
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 15 October 2019
Sec. Population, Community, and Ecosystem Dynamics
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00375
This article is part of the Research Topic Arthropod Interactions and Responses to Disturbance in a Changing World View all 20 articles
Eusociality, a form of animal social organization involving sterile and reproductive castes, is a rare, but highly ecologically successful form of life. There are striking examples of eusocial species with populations that are ecologically dominant in their native ranges, as well as remarkably successful globally as invasive species; prominent examples include fire ants and yellowjacket wasps. At the same time, there have been startling population declines in other social insects, notably bumble bees. Here, we explore the possible role of phenotypic plasticity in invasion biology and declines of social insect species. This topic is of particular interest, because social insects exhibit extreme behavioral, developmental, physiological, and morphological plasticity. It has been suggested that this plasticity may contribute to ecological dominance in some species, but could be a liability or cost to others. In this review, we explore the relationship between phenotypic plasticity, invasion biology, and vulnerability to global change in social insects. By considering plasticity at three levels–molecular, individual, and colony—we suggest ways in which considerations of phenotypic plasticity may help in managing social insect populations.
Phenotypic plasticity is defined as the ability of an individual organism to respond to the environment by producing alternative phenotypes based on the same genotype (Baldwin, 1896). Phenotypic plasticity is of great interest in ecology and evolution because it allows an organism to actively adjust its phenotype in response to environmental conditions; thus it is a major mechanism of ecological adaptation (Via et al., 1995; Lande, 2015; Beaman et al., 2016; Colautti et al., 2017). In addition, phenotypic plasticity has been suggested to be an important driver of evolutionary change; animals with more flexibility in how they respond to the environment may have greater potential to enter and survive in novel and/or changing habitats (Baldwin, 1896; West-Eberhard, 1989).
Eusocial insects show some of the most striking known examples of phenotypic plasticity known in the animal world, on both the individual and colony levels (Kennedy et al., 2017). These animals are defined by the presence of distinct castes within colonies—individuals that are specialized for specific types of activity, the most prominent being the division of labor between reproductive “royal” castes (e.g., queens) and non-reproductive castes (e.g., workers, Wilson, 1971). In many species, reproductive caste differences are independent of genotype (Schwander et al., 2010)—e.g., in many eusocial bees, ants, and wasps, any egg has the potential to develop into a queen or a worker. As adults, there is further phenotypic plasticity, with specialized subcastes of individuals involved in different behavioral and physiological activities (e.g., foraging, nursing brood). One key aspect of colony level plasticity is the ability to alter division of labor (e.g., ratio of foragers and nurses) according to colony nutritional needs, colony demography, and environmental conditions (Robinson, 1992; Gordon, 1996; Traniello and Rosengaus, 1997). In some species, there is further phenotypic plasticity in the form of specialized forms of foraging preference (e.g., pollen vs. nectar foraging in honey bees), dominance status (e.g., in Polistes workers), and task-related behaviors including learning and memory abilities (Reeve and Nonacs, 1992; Robinson, 1992; O'Donnell et al., 2004). Thus, there is no doubt that social insects are champions of phenotypic plasticity.
Eusocial insects represent some of the most important known invasive species in the world. For example, in a list of the Top 100 most invasive species, 41% of invasive invertebrates are social insects (Lowe et al., 2000). This figure suggests a large overrepresentation of social insect as successful invaders, given the fact that only 2% of insect species are estimated to be eusocial. Although the aforementioned list is somewhat anecdotal, it is a well-established fact that some social insect species are spectacularly successful invasive species (Evans, 2010; Lach and Hooper-Bui, 2010). Are these two phenomena related? That is, is the extreme phenotypic plasticity exhibited by social insects one of the secrets to their worldwide success, and their ability to invade new environments and dominate ecosystems? Studies from other organisms suggest that indeed, the extent of phenotypic plasticity exhibited by a species can contribute to its ability to become an invasive species (Richards et al., 2006; Wilson, 2012).
On the other hand, there have been dramatic declines in insect populations worldwide over the past several decades (Sánchez-Bayo and Wyckhuys, 2019). Prominent examples of insect decline include, notably, several species of bees, which are highly valued as pollinators (Potts et al., 2010). Some of these are social bees, the best studied examples being various species of bumble bees. If plasticity is related to invasion success and the ability of social insect species to survive in novel environments, then why are other social insects in decline and so prominently of conservation concern? Could variation in the extent of phenotypic plasticity, specifically, a relative lack of plasticity in some social insect species, be related to species decline? And, is colony or individual level plasticity playing a role, or both? Evolutionary modeling studies (Chevin and Lande, 2010; Chevin et al., 2010) and studies in non-social insect species (reviewed in the sections that follow) have provided ample evidence that the extent of phenotypic plasticity can play a role in species extinction and robustness to environmental change.
The goal of this article is to explore the potential relationships between phenotypic plasticity, eusocial insect invasions, and eusocial insect declines. Specifically, we explore the hypothesis that drastic environmental change, such as current global climate and anthropogenic disturbance, will favor more phenotypically plastic social insect species, fueling their invasions, while harming less plastic species, contributing to their declines (Figure 1). A related hypothesis is that social insects themselves, due to their inherent plasticity because of their eusocial lifestyle, are buffered from environmental change. Because direct comparative studies of the population status and plasticity of social and non-social species are lacking, this review focuses on addressing the former hypothesis, with the goal of elucidating a path forward to answer the latter. Previous reviews have discussed traits related to social insect invasions (especially in ants, see Holway et al., 2002) and declines (especially in bees, see Williams et al., 2010). However, to our knowledge, there has been no review to synthesize this information in a more general framework, despite the fact that species traits and robustness to global change has been broadly explored outside of social insects (Jiguet et al., 2007; Pyšek et al., 2012). Here, we first briefly review the literature related to phenotypic plasticity in the context of organisms outside social insects, identifying traits that are potentially phenotypically plastic in other systems that have been proposed to contribute to invasive potential. Similarly, we review the literature related to life history traits associated with declines of species outside of social insects, and assess these traits in the context of potential relationships to phenotypic plasticity. We then review evidence from selected social insect taxa that represent both invasive and declining species, and examine whether this information supports/refutes the hypothesis that phenotypic plasticity contributes to population stability of social insects in the face of environmental change.
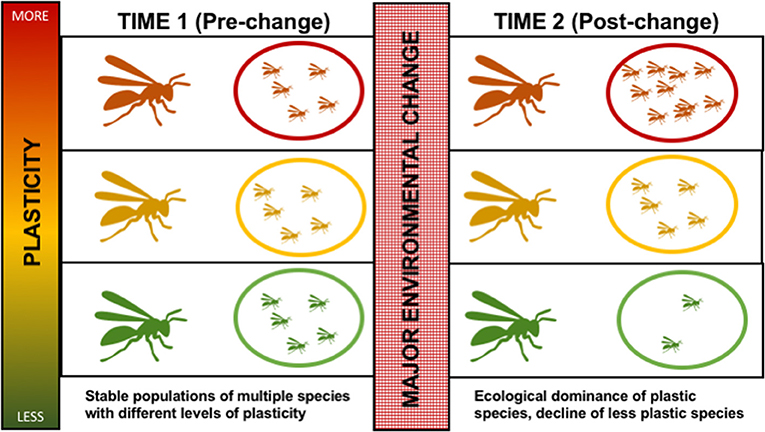
Figure 1. Schematic representing how phenotypic plasticity may contribute to species declines or species invasions in the face of a major environmental change. Three hypothetical species are represented with variation in their level of phenotypic plasticity (overall plasticity, or related to specific traits) (see text). These hypothetical species vary in their level of phenotypic plasticity at both individual (insect drawing) and colony (nest drawing) levels, with green representing lowest plasticity, rust representing highest plasticity, and yellow representing intermediate plasticity. At time 1, all three species are able to coexist with stable populations (ovals), each taking advantage of different ecological niches with the less plastic species being successful despite lacking plasticity due to being able to specialize on certain aspects of its environment. A major environmental change (red stippled box in center) occurs, such as major habitat loss, climatic shift, arrival in a new exotic region, or stress from disease, toxins, or new competitors. At time 2, the major environmental change has served as a filter, with a higher probability of more plastic species (rust) prospering and increasing their populations (rust oval) with this change due to their ability to rapidly adapt to change and/or enter new niches. At the same time, the less plastic and more specialized species (green) is less likely to fit this new environment or withstand a large change and experiences population declines (green oval).
Before considering the potential role of phenotypic plasticity in any context, it is important to define phenotypic plasticity and clarify what makes it distinct from other processes that might produce phenotypic variation within a species. For example, inter-individual variation can also be the result of standing genetic variation in a trait (e.g., the result of balancing selection in which there is no single trait value that optimizes fitness), as well as stochastic, developmental variation that is not based on adaptive differences in gene expression or genotype (Gianoli and Valladares, 2011). In addition, variation in size and age among individuals within a population can be confounding factors when analyzing the extent of phenotypic plasticity in a population of organisms. Thus, how do we separate out “real” phenotypic plasticity from other sources of phenotypic variation? In many cases, whether a trait is truly the result of phenotypic plasticity is not known, especially if the species has not been extensively studied. However, one aspect that might be useful to discriminate phenotypic plasticity from other mechanisms is the timescale of the phenomenon that we observe. By definition, a plastic trait must experience variation within a short timespan (though this might be different from organism to organism), hence complex traits that have undergone change over a long period of time are not plastic. Also, plasticity might be a transient trait that is displayed by a species in a specific moment in history—for example immediately after settling into a new range—and then disappears when it is no longer needed. For the purposes of this study, we followed the principle that the study of phenotypic plasticity in an ecological context goes beyond addressing the question of whether plasticity exists (or not) in each specific case—because most traits are plastic to a certain level (Gianoli and Valladares, 2011).
Previous studies have provided some support for the idea that phenotypic plasticity plays a role in the success of invasive organisms. In a meta-analysis of plants, Davidson et al. (2011) found that invasive plants display higher phenotypic plasticity than their non-invasive counterparts—though this does not always correlate with increased fitness. However, Palacio-López and Gianoli (2011) did not find any difference in the extent of phenotypic plasticity between invasive and non-invasive plants. They hypothesize that the success in invasion might be linked instead to a better ability to adapt to specific ecological niches, but acknowledge that plasticity should be investigated very early in the process of invasion when adaptation has not occurred yet. Although no large-scale meta-analyses have been conducted on invasion potential and phenotypic plasticity in animals, below, we provide a brief review of potentially plastic traits that have been proposed to be associated with invasiveness in animals, highlighting some specific examples that lead to hypotheses about invasive traits in social insects.
There is a large literature on plasticity in morphology (external and internal) and physiology as related to invasiveness in animals. Invasive species are often released from natural competitors and enemies (see Box 1) that are absent in the new range (Liu and Stiling, 2006). It has been hypothesized that this release could be followed by a reduction of competition effort or defense against enemies, accompanied by an increased investment into reproduction (Blossey and Notzold, 1995). The ability to move resources from one physiological compartment to another (possibly a form of phenotypic plasticity) is thought to give invasive species an advantage compared to new competitors that they might encounter in the invasive range. Plastic morphological traits in invasive organisms are well-documented, such as the appearance of head spines in invasive Daphnia in North America when predator pressure is high (Engel and Tollrian, 2009), or adaptive adjustments in body size in brown anoles after invading two Floridian islands (Campbell and Echternacht, 2003). In considering social insects, body size plasticity among workers is a well-known aspect usually linked to division of labor in various species of ants and bumble bees (i.e., workers of different size undertaking different size-specific tasks, see for example Wilson, 1978). Thus, one might predict that invasive populations of social insects might display a wider range of body size plasticity (either within a colony or at the population level) compared to non-invasive populations—or an invasive species overall might display wider body size plasticity compared to other species that are non-invasive.
Box 1. The role of natural enemies in biological invasions.
An intriguing hypothesis often invoked to explain the evolutionary ecology of biological invasions is the “Enemy Release Hypothesis” (ERH). It postulates that invasive species might leave behind their natural enemies (predators, competitors, parasites, and pathogens) when establishing in a new range (reviewed in Liu and Stiling, 2006). This gives them an advantage with respect to sympatric species in the new range, as they can redirect resources originally allocated for fighting natural enemies toward different physiological compartments, for example growth and reproduction (“Evolution of Increased Competitive Ability” or EICA, Blossey and Notzold, 1995). EICA is often described as a natural consequence of the ERH. However, it is not fully clear whether specific conditions are needed for the EICA to be triggered and, in particular, whether it requires a certain amount of plasticity in the physiology of the organism. This could be a key feature that differentiates between successful and failed invaders.
The ERH has been documented for several species of social insects in relation to parasites and pathogens: for example, fire ants have been released from Wolbachia, two microsporidia and one RNA virus after invading South-East Asia and Oceania (Yang et al., 2010), Argentine ants have lost Wolbachia in seven out of eight regions where they have been introduced (Reuter et al., 2005), and paper wasps have left behind two insect parasitoids after invasion into North America (Cervo et al., 2000). However, there are also remarkable cases that refute the ERH: for example, many common pathogens have been detected in bumble bees that were introduced to South America (Arbetman et al., 2013), and a study detected high microsporidian infection rates in yellowjackets that invaded New Zealand (Lester et al., 2014). Furthermore, the ERH is just one side of the coin, as there are instances where invasive species appear more susceptible to new enemies than native sympatric species (often referred to as the “Increased Susceptibility Hypothesis” or “Exotic Prey Naïveté” hypothesis,” Li et al., 2011). This has been documented for paper wasps in South Africa, where invasive Polistes dominula experience more parasite pressure than native sympatric Polistes marginalis (Roets et al., 2019), and for Asian hornets that invaded southern France, infected by conopid flies and mermithin nematodes (Villemant et al., 2015) that represent “new enemies.”
In this complex scenario, plasticity might be a key element that underpins the ability of successful invaders to “forget” old enemies, when they are no longer around, while maintaining high levels of defense against new enemies that might be encountered. When dealing with parasites and pathogens, social insects display the potential for high levels of plasticity in their defense responses. They can choose, for example, between response that are at the individual or group levels (known as “social immunity,” Cremer et al., 2007), physiological or behavioral, constitutive, or induced (Schmid-Hempel and Ebert, 2003), and they can also respond as immatures or adults. One pioneer study in this field has shown how invasive paper wasps in the USA have reduced their defense against new general pathogens (at both the individual and group levels) while maintaining a strong behavioral group response against an old parasitic enemy that was lost after invasion (Manfredini et al., 2013). This exemplifies how plasticity in immunocompetence might mediate the successful invasion of a social insect.
Plasticity in response to temperature variation is undoubtedly one of the key aspects to consider in how species are able to survive and compete in novel or rapidly changing environments. For example, Trinidadian guppies have extensive plasticity in modulating their reproductive activity according to daily variation of temperature and light (Reeve et al., 2014). Because social insects possess various different castes with different body sizes and physiologies, it has been proposed that thermal plasticity could be an advantage in social insects, i.e., that a single colony may possess the ability to send out “thermal explorers” into an environment that would be more thermally limiting for a monomorphic species (Baudier and O'Donnell, 2017).
There is no doubt about the fact that one key ingredient for a successful invasion is the ability of a given species to spread beyond the native range. A series of elegant studies on invasive cane toads in Australia has shown how the geographic spread of this species varies along a gradient of dispersal abilities from the range-core to the invasion-front, showing incredible plasticity (Rollins et al., 2015). In social insects, plasticity during dispersal may be essential at several points in the colony cycle including queen dispersal during colony founding, male dispersal during mating season, or in mature colonies, potential for the production of sexuals (e.g., production of one vs. multiple broods of sexual, dispersing individuals).
Behavior encompasses a complex set of traits that are extremely important during biological invasions, as behavior is often the first line of response to environmental challenges. Aggression, exploratory behavior and plasticity in sexual/reproductive behavior are often important traits associated with successful invasions. For example, virile crayfish shifted towards more aggressive behavior in their invasive range compared to their native range (Glon et al., 2018). Dark-eyed junco songbirds changed their sexual behavior after introduction into Southern California, accompanied by changes in testosterone levels (Atwell et al., 2014). Social insects are well-known to show wide inter- and intraspecies variation in aggression, exploratory behavior, and reproductive strategies (e.g., single vs. multi-queen colony organization, Bengston and Jandt, 2014). Some of these behavioral traits have also been proposed to be related to invasion success in social insects, in particular high levels of aggression (Human and Gordon, 1999) and plasticity in queen number (Ingram, 2002).
There are many forms of genomic plasticity, spanning from complex mechanisms that involve large portions of the genome and produce changes over evolutionary time (e.g., structural polymorphisms, copy-number variations, and chromosome rearrangements Leitch and Leitch, 2008), to forms of plasticity that involve the functioning of specific genomic elements and produce variation at the phenotypic level in a much shorter amount of time (e.g., the life span of an individual or few generations): these include, for example, epistatic interactions between genes in the context of gene networks, interactions between transcription factors and regulatory elements, and epigenetic modifications. Some changes in genome structure can occur incredibly quickly and might be important in invasions. For example, autopolyploidy caused rapid speciation of the marbled crayfish from the slough crayfish and clonality mediated the incredible success of marbled crayfish as an invasive species in Madagascar (Gutekunst et al., 2018). Hybridization instead is thought to be one of the key factors at the basis of big-headed carps invasion into the Mississippi river and Lake Balaton (Cooke, 2016). Some social insects display examples of structural polymorphisms in their genomes that are associated with key social traits possibly linked to successful invasions, notably, the fire ant supergene associated with the presence of multiple queens in the colony, or “polygyny” (Wang et al., 2013). However, a direct correlation between the supergene and fire ant invasions has not been established, and a similar feature has also been detected in another ant that is not invasive (Formica selysi, Purcell et al., 2014).
In terms of genome function, changes in the expression of several key genes after invasion were reported for cane toads in Australia (Rollins et al., 2015) and goby fish in the Great Lakes (Wellband and Heath, 2017). Epigenetic mechanisms have also been suggested to be relevant in facilitating phenotypic changes associated with invasion; for example in house sparrows increased variation in genome-wide DNA methylation was detected in invasive populations in Kenya (Liebl et al., 2013), and in invasive ascidians and corals, methylation changes quickly occurred in response to environmental challenges (Putnam et al., 2016; Huang et al., 2017). Epigenetic mechanisms are particularly relevant for the success of biological invasions as they can produce new variation (and therefore adaptation) even in the lack of genetic diversity (Hawes et al., 2018), and even more so when combined with plasticity in other regulators of genetic activity, such as non-coding RNAs or transposable elements (Stapley et al., 2015; Marin et al., 2019). Epigenetic mechanisms have also been a topic of substantial interest in social insects, where they have been demonstrated to be relevant to caste plasticity, worker division of labor, and learning and memory in social insects (Glastad et al., 2019). In the context of social insects we predict that epigenetic modifications may facilitate phenotypic plasticity in the case of invasion potential.
The Earth is experiencing massive species declines worldwide, which according to the IPBS are “already at least tens to hundreds of times higher than… averaged over the past 10 million years” (IPBES et al., 2019). Thus, understanding major factors contributing to species declines is key for conservation purposes. Although there might not be a single or simple reason for the decline of any one species, some general causes, and or correlations with decline have pervaded the conservation literature (Didham et al., 2007). In general, the survival and/or persistence of species are thought to result from a combination of both extrinsic (e.g., environmental change) and intrinsic (e.g., species characteristics) factors (but see Fisher et al., 2003). Most research related to decline focuses on the extrinsic factors affecting species, including habitat (land/sea) use change, direct exploitation of species by humans, climate change, pollution, and negative impacts of invasive species. Although there has been some work trying to integrate intrinsic traits with extrinsic pressures (Murray et al., 2010), there is less understanding about the importance of plasticity of species' intrinsic traits. Understanding the true causes of decline of a species is often a complex situation, as a single extrinsic factor affects very many different intrinsic traits. For example, “climate change” can challenge a species' physiology, acclimatization, dispersal ability, and also behavioral traits. Here we summarize and give examples of specific traits associated with decline in different organisms, and explore whether phenotypic plasticity could be related to such declines.
Phenotypic plasticity can allow an organism to rapidly respond to a dynamic and temporally variable environment (e.g., climate change, habitat change use), whereas the lack of a plastic response has the potential to push a species to reduce its area of occupancy or result in a decrease in the number of individuals (i.e., species decline). It has been proposed that large-bodied species are more prone to decline than small-bodied species; for example, large-bodied carabid beetles have declined in Belgium more than smaller ones (Kotze and O'hara, 2003). In amphibians, a group of prime conservation concern, it has been reported that Rana sylvatica have a plastic response to desiccation, that results in decreases in post-metamorphic immune function. This physiological plasticity may thus result in a tradeoff with immune system functions, potentially contributing to increased sensitivity of amphibians to disease and decline (Gervasi and Foufopoulos, 2008). A recent study found that wild bees decreased in body size in the absence of native floral resource availability and that bees with particularly small or large body were most susceptible, suggesting that body size could plastically buffer bees from dearth and habitat disturbance (Grab et al., 2019).
The ability of species to adapt to changing environments, particularly shifts in temperature, is one of the main challenges in a warming world. Some species show superior abilities to adapt to temperature changes, while others are adversely affected. For example, it has been shown that in an endangered turtle (Podocnemis lewyana), environmental sex determination (a form of phenotypic plasticity) is sensitive to temperature stability during the egg incubation period (Gómez-Saldarriaga et al., 2016). Another study demonstrated that declining species of springtails were more sensitive to desiccation under warmer temperatures as compared to invasive springtails (Chown et al., 2007). In the case of social insects, among bumble bees in the genus Bombus, some declining species may also be suffering from rising global temperatures, as evidenced by shrinking distributions in warmer areas along with a failure to move closer to polar regions (Kerr et al., 2015).
The ability to disperse is an important challenge for declining species, particularly in situations of population fragmentation. If species have limited dispersal abilities or cannot plastically adjust their dispersal strategies in response to habitat loss, they may be unable to recolonize habitat fragments and become more susceptible to local extinctions (Kokko and López-Sepulcre, 2006). For example, dispersal ability may be associated with declines in large-bodied carabid beetles, which have poorer dispersal abilities as compared to smaller, more mobile species (Kotze and O'hara, 2003). Limited dispersal of sexuals (reproductive males and females) from their natal colonies has also been suggested to be one of the factors responsible for the rarity of wood ants in a fragmented landscape (Gyllenstrand and Seppä, 2003).
Behavioral traits, as dynamic responses to environmental stimuli, are by their very nature plastic. However, species can vary greatly in the extent of behavioral flexibility of different traits, including numerous traits relevant to coping with major environmental change; e.g., cognitive capacity, foraging preferences, exploratory behavior, and territoriality. Host behavior can also influence the transmission of novel and/or damaging pathogens; e.g., in Australian rainforest frogs in the genus Litoria, species differences in contact frequency (with contaminated frogs and water) were associated with differences in chytridiomycosis infection rate, and these reflected their conservation status (Rowley and Alford, 2007). In the eusocial bee Plebeia droryana, behavioral emergence from diapause occurs at a specific temperature (suggesting a lack of plasticity). This trait has been suggested to be a potential liability, as climate patterns producing milder winters or sudden extreme colds could prevent normal colony initiation in this species of conservation concern (Dos Santos et al., 2015).
Below, we highlight prominent case studies covering various species and genera of social insects related to population invasions and declines. The purpose of this is not to provide a comprehensive review of the literature, but rather to highlight some of the most prominent, well-known declining and invading species and consider what is known about the traits contributing to each of these phenomena in light of phenotypic plasticity. We focus on hymenopteran social insects (ants, wasps, and bees) because this allows us to most coherently explore shared life history traits associated with these phenomena. Although termites are eusocial insects with numerous highly invasive species found throughout the world (Evans et al., 2013), in this review we do not provide specific examples of termites because they are taxonomically distant from Hymenoptera and also because there is less known about the ecology of termite invasions as much of this research has occurred in urban environments (Buczkowski and Bertelsmeier, 2017).
Ants are among the most successful invaders. There are 5 ants in the list of the world's top 100 invasive species (Lowe et al., 2000), and among these Solenopsis invicta (fire ants) and Linepithema humile (Argentine ants) are the two best studied examples. Some traits that S. invicta and L. humile have in common (also shared by other top ant invaders) have been linked to their successful invasions (Holway et al., 2002): first, the South American origin, where a rich ant biodiversity promotes high competition for resources and recurrent floods create a permanently unsettled environment; second, a highly omnivorous diet; and third, a flexible social structure, that can range from individual colonies to multi/unicoloniality. On the flip side, there are also examples of critically endangered ants, such as red wood ants in Northern Europe and dinosaurs ants in Australia (Lach et al., 2010). However, well-documented examples of declining ant species are few. This could be a consequence of a lack of research on the topic, due to the predominating view of ants as problematic invasive organisms, or the fact that ants are in fact less likely to experience declines because the group possesses shared life history traits that allow them to better cope with environmental changes.
Ants in the genus Solenopsis are commonly referred to as “fire ants” and they are all native to the Americas. Several fire ants like S. invicta, Solenopsis geminata, and Solenopsis richteri have become invasive in different parts of the world at different times in history (Ascunce et al., 2011; Gotzek et al., 2015), while others have remained localized to their native range (e.g., Solenopsis xyloni). Therefore, this is an interesting cluster of closely related ants that offers a unique opportunity to investigate specific traits associated with invasiveness. The best characterized invasive fire ant is S. invicta, commonly known as the “red imported fire ant”. S. invicta first became invasive in the USA, where it is currently outcompeting the earlier invaders S. geminata and S. richteri, and subsequently invaded in many other parts of the world. In the USA, S. invicta displays better acclimatization than S. geminata to extremes in temperature, relative humidity and light levels (Wuellner and Saunders, 2003), and it also shows more tolerance than S. richteri to heat and desiccation (Chen et al., 2014). A series of studies also revealed that S. invicta better responds to habitat disturbance than S. geminata (Plowes et al., 2007; LeBrun et al., 2012; Axen et al., 2014) and displays higher potential of dispersal than S. geminata, being able to engage in mating flights earlier in the season (Tschinkel, 2006) and to produce larger colonies on average (Trible et al., 2018). Finally, S. invicta outcompetes S. geminata at food sites (Morrison, 2000), experiences lower mortality during interspecific aggressive interactions (Lai et al., 2015), and quickly responds to the loss of the functional queen with dealation among female sexuals and onset of egg-laying (Vargo and Porter, 1993), a mechanism of developmental plasticity that S. geminata lacks.
Linepithema humile, commonly known as the “Argentine ant,” shares with S. invicta the ability to acclimatize to a wide range of conditions of temperature, humidity, and disturbance (Di Girolamo and Fox, 2006; Suarez et al., 2008). In particular, L. humile is extremely flexible and opportunistic when choosing a nesting site and can easily relocate (Heller and Gordon, 2006): this trait is a consequence of its nomadic nature that evolved in the native range as a response to repeated flooding events (Suarez et al., 2008). In common with S. invicta, Argentine ants have also a great potential to disperse (Aron, 2001; Abril et al., 2013; Abril and Gómez, 2014) and the ability to outcompete other ants at food sites (Rowles and O'Dowd, 2007). Most interestingly, during interspecific interactions L. humile adopt a typically plastic behavior called “the bourgeois strategy,” whereby the extent of the aggression displayed is proportional to the size of the propagule (Carpintero and Reyes-López, 2008; Sagata and Lester, 2009). A unique plastic trait that has been observed in Argentine ants so far is the shift in diet that followed invasion. Shik and Silverman (2013) documented a shift from protein-rich to carbohydrate-rich diet mediated by the mutualistic interactions with honeydew-producing aphids, while Hu et al. (2017) reported a shift from nitrogen-rich to nitrogen-poor (sugar-rich) diet. Hence, in this scenario, plasticity emerged by the combination of an omnivorous diet with the ability to modify sugar/protein ratios according to new colony needs.
Research on endangered ants, despite being limited, has identified few common traits across species that appear to be linked to reduced plasticity. Habitat disturbance has serious negative consequence on red wood ants in the genus Formica. Deforestation has the most drastic impact, not only because it reduces wood availability that these ants rely on for nesting and feeding (Stockan et al., 2010), but also because wood ants are extremely sensitive to conditions of light, shading, vegetation, tree cover and increased human pressure, that usually increases with deforestation (Sorvari and Hakkarainen, 2007; Dekoninck et al., 2010). Limited dispersal of reproductive individuals is another factor potentially associated with decline among endangered ants. The dinosaur ant Nothomyrmecia macrops relies exclusively on solitary founding to start a new colony (Sanetra and Crozier, 2002), and both dinosaur and wood ants produce female sexuals that display restricted dispersal ranges (Gyllenstrand and Seppä, 2003; Sanetra and Crozier, 2003).
Unlike ants, which are all eusocial, species of wasps in the family Vespidae span the full range of behaviors from solitary, to small colony “primitively eusocial” species, to species with moderate size populations, to highly social species with perennial nests containing up to 1 million of individuals (Jandt and Toth, 2015). One of the top 100 invasive species is a yellowjacket wasp (Vespula vulgaris, discussed below), and nearly all of the invasive wasps are temperate species with small to moderately large colony sizes and annual colony cycles (Lester et al., 2013). As with ants, there are nearly no data on species or population declines in social wasps; thus it is inconclusive whether this group is truly lacking in declining species or that the group as a whole is less susceptible to environmental change.
Polistes paper wasps are a successful and cosmopolitan genus of primitively eusocial wasps (Ross and Matthews, 1991). They are well-known as model organisms for the study of sociality, valued for their phenotypic plasticity in the context of their social biology (Jandt et al., 2014). Most members of the genus Polistes are characterized by high levels of physiological and behavioral plasticity in terms of social caste and dominance status. For example, the typical female Polistes larva has multiple layers of plasticity: (1) like many social insect species, any egg has the potential to develop into a queen or worker and caste bias is determined pre-imaginally via differential nutrition (Hunt, 2007), (2) more uniquely, there are no external morphological differences between queens and workers and all females are totipotent with respect to whether they act as workers or queens, depending on social context, and (3) adults engage in dominance contests which serve to structure a social hierarchy with the most dominant wasp being main egg-layer on the nest.
There are over 300 members of the genus found worldwide, yet one species, Polistes dominula, native to Europe, is notable in that it has successfully invaded many different geographic regions around the globe, including much of the United States and Canada in North America, Patagonia in South America (Sackmann et al., 2003), and South Africa (Roets et al., 2019). What can explain its success as an invader (compared to other species of Polistes), and are any of these traits related to phenotypic plasticity? To begin to explore this question, it is useful to compare traits within the genus Polistes, and to date the best information we have is on P. dominula in the context of its well-studied invasion in North America.
In the invasive range of P. dominula in the United States, multiple native species of Polistes are present across different parts of the range. In particular, in the Northern and Eastern United States, Polistes fuscatus and Polistes metricus are two native congeners that appear to have been displaced and/or outcompeted by P. dominula. Comparing the nesting and social biology of P. dominula with the two native species, the invasive species has a more diverse diet (Cervo et al., 2000), the option to reuse nests (Giovanetti et al., 1996), shorter brood development time leading to larger nests containing more individuals (Cervo et al., 2000), a large range of number of foundresses (especially a higher upper limit, whereas P. fuscatus and P. metricus are more often single-founded and/or with a smaller number of co-foundresses; Liebert et al., 2006), more variable nest sites and architectures (Gamboa et al., 2002), more variable nesting locations (e.g., ability to colonize new habitats Cervo et al., 2000), and a larger thermal tolerance range during flight (Weiner et al., 2011). In addition, P. dominula has also invaded in South Africa, likely outcompeting the native Polistes marginalis. There are likely to be some common features with the North American situation, e.g., higher colony productivity and broader seasonal activity patterns in P. dominula related to the South African invasion (Roets et al., 2019).
Part of the reason that this species may have such flexibility in its habits is that it is one of the most abundant and widespread Old World social wasps (Cervo et al., 2000; Höcherl and Tautz, 2015); thus the species possesses characteristics allowing it to thrive in a wide variety of habitats in its native range. This adaptability may be the result of genetic diversity and/or phenotypic plasticity. Because genetic diversity in the invasive range in the USA is fairly high (Johnson and Starks, 2004), it is not yet obvious whether the aforementioned variable traits that may be related to the invasive potential of P. dominula are attributable to phenotypic plasticity vs. genetic diversity.
Vespula wasps, commonly known as yellowjackets, are native to the Northern hemisphere and are characterized by (typically) annual colonies founded by single queens, morphological differences between queens and workers, and moderate to large colony sizes (Ross and Matthews, 1991). These wasps have an age-based division of labor among workers and possess complex communication systems related to foraging and recruitment to food sources (Overmyer and Jeanne, 1998; Hurd et al., 2007). They have a highly generalized diet consisting of insect and small vertebrate prey of various types, carrion, nectar from flowers, and rotting/fermenting fruit (Archer, 2012). The genus Vespula includes 23 species, with three that are well-known invasive species that have successfully established on several continents (Lester and Beggs, 2019). The German yellowjacket (Vespula germanica) is native to much of the Northern Old World (Europe, Asia, and Africa) and invasive in North and South America, Australia, and New Zealand. The common wasp (Vespula vulgaris) is native and widespread in Eurasia, and invasive in Hawaii, Oceania, and South America. The western yellowjacket (Vespula pensylvanica) has a Nearctic native range, and has become invasive in Hawaii. Because multiple Vespula species have become important invaders, below, we provide an overview and discuss traits and examples from all three species.
Several studies have investigated possible factors and traits contributing to the great success of Vespula sp. as worldwide invaders, indicating that plasticity in key life history traits might have fueled their invasiveness. A common trait of invasive Vespula species is that they have rapidly (suggesting plasticity) shifted from an annual colony cycle to the formation of perennial colonies that last for multiple years, likely due to their ability to persist in the milder climates that characterize some areas of their invaded ranges. This has been reported for different invasive Vespula species: V. vulgaris in Tasmania (Spradbery, 1973), V. germanica in New Zealand (Thomas, 1960), and V. pensylvanica in Hawaii (Wilson et al., 2009). In all these areas, invasive wasps have become able to produce massive colonies that are significantly larger than what is observed in the native range, denoting a great amount of plasticity in the social structure of the colony.
Interestingly, invasive V. pensylvanica in Hawaii demonstrate the concomitant occurrence of several plastic life history traits that may have mediated its successful invasion: a shift from annual to perennial colony structure, wide diet breadth, and wide variation in individual longevity (Wilson et al., 2009). However, another study reported that V. pensylvanica displays a certain level of plasticity in queen number and duration of colony cycle also in its native range in California (Visscher and Vetter, 2003): therefore it remains to be tested whether plasticity in this case was a pre-existing condition that facilitated invasion or rapidly evolved in invasive populations of these wasps.
Another major factor potentially contributing to the invasion success of Vespula wasps is their behavioral and cognitive plasticity, especially in terms of foraging behavior (Lester and Beggs, 2019). Vespula wasps are also known for their ability to navigate new routes and recruit nest mates to new food sources, likely enhanced by their excellent learning and memory abilities (D'adamo and Lozada, 2003). These wasps are highly generalist foragers and their flexible diet has enabled them to take advantage of a huge variety of food sources in the invasive range, often dominating, and restructuring local ecosystems (Beggs and Rees, 1999). A striking example has been the ability of V. vulgaris to aggressively monopolize honeydew resources of the Nothofagus forest in New Zealand (Harris et al., 1991; Beggs, 2001). The honeydew provides opportunities for massive carbohydrate intake, which has been linked to the fast growth and competitive advantage of V. vulgaris colonies, which strikingly resembles what has been observed in invasive Argentine ants (see above).
Finally, two additional (related) traits that show plasticity and might have mediated wasp invasions are spring emergence time and thermal tolerance. For example, spring emergence occurs earlier in V. vulgaris than in V. germanica, with the former dominating at the extreme southern and northern ranges of their distribution. Plasticity in time of emergence and nesting strategy may provide an advantage for V. vulgaris in the context of nest site competition with sympatric congeneric species in colder regions. V. vulgaris also display a larger range of temperature and habitat tolerance, and are found at significantly higher latitudes than congener V. germanica (Lester and Beggs, 2019). Milder temperatures have been associated with Vespula wasps extending their distribution northward in Finland (Sorvari, 2013).
Unlike ants and wasps which are often viewed as pest species, social bees are generally considered beneficial insects due to their pollination services and, in the case of honey bees, honey production. They have been the major subjects of a large body of recent research into population declines and losses of managed colonies. By some estimates, at least half of species in certain bee groups (e.g., bumble bees) are in decline (e.g., Wood et al., 2019), and rates of honey bee colony losses have been historically high in many areas of the world (Ratnieks and Carreck, 2010). At the same time, there are some notable examples of successful exotic and invasive social bees. Thus, bees span the gamut from invasive to declining, sometimes with examples of both within the same genus or even species. This provides a unique opportunity to examine the traits of species associated with winning or losing in the face of global change.
Species in the bumble bee genus Bombus are considered a key group for both wild plant and crop pollination. These bees have an annual colony cycle, with morphologically differentiated queens and workers, and moderate colony sizes (Goulson, 2003). Like all bees, they have a diet comprised entirely of pollen and nectar, but many species of bumble bees are fairly generalist in foraging on a wide variety of different flower species. The genus comprises about 260 species of primitively eusocial insects (Cameron et al., 2007) spanning from invasive (B. hortorum, B. hypnorum, B. impatiens, B. lucorum, B. ruderatus, B. subterraneus, and B. terrestris) to “Critically Endangered” species (B. affinis, B. franklini, B. rubriventris, B. suckleyi, and B. variabilis) based on global IUCN categorization (IUCN Red List of Threatened Species, 2016), and including possible extinctions (e.g., B. cullumanus) (Williams et al., 2013).
Bombus terrestris is an important managed pollinator that has been introduced for crop pollination in many parts of the world; despite introductions of other Bombus species, B. terrestris has been the most successful worldwide invader. This European species has become invasive in New Zealand (Macfarlane and Gurr, 1995), Japan (Matsumura et al., 2004), Tasmania (Hingston, 2006), Chile (Ruz and Herrera, 2001), and Argentina (Torretta et al., 2006). This wide range shows the ability of this species to survive and reproduce in a wide range of climates, geographies, and living among different congeners (both native and introduced). Because the speed and spread of the invasions is among the fastest recorded worldwide (e.g., Morales et al., 2013), and genetic diversity of some introduced populations is known (Schmid-Hempel et al., 2007), it might be possible to test whether the invasion success of this species is due to phenotypic plasticity.
Several traits have been suggested to be related to invasions in B. terrestris and represent interesting candidates to consider in the context of phenotypic plasticity. One trait that may provide an advantage to B. terrestris over native sympatric congeners is that its queens emerge earlier in the spring (Dafni et al., 2010), suggesting possible thermal adaptations. Early emergence (and long cycles of activity in general) have also been documented in introduced B. terrestris populations in Japan and Argentina (Inoue et al., 2008). In the Patagonia region of South America, B. terrestris also show an extended period of activity (early in the spring and late in the fall) compared to two bumble bee species that it has apparently displaced in this region, the introduced Bombus ruderatus and the native Bombus dahlbomii (Arbetman, 2017). Earlier emergence than other species may provide B. terrestris with the opportunity to take sole advantage of the earliest flowering resources as well as occupy optimal nesting sites before other species. Although to date there is no definitive evidence that spring emergence time is a plastic trait, this possibility deserves to be directly tested, given the possibility that this trait appears to provide an advantage over native species.
Diet breadth is another plastic trait that might be linked to successful invasion in bumble bees. In terms of its foraging behavior, B. terrestris is able to utilize a wide variety of native and non-native plants (Ruz and Herrera, 2001), readily foraging on multiple different crops, and is also known to “nectar rob” at high rates to access flowers with long corollas (Ishii et al., 2008). In New Zealand, the less successful introduced species B. ruderatus uses a narrower range of flowers and is more restricted to areas with warmer, drier climates as compared to B. terrestris (Macfarlane and Gurr, 1995). B. hortorum, another New Zealand introduced species, has not adapted well to areas with regular early summer droughts and a limited availability of flowers (Macfarlane and Gurr, 1995 and references therein). The high worker number in B. terrestris colonies, compared to other native and introduced species, may also allow this species to effectively outcompete other congenerics in terms of exploiting available foraging resources (Ings et al., 2006).
Numerous bumble bees have been well-documented to be in decline, by some estimates up to 50% of native Bombus species in some regions of the world (Wood et al., 2019). It seams that between 1/3 to 1/2 of Bombus species are declining (Arbetman et al., 2017; Wood et al., 2019). Several hypotheses have been put forward to understand the factors and life history traits associated with decline in certain bumble bee species, some of which may relate to phenotypic plasticity. For example, in the US, declining species show a narrower range of plants used (Wood et al., 2019) suggesting a less flexible diet may make some bumble bee species more prone to decline in the face of habitat loss. A European study suggested that declining species were unable to shift floral plant species usage through decades of land use change, whereas stable species were (Kleijn et al., 2008). Some bumble bee species have been documented to be narrowing their geographical areas of occupancy. This may be related to climate change and could happen as a result of higher thermal sensitivity or lower thermal tolerance. These patterns have been observed in those bumble bee species that are cold-adapted and originated in temperate regions of the globe (Kerr et al., 2015). Declining species may also have narrower seasonal activity compared to stable or invading species, again suggesting there may be a connection to thermal tolerance and/or thermal activity ranges (Arbetman, 2017). Another important factor in declines of native Bombus species has likely been pathogen and parasite spillover from commercially produced and introduced bees (Meeus et al., 2011; Arbetman et al., 2013). It is not known whether this relates simply to naïveté to specific pathogens, or to limited immune system investment in declining species. There also appears to be a phylogenetic component to patterns of Bombus decline, with some subgenera of Bombus more prone to decline, especially species with small geographic ranges (Arbetman et al., 2017). This suggests that narrow habitat requirements could make some bumble bee species more susceptible to decline.
The honey bee Apis mellifera is perhaps the best studied single species of social insects; as a semi-domesticated pollinator, honey producer, and fascinating behavioral model system. A. mellifera are highly social bees with a perennial life cycle, strongly differentiated reproductive castes, a highly-structured worker division of labor based on age polyethism. A. mellifera has a vast native geographical distribution across large parts of Africa, Europe, and the Middle East. Interestingly, because of its value for pollination and honey production, this species has been introduced (and is managed by humans) in nearly every country, all over the world. While in the native range there are multiple subspecies that vary in behavior, colony structure, and morphology, the introduced varieties of A. mellifera have been mostly restricted to fewer subspecies or mixed race bees with desirable traits such as gentleness and high honey production. While many honey bee colonies are managed by humans, in many areas of its invasive and native range, wild honey bee colonies can be found.
As a semi-domesticated, managed species that has been introduced repeatedly in many, many places across the globe, perhaps the most surprising observation about A. mellifera is the fact that it has not become highly invasive in many parts of its introduced range. On the contrary, in many regions, including parts of its native range (in Europe) and some areas of its introduced range (North America), A. mellifera colonies have sustained high mortality and beekeepers are struggling to keep bees alive (Neumann and Carreck, 2010), in the face of multiple, interacting environmental stressors such as disease, poor nutrition, and pesticides (Smith et al., 2013). Most of these are mixes of European subspecies, especially A. mellifera ligustica and A. mellifera. An exception to this pattern has occurred with the so-called “killer” or Africanized bees (AHB), which are genetic mixes between A. mellifera scutellata, a subspecies native to Africa, and European races of A. mellifera. These bees appear to be more resistant to some of the stressors that are challenging European honey bees (EHB); in fact, the population status of the AHB has not been documented to decline, instead, these Africanized honey bees have undergone a rapid expansion out of Brazil (where they were originally accidentally released) and become well-established across large parts of South, Central, and North America, where they appear to be continuing to spread (Lin et al., 2018).
Thus, in A. mellifera we have different genetic backgrounds of bees, one in decline and one that is more invasive, sometimes living side by side in the same environment. This provides a unique opportunity to dissect the traits that may differentiate winners and losers in the face of an environmental change. Differences in traits between AHB and EHB that have been cited include: less honey storage in AHB vs. EHB (Winston et al., 1983), higher propensity to abscond and initiate a new nest in AHB vs. EHB (Winston et al., 1979), and higher resistance to Varroa mites in AHB vs. EHB due to more efficient hygienic behavior (Rosenkranz, 1999). It is possible that some of these traits may relate to phenotypic plasticity, but studies have not directly addressed how plastic or fixed these traits are in AHB compared to EHB.
On the contrary, in a different environment, e.g., China, A, mellifera ligustica has become an invasive species, outcompeting, and displacing native Apis cerana honey bees (Ji et al., 2003). Also, in the Bonin Islands off of Japan, A. mellifera (subspecies not specified), has also become invasive with likely negative impacts on biodiversity (Kato et al., 1999). Stable feral populations of A. mellifera have also established in Australia (Oldroyd et al., 1997), and managed colonies have been more resilient than in other parts of the world, which may be partially due to lack of Varroa mites (Neumann and Carreck, 2010). These differences in the status of A. mellifera ligustica across different regions of the world demonstrate the important role of the environmental conditions in determining whether a species becomes invasive. This could relate to specific forms of phenotypic plasticity being advantageous in certain environments.
This review synthesizes recurrent traits that have been linked to successful invasions and declines in social insects across multiple species of ants, bees, and wasps (summarized in Figure 2). Some of these traits exhibit evidence of high levels of plasticity in invasive social insects. These include flexible dispersal and colony founding strategies, broad diet breadth, and rapid colony growth in response to high resource availability, and flexible preferences for nesting sites. Other traits linked to invasiveness in social insects, but that have not been directly investigated in relation to plasticity include thermal tolerance, reproductive potential, and genomic plasticity and/or structure [but see Felden et al. (2019)]. However, these traits have been shown to be plastic in other invasive organisms (reviewed above) and therefore have the potential to also be important in invasive social insects. On the flip side, related to declines of social insects, we uncovered evidence for a small group of recurring traits in declining ants and bumble bees for which a lack of plasticity might be linked to the decline of some species, including specialized diets and restricted habitat and nesting requirements (Figure 2). Below, we discuss some of the most relevant plasticity-related traits in the context of population management strategies; i.e., mitigation strategies to reduce invasive species and conserve declining species.
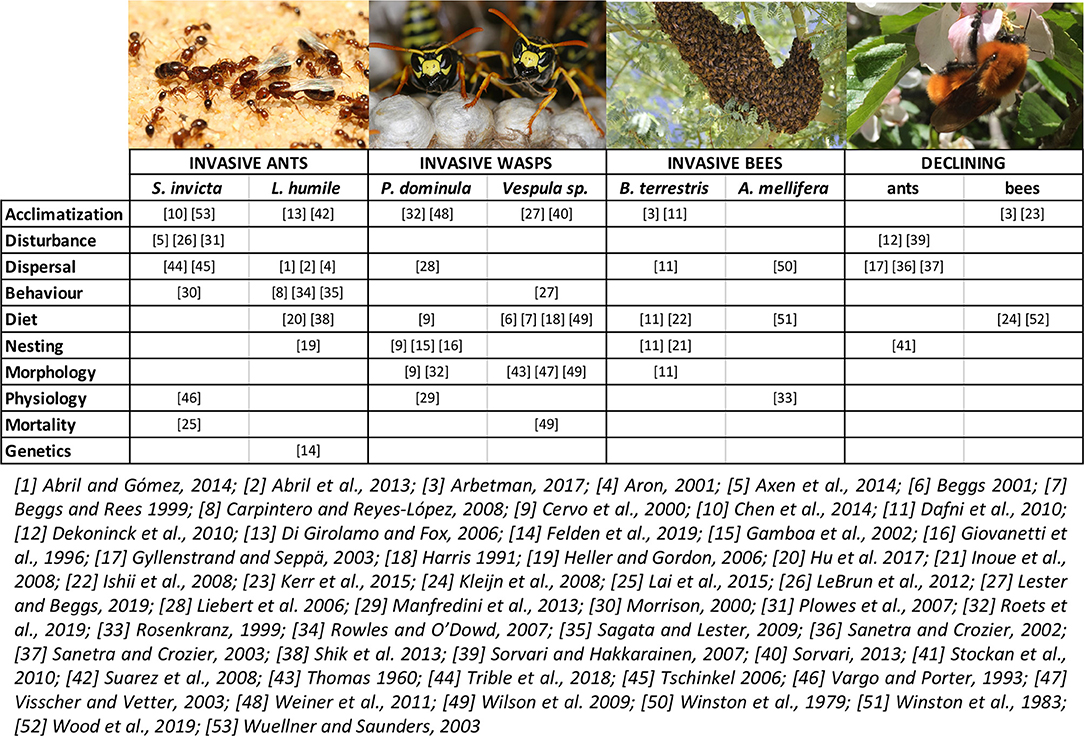
Figure 2. Summary of research studies (to date) that have investigated traits that show the potential for plasticity (or lack thereof) in association with invasions or declines of various taxa of social insects. The traits reported in the figure are the same as discussed in the main text (with the relevant references). Individual traits have been classified and clumped together according to broadly defined macro-categories in line with what reported for other organisms (sections Phenotypic plasticity in various traits as facilitators of biological invasions and Limited phenotypic plasticity in the context of species declines). Some macro-categories include traits that are highly relevant/specific for social insects: “Dispersal” includes range of mating flights, colony founding strategy, time of queens emergence from diapause and time of release of sexuals; “Behavior” includes intraspecific competition for food resources; “Morphology” includes colony size; “Mortality” refers to individual mortality of single insects. Pictures shown above are (from left to right): the fire ant Solenopsis invicta, the paper wasp Polistes dominula, the Africanized honey bee Apis mellifera, and the bumble bee Bombus dahlbomii (Photo credits: Wikimedia Commons, except bumble bee by Amy Toth).
Our review strongly suggests a recurring pattern in which invasive social insects display a high dispersal range and mode, while declining social insects are often limited in the capacity to disperse and in their repertoire of modalities with which to do so. This observation suggests the following strategies may be effective:
• Actions to target invasive social insects in temperate climates should be done early in the spring season, as these organisms often emerge earlier than native sympatric competitors. Targeting queens that emerge from winter diapause or newly mated queens after mating flights would be more effective than targeting whole mature colonies later in the season. Although targeted species may respond by delaying dispersal/colony founding (due to plasticity), this strategy at least has the potential to reduce their success and place them on more of an “even playing field” with native species.
• Endangered social insects need great support at the dispersal stage, because colony founding is a critical moment in their life cycle with a high rate of failure and mortality. To support dispersing queens, actions should be taken to provide foraging and nest site options during the time period when new queens emerge/found a new colony (and not sooner, in the case of competition with earlier emerging invasive species). This might involve managing the landscape to introduce flowering/seed producing plants that bloom during critical periods of the life cycle of threatened species, providing nest boxes and/or habitats adequate for nesting, and providing appropriately placed nesting sites for species with limited ranges of dispersal.
This review strongly suggests that the ability to find and secure nesting sites may be an important “filter” separating invasive and declining social insects. Plasticity in nesting strategies becomes particularly relevant in association with anthropogenic habitat disturbance, which in general results in limiting nesting possibilities for declining species while opening new possibilities for more flexible invasive species.
• Invasive social insects often utilize human-altered or disturbed environments heavily for nesting substrates (e.g., P. dominula paper wasps nesting on buildings). We suggest sudden, large scale disruption of natural habitats are likely to favor invasive species. We suggest that smaller scale and more patchy patterns of change, leaving some native habitat refuges in place within the matrix of a rapidly developing area, may both reduce the spread of invaders and buffer against damages created to the native community in the area. If large scale disturbance is unavoidable, it becomes important to compensate with measures that create required habitat niches (e.g., nest boxes) for native species most likely to be affected by loss of nesting habitat.
• In addition, more research is needed to identify the key nesting needs (microhabitat, substrate, size) of endangered social insects, if possible, pinpointing what makes them distinct from invasive competitors. For example, a successful strategy in insect conservation has been accomplished with the creation of the “bee hotels” for solitary bees and wasps.
In reality, many/most modern ecosystems are already composed of invasive and endangered species coexisting at the same time. At the same time, new invaders are moving into more and more regions across the globe, and are often not reported or noticed until substantial populations have established. In general, we suggest that preserving and boosting populations of declining species should be a general priority, as a solid and diversified native community can help prevent the establishment/spread of invasive species.
There is no doubt that exploring the role of phenotypic plasticity in invasions and declines of social insects is a fascinating area of research. It is also evident that we have just started to uncover the wide range of forms that this association can take, and more work needs to be done in the future. First of all, a main priority is to understand where plasticity (or the lack thereof) originates. For example, for successful invaders, is plasticity in key traits that fostered invasiveness a pre-existing condition that was widespread among native populations? Or did plastic traits evolved after invasion only in those populations that became invasive? This was one of three fundamental issues on the nature of phenotypic plasticity identified by Kennedy et al. (2017). Addressing this question will require comparing in a rigorous way (e.g., common garden experiments) the extent of plasticity (i.e., amplitude of the range of responses) for the trait of interest in populations of conspecifics from the native and invasive ranges. We foresee that, though this approach might be relatively easy to perform for temperate species (e.g., many wasps and bees), the logistic will be sometimes challenging for tropical or subtropical species like many invasive ants, whose native range is in most cases thick forest or remote and frequently flooded areas in South America and South East Asia. Complementary to this approach, the study of failed invasions (Box 2) will be fundamental to test whether failure to invade is linked to lack of plasticity—which can be consequently used to refute the hypothesis of plasticity as a species-level pre-condition for invasiveness.
Box 2. What do we know about failed invasions?
The separation between invasive and non-invasive species is far from being clear-cut and this dichotomy is often used to simplify nomenclature. There are species that might be invasive in some parts of the world while remaining stable somewhere else (e.g., B. terrestris), and other species that might be at a pre-invasion stage (or lag-phase, Chapple et al., 2012) and therefore are not yet referred to as invasive. Particularly intriguing are those cases of “failed invasions,” when species that are usually described as successful invaders fail to establish in an area where they have been introduced. Failed invasions represent a unique opportunity to understand the underpinnings of biological invasions as they often highlight the key components (e.g., phenotypic plasticity) that are missing for a successful invasion.
Two types of failure have been identified (Zenni and Nuñez, 2013): (a) failure to naturalize, whereby an alien species is unable to survive, reproduce or grow to a minimum size in the new range; and (b) failure to invade after naturalization, which correspond to inability to spread. Unsurprisingly option (a) is more difficult to observe, in particular with reference to unintentional introductions. As a matter of fact, taxonomic, and geographic biases have been reported to explain our limited knowledge on failed invasions: for example, reports on plants and insects are underrepresented compared to vertebrates, and Asia and Africa have significantly fewer records compared to the Western world. In terms of mechanisms that could be at the basis of failed invasions, five categories have been identified: (1) abiotic resistance, including both macroclimatic, and local-scale factors; (2) biotic resistance, such as competition with or predation by resident species; (3) genetic constraints, that might selectively affect only a subset of populations within a species; (4) propagule pressure, linked to the size of the initial settlements; and (5) mutualist release, which acts exactly opposite to the “enemy release” described in Box 1.
A few examples of failed invasions have been reported among social insects. Notably, four ant species (three of which are worldwide successful invaders) failed to invade New Zealand: the yellow crazy ant, the red fire ant, the Papuan thief ant and the electric ant. Analogously, seven species of bumble bees were introduced in New Zealand, but only B. terrestris managed to establish and spread successfully all over the region. The lack of invasion is striking, considering that New Zealand has been highly permeable to the invasion of other social insects such as yellowjacket wasps. Interestingly, a subspecies of B. terrestris, the Sardinian bumble bee (B. terrestris sassaricus), failed to establish in southern France, despite the fact that it had been introduced intentionally for crop pollination (Ings et al., 2010). Studies of failed invasions, especially using contrasts between closely related species or the same species in different regions, have great potential for helping us to better understand the environmental and phenotypic drivers of invasiveness.
On the other hand, if it is true that some social insects are lacking of adequate levels of phenotypic plasticity and therefore fail to cope with environmental changes, the obvious question is how did these species survive until now? Probably lower plasticity might be advantageous in more stable environments, due to the fact that maintaining plasticity when it is not needed might be costly (reviewed in Auld et al., 2009). We need to address this compelling question in social insects, and keep in mind that there are two ways of quantifying costs in these organisms: at the individual level (as a proxy for energy expenditure), and at the colony level (which instead reflects real fitness costs, as the colony is the real reproductive unit in social insects).
Finally, we suggest two additional aspects should be prioritized in the investigation of phenotypic plasticity in invasive and endangered social insects: social structure and genetics. These traits are particularly relevant for social insects and are tightly interconnected. We have reported here that social structure can change after invasion: for example, transitions to unicoloniality and polygyny in ants, or from annual to perennial colonies in wasps. All these phenomena suggest pre-exisiting plasticity in social structures of these species, but in most cases we lack evidence of such flexibility in native populations of these invaders. Understanding how major transitions in social structure occur relates to fundamental questions of sociobiology, for they must accommodate substantial changes in the “rules” of the nest, such as multiple queens coexisting together instead of one, or the elimination of colony boundaries and the loss of aggressive behavior toward neighboring conspecifics. How is plasticity at this complex behavioral level achieved? Is it encoded at the genetic level and, if so, does it relate to the unique haplodiploid reproductive system that is such a key trait of all social insects? An important step in this direction has been achieved with the discovery of the Gp-9 region in fire ants, a large portion of a chromosome tightly linked to social structure (Wang et al., 2013). One of the two variants of this region is associated with polygyny, and polygyny is the social form that appears to facilitate invasiveness. We suggest, moving forward, that the study of phenotypic plasticity and its genetic basis in the context of social insect invasions can help understanding major evolutionary transitions in social organization.
AT, FM, and MA developed the idea for the review and wrote the paper.
This work was supported by a Fulbright Science and Technology Scholarship to AT and National Geographic Species Recovery Grant Number NGS-57001R-19 to MA and AT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abril, S., Diaz, M., Enríquez, M. L., and Gómez, C. (2013). More and bigger queens: a clue to the invasive success of the Argentine ant (Hymenoptera: Formicidae) in natural habitats. Myrmecol. News 18, 19–24.
Abril, S., and Gómez, C. (2014). Strength in numbers: large and permanent colonies have higher queen oviposition rates in the invasive Argentine ant (Linepithema humile, Mayr). J. Insect. Physiol. 62, 21–25. doi: 10.1016/j.jinsphys.2014.01.004
Arbetman, M. P. (2017). La declinación de Abejorros (bombus) y sus Potenciales Causas, a Distintas Escalas Espaciales (Ph.D. thesis). Universidad Nacional del Comahue, Argentina. Available online at: http://rdi.uncoma.edu.ar:8080/handle/123456789/183
Arbetman, M. P., Gleiser, G., Morales, C. L., Williams, P., and Aizen, M. A. (2017). Global decline of bumblebees is phylogenetically structured and inversely related to species range size and pathogen incidence. Proc. R. Soc. B Biol. Sci. 284:20170204. doi: 10.1098/rspb.2017.0204
Arbetman, M. P., Meeus, I., Morales, C. L., Aizen, M. A., and Smagghe, G. (2013). Alien parasite hitchhikes to Patagonia on invasive bumblebee. Biol. Invasions 15, 489–494. doi: 10.1007/s10530-012-0311-0
Archer, M. E. (2012). Vespine Wasps of the World: Behaviour, Ecology & Taxonomy of the Vespinae. Siri Scientific Press.
Aron, S. (2001). Reproductive strategy: an essential component in the success of incipient colonies of the invasive Argentine ant. Insectes Soc. 48, 25–27. doi: 10.1007/PL00001740
Ascunce, M. S., Yang, C.-C., Oakey, J., Calcaterra, L., Wu, W.-J., Shih, C.-J., et al. (2011). Global invasion history of the fire ant Solenopsis invicta. Science 331, 1066–1068. doi: 10.1126/science.1198734
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Price, T. D., and Ketterson, E. D. (2014). Hormonal, behavioral, and life-history traits exhibit correlated shifts in relation to population establishment in a novel environment. Am. Nat. 184, E147–E160. doi: 10.1086/678398
Auld, J. R., Agrawal, A. A., and Relyea, R. A. (2009). Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B Biol. Sci. 277, 503–511. doi: 10.1098/rspb.2009.1355
Axen, H. J., Wildermuth, A., and Helmscahan, S. (2014). Environmental filtering of foraging strategies mediates patterns of coexistence in the fire ants Solenopsis geminata and Solenopsis xyloni, and their interspecific hybrids. Ecol. Entomol. 39, 290–299. doi: 10.1111/een.12100
Baudier, K. M., and O'Donnell, S. (2017). Weak links: how colonies counter the social costs of individual variation in thermal physiology. Curr. Opin. Insect. Sci. 22, 85–91. doi: 10.1016/j.cois.2017.06.004
Beaman, J. E., White, C. R., and Seebacher, F. (2016). Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. doi: 10.1016/j.tree.2016.01.004
Beggs, J. (2001). The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol. Conserv. 99, 17–28. doi: 10.1016/S0006-3207(00)00185-3
Beggs, J. R., and Rees, J. S. (1999). Restructuring of Lepidoptera communities by introduced Vespula wasps in a New Zealand beech forest. Oecologia 119, 565–571. doi: 10.1007/s004420050820
Bengston, S. E., and Jandt, J. M. (2014). The development of collective personality: the ontogenetic drivers of behavioral variation across groups. Front. Ecol. Evol. 2:81. doi: 10.3389/fevo.2014.00081
Blossey, B., and Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecol. 83, 887–889. doi: 10.2307/2261425
Buczkowski, G., and Bertelsmeier, C. (2017). Invasive termites in a changing climate: a global perspective. Ecol. Evol. 7, 974–985. doi: 10.1002/ece3.2674
Cameron, S. A., Hines, H. M., and Williams, P. H. (2007). A comprehensive phylogeny of the bumble bees (Bombus). Biol. J. Linn. Soc. 91, 161–188. doi: 10.1111/j.1095-8312.2007.00784.x
Campbell, T. S., and Echternacht, A. C. (2003). Introduced species as moving targets: changes in body sizes of introduced lizards following experimental introductions and historical invasions. Biol. Invasions 5, 193–212. doi: 10.1023/A:1026172314139
Carpintero, S., and Reyes-López, J. (2008). The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile). Biol. Invasions 10, 25–35. doi: 10.1007/s10530-007-9103-3
Cervo, R., Zacchi, F., and Turillazzi, S. (2000). Polistes dominulus (Hymenoptera, Vespidae) invading North America: some hypotheses for its rapid spread. Insect. Soc. 47, 155–157. doi: 10.1007/PL00001694
Chapple, D. G., Simmonds, S. M., and Wong, B. B. (2012). Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64. doi: 10.1016/j.tree.2011.09.010
Chen, J., Rashid, T., and Feng, G. (2014). A comparative study between Solenopsis invicta and Solenopsis richteri on tolerance to heat and desiccation stresses. PLoS ONE 9:e96842. doi: 10.1371/journal.pone.0096842
Chevin, L.-M., Lande, R., and Mace, G. M. (2010). Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8:e1000357. doi: 10.1371/journal.pbio.1000357
Chevin, L. M., and Lande, R. (2010). When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evol. Int. J. Org. Evol. 64, 1143–1150. doi: 10.1111/j.1558-5646.2009.00875.x
Chown, S. L., Slabber, S., McGeoch, M., Janion, C., and Leinaas, H. P. (2007). Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B Biol. Sci. 274, 2531–2537. doi: 10.1098/rspb.2007.0772
Colautti, R. I., Alexander, J. M., Dlugosch, K. M., Keller, S. R., and Sultan, S. E. (2017). Invasions and extinctions through the looking glass of evolutionary ecology. Philos. Trans. R. Soc. B Biol. Sci. 372:20160031. doi: 10.1098/rstb.2016.0031
Cooke, S. L. (2016). Anticipating the spread and ecological effects of invasive bigheaded carps (Hypophthalmichthys spp.) in North America: a review of modeling and other predictive studies. Biol. Invasions 18, 315–344. doi: 10.1007/s10530-015-1028-7
Cremer, S., Armitage, S. A. O., and Schmid-Hempel, P. (2007). Social immunity. Curr. Biol. 17, R693–R702. doi: 10.1016/j.cub.2007.06.008
D'adamo, P., and Lozada, M. (2003). The importance of location and visual cues during foraging in the German wasp (Vespula germanica F.)(Hymenoptera: Vespidae). N. Zeal. J. Zool. 30, 171–174. doi: 10.1080/03014223.2003.9518336
Dafni, A., Kevan, P., Gross, C. L., and Goka, K. (2010). Bombus terrestris, pollinator, invasive and pest: an assessment of problems associated with its widespread introductions for commercial purposes. Appl. Entomol. Zool. 45, 101–113. doi: 10.1303/aez.2010.101
Davidson, A. M., Jennions, M., and Nicotra, A. B. (2011). Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 14, 419–431. doi: 10.1111/j.1461-0248.2011.01596.x
Dekoninck, W., Hendrickx, F., Grootaert, P., and Maelfait, J.-P. (2010). Present conservation status of red wood ants in north-western Belgium: worse than previously, but not a lost cause. Eur. J. Entomol. 107, 209–218. doi: 10.14411/eje.2010.028
Di Girolamo, L. A., and Fox, L. R. (2006). The influence of abiotic factors and temporal variation on local invasion patterns of the Argentine ant (Linepithema humile). Biol. Invasions 8, 125–135. doi: 10.1007/s10530-004-1572-z
Didham, R. K., Tylianakis, J. M., Gemmell, N. J., Rand, T. A., and Ewers, R. M. (2007). Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496. doi: 10.1016/j.tree.2007.07.001
Dos Santos, C. F., Nunes-Silva, P., and Blochtein, B. (2015). Temperature rise and its influence on the cessation of diapause in Plebeia droryana, a eusocial bee (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 109, 29–34. doi: 10.1093/aesa/sav096
Engel, K., and Tollrian, R. (2009). Inducible defences as key adaptations for the successful invasion of Daphnia lumholtzi in North America? Proc. R. Soc. Biol. Sci. 276, 1865–1873. doi: 10.1098/rspb.2008.1861
Evans, T. A. (2010). “Invasive termites,” in Biology of Termites: A Modern Synthesis, eds D. Bignell, Y. Roisin, N. Lo (Dordrecht: Springer), 519–562. doi: 10.1007/978-90-481-3977-4_19
Evans, T. A., Forschler, B. T., and Grace, J. K. (2013). Biology of invasive termites: a worldwide review. Annu. Rev. Entomol. 58, 455–474. doi: 10.1146/annurev-ento-120811-153554
Felden, A., Paris, C., Chapple, D. G., Suarez, A. V., Tsutsui, N. D., Lester, P. J., et al. (2019). Native and introduced Argentine ant populations are characterised by distinct transcriptomic signatures associated with behaviour and immunity. NeoBiota 49, 105–126. doi: 10.3897/neobiota.49.36086
Fisher, D. O., Blomberg, S. P., and Owens, I. P. (2003). Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proc. R. Soc. London. Ser. B Biol. Sci. 270, 1801–1808. doi: 10.1098/rspb.2003.2447
Gamboa, G. J., Greig, E. I., and Thom, M. C. (2002). The comparative biology of two sympatric paper wasps, the native Polistes fuscatus and the invasive Polistes dominulus (Hymenoptera, Vespidae). Insect. Soc. 49, 45–49. doi: 10.1007/s00040-002-8278-y
Gervasi, S. S., and Foufopoulos, J. (2008). Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 22, 100–108. doi: 10.1111/j.1365-2435.2007.01340.x
Gianoli, E., and Valladares, F. (2011). Studying phenotypic plasticity: the advantages of a broad approach. Biol. J. Linn. Soc. 105, 1–7. doi: 10.1111/j.1095-8312.2011.01793.x
Giovanetti, M., Cervo, R., and Turillazzi, S. (1996). “Comb reutilization in Polistes dominulus (Hymenoptera: Vespidae),” in Proceedings of the VI Conference of the Italian Section of the International Union for the Study of Social Insects, Vol. 1. eds F. Le Moli, A. Mori, and D. Grasso (Insect Soc. Life), 101–105.
Glastad, K. M., Hunt, B. G., and Goodisman, M. A. D. (2019). Epigenetics in insects: genome regulation and the generation of phenotypic diversity. Annu. Rev. Entomol. 64, 185–203. doi: 10.1146/annurev-ento-011118-111914
Glon, M. G., Reisinger, L. S., and Pintor, L. M. (2018). Biogeographic differences between native and non-native populations of crayfish alter species coexistence and trophic interactions in mesocosms. Biol. Invasions 20, 3475–3490. doi: 10.1007/s10530-018-1788-y
Gómez-Saldarriaga, C., Valenzuela, N., and Ceballos, C. P. (2016). Effects of incubation temperature on sex determination in the endangered magdalena river turtle, Podocnemis lewyana. Chelonian Conserv. Biol. 15, 43–53. doi: 10.2744/CCB-1170.1
Gordon, D. M. (1996). The organization of work in social insect colonies. Nature 380, 121–124. doi: 10.1038/380121a0
Gotzek, D., Axen, H. J., Suarez, A. V., Helms Cahan, S., and Shoemaker, D. (2015). Global invasion history of the tropical fire ant: a stowaway on the first global trade routes. Mol. Ecol. 24, 374–388. doi: 10.1111/mec.13040
Grab, H., Brokaw, J., Anderson, E., Gedlinske, L., Gibbs, J., Wilson, J., et al. (2019). Habitat enhancements rescue bee body size from the negative effects of landscape simplification. J. Appl. Ecol. 56, 2144–2154. doi: 10.1111/1365-2664.13456
Gutekunst, J., Andriantsoa, R., Falckenhayn, C., Hanna, K., Stein, W., Rasamy, J., et al. (2018). Clonal genome evolution and rapid invasive spread of the marbled crayfish. Nat. Ecol. Evol. 2, 567–573. doi: 10.1038/s41559-018-0467-9
Gyllenstrand, N., and Seppä, P. (2003). Conservation genetics of the wood ant, Formica lugubris, in a fragmented landscape. Mol. Ecol. 12, 2931–2940. doi: 10.1046/j.1365-294X.2003.01975.x
Harris, R. J., Thomas, C. D., and Moller, H. (1991). The influence of habitat use and foraging on the replacement of one introduced wasp species by another in New Zealand. Ecol. Entomol. 16, 441–448. doi: 10.1111/j.1365-2311.1991.tb00237.x
Hawes, N. A., Fidler, A. E., Tremblay, L. A., Pochon, X., Dunphy, B. J., and Smith, K. F. (2018). Understanding the role of DNA methylation in successful biological invasions: a review. Biol. Invasions 20, 2285–2300. doi: 10.1007/s10530-018-1703-6
Heller, N. E., and Gordon, D. M. (2006). Seasonal spatial dynamics and causes of nest movement in colonies of the invasive Argentine ant (Linepithema humile). Ecol. Entomol. 31, 499–510. doi: 10.1111/j.1365-2311.2006.00806.x
Hingston, A. B. (2006). Is the exotic bumblebee Bombus terrestris really invading Tasmanian native vegetation? J. Insect. Conserv. 10, 289–293. doi: 10.1007/s10841-006-6711-7
Höcherl, N., and Tautz, J. (2015). Nesting behavior of the paper wasp Polistes dominula in Central Europe—a flexible system for expanding into new areas. Ecosphere 6, 1–11. doi: 10.1890/ES15-00254.1
Holway, D. A., Lach, L., Suarez, A. V., Tsutsui, N. D., and Case, T. J. (2002). The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 33, 181–233. doi: 10.1146/annurev.ecolsys.33.010802.150444
Hu, Y., Holway, D. A., Łukasik, P., Chau, L., Kay, A. D., LeBrun, E. G., et al. (2017). By their own devices: invasive Argentine ants have shifted diet without clear aid from symbiotic microbes. Mol. Ecol. 26, 1608–1630. doi: 10.1111/mec.13991
Huang, X., Li, S., Ni, P., Gao, Y., Jiang, B., Zhou, Z., et al. (2017). Rapid response to changing environments during biological invasions: DNA methylation perspectives. Mol. Ecol. 26, 6621–6633. doi: 10.1111/mec.14382
Human, K. G., and Gordon, D. M. (1999). Behavioral interactions of the invasive Argentine ant with native ant species. Insect. Soc. 46, 159–163. doi: 10.1007/s000400050127
Hurd, C. R., Jeanne, R. L., and Nordheim, E. V (2007). Temporal polyethism and worker specialization in the wasp, Vespula germanica. J. Insect Sci. 7, 1–13. doi: 10.1673/031.007.4301
Ingram, K. K. (2002). Plasticity in queen number and social structure in the invasive Argentine ant (Linepithema humile). Evolution 56, 2008–2016. doi: 10.1111/j.0014-3820.2002.tb00127.x
Ings, T. C., Ings, N. L., Chittka, L., and Rasmont, P. (2010). A failed invasion? Commercially introduced pollinators in Southern France. Apidologie 41, 1–13. doi: 10.1051/apido/2009044
Ings, T. C., Ward, N. L., and Chittka, L. (2006). Can commercially imported bumble bees out-compete their native conspecifics? J. Appl. Ecol. 43, 940–948. doi: 10.1111/j.1365-2664.2006.01199.x
Inoue, M. N., Yokoyama, J., and Washitani, I. (2008). Displacement of Japanese native bumblebees by the recently introduced Bombus terrestris (L.)(Hymenoptera: Apidae). J. Insect Conserv. 12, 135–146. doi: 10.1007/s10841-007-9071-z
IPBES, E. S., Brondizio, J., Settele, S., and Díaz, H. T. N. (editors). (2019). Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science- Policy Platform on Biodiversity and Ecosystem Services.
Ishii, H. S., Kadoya, T., Kikuchi, R., Suda, S.-I. I., and Washitani, I. (2008). Habitat and flower resource partitioning by an exotic and three native bumble bees in central Hokkaido, Japan. Biol. Conserv. 141, 2597–2607. doi: 10.1016/j.biocon.2008.07.029
IUCN Red List of Threatened Species (2016). IUCN. Available online at: www.iucnredlist.org (accessed June 6, 2016).
Jandt, J. M., Bengston, S., Pinter-Wollman, N., Pruitt, J. N., Raine, N. E., Dornhaus, A., et al. (2014). Behavioural syndromes and social insects: personality at multiple levels. Biol. Rev. 89, 48–67. doi: 10.1111/brv.12042
Jandt, J. M., and Toth, A. L. (2015). “Physiological and genomic mechanisms of social organization in wasps (Family: Vespidae),” in Advances in Insect Physiology, eds A. Zayed and C. Kent (Elsevier), 95–130. doi: 10.1016/bs.aiip.2015.01.003
Ji, R., Xie, B., Yang, G., and Li, D. (2003). From introduced species to invasive species–a case study on the Italian bee Apis mellifera L. Chinese J. Ecol. 22, 70–73.
Jiguet, F., Gadot, A., Julliard, R., Newson, S. E., and Couvet, D. (2007). Climate envelope, life history traits and the resilience of birds facing global change. Glob. Chang. Biol. 13, 1672–1684. doi: 10.1111/j.1365-2486.2007.01386.x
Johnson, R. N., and Starks, P. T. (2004). A surprising level of genetic diversity in an invasive wasp: polistes dominulus in the northeastern United States. Ann. Entomol. Soc. Am. 97, 732–737. doi: 10.1603/0013-8746(2004)097[0732:ASLOGD]2.0.CO;2
Kato, M., Shibata, A., Yasui, T., and Nagamasu, H. (1999). Impact of introduced honeybees, Apis mellifera, upon native bee communities in the Bonin (Ogasawara) Islands. Popul. Ecol. 41, 217–228. doi: 10.1007/s101440050025
Kennedy, P., Baron, G., Qiu, B., Freitak, D., Helanterä, H., Hunt, E. R., et al. (2017). Deconstructing superorganisms and societies to address big questions in biology. Trends Ecol. Evol. 32, 861–872. doi: 10.1016/j.tree.2017.08.004
Kerr, J. T., Pindar, A., Galpern, P., Packer, L., Potts, S. G., Roberts, S. M., et al. (2015). Climate change impacts on bumblebees across continents. Science 349, 177–180. doi: 10.1126/science.aaa7031
Kleijn, D., Kleijn, D., Raemakers, I., and Raemakers, I. (2008). A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology 89, 1811–1823. doi: 10.1890/07-1275.1
Kokko, H., and López-Sepulcre, A. (2006). From individual dispersal to species ranges: perspectives for a changing world. Science 313, 789–791. doi: 10.1126/science.1128566
Kotze, D. J., and O'hara, R. B. (2003). Species decline—but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia 135, 138–148. doi: 10.1007/s00442-002-1174-3
Lach, L., and Hooper-Bui, L. M. (2010). Consequences of ant invasions. Ant Ecol. 2010, 261–286. doi: 10.1093/acprof:oso/9780199544639.003.0015
Lai, L.-C., Hua, K.-H., and Wu, W.-J. (2015). Intraspecific and interspecific aggressive interactions between two species of fire ants, Solenopsis geminata and S. invicta (Hymenoptera: Formicidae), in Taiwan. J. Asia. Pac. Entomol. 18, 93–98. doi: 10.1016/j.aspen.2014.09.003
Lande, R. (2015). Evolution of phenotypic plasticity in colonizing species. Mol. Ecol. 24, 2038–2045. doi: 10.1111/mec.13037
LeBrun, E. G., Plowes, R. M., and Gilbert, L. E. (2012). Imported fire ants near the edge of their range: disturbance and moisture determine prevalence and impact of an invasive social insect. J. Anim. Ecol. 81, 884–895. doi: 10.1111/j.1365-2656.2012.01954.x
Leitch, A. R., and Leitch, I. J. (2008). Genomic plasticity and the diversity of polyploid plants. Science 320, 481–483. doi: 10.1126/science.1153585
Lester, P. J., and Beggs, J. R. (2019). Invasion success and management strategies for social Vespula wasps. Annu. Rev. Entomol. 64, 51–71. doi: 10.1146/annurev-ento-011118-111812
Lester, P. J., Beggs, J. R., Brown, R. L., Edwards, E. D., Groenteman, R., Toft, R. J., et al. (2013). The outlook for control of New Zealand's most abundant, widespread and damaging invertebrate pests: social wasps. NZ Sci. Rev. 70, 56–62. Available online at: http://hdl.handle.net/2292/35313
Lester, P. J., Gruber, M. A. M., Brenton-Rule, E. C., Archer, M., Corley, J. C., Dvorák, L., et al. (2014). Determining the origin of invasions and demonstrating a lack of enemy release from microsporidian pathogens in common wasps (Vespula vulgaris). Divers. Distrib. 20, 964–974. doi: 10.1111/ddi.12223
Li, Y., Ke, Z., Wang, S., Smith, G. R., and Liu, X. (2011). An exotic species is the favorite prey of a native enemy. PLoS ONE 6:e24299. doi: 10.1371/journal.pone.0024299
Liebert, A. E., Gamboa, G. J., Stamp, N. E., Curtis, T. R., Monnet, K. M., Turillazzi, S., et al. (2006). Genetics, behavior and ecology of a paper wasp invasion: polistes dominulus in North America. Ann Zoologici Fennici 43, 595–624.
Liebl, A. L., Schrey, A. W., Richards, C. L., and Martin, L. B. (2013). Patterns of DNA methylation throughout a range expansion of an introduced songbird. Integr. Comp. Biol. 53:351–358. doi: 10.1093/icb/ict007
Lin, W., McBroome, J., Rehman, M., and Johnson, B. R. (2018). Africanized bees extend their distribution in California. PLoS ONE 13:e0190604. doi: 10.1371/journal.pone.0190604
Liu, H., and Stiling, P. (2006). Testing the enemy release hypothesis: a review and meta-analysis. Biol. Invasions 8, 1535–1545. doi: 10.1007/s10530-005-5845-y
Lowe, S., Browne, M., Boudjelas, S., and De Poorter, M. (2000). “100 of the world's worst invasive alien species a selection from the global invasive species database,” in Publ. by Invasive Species Spec. Gr. a Spec. Gr. Species Surviv. Comm. World Conserv. Union (IUCN) (Hollands Printing Ltd.), 12p. First Publ. as Spec. lift-out Aliens 12.
Macfarlane, R. P., and Gurr, L. (1995). Distribution of bumble bees in New Zealand. N. Zeal. Entomol. 18, 26–36. doi: 10.1080/00779962.1995.9721999
Manfredini, F., Grozinger, C. M., and Beani, L. (2013). Examining the “evolution of increased competitive ability” hypothesis in response to parasites and pathogens in the invasive paper wasp Polistes dominula. Naturwissenschaften 100, 219–228. doi: 10.1007/s00114-013-1014-9
Marin, P., Genitoni, J., Barloy, D., Maury, S., Gibert, P., Ghalambor, C. K., et al. (2019). Biological invasion: the influence of the hidden side of the (Epi) genome. Funct. Ecol. doi: 10.1111/1365-2435.13317
Matsumura, C., Yokoyama, J., and Washitani, I. (2004). Invasion status and potential ecological impacts of an invasive alien bumblebee, Bombus terrestris L. (Hymenoptera: Apidae) naturalized in Southern Hokkaido, Japan. Glob. Environ. Res. Ed. 8, 51–66.
Meeus, I., Brown, M. J., De Graaf, D. C., and Smagghe, G. (2011). Effects of invasive parasites on bumble bee declines. Conserv. Biol. 25, 662–671. doi: 10.1111/j.1523-1739.2011.01707.x
Morales, C. L., Arbetman, M. P., Cameron, S. A., and Aizen, M. A. (2013). Rapid ecological replacement of a native bumble bee by invasive species. Front. Ecol. Environ. 11, 529–534. doi: 10.1890/120321
Morrison, L. W. (2000). Mechanisms of interspecific competition among an invasive and two native fire ants. Oikos 90, 238–252. doi: 10.1034/j.1600-0706.2000.900204.x
Murray, K. A., Rosauer, D., McCallum, H., and Skerratt, L. F. (2010). Integrating species traits with extrinsic threats: closing the gap between predicting and preventing species declines. Proc. R. Soc. B Biol. Sci. 278, 1515–1523. doi: 10.1098/rspb.2010.1872
Neumann, P., and Carreck, N. L. (2010). Honey Bee Colony Losses. J. Apicult. Re. 49, 1–6. doi: 10.3896/IBRA.1.49.1.01
O'Donnell, S., Donlan, N. A., and Jones, T. A. (2004). Mushroom body structural change is associated with division of labor in eusocial wasp workers (Polybia aequatorialis, Hymenoptera: Vespidae). Neurosci. Lett. 356, 159–162. doi: 10.1016/j.neulet.2003.11.053
Oldroyd, B. P., Thexton, E. G., Lawler, S. H., and Crozier, R. H. (1997). Population demography of Australian feral bees (Apis mellifera). Oecologia 111, 381–387. doi: 10.1007/s004420050249
Overmyer, S. L., and Jeanne, R. L. (1998). Recruitment to food by the German yellowjacket, Vespula germanica. Behav. Ecol. Sociobiol. 42, 17–21. doi: 10.1007/s002650050407
Palacio-López, K., and Gianoli, E. (2011). Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120, 1393–1401. doi: 10.1111/j.1600-0706.2010.19114.x
Plowes, R. M., Dunn, J. G., and Gilbert, L. E. (2007). The urban fire ant paradox: native fire ants persist in an urban refuge while invasive fire ants dominate natural habitats. Biol. Invasions 9, 825–836. doi: 10.1007/s10530-006-9084-7
Potts, S. G., Roberts, S. P. M., Dean, R., Marris, G., Brown, M. A., Jones, R., et al. (2010). Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 49, 15–22. doi: 10.3896/IBRA.1.49.1.02
Purcell, J., Brelsford, A., Wurm, Y., Perrin, N., and Chapuisat, M. (2014). Convergent genetic architecture underlies social organization in ants. Curr. Biol. 24, 2728–2732. doi: 10.1016/j.cub.2014.09.071
Putnam, H. M., Davidson, J. M., and Gates, R. D. (2016). Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol. Appl. 9, 1165–1178. doi: 10.1111/eva.12408
Pyšek, P., Jarošík, V., Hulme, P. E., Pergl, J., Hejda, M., Schaffner, U., et al. (2012). A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species' traits and environment. Glob. Chang. Biol. 18, 1725–1737. doi: 10.1111/j.1365-2486.2011.02636.x
Ratnieks, F. L., and Carreck, N. L. (2010). Clarity on honey bee collapse? Science 327, 152–153. doi: 10.1126/science.1185563
Reeve, A. J., Ojanguren, A. F., Deacon, A. E., Shimadzu, H., Ramnarine, I. W., and Magurran, A. E. (2014). Interplay of temperature and light influences wild guppy (Poecilia reticulata) daily reproductive activity. Biol. J. Linn. Soc. 111, 511–520. doi: 10.1111/bij.12217
Reeve, H. K., and Nonacs, P. (1992). Social contracts in wasp societies. Nature 359, 823–825. doi: 10.1038/359823a0
Reuter, M., Pedersen, J. S., and Keller, L. (2005). Loss of Wolbachia infection during colonisation in the invasive Argentine ant Linepithema humile. Heredity 94, 364–369. doi: 10.1038/sj.hdy.6800601
Richards, C. L., Bossdorf, O., Muth, N. Z., Gurevitch, J., and Pigliucci, M. (2006). Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 9, 981–993. doi: 10.1111/j.1461-0248.2006.00950.x
Robinson, G. E. (1992). Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665. doi: 10.1146/annurev.en.37.010192.003225
Roets, F., Benadé, P. C., Samways, M. J., and Veldtman, R. (2019). Better colony performance, not natural enemy release, explains numerical dominance of the exotic Polistes dominula wasp over a native congener in South Africa. Biol. Invasions 21, 925–933. doi: 10.1007/s10530-018-1870-5
Rollins, L. A., Richardson, M. F., and Shine, R. (2015). A genetic perspective on rapid evolution in cane toads (Rhinella marina). Mol. Ecol. 24, 2264–2276. doi: 10.1111/mec.13184
Rosenkranz, P. (1999). Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30, 159–172. doi: 10.1051/apido:19990206
Ross, K. G., and Matthews, R. W. (1991). The Social Biology of Wasps. Ithaca, NY: Cornell University Press.
Rowles, A. D., and O'Dowd, D. J. (2007). Interference competition by Argentine ants displaces native ants: implications for biotic resistance to invasion. Biol. Invasions 9, 73–85. doi: 10.1007/s10530-006-9009-5
Rowley, J. J., and Alford, R. A. (2007). Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis. Aquat. Organ. 77, 1–9. doi: 10.3354/dao01830
Ruz, L., and Herrera, R. (2001). Preliminary observations on foraging activities of Bombus dahlbomii and Bombus terrestris (Hym: Apidae) on native and non native vegetation in Chile. Acta Hortic 561, 165–169. doi: 10.17660/ActaHortic.2001.561.24
Sackmann, P., Villacide, J. M., and Corley, J. (2003). Presencia de una nueva avispa social exótica, Polistes dominulus (Hymenoptera: Vespidae) en la Patagonia argentina. Pan-Pac. Entomol. 75, 58–59.
Sagata, K., and Lester, P. J. (2009). Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. J. Appl. Ecol. 46, 19–27. doi: 10.1111/j.1365-2664.2008.01523.x
Sánchez-Bayo, F., and Wyckhuys, K. A. G. (2019). Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27. doi: 10.1016/j.biocon.2019.01.020
Sanetra, M., and Crozier, R. H. (2002). Daughters inherit colonies from mothers in the'living-fossil'ant Nothomyrmecia macrops. Naturwissenschaften 89, 71–74. doi: 10.1007/s00114-001-0288-5
Sanetra, M., and Crozier, R. H. (2003). Patterns of population subdivision and gene flow in the ant Nothomyrmecia macrops reflected in microsatellite and mitochondrial DNA markers. Mol. Ecol. 12, 2281–2295. doi: 10.1046/j.1365-294X.2003.01900.x
Schmid-Hempel, P., and Ebert, D. (2003). On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 18, 27–32. doi: 10.1016/S0169-5347(02)00013-7
Schmid-Hempel, P., Schmid-Hempel, R., Brunner, P. C., Seeman, O. D., and Allen, G. R. (2007). Invasion success of the bumblebee, Bombus terrestris, despite a drastic genetic bottleneck. Heredity 99, 414–422. doi: 10.1038/sj.hdy.6801017
Schwander, T., Lo, N., Beekman, M., Oldroyd, B. P., and Keller, L. (2010). Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol. 25, 275–282. doi: 10.1016/j.tree.2009.12.001
Shik, J. Z., and Silverman, J. (2013). Towards a nutritional ecology of invasive establishment: aphid mutualists provide better fuel for incipient Argentine ant colonies than insect prey. Biol. Invasions 15, 829–836. doi: 10.1007/s10530-012-0330-x
Smith, K. M., Loh, E. H., Rostal, M. K., Zambrana-Torrelio, C. M., Mendiola, L., and Daszak, P. (2013). Pathogens, pests, and economics: drivers of honey bee colony declines and losses. Ecohealth 10, 434–445. doi: 10.1007/s10393-013-0870-2
Sorvari, J. (2013). Social wasp (Hymenoptera: Vespidae) beer trapping in Finland 2008-2012: a German surprise. Entomol. Fenn. 24, 156–164. doi: 10.33338/ef.8983
Sorvari, J., and Hakkarainen, H. (2007). Wood ants are wood ants: deforestation causes population declines in the polydomous wood ant Formica aquilonia. Ecol. Entomol. 32, 707–711. doi: 10.1111/j.1365-2311.2007.00921.x
Spradbery, J. P. (1973). Wasps. An Account of the Biology and Natural History of Social and Solitary Wasps, With Particular Reference to Those of the British Isles. Seattle: University of Washingon Press.
Stapley, J., Santure, A. W., and Dennis, S. R. (2015). Transposable elements as agents of rapid adaptation may explain the genetic paradox of invasive species. Mol. Ecol. 24, 2241–2252. doi: 10.1111/mec.13089
Stockan, J. A., Rao, S., and Pakeman, R. (2010). Nesting preferences of the threatened wood ant Formica exsecta (Hymenoptera: Formicidae); implications for conservation in Scotland. J. insect Conserv. 14, 269–276. doi: 10.1007/s10841-009-9255-9
Suarez, A. V., Holway, D. A., and Tsutsui, N. D. (2008). Genetics and behavior of a colonizing species: the invasive Argentine ant. Am. Nat. 172, S72–S84. doi: 10.1086/588638
Thomas, C. R. (1960). The European wasp (Vespula germanica Fab.) in New Zealand. Wellington, NZ: Department of Scientific and Industrial Research.
Torretta, J. P., Medan, D., and Abrahamovich, A. H. (2006). First record of the invasive bumblebee Bombus terrestris (L.) (Hymenoptera, Apidae) in Argentina. Trans. Am. Entomol. Soc. 132, 285–289. doi: 10.3157/0002-8320(2006)132[285:FROTIB]2.0.CO;2
Traniello, J. F. A., and Rosengaus, R. B. (1997). Ecology, evolution and division of labour in social insects. Anim. Behav. 53, 209–213. doi: 10.1006/anbe.1996.0289
Trible, W., Shoemaker, D. D., and Gotzek, D. (2018). Sociometry of Solenopsis geminata (Hymenoptera: Formicidae) reveals variation in colony-level phenotypes in fire ants. Myrmecol. News 26, 47–53. doi: 10.25849/myrmecol.news_026:047
Vargo, E. L., and Porter, S. D. (1993). Reproduction by virgin queen fire ants in queenless colonies: comparative study of three taxa (Solenopsis richteri, hybridS. invicta/richteri, S. geminata) (Hymenoptera: Formicidae). Insect. Soc. 40, 283–293. doi: 10.1007/BF01242364
Via, S., Gomulkiewicz, R., De Jong, G., Scheiner, S. M., Schlichting, C. D., and Van Tienderen, P. H. (1995). Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217. doi: 10.1016/S0169-5347(00)89061-8
Villemant, C., Zuccon, D., Rome, Q., Muller, F., Poinar, G. O. Jr, and Justine, J.-L. (2015). Can parasites halt the invader? Mermithid nematodes parasitizing the yellow-legged Asian hornet in France. PeerJ. 3:e947. doi: 10.7717/peerj.947
Visscher, P. K., and Vetter, R. S. (2003). Annual and multi-year nests of the western yellowjacket, Vespula pensylvanica, in California. Insect. Soc. 50, 160–166. doi: 10.1007/s00040-003-0636-x
Wang, J., Wurm, Y., Nipitwattanaphon, M., Riba-Grognuz, O., Huang, Y.-C., Shoemaker, D., et al. (2013). A Y-like social chromosome causes alternative colony organization in fire ants. Nature 493, 664–668. doi: 10.1038/nature11832
Weiner, S. A., Noble, K., Upton, C. T., Woods, W. A., and Starks, P. T. (2011). A role for thermoregulation in the Polistes dominulus invasion: a comparison of the thermoregulatory abilities of the invasive wasp P. dominulus and the native wasp P. fuscatus. Insect. Soc. 58, 185–190. doi: 10.1007/s00040-010-0136-8
Wellband, K. W., and Heath, D. D. (2017). Plasticity in gene transcription explains the differential performance of two invasive fish species. Evol. Appl. 10, 563–576. doi: 10.1111/eva.12463
West-Eberhard, M. J. (1989). Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278. doi: 10.1146/annurev.es.20.110189.001341
Williams, N. M., Crone, E. E., T'ai, H. R., Minckley, R. L., Packer, L., and Potts, S. G. (2010). Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 143, 2280–2291. doi: 10.1016/j.biocon.2010.03.024
Williams, P. H., Byvaltsev, A., Sheffield, C., and Rasmont, P. (2013). Bombus cullumanus- An extinct European bumblebee species? Apidologie 44, 121–132. doi: 10.1007/s13592-012-0161-x
Wilson, E. E., Mullen, L. M., and Holway, D. A. (2009). Life history plasticity magnifies the ecological effects of a social wasp invasion. Proc. Natl. Acad. Sci. U.S.A. 106, 12809–12813. doi: 10.1073/pnas.0902979106
Wilson, E. O. (1978). Division of labor in fire ants based on physical castes (Hymenoptera: Formicidae: Solenopsis). J. Kansas Entomol. Soc. 51, 615–636.
Winston, M. L., Otis, G. W., and Taylor, O. R. Jr (1979). Absconding behaviour of the Africanized honeybee in South America. J. Apic. Res. 18, 85–94. doi: 10.1080/00218839.1979.11099951
Winston, M. L., Taylor, O. R., and Otis, G. W. (1983). Some differences between temperate European and tropical African and South American honeybees. Bee World 64, 12–21. doi: 10.1080/0005772X.1983.11097902
Wood, T. J., Gibbs, J., Graham, K. K., and Isaacs, R. (2019). Narrow pollen diets are associated with declining Midwestern bumble bee species. Ecology 100:e02697. doi: 10.1002/ecy.2697
Wuellner, C. T., and Saunders, J. B. (2003). Circadian and circannual patterns of activity and territory shifts: comparing a native ant (Solenopsis geminata, Hymenoptera: Formicidae) with its exotic, invasive congener (S. invicta) and its parasitoids (Pseudacteon spp., Diptera: Phoridae) at a centra. Ann. Entomol. Soc. Am. 96, 54–60. doi: 10.1603/0013-8746(2003)096[0054:CACPOA]2.0.CO;2
Yang, C.-C., Yu, Y.-C., Valles, S. M., Oi, D. H., Chen, Y.-C., Shoemaker, D., et al. (2010). Loss of microbial (pathogen) infections associated with recent invasions of the red imported fire ant Solenopsis invicta. Biol. Invasions 12, 3307–3318. doi: 10.1007/s10530-010-9724-9
Keywords: social insect, phenotypic plasticity, species conservation, invasive species, global change
Citation: Manfredini F, Arbetman M and Toth AL (2019) A Potential Role for Phenotypic Plasticity in Invasions and Declines of Social Insects. Front. Ecol. Evol. 7:375. doi: 10.3389/fevo.2019.00375
Received: 18 June 2019; Accepted: 17 September 2019;
Published: 15 October 2019.
Edited by:
Shannon Murphy, University of Denver, United StatesReviewed by:
Adam R. Smith, George Washington University, United StatesCopyright © 2019 Manfredini, Arbetman and Toth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Manfredini, Zm1hbmZyZWRpbmk3OUBnbWFpbC5jb20=; Marina Arbetman, bWFyYmV0bWFuQGNvbWFodWUtY29uaWNldC5nb2IuYXI=; Amy L. Toth, YW15dG90aEBpYXN0YXRlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.