- Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Geelong, VIC, Australia
Acoustic communication is central to many social interactions between family members. Whilst song and begging calls have been extensively studied, in this review I focus on familial interactions, where acoustic communication plays a critical role but has often been overlooked. I show that considering acoustic information transfer challenges the traditional views on sexual and parent-offspring conflicts. In particular, I first discuss the role of acoustic communication between breeding partners in parental care negotiation and coordination. I consider the potential for vocalisations to signal partners' state, in terms of current satiation or energy levels during parental care provisioning. Secondly, I review the occurrence of parent-embryo acoustic communication and highlight the possibility for acoustic developmental programming to facilitate the matching of offspring phenotype with parental provisioning capacities. I also discuss how acoustic information available to avian embryos from the environment may empower them to direct their development, independently of their parents. Thirdly, I bring together evidence on sib-sib acoustic communication before and after birth, and highlight its function in sibling cooperation for hatching synchronisation and resource partitioning. Overall, this synthesis demonstrates the importance of considering acoustic information to understand the evolution of parental care and cooperation.
Introduction
Interactions within the family are regulated by both cooperation and conflict. Whilst parents cooperate with each other to rear offspring, and provide care to their offspring at a cost to themselves, conflict arises between family members over the amount of care to be provided (Trivers, 1972, 1974).
Notably, sexual conflict over parental care, as first formulated by Trivers (1972), occurs when parents share equally the benefit of caring (through increased offspring fitness), but only pay the cost of their own investment in parental care. To maximise its benefit-to-cost ratio, a parent should therefore decrease its own effort and let its partner compensate for the shortfall (Trivers, 1972). Theory generally predicts that, in order for bi-parental care to be evolutionary stable, individuals should only partially compensate for their partner's shortfall (Houston and Davies, 1985; McNamara et al., 1999). Whilst most empirical studies conform to this prediction, many others find full compensation, no change, or instead a decrease in investment as individuals match their partner's effort (reviewed in Hinde, 2006; Harrison et al., 2009). Theory predicts that some of this variation may arise from partners' disparity in access to information about offspring needs, if the least informed parent cues on its partner, thereby matching its effort (Johnstone and Hinde, 2006; Hinde and Kilner, 2007).
Mariette and Griffith (2012, 2015) further proposed that the coordination of parental care could not only lead to investment matching, but may also improve parental care efficiency. Indeed, even though theoretical models generally assume otherwise (but see Johnstone and Savage, 2019), partners may partly share the cost of parental care when it impairs their continued investment within the current breeding attempt or in following attempts with the same partner (Mariette and Griffith, 2015). If so, optimising provisioning may prevail over exploiting partner's efforts, and it is expected that, by providing a simple reciprocity rule, coordination may facilitate negotiation and decrease the cost of sexual conflict (Johnstone et al., 2014). Accordingly, we showed that wild zebra finch parents (Taeniopygia guttata) synchronise provisioning by visiting the nest together during nestling rearing, and that better coordinated pairs produce more fledglings (Mariette and Griffith, 2012). Furthermore, consistent with an efficiency benefit of coordination, partners increased nest visit synchrony when parental workload (brood size) was experimentally increased, and nestling condition increased with parental coordination during foraging (Mariette and Griffith, 2015). Interestingly, we also showed that acoustic communication plays a central role in breeding partners interactions at the nest (Elie et al., 2010), including for the coordination of parental care (Boucaud et al., 2016a, 2017). Likewise, parental care coordination, either by alternating or synchronising nest visits, has been evidenced in several other avian species (e.g., Raihani et al., 2010; Bebbington and Hatchwell, 2016; Koenig and Walters, 2016; Savage et al., 2017; but see Khwaja et al., 2017), including in bi-parental care species with short-term pair bonds (Johnstone et al., 2014; Lejeune et al., 2019) and complex acoustic communication between partners (Gorissen and Eens, 2005). Although the effect on nestling growth is largely unknown (but see Iserbyt et al., 2017; Wojczulanis-Jakubas et al., 2018) and varies with ecological conditions (Lejeune et al., 2019) and foraging behaviour (Mariette and Griffith, 2015), the accumulation of recent studies clearly show that parental care coordination and equitable negotiation are more common than previously assumed.
Here, I further show how multiple other aspects of cooperation have been overlooked, by failing to consider the communication between family members, and the information it conveys. For example, there is some evidence in birds that skin or bill colour signals individual condition, which parents may cue on to adjust their reproductive investment relative to their partner's current provisioning capacity or offspring needs (e.g., foot colour in blue-footed boobies: Velando et al., 2006; Dentressangle et al., 2008; nestling mouth coloration: Ewen et al., 2008). However, even though visual and chemical signals are used, acoustic signals are likely to play a particularly prominent role in avian family communication. Indeed, acoustic signals can readily indicate individuals' immediate state and perhaps their short-term intentions. In addition, interactive acoustic communication, whereby individuals adjust their vocalisations in response to others' signalling, is especially well-suited for real-time negotiation (e.g., Ducouret et al., 2019). Lastly, unlike other senses, the acoustic (or vibratory) channel allows a sophisticated level of communication and negotiation to occur prenatally, in parent-embryo and embryo-embryo interactions (Mariette and Buchanan, 2019b; Noguera and Velando, 2019).
Surprisingly however, with the notable exception of nestling begging calls, the importance of acoustic communication for cooperation within the family has received very little attention. Here, I highlight the role of acoustic communication between breeding partners in parental care coordination. I then point to the multiple ways in which acoustic communication may alter parent-offspring cooperation and co-adaptation, before and after birth. Lastly, I discuss the role of begging calls and other offspring vocalisations in sib-sib interactions, including prenatally.
Vocal Negotiation Between Breeding Partners
The hypothesis that acoustic communication allows partners to negotiate their relative efforts in real-time was first proposed by Boucaud et al. (2016a) (Figure 1). In this study on captive zebra finches, where both partners incubate, we experimentally delayed the return to the nest of the male partner during incubation, and recorded vocal interactions at the nest. As predicted, partners' acoustic interactions were altered by the male delay. More strikingly however, the time the female subsequently stayed off the nest was predicted by her partner's calling rate when he returned, rather than by how long she had been incubating for. Likewise, the female's calling rate when her belated mate returned was the best predictor of the duration of her next incubation bout (Boucaud et al., 2016a). This suggests the male also cued on its partner's vocal behaviour rather than on the female's recent investment, although the reciprocal experiment (delaying female's return) would be interesting to carry. Overall, it therefore appears that individuals signal their need to be off the nest by calling more during nest reliefs, and that the partner respond to this signal by coming back early to relieve its partner. Acoustic communication may thus inform individuals on their partner's state, and hence on the cost of a prolonged incubation bout in that particular moment. Having access to such information, partners may not simply match their investment (i.e., time spent incubating), but instead match the cost of that parental care investment (e.g., as in Griffioen et al., 2019). If so, focussing research on the matching of investments rather than on their costs may misrepresent individuals' investment decisions, and importantly underestimate the level of cooperation within pairs (see also, asymetries in “recovery rate” in Johnstone and Savage, 2019).
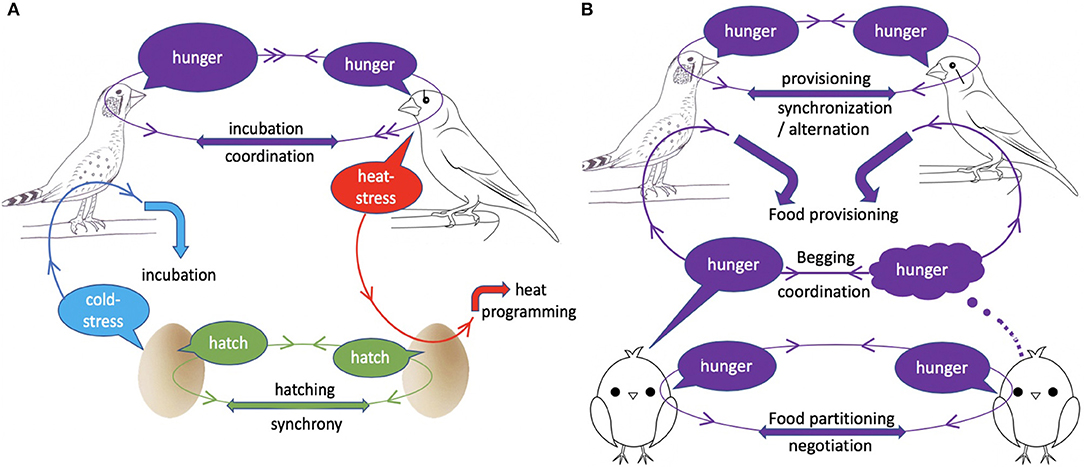
Figure 1. The place of acoustic communication between family members during incubation (A) and nestling rearing (B). During incubation (A), parents coordinate incubation by signalling their hunger levels through calls; vocalisations may also signal (i) parental heat-stress to embryos, which alters offspring developmental response to heat, (ii) embryonic cold-stress to parents, which may optimise incubation temperature, and (iii) eminence of hatching, which allows hatching synchronisation. During nestling rearing (B), offspring vocally negotiate food partitioning among themselves and coordinate begging to increase total provisioning from the parents, whilst parents coordinate and negotiate provisioning. “Information loops” (circles with arrows) show the receiver of each signal and the response it triggered.
In follow up studies, Boucaud, Vignal, and collaborators further demonstrated that females may signal their immediate needs to their partner by vocalizing from inside the nest when the male arrives in the vicinity (Boucaud et al., 2016c, 2017). In wild zebra finches, female calling rate and her calls' acoustic structure on her mate arrival predicted whether or not the male relieved the female for incubation (Boucaud et al., 2017). Likewise, in wild great tits (Parus major), with female-only incubation, female's vocalisations, and the pair's vocal interactions differed depending on whether or not the male entered the nest to feed the female (Gorissen and Eens, 2005; Boucaud et al., 2016c). Moreover, when great tit females were experimentally supplemented with food, they altered their vocalisations, uttered before and after the male entered the nest (Boucaud et al., 2016b). This suggests that female vocalisations may honestly signal her needs, and that the male potentially receives information on female's state even from outside the nest. What would appear as a male-only decision (whether or not to relieve/feed the female) when acoustic information is ignored, may in fact be a negotiated decision, incorporating the female's needs. These studies again reveal a higher level of cooperation between partners than is generally assumed, including in species with short-term pair-bonds.
Acoustic Communication Between Parents and Nestlings
By far the most studied aspect of acoustic communication within the family is offspring begging calls and their importance for parent-offspring negotiation and conflict mitigation (reviewed in Kilner and Johnstone, 1997; Royle et al., 2002; Kilner and Hinde, 2012). Parent-offspring conflict arises because offspring's provisioning rate optimum is expected to be higher than that of their parents, who trade-off current with future reproductive investment, as well as offspring quality with quantity (Trivers, 1974; Stearns, 1992; Kilner and Hinde, 2012). Yet, the costs of begging on the offspring maintain begging as an honest signal of needs, that parents may cue on (Kilner and Johnstone, 1997; Kilner, 2001). Moreover, begging sensitivity to maternal hormones provides a mechanism for mothers to control their offspring's begging display (Eising et al., 2001), potentially allowing the co-adaptation of begging display and parental provisioning capacity (Hinde et al., 2009, 2010). It is clear from this large body of work that considering information transfer through acoustic signals can drastically alter our understanding of conflict and cooperation within the family.
Beside offspring vocalisations, parents are also known to communicate vocally with their nestlings, particularly using alarm or food calls (Madden et al., 2005; Magrath et al., 2007 and references therein). These parental vocalisations have been hypothesised to reduce detection of nests by predators cueing on loud nestling begging calls. Specifically, parental alarm calls for nest predators are found to supress begging (Madden et al., 2005; Platzen and Magrath, 2005), whereas in some species, parents produce food calls when arriving at the nest, which generally indicates to nestlings that it is safe to beg (Madden et al., 2005; Magrath et al., 2007). Indeed, whilst fledglings may blackmail parents by begging in dangerous locations (Thompson et al., 2013), we may expect parents and nestlings to cooperate more closely, as both equally benefit from avoiding detection by predators when nestlings are not yet mobile.
Parent-Embryo Acoustic Communication
Occurrence of Prenatal Acoustic Communication
Prenatal acoustic and vibratory communication is widespread across taxa ranging from insects to humans (Gottlieb, 1965; Grier et al., 1967; DeCasper and Fifer, 1980; Endo et al., 2019). In birds and humans in particular, late-stage embryos have been found to perceive, respond and even learn acoustic signals from their parents and the external environment (Gottlieb, 1965; Grier et al., 1967; Partanen et al., 2013). A large part of the field have focused on the cognitive effects of prenatal acoustic experience, including its role in imprinting and individual recognition (Gottlieb, 1965; Grier et al., 1967; Lickliter and Lewkowicz, 1995), as well as in vocal learning (Mampe et al., 2009; Colombelli-Négrel et al., 2012). A few studies however, have revealed functions of prenatal acoustic communication – namely for developmental programming and embryonic thermoregulation – that warrant further investigation in the context of intra-familial cooperation.
Acoustic Developmental Programming
Extensive research in birds and mammals have demonstrated the importance of maternal hormones in programming offspring development (Schwabl, 1996; Mousseau and Fox, 1998; Groothuis et al., 2005). However, it is not clear how much avian mothers may control the transfer of hormones to their eggs (Groothuis et al., 2019); and post-natal environments, such as climatic conditions or predation risk, may not always be predictable at laying when hormones are deposited into the eggs. Recently, we proposed that prenatal acoustic communication, which mostly occurs late in the incubation period, may provide an alternative mechanism for adaptive developmental programming (Mariette and Buchanan, 2016).
We discovered that adult zebra finch produce a peculiar call at high ambient temperatures, particularly in the late stage of incubation (Mariette and Buchanan, 2016, 2019a). Using playback to embryos in artificial incubators, we demonstrated that this call alone adaptively alters nestlings growth in a temperature-dependent manner (Figure 1). Individuals exposed prenatally to heat calls rather than control calls were lighter in hot nests throughout the nestling period, but then produced more fledglings as adults, consistently across breeding seasons (Mariette and Buchanan, 2016). However, whilst embryonic programming by parental heat calls is adaptive, we later demonstrated that heat calls are not exclusively uttered for embryos. Instead, heat calls are also spontaneously produced (albeit less often) outside the late incubation period, when adults are in roost nests (without eggs) in the wild, or on a perch in a heated chamber in the lab (Mariette et al., 2018). Importantly nonetheless, the temperature threshold triggering calling is highly repeatable within individual, and predicted by body mass, which suggests heat-calls provide an honest signal of parental heat-stress to embryos (Mariette et al., 2018). Whether heat-calling is associated with a particular thermoregulatory behaviour of the parent remains to be established. Nevertheless, it is possible that embryonic eavesdropping on parental heat-stress could benefit both parents and offspring, if it prepares offspring to withstand long periods of fasting during heat events, when their heat-stressed parents have to interrupt provisioning.
Very recently, a second study, in another avian order and environmental context, also provided evidence that embryos eavesdrop on external sounds to channel their development (Mariette and Buchanan, 2019b; Noguera and Velando, 2019). They demonstrated that prenatal exposure to parental alarm calls in yellow-legged gulls shaped the development of embryos and hatchlings, compared to individuals exposed to silence (Noguera and Velando, 2019). Remarkably, prenatal sound altered a wide range of traits, including early skeletal growth, physiology (corticosterone levels), molecular traits (mitochondrial DNA) and behaviour (prenatal vibration and call rates, crouching behaviour). Whether this programming can be considered as parent-offspring cooperation remains to be established, by first testing whether the observed effects are specific to alarm calls, and bring a fitness advantage in a high predation environment (Mariette and Buchanan, 2019b). Fascinatingly however, that same study showed that embryos may cooperate with each other and coordinate their developmental trajectories, by exchanging information, most likely through the changes in vibration or call rates (Noguera and Velando, 2019).
These recent findings clearly show that acoustic signals and cues provide an alternative source of information to embryos, beyond endocrine and nutritional maternal effects. Whilst parents may exploit this information channel (as suggested in zebra finches by the intensification of heat-calling in late incubation compared to other breeding stages or roost nests: (Mariette et al., 2018; Mariette and Buchanan, 2019a), audition can potentially also allow embryos to by-pass parental control by collecting information directly from the environment. Acoustic eavesdropping, similarly to embryonic metabolism of maternal hormones (Groothuis et al., 2019), may therefore empower embryos to control their own developmental trajectory (Mariette et al., 2018). This challenges the traditional views of embryos as a passive agent during their development, and may lead to either parent-offspring conflict or cooperation, depending on the degree to which offspring's interests align with those of their parents.
Vocal Thermoregulation: Honest Signal of Embryonic Thermal Needs?
Sub-optimal incubation temperatures are known to delay hatching and increase embryonic mortality, but also have negative carry-over effects on nestling growth, immune functions and metabolism (Ardia et al., 2010; Nord and Nilsson, 2011; Martin et al., 2013). Therefore, whilst incubation is costly to the parent (Nord and Williams, 2015), it may pay to invest sufficiently in incubation to produce fast-growing nestlings. Accordingly, the majority of avian studies suggest that parents work at the maximum of their capacity during incubation (Chalfoun and Martin, 2007; Ardia et al., 2009; Nord and Williams, 2015; but see Bulla et al., 2014). On the other hand however, experimentally increased parental effort during incubation (by reducing nest temperature) negatively impacts the parents' subsequent investment in nestling provisioning, thereby impairing offspring growth (Ardia et al., 2010). Overall, therefore, because parents have to trade-off the energy they may save during incubation against the additional cost of raising low-efficiency nestlings, and offspring have to trade-off the level of care they receive before vs. after hatching, the optimal incubation temperature for parents and offspring may closely align (Evans et al., 1995). However, since the costs and benefits of parental incubation varies with nest temperature (e.g., Ardia et al., 2009, 2010), we may expect embryos to signal their thermal needs to the incubating parent. In a series of experiments in the 1990s, Evans showed that avian embryos do so using acoustic signals. In a number of precocial and altricial species, embryos increase calling rate when their temperature deviates from the optimal incubation temperature (Evans, 1990; Evans et al., 1995; Bugden and Evans, 1997) (Figure 1). Evans demonstrated that if parents respond to calls by resuming incubation, embryonic vocalisations can effectively maintain optimal incubation temperature (Evans, 1990; Evans et al., 1995). This likely represents a case of parent-offspring cooperation for optimal incubation investment.
Acoustic Cooperation Between Siblings
Vocally Synchronised Hatching
Embryonic vibrations, clicks and calls have long been known to alter hatching time in order to synchronise hatching within clutches (Vince, 1964). In particular, early studies in birds and a more recent one in reptile have shown that late eggs accelerate hatching when exposed to calls or clicks from more developed siblings (Vince, 1964; Woolf et al., 1976; Schwagmeyer et al., 1991; Vergne and Mathevon, 2008) (Figure 1). Most strikingly, a recent study in yellow-legged gulls, where unmanipulated embryos were in physical contact with clutchmates independently exposed to alarm calls or silence, found that prenatal communication between siblings altered not only hatching time, but also a suite of behavioural and physiological traits (Noguera and Velando, 2019; see above). Whether vocally synchronised hatching represents a case of sibling cooperation may depend on the study system. On the one hand, late embryos are understood to accelerate hatching to avoid poor incubation conditions, and associated mortality risk, after hatching of the first eggs (Evans et al., 1995). Early embryos, on the other hand, likely benefit from synchronous hatching through a predation dilution effect, at least in precocial species (Vergne and Mathevon, 2008). However, by eroding the disparity in competitiveness between older and younger siblings, synchronous hatching, particularly in altricial species, increases sibling competition over food, to the detriment of older siblings, and the benefit of the younger ones (Roulin and Dreiss, 2012). Overall therefore, embryos in precocial species may cooperate to synchronise hatching, whereas in altricial species, synchronous hatching may be mostly driven by the benefits to the younger siblings.
Post-hatch Vocal Negotiation and Cooperation Between Siblings
Since Trivers' landmark model (Trivers, 1974), sib-sib interactions post-hatch have been mostly viewed as competitive interactions over parental resources, either through scramble competition between evenly competitive siblings, or through a dominance hierarchy generally following birth order (Mock and Parker, 1997; Roulin and Dreiss, 2012). Nonetheless, following theoretical predictions (Johnstone and Roulin, 2003), there is emerging evidence of negotiation and cooperation among siblings, notably through acoustic communication (Roulin and Dreiss, 2012) (Figure 1). Most remarkably, in the barn owl (Tyto alba), a series of original experiments by Dreiss, Roulin, and collaborators has revealed the vocal negotiation occurring between siblings, before parents bring a single indivisible prey back to the nest (Roulin and Dreiss, 2012). They have shown that owlets challenge each other vocally in an interactive process by adjusting their calls to those of their siblings, either intensifying or reducing begging calls depending on their level of need (Roulin et al., 2009) and their opponents' vocalisations (Ducouret et al., 2019). The most vocal chick in parent's absence is more likely to get the next prey from the parents (Roulin et al., 2009), whilst others refrain from begging to the parents (Dreiss et al., 2010). Interestingly, negotiation rules are dynamic, as individuals become more cooperative with age, being more likely to withdraw from a vocal contest with an hungry sibling (Dreiss et al., 2017). Beside the barn owl, parent-absent vocalisations have also been found to increase with hunger levels and predict nestling begging to parents in both spotted starlings (Sturnus vulgarus) and barn swallows (Hirundo rustica, Bulmer et al., 2008; Romano et al., 2013, 2015), which suggests that sib-sib vocal negotiation may be more widespread than currently acknowledged.
In addition, beside negotiation for food partitioning among siblings, theory predicts that siblings may also cooperate to obtain more resources overall for the brood (Johnstone, 2004) (Figure 1). In both birds and mammals, siblings have been found to coordinate their begging calls, which then increased parental provisioning (Mathevon and Charrier, 2004; Bell, 2007; Madden et al., 2009; Blanc et al., 2010). For example in meerkat, playback of alternating begging calls triggers more provisioning than when calls of the same two individuals overlap (Madden et al., 2009). In addition, the coordination of begging calls within brood or litter may reduce the per-capita cost of begging, as siblings decrease begging call rate when their siblings' calling rate is high (Mathevon and Charrier, 2004; Bell, 2007; Madden et al., 2009).
Conclusion and Perspectives
Even though negotiation has been identified as a key process for the evolution of cooperation and parental care (McNamara et al., 1999, 2003; Johnstone and Hinde, 2006), we know surprisingly little on how negotiation operates, particularly on a behavioural time scale. The evidence brought together here strongly suggests that acoustic communication is likely to play a central role in family negotiation and cooperation. Interestingly, the evidence above on vocal negotiation between incubating partners, temperature-dependent calls in both parents or embryos, and vocal cooperation between siblings pre and post-natally, consistently demonstrates that considering acoustic communication often reveals previously unsuspected cooperative interactions where conflict had instead been considered as the driving force. This likely stems from the capacity of acoustic signals to convey large amount of information, notably on individual's hunger or thermal state in both parents and offspring. Access to this information can drastically alter investment decisions, by allowing the optimisations of costs and benefits to family members. A theoretical approach will be highly valuable in predicting the impact of prenatal and postnatal acoustic communication on the evolution of cooperation and parental care.
In addition, more empirical studies are clearly needed on a range of species to understand the generality of the patterns highlighted here. Beside their ubiquity in many taxa, acoustic signals are particularly amenable to fine experimental research using playbacks and continuous recordings, including automatic interactive playbacks (Ducouret et al., 2019) and playbacks targeting specific individuals (e.g., Hinde and Kilner, 2007). Integrating acoustic communication to the study of cooperation is therefore a highly promising field of research.
Author Contributions
MM conceived the manuscript, reviewed published literature, and wrote the manuscript.
Funding
This research was funded by the Australian Research Council grants DE170100824 and DP180101207 to MM.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I am grateful to Kate Buchanan and members of the Centre for Integrative Ecology for discussions on this topic over the years and to Raoul Ribot and Justin Eastwood for technical assistance.
References
Ardia, D. R., Pérez, J. H., Chad, E. K., Voss, M. A., and Clotfelter, E. D. (2009). Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behaviour. J. Anim. Ecol. 78, 4–13. doi: 10.1111/j.1365-2656.2008.01453.x
Ardia, D. R., Pérez, J. H., and Clotfelter, E. D. (2010). Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc. R.Soc. B-Biol. Sci. 277, 1881–1888. doi: 10.1098/rspb.2009.2138
Bebbington, K., and Hatchwell, B. J. (2016). Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird. Behav. Ecol. 27, 652–659. doi: 10.1093/beheco/arv198
Bell, M. B. (2007). Cooperative begging in banded mongoose pups. Curr. Biol. 17, 717–721. doi: 10.1016/j.cub.2007.03.015
Blanc, A., Ogier, N., Roux, A., Denizeau, S., and Mathevon, N. (2010). Begging coordination between siblings in black-headed gulls. C. R. Biol. 333, 688–693. doi: 10.1016/j.crvi.2010.06.002
Boucaud, I. C. A., Mariette, M. M., Villain, A. S., and Vignal, C. (2016a). Vocal negotiation over parental care? Acoustic communication at the nest predicts partners' incubation share. Biol. J. Linn. Soc. 117, 322–336. doi: 10.1111/bij.12705
Boucaud, I. C. A., Perez, E. C., Ramos, L. S., Griffith, S. C., and Vignal, C. (2017). Acoustic communication in zebra finches signals when mates will take turns with parental duties. Behav. Ecol. 28, 645–656. doi: 10.1093/beheco/arw189
Boucaud, I. C. A., Smith, M., Valere, P. A., and Vignal, C. (2016b). Incubating females signal their needs during intrapair vocal communication at the nest: a feeding experiment in great tits. Anim. Behav. 122, 77–86. doi: 10.1016/j.anbehav.2016.09.021
Boucaud, I. C. A., Valere, P. A., Smith, M., Doligez, B., Cauchard, L., Rybak, F., et al. (2016c). Interactive vocal communication at the nest by parent great tits parus major. IBIS 158, 630–644. doi: 10.1111/ibi.12374
Bugden, S. C., and Evans, R. M. (1997). Vocal solicitation of heat as an integral component of the developing thermoregulatory system in young domestic chickens. Can. J. Zool. 75, 1949–1954. doi: 10.1139/z97-826
Bulla, M., Creswell, W., Rutten, A. L., Vaclu, M., and Kempenaers, B. (2014). Biparental incubation-scheduling: no experimental evidence for major energetic constraints. Behav. Ecol. 26:30–37. doi: 10.1093/beheco/aru156
Bulmer, E., Celis, P., and Gil, D. (2008). Parent-absent begging: evidence for sibling honesty and cooperation in the spotless starling (sturnus unicolor). Behav. Ecol. 19, 279–284. doi: 10.1093/beheco/arm134
Chalfoun, A. D., and Martin, T. E. (2007). Latitudinal variation in avian incubation attentiveness and a test of the food limitation hypothesis. Anim. Behav. 73, 579–585. doi: 10.1016/j.anbehav.2006.09.010
Colombelli-Négrel, D., Hauber, M. E., Robertson, J., Sulloway, F. J., Hoi, H., Griggio, M., et al. (2012). Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. doi: 10.1016/j.cub.2012.09.025
DeCasper, A. J., and Fifer, W. P. (1980). Of human bonding - newborns prefer their mothers voices. Science 208, 1174–1176. doi: 10.1126/science.7375928
Dentressangle, F., Boeck, L., and Torres, R. (2008). Maternal investment in eggs is affected by male feet colour and breeding conditions in the blue-footed booby, sula nebouxii. Behav. Ecol. Sociobiol. (Print). 62, 1899–1908. doi: 10.1007/s00265-008-0620-6
Dreiss, A., Lahlah, N., and Roulin, A. (2010). How siblings adjust sib-sib communication and begging signals to each other. Anim. Behav. 80, 1049–1055. doi: 10.1016/j.anbehav.2010.09.012
Dreiss, A. N., Ruppli, C. A., Delarbre, A., Faller, C., and Roulin, A. (2017). Responsiveness to siblings' need increases with age in vocally negotiating barn owl nestlings. Behav. Ecol. Sociobiol. (Print). 71, 109. doi: 10.1007/s00265-017-2342-0
Ducouret, P., Romano, A., Dreiss, A. N., Marmaroli, P., Falourd, X., and Roulin, A. (2019). The art of diplomacy in vocally negotiating barn owl siblings. Front. Ecol. Evol. 7:351. doi: 10.3389/fevo.2019.00351
Eising, C. M., Eikenaar, C., Schwabl, H., and Groothuis, T. G. (2001). Maternal androgens in black-headed gull (larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. Lond. Ser. B Biol. Sci. 268, 839–846. doi: 10.1098/rspb.2001.1594
Elie, J. E., Mariette, M. M., Soula, H. A., Griffith, S. C., Mathevon, N., and Vignal, C. (2010). Vocal communication at the nest between mates in wild zebra finches: A private vocal duet? Anim. Behav. 80, 597–605. doi: 10.1016/j.anbehav.2010.06.003
Endo, J., Takanashi, T., Mukai, H., and Numata, H. (2019). Egg-cracking vibration as a cue for stink bug siblings to synchronize hatching. Curr. Biol. 29, 143–148. doi: 10.1016/j.cub.2018.11.024
Evans, R. M. (1990). Vocal regulation of temperature by avian embryos - a laboratory study with pipped eggs of the american white pelican. Anim. Behav. 40, 969–979. doi: 10.1016/S0003-3472(05)80999-6
Evans, R. M., Wiebe, M. O., Lee, S. C., et al. (1995). Embryonic and parental preferences for incubation temperature in herring gulls - implications for parent offsrping conflict. Behav. Ecol. Sociobiol. (Print). 36, 17–23. doi: 10.1007/s002650050120
Ewen, J. G., Thorogood, R., Karadas, F., Cassey, P., Ewen, J. G., Thorogood, R., et al. (2008). Condition dependence of nestling mouth colour and the effect of supplementing carotenoids on parental behaviour in the hihi (notiomystis cincta). Oecologia 157, 361–368. doi: 10.1007/s00442-008-1073-3
Gorissen, L., and Eens, M. (2005). Complex female vocal behaviour of great and blue tits inside the nesting cavity. Behaviour 142, 489–506. doi: 10.1163/1568539054012056
Gottlieb, G. (1965). Prenatal auditory sensitivity in chickens and ducks. Science 147, 1596–1598. doi: 10.1126/science.147.3665.1596
Grier, J. B., Counter, S. A., and Shearer, W. M. (1967). Prenatal auditory imprinting in chickens. Science 155, 1692–1693. doi: 10.1126/science.155.3770.1692
Griffioen, M., Iserbyt, A., and Muller, W. (2019). Handicapping males does not affect their rate of parental provisioning, but impinges on their partners' turn taking behavior. Front. Ecol. Evol. 7:347. doi: 10.3389/fevo.2019.00347
Groothuis, T. G., Müller, W., von Engelhardt, N., Carere, C., and Eising, C. (2005). Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352. doi: 10.1016/j.neubiorev.2004.12.002
Groothuis, T. G. G., Hsu, B. Y., Kumar, N., and Tschirren, B. (2019). Revisiting mechanisms and functions of prenatal hormone-mediated maternal effects using avian species as a model. Philos. Trans. R. Soc. B Biol. Sci. 374:20180115. doi: 10.1098/rstb.2018.0115
Harrison, F., Barta, Z., Cuthill, I., and Székely, T. (2009). How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800–1812. doi: 10.1111/j.1420-9101.2009.01792.x
Hinde, C. A. (2006). Negotiation over offspring care? A positive response to partner-provisioning rate in great tits. Behav. Ecol. 17, 6–12. doi: 10.1093/beheco/ari092
Hinde, C. A., Buchanan, K. L., and Kilner, R. M. (2009). Prenatal environmental effects match offspring begging to parental provisioning. Proc. R.Soc. B Biol. Sci. 276, 2787–2794. doi: 10.1098/rspb.2009.0375
Hinde, C. A., Johnstone, R. A., and Kilner, R. M. (2010). Parent-offspring conflict and coadaptation. Science 327, 1373–1376. doi: 10.1126/science.1186056
Hinde, C. A., and Kilner, R. M. (2007). Negotiations within the family over the supply of parental care. Proc. R.Soc. B-Biol. Sci. 274, 53–60. doi: 10.1098/rspb.2006.3692
Houston, A. I., and Davies, N. B. (1985). The Evolution of Cooperation and Life History in the Dunnock Prunella Modularis. Oxford: Blackwell Scientific Publications.
Iserbyt, A., Fresneau, N., Kortenhoff, T., Eens, M., and Müller, W. (2017). Decreasing parental task specialization promotes conditional cooperation. Sci. Rep. 7:6565. doi: 10.1038/s41598-017-06667-1
Johnstone, R. A. (2004). Begging and sibling competition: how should offspring respond to their rivals? Am. Nat. 163, 388–406. doi: 10.1086/375541
Johnstone, R. A., and Hinde, C. A. (2006). Negotiation over offspring care - how should parents respond to each other's efforts? Behav. Ecol. 17, 818–827. doi: 10.1093/beheco/arl009
Johnstone, R. A., Manica, A., Fayet, A. L., Stoddard, M. C., Rodriguez-Gironés, M. A., Hinde, C. A., et al. (2014). Reciprocity and conditional cooperation between great tit parents. Behav. Ecol. 25, 216–222. doi: 10.1093/beheco/art109
Johnstone, R. A., and Roulin, A. (2003). Sibling negotiation. Behav. Ecol. 14, 780–786. doi: 10.1093/beheco/arg024
Johnstone, R. A., and Savage, J. L. (2019). Conditional cooperation and turn-taking in parental care. Front. Ecol. Evol. 7:335. doi: 10.3389/fevo.2019.00335
Khwaja, N., Preston, S. A. J., Hatchwell, B. J., Briskie, J. V., Winney, I. S., Savage, J. L., et al. (2017). Flexibility but no coordination of visits in provisioning riflemen. Anim. Behav. 125, 25–31. doi: 10.1016/j.anbehav.2016.12.021
Kilner, R., and Johnstone, R. A. (1997). Begging the question: are offspring solicitation behaviours signals of needs. Trends Ecol. Evol. (Amst). 12, 11–15. doi: 10.1016/S0169-5347(96)10061-6
Kilner, R. M. (2001). A growth cost of begging in captive canary chicks. Proc. Natl. Acad. Sci. U.S.A. 98, 11394–11398. doi: 10.1073/pnas.191221798
Kilner, R. M., and Hinde, C. A. (2012). “Parent-offspring conflict,” in Evolution of parental care. eds N. J. Royle, P. T. Smiseth, and M. Kolliker (Oxford: Oxford University Press), 119–132.
Koenig, W. D., and Walters, E. L. (2016). Provisioning patterns in the cooperatively breeding acorn woodpecker: does feeding behaviour serve as a signal? Anim. Behav. 119, 125–134. doi: 10.1016/j.anbehav.2016.06.002
Lejeune, L., Savage, J. L., Brundl, A. C., Thiney, A., Russell, A. F., and Chaine, A. S. (2019). Environmental effects on parental care visitation patterns in blue tits cyanistes caeruleus. Front. Ecol. Evol. 7:356. doi: 10.3389/fevo.2019.00356
Lickliter, R., and Lewkowicz, D. J. (1995). Intersensory experience and early perceptual development - attenuated prenatal sensory stimulation affects postnatal auditory and visual responsiveness in bobwhite quail chicks (Colinus virginianus). Dev. Psychol. 31, 609–618. doi: 10.1037/0012-1649.31.4.609
Madden, J. R., Kilner, R. M., and Davies, N. B. (2005). Nestling responses to adult food and alarm calls: 1. Species-specific responses in two cowbird hosts. Anim. Behav. 70, 619–627. doi: 10.1016/j.anbehav.2004.11.019
Madden, J. R., Kunc, H. P., English, S., Manser, M. B., and Clutton-Brock, T. H. (2009). Calling in the gap: competition or cooperation in littermates' begging behaviour? Proc. R.Soc. B-Biol. Sci. 276, 1255–1262. doi: 10.1098/rspb.2008.1660
Magrath, R. D., Pitcher, B. J., and Dalziell, A. H. (2007). How to be fed but not eaten: nestling responses to parental food calls and the sound of a predator's footsteps. Anim. Behav. 74, 1117–1129. doi: 10.1016/j.anbehav.2007.01.025
Mampe, B., Friederici, A. D., Christophe, A., and Wermke, K. (2009). Newborns' cry melody is shaped by their native language. Curr. Biol. 19, 1994–1997. doi: 10.1016/j.cub.2009.09.064
Mariette, M. M., and Buchanan, K. L. (2016). Prenatal acoustic communication programs offspring for high posthatching temperatures in a songbird. Science 353, 812–814. doi: 10.1126/science.aaf7049
Mariette, M. M., and Buchanan, K. L. (2019a). Calling in the heat: the zebra finch incubation call depends on heat and reproductive stage: a comment on mcdiarmid et al. 2018. Behav. Ecol. 30, 1–3. doi: 10.1093/beheco/arz045
Mariette, M. M., and Buchanan, K. L. (2019b). Good vibrations in the nest. Nat. Ecol. Evol. 3, 1144–1145. doi: 10.1038/s41559-019-0955-6
Mariette, M. M., and Griffith, S. C. (2012). Nest visit synchrony is high and correlates with reproductive success in the wild zebra finch taeniopygia guttata. J. Avian Biol. 43, 131–140. doi: 10.1111/j.1600-048X.2012.05555.x
Mariette, M. M., and Griffith, S. C. (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185, 270–280. doi: 10.1086/679441
Mariette, M. M., Pessato, A., Buttemer, W. A., McKechnie, A. E., Udino, E., Collins, R. N., et al. (2018). Parent-embryo acoustic communication: a specialised heat vocalisation allowing embryonic eavesdropping. Sci. Rep. 8:17721. doi: 10.1038/s41598-018-35853-y
Martin, T. E., Ton, R., and Niklison, A. (2013). Intrinsic vs. Extrinsic influences on life history expression: Metabolism and parentally induced temperature influences on embryo development rate. Ecol. Lett. 16, 738–745. doi: 10.1111/ele.12103
Mathevon, N., and Charrier, I. (2004). Parent-offspring conflict and the coordination of siblings in gulls. Proc. R.Soc. B Biol. Sci. 271, S145–S147. doi: 10.1098/rsbl.2003.0117
McNamara, J. M., Gasson, C. E., and Houston, A. I. (1999). Incorporating rules for responding into evolutionary games. Nature 401, 368–371. doi: 10.1038/43869
McNamara, J. M., Houston, A. I., Barta, Z., et al. (2003). Should young ever be better off with one parent than with two? Behav. Ecol. 14, 301–310. doi: 10.1093/beheco/14.3.301
Mock, D. W., and Parker, G. A. (1997). The Evolution of Sibling Rivalry. Oxford: Oxford University Press.
Mousseau, T. A., and Fox, C. W. (1998). The adaptive significance of maternal effects. Trends Ecol. Evol. (Amst). 13, 403–407. doi: 10.1016/S0169-5347(98)01472-4
Noguera, J. C., and Velando, A. (2019). Bird embryos perceive vibratory cues of predation risk from clutch mates. Nat. Ecol. Evol. 3, 1225–1232. doi: 10.1038/s41559-019-0929-8
Nord, A., and Nilsson, J. Å. (2011). Incubation temperature affects growth and energy metabolism in blue tit nestlings. Am. Nat. 178, 639–651. doi: 10.1086/662172
Nord, A., and Williams, J. B. (2015). “The energetic costs of incubation,” in Nests, Eggs and Incubation: New Ideas About Avian Reproduction. eds D. C. Deeming, and S. J. Reynolds (Oxford: Oxford University Press), 152–170.
Partanen, E., Kujala, T., Näätänen, R., Liitola, A., Sambeth, A., and Huotilainen, M. (2013). Learning-induced neural plasticity of speech processing before birth. Proc. Natl. Acad. Sci. U.S.A. 110, 15145–15150. doi: 10.1073/pnas.1302159110
Platzen, D., and Magrath, R. D. (2005). Adaptive differences in response to two types of parental alarm call in altricial nestlings. Proc. R. Soc. B Biol. Sci. 272, 1101–1106. doi: 10.1098/rspb.2005.3055
Raihani, N. J., Nelson-Flower, M. J., Moyes, K., Browning, L. E., and Ridley, A. R. (2010). Synchronous provisioning increases brood survival in cooperatively breeding pied babblers. J. Anim. Ecol. 79, 44–52. doi: 10.1111/j.1365-2656.2009.01606.x
Romano, A., Boncoraglio, G., Rubolini, D., and Saino, N. (2013). Parent-absent signalling of need and its consequences for sibling competition in the barn swallow. Behav. Ecol. Sociobiol. (Print). 67, 851–859. doi: 10.1007/s00265-013-1508-7
Romano, A., Rubolini, D., Caprioli, M., Musitelli, F., Ambrosini, R., and Saino, N. (2015). Parent-absent begging in barn swallow broods: causes of individual variation and effects on sibling interactions and food allocation. Evol. Biol. 42, 432–442. doi: 10.1007/s11692-015-9336-5
Roulin, A., and Dreiss, A. (2012). “Sibling competition and cooperation over parental care,” in Evolution of Parental Care. eds N. J. Royle, P. T. Smiseth, and M. Kolliker (Oxford: Oxford University Press), 133–149.
Roulin, A., Dreiss, A., Fioravanti, C., and Bize, P. (2009). Vocal sib-sib interactions: how siblings adjust signalling level to each other. Anim. Behav. 77, 717–725. doi: 10.1016/j.anbehav.2008.12.004
Royle, N. J., Hartley, I. R., and Parker, G. A. (2002). Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. (Amst). 17, 434–440. doi: 10.1016/S0169-5347(02)02565-X
Savage, J. L., Browning, L. E., Manica, A., Russell, A. F., Johnstone, R. A., Savage, J. L., et al. (2017). Turn-taking in cooperative offspring care: By-product of individual provisioning behavior or active response rule? Behav. Ecol. Sociobiol. 71:162. doi: 10.1007/s00265-017-2391-4
Schwabl, H. (1996). Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. A Physiol. 114, 271–276. doi: 10.1016/0300-9629(96)00009-6
Schwagmeyer, P. L., Mock, D. W., Lamey, T. C., Lamey, C. S., and Beeher, M. D. (1991). Effects of sibling contact on hatch timing in an asynchronously hatching bird. Anim. Behav. 41, 887–894. doi: 10.1016/S0003-3472(05)80355-0
Thompson, A. M., Raihani, N. J., Hockey, P. A. R., Britton, A., Finch, F. M., and Ridley, A. R. (2013). The influence of fledgling location on adult provisioning: a test of the blackmail hypothesis. Proc. R. Soc. B Biol. Sci. 280:20130558. doi: 10.1098/rspb.2013.0558
Trivers, R. L. (1972). “Parental investment and sexual selection,” in Sexual Selection and the Descent of Man. ed Cambell B (London: Heinemann), 136–207.
Velando, A., Beamonte-Barrientos, R., Torres, R., Velando, A., Beamonte-Barrientos, R., and Torres, R. (2006). Pigment-based skin colour in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149, 535–542. doi: 10.1007/s00442-006-0457-5
Vergne, A. L., and Mathevon, N. (2008). Crocodile egg sounds signal hatching time. Curr. Biol. 18, R513–R514. doi: 10.1016/j.cub.2008.04.011
Vince, M. A. (1964). Social facilitation of hatching in the bobwhite quail. Nature 12, 531–534. doi: 10.1016/0003-3472(64)90075-2
Wojczulanis-Jakubas, K., Araya-Salas, M., and Jakubas, D. (2018). Seabird parents provision their chick in a coordinated manner. PLoS ONE 13:e0189969. doi: 10.1371/journal.pone.0189969
Keywords: cooperation, coordination, negotiation, conflict, acoustic communication, prenatal interactions, acoustic developmental programming
Citation: Mariette MM (2019) Acoustic Cooperation: Acoustic Communication Regulates Conflict and Cooperation Within the Family. Front. Ecol. Evol. 7:445. doi: 10.3389/fevo.2019.00445
Received: 04 July 2019; Accepted: 04 November 2019;
Published: 22 November 2019.
Edited by:
James Luke Savage, University of Sheffield, United KingdomReviewed by:
Pauline Ducouret, Université de Lausanne, SwitzerlandDiane Colombelli-Negrel, Flinders University, Australia
Copyright © 2019 Mariette. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mylene M. Mariette, bS5tYXJpZXR0ZUBkZWFraW4uZWR1LmF1
 Mylene M. Mariette
Mylene M. Mariette