- 1Department of Biology, University of Missouri-St. Louis, St. Louis, MO, United States
- 2Department of Biological Sciences, George Washington University, Washington, DC, United States
- 3Department of Biology, George Mason University, Fairfax, VA, United States
- 4Institut de Recherche en Horticulture et Semences, INRA-Université d'Angers, Beaucouzé, France
- 5Department of Entomology, University of Illinois at Urbana-Champaign, Urbana, IL, United States
Extreme weather events dramatically impact populations of individual insect species but the consequences of such events for entire insect communities are not well documented. We present evidence that mid spring frosts and summer drought negatively affect the community of insects found on Missouri oaks (Quercus alba and Q. velutina), amounting to a 23–186 fold decrease depending on the oak species, insect body size and feeding guild, and the specific weather event. Depending on the event, spring faunas required 1–5 years and summer faunas 1–4 years following spring frosts to reach pre-event levels. The impact of summer drought on leaf tying caterpillars also lasted over an extended period of time; it was 5 years before numbers of leaf ties reached pre-drought levels. Smaller-bodied species of leaf tying Lepidoptera took longer to recover than larger-bodied species following the drought. Overall, we found no evidence for a general decline in abundance, even a modest one, during the 20 years of study of faunas on oak trees in southeastern Missouri. However, the risk of mid-season frost damage to trees is expected to increase with predicted earlier onset of spring. Similarly, the effects of drought reported here are likely to increase with time, as the climate in the Midwest U.S. is only expected to become warmer and drier during the summer months. Understanding the impact of such weather events on insect communities influences our ability to predict how habitat and landscape management, or lack thereof, will influence future patterns of insect abundance and diversity.
Introduction
Many foliage-feeding insect species, particularly those in temperate forests, undergo dramatic fluctuations in abundance across years (Barbosa and Schultz, 1987; Royama, 1997; Barbosa et al., 2012). Insects whose numbers reach sufficiently high levels that they cause noticeable defoliation are labeled as “pests” (Nothnagle and Schultz, 1987). Other species do not go through such eruptions and have been classified as non-outbreak species (Mason, 1987). Intrinsic characteristics of the insect species including body size, gregariousness in the larval stage, host range, and maternal effects have been used to categorize particular insect species with respect to the likelihood of going through an outbreak. Host plant characteristics, natural enemy impacts, and weather all have been suggested as factors extrinsic to the insect species that might lead to insect outbreaks (Hunter, 1991; Koricheva et al., 2012).
The opposite of outbreaks are, of course, population declines. Determining which factors drive declines of insect communities is necessary for predicting how insects will respond to current and future anthropogenic activities. The fact that some species of insects go through periodic increases and then decreases apparently in the absence of human influence makes it difficult to distinguish these population cycles from actual declines due to anthropogenic effects. For example, in our study system of Lepidoptera larvae on Quercus in Missouri, USA, fluctuating abundances from year to year are linked to the time of year when a particular insect species is feeding. Spring-feeding Lepidoptera are more variable in their population dynamics than summer-feeding species because of the greater unpredictability of the timing of spring foliage availability than that of summer foliage (Forkner et al., 2008). Based on studies for which there is a minimum 10 years of continuous sampling for multiple species, there is increasing evidence that some insect populations are declining worldwide (Hallmann et al., 2017; Sanchez-Bayo and Wyckhuys, 2019). Climate change as it directly affects insect survivorship has been suggested as the cause of insect declines in the wet tropical forest of Puerto Rico (Lister and Garcia, 2018).
The impacts of local weather events on insect survival may have both short term and long term effects. Hurricanes can increase the number of leaf-feeding Lepidoptera as result of the ensuing new leaf flush (Torres, 1992), and spring frosts contribute to the population dynamics of some Lepidoptera (LeRoux et al., 1963). Drought has been proposed to influence forest insect populations (Mattson and Haack, 1987), although support is mixed (Martinet and Allen, 1987; Kolb et al., 2016). Outbreaks of the variable oakleaf caterpillar (Lochmeus manteo) were associated with multiple year droughts in the Missouri Ozarks in 1970 (Law and Gott, 1987) and again in 1980 (Gass, 1971; Law and Gott, 1987). Similarly, years with warmer-than-average summer temperatures in Canada were associated with population outbreaks of fall webworm (Hyphantria cunea: Morris, 1964). Even within the same species and during the same drought period, one population of the checkerspot butterfly responded by increasing while all others declined (Ehrlich et al., 1980). Declines in species richness of multiple adult moth species in Indiana oak-hickory forests were associated with the severe 2012 drought in the Midwest, USA (Summerville and Marquis, 2016). Perhaps even more threatening is the possibility of multiple events (e.g., spring frosts and summer droughts) occurring in the same year.
Long term data are important with regard to questions of impacts of climate and human management on insect populations (Summerville and Marquis, 2016; Sanchez-Bayo and Wyckhuys, 2019). Such data are necessary because they give evidence for general trends of increases or declines. In addition, the longer the data set, the more likely it is to capture infrequent climatic events, which may have persistent effects, and to estimate recovery times following such events. Importantly, they help us distinguish the impact of local weather events, which may be short term, from long term trends, that might indicate the influence of extensive habitat modification and/or climate change.
We focus here are on two types of climatic events, spring frosts and summer droughts. Both were linked to immediate declines in insect numbers for leaf-feeding insects on oaks (Quercus) in Missouri, USA. Depending on the event, these declines in numbers persisted for a number of years afterward. Spring frosts can directly affect insects, by disrupting metabolism and killing cells through the formation of ice crystals (Bale, 1996; Sinclair et al., 2013), and indirectly affect them by killing the young leaves (Augspurger, 2013) on which the insects feed. Summer drought could also have direct and indirect effects. Exposure to desiccating humidity can kill insects (Willmer, 1980). This desiccation effect might by mitigated by body size (larger insects would be less vulnerable) (Le Lagadec et al., 1998; Kærsgaard et al., 2004) or by specific adaptations, such as body hairs or physiological mechanisms (Philip et al., 2008). Indirectly, drought can reduce water content of plant tissues upon which the insects feed (Mattson and Haack, 1987).
We used long term census data to determine the effects of mid spring frosts and summer droughts on the abundances of these insects. Specifically, we asked: (1) What is the impact at the community level on abundances following such events relative to pre-event abundances? (2) How long do communities require to return to pre-event levels? and (3) What climatic factors are linked to the observed declines? Here, the “community” consists of the leaf-chewing insects (mid spring frost study), or a subset of them (summer drought study), found on Quercus in Missouri, USA. In the case of the mid spring frost events, we hypothesized that abundances would decline in the year of the frost, and that the time to recovery would be greater for the mostly univoltine spring fauna than for the more commonly multivoltine summer fauna. The spring and summer fauna of leaf chewing insects on Missouri oaks are each themselves quite diverse (75+ species each) but compositionally distinct, sharing fewer than 5 species (Marquis et al., 2019). Following the 2012 drought, we hypothesized that recovery would require multiple years, and that large-bodied species would recover before small-bodied species because of the smaller surface area to volume ratio in the former. To illustrate the impacts of two mid spring frost events, we used data originally collected to estimate impacts of forest management on biodiversity and sustainability on forest productivity during the years 1991–2010 (Forkner et al., 2006). To explore the impact of a major drought in 2012, we used data collected sporadically on the abundances of leaf tying caterpillars prior to the drought year and then continuously through 2018 following the drought. We describe how local climate data are associated with the observed population events, and then discuss the results in light of predicted future changes in local weather, i.e., climate change, for our region. Although the time scale of the two studies was different and the studies occurred in different locations, the insects associated with the two oak species in the two locations are much the same (Marquis et al., 2019).
Materials and Methods
Mid Spring Frosts
We sampled leaf-chewing insect abundance on Quercus alba and Q. velutina for the years 1991–2010 as part of the Missouri Ozark Forest Ecosystem Project (MOFEP), located in southeastern Missouri. The MOFEP experiment consisted of three treatments (control, uneven-aged management, and even-aged management) applied at the landscape scale, with three replicates (“plots”) per treatment, each replicate ~400 ha in size. We present data here only from control plots, which were not directly impacted by local forest management activities. See Brookshire et al. (1997) and Sheriff and He (1997) for more detailed descriptions of the experimental design. Methods and results based on insect sampling in the MOFEP are presented in detail in previous publications (Forkner and Marquis, 2004; Forkner et al., 2006; Summerville and Marquis, 2016). Specifically for each of the three control treatment plots, we sampled 1–6 stands depending on the year. All sampled plants of each stand were on the same slope, and no more than 25 m apart. For each stand, we sampled a minimum 5 plants and up to 12 plants per tree species per stand, to reach a sample size of ~3,000 Q. alba leaves and ~1,200 Q. velutina leaves per stand. Plants sampled were either saplings or trees with low-hanging branches. We sampled all leaves on saplings or all leaves of marked branches of larger trees. We do not know the possible effect of plant size on the interactions described here. Initial studies and species accumulation curve analyses indicated that this was sufficient sampling to estimate abundance and diversity of leaf-chewing oak insects in these areas (Marquis and Le Corff, 1997). For the years 1991–2008, we systematically sampled four times during the growing season (early May, late June, late July, and late August-early September) to account for seasonal turnover in the identity of species present. For 2009–2010, we sampled only the spring fauna, in May, because of funding constraints. Number of stands sampled per plot in each year were the following: 1991 (1), 1992–2008 (6), 2009–2010 (2). We inspected the top and bottom of each leaf of each marked branch or tree, and all twigs, branches, and trunks associated with entire saplings, or the marked branches of larger trees, recording the number and identity of all encountered leaf chewing insect species. Once per year, the number of leaves/tree that were censused was recorded allowing computation of insect densities (caterpillars/m2 foliage). Sampled insects were left undisturbed on the trees unless it was necessary to rear them in the laboratory for identification. Sampling was prohibited in 1996 (Figure 1), the first year of timber harvest in MOFEP.
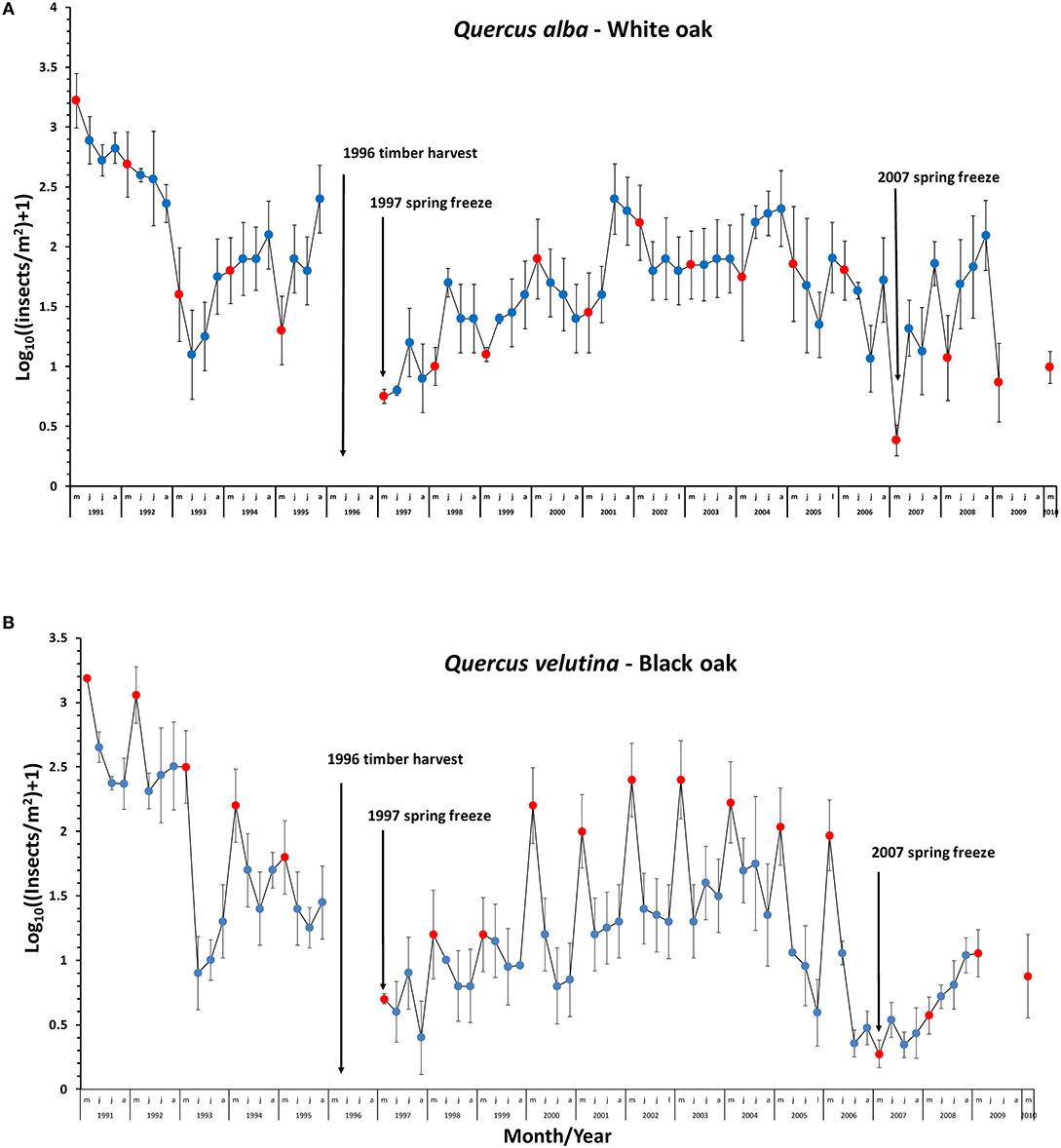
Figure 1. Effect of mid spring freezes (indicated by vertical black arrows, 1997 and 2007) on mean abundance of leaf chewing insects on (A) Quercus alba and (B) Q. velutina in Missouri Ozark Forest Ecosystem Project (MOFEP) control plots, southeastern Missouri. Red dots and blue dots indicate values for the spring (May) and summer (June-August) communities, respectively. Timber harvest in MOFEP occurred in 1996, during which data collection was prohibited. Values for each point are means of means (site/stand) for one (1991), six (1992–2008), or two stands (2009–2010) for each of three control sites in the MOFEP. Error bars are 95% confidence intervals for each point. Note that data are presented on a log10 scale. No sampling occurred September through April, when there are few insects, and/or no leaves on trees (mid-October to March or later).
Summer Drought
At Cuivre River State Park (CRSP) in east central Missouri, we have been studying the impact of lepidopteran larvae that silk together neighboring leaves, leaf blade to leaf blade, to form a leaf tie, on the resulting secondary inhabitants of these leaf ties (Lill and Marquis, 2003; Lill, 2004; Marquis and Lill, 2010; Reinhardt, 2011; Wang et al., 2012; Baer and Marquis, 2014). There are 15 species of Lepidoptera at CRSP that construct leaf ties. The vast majority of leaf ties are constructed in the summer, after leaves have hardened, often persisting after the original tie maker has completed its larval life stage.
In 2012, a major drought occurred throughout the Midwestern U.S. This drought was associated with back-to-back La Niña events in 2010–2011 and then again in 2011–2012 (Rippey, 2015). We first noticed almost a complete absence of leaf ties on oaks in the St. Louis region in June-August 2013. Thus, we began sampling in 2013 at CRSP, continuing to 2018. We counted the number of leaf ties in August to October (depending on the year) that had accumulated through the summer on 15–30 understory Q. alba saplings, 1.5–3 m tall. Sample size varied depending on the design of the experiment taking place in a given year. We sampled where our pre-drought studies had taken place, i.e., in closed canopy forest north of the Assistant's Road and in the North Woods Wild Area of CRSP, both of which had not been subjected to prescribed burning. We recorded the lepidopteran inhabitants of all of these late season ties. In addition, we haphazardly sampled leaf ties through each of the summer months from 2013 to 2018 (75–100 h per summer), recording inhabitants, with the goal of specifically determining when species recorded in leaf ties (pre-drought) first re-appeared subsequent to the drought. We also recorded sightings of Limacodidae slug caterpillars, who we thought might be particularly vulnerable to the drought because of their relatively unsclerotized ventral surface (Epstein, 1996). A series of short term studies conducted at CRSP prior to 2012 provide estimates of leaf tie abundance and leaf tying caterpillar identity pre-drought (Lill and Marquis, 2003; Lill, 2004; Marquis and Lill, 2010; Reinhardt, 2011). Specifically, we had estimates of leaf tie abundance on Q. alba for 8 of the 16 years from 1996 to 2012: 1996 (Lill, 2004), 1997 (Marquis and Lill, 2010), 1999–2000 (Lill and Marquis, 2003), 2005–2006 (Marquis, unpublished), 2009 (Reinhardt, 2011), 2010 (Wang et al., unpublished). To estimate the effect of body size on the year of first appearance of a species in sampling following the 2012 drought, we measured the length of 10 adult voucher specimens per species, reared from larvae collected on Quercus in Missouri (in the collection of R.J. Marquis at University Missouri-St. Louis).
Climate Data
Temperature and precipitation data were extracted from the local weather stations closest to the study sites for the study years (National Centers for Environmental Information: https://www.ncei.noaa.gov/). For the years 1991–2002, we used spring temperature data (February 1 to May 31) principally from the Summersville weather station (border of Shannon and Texas Counties, MO, 37.14002°N, −91.62797°W, 376.7 m elevation), supplemented with data from the weather station in Van Buren, MO, Carter County (36.975°N, −91.0186°W, 304.8 m elevation) when data were missing from Summersville. For the years 2003–2010, we used temperature data from the Big Spring weather station in Carter County (southern boundary of MOFEP, 36.975°N, −91.0181°W, 304.6 m elevation). Data for the same years from the Carr Creek weather station in Shannon County at the northern boundary of MOFEP (37.1806°N, −91.1181°W 426.7 m elevation), an alternative source, were highly correlated (Pearson) with those of the same years from Big Spring (maximum daily temperature for February 1 to May 31 2007 r = 0.995, P < 0.0001, minimum daily temperature for February 1-May 31 2007 r = 0.9751, P < 0.0001).
Temperature and precipitation data were extracted for the years 1996–2018 from Troy, MO, the nearest data station to CRSP (Lincoln County, 38.95°N, −91°W, 170.7 m elevation). Long term (1895–2019) temperature and precipitation data used to represent local climate at CRSP were extracted from various weather stations across Lincoln County (https://www.ncei.noaa.gov/).
Statistical Analysis
To estimate the time to recovery after the two spring freezes and the 2012 drought, we calculated the 95% confidence intervals for each census. We concluded that when the confidence interval for the insect abundance in a particular post-event year did not overlap with the 95% confidence interval for the most immediate pre-event year, recovery to “normal” had yet to take place. For the leaf tying caterpillar data set, body size (average adult body length measured on 10 reared adults) was correlated with the number of years passing before a species was recorded post-drought.
We followed the lead of Augspurger (2013) in seeking weather variables linked to declines in insect abundance for the MOFEP over the 1991–2010 period (see also Story and Congalton, 1986). Augspurger (2013) found that the magnitude of leaf freeze occurring in Trelease Woods, ~480 km to the northeast of MOFEP, was most strongly linked to the number of days >20°C in March, in combination with the number of days experiencing <0°C in April. We tested the predictive reliability of various one-factor error matrices, and one two-factor error matrix, extracting measures from the climate data sets available for the years 1991–2010. The data set included the number of growing degree days (GDD, the mean of daily maximum and minimum temperature minus 10°C, summed over all days from February 1 to the day in April with the lowest minimum temperature), the number of warm days in March, and the number of cold days in April. The complete data set and the annual pattern of spring temperatures for 1991–2010 are given in Table S1.
A similar analysis was not feasible for the drought-associated decline in insect abundance at CRSP, with only one event. We were able, however, to compare precipitation and temperature data for 2012 with past and future data (years 1895–2018) to estimate how frequently such an event might have occurred previously. All analyses were conducted in SAS version 9.4.
Results
Mid Spring Frosts: Leaf-Chewing Insects on Quercus alba and Q. velutina in Southeastern Missouri
There were three major declines in the May fauna (Figure 1, note log scale). In May 1993, abundances on Q. alba declined by 91.8% and by 72.2% on Q. velutina. The summer faunas (comparing August values) also declined for both species (Q. alba: 75.7%, Q. velutina: 91.8%). The spring fauna on Q. alba did not reach its former 1992 level until 2002, and the summer values by 1994 (based on overlapping 95% confidence intervals). The spring fauna on Q. velutina reached its former 1992 level by 2000, but by 2008 (the last year of our sampling), the summer fauna on Q. velutina still had not reached the high levels of 1991–1992 (Figure 1B). We saw no frost-killed leaves in our May 1993 sampling. There was only one April day in 1993 when the temperature was below 0°C (Figure 2A) (National Centers for Environmental Information: https://www.ncei.noaa.gov/).
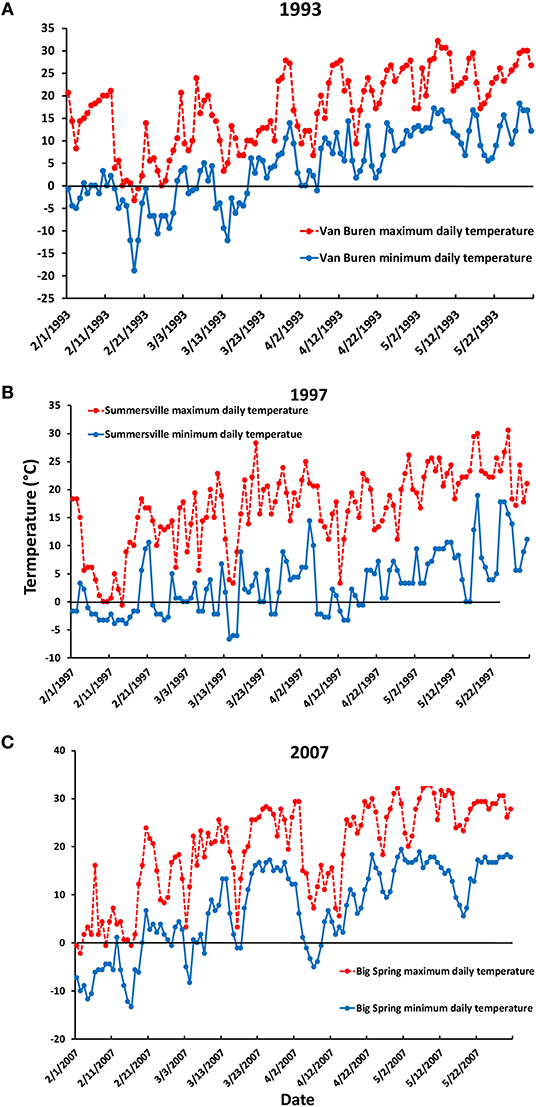
Figure 2. Daily maximum and minimum temperatures for (A) 1993, Van Buren weather station, (B) 1997, Summersville weather station, (C) 2007, Big Spring weather station (https://www.ncei.noaa.gov). See text for station locations.
The other two major declines during the 1991–2010 period occurred in 1997 and 2007 (Figure 1). Declines were 71.8–98% for the spring faunas (compared to the previous May values), and 0–97% for the summer faunas, depending on the year and the oak species. By 1998, the spring fauna on both oak species had recovered following the 1997 decline. Following the 2007 decline, by 2010 (at the end of our sampling), the spring fauna still had not recovered to 2006 levels on either oak species. June and July levels on Q. alba in 1998 were similar to those in 1995, while August numbers did not reach 1995 levels until 2001. June and July numbers on Q. velutina recovered by 1999 and August values by 2001. Following the 2007 event, June numbers of insects on Q. alba and Q. velutina had recovered by 2008. July and August numbers did not decline in 1997 for either host species.
There were early April spring frosts (temperatures below 0°C) in both 1997 and 2007, lasting 7 days in 1997 and 5 days in 2007 (Figures 2B,C). Our observations are that these frosts killed young leaves over wide expanses of the MOFEP experimental region in both years (see also Gu et al., 2008). In MOFEP sampled stands, frozen leaves occurred over approximately 50 percent of the landscape in 1997, while 50–90% of all leaves in all marked stands were frozen in 2007. May sampling in 2007 was delayed by 1 week until new leaves appeared.
In addition to the 1997 and 2007 April freeze events, there were high daily maximum temperatures in March (9 days over 20°C in March of both years) (National Centers for Environmental Information: https://www.ncei.noaa.gov/). During the 20 years of this study, this combination of high March temperatures followed by an extended freeze of 5 days or more only occurred in 1997 and 2007. In contrast, in 1993, when insect numbers declined markedly on Q. alba compared to 1992, only 2 days in March were over 20°C, and there was only 1 day in April when the minimum temperature was <0°C (−1.1°C, Figure 2A). Accordingly, high temperatures in March and low temperatures in April together perfectly predicted that occurrence of leaf-killing frosts in 1997 and 2007, and never predicted such an event when it did not occur (Table 1). None of the single variable models tested proved as accurate (Table 1). One sample t-tests revealed that 9 days of temperatures >20°C was significantly greater than the mean value for the other years (mean ± s.e. = 7.00 ± 1.30, t = −1.94, P = 0.0366), and 5 days <0°C was significantly greater than the mean value for the other years excluding 1997 (mean ± s.e. = 1.47 ± 0.36) (t = −9.72, P < 0.0001).
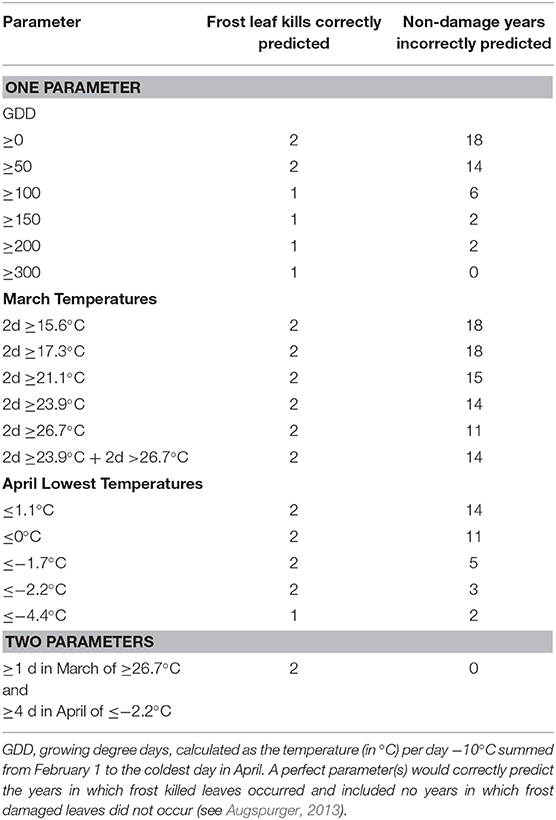
Table 1. Error matrix for parameter(s) used to predict number of years between 1991 and 2010 with observed frost damage to leaves.
Summer Drought: Leaf Tying Caterpillars on Quercus alba at Cuivre River State Park
The average density (± s.e.) of leaf ties at the end of the season on Quercus alba at CRSP prior to the 2012 drought was 5.6 ± 0.2 (N = 8 years) (Figure 3). During 2012, especially in the late summer (when the second generation of leaf tying caterpillars typically peaks; Lill, 2004), our casual observation was that tie abundance was not noticeably low but there were few or no living caterpillars in the ties in August and September. In 2013, there were very few ties (none in the area in which plants were censused). There were only three leaf ties on census plants in 2014. Subsequent to 2014, the density of ties steadily increased. The density of ties post-drought, however, did not reach the pre-2012 drought level average until 2017, a full 5 years after the drought year (Figure 3). Only two species of leaf tying caterpillars were encountered in 2013, and these were the two largest species of this guild at CRSP, Psilocorsis reflexella and Antaeotricha schlaegeri (both Depressariidae). Other species became sufficiently abundant to be recorded in censuses and casual sampling of leaf ties at staggered intervals: Pseudotelphusa quercinigracella (non-banded form) (Gelechiidae), Psilocorsis cryptolechiella, Rectiostoma xanthobasis (Depressariidae), Pococera expandens (Pyralidae) (first re-recorded in 2015), Arogalea cristifasciella (Gelechiidae) (first re-recorded in 2017), and Chionodes fuscomaculella (Gelechiidae) (first re-recorded in 2018). As of 2018, there were still five species known to occur at CRSP prior to the drought that had yet to be recorded in annual censuses and by casual sampling: Antaeotricha ossella, A. humilis, Coleotechnitis quercivorella (Gelechiidae), Aristotelia sp. (Gelechiidae), and Pseudotelphusa quercinigracella (banded form). Body size (adult forewing length) was negatively correlated with the number of years before the species was first recorded post-drought (r = −0.797, P = 0.0178, N = 8 species). In addition, often occurring at relative low numbers at our study sites (Lill et al., 2006), we have seen only five individuals of Limacodidae (slug caterpillars) during the years 2012–2018 [one Isa textula (2014) and four Acharia stimulea (2018)] at CRSP (at a site that would typically host at least a dozen species in any given year).
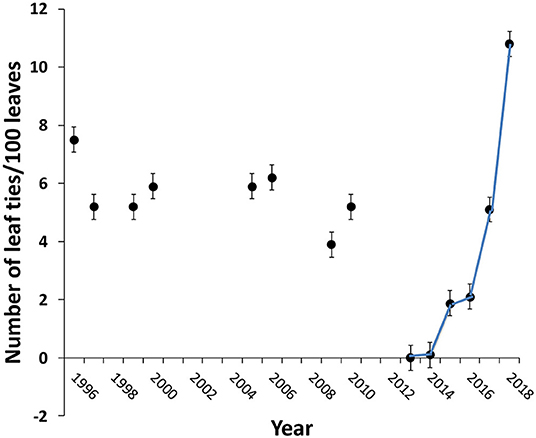
Figure 3. Impact of 2012 drought on the number of leaf ties on Quercus alba at Cuivre River State Park, near Troy, MO. Data for years 2013–2018, this study. Error bars are 95% confidence intervals for each point. Data sources for years prior to 2012: 1996 (Lill, 2004), 1997 (Marquis and Lill, 2010), 1999–2000 (Lill and Marquis, 2003), 2005–6 (Marquis, unpublished), 2009 (Reinhardt, 2011), 2010 (Wang et al., unpublished). N = 15–30 saplings per year sampled between August and October, depending on the year.
The decline in abundance of insects at CRSP was associated with a major drought coupled with abnormally high temperatures (Figure 4). Rainfall on average was about 8% lower than the annual average throughout the Midwest (Rippey, 2015). But this lower rainfall was coupled with high summer temperatures not previously seen since 1936. Rainfall in Lincoln County for the months of May–August 2012 was 72% lower than the 1895–2018 average (158 vs. 410 mm), and daily temperature for those same months was 8% higher than 1895–2018 average (24.39°C vs. 22.42°C) (NCEI records).
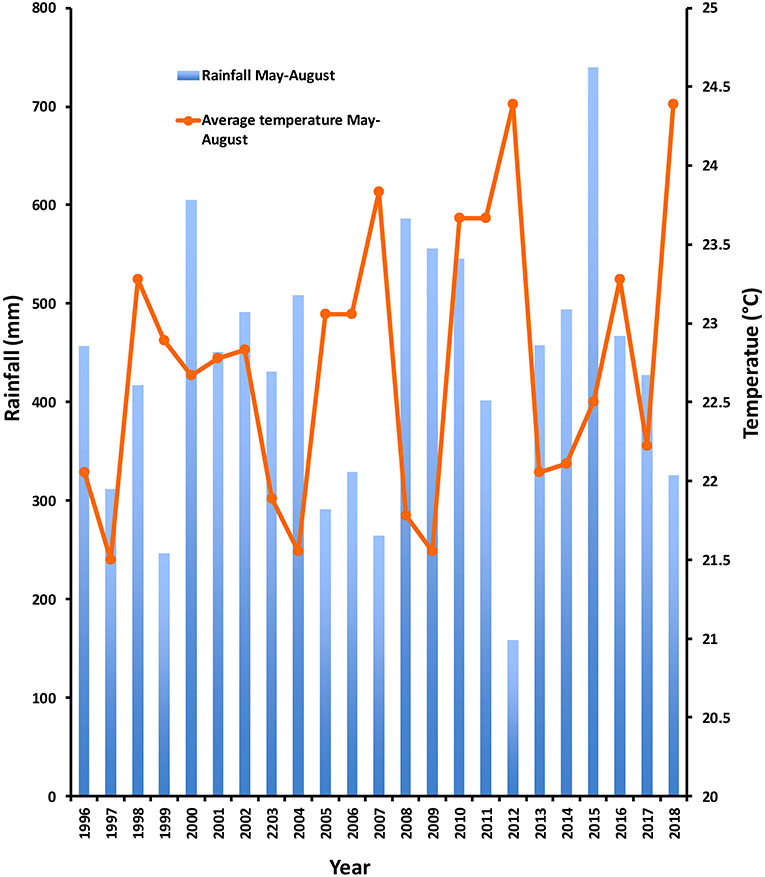
Figure 4. Total rainfall and mean daily temperature for the months May-August over the years 1996–2018 for the Troy, MO, weather station (https://www.ncei.noaa.gov), the closest weather station to Cuivre River State Park (~6.6 km).
This combination of low summer rainfall and high summer temperatures has only occurred five other times in Lincoln County, MO, between 1895 and 2018: 1913, 1914, 1930, 1934, and 1936. The fact that there was no decline in leaf-tying caterpillar abundance in 2018 at CRSP (Figure 3) despite the high May-August temperatures there (average 24.4°C) (Figure 4), strongly supports the hypothesis that both high temperatures and low rainfall are required to impact these insect communities. Rainfall during May-August in 2018 was only 19% lower than the 123 years average. The drought conditions of 2012 were alleviated by a return to normal rainfall in early 2013 and more moderate temperatures the following summer.
Discussion
Our sampling reveals that insect abundance on Missouri oak trees varies dramatically over relatively short periods of time. From 1991 to 2007 (the highest and lowest sampled years, Figure 1) in southeastern Missouri, the range in numbers of insects per plant was 645-fold on Quercus alba and 890-fold on Q. velutina. Part of this variation was due to declines brought on by mid spring frosts that killed large numbers of young leaves. These frosts reduced both spring and summer feeding insects despite the re-flush of new leaves within a relatively short period of time. In addition, at a more local site in east central Missouri, leaf ties made by leaf tying Lepidoptera all but disappeared following an extreme summer drought the previous year (Figure 3). Subsequent to the spring freezes and summer drought, insect populations recovered, taking 1–5 years (or more following the 2007 freeze event) depending on the oak species and the weather event. The fact that these populations did recover and that initial declines were coincident with these weather events suggests that the declines were due to the weather events themselves and not to some unrecognized human-driven cause. Much of the southern half of Missouri is contiguous forest, the majority of which is mature second growth oak-hickory forest (Spencer et al., 1992). In addition, the study sites are contiguous with a portion of the Mark Twain National Forest, which contains 708 km2 of forest. Ninety percent of the 2,600 ha of Cuivre River State Park is in forest cover, and although the park is surrounded by agricultural land, the study sites were about 2 km from the nearest park boundaries, making it unlikely that insect declines were due to the drift of sprayed insecticides from neighboring agricultural lands (Hallmann et al., 2017).
The immediate effect of April frost on the spring fauna was most likely a loss of food, as opposed to a direct effect of freezing on the insects themselves, although early instar larvae could be susceptible. At a location near St. Louis (the Tyson Research Center), a population of fall cankerworm, Alsophila pometaria (Geometridae), had been increasing over the years prior to 2007, but was decimated by the spring freeze. Caterpillars of A. pometaria were seen searching for food on twigs containing only frozen leaves (Marquis et al., 2019). The mechanism underlying recorded impacts of the spring freeze on the summer fauna is less clear. Perhaps overwintering pupae were sensitive to the abnormally low temperatures during a time when they were close to breaking diapause or quality of re-flush leaves was low (Rooke and Bergström, 2007). Whatever the cause, the more rapid recovery of the summer fauna (relative to the spring fauna) subsequent to the 2007 freezing event could be due in part to the much higher numbers of multivoltine species comprising the summer assemblage, as almost all of the spring fauna is univoltine.
One of the hypothesized advantages of building shelters by insects is protection against abiotic factors (Lill and Marquis, 2007). For example, low humidity has been experimentally shown to kill leaf shelter building caterpillars removed from their shelters (Hunter and Wilmer, 1989). If indeed leaf shelter construction routinely buffers desiccation prone inhabitants from stress, the high temperatures and low humidity associated with the extreme drought of 2012 may have surpassed their ability to do so. The loss of ties could have triggered declines in other species, given that the ties are secondarily colonized by an array of other arthropod species, including most of the other species of leaf tying caterpillars (e.g., Marquis and Lill, 2010). There is some evidence that leaf ties on Q. alba provide refuge for the Asiatic oak weevil, Cyrtepistomus castaneus, during years of low abundance (Lill and Marquis, 2003). The fact that smaller-bodied species were more heavily affected, and that Limacodidae, which generally do not secondarily occupy leaf ties, continued to be exceedingly low in abundance 5 years after the drought strongly suggests that temperature and low humidity together were lethal. We saw no evidence of high abundances of either Lochmaeus manteo or Cecrita (Heterocampa) guttivitta (both Notodontidae) subsequent to the 2012 drought, at least in the St. Louis region. Drought has been suggested to trigger outbreaks in both species (Law and Gott, 1987; Martinet and Allen, 1987, respectively). Perhaps multiple years of drought are required to trigger such outbreaks.
The high abundance of oak feeding insects seen in our 1991 sampling in MOFEP, and less so in 1992, may be an example of a coincidence of high populations of many spring feeding species. These spring feeding species are called the “leaf roller and looper complex” in numerous pest reports by the Missouri Department of Conservation produced over the period 1968–1980 (e.g., Gass, 1973). In 1991, there were extremely high numbers of Geometridae, particularly Phigalia strigataria, various Totricidae, including Cenopis pettitana, Pseudexentera faracana, and P. spoliana, and Gelechiidae, particularly Chionodes adamas and C. pereyra (Gelechiidae) (R. J. Marquis and J. Whitfield, unpublished data). Similar outbreaks of several species occurred in the St. Louis region in 2013, resulting in patches of nearly complete defoliation of canopy trees (R. J. Marquis, pers. obs.). Such events are also reported from Virginia (Asaro and Chamberlin, 2015). Law and Gott (1987) suggest that such outbreaks are triggered by extended droughts in previous years. A sharp decline in abundance in 1993 from the previous high levels in years 1991–1992 occurred for insects on both oak species, but particularly on Q. alba (Figure 1). Unlike the declines in 1997 and 2007, daily high temperatures in March 1993 only once went above 20°C, and only once below 0°C (Figure 2A). Instead of climatic factors, avian and arthropod predators and parasitoids might have played a role. Both predation of caterpillars by birds (Marquis and Whelan, 1994) and parasitism of caterpillars (Le Corff et al., 2000) can be high in the Missouri Ozarks.
Given the observed declines in insect numbers at many locations across the globe (Sanchez-Bayo and Wyckhuys, 2019), one might be tempted to make the same conclusion from our data (Figure 1). At least superficially, there seems to be an overall trend of a modest decreasing abundance from 1991 to 2010 on both Q. alba and Q. velutina. At least for these faunas on Missouri oaks, however, one must recognize that local weather events have dramatic effects on insect numbers. These weather events cause declines, which are then followed by rebounds. Continuous sampling for a few years, interspersed with large numbers of years in which no data were gathered (e.g., Lister and Garcia, 2018), could mask these important dynamics as evidenced by the current study. Moreover, the impacts of these local weather events must be understood in order that they might not be mistaken for the effects of humans on insects, perhaps via habitat destruction or climate change. Continuous data are very important. The low numbers in 1997 might be interpreted as a part of a general decline from 1991 to 1995, particularly for Q. alba (Figure 1A). Missing data from 1996 call into question whether numbers in 1997 were low due to the spring freeze in 1997 or were already low in 1996. Fortunately, our data captured a second freeze event in 2007, showing a dramatic decline in May 2007 numbers compared to May 2006 for both host plant species (Figure 1). Furthermore, temperatures did not go below 0°C in either April or May 1996 at any of the weather stations in the region.
The picture that emerges for our study sites in southeastern Missouri is that there is little evidence that overall abundances are declining during the years 1991–2010. In fact, there is a low likelihood for humans to have caused such changes in the southern half of Missouri, in the form of habitat destruction and pesticides, because forest cover is so continuous and widespread. Impacts of climate change are a possibility, but the data presented here show no overall decline. In fact, just the opposite seems to be the case. Our sampling in a chronosequence suggests that insect abundance and species richness are only increasing as these forests continue to age following widespread deforestation in the late 1800's and early 1900's (Jeffries et al., 2006).
Although we do not see evidence for a decline in abundance over the years studied, we suggest two cautionary notes regarding future climate impacts on herbivorous insect faunas in north temperate regions. First, evidence suggests that the likelihood of mid spring frosts has increased over the last 100+ years in nearby Illinois (Augspurger, 2013) and is only expected to increase in the future (Gu et al., 2008). In addition because of warmer winters, plants are likely to be more susceptible to these frosts when they do occur (Gu et al., 2008). Finally, different plant species appear to be differentially susceptible to the same frost event (e.g., oaks more than sugar maple) (Gu et al., 2008; Augspurger, 2013; Muffler et al., 2016). Thus, the faunas on oaks may be more susceptible than those found on other host plant species.
The second cautionary note is that climate in the Midwest of the U.S. is predicted to become warmer and drier in the summer months in the near future (Hayhoe et al., 2018). Based on our results from Cuivre River State Park, we predict that drought will decrease populations of insects both directly because of high temperatures, low humidity, and low host plant water content, and indirectly by disrupting positive interactions involving shelter building insects, and the secondary inhabitants of those shelters.
In light of these predictions for future climate change, our results suggest mid spring frosts and summer droughts of magnitudes high enough to negatively affect insect abundance on oaks in our region are likely to increase, ultimately leading to long term declines. In addition, insect declines associated with both mid spring frosts and summer droughts are likely to have bottom up effects on the natural enemies that simultaneously use those insects as a food resource and serve to control their populations in more typical years. The magnitude of such bottom up effects on the third trophic level is unknown at this time.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
RM and RF conducted the statistical analyses. RM wrote the original manuscript draft. JTL, RF, JL, JML, and JW edited subsequent drafts. All authors helped with data collection.
Funding
This work was supported by the Missouri Department of Conservation, USDA Grant No. 02DG 1124225 430, and National Science Foundation grant 6164397.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank numerous undergrad and graduate students at the University of Missouri-St. Louis for help with insect sampling. In addition to the many students, we thank F. DiTirro, N. Greig, J. McGrath, and G. Wang who all helped with data collection. R. Jensen helped with logistics on the ground in MOFEP, and B. Schuette did the same at CRSP. Patrick Guinan provided information on the NCEI climate data base, and V. Redensek and S. Bahar provided many helpful suggestions regarding climate data analysis. The Missouri Department of Conservation, the USDA, and the National Science Foundation provided funding. The Missouri Department of Natural Resources provided access to Cuivre River State Park, and Washington University gave us access to the Tyson Research Center.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00396/full#supplementary-material
References
Asaro, N., and Chamberlin, L. A. (2015). Outbreak history (1953-2014) of spring defoliators impacting oak-dominated forests in Virginia, with emphasis on gypsy moth (Lymantria dispar L.) and fall cankerworm (Alsophila pometaria Harris). Amer. Entomol. 61, 174–185. doi: 10.1093/ae/tmv043
Augspurger, C. K. (2013). Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage is increasing. Ecology 94, 41–50. doi: 10.1890/12-0200.1
Baer, C. S., and Marquis, R. J. (2014). Native leaf-tying caterpillars influence host plant use by the invasive Asiatic oak weevil through ecosystem engineering. Ecology 95, 1472–1478. doi: 10.1890/13-1689.1
Barbosa, P., Letourneau, D. K., and Agrawal, A. A. (2012). Insect Outbreaks Revisited. Chichester, UK: J. Wiley-Blackwell.
Brookshire, B. L., Jensen, R., and Dey, D. C. (1997). “The Missouri ozark forest ecosystem project: past, present, and future,” in Proceedings of the Missouri Ozark Forest Ecosystem Project Symposium: An Experimental Approach to Landscape Research, St. Louis, MO, General Technical Report NC-193, eds B. L. Brookshire, and S. Shifley (Saint Paul, MN: USDA Forest Service; North Central Forest Experiment Station, 1–25.
Ehrlich, P. R., Murphy, D. D., Singer, M. C., Sherwood, C. B., White, R. R., and Brown, I. L. (1980). Extinction, reduction, stability and increase: the responses of checkerspot butterfly (Euphydryas) populations to the California drought. Oecologia 46, 101–105. doi: 10.1007/BF00346973
Epstein, M. E. (1996). Revision and Phylogeny of the Limacodid-group Families, with Evolutionary Studies on Slug Caterpillars (Lepidoptera: Zygaenoidea). Smithsonian Contributions to Zoology 582. Washington, DC: Smithsonian Institution Press.
Forkner, R. E., and Marquis, R. J. (2004). Uneven-aged and even-aged logging alter foliar phenolics of oak trees remaining in forested habitat matrix. For. Ecol. Manage. 199, 21–37. doi: 10.1016/j.foreco.2004.03.044
Forkner, R. E., Marquis, R. J., Lill, J. T., and Le Corff, J. (2006). Impacts of forest management on oak-feeding caterpillar populations in the Missouri Ozarks. Cons. Biol. 20, 429–440. doi: 10.1111/j.1523-1739.2006.00346.x
Forkner, R. E., Marquis, R. J., Lill, J. T., and Le Corff, J. (2008). Timing is everything? Phenological synchrony and population variability in leaf-chewing herbivores of Quercus. Ecol. Entomol. 33, 276–285. doi: 10.1111/j.1365-2311.2007.00976.x
Gass, R. D. (1971). Life History and Ecology of the Variable Oak Leaf Caterpillar, Heterocampa manteo (Dbldy.), in Missouri (Lepidoptera: Notodontidae). Jefferson City, MO: Missouri Department of Conservation.
Gass, R. D. (1973). Missouri Forest Pest Report. Jefferson City, MO: Missouri Department of Conservation.
Gu, L., Hanson, P. J., Post, W. M., Kaiser, D. P., Yang, B., Nemani, R., et al. (2008). The 2007 eastern U. S. spring freeze: increased cold damage in a warming world? Bioscience 58, 253–262. doi: 10.1641/B580311
Hallmann, C. A., Sorg, M., Jongejans, E., Siepe, H., Hofland, N., Schwan, H., et al. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12:e0185809. doi: 10.1371/journal.pone.0185809
Hayhoe, K., Wuebbles, D. J., Easterling, D. R., Fahey, D. W., Doherty, S., Kossin, J., et al. (2018). “Our changing climate,” in Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Vol II, eds D. R. Reidmiller, C. W. Avery, D. R. Easterling, K. E. Kunkel, K. L. M. Lewis, T. K. Maycock, and B. C. Stewar (Washington, DC: U.S. Global Change Research Program, 72–144.
Hunter, A. F. (1991). Traits that distinguish outbreaking and nonoutbreaking Macrolepidoptera feeding on northern hardwood trees. Oikos 60, 275–282.
Hunter, M. D., and Wilmer, P. G. (1989). The potential for interspecific competition between two abundant defoliators on oak: leaf damage and habitat quality. Ecol. Ent. 12, 373–382. doi: 10.1111/j.1365-2311.1987.tb01018.x
Jeffries, J., Marquis, R. J., and Forkner, R. E. (2006). Community composition, species richness, and abundance of oak herbivore insects in a chronoseries of temperate forest. Ecol. Appl. 16, 901–912. doi: 10.1890/1051-0761(2006)016[0901:FAIOIH]2.0.CO;2
Kærsgaard, C. W., Holmstrup, M., Malte, H., and Bayley, M. (2004). The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Phys. 50, 5–15. doi: 10.1016/j.jinsphys.2003.09.003
Kolb, T. E., Fettig, C. J., Ayres, M. P., Bentz, B. J., Hicke, J. A., Mathiasen, R., et al. (2016). Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 380, 321–334. doi: 10.1016/j.foreco.2016.04.051
Koricheva, J., Klapwijk, M. J., and Bjorkman, C. (2012). “Life history traits and host plant use in defoliators and bark beetles: implications for population dynamics,” in Insect Outbreaks Revisited, eds P. D. Barbosa, D. K. Letourneau, and A. A. Agrawal (Chichester, UK: J. Wiley-Blackwell, 177–196.
Law, J. R., and Gott, J. D. (1987). Oak Mortality in the Missouri Ozarks. Knoxville, TN: Sixth Central Hardwood Forest Conference.
Le Corff, J., Marquis, R. J., and Whitfield, J. B. (2000). Temporal and spatial variation in a parasitoid community associated with the herbivores that feed on Missouri Quercus. Env. Entomol. 24, 46–58. doi: 10.1603/0046-225X(2000)029[0181:TASVIA]2.0.CO;2
Le Lagadec, M. D., Chown, S. L., and Scholtz, C. H. (1998). Desiccation resistance and water balance in southern African keratin beetles (Coleoptera, Trogidae): the influence of body size and habitat. J. Comp. Phys. B 168, 112–122. doi: 10.1007/s003600050127
LeRoux, E. J., Paradis, R. O., and Hudon, M. (1963). Major mortality factors in the population dynamics of the eye-spotted bud moth, the pistol casebearer, the fruit-tree leaf roller, and the European corn borer in Quebec. Mem. Entomol. Soc. Canada 95, 67–92. doi: 10.4039/entm9532067-1
Lill, J. T. (2004). Seasonal dynamics of leaf-tying caterpillars on white oak. J. Lep. Soc. 58, 1–6.
Lill, J. T., and Marquis, R. J. (2003). Ecosystem engineering by caterpillars increases insect herbivore density on white oak. Ecology 84, 682–690. doi: 10.1890/0012-9658(2003)084[0682:EEBCII]2.0.CO;2
Lill, J. T., and Marquis, R. J. (2007). “Microhabitat manipulation: ecosystem engineering by shelter-building insects,” in Ecosystem Engineers: Concepts, Theory, and Applications in Ecology, eds K. M. D. Cuddington, J. E. Byers, A. Hastings, and W. G. Wilson (San Diego, CA: Elsevier Press, 107–138.
Lill, J. T., Marquis, R. J., Forkner, R. E., Le Corff, J., Holmberg, N., and Barber, N. A. (2006). Leaf pubescence affects distribution and abundance of generalist slug caterpillars (Lepidoptera: Limacodidae). Environ. Ent. 35, 797–806. doi: 10.1603/0046-225X-35.3.797
Lister, B. C., and Garcia, A. (2018). Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. U.S.A. 115, E10397–E10406. doi: 10.1073/pnas.1722477115
Marquis, R. J., and Le Corff, J. (1997). “Estimating pre-treatment variation in the oak leaf-chewing insect fauna of the Missouri ozark forest ecosystem project,” in Proceedings of the Missouri Ozark Forest Ecosystem Project Symposium: An Experimental Approach to Landscape Research, eds B. L. Brookshire, and S. R. Shifley (St. Paul, MN: USDA Forest Service, North Central Forest Experiment Station. General Technical Report NC-193, 332–346.
Marquis, R. J., and Lill, J. T. (2010). Impact of plant architecture versus leaf quality on attack by leaftying caterpillars on five oak species. Oecologia 163, 203–213. doi: 10.1007/s00442-009-1519-2
Marquis, R. J., Passoa, S. C., Lill, J. T., Whitfield, J. B., Le Corff, J., Forkner, R. E., et al. (2019). An Introduction to the Immature Lepidopteran Fauna of Oaks in Missouri. Morgantown, WV: USDA Forest Service, Forest Health Assessment and Applied Sciences Team, FHAAST-2018–T-2005.
Marquis, R. J., and Whelan, C. J. (1994). Insectivorous birds increase plant growth through their impact on herbivore communities of white oak. Ecology 75, 2007–2014. doi: 10.2307/1941605
Martinet, P. J., and Allen, D. C. (1987). Relationship between outbreaks of saddled prominent, Heterocampa guttivitta (Lepidoptera: Notodontidae), and drought. Environ. Entomol. 16:246–249. doi: 10.1093/ee/16.1.246
Mason, R. R. (1987). “Nonoutbreak species of forest Lepidoptera,” in Insect Outbreaks, eds P. Barbosa, and J. C. Schultz (San Diego, CA: Academic Press, 31–58.
Mattson, W. J., and Haack, R. A. (1987). The role of drought in outbreaks of plant-eating insects. Bioscience 37, 110–118. doi: 10.2307/1310365
Morris, R. F. (1964). The value of historical data in population research, with particular reference to Hyphantria cunea Drury. Can. Ent. 96, 356–368. doi: 10.4039/Ent96356-1
Muffler, L., Beierkuhnlein, C., Aas, G., Jentsch, A., Scheiger, A. H., Zohner, C., et al. (2016). Distribution ranges and spring phenology explain late frost sensitivity in 170 woody plants from the Northern Hemisphere. Glob. Ecol. Biogeogr. 25, 1061–1071. doi: 10.1111/geb.12466
Nothnagle, P. J., and Schultz, J. C. (1987). “What is a forest pest?,” in Insect Outbreaks, eds P. Barbosa, and J. C. Schultz (San Diego, CA: Academic Press, 59–80.
Philip, B. N., Yi, S. X., Elnitsky, M. A., and Lee, R. E. (2008). Aquaporins play a role in desiccation and freeze tolerance in larvae of the goldenrod gall fly, Eurosta solidaginis. J. Exp. Biol. 211, 1114–1119. doi: 10.1242/jeb.016758
Reinhardt, J. R. (2011). Ecosystem engineering and environmental context: the impact of leaftying caterpillars and foliage quality on the arthropod community associated with white oak (Quercus alba, L.) (masters thesis). St. Louis, MO: University of Missouri-St. Louis.
Rippey, B. R. (2015). The, U. S. drought of 2012. Weather Climate Extreme 10, 57–64. doi: 10.1016/j.wace.2015.10.004
Rooke, T., and Bergström, R. (2007). Growth, chemical responses and herbivory after simulated leaf browsing in Combretum apiculatum. Plant Ecol. 189, 201–212. doi: 10.1007/s11258-006-9177-5
Royama, T. (1997). “Population dynamics of forest insects: are they governed by single or multiple factors?,” in Forests and Insects, eds A. D. Watt, N. E. Stork, and M. D. Hunter (London: Chapman and Hall, 37–48.
Sanchez-Bayo, R., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: a review of its drivers. Biol. Cons. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Sheriff, S. L., and He, Z. (1997). “The experimental design of the Missouri Ozark Forest Ecosystem Project,” in Proceedings of the Missouri Ozark Forest Ecosystem Project Symposium: An Experimental Approach to Landscape Research, St. Louis, MO, June 3–5. General Technical Report NC-193, eds B. L. Brookshire, and S. Shifley (St. Paul, MN: USDA Forest Service, North Central Forest Experiment Station, 26–40.
Sinclair, B. J., Ferguson, L. V., Salehipour-shirazi, G., and MacMillan, H. A. (2013). Cross-tolerance and cross-talk in the cold: relating low temperatures to desiccation and immune stress in insects. Integrat. Comp. Biol. 53, 545–556. doi: 10.1093/icb/ict004
Spencer, J. S. Jr., Roussopoulos, S. M., and Massengale, R. A. (1992). Missouri's Forest Resource, 1989: An Analysis. St. Paul, MN: USDA Forest Service, North Central Forest Experiment Station, NC-139.
Story, M., and Congalton, R. G. (1986). Accuracy assessment: a user's perspective. Photogr. Eng. Remote Sens. 52, 397–399.
Summerville, K. S., and Marquis, R. J. (2016). Comparing the responses of larval and adult Lepidopteran communities to timber harvest using long-term, landscape-scale studies in oak-hickory forests. For. Ecol. Manag. 387, 64–72. doi: 10.1016/j.foreco.2016.08.050
Torres, J. A. (1992). Lepidoptera outbreaks in response to successional changes after the passage of Hurricane Hugo in Puerto Rico. J. Trop. Ecol. 8, 285–298. doi: 10.1017/S0266467400006544
Wang, H. G., Marquis, R. J., and Baer, C. S. (2012). Both host plant and ecosystem engineer identity influence leaf-tie impacts on the arthropod community of Quercus. Ecology 93, 2186–2197. doi: 10.1890/11-1838.1
Keywords: insect decline, climate change, insect populations, drought, spring frosts, Quercus, herbivorous insects
Citation: Marquis RJ, Lill JT, Forkner RE, Le Corff J, Landosky JM and Whitfield JB (2019) Declines and Resilience of Communities of Leaf Chewing Insects on Missouri Oaks Following Spring Frost and Summer Drought. Front. Ecol. Evol. 7:396. doi: 10.3389/fevo.2019.00396
Received: 11 May 2019; Accepted: 04 October 2019;
Published: 22 October 2019.
Edited by:
Lora A. Richards, University of Nevada, Reno, United StatesReviewed by:
Ken Oyama, National Autonomous University of Mexico, MexicoBastien Castagneyrol, INRA Centre Bordeaux-Aquitaine, France
Copyright © 2019 Marquis, Lill, Forkner, Le Corff, Landosky and Whitfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert J. Marquis, cm9iZXJ0X21hcnF1aXNAdW1zbC5lZHU=
 Robert J. Marquis
Robert J. Marquis John T. Lill
John T. Lill Rebecca E. Forkner
Rebecca E. Forkner Josiane Le Corff
Josiane Le Corff John M. Landosky
John M. Landosky James B. Whitfield5
James B. Whitfield5