexibility in infants. Proc. Natl. Acad. Sci. U.S.A. 112, 12882–12886. doi: 10.1073/pnas.1508345112
Smit, J. A. H., and van Oers, K. (2019). Personality types vary in their personal and social information use. Anim. Behav. 151, 185–193. doi: 10.1016/j.anbehav.2019.02.002
Snijders, L., Naguib, M., and van Oers, K. (2016). Dominance rank and boldness predict social attraction in great tits. Behav. Ecol. 28, 398–406. doi: 10.1093/beheco/arw158
Sonnenberg, B. R., Branch, C. L., Pitera, A. M., Bridge, E., and Pravosudov, V. V. (2019). Natural selection and spatial cognition in wild food-caching mountain chickadees. Curr. Biol. 29, 670–676.e3. doi: 10.1016/j.cub.2019.01.006
Sörqvist, P. (2016). Grand challenges in environmental psychology. Front. Psychol. 7:583. doi: 10.3389/fpsyg.2016.00583
Stern, P. C. (2000). Psychology and the science of human-environment interactions. Am. Psychol. 55, 523–530. doi: 10.1037//0003-066X.55.5.523
Suchak, M., Watzek, J., Quarles, L. F., and de Waal, F. B. M. (2018). Novice chimpanzees cooperate successfully in the presence of experts, but may have limited understanding of the task. Anim. Cogn. 21, 87–98. doi: 10.1007/s10071-017-1142-2
Sundström Poromaa, I., and Gingnell, M. (2014). Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 8:380. doi: 10.3389/fnins.2014.00380
Taborsky, B., and Oliveira, R. F. (2013). Social competence vs responsiveness: similar but not same. A reply to Wolf and McNamara. Trends Ecol. Evol. 28, 254–255. doi: 10.1016/j.tree.2013.02.005
Taylor, L., Watkins, S. L., Marshall, H., Dascombe, B. J., and Foster, J. (2015). The impact of different environmental conditions on cognitive function: a focused review. Front. Physiol. 6:372. doi: 10.3389/fphys.2015.00372
Tognetti, A., Dubois, D., Faurie, C., and Willinger, M. (2016). Men increase contributions to a public good when under sexual competition. Sci. Rep. 6:29819. doi: 10.1038/srep29819
Tramontin, A. D., and Brenowitz, E. A. (2000). Seasonal plasticity in the adult brain. Trends Neurosci. 23, 251–258. doi: 10.1016/s0166-2236(00)01558-7
Turner, K. M., and Burne, T. H. J. (2013). Interaction of genotype and environment: effect of strain and housing conditions on cognitive behavior in rodent models of schizophrenia. Front. Behav. Neurosci. 7:97. doi: 10.3389/fnbeh.2013.00097
Van Bavel, J. J., Mende-Siedlecki, P., Brady, W. J., and Reinero, D. A. (2016). Contextual sensitivity in scientific reproducibility. Proc. Natl. Acad. Sci. U.S.A. 113, 6454–6459. doi: 10.1073/pnas.1521897113
Voelkl, B., and Würbel, H. (2016). Reproducibility crisis: are we ignoring reaction norms? Trends Pharmacol. Sci. 37, 509–510. doi: 10.1016/j.tips.2016.05.003
West-Eberhard, M. J. (2003). Developmental Plasticity and Evolution. Oxford: Oxford University Press.
Keywords: cognition, cognitive plasticity, evolution, environment, reaction norm
Citation: Cauchoix M, Chaine AS and Barragan-Jason G (2020) Cognition in Context: Plasticity in Cognitive Performance in Response to Ongoing Environmental Variables. Front. Ecol. Evol. 8:106. doi: 10.3389/fevo.2020.00106
Received: 31 January 2020; Accepted: 31 March 2020;
Published: 28 April 2020.
Edited by:
Laure Cauchard, University of Aberdeen, United KingdomReviewed by:
Claudia A.F. Wascher, Anglia Ruskin University, United KingdomJean-Nicolas Audet, The Rockefeller University, United States
Copyright © 2020 Cauchoix, Chaine and Barragan-Jason. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maxime Cauchoix, bWF4aW1lLmNhdWNob2l4QHNldGUuY25ycy5mcg==; bWNhdWNob2l4eHhAZ21haWwuY29t
 Maxime Cauchoix
Maxime Cauchoix Alexis S. Chaine
Alexis S. Chaine Gladys Barragan-Jason
Gladys Barragan-Jason