
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol., 03 July 2019
Sec. Conservation and Restoration Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00258
This article is part of the Research TopicNorth American Monarch Butterfly Ecology and ConservationView all 35 articles
Western monarch butterflies dropped by ~97% of their average historic abundance between the 1980s and mid-2010s. In winter 2018–2019, the population plummeted even farther, to fewer than 30,000 monarchs, which represents a single year drop of 86% and a drop of >99% since the 1980s. The population may now be hovering at its quasi-extinction threshold. In this Perspectives piece, we: (1) Place the current status in context, (2) Highlight the most likely window during the annual life cycle when the population declined, (3) Review probable causes of long-term declines, and (4) Recommend steps that the public, policy makers, and land managers can take to recover western monarchs. The available studies reinforce the hypotheses that overwintering habitat loss and loss of central California breeding habitat, as well as pesticide use, are likely important contributors to the western monarch's long-term decline. The most limiting part of the migratory cycle appears to be concentrated during the overwintering stage and/or in early spring. If western monarchs are in fact entering an extinction vortex, they need extraordinary efforts—focused on the most vulnerable periods of the annual cycle— to save the migration. Critical short-term conservation priorities are to (1) Protect, manage and restore overwintering habitat, (2) Protect monarchs and their habitat from pesticides, (3) Restore breeding and migratory habitat in California, (4) Protect, manage, and restore summer breeding and fall migration monarch habitat throughout the western monarch's range, and (5) Fill research gaps to inform western monarch recovery strategies.
Monarch butterflies (Danaus plexippus plexippus) across North America have been undergoing a multi-decade decline (Semmens et al., 2016; Schultz et al., 2017). Nonetheless, the crash of the western population (Figure 1) in winter 2018–2019 was particularly stunning. In 2017, we estimated that the overwintering population had dropped by 97% of its average historic abundance, from ~3 to 10 million to ~200–300 thousand butterflies (Schultz et al., 2017). In winter 2018-2019, the population plummeted to fewer than 30,000 monarchs, which represents a single year drop of 86%, and a >99% drop since the 1980s (Figure 2A).
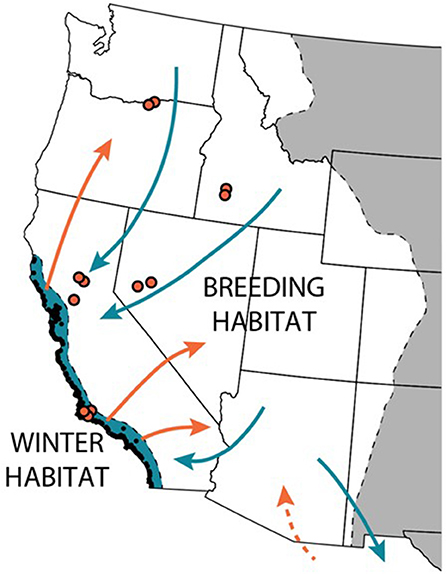
Figure 1. Western monarchs breed west of the Rocky mountains and primarily overwinter at over 200 sites (black points) along the Pacific coast in California. During the spring, monarchs leave the overwintering habitat (colored blue) to disperse (orange arrows) across the West. The butterflies breed continuously across the West during the summer (colored white); in the fall, they return (blue arrows) to the overwintering grounds. [Tag recoveries in Mexico show that at least some western monarchs migrate to central Mexico, mixing with the eastern monarch overwintering population; whether or not monarchs from Mexico return to the West in the spring has not been documented, but is suspected (dashed orange arrow)]. The authors have monitored monarch breeding phenology and milkweed at 12 sites throughout the West (orange points) as part of a multi-year study.
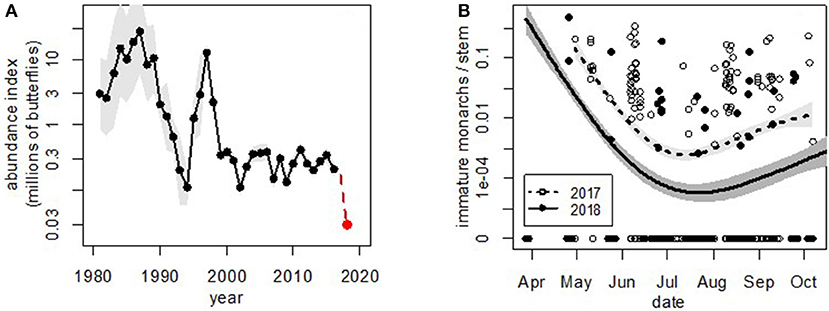
Figure 2. Western monarch abundance at (A) overwintering and (B) breeding sites. In both panels, shaded areas show 95% confidence limits. (A) Western monarch butterfly 1981–2018 estimates for overwintering abundance during the Thanksgiving Count time period in coastal California. Estimates for 1981–2017 were calculated with state space models (Schultz et al., 2017), scaled to be comparable to the raw count from 2018 (shown). (B) Monarch egg and larva counts per stem at all 12 monitoring sites (shown in Figure 1) throughout the season in 2017 and 2018. Curves were fitted with generalized additive models (Wood, 2011) to show general trends in abundance. The fact that the two curves are parallel suggests that densities were lower by the time monarchs arrived in 2018; the decline does not appear to be due to different dynamics during breeding. Note the log scale and 10-fold difference among years.
In this Perspective, we: (1) Place the current status in context, both how trends compare to the eastern population and potential implications of dropping to unprecedentedly low abundance in the West, (2) Highlight the most likely window during the annual life cycle when the population declined, (3) Review probable causes of long-term declines, and (4) Use our understanding of drivers of declines to recommend steps that the public, policy makers, and land managers can take including identifying knowledge gaps for which focused mechanistic studies could contribute to developing more effective and efficient conservation actions.
Since 1997, volunteers have estimated the overwintering population in California each fall at coastal groves (Xerces Society Western Monarch Thanksgiving Count, 2019). The 2018 Xerces Thanksgiving Count revealed a new low—only 28,429 monarchs were tallied—<1% of the historic population (Figure 2A). The current trend in western monarchs is in contrast to eastern monarchs, which hit the highest estimated population size in the last decade in winter 2018–2019 with 6.05 hectares occupied (Rendón-Salinas et al., 2019).
We know from our past analyses that a western population of <30,000 butterflies is unprecedented. The 2018 Thanksgiving count mirrors a textbook extinction vortex (Gilpin and Soule, 1986), in the sense that fluctuations in abundance—which have been happening throughout the past 30 years—become riskier as the population becomes smaller. As populations become smaller, “ordinary” environmental variation can cause a population to drop below a point from which extinction is inevitable, unless extraordinary measures are taken. We call this point the quasi-extinction threshold. In 2016, a group of experts proposed 30,000 butterflies as the quasi-extinction threshold for western monarchs (Schultz et al., 2017). Now, it is suddenly imperative to know if the experts were correct, and, if so, what extraordinary measures need to be taken to preserve the population.
In general, we know very little about what happens when formerly large populations become small. Individuals in small populations may have reduced mating success, suffer increased predation, and lose other benefits of schooling or flocking (Courchamp et al., 1999). These effects due to small population size are known as “Allee effects” and are difficult to estimate in wild populations because they are only expressed after a population has begun to decline to extinction (Liermann and Hilborn, 1997). Therefore, setting quasi-extinction thresholds is one of the most subjective steps of population viability analysis (e.g., Frick et al., 2010; McGowan et al., 2017). If the published quasi-extinction threshold is correct, then positive density-dependent processes associated with Allee effects could lead to further rapid decline. If the quasi-extinction threshold is incorrect, we will see the western monarch recover to a larger population size. Regardless, this serves as a call to intensify efforts to boost abundance to healthy enough numbers in the wild for the population to be able to sustain itself through normal ups and downs in the population size.
Given the large drop in western monarchs from 2017 to 2018, some are tempted to blame the weather for the low numbers. Late rainy season storms swept across California in March. There was a severe and extended wildfire season in the West and smoke was widespread at times. California is still recovering from a historic drought. Large amplitude inter-annual fluctuations are an intrinsic aspect of butterfly population dynamics, and causes of year-to-year variation are not necessarily the same as the causes of long-term declines. Nonetheless, it is important to try to understand western monarch abundance throughout the year from winter 2017–2018 through winter 2018–2019, when the decline occurred.
Starting in winter 2016–2017, the Xerces Society and volunteers began a second count at overwintering sites, the New Year's count (centered around New Year's Day, to complement the Thanksgiving Count 6 weeks earlier). Monarch abundance at the New Year's Count had declined by 43% on average in 2017 (n = 44 sites), 49% on average in 2018, (n = 115 sites) and 36% in 2019 (n = 130 sites), when compared to monarch abundance at those same sites during the Thanksgiving Count. These data suggest that monarch butterflies did not have exceptionally low survival between November 2017 and January 2018, compared to the previous year.
In addition to counts at overwintering sites, we started monitoring summer breeding of western monarchs in 2017 at 12 sites throughout the West (Figure 1). Across these 2 years, the density of monarch eggs and larvae was consistently lower in 2018 than 2017 (Figure 2B), with about a 10-fold decline between the 2 years (average immature monarchs/stem = 0.0273 [95% CI = 0.0025, 0.2953] in 2017 and 0.0022 [95% CI = 0.0001, 0.0429] in 2018; paired t-test of site averages between years: t = −2.53, df = 10, P = 0.030). We therefore suggest that the drop measured at Thanksgiving 2018 originated before the beginning of the 2018 breeding season, either late during the overwintering season or very early in the breeding season.
This inference is consistent with Espeset et al. (2016) who concluded that western monarch declines were concentrated in early spring. Of the environmental events that seemed “unusual” in 2017–2018, this pattern points to the possible negative effects of unusually heavy rains in March 2018 with the caveat that many other factors may have caused the population drop, including the interaction of weather with habitat quality at overwintering sites, and habitat inland from the coast in California, where the first generation breeds.
In the larger eastern population, declines have largely been attributed to overwintering habitat loss (Brower et al., 2012; Vidal et al., 2013) and breeding habitat loss, especially through the use of herbicides (e.g., Pleasants and Oberhauser, 2012; Flockhart et al., 2014). We (Crone et al., in press) recently evaluated climate and land use factors simultaneously as potential drivers of western monarch abundance. Trends in abundance were more strongly associated with land use variables including coastal development in overwintering areas and pesticide use (glyphosate and neonicotinoid insecticides) in breeding areas than climate variables in both overwintering and breeding areas (Crone et al., in press). These results are consistent with the hypotheses that overwintering habitat loss and loss of central California breeding habitat are important for western monarchs (see Espeset et al., 2016) and that trends in pesticide use likely contribute to declining monarch populations as well as declines in other butterfly taxa (see also Forister et al., 2016).
In addition to this broad scale analysis, we estimated daily survival using data from Tuskes and Brower (1978), for comparison with population declines estimated from Thanksgiving and New Year's counts. Daily survival at Natural Bridges near Santa Cruz was 0.995 (95% CI 0.988, 0.997) and at Santa Barbara was 0.991 (0.989, 0.993). Over 6 weeks (the approximate time between Thanksgiving to New Year's counts), this historical estimate translates into a 29% drop (95% CI 12–40%) using estimates from Santa Cruz and a 32% drop (95% CI 26–37%) using estimates from Santa Barbara. Hence, based on the best available evidence, apparent survival during winter in recent years (36–49% drop) has been lower than it was in the past. This change reinforces the importance of overwintering habitat quality on the long-term decline of the western monarch population. At the present time, we have not found comparable data to evaluate whether breeding season survival or reproduction have changed in western monarchs.
To date, western monarchs have received far less conservation attention and financial resources than the larger eastern population. Nonetheless, the western monarch breeds across most of the US west of the Rocky Mountains, a significant portion of the monarch's overall North American range. It makes an important contribution to the resilience, redundancy, and representation of the species as a whole (see definition in Shaffer and Stein, 2000).
While the precise causes of the recent dramatic drop in the western population, as well as the longer term decline, remain unknown, this knowledge gap should not prevent conservation action. We suggest that a precautionary approach be taken to remediate potential causes of decline. Specifically we recommend efforts (1) to protect, enhance, and actively manage overwintering sites; (2) to protect monarch habitat from pesticides, particularly systemic insecticides (including neonicotinoids); (3) to supplement larval and adult resources-especially in the early spring-in California; (4) to identify, protect, and enhance monarch habitats throughout the West, and (5) to prioritize research efforts to answer questions critical to developing an effective and efficient recovery strategy. Here, we briefly explain our recommendations, and their relationship to the causes of western monarch declines, described above. These recommendations and relevant resources are expanded in in our “Western Monarch Call to Action.”1
Our analyses (“Environmental drivers” above) point to the importance of monarch habitat in winter and early spring, prior to the breeding season. Conservation biologists have long known that efforts focused only on one stage of a species' life cycle (e.g., breeding) may not be sufficient if populations are limited by another life stage [e.g., overwintering (Brown et al., 2017)]. Despite the importance of monarchs to Californians and the state's tourism economy, few overwintering sites are meaningfully protected (International Environmental Law Project and the Xerces Society, 2012) and sites continue to be destroyed—indeed, from 2017 to present, over one dozen sites have either been newly destroyed or are reported to be threatened by inappropriate tree trimming, removal, and/or development (Xerces Society Western Monarch Overwintering Sites Database 2019, unpublished). To protect remaining habitat, overwintering sites could be designated as Environmentally Sensitive Habitat Areas (ESHAs) by the California Coastal Commission, protected as Critical Habitat if monarchs were listed under the federal Endangered Species Act, protected by California Department of Fish and Wildlife if monarchs were listed as endangered under the California Endangered Species Act, or a new law could be created by the California state legislature to protect overwintering sites from destruction.
To address the need for active management of overwintering sites, the majority of which occur on publicly owned land, a greater financial investment is needed. The Monarch Butterfly and Pollinator Rescue Program (California Assembly Bill 2421), was signed into law in 2018, and $3 million was allocated to this program. An additional $3.9 million was recently allocated for restoration of overwintering sites owned by the City of Goleta. While these represent important steps forward, more resources are needed to restore and manage the over 200 actively used overwintering sites. While there are no published estimates, restoring a significant number of overwintering sites would easily require tens of millions of dollars and, more importantly, would benefit from sustained funding to continue to manage the groves for monarchs in the long-term. Of the Top 50 priority sites identified by Pelton et al. (2016) many of the most important sites are owned by the California Department of Parks and Recreation, followed by cities, U. S. Department of Defense, East Bay Regional Parks District, and county, university, and other state and federal agencies as well as private entities. Some of these owners do not encourage or permit the planting of eucalyptus (the dominant tree used by monarchs in California during overwintering), nor are these land managers necessarily focused on managing for monarch overwintering habitat—and, in some cases, may be unaware of the full extent of overwintering habitat within their jurisdiction.
In our analyses of long-term trends, insecticide and herbicide use were almost as tightly associated with monarch declines as overwinter habitat loss. Restricting insecticide and herbicide use increases adult Lepidoptera abundance (Frampton and Dorne, 2007). Broadcast herbicide use can kill host and nectar plants and have non-target effects on butterflies (Stark et al., 2012). We advise protecting the most important monarch breeding and overwintering habitats from insecticide and herbicide use. Specifically, we recommend avoiding herbicide applications that damage monarch breeding and migratory habitat such as milkweed and wildflowers. These recommendations apply to home gardens and lawns, as well as lands used for agriculture and other purposes. If herbicides are used, we advise using targeted application methods, avoiding large-scale broadcast applications of herbicides, and taking precautions to limit off-site movement of herbicides. Neonicotinoid insecticides, in particular, should be avoided at all times in monarch habitat due to their persistence, systemic nature, and toxicity. When purchasing milkweeds or wildflowers from nurseries, we recommend ensuring that they have not been treated with neonicotinoids or other systemic insecticides.
Enhancing monarch breeding habitat may be able to partly mitigate reductions in overwintering habitat quality because larger populations at the end of the summer can potentially withstand higher mortality. Numerous studies have quantified the importance of host and nectar plants for butterfly populations (Dennis et al., 2006; Dennis, 2010), and restoration efforts which enhance host and nectar have been effective approaches for the conservation of rare butterflies (Carleton and Schultz, 2013). We recommend planting native milkweeds in areas where they historically grew in California, and, in particular, in the Coast Range, Central Valley, and the foothills of the Sierra Nevada, areas where the first generation of monarchs are produced each spring. Early emerging native species that may be particularly important in spring include woollypod (Asclepias eriocarpa), California (A. californica), and heartleaf milkweed (A. cordifolia). However, commercial availability of these species is limited. Later-emerging native California milkweed species that are more readily available, and may also help, include narrowleaf (A. fascicularis) and showy milkweed (A. speciosa). In the desert southwest of California, we recommend rush (A. subulata) and desert milkweed (A. erosa). We recommend only planting milkweed >5 miles inland from overwintering sites, as milkweed does not naturally grow close to the coast north of Santa Barbara and milkweed at overwintering sites can interrupt natural overwintering behavior. Tropical milkweed (A. curassavica) is exotic to California, disrupts the monarch's migratory cycle, and serves as a reservoir for monarch pathogens (Satterfield et al., 2016). As such we recommend against planting tropical milkweed. In places where tropical milkweed already exists, we recommend cutting it back to the ground in the fall (October/November) and repeatedly throughout the winter to mimic native milkweed phenology and break the disease cycle; ideally, it should be replaced by native milkweed.
In addition, we recommend planting nectar-rich wildflowers, especially those that bloom early in the spring (February–April) and fall (September-October). If located close to the coast, plants which bloom in the winter (November-January) may also be useful.
Identifying key areas of breeding and migrating habitat for monarchs in the West remains a knowledge gap. Some geographic regions contribute disproportionately to the eastern monarch overwintering population in Mexico (e.g., Flockhart et al., 2017), and it is important to know whether the same is true for western monarchs. No data exist from which we could meaningfully evaluate their importance for short- or long-term population declines. Thus, while some of the most important monarch habitat within its western breeding (Yang et al., 2016; Dilts et al., 2019) and overwintering (Pelton et al., 2016) range has already been identified, additional work is needed to identify and rank these areas. We recommend identifying existing monarch habitat, ensuring that it is managed to protect monarchs (Xerces Society, 2018) and in some regions and landscape types, we recommend habitat enhancement or restoration. Habitat restoration in regions where monarch habitat historically occurred, but have likely been lost (such as the Columbia Plateau and Snake River Plain), as well as riparian areas, are high priority areas outside of California. Such restoration would likely benefit from habitat elements beyond milkweed and nectar, such as shrubs or trees for roosting and shade.
Breeding and migrating habitat are only a few of the gaps in our knowledge of western monarchs. We especially need observations of monarch biology in places where human populations are low (e.g., the Great Basin desert) and at times of year when monarch butterflies are sparse (e.g., early spring in western California, just as they leave the overwintering grounds). We urge volunteers across the West to collect observations of monarchs and milkweeds, especially in the early spring (February–April), the period in which monarchs typically leave the overwintering sites. Together these observations will help answer questions about monarch breeding phenology. In this year, when numbers are low in the West and high in the East, targeted observations of monarch adults and larvae may also tell us whether the West sees an influx of monarchs arriving from Mexico (see Pyle, 2015). Monarch adult, larva, egg, nectaring, and milkweed sightings can be reported to the Western Monarch Milkweed Mapper2 and first adults observed can be reported to Journey North3 as well. More robust monitoring may be achieved through increased western participation in the Integrated Monarch Monitoring Program4.
We urge academic ecologists to conduct targeted experimental and observational studies to complement large-scale observations like the ones described above. In both the eastern and western monarch populations, filling knowledge gaps about demography throughout the life cycle would allow us to design quantitative thresholds for conservation and restoration. For example, it may be possible for targeted actions at one point in the life cycle to make up for stresses at other points. If climate change is making the landscape less favorable, can we make up for this with improved breeding or overwintering habitat quality and/or area? Can more breeding habitat in the outer parts of the breeding distribution make up for habitat loss at breeding or overwintering sites in California? Intuitively, the answer is probably “yes, but only partly.” To answer this in a more quantitative way, we need a better understanding of how the life cycle pieces fit together.
In closing, western monarchs are currently in peril. Their status reflects a long-term decline due to some combination of habitat loss and degradation in their overwintering and breeding range, increased pesticide use, and possibly climate change. The recent dramatic drop reflects conditions when the least is known about western monarchs—where they are, what habitat they are using, and what they need to survive, migrate and reproduce. In spite of their current status, monarchs are resilient; we believe that rapid conservation actions can recover the population. This recovery will require the protection of monarchs and their habitat, as well as targeted research to understand the unique life cycle of western monarch butterflies. If we are going to take these actions, the time is now.
The datasets for this study will not be made publicly available because restrictions apply to some of the datasets. Some of the datasets are in a publicly accessible repository:
The Xerces Society Western Monarch Thanksgiving and New Year's Counts analyzed in this study can be found at www.westernmonarchcount.org/data.
Restrictions apply to some of the datasets:
The Xerces Society Western Monarch Overwintering Sites Database 2019 is not publicly available because of privacy concerns with a subset of the information. Requests to access the database should be directed to Emma Pelton, bW9uYXJjaHNAeGVyY2VzLm9yZw==.
The western monarch and milkweed phenology dataset summarized in this manuscript are not publicly available because it is part of a study currently in-progress. Requests to access the datasets should be directed to Cheryl Schultz, c2NodWx0emNAd3N1LmVkdQ==.
EP, SJ, and SB (along with others—see Acknowledgments) oversee Thanksgiving and New Year's Counts and maintain the overwintering sites database. All authors contributed to funding and implementing the 2017–2018 surveys in the breeding range. EC conceived and ran all analyses with input from CS and EP. All authors wrote and revised the manuscript.
Funds for the 2017–2018 breeding and phenology surveys and analysis were provided by Department of Defense Legacy Natural Resources Program (NR 16 Western Monarch) and the U.S. Fish & Wildlife Service Coastal Program. Authors were supported by their institutions (WSU, Tufts and Xerces Society) and EC, SJ, EP, and CS were partly supported by the National Science Foundation (NSF DEB 1920834).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Thank you to the Western Monarch Thanksgiving Count volunteers, particularly our regional coordinators, Mia Monroe, and Katie Hietala-Henschell of the Xerces Society; Stephanie McKnight of the Xerces Society and Cameron Thomas of Washington State University for conducting the fieldwork for the breeding and milkweed phenology project; fellow western monarch researchers and conservation practitioners for conversations that led to the development to the Western Monarch Call to Action; US Fish and Wildlife Service Coastal Program, Department of Defense, National Science Foundation, and Xerces Society members and other funders for supporting the work presented in this Perspective.
1. ^www.savewesternmonarchs.org
2. ^www.monarchmilkweedmapper.org
Brower, L. P., Taylor, O. R., Williams, E. H., Slayback, D. A., Zubieta, R. R., and Ramirez, M. I. (2012). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Div. 5, 95–100. doi: 10.1111/j.1752-4598.2011.00142.x
Brown, L. M., Breed, G. A., Severns, P. M., and Crone, E. E. (2017). Losing a battle but winning the war: moving past preference-performance to understand native herbivore-novel host plant interactions. Oecologia 183, 441–453. doi: 10.1007/s00442-016-3787-y
Carleton, A., and Schultz, C. B. (2013). Restoration action and species response: oviposition habits of Plebejus icarioides fenderi (Lepidoptera: Lycaenidae) across a restoration chronosequence in the Willamette Valley, Oregon, USA. J. Insect Conserv. 17, 511–520. doi: 10.1007/s10841-012-9535-7
Courchamp, F., Clutton-Brock, T., and Grenfell, B. (1999). Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410. doi: 10.1016/S0169-5347(99)01683-3
Crone, E. E., Pelton, E. M., Brown, L. M., Thomas, C. C., and Schultz, C. B. (in press). Why are monarch butterfly populations declining in western North America? Ecol. Appl.
Dennis, R., Shreeve, T., and Van Dyck, H. (2006). Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers. Conserv. 15, 1943–1966. doi: 10.1007/s10531-005-4314-3
Dennis, R. L. H. (2010). A Resource-Based Habitat View for Conservation: Butterflies in the British Landscape. Oxford: John Wiley & Sons.
Dilts, T. E., Steele, M. O., Engler, J. D., Pelton, E. M., Jepsen, S. J., McKnight, S., et al. (2019). Host plants and climate structure habitat associations of the western monarch butterfly. Front. Ecol. Evol. 7:188. doi: 10.3389/fevo.2019.00188
Espeset, A. E., Harrison, J. G., Shapiro, A. M., Nice, C. C., Thorne, J. H., Waetjen, D. P., et al. (2016). Understanding a migratory species in a changing world: climatic effects and demographic declines in the western monarch revealed by four decades of intensive monitoring. Oecologia 181, 819–830. doi: 10.1007/s00442-016-3600-y
Flockhart, D. T., Brower, L. P., Ramirez, M. I., Hobson, K. A., Wassenaar, L. I., Altizer, S., et al. (2017). Regional climate on the breeding grounds predicts variation in the natal origin of monarch butterflies overwintering in Mexico over 38 years. Glob. Change Biol. 23, 2565–2576. doi: 10.1111/gcb.13589
Flockhart, D. T., Pichancourt, J. B., Norris, D. R., and Martin, T. G. (2014). Unraveling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Ani. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Forister, M. L., Cousens, B., Harrison, J. G., Anderson, K., Thorne, J. H., Waetjen, D., et al. (2016). Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol. Lett. 12:20160475. doi: 10.1098/rsbl.2016.0475
Frampton, G. K., and Dorne, J. L. C. (2007). The effects on terrestrial invertebrates of reducing pesticide inputs in arable crop edges: a meta-analysis. J. Appl. Ecol. 44, 362–373. doi: 10.1111/j.1365-2664.2007.01277.x
Frick, W. F., Pollock, J. F., Hicks, A. C., Langwig, K. E., Reynolds, D. S., Turner, G. G., et al. (2010). An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682. doi: 10.1126/science.1188594
Gilpin, M. E., and Soule, M. E. (1986). “Minimum viable populations: processes of species extinction,” in Conservation Biology: The Science of Scarcity and Diversity, ed M. E. Soule (Sunderland, MA: Sinauer Associates), 19–34.
International Environmental Law Project the Xerces Society (2012). The Legal Status of Monarch Butterflies in California. Portland, OR: International Environmental Law Project; The Xerces Society. Available online at: www.xerces.org
Liermann, M., and Hilborn, R. (1997). Depensation in fish stocks: a hierarchic Bayesian meta-analysis. Can. J. Fish. Aqua. Sci. 5, 1976–1984. doi: 10.1139/f97-105
McGowan, C. P., Allan, N., Servoss, J., Hedwall, S., and Wooldridge, B. (2017). Incorporating population viability models into species status assessment and listing decisions under the US Endangered Species Act. Glob. Ecol. Conserv. 12, 119–130. doi: 10.1016/j.gecco.2017.09.004
Pelton, E., Jepsen, S., Schultz, C., Fallon, C., and Black, S. H. (2016). State of the Monarch Butterfly Overwintering Sites in California. Portland, OR: The Xerces Society for Invertebrate Conservation. Available online at: www.xerces.org
Pleasants, J. M., and Oberhauser, K. S. (2012). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Div. 6,135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Pyle, R. M. (2015). “Monarchs in the mist: new perspectives on monarch distribution in the Pacific Northwest,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser, S. Altizer, and K. Nail (Ithaca, NY: Cornell University Press, 236–246.
Rendón-Salinas, E., Martínez-Meza, F., Mendoza-Pérez, M., Cruz-Piña, M., Mondragon-Contreras, G., and Martínez-Pacheco, A. (2019).Superficie Forestall Ocupada por las Colonias de Mariposas Monarca en México Durante la Hibernación de 2018-2019. Available online at http://d2ouvy59p0dg6k.cloudfront.net/downloads/2018_reporte_monitoreo_mariposa_monarca_mexico_2018_2019.pdf
Satterfield, D. A., Villablanca, F. X., Maerz, J. C., and Altizer, S. (2016). Migratory monarchs wintering in California experience low infection risk compared to monarchs breeding year-round on non-native milkweed. Integr. Comp. Biol. 56, 343–352. doi: 10.1093/icb/icw030
Schultz, C. B., Brown, L. M., Pelton, E., and Crone, E. E. (2017). Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 214, 343–346. doi: 10.1016/j.biocon.2017.08.019
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Shaffer, M. L., and Stein, B. A. (2000). “Safeguarding our precious heritage,” in Precious Heritage: The Status of Biodiversity in the United States, ed M. Schaffer (New York, NY: Oxford University Press, 301–321.
Stark, J. D., Chen, X. D., and Johnson, C. S. (2012). Effects of herbicides on Behr's metalmark butterfly, a surrogate species for the endangered butterfly, Lange's metalmark. Environ. Pollut. 164, 24–27. doi: 10.1016/j.envpol.2012.01.011
Tuskes, P. M., and Brower, L. P. (1978). Overwintering ecology of the monarch butterfly, Danaus plexippus L., in California. Ecol. Entomol. 3, 141–153. doi: 10.1111/j.1365-2311.1978.tb00912.x
Vidal, O., Lopez-Garcia, J., and Rendón-Salinas, E. (2013). Trends in deforestation and forest degradation after a decade of monitoring in the Monarch Butterfly Biosphere Reserve in Mexico. Conserv. Biol. 28, 177–186. doi: 10.1111/cobi.12138
Wood, S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 73, 3–36. doi: 10.1111/j.1467-9868.2010.00749.x
Xerces Society (2018). Managing for Monarchs in the West: Best Management Practices for Conserving the Monarch Butterfly and its Habitat. Portland, OR:The Xerces Society for Invertebrate Conservation. Available online at: www.xerces.org
Xerces Society Western Monarch Thanksgiving Count (2019). Western Monarch Thanksgiving Count Data from 1997–2018. Available online at: www.westernmonarchcount.org
Keywords: Danaus plexippus plexippus, western monarchs, quasi-extinction, conservation, population trends
Citation: Pelton EM, Schultz CB, Jepsen SJ, Black SH and Crone EE (2019) Western Monarch Population Plummets: Status, Probable Causes, and Recommended Conservation Actions. Front. Ecol. Evol. 7:258. doi: 10.3389/fevo.2019.00258
Received: 05 March 2019; Accepted: 18 June 2019;
Published: 03 July 2019.
Edited by:
Jay E. Diffendorfer, United States Geological Survey, United StatesReviewed by:
Tyler Flockhart, University of Maryland Center for Environmental Science (UMCES), United StatesCopyright © 2019 Pelton, Schultz, Jepsen, Black and Crone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth E. Crone, ZWxpemFiZXRoLmNyb25lQHR1ZnRzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.