
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 16 July 2019
Sec. Population, Community, and Ecosystem Dynamics
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00269
This article is part of the Research Topic Essential Biomolecules Caught in the Food Web? View all 8 articles
We here review the ecological role of essential nutritional biomolecules [fatty acids (FA), amino acids (AA), sterols, vitamins] in aquatic and terrestrial food webs, encompassing the forces behind their environmental distribution. Across ecosystems, mutualistic relationships frequently ensure exchanges of vitamins between producer and demander, especially between B12 and other B vitamins as well as the AA methionine. In contrast, FA, sterols and most AA are transferred up the food chain via classical predator-prey interactions, and therefore have good biomarker potential for trophic interactions. As biomass-flow depends on the absolute amounts of potential limiting resources, considering solely the relative share in the respective biochemical group may under- or overestimate the availability to consumers. Moreover, if not accounted for, “hidden” trophic channels, such as gut symbionts as well as metabolic conversion of precursor molecules, can hamper food web analyses. Fundamental differences exist between aquatic and terrestrial ecosystems: Vitamin B12 produced by ammonium oxidizing Archaea is essential to many aquatic algae, whereas terrestrial plants escaped this dependency by using B12 independent enzymes. Long-chain ω3 polyunsaturated FA (LC-ω3PUFA) in aquatic systems mainly originate from planktonic algae, while in terrestrial systems, belowground invertebrates can well be a source, also supporting aboveground biota. Interlinks from terrestrial to aquatic ecosystems are of a biochemically totally different nature than vice versa. While biomass rich in proteins and LC-ω3PUFA is transferred to land, e.g., by trophic relationships, the link from terrestrial to aquatic ecosystems provides recalcitrant plant carbon, mainly devoid of essential nutrients, fuelling detrital food chains. Recent global changes influence food webs via altered input and transfer of essential biomolecules, but separating the effects of nutrients, CO2, and warming is not trivial. Current evolutionary concepts (e.g., Black Queen, relaxed selection) considering the costs of metabolic production partly explain food web dynamics, especially for vitamins, whereas adaptations to potential oxidative stress seem to be more important for LC-PUFA. Overall, the provision with essential biomolecules is precious for both heterotrophs and auxotrophs. These nutritional valuable molecules often are kept unaltered in consumer metabolism, including their stable isotope composition, offering a great advantage for their use as trophic markers.
Despite the importance for trophic interactions and consumer fitness, a general comprehension of essential biomolecules structuring food webs and driving consumer adaptations is still lacking to date. For example, little is known about the environmental distribution of essential FA, AA, sterols and vitamins, and how ecosystem functions are constrained by their bioavailability. Essential biochemical resources can directly influence consumers' growth and reproduction, and in turn trophic relationships, including food intake or metabolic requirements. Thus, their availability can act as selection pressure on the evolution of consumer populations.
This paper conceptually summarizes current knowledge and potential implications of essential biomolecules on ecosystem dynamics, i.e., their biochemistry and functionality, their availability and transfer within food webs, and their use as trophic markers. A major focus is set on the forces behind environmental distributions, interlinks between ecosystems and alterations due to anthropogenic impacts. Further, evolutionary aspects in the provision of biomolecules and metabolic adaptations are highlighted.
Among FA, ω3- and ω6PUFA are essential for many animals. Unlike plants and higher fungi, whose methyl-end desaturases introduce double bonds in C16- and C18FA at the ω3 and ω6 position, animal desaturases work toward the carboxyl-end beyond the ω6 position (Sprecher et al., 1995; Monroig and Kabeya, 2018). This results in characteristic “ω families,” i.e., ω3, ω6, ω9 (Figure 1). For animals the primary essential dietary derived PUFA of the ω6 family is linoleic acid (LA: 18:2ω6), and of the ω3 family α-linolenic acid (ALA: 18:3ω3). LA and ALA are the precursors for the respective LC-PUFA, also called highly-unsaturated fatty acids (HUFA), obtained by chain elongation and further desaturation (Figure 1). Conversion rates among PUFA, especially from ALA to eicosapentaenoic acid (EPA: 20:5ω3) or docosahexaenoic acid (DHA: 22:6ω3), are low (Stanley-Samuelson et al., 1987; Bell and Tocher, 2009). For instance, only around 5% of ALA are converted to EPA in omnivorous vertebrates, including humans (Davis and Kris-Etherton, 2003). Dietary provision with EPA and DHA can directly enhance consumers' growth, health and fitness (e.g., humans—Hulbert et al., 2014; Nwachukwu et al., 2017; insectivorous birds—Twining et al., 2016; soil microarthropods—Menzel et al., 2018). Therefore, LC-ω3PUFA are classified as semi-essential (Müller-Navarra, 1995a), and LC-ω3PUFA supplementation, e.g., produced via metabolic engineering (Gong et al., 2014), is recommended for humans.
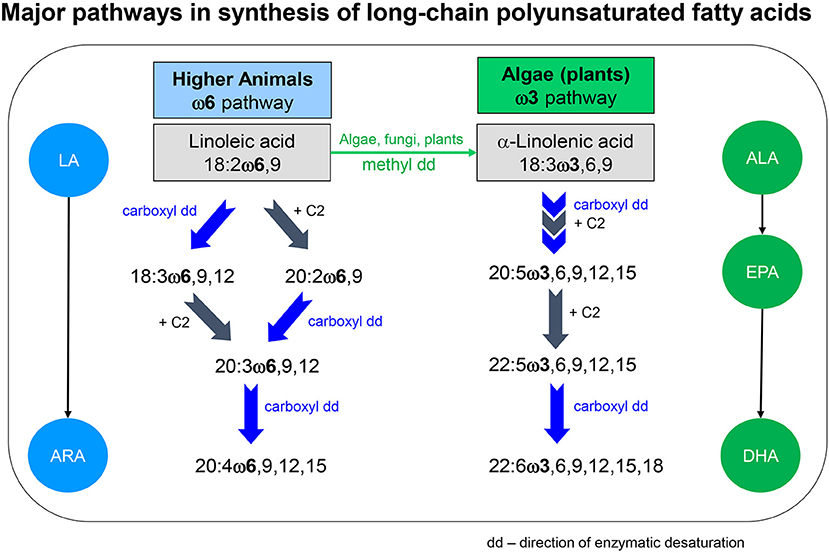
Figure 1. Major pathways in synthesis of long-chain polyunsaturated fatty acids (LC-PUFA) in eukaryotic organisms (modified after Sprecher et al., 1995). LA, linoleic acid; ALA, α-linoleic acid; ARA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; dd, direction of the enzymatic desaturation in the fatty acids molecule; methyl-end desaturases introduce double bonds in C16- and C18-FA at ω3 and ω6 position, animal desaturases work toward the carboxyl-end beyond the ω6 position.
LC-PUFA have several key physiological functions in animals. They are an integral part of biological membranes and maintain their adequate viscosity and selective permeability (Vance and Vance, 2002). Further, γ-linolenic acid (GLA: 18:3ω6), arachidonic acid (ARA: 20:4ω6) and EPA serve as precursor for tissues hormones such as eicosanoids, which mediate inflammatory processes, immune response or cell growth (Stanley and Kim, 2019). Moreover, EPA and DHA are concentrated in nervous tissue, being the dominant FA of brain lipids (Wiktorowska-Owczarek et al., 2015). Differences in PUFA requirements exist depending on the consumer taxonomic affiliation, its ontogenetic stage and especially egg production (for zooplankton: Brett et al., 2009), which can affect trophic interactions (Müller-Navarra, 2008). Overall, the dietary LC-PUFA content is critical for growth, reproduction, and neural development for many aquatic and terrestrial invertebrates as well as vertebrates, including humans (e.g., Stanley-Samuelson et al., 1988; Parrish, 2009; Hulbert and Abbott, 2012).
The main ω3- and ω6PUFA producer in aquatic systems are eukaryotic algae (Table 1). In particular, the synthesis of LC-ω3PUFA in algae is tightly connected to photosynthesis, because they are part of the glycolipids in thylakoid membranes of chloroplasts (Guschina and Harwood, 2009). Similar to accessory pigments, LC-PUFA play a role in the light harvesting process. Systematic affiliation seems to be the strongest determinant of PUFA distribution in seston (Müller-Navarra et al., 2004), a finding also reported from cultures (Cobelas and Lechado, 1989; Ahlgren et al., 1992; Galloway and Winder, 2015; Taipale et al., 2016a). However, environmental factors including nutrients, temperature, salinity and light also directly affect the PUFA pattern of algae (Guschina and Harwood, 2009). While LC-ω3PUFA compositions in algae based on relative proportions already assigns group specific pattern (Galloway and Winder, 2015; Peltomaa et al., 2017), differences based on absolute amounts are much more pronounced (data by Cobelas and Lechado, 1989; Ahlgren et al., 1992; Müller-Navarra, 1995b; Jónasdóttir, 2019) and important for the availability to consumers.
Table 1. Essential biomolecules within the groups of fatty acids, sterols, vitamins, and amino acids.
Due to their high absolute EPA content, diatoms are the greatest EPA supplier, both in most marine and freshwater (except dystrophic) systems, followed by chryptophytes, haptophytes, dinophytes and eustigmatophytes (Table 1). From their absolute DHA content, dinophytes are the main supplier, followed by haptophytes. However, certain diatoms as well as most dinophytes are too big to be ingested by most zooplankton. Moreover, heterotrophic stramenopiles (e.g., thraustochytrids) used in pharmaceutical DHA production (Gupta et al., 2012) may be a so far underestimated DHA provider. Heterotrophic eukaryotes can also contribute to sestonic EPA (e.g., ciliates—Bec et al., 2010; Hartwich et al., 2012), converting ALA mainly supplied by chlorophytes and cryptophytes to EPA, i.e., trophically up-grading sestonic food quality for zooplankton. Cyanobacteria generally do not contain LC-PUFA, although some taxa comprise C18-PUFA (e.g., ALA) but at low absolute concentrations (Table 2). Prokaryotes are usually not a source of LC-PUFA, although they occur in bacteria in deep-sea isolates, which is probably an adaptation to low temperature and high pressure in the habitat (DeLong and Yayanos, 1986). Macro-algal chlorophytes display a FA pattern similar to their micro-algal counterpart, while marine rhodophytes have an especially high share of EPA (but none or low DHA) and phaeophytes comprise intermediate levels of LC-PUFA (Fleurence et al., 1994; Pereira et al., 2012).
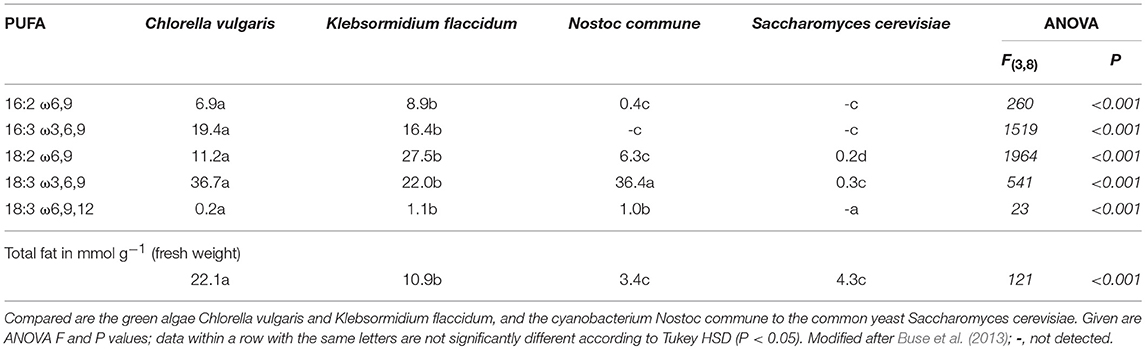
Table 2. Proportion of polyunsaturated fatty acids (PUFA in % of total fatty acids) in the neutral lipids of photoautrotrophic microbiota dominant in terrestrial soil crusts.
Vascular plants are considered as the major source for PUFA in terrestrial food webs and predominantly contain C18-PUFA, i.e., ALA and LA. Herbivores such as ruminants can acquire LC-ω3PUFA via ciliates as gut symbionts (Veira, 1986; Newbold et al., 2015), which may be able to convert ALA to EPA, like certain ciliates in aquatic systems. Primary producers in biological soil crusts (green algae and cyanobacteria) synthesize ALA, with green algae additionally comprising the PUFA 16:3ω3 as a potential precursor (Buse et al., 2013; Table 2). However, the role of biological soil crusts as ω3PUFA supply for terrestrial food webs has not been explored yet. Among heterotrophs higher fungal taxa commonly synthesize ALA (Ruess and Chamberlain, 2010), moreover lower soil fungi can supply LC-ω3PUFA, predominantly Chytridiomycetes and Oomycetes (Lösel, 1988), and arbuscular mycorrhiza fungi (Gladyshev et al., 2013). Further, there is evidence that many soil invertebrates provide LC-ω3PUFA (see Figure 3, and chapter ω3PUFAs—aquatic vs. terrestrial food webs).
The importance of EPA and DHA for food quality affects biomass trophic transfer in aquatic ecosystems (Müller-Navarra, 1995a; Brett and Müller-Navarra, 1997; Müller-Navarra et al., 2000). PUFA itself can be as twice as efficiently transferred from the first to the second trophic level compared to bulk carbon (Gladyshev et al., 2011; Mayor et al., 2011). LC-ω3PUFA are particularly retained and accumulated in consumer lipids of aquatic food webs (Strandberg et al., 2015; Guo et al., 2016), which is also a central issue in aquaculture (e.g., Navarro et al., 2014). Generally, fatty acids from the diet can be incorporated into consumer body tissue without major modification, a fact called “dietary routing” (Stott et al., 1997). Based on this, ω3PUFA were used as qualitative markers for trophic interactions in freshwater plankton (Desvilettes et al., 1997), and semi-quantitatively in food webs in marine pelagial (Dalsgaard et al., 2003) and benthos (Kelly and Scheibling, 2012). By applying metabolic conversion factors, dietary routing was traced quantitatively (Iverson et al., 2004) in vertebrates connected to the marine food chain, e.g., for arctic mammals (polar bears—Thiemann et al., 2008) or sea birds (Williams and Buck, 2010). Further, Galloway et al. (2015) employed a Bayesian approach (FA source-tracking algorithm) comparable to the use of stable isotopes in food web ecology. This wide application of lipids as trophic markers in aquatic habitats has benefitted from the frequent occurrence of ω3PUFA in primary producers at the food web base.
In contrast, the use of FA biomarker in terrestrial food webs has lagged behind. First trophic transfer of fatty acids was assigned in detrital food chains in soils from bacteria and fungi to microbial grazers (nematodes—Ruess et al., 2002; Collembola—Haubert et al., 2006) and further up to omnivores and predators (Collembola—Ruess et al., 2004; spiders—Pollierer et al., 2010). Opposite to aquatic ecosystems, this was based on the dietary routing of non-essential FAs, e.g., methly-branched and cyclic forms derived from bacteria (Ruess and Chamberlain, 2010). The achievement of comparable results in the herbivore food chain was hindered by the lack of LC-ω3PUFA in terrestrial primary producers, resulting in ALA as major biomarker for plant feeding (Ruess and Chamberlain, 2010) and C16-ω3PUFA for algal feeding (Buse et al., 2013). However, this drawback of lower diversity in FA markers can be overcome by applying stable isotope fingerprints, tracing FA dietary routing via their δ13C signal. Here, main approaches are 13CO2 pulse-labeling of plants (Pausch et al., 2016) or using natural differences in 13/12C ratios of C3 and C4 plants (Haubert et al., 2009; Ngosong et al., 2011) (Figure 2). However, a broader application of FA in food web ecology of vertebrates, as common in marine systems, is still lacking, except of the usage of FA markers in food chemistry (Molketin and Giesemann, 2007). For an overview on trophic transfer and biomarkers in terrestrial systems see reviews of Ruess and Chamberlain (2010) and Traugott et al. (2013).
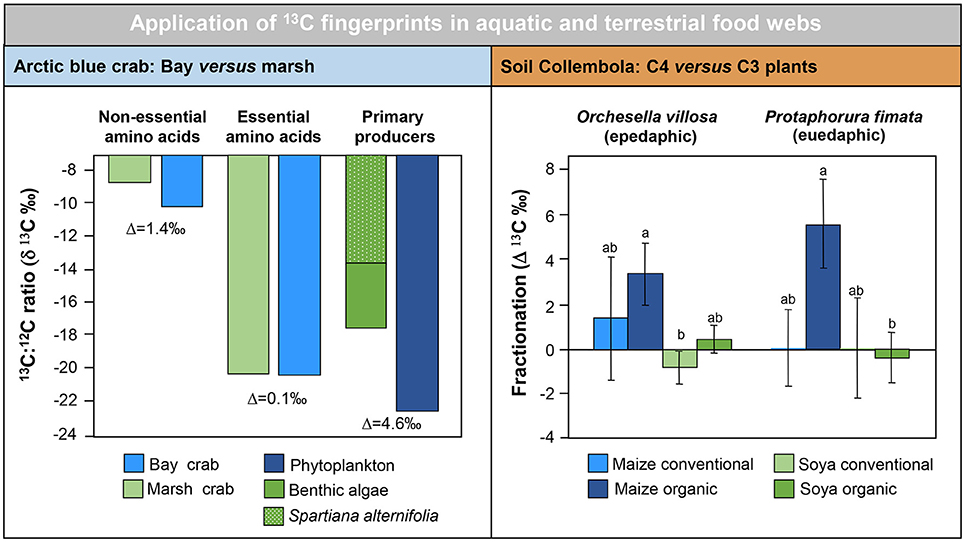
Figure 2. Carbon stable isotope fingerprinting in aquatic and terrestrial food webs based on differences in δ13C of primary producers. Left—δ13C content in bulk tissue of major resources and in non-essential and essential amino acids of Atlantic blue crabs (Callinectes sapdius) in bay and marsh habitats (data from Fantle et al., 1999). The difference in δ13C between primary producers or amino acid fractions is expressed as Δ13C. Right—fractionation (Δ13C) in linoleic acid (18:2ω6,9) between Collembola (Orchesella villosa, Protaphorura fimata) and soil fungi in a C3 (soya) and C4 (maize) arable system with different farming practice (data from Haubert et al., 2009). No fractionation indicates dietary routing of linoleic acid and hence fungal-feeding. Values with the same letters are not significantly different according to Tukey HSD (P < 0.05).
Sterols are an important lipophilic part of cell membranes in eukaryotic organisms, embedded within the membrane phospholipids. Moreover, they are frequently esterified to membrane LC-PUFA. The central sterol synthesized in animals is cholesterol, a C27 sterol. However, not all animals are capable of de novo sterol synthesis. For example, arthropods lack this ability (Goad, 1981; Martin-Creuzburg and von Elert, 2009), and many insects and crustaceans prefer Δ5 and Δ5, 7 over Δ7 and Δ22 sterols (e.g., Teshima, 1971; Behmer and Nes, 2003). Known from herbivorous insects and crustaceans, dietary phytosterols are typically altered by de-alkylation and saturation, e.g., of C24 methyl and ethyl sterols, to form cholesterol (Svoboda and Thompson, 1985). Despite this, sterol limitation was shown for crustaceans feeding on laboratory cultures of heterotrophic and autotrophic bacteria (e.g. Martin-Creuzburg et al., 2011 for Daphnia) or diatoms (Hassett, 2004 for Acartia, Calanus). However, interacting effects of sterols with the provision of EPA (Martin-Creuzburg et al., 2009 for Daphnia) or AA (Wacker and Martin-Creuzburg, 2012 for a rotifer) were also demonstrated. Nevertheless, a minor part of sterols (less than 1%) is required as precursor for molting hormones in arthropods (e.g., ecdysteroids; Svoboda and Thompson, 1985).
In vertebrates, 7-dehydrocholesterol is converted to cholecalciferol (vitamin D3) under UV irradiation and functions as steroid hormone in the Ca-metabolism, with special importance for skeleton structures, e.g., in humans (DeLuca, 1998). Except for hormone production, sterol requirements of many organisms seem not to be specific. Additionally, the metabolic modification of dietary sterols widens sources for organisms with a low or lost ability of de novo synthesis.
In planktonic food webs, eukaryotic macro- and micro-algae are very variable sources of over 250 different sterols (mainly C28 phytosterols) (Santos et al., 2015) (Table 1). However, their distribution within an algal class is not as uniform as for PUFA (e.g., Volkman, 1986, Martin-Creuzburg and Merkel, 2016; Taipale et al., 2016a). Prokaryotic planctomycetes and poribacteria have the genetic infrastructure for sterol synthesis (Pearson et al., 2003) but their role in dietary sterol supply is unknown. Eukaryotic fungi are potentially an important resource in aquatic ecosystems, especially in streams (Krauss et al., 2011). The same applies to chytrids, ubiquitous fungal parasites in aquatic ecosystems, providing a sterol source to their grazers even in absence of their phytoplankton hosts (Gerphagnon et al., 2019). Cyanobacteria as prokaryotes do not produce sterols at all (Volkman, 2003).
In terrestrial ecosystems, higher plants deliver mainly C28 and C29 sterols (Moreau et al., 2018) e.g., by leaves, fruits, roots and bulbs to herbivores, both in above- and belowground food webs (Table 1). Fungi are also frequent sterol producers in soil systems (Burden et al., 1989), contributing ergosterol as a major component of their cell membranes (Joergensen and Wichern, 2008). If and how these fungal sterols can act as a nutrition source for soil animals has yet to be explored. Finally, symbiotic fungi in the gut may provide a substantial part of sterols to their insect host (reviewed by Douglas, 2009).
Phytosterols are metabolic converted by several herbivorous consumers to suit specific physiological requirements, hampering their trophic biomarker usage. Above, many animals—especially vertebrates—are capable of de novo sterol synthesis, which makes sterol limitation further up the food chain unlikely, especially in the 3RD and 4TH trophic level of both, aquatic and terrestrial food webs. However, a comprehensive understanding is still lacking.
Metabolic sterol processing was reported, e.g., for heterotrophic protists feeding on macro-algae (Chu et al., 2009), soil nematodes feeding on plant roots (Chitwood and Lusby, 1991; Chitwood, 1999), millipedes feeding on terrestrial plant litter (Rawlins et al., 2006), and crustaceans feeding on phytoplankton (Martin-Creuzburg and Merkel, 2016 for Daphnia). Additionally, biochemical sterol modulation generally occurs is herbivore insects (Svoboda and Thompson, 1985). However, the suitability of phytosterols may differ (Martin-Creuzburg et al., 2014 for Daphnia; Gergs et al., 2015 for gammarids), and not all zooplankton consumers do convert those (Wacker and Martin-Creuzburg, 2012 for rotifers). Although the less group-specific occurrence of phytosterols in algae and their conversion in many herbivores are hampering their sole application as trophic markers in pelagic food webs, sterols together with FA as markers seem promising to track food intake, as shown for dwelling ophiurids and holothurians in a deep-sea environment (Drazen et al., 2008). In general, sterols are widely used as biomarkers in sediments due to their chemical persistence, e.g., separating allochtonous and autochtonous produced biomass (Bianchi and Canuel, 2011).
Vitamins are a diverse class of rather complex compounds, and from the two major groups only the most important in food web ecology are considered here:
Vitamin A is essential for animals and primarily needed for vision. Carotenoides (provitamin A) are synthesised in chloro- and chromoplasts of photoautotrophic organisms as photoprotecting agent (Saini et al., 2015; Huang et al., 2017). Vitamin E is an anti-oxidant protecting susceptible LC-PUFA, and requirements in animals are connected to PUFA provision (Zhang et al., 2017). This protective function may also be related to the transition from parthenogenetic to sexual reproduction, male fertility (spermatogenesis) and enhanced growth of the mictic offspring as shown for the rotifer Asplanchna by Gilbert (1980). Vitamin D is a derivate from cholesterol and considered in the chapter on sterols above.
B1 (thiamin) is a cofactor of several core enzymes, e.g., in the transfer of C2-groups (dehydrogenases, decarboxylases) in the carbohydrate metabolism. Requirements of external B1 are mostly due to the lack of either synthesis of the pyrimidine or the thiazole moiety in algae (Lewin, 1961). If capable of biosynthesis, thiamin is only produced on demand via riboswitches, both in prokaryotes and eukaryotes (McRose et al., 2014), making the process very efficient. Physiological interconnections exist with other vitamins, e.g., folic acid (B9) deficiency influences B1 adsorption and metabolism (Wolak et al., 2017). Further, supply in B1 can alter the FA pattern towards EPA and DHA, e.g., in the alga Rhodomonas baltica (Chi et al., 2018).
B7 (biotin) requirements are known for some crysophytes, dinophytes, and euglenoid algae (Provasoli and Carlucci, 1974). Folic acid (B9) is synthesized by plants, including algae (Baker et al., 1981), but is essential to animals as a cofactor of thymidyl-synthase, catalyzing nucleotide syntheses, which governs DNA repair and replication.
B12 (cobalamin) is a cofactor in enzymes carrying activated methyl groups or providing carbon-based free radicals for reactions removing non-acid hydrogen atoms (Marsh, 1999). It is involved in the synthesis of AA and DNA, and in shuffling C1-groups into the citrate circle. Two synthesis pathways exist, i.e., the more ancient anaerobic pathway of bacteria and Archaea leading to cobalamin, and the aerobic pathway of cyanobacteria resulting in pseudo-cobalamin (Raux et al., 1999). Cobalamin cannot be synthesized by most eukaryotes, including animals (Gräsbeck and Salonen, 1976). Overcoming potential limitations due to B12 auxotrophy seems to be governed by a non-cobalamin methionine synthase in higher plants and certain algal taxa (Croft et al., 2006). Moreover, B1 deficiency syndromes in fish (Marcquenski and Brown, 1997) and sea birds (Balk et al., 2009) may be coupled to B12 shortage in resources. Overall, the metabolic pathways of B1, B9, B12 and the AA methionine are interwoven, and also relevant on the ecological level by impacting nitrogen (N) and sulfur metabolism of eukaryotic phytoplankton (Bertrand and Allen, 2012). This makes it difficult to determine the vitamin that is the most limiting (Table 1).
The syntheses of the lipophilic vitamins A and E are tightly connected to the metabolic capacity of algae in aquatic (Huang et al., 2017) and of plants in terrestrial (Saini et al., 2015) ecosystems. In marine and freshwater, microalgae as well as makrophytes represent the dominant source (Cuvelier et al., 2003; Huang et al., 2017) (Table 1). Pro-vitamin A dominates in leaves and fruits, whereas seeds comprise the highest amounts of vitamin E, and leaves of vitamin K (Aseni-Fabado and Munné-Bosch, 2010). Fungi and yeast are major producer of ergosterol, the precursor of ergocalciferol (i.e., pro-vitamin D2), but UVB exposure is necessary for conversion, restricting the supply to aboveground habitats. Traditionally higher plants were thought to contain only negligible amounts of D3 but recent work raises evidence for a broader distribution (Jäpelt and Jakobsen, 2013). Whether these contents are sufficient to subsidize decomposer food webs in lightless soil habitats remains to be explored.
In aquatic systems, red and green algae are a good source of B vitamins, especially B1, and B9 (Croft et al., 2006) (Table 1). In contrast, main B12 producer seem to be ammonium oxidizing thaumarchaeota, primarily under cold conditions, i.e., at greater depth, during winter and in polar-regions (Doxey et al., 2015). Interestingly, most algae—especially those groups known to provide LC-ω3PUFA to food webs—are only minor vitamin B suppliers, as many of them are auxotroph in respect to B1 (20%), B7 (5%) and especially B12 (50%) (Provasoli and Carlucci, 1974; Croft et al., 2006). Although cyanobacteria are rich in B1, they are an inefficient source of B12 as they only contain the nutritionally inferior pseudo-cobalamin (Helliwell et al., 2016).
In terrestrial systems, higher plants are the major producers of B-vitamins (Roje, 2007), delivering these water-soluble vitamins via root exudation into the rhizosphere and additionally supporting their mycorrhiza symbionts (Grayston et al., 1996) (Table 1). The second important vitamin B sources are soil bacteria, with phosphate-solubilising taxa azotobacters and rhizobia as significant producers (Vranova et al., 2013). The cumulative vitamin production by bacteria in the rhizosphere enhances general bioavailability and complements root exudates (Baya et al., 1981), likely important for the associated fungi and fauna.
Lipophilic vitamins synthesized by algae or vascular plants are generally transferred to heterotrophic consumers via predator-prey interactions. In contrast, syntrophic relationships with microbes seem to be important to overcome certain vitamin B limitations, as shown for marine algae co-cultured with bacteria (Paerl et al., 2015). Beneficial microbe-algae (Croft et al., 2005) and algae-algae interactions (Carlucci and Bowes, 1970; Baker et al., 1981) seem rather to be the rule than the exception. However, their role in overcoming B12 limitation sustaining global primary production is debated (e.g., Droop, 2007). In nature, B12 is acquired from soluble surroundings with specific transporters by B12 auxotrophic algal cells (Bertrand and Allen, 2012), ensuring rapid uptake. For food webs of marine pelagic systems there is evidence that B1 is not accumulated but rather diluted from phytoplankton to zooplankton to fish (Sylvander et al., 2013).
In aquatic ecosystems, B12 provision by endosymbionts is known for certain ciliates (Baker et al., 1981). More recently, Fiore et al. (2015) showed symbiotic interactions with Archaea and bacteria to be crucial for the Caribbean giant barrel sponge (Xestospongia muta) in obtaining B-vitamins and essential AA. So far, mutualistic relationships of sponges or corals are rather viewed in the light of carbon acquisition but should also be connected to the acquisition of essential biomolecules. In contrast to aquatic ecosystems, the importance of gut symbionts as suppliers of vitamins to their terrestrial vertebrate and invertebrate hosts has been recognized for a long time, especially for bacteria or ciliates as endosymbionts in social insects such as wood feeding termites or insects that suck on plant sap, which is poor in vitamins (Moran et al., 2003; McCutcheon et al., 2009). B2 is provided by gut bacteria to earthworms (Sulik et al., 2012) or by the mutualistic Wolbachia bacteria to nematodes (Taylor et al., 2012). Moreover, the intestinal flora plays a major role in the provision with B7, as shown for humans (Magnúsdóttir et al., 2015). Overall, the provision with B vitamins by mutualistic prokaryotes in invertebrates and vertebrates as “hidden” trophic interactions complicates determination of their trophic transfer in food web studies.
AA are the structural elements of enzymes, regulating key metabolic pathways important for health, survival and growth, e.g., neurological and immunological functions (Wu, 2010). Traditionally, AA are classified as none-essential (dispensible) and essential (indispensible). The former can be de novo synthesized by the consumer, whereas the later must be provided with the diet. Nine AA have been assigned as essential in humans and other vertebrates, i.e., isoleucine, histidine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine (Young, 1994), yet requirements vary between taxonomic entities. In addition, arginine is essential for terrestrial carnivores (Morris, 1985). A third category is named conditionally indispensable or “functional” AA, which are semi-essential as synthesis rates in certain taxa are limiting (Wu, 2010). A relevant example is arginine in many mammals (Wu et al., 2013). The metabolic flexibility in consumers, such as compensatory mechanisms can affect AA limitation. Only recently, the upregulation of AA retention efficiency as a response to nitrogen deficiency was shown in copepods (Burian et al., 2018). In conclusion, we are still at the start of understanding which AA are essential in different animal groups, and what implication this dependency has for food web ecology.
In aquatic food webs, phytoplankton, particularly protein-rich algae (above 50% dry weight - Bleakley and Hayes, 2017) are a main source for AA (Table 1). Aquatic macrophytes, in contrast, display a much lower protein (Rather and Nazir, 2015) and consequently AA content, due to more structural components (cellulose, hemicellulose, lignin). Terrestrial plants, their leaves, flowers and fruits provide AA to herbivores in terrestrial food webs (Takizawa et al., 2017). However, AA levels are typically low and supply often needs to be enriched via mutualistic interactions with gut microbiota in herbivores (Cantalapiedra-Hijar et al., 2016 for lambs). Microbial derived proteins further represent an important resource for essential AA in detritivore food webs as shown by Steffan et al. (2017) analyzing invertebrates (moths, beetles) and vertebrates (fish). Degradation of plant litter by bacteria and fungi provide AA for detrivores (Larsen et al., 2009). Protein is hydrolysed via extracellular enzymes, followed by adsorption of free AA from the soil solution (Czimcik et al., 2005). Particularly bacteria, which can acquire AA by both, de novo synthesis using inorganic nitrogen, and by direct incorporation of organic substrates, comprise diverse AA (McMahon and McCarthy, 2016). Moreover, AA can be derived from endo-symbiotic gut bacteria, e.g., in earthworms and enchytraeids (Larsen et al., 2016) or intracellular co-symbionts like Buchnera in nematodes and insects (Moran et al., 2003; McCutcheon et al., 2009).
In contrast to terrestrial systems, protein limitation per se is rather unlikely in pelagic aquatic systems, but certain essential AA may become in short supply (review by Müller-Navarra, 2008). Moreover, trophic transfer of AA can be impaired by co-limiting resources such as sterols (Wacker and Martin-Creuzburg, 2012 for a rotifer). In addition, a meta-analysis of zooplankton across 29 oligotrophic lakes by Guisande et al. (2003) revealed taxa specific AA composition, likely indicating distinct food sources.
The AA composition of primary producers can be markedly different to their animal consumers or microbial degraders (Steffan et al., 2015; Takizawa et al., 2017), offering great potential to follow the transfer of specific AA along the trophic cascade. Two AA categories are distinguished as biomarker in food web ecology: The so called “source” AA exhibiting small 15N-discrimination but preserve the 15N/14N ratio (δ15N) of the food (McMahon and McCarthy, 2016), while the amino-N of “trophic” AA is routinely exchanged in the metabolic pool (O'Conell, 2017). Thus, 15N of “trophic” AA is enriched along the trophic cascade (McMahon et al., 2016) (see Table 3). However, it is important to note that the categorization in “source” and “trophic” AA is based on differences in cycling of the N moiety and not on changes within the carbon skeleton. The latter is distinguishing essential and non-essential AA, respectively. The respective parameters and calculations to assign trophic positions by the stable isotope signal of AA are summarized in Table 3.
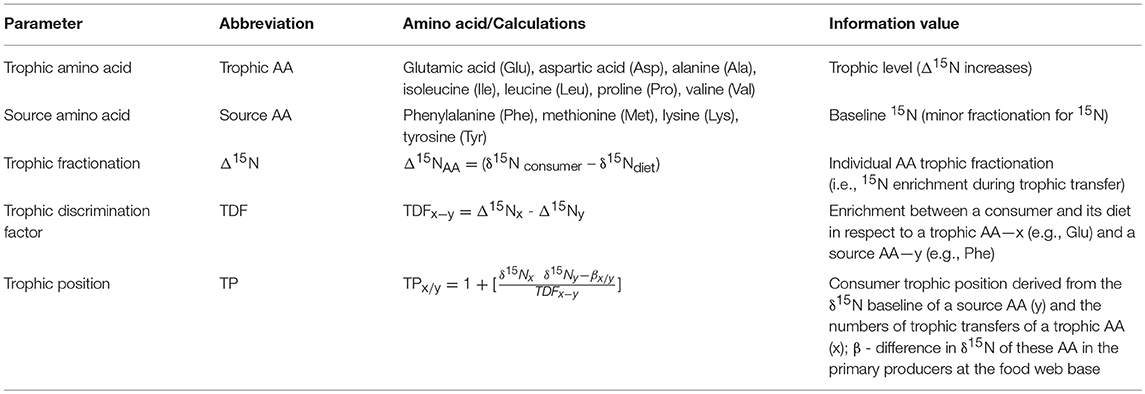
Table 3. “Trophic” and “source” amino acids, fractionation factors, and formula used in in food web studies (Modified after McMahon and McCarthy, 2016).
The broadest biomarker application received the AA couple glutamic acid (Glu; trophic) and phenylalanine (Phe; source). The underlying assumption is that the isotopic fractionation is constant across binary links with the trophic discrimination factor (TDFGlu−Phe) being 7.6‰ (Chikaraishi et al., 2007) (Table 3). TDFGlu−Phe was successfully applied to assign trophic positions of consumers in very different trophic relationships. These comprised arthropod functional groups in orchard food webs, ranging from sap-feeding herbivores over parasitoids and hyperparasitoids to predators (Steffan et al., 2013) as well as food chains with a broad phylogenetic spectrum, ranging from bacteria and fungi over insects to mammals (Steffan et al., 2015). However, recent work questions a single fixed value for TDFGlu−Phe and reports variability from 0 to 10‰ depending on diet quality, consumer nitrogen excretion, and trophic level (Nielsen et al., 2015; McMahon and McCarthy, 2016; O'Conell, 2017; Pollierer et al., 2019).
Another premise in applying δ15N of AA in food web ecology is the absolute term “β”, the difference in δ15N of AA in primary producers at the base of food webs (Table 3). For example, in primary producers, δ15N of AA in floral biomass seem to depend on the absence or presence of active photosynthesis (Takizawa et al., 2017). At the consumer level interspecific differences in prey organisms may also lead to AA imbalance and subsequently variation in AA δ15N (Ventura and Catalan, 2010 for different crustaceous zooplankton) or increased AA losses by N excretion (Saavedra et al., 2015). The βGlu/Phe (Table 3) was initially proposed to be −3.4‰ for coastal marine and +8.4‰ for terrestrial primary producers (Chikaraishi et al., 2010) but variation in “β” occurs, and its combination with δ13C in bulk tissue is recommended to enhance resolution (Choi et al., 2017).
Generally, inter-trophic AA 15N discrimination seem to be similar among bacteria, fungi and animals (Steffan et al., 2015), whereas the δ15C of essential AA exhibited very different pattern between plants and bacteria or fungi (Larsen et al., 2009). This allows enhancing the resolution in the application of AA as trophic markers by using their isotopic C signal. Stable isotope fingerprinting of 13C/12C in AA is particularly successful in habitats where primary producers display distinctly different 13C signals (Figure 2). This approach was used to identify plant, fungal or bacterial origin of AA (Larsen et al., 2009), feeding habits of the Atlantic blue crab (Fantle et al., 1999) as well as movement and foraging ecology of birds (McMahon et al., 2015b). Only recently, this approach was employed to quantify the trophic levels and the basal resources in soil detritivores (earthworms, Potapov et al., 2019) as well as predators (gamasid mites, spiders; Pollierer et al., 2019). Overall, there is sufficient evidence that the “trophic” and “source” concept is pertinent for many organisms, yet a quest for a single TDF or “β” value may not hold across food webs and ecosystems. Factors such as AA limitation or excretion can lead to mismatches in biochemical stoichiometry, which calls for more physiological and environmental studies to improve the application of AA in trophic ecology.
There are vast differences in the comprehension of what drives the distribution of essential biochemicals in ecosystems with the least known about AA and for terrestrial systems. In aquatic habitats, strong negative correlations between total phosphorus and the lakes' summer seston content of EPA and DHA—but not ALA—were reported (Müller-Navarra et al., 2004; Table 1). Those were connected to the relative abundances of algal groups (Müller-Navarra et al., 2004; Sushchik et al., 2014) and—via trophic transfer—could even be detected in top predators, such as perch (Taipale et al., 2016b, 2018). The whole years' distribution of PUFA is, however, more complex (Gladyshev et al., 2007). In addition, temperature and light can alter seston PUFA directly as shown for EPA in a clear water lake by Hartwich et al. (2012). Humic substances in dystrophic lakes may shield susceptible LC-PUFA from UV, but also exert direct toxic effects to consumers, such as daphnids (CasaNova et al., 2018 and references therein). Salinity gradients structuring estuaries can affect trophic dynamics of LC-ω3PUFA, especially in larval fish (Litchi et al., 2017).
Vertical distributions of PUFA and sterols differ in particulate organic matter of aquatic ecosystems. Generally, LC-PUFA are depleted and sterols enriched during sinking, mainly because of the high susceptibility of LC-PUFA but high persistency of sterols against degradation (Wakeham and Canuel, 1986). Many profundal habitats depend on pelagic provision with PUFA, e.g., when algae blooms perish (Ahlgren et al., 1997), and consumers have adapted to this low availability. For example, benthic harpactoid copepods have a more efficient conversion of C18-PUFA to the respective LC-PUFA than pelagic calanoid copepods (Nanton and Castell, 1998). Aquatic phanerogams contain only ALA and allochtonous carbon is devoid of nutritional LC-PUFA alongside protein (Brett et al., 2017).
Pronounced differences also exist in the distribution of B12 in aquatic ecosystems with Archaea preferentially producing B12 in cold areas and times of the year (Doxey et al., 2015). Primary production can be B12 limited in high-nutrient low-chlorophyll areas (Koch et al., 2011 for Gulf of Alaska), and the competition for B12 between eukaryotic algae and heterotrophic bacteria impact total phytoplankton quantity, species composition and succession (Koch et al., 2012). The vitamins B1, B7, and B12 seem to co-act in controlling algal succession and blooms in the ocean (Sañudo-Wilhelmy et al., 2006, Gobler et al., 2007). Occurrence of harmful algae blooms in marine systems may be favored by high vitamin B presence, as especially toxin-producing chryso- and dinophytes are vitamin B auxotrophs (75% B1, 37% B7, and 100% B12; Tang et al., 2010). In freshwater systems, algal blooms dominated by cyanobacteria reduce resource quality at the food web base. Cyanobacteria lack LC-ω3PUFA and sterols, their pseudo-cobalamin is no source of B12 for most herbivores, and, moreover, they potentially contain toxins.
In contrast to aquatic systems with a predominant supply of LC-ω3PUFA (EPA, DHA) by micro-algae, higher plants as major primary producers in terrestrial ecosystems provide only C18-ω3PUFA (ALA; Figure 3). This questions the main origin of LC-ω3PUFAs in terrestrial food webs. The potential of aquatic derived LC-ω3PUFA to subsidize terrestrial ecosystems was first estimated by Gladyshev et al. (2009). Trophic transfer to land can occur when terrestrial predators (bears, herons, eagles; Figure 3) directly feed on aquatic animals (molluscs, crustaceans, fish). The same applies when terrestrial predators (swallows; Twining et al., 2016) feed on terrestrial stages of prey with both, aquatic and terrestrial life stages (e.g., after insects' emergence; Borisova et al., 2016). In addition, the wax and wane of flooding events (Junk et al., 1989), with algal production in temporary shallow water habitats of riparian areas, is a crucial source of LC-ω3PUFA. In sum, aquatic systems are currently considered a major origin of LC-ω3PUFA for residential terrestrial animals, including humans (Gladyshev et al., 2013; Hixson et al., 2015; Colombo et al., 2017) (Figure 3).
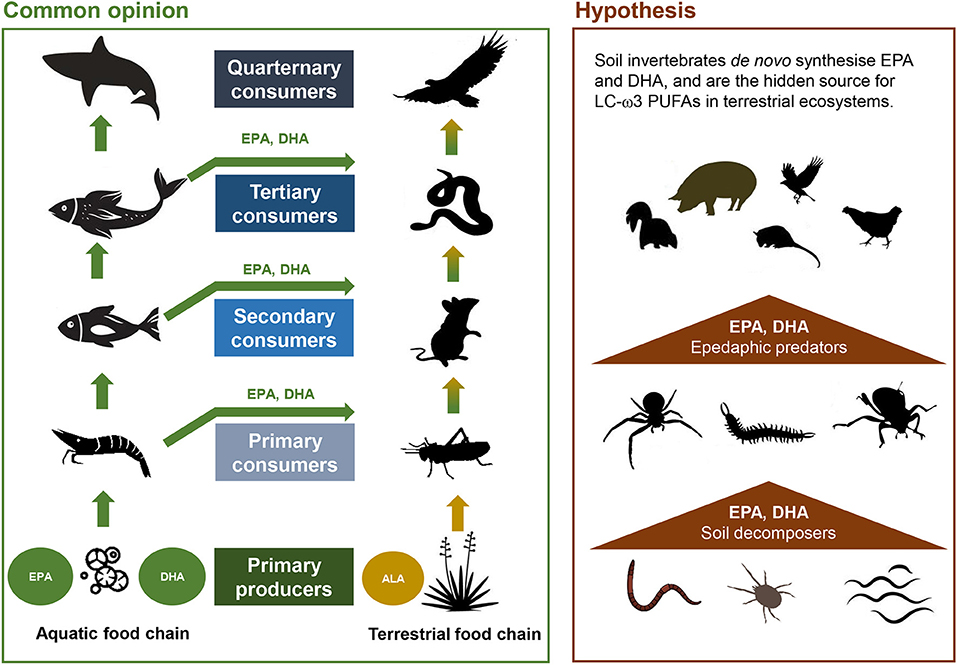
Figure 3. Proposed origin of long-chain polyunsaturated fatty acids (LC-PUFA) in terrestrial ecosystems. Left—transfer to land via different trophic levels of the aquatic food chain is commonly regarded as major input; right—soil decomposers are a neglected source. Please note that “hidden” channels such as mutualistic gut microbiota in e.g., earthworms or ruminants, are not specifically emphasized. ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. Illustration credit: https://vecteezy.com.
However, this hypothesis is controversial. For example, the highest DHA contents were not found in water birds, such as waterfowls feeding in aquatic habitats, but instead, in terrestrial feeders such as Passeriformes species (Gladyshev et al., 2016). Correspondingly, Fontaneto et al. (2011) suggested terrestrial insects, which besides ARA and ALA also comprise EPA, as a better source for LC-PUFA than aquatic insects due to their higher abundance and accessibility to terrestrial consumers. Moreover, the contribution of ω3PUFA derived from photosynthetic microalgae in biological soil crusts (Table 2) to food webs in nutrient poor and dry desert, dune and tundra habitats remains to be explored.
A so far neglected source of LC-ω3PUFA in terrestrial ecosystems is the soil fauna (Figure 3). Across a wide taxonomic range of soil invertebrates the capability to synthesize LC-ω3PUFA de novo was shown. The entire set of desaturases and elongases to produce LC-ω3PUFA occurs in the nematode Caenorhabditis elegans (Watts and Browse, 2002). Recent molecular studies proved the presence of ω3FA desaturase enzymes in several other free-living soil nematode taxa (Menzel et al., 2019) as well as in terrestrial oligochaetes and insects (Kabeya et al., 2018). EPA synthesis was further indicated for soil Collembola (Menzel et al., 2018) and earthworms (Petersen and Holmstrup, 2000).
Proportions of EPA in total fatty acids reported are 3.5% for free-living soil nematodes (Kühn et al., 2018) and up to 17.4% for yeast feed Collembola (Chamberlain and Black, 2005). In particular, earthworms (their body, gut and burrow lining) are a rich source for LC-ω3PUFA, with EPA being 7.5 times higher than in bulk soil (Sampredo et al., 2006). This represents a considerable source irrespective of whether LC-ω3PUFA are acquired by the animal itself or from gut microbiota. Comparable to aquatic food webs, EPA seems to be selectively retained in soil decomposer food chains as suggested by the occurrence in top predator such as centipedes and spiders (Pollierer et al., 2010; Ferlian et al., 2012). In sum, there is sufficient evidence that belowground biota build central deposits for essential LC-ω3PUFA, which can fuel higher trophic levels above ground (Figure 3).
Since humans have settled, they preferentially modulated riparian areas, which have a central role in LC-ω3PUFA production and export to land, thereby altering the ecological availability of these nutritional valuable biomolecules. A more recent anthropogenic impact is global climate change, i.e., elevated atmospheric CO2 and temperature. Temperature changes act predominantly on PUFA, due to their central role in maintaining membrane fluidity in poikilothermic species (Hazel, 1995). The proposed food web impacts of increased temperature focus on a general decline of LC-ω3PUFA in aquatic primary producers (e.g., Hixson and Arts, 2016). However, extrapolating temperature effects observed for algal cultures to a potential worldwide reduction in sestonic LC-ω3PUFA may be misleading. As algal group composition mainly drives ω3PUFA content, all factors altering community structure, including nutrient regimes and their interactions (Suikkanen et al., 2013) need to be considered, not solely temperature. A recent statistical model predicts a 12% decline of EPA in top predator fish at increasing ocean surface temperatures off Australia, in an area also prone to alterations in nutrient regime (Pethybridge et al., 2015). Moreover, trophic transfer can be impacted by the mismatch in timing rather than by the biochemical composition of the prey (e.g., Seldon et al., 2018). In any case, melting of polar ice with increasing temperatures will affect the occurrence of ice-algae as reliable source of EPA for zooplankton productivity in the Arctic (Leu et al., 2011) and Antarctic (Kohlbach et al., 2017).
In terrestrial plants, the decline of the LC-ω3PUFA precursor ALA with increasing temperature can reach 50% in soybeans (Byfield and Upchurch, 2007) and 77% in rapeseed (Namazkar et al., 2016). On one hand, this is due to low expression of ω3-FA desaturase genes, on the other hand it is induced by general temperature stress that reduces the overall fat and seed biomass. Higher growing temperatures also lower vitamin contents in fruits and vegetables, as shown for vitamin C in kiwi (Richardson et al., 2004) or broccoli (Kumar et al., 2016). With elevated temperature, alterations in PUFA pattern and vitamin contents are expected in herbivores (Adler et al., 2013 for cow milk) accompanied by changes of plant community structure from C3 to less nutritious C4 (Warne et al., 2010). Finally, temperature increase can provoke proteomic responses of microorganisms affecting biosynthesis of essential nutrients in decomposer food chains (Mosier et al., 2015).
The ecological influences of elevated atmospheric CO2 on essential biomolecules in aquatic ecosystems are not uniform, as CO2 can be a potential limiting resource for primary producer but also a stressor by lowering the pH. Ocean acidification can induce phytoplankton community shifts, which negatively influences trophic transfer of essential compounds (e.g., Rosoll et al., 2012). However, larval fish exhibited more whole-body fat at higher CO2, including essential FAs (Díaz-Gill et al., 2015). Studies combining effects of warming and acidification found minor or no changes in the essential AA content in marine diatoms (Bermúdez et al., 2015) but an increase in EPA in primary producer and mesozooplankton consumer (Wang et al., 2017). By using AA-specific δ13C measured in long living deep-sea corals, McMahon et al. (2015a) could detect a shift toward non N2-fixing cyanobacteria during warmer times, i.e., in the Medieval Climate Anomaly and the Industrial Era.
In terrestrial ecosystems, elevated CO2 per se and in combination with temperature increase, reduced ALA and vitamin C contents in fruits, oils and seeds of plants (Kahn et al., 2013; Namazkar et al., 2016). Increased droughts can up-regulate the metabolic pathways for thiamin (B1) reported for Zea mays (Rapala-Kozik et al., 2008), a response to enhance stress tolerance of the plant. Moreover, changes in land use affect the interactions between plants and the soil microbiome, altering vitamin exudation by roots as well as the production by rhizosphere bacteria. For example, herbicides reduced the production of B vitamins in the soil bacterium Azotobacter (Murcia et al., 1997). Overall, such changes in the rhizosphere as a hot spot for diverse microbial communities and, thus, metabolic pathways and substrates, have considerable impact on the occurrence and availability of essential biomolecules for higher trophic levels.
Essential biomolecule physiology recently achieved attention as a trait within evolutionary concepts (reviewed by Ellers et al., 2012). For example, the loss of de novo synthesis of vitamin C in many mammals is connected to ample access by frugivory, leading to “relaxed selection” sensu Lahti et al. (2009). Under “environmental compensation” lost traits can be functionally compensated by others, e.g., in the case of vitamin D dietary provision is substituted by light. According to the “Black Queen Hypothesis,” genes and related traits can be lost as the metabolites are provided as common goods by other members of a consortium (Morris et al., 2012).
Whether these evolutionary concepts will explain the environmental distribution in nutritional valuable molecules on a food web level warrants further investigation. With comparative genomics, Mee and Wang (2012) showed that AA de novo synthesis of Eukarya is restricted to 4.1 AA (humans 11 AA), whereas Archaean on average de novo synthesize 8.3 and bacteria 7.9 out of 20 AA, respectively. Corresponding to the constitutive costs proposed by Lahti et al. (2009), most organisms do not synthesize those AA, which require many biochemical steps, thus are metabolically more costly to produce. Moreover, intra- and extracellular production of AA and vitamins differ between free-living bacteria and biofilms in marine benthos (Johnson et al., 2016), suggesting provision of essential biomolecules by the consortium in line with the “Black Queen Hypothesis.” Essential extracellular metabolites such as AA and vitamins may even be a valuable “currency” in microbial cross-feeding (Seth and Taga, 2014) and for microbial grazers at the base of aquatic and terrestrial decomposer food webs.
The dynamics of ω3- and ω6PUFA in food webs, however, do not fit those evolutionary trait considerations of dependencies through adaptive gene loss. In a phylogenetic study across 54 invertebrate taxa, including Acari, Crustacea, Insecta, and Nematoda, no indication of a link between the dietary availability of LA and its de novo synthesis was detected, rather LA synthesis was facilitated by the bi-functionality of desaturase enzymes (Malcicka et al., 2018). Generally, fewer steps are involved in the synthesis of LC-PUFA than for many other essential biomolecules. LC-PUFA are prone to peroxidation if not protected by antioxidants like vitamin E, with peroxidation products being harmful (Finkel and Holbrook, 2000). This suggests that, in contrast to other nutritional biomolecules, the response to oxidative stress may be the trait here, and the costs are related to features mitigating the damaging effects of LC-PUFA peroxidation (Monaghan et al., 2009). Nevertheless, holding genes such as the Fads2 desaturase to de novo synthesize DHA, is considered important for recurrent freshwater colonization and radiation in fishes (Ishikawa et al., 2019).
Overall, synthesis of specific nutritional biomolecules can be lost by species or whole systematic groups if it is more efficient to obtain those from the environment. This converts them into essential dietary compounds, yet constraints appear to differ according to biochemical susceptibility and synthesis pathways.
Essential biomolecules play an important role in trophic interactions across taxa in aquatic and terrestrial ecosystems. Their availability at the base of the food web matters for the mode of trophic transfer, affecting food web structure as well as response to environmental changes. For example, algae and higher plants as primary carbon fixers do not provide all essential biomolecules, and e.g., Archaea are the main producer of B12. As its' biochemical functioning is tightly connected to other essential biomolecules (B vitamins and methionine), mutual to symbiotic trophic interactions are characteristic for provision. In contrast, LC-ω3PUFA and AA are mainly transferred via predator-prey interactions and their minor metabolic alterations predetermine them for dietary tracing. Particularly in combination with compound-specific stable isotope analyses they provide quantitative estimates of resource flows. Terrestrial and aquatic food webs differ considerably in the amount and distribution of essential biomolecules, e.g., in FAs, and interlinks are important, especially from water to land.
However, our knowledge on the function of essential biomolecules in food webs is far from complete. Future food web research questions to be addressed are: (1) How are food web assemblages in different biomes adapted to the local sources of essential nutritional compounds? (2) Are there considerable exchanges/fluxes in essential compounds between terrestrial herbivore and detritivore food chains? (3) How do food chain loops, such as the microbial loop, contribute to the dynamics of essential biomolecules, besides known trophic-upgrading within PUFA? (4) How does the availability of essential biomolecules affect the organization of biotic consortia, e.g., aquatic biofilms or rhizosphere microbiomes? (5) What is the impact of hidden sources, e.g., gut microbiota, on the reconstruction of food webs using essential biomolecules as trophic markers? (6) What is the general role of essential biomolecules in food web dynamics at higher trophic levels compared to the frequently investigated plant-herbivore interface? Finally, as the capability for de novo synthesis can act as trait in evolution, a new research field opens up, connected to questions on how essential biomolecules can impact adaptations and radiation of species, including the divers trophic interactions—from mutualism to parasitism.
LR and DM-N contributed equally to the conception and writing of the work as well as to its content and interpretation.
We acknowledge support by the German Research Foundation (DFG) and the Open Access Publication Fund of Humboldt-Universität zu Berlin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adler, S. A., Dahl, A. V., Jensen, S. K., Thuen, E., Gustavsson, A. M., and Steinshamm, H. (2013). Fatty acid composition, fat-soluble concentrations and oxidative stability in bovine milk produced on two pastures with different botanical composition. Livestock Sci. 154, 93–102. doi: 10.1016/j.livsci.2013.03.013
Ahlgren, G., Goedkoop, W., Markensten, H., Sonesten, L., and Boberg, M. (1997). Seasonal variations in food quality for pelagic and benthic invertebrates in lake Erken – the role of fatty acids. Freshw. Biol. 38, 555–570. doi: 10.1046/j.1365-2427.1997.00219.x
Ahlgren, G., Gustafsson, I. B., and Boberg, M. (1992). Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 28, 37–50. doi: 10.1111/j.0022-3646.1992.00037.x
Aseni-Fabado, M. A., and Munné-Bosch, S. (2010). Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 15, 582–592. doi: 10.1016/j.tplants.2010.07.003
Baker, E. R., Mc Laughlin, J. J. A., Hutner, S. H., DeAngelis, B., Feingold, S., Frank, O., et al. (1981). Water soluble vitamins in cells and spent culture supernatants of Proteriochromos stipitata, Euglena gracilis, and Tetrahymena thermophila. Arch. Microbiol. 129, 310–313. doi: 10.1007/BF00414703
Balk, L., Hägerroth, P. Å., Åkerman, G., Hanson, M., Tjärnlund, U., Hansson, T., et al. (2009). Wild birds of declining European species are dying from a thiamin deficiency syndrome. Proc. Nat. Acad. Sci. U.S.A. 106, 12001–12006. doi: 10.1073/pnas.0902903106
Baya, A. M., Boethling, R. S., and Ramos-Cormenzana, A. (1981). Vitamin production in relation to phosphate solubilization by soil bacteria. Soil Biol. Biochem. 13, 527–531. doi: 10.1016/0038-0717(81)90044-4
Bec, A., Perga, M.-E., Desvilettes, C., and Bourdier, G. (2010). How well can the fatty acid content of lake seston be predicted from its taxonomic composition? Freshw. Biol. 55, 1958–1972. doi: 10.1111/j.1365-2427.2010.02429.x
Behmer, S. T., and Nes, W. D. (2003). Insect sterol nutrition and physiology: a global overview. Adv. Insect. Physiol. 31, 1–72. doi: 10.1016/S0065-2806(03)31001-X
Bell, M. V., and Tocher, D. R. (2009). “Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: General pathways and new directions,” in Lipids in Aquatic Ecosystems, eds M.T. Arts, M.T. Brett, and M. Kainz (New York, NY: Springer), 211–236. doi: 10.1007/978-0-387-89366-2_9
Bermúdez, R., Feng, Y., Roleda, M. Y., Tatters, A. O., Hutchins, D. A., Larsen, T., et al. (2015). Long-term condition to elevated pCO2 and warming influences the fatty and amino acid composition of the diatom Cylindrotheca fusiformis. PLoS ONE 10:e0123945. doi: 10.1371/journal.pone.0123945
Bertrand, E. M., and Allen, A. E. (2012). Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front. Microbiol. 3:375. doi: 10.3389/fmicb.2012.00375
Bianchi, T. S., and Canuel, E. A. (2011). Chemical Biomarkers in Aquatic Ecosystems. Princeton: Princeton University Press.
Bleakley, S., and Hayes, M. (2017). Algal proteins: Extraction, application, and challenges concerning production. Foods 6:33. doi: 10.3390/foods6050033
Borisova, E. V., Makhutova, O. N., Gladyshev, M. I., and Sushchik, N. N. (2016). Fluxes of biomass and essential polyunsaturated fatty acids from water to land via chironomid emergence from a mountain lake. Contemp. Problems Ecol. 9, 446–457. doi: 10.1134/S199542551604003X
Brett, M. T., Bunn, S. E., Chandra, S., Galloway, A. W., Guo, F., Kainz, M. J., et al. (2017). How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 62, 833–853. doi: 10.1111/fwb.12909
Brett, M. T., and Müller-Navarra, D. C. (1997). The role of highly unsaturated fatty acids in aquatic food web processes. Freshw. Biol. 38, 483–499. doi: 10.1046/j.1365-2427.1997.00220.x
Brett, M. T., Müller-Navarra, D. C., and Person, J. (2009). “Crustacean zooplankton fatty acids composition,” in Lipids in Aquatic Ecosystems, eds M.T. Arts, M.T. Brett, and M. Kainz, (New York, NY: Springer, 115–146.
Burden, R. S., Cooke, D. T., and Carter, G. A. (1989). Inhibitors of sterol biosynthesis and growth in plants and fungi. Phytochemistry 28, 1791–1804. doi: 10.1016/S0031-9422(00)97862-2
Burian, A., Grosse, J., Winder, M., and Boschker, H. T. S. (2018). Nutrient deficiencies and the restriction of compensatory mechanisms in copepods. Funct. Ecol. 32, 636–647. doi: 10.1111/1365-2435.13016
Buse, T., Ruess, L., and Filser, J. (2013). New trophic biomarkers for Collembola reared on algal diets. Pedobiologia 56, 153–159. doi: 10.1016/j.pedobi.2013.03.005
Byfield, G. E., and Upchurch, R. G. (2007). Effect of temperature on microsomal omega-3 linoleate desaturase gene expression and linoleic acid content in developing soybean seeds. Crop Sci. 47, 2445–2452. doi: 10.2135/cropsci2007.05.0263
Cantalapiedra-Hijar, G., Ortigues-Marty, I., Schiphorst, A. M., Robins, R. J., Tea, I., and Prache, S. (2016). Natural 15N abundance in key amino acids from lamb muscle: Exploring a new horizon in diet authentication and assessment of fee efficiency in ruminants. J. Agric. Food Chem. 64, 4058–4067. doi: 10.1021/acs.jafc.6b00967
Carlucci, A. F., and Bowes, P. M. (1970). Vitamin production and utilization by phytoplankton in mixed culture. J. Phycol. 6, 393–400. doi: 10.1111/j.1529-8817.1970.tb02413.x
CasaNova, C., Bozelli, R., Spitzy, A., and Müller-Navarra, D. C. (2018). Living in a browning environment: effects on Daphnia's growth and fatty acid pattern. Limnol. Oceanogr. 64, 18–31. doi: 10.1002/lno.11016
Chamberlain, P. M., and Black, H. I. J. (2005). Fatty acid composition of collembola: unusually high proportions of C20 polyunsaturated fatty acids in a terrestrial invertebrate. Comp. Biochem. Physiol. 140, 299–307. doi: 10.1016/j.cbpc.2004.10.016
Chi, X., Javidpour, J., Sommer, U., and Müller-Navarra, D. C. (2018). Tracking fatty acids from phytoplankton to jellyfish polyps under different stress regimes: a three trophic levels experiment. Front. Ecol. Evol. 6:118. doi: 10.3389/fevo.2018.00118
Chikaraishi, Y., Kashiyama, Y., Ogawa, N. O., Kitazato, H., and Ohkouchi, N. (2007). Metabolic control of nitrogen stable isotope composition of amino acids in macroalgae and gastropods: implications for aquatic food web studies. Marin. Ecol. Progr. Ser. 342, 85–90. doi: 10.3354/meps342085
Chikaraishi, Y., Ogawa, N. O., and Ohkouchi, N. (2010). “Further evaluation on the trophic level estimation based on nitrogen isotopic composition of amino acids,” in Earth, Life, and Isotopes, eds N. Ohkouchi, I, Tayusu, and K. Koba (Kyoto: Kyoto University Press, 37–51.
Chitwood, D. J. (1999). Biochemistry and function of nematode steroids. Critic. Rev. Biochem. Mol. Biol. 34, 273–284. doi: 10.1080/10409239991209309
Chitwood, D. J., and Lusby, W. R. (1991). Metabolism of plant sterols by nematodes. Lipids 26, 619–627. doi: 10.1007/BF02536426
Choi, B., Ha, S.-Y., Lee, J. S., Chikaraishi, Y., Ohkouchi, N., and Shin, K.-H. (2017). Trophic interaction among organisms in a seagrass meadow ecosystem as revealed by bulk δ13C and amino acid δ15N analyses. Linmol. Oceanogr. 62, 1426–1435. doi: 10.1002/lno.10508
Chu, F.-L., Lund, E. D., Litteral, P. R., Ruck, K. E., and Harvey, E. (2009). Species-specific differences in long-chain n-3 essential fatty acid, sterol, and steroidal ketone production in six heterotrophic protist species. Aquat. Biol. 6, 159–172. doi: 10.3354/ab00174
Cobelas, M. A., and Lechado, J. Z. (1989). Lipids in microalgae. a review. I. Biochemistry. Grasas y Aceites 40, 118–145.
Colombo, S. M., Wacker, A., Parrish, C. C., Kainz, M. J., and Arts, M. T. (2017). A fundamental dichotomy in long-chain polyunsaturated fatty acid abundance between and within marine and terrestrial ecosystems. Environ. Rev. 25, 163–174. doi: 10.1139/er-2016-0062
Croft, M. T., Lawrence, A. D., Raux-Deery, E., Warren, M. J., and Smith, A. G. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 438, 90–93. doi: 10.1038/nature04056
Croft, M. T., Warren, M. J., and Smith, A. G. (2006). Algae need their vitamins. Eukaryot. Cell 5, 1175–1183. doi: 10.1128/EC.00097-06
Cuvelier, C., Dotreppe, O., and Istasse, L. (2003). Chemistry, nutritional sources and analyses of vitamin E. Ann. Med. Veterin. 147, 315–324.
Czimcik, C. I., Treseder, K. K., Carbone, M. S., and Trumbore, S. E. (2005). Radiocarbon – a low-impact tool to study nutrient transport by soil fungi under field conditions. New Phytol. 166, 595–600. doi: 10.1111/j.1469-8137.2005.01326.x
Dalsgaard, J., St. John, M., Kattner, G., Müller-Navarra, D. C., and Hagen, W. (2003). Fatty acid trophic markers in the pelagic marine environment. Adv. Marin Biol. 46, 225–340. doi: 10.1016/S0065-2881(03)46005-7
Davis, B. C., and Kris-Etherton, P. M. (2003). Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am. J. Clin. Nutr. 78, 640s-646s. doi: 10.1093/ajcn/78.3.640S
DeLong, E. F., and Yayanos, A. A. (1986). Biochemical function and significance of novel bacterial lipids in deep-sea procaryotes. Appl. Environ. Microbiol. 51, 730–737.
DeLuca, H. F. (1998). The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 2, 224–236. doi: 10.1096/fasebj.2.3.3280376
Desvilettes, C., Bourdier, G., Amblard, C., and Barth, B. (1997). Use of fatty acids for the assessment of zooplankton grazing on bacteria, protozoans and microalgae. Freshw. Biol. 38, 629–637. doi: 10.1046/j.1365-2427.1997.00241.x
Díaz-Gill, C., Catalán, I. A., Palmer, M., Faulk, C. K., and Fuiman, L. A. (2015). Ocean acidification increases fatty acids levels of larval fish. Biol. Lett. 11:20150331. doi: 10.1098/rsbl.2015.0331
Douglas, A. E. (2009). The microbial dimension of insect nutritional ecology. Funct. Ecol. 23, 38–47. doi: 10.1111/j.1365-2435.2008.01442.x
Doxey, A. C., Kurtz, D. A., Lynch, M. D., Sauder, L. A., and Neufeld, J. D. (2015). Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J. 9, 461–471. doi: 10.1038/ismej.2014.142
Drazen, J. C., Phleger, C. F., Guest, M. A., and Nichols, P. D. (2008). Lipid, sterols and fatty acids of abyssal holothurians and ophiuroids from the North-East Pacific Ocean: food web implications. Comp. Biochem. Physiol, Part B 151, 79–87. doi: 10.1016/j.cbpb.2008.05.013
Droop, M.R. (2007). Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J. Plankt. Res. 29, 107–113. doi: 10.1093/plankt/fbm009
Ellers, J., Kiers, E. T., Currie, C. R., McDonald, B. R., and Visser, B. (2012). Ecological implications drive evolutionary loss of traits. Ecol. Lett. 15, 1017–1082. doi: 10.1111/j.1461-0248.2012.01830.x
Fantle, M. S., Dittel, A. I., Schwalm, S. M., Epifanio, C. E., and Fogel, M. L. (1999). A food web analysis of the juvenile blue crab, Callinectes sapidus, using stable isotopes in whole animals and individual amino acids. Oecologia 120, 416–426. doi: 10.1007/s004420050874
Ferlian, O., Scheu, S., and Pollierer, M. (2012). Trophic interactions in centipedes (Chilopoda, Myriapoda) as indicates by fatty acid patterns: Variation with life stage, forest and season. Soil Biol. Biochem. 52, 33–42. doi: 10.1016/j.soilbio.2012.04.018
Finkel, T., and Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247. doi: 10.1038/35041687
Fiore, C.L., Labrie, M., Jarett, J.K., and Lesser, M.P. (2015). Transcriptional activity of the giant barrel sponge Xestos pongiamuta holobiont: molecular evidence for metabolic exchange. Front. Microbiol. 6:364. doi: 10.3389/fmicb.2015.00364
Fleurence, J., Gutbier, G., Mabeau, S., and Leray, C. (1994). Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 6, 527–532. doi: 10.1007/BF02182406
Fontaneto, D., Tommaseo-Ponzetta, M., Galli, C., Ris,é, P., Glew, R.H., and Paoletti, M.G. (2011). Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecol. Food Nutr. 50, 351–367. doi: 10.1080/03670244.2011.586316
Galloway, A. W., Brett, M. T., Holtgrieve, G. W., Ward, E. J., Ballantyne, A. P., Burns, C. W., et al. (2015). A fatty acid based Bayesian approach for inferring diet in aquatic consumers. PLOS ONE 10:e0129723. doi: 10.1371/journal.pone.0129723
Galloway, A. W. E., and Winder, M. (2015). Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS ONE 10:e0130053. doi: 10.1371/journal.pone.0130053
Gergs, R., Steinberger, N., Beck, B., Basen, T., Yohannes, E., Schulz, R., et al. (2015). Compound-specific δ13C analyses reveal sterol metabolic constraints in an aquatic invertebrate. Rapid Com. Mass Spectrom. 29, 1789–1794. doi: 10.1002/rcm.7279
Gerphagnon, M., Agha, R., Martin-Creuzburg, D., Bec, A., Perriere, F., Rad-Menéndez, C., et al. (2019). Comparison of sterol and fatty acid profiles of chytrids and their hosts reveals trophic upgrading of nutritionally inadequate phytoplankton by fungal parasites. Environ. Microbiol. 21, 949–958. doi: 10.1111/1462-2920.14489.
Gilbert, J. J. (1980). Female polymorphism and sexual reproduction in the rotifer Asplanchna: evolution of their relationship and control by dietary tocopherol. Amer. Nat. 116, 409–431. doi: 10.1086/283635
Gladyshev, M. I., Popova, O. N., Makhutova, O. N., Zinchenko, T. D., Golovatyuk, L. V., Yurchenko, Y. A., et al. (2016). Comparison of fatty acid composition in birds feeding in aquatic and terrestrial ecosystems. Contemp. Problems Ecol. 9, 503–513. doi: 10.1134/S1995425516040065
Gladyshev, M. I., Suschik, N. N., Kolmakova, A. A., Kalachova, G. S., Kravchuk, E. S., Ivanova, E. A., et al. (2007). Seasonal correlations of elemental and ω3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Sibirian reservoir. Aquat. Ecol. 41, 9–23. doi: 10.1007/s10452-006-9040-8
Gladyshev, M. I., Sushchik, N. N., and Makhutova, O. N. (2013). Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostag. oth. Lipid M. 107, 117–126. doi: 10.1016/j.prostaglandins.2013.03.002
Gladyshev, M. J., Arts, M. T., and Sushchik, N. N. (2009). “Preliminary estimates of the export of omega-3 highly unsaturated fatty acids (EPA + DHA) from aquatic to terrestrial ecosystems,” in Lipids in Aquatic Ecosystems, eds M. T. Arts, M. T. Brett, and M. Kainz, (New York, NY: Springer, 179–209.
Gladyshev, M. J., Sushchik, N. N., Anishchenko, O. V., Makhuttova, O. N., Kolmakov, V. I., Kalachova, G. S., et al. (2011). Efficiency of transfer of essential polyunsaturated fatty acids versus organic carbon from producers to consumers in a eutrophic reservoir. Oecologia 163, 521–531. doi: 10.1007/s00442-010-1843-6
Goad, I. J. (1981). Sterol biosynthesis and metabolism in marine invertebrates. Pure Appl. Chem. 51, 837–852. doi: 10.1351/pac198153040837
Gobler, C.J., Norman, C., Panzea, C., Taylor, G.T., and Sañudo-Wilhelmy, S. A. (2007). Effect of B-vitamins (B1, B12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquat. Microb. Ecol. 49, 181–194. doi: 10.3354/ame01132
Gong, Y., Wan, X., Jiang, M., Hu, C., Hu, H., and Huang, F. (2014). Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog. Lipid Res. 56, 19–35. doi: 10.1016/j.plipres.2014.07.001
Grayston, S. J., Vaughan, D., and Jones, D. (1996). Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5, 29–56. doi: 10.1016/S0929-1393(96)00126-6
Guisande, C., Bartumeus, F., Ventura, M., and Catalan, J. (2003). Role of food partitioning in structuring the zooplankton community in mountain lakes. Oecologia 136, 627–634. doi: 10.1007/s00442-003-1306-4
Guo, F., Kainz, M. J., Sheldon, F., and Bunn, S. E. (2016). The importance of high-quality algal food sources in stream food webs – current status and future perspectives. Freshw. Biol. 61, 815–831. doi: 10.1111/fwb.12755
Gupta, A., Barrow, C. J., and Puri, M. (2012). Omega biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol Adv. 30, 1733–1745. doi: 10.1016/j.biotechadv.2012.02.014
Guschina, I. A., and Harwood, J. I. (2009). “Algal lipid and effects of the environment on their biochemistry,” in Lipids in Aquatic Ecosystems, eds M. T. Arts, M. T. Brett, and M. Kainz (New York, NY: Springer), 1–24. doi: 10.1007/978-0-387-89366-2_1
Hartwich, M., Straile, D., Gaedke, U., and Wacker, A. (2012). Use of ciliate and phytoplankton taxonomic composition for the estimation of eicosapentaenoic acid in lakes. Freshw. Biol. 57, 1385–1398. doi: 10.1111/j.1365-2427.2012.02799.x
Hassett, R. P. (2004). Supplementation of a diatom diet with cholesterol can enhance copepod egg-production rates. Limnol. Oceanogr. 49, 488–494. doi: 10.4319/lo.2004.49.2.0488
Haubert, D., Birkhofer, K., Fließbach, A., Gehre, M., Scheu, S., and Ruess, L. (2009). Trophic structure and major trophic links in conventional versus organic farming systems as indicated by carbon stable isotope ratios of fatty acids. Oikos 118, 1579–1589. doi: 10.1111/j.1600-0706.2009.17587.x
Haubert, D., Häggblom, M. M., Langel, R., Scheu, S., and Ruess, L. (2006). Trophic shift of stable isotopes and fatty acids in Collembola on bacterial diets. Soil Biol. Biochem. 38, 2004–2007. doi: 10.1016/j.soilbio.2005.11.031
Hazel, J. R. (1995). Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Ann. Rev. Physiol. 57, 19–42. doi: 10.1146/annurev.ph.57.030195.000315
Helliwell, K. E., Lawrence, A. D., Holzer, A., Kudahl, U. J., Sasso, S., Kräutler, B., et al. (2016). Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr. Biol. 26, 999–1008. doi: 10.1016/j.cub.2016.02.041
Hixson, S. M., and Arts, M. T. (2016). Climate warming is predicted to reduce omega-3 long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Change Biol. 22, 2744–2755. doi: 10.1111/gcb.13295
Hixson, S. M., Sharma, B., Kainz, M. J., Wacker, A., and Arts, M. T. (2015). Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: a fundamental dichotomy between freshwater and terrestrial ecosystems. Environ. Rev. 23, 414–424. doi: 10.1139/er-2015-0029
Huang, J. J., Lin, S., Xu, W., and Cheung, P. C. K. (2017). Occurrence and biosynthesis of carotenoids in phytoplankton. Biotech. Adv. 35, 597–618. doi: 10.1016/j.biotechadv.2017.05.001
Hulbert, A. J., and Abbott, S. K. (2012). Nutritional ecology of essential fatty acids: an evolutionary perspective. Aust. J. Zool. 59:369–379. doi: 10.1071/ZO11064
Hulbert, A. J., Kelly, M. A., and Abbot, S. K. (2014). Polyunsaturated fats, membrane lipids and animal longevity. J. Comp. Physiol. B 184, 149–166. doi: 10.1007/s00360-013-0786-8
Hunter, D. J., Torkelson, J. L., Mortazavi, B., Laurent, T., Deason, J., Thephavongsa, K., et al. (2015). The rickettsia endosymbiont of Ixodes pacificus contains all the genes of de novo folate biosynthesis. PLoS ONE 12:e0144552. doi: 10.1371/journal.pone.0144552
Ishikawa, A., Kabeya, N., Ikeya, K., Kakioka, R., Cech, J. N., Osada, N., et al. (2019). A key metabolic gene for recurrent freshwater colonization and radiation in fishes. Science 364, 886–889. doi: 10.1126/science.aau5656
Iverson, S. J., Field, C., Bowen, W. D., and Blanchard, W. (2004). Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol. Monogr. 74, 211–235. doi: 10.1890/02-4105
Jäpelt, R. B., and Jakobsen, J. (2013). Vitamin D in plants: a review of occurrence, analysis and biosynthesis. Front. Plant Sci. 4:123. doi: 10.3389/fpls.2013.00136
Joergensen, R. G., and Wichern, F. (2008). Quantitative assessemnt of the fungal contribution to microbial tissue in soil. Soil Biol. Biochem. 40, 2977–2991. doi: 10.1016/j.soilbio.2008.08.017
Johnson, W. M., Kido Soule, M. C., and Kujawinski, E. B. (2016). Evidence for quorum sensing and differential metabolite production by a marine bacterium in response to DMSP. ISME J. 19, 2304–2316. doi: 10.1038/ismej.2016.6
Jónasdóttir, S. H. (2019). Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 17:151. doi: 10.3390/md17030151
Junk, W., Bayley, P. B., and Sparks, R. E. (1989). The flood pulse concept in river-floodplain systems. Can J. Fish. Aquat. Sci. 106, 110–127.
Kabeya, N., Fonseca, M. M., Ferrier, D. E. K., Navarro, J. C., Bay, L. K., Francis, D. S., et al. (2018). Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci. Adv. 4:eaar6849. doi: 10.1126/sciadv.aar6849
Kahn, I., Azam, A., and Mahmood, A. (2013). The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acids and vitamin C contents of tomato (Lycopersicon esculentum). Environ. Monitor. Assess. 185, 205–214. doi: 10.1007/s10661-012-2544-x
Kelly, J. R., and Scheibling, R. E. (2012). Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 446, 1–22. doi: 10.3354/meps09559
Koch, F., Hattenrath-Lehmann, T. K., Goleski, J. A., Sañudo-Wilhelmy, S. A., Fisher, N. S., and Gobler, C. J. (2012). Vitamin B1 and B12 uptake and the cycling by plankton communities in coastal ecosystems. Front. Microbiol. 3, 363. doi: 10.3389/fmicb.2012.00363
Koch, F., Marcoval, M. A., Panzeca, C., Bruland, K. W., Sañudo-Wilhelmy, S. A., and Gobler, C. J. (2011). The effect of vitamin B12 on phytoplankton growth and community structure in the Gulf of Alaska. Limnol. Oceanogr. 56, 1023–1034. doi: 10.4319/lo.2011.56.3.1023
Kohlbach, D., Lange, B. A., Schaafsma, F. L., David, C., Vortkamp, M., Graeve, M., et al. (2017). Ice algae-produced carbon is critical for overwintering of Antarctic krill Euphausia superba. Front Mar. Sci. 4:310. doi: 10.3389/fmars.2017.00310
Krauss, G. J., Sol,é, M., Krauss, G., Schlosser, D., Wesenberg, D., and Bärlocher, F. (2011). Fungi in freshwaters: ecology, physiology, and biochemical potential. FEMS 35, 620–651. doi: 10.1111/j.1574-6976.2011.00266.x
Kühn, J., Richter, A., Kahl, T., Bauhus, J., Schöning, I., and Ruess, L. (2018). Community level lipid profiling of consumers as a tool for soil food web diagnostics. Methods Ecol. Evol. 9, 1265–1275. doi: 10.1111/2041-210X.12966
Kumar, M., Kumari, P., Singh, S. K., Prasad, K. K., and Singh, P. K. (2016). Quality and yield response of broccoli to air temperature under integrated nutrient management. Ann. Biol. 32, 36–40.
Lahti, D. C., Johnson, N. A., Ajie, B. C., Otto, S. P., Hendry, A. P., Blumenstein, D. T., et al. (2009). Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496. doi: 10.1016/j.tree.2009.03.010
Larsen, T., Pollierer, M. M., Holmstrup, M., D'Annibale, A., Maraldo, K., Andersen, N., et al. (2016). Substantial nutritional contribution of bacterial amino acids to earthworms and enchytraeids: a case study from organic grassland. Soil Biol. Biochem. 99, 21–27. doi: 10.1016/j.soilbio.2016.03.018
Larsen, T., Taylor, D. L., Leigh, M. B., and O'Brien, D. M. (2009). Stable isotope fingerprinting: a novel method for identifying plant, fungal, or bacterial origins of amino acids. Ecology 90, 3526–3535. doi: 10.1890/08-1695.1
Leu, E., Søreide, J. E., Hessen, D. O., Falk-Petersen, S., and Berge, J. (2011). Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: timing, quantity, and quality. Prog. Oceanogr. 90, 18–32. doi: 10.1016/j.pocean.2011.02.004
Lewin, R. A. (1961). “Phytoflagellates and algae,” in Growth and growth substances. Encyclopedia of plant physiology XIV, ed H. Burgström (Berlin, Göttingen, Heidelberg: Springer, 401–417.
Litchi, D. A., Rinchard, J., and Kimmel, D. G. (2017). Changes in zooplankton community, and seston and zooplankton fatty acid profiles at the freshwater/saltwater interface of the Chowan River, North Carolina. Peer J. 5:e3667. doi: 10.7717/peerj.3667
Lösel, D. M. (1988). “Fungal Lipids,” in Microbial Lipids, eds C. Ratledge, and S.G. Wilkinson (London: Academic Press, 699–806.
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V., and Thiele, I. (2015). Systematic genome assessment of B-vitamin biosynthesis suggests cooperation among gut microbes. Front. Microbiol. 6:148. doi: 10.3389/fgene.2015.00148
Malcicka, M., Visser, B., and Ellers, J. (2018). An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 45, 15–26. doi: 10.1007/s11692-017-9436-5
Marcquenski, S. V., and Brown, S. B. (1997). “Early mortality syndrome in the Great Lakes,” in Chemically Induced Alterations in Functional Development and Reproduction in Fishes, eds R. M. Rolland, M. Gilbertson, and R. E. Peterson. (Pensacola, FL: Soc. Environm. Tox. Chem., 135–152.
Marsh, E. N. (1999). Coenzyme B12 (cobalamin)-dependent enzymes. Essays Biochem. 34, 139–154. doi: 10.1042/bse0340139
Martin-Creuzburg, D., Beck, B., and Freese, H. M. (2011). Food quality of heterotrophic bacteria for Daphnia magna: evidence for a limitation by sterols. FEMS Microbiol. Ecol. 76, 592–601. doi: 10.1111/j.1574-6941.2011.01076.x
Martin-Creuzburg, D., and Merkel, P. (2016). Sterols of freshwater microalgae: Potential implications for zooplankton nutrition. J. Plankt. Res. 38, 865–877. doi: 10.1093/plankt/fbw034
Martin-Creuzburg, D., Oexle, S., and Wacker, A. (2014). Thresholds for sterol-limited growth of Daphnia magna: a comparative approach using 10 different sterols. J. Chem. Ecol. 40, 1039–1050. doi: 10.1007/s10886-014-0486-1
Martin-Creuzburg, D., Sperfeld, E., and Wacker, A. (2009). Co-limitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc. R. Soc. B 276, 1805–1814. doi: 10.1098/rspb.2008.1540
Martin-Creuzburg, D., and von Elert, E. (2009). “Ecological significance of sterols in aquatic food webs,” in Lipids in Aquatic Ecosystems, eds M. T. Arts, M. Brett and M. Kainz (New York, NY: Springer, 43–64.
Mayor, D. J., Cook, K., Thornton, B., Walsham, P., Witte, U. F. M., Zuur, A. F., et al. (2011). Absorption efficiencies and basal turnover of C, N and fatty acids in a marine Calanoid copepod. Funct. Ecol. 25, 509–518. doi: 10.1111/j.1365-2435.2010.01791.x
McCutcheon, J. P., McDonald, B. R., and Moran, N. A. (2009). Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Nat Acad Sci U.S.A. 106, 36. doi: 10.1073/pnas.0906424106
McMahon, K. W., and McCarthy, M. D. (2016). Embracing variability in amino acid δ15N fractionation: mechanisms, implications, and applications for trophic ecology. Ecosphere 7, e01511. doi: 10.1002/ecs2.1511
McMahon, K. W., McCathy, M. D., Sherwood, O. A., Larsen, T., and Guilderson, T. P. Millenial-scale plankton regime shifts in the subtropical North Pacific Ocean. (2015a). Science 350, 1530–1533. doi: 10.1126/science.aaa9942
McMahon, K. W., Polito, M. J., Abel, S., McCarthy, M. D., and Thorrold, S. R. (2015b). Carbon and nitrogen isotope fractionation of amino acids in an avian marine predator, the Gentoo penguin (Pygoscelis papua). Ecol. Evol. 5, 1278–1290. doi: 10.1002/ece3.1437
McMahon, K. W., Thorrold, S. R., Houghton, L. A., and Berumen, M. L. (2016). Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia 180, 809–821. doi: 10.1007/s00442-015-3475-3
McRose, D., Guo, J., Monier, A., Sudek, S., Wilken, S., Yan, S., et al. (2014). Alternatives to vitamin B1 uptake revealed with discovery of riboswitches in multiple marine eukaryotic lineages. ISME J. 8, 2517–2529. doi: 10.1038/ismej.2014.146
Mee, M. T., and Wang, H. H. (2012). Engineering ecosystems and synthetic ecologies. Mol. BioSystems 8, 2470–2483. doi: 10.1039/c2mb25133g
Menzel, R., Geweiler, D., Sass, A., Simsek, D., and Ruess, L. (2018). Nematodes as important source for omega-3 long-chain fatty acids in the soil food web and the impact in nutrition for higher trophic levels. Front. Ecol. Evol. 6:96. doi: 10.3389/fevo.2018.00096
Menzel, R., von Chrzanowski, H., Tonat, T., van Riswyck, K., Schliesser, P., and Ruess, L. (2019). Presence or absence? Primary structure, regioselectivity and evolution of Δ12/Δ15 fatty acid desaturases in nematodes. BBA – Mol. Cell Biol. Lipids 1864, 1194–1205. doi: 10.1016/j.bbalip.2019.05.001
Molketin, J., and Giesemann, A. (2007). Differentiation of organically and conventionally produced milk by stable isotope and fatty acid analysis. Anal. Bioana. Chem. 388, 297–305. doi: 10.1007/s00216-007-1222-2
Monaghan, P., Metcalfe, N. B., and Torres, R. (2009). Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. doi: 10.1111/j.1461-0248.2008.01258.x
Monroig, Ó., and Kabeya, N. (2018). Desaturases and elongases in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: a comprehensive review. Fish. Sci. 84, 911–928. doi: 10.1007/s12562-018-1254-x
Moran, N. A., Plague, G. R., Sandström, J. P., and Wilcox, J. L. (2003). A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc Nat Acad Sci U.S.A. 100, 14543–14548. doi: 10.1073/pnas.2135345100
Moreau, R. A., Nyström, L., Whitaker, B. D., Winkler-Moser, J. K., Baer, D. J., Gebauer, S. K., et al. (2018). Phytosterols and their derivates: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Progr. Lipid Res. 70, 35–61. doi: 10.1016/j.plipres.2018.04.001
Morris, J. G. (1985). Nutritional and metabolic responses to arginine deficiency in carnivores. J. Nutr. 115, 524–531. doi: 10.1093/jn/115.4.524
Morris, J. J., Lenski, R. E., and Zinser, E. R. (2012). The black queen hypothesis: evolution of dependencies through adaptive gene loss. mBio 3, e00036–e00012. doi: 10.1128/mBio.00036-12
Mosier, A. C., Zhou, L., Thomas, B. C., Hettich, R. L., Pan, C., and Banfield, J. F. (2015). Elevated temperature alters proteomic responses in individual organisms within a biofilm community. ISME J. 9, 180–194. doi: 10.1038/ismej.2014.113
Müller-Navarra, D. (1995a). Evidence that a highly unsaturated fatty acid limits Daphnia in nature. Arch. Hydrobiol. 132, 297–307.
Müller-Navarra, D. C. (1995b). Biochemical versus mineral limitation in Daphnia. Limnol. Oceanogr. 40, 1209–1214. doi: 10.4319/lo.1995.40.7.1209
Müller-Navarra, D. C. (2008). Food web paradigms: the biochemical view on trophic interactions. Internat. Rev. Hydrobiol. 93, 489–505. doi: 10.1002/iroh.200711046
Müller-Navarra, D. C., Brett, M. T., Liston, A. M., and Goldman, C. R. (2000). A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403, 74–77. doi: 10.1038/47469
Müller-Navarra, D. C., Brett, M. T., Park, S.-K., Chandra, S., Ballantyne, A. P., Zorita, E., et al. (2004). Unsaturated fatty acid content in seston and trophy-dynamic coupling in lakes. Nature 427, 69–72. doi: 10.1038/nature02210
Murcia, R., Rodelas, B., Salmerón, M. V., Martínez-Toleda, J., and González-López, J. (1997). Effects of the herbicide simazine on vitamin production by Azotobacter chroococcum and Azotobacter vinelandii. Appl. Soil Ecol. 6, 187–193. doi: 10.1016/S0929-1393(96)00143-6
Namazkar, S., Stockmarr, A., Frenck, G., Egsgaard, H., Terkelsen, T., Mikkelsen, T., et al. (2016). Concurrent elevation of CO2, O3 and temperature severely affects oil quality in rapeseed. J. Exp. Botany 67, 4117–4125. doi: 10.1093/jxb/erw180
Nanton, D. A., and Castell, J. D. (1998). The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod Tisbe sp. for use as a live food for marine fish larvae. Aquaculture 163, 251–261. doi: 10.1016/S0044-8486(98)00236-1
Navarro, J. C., Monroig, O., and Sykes, A. V. (2014). “Nutrition as a key factor for cephalopod aquaculture,” in Cephalopod Culture, eds J. Iglesias, L. Fuentes, and R. Villanueva. (New York, NY: Springer, 77–96.
Newbold, C. J., de la Fuente, G., Belanche, A., Ramos-Mrales, E., and McEwan, N. R. (2015). The role of ciliate protozoa in the rumen. Front. Microbiol. 6:1313. doi: 10.3389/fmicb.2015.01313
Ngosong, C., Raupp, J., Richnow, H. H., and Ruess, L. (2011). Tracking Collembola feeding strategies by the natural 13C signal of fatty acids in an arable soil with different fertilizer regimes. Pedobiologia 54, 225–233. doi: 10.1016/j.pedobi.2011.02.004
Nielsen, J. M., Popp, B. N., and Winder, M. (2015). Meta-analysis of amino acid stable nitrogen isotope ratios for estimating trophic position in marine organisms. Oecologia 178, 631–642. doi: 10.1007/s00442-015-3305-7
Nwachukwu, I. D., Kouritzin, T. M., Aluko, R. E., and Myrie, S. B. (2017). The role of omega-3 fatty acids in skeletal muscle anabolism, strength, and function in healthy and diseased states. Food Biochem. 41:e12435. doi: 10.1111/jfbc.12435
O'Conell, T. C. (2017). ‘Trophic' and ‘source' amino acids in trophic estimation: a likely metabolic explanation. Oecologia 184, 317–326. doi: 10.1007/s00442-017-3881-9
Paerl, R. W., Bertrand, E. M., Allen, A. E., Palenik, B., and Azam, F. (2015). Vitamin B1 ecophysiology of marine picoeukaryotic algae: Strain-specific differences and a new role for bacteria in vitamin cycling. Limnol. Oceanogr. 60, 215–228. doi: 10.1002/lno.10009
Parrish, C. C. (2009). “Essential fatty acids in aquatic food webs,” in Lipids in Aquatic Ecosystems, eds M. T. Arts, M. T. Brett, and M. Kainz, (New York, NY: Springer), 309–326. doi: 10.1007/978-0-387-89366-2_13
Pausch, J., Kramer, S., Scharroba, A., Scheunemann, N., Butenschoen, O., Kandeler, E., et al. (2016). Small but active pool size does not matter for carbon incorporation in below-ground food webs. Funct. Ecol. 30, 479–489. doi: 10.1111/1365-2435.12512
Pearson, A., Budin, M., and Brocks, J. J. (2003). Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc Nat Acad Sci U.S.A. 100, 15352–15357. doi: 10.1073/pnas.2536559100
Peltomaa, E., Johnson, M. D., and Taipale, S. J. (2017). Marine crypotphytes are great sources of EPA and DHA. Mar. Drugs 16:E3. doi: 10.3390/md16010003
Pereira, H., Barreira, L., Figueiredo, F., Custódie, L., Vizetto-Duarte, C., Polo, C., et al. (2012). Polyunsaturated fatty acids of marine macroalgae: potential for nutritional and pharmaceutical applications. Mar. Drugs 10, 1920–1935. doi: 10.3390/md10091920
Petersen, S. O., and Holmstrup, M. (2000). Temperature effects on lipid composition of the earthworms Lumbricus rubellus and Eisenia nordenskioeldi. Soil Biol. Biochem. 32, 1787–1791. doi: 10.1016/S0038-0717(00)00059-6
Pethybridge, H. R., Parrish, C. C., Morrongiello, J., Young, J. W., Farley, J. H., Gunasekera, R. M., et al. (2015). Spatial pattern and temperature predictions of tuna fatty acids: tracing essential nutrients and changes in primary producers. PLOS ONE 10:e0131598. doi: 10.1371/journal.pone.0131598
Pilorget, L., Buckner, J., and Lundgren, J. G. (2010). Sterol limitation in a pollen-fed omnivorous lady beetle. J. Insect Physiol. 56, 81–87. doi: 10.1016/j.jinsphys.2009.09.006
Pollierer, M. M., Larsen, T., Potapov, A., Brückner, A., Heethoff, M., Dyckmans, J., et al. (2019). Compound-specific isotope analysis of amino acids as a new tool to uncover trophic chains in soil food webs. Ecol. Monogr. e01384. doi: 10.1002/ecm.1384
Pollierer, M. M., Scheu, S., and Haubert, D. (2010). Taking it to the next level: Trophic transfer of marker fatty acids from basal resources to predators. Soil Biol. Biochem. 42, 919–925. doi: 10.1016/j.soilbio.2010.02.008
Potapov, A. M., Tiunov, A. V., Scheu, S., Larsen, T., and Pollierer, M. M. (2019). Combining bulk and amino acid stable isotope analyses to quantify trophic level and basal resources of detritivores: a case study. Oecologia. 189, 447–460. doi: 10.1007/s00442-018-04335-3
Provasoli, L., and Carlucci, A. F. (1974). Algal physiology and biochemistry. Bot. Monogr. 10, 741–787.
Rapala-Kozik, M., Kowalska, E., and Ostrowska, K. (2008). Modulation of thiamin metabolisms in Zea mays seedlings under conditions of abiotic stress. J. Exp. Bot. 59, 4133–4143. doi: 10.1093/jxb/ern253
Rather, Z. A., and Nazir, R. (2015). Biochemical composition of selected macrophytes of Dal Lake, Kashmir Himalaya. J. Ecosystem Ecography 5, 1–5. doi: 10.4172/2157-7625.1000158
Raux, E., Schubert, H. L., Roper, J. M., Wilson, K. S., and Warren, M. J. (1999). Vitamin B12: insights into biosynthesis's mount improbably. Bioorganic Chem. 27, 100–118. doi: 10.1006/bioo.1998.1125
Rawlins, A. J., Bull, I. D., Poirier, N., Ineson, P., and Evershed, R. P. (2006). The biochemical transformation of oak (Quercus robur) leaf litter consumed by the pill millipede (Glomeris marginata). Soil Biol. Biochem. 38, 1063–1076. doi: 10.1016/j.soilbio.2005.09.005
Richardson, A. C., Marsh, K. B., Boldingh, H. L., Pickering, A. H., Bulley, S. M., Frearson, N. J., et al. (2004). High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ. 27, 423–435. doi: 10.1111/j.1365-3040.2003.01161.x
Roje, S. (2007). Vitamin B biosynthesis in plants. Phytochemistry 68, 1904–1921. doi: 10.1016/j.phytochem.2007.03.038
Rosoll, D., Bermúndez, R., Hauss, H., Schulz, K. G., Riebesell, U., Sommer, U., et al. (2012). Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 7:e34737. doi: 10.1371/journal.pone.0034737
Ruess, L., and Chamberlain, P. M. (2010). The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biol. Biochem. 42, 1898–1910. doi: 10.1016/j.soilbio.2010.07.020
Ruess, L., Häggblom, M. M., Grazía Zapata, E. J., and Dighton, J. (2002). Fatty acids of fungi and nematodes - possible biomarkers in the soil food chain? Soil Biol. Biochem. 34, 745–756. doi: 10.1016/S0038-0717(01)00231-0
Ruess, L., Langel, R., Häggblom, M. M., and Scheu, S. (2004). Nitrogen isotope ratios and fatty acids composition as indicators of animal diet in belowground systems. Oecologia 139, 336–346. doi: 10.1007/s00442-004-1514-6
Saavedra, M., Candeias-Mendes, A., Castanho, S., Teixeira, B., Mendes, R., and Pousao-Ferreira, P. (2015). Amino acid profiles of meagre (Argyrosomus regius) larvae: Towards the formulation of an amino acid balanced diet. Aquaculture 448, 315–320. doi: 10.1016/j.aquaculture.2015.06.003
Saini, R. K., Nile, S. H., and Park, S. W. (2015). Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Inter. 76, 735–750. doi: 10.1016/j.foodres.2015.07.047
Sampredo, L., Jeannotte, R., and Whalen, J. K. (2006). Trophic transfer of fatty acids from gut microbiota to the earthworm Lumbricus terrestris L. Soil Biol. Biochem. 38, 2188–2198. doi: 10.1016/j.soilbio.2006.02.001
Santos, S. A., Viela, C., Freire, C. S., Abreu, M. H., Rocha, S. M., and Silvestre, A. J. (2015). Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem. 183, 122–128. doi: 10.1016/j.foodchem.2015.03.006
Sañudo-Wilhelmy, S. A., Gobler, C. J., Okbamichael, M., and Taylor, G. T. (2006). Regulation of phytoplankton dynamics by vitamin B12. Geophys. Res. Lett. 33:L04604. doi: 10.1029/2005GL025046
Seldon, R. L., Batt, R. D., Saba, V. S., and Pinsky, M. L. (2018). Diversity in thermal affinity among key piscivores buffers impacts of ocean warming on predator–prey interactions. Glob. Change Biol. 24, 117–131. doi: 10.1111/gcb.13838
Seth, E. C., and Taga, M. E. (2014). Nutrient cross-feeding in the microbial world. Front. Microbiol. 5:350. doi: 10.3389/fmicb.2014.00350
Sprecher, H., Luthria, D. L., Mohammed, B. S., and Baykousheva, S. P. (1995). Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J. Lipid Res. 36, 2471–2477. doi: 10.1201/9781439831939.ch2
Stanley, D., and Kim, Y. (2019). Insect prostaglandin and other eicosanoids: From molecular to physiological actions. Advanc. Insect Physiol. 56, 283–343. doi: 10.1016/bs.aiip.2019.01.003
Stanley-Samuelson, D. W., Jurenka, R. A., Cripps, C., Blomquist, G. J., and de Renobales, M. (1988). Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 9, 1–33. doi: 10.1002/arch.940090102
Stanley-Samuelson, D. W., Jurenka, R. A., Loher, W., and Blomquist, G. J. (1987). Metabolism of polyunsaturated fatty acids by larvae of the waxmoth, Galleria mellonella. Arch. Insect. Biochem. Physiol. 6, 141–149. doi: 10.1002/arch.940060302
Steffan, S. A., Chikaraishi, Y., Currie, C. R., Horn, H., Gaines-Day, H. R., Pauli, J. N., et al. (2015). Microbes are trophic analogs of animals. Proc Nat Acad Sci U.S.A. 112, 15119–15124. doi: 10.1073/pnas.1508782112
Steffan, S. A., Chikaraishi, Y., Dharampal, P. S., Pauli, J. N., Guédot, C., and Ohkouchi, N. (2017). Unpacking brown food webs: animal trophic identity reflects rampant microbivory. Ecol. Evol. 7, 3532–3541. doi: 10.1002/ece3.2951
Steffan, S. A., Chikaraishi, Y., Horton, D., Ohkouchi, N., Singleton, M. E., Miliczky, E., et al. (2013). Trophic hierarches illuminated via amino acids isotopic analysis. PLoS ONE 8:e76152. doi: 10.1371/journal.pone.0076152
Stott, A. W., Davies, E., and Evershed, R. P. (1997). Monitoring the routing of dietary and biosynthesised lipids through compound specific isotope (δ13C) measurements at natural abundance. Naturwissenschaften 84, 82–86. doi: 10.1007/s001140050354
Strandberg, U., Hiltunen, M., Jelkänen, E., Taipale, S. J., Kainz, M. J., Brett, M. T., et al. (2015). Selective transfer of polyunsaturated fatty acids from phytoplankton to planktivorous fish in large boreal lakes. Sci. Total Environm. 536, 858–865. doi: 10.1016/j.scitotenv.2015.07.010
Suikkanen, S., Pulina, S., Engström-Öst, J., Lehtiniemi, M., Lehtinen, S., and Brutemark, A. (2013). Climate change and eutrophication induced shifts in northern summer plankton communities. PLoS ONE 8:e66475. doi: 10.1371/journal.pone.0066475
Sulik, P., Klimek, M., Talik, P., Kruk, J., Morgan, A. J., and Plytycz, B. (2012). Searching for external sources of the riboflavin stored in earthworm eleocytes. Inv. Survival J. 9, 169–177.
Sushchik, N. N., Gladyshev, M. I., Makhutova, O. N., Kalachova, G. S., Kravchuk, E. S., Ivanova, E., et al. (2014). Associating particulate essential fatty acids of the ω3 family with phytoplankton species composition in a Siberian reservoir. Freshw. Biol. 49:1206. doi: 10.1111/j.1365-2427.2004.01263.x
Svoboda, J. A., and Thompson, M. J. (1985). “Steroids,” in Comprehensive Insect Physiology, Biochemistry and Pharmacology, eds G. A. Kerkut, and L. I. Gilbert (New York, NY: Pergamon), 137–175. doi: 10.1016/B978-0-08-030811-1.50011-8
Sylvander, P., Häubner, N., and Snoeijs, P. (2013). The thiamin content of phytoplankton cells is affected by abiotic stress and growth rate. Micr. Ecol. 65, 566–577. doi: 10.1007/s00248-012-0156-1
Taipale, S. J., Hiltunen, M., Vuorio, K., and Peltomaaa, E. (2016a). Suitability of phytosterols alongside fatty acids as chemotaxonomic biomarkers for phytoplankton. Front. Plant Sci. 7:212. doi: 10.3389/fpls.2016.00212
Taipale, S. J., Kahilainen, K. K., Holtgrieve, G. W., and Peltomaaa, E. T. (2018). Simulated eutrophication and browning alters zooplankton nutritional quality and determines juvenile fish growth and survival. Ecol. Evol. 8, 2671–2687. doi: 10.1002/ece3.3832
Taipale, S. J., Vuorio, K., Strandberg, U., Kahilainen, K. K., Järvinen, M., Hiltunen, M., et al. (2016b). Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 96, 156–166. doi: 10.1016/j.envint.2016.08.018
Takizawa, Y., Dharampal, P. S., Steffan, S. A., Takano, Y., Ohkouchi, N., and Chikarashi, Y. (2017). Inter-trophic isotopic discrimination of 15N/14N for amino acids in autotrophs: implications for nitrogen dynamics in ecological studies. Ecol. Evol. 7, 2916–2924. doi: 10.1002/ece3.2866
Tang, Y. Z., Koch, F., and Gobler, C. J. (2010). Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc Nat Acad Sci U. S. A. 107, 20756–20761. doi: 10.1073/pnas.1009566107
Taylor, M., Mediannikov, O., Raoult, D., and Greub, G. (2012). Endosymbiontic bacteria associated with nematodes, ticks and amoebae. FEMS Immunol. Med. Microbiol. 64, 21–31. doi: 10.1111/j.1574-695X.2011.00916.x
Teshima, S.-I. (1971). Bioconversion of β-sitosterol and 24-methylcholesterol to cholesterol in marine crustacea. Comp. Biochem. Physiol. B 39, 815–822. doi: 10.1016/0305-0491(71)90105-2
Thiemann, G. W., Iverson, S. J., and Stirling, I. (2008). Polar bear diets and arctic marine food webs: insights from fatty acid analysis. Ecol. Monogr. 78, 591–613. doi: 10.1890/07-1050.1
Traugott, M., Kamenova, S., Ruess, L., Seeber, J., and Plantegenest, W. (2013). “Characterising trophic networks: What emerging DNA based methods, stable isotopes and fatty acid analyses can offer”, in Advances in Ecological Research: Ecological networks in an agricultural world, eds G. Woodward, and D. A. Bohan (Amsterdam: Academic Press), 177–224. doi: 10.1016/B978-0-12-420002-9.00003-2
Twining, C. W., Brenna, J. T., Lawrence, P., Shipley, J. R., Tollefson, T. N., and Winkler, D. W. (2016). Omega-3 long-chain polyunsaturated fatty acids support aerial insectivore performance more than food quantity. Proc Nat Acad Sci U.S.A. 113, 10920–10925. doi: 10.1073/pnas.1603998113
Vance, D. D., and Vance, J. E. (2002). Biochemistry of Lipids, Lipoproteins and Membranes, 4th edn. Amsterdam: Elsevier.
Veira, D. M. (1986). The role of ciliate protozoa in nutrition of the ruminant. J. Anum. Sci. 63, 1547–1560. doi: 10.2527/jas1986.6351547x
Ventura, M., and Catalan, J. (2010). Variability in amino acid composition of alpine crustacean zooplankton and its relationship with nitrogen-15 fractionation. J. Plankton Res. 32, 1583–1597. doi: 10.1093/plankt/fbq066
Volkman, J. K. (1986). A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 9, 83–99. doi: 10.1016/0146-6380(86)90089-6
Volkman, J. K. (2003). Sterols in microorganisms. Appl. Microbiol. Biotechnol. 60, 495–506. doi: 10.1007/s00253-002-1172-8
Von Elert, E., Martin-Creuzburg, D., and Le Coz, J. R. (2003). Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. Royal. Soc. London Ser. B 270, 1209–1214. doi: 10.1098/rspb.2003.2357
Vranova, V., Rejsek, K., and Formanek, P. (2013). Aliphatic, cyclic, and aromatic organic acids, vitamins, and carbohydrates in soil: a review. Sci. World J. 2013:524239. doi: 10.1155/2013/524239
Vučić, V., and Cvetkovi,ć, Z. (2015). Cholesterol: Adsorption, function and metabolism. Encylop. Food Health, 47–52. doi: 10.1016/B978-0-12-384947-2.00151-3
Wacker, A., and Martin-Creuzburg, D. (2012). Biochemical nutrient requirements of the rotifer Brachionus calyciflorus: co-limitation by sterols and amino acids. Funct. Ecol. 26, 1135–1143. doi: 10.1111/j.1365-2435.2012.02047.x
Wakeham, S. G., and Canuel, E. A. (1986). Lipid composition of the pelagic crab Pleuroncodes planipes, its faeces, and sinking particulate organic matter in the Equatorial North Pacific Ocean. Org. Geochem. 9, 331–343. doi: 10.1016/0146-6380(86)90114-2
Wang, T., Tonga, S., Liu, N., Li, F., Wells, M. L., and Gao, K. (2017). The fatty acid content of plankton is changing in subtropical coastal waters as a result of OA: Results from a mesocosm study. Mar. Env. Res. 132, 51–62 doi: 10.1016/j.marenvres.2017.10.010
Warne, R. W., Pershall, A. D., and Wolf, B. O. (2010). Linking precipitation and C3-C4 plant production to resource dynamics in higher trophic levels consumers. Ecology 91, 1628–1638. doi: 10.1890/08-1471.1
Watts, J. L., and Browse, J. (2002). Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 99, 5854–5859. doi: 10.1073/pnas.092064799
Wiktorowska-Owczarek, A., Berezinska, M., and Nowak, J. Z. (2015). PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 24, 931–941. doi: 10.17219/acem/31243
Williams, C. T., and Buck, C. L. (2010). Using fatty acids as dietary tracers in seabird trophic ecology: theory, application and limitations. J. Ornithol. 151, 531–543. doi: 10.1007/s10336-010-0513-0
Wolak, N., Zawrotniak, M., Gogol, M., Kozik, A., and Rapala-Kozik, M. (2017). Vitamins B1, B2, B3, and B9 – occurrence, biosynthesis pathways and functions in human nutrition. Mini Rev. Med. Chem. 17, 1075–1111. doi: 10.2174/1389557516666160725095729
Wu, G. (2010). Functional amino acids in growth, reproduction, and health. Am. Soc. Nutr. 1, 31–37. doi: 10.3945/an.110.1008
Wu, G., Wu, Z., Dai, Z., Yang, Y., Wang, W., Liu, C., et al. (2013). Dietary requirements of nutritionally non-essential amino acids by animals and humans. Amino Acids 44, 1107–1113. doi: 10.1007/s00726-012-1444-2
Young, V. R. (1994). Adult amino acid requirements: the case for a major revision in current recommendations. J. Nutr. 124, 1517s-1523s. doi: 10.1093/jn/124.suppl_8.1517S
Zhang, Y., Li, Y., Liang, X., and Gao, J. (2017). Effects of dietary vitamin E supplementation on growth performance, fatty acid composition, lipid peroxidation and peroxisome proliferator-activated receptors (PPAR) expressions in juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol. Biochem. 43, 913–922. doi: 10.1007/s10695-016-0224-4
Keywords: food web, essential biomolecules, fatty acids, amino acids, sterols, vitamins, trophic transfer, environmental distribution
Citation: Ruess L and Müller-Navarra DC (2019) Essential Biomolecules in Food Webs. Front. Ecol. Evol. 7:269. doi: 10.3389/fevo.2019.00269
Received: 02 November 2018; Accepted: 26 June 2019;
Published: 16 July 2019.
Edited by:
Monika Winder, Stockholm University, SwedenReviewed by:
Sami Johan Taipale, University of Eastern Finland, FinlandCopyright © 2019 Ruess and Müller-Navarra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liliane Ruess, bGlsaWFuZS5ydWVzc0BiaW9sb2dpZS5odS1iZXJsaW4uZGU=; Dörthe C. Müller-Navarra, ZG9lcnRoZS5tdWVsbGVyLW5hdmFycmFAdW5pLWhhbWJ1cmcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.