- 1Mara Hyena Project, Department of Integrative Biology and Program in Ecology, Evolutionary Biology and Behavior, Michigan State University, East Lansing, MI, United States
- 2Animal Behavior & Cognition Lab, Department of Zoology and Physiology and Program in Ecology, University of Wyoming, Laramie, WY, United States
Innovation is a well-studied cognitive phenomenon related to general intelligence and brain size. Innovative ability varies considerably within species and it is widely assumed that this variation must have important fitness consequences. However, direct evidence for a link between innovative ability and fitness has rarely been shown. Previous research examined variation in innovative problem-solving in wild spotted hyenas when confronting a novel puzzle box baited with meat. The earlier work revealed that variation in innovativeness in spotted hyenas was not related to age, sex, or social rank, but was predicted by neophobia, persistence, and diversity of motor responses to the puzzle. Here, we used the same dataset from wild spotted hyenas to investigate potential links between innovativeness and fitness. We found that innovative hyenas had lower offspring survivorship than non-innovators, but higher annual cub production (ACP). To test the hypothesis that high ACP can compensate for low offspring survival, we also measured annual cub survivorship (ACS) counting only offspring that survived at least 1 year. Here, there was no significant difference between innovators and non-innovators, which suggests that higher ACP does compensate for lower offspring survival, at least to 1 year of age. Overall, our data suggest that innovativeness may have both costs and benefits for fitness in wild spotted hyenas.
Introduction
Innovation, solving a novel problem, or using a novel behavior to solve a familiar problem, is widely studied in humans and animals (Kummer and Goodall, 1985; Reader and Laland, 2003). Innovation has been strongly linked to brain size across bird, primate, and carnivore species (Reader and Laland, 2002; Lefebvre et al., 2004; Benson-Amram et al., 2016). Innovation is also thought to be an important marker of high general intelligence (Ramsey et al., 2007; Reader et al., 2016) across a diverse array of taxa. Whereas the socio-ecological causes of inter- and intraspecific variation in innovative ability have been well-studied (reviewed in Reader and Laland, 2003; Reader et al., 2016), the fitness consequences of variation in innovativeness have rarely been examined despite growing interest (Morand-Ferron et al., 2015; Ashton et al., 2018; Boogert et al., 2018).
Innovative ability is typically measured on the species level by observing the rate of spontaneous innovations demonstrated in the wild (Lefebvre et al., 2013), and on the individual level by experimentally presenting captive or wild subjects with novel problem-solving tasks (Griffin and Guez, 2014; Reader et al., 2016), which typically require performance of a novel behavior to obtain a reward. Research suggests that innovativeness may be beneficial for adjusting to novelty and environmental change (Sol et al., 2016), an idea supported by correlations between innovation rates and generalist dietary or habitat preferences (Overington et al., 2011b; Ducatez et al., 2014). Furthermore, species that are more innovative appear more likely to invade novel habitats, including urban ones (Sol et al., 2005; Griffin and Diquelou, 2015). Finally, more innovative bird species tend to have slower life-histories and longer lifespans (Sol, 2009; Sol et al., 2016). Overall, this work suggests that innovativeness is likely adaptive for individuals responding to environmental change and novelty by enabling those individuals to express novel behaviors, exploit novel food sources, or avoid novel sources of mortality. Likewise, innovativeness is generally assumed to increase fitness through enhanced survival or reproductive success by buffering individuals against mortality-causing events (Sol, 2009; Sol et al., 2016), by increasing mating success (e.g., Keagy et al., 2009; Chen et al., 2019), or by increasing foraging rate, efficiency or quality. However, direct evidence supporting these assumptions is scarce.
Although innovation and general intelligence in humans have been consistently related to positive life outcomes (Plomin and Deary, 2015), the relationship between innovative problem-solving and fitness in non-human animals is much less clear. Across bird species, five studies have found a positive link between innovative problem-solving and fitness measures that included mating success, clutch size, hatching success, fledgling survival, provisioning rates, and offspring survival (Keagy et al., 2009; Cauchard et al., 2013; Preiszner et al., 2017; Wetzel, 2017; Chen et al., 2019). However, other studies of birds found no relationship between innovative problem-solving and mating success (Isden et al., 2013), or found that innovative problem-solving was correlated with lower competitiveness and higher nest desertion (Cole et al., 2012a,b). In the only study that has looked at innovation and fitness in a mammal, Huebner et al. (2018) found no link between more efficient problem-solving and any measure of fitness in mouse lemurs. Overall, the literature linking innovation and fitness in animals is very small, with limited taxonomic representation, and with largely mixed results. Here our goal was to examine the relationship between innovativeness and fitness in wild spotted hyenas.
Spotted hyenas are large African carnivores that have previously been established as a good model system for testing hypotheses about the evolution of cognition (Holekamp et al., 2007). Unlike most large carnivores in Africa, spotted hyenas are not endangered; their success may have been facilitated by their impressive behavioral flexibility. Spotted hyenas are generalist feeders; they eat everything from termites to elephants (Cooper et al., 1999; Hayward, 2006) and have established themselves in nearly every habitat in sub-Saharan Africa (Holekamp and Dloniak, 2010) including urban ones (Yirga et al., 2017). Earlier research found that spotted hyenas show innovative ability similar to that of wild vervet monkeys (Benson-Amram and Holekamp, 2012), and that hyenas also show high levels of innovativeness relative to other carnivores (Benson-Amram et al., 2016). In the present study we aimed to test the idea that innovativeness might be an adaptive trait in spotted hyenas by comparing their problem-solving performance to three measures of fitness. To do this, we used a subset of the data from Benson-Amram and Holekamp (2012) on innovative problem-solving in female spotted hyenas, and analyzed it in relation to our long-term data on reproduction and survival for each female. This subset included 29 female spotted hyenas, of which five were considered innovative.
Hyena fitness has been linked to both social and ecological variables. Dominance rank has large effects on lifetime reproductive success in hyenas; the highest ranking female in a clan may have up to five times more offspring than the lowest ranking female due to better access to food, younger ages at first parturition, shorter interbirth intervals, better offspring survival, and longer reproductive lifespans (Frank, 1986; Holekamp et al., 1996). Finally, ecological variables such as prey abundance and competition with lions also affect reproductive success and juvenile survival (Watts and Holekamp, 2009). Our goal here was to test the hypothesis that innovativeness is adaptive in regard to both reproductive success and survival in wild spotted hyenas; if true, we expected to see a direct positive relationship between innovative problem-solving and our measures of fitness.
Materials and Methods
Subjects, Population, Location
The subjects were individuals from two neighboring clans (the Talek West clan and the Fig Tree clan) of spotted hyenas in the Maasai Mara National Reserve, Kenya. Individuals were identified by their unique spot patterns and other natural markings. Observations were conducted daily from 0530 to 0900 h and from 1700 h to 2000 h, on an average of 23.5 days per month. The Talek West clan was monitored continuously from May 1988 to December 2016, and the Fig Tree clan was monitored continuously from April 2007 to May 2015. All innovation testing took place between May 2007 and May 2008; during this period, the Talek West clan contained 46–48 members, including 12–13 adult females with their juvenile offspring and 10 adult males, and the Fig Tree clan contained 36–38 members, including 10 adult females with their juvenile offspring and 7–8 adult immigrant males. Additional information about the study subjects, methods and materials can be found in Benson-Amram and Holekamp (2012). Although innovativeness was tested in both male and female hyenas, in the current analysis we only included female hyenas for which we had reproductive data.
Problem-Solving Apparatus
We used a novel problem-solving apparatus to test innovative ability. The “puzzle box” used here measured 60 × 31 × 37 cm and was built from welded 10.5 mm steel rebar (Figure 1). The box had a single 30 × 34 cm door on one long side, large enough for a hyena to put its head inside the box, and handles in the center of each short side. When it was baited with roughly 2 kg of raw meat, the box weighed more than 35 kg. To obtain access to the meat, a subject had to slide a 12 cm steel bolt latch laterally using the mouth or forepaws, and the door would swing open. For more detail on the apparatus, see Benson-Amram and Holekamp (2012). Successful trials were those in which the puzzle box was opened. Unsuccessful trials included those in which the hyena contacted the box, but failed to open it, as well as those in which the hyena did not interact with the box, despite spending time within 5 m of it (average duration in minutes spent within 5 m on the first trial ±SD = 11.95 ± 13.47, N = 29). Previously, we found that 14.5% of all hyenas tested with this problem-solving task had at least one successful trial. Within this group of successful hyenas, 78% were successful on subsequent trials. Trial number was a significant predictor of latency to solve the problem, with hyenas generally solving the box faster in later trials, which suggests that hyenas learned how to open the box (see Figure 2 in Benson-Amram and Holekamp, 2012). Successful problem-solving also showed modest but significant repeatability after controlling for the effect of trial number (R = 0.24, SE = 0.12, CI = 0.03–0.41, P < 0.0001) (rptR package; Stoffel et al., 2017). Additionally, another study, with the same population of wild spotted hyenas, found that innovation was significantly repeatable across four novel problem-solving tasks (R = 0.96; Johnson-Ulrich et al., in review). Therefore, in the present study hyenas were defined as innovative if they had at least one successful trial and non-innovative if they had only unsuccessful trials.
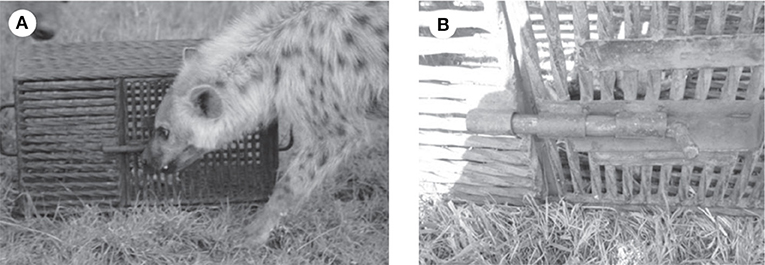
Figure 1. (A) An image of the puzzle box apparatus used in the experiment. (B) A close-up image of the latch bolt that hyenas had to move laterally in order to access the meat inside the puzzle box. Republished from Benson-Amram and Holekamp (2012).
Data Collection Protocols
Because we were working with a wild population, subjects for these experiments were chosen opportunistically, based on which animals were available at the time. However, every attempt was made to conduct equal numbers of trials with all the individuals in each clan, and to balance the number of participants in each age, sex and social rank category. When an appropriate subject animal was sighted in an accessible location, we parked our research vehicle ~100 m upwind of the hyena. The box was placed on the ground on the opposite side of the vehicle from the hyena in a location with good visual access, both for the subject, and for observers. The box was oriented with the door toward the hyena, with the latch protruding at 90° from the box, parallel to the ground. We then pulled the vehicle back ~50 m from the box and initiated observations. A trial began when a hyena approached to within 5 m of the box (thereby becoming a “focal hyena”); the trial ended when the hyena left the 5 m radius and remained outside of it for 5 min, or when it moved to at least 200 m from the box. All attempts were made to test subjects only when they were alone, but occasionally conspecifics approached and participated in a trial. However, the presence or absence of conspecifics did not significantly affect the likelihood of a hyena successfully opening the box (Benson-Amram and Holekamp, 2012), so this variable was not analyzed in the present study. All trials were videotaped in their entirety from our vehicle. For more detail on data collection protocols see Benson-Amram and Holekamp (2012).
Fitness Variables
Demography
We used several demographic variables to calculate survivorship and annual reproductive success. First, cub ages were estimated to ± 1 week based on their appearance when first observed (Holekamp et al., 1996), and date of birth (DOB) was calculated on that basis. Date of first conception (DFC) for each adult female was estimated by subtracting 110 days, the length of gestation in spotted hyenas, from the DOB of a female's first observed litter. Some females in the Fig Tree clan were adults when monitoring began; therefore DFC represents the first conception that researchers observed, but might not represent every female's first conception after reaching sexual maturity. Finally, date last seen (DLS) was recorded as the last day on which a hyena was seen alive or, if its body was found, the date on which it was found dead. Female hyenas remain in their natal clans throughout their lives (Kruuk, 1972) so females that had not been seen for at least 6 months were considered to be dead. Standardized social rank was measured as a continuous variable on a scale of 1 to −1 where a rank of 1 indicated the highest-ranking female in the clan and a rank of −1 indicated the lowest-ranking female in the clan. All individuals in a clan were assigned their own rank except for pre-weaning cubs and subadults who were assigned the rank of their mother. Ranks were assigned based on the clan hierarchy during the period from May 2007–2008, when innovation was tested. This hierarchy was generated using a dominance matrix ordering observations of aggressive or submissive behaviors within dyads of adult hyenas (Martin and Bateson, 1993; Holekamp et al., 2012). Rank hierarchies among spotted hyenas are convention-based such that offspring acquire ranks immediately below those of their mothers through a process of maternal interventions and social learning. Social ranks in spotted hyenas are relatively stable and rank reversals are rare (Strauss and Holekamp, 2019).
Offspring Survivorship
Offspring survivorship was calculated from birthdate and mortality data during the first 24 months of life. Mortality was recorded as a binary variable: dead vs. alive. Using this mortality data, the proportion of offspring surviving was estimated at each age (in months) up to 24 months of age. If offspring disappear before 24 months of age, this represents mortality, but this is not necessarily true after 24 months because 24 months represents the age at which hyenas reach sexual maturity and male hyenas begin to disperse then from their natal clans (Van Horn et al., 2003). Thus, disappearance after 24 months of age for male hyenas may be due to either mortality or dispersal.
Annual Reproductive Success
Offspring survivorship does not necessarily correlate with lifetime reproductive success because it doesn't account for the number of offspring produced. Therefore, the next measure of fitness we examined was annual reproductive success. We included two measures of annual reproductive success: annual cub production (ACP) and annual cub survival (ACS). ACP was calculated by dividing the total number of cubs born to a female during the study divided by her observed reproductive lifespan. Observed reproductive lifespans were calculated by subtracting a female's DFC from her DLS or the end date of the study. Annual cub survival (ACS) was calculated in the same manner as ACP, but instead of using the number of cubs born, only the number of cubs surviving to 1 year of age were counted.
Statistical Analysis
All statistical analyses were done using R version 3.5.0 (R Core Team, 2019). To analyze offspring survival we used a Cox proportional hazards model, which is ideal for analyzing right-censored time-to-event data. This model estimated the probably that subjects would survive to specific ages by using both the lifespan and mortality variables described in section Offspring Survivorship. Cox regression was conducted using the R packages “survival” (Therneau and Grambsch, 2000; Therneau, 2015) and “survminer” (Kassambara and Kosinski, 2018). For all other fitness analyses we used linear regression models built using the R package “glmmTMB” (Brooks et al., 2017). The dependent variables in each of our models were survival, ACP, or ACS. Every model included innovativeness as the independent variable. Subject rank, number of trials, and an interaction effect between innovativeness and number of trials were included as potential confounds in all models. We included rank to control for its previously demonstrated effect on reproductive success in spotted hyenas (Holekamp et al., 1996). We included the number of trials each hyena received prior to her first successful trial in each model to control for the number of opportunities each hyena had to open the puzzle box. If the hyena had no successful trials, this number represented the total number of trials in which she participated. Likewise, we added an interaction effect because subjects who solved the puzzle box on their first trial were potentially demonstrating a higher level of innovative ability than those who solved the box after many trials. That is, the effect of innovativeness on fitness might depend on the trial number. We also included a random effect of maternal ID in the Cox regression. Because proportions such as ACP and ACS might not fully account for the potentially confounding effect of length of the observed reproductive lifespan we also included the length of the observed reproductive lifespan as a covariate in these two models. Full output from each model is available in the Supplementary Material (Supplementary Tables 1–3). Model fit for each model was assessed using the R package “DHARMa” (Hartig, 2019). All models showed good fits as indicated by normally distributed residuals, non-significant DHARMa non-parametric dispersion tests, and non-significant Durbin-Watson tests for temporal autocorrelation.
Results
Innovative Problem-Solving
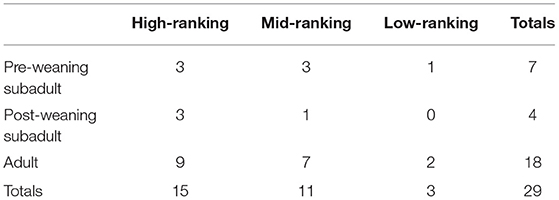
Thirty-three female hyenas participated in trials with the puzzle box; however, the exact social ranks of two females were unknown, and two other females had incomplete reproductive data, so they were dropped from the analysis, yielding a sample size of 29 (Table 1). Of these 29 females, five females were able to open the box at least once, and were thus considered to be innovative. On average, female hyenas received 4.48 ± 4.16 trials (range = 1–14 trials) and opened the box an average of 1.62 ± 4.53 times (range = 0–18).
Offspring Survivorship
These 29 females produced 288 offspring across the study period and we confirmed 114 cases of mortality within the first 24 months of age. Using a Cox proportional hazards regression model, we found that offspring of innovative mothers had significantly lower survival rates during the first 24 months than offspring of non-innovative mothers (cox: z = 2.31, P < 0.02; Figure 2; Supplementary Table 1). None of the other covariates were significant in this model.
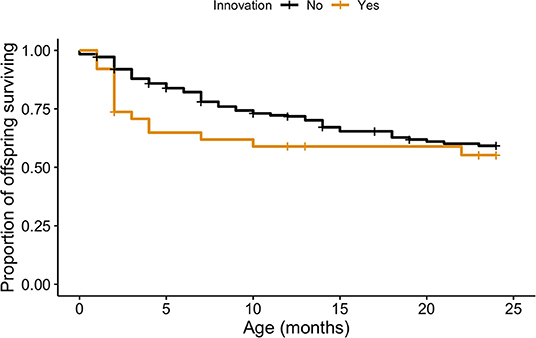
Figure 2. Unadjusted survival curves calculated showing the proportion of offspring surviving at each age point between 0 and 24 months of age for non-innovative and innovative mothers.
Annual Reproductive Success
On average, subjects gave birth to 9.6 ± 4.48 cubs (range = 3–19) during their observed reproductive lifespan. The average length of the observed reproductive lifespan was 8.24 ± 4.57 years (range = 1.73–20.00). Across all 29 females, without controlling for covariates, average ACP was 1.29 ± 0.35 cubs per year (range = 0.60–1.93). In our model, innovative females produced significantly more cubs annually than did non-innovative females (LM: z = 2.85, P = 0.004; Figure 3A; Supplementary Table 2). Innovative females produced an average of 1.35 ± 0.24 (estimated marginal mean ± SE) cubs annually, whereas non-innovative females produced only 1.2 ± 0.07 cubs annually. The length of the observed reproduction lifespan (LM: z = −3.42, P < 0.001) and the interaction between innovativeness and trial number were also significant in this model (Supplementary Table 2). The effect of innovativeness on ACP was highest for female hyenas with the fewest trials (LM: z = −0.44, P = 0.01). When this interaction effect was not included the model, the effect of innovation on ACP was not significant (LM: z = 1.04, P = 0.30).
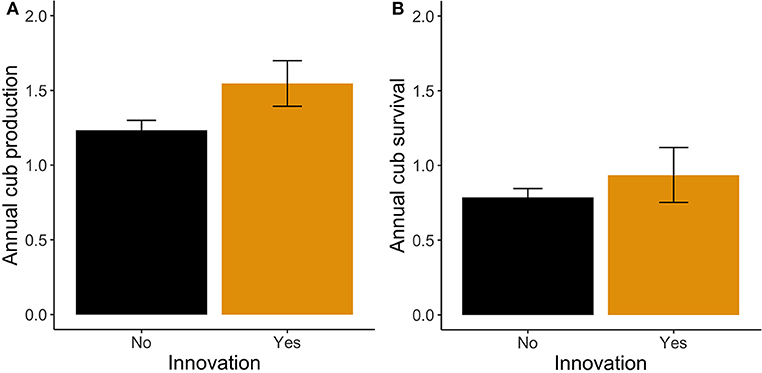
Figure 3. Annual reproductive success for innovative and non-innovative females. (A) Average number of cubs produced annually by female hyenas. (B) Overall number cubs that survive to 1 year of age for female hyenas. Errors bars show standard error.
Next, we investigated the possibility that high ACP could compensate for low offspring survival by comparing the overall number of cubs surviving to 1 year of age between innovative and non-innovative females. On average, without controlling for covariates, females produced 0.81 ± 0.31 surviving cubs each year (range = 0–1.62). In our model, innovative females produced 0.94 ± 0.18 cubs annually and non-innovative females produced 0.79 ± 0.06 cubs annually, but this difference was not significant (LM: z = 1.28, P = 0.20), suggesting that innovative and non-innovative females produce similar numbers of cubs that survive to 1 year of age (Figure 3B; Supplementary Table 3). No other covariates were significant in this model (Supplementary Table 3).
Discussion
We found that innovativeness was linked to fitness in variable ways in wild spotted hyenas. Innovative females had lower offspring survivorship, but gave birth to more offspring annually, than did non-innovative females. In addition, innovative and non-innovative females gave birth to similar numbers of cubs that survived to 1 year of age. This suggests that innovative females might be able to offset lower offspring survival with higher reproductive success. However, given our very small sample size of 29 hyenas (of which, only five were able to solve the puzzle box), our results should be interpreted cautiously. Our results are similar to those from other species suggesting that cognitive abilities may have both fitness costs and benefits. For example, guppies that were artificially selected for larger brains had better performance on cognitive tasks, but produced fewer offspring (Kotrschal et al., 2013), and more innovative great tits had larger clutches but also had higher levels of nest desertion (Cole et al., 2012a). Fitness trade-offs involving non-cognitive traits appear to be fairly common in animals (e.g., Sinervo et al., 2000; Ricklefs and Wikelski, 2002; Wolf et al., 2007; Barrickman et al., 2008; Lewin et al., 2017; Ducatez et al., 2019), so it should probably not surprise us to find that this may be true in regard to cognitive traits as well.
Life History Trade-Offs
Because innovative females had lower offspring survival (Figure 2), but higher ACP (Figure 3A) than non-innovative females, we considered the possibility that these correlations represented an adaptive trade-off between two alternative life history strategies where innovative female hyenas pursue a faster, quantity over quality, life history strategy and non-innovative females pursue a slower, quality over quantity, life history strategy. Previous research in wild spotted hyenas found that in juveniles, high insulin-like growth factor 1 (IGF-1) concentrations correlated with fast growth and earlier reproduction, but shorter lifespans (Lewin et al., 2017), suggesting that hyenas may invest differentially in reproduction and survival. However, innovation is generally thought to be associated with slower life histories across species (Sol et al., 2016). Instead of, or in addition to, alternative life-history strategies, it is also worth considering the possibility that innovativeness is an evolutionary stable strategy where the adaptive benefits of innovativeness are only realized at a specific ratio of innovative to non-innovative hyenas in the study population due to frequency-dependent selection. It is conceivable that the presence of a small ratio of innovative females could be beneficial at the scale of the entire clan if innovative females make previously unexploited resources available to the entire clan. Although spotted hyenas show only limited social learning of novel behaviors, feeding itself is highly socially facilitated (Yoerg, 1991; Benson-Amram et al., 2014).
Our result showed that innovative and non-innovative females have similar numbers of cubs surviving to 1 year, which suggests that higher birth rates in innovative females do indeed offset the significantly lower offspring survival rates. For spotted hyenas, mortality rates are highest in the first year of life; nearly half of all cubs born perish during their first year (Watts and Holekamp, 2009). Therefore, it seems reasonable to assume that the proportion of cubs that survive to 1 year would correlate with the overall proportion of offspring surviving to sexual maturity for female hyenas. This could be interpreted as evidence for equal adaptive value between being innovative vs. non-innovative; however, previous research on spotted hyenas found that, whereas annual reproductive success significantly predicted lifetime fitness, the length of the reproductive lifespan was the strongest determinant of lifetime fitness in spotted hyenas (Swanson et al., 2011). We were unable to calculate the actual reproductive lifespans of our female subjects because our dataset was both right and left-censored temporally; some of our subjects were adults females without known DOBs or dates of sexual maturity, and many were still alive at the end of the study period. However, the average observed reproductive lifespan in our dataset was 8.24 ± 4.57 years which, although censored for some subjects, is not significantly different from the average reproductive lifespan in our study population (7.13 ± 3.34 years, N = 170) (Swanson et al., 2011). Therefore, it is possible that innovative and non-innovative females have similar lifetime fitness, but, without actual lifetime fitness data on a larger sample of females, we cannot conclude this with any certainty.
Mediators of the Link Between Innovativeness and Fitness
Not only are researchers interested in the links between cognition and fitness, but also the mechanisms mediating such linkages. However, it is often unclear just why a specific cognitive ability might improve reproductive success or survival. The largest natural source of mortality for wild spotted hyenas comes from lions, both directly through conflict and indirectly through competition over food resources (Watts and Holekamp, 2009). In hyenas and other animals innovative problem-solving has been linked to greater boldness or risk-taking behavior (Webster and Lefebvre, 2001; Overington et al., 2011a; Benson-Amram and Holekamp, 2012; Audet et al., 2016; van Horik et al., 2017; Johnson-Ulrich et al., 2018), which in turn are correlated with higher mortality in wild hyenas. Hyenas that are bolder in the presence of lions, in particular, have a higher risk of mortality than conspecifics with intermediate or low levels of boldness (Yoshida et al., 2016), so lower survivorship among offspring of innovative females may be mediated by high boldness during conflict or competition with lions. In addition, greater risk-taking behavior in spotted hyenas, measured with a “mock intruder” test, is also correlated with a higher risk of mortality (Turner et al., 2019). Overall, if more proactive, bold, or risk-taking behavior, demonstrated by hyenas while interacting with problem-solving apparatuses, is correlated with their behavior in other contexts, it is possible that these traits mediate the link between innovation and survival.
The relationship between innovative problem-solving and reproductive success, on the other hand, has been linked in some bird species to the ability to forage more efficiently (Cauchard et al., 2017; Preiszner et al., 2017; Wetzel, 2017) but see Cole et al. (2012a). Access to food is a strong determinant of reproductive success among female hyenas; social rank is the strongest determinant of reproductive success because high ranking individuals enjoy the best access to high quality food resources (Holekamp et al., 1996). In addition, both average fatness, which usually indicates how recently a hyena has fed, and per capita prey availability also correlate with reproductive success in hyenas (Watts and Holekamp, 2009; Swanson et al., 2011). Social rank doesn't predict innovativeness in spotted hyenas (Benson-Amram and Holekamp, 2012); therefore, if innovativeness is correlated with the ability to access food in hyenas, then it is plausible that this would directly increase reproductive success.
Assumptions and Limitations
Our analysis of the relationship between offspring survival and female innovativeness is based on only a small sample and hinges on several assumptions. First, for innovativeness to be related to offspring survival, innovativeness must be transmitted from mother to offspring through genetic heritability or social learning. However, few studies have assessed the heritability of innovative problem-solving and one that has, in great tits, found no evidence for heritability (Quinn et al., 2016). If instead the relationship between innovative ability and offspring survival is mediated by a trait such as boldness, then boldness must be transmissible. Although the heritability of innovativeness in wild spotted hyenas has not been tested, previous research indicates that their boldness is heritable (Yoshida et al., 2016). Alternatively, it is also possible that innovative ability is entirely stochastic and has a direct effect on offspring survival through early-life effects or ongoing social support. Cubs usually wean between 12 and 18 months of age, but female hyenas provide ongoing social support to their mature female offspring throughout their lives during feeding competition and other social interactions with clan members (Watts et al., 2009; Smith et al., 2010; Vullioud et al., 2019). Thus, it is possible that variation in this support, if related to the ability to innovate, results in differential survival between offspring of innovative and non-innovative females.
Although our analysis of annual reproductive success in spotted hyenas doesn't hinge on assumptions about heritability, it is less robust than our analysis of offspring survival due to an extremely small sample size and censored windows of time during which we were able to monitor reproductive output for many subjects. Our total sample size consisted of 29 female hyenas, of which only five were innovators. Of these five individuals three were high ranking, one was mid-ranking, and one was low-ranking. In addition, three were adults at the time innovativeness was measured and two were pre-weaning subadults. Thus, these five innovative hyenas did not differ in any substantial measurable way from our overall sample of females, but our results should still be interpreted with caution because we cannot be sure that these five are not outliers in ways we did not measure. Maternal age might also have affected our measures of fitness. For individuals with known birth dates, the length of the reproductive lifespan would have controlled for this. Unfortunately, without knowing the birth dates for some of the females sampled (N = 2 innovators, N = 5 non-innovators), we have no way of knowing what their age was during the observed portions of their reproductive lives.
Conclusion
In summary, innovative female spotted hyenas were found to have lower offspring survival, but higher annual cub production, than non-innovative females. These results suggest there might be trade-offs among the costs and benefits of innovativeness, or that innovative and non-innovative females pursue different life history strategies. We would benefit from further study of the relationship between fitness and innovativeness in wild spotted hyenas. Ideally, long-term study would allow for measurement of lifetime reproductive success and assessing the heritability of innovativeness across generations. In addition, future work might investigate the mediators of the relationship between innovativeness and fitness by comparing innovativeness to other variables such as foraging ability, boldness, and social relationships.
Data Availability Statement
The datasets and corresponding R code analyzed for this study can be found in the Knowledge Network for Biocomplexity (KNB) Repository: doi: 10.5063/F1D21VXP.
Ethics Statement
The animal study was reviewed and approved by Michigan State University Institutional Animal Care & Use Committee, Application no. 07/08-099-00.
Author Contributions
LJ-U, SB-A, and KH designed the research and wrote the manuscript. SB-A collected the data on innovative problem-solving by spotted hyenas using an apparatus designed by KH. KH collected the data on spotted hyena demography. LJ-U performed all statistical analyses. This manuscript was submitted with the full knowledge and approval of LJ-U, SB-A, and KH.
Funding
This work was funded by NSF grants IOS0819437, OISE1853934, and IOS 1755089 to KH. LJ-U was otherwise supported by a Graduate Research Fellowship from NSF.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Kenyan National Commission for Science, Technology, and Innovation, the Narok County Government, and the Kenya Wildlife Service for permission to conduct this research. We also thank all those who assisted with long-term data collection in the field.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00443/full#supplementary-material
References
Ashton, B. J., Thornton, A., and Ridley, A. R. (2018). An intraspecific appraisal of the social intelligence hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 373:20170288. doi: 10.1098/rstb.2017.0288
Audet, J.-N., Ducatez, S., and Lefebvre, L. (2016). The town bird and the country bird: problem solving and immunocompetence vary with urbanization. Behav. Ecol. 27, 637–644. doi: 10.1093/beheco/arv201
Barrickman, N. L., Bastian, M. L., Isler, K., and van Schaik, C. P. (2008). Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J. Hum. Evol. 54, 568–590. doi: 10.1016/j.jhevol.2007.08.012
Benson-Amram, S., Dantzer, B., Stricker, G., Swanson, E. M., and Holekamp, K. E. (2016). Brain size predicts problem-solving ability in mammalian carnivores. Proc. Natl. Acad. Sci. U.S.A. 113, 2532–2537. doi: 10.1073/pnas.1505913113
Benson-Amram, S., Heinen, V. K., Gessner, A., Weldele, M. L., and Holekamp, K. E. (2014). Limited social learning of a novel technical problem by spotted hyenas. Behav. Processes 109, 111–120. doi: 10.1016/j.beproc.2014.09.019
Benson-Amram, S., and Holekamp, K. E. (2012). Innovative problem solving by wild spotted hyenas. Proc. R. Soc. B Biol. Sci. 279, 4087–4095. doi: 10.1098/rspb.2012.1450
Boogert, N. J., Madden, J. R., Morand-Ferron, J., and Thornton, A. (2018). Measuring and understanding individual differences in cognition. Philos. Trans. R. Soc. B Biol. Sci. 373:20170280. doi: 10.1098/rstb.2017.0280
Brooks, M. E., Kristensen, K., Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9:378. doi: 10.32614/RJ-2017-066
Cauchard, L., Angers, B., Boogert, N. J., Lenarth, M., Bize, P., and Doligez, B. (2017). An experimental test of a causal link between problem-solving performance and reproductive success in wild great tits. Front. Ecol. Evol. 5, 1–8. doi: 10.3389/fevo.2017.00107
Cauchard, L., Boogert, N. J., Lefebvre, L., Dubois, F., and Doligez, B. (2013). Problem-solving performance is correlated with reproductive success in a wild bird population. Anim. Behav. 85, 19–26. doi: 10.1016/j.anbehav.2012.10.005
Chen, J., Zou, Y., Sun, Y.-H., and Ten Cate, C. (2019). Problem-solving males become more attractive to female budgerigars. Science. 363, 166–167. doi: 10.1126/science.aau8181
Cole, E. F., Morand-Ferron, J., Hinks, A. E., and Quinn, J. L. (2012a). Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808–1812. doi: 10.1016/j.cub.2012.07.051
Cole, E. F., Quinn, J. L., Cole, E. F., and Quinn, J. L. (2012b). Personality and problem-solving performance explain competitive ability in the wild. Proc. R. Soc. B Biol. Sci. 279, 1168–1175. doi: 10.1098/rspb.2011.1539
Cooper, S. M., Holekamp, K. E., and Smale, L. (1999). A seasonal feast: long-term analysis of feeding behaviour in the spotted hyaena (Crocuta crocuta). Afr. J. Ecol. 37, 149–160. doi: 10.1046/j.1365-2028.1999.00161.x
Ducatez, S., Audet, J.-N., and Lefebvre, L. (2019). Speed–accuracy trade-off, detour reaching and response to PHA in Carib grackles. Anim. Cogn. 22:625–33. doi: 10.1007/s10071-019-01258-1
Ducatez, S., Clavel, J., and Lefebvre, L. (2014). Ecological generalism and behavioural innovation in birds: technical intelligence or the simple incorporation of new foods? J. Anim. Ecol. 84, 79–89. doi: 10.1111/1365-2656.12255
Frank, L. G. (1986). Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510–1527. doi: 10.1016/S0003-3472(86)80221-4
Griffin, A. S., and Diquelou, M. C. (2015). Innovative problem solving in birds: a cross-species comparison of two highly successful passerines. Anim. Behav. 100, 84–94. doi: 10.1016/j.anbehav.2014.11.012
Griffin, A. S., and Guez, D. (2014). Innovation and problem solving: a review of common mechanisms. Behav. Processes 109, 121–134. doi: 10.1016/j.beproc.2014.08.027
Hartig, F. (2019). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. Available online at: http://florianhartig.github.io/DHARMa/
Hayward, M. W. (2006). Prey preferences of the spotted hyaena (Crocuta crocuta) and degree of dietary overlap with the lion (Panthera leo). J. Zool. 270, 606–614. doi: 10.1111/j.1469-7998.2006.00183.x
Holekamp, K. E., and Dloniak, S. M. (2010). “Intraspecific variation in the behavioral ecology of a tropical carnivore, the spotted hyena,” in Behavioral Ecology of Tropical Animals Advances in the Study of Behavior (Burlington, MA: Academic Press), 189–229. doi: 10.1016/S0065-3454(10)42006-9
Holekamp, K. E., Sakai, S. T., and Lundrigan, B. L. (2007). The spotted hyena (Crocuta crocuta) as a model system for study of the evolution of intelligence. J. Mammal. 88, 545–554. doi: 10.1644/06-MAMM-S-361R1.1
Holekamp, K. E., Smale, L., and Szykman, M. (1996). Rank and reproduction in the female spotted hyaena. Reproduction 108, 229–237. doi: 10.1530/jrf.0.1080229
Holekamp, K. E., Smith, J. E., Strelioff, C. C., Van Horn, R. C., and Watts, H. E. (2012). Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613–632. doi: 10.1111/j.1365-294X.2011.05240.x
Huebner, F., Fichtel, C., and Kappeler, P. M. (2018). Linking cognition with fitness in a wild primate: fitness correlates of problem-solving performance and spatial learning ability. Philos. Trans. R. Soc. B Biol. Sci. 373:20170295. doi: 10.1098/rstb.2017.0295
Isden, J., Panayi, C., Dingle, C., and Madden, J. (2013). Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim. Behav. 86, 829–838. doi: 10.1016/j.anbehav.2013.07.024
Johnson-Ulrich, L., Johnson-Ulrich, Z., and Holekamp, K. (2018). Proactive behavior, but not inhibitory control, predicts repeated innovation by spotted hyenas tested with a multi-access box. Anim. Cogn. 21, 379–392. doi: 10.1007/s10071-018-1174-2
Kassambara, A., and Kosinski, M. (2018). Drawing Survival Curves Using “ggplot2.” Available online at: https://cran.r-project.org/package=survminer
Keagy, J., Savard, J.-F., and Borgia, G. (2009). Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817. doi: 10.1016/j.anbehav.2009.07.011
Kotrschal, A., Rogell, B., Bundsen, A., Svensson, B., Zajitschek, S., Brännström, I., et al. (2013). Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. doi: 10.1016/j.cub.2012.11.058
Kruuk, H. (1972). The Spotted Hyena: A Study of Predation and Social Behavior. Brattleboro, VT: Echo Point Books & Media.
Kummer, H., and Goodall, J. (1985). Conditions of innovative behaviour in primates. Philos. Trans. R. Soc. B Biol. Sci. 308, 203–214. doi: 10.1098/rstb.1985.0020
Lefebvre, L., Reader, S. M., and Sol, D. (2004). Brains, innovations and evolution in birds and primates. Brain. Behav. Evol. 63, 233–246. doi: 10.1159/000076784
Lefebvre, L., Reader, S. M., and Sol, D. (2013). Innovating innovation rate and its relationship with brains, ecology and general intelligence. Brain. Behav. Evol. 81, 143–145. doi: 10.1159/000348485
Lewin, N., Swanson, E. M., Williams, B. L., and Holekamp, K. E. (2017). Juvenile concentrations of IGF-1 predict life-history trade-offs in a wild mammal. Funct. Ecol. 31, 894–902. doi: 10.1111/1365-2435.12808
Martin, P., and Bateson, P. (1993). Measuring Behaviour. New York, NY: Cambridge University Press. doi: 10.1017/CBO9781139168342
Morand-Ferron, J., Cole, E. F., and Quinn, J. L. (2015). Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. Camb. Philos. Soc. 25, 2795–2803. doi: 10.1111/brv.12174
Overington, S. E., Cauchard, L., Côté, K.-A., and Lefebvre, L. (2011a). Innovative foraging behaviour in birds: what characterizes an innovator? Behav. Processes 87, 274–285. doi: 10.1016/j.beproc.2011.06.002
Overington, S. E., Griffin, A. S., Sol, D., and Lefebvre, L. (2011b). Are innovative species ecological generalists? A test in North American birds. Behav. Ecol. 22, 1286–1293. doi: 10.1093/beheco/arr130
Plomin, R., and Deary, I. J. (2015). Genetics and intelligence differences: Five special findings. Mol. Psychiatry 20, 98–108. doi: 10.1038/mp.2014.105
Preiszner, B., Papp, S., Pipoly, I., Seress, G., Vincze, E., Liker, A., et al. (2017). Problem-solving performance and reproductive success of great tits in urban and forest habitats. Anim. Cogn. 20, 53–63. doi: 10.1007/s10071-016-1008-z
Quinn, J. L., Cole, E. F., Reed, T. E., and Morand-Ferron, J. (2016). Environmental and genetic determinants of innovativeness in a natural population of birds. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 371:20150184. doi: 10.1098/rstb0.2015.0184
R Core Team (2019). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/
Ramsey, G., Bastian, M. L., and van Schaik, C. (2007). Animal innovation defined and operationalized. Behav. Brain Sci. 30, 393–407. doi: 10.1017/S0140525X07002373
Reader, S. M., and Laland, K. N. (2002). Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl. Acad. Sci. U. S. A. 99, 4436–4441. doi: 10.1073/pnas.062041299
Reader, S. M., and Laland, K. N., (eds.). (2003). Animal Innovation. New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780198526223.001.0001
Reader, S. M., Morand-Ferron, J., and Flynn, E. (2016). Animal and human innovation: novel problems and novel solutions. Philos. Trans. R. Soc. B Biol. Sci. 371:20150182. doi: 10.1098/rstb.2015.0182
Ricklefs, R. E., and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. doi: 10.1016/S0169-5347(02)02578-8
Sinervo, B., Svensson, E., and Comendant, T. (2000). Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406, 985–988. doi: 10.1038/35023149
Smith, J. E., Van Horn, R. C., Powning, K. S., Cole, A. R., Graham, K. E., Memenis, S. K., et al. (2010). Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 84–303. doi: 10.1093/beheco/arp181
Sol, D. (2009). “The cognitive-buffer hypothesis for the evolution of large brains,” in Cognitive Ecology II, eds R. Dukas and J. M. Ratcliffe (Chicago, IL: The University of Chicago Press), 111–134. doi: 10.7208/chicago/9780226169378.003.0007
Sol, D., Duncan, R. P., Blackburn, T. M., Cassey, P., and Lefebvre, L. (2005). Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. U. S. A. 102, 5460–5465. doi: 10.1073/pnas.0408145102
Sol, D., Sayol, F., Ducatez, S., and Lefebvre, L. (2016). The life-history basis of behavioural innovations. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 371:20150187. doi: 10.1098/rstb.2015.0187
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Strauss, E. D., and Holekamp, K. E. (2019). Social alliances improve rank and fitness in convention-based societies. Proc. Natl. Acad. Sci. U.S.A. 116, 8919–8924. doi: 10.1073/pnas.1810384116
Swanson, E. M., Dworkin, I., and Holekamp, K. E. (2011). Lifetime selection on a hypoallometric size trait in the spotted hyena. Proc. R. Soc. B Biol. Sci. 278, 3277–3285. doi: 10.1098/rspb.2010.2512
Therneau, T. M. (2015). A Package for Survival Analysis in S. Available online at: https://cran.r-project.org/package=survival
Therneau, T. M., and Grambsch, P. M. (2000). Modeling Survival Data: Extending the Cox Model. New York, NY: Springer. doi: 10.1007/978-1-4757-3294-8
Turner, J. W., Lafleur, R. M., Richardson, A. T., and Holekamp, K. E. (2019). Risk-taking in free-living spotted hyenas is associated with anthropogenic disturbance, predicts survivorship, and is consistent across experimental contexts. Ethology. doi: 10.1111/eth.12964. [Epub ahead of print]
van Horik, J. O., Langley, E. J., Whiteside, M. A., and Madden, J. R. (2017). Differential participation in cognitive tests is driven by personality, sex, body condition and experience. Behav. Processes 134, 22–30. doi: 10.1016/j.beproc.2016.07.001
Van Horn, R. C., McElhinny, T. L., and Holekamp, K. E. (2003). Age estimation and dispersal in the spotted hyena (Crocuta crocuta). J. Mammal. 84, 1019–1030. doi: 10.1644/BBa-023
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. Evol. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Watts, H. E., and Holekamp, K. E. (2009). Ecological determinants of survival and reproduction in the spotted hyena. J. Mammal. 90, 461–471. doi: 10.1644/08-MAMM-A-136.1
Watts, H. E., Tanner, J. B., Lundrigan, B. L., and Holekamp, K. E. (2009). Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc. Biol. Sci. 276, 2291–2298. doi: 10.1098/rspb.2009.0268
Webster, S. J., and Lefebvre, L. (2001). Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim. Behav. 62, 23–32. doi: 10.1006/anbe.2000.1725
Wetzel, D. P. (2017). Problem-solving skills are linked to parental care and offspring survival in wild house sparrows. Ethology 123, 475–483. doi: 10.1111/eth.12618
Wolf, M., van Doorn, G. S., Leimar, O., and Weissing, F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. doi: 10.1038/nature05835
Yirga, G., Leirs, H., De Iongh, H. H., Asmelash, T., Gebrehiwot, K., Vos, M., et al. (2017). Densities of spotted hyaena (Crocuta crocuta) and African golden wolf (Canis anthus) increase with increasing anthropogenic influence. Mamm. Biol. 85, 60–69. doi: 10.1016/j.mambio.2017.02.004
Yoerg, S. I. (1991). Social feeding reverses learned flavor aversions in spotted hyenas. J. Comp. Psychol. 105, 185–189. doi: 10.3389/fevo.2019.00443
Keywords: innovation, fitness, spotted hyenas, survival, reproduction
Citation: Johnson-Ulrich L, Benson-Amram S and Holekamp KE (2019) Fitness Consequences of Innovation in Spotted Hyenas. Front. Ecol. Evol. 7:443. doi: 10.3389/fevo.2019.00443
Received: 11 September 2019; Accepted: 31 October 2019;
Published: 22 November 2019.
Edited by:
Laure Cauchard, University of Aberdeen, United KingdomReviewed by:
Marion Valeix, Centre National de la Recherche Scientifique (CNRS), FranceJulie Morand-Ferron, University of Ottawa, Canada
Copyright © 2019 Johnson-Ulrich, Benson-Amram and Holekamp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lily Johnson-Ulrich, am9objM5MjNAbXN1LmVkdQ==
 Lily Johnson-Ulrich
Lily Johnson-Ulrich Sarah Benson-Amram
Sarah Benson-Amram Kay E. Holekamp1
Kay E. Holekamp1