- Biology Department, Appalachian State University, Boone, NC, United States
Cooperation between parents in species with biparental care can reduce sexual conflict and increase reproductive success. If parents cooperate in a conditional way – that is, alternate feeding visits to offspring – this should equalize parental investment and may improve nestling growth. Environmental variation, including competition for limited resources, may influence the need for, and benefits of, parental cooperation. We measured the benefits of partner coordination in offspring provisioning behavior among eastern bluebird partners (Sialia sialis) in which the strength of interspecific density varied spatially. Tree swallows (Tachycineta bicolor) are a recent (<40 years) arrival in our study area, are aggressive nestbox competitors with eastern bluebirds, and their density varies across the field site. Nesting among higher densities of tree swallows led to reduced parental feeding rates and reproductive success of bluebirds. Partner alternation, however, did not vary with tree swallow density. Additionally, alternation level and provisioning rate only influenced nestling growth in areas of high swallow density. It may be that the benefits of parental coordination may only be apparent when environmental conditions are poor. This study provides an important new perspective on the resolution of negotiations between breeding partners; environmental variation could influence the benefits of parental cooperation in a wide variety of animals.
Introduction
Biparental care involves the cooperation of two unrelated individuals that share fitness benefits in the current breeding attempt but, because each individual pays the costs of reproduction, should have conflicting interests in parental investment (reviewed in Westneat and Sargent, 1996). Trivers (1972) argued that, in biparental care systems, each parent should save energy for future reproductive attempts by decreasing their current parental effort. Because individuals benefit when their offspring are fed at high rates, the perception of a partner withholding energy in the current breeding attempt should lead to conflict between mated partners (Stearns, 1989) particularly when partners are unlikely to breed together in the future (Griffith, 2019; Johnstone and Savage, 2019).
One mechanism to resolve conflict could be to flexibly adjust parental care in response to their partner’s behavior (Hinde, 2006; Johnstone and Hinde, 2006). Coordination of provisioning to offspring could consist of synchronous or alternated feeding by the parents. For partner synchrony or alternation to occur, birds must keep track of their partners (Mariette and Griffith, 2015). For example, among wild breeding zebra finches (Taeniopygia guttata) partners that synchronize provisioning visits by arriving at the nest at the same time can equalize their parental effort (Mariette and Griffith, 2012; Johnstone and Savage, 2019).
Johnstone et al. (2014) proposed a model where individuals may benefit from using a strategy of “conditional cooperation” and thus increase provisioning effort if their partner was the last to feed. Empirical data suggest that great tits (Parus major) keep track of partner visits and tend to alternate provisioning visits (Johnstone et al., 2014).
The extent to which offspring benefit from increased parental coordination, however, is still unclear. Some studies show no effects of increased coordination on nestling quality or fledging success (van Rooij and Griffith, 2013; Iserbyt et al., 2017; Griffioen et al., 2019), whereas others have found positive effects on nestling growth (Mariette and Griffith, 2015) or higher fledging rates (Mariette and Griffith, 2012; Bebbington and Hatchwell, 2016; Leniowski and Wȩgrzyn, 2018) or increased brood survival (Raihani et al., 2010). Relationships between partner coordination of parental care and fitness may be influenced by variation in environmental conditions.
Although it has long been known that environmental conditions influence food availability, risk of predation and competition, and parental provisioning (reviewed in Kamil et al., 2012), comparatively little research has focused on how ecological conditions influence the degree to which parents coordinate provisioning or how parental coordination influences reproductive success. Recently, Lejeune et al. (2019) showed that great tit partners alternate nest visits more and feed more often at lower elevations and show more synchronized nestling provisioning in edge versus interior forests. Heightened predation or nest usurpation risk could also favor partner coordination. Partner coordination during parental care may also reflect their coordination in other contexts such as when mates defend their territory or offspring against intruders or predators (Curio and Regelmann, 1986; Black, 2001; Krams et al., 2006). Finally, it is possible that environmental variation may exacerbate or mask the relationship between parental coordination and offspring condition.
The goals of this study were to understand how interspecific density influences parental coordination and reproductive success. First, we hypothesized that parental coordination influenced nestling growth rates, with the expectation that parents with greater coordination (defined as greater parental alternation of feeding visits) should rear faster growing offspring. Second, we tested the hypothesis that the relationship between parental coordination and reproductive success of mated partners may be influenced by the density of interspecific competitors for nesting sites. Third, we explore how reproductive parameters differ with the density of interspecific competitors. We focus on parental coordination of eastern bluebirds (Sialia sialis), a species that experiences high competition for nesting sites with tree swallows (Tachycineta bicolor). In the year the data were collected (2015), 44% of early nesting bluebird partners were evicted by tree swallows (Albers et al., 2017). Finally, because tree swallows preferentially settle near water, we test whether distance to water or tree swallow density has a greater influence on bluebird behavior and reproductive success.
Materials and Methods
Study Species
Eastern bluebirds (S. sialis; mass ∼30 g) are a secondary cavity nesting species that readily nest in human-constructed nestboxes. They are socially monogamous and both parents defend the nest during the incubation and nestling rearing stages, females incubate the eggs and both parents provision the young (Gowaty and Plissner, 2015). Over the last 40 years, tree swallows (mass: ∼20 g) have expanded their breeding range to the southeastern United States and were not documented breeding in North Carolina until the 1980s (Lee, 1993). Eastern bluebirds and tree swallows act aggressively toward one another (Winkler et al., 2011; Gowaty and Plissner, 2015) and tree swallows often outcompete bluebirds and evict them from nesting cavities (Hersey, 1933; Weibe, 2016). These species do not compete for food; tree swallows are semi-colonial nesters that forage on emergent aquatic insects primarily within an ∼300 m radius of their nest (McCarty and Winkler, 1999) while bluebirds forage on terrestrial arthropods and defend ∼75 m radius of their nest (Gowaty and Plissner, 2015). At our western North Carolina field site, eastern bluebirds are non-migratory (or short distance migrants) and settle on territories earlier than do the migratory tree swallows (Knight et al., 2018). Bluebirds lay eggs on average, 3 weeks before tree swallows (the bluebird first egg date in 2015 was April 7 while swallow first egg date in 2015 was May 1). Eviction occurs predominately during the time of tree swallow settlement when bluebirds are in the laying and incubation stages and is rare during the nestling rearing stage. However, even after tree swallows have established their own nests, it is common to observe tree swallows (often more than two) sitting on nest boxes, tussling with bluebirds and circling the nest box (pers. obs.). At our field site, in areas of high density of tree swallows, bluebird partners that displayed similar levels of aggression fledged offspring with higher mass than dissimilar partners (Harris and Siefferman, 2014), thus interspecific competition may select for coordinated parental defense and parental provisioning behaviors. Other competitors for nestboxes at our field site are unlikely to significantly influence bluebird nest success including: house wrens (Troglodytes aedon; usurped 0.09% of bluebird nests), house sparrows (Passer domesticus; usurped 0% of bluebird nests) and bluebirds (usurped 0% of conspecific nests).
General Field Methods
We monitored nest building, egg laying, hatching, and fledging success of 110 eastern bluebird nests and 109 tree swallow nests in Watauga County, NC during the breeding season of 2015. Of the 49 nests in which nestlings hatched, we measured nestling mass (±0.1 g) when bluebird nestlings were 2, 5, 8, 11, and 14 days old (day 1 = hatch day) and we use these data in a repeated measures approach to understand how parental care and tree swallow density influence nestling growth. Nestling bluebird growth asymptotes at 13 days old (Pinkowski, 1975), therefore, the mass of the nestlings at 14 days old is indicative of mass at fledging (Gowaty and Plissner, 2015). Adult bluebirds were captured in nest boxes using trapdoors and banded with a numbered USGS aluminum band, along with three colored plastic bands for remote identification. Nestlings were also fitted with a USGS aluminum band at 8 days old.
Provisioning
We recorded offspring provisioning at 37 nests using video cameras placed at least 2 m from the nestbox between 7 and 10 am. We took videos of each nest twice, first when nestlings were between 3 and 7 days old (early nestling period), and again when nestlings were between 9 and 13 days old (late nestling period; hatch day = 1 day old). Each observation lasted 2 h. Provisioning videotapes did not yield useful data on tree swallow harassment at the nest, however, because the field of view was narrow and focused on the nestbox hole. We recorded the total number of visits to the nest for each parent and calculated the provisioning rate (visits/h). Alternation was calculated based on the number and order of parental feeding visits to the nest. The proportion of alternated visits was calculated following Bebbington and Hatchwell (2016); observed alternation, A, as A = F/(t − 1), where F is the number of times a bird fed after the other and t is the total number of feeds in the observation. As both provisioning rates and alternation was highly repeatable (Burdick, 2018), we use the average feeding rate and alternation in subsequent analyses.
Interspecific Density
We quantified interspecific density during the bluebird nestling stage as the number of active swallow nests within a 300 m radius of the focal bluebird nest using Point Distance Tool in ArcGIS 10.4.1 (ESRI, 2016). We categorized density as low (0–1 swallow nests) or high (2 or more swallow nests; range: 2–12) because the distribution of tree swallow density was bimodal (Figure 1). To attempt to understand whether tree swallow density or distance of nestbox to water had a greater influence of bluebird behavior and reproductive output, we categorized all active bluebird nests as either within or beyond 50 m of water. Indeed, bluebird nests that were classified as high tree swallow density tended to also be within 50 m of water (13 of 21 nests compared to the bluebirds that settle in locations that subsequently experienced low density of swallows (6 of 28).
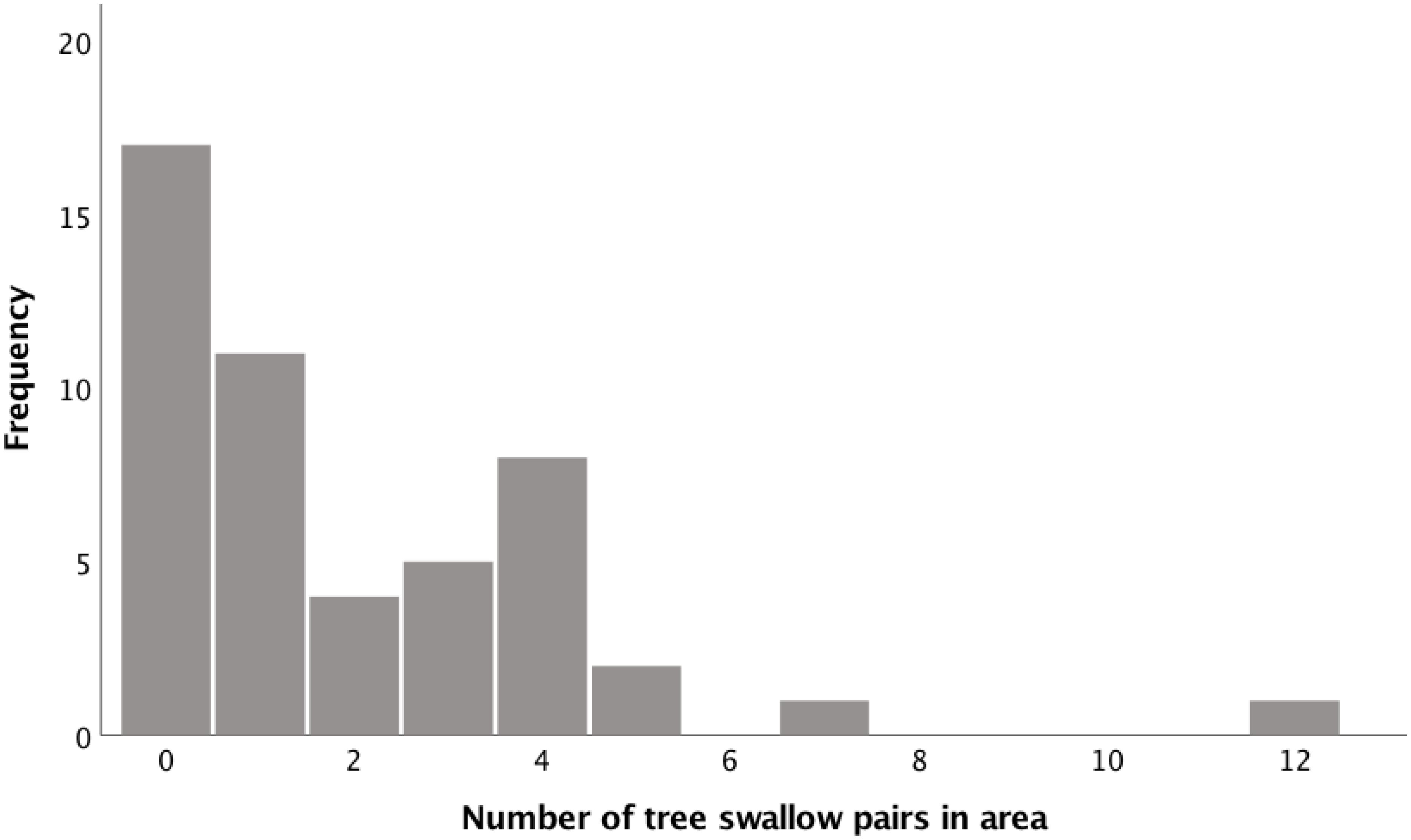
Figure 1. Distribution of the number of active tree swallow nests within the 75 m2 territory of breeding eastern bluebirds. Low density is designated as 0 or 1 tree swallow nest while high density is designated as 2 or more tree swallow nests.
Statistical Methods
Statistical analyses were conducted using SPSS v.24 statistical software (IBM Corp, 2017). We then performed General Linear Mixed Models (LMM) to investigate parameters influenced nestling mass. We used nestling identity and brood identity as random effects to investigate the effects of nestling age (2, 5, 8, 11, 14 days post-hatch), brood size, hatch date, tree swallow density, average provisioning rates of parent birds, and average alternation (predictors) on nestling mass (dependent variable). We measured 130 nestlings from 49 nests. We plot nestling growth rate (mean mass gain per day). Next, we used t-tests to explore how reproductive parameters and parental provisioning varied between areas of high and low tree swallow density. Finally, to attempt to disentangle influences of interspecific density from that of habitat, we compared the Akaike’s Information Criterion (AIC) of models in which we swapped the predictor variable “tree swallow density” with that of “distance to water” for each response variable.
Ethics Statement
This study was carried out in accordance with the recommendations for the Care and Use of Animals for Research, Teaching, or Demonstrations provided by the Appalachian State University through Institutional Animal Care and Use Committee (IACUC #12-09) under USFWS Master Banding Permit #23563. All animals were handled in such a way to reduce stress and avoid physical harm and were released in their home territory.
Results
Effect of Tree Swallow Density and Parent Provisioning on Nestling Mass
Brood size and hatch date did not contribute significantly to models and thus were excluded from further analyses (F < 0.5, p > 0.1). We observed a significant interaction between swallow density level and average provisioning rate of the parents on nestling mass (F = 8.33, df = 3, 175.3, n = 130 nestlings, p = 0.004). We therefore examined data from high- and low-density areas separately in subsequent analyses. In high density areas, average provisioning rate positively predicted mass (Effect size ± 1 SE = 0.52 ± 0.17, F = 9.43, df = 1, 75.4, n = 47 nestlings, p = 0.003), whereas we found no evidence that provisioning rates influenced nestling mass amongst partners breeding in low-density areas (Effect size ± 1 SE = 0.11 ± 0.08, F = 1.33, df = 1, 101, n = 83 nestlings, p = 0.25, Figure 2). In all models, older chicks were significantly heavier (all p < 0.001). Comparing AIC values of models in which we swapped distance to water with interspecific density suggests that the density model had better fit compared with the distance to water model (ΔAIC = 320.7).
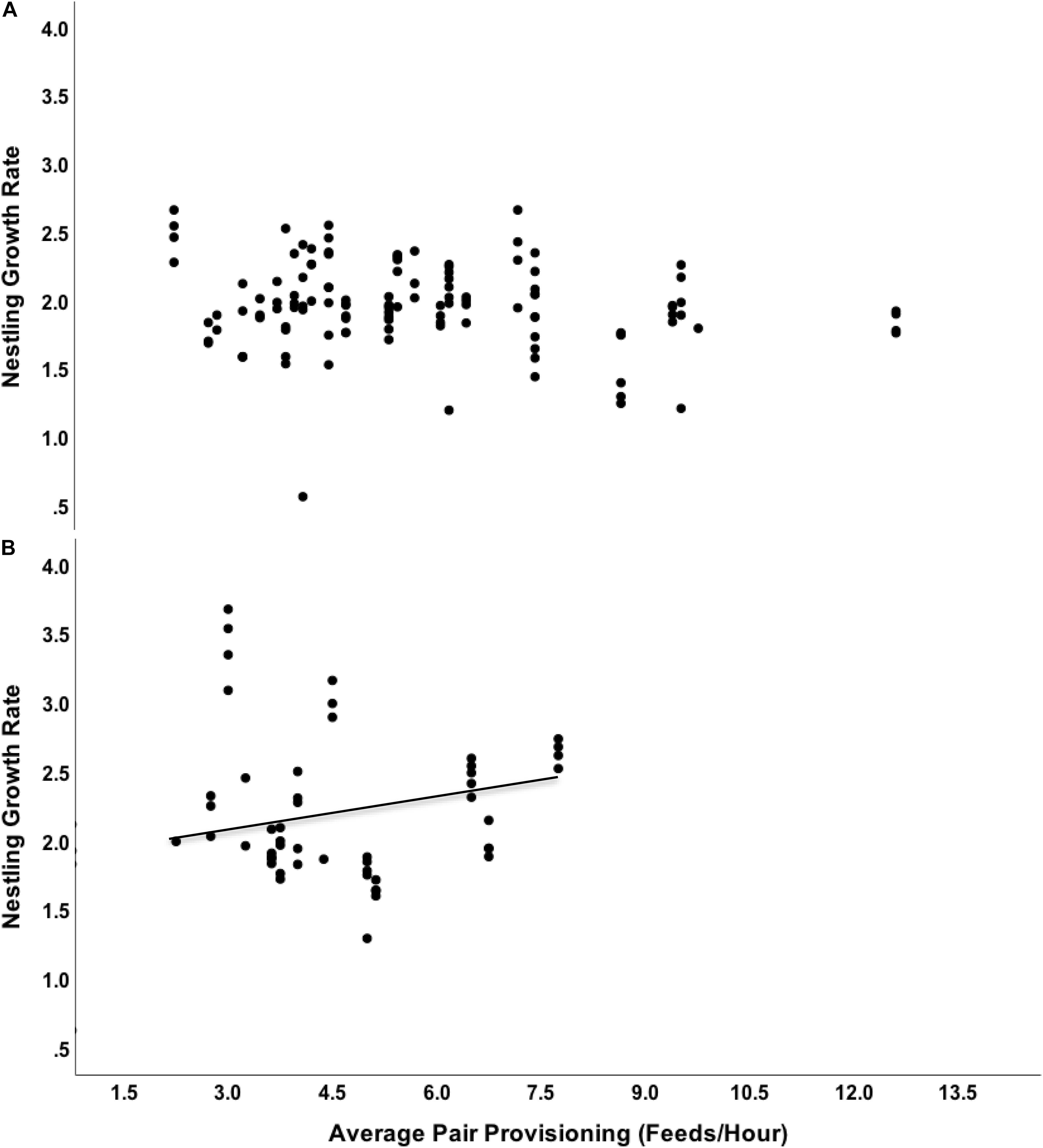
Figure 2. Relationship between average provisioning rate of the pair (feeds/hour) on eastern bluebird nestling growth rate (g/day) split by areas of low (A) and high (B) tree swallow density.
We found significant interaction between swallow density level and alternation level on nestling mass (F = 8.49, df = 3,175.3, n = 130 nestlings, p = 0.004). After splitting the data by density areas, we found a significant positive relationship between parental alternation and nestling mass in both low and high-density areas, although the trend was stronger in nestlings raised in high-density environments (High Density: Effect size ± 1 SE = 4.29 ± 1.45, F = 8.69, df = 1, 75.4, n = 47 nestlings, p = 0.004, Low Density: Effect size ± 1 SE = 2.74 ± 1.18, F = 5.43, df = 1, 101, n = 83 nestlings, p = 0.022, Figure 3). In all models, older chicks were significantly heavier (all F > 1700, all p < 0.001). Using AIC values of models in which we swapped distance to water with interspecific density, we found very similar results (ΔAIC = 1.2).
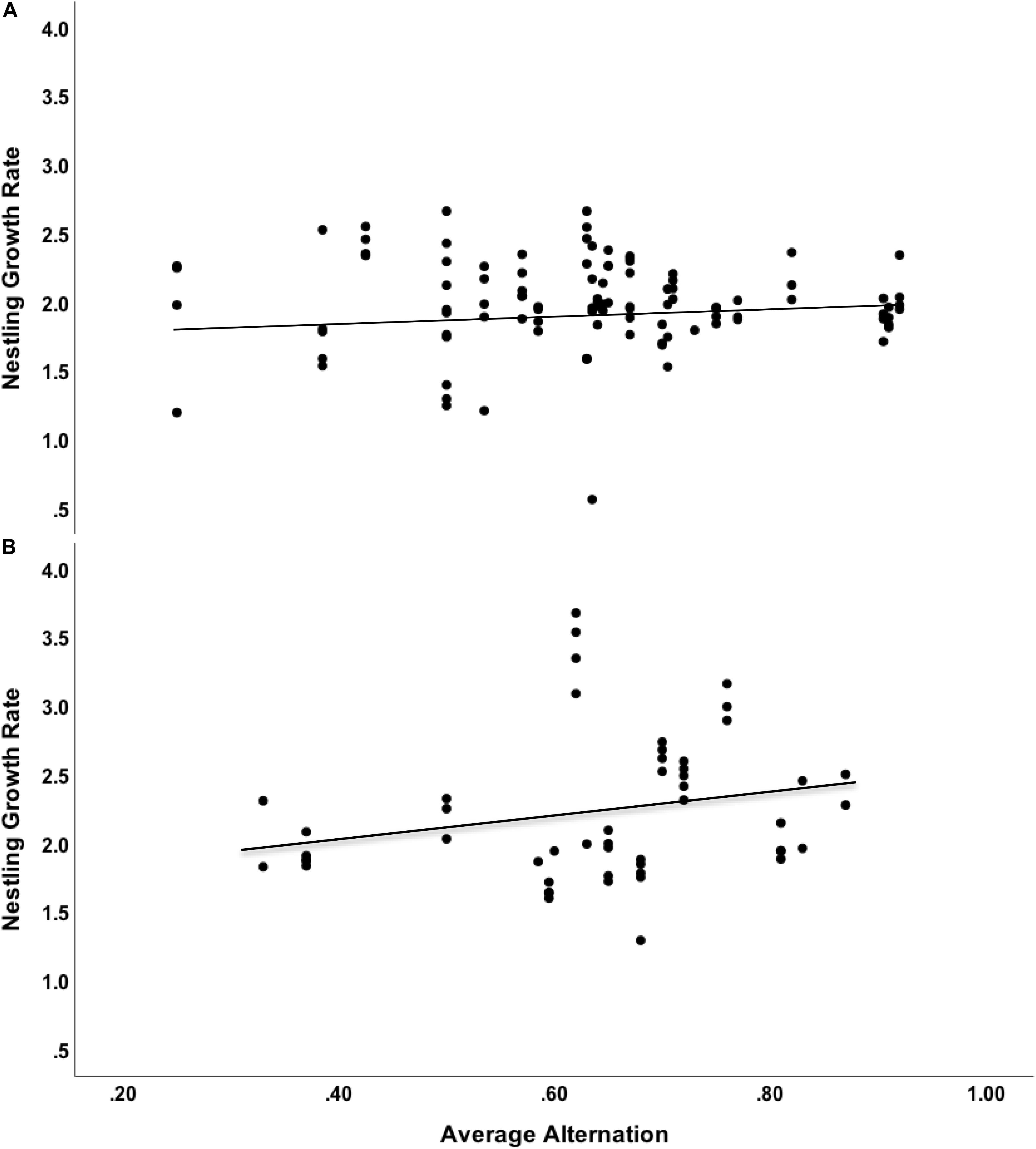
Figure 3. Relationship between average alternation level (proportion of all visits that are alternated within pairs) and nestling growth rate (g/day) of eastern bluebirds, split by areas of low (A) and high (B) density of tree swallows.
Effect of Swallow Density on Bluebird Nest Parameters
Of the nests that successfully fledged at least one nestling, t-tests revealed that neither initial clutch nor brood size were significantly different in areas of high and low swallow density (Table 1). However, in low-density areas the number of offspring fledged was significantly greater than in areas of high density (Table 1). Despite this, there was no significant difference in average fledging mass between areas of high and low swallow density (Table 1). Using AIC values of models in which we swapped distance to water with interspecific density to compare their effects on number of offspring fledged, we found the density model had slightly better fit compared with the distance to water model (ΔAIC = 2.1).
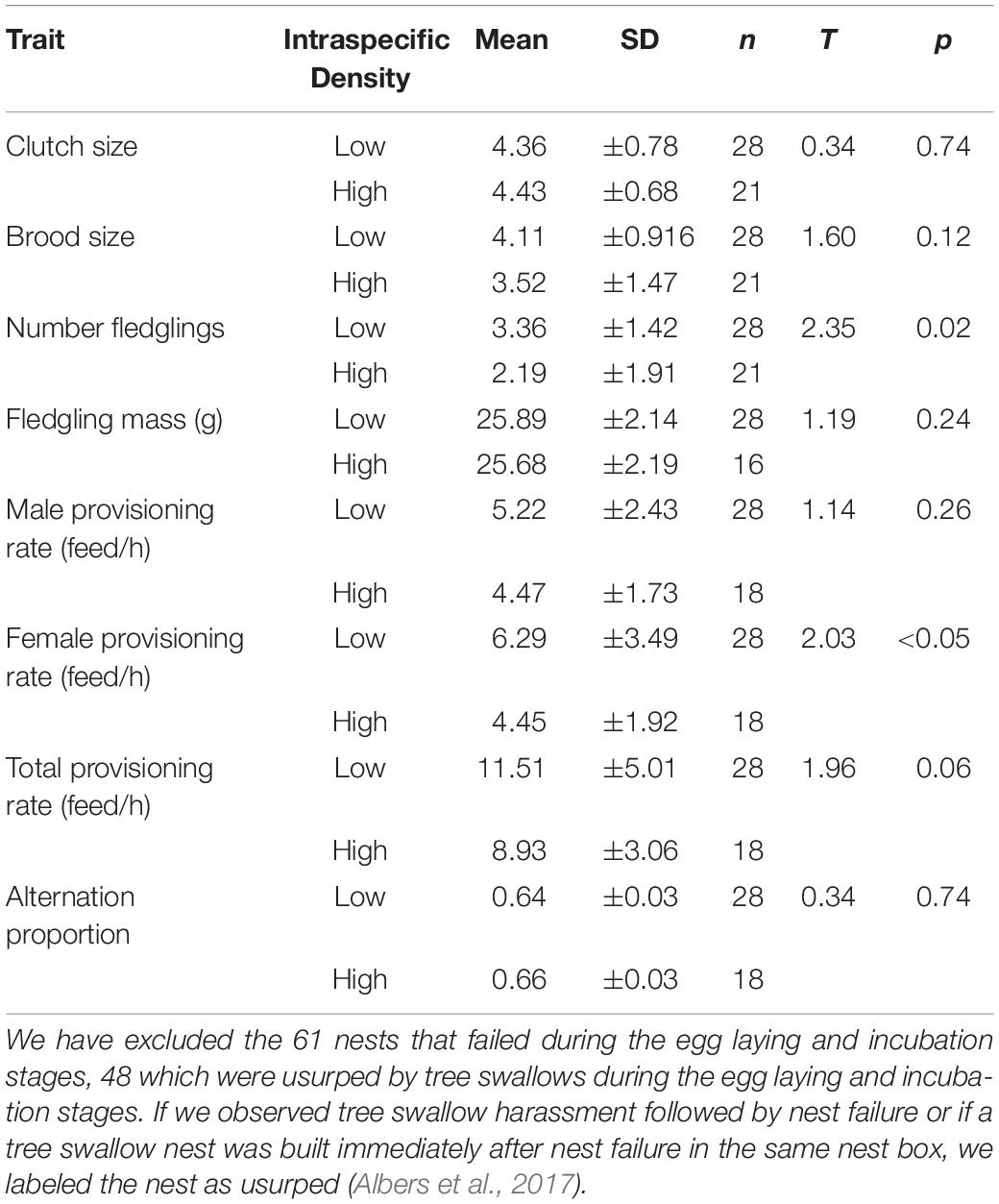
Table 1. Comparison of reproductive parameters of the bluebird nests in relation to tree swallow density (i.e., low versus high density areas).
Alternation values were not significantly different between high- and low-density partners (Table 1). Using AIC values of models in which we swapped distance to water with interspecific density to compare their effects on female provisioning, we found the density model had better fit compared with the distance to water model (ΔAIC = 4.27). Likewise, there was no significant effect of density level on male provisioning rates (Table 1) and the distance to water had slightly better fit compared with the density model (ΔAIC = 1.18). However, females in high-density areas provisioned offspring at significantly lower rates when compared to females in low density areas (Table 1). Using AIC values of models in which we swapped distance to water with interspecific density to compare their effects on female provisioning, we found the density model had better fit compared with the distance to water model (ΔAIC = 4.13). The average provisioning rate, however, did not differ significantly between partners in areas of high and low density (Table 1) and that the density model was a slightly better fit compared with the distance to water model (ΔAIC = 3.76).
Discussion
In this population of eastern bluebirds, although parents alternated provisioning trips similarly when nesting in areas of high and low density of tree swallows, the benefits of parental alternation to offspring were only apparent when the bluebirds nested among high densities of tree swallows. Tree swallows are a relatively new nest competitor in the southern Appalachian Mountains population and prior to ∼1980 were uncommon and only observed during migration (Lee, 1993). Today, competition from tree swallows is fierce as 45% of the early season bluebird nestboxes were usurped by tree swallows (Albers et al., 2017). The swallow density had clear effects on parental provisioning strategies and how provisioning influenced nestling mass. Bluebirds (particularly the females) nesting in high-density areas provisioned their offspring less often and fewer nestlings survived to fledging age. However, offspring that did survive achieved a similar fledging mass to those in low-density areas. Finally, when bluebirds bred in high-density areas, those that provisioned offspring more frequently and had a high level of partner alternation reared nestlings that grew faster, suggesting that paying attention to partner behavior increases parental fitness. A concordant relationship between partner provisioning, alternation and nestling growth was not apparent in areas of low density of swallows. It seems likely that effects of partner behavior on nestling fitness may only occur when nestlings experience some level of nutritional stress. Interactions with tree swallows force the bluebirds to defend the nest more often (Authors, pers. obs.) and this likely leads to reduced provisioning and thus demonstrates the importance of partner coordination on nestling growth.
Harassment from tree swallows occurs more often in areas of high density of breeding tree swallows (Authors, pers. obs.) and likely this causes the bluebirds to experience short term temporal disturbances. Tree swallows arrive on breeding grounds and establish territories nearly a month after bluebirds have paired for the season. When the need for nest vigilance (i.e., territorial defense aggression) increases, partner coordination may present a selective advantage and promote partner investment matching. Thus, equity in partner investment (i.e., increasing cooperation rather than intensifying sexual conflict – see Mariette and Griffith, 2015) may help bluebirds successfully rear young under harassment from tree swallows. This further supports the idea that nest visit coordination can reduce conflict and increase reproductive success when partners respond to environmental cues such as increased brood size (Mariette and Griffith, 2015), offspring competition (Shen et al., 2010) or risk of nest predation (Raihani et al., 2010; Bebbington and Hatchwell, 2016) or habitat (and presumably food availability; Lejeune et al., 2019).
Here, we show some of the first evidence that environmental conditions influence the degree to which a mated pair’s coordinated parental provisioning can affect offspring fitness. Although partners in this population of bluebirds alternate provisioning more often than would be expected by chance (Burdick, 2018), the effects of alternation and parental feeding rates on offspring growth were more apparent in areas of high density of swallows. It may be that parents in high-density areas are forced to spend time and energy defending territories and thus nestlings experience some level of nutritional stress. How environmental variation influences parental cooperation has not yet been well researched. Yet, a recent study compares cooperation (alternation and synchrony of partner provisioning) of great tit pairs breeding in habitat that varies with elevation and forest cover (Lejeune et al., 2019). Lejeune et al. (2019) found that, (1) alternation and provisioning rate was greater among pairs nesting at low elevation, (2) provisioning synchrony was greater in areas less forest and more edge, and (3) that nestlings were heavier when reared by synchronous pairs only in forested habitat. Our results differ from theirs in that we found little evidence that partner coordination is impacted by environmental variance. However, our data corroborate theirs in that the relationship between parental coordination and nestling condition appears to be differently influenced by environmental clines – in both studies the relationship between partner cooperation and nestling size was more apparent under poorer environmental conditions. In the wild, relationships between fitness and parental coordination often may be context dependent.
One important limitation of our study, however, is the correlative approach so no causal effect of tree swallows can be assured. It is possible that these patterns may result from differences in environmental conditions in areas of high and low tree swallow density (for example, tree swallows prefer to settle near water while both species prefer open habitat (Winkler et al., 2011; Jones et al., 2014) or in differences in the type of bluebird pairs that persist (i.e., are able to retain their nestbox) in areas of high tree swallow density. Thus, here we tested the alternative idea that habitat rather than tree swallow density could be influence bluebird coordination and reproductive success. Model comparisons suggest, however, that distance to water is not, generally, as good a predictor of bluebird behavior and reproductive parameters as is swallow density. A more powerful approach would be to experimentally harass bluebird partners to separate potential influences of interspecific density and habitat quality on partner coordinated behaviors and nestling quality. It is also possible that the results could be confounded by age or breeding experience of the birds or partners and birds with prior experience should be more successful (e.g., Sanchez-Macouzet et al., 2014) and may be better able to coordinate provisioning. However, annual survival is low in this population (<20% of birds breed in two or more consecutive years and no partners in these analyses had previously bred together).
Other coordinated parental care behaviors in this population of bluebirds appear to help partners improve reproductive success in areas of high densities of tree swallows. Similarity of partner territorial aggression (as measured by controlled simulated territorial intrusions) leads to heavier nestlings when bluebirds breed in high-density areas, however this relationship is not apparent in low-density areas (Harris and Siefferman, 2014). Together these studies suggest that coordination of both territory defense and provisioning rate may benefit reproductive success, but the effects may only become apparent when tree swallows are present in high numbers.
Thus, our study provides an important new perspective on the resolution of parental negotiations in that it reminds us that fitness benefits are often context dependent. Cooperative investment may be important for parents to ease their sexual conflict and to improve reproductive success under adverse environmental conditions. Such maintenance of partner alternation levels could be achieved if each parent where to match each other’s visit rate. Nonetheless, it is important to note that these alternated nest visits may only reflect similarities in how individuals respond to environmental conditions, offspring demand or to their own individual quality.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
LS conceived the study. CB transcripted video footage and took the lead in field work. Both authors have contributed equally to the analyses, writing, and revisions, gave final approval for publication, and agreed to be held accountable for the work performed therein.
Funding
This research was supported by the Appalachian State University, Office of Student Research and the Biology Department.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Eric Rayfield for help with field work and Kristen Content for help with the data analysis. We thank Jennifer C. Geib and Michael Osbourn and members of the Siefferman and Gangloff Labs for providing comments to early drafts of this manuscript.
References
Albers, A. N., Jones, J. A., and Siefferman, L. (2017). Behavioral differences among eastern bluebird populations could be a consequence of tree swallow presence: a pilot study. Front. Ecol. Evol. 5:116. doi: 10.3389/fevo.2017.00116
Bebbington, K., and Hatchwell, B. J. (2016). Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird. Behav. Ecol. 27, 652–659. doi: 10.1093/beheco/arv198
Black, J. M. (2001). Fitness consequences of long-term pair bonds in barnacle geese: monogamy in the extreme. Behav. Ecol. 12, 640–645. doi: 10.1093/beheco/12.5.640
Burdick, C. (2018). Personalities of Mated Pairs and Parental Provisioning Coordination in Eastern Bluebirds. Thesis, Appalachian State University, Boone.
Curio, E., and Regelmann, K. (1986). Predator harassment implies a real deadly risk: a reply to hennessy. Ethology 72, 75–78. doi: 10.1111/j.1439-0310.1986.tb00607.x
Gowaty, P. A., and Plissner, J. H. (2015). “Eastern bluebird (Sialia sialis),” in The Birds of North America, ed. A. Poole, (Ithaca, NY: Cornell Lab of Ornithology).
Griffioen, M., Müller, W., and Iserbyt, A. (2019). A fixed agreement–consequences of brood size manipulation on alternation in blue tits. PeerJ 7:e6826. doi: 10.7717/peerj.6826
Griffith, S. C. (2019). Cooperation and coordination in socially monogamous birds: moving away from a focus on sexual conflict. Front. Ecol. Evol. 7:455. doi: 10.3389/fevo.2019.00455
Harris, M. R., and Siefferman, L. (2014). Interspecific competition influences fitness benefits of assortative mating for territorial aggression in eastern bluebirds (Sialia sialis). PLoS One 9:e88668. doi: 10.1371/journal.pone.0088668
Hinde, C. A. (2006). Negotiation over offspring care? - A positive response to partner-provisioning rate in great tits. Behav. Ecol. 17, 6–12. doi: 10.1093/beheco/ari092
Iserbyt, A., Fresneau, N., Kortenhoff, T., Eens, M., and Müller, W. (2017). Decreasing parental task specialization promotes conditional cooperation. Sci. Rep. 7:6565. doi: 10.1038/s41598-017-06667-1
Johnstone, R. A., and Hinde, C. A. (2006). Negotiation over offspring care - how should parents respond to each other’s efforts? Behav. Ecol. 17, 818–827. doi: 10.1093/beheco/arl009
Johnstone, R. A., Manica, A., Fayet, A. L., Stoddard, M. C., Rodriguez-Gironés, M. A., and Hinde, C. A. (2014). Reciprocity and conditional cooperation between great tit parents. Behav. Ecol. 25, 216–222. doi: 10.1093/beheco/art109
Johnstone, R. A., and Savage, J. L. (2019). Conditional cooperation and turn-taking in parental care. Front. Ecol. Evol. 7:335. doi: 10.3389/fevo.2019.00335
Jones, J. A., Harris, M. R., and Siefferman, L. (2014). Physical habitat quality and interspecific competition interact to influence territory settlement and reproductive success in a cavity nesting bird. Front. Ecol. Evol. 2:71. doi: 10.3389/fevo.2014.00071
Kamil, A. C., Krebs, J. R., and Pulliam, H. R. (eds) (2012). Foraging Behavior. Berlin: Springer Science & Business Media.
Knight, S. M., Bradley, D. W., Clark, R. G., Gow, E. A., Bélisle, M., Berzins, L. L., et al. (2018). Constructing and evaluating a continent-wide migratory songbird network across the annual cycle. Ecol. Monograph. 88, 445–460. doi: 10.1002/ecm.1298
Krams, I., Krama, T., and Igaune, K. (2006). Mobbing behaviour: reciprocity-based co-operation in breeding pied flycatchers Ficedula hypoleuca. Ibis 148, 50–54. doi: 10.1111/j.1474-919x.2006.00480.x
Lee, D. S. (1993). Range expansion of the tree swallow, tachycineta bicolor (Passeriformes: Hirundinidae), in the southeastern United States. Brimleyana 18, 103–113.
Lejeune, L. A., Savage, J. L., Bründl, A. C., Thiney, A., Russell, A. F., and Chaine, A. S. (2019). Environmental effects on parental care visitation patterns in blue tits Cyanistes caeruleus. Front. Ecol. Evol. 7:356. doi: 10.3389/fevo.2019.00356
Leniowski, K., and Wȩgrzyn, E. (2018). Synchronisation of parental behaviours reduces the risk of nest predation in a socially monogamous passerine bird. Sci. Rep. 8:7385. doi: 10.1038/s41598-018-25746-5
Mariette, M. M., and Griffith, S. C. (2012). Nest visit synchrony is high and correlates with reproductive success in the wild zebra finch Taeniopygia guttata. J. Avian Biol. 43, 131–140. doi: 10.1111/j.1600-048x.2012.05555.x
Mariette, M. M., and Griffith, S. C. (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185, 270–280. doi: 10.1086/679441
McCarty, J. P., and Winkler, D. W. (1999). Foraging ecology and diet selectivity of tree swallows feeding nestlings. Condor 101, 246–254. doi: 10.2307/1369987
Raihani, N. J., Nelson-Flower, M. J., Moyes, K., Browning, L. E., and Ridley, A. R. (2010). Synchronous provisioning increases brood survival in cooperatively breeding pied babblers. J. Anim. Ecol. 79, 44–52. doi: 10.1111/j.1365-2656.2009.01606.x
Sanchez-Macouzet, O., Rodriguez, C., and Drummond, H. (2014). Better stay together: pair bond duration increases individual fitness independent of age-related variation. Proc. R. Soc. B Biol. Sci. 281:20132843. doi: 10.1098/rspb.2013.2843
Shen, S. F., Chen, H. C., Vehrencamp, S. L., and Yuan, H. W. (2010). Group provisioning limits sharing conflict among nestlings in joint-nesting Taiwan yuhinas. Biol. Lett. 6, 318–321. doi: 10.1098/rsbl.2009.0909
van Rooij, E. P., and Griffith, S. C. (2013). Synchronised provisioning at the nest: parental coordination over care in a socially monogamous species. PeerJ 1:e232. doi: 10.7717/peerj.232
Weibe, K. L. (2016). Intraspecific compeition for nests: prior ownership trumps resource holding potential for mountian bluebird competing with tree swallows. Auk 133, 512–519. doi: 10.1642/auk-16-25.1
Westneat, D. F., and Sargent, R. C. (1996). Sex and parenting: the effects of sexual conflict and parentage on parental strategies. Tree 11, 87–91. doi: 10.1016/0169-5347(96)81049-4
Keywords: aggression, conditional cooperation, fitness, pair alternation, pair coordination
Citation: Burdick C and Siefferman L (2020) Interspecific Density Influences the Adaptive Significance of Provisioning Coordination Between Breeding Partners. Front. Ecol. Evol. 8:29. doi: 10.3389/fevo.2020.00029
Received: 24 June 2019; Accepted: 31 January 2020;
Published: 19 February 2020.
Edited by:
James Luke Savage, University of Sheffield, United KingdomReviewed by:
Walter D Koenig, Cornell University, United StatesMylene M Mariette, Deakin University, Australia
Copyright © 2020 Burdick and Siefferman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lynn Siefferman, c2llZmZlcm1hbmxtQGFwcHN0YXRlLmVkdQ==
 Chloe Burdick
Chloe Burdick Lynn Siefferman
Lynn Siefferman