
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Ecol. Evol. , 08 August 2019
Sec. Behavioral and Evolutionary Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00295
This article is part of the Research Topic How Enemies Shape Communication Systems: Sensory Strategies of Prey to Avoid Eavesdropping Predators and Parasites View all 13 articles
In sexual reproduction, the search for mating partners elevates the individual's risks of predation and parasitism. One way to increase mate search effectiveness and reduce search costs is acoustic signaling. However, acoustic orienting parasitoid flies exploit singing hosts, leading to high parasitism rates. Aggregations of males and females at mating and singing in choruses might reduce individual risks by dilution and predator saturation. This mini-review reflects on consequences for host's acoustic signaling in choruses using the examples of cicadas and bushcrickets. It concludes that despite antagonistic selection pressure by parasitoids, singing in choruses might select for increased, not reduced signaling in males. The time joining and leaving a chorus might be crucial: once mated, a refractory period will drop males off the signaling pool, preventing parasitism. In a chorus, fast and loud singing might be highly advantageous, supporting the fittest males. Natural selection might have shaped signaling strategies in choruses, which can probably only be understood when applying individual based dynamic modeling.
Mating requires the finding of a partner and the search can expose participants to increased risk of predation, and parasitism (Andersson, 1994). Many animals have, therefore, evolved signaling as a strategy to increase the effectiveness, and simultaneously reduce search costs (Greenfield, 2002). Acoustic communication is a very effective way of sending information and is employed by different insect taxa (Gerhardt and Huber, 2002; Strauß and Lakes-Harlan, 2014). The diversity of potential acoustic options allows for the selection of very specific signals that can create private channels, interfering little with other broadcasters and receivers. Despite the uniqueness of acoustic signals, some parasitoid flies have evolved sensory systems to break into the private communication channel of their hosts and become unintended signal exploiters (Zuk and Kolluru, 1998). Acoustically hunting flies belong to the Tachinidae and Sarcophagidae, with only a few specialized taxa in both fly families. Interestingly, the flies hunt for sound producing hosts from two distinct host taxa: flies of the tribus Ormiini (Family Tachinidae) parasitize singing crickets and bushcrickets (Orthoptera: Ensifera; Lehmann, 2003), while flies of the tribus Emblemasomatini (Family Sarcophagidae) attack cicadas (Homoptera; Lakes-Harlan and Lehmann, 2015). This clear separation of host taxa indicates at least two independent evolutionary processes, each with different adaptations. Neuroanatomy and neurophysiology underline the separate evolution of the hearing sense for host detection (Lakes-Harlan and Heller, 1992; Robert et al., 1992; Lakes-Harlan et al., 2007). The parasitoids are well-adapted to their hosts and are very successful acoustic hunters, resulting in high rates of parasitism (Lehmann, 2008; Lakes-Harlan and Lehmann, 2015).
Within the Ormiini, some radiation might have occurred leading to around 70 parasitoid taxa (including Ormia, Therobia, and Homotrixa) which are specialized on a single species or a limited set of crickets, mole crickets and bushcricket host species (Lehmann, 2003). The radiation could simply be caused by an availability of different host species. Alternatively, avoidance and defense adaptations of hosts to parasitoid pressure could force parasitoid species to a more general host spectrum or a switch of host species. Such adaptations could be realized by satellite males and other alternative behavior (Zuk et al., 1993) and have mainly been reported for crickets and their parasitoids, especially Ormia ochracea. Within the Emblemasomatini some parasitoids have multiple host species (Stucky, 2015), whereas the well-investigated Emblemasoma auditrix seems to be more selective as it has almost exclusively been found in the cicada Okanagana rimosa (Lakes-Harlan et al., 2000). E. auditrix shows several host specific adaptations, from ecological preferences to tuning to the auditory cue for host localization and a highly specific host infection behavior (Schniederkötter and Lakes-Harlan, 2004).
We highlight possible processes in the two independent host systems of bushcrickets and cicadas, which are convergently under the risk of parasitism yet share some similarities in the sensory exploitation. One obvious fact is that despite the high parasitism rates, host sensory counter adaptations seem to be less effective in both, the bushcrickets and the cicadas, as host individuals seldom detect approaching parasitoid flies until direct contact. Furthermore, contrary to the selection to reduce calling in some of the Ormia-cricket systems, neither the bushcrickets nor the cicadas show signaling reduction as response to the acoustic parasitoids (Lakes-Harlan and Lehmann, 2015). The two host systems share however important life-history aspects; (1) a highly synchronized occurrence of males and females (Williams and Simon, 1995; Lehmann, 2012), including an operational sex ratio close to one (Heller and von Helversen, 1991), and (2) an aggregated occurrence at mating time including chorusing (Lehmann, 1998; Stölting et al., 2004). We review what is known in these systems, and emphasize the importance of social aspects to reduce individual parasitism risk, especially of superior signaling males. Several bushcrickets species of the genus Poecilimon, especially those of the P. propinquus-group, are well-known hosts of the Ormiini Therobia leonidei (Lehmann, 2003). Because of singing, male bushcrickets are under a steady rate of attack from parasitoids, reaching up to a parasitism rate of 65% (Lehmann and Heller, 1998; Lehmann, 2008). Interestingly, bushcricket species of the genus Poecilimon hatch, and develop in a highly synchronized manner. Similarly, males show only little protandry, with females following 0.6–3 days after the males, which is extremely short in comparison to other bushcricket species (Lehmann, 2012). Consequently, a close match is found for the time to reach sexual maturation (Lehmann and Lehmann, 2008). However, this pattern of high synchronization is found in all three Poecilimon species, with one being a highly parasitized species, the second one a probably parasitized and the third one a species uninfected by the acoustic parasitoid. Hence, environmental factors like the summer drought in Mediterranean habitats depriving the herbivore bushcrickets of their food may select for such synchronization (Lehmann and Lehmann, 2006). Regardless of the ecological drivers, the Poecilimon species with unidirectional communication system of signaling males and silent females (Heller, 1984, 1992; Strauß et al., 2014) sing temporarily and locally aggregated. The aggregated males increase singing performance as response to acoustic rivals (Anichini et al., 2018), hence stimulate each other into unstructured choruses (Lehmann, 1998). Such aggregations might increase the total risk for the population but lower the per capita risk of an individual compared to singing in isolation, as found for the cricket-Ormia system (Cade, 1981). It has been shown that the acoustically orienting parasitoid O. ochracea reduce the propensity of cricket males to sing, especially in the host-parasitoid system on Hawaii (Zuk et al., 2006) where the parasitoid fly seems to be introduced rather recently and have adapted to a new host (Gray et al., 2019). Although no differences in signaling behavior were found between low-risk and high-risk populations in some north American mainland areas attacked by the same parasitoid (Beckers and Wagner, 2012) and predominant parasitism late in the season might even increase not reduce reproductive trait investment (Beckers and Wagner, 2018).
Even with a balancing selection by acoustic parasitoids (Lehmann et al., 2001), Poecilimon males increase their investment into songs depending on social environment, such as the number and fitness of competitors, expressed through song parameters (Anichini et al., 2018). Conditionally fitter males (expressed as body mass) produce not only the larger nuptial gifts (Lehmann, 2008; Lehmann and Lehmann, 2009), they also have larger morphological structures for stridulation (Anichini et al., 2017), win song contests against weaker males (Anichini et al., 2018), and are preferred by phonotactic approaching females (Lehmann and Lehmann, 2008). The attractive singers to females might face a dilemma as they are also preferred by the parasitoid flies (Lehmann et al., 2001). We do not know which individual song characters are preferred by females and flies (Lehmann et al., 2001), but in a cross-species comparison the one with the longer songs including several repeated song elements (vs.) is at a much higher risk of infection (Lehmann and Heller, 1998). At first glance this is a paradox as both, sexual and natural selection, seem to act in opposite directions on song characteristics. The solution might lie in the social aspect of a chorus: once a male has attracted a female and mated, it shows a refractory period to produce the massive spermatophore (Lehmann and Lehmann, 2000), which can make up to 30% of a male's body mass as reviewed for the genus (McCartney et al., 2008); consequently it will keep silent for a few days until it is able to remate (Heller and von Helversen, 1991; Reinhold and von Helversen, 1997; Lehmann and Lehmann, 2000). In phonotactic experiments females reach a singing male in less than a minute up to a meter apart (Lehmann and Lehmann, 2006; Lehmann, 2008). However, parasitoid flies are also able to very precisely track and localize a singing bushcricket (summarized in Lakes-Harlan and Lehmann, 2015). Therefore, quick and risky singing might be highly advantageous, if better singers are able to attract conspecific females before a parasitoid localizes them. Once mated, those males drop out of the chorus and are no target of parasitoids anymore lighter, less fit rivals in turn need to continue singing until they attract a female. Mate choice in bushcrickets is best described as a best-of-n search strategy (Lehmann, 2007; Lehmann and Lehmann, 2007); once the fittest male drops out of the chorus, the second best singer will become the favored male (Lehmann, 2007). Females will mate when giving access to a single male (Lehmann and Lehmann, 2008), even if it was the non-preferred singer in a previous phonotactic experiment (Lehmann and Lehmann, unpubl. data). Consequently, the parasitism risk for low condition singers of being parasitized might in fact be even higher when integrated over the reproductive season (Figure 1). As T. leonidei flies are rarely attracted to loudspeakers (Lehmann and Heller, 1998), we have no direct test for the duration until flies approach their hosts in the field. However, a comparison of the number of mated males per night, estimated to be around 20% (Heller and von Helversen, 1991), with the nightly parasitoid attack rate of 3–7% (Lehmann and Heller, 1998) shows the advantage of mating over parasitism. So, in a simple approximation it is three to six times more likely for a high condition male to attract a female than to be parasitized. This easily would mask the parasitism risk and could also be a pattern found in other taxa.
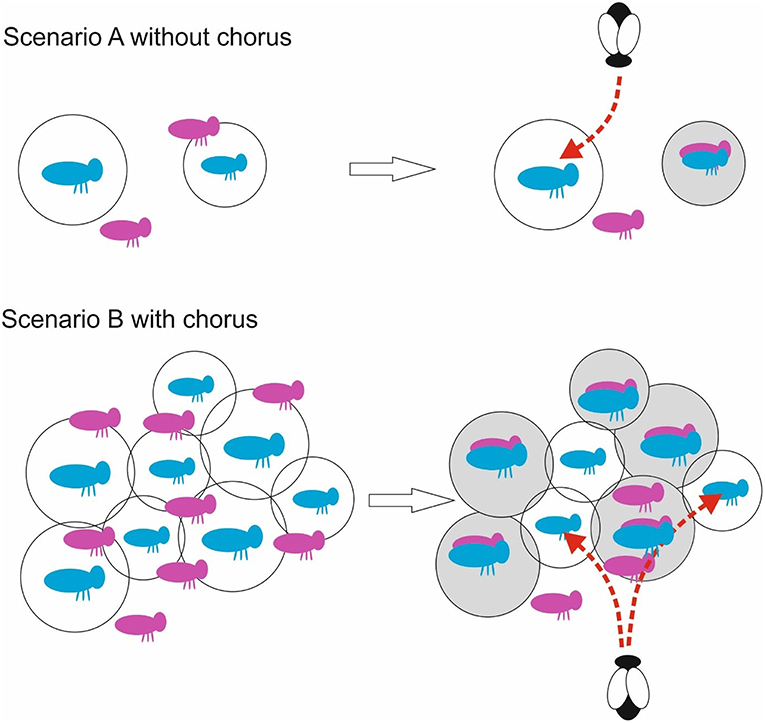
Figure 1. Schematic illustration of possible chorus effect on parasitoids. Singing males (blue) are classified by their sound propagation as superior (large radius) and inferior (smaller radius). Scenario A: In a non-chorus situation, females (pink) may have limited choices, and might mate with an inferior male nearby, due to better sound representation in her nervous system. Overlaid figures indicate mating pairs. This male then stops singing for some time (indicated by a gray circle) and cannot be detected by the acoustically hunting parasitoid. A dipteran parasitoid might then attack the next sound producing male, which might be by chance a superior individual. Scenario B: In a chorus situation males and females are densely packed. Females which are in the broadcasting range of more than one male can select a superior one. As result more superior than inferior males mate and cease song production (gray circle). The parasitoid will affect mostly inferior singers. Some females might remain unmated, as more than one choose the same male (e.g., Lehmann and Lehmann, 2007) or are not in the broadcasting range.
The cicada O. rimosa, as hosts of the fly E. auditrix, might show the similar behavioral, and social traits as the bushcrickets. It is under attack by an acoustically orienting parasitoid, possibly influencing signaling behavior. In contrast to Poecilimon not much is known about intraspecific competition and female choice. Here we highlight the commonalities and the differences in aggregation calling. Even stronger than in the bushcrickets, the cicada life cycles might have evolved under predation and/or parasitism pressure, having a multi-year life cycle with nymphal stages living several years in the ground (Williams and Simon, 1995). At the extreme end are the periodical cicadas, which emerge in large numbers every 13 or 17 years at a given location (Williams and Simon, 1995). With such prime numbered periodicity, it may be possible to reduce the pressure from predators and parasitoids (Hoppensteadt and Keller, 1976). Modeling confirmed that this prime numbered periodicity could have arisen from such processes (Goles et al., 2001). Connected to the periodical life cycle are mass emergences of adult cicadas, often resulting in large and loud choruses (Williams and Simon, 1995). Such choruses are interpreted as adaptations to predation pressure, as for example birds are saturated due to the high numbers. Chorus synchronization has been reported for the periodical cicada species Magicicada cassini (Huber et al., 1990). While not fully periodical, O. rimosa populations fluctuate in abundances from year to year making them proto-periodic (Lakes-Harlan and de Vries, 2014). The abundance fluctuations seen today might be an effective measure to reduce parasitization pressure for the cicada, as in poor cicada years the parasitoids quickly become host saturated, which reduces the parasitism risk in the following years. Like the Poecilimon bushcrickets, the cicada O. rimosa form unstructured choruses where the temporal pattern of the specific calling song is obscured (Stölting et al., 2004). Surprisingly little is known about the auditory behavior of female cicadas acting as receivers. Even simple female phonotaxis is rarely studied in cicadas (but see Doolan and Young, 1989; Daws et al., 1997), and female sexual selection less so. Nevertheless, calling is a prerequisite for O. rimosa males to attract females (Stölting et al., 2004) and this signal is similarly exploited by the parasitoid E. auditrix (Lakes-Harlan et al., 2000; Tron et al., 2015). Interestingly, the cicada choruses might distract the acoustically hunting parasitoid, as it's phonotaxis is tuned to the temporal structure of an isolated calling song (Lakes-Harlan et al., 2000). Individually calling of single males are noted either in years with low population densities, or early in the season. Such males face strong parasitism pressure, as early in season up to 80% of males have been found to be infected (Schniederkötter and Lakes-Harlan, 2004). O. rimosa does not exhibit defense strategies like wing flips or other behaviors to expel the parasitoid (Schniederkötter and Lakes-Harlan, 2004). Individual cicada males cannot reduce the risk of being attacked by a parasitoid by shortening songs, as the parasitoid can detect signals of only 1 s duration (de Vries and Lakes-Harlan, 2003). Chorusing might provide the only possibility to reduce an individual's risk of parasitism (Figure 1). The adaptation for chorus calling can be shown by experimentally broadcasting the calling song in silent cicada habitats. If males are present, they produce short calling songs triggered by the stimulus (Stölting et al., 2004), which in turn might result eventually in a chorus. The number of calls increases drastically with start of the chorus, and the number of fly larvae per female E. auditrix drops simultaneously by 50–75% (de Vries and Lakes-Harlan, 2005). Interestingly, the success rate of host infection by the parasitoid seems to slow down as the number of larvae per female fly stay constant for the next few days. This finding might be another indication of a protective character of the chorus.
Thus, the cicada chorus with its acoustically hunting parasitoid has several commonalities to the Poecilimon-Therobia system and it might serve as testing system for the hypothesis, that successful males are more protected than unsuccessful males. Therefore, we need data on the individual song characteristics of males, the female preferences, interactions within the chorus and preferences of the parasitoid. The same system might also provide the control, as the different abundances between years vary from low density populations with singly calling to high density populations with chorus.
Acoustic communication of bushcrickets and cicadas is shaped through selection of acoustic orienting parasitoids. Similar social signaling strategies evolved in the two distinct taxa, which form large choruses. Fitter males might face an advantage through the chorus by mating faster and risking fewer parasitoid attacks as they drop out of the pool of signalers. Thus, this type of sexual communication can be best understood when analyzed in a socially dynamic network, including the individual risk of -and the fitness deprivation by- parasitism. We suggest further cicada experiments to test for a mating advantage of better singers.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Anichini, M., Frommolt, K.-H., and Lehmann, G. U. C. (2018). To compete or not to compete: outcome of bushcricket song contests reveals the singing plasticity according to male condition and inter-rival distance. Anim. Behav. 142, 59–68. doi: 10.1016/j.anbehav.2018.05.022
Anichini, M., Kuchenreuther, S., and Lehmann, G. U. C. (2017). Allometry of male sound-producing structures indicates sexual selection on wing size and stridulatory teeth density in a bushcricket. J. Zool. 301, 271–279. doi: 10.1111/jzo.12419
Beckers, O. M., and Wagner, W. E. (2012). Eavesdropping parasitoids do not cause the evolution of less conspicuous signaling behavior in a field cricket. Anim. Behav. 84, 1457–1462. doi: 10.1016/j.anbehav.2012.09.016
Beckers, O. M., and Wagner, W. E. Jr. (2018). Males and females evolve riskier traits in populations with eavesdropping parasitoids. Behav. Ecol. Sociobiol. 72:174. doi: 10.1007/s00265-018-2588-1
Cade, W. H. (1981). Field cricket spacing, and the phonotaxis of crickets and parasitoid flies to clumped and isolated cricket songs. Ethology 55, 365–375. doi: 10.1111/j.1439-0310.1981.tb01278.x
Daws, A. G., Hennig, R. M., and Young, D. (1997). Phonotaxis in cicadas Cystosoma saundersii and Cyclochila australasiae. Bioacoustics 7, 173–188. doi: 10.1080/09524622.1997.9753330
de Vries, T., and Lakes-Harlan, R. (2003). “Phonotaxis to discontinuous signals”, in The Neurosciences From Basic Research to Therapy, eds N. Elsner and H. Zimmermann (Stuttgart; New York, NY: Thieme Verlag, 410–411.
de Vries, T., and Lakes-Harlan, R. (2005). Phonotaxis of the female parasitoid Emblemasoma auditrix (Diptera, Sarcophagidae) in relation to number of larvae and age. Zoology 108, 239–246. doi: 10.1016/j.zool.2005.04.004
Doolan, J. M., and Young, D. (1989). Relative importance of song parameters during flight phonotaxis and courtship in the bladder cicada Cystosoma saundersii. J. Exp. Biol. 141, 113–131.
Gerhardt, H. C., and Huber, F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: Chicago University Press.
Goles, E., Schulz, O., and Markus, M. (2001). Prime number selection of cycles in a predator-prey model. Complexity 6, 33–38. doi: 10.1002/cplx.1040
Gray, D. A., Kunerth, H. D., Zuk, M., Cade, W. H., and Balenger, S. L. (2019). Molecular biogeography and host relations of a parasitoid fly. bioRxiv:576892. doi: 10.1101/576892
Greenfield, M. (2002). Signalers and Receivers: Mechanisms and Evolution of Arthropod Communication. Oxford: Oxford University Press.
Heller, K. G. (1984). Zur Bioakustik und Phylogenie der Gattung Poecilimon (Orthoptera, Tettigoniidae, Phaneropterinae). Zool. Jb. Syst. 111, 69–117.
Heller, K. G. (1992). Risk shift between males and females in the pair-forming behavior of bushcrickets. Naturwissenschaften 79, 89–91. doi: 10.1007/BF01131812
Heller, K.-G., and von Helversen, D. (1991). Operational sex ratio and individual mating frequencies in two bushcricket species (Orthoptera, Tettigonioidea, Poecilimon). Ethology 89, 211–228. doi: 10.1111/j.1439-0310.1991.tb00305.x
Hoppensteadt, F. C., and Keller, J. B. (1976). Synchronization of periodical cicada emergences. Science 194, 335–337. doi: 10.1126/science.987617
Huber, F., Kleindienst, H.-U., Moore, T. E., Schildberger, K., and Weber, T. (1990). “Acoustic communication in periodical cicadas: neuronal responses to songs of sympatric species,” in Sensory Systems and Communication in Arthropods, eds F. G. Gribakin, K. Wiese, and A. V. Popov (Basel; Boston, MA; Berlin: Birkhäuser Verlag, 217–228.
Lakes-Harlan, R., and de Vries, T. (2014). Experimental infection of a periodical cicada (Magicicada cassinii) with a parasitoid (Emblemasoma auditrix) of a proto-periodical cicada (Okanagana rimosa). BMC Ecol. 14:31. doi: 10.1186/s12898-014-0031-7
Lakes-Harlan, R., and Heller, K.-G. (1992). Ultrasound-sensitive ears in a parasitoid fly. Naturwissenschaften 79, 224–226. doi: 10.1007/BF01227133
Lakes-Harlan, R., Jacobs, K., and Allen, G. (2007). Comparison of auditory sense organs in parasitoids Tachinidae (Diptera) hosted by Tettigoniidae (Orthoptera) and homologous structures in a non-hearing Phoridae (Diptera). Zoomorphology 126, 229–243. doi: 10.1007/s00435-007-0043-3
Lakes-Harlan, R., and Lehmann, G. U. (2015). Parasitoid flies exploiting acoustic communication of insects – comparative aspects of independent functional adaptations. J. Comp. Physiol. A 201, 123–132. doi: 10.1007/s00359-014-0958-3
Lakes-Harlan, R., Stölting, H., and Moore, T. E. (2000). Phonotactic behavior of a parasitoid (Emblemasoma auditrix, Sarcophagidae, Diptera) in response to the calling song of the host (Okanagana rimosa, Homoptera, Cicada). Zoology 103, 31–39.
Lehmann, G. U. C. (1998). Der einfluss der phonotaktischen parasitoiden fliege therobia leonidei (Tachinidae: Ormiini) auf die akustische kommunikation von laubheuschrecken (Tettigonioidea, Phaneropteridae) (Doctoral thesis). University Erlangen-Nürnberg, Erlangen, Germany.
Lehmann, G. U. C. (2003). Review of biogeography, host range and evolution of acoustic hunting in Ormiini (Insecta, Diptera, Tachinidae), parasitoids of night-calling bushcrickets and crickets (Insecta, Orthoptera, Ensifera). Zool. Anz. 242, 107–120. doi: 10.1078/0044-5231-00091
Lehmann, G. U. C. (2007). Density-dependent plasticity of sequential mate choice in a bushcricket. Austr. J. Zool. 55, 123–130. doi: 10.1071/ZO06105
Lehmann, G. U. C. (2008). “How different host species influence parasitism patterns and larval competition of acoustically-orienting parasitoid flies (Tachinidae: Ormiini),” in Animal Behaviour: New Research, eds Weber E. A. and L. H. Krause (New York, NY: Nova Science Publishers, 93–132.
Lehmann, G. U. C. (2012). Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Front. Zool. 9:19. doi: 10.1186/1742-9994-9-19
Lehmann, G. U. C., and Heller, K.-G. (1998). Bushcricket song structure and predation by the acoustically orienting parasitoid fly Therobia leonidei (Diptera: Tachinidae: Ormiini). Behav. Ecol. Sociobiol. 43, 239–245. doi: 10.1007/s002650050488
Lehmann, G. U. C., Heller, K.-G., and Lehmann, A. W. (2001). Male bushcrickets favoured by parasitoid flies when acoustically more attractive for conspecific females (Orthoptera: Phaneropteridae / Diptera: Tachinidae). Entomol. Gener. 25, 135–140. doi: 10.1127/entom.gen/25/2001/135
Lehmann, G. U. C., and Lehmann, A. W. (2000). Spermatophore characteristics in bushcrickets vary with parasitism and remating interval. Behav. Ecol. Sociobiol. 47, 393–399. doi: 10.1007/s002650050682
Lehmann, G. U. C., and Lehmann, A. W. (2006). Potential lifetime reproductive success of male bushcrickets parasitized by a phonotactic fly. Anim. Behav. 71, 1103–1110. doi: 10.1016/j.anbehav.2005.08.009
Lehmann, G. U. C., and Lehmann, A. W. (2007). Sex differences in “time out” from reproductive activity and sexual selection in male bushcrickets (Orthoptera: Zaprochilinae: Kawanaphila mirla). J. Insect Behav. 20, 215–227. doi: 10.1007/s10905-007-9076-1
Lehmann, G. U. C., and Lehmann, A. W. (2008). Bushcricket song as a clue for spermatophore size? Behav. Ecol. Sociobiol. 62, 569–578. doi: 10.1007/s00265-007-0481-4
Lehmann, G. U. C., and Lehmann, A. W. (2009). Condition-dependent spermatophore size is correlated with male's age in a bushcricket (Orthoptera: Phaneropteridae). Biol. J. Linn. Soc. 96, 354–360. doi: 10.1111/j.1095-8312.2008.01129.x
McCartney, J., Potter, M. A., Robertson, A. W., Telscher, K., Lehmann, G. U. C., Lehmann, A. W., et al. (2008). Understanding nuptial gift size in bush-crickets: an analysis of the genus Poecilimon (Tettigoniidae; Orthoptera). J. Orthoptera Res. 17, 231–242. doi: 10.1665/1082-6467-17.2.231
Reinhold, K., and von Helversen, D. (1997). Sperm number, spermatophore weight and remating in the bushcricket Poecilimon veluchianus. Ethology 103, 12–18. doi: 10.1111/j.1439-0310.1997.tb00002.x
Robert, D., Amoroso, J., and Hoy, R. R. (1992). The evolutionary convergence of hearing in a parasitoid fly and its cricket host. Science 258, 1135–1137. doi: 10.1126/science.1439820
Schniederkötter, K., and Lakes-Harlan, R. (2004). Infection behavior of a parasitoid fly, Emblemasoma auditrix and its host cicada Okanagana rimosa. J. Ins. Sci. 4:36. doi: 10.1673/031.004.3601
Stölting, H., Moore, T. A., and Lakes-Harlan, R. (2004). Acoustic communication of Okanagana rimosa (Say) (Homoptera: Cicadidae). Zoology 107, 243–257. doi: 10.1016/j.zool.2004.07.003
Strauß, J., and Lakes-Harlan, R. (2014). Evolutionary and phylogenetic origins of tympanal hearing organs in insects”, in Insect Hearing And Acoustic Communication, ed B. Hedwig (Berlin; Heidelberg: Springer, 5–26.
Strauß, J., Lehmann, A. W., and Lehmann, G. U. C. (2014). Sensory evolution of hearing in tettigoniids with differing communication systems. J. Evol. Biol. 27, 200–213. doi: 10.1111/jeb.12294
Stucky, B. (2015). Infection behavior, life history, and host parasitism rates of Emblemasoma erro (Diptera: Sarcophagidae), an acoustically hunting parasitoid of the cicada Tibicen dorsatus (Hemiptera: Cicadidae). Zool. Stud. 54:30. doi: 10.1186/s40555-015-0105-z
Tron, N., Beuter, L.-K., and Lakes-Harlan, R. (2015). Phonotactic behaviour and vertical sound source localization of the parasitoid fly Emblemasoma auditrix (Diptera, Sarcophagidae). Ecol. Entomol. 40, 707–716. doi: 10.1111/een.12246
Williams, K. S., and Simon, C. (1995). The ecology, behavior, and evolution of periodical cicadas. Annu. Rev. Entomol. 40, 269–295. doi: 10.1146/annurev.en.40.010195.001413
Zuk, M., and Kolluru, G. R. (1998). Exploitation of sexual signals by predators and parasitoids. Quart. Rev. Biol. 73, 415–438. doi: 10.1086/420412
Zuk, M., Rotenberry, J. T., and Tinghitella, R. M. (2006). Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2:4. doi: 10.1098/rsbl.2006.0539
Keywords: acoustic communication, parasitoid, host finding, host infection, signal plasticity
Citation: Lehmann GUC and Lakes-Harlan R (2019) Adaptive Strategies in Life-History of Bushcrickets (Orthoptera) and Cicadas (Homoptera) to Parasitoids Pressure on Their Acoustic Communication Systems—A Case for Sociality? Front. Ecol. Evol. 7:295. doi: 10.3389/fevo.2019.00295
Received: 03 January 2019; Accepted: 24 July 2019;
Published: 08 August 2019.
Edited by:
Rachel A. Page, Smithsonian Tropical Research Institute (SI), PanamaReviewed by:
Andreas Stumpner, University of Göttingen, GermanyCopyright © 2019 Lehmann and Lakes-Harlan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerlind U. C. Lehmann, Z2VybGluZC5sZWhtYW5uQGJpb2xvZ2llLmh1LWJlcmxpbi5kZQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.