- 1Flathead Lake Biological Station, University of Montana, Polson, MT, United States
- 2Laboratorio de Limnología, INIBIOMA, CONICET—Universidad Nacional del Comahue, Bariloche, Argentina
- 3Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO, United States
- 4Department of Biology, University of Maryland, College Park, MD, United States
- 5School of Biological Sciences, Washington State University, Pullman, WA, United States
Roughly 10% of the Earth's surface is permanently covered by glaciers and ice sheets and in mountain ecosystems, this proportion of ice cover is often even higher. From an ecological perspective, ice-dominated ecosystems place harsh controls on life including cold temperature, limited nutrient availability, and often prolonged darkness due to snow cover for much of the year. Despite these limitations, glaciers, and perennial snowfields support diverse, primarily microbial communities, though macroinvertebrates and vertebrates are also present. The availability and mass balance of key elements [(carbon (C), nitrogen (N), phosphorous (P)] are known to influence the population dynamics of organisms, and ultimately shape the structure and function of ecosystems worldwide. While considerable attention has been devoted to patterns of biodiversity in mountain cryosphere-influenced ecosystems, the ecological stoichiometry of these habitats has received much less attention. Understanding this emerging research arena is particularly pressing in light of the rapid recession of glaciers and perennial snowfields worldwide. In this review, we synthesize existing knowledge of ecological stoichiometry, nutrient availability, and food webs in the mountain cryosphere (specifically glaciers and perennial snowfields). We use this synthesis to develop more general understanding of nutrient origins, distributions, and trophic interactions in these imperiled ecosystems. We focus our efforts on three major habitats: glacier surfaces (supraglacial), the area beneath glaciers (subglacial), and adjacent downstream habitats (i.e., glacier-fed streams and lakes). We compare nutrient availability in these habitats to comparable habitats on continental ice sheets (e.g., Greenland and Antarctica) and show that, in general, nutrient levels are substantially different between the two. We also discuss how ongoing climate warming will alter nutrient and trophic dynamics in mountain glacier-influenced ecosystems. We conclude by highlighting the pressing need for studies to understand spatial and temporal stoichiometric variation in the mountain cryosphere, ideally with direct comparisons to continental ice sheets, before these imperiled habitats vanish completely.
Introduction
Approximately 75% of the freshwater on Earth is frozen at any given time (Jain, 2014; Talalay et al., 2014). This global “cryosphere” includes continental ice sheets, mountain glaciers, snowfields, permafrost, and sea ice. Beyond extreme cold and frequent subzero temperatures, frozen environments place additional harsh controls on life including a lack of available water, high ultraviolet radiation, and highly dynamic, but generally limited, nutrient supplies (Anesio and Laybourn-Parry, 2012). Nevertheless, the cryosphere supports diverse biological communities on the surface of glaciers and continental ice sheets (Anesio et al., 2009; Hotaling et al., 2017a), beneath them (Hamilton et al., 2013), in their meltwater (Hotaling et al., 2019a), in permafrost (Jansson and Tas, 2014), and in sea ice (Boetius et al., 2015). Even though these biotic communities are dominated by unicellular microbial life (e.g., bacteria and algae: Boetius et al., 2015; Anesio et al., 2017; Hotaling et al., 2017a), macroinvertebrates (e.g., ice worms and rotifers; Shain et al., 2016; Hotaling et al., 2019b) and vertebrates (e.g., gray-crowned rosy finches; Rosvold, 2016) are also represented. However, rising global temperatures are driving widespread recession of the cryosphere (Lyon et al., 2009; Notz and Stroeve, 2016; Roe et al., 2017) with profound implications for biodiversity (Hotaling et al., 2017b), human populations (Pritchard, 2017), and ecological feedbacks (e.g., trophic interactions and biogeochemical cycles, Hood et al., 2009; Sommaruga, 2015; Milner et al., 2017). Therefore, understanding the interplay between resource availability and biological communities across cryosphere-associated habitats, as well as how climate change may alter them, is a pressing research need.
The field of ecological stoichiometry, which seeks to understand the balance of energy and chemical elements in ecological interactions and processes (Sterner and Elser, 2002), provides a valuable framework for understanding how ecosystems function. The availability and mass balance of key elements [carbon (C), nitrogen (N), and phosphorous (P)] place crucial controls on the population dynamics of organisms (Cross et al., 2005), ultimately regulating the structure, function, and processes of ecosystems (Sterner and Elser, 2002; Elser et al., 2007). On mountain glaciers and snowfields, solar radiation, atmospheric CO2, allochthonous (e.g., arthropod fallout, Edwards, 1987; black carbon, Skiles et al., 2018), and autochthonous (e.g., cryoconite hole metabolism, Anesio et al., 2009; snow algae primary productivity, Hamilton and Havig, 2018) inputs all interact to shape elemental balances and the structure of food webs. Furthermore, glacier environments support higher trophic levels (Tynen, 1970; Kohshima, 1984; De Smet and Van Rompu, 1994; Kikuchi, 1994), highlighting the key role that stoichiometric dynamics likely play in shaping ice-associated food webs. Spatial links also exist among cryospheric habitats; for instance, primary production and atmospheric deposition occuring on the surface of glaciers (supraglacial zone) influences stoichiometric processes beneath them in the subglacial zone. Glacier-associated biological and biogeochemical processes ultimately affect downstream lakes and streams as far as the world's oceans (Hotaling et al., 2017a). Thus, ecological stoichiometry can provide mechanistic insight into connections among different environments, interactions between trophic levels, and can act as a means to link the influences of glacier melt dynamics on ecosystem structure and function in cryosphere-associated environments and beyond.
In this review, we provide the first synthesis of ecological stoichiometry in the mountain cryosphere. We identify mountains (and by proxy, mountain ecosystems) in the same way as a related review (Moser et al., 2019), which used the definition provided by Körner et al. (2017): mountainous areas exhibit more than 200 m of elevation difference within a 2.5′ grid cell, irrespective of elevation. Our motivation for focusing on the mountain cryosphere was 3-fold. First, much is known of the general stoichiometry of continental ice sheets (i.e., Greenland and Antarctica), however there has been considerably less focus on similar mountain ecosystems and thus, it is unclear how comparable the two environments are. Second, mountain glaciers and snowfields harbor extensive biological diversity, including multiple trophic levels from microbes to birds (e.g., Anesio and Laybourn-Parry, 2012; Hotaling et al., 2019a). Third, many human populations rely on mountain glacier meltwater for agriculture, hydropower, and drinking water (Milner et al., 2017), highlighting the potential relevance of ecological processes occurring in headwaters beyond adjacent biotic communities.
Within the mountain cryosphere, we focus on three distinct, but interconnected, habitats: the supraglacial zone, subglacial zone, and downstream glacier-fed streams and lakes (Figure 1). The supraglacial zone encompasses the interface between surface ice/snow and atmospheric conditions. The subglacial zone comprises the area where glacier ice interacts directly with bedrock and meltwater. And, glacier-fed streams and lakes include the downstream habitats which are directly adjacent to glaciers (Figure 1). By considering the mountain cryosphere in a stoichiometric framework, we clarify key environment-organism interactions, including the effects of nutrients on psychrophiles (organisms capable of living in extremely low temperatures) and their reciprocal effects on biogeochemical processes (e.g., respiration, nitrification) and habitat characteristics (e.g., snow albedo, light attenuation). For each habitat, we address two stoichiometric questions: (1) What is the origin, availability, and variation (spatial and temporal) of C, N, and P? What dynamics of stoichiometric ratios exist? (2) To what extent are complex food webs (i.e., multiple trophic levels) present? What does stoichiometry mean for their trophic interactions and biogeochemical cycling? As part of this, we explicitly test the degree to which nutrient concentrations in the mountain cryosphere are comparable to those measured for continental ice sheets to better understand how insight gained from ice sheets can be translated to mountain systems. We conclude by discussing how contemporary climate change, and particularly rapid glacier decline, will impact these ecosystems. As the cryosphere continues to change, a stoichiometric understanding will allow for refined ecological predictions of nutrient dynamics and consequences for trophic interactions.
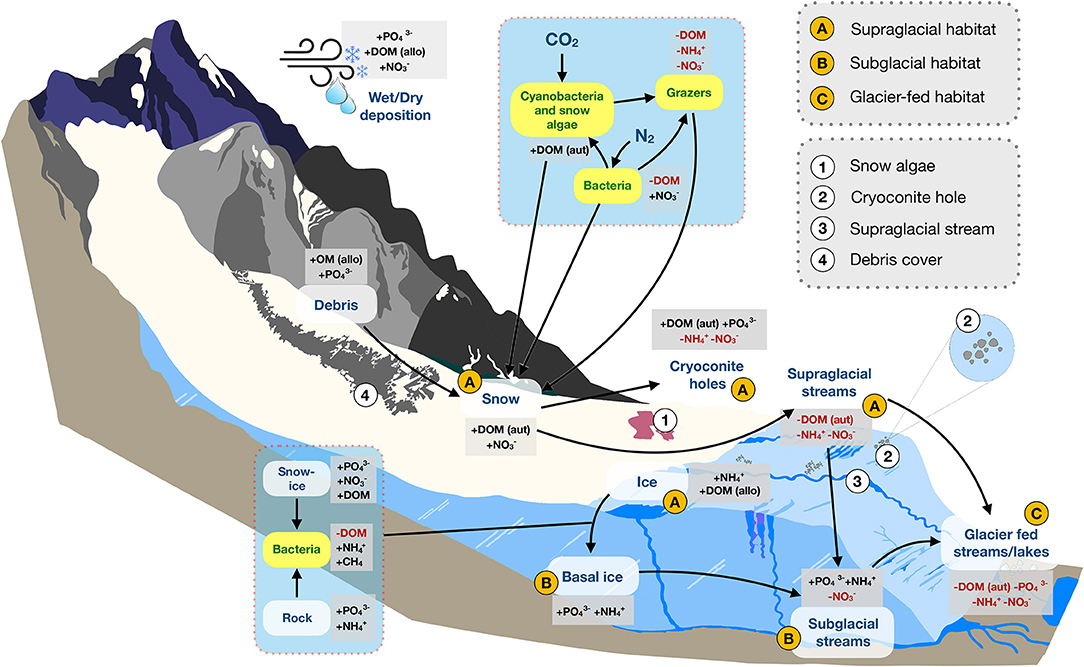
Figure 1. A conceptual diagram of habitats and nutrient dynamics in mountain glacier ecosystems. Major (A–C) and minor (1, 2, 3, 4) components of the mountain cryosphere discussed in this review are shown. Arrows indicate nutrient flow paths, plus signs (+) indicate sources, while red text and minus signs “–” indicate sinks. Yellow boxes with green text represent organisms. Abbreviations include: allo, allochthonous; aut, autochthonous; DOM, dissolved organic matter; , phosphate; , ammonium; CH4, methane; , nitrate. Figure is not drawn to scale.
Living on Ice: the Supraglacial Zone
During melt, considerable primary production occurs in the supraglacial zone, primarily due to snow algal blooms (e.g., Chlamydomonas nivalis and cyanobacteria, Anesio et al., 2017), in spite of high levels of ultraviolet radiation (Morgan-Kiss et al., 2006; Yallop et al., 2012) and often limited nutrient availability (Hawkings et al., 2016; Wadham et al., 2016). This biomass production has key implications for local biota (e.g., heterotrophs), hydrologically connected habitats downstream, and even glacier albedo (Ganey et al., 2017; Figure 1). While surface ice and snow represent the bulk of available habitat in the supraglacial zone, and thus are where most of the biomass on mountain glaciers resides, the supraglacial zone is highly heterogeneous (e.g., with respect to debris cover) and habitat types within it (e.g., cryoconite holes) must also be considered (Figure 1).
Nutrients
Supraglacial environments are typically nutrient poor (Figure 2; Table S1) and available nutrients are often present in organic forms (e.g., residues of microbial and/or plant cells, decayed organic matter, microbial exudates; Antony et al., 2017). For reference eutrophic lakes typically have total N concentrations of 650–1,200 μg L−1 and total P concentrations of 30–100 μg L−1 (Dodds and Whiles, 2010). Mountain glaciers typically only contain a fraction of that level of nutrient concentrations (Figure 2). Surface ice and snow in particular exhibit low nutrient concentrations when compared to other microhabitats (e.g., cryoconite holes; Figure 2). Through C fixation, supraglacial primary production promotes the accumulation of autochthonous organic C (Musilova et al., 2017; Williamson et al., 2019), thereby driving C and N cycling (Stibal et al., 2012; Havig and Hamilton, 2019). Due to near constant melt during periods of warm temperature and/or high solar radiation, any C that is fixed on the supraglacial surface and not consumed by local heterotrophs (e.g., bacteria, fungi, ice worms) is exported to subglacial and downstream habitats. Thus, metabolic activity of supraglacial organisms may alter the environmental availability of nutrients and shape trophic relationships in hydrologically connected habitats.
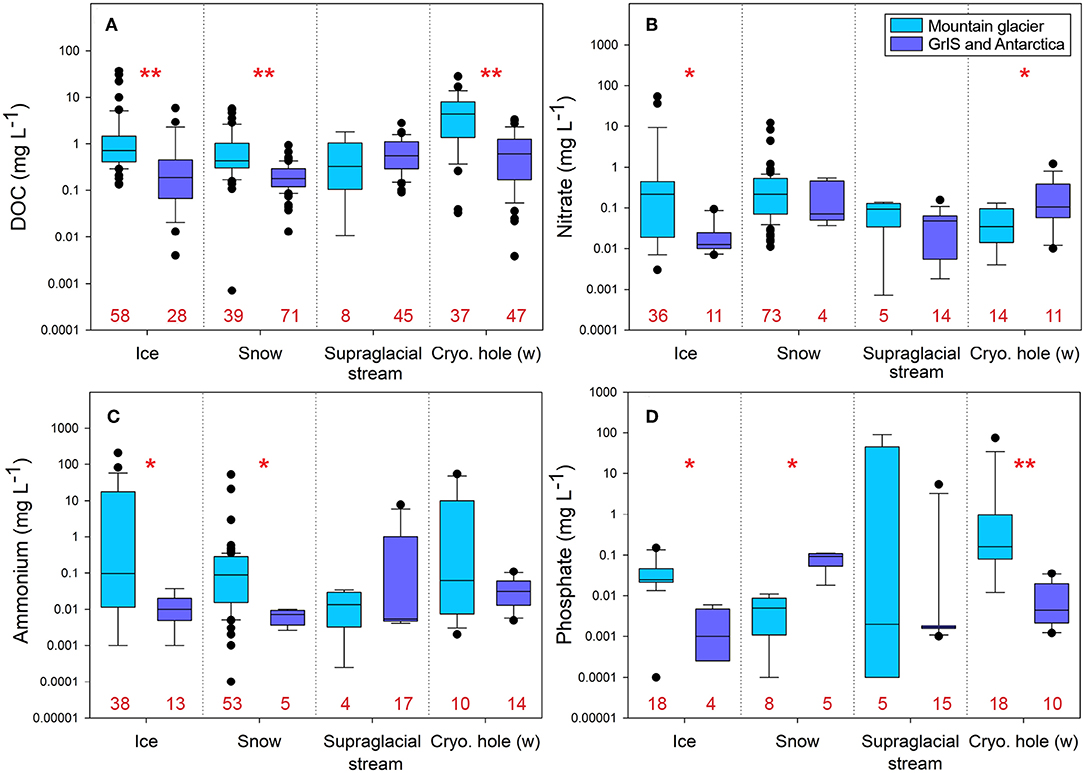
Figure 2. Nutrient comparisons between mountain glaciers and continental ice sheets for: (A) dissolved organic carbon (DOC), (B) nitrate, (C) ammonium, and (D) phosphate. Data were log-transformed for ease of visualization. Numbers in red along the x-axis indicate sample sizes for each group. Pairwise comparisons were made between mountain glaciers and continental ice sheets for each nutrient using Mann-Whitney or t-tests (see Table S2). Single asterisks denote significance at p < 0.02 and double asterisks denote significance at p < 0.001. Analyses were performed from data summarized in Table S1. Table S2 includes detailed information of the statistical analyses. Abbreviations include: GrIS, Greenland Ice Sheet; cryo hole (w), cryoconite hole water.
The presence and growth of supraglacial microbes, and in particular snow algae (Figure 1), can reduce local albedo and induce additional melting (Ganey et al., 2017). Since meltwater itself is often a limited resource in the supraglacial zone, the release of additional water can locally stimulate further microbial growth, which drives more melt in a bio-albedo feedback loop (Anesio et al., 2017). Melting can also promote the formation of cryoconite holes in the supraglacial zone. Cryoconite holes form when particles with a lower albedo than the surrounding area induce locally elevated melt rates which in turn form small melt depressions (< 10 cm deep and < 1 m wide)—“cryoconite holes”—on the glacier surface (Fountain et al., 2004; Figure 1). These unique microhabitats are hotspots of microbial activity (Anesio and Laybourn-Parry, 2012) but also typically harbor larger organisms (e.g., De Smet and Van Rompu, 1994).
Overall, average dissolved organic carbon (DOC) concentrations are significantly higher in mountain glacier ice and snow than the same habitats on continental ice sheets (Figure 2; Table S2). While the mechanism(s) underlying this pattern remain unclear, it likely exists because mountain glaciers are generally closer to terrestrial sources of C vs. continental ice sheets. Related factors may also include higher ambient temperatures (and thus more meltwater) as well as more primary production on mountain glaciers. In general, supraglacial ecosystems accumulate organic matter through in situ primary production as well as deposition from terrestrial and anthropogenic sources (Hood et al., 2009; Singer et al., 2012; Stibal et al., 2012; Figure 2). Both autochthonous and allochthonous dissolved organic matter (DOM) are actively transformed by microbial communities through degradation and synthesis (Antony et al., 2017). Through this microbial processing, low quality DOM (i.e., high C:N, C:P) can be processed and “upgraded” (converted to lower C:N and/or C:P), thereby becoming more bioavailable to heterotrophs both within and downstream of the supraglacial zone (Musilova et al., 2017). Due to the tight recycling of DOM in the supraglacial zone, DOM in downstream systems can still be dominated by allochthonous C sources derived from aerial and terrestrial deposition (Stubbins et al., 2012). However, recent work from the outflow of surface and rock glaciers in the western United States demonstrated that exported DOM is still readily consumed by bacteria (Fegel et al., 2019). This suggests that even among mountain glacier ecosystems, there is substantial variation in exported DOC quality and quantity.
The diversity and activity of supraglacial microorganisms also exert a major influence on the cycling of N and P (Tranter et al., 2004; Hodson et al., 2005). Supraglacial meltwaters are low in nutrients (Säwström et al., 2007a; Grzesiak et al., 2015; Figure 2) and bacterial activity on glaciers is largely P-limited (Mindl et al., 2007; Säwström et al., 2007b; Stibal et al., 2008). This is reasonable given that P concentrations in snow, ice, and supraglacial streams are typically very low with an average of 0.005, 0.043, and 0.002 mg L−1, respectively (Figure 2). Cryoconite holes are an exception and exhibit higher P concentrations (0.31 mg L−1), likely due to leakage of nutrients from algal cells, cell lysis, and general breakdown of organic matter (Figure 2). Thus, P demand likely exceeds supply in the supraglacial zone (except in cryoconite holes) because atmospheric deposition and microbial rock weathering alone cannot support existing needs (Mueller et al., 2001; Stibal et al., 2009). However, in addition to terrestrial inputs, debris cover on glaciers, and weathering from atmospheric deposition or rock fall can also supply P (Modenutti et al., 2018). These additional P sources can greatly alter nutrient dynamics in glaciers, and therefore productivity. However, since concentrations of P are much less frequently reported, spatial variation in P and the degree to which additional P inputs alter supraglacial productivity on local, regional, and global scales remains unclear.
Supraglacial microbial communities cycle N through the oxidation of ammonium to nitrate, chemical decomposition of organic matter (Hodson et al., 2005; Wynn et al., 2007), and nitrogen fixation (Telling et al., 2011). Terrestrial debris and anthropogenic N deposition are significant sources of reactive N to glacier surfaces worldwide (Anderson et al., 2017; Havig and Hamilton, 2019); this is particularly true in mountainous areas located near centers of industrial and/or agricultural activity and may partially explain the large differences in nitrate () and ammonium () concentrations between mountain glaciers and continental ice sheets (Figure 2B). For example, rates of total N deposition in the Rocky Mountains (~3–5 kg N ha−1 yr−1; NRSP-3, 2019) and the southern Andes (~8.2 kg N ha−1 yr−1; Godoy et al., 2003) are nearly an order of magnitude higher than Greenland (~0.5–1 kg N ha−1 yr−1; AMAP, 2006). Since deposition is the most important factor influencing supraglacial N (Tranter et al., 1993; Hodson et al., 2005), the highest N concentrations are typically observed in snow and ice (Figure 2). Most N fixation occurs in the ablation zone of glaciers, where more ice mass is lost due to melting, evaporation, and related processes than accumulates via new snowfall. The zone of N fixation progressively migrates upslope during melt seasons as temperatures rise, solar radiation increases, and lower elevation N reserves are taken up (Telling et al., 2011). Due to high microbial metabolism, cryoconite holes act as sinks for dissolved inorganic N, and typically exhibit the lowest levels of dissolved inorganic N (i.e., ammonium and nitrate) in the supraglacial zone (Figure 2). Breakdown of organic matter, either from allochthonous deposition or historical autochthonous production, within cryoconite holes is likely an important additional source of recycled N to microbial communities (Stibal et al., 2012).
Trophic Interactions
To a greater extent than continental ice sheets, mountain glaciers support multi-trophic food webs which often include microbial diversity, macroinvertebrates, and, in some instances, vertebrates. The structure of supraglacial food webs varies geographically and the reason for this remains largely unknown (Zawierucha et al., 2015; Hotaling et al., 2019a). Recently, the gut microbiome of the Patagonian dragon (Andiperla willinki), a ~2.5 cm stonefly which lives on the surface of Patagonian glaciers, revealed glacier-specific community modifications (Murakami et al., 2018). Indeed, bacterial taxa associated with glacier ice (e.g., Polaromonas and Rhodoferax) were present and are speculated to contribute to both host nutrition as well as the recycling and breakdown of organic matter where A. willinki is present. The gut bacteria-host relationship between A. willinki and glacier microbiota highlights the potential for direct trophic relationships between glacier-endemic bacteria and eukaryotes (Murakami et al., 2018).
In North America, millions of glacier ice worms (Mesenchytraeus solifugus) inhabit coastal glaciers from Oregon to Alaska, USA (Hotaling et al., 2019a). Ice worms feed on glacier bacteria and snow algae (Murakami et al., 2015) and are, in turn, heavily predated upon by a variety of high-elevation nesting birds, including Gray-crowned Rosy finches (Leucosticte tephrocotis, Hotaling et al., 2019a). Thus, nutrient limitations occurring at lower trophic levels have the potential to be transferred from microbial communities to vertebrates. This could be particularly true on mountain glaciers because stoichiometric flexibility may be reduced at low temperatures (Godwin and Cotner, 2015). The potential for nutrient variation at the base of the food web to alter higher level trophic ecology, however, is not specific to glaciers in North or South America. In addition to A. willinki and M. solifugus, at least three other species of macroinvertebrates live on glaciers, including another ice worm in Tibet (Sinenchytraeus glacialis, Liang, 1979) and two chironomid midges, one in the Himalayas (Diamesa kohshima, Kohshima, 1984) and another in New Zealand (Zealandochlus latipalpis, Boothroyd and Cranston, 1999). Vertebrates feeding on glacier-endemic invertebrates may provide nutrient subsidies to supraglacial zones, potentially driving ecological stoichiometry and food web structure in a manner similar to the role of bird guano on remote islands (e.g., Anderson and Polis, 1999; Vizzini et al., 2016). Collectively, the presence of these unique, understudied organisms highlights the need for global, multi-trophic studies of ecological stoichiometry in mountain glacier ecosystems to better understand how nutrient inputs (and limitations) flow through these vanishing habitats.
Living Below Ice: the Subglacial Zone
Though perpetually dark and long assumed to contain little to no life, an extensive microbial community has been documented below mountain glaciers and continental ice sheets around the world (Hamilton et al., 2013; Anesio et al., 2017; Hotaling et al., 2017a; Figure 1). The subglacial zone exists at the ice-bedrock interface and includes any habitat that is perpetually covered by glacier ice. However, current understanding of biodiversity, nutrient availability, and associated stoichiometric composition of life in these habitats remains limited compared to supraglacial and downstream environments. This lack of understanding is likely due in large part to the extreme challenges associated with sampling habitats below tens to hundreds of meters of ice in rugged terrain (Anesio et al., 2017). To our knowledge, all studies of microbial ecology and ecological stoichiometry in mountain subglacial environments have only included samples collected near glacier snouts where subglacial runoff and sediments (e.g., within ice caves) are accessible rather than coring through ice to the bedrock itself.
Nutrients
During the melt season, water flows over and within the surface of glaciers, inducing a dynamic drainage network that moves water from the glacier surface to the base where much of it flows into the subglacial zone, and eventually into headwater streams and lakes (Fountain et al., 2005). The subglacial zone is rich in biogeochemical activity (Hamilton et al., 2013) that mobilizes nutrients and organic matter of both supraglacial and subglacial origin. Through this processing, microbially produced DOC is exported downstream, ultimately contributing to cycling of C in downstream and adjacent ecosystems (e.g., glacier forefields and headwater streams; Anesio et al., 2009, 2010; Edwards et al., 2014). Unlike continental ice sheets, the steep gradients of mountain systems generally preclude the development of subglacial lakes (though they can occur, e.g., Capps et al., 2010). However, substantial water is still present at the bedrock-ice interface which provides key habitat and resources for an active, diverse subglacial microbiome (Hamilton et al., 2013). Indeed, flowing water is the primary driver of nutrient movement both within glaciers (e.g., from the surface to subglacial habitats, Hotaling et al., 2017a) and into glacier outflows (e.g., delivery of labile C to glacier-influenced streams, Hood et al., 2009). Subglacial microbial communities may have even acted as refugia for microbial biodiversity during glacial periods due to the relative stability of subglacial habitats compared to their surface counterparts paired with a continual exposure to fresh mineral surfaces due to the grinding of bedrock by glaciers (Hodson et al., 2008).
An array of energy sources sustain life beneath glaciers (Hotaling et al., 2017a) including the aforementioned bedrock grinding and supraglacial inputs (e.g., primary productivity) flowing to the subsurface, as well as in some instances, geothermal energy (Hodson et al., 2008; Boyd et al., 2014; Telling et al., 2015). There is also considerable evidence for chemolithoautotrophic primary productivity (e.g., via methane production pathways; Boyd et al., 2010; Hamilton et al., 2013). In polar and subpolar regions, chemolithoautotrophs can fix several micrograms of C per square meter per day (Christner et al., 2014). Though C fixation by chemolithoautotrophs has not been estimated for any mountain glacier ecosystem, their abundance below mountain glaciers (e.g., Hamilton et al., 2013) suggests this activity likely rises to non-negligible levels with direct implications for food webs both near and far. In the Canadian Rockies, subglacial primary production is likely sustained in large part by the oxidation of pyrite and nitrification (Boyd et al., 2011, 2014). However, the full scope of bedrock lithologies in mountain ecosystems remains unsampled, making it difficult to generalize beyond specific study regions and local geology (Hotaling et al., 2017a). For N, microbially mediated nitrification and denitrification occur in subglacial environments (e.g., Boyd et al., 2010) and similar to C, its export during times of glacial melt affects downstream communities (see below; Hodson et al., 2010).
Trophic Interactions
Subglacial food webs appear to generally be sustained by microbially-mediated chemical weathering of bedrock through sulfide oxidation (e.g., Wadham et al., 2010; Boyd et al., 2014) or carbonic acid weathering (e.g., Havig and Hamilton, 2019). This chemical weathering releases organic C (Wadham et al., 2004) as well as N and P (Hodson et al., 2005) from the bedrock, thereby providing crucial nutrients. However, the degree to which nutrient limitations affect subglacial food webs, whether chronically or seasonally, remains largely unknown. For instance, bacterial and archaeal sediment communities beneath a mid-latitude, temperate Canadian glacier, with a ~210:1 C:N of dissolved organic matter, appear N limited (Boyd et al., 2011). A particulate organic C:N of ~137:1 beneath the same Canadian glacier is elevated relative to comparable geologies (Ingall et al., 1993), whether or not this elevated C:N ratio is the product of contemporary or historical processes is not known (Boyd et al., 2011). Furthermore, the degree to which nutrients influence higher trophic levels (e.g., fungi, the likely largest organisms in subglacial ecosystems; Hotaling et al., 2017a) below glaciers has not been investigated.
Living Downstream of Ice: Glacier-Fed Streams and Lakes
Glacier-fed streams and lakes reflect the integration of both upstream and in situ processes (Figure 1; Brittain and Milner, 2001; Mindl et al., 2007; Robinson et al., 2016; Hotaling et al., 2017b; Ren and Gao, 2019). Melting glaciers supply key water, sediment, and nutrients to aquatic ecosystems, determining their optical properties, resource availability, and the structure and composition of biological communities (Laspoumaderes et al., 2013; Martyniuk et al., 2014; Rose et al., 2014; Hotaling et al., 2017b; Ren et al., 2017a; Figures 4, 5). In general, glacier-fed streams and lakes are characterized by considerable sediment input from upstream grinding of bedrock, cold temperatures (e.g., < 10° even in summer), and dynamic water levels. Climate change is accelerating glacier retreat (Masiokas et al., 2008; Zemp et al., 2015) and will alter downstream ecosystems from biogeochemical cycling to elemental flow through food webs (Baron et al., 2009; Slemmons and Saros, 2012; Fell et al., 2017; Figures 4, 5).
Glacier-Fed Streams
Nutrients
Glaciers export organic C, inorganic and organic N, and soluble P to adjacent stream environments (Fegel et al., 2016; Milner et al., 2017; Colombo et al., 2019). In headwaters, glaciers are a major source of DOC (Hood et al., 2009; Milner et al., 2017; Hemingway et al., 2019), which is assimilated into multiple levels of stream food webs (Fellman et al., 2015). Concentrations of DOC in glacier-fed streams vary widely from near zero to more than 3 mg L−1 (Zah et al., 2001; Hood et al., 2009; Wilhelm et al., 2013; Martyniuk et al., 2014; Robinson et al., 2016; Hemingway et al., 2019) and are generally comparable to values observed for snow and ice (Figures 2, 3). Previously ice-locked DOC is particularly valuable to glacier-fed stream food webs as it is highly bioavailable for heterotrophic consumption (i.e., composed of > 50% bioavailable DOM; Hood et al., 2009, 2015; Singer et al., 2012; Fegel et al., 2019).
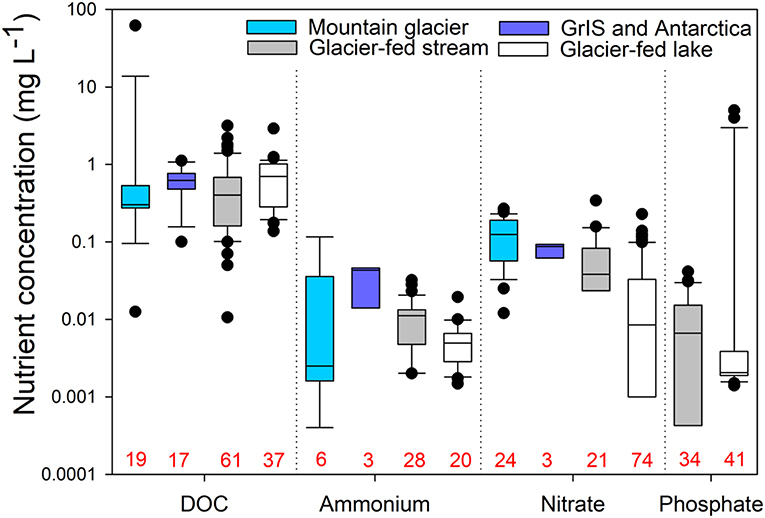
Figure 3. Comparisons of nutrient concentrations for subglacial water from mountain glaciers (light blue) and the Greenland Ice Sheet (GrIS) and Antarctica (dark blue), as well as water from mountain glacier-fed streams and lakes. No data were available for phosphate in subglacial systems. Numbers in red along the x-axis indicate sample sizes for each group. The data for this figure, including details of the studies represented, are provided in Table S1.
Three substantial pools of N exist near glacier-fed streams and lakes: glacier ice, subglacial sediment, and nearby soils (Malard et al., 2000; Hood and Scott, 2008; Robinson et al., 2016). In glacier-fed streams, nitrate concentrations range from 0.08 to 0.22 mg L−1, which is lower than supraglacial concentrations, potentially reflecting uptake upon export (Rinke et al., 2001; Robinson et al., 2016; Figure 3). While soluble reactive P is often below the detection limit in glacier-fed streams (i.e., < 2 μg L−1; Rinke et al., 2001; Robinson et al., 2016), depending on the underlying geology, dissolved and particulate P can be quite high. For example, Patagonian glacier-fed streams, with basaltic, granitic, pyritisic, and silicic metamorphic bedrock, have up to ~40 μg of dissolved P L−1 (Martyniuk et al., 2014; Miserendino et al., 2018). It is not clear, however, how bioavailable particulate P released from bedrock weathering is though very small grain sizes of P can be readily consumed by microbial life (Smith et al., 1977; Brahney et al., 2015a). Generally, high concentrations of DOC and N but limited P drive correspondingly high ratios of C:P and N:P in glacier-fed streams (Rinke et al., 2001; Robinson et al., 2016; Figure 4).
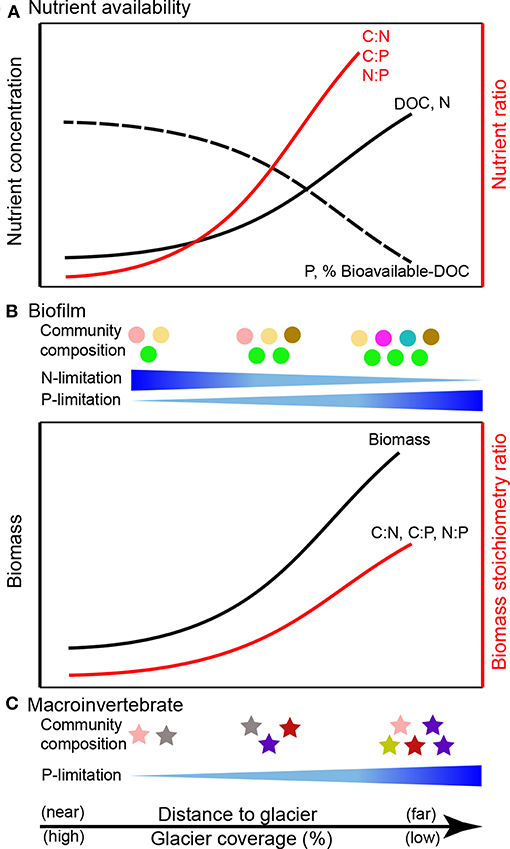
Figure 4. Predicted consequences of glacier retreat for ecological stoichiometry in glacier-fed streams: (A) nutrient availability (modified from Milner et al., 2017), (B) biofilms (predicted following Sterner and Elser, 2002; Frost et al., 2005; Kohler et al., 2011), and (C) macroinvertebrates (predicted following Jacobsen et al., 2014). DOC, dissolved organic carbon.
Trophic Interactions
Glacier-fed stream biofilms are generally limited by both N and P (Figure 4), but they exhibit stoichiometric flexibility and are capable of producing biomass with high C:P and N:P, which can in turn drive P limitation of consumers (e.g., macroinvertebrates, fish; Figure 4). Experimental addition of P increases microbial richness in glacier-fed streams (Kohler et al., 2016). Combined N and P additions stimulate biofilm primary production and shift microbial community composition (Robinson et al., 2003; Kohler et al., 2016; Figure 4). However, hydrological characteristics (e.g., flow and proportion of glacier influence) may override the influence of nutrient additions in some areas, such as the Swiss Alps (Rinke et al., 2001) and the Tian Shan Mountains of central Asia (Ren et al., 2017b). Other physical factors (e.g., suspended solids) can enhance C fixation by algae, thereby increasing C:P and lowering food quality (Martyniuk et al., 2014). In light-limited streams with high suspended solids, this reduction in food quality (e.g., higher biofilm C:nutrient) may consequently lead to elemental imbalances between primary producers and invertebrate consumers (Martyniuk et al., 2019).
Glacier-Fed Lakes
Nutrients
In glacierized regions, mountain lakes are typically fed either directly by glacier meltwater or indirectly by glacier-fed streams. Either way, glaciers drive lake ecosystem processes through the regulation of nutrients, temperature, and contaminant inputs (Saros et al., 2010; Slemmons and Saros, 2012; Rose et al., 2014). However, unlike nearby streams, mountain lakes are more likely to be fed by multiple hydrological sources than streams. To this end, an important distinction must be made between meltwater from glaciers and perennial snowfields. Glacier-derived meltwater stems from a permanent, moving body of ice (i.e., the glacier) and is often rich with suspended sediments and may reflect longer term accumulation of nutrients (e.g., N from atmospheric deposition). Perennial snowfields encompass any other permanent bodies of snow. In general, glacier-derived meltwater exhibits higher nitrate concentrations than snowmelt (Hodson, 2006; Wynn et al., 2007; Saros et al., 2010), which in turn leads to more nitrate in primarily glacier-fed vs. snow-fed lakes (Saros et al., 2010; Slemmons and Saros, 2012; Williams et al., 2016; Warner et al., 2017). For example, Williams et al. (2016) reported average nitrate concentrations of 13 μg L−1 in glacier-fed lakes vs. 5 μg L−1 in snow-fed lakes of the northern Rocky Mountains, USA. In the southern Rocky Mountains, nitrate concentrations are remarkably higher in glacier-fed lakes (> 50 μg L−1) compared to nearby snow-fed lakes (< 15 μg L−1; Saros et al., 2010; Slemmons and Saros, 2012). In western North America, rock and debris-covered glaciers are an additional, generally overlooked, resource subsidy to headwater aquatic ecosystems. In addition to providing another source of meltwater, rock glacier outflows are substantially higher in total N and other solutes vs. surface glaciers (Fegel et al., 2016).
In the absence of significant anthropogenic N inputs (pollution and atmospheric deposition) and meltwater input from glaciers, mountain lakes are typically low in N (Bergstrom and Jansson, 2006; Elser et al., 2009). Phytoplankton in mountain lakes are thus generally N-limited and increased N input can stimulate their growth and easily shift them to P-limitation (Elser et al., 2009, 2010). Meltwater from glaciers and snowfields provide additional N to mountain lakes, alleviating N-limitation and escalating P-limitation of phytoplankton. In addition to providing a source of N (Saros et al., 2010; Slemmons and Saros, 2012; Williams et al., 2016), glacier meltwater carries large amounts of suspended P-rich silt and clay, which is mainly generated by comminution (i.e., grinding) of the underlying bedrock, and supplies important, albeit less bioavailable, P to glacier-fed lakes (Hodson et al., 2004, 2005). In practice, nutrient limitation experiments have demonstrated that phytoplankton communities are limited by P in glacier-fed lakes while co-limitation of P and N occurs in snow-fed lakes (Slemmons and Saros, 2012). While total phytoplankton biomass is not consistently higher in glacier- vs. snow-fed lakes (Saros et al., 2010; Slemmons and Saros, 2012), phytoplankton communities in glacier-fed lakes tend to be more similar to one another than their snow-fed counterparts (Warner et al., 2017). At higher trophic levels, heterotrophic production appears primarily limited by P in glacier-fed lakes (Mindl et al., 2007).
Suspended sediments in glacial meltwater affect the optical properties of the lake water column by intensifying light attenuation via absorption and reflectance (Gallegos et al., 2008; Laspoumaderes et al., 2013; Sommaruga, 2015), inducing a shallower photosynthetic zone, reduced photosynthetically active radiation (PAR), and ultimately reduced photosynthetic rates (Modenutti et al., 2000; Rose et al., 2014; Figure 5). According to the light:nutrient hypothesis, the variation of PAR intensity and phosphorus availability (light:P) determine phytoplankton stoichiometry (Sterner et al., 1997; Huisman et al., 2004). Under high light:P, phytoplankton are severely P-limited and exhibit high biomass C:P, while low light:P results in low biomass C:P. The light:nutrient hypothesis is supported in glacier-fed lakes. Phytoplankton tend to have low C:P in turbid water (higher contribution of glacier meltwater) while high C:P ratios are associated with clearer water (i.e., less contribution of glacier meltwater; Laspoumaderes et al., 2013, 2017; Figure 5).
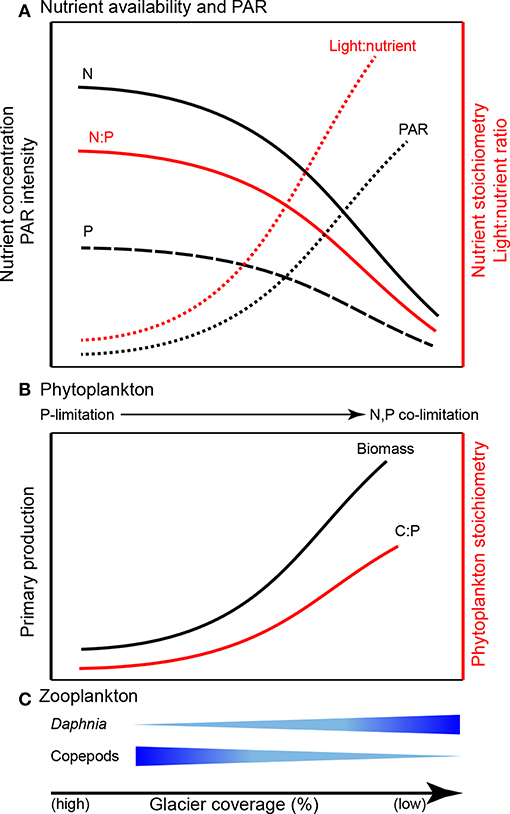
Figure 5. Predicted consequences of glacier retreat for ecological stoichiometry in glacier-fed lakes: (A) nutrient availability and PAR (photosynthetically active radiation; synthesized from Modenutti et al., 2000; Hodson et al., 2004, 2005; Piwosz et al., 2009; Saros et al., 2010; Slemmons and Saros, 2012; Rose et al., 2014; Salerno et al., 2016), (B) phytoplankton (synthesized from Sterner et al., 1997; Huisman et al., 2004; Mindl et al., 2007; Lami et al., 2010; Slemmons and Saros, 2012; Laspoumaderes et al., 2013, 2017), and (C) zooplankton (synthesized from Elser et al., 1996; Sterner and Elser, 2002; Urabe et al., 2002; Laspoumaderes et al., 2013, 2017; Sommaruga, 2015).
Trophic Interactions
Both nutrient availability and physical characteristics of glacier-fed lakes drive trophic interactions (Figure 5). Nutrient availability ultimately governs phytoplankton C:P (an indicator of food quality for zooplankton) and zooplankton taxonomic groups differ significantly in their energetic P-demand which results in decoupled community responses to altered food quality (Elser et al., 1996; Elser and Urabe, 1999; Sterner and Elser, 2002; Urabe et al., 2002). For example, Daphnia require relatively high P food sources and exhibit consistent, low C:P body content. In contrast, some copepods exhibit more variable, but generally high C:P ratios. Thus, with declining food quality (high C:P), P-rich species are less competitive than P-poor species (Sterner and Elser, 2002). Consequently, as glaciers recede, increasing phytoplankton C:P could alter grazer communities by increasing the abundance of P-poor copepods (e.g., Boeckella gracillipes, Sommaruga, 2015; Laspoumaderes et al., 2017) but decreasing P-rich Daphnia (e.g., Daphia commutate; Laspoumaderes et al., 2013, 2017). Temperature also affects food quality and may intensify P limitation in P-rich consumers of glacier-fed lakes (Laspoumaderes et al., 2013). According to the stoichiometric growth rate hypothesis, high temperature accelerates consumer growth rates, leading to elevated demand for P in ribosomal RNA to support rapid growth (Main et al., 1997; Acharya et al., 2004). However, the growth rate hypothesis has not been tested in glacier-fed lakes. Although an important component of lake ecosystems (Vadeboncoeur et al., 2002), sediment stoichiometry and benthic communities are also largely overlooked (but see Lepori and Robin, 2014; Oleksy, 2019). The framework of ecological stoichiometry provides a means for testing these hypotheses to develop new understanding of how nutrient availability, elemental ratios, and light availability alter ecosystem structure and function in glacier-fed lakes (e.g., the predictions shown in Figure 5).
Climate Change Implications
Climate change is dramatically altering mountain landscapes. At high elevations, atmospheric temperatures are rising up to three times more quickly than the global average (Nogués-Bravo et al., 2007). Rapid warming is driving the most obvious physical change in mountain ecosystems worldwide: the ongoing recession of glaciers and perennial snowfields (e.g., Zemp et al., 2015). Considerable attention has been devoted to understanding the effects of cryosphere decline on the ecology of mountain ecosystems, and this work has been summarized in recent syntheses and reviews, focused on streams (Hotaling et al., 2017b), lakes (Moser et al., 2019), and terrestrial plants (Vitasse et al., 2018). Generally, the story is clear; climate-induced recession of the mountain cryosphere will alter hydrological regimes, nutrient fluxes, and aquatic biogeochemistry (Huss et al., 2017) and induce global biodiversity loss across taxonomic scales from genetic diversity (e.g., Finn et al., 2013; Jordan et al., 2016) to species (e.g., Giersch et al., 2017) and communities (e.g., Fell et al., 2018; Hotaling et al., 2019a). Efforts to predict biodiversity loss in response to a fading cryosphere, while valuable, represent an end-member focus (e.g., the loss of a species) which overlooks an intermediary discussion of how factors that underlie biotic responses (e.g., nutrient limitations) may be affected.
As we seek to predict how the ecological stoichiometry of cryosphere-influenced headwater lakes and streams will change, additional factors must be considered. First, even though atmospheric temperatures are rising more quickly at high elevations than almost anywhere on Earth (Nogués-Bravo et al., 2007), mountain lakes may actually be warming more slowly than those in low elevations (Christianson et al., 2019). The same is likely also true for headwater streams. This lag in aquatic temperature change as they relate to atmospheric temperatures is likely due to buffering from dwindling glaciers and perennial snowfields (Zhang et al., 2014; Zemp et al., 2015). However, this buffering effect may soon be lost, and in combination with earlier melting of snowpack and longer ice-free stretches in headwaters (Preston et al., 2016), increased productivity in glacier-influenced lakes and streams is almost sure to follow. Second, nutrient deposition legacies and spatial variation in deposition rates matter. Globally, deposition rates of N, P, and dust are geographically variable (Mahowald et al., 2008) and are known to alter aquatic stoichiometry even in remote locations (Mladenov et al., 2011; Brahney et al., 2015b). For instance, historical N deposition leads to P limitation in lakes (Elser et al., 2009), thus N deposition legacies must be considered when trying to anticipate how N:P stoichiometry might change.
Nutrients
Warming temperatures, and altered precipitation regimes (e.g., more winter precipitation falling as rain rather than snow, Knowles et al., 2006), will directly influence the supraglacial zone and lead to declines in glacier volume, shorter periods of seasonal snow cover, and increased outflows in the near-term that will dwindle as ice sources fade (Huss and Hock, 2018). A longer “growing season” for microbial communities in the supraglacial zone paired with rising atmospheric CO2, which will stimulate snow algae growth (Hamilton and Havig, 2018; Figure 1), may yield a concurrent increase in autochthonous organic C production by primary producers (e.g., algae and cyanobacteria). Such a rise in primary production is likely to escalate existing glacial-melt feedback loop by decreasing surface ice albedo (e.g., Ganey et al., 2017). While N deposition rates are decreasing or stabilizing in some regions (Engardt et al., 2017; Yu et al., 2019), N emissions are rising globally and resulting deposition will increase N subsidies in remote mountainous areas with reactive N (Holtgrieve et al., 2011; Battye et al., 2017; Milner et al., 2017). In general, DOC and N concentrations will increase in downstream environments as glaciers recede due to ice-locked pools of both nutrients being released while P concentrations will decline as the loss of ice mass reduces the erosive power of the glacier (Hood and Scott, 2008; Hood and Berner, 2009; Hood et al., 2015; Figure 4).
In the short term, increased N and DOC inputs from glacial meltwaters may alleviate N limitation of primary producers and heterotrophic microorganisms in biofilms, increasing gross primary production and ecosystem respiration in glacier-fed streams (Uehlinger et al., 2010; Cauvy-Fraunié et al., 2016; Kohler et al., 2016; Figure 4). However, while DOC in glacier-fed streams may initially increase due to accelerated glacier melting, the lability (i.e., potential rate of turnover) will substantially decrease as the proportion of ice-locked DOC declines relative to less labile terrestrially derived DOC (Singer et al., 2012; Milner et al., 2017; Hemingway et al., 2019). Glacier-fed lakes and streams should continue to be P-limited in the near term (Figures 4, 5); however, as glaciers completely recede, downstream environments may become co-limited by both N and P (Saros et al., 2010; Slemmons and Saros, 2012; Figures 4, 5). Ultimately, it is clear that future climate conditions will favor increased in situ C fixation in the mountain cryosphere. Assuming no corresponding increases in N or P, via atmospheric deposition or otherwise, supraglacial communities will become increasingly N- and P-limited as climate change proceeds. However, heterogeneity in N and P deposition rates make general predictions difficult.
Changes in downstream ecosystems induced by atmospheric warming will not be solely dependent on upstream deglaciation. Indeed, warmer temperatures will increase chemical weathering of bedrock minerals (e.g., calcite and apatite) which will subsequently alter water chemistry, particularly via increased N and P (Heath and Baron, 2014; Price et al., 2017). Rock glaciers, which are likely to be less affected by atmospheric warming and will therefore persist on the landscape longer than their surface counterparts (Knight et al., 2019), release solute-rich outflows into headwaters and labile C that can fuel heterotrophic production (Fegel et al., 2016, 2019). Thus, the ratio of surface:rock glacier meltwater in headwaters is likely to decline with solute concentrations in available water shifting toward levels typical of rock glaciers occurring simultaneously.
As climate change proceeds, treeline dynamics will also alter ecological stoichiometry in mountain headwaters (Martyniuk et al., 2016). Generally speaking, lakes and streams surrounded by terrestrial vegetation have higher water column C:P than those above treeline (Stenzel et al., 2017). In addition to changing quality and quantity of C melting from glaciers, lakes above treeline are typically net autotrophic, however, as treelines advance, they will become more influenced by terrestrial allochthonous inputs (Rose et al., 2015). This will fundamentally alter how C is cycled, since presently DOM is mainly of autochthonous origin, which drives a greater proportion of highly labile, easily respired organic C (Kortelainen et al., 2013). Shifts in DOC quality coupled with climate-driven warming may therefore alter algal-bacterial interactions, which will ultimately cascade through the trophic food web and shift community compositions (González-Olalla et al., 2018). Thus, as treeline encroachment occurs, C and P inputs will increase for lakes and streams (Kopàček et al., 2011), however, a corresponding increase in N uptake potential will occur in the surrounding landscape which will influence the amount of N export.
Trophic Interactions
Changing nutrient dynamics in the supraglacial zone under climate warming have the potential to alter linked habitats through changes in the amount and quality of nutrients being exported. Through the biology-albedo feedback loop described earlier, an increase in microbial growth may induce both elevated C fixation on the glacier surface and greater export of nutrients to the subglacial zone as well as glacier-fed streams and lakes. However, temporal dynamics of microbial communities on glaciers and snowfields—and particularly the interplay between primary producers (e.g., cyanobacteria) and consumers (e.g., fungi, invertebrates)—are largely unknown. Evidence from cryoconite hole bacterial communities of the Italian Alps suggests that early season melting gives rise to a wave of primary productivity (e.g., cyanobacteria) which seeds a later season heterotrophic community (Pittino et al., 2018). The ratio of producers to consumers throughout the melt season, and specifically that of resource production and consumption, paired with potential albedo variation among groups (e.g., if snow algae reduce albedo to a greater degree than fungi), are important avenues for future study. This is particularly true as we seek to move from observational studies of supraglacial biological activity to a more predictive framework that also includes the ecological stoichiometry of adjacent downstream habitats.
With limited knowledge of biological and chemical diversity of subglacial habitats on global scales, including the degree to which they are nutrient-limited in space and time, it is difficult to make stoichiometric predictions of their future. As discussed above, climate change will drive an increase in meltwater flowing from the surface to the subglacial zone with a concomitant increase in nutrients (particularly organic C) in tow. However, given that subglacial communities appear N-limited (Boyd et al., 2011), an increase in available C may not dramatically alter existing stoichiometric ratios in these habitats. Furthermore, in the short-term, glacial sediment loads will increase, leading to increases in algal biomass and trends toward increased C:P ratios and thus lower food quality for higher trophic levels (Martyniuk et al., 2014). From a hydrological perspective, extended melt seasons at the glacier surface may translate to more stable, and open, flow paths within the glacier, perhaps including a reduction in anoxic conditions at the glacier-bedrock interface and elevated potential for direct nutrient transport. Because so little is known of trophic ecology at higher levels (e.g., ice worms, birds) on mountain glaciers, it is also difficult to predict how their interactions will be altered in the future. However, for high-elevation birds (e.g., Gray-crowned Rosy finches) which appear to derive substantial food resources from ice worms (S.H., pers. obs.), loss of glacier habitat may induce a major diet shift across portions of their range, fundamentally altering their present-day stoichiometry.
Conclusions and Future Directions
Although frozen environments place severe constraints on life, the mountain cryosphere harbors complex, dynamic biological communities which often experience some degree of nutrient limitation. In this review, we synthesized the ecological stoichiometry of three major components of the mountain cryosphere—supraglacial, subglacial, and adjacent downstream habitats—which vary in their nutrient inputs and availability. These habitats have received varying levels of research attention to date.
Habitats
Supraglacial environments are generally nutrient-limited however the vast majority of our understanding of these habitats stems from continental ice sheets (e.g., Greenland, Antarctica), which differ from mountain glaciers in many ways, including geomorphology, nutrient availability (Figure 2), and biological diversity (e.g., presence of higher trophic levels). Chemical and biological differences between mountain glaciers and continental ice sheets may be due in large part to differing proximity to terrestrial and anthropogenic nutrient sources (e.g., N deposition). Moreover, chemical contrasts between mountain glaciers and continental ice sheets suggest that the former cannot simply serve as an analog for the latter, and that mountain glaciers must be treated as unique components of the global cryosphere. However, given the spatial variation of samples included in our analysis, we consider our conclusions to be intriguing but largely preliminary as unforeseen biases (e.g., in sampling locations) may exist in the data being compared.
Below mountain glaciers in the subglacial zone, much less is known of ecological stoichiometry beyond a handful of studies (e.g., Boyd et al., 2011) which point to high water C:N driving N limitation in sediment communities. However, due to sampling challenges (i.e., extremely difficult access), the nature of biogeochemical cycling, the general rules governing subglacial life, and the nutrient limitations on subglacial food webs are largely unknown. Similarly, the englacial environment—i.e., the portion of the glacier below the supraglacial but above the subglacial—remains a black box with presumably little to no biodiversity present (Hotaling et al., 2017a). However, future efforts to generate ice cores in mountain glaciers from the supraglacial zone to bedrock would provide an excellent opportunity to explore patterns of diversity, nutrient availability, and stoichiometry along a spatial continuum in these enigmatic habitats. This type of effort may also provide new insights on a challenge that has long puzzled glacier biogeochemists: why is N export from glaciers high even in regions with relatively low rates of N deposition (Slemmons et al., 2013)?
Like supraglacial environments, the ecological stoichiometry of glacier-fed lakes and streams has received relatively intense study. As the most influential factor mediating biological processes in these habitats, it is perhaps unsurprising that glacier coverage largely drives nutrient availability, elemental ratios, and light availability. However, the biological and biogeochemical connections between upstream (supraglacial and subglacial) and downstream environments remain unclear, with an overwhelming stoichiometric focus on the effects of N loads and turbidity. To this end, future efforts should focus on the relative ratios of N:P, overall ecosystem function, and temperature-nutrient interactions, as these avenues are likely to be particularly important to understanding algal community structure and productivity (Oleksy, 2019).
Looking Ahead
Glaciers and perennial snowfields will continue to disappear in the decades to come (Zemp et al., 2015), particularly at low latitudes (Hall and Fagre, 2003). As the mountain cryosphere fades, the entire biome they support including most, if not all, of the existing genetic, species, and stoichiometric diversity will also be lost. However, while some habitats will be lost entirely (e.g., supraglacial and subglacial), others will persist in fundamentally altered form (e.g., high-elevation lakes). In the short-term, glacier recession will generally lead to elevated levels of C and N, and reduced P, in headwaters. Eventually, however, downstream environments will likely transition to co-limitation by N and P as the large store of N currently present in mountain glaciers is depleted.
In this review, we identified gaps in stoichiometric knowledge of the mountain cryosphere, as well as what appear to be key, systemic differences in nutrient concentrations between mountain glaciers and continental ice sheets (Figure 2). These differences are important because they highlight the need for targeted studies of mountain glacier ecosystems since for the most part, they indicate that patterns of ecological stoichiometry in mountains cannot be inferred from existing ice sheet-focused research efforts. Furthermore, given the potential for substantial variation in nutrient concentrations in mountain cryosphere-influenced habitats (Figure 3), more comparative studies that explicitly integrate spatial and temporal sampling will shed light on this variation and refine understanding of the mechanism(s) underlying it. Multi-trophic stoichiometric perspectives are also needed. For instance, in North America, it is currently unknown how nutrient dynamics that shape elemental ratios at the microbial scale translate to heterotrophic fungi, macroinvertebrate consumers (e.g., ice worms), and ultimately birds. Finally, refined understanding of ecological stoichiometry in the mountain cryosphere can also improve general understanding of stoichiometric principles. Indeed, with nutrient limitations that vary in space and time paired with dramatic differences in turbidity and light availability across aquatic habitats, the mountain cryosphere and the habitats it directly influences provide a natural laboratory for testing fundamental stoichiometric hypotheses at environmental extremes.
Author Contributions
ZR conceived of the review. ZR, NM, IO, AS, and SH wrote the manuscript. All authors read and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, EH, declared a shared affiliation, though no collaboration, with one of the authors, IO, to the handling Editor.
Acknowledgments
This manuscript stemmed from the fourth Woodstoich meeting at Flathead Lake Biological Station (FLBS) and was supported by the National Science Foundation (#DEB-1840408). We thank FLBS staff for their logistical support. Trinity Hamilton, Jen Schweitzer, and three reviewers provided feedback that improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00360/full#supplementary-material
References
Acharya, K., Kyle, M., and Elser, J. J. (2004). Effects of stoichiometric dietary mixing on Daphnia growth and reproduction. Oecologia 138, 333–340. doi: 10.1007/s00442-003-1444-8
Anderson, N. J., Saros, J. E., Bullard, J. E., Cahoon, S. M., McGowan, S., Bagshaw, E. A., et al. (2017). The Arctic in the twenty-first century: changing biogeochemical linkages across a paraglacial landscape of Greenland. BioScience 67, 118–133. doi: 10.1093/biosci/biw158
Anderson, W. B., and Polis, G. A. (1999). Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118, 324–332. doi: 10.1007/s004420050733
Anesio, A. M., Hodson, A. J., Fritz, A., Psenner, R., and Sattler, B. (2009). High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biol. 15, 955–960. doi: 10.1111/j.1365-2486.2008.01758.x
Anesio, A. M., and Laybourn-Parry, J. (2012). Glaciers and ice sheets as a biome. Trends Ecol. Evol. 27, 219–225. doi: 10.1016/j.tree.2011.09.012
Anesio, A. M., Lutz, S., Chrismas, N., and Benning, L. G. (2017). The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes 3, 1–10. doi: 10.1038/s41522-017-0019-0
Anesio, A. M., Sattler, B., Foreman, C., Telling, J., Hodson, A., Tranter, M., et al. (2010). Carbon fluxes through bacterial communities on glacier surfaces. Ann. Glaciol. 51, 32–40. doi: 10.3189/172756411795932092
Antony, R., Willoughby, A. S., Grannas, A. M., Catanzano, V., Sleighter, R. L., Thamban, M., et al. (2017). Molecular insights on dissolved organic matter transformation by supraglacial microbial communities. Environ. Sci. Technol. 51, 4328–4337. doi: 10.1021/acs.est.6b05780
Baron, J. S., Schmidt, T. M., and Hartman, M. D. (2009). Climate-induced changes in high elevation stream nitrate dynamics. Glob. Change Biol. 15, 1777–1789. doi: 10.1111/j.1365-2486.2009.01847.x
Battye, W., Aneja, V. P., and Schlesinger, W. H. (2017). Is nitrogen the next carbon? Earth's Future 5, 894–904. doi: 10.1002/2017EF000592
Bergstrom, A. K., and Jansson, M. (2006). Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob. Change Biol. 12, 635–643. doi: 10.1111/j.1365-2486.2006.01129.x
Boetius, A., Anesio, A. M., Deming, J. W., Mikucki, J. A., and Rapp, J. Z. (2015). Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat. Rev. Microbiol. 13, 677–790. doi: 10.1038/nrmicro3522
Boothroyd, I., and Cranston, P. (1999). The'ice worm'-the immature stages, phylogeny and biology of the glacier midge Zelandochlus (Diptera: Chironomidae). Aquat. Insect. 21, 303–316. doi: 10.1076/aqin.21.4.303.4513
Boyd, E. S., Hamilton, T. L., Havig, J. R., Skidmore, M. L., and Shock, E. L. (2014). Chemolithotrophic primary production in a subglacial ecosystem. Appl. Environ. Microb. 80, 6146–6153. doi: 10.1128/AEM.01956-14
Boyd, E. S., Lange, R. K., Mitchell, A. C., Havig, J. R., Hamilton, T. L., Lafreniere, M. J., et al. (2011). Diversity, abundance, and potential activity of nitrifying and nitrate-reducing microbial assemblages in a subglacial ecosystem. Appl. Environ. Microb. 77, 4778–4787. doi: 10.1128/AEM.00376-11
Boyd, E. S., Skidmore, M., Mitchell, A. C., Bakermans, C., and Peters, J. W. (2010). Methanogenesis in subglacial sediments. Env. Microbiol. Rep. 2, 685–692. doi: 10.1111/j.1758-2229.2010.00162.x
Brahney, J., Ballantyne, A. P., Kociolek, P., Leavitt, P. R., Farmer, G. L., and Neff, J. C. (2015a). Ecological changes in two contrasting lakes associated with human activity and dust transport in western Wyoming. Limnol. Oceanogr. 60, 678–695. doi: 10.1002/lno.10050
Brahney, J., Mahowald, N., Ward, D. S., Ballantyne, A. P., and Neff, J. C. (2015b). Is atmospheric phosphorus pollution altering global alpine Lake stoichiometry? Glob. Biogeochem. 29, 1369–1383. doi: 10.1002/2015GB005137
Brittain, J. E., and Milner, A. M. (2001). Ecology of glacier-fed rivers: current status and concepts. Freshw. Biol. 46, 1571–1578. doi: 10.1046/j.1365-2427.2001.00845.x
Capps, D. M., Rabus, B., Clague, J. J., and Shugar, D. H. (2010). Identification and characterization of alpine subglacial lakes using interferometric synthetic aperture radar (InSAR): Brady Glacier, Alaska, USA. J. Glaciol. 56, 861–870. doi: 10.3189/002214310794457254
Cauvy-Fraunié, S., Andino, P., Espinosa, R., Calvez, R., Jacobsen, D., and Dangles, O. (2016). Ecological responses to experimental glacier-runoff reduction in alpine rivers. Nat. Commun. 7:e12025. doi: 10.1038/ncomms12025
Christianson, K. R., Johnson, B. M., Hooten, M. B., and Roberts, J. J. (2019). Estimating lake-climate responses from sparse data: an application to high elevation lakes. Limnol. Oceanogr. 64, 1371–1385. doi: 10.1002/lno.11121
Christner, B. C., Priscu, J. C., Achberger, A. M., Barbante, C., Carter, S. P., Christianson, K., et al. (2014). A microbial ecosystem beneath the West Antarctic ice sheet. Nature 512, 310–313. doi: 10.1038/nature13667
Colombo, N., Salerno, F., Martin, M., Malandrino, M., Giardino, M., Serra, E., et al. (2019). Influence of permafrost, rock and ice glaciers on chemistry of high-elevation ponds (NW Italian Alps). Sci. Total Environ. 685, 886–901. doi: 10.1016/j.scitotenv.2019.06.233
Cross, W. F., Benstead, J. P., Frost, P. C., and Thomas, S. A. (2005). Ecological stoichiometry in freshwater benthic systems: recent progress and perspectives. Freshw. Biol. 50, 1895–1912. doi: 10.1111/j.1365-2427.2005.01458.x
De Smet, W. H., and Van Rompu, E. (1994). Rotifera and tardigrada from some cryoconite holes on a Spitsbergen (Svalbard) glacier. Belg. J. Zool. 124, 27–27.
Dodds, W. K., and Whiles, M. R. (2010). Freshwater Ecology: Concepts and Environmental Applications of Limnology, 2nd Edn. Cambridge, MA: Academic press. doi: 10.1016/B978-0-12-374724-2.00024-6
Edwards, A., Mur, L. A., Girdwood, S. E., Anesio, A. M., Stibal, M., Rassner, S. M., et al. (2014). Coupled cryoconite ecosystem structure–function relationships are revealed by comparing bacterial communities in alpine and Arctic glaciers. FEMS Microbiol. Ecol. 89, 222–237. doi: 10.1111/1574-6941.12283
Edwards, J. S. (1987). Arthropods of alpine aeolian ecosystems. Annu. Rev. Entomol. 32, 163–179. doi: 10.1146/annurev.en.32.010187.001115
Elser, J. J., Andersen, T., Baron, J. S., Bergstroem, A., Jansson, M., Kyle, M., et al. (2009). Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326, 835–837. doi: 10.1126/science.1176199
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x
Elser, J. J., Dobberfuhl, D. R., MacKay, N. A., and Schampel, J. H. (1996). Organism size, life history, and N:P stoichiometry. BioScience 46, 674–684. doi: 10.2307/1312897
Elser, J. J., Peace, A. L., Kyle, M., Wojewodzic, M., McCrackin, M. L., Andersen, T., et al. (2010). Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol. Lett. 13, 1256–1261. doi: 10.1111/j.1461-0248.2010.01519.x
Elser, J. J., and Urabe, J. (1999). The stoichiometry of consumer-driven nutrient recycling: theory, observations, and consequences. Ecology 80, 735–751. doi: 10.1890/0012-9658(1999)080[0735:TSOCDN]2.0.CO;2
Engardt, M., Simpson, D., Schwikowski, M., and Granat, L. (2017). Deposition of sulphur and nitrogen in Europe 1900–2050. Model calculations and comparison to historical observations. Tellus B 69:e1328945. doi: 10.1080/16000889.2017.1328945
Fegel, T., Boot, C. M., Broeckling, C. D., Baron, J. S., and Hall, E. K. (2019). Assessing the chemistry and bioavailability of dissolved organic matter from glaciers and rock glaciers. J. Geophys. Res. 124, 1988–2004. doi: 10.1029/2018JG004874
Fegel, T. S., Baron, J. S., Fountain, A. G., Johnson, G. F., and Hall, E. K. (2016). The differing biogeochemical and microbial signatures of glaciers and rock glaciers. J. Geophys. Res. 121, 919–932. doi: 10.1002/2015JG003236
Fell, S. C., Carrivick, J. L., and Brown, L. E. (2017). The multitrophic effects of climate change and glacier retreat in mountain rivers. BioScience 67, 897–911. doi: 10.1093/biosci/bix107
Fell, S. C., Carrivick, J. L., Kelly, M. G., Füreder, L., and Brown, L. E. (2018). Declining glacier cover threatens the biodiversity of alpine river diatom assemblages. Glob. Change Biol. 24, 5828–5840. doi: 10.1111/gcb.14454
Fellman, J. B., Hood, E., Raymond, P. A., Hudson, J., Bozeman, M., and Arimitsu, M. (2015). Evidence for the assimilation of ancient glacier organic carbon in a proglacial stream food web. Limnol. Oceanogr. 60, 1118–1128. doi: 10.1002/lno.10088
Finn, D. S., Khamis, K., and Milner, A. M. (2013). Loss of small glaciers will diminish beta diversity in Pyrenean streams at two levels of biological organization. Glob. Ecol. Biogeogr. 22, 40–51. doi: 10.1111/j.1466-8238.2012.00766.x
Fountain, A. G., Jacobel, R. W., Schlichting, R., and Jansson, P. (2005). Fractures as the main pathways of water flow in temperate glaciers. Nature 433, 618–621. doi: 10.1038/nature03296
Fountain, A. G., Tranter, M., Nylen, T. H., Lewis, K. J., and Mueller, D. R. (2004). Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. J. Glaciol. 50, 35–45. doi: 10.3189/172756504781830312
Frost, P. C., Hillebrand, H., and Kahlert, M. (2005). Low algal carbon content and its effect on the C:P stoichiometry of periphyton. Freshwater Biol. 50, 1800–1807. doi: 10.1111/j.1365-2427.2005.01449.x
Gallegos, C., Davies-Colley, R., and Gall, M. (2008). Optical closure in lakes with contrasting extremes of reflectance. Limnol. Oceanogr. 53, 2021–2034. doi: 10.4319/lo.2008.53.5.2021
Ganey, G. Q., Loso, M. G., Burgess, A. B., and Dial, R. J. (2017). The role of microbes in snowmelt and radiative forcing on an Alaskan icefield. Nat. Geosci. 10, 745–759. doi: 10.1038/ngeo3027
Giersch, J. J., Hotaling, S., Kovach, R. P., Jones, L. A., and Muhlfeld, C. C. (2017). Climate-induced glacier and snow loss imperils alpine stream insects. Glob. Change Biol. 23, 2577–2589. doi: 10.1111/gcb.13565
Godoy, R., Paulino, L., Oyarzún, C., and Boeckx, P. (2003). Atmospheric N deposition in central and southern Chile. An overview. Gayana Botanica 60, 47–53. doi: 10.4067/S0717-66432003000100008
Godwin, C. M., and Cotner, J. B. (2015). Stoichiometric flexibility in diverse aquatic heterotrophic bacteria is coupled to differences in cellular phosphorus quotas. Front. Microbiol. 6:159. doi: 10.3389/fmicb.2015.00159
González-Olalla, J. M., Medina-Sánchez, J. M., Lozano, I. L., Villar-Argaiz, M., and Carrillo, P. (2018). Climate-driven shifts in algal-bacterial interaction of high-mountain lakes in two years spanning a decade. Sci. Rep. 8:e10278. doi: 10.1038/s41598-018-28543-2
Grzesiak, J., Gorniak, D., Swiatecki, A., Aleksandrzak-Piekarczyk, T., Szatraj, K., and Zdanowski, M. K. (2015). Microbial community development on the surface of Hans and Werenskiold Glaciers (Svalbard, Arctic): a comparison. Extremophiles 19, 885–897. doi: 10.1007/s00792-015-0764-z
Hall, M. H., and Fagre, D. B. (2003). Modeled climate-induced glacier change in Glacier National Park, 1850–2100. BioScience 53, 131–140. doi: 10.1641/0006-3568(2003)053[0131:MCIGCI]2.0.CO;2
Hamilton, T. L., and Havig, J. R. (2018). Inorganic carbon addition stimulates snow algae primary productivity. ISME J. 29. doi: 10.1038/s41396-018-0048-6
Hamilton, T. L., Peters, J. W., Skidmore, M. L., and Boyd, E. S. (2013). Molecular evidence for an active endogenous microbiome beneath glacial ice. ISME J. 7, 1402–12. doi: 10.1038/ismej.2013.31
Havig, J. R., and Hamilton, T. L. (2019). Snow algae drive productivity and weathering at volcanic rock-hosted glaciers. Geochim. Cosmochim. 247, 220–242. doi: 10.1016/j.gca.2018.12.024
Hawkings, J., Wadham, J., Tranter, M., Telling, J., Bagshaw, E., Beaton, A., et al. (2016). The greenland ice sheet as a hot spot of phosphorus weathering and export in the Arctic. Glob. Biogeochem. 30, 191–210. doi: 10.1002/2015GB005237
Heath, J., and Baron, J. S. (2014). Climate, not atmospheric deposition, drives the biogeochemical mass-balance of a mountain watershed. Aquat. Geochem. 20, 167–181. doi: 10.1007/s10498-013-9199-2
Hemingway, J. D., Spencer, R. G. M., Podgorski, D. C., Zito, P., Sen, I. S., and Galy, V. V. (2019). Glacier meltwater and monsoon precipitation drive Upper Ganges Basin dissolved organic matter composition. Geochim. Cosmochim. 244, 216–228. doi: 10.1016/j.gca.2018.10.012
Hodson, A. (2006). Biogeochemistry of snowmelt in an Antarctic glacial ecosystem. Water Resour. Res. 42:W11406. doi: 10.1029/2005WR004311
Hodson, A., Anesio, A. M., Tranter, M., Fountain, A., Osborn, M., Priscu, J., et al. (2008). Glacial ecosystems. Ecol. Monogr. 78, 41–67. doi: 10.1890/07-0187.1
Hodson, A., Mumford, P., and Lister, D. (2004). Suspended sediment and phosphorus in proglacial rivers: bioavailability and potential impacts upon the P status of ice-marginal receiving waters. Hydrol. Process. 18, 2409–2422. doi: 10.1002/hyp.1471
Hodson, A., Roberts, T. J., Engvall, A., Holmén, K., and Mumford, P. (2010). Glacier ecosystem response to episodic nitrogen enrichment in Svalbard, European High Arctic. Biogeochemistry 98, 171–184. doi: 10.1007/s10533-009-9384-y
Hodson, A. J., Mumford, P. N., Kohler, J., and Wynn, P. M. (2005). The high arctic glacial ecosystem: new insights from nutrient budgets. Biogeochemistry 72, 233–256. doi: 10.1007/s10533-004-0362-0
Holtgrieve, G. W., Schindler, D. E., Hobbs, W. O., Leavitt, P. R., Ward, E. J., Bunting, L., et al. (2011). A coherent signature of anthropogenic nitrogen deposition to remote watersheds of the northern hemisphere. Science 334, 1545–1548. doi: 10.1126/science.1212267
Hood, E., Battin, T. J., Fellman, J., O'Neel, S., and Spencer, R. G. M. (2015). Storage and release of organic carbon from glaciers and ice sheets. Nat. Geosci. 8, 91–96. doi: 10.1038/ngeo2331
Hood, E., and Berner, L. (2009). Effects of changing glacial coverage on the physical and biogeochemical properties of coastal streams in southeastern Alaska. J. Geophys. Res. 114:G03001. doi: 10.1029/2009JG000971
Hood, E., Fellman, J., Spencer, R. G., Hernes, P. J., Edwards, R., D'Amore, D., et al. (2009). Glaciers as a source of ancient and labile organic matter to the marine environment. Nature 462, 1044–1047. doi: 10.1038/nature08580
Hood, E., and Scott, D. (2008). Riverine organic matter and nutrients in southeast Alaska affected by glacial coverage. Nat. Geosci. 1, 583–587. doi: 10.1038/ngeo280
Hotaling, S., Finn, D. S., Giersch, J. J., Weisrock, D. W., and Jacobsen, D. (2017b). Climate change and alpine stream biology: progress, challenges, and opportunities for the future. Biol. Rev. 92, 2024–2045. doi: 10.1111/brv.12319
Hotaling, S., Foley, M. E., Zeglin, L. H., Finn, D. S., Tronstad, L. M., Giersch, J. J., et al. (2019a). Microbial assemblages reflect environmental heterogeneity in alpine streams. Glob. Change Biol. 25, 2576–2590. doi: 10.1111/gcb.14683
Hotaling, S., Hood, E., and Hamilton, T. L. (2017a). Microbial ecology of mountain glacier ecosystems: biodiversity, ecological connections, and implications of a warming climate. Environ. Microbiol. 19, 2935–2948. doi: 10.1111/1462-2920.13766
Hotaling, S., Shain, D. H., Lang, S. A., Bagley, R. K., Tronstad, L. M., Weisrock, D. W., et al. (2019b). Long-distance dispersal, ice sheet dynamics and mountaintop isolation underlie the genetic structure of glacier ice worms. Proc. R. Soc. 286:20190983. doi: 10.1098/rspb.2019.0983
Huisman, J., Sharples, J., Stroom, J. M., Visser, P. M., Kardinaal, W. E. A., Verspagen, J. M., et al. (2004). Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960–2970. doi: 10.1890/03-0763
Huss, M., Bookhagen, B., Huggel, C., Jacobsen, D., Bradley, R. S., Clague, J. J., et al. (2017). Toward mountains without permanent snow and ice. Earth's Future 5, 418–435. doi: 10.1002/2016EF000514
Huss, M., and Hock, R. (2018). Global-scale hydrological response to future glacier mass loss. Nat. Clim. Change 8, 135–140. doi: 10.1038/s41558-017-0049-x
Ingall, E. D., Bustin, R. M., and Van Cappellen, P. (1993). Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. 57, 303–316. doi: 10.1016/0016-7037(93)90433-W
Jacobsen, D., Cauvy-Fraunie, S., Andino, P., Espinosa, R., Cueva, D., and Dangles, O. (2014). Runoff and the longitudinal distribution of macroinvertebrates in a glacier-fed stream: implications for the effects of global warming. Freshw. Biol. 59, 2038–2050. doi: 10.1111/fwb.12405
Jain, S. (2014). “Glaciers,” in Fundamentals of Physical Geology, eds P. Smithson, K. Addison, and K. Atkinson (New Delhi: Springer, 241–262.
Jansson, J. K., and Tas, N. (2014). The microbial ecology of permafrost. Nat. Rev. Microbiol. 12, 414–425. doi: 10.1038/nrmicro3262
Jordan, S., Giersch, J. J., Muhlfeld, C. C., Hotaling, S., Fanning, L., Tappenbeck, T. H., et al. (2016). Loss of genetic diversity and increased subdivision in an endemic alpine stonefly threatened by climate change. PLoS ONE 11:e0157386. doi: 10.1371/journal.pone.0157386
Kikuchi, Y. (1994). Glaciella, a new genus of freshwater Canthocamptidae (Copepoda, Harpacticoida) from a glacier in Nepal, Himalayas. Hydrobiologia 292, 59–66. doi: 10.1007/BF00229923
Knight, J., Harrison, S., and Jones, D. B. (2019). Rock glaciers and the geomorphological evolution of deglacierizing mountains. Geomorphology 324, 14–24. doi: 10.1016/j.geomorph.2018.09.020
Knowles, N., Dettinger, M. D., and Cayan, D. R. (2006). Trends in snowfall versus rainfall in the western United States. J. Climate 19, 4545–4559. doi: 10.1175/JCLI3850.1
Kohler, T. J., Murdock, J. N., Gido, K. B., and Dodds, W. K. (2011). Nutrient loading and grazing by the minnow Phoxinus erythrogaster shift periphyton abundance and stoichiometry in mesocosms. Freshwater Biol. 56, 1133–1146. doi: 10.1111/j.1365-2427.2010.02557.x
Kohler, T. J., Van Horn, D. J., Darling, J. P., Takacs-Vesbach, C. D., and McKnight, D. M. (2016). Nutrient treatments alter microbial mat colonization in two glacial meltwater streams from the McMurdo Dry Valleys, Antarctica. FEMS Microbiol. Ecol. 92:fiw049. doi: 10.1093/femsec/fiw049
Kohshima, S. (1984). A novel cold-tolerant insect found in a Himalayan glacier. Nature 310, 225–227. doi: 10.1038/310225a0
Kopàček, J., Hejzlar, J., Vrba, J., and Stuchlík, E. (2011). Phosphorus loading of mountain lakes: terrestrial export and atmospheric deposition. Limnol. Oceanogr. 56, 1343–1354. doi: 10.4319/lo.2011.56.4.1343
Körner, C., Jetz, W., Paulsen, J., Payne, D., Rudmann-Maurer, K., and Spehn, E. M. (2017). A global inventory of mountains for bio-geographical applications. Alpine Bot. 127, 1–15. doi: 10.1007/s00035-016-0182-6
Kortelainen, P., Rantkari, M., Pajunen, H., Huttunen, J. T., Mattsson, T., Juutinen, S., et al. (2013). Carbon evasion/accumulation ratio in boreal lakes is linked to nitrogen. Global Biogeochem. 27, 363–374. doi: 10.1002/gbc.20036
Lami, A., Turner, S., Musazzi, S., Gerli, S., Guilizzoni, P., Rose, N. L., et al. (2010). Sedimentary evidence for recent increases in production in Tibetan plateau lakes. Hydrobiologia 648, 175–187. doi: 10.1007/s10750-010-0263-2
Laspoumaderes, C., Modenutti, B., Sol Souza, M., Bastidas Navarro, M., Cuassolo, F., and Balseiro, E. (2013). Glacier melting and stoichiometric implications for lake community structure: zooplankton species distributions across a natural light gradient. Glob. Change Biol. 19, 316–326. doi: 10.1111/gcb.12040
Laspoumaderes, C., Sol Souza, M., Modenutti, B., and Balseiro, E. (2017). Glacier melting and response of Daphnia oxidative stress. J. Plankton Res. 39, 675–686. doi: 10.1093/plankt/fbx028
Lepori, F., and Robin, J. (2014). Nitrogen limitation of the phytobenthos in Alpine lakes: results from nutrient-diffusing substrata. Freshw. Biol. 59, 1633–1645. doi: 10.1111/fwb.12370
Liang, Y. (1979). A new genus and species of Enchytraeidae from Tibet. Acta Zootaxonom. Sinica 4, 312–315.
Lyon, S., Destouni, G., Giesler, R., Humborg, C., Mörth, C., Seibert, J., et al. (2009). Estimation of permafrost thawing rates in a sub-arctic catchment using recession flow analysis. Hydrol. Earth Syst. Sc. 13, 595–604. doi: 10.5194/hess-13-595-2009
Mahowald, N., Jickells, T. D., Baker, A. R., Artaxo, P., Benitez Nelson, C. R., Bergametti, G., et al. (2008). Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem. Cy. 22:GB4026. doi: 10.1029/2008GB003240
Main, T. M., Dobberfuhl, D. R., and Elser, J. J. (1997). N:P stoichiometry and ontogeny of crustacean zooplankton: a test of the growth rate hypothesis. Limnol. Oceanogr. 42, 1474–1478. doi: 10.4319/lo.1997.42.6.1474
Malard, F., Tockner, K., and Ward, J. (2000). Physico-chemical heterogeneity in a glacial riverscape. Landscape Ecol. 15, 679–695. doi: 10.1023/A:1008147419478
Martyniuk, N., Modenutti, B., and Balseiro, E. (2014). Can increased glacial melting resulting from global change provide attached algae with transient protection against high irradiance? Freshw. Biol. 59, 2290–2302. doi: 10.1111/fwb.12431
Martyniuk, N., Modenutti, B., and Balseiro, E. (2016) Forest structure affects the stoichiometry of periphyton primary producers in mountain streams of northern Patagonia. Ecosystems. 19, 1225–1239. doi: 10.1007/s10021-016-9996-8
Martyniuk, N., Modenutti, B., and Balseiro, E. (2019). Light intensity regulates stoichiometry of benthic grazers through changes in the quality of stream periphyton. Freshw. Sci. 38, 391–405. doi: 10.1086/703441
Masiokas, M. H., Villalba, R., Luckman, B. H., Lascano, M. E., Delgado, S., and Stepanek, P. (2008). 20th-century glacier recession and regional hydroclimatic changes in northwestern Patagonia. Global Planet. Change 60, 85–100. doi: 10.1016/j.gloplacha.2006.07.031
Milner, A. M., Khamis, K., Battin, T. J., Brittain, J. E., Barrand, N. E., Fuereder, L., et al. (2017). Glacier shrinkage driving global changes in downstream systems. Proc. Natl. Acad. Sci. U.S.A. 114, 9770–9778. doi: 10.1073/pnas.1619807114
Mindl, B., Anesio, A. M., Meirer, K., Hodson, A. J., Laybourn-Parry, J., Sommaruga, R., et al. (2007). Factors influencing bacterial dynamics along a transect from supraglacial runoff to proglacial lakes of a high Arctic glacieri. FEMS Microbiol. Ecol. 59, 307–317. doi: 10.1111/j.1574-6941.2006.00262.x
Miserendino, M. L., Brand, C., Epele, L. B., Di Prinzio, C. Y., Omad, G. H., Archangelsky, M., et al. (2018). Biotic diversity of benthic macroinvertebrates at contrasting glacier-fed systems in Patagonia Mountains: the role of environmental heterogeneity facing global warming. Sci. Total Environ. 622, 152–163. doi: 10.1016/j.scitotenv.2017.11.320
Mladenov, N., Sommaruga, R., Morales-Baquero, R., Laurion, I., Camarero, L., Dieguez, M. C., et al. (2011). Dust inputs and bacteria influence dissolved organic matter in clear alpine lakes. Nat. Commun. 2:405. doi: 10.1038/ncomms1411
Modenutti, B., Bastidas Navarro, M., Martyniuk, N., and Balseiro, E. (2018). Melting of clean and debris-rich ice differentially affect nutrients, dissolved organic matter and bacteria respiration in the early ontogeny of the newly formed proglacial Ventisquero Negro Lake (Patagonia Argentina). Freshw. Biol. 63, 1341–1351. doi: 10.1111/fwb.13161
Modenutti, B., Perez, G., Balseiro, E., and Queimalinos, C. (2000). The relationship between light attenuation, chlorophyll a and total suspended solids in a Southern Andes glacial lake. Int. Verein. Theoret. Angew. Limnol. 27, 2648–2651. doi: 10.1080/03680770.1998.11898147
Morgan-Kiss, R. M., Priscu, J. C., Pocock, T., Gudynaite-Savitch, L., and Huner, N. P. (2006). Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. 70, 222–252. doi: 10.1128/MMBR.70.1.222-252.2006
Moser, K. A., Baron, J. S., Brahney, J., Oleksy, I. A., Saros, J. E., Hundey, E. J., et al. (2019). Mountain lakes: eyes on global environmental change. Glob. Planet. Change 178, 77–95. doi: 10.1016/j.gloplacha.2019.04.001
Mueller, D. R., Vincent, W. F., Pollard, W. H., and Fritsen, C. H. (2001). Glacial cryoconite ecosystems: a bipolar comparison of algal communities and habitats. Nova Hedw. Beiheft 123, 173–198.
Murakami, T., Segawa, T., Bodington, D., Dial, R., Takeuchi, N., Kohshima, S., et al. (2015). Census of bacterial microbiota associated with the glacier ice worm Mesenchytraeus solifugus. FEMS Microbiol. Ecol. 91:iv003. doi: 10.1093/femsec/fiv003
Murakami, T., Segawa, T., Takeuchi, N., Sepulveda, G. B., Labarca, P., Kohshima, S., et al. (2018). Metagenomic analyses highlight the symbiotic association between the glacier stonefly Andiperla willinki and its bacterial gut community. Environ. Microbiol. 20, 4170–4183. doi: 10.1111/1462-2920.14420
Musilova, M., Tranter, M., Wadham, J., Telling, J., Tedstone, A., and Anesio, A. M. (2017). Microbially driven export of labile organic carbon from the Greenland ice sheet. Nat. Geosci. 10, 360–365. doi: 10.1038/ngeo2920
Nogués-Bravo, D., Araújo, M. B., Errea, M. P., and Martinez-Rica, J. P. (2007). Exposure of global mountain systems to climate warming during the 21st Century. Glob. Environ. Change 17, 420–428. doi: 10.1016/j.gloenvcha.2006.11.007
Notz, D., and Stroeve, J. (2016). Observed Arctic sea-ice loss directly follows anthropogenic CO2 emission. Science 354, 747–750. doi: 10.1126/science.aag2345
NRSP-3 (2019). National Atmospheric Deposition Program. Madison, WI: NADP Program Office, Wisconsin State Laboratory of Hygiene.
Oleksy, I. A. (2019). Algal Blooms in the Alpine: Investigating the Coupled Effects of Warming and Nutrient Deposition on Mountain Lakes (Doctoral Dissertation). Colorado State University, Fort Collins, CO. Available from ProQuest Dissertations and Theses database.
Pittino, F., Maglio, M., Gandolfi, I., Azzoni, R. S., Diolaiuti, G., Ambrosini, R., et al. (2018). Bacterial communities of cryoconite holes of a temperate alpine glacier show both seasonal trends and year-to-year variability. Ann. Glaciol. 59, 1–9. doi: 10.1017/aog.2018.16
Piwosz, K., Walkusz, W., Hapter, R., Wieczorek, P., Hop, H., and Wiktor, J. (2009). Comparison of productivity and phytoplankton in a warm (Kongsfjorden) and a cold (Hornsund) Spitsbergen fjord in mid-summer 2002. Polar Biol. 32, 549–559. doi: 10.1007/s00300-008-0549-2
Preston, D. L., Caine, N., McKnight, D. M., Williams, M. W., Hell, K., Miller, M. P., et al. (2016). Climate regulates alpine lake ice cover phenology and aquatic ecosystem structure. Geophys. Res. Lett. 43, 5353–5360. doi: 10.1002/2016GL069036
Price, J. R., Moore, J., and Kerans, D. (2017). Monazite chemical weathering, rare earth element behavior, and paleoglaciohydrology since the last glacial maximum for the Loch Vale watershed, Colorado, USA. Quat. Res. 87, 191–207. doi: 10.1017/qua.2016.16
Pritchard, H. D. (2017). Asia's glaciers are a regionally important buffer against drought. Nature 545, 169–174. doi: 10.1038/nature22062
Ren, Z., and Gao, H. K. (2019). Ecological networks reveal contrasting patterns of bacterial and fungal communities in glacier-fed streams in Central Asia. Peer J. 7:e7715. doi: 10.7287/peerj.preprints.27887
Ren, Z., Gao, H. K., and Elser, J. J. (2017b). Longitudinal variation of microbial communities in benthic biofilms and association with hydrological and physicochemical conditions in glacier-fed streams. Freshw. Sci. 36, 479–490. doi: 10.1086/693133
Ren, Z., Gao, H. K., Elser, J. J., and Zhao, Q. D. (2017a). Microbial functional genes elucidate environmental drivers of biofilm metabolism in glacier-fed streams. Sci. Rep. 7:e12668. doi: 10.1038/s41598-017-13086-9
Rinke, K., Robinson, C. T., and Uehlinger, U. (2001). A note on abiotic factors that constrain periphyton growth in alpine glacier streams. Int. Rev. Hydrobiol. 86, 361–366. doi: 10.1002/1522-2632(200106)86:3<361::AID-IROH361>3.0.CO;2-Z
Robinson, C. T., Tonolla, D., Imhof, B., Vukelic, R., and Uehlinger, U. (2016). Flow intermittency, physico-chemistry and function of headwater streams in an Alpine glacial catchment. Aquat. Sci. 78, 327–341. doi: 10.1007/s00027-015-0434-3
Robinson, C. T., Uehlinger, U., and Gessner, M. O. (2003). “Nutrient Limitation,” in Ecology of a Glacial Flood Plain, eds J. V. Ward and U. Uehlinger (Springer, Netherlands), 231–241. doi: 10.1007/978-94-017-0181-5_14
Roe, G. H., Baker, M. B., and Herla, F. (2017). Centennial glacier retreat as categorical evidence of regional climate change. Nat. Geosci. 10, 95–99. doi: 10.1038/ngeo2863
Rose, K. C., Hamilton, D. P., Williamson, C. E., McBride, C. G., Fischer, J. M., Olson, M. H., et al. (2014). Light attenuation characteristics of glacially-fed lakes. J. Geophys. Res. 119, 1446–1457. doi: 10.1002/2014JG002674
Rose, K. C., Williamson, C. E., Kissman, C. E., and Saros, J. E. (2015). Does allochthony in lakes change across an elevation gradient? Ecology 96, 3281–3291. doi: 10.1890/14-1558.1
Rosvold, J. (2016). Perennial ice and snow-covered land as important ecosystems for birds and mammals. J. Biogeogr. 43, 3–12. doi: 10.1111/jbi.12609
Salerno, F., Rogora, M., Balestrini, R., Lami, A., Tartari, G. A., Thakuri, S., et al. (2016). Glacier melting increases the solute concentrations of Himalayan glacial lakes. Environ. Sci. Technol. 50, 9150–9160. doi: 10.1021/acs.est.6b02735
Saros, J. E., Rose, K. C., Clow, D. W., Stephens, V. C., Nurse, A. B., Arnett, H. A., et al. (2010). Melting alpine glaciers enrich high-elevation lakes with reactive nitrogen. Environ. Sci. Technol. 44, 4891–4896. doi: 10.1021/es100147j
Säwström, C., Anesio, M. A., Granéli, W., and Laybourn-Parry, J. (2007a). Seasonal viral loop dynamics in two large ultraoligotrophic Antarctic Freshwater Lakes. Microb. Ecol. 53, 1–11. doi: 10.1007/s00248-006-9146-5
Säwström, C., Laybourn-Parry, J., Granéli, W., and Anesio, A. M. (2007b). Heterotrophic bacterial and viral dynamics in Arctic freshwaters: results from a field study and nutrient-temperature manipulation experiments. Polar Biol. 30, 1407–1415. doi: 10.1007/s00300-007-0301-3
Shain, D. H., Halldórsdóttir, K., Pálsson, F., Aðalgeirsdóttir, G., Gunnarsson, A., Jónsson, Þ., et al. (2016). Colonization of maritime glacier ice by bdelloid Rotifera. Mol. Phylogenet. Evol. 98, 280–287. doi: 10.1016/j.ympev.2016.02.020
Singer, G. A., Fasching, C., Wilhelm, L., Niggemann, J., Steier, P., Dittmar, T., et al. (2012). Biogeochemically diverse organic matter in Alpine glaciers and its downstream fate. Nat. Geosci. 5, 710–714. doi: 10.1038/ngeo1581
Skiles, S. M., Flanner, M., Cook, J. M., Dumont, M., and Painter, T. H. (2018). Radiative forcing by light-absorbing particles in snow. Nat. Clim. Change 8, 964–971. doi: 10.1038/s41558-018-0296-5
Slemmons, K. E. H., and Saros, J. E. (2012). Implications of nitrogen-rich glacial meltwater for phytoplankton diversity and productivity in alpine lakes. Limnol. Oceanogr. 57, 1651–1663. doi: 10.4319/lo.2012.57.6.1651
Slemmons, K. E. H., Saros, J. E., and Simon, K. (2013). The influence of glacial meltwater on alpine aquatic ecosystems: a review. Environ. Sci. Proc. Imp. 15, 1794–1806. doi: 10.1039/C3EM00243H
Smith, E. A., Mayfield, C. I., and Wong, P. T. S. (1977). Naturally occurring apatite as a source of orthophosphate for growth of bacteria and algae. Microb. Ecol. 4, 105–117. doi: 10.1007/BF02014281
Sommaruga, R. (2015). When glaciers and ice sheets melt: consequences for planktonic organisms. J. Plankton Res. 37, 509–518. doi: 10.1093/plankt/fbv027
Stenzel, B., Rofner, C., Pérez, M. T., and Sommaruga, R. (2017). Stoichiometry of natural bacterial assemblages from lakes located across an elevational gradient. Sci. Rep. 7:5875. doi: 10.1038/s41598-017-06282-0
Sterner, R. W., and Elser, J. J. (2002). Ecological Stoichiometry: the Biology of Elements From Molecules To The Biosphere. Princeton, NJ: Princeton University Press. doi: 10.1515/9781400885695
Sterner, R. W., Elser, J. J., Fee, E. J., Guildford, S. J., and Chrzanowski, T. H. (1997). The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am. Nat. 150, 663–684. doi: 10.1086/286088
Stibal, M., Anesio, A. M., Blues, C. J. D., and Tranter, M. (2009). Phosphatase activity and organic phosphorus turnover on a high Arctic glacier. Biogeosciences 6, 913–922. doi: 10.5194/bg-6-913-2009
Stibal, M., Šabacka, M., and Žárský, J. (2012). Biological processes on glacier and ice sheet surfaces. Nat. Geosci. 5, 771–774. doi: 10.1038/ngeo1611
Stibal, M., Tranter, M., Telling, J., and Benning, L. G. (2008). Speciation, phase association and potential bioavailability of phosphorus on a Svalbard glacier. Biogeochemistry 90, 1–13. doi: 10.1007/s10533-008-9226-3
Stubbins, A., Hood, E., Raymond, P. A., Aiken, G. R., Sleighter, R. L., Hernes, P. J., et al. (2012). Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers. Nat. Geosci. 5, 198–201. doi: 10.1038/ngeo1403
Talalay, P., Fan, X., Xu, H., Yu, D., Han, L., Han, J., et al. (2014). Drilling fluid technology in ice sheets: hydrostatic pressure and borehole closure considerations. Cold Reg. Sci. Technol. 98, 47–54. doi: 10.1016/j.coldregions.2013.10.012
Telling, J., Anesio, A. M., Tranter, M., Irvine-Fynn, T., Hodson, A., Butler, C., et al. (2011). Nitrogen fixation on Arctic glaciers, Svalbard. J. Geophys. Res. 116:G03039. doi: 10.1029/2010JG001632
Telling, J., Boyd, E. S., Bone, N., Jones, E. L., Tranter, M., MacFarlane, J. W., et al. (2015). Rock comminution as a source of hydrogen for subglacial ecosystems. Nat. Geosci. 8, 851–855. doi: 10.1038/ngeo2533
Tranter, M., Brown, G., Raiswell, R., Sharp, M., and Gurnell, A. (1993). A conceptual model of solute acquisition by Alpine glacial meltwaters. J. Glaciol. 39, 573–581. doi: 10.1017/S0022143000016464
Tranter, M., Fountain, A. G., Fritsen, C. H., Berry Lyons, W., Priscu, J. C., Statham, P. J., et al. (2004). Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrol. Process. 18, 379–387. doi: 10.1002/hyp.5217
Tynen, M. J. (1970). The geographical distribution of ice worms (O1igochaeta: Enchytraeidae). Can. J. Zool. 48, 1363–1367. doi: 10.1139/z70-233
Uehlinger, U., Robinson, C. T., Hieber, M., and Zah, R. (2010). The physico-chemical habitat template for periphyton in alpine glacial streams under a changing climate. Hydrobiologia 657, 107–121. doi: 10.1007/s10750-009-9963-x
Urabe, J., Elser, J. J., Kyle, M., Yoshida, T., Sekino, T., and Kawabata, Z. (2002). Herbivorous animals can mitigate unfavourable ratios of energy and material supplies by enhancing nutrient recycling. Ecol. Lett. 5, 177–185. doi: 10.1046/j.1461-0248.2002.00303.x
Vadeboncoeur, Y., Vander Zanden, M. J., and Lodge, D. M. (2002). Putting the lake back together: reintegrating benthic pathways into lake food web models. BioScience 52, 44–54. doi: 10.1641/0006-3568(2002)052[0044:PTLBTR]2.0.CO;2
Vitasse, Y., Signarbieux, C., and Fu, Y. H. (2018). Global warming leads to more uniform spring phenology across elevations. Proc. Natl. Acad. Sci. U.S.A. 115, 1004–1008. doi: 10.1073/pnas.1717342115
Vizzini, S., Signa, G., and Mazzola, A. (2016). Guano-derived nutrient subsidies drive food web structure in coastal ponds. PLoS ONE 11:e0151018. doi: 10.1371/journal.pone.0151018
Wadham, J. L., Bottrell, S., Tranter, M., and Raiswell, R. (2004). Stable isotope evidence for microbial sulphate reduction at the bed of a polythermal high Arctic glacier. Earth Planet. Sc. Lett. 219, 341–355. doi: 10.1016/S0012-821X(03)00683-6
Wadham, J. L., Hawkings, J., Telling, J., Chandler, D., Alcock, J., O'Donnell, E., et al. (2016). Sources, cycling and export of nitrogen on the Greenland Ice Sheet. Biogeosci. 13, 6339–6352. doi: 10.5194/bg-13-6339-2016
Wadham, J. L., Tranter, M., Skidmore, M., Hodson, A. J., Priscu, J., Lyons, W. B., et al. (2010). Biogeochemical weathering under ice: Size matters. Global Biogeochem. Cy. 24:GB3025. doi: 10.1029/2009GB003688
Warner, K. A., Saros, J. E., and Simon, K. S. (2017). Nitrogen subsidies in glacial meltwater: implications for high elevation aquatic chains. Water Resour. Res. 53, 9791–9806. doi: 10.1002/2016WR020096
Wilhelm, L., Singer, G. A., Fasching, C., Battin, T. J., and Besemer, K. (2013). Microbial biodiversity in glacier-fed streams. ISME J. 7, 1651–1660. doi: 10.1038/ismej.2013.44
Williams, J. J., Nurse, A., Saros, J. E., Riedel, J., and Beutel, M. (2016). Effects of glaciers on nutrient concentrations and phytoplankton in lakes within the Northern Cascades Mountains (USA). Biogeochemistry 131, 373–385. doi: 10.1007/s10533-016-0264-y
Williamson, C. J., Cameron, K. A., Cook, J. M., Zarsky, J. D., Stibal, M., and Edwards, A. (2019). Glacier algae: a dark past and a darker future. Front. Microbiol. 10:e524. doi: 10.3389/fmicb.2019.00524
Wynn, P. M., Hodson, A. J., Heaton, T. H. E., and Chenery, S. R. (2007). Nitrate production beneath a High Arctic Glacier, Svalbard. Chem. Geol. 244, 88–102. doi: 10.1016/j.chemgeo.2007.06.008
Yallop, M. L., Anesio, A. M., Perkins, R. G., Cook, J., Telling, J., Fagan, D., et al. (2012). Photophysiology and albedo-changing potential of the ice algal community on the surface of the Greenland ice sheet. ISME J. 6, 2302–2313. doi: 10.1038/ismej.2012.107
Yu, G., Jia, Y., He, N., Zhu, J., Chen, Z., Wang, Q., et al. (2019). Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 12, 424–429. doi: 10.1038/s41561-019-0352-4
Zah, R., Burgherr, P., Bernasconi, S. M., and Uehlinger, U. (2001). Stable isotope analysis of macroinvertebrates and their food sources in a glacier stream. Freshw. Biol. 46, 871–882. doi: 10.1046/j.1365-2427.2001.00720.x
Zawierucha, K., Kolicka, M., Takeuchi, N., and Kaczmarek, L. (2015). What animals can live in cryoconite holes? A faunal review. J. Zool. 295, 159–169. doi: 10.1111/jzo.12195
Zemp, M., Frey, H., Gaertner-Roer, I., Nussbaumer, S. U., Hoelzle, M., Paul, F., et al. (2015). Historically unprecedented global glacier decline in the early 21st century. J. Glaciol. 61, 745–762. doi: 10.3189/2015JoG15J017
Keywords: alpine, glacier biology, nutrient dynamics, C:N, supraglacial, subglacial, glacier-fed lakes, glacier-fed streams
Citation: Ren Z, Martyniuk N, Oleksy IA, Swain A and Hotaling S (2019) Ecological Stoichiometry of the Mountain Cryosphere. Front. Ecol. Evol. 7:360. doi: 10.3389/fevo.2019.00360
Received: 22 July 2019; Accepted: 11 September 2019;
Published: 26 September 2019.
Edited by:
Michelle Evans-White, University of Arkansas, United StatesReviewed by:
Rana El-Sabaawi, University of Victoria, CanadaEdward Hall, Colorado State University, United States
James Cotner, University of Minnesota Twin Cities, United States
Copyright © 2019 Ren, Martyniuk, Oleksy, Swain and Hotaling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott Hotaling, c2NvdHQuaG90YWxpbmdAd3N1LmVkdQ==
†These authors have contributed equally to this work
‡Present address: Isabella A. Oleksy, Cary Institute of Ecosystem Studies, Millbrook, NY, United States
§ORCID: Ze Ren orcid.org/0000-0002-1473-2697
Nicolas Martyniuk orcid.org/0000-0002-2423-7040
Isabella A. Oleksy orcid.org/0000-0003-2572-5457
Anshuman Swain orcid.org/0000-0002-9180-2222
Scott Hotaling orcid.org/0000-0002-5965-0986
 Ze Ren
Ze Ren Nicolas Martyniuk
Nicolas Martyniuk Isabella A. Oleksy
Isabella A. Oleksy Anshuman Swain
Anshuman Swain Scott Hotaling
Scott Hotaling