- 1Centre for Pain Research, Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD, Australia
- 2Departamento de Ciências Patológicas, Centro de Ciências Biológicas, Universidade Estadual de Londrina, Londrina, Brazil
- 3Division of BioAnalytical Chemistry, Amsterdam Institute for Molecules Medicines and Systems, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 4Centre for Snakebite Research & Interventions, Liverpool School of Tropical Medicine, Liverpool, United Kingdom
Animal venoms have evolved over millions of years for prey capture and defense from predators and rivals. Snake venoms, in particular, have evolved a wide diversity of peptides and proteins that induce harmful inflammatory and neurotoxic effects including severe pain and paralysis, hemotoxic effects, such as hemorrhage and coagulopathy, and cytotoxic/myotoxic effects, such as inflammation and necrosis. If untreated, many envenomings result in death or severe morbidity in humans and, despite advances in management, snakebite remains a major public health problem, particularly in developing countries. Consequently, the World Health Organization recently recognized snakebite as a neglected tropical disease that affects ~2.7 million p.a. The major protein classes found in snake venoms are phospholipases, metalloproteases, serine proteases, and three-finger peptides. The mechanisms of action and pharmacological properties of many snake venom toxins have been elucidated, revealing a complex multifunctional cocktail that can act synergistically to rapidly immobilize prey and deter predators. However, despite these advances many snake toxins remain to be structurally and pharmacologically characterized. In this review, the multifunctional features of the peptides and proteins found in snake venoms, as well as their evolutionary histories, are discussed with the view to identifying novel modes of action and improving snakebite treatments.
Introduction
The composition and evolutionary histories of animal venoms have fascinated the scientific community for centuries. Venoms have evolved over millions of years to facilitate prey capture and/or defense from predators and rivals. Snake venoms, in particular, likely originated in the Cenozoic Era (Fry, 2005; Fry et al., 2006), and they are amongst the most well-characterized of animal venoms, comprising a complex mixture of toxic, and pharmacologically-active proteins and peptides (Casewell et al., 2013; Chan et al., 2016). Tragically, snake envenomation is a significant health and economic burden worldwide. It is estimated 1.8–2.7 million snakebites and 81,410–137,880 deaths occur annually worldwide (Kasturiratne et al., 2008; Gutierrez et al., 2017), a problem which is mostly associated with agricultural work, especially in South and Southeast Asia, sub-Saharan Africa, and Central and South America (Kasturiratne et al., 2008; Harrison et al., 2019). In 2017, the World Health Organization (WHO) finally recognized the snakebite as a priority neglected tropical disease in which morbidity and mortality affects mostly individuals under 30 years old, who are often the most economically productive members of a community (WHO, 2018).
Snake venoms have a distinct complexity when compared to venoms from other animals such as spiders, scorpions, and cone snails (Zelanis and Tashima, 2014). In these animal venoms, the pharmacological effects are primarily caused by disulfide bridged peptides, whilst snake venoms consist of a more diverse array of larger proteins and peptides which results in a wider variety of pharmacological and toxicological effects (Zhang, 2015). These venoms comprise 50–200 components distributed in dominant and secondary families which can be presented in multiple proteins and peptides isoforms (Vonk et al., 2011; Slagboom et al., 2017; Tasoulis and Isbister, 2017). The dominant families are secreted phospholipases A2 (PLA2s), snake venom metalloproteinases (SVMP), snake venom serine proteases (SVSP), and three-finger peptides (3FTX), while the secondary families comprise cysteine-rich secretory proteins, L-amino acid oxidases, kunitz peptides, C-type lectins, disintegrins, and natriuretic peptides (Slagboom et al., 2017; Tasoulis and Isbister, 2017; Munawar et al., 2018). Interestingly, snake venom composition varies interspecifically (Fry et al., 2008; Tasoulis and Isbister, 2017), as well as intraspecifically, with many factors influencing this diversity including age (Dias et al., 2013), gender (Menezes et al., 2006; Zelanis et al., 2016), location (Durban et al., 2011; Goncalves-Machado et al., 2016), diet (Barlow et al., 2009), and season (Gubensek et al., 1974). This variability phenomenon underpins toxin diversity and multifunctionality, and is of great importance to be considered in antivenom production and envenomation treatment (Gutierrez et al., 2017).
The pharmacological effects of snake venoms are classified into three main types, hemotoxic, neurotoxic, and cytotoxic (WHO, 2010). The major toxins involved in these effects are the PLA2s, SVMPs, SVSPs, and 3FTXs, that alone or in combination, are responsible for the multiple pharmacological effects occurring in snakebite victims. For example, some PLA2s and 3FTX are able to act on pre- or post-synaptic junctions as antagonist of ion channels and nicotinic or muscarinic receptors to induce severe neurotoxicity such as paralysis and respiratory failure (Fry et al., 2003; Lynch, 2007; Casewell et al., 2013; Harris and Scott-Davey, 2013; Tsetlin, 2015). In addition, other PLA2s and 3FTXs, along with SVMPs, cause local tissue damage resulting in swelling, blistering, bruising, and necrosis, and systemic effects such as hypovolemic shock (Gutierrez and Rucavado, 2000; Gutierrez et al., 2005; Harris and Scott-Davey, 2013; Rivel et al., 2016). Furthermore, SVSPs and SVMPs induce hemostatic and cardiovascular effects as coagulopathy, hypotension and hemorrhage (Slagboom et al., 2017). Interestingly, some PLA2s, SVSPs, and SVMPs are also capable of triggering severe pain by modulating pain pathways through activation of ion channels, such as transient receptor potential vanilloid type 1 (TRPV1) and acid-sensing ion channel (ASIC) (Bohlen et al., 2011; Zhang et al., 2017); and/or by pain sensitization through inflammatory mediators (Zychar et al., 2010; Menaldo et al., 2013; Ferraz et al., 2015; Mamede et al., 2016; Zambelli et al., 2017b). The inflammation induced by the elapid and viper venoms is widely reported to produce pain or hyperalgesia in human and in experimental models (Hifumi et al., 2015; Bucaretchi et al., 2016; Kleggetveit et al., 2016; Mamede et al., 2016). Unfortunately, these are not completely reversed by antivenom and anti-inflammatory therapies (Picolo et al., 2002; Ferraz et al., 2015; Hifumi et al., 2015).
The toxicological effects induced by snakebite are currently treated with intravenous administration of antivenom in combination to analgesics, fluid therapy, hemodialysis and/or antibiotics (Gutierrez et al., 2017). Although sufficient in most cases, snakebite treatments have been challenged by the continuous high numbers of clinical illness and mortality associated with snakebites worldwide (WHO, 2018). Furthermore, chronic morbidity following snakebites have been underestimated, with many victims reporting chronic symptoms in the bitten region, including complex regional pain syndrome (CRPA) (Seo et al., 2014; Kleggetveit et al., 2016) and musculoskeletal disabilities (Jayawardana et al., 2016). Available snakebite treatments face challenges associated with limited para-specificity, poor antibody specificity, high incidences of adverse reactions, low availability and poor affordability to those who need them, along with poor efficacy against local tissue effects (Williams et al., 2011; Gutierrez et al., 2017; Ainsworth et al., 2018; Harrison et al., 2019). Therefore, current research efforts are directed to the development of more effective snakebite therapies able to generically fully inhibit the major toxic components of snake venoms in order to better overcome severe acute and chronic effects caused by snakebite.
In light of the public health importance and the complexity of snake venoms, in this review we highlight the multifunctionality, structure-activity relationships and evolution of proteins and peptides in snake venoms. We aim to provide a better understanding of their action mechanisms and effects, and to bring attention to their undetermined targets and a host of potential novel therapeutic targets that might have implications for improving the treatments of snakebites.
Phospholipases (PLA2s)
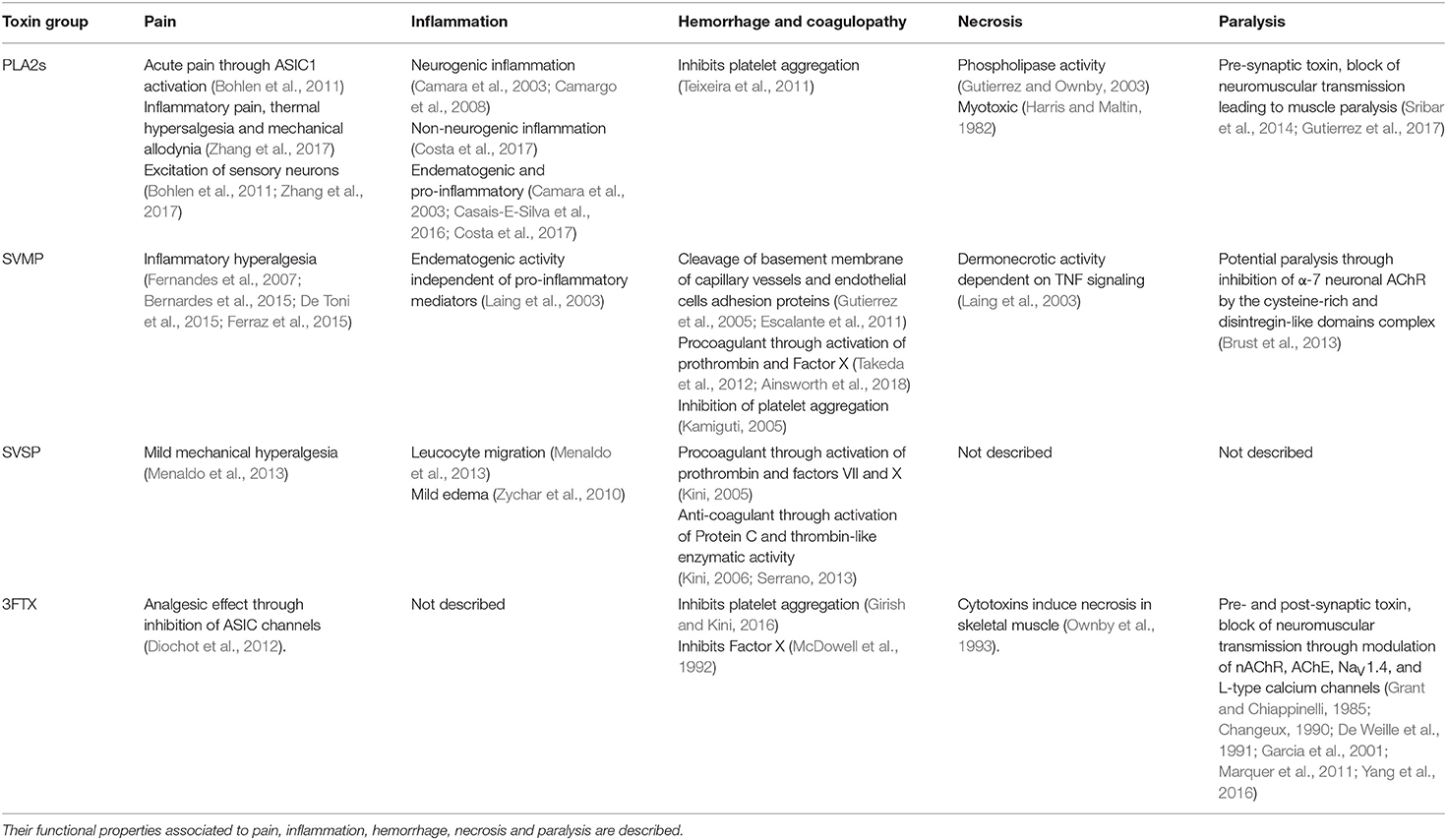
Phospholipases A2 play an important role in the neurotoxic and myotoxic effects of snakebites (Harris and Scott-Davey, 2013). These proteins have molecular masses of 13–15 kDa and are classified into groups I and II, which are found as major components in the venoms of Elapidae and Viperidae, respectively (Six and Dennis, 2000; Harris and Scott-Davey, 2013). In addition, a third group of PLA2s, termed IIE, have been predominately recovered from the venoms of non-front fanged snakes, although their importance in the venom arsenal remains unclear (Fry et al., 2012; Perry et al., 2018). Studies reconstructing the evolutionary history of this multi-locus gene family have demonstrated that each of these PLA2s types (I, II, and IIE) have been independently recruited into snake venom systems (Fry et al., 2012; Junqueira-De-Azevedo et al., 2015), suggesting they have evolved their toxic properties by convergent evolution (Lomonte and Rangel, 2012). Although PLA2s from vipers and elapids share similar enzymatic properties, both types have undergone extensive gene duplication over evolutionary time, seemingly facilitating the evolution of new toxic functions, and resulting in different patterns of residue conservation (Lynch, 2007; Vonk et al., 2013; Dowell et al., 2016; Figure 1A). In addition, the venoms of viper snakes contain isoforms of group II PLA2s that are catalytically-active (e.g., Asp49) and catalytically-less active (e.g., Lys49) (Lomonte and Rangel, 2012).
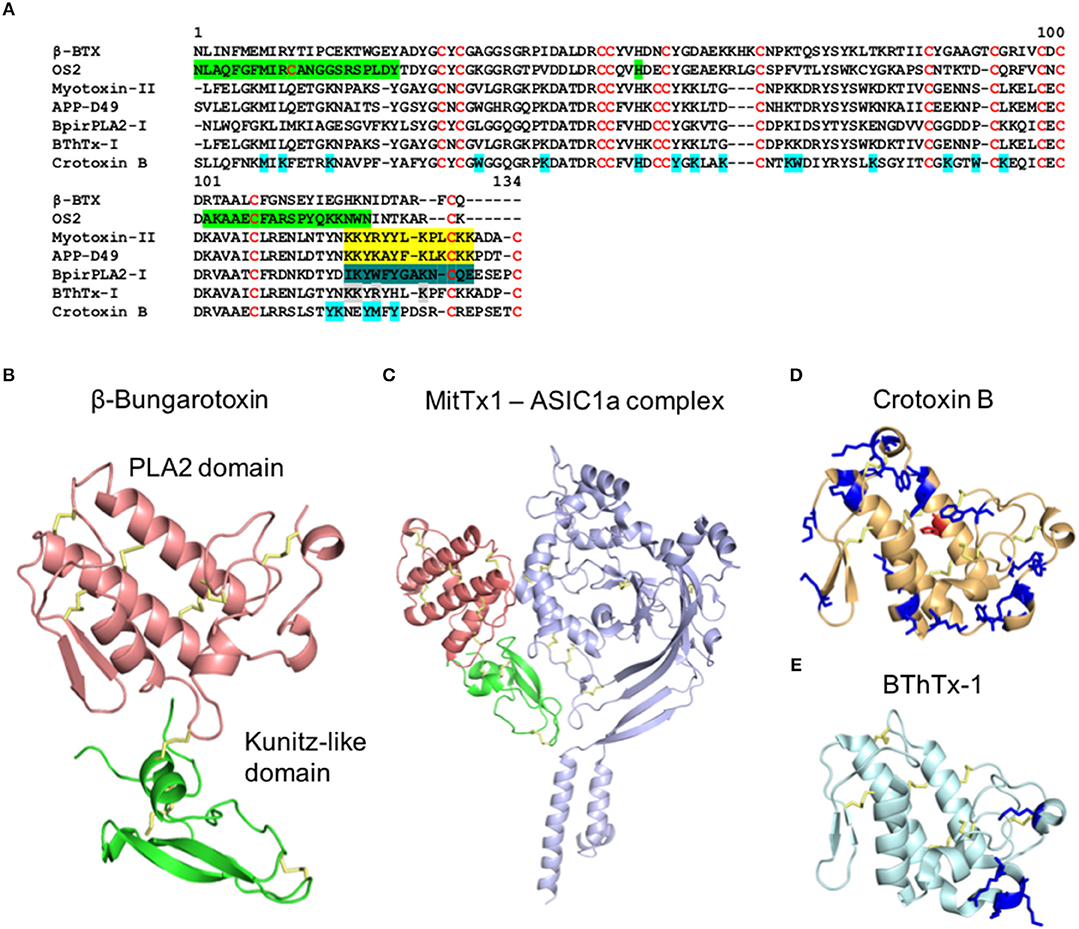
Figure 1. Structure of PLA2s from snake venoms. (A) Alignment of the primary structure of PLA2s from snakes belong to Elapidae (Class I) and Viparinae (Class II). β-Bungarotoxin (β-BTX) from Bungarus multicinctus (Class I, Basic) (Uniprot P00617), OS2 from Oxyuranus scutellatus (Class I, Basic) (Uniprot Q45Z47), Myotoxin II from Bothrops asper (Class II, Basic) (Uniprot P24605), APP-D49 from A. piscivorus (Class II, Basic) (Uniprot P51972), BpirPLA2-I from Bothrops pirajai (Class II, Acid) (Uniprot C9DPL5), Bothropstoxin-1 (BThTx-I) from Bothrops jararacussu (Class II, Basic) (Uniprot Q90249), and Crotoxin B from Crotalus durissus terrificus (Class II, Basic) (Uniprot P24027). Green: N-terminal region critical for enzymatic and neurotoxic properties, C-terminal region essential for enzymatic activity and central Histidine in the catalytic site (Rouault et al., 2006), Yellow: C-terminal region with myotoxic properties (Lomonte et al., 1994; Nunez et al., 2001), Dark green: C-terminal region with anti-platelet aggregation activity (Teixeira et al., 2011), Gray: Lysin residues involved in the nociceptive, and/or endematogenic properties (Zambelli et al., 2017a) and Cyan: modified residues in the Crotoxin B which lead to alterations in the enzymatic, toxic, and pharmacological properties (Soares et al., 2001). (B–E) Cartoon representation of the three-dimensional structure of (B) β-Bungarotoxin with the PLA2 domain in red and knutiz domain in green (PDB 1BUN), (C) MitTx1 and ASIC1a channel complex with PLA2 domain in red, knutiz domain in green and ASIC1a channel in blue (PDB 4NTY), (D) Crotoxin B (PDB 2QOG) and (E) BThrTx1 (PDB 3HZD). (D,E) Highlighted in blue are the amino acids positions involved in the enzymatic, toxic and pharmacological properties of Crotoxin B (Soares et al., 2001) and in the nociceptive and/or endematogenic properties in BThrTx1 (Zambelli et al., 2017a). The central histidine in the catalytic site of Crotoxin B is highlighted in red.
A number of PLA2s exert strong myotoxic effects which often lead to severe necrosis (Harris and Maltin, 1982; Gutierrez and Ownby, 2003), and many of these toxins also promote inflammation, including edema formation, cytokine production and leukocyte recruitment, pain by inducing thermal allodynia and mechanical hyperalgesia, paralysis through block of neuromuscular transmission and intensify hemorrhage by inhibiting coagulation (Table 1) (Camara et al., 2003; Chacur et al., 2003; Camargo et al., 2008; Teixeira et al., 2011; Lomonte and Rangel, 2012; Harris and Scott-Davey, 2013; Casais-E-Silva et al., 2016; Costa et al., 2017; Zambelli et al., 2017b; Zhang et al., 2017). Neurotoxic effects caused by these toxins, as well as some of their proinflammatory effects, occurs via the modulation of pre-synaptic terminals as well as sensory nerve-endings (Camara et al., 2003; Harris and Scott-Davey, 2013; Sribar et al., 2014; Zhang et al., 2017). The PLA2s pre-synaptic effects are characteristic of β-neurotoxins and target the motor nerve terminals at the neuromuscular junction (Sribar et al., 2014; Gutierrez et al., 2017). The mechanisms in which certain PLA2s exert their pre-synaptic effects are not fully understood, and the primary targets remain unidentified, although the PLA2 β-bungarotoxin is known to bind to K+ channels at the pre-synaptic terminals via an accessory kunitz subunit (Benishin, 1990; Sribar et al., 2014; Figure 1B). Overall, these pre-synaptic effects induce robust exocytosis of the neurotransmitters vesicles reserves which consequently lead to the depletion of neurotransmitter release in the neuromuscular junction to promote muscle paralysis (Harris et al., 2000; Sribar et al., 2014; Gutierrez et al., 2017).
The inflammation induced by PLA2s has non-neurogenic and neurogenic (substance-P dependent) components (Camara et al., 2003; Camargo et al., 2008; Costa et al., 2017; Zhang et al., 2017). The non-neurogenic component is mostly caused by the hydrolysis of membrane lipids that generate potent pro-inflammatory lipid mediators (Costa et al., 2017). Additional non-neurogenic and neurogenic inflammations induced by PLA2s use more complex mechanisms still not fully understood. For example, leukocyte recruitment (De Castro et al., 2000), mastocytes degranulation (Menaldo et al., 2017), and macrophage activation (Triggiani et al., 2000; Granata et al., 2006; Giannotti et al., 2013) were demonstrated to occur independently of the generic PLA2s lipid hydrolysis catalytic activity. Furthermore, substance-P mediated neurogenic inflammation has been described to be induced by PLA2s from Crotalus durissus cascavella (Camara et al., 2003) and from Naja mossambica (Camargo et al., 2008). Interestingly, the C-terminal of Myotoxin-II (a Lys49-PLA2) isolated from Bothrops asper was able to activate macrophages, showing this region maybe be crucial for the observed enzymatic-independent inflammation (Giannotti et al., 2013) (Figure 1A).
The pain induced by PLA2s is driven by inflammatory processes and sensory neuronal activation. Bradykinin is an important mediator of the inflammatory pain induced by PLA2s (Moreira et al., 2014; Urs et al., 2014; Mamede et al., 2016; Zambelli et al., 2017b). It induces mechanical hyperalgesia dependent on the production of TNF-α, IL-1β, and prostaglandins (Cunha et al., 1992). This suggests that PLA2s contribute to an increase in arachidonic acid release from cell membranes and its availability to be processed by cyclooxygenase resulting in prostaglandin production (Verri et al., 2006). Corroborating this hypothesis, studies performed in rodents have demonstrated that PLA2s isolated from different snake venoms induced hyperalgesia mediated by biogenic amines, cytokines, prostaglandins, sympathomimetic amines, ATP, K+ release, purinergic receptor activation, and glial cell activation (Nunez et al., 2001; Chacur et al., 2003, 2004; Zhang et al., 2017). Interestingly, the presence of a strong catalytic activity in the PLA2s is not essential for its nociceptive activity as observed by the nociceptive effects of PLA2s “Lys49” variants (Lomonte et al., 1994; Rong et al., 2016; Zhang et al., 2017). Direct activation of sensory neurons was demonstrated by MitTx from Micrurus tener tener, a heteromeric complex between a PLA2 and a kunitz peptide (Bohlen et al., 2011), and by the Lys49 PLA2 BomoTx from the Brazilian viper Bothrops moojeni (Zhang et al., 2017). MitTx activates somatosensory neurons and was found to be a potent and selective agonist of ASIC channels (Figure 1C). This agonistic effect induces robust pain behavior in mice via activation of ASIC1 channels on capsaicin-sensitive nerve fibers (Bohlen et al., 2011). BomoTx also activated a cohort of sensory neurons to induce ATP release followed by activation of purinergic receptors (Zhang et al., 2017). Unfortunately, the primary target of this neuronal activation is still unknown. This same toxin induced non-neurogenic inflammatory pain, thermal hyperalgesia dependent on TRPV1 channels-expressing nerve fibers, and mechanical allodynia dependent on P2X2/P2X3-expressing fibers (Zhang et al., 2017).
The multifunctionality of PLA2s is evidenced by their myotoxic, neurotoxic and enzymatic functions, as well as by their inflammatory properties. There is evidence that separate domains and regions of the PLA2s structure participate in these various activities (Figures 1A,B). For example, for the Lys49-PLA2 from Bothrops asper and Agkistrodon piscivorus piscivorus, the C-terminal region of these toxins (residues 115–129) were identified as the active sites responsible for their myotoxic effects (Lomonte et al., 1994; Nunez et al., 2001) (Figure 1A). Interestingly, the same C-terminal region in BpirPLA2-I isolated from Bothrops pirajai had anticoagulant activity through inhibition of platelet aggregation (Teixeira et al., 2011). Crotoxin B, an Asp49-PLA2, and a major component of the venom of Crotalus durissus terrificus, has toxic active sites fully independent of its enzymatic activity (Soares et al., 2001; Figure 1D), while Lemnitoxin, a basic class I PLA2 isolated from Micrurus lemniscatus, has strong myotoxic and proinflammatory effects but no neurotoxic activity (Casais-E-Silva et al., 2016). A detailed mutational study using the PLA2 OS2 from the Australian Taipan snake Oxyuranus scutellatus scutellatus revealed that a 500-fold loss in enzymatic activity had only a minor effect on its neurotoxicity (Rouault et al., 2006). Furthermore, the enzymatic activity of OS2 was dependent of the N- and C-terminal regions, and the N-terminal region had a major role in the central nervous system neurotoxicity. An alanine scan of the Lys49-PLA2 from Bothrops jararacussu (BThTx-I) demonstrated distinct regions involved in the hyperalgesia and edema (Zambelli et al., 2017a). In this study, the mutant Arg118Ala lost both nociceptive and edematogenic properties, Lys115Ala and Lys116Ala lost the nociceptive effects without interfering with the edema formation and Lys122Ala lost the nociceptive properties and had weak inflammatory effects (Figure 1E). Similarly, an independent study showed the Lys122Ala substitution led to reduced membrane damaging and myotoxic activities (Ward et al., 2002). This C-terminal region is characterized by the presence of basic and hydrophobic residues which have been strongly associated with the ability of PLA2s to interact and penetrate the lipid bilayer (Delatorre et al., 2011; Gutierrez and Lomonte, 2013).
The variability of PLA2 isoforms observed in the venom of different snakes may reflect a variety of factors, including the evolutionary history, phylogeography, diet, and/or environmental conditions relating to a species or populations within a species (Zancolli et al., 2019). Many snake venom toxins are known to be encoded by multi-locus gene families (Casewell et al., 2013), and the resulting genes within those families have been found clustered together in arrays on microchromosomes, likely as the result of tandem gene duplication events (Vonk et al., 2013). The process of gene duplication and loss underpins the evolution of many snake venom toxin families, including the PLA2s (Lynch, 2007; Vonk et al., 2013; Casewell et al., 2014; Dowell et al., 2016), with duplications likely initially stimulating a gene dosing effect while also freeing duplicates from evolutionary constraints, and thus enabling a scenario that may facilitate protein sub- and/or neo-functionalization (Lynch and Conery, 2000). Indeed, studies have demonstrated that extremely divergent venom phenotypes (e.g., neurotoxic vs. haemorrhagic) observed within populations of the same snake species, or between closely related species, are at least partially the result of major genomic differences in PLA2 toxin loci, with variation at different gene complexes resulting in markedly different haplotypes (Dowell et al., 2018; Zancolli et al., 2019). It remains unclear as to the specific processes that underpin such diversity, although natural selection driven by environmental factors and hybridization events have both been proposed (Dowell et al., 2018).
Snake Venom Metalloproteinases (SVMPs)
Snake Venom MetalloProteinases (SVMPs) are zinc-dependent proteinases ranging from 20 to 110 kDa in size and are categorized into P-I, P-II, and P-III classes according to their structural domains (Hite et al., 1994; Jia et al., 1996; Fox and Serrano, 2005) (Figure 2). These toxins are major components of viper venoms and play a key role in the toxicity of these snake venoms (Table 1; Tasoulis and Isbister, 2017). Venom SVMPs have evolved from ADAM (a disintegrin and metalloproteinase) proteins, specifically ADAM28 (Casewell, 2012), with the P-III being the most basal structural variant consisting of metalloproteinase, disintegrin-like, and cysteine-rich domains (Moura-Da-Silva et al., 1996; Casewell et al., 2011; Figure 2B). Subsequently, P-II SVMPs diverged from P-IIIs and consist of a metalloproteinase and disintegrin domain, with the latter typically detected in venom as a proteolytically processed product (Fox and Serrano, 2008; Casewell et al., 2011). The final class, P-I SVMPs which consist only of the metalloproteinase domain, appeared to have evolved on multiple independent occasions in specific lineages as a result of loss of the P-II disintegrin-encoding domain (Casewell et al., 2011). Throughout this diverse evolutionary history, SVMPs show evidence of extensive gene duplication events, coupled with bursts of accelerated molecular evolution (Casewell et al., 2011; Vonk et al., 2013). However, while all three classes of SVMPs have been described from viper venoms, only P-III SVMPs have been detected in elapid and “colubrid” venoms (Casewell et al., 2015). While these P-III SVMPs are typically relatively lowly abundant venom components in elapid snakes (e.g., <10% of venom toxins), they can be major components in “colubrids” (Mackessy and Saviola, 2016; Pla et al., 2017; Tasoulis and Isbister, 2017; Modahl et al., 2018a). These abundance differences likely underpin the distinct pathologies observed following envenomings by snakes found in these families. SVMPs contribute extensively to the hemorrhagic and coagulopathic venom activities following bites by viperid snakes, and the diversity of SVMPs isoforms often present in their venom likely facilitate synergistic effects, such as simultaneous action on multiple steps of the blood clotting cascade (Kini and Koh, 2016; Slagboom et al., 2017). Certain “colubrid” snakes, whether medically important or not, also show evidence of having multiple SVMP toxins in their venom, even if they do not show the sub-class diversity observed in the vipers (Mackessy and Saviola, 2016; Pla et al., 2017; Modahl et al., 2018a; Perry et al., 2018). However, it is relatively uncommon for elapid snakebites to cause systemic hemotoxicity (Slagboom et al., 2017) and this is likely a consequence of those venoms exhibiting little diversity or abundance of SVMPs, and instead usually being dominated by neurotoxic toxin families such as the 3FTXs and PLA2s (Tasoulis and Isbister, 2017).
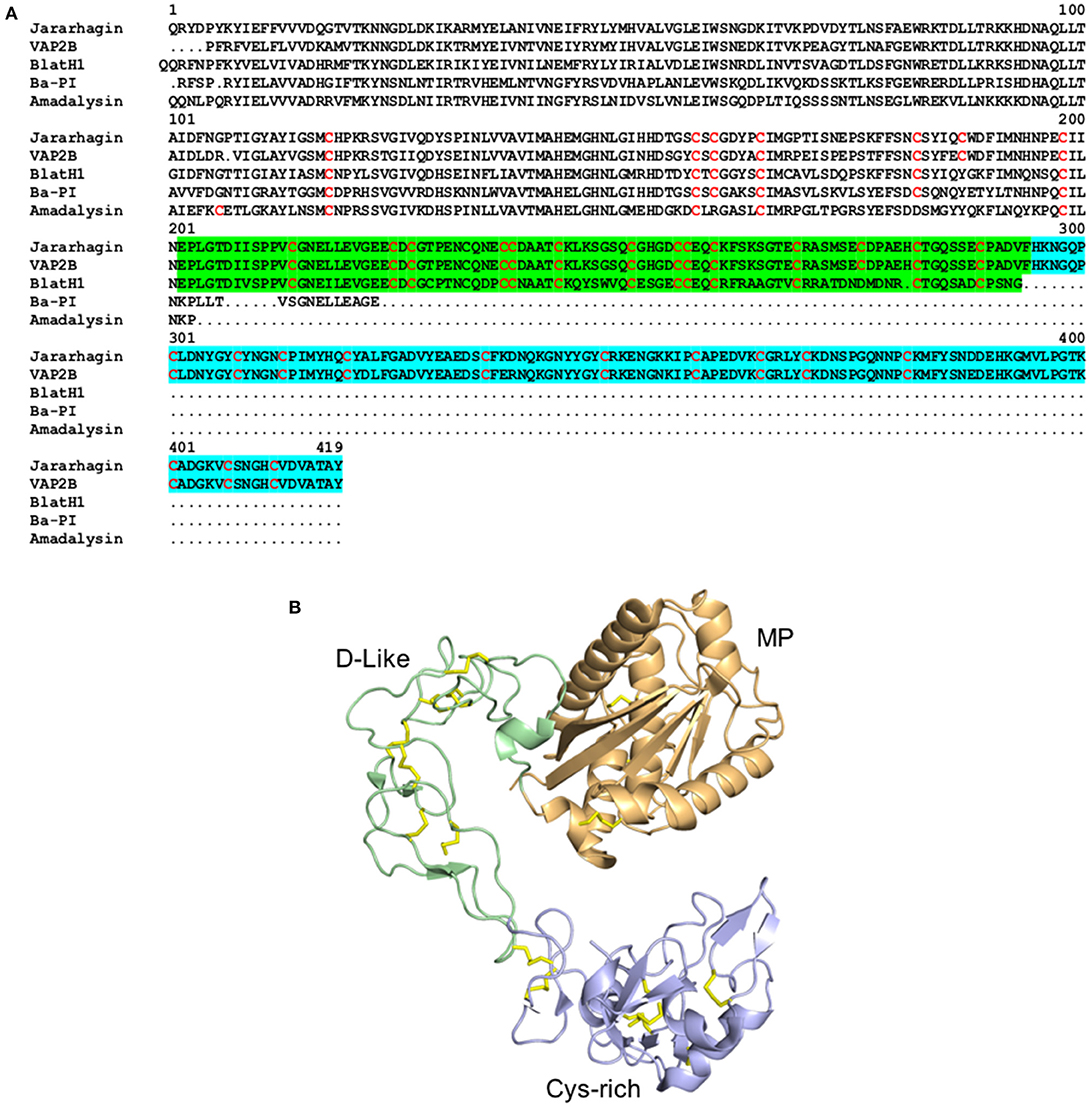
Figure 2. Structure of metalloproteinaises from snake venoms. (A) Alignment of the primary structure of the SVMPs Jararhagin from Bothrops jararaca (Uniprot P30431) and VAPB2 from Crotalus atrox (Uniprot Q90282) belonging to the class P-III, BlatH1 from Bothriechis lateralis (Uniprot U5PZ28) belonging to the class P-II, and Ba-PI from Bothrops asper (Uniprot P83512) and Adamalysin from Crotalus adamanteus (Uniprot P34179) belonging to classes P-I. Cysteines are colored in red, the disintegrin-like domain is highlighted in green and the cysteine-rich domain is highlighted in blue. (B) Cartoon representation of the three-dimensional structure of the class P-III metalloproteinase VAPB2 from Crotalus atrox (PDB 2DW0). The metalloproteinase domain is colored in orange, the disintegrin-like domain (D-like) is colored in green and the cysteine-rich domain (Cys-rich) is colored in blue. The disulphide bridges are colored in yellow.
As mentioned above, the SVMPs are known for their hemorrhagic activity as well as for their ability to influence multiple steps of the blood clotting cascade, resulting in a lethal combination of systemic hemorrhage and incoagulable blood in prey and/or victims (Markland and Swenson, 2013). Research has revealed that the effects of SVMP-induced hemorrhage relies on a mechanism that occurs in two steps (Gutierrez et al., 2005; Escalante et al., 2011). First, SVMPs cleave the basement membrane and adhesion proteins of endothelial cells-matrix complex to weaken the capillary vessels. During the second stage, the endothelial cells detach from the basement membrane and become extremely thin, resulting in disruption of the capillary walls and effusion of blood from the fragile capillary walls. In addition to the proteinase activity, SVMPs impact on homeostasis by altering coagulation, which contributes to their toxic hemorrhagic effects (Markland, 1998; Takeda et al., 2012; Slagboom et al., 2017). This occurs through modulation of factors such as fibrinogenase and fibrolase that mediate the coagulation cascade, depletion of pro-coagulation factors through consumption processes (e.g., Factor X, prothrombin and fibrinogen), platelet aggregation inhibition and inflammatory activities (Kamiguti, 2005; Kini, 2005; Takeda et al., 2012; Kini and Koh, 2016; Slagboom et al., 2017; Ainsworth et al., 2018).
Some SVMPs also induce inflammation, including edema, and pain by triggering hyperalgesia (Dale et al., 2004; da Silva et al., 2012; Bernardes et al., 2015). The inflammation induced by jararhagin, a class P-IIIb metalloproteinase with potent hemorrhagic and dermonecrotic activity isolated from Bothrops jararaca, produced TNF-α and IL-1β in vivo (Laing et al., 2003; Clissa et al., 2006), while BJ-PI2, isolated from the same snake species, lacked hemorrhagic and necrotic activities but still induced vascular permeability and inflammatory cell migration (da Silva et al., 2012). Curiously, the edema formation induced by jararhagin was independent of pro-inflammatory mediators such as TNF, IL-1β, and IL-6 (Laing et al., 2003). Neurogenic inflammation was also implicated in the local hemorrhage induced by Bothrops jararaca which was shown to be dependent on serotonin and other neuronal factors (Goncalves and Mariano, 2000). The mechanisms on how neurogenic inflammation is triggered by the snake venom components and how it participates in the hemorrhagic process are still not understood. Pain induced by SVMPs is characterized by hyperalgesia and inflammatory pain, which is dependent on the production of cytokines, nitric oxide, prostaglandins, histamine, leukotrienes, and migration of leukocytes, mast cell degranulation and NFkB activation (Fernandes et al., 2007; Bernardes et al., 2015; De Toni et al., 2015; Ferraz et al., 2015). However, the mechanisms underlying SVMP-induced pain are still poorly understood, with neurogenic inflammation and neuronal excitatory properties still underexplored. The research reported to date indicates that SVMPs induce the production of inflammatory mediators and activate cytokine/chemokine cascades and the release of prostaglandins and sympathetic amines to engender nociceptor sensitization (Verri et al., 2006; De Toni et al., 2015; Ferraz et al., 2015).
The multifunctional properties of SMVPs are also well-described. Class P-III SVMPs tend to display stronger hemorrhagic activity compared to P-I and P-II SVMPs, possibly due to the disintegrin-like and cysteine-rich domains enabling binding to relevant targets in the extracellular matrix of capillary vessels. The functions of these domains have been investigated in inflammation, revealing that these domains are sufficient to induce pro-inflammatory responses through production of TNF-α, IL-1β and IL-6, and leukocyte migration, in which mechanisms and primary targets are still unknown (Clissa et al., 2006; Ferreira et al., 2018). These observations suggest that these domains are involved in the inflammatory hyperalgesia induced by SVMPs. Furthermore, the pronounced hemorrhagic and necrotic activities are strongly dependent on biological effects driven by the disintegrin-like and cysteine-rich domains, as observed for BJ-PI2 (da Silva et al., 2012). The hemorrhagic activity of Bothrops jararaca venom was also shown dependent on neurogenic inflammation (Goncalves and Mariano, 2000). These findings implicate the disintegrin-like and/or cysteine-rich domains as key player(s) in these neurogenic mechanisms, and possibly in non-inflammatory pain, from which the neuronal targets are still to be identified.
Snake Venom Serine Proteinases (SVSPs)
Snake Venom Serine Proteinases (SVSPs) belong to the S1 family of serine proteinases and display molecular masses ranging from 26 to 67 kDa and two distinct structural domains (Figure 3). These venom toxins have evolved from kallikrein-like serine proteases and, following their recruitment for use in the venom gland, have undergone gene duplication events giving rise to multiple isoforms (Fry et al., 2008; Vaiyapuri et al., 2012). SVSPs catalyze the cleavage of polypeptide chains on the C-terminal side of positively charged or hydrophobic amino acid residues (Page and Di Cera, 2008; Serrano, 2013). Similar to SVMPs, SVSPs have been described in the venom of a wide variety of snake families, although they are typically only abundant in viper venoms, and much less common in the venoms of elapid and “colubrid” snakes (Tasoulis and Isbister, 2017; Modahl et al., 2018a). Whilst the SVMPs are well-known for their ability to rupture capillary vessels, SVSPs execute their primary toxicity by altering the hemostatic system of their victims, and by inducing edema and hyperalgesia through mechanisms still poorly understood (Table 1). Hemotoxic effects caused by SVSPs include perturbations of blood coagulation (pro-coagulant or anti-coagulant), fibrinolysis, platelet aggregation and blood pressure, with potential deadly consequences for snakebite victims (Murakami and Arni, 2005; Kang et al., 2011; Serrano, 2013; Slagboom et al., 2017).
Figure 3. Structure of Serine proteinases from snake venoms. (A) Alignment of the primary structure of the SVSPs Dav-PA from Deinagkistrodon acutus (Uniprot Q9I8X1), BPA from Bothrops jararaca (Uniprot Q9PTU8), and ACC-C from Agkistrodon contortrix (Uniprot P09872). In Dav-PA, the N- and C-terminal domains are highlighted in blue and green, respectively. (B) Cartoon representation of the three-dimensional structure of the SVSP Dav-PA from Deinagkistrodon acutus (PDB 1OP0), with N- and C-terminal domains colored in blue and green, respectively.
Pro-coagulant SVSPs have been described to activate multiple coagulation factors, including prothrombin and factors V, VII, and X (Kini, 2005; Serrano, 2013). For example, the activation of prothrombin produces thrombin which in turn produces fibrin polymers that are cross-linked. Thrombin also activates aggregation of platelets which, together with the formation of fibrin clots, results in coagulation (Murakami and Arni, 2005). In addition, platelet-aggregating SVSPs will activate the platelet-receptors to promote binding to fibrinogen and clot formation (Yip et al., 2005). These procoagulant and platelet-aggregating activities will lead to the rapid consumption of key factors in the coagulation cascade and clot formation. On the other hand, anticoagulant SVSPs effects involve the activation of Protein C, which subsequently inactivates the coagulant factors Va and VIIIa (Kini, 2006). Furthermore, fibrinolytic SVSPs play an important role in the elimination of blood clots by acting as thrombin-like enzymes or plasminogen activators, which eliminates the fibrin in the clots and contributes significantly to the establishment of the coagulopathy (Kang et al., 2011; Serrano, 2013). Through the activation/depletion and inactivation of these coagulation factors, the clotting of blood is prevented, leading to uncoagulable blood, and external and internal bleeding.
Little is known about inflammatory responses and hyperalgesia induced by SVSPs. Studies suggest SVMPs and PLA2s have a pivotal role in the inflammatory responses and pain induced by snake venoms, while SVSPs have an important role in inflammation and a minor role in pain (Zychar et al., 2010; Menaldo et al., 2013). SVSPs in the venoms of Bothrops jararaca and Bothrops pirajai induce inflammation through edema formation, leucocyte migration (mainly neutrophils) and mild mechanical hyperalgesia, however, the mediators involved in these effects are still unknown (Zychar et al., 2010; Menaldo et al., 2013).
Three-Finger Toxins (3FTXs)
Three-fingers toxins (3FTXs) are non-enzymatic neurotoxins ranging from 58 to 81 residues that contain a three-finger fold structure stabilized by disulfide bridges (Osipov and Utki, 2015; Kessler et al., 2017; Figure 4A). They are present mostly in the venoms of elapid and colubrid snakes, and exert their neurotoxic effects by binding postsynaptically at the neuromuscular junctions to induce flaccid paralysis in snakebite victims (Barber et al., 2013). Three-finger toxins differ in length, with short-chain 3FTXs including α-neurotoxins, β-cardiotoxins, cytotoxins, fasciculins and mambalgins, which comprise of 57–62 residues and 4 disulfide bridges, and long-chain 3FTXs which include α-neurotoxins and γ-neurotoxins, hannalgesin and κ-neurotoxins, and comprise 66–74 residues and five disulfide bridges. Furthermore, they can exist as monomers and as covalent or non-covalent homo or heterodimers. The diversity of 3FTX isoforms described above are a direct result of a diverse evolutionary history, whereby ancestral 3FTXs have diversified by frequent gene duplication and accelerated rates of molecular evolution. These processes, which are broadly similar to those underpinning the evolution of the other toxin families described above, are particularly associated with the evolution of a high-pressure hollow-fanged venom delivery system observed in elapid snakes (Sunagar et al., 2013). For example, gene duplication events have resulted in the expansion of 3FTX loci from one in non-venomous snakes like pythons, to 19 in the elapid Ophiophagus hannah (king cobra) (Vonk et al., 2013), and selection appears to have acted extensively on surface exposed amino acid residues in these resulting paralogous elapid 3FTX genes (Sunagar et al., 2013). The consequences of this evolutionary history are the differential production of numerous 3FTX isoforms that often exhibit considerable structural differences and distinct biological functions (Figures 4B–E). Although many elapid snakes exhibit broad diversity of these functionally varied toxins in their venom (e.g., multiple short- and long-chain 3FTX isoforms), it remains unclear why particular functional variants are enriched in the venoms of certain elapid lineages, such as the cytotoxin-rich venoms of cobras (genus Naja) or the neurotoxin-rich venoms of mambas (genus Dendroaspis) (Tan et al., 2015; Lauridsen et al., 2017; Ainsworth et al., 2018). However, evidence of taxon-specific 3FTXs in the venoms of certain “colubrid” snakes (Pawlak et al., 2006; Mackessy and Saviola, 2016; Modahl et al., 2018a), coupled with pseudogenization of 3FTXs in species that no longer rely on their venom (Li et al., 2005), suggests that selection for prey capture may be at least partially responsible for influencing differential 3FTX representation.
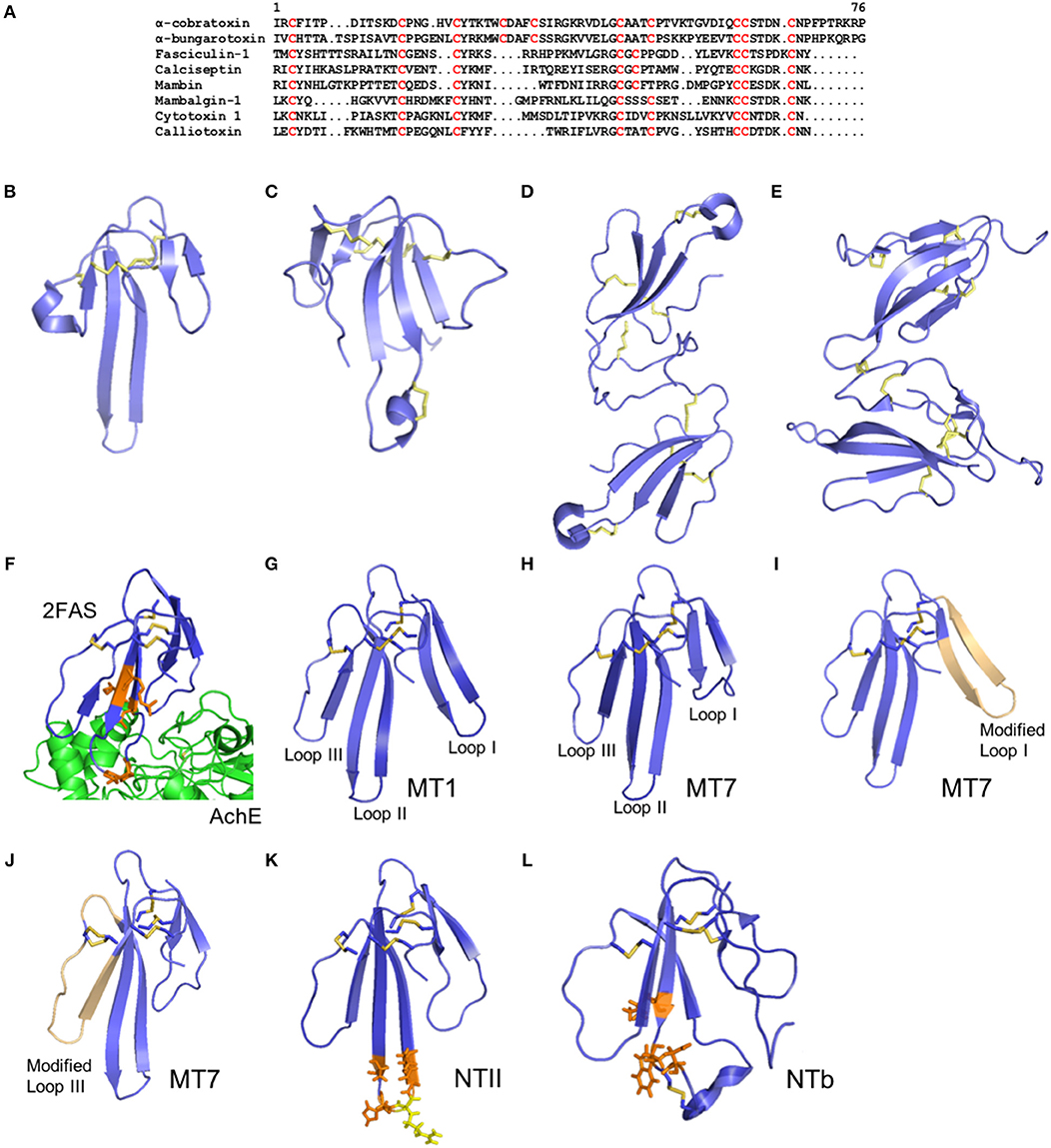
Figure 4. Structure of three-finger toxins from snake venoms. (A) Alignment of the primary structure of α-cobratoxin from Naja kaouthia (Uniprot P01391), α-bungarotoxin from Bungarus multicinctus (Uniprot P60615), fasciculin from Dendroaspis angusticeps (Uniprot P0C1Y9), Calciseptin from Dendroaspis polylepis polylepis (Uniprot P22947), mambin from Dendroaspis jamesoni kaimosae (Uniprot P28375), mambalgin-1 from Dendroaspis polylepis polylepis (Uniprot P0DKR6), cytotoxin 1 from Naja atra (Uniprot P60304), and calliotoxin from Calliophis bivirgatus (Uniprot P0DL82). (B–E) Cartoon representation of the three-dimensional structure of the 3FTXs short-chain mambalgin-1 from Dendroaspis polylepis polylepis (PDB 5DU1) (B), long-chain α-bungarotoxin from Bungarus multicinctus (PDB 1ABT) (C), non-covalent homodimer α-cobratoxin from Naja kaouthia (PDB 4AEA) (D), and covalent heterodimer irditoxin from Boiga irregularis (PDB 2H7Z) (E). (F) Cartoon representation of the three-dimensional structure of Fascilulin-2 bound to the AChE (PDB 4BDT). The residues Arg24, Lys25, Pro31 and Leu35 which form hydrogen bonds with AChE are shown in orange. (G–J) Cartoon representation of the three-dimensional structure of the muscarinotoxin 1 (MT1, PDB 4DO8) (G) and muscarinotoxin 7 (MT7, PDB 2VLW) (H), and respective analogs displaying the modified loop 1 (PDB 3FEV) (I) and loop 3 (PDB 3NEQ) (J) in light orange color. (K) Neurotoxin II from N. oxiana (PDB 1NOR). The residues Ser29, His31, Gly33 and Thr34 which form hydrogen bonds with the α-subunit of nAChR are shown in orange, and the residue Arg32 forming ionic interactions with the α-subunit of nAChR is shown in yellow. (L) Neurotoxin b (NTb) from O. Hannah (PDB 1TXA). The residues Lys24, Trp26, and Asp28 that form hydrogen bonds with the α-subunit nAChR are shown in orange.
Despite the shared three-finger fold, the 3FTXs have diverse targets and biological activities. For example, α-neurotoxins inhibit muscle acetylcholine receptors (nAChR) (Changeux, 1990), κ-neurotoxins inhibits neuronal AChR (Grant and Chiappinelli, 1985), muscarinic toxins inhibit muscarinic receptors (Marquer et al., 2011), fasciculins inhibit acetylcholinesterase (AChE) (Marchot et al., 1998), calciseptine modulates L-type calcium channels (De Weille et al., 1991; Garcia et al., 2001), cardiotoxins interact non-specifically with phospholipids (Konshina et al., 2017), or induce insulin secretion (Nguyen et al., 2012), mambin interacts with platelet receptors (McDowell et al., 1992), exactin inhibits Factor X (Girish and Kini, 2016), β-cardiotoxins inhibit β-adrenoreceptors (Rajagopalan et al., 2007), MTα inhibits α-adrenoreceptors (Koivula et al., 2010), mambalgins inhibit ASIC channels (Diochot et al., 2012), Tx7335 that activates potassium channels (Rivera-Torres et al., 2016) and calliotoxin activates voltage-gated sodium channels (NaV) (Yang et al., 2016). Their toxic biological effects include flaccid or spastic paralysis due to the inhibition of AChE and ACh receptors (Grant and Chiappinelli, 1985; Changeux, 1990; Marchot et al., 1998; Marquer et al., 2011), and activation of NaV1.4 (Yang et al., 2016) and L-type calcium channels (Garcia et al., 2001) in the periphery, necrosis through the action of cardiotoxins (cytotoxins) (Konshina et al., 2017), alteration of the cardiac rate through modulation of α- and β-adrenoreceptors (Rajagopalan et al., 2007; Koivula et al., 2010), and altered homeostasis through inhibition of platelet aggregation (McDowell et al., 1992) and Factor X (Girish and Kini, 2016). In addition to their multitude of bio-activities, 3FTXs can remarkably display toxicities that target distinct classes of organisms as demonstrated in non-front fanged snake venoms that produce 3FTX isoforms which are non-toxic to mice but highly toxic to lizards, and vice-versa (Modahl et al., 2018b).
Some 3FTXs are able to induce analgesia through inhibition of ASIC channels (Salinas et al., 2014), while no 3FTXs are known to be involved in inflammation and hyperalgesia as commonly reported for other snake toxins. Furthermore, 3FTXs are relatively small compared to the other snake toxins discussed herein, and do not exhibit multiple domains to produce their multiple toxic functions. Nevertheless, the number of receptors, ion channels, and enzymes targeted by snake 3FTXs highlights the unique capacity of this fold to modulate diverse biological functions and the arsenal of toxic effects that are induced by 3FTXs. The unique multifunctionality of the 3FTX scafold occurs because of their resistance to degradation and tolerance to mutations and large deletions (Kini and Doley, 2010). Therefore, the structure-activity relationship of the 3FTXs is complex and yet to be fully understood. Their functional sites are located on various segments of the molecule surface. Conserved regions determine structural integrity and correct folding of 3FTXs to form the three loops, including eight conserved cysteine residues found in the core region. Aromatic residues (Tyr25 or Phe27) are conserved in most 3FTXs and essential to their folding. Another conserved features are the antiparallel β-sheet structure and charged amino acid residues also essential to stabilize the native conformation of the protein by forming hydrogen and ionic interactions, respectively (Torres et al., 2001). Additional disulfide bonds can be observed either in the loop I or loop II which can potentially change the activity of the 3FTX in some cases.
Specific amino acid residues in critical segments of the 3FTXs have been identified to be important for binding to their targets. For example, the interactions between fasciculin and AChE enzyme has been studied. The first loop or finger of fasciculin reaches down the outer surface of the enzyme, while the second loop inserts into the active site and exhibit hydrogen bonds and hydrophobic interaction (Harel et al., 1995; Figure 4F). Several basic residues in fasciculin make key contacts with AChE. From docking studies, hydrogen bonds, and hydrophobic interactions where shown to establish receptor-toxin assembly. Six amino acid residues (Lys25, Arg24, Asn47, Pro31, Leu35 and Ala12) of fasciculin interact with the AChE residues by forming hydrogen bonds at its active site. Hydrophobic interactions are also observed between eight amino acid residues (Lys32, Cys59, Val34, Leu48, Ser26, Gly36, Thr15, Asn20) from fasciculin and the enzyme active site (Waqar and Batool, 2015). These interactions involve charged residues but lacks intermolecular salt linkages.
Muscarinic toxins from mamba venoms, such as MT1 and MT7 (Figures 4G,H), act as highly potent and selective antagonists of M1 receptor subtype through allosteric interactions with the M1 receptor. Fruchart-Gaillard et al. (2012) synthesized seven chimeric 3FTXs based on MT1 and MT7 proteins that have remarkable affinity for α1A-adrenoceptor receptor subtypes but low affinity for M1 receptor. In this study, substitution within loop 1 and loop 3 weaken the toxin interactions with the M1 receptor, resulting in a 2-fold decrease in affinity (Figures 4I,J). Furthermore, modifications in loop 2 of the MT1 and MT7 significantly reduce the affinity for the M1 receptor. Interestingly, a significant increase in affinity was achieved on the α1A-adrenoceptor by combined modifications in loops 1 and 3, where loop 1 forms a critical interaction with the receptor (Fruchart-Gaillard et al., 2012). Another muscarinic toxin named MTβ was designed based on ρ-Da1a protein from Black Mamba which is known to have affinity for the muscarinic receptor. The Ser/Ile38 and Ala/Val43 substitutions in ρ-Da1a could be responsible for the increased affinity of MTβ for α1B-adrenoreceptor and α1D-adrenoreceptor (Blanchet et al., 2013). These two residues were not located at the tip of the toxin loop, however, they played a critical role in the interactions with their molecular targets (Bourne et al., 2005). These mutations seem to induce a significant change in the structure of the toxin which is mostly due to additional hydrophobic interactions between Ile38 and the aliphatic side chains of the toxin that may induce a slight movement of the side chain surrounding Ser/Ile38.
Neurotoxin II (NTII) from Naja oxiana is a potent blocker of nAChR. NTII is a short chain α-neurotoxin which consists of 61 amino acid residues and four disulfide bridges (Figure 4K). A computational model for examining the interactions of NTII with the Torpedo californica nAChR has been studied (Mordvintsev et al., 2005). The model showed that the binding of the short α-neurotoxin occurs by rearranging the aromatic residues in the binding pocket. The insertion of the loop II into the binding pocket of a nAChR induces the neurotoxin activity and significantly determines the toxin-receptor interactions, while loop I and III contact the receptor residues by their tips only and determine the immunogenicity of the short neurotoxins. In the model, the Arg32 NTII residue forms an ionic pair with Trp149 from the nAChR and is observed as the strongest interaction. Hydrogen bond interactions such as Asp30 from loop II with Tyr198 of the nAChR and Lys46 from loop III with Thr191 of the nAChR complement the ionic interaction between NTII and its target receptor. Aside from hydrogen bonds, van-der-Waals interactions were also observed at the fingertip of loop II amino acid residues (Lys25, Trp27, Trp28, Ser29, His31, Gly33, Thr34 and Arg38) (Mordvintsev et al., 2005).
The structure of neurotoxin b (NTb), a long neurotoxin from Ophiophagus hannah, has been elucidated (Peng et al., 1997; Figure 4L). Conserved residues in loop II also play an important role in the toxicity of the long neurotoxins by making ionic interactions between toxin and receptor. Positively charged residues Trp27, Lys24 and Asp28 are highly conserved residues in the long neurotoxins. Furthermore, a modification of the Trp27 in the long neurotoxin analog of NTb from king cobra venom led to a significant loss in neurotoxicity. The additional disulphide bridge in loop II of long neurotoxins does not affect the toxin activity. Nevertheless, cleavage of the additional disulphide bridge in loop II can disrupt the positively charged cluster at the tip of loop II. Changes in loop II conformation will affect the binding of the long neurotoxin to the target receptor resulting the loss of neurotoxicity (Peng et al., 1997).
Long and short neurotoxins show sequence homology and similar structure. Previous studies show that many residues located at the tip of loop II are conserved in both short and long neurotoxins. It is consisting of the long central β-sheets forming three loops and globular core. From the studies of α-bungarotoxin and α-cobratoxin, the least conserved regions of the long neurotoxin are the C-terminal and the first loop (Walkinshaw et al., 1980; Juan et al., 1999; Dutertre et al., 2017). However, significant differences between long-chain neurotoxin and short chain neurotoxin are indicated by the immunological reactivity. Many of the residues involved in the antibody-long neurotoxins binding are located in loop II, loop III, and in the C-terminal, while in short neurotoxins the antibody's epitope makes interactions with the loop I and loop II (Engmark et al., 2016).
Therapeutic Implications
Treating Snake Envenomation
Animal-derived antivenoms are considered the only specific therapy available for treating snakebite envenoming (Maduwage and Isbister, 2014; Slagboom et al., 2017; Ainsworth et al., 2018). These consist of polyclonal immunoglobulins, such as intact IgGs or F(ab')2, or Fab fragments (Ouyang et al., 1990; Maduwage and Isbister, 2014; Roncolato et al., 2015), derived from hyperimmune animal serum/plasma (typically horse or sheep). When used rapidly and appropriately, they are capable of neutralizing life-threatening systemic envenoming, for example pathologies such as venom-induced coagulopathy, hemorrhage, neurotoxic effects, and/or hypotensive shock (Warrell, 1992; Calvete et al., 2009; Maduwage and Isbister, 2014; Slagboom et al., 2017; Ainsworth et al., 2018).
Antivenoms can be classified as monovalent or polyvalent depending on the immunogen used during production. Monovalent antivenoms are produced by immunizing animals with venom from a single snake species, whereas polyvalent antivenoms contain antibodies produced from a cocktail of venoms of several medically relevant snakes from a particular geographical region. Polyvalent antivenoms are therefore designed to address the limited paraspecific cross-reactivity of monovalent antivenoms by stimulating the production of antibodies against diverse venom toxins found in different snake species, and to avoid issues relating to the wrong antivenom being given due to a lack of existing snakebite diagnostic tools (O'leary and Isbister, 2009; Abubakar et al., 2010). However, polyvalent therapies come with disadvantages—larger therapeutic dose are required to effect cure, potentially resulting in an increased risk of adverse reactions, and in turn increasing cost to impoverished snakebite victims (Hoogenboom, 2005; O'leary and Isbister, 2009; Deshpande et al., 2013; Roncolato et al., 2015).
Variation in venom constituents therefore causes a great challenge for the development of broadly effective snakebite therapeutics. The diversity of toxins found in the venom of any one species represents considerable complexity, which is further enhanced when trying to neutralize the venom of multiple species, particularly given variations in the immunogenicity of the multi-functional toxins described in this review. Antivenom efficacy is therefore, typically limited to those species whose venoms were used as immunogens and, in a number of cases, closely-related snake species that share sufficient toxin overlap for the generated antibodies to recognize and neutralize the key toxic components (Casewell et al., 2010; Segura et al., 2010; Williams et al., 2011; Ainsworth et al., 2018).
Because variation in venom composition is ubiquitous at every level of snake taxonomy (e.g., interspecifically, intraspecifically, and even ontogenically Chippaux et al., 1991; Gibbs et al., 2013; Casewell et al., 2014; Durban et al., 2017; Ainsworth et al., 2018), gaining an understanding of venom composition in medically important snake species is valuable, and can inform predictions of the likely paraspecific neutralizing capability of an antivenom, and therefore the geographical applicability of a particular therapeutic. Consequently, venom toxicity/pathology analyses in combination with venom proteomics, antivenomics, and/or immunological analyses have been integrated to investigate the paraspecificity of antivenoms (Calvete et al., 2009; Madrigal et al., 2012; Pla et al., 2013; Tan et al., 2015). Such studies have revealed surprising cross-reactivity of antivenoms against distinct, non-targeted, snake species, such as: (i) the potential utility of Asian antivenoms developed against terrestrial elapid snakes at neutralizing the venom toxicity of potent sea snake venoms (Tan et al., 2015), (ii) the seeming utility of African polyvalent antivenom at neutralizing the venom of a genus of elapid snakes not including in the immunizing mixture (Whiteley et al., 2019), and (iii) the potential for saw-scaled viper antivenom to be used as an alternative treatment for bites by the boomslang (Dispholidus typus) in regions where the appropriate species-specific antivenom is unavailable or unaffordable (Ainsworth et al., 2018). The later of these studies demonstrated cross-neutralization between distinct snake lineages (e.g., viper and colubrid), and this surprising finding was ultimately informed by venom comparisons indicating that these snakes had converged upon similar toxin compositions. Thus, detailed knowledge of venom composition can greatly inform studies assessing the geographical utility of antivenoms.
The application of “venomic” (e.g., transcriptomic, proteomic) approaches to characterize venom composition have revealed that many abundant venom proteins belong to only a few major families of toxins, which despite their diversity, often share antigenic determinants (Calvete et al., 2007, 2009; Gutiérrez et al., 2009). Such studies have stimulated much research into the development of novel therapeutic approaches to tackle snakebite. These include the use of monoclonal antibody technologies to target key pathogenic toxins found in certain snake species (Laustsen et al., 2018; Silva et al., 2018), pathology rather than geographically-focused approaches to neutralizing venom toxins (Ainsworth et al., 2018), and the use of small molecule inhibitors designed to generically target specific toxin classes (Arias et al., 2017; Bryan-Quiros et al., 2019). The priority targets for these “next generation” snakebite therapeutics are the multifunctional toxins described herein (PLA2s, SVMP, SVSP, 3FTX) because of the major pathologies that they cause in snakebite victims. It is anticipated that in the future these new therapeutics may offer superior specificities, neutralizing capabilities, affordability and safety over conventional antivenoms. However, the translation of their early research promise into the mainstay of future snakebite treatments will ultimately rely on further research on the toxins that they are designed to neutralize. Specifically, the selection, testing and optimization of new tools to combat snake envenoming is reliant upon the characterization of key pathogenic, and often multifunctional, toxins found in the venom of a diverse array of medically important snake species.
Snake Toxins as Therapeutics, Cosmetics and in Diagnostics
The first drug derived from animal venoms approved by the FDA is captopril, a potent inhibitor of the angiotensin converting enzyme (sACE) used to treat hypertension and congestive heart failure (Cushman et al., 1977; Cushman and Ondetti, 1999). Captopril was derived from proline-rich oligopeptides from the venom of the Brazilian snake Bothrops jararaca (Ferreira et al., 1970; Gavras et al., 1974). This milestone in translational science in the late 70's revealed the exceptional potential of snake venoms, and possibly other animal venoms such as from spider and cone snails, as an exquisite source of bioactive molecules with applications in drug development. More recently, an anti-platelet drug derived from the venom of the southeastern pygmy rattlesnake Sistrurus miliarius barbouri was commercialized as Integrillin by Millenium Pharmaceuticals, and is used to prevent acute cardiac ischemia (Lauer et al., 2001). Furthermore, a group of snake α-neurotoxins named waglerins from the viper Tropidolaemus wagleri (Schmidt and Weinstein, 1995; Debono et al., 2017) was used in the development of anti-wrinkle cosmetics by Pentapharm Ltd., a Swiss-based chemical company. The resulting product is now commercialized as Syn-AKE. The same company commercialized Defibrase®, a SVSP purified from Bothrops moojeni, for use in acute cerebral infarction, angina pectoris and sudden deafness, and Haemocoagulase, purified from Bothrops atrox, for the treatment of hemorrhages of various origins.
Snake toxins have been applied with great success in diagnostics. For example, the Textarin: Ecarin test is commonly used to detect Lupus Anticoagulant (LA) (Triplett et al., 1993), and is composed by a SVSP from the venom of the Australian Eastern brown snake Pseudonaja textilis (Textarin) (Stocker et al., 1994), and a SVMP from the venom of the saw-scaled viper Echis carinatus (Ecarin) (Stocker et al., 1986). Snake toxins also have the potential to become novel painkillers. The toxin crotalphine, from the venom of Crotalus durissus, is a 14 residues peptide able to induce analgesia through modulation of κ-opioids receptors and TRPV1 channels (Gutierrez et al., 2008; Konno et al., 2008; Bressan et al., 2016), while mambalgin, a 3FTX from Dendroaspis polylepis, induces analgesia by inhibiting ASIC channels (Diochot et al., 2012). Other 3FTXs have been applied in studies of novel treatments for blood pressure disorders (MTα), blood coagulation disorders (KT-6.9), diabetes type-2 (cardiotoxin 1) and infertility (actiflagelin) (Utkin, 2019). These findings, alongside current research into venom toxins, suggest an exciting future for the use of snake venoms in the field of drug discovery.
Conclusions
Snake venoms are amongst the most fascinating animal venoms regarding their complexity, evolution, and therapeutic applicability. They also offer one of the most challenging drugs targets due to the variable toxin compositions injected following snakebite. The multifunctional approach adopted by the major components of their venoms, by using multidomain proteins and peptides with promiscuous folds (e.g., three-finger fold), as well as their diversity of toxic effects, are unique and yet to be identified in other animal venoms at such level of complexity. Gaining a better understanding of the evolution, structure-activity relationships and pathological mechanisms of these toxins is essential to develop better snakebite therapies and novel drugs.
Recent developments in genomics, proteomics and bioactivity assays, as well as in the understanding of human physiology in health and disease, are enhancing the quality and speed of research into snake venoms. We hope to improve the therapies used to neutralize the toxic effects of PLA2s, SVMPs, SVSPs and 3FTXs, and to develop drugs as new antidotes for a broad-spectrum of snake venoms that could also be effective in preventing the described inflammatory reactions and pain induced by snakebite. Finally, a diversity of biological functions in snake venoms is yet to be explored, including their inflammatory properties and their intriguing interactions with sensory neurons and other compartments of the nervous system, which will certainly lead to the elucidation of new biological functions and the development of useful research tools, diagnostics and therapeutics.
Author Contributions
FC provided theme, scope, and guidance. FC, CF, AA, CX, NC, RL, and JK wrote the manuscript. FC, NC, RL, and JK critically reviewed the manuscript.
Funding
Australian National Health and Medical Research Council (APP1119056) provided a Fellowship to RL; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) provided an international scholarship to CF; Sir Henry Dale Fellowship to NC (200517/Z/16/Z) funded by the Wellcome Trust and the Royal Society.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abubakar, I. S., Abubakar, S. B., Habib, A. G., Nasidi, A., Durfa, N., Yusuf, P. O., et al. (2010). Randomised controlled double-blind non-inferiority trial of two antivenoms for saw-scaled or carpet viper (Echis ocellatus) envenoming in Nigeria. PLoS Negl. Trop. Dis. 4:e767. doi: 10.1371/journal.pntd.0000767
Ainsworth, S., Slagboom, J., Alomran, N., Pla, D., Alhamdi, Y., King, S. I., et al. (2018). The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun. Biol. 1:34. doi: 10.1038/s42003-018-0039-1
Arias, A. S., Rucavado, A., and Gutierrez, J. M. (2017). Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 132, 40–49. doi: 10.1016/j.toxicon.2017.04.001
Barber, C. M., Isbister, G. K., and Hodgson, W. C. (2013). Alpha neurotoxins. Toxicon 66, 47–58. doi: 10.1016/j.toxicon.2013.01.019
Barlow, A., Pook, C. E., Harrison, R. A., and Wuster, W. (2009). Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. Biol. Sci. 276, 2443–2449. doi: 10.1098/rspb.2009.0048
Benishin, C. G. (1990). Potassium channel blockade by the B subunit of β-bungarotoxin. Mol. Pharmacol. 38, 164–169.
Bernardes, C. P., Menaldo, D. L., Mamede, C. C. N., Zoccal, K. F., Cintra, A. C. O., Faccioli, L. H., et al. (2015). Evaluation of the local inflammatory events induced by BpirMP, a metalloproteinase from Bothrops pirajai venom. Mol. Immunol. 68, 456–464. doi: 10.1016/j.molimm.2015.09.023
Blanchet, G., Upert, G., Mourier, G., Gilquin, B., Gilles, N., and Servent, D. (2013). New α-adrenergic property for synthetic MTbeta and CM-3 three-finger fold toxins from black mamba. Toxicon 75, 160–167. doi: 10.1016/j.toxicon.2013.04.017
Bohlen, C. J., Chesler, A. T., Sharif-Naeini, R., Medzihradszky, K. F., Zhou, S., King, D., et al. (2011). A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 479, 410–414. doi: 10.1038/nature10607
Bourne, Y., Talley, T. T., Hansen, S. B., Taylor, P., and Marchot, P. (2005). Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake α-neurotoxins and nicotinic receptors. EMBO J. 24, 1512–1522. doi: 10.1038/sj.emboj.7600620
Bressan, E., Touska, F., Vetter, I., Kistner, K., Kichko, T. I., Teixeira, N. B., et al. (2016). Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain 157, 2504–2516. doi: 10.1097/j.pain.0000000000000669
Brust, A., Sunagar, K., Undheim, E. A., Vetter, I., Yang, D. C., Casewell, N. R., et al. (2013). Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol. Cell Proteom. 12, 651–663. doi: 10.1074/mcp.M112.023135
Bryan-Quiros, W., Fernandez, J., Gutierrez, J. M., Lewin, M. R., and Lomonte, B. (2019). Neutralizing properties of LY315920 toward snake venom group I and II myotoxic phospholipases A2. Toxicon 157, 1–7. doi: 10.1016/j.toxicon.2018.11.292
Bucaretchi, F., Capitani, E. M., Vieira, R. J., Rodrigues, C. K., Zannin, M., Da Silva, N. J. Jr., et al. (2016). Coral snake bites (Micrurus spp.) in Brazil: a review of literature reports. Clin. Toxicol. 54, 222–234. doi: 10.3109/15563650.2015.1135337
Calvete, J. J., Juárez, P., and Sanz, L. (2007). Snake venomics. Strategy and applications. J. Mass Spectr. 42, 1405–1414. doi: 10.1002/jms.1242
Calvete, J. J., Sanz, L., Angulo, Y., Lomonte, B., and Gutiérrez, J. M. (2009). Venoms, venomics, antivenomics. FEBS Lett. 583, 1736–1743. doi: 10.1016/j.febslet.2009.03.029
Camara, P. R., Esquisatto, L. C., Camargo, E. A., Ribela, M. T., Toyama, M. H., Marangoni, S., et al. (2003). Inflammatory oedema induced by phospholipases A2 isolated from Crotalus durissus sp. in the rat dorsal skin: a role for mast cells and sensory C-fibers. Toxicon 41, 823–829. doi: 10.1016/S0041-0101(03)00037-0
Camargo, E. A., Ferreira, T., Ribela, M. T., De Nucci, G., Landucci, E. C., and Antunes, E. (2008). Role of substance P and bradykinin in acute pancreatitis induced by secretory phospholipase A2. Pancreas 37, 50–55. doi: 10.1097/MPA.0b013e3185d9b9b
Casais-E-Silva, L. L., Teixeira, C. F. P., Lebrun, I., Lomonte, B., Alape-Giron, A., and Gutierrez, J. M. (2016). Lemnitoxin, the major component of Micrurus lemniscatus coral snake venom, is a myotoxic and pro-inflammatory phospholipase A2. Toxicol. Lett. 257, 60–71. doi: 10.1016/j.toxlet.2016.06.005
Casewell, N. R. (2012). On the ancestral recruitment of metalloproteinases into the venom of snakes. Toxicon 60, 449–454. doi: 10.1016/j.toxicon.2012.02.006
Casewell, N. R., Cook, D. A., Wagstaff, S. C., Nasidi, A., Durfa, N., Wüster, W., et al. (2010). Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 4:e851. doi: 10.1371/journal.pntd.0000851
Casewell, N. R., Wagstaff, S. C., Harrison, R. A., Renjifo, C., and Wuster, W. (2011). Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Mol. Biol. Evol. 28, 2637–2649. doi: 10.1093/molbev/msr091
Casewell, N. R., Wagstaff, S. C., Wüster, W., Cook, D. A., Bolton, F. M., King, S. I., et al. (2014). Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. 111, 9205–9210. doi: 10.1073/pnas.1405484111
Casewell, N. R., Wuster, W., Vonk, F. J., Harrison, R. A., and Fry, B. G. (2013). Complex cocktails: the evolutionary novelty of venoms. Trends Ecol. Evol. 28, 219–229. doi: 10.1016/j.tree.2012.10.020
Casewell, N. R. S., Takacs, Z., Calvete, J. J., Jackson, T. N. W., and Fry, B. G. (2015). “Snake venom metalloprotease enzymes,” in Venomous, Reptiles and Their Toxins. Evolution, Pathophysiology and Biodiscovery, ed B. G. Fry, 347–363.
Chacur, M., Gutierrez, J. M., Milligan, E. D., Wieseler-Frank, J., Britto, L. R. G., Maier, S. F., et al. (2004). Snake venom components enhance pain upon subcutaneous injection: an initial examination of spinal cord mediators. Pain 111, 65–76. doi: 10.1016/j.pain.2004.06.001
Chacur, M., Longo, I., Picolo, G., Gutierrez, J. M., Lomonte, B., Guerra, J. L., et al. (2003). Hyperalgesia induced by Asp49 and Lys49 phospholipases A2 from Bothrops asper snake venom: pharmacological mediation and molecular determinants. Toxicon 41, 667–678. doi: 10.1016/S0041-0101(03)00007-2
Chan, Y. S., Cheung, R. C. F., Xia, L. X., Wong, J. H., Ng, T. B., and Chan, W. Y. (2016). Snake venom toxins: toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 100, 6165–6181. doi: 10.1007/s00253-016-7610-9
Changeux, J. P. (1990). The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 11, 485–492. doi: 10.1016/0165-6147(90)90049-E
Chippaux, J. P., Williams, V., and White, J. (1991). Snake venom variability: methods of study, results and interpretation. Toxicon 29, 1279–1303. doi: 10.1016/0041-0101(91)90116-9
Clissa, P. B., Lopes-Ferreira, M., Della-Casa, M. S., Farsky, S. H., and Moura-Da-Silva, A. M. (2006). Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon 47, 591–596. doi: 10.1016/j.toxicon.2006.02.001
Costa, S. K. P., Camargo, E. A., and Antunes, E. (2017). Inflammatory action of secretory phospholipases A2 from snake venoms. Toxins Drug Disc. 35–52. doi: 10.1007/978-94-007-6452-1_10
Cunha, F. Q., Poole, S., Lorenzetti, B. B., and Ferreira, S. H. (1992). The pivotal role of tumor-necrosis-factor-alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 107, 660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x
Cushman, D. W., Cheung, H. S., Sabo, E. F., and Ondetti, M. A. (1977). Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 16, 5484–5491. doi: 10.1021/bi00644a014
Cushman, D. W., and Ondetti, M. A. (1999). Design of angiotensin converting enzyme inhibitors. Nat. Med. 5, 1110–1113. doi: 10.1038/13423
da Silva, I. R. F., Lorenzetti, R., Renno, A. L., Baldissera, L., Zelanis, A., Serrano, S. M. D., et al. (2012). BJ-PI2, A non-hemorrhagic metalloproteinase from Bothrops jararaca snake venom. Biochim. Biophys. Acta 1820, 1809–1821. doi: 10.1016/j.bbagen.2012.07.011
Dale, C. S., Goncalves, L. R., Juliano, L., Juliano, M. A., Da Silva, A. M., and Giorgi, R. (2004). The C-terminus of murine S100A9 inhibits hyperalgesia and edema induced by jararhagin. Peptides 25, 81–89. doi: 10.1016/j.peptides.2003.12.008
De Castro, R. C., Landucci, E. C., Toyama, M. H., Giglio, J. R., Marangoni, S., De Nucci, G., et al. (2000). Leucocyte recruitment induced by type II phospholipases A2 into the rat pleural cavity. Toxicon 38, 1773–1785. doi: 10.1016/S0041-0101(00)00107-0
De Toni, L. G. B., Menaldo, D. L., Cintra, A. C. O., Figueiredo, M. J., De Souza, A. R., Maximiano, W. M. A., et al. (2015). Inflammatory mediators involved in the paw edema and hyperalgesia induced by Batroxase, a metalloproteinase isolated from Bothrops atrox snake venom. Int. Immunopharmacol. 28, 199–207. doi: 10.1016/j.intimp.2015.06.001
De Weille, J. R., Schweitz, H., Maes, P., Tartar, A., and Lazdunski, M. (1991). Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl. Acad. Sci. U. S. A. 88, 2437–2440. doi: 10.1073/pnas.88.6.2437
Debono, J., Xie, B., Violette, A., Fourmy, R., Jaeger, M., and Fry, B. G. (2017). Viper venom Botox: The molecular origin and evolution of the waglerin peptides used in anti-wrinkle skin cream. J. Mol. Evol. 84, 8–11. doi: 10.1007/s00239-016-9764-6
Delatorre, P., Rocha, B. A., Santi-Gadelha, T., Gadelha, C. A., Toyama, M. H., and Cavada, B. S. (2011). Crystal structure of Bn IV in complex with myristic acid: a Lys49 myotoxic phospholipase A2 from Bothrops neuwiedi venom. Biochimie 93, 513–518. doi: 10.1016/j.biochi.2010.11.003
Deshpande, R. P., Motghare, V. M., Padwal, S. L., Pore, R. R., Bhamare, C. G., Deshmukh, V. S., et al. (2013). Adverse drug reaction profile of anti-snake venom in a rural tertiary care teaching hospital. J. Young Pharm. 5, 41–45. doi: 10.1016/j.jyp.2013.02.003
Dias, G. S., Kitano, E. S., Pagotto, A. H., Sant'anna, S. S., Rocha, M. M., Zelanis, A., et al. (2013). Individual variability in the venom proteome of juvenile Bothrops jararaca specimens. J. Proteome Res. 12, 4585–4598. doi: 10.1021/pr4007393
Diochot, S., Baron, A., Salinas, M., Douguet, D., Scarzello, S., Dabert-Gay, A. S., et al. (2012). Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 490, 552–555. doi: 10.1038/nature11494
Dowell, N. L., Giorgianni, M. W., Griffin, S., Kassner, V. A., Selegue, J. E., Sanchez, E. E., et al. (2018). Extremely divergent haplotypes in two toxin gene complexes encode alternative venom types within rattlesnake species. Curr. Biol. 28, 1016–1026.e1014. doi: 10.1016/j.cub.2018.02.031
Dowell, N. L., Giorgianni, M. W., Kassner, V. A., Selegue, J. E., Sanchez, E. E., and Carroll, S. B. (2016). The deep origin and recent loss of venom toxin genes in rattlesnakes. Curr. Biol. 26, 2434–2445. doi: 10.1016/j.cub.2016.07.038
Durban, J., Juarez, P., Angulo, Y., Lomonte, B., Flores-Diaz, M., Alape-Giron, A., et al. (2011). Profiling the venom gland transcriptomes of Costa Rican snakes by 454 pyrosequencing. BMC Genom. 12:259. doi: 10.1186/1471-2164-12-259
Durban, J., Sanz, L., Trevisan-Silva, D., Neri-Castro, E., Alagon, A., and Calvete, J. J. (2017). Integrated venomics and venom gland transcriptome analysis of juvenile and adult Mexican rattlesnakes Crotalus simus, C. tzabcan, and C. culminatus revealed miRNA-modulated ontogenetic shifts. J. Proteome Res. 16, 3370–3390. doi: 10.1021/acs.jproteome.7b00414
Dutertre, S., Nicke, A., and Tsetlin, V. I. (2017). Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 127, 196–223. doi: 10.1016/j.neuropharm.2017.06.011
Engmark, M., Andersen, M. R., Laustsen, A. H., Patel, J., Sullivan, E., De Masi, F., et al. (2016). High-throughput immuno-profiling of mamba (Dendroaspis) venom toxin epitopes using high-density peptide microarrays. Sci. Rep. 6:36629. doi: 10.1038/srep36629
Escalante, T., Rucavado, A., Fox, J. W., and Gutierrez, J. M. (2011). Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteom. 74, 1781–1794. doi: 10.1016/j.jprot.2011.03.026
Fernandes, C. M., Teixeira, C. D. F. P., Leite, A. C. R. M., Gutierrez, J. M., and Rocha, F. A. C. (2007). The snake venom metalloproteinase BaP1 induces joint hypernociception through TNF-α and PGE2-dependent mechanisms. Br. J. Pharmacol. 151, 1254–1261. doi: 10.1038/sj.bjp.0707351
Ferraz, C. R., Calixto-Campos, C., Manchope, M. F., Casagrande, R., Clissa, P. B., Baldo, C., et al. (2015). Jararhagin-induced mechanical hyperalgesia depends on TNF-α, IL-1β and NFκB in mice. Toxicon 103, 119–128. doi: 10.1016/j.toxicon.2015.06.024
Ferreira, B. A., Deconte, S. R., De Moura, F. B. R., Tomiosso, T. C., Clissa, P. B., Andrade, S. P., et al. (2018). Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. Int. J. Biol. Macromol. 119, 1179–1187. doi: 10.1016/j.ijbiomac.2018.08.051
Ferreira, S. H., Bartelt, D. C., and Greene, L. J. (1970). Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 9, 2583–2593. doi: 10.1021/bi00815a005
Fox, J. W., and Serrano, S. M. (2005). Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 45, 969–985. doi: 10.1016/j.toxicon.2005.02.012
Fox, J. W., and Serrano, S. M. (2008). Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 275, 3016–3030. doi: 10.1111/j.1742-4658.2008.06466.x
Fruchart-Gaillard, C., Mourier, G., Blanchet, G., Vera, L., Gilles, N., Menez, R., et al. (2012). Engineering of three-finger fold toxins creates ligands with original pharmacological profiles for muscarinic and adrenergic receptors. PLoS ONE 7:e39166. doi: 10.1371/journal.pone.0039166
Fry, B. G. (2005). From genome to “venome”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 15, 403–420. doi: 10.1101/gr.3228405
Fry, B. G., Scheib, H., de L. M. Junqueira de Azevedo, I., Silva, D. A., and Casewell, N.R. (2012). Novel transcripts in the maxillary venom glands of advanced snakes. Toxicon 59, 696–708. doi: 10.1016/j.toxicon.2012.03.005
Fry, B. G., Scheib, H., Van Der Weerd, L., Young, B., Mcnaughtan, J., Ramjan, S. F., et al. (2008). Evolution of an arsenal: structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell Proteom. 7, 215–246. doi: 10.1074/mcp.M700094-MCP200
Fry, B. G., Vidal, N., Norman, J. A., Vonk, F. J., Scheib, H., Ramjan, S. F. R., et al. (2006). Early evolution of the venom system in lizards and snakes. Nature 439, 584–588. doi: 10.1038/nature04328
Fry, B. G., Wuster, W., Kini, R. M., Brusic, V., Khan, A., Venkataraman, D., et al. (2003). Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 57, 110–129. doi: 10.1007/s00239-003-2461-2
Garcia, M. C., Hernandez-Gallegos, Z., Escamilla, J., and Sanchez, J. A. (2001). Calciseptine, a Ca2+ channel blocker, has agonist actions on L-type Ca2+ currents of frog and mammalian skeletal muscle. J. Membr. Biol. 184, 121–129. doi: 10.1007/s00232-001-0080-7
Gavras, H., Brunner, H. R., Laragh, J. H., Sealey, J. E., Gavras, I., and Vukovich, R. A. (1974). An angiotensin converting-enzyme inhibitor to identify and treat vasoconstrictor and volume factors in hypertensive patients. N. Engl. J. Med. 291, 817–821. doi: 10.1056/NEJM197410172911603
Giannotti, K. C., Leiguez, E., Moreira, V., Nascimento, N. G., Lomonte, B., Gutierrez, J. M., et al. (2013). A Lys49 phospholipase A2, isolated from Bothrops asper snake venom, induces lipid droplet formation in macrophages which depends on distinct signaling pathways and the C-terminal region. Biomed. Res. Int. 2013:807982. doi: 10.1155/2013/807982
Gibbs, H. L., Sanz, L., Sovic, M. G., and Calvete, J. J. (2013). Phylogeny-based comparative analysis of venom proteome variation in a clade of rattlesnakes (Sistrurus sp.). PloS ONE 8:e67220. doi: 10.1371/journal.pone.0067220
Girish, V. M., and Kini, R. M. (2016). Exactin: a specific inhibitor of Factor X activation by extrinsic tenase complex from the venom of Hemachatus haemachatus. Sci. Rep. 6:32036. doi: 10.1038/srep32036
Goncalves, L. R., and Mariano, M. (2000). Local haemorrhage induced by Bothrops jararaca venom: relationship to neurogenic inflammation. Mediators Inflamm. 9, 101–107. doi: 10.1080/096293500411569
Goncalves-Machado, L., Pla, D., Sanz, L., Jorge, R. J. B., Leitao-De-Araujo, M., Alves, M. L. M., et al. (2016). Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteomics 135, 73–89. doi: 10.1016/j.jprot.2015.04.029
Granata, F., Frattini, A., Loffredo, S., Del Prete, A., Sozzani, S., Marone, G., et al. (2006). Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur. J. Immunol. 36, 1938–1950. doi: 10.1002/eji.200535567
Grant, G. A., and Chiappinelli, V. A. (1985). kappa-Bungarotoxin: complete amino acid sequence of a neuronal nicotinic receptor probe. Biochemistry 24, 1532–1537. doi: 10.1021/bi00327a036
Gubensek, F., Sket, D., Turk, V., and Lebez, D. (1974). Fractionation of Vipera ammodytes venom and seasonal variation of its composition. Toxicon 12, 167–171. doi: 10.1016/0041-0101(74)90241-4
Gutierrez, J. M., Calvete, J. J., Habib, A. G., Harrison, R. A., Williams, D. J., and Warrell, D. A. (2017). Snakebite envenoming. Nat. Rev. Dis. Primers 3:17079. doi: 10.1038/nrdp.2017.79
Gutierrez, J. M., and Lomonte, B. (2013). Phospholipases A2: unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 62, 27–39. doi: 10.1016/j.toxicon.2012.09.006
Gutiérrez, J. M., Lomonte, B., Leon, G., Alape-Giron, A., Flores-Diaz, M., Sanz, L., et al. (2009). Snake venomics and antivenomics: proteomic tools in the design and control of antivenoms for the treatment of snakebite envenoming. J. Proteomics 72, 165–182. doi: 10.1016/j.jprot.2009.01.008
Gutierrez, J. M., and Ownby, C. L. (2003). Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon 42, 915–931. doi: 10.1016/j.toxicon.2003.11.005
Gutierrez, J. M., and Rucavado, A. (2000). Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie 82, 841–850. doi: 10.1016/S0300-9084(00)01163-9
Gutierrez, J. M., Rucavado, A., Escalante, T., and Diaz, C. (2005). Hemorrhage induced by snake venom metalloproteinases: biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 45, 997–1011. doi: 10.1016/j.toxicon.2005.02.029
Gutierrez, V. P., Konno, K., Chacur, M., Sampaio, S. C., Picolo, G., Brigatte, P., et al. (2008). Crotalphine induces potent antinociception in neuropathic pain by acting at peripheral opioid receptors. Eur. J. Pharmacol. 594, 84–92. doi: 10.1016/j.ejphar.2008.07.053
Harel, M., Kleywegt, G. J., Ravelli, R. B., Silman, I., and Sussman, J. L. (1995). Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure 3, 1355–1366. doi: 10.1016/S0969-2126(01)00273-8
Harris, J. B., Grubb, B. D., Maltin, C. A., and Dixon, R. (2000). The neurotoxicity of the venom phospholipases A2, notexin and taipoxin. Exp. Neurol. 161, 517–526. doi: 10.1006/exnr.1999.7275
Harris, J. B., and Maltin, C. A. (1982). Myotoxic activity of the crude venom and the principal neurotoxin, taipoxin, of the Australian taipan, Oxyuranus scutellatus. Br. J. Pharmacol. 76, 61–75. doi: 10.1111/j.1476-5381.1982.tb09191.x
Harris, J. B., and Scott-Davey, T. (2013). Secreted phospholipases A2 of snake venoms: Effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins 5, 2533–2571. doi: 10.3390/toxins5122533
Harrison, R. A., Casewell, N. R., Ainsworth, S. A., and Lalloo, D. G. (2019). The time is now: a call for action to translate recent momentum on tackling tropical snakebite into sustained benefit for victims. Trans. R. Soc. Trop. Med. Hyg. doi: 10.1093/trstmh/try134. [Epub ahead of print].
Hifumi, T., Sakai, A., Kondo, Y., Yamamoto, A., Morine, N., Ato, M., et al. (2015). Venomous snake bites: clinical diagnosis and treatment. J. Intensive Care 3:16. doi: 10.1186/s40560-015-0081-8
Hite, L. A., Jia, L. G., Bjarnason, J. B., and Fox, J. W. (1994). cDNA sequences for 4 snake-venom metalloproteinases–structure, classification, and their relationship to mammalian reproductive proteins. Arch. Biochem. Biophys. 308, 182–191. doi: 10.1006/abbi.1994.1026
Hoogenboom, H. R. (2005). Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 23:1105. doi: 10.1038/nbt1126
Jayawardana, S., Gnanathasan, A., Arambepola, C., and Chang, T. (2016). Chronic musculoskeletal disabilities following snake envenoming in Sri Lanka: a population-based study. PLoS Negl. Trop. Dis. 10:e0005103. doi: 10.1371/journal.pntd.0005103
Jia, L. G., Shimokawa, K., Bjarnason, J. B., and Fox, J. W. (1996). Snake venom metalloproteinases: structure, function and relationship to the ADAMs family of proteins. Toxicon 34, 1269–1276. doi: 10.1016/S0041-0101(96)00108-0
Juan, H. F., Hung, C. C., Wang, K. T., and Chiou, S. H. (1999). Comparison of three classes of snake neurotoxins by homology modeling and computer simulation graphics. Biochem. Biophys. Res. Commun. 257, 500–510. doi: 10.1006/bbrc.1999.0437
Junqueira-De-Azevedo, I. L., Bastos, C. M., Ho, P. L., Luna, M. S., Yamanouye, N., and Casewell, N. R. (2015). Venom-related transcripts from Bothrops jararaca tissues provide novel molecular insights into the production and evolution of snake venom. Mol. Biol. Evol. 32, 754–766. doi: 10.1093/molbev/msu337
Kamiguti, A. S. (2005). Platelets as targets of snake venom metalloproteinases. Toxicon 45, 1041–1049. doi: 10.1016/j.toxicon.2005.02.026
Kang, T. S., Georgieva, D., Genov, N., Murakami, M. T., Sinha, M., Kumar, R. P., et al. (2011). Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. Febs. J. 278, 4544–4576. doi: 10.1111/j.1742-4658.2011.08115.x
Kasturiratne, A., Wickremasinghe, A. R., De Silva, N., Gunawardena, N. K., Pathmeswaran, A., Premaratna, R., et al. (2008). The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 5:e218. doi: 10.1371/journal.pmed.0050218
Kessler, P., Marchot, P., Silva, M., and Servent, D. (2017). The three-finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 142(Suppl 2), 7–18. doi: 10.1111/jnc.13975
Kini, R. M. (2005). The intriguing world of prothrombin activators from snake venom. Toxicon 45, 1133–1145. doi: 10.1016/j.toxicon.2005.02.019
Kini, R. M. (2006). Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem. J. 397, 377–387. doi: 10.1042/BJ20060302
Kini, R. M., and Doley, R. (2010). Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56, 855–867. doi: 10.1016/j.toxicon.2010.07.010
Kini, R. M., and Koh, C. Y. (2016). Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: definition and nomenclature of interaction sites. Toxins 8:E284. doi: 10.3390/toxins8100284
Kleggetveit, I. P., Skulberg, P. K., and Jorum, E. (2016). Complex regional pain syndrome following viper-bite. Scand. J. Pain 10, 15–18. doi: 10.1016/j.sjpain.2015.07.005
Koivula, K., Rondinelli, S., and Nasman, J. (2010). The three-finger toxin MTα is a selective α(2β)-adrenoceptor antagonist. Toxicon 56, 440–447. doi: 10.1016/j.toxicon.2010.05.001
Konno, K., Picolo, G., Gutierrez, V. P., Brigatte, P., Zambelli, V. O., Camargo, A. C., et al. (2008). Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides 29, 1293–1304. doi: 10.1016/j.peptides.2008.04.003
Konshina, A. G., Krylov, N. A., and Efremov, R. G. (2017). Cardiotoxins: functional role of local conformational changes. J. Chem. Inf. Model. 57, 2799–2810. doi: 10.1021/acs.jcim.7b00395
Laing, G. D., Clissa, P. B., Theakston, R. D., Moura-Da-Silva, A. M., and Taylor, M. J. (2003). Inflammatory pathogenesis of snake venom metalloproteinase-induced skin necrosis. Eur. J. Immunol. 33, 3458–3463. doi: 10.1002/eji.200324475
Lauer, M. A., Houghtaling, P. L., Peterson, J. G., Granger, C. B., Bhatt, D. L., Sapp, S. K., et al. (2001). Attenuation of rebound ischemia after discontinuation of heparin therapy by glycoprotein IIb/IIIa inhibition with eptifibatide in patients with acute coronary syndromes: observations from the platelet IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial. Circulation 104, 2772–2777. doi: 10.1161/hc4801.100358
Lauridsen, L. P., Laustsen, A. H., Lomonte, B., and Gutierrez, J. M. (2017). Exploring the venom of the forest cobra snake: toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteomics 150, 98–108. doi: 10.1016/j.jprot.2016.08.024
Laustsen, A. H., Karatt-Vellatt, A., Masters, E. W., Arias, A. S., Pus, U., Knudsen, C., et al. (2018). In vivo neutralization of dendrotoxin-mediated neurotoxicity of black mamba venom by oligoclonal human IgG antibodies. Nat. Commun. 9:3928. doi: 10.1038/s41467-018-06086-4
Li, M., Fry, B. G., and Kini, R. M. (2005). Eggs-only diet: its implications for the toxin profile changes and ecology of the marbled sea snake (Aipysurus eydouxii). J. Mol. Evol. 60, 81–89. doi: 10.1007/s00239-004-0138-0
Lomonte, B., Moreno, E., Tarkowski, A., Hanson, L. A., and Maccarana, M. (1994). Neutralizing interaction between heparins and myotoxin-II, a Lysine-49 Phospholipase A2 from Bothrops Asper Snake Venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. J. Biol. Chem. 269, 29867–29873. doi: 10.1016/0041-0101(94)90408-1
Lomonte, B., and Rangel, J. (2012). Snake venom Lys49 myotoxins: from phospholipases A2 to non-enzymatic membrane disruptors. Toxicon 60, 520–530. doi: 10.1016/j.toxicon.2012.02.007
Lynch, M., and Conery, J. S. (2000). The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155. doi: 10.1126/science.290.5494.1151
Lynch, V. J. (2007). Inventing an arsenal: adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol. Biol. 7:2. doi: 10.1186/1471-2148-7-2
Mackessy, S. P., and Saviola, A. J. (2016). Understanding biological roles of venoms among the Caenophidia: the importance of rear-fanged snakes. Integr. Comp. Biol. 56, 1004–1021. doi: 10.1093/icb/icw110
Madrigal, M., Sanz, L., Flores-Díaz, M., Sasa, M., Núñez, V., Alape-Girón, A., et al. (2012). Snake venomics across genus Lachesis. Ontogenetic changes in the venom composition of Lachesis stenophrys and comparative proteomics of the venoms of adult Lachesis melanocephala and Lachesis acrochorda. J. Proteomics 77, 280–297. doi: 10.1016/j.jprot.2012.09.003
Maduwage, K., and Isbister, G. K. (2014). Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 8:e3220. doi: 10.1371/journal.pntd.0003220
Mamede, C. C., De Sousa, B. B., Pereira, D. F., Matias, M. S., De Queiroz, M. R., De Morais, N. C., et al. (2016). Comparative analysis of local effects caused by Bothrops alternatus and Bothrops moojeni snake venoms: enzymatic contributions and inflammatory modulations. Toxicon 117, 37–45. doi: 10.1016/j.toxicon.2016.03.006
Marchot, P., Bourne, Y., Prowse, C. N., Bougis, P. E., and Taylor, P. (1998). Inhibition of mouse acetylcholinesterase by fasciculin: crystal structure of the complex and mutagenesis of fasciculin. Toxicon 36, 1613–1622. doi: 10.1016/S0041-0101(98)00154-8
Markland, F. S. (1998). Snake venoms and the hemostatic system. Toxicon 36, 1749–1800. doi: 10.1016/S0041-0101(98)00126-3
Markland, F. S. Jr., and Swenson, S. (2013). Snake venom metalloproteinases. Toxicon 62, 3–18. doi: 10.1016/j.toxicon.2012.09.004
Marquer, C., Fruchart-Gaillard, C., Letellier, G., Marcon, E., Mourier, G., Zinn-Justin, S., et al. (2011). Structural model of ligand-G protein-coupled receptor (GPCR) complex based on experimental double mutant cycle data: MT7 snake toxin bound to dimeric hM1 muscarinic receptor. J. Biol. Chem. 286, 31661–31675. doi: 10.1074/jbc.M111.261404
McDowell, R. S., Dennis, M. S., Louie, A., Shuster, M., Mulkerrin, M. G., and Lazarus, R. A. (1992). Mambin, a potent glycoprotein IIb-IIIa antagonist and platelet aggregation inhibitor structurally related to the short neurotoxins. Biochemistry 31, 4766–4772. doi: 10.1021/bi00135a004
Menaldo, D. L., Bernardes, C. P., Pereira, J. C., Silveira, D. S., Mamede, C. C., Stanziola, L., et al. (2013). Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int. Immunopharmacol. 15, 764–771. doi: 10.1016/j.intimp.2013.02.023
Menaldo, D. L., Bernardes, C. P., Zoccal, K. F., Jacob-Ferreira, A. L., Costa, T. R., Del Lama, M. P., et al. (2017). Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 85, 238–247. doi: 10.1016/j.molimm.2017.03.008
Menezes, M. C., Furtado, M. F., Travaglia-Cardoso, S. R., Camargo, A. C., and Serrano, S. M. (2006). Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 47, 304–312. doi: 10.1016/j.toxicon.2005.11.007
Modahl, C. M., Frietze, S., and Mackessy, S. P. (2018a). Transcriptome-facilitated proteomic characterization of rear-fanged snake venoms reveal abundant metalloproteinases with enhanced activity. J. Proteomics 187, 223–234. doi: 10.1016/j.jprot.2018.08.004
Modahl, C. M., Mrinalini, F., rietze, S., and Mackessy, S.P. (2018b). Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc. Biol. Sci. 285:20181003. doi: 10.1098/rspb.2018.1003
Mordvintsev, D. Y., Polyak, Y. L., Levtsova, O. V., Tourleigh, Y. V., Kasheverov, I. E., Shaitan, K. V., et al. (2005). A model for short α-neurotoxin bound to nicotinic acetylcholine receptor from Torpedo californica: comparison with long-chain α-neurotoxins and α-conotoxins. Comput. Biol. Chem. 29, 398–411. doi: 10.1016/j.compbiolchem.2005.08.007
Moreira, V., Lomonte, B., Vinolo, M. A., Curi, R., Gutierrez, J. M., and Teixeira, C. (2014). An Asp49 phospholipase A2 from snake venom induces cyclooxygenase-2 expression and prostaglandin E2 production via activation of NF-κB, p38MAPK, and PKC in macrophages. Mediators Inflamm. 2014:105879. doi: 10.1155/2014/105879
Moura-Da-Silva, A. M., Theakston, R. D., and Crampton, J. M. (1996). Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases: gene duplication and divergence of a common ancestor rather than convergent evolution. J. Mol. Evol. 43, 263–269. doi: 10.1007/BF02338834
Munawar, A., Ali, S. A., Akrem, A., and Betzel, C. (2018). Snake venom peptides: tools of biodiscovery. Toxins 10:E474. doi: 10.3390/toxins10110474
Murakami, M. T., and Arni, R. K. (2005). Thrombomodulin-independent activation of protein C and specificity of hemostatically active snake venom serine proteinases: crystal structures of native and inhibited Agkistrodon contortrix contortrix protein C activator. J. Biol. Chem. 280, 39309–39315. doi: 10.1074/jbc.M508502200
Nguyen, T. T., Folch, B., Letourneau, M., Vaudry, D., Truong, N. H., Doucet, N., et al. (2012). Cardiotoxin-I: an unexpectedly potent insulinotropic agent. Chembiochem 13, 1805–1812. doi: 10.1002/cbic.201200081
Nunez, C. E., Angulo, Y., and Lomonte, B. (2001). Identification of the myotoxic site of the Lys49 phospholipase A2 from Agkistrodon piscivorus piscivorus snake venom: synthetic C-terminal peptides from Lys49, but not from Asp49 myotoxins, exert membrane-damaging activities. Toxicon 39, 1587–1594. doi: 10.1016/S0041-0101(01)00141-6
O'leary, M. A., and Isbister, G. K. (2009). Commercial monovalent antivenoms in Australia are polyvalent. Toxicon 54, 192–195. doi: 10.1016/j.toxicon.2009.04.004
Osipov, A. V., and Utki, Y. N. (2015). “Snake venom toxins targeted at the nervous system,” in Snake Venoms, eds H. I. P. Gopalakrishnakone, A. K. Mukherjee, T. R. Rahmy, C.-W. Vogel, (Springer Science+Business Media Dordrecht) 1–21. doi: 10.1007/978-94-007-6648-8_23-1
Ouyang, C., Teng, C.-M., and Huang, T.-F. (1990). “Characterization of snake venom principles affecting blood coagulation and platelet aggregation,” in Fibrinogen, Thrombosis, Coagulation, and Fibrinolysis, (Springer) 151–163. doi: 10.1007/978-1-4615-3806-6_15
Ownby, C. L., Fletcher, J. E., and Colberg, T. R. (1993). Cardiotoxin 1 from cobra (Naja naja atra) venom causes necrosis of skeletal muscle in vivo. Toxicon 31, 697–709. doi: 10.1016/0041-0101(93)90376-T
Page, M. J., and Di Cera, E. (2008). Serine peptidases: classification, structure and function. Cell Mol. Life Sci. 65, 1220–1236. doi: 10.1007/s00018-008-7565-9
Pawlak, J., Mackessy, S. P., Fry, B. G., Bhatia, M., Mourier, G., Fruchart-Gaillard, C., et al. (2006). Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 281, 29030–29041. doi: 10.1074/jbc.M605850200
Peng, S. S., Kumar, T. K., Jayaraman, G., Chang, C. C., and Yu, C. (1997). Solution structure of toxin b, a long neurotoxin from the venom of the king cobra (Ophiophagus hannah). J. Biol. Chem. 272, 7817–7823. doi: 10.1074/jbc.272.12.7817
Perry, B. W., Card, D. C., Mcglothlin, J. W., Pasquesi, G. I. M., Adams, R. H., Schield, D. R., et al. (2018). Molecular adaptations for sensing and securing prey and insight into amniote genome diversity from the garter snake genome. Genome Biol. Evol. 10, 2110–2129. doi: 10.1093/gbe/evy157
Picolo, G., Chacur, M., Gutierrez, J. M., Teixeira, C. F., and Cury, Y. (2002). Evaluation of antivenoms in the neutralization of hyperalgesia and edema induced by Bothrops jararaca and Bothrops asper snake venoms. Braz. J. Med. Biol. Res. 35, 1221–1228. doi: 10.1590/S0100-879X2002001000016
Pla, D., Sanz, L., Molina-Sánchez, P., Zorita, V., Madrigal, M., Flores-Díaz, M., et al. (2013). Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteomics 89, 112–123. doi: 10.1016/j.jprot.2013.05.028
Pla, D., Sanz, L., Whiteley, G., Wagstaff, S. C., Harrison, R. A., Casewell, N. R., et al. (2017). What killed Karl Patterson Schmidt? Combined venom gland transcriptomic, venomic and antivenomic analysis of the South African green tree snake (the boomslang), Dispholidus typus. Biochim. Biophys. Acta Gen. Subj. 1861, 814–823. doi: 10.1016/j.bbagen.2017.01.020
Rajagopalan, N., Pung, Y. F., Zhu, Y. Z., Wong, P. T., Kumar, P. P., and Kini, R. M. (2007). Beta-cardiotoxin: a new three-finger toxin from Ophiophagus hannah (king cobra) venom with β-blocker activity. FASEB J. 21, 3685–3695. doi: 10.1096/fj.07-8658com
Rivel, M., Solano, D., Herrera, M., Vargas, M., Villalta, M., Segura, A., et al. (2016). Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: an experimental study in mice. Toxicon 119, 171–179. doi: 10.1016/j.toxicon.2016.06.006
Rivera-Torres, I. O., Jin, T. B., Cadene, M., Chait, B. T., and Poget, S. F. (2016). Discovery and characterisation of a novel toxin from Dendroaspis angusticeps, named Tx7335, that activates the potassium channel KcsA. Sci. Rep. 6:23904. doi: 10.1038/srep23904
Roncolato, E. C., Campos, L. B., Pessenda, G., E., Silva, L. C., Furtado, G. P., and Barbosa, J. E. (2015). Phage display as a novel promising antivenom therapy: a review. Toxicon 93, 79–84. doi: 10.1016/j.toxicon.2014.11.001
Rong, L., Zhang, C., Lei, Q., Hu, M. M., Feng, J., Shu, H. B., et al. (2016). Hydrogen peroxide detection with high specificity in living cells and inflamed tissues. Regen. Biomater. 3, 217–222. doi: 10.1093/rb/rbw022
Rouault, M., Rash, L. D., Escoubas, P., Boilard, E., Bollinger, J., Lomonte, B., et al. (2006). Neurotoxicity and other pharmacological activities of the snake venom phospholipase A2 OS2: the N-terminal region is more important than enzymatic activity. Biochemistry 45, 5800–5816. doi: 10.1021/bi060217r
Salinas, M., Besson, T., Delettre, Q., Diochot, S., Boulakirba, S., Douguet, D., et al. (2014). Binding site and inhibitory mechanism of the mambalgin-2 pain-relieving peptide on acid-sensing ion channel 1a. J. Biol. Chem. 289, 13363–13373. doi: 10.1074/jbc.M114.561076
Schmidt, J. J., and Weinstein, S. A. (1995). Structure-function studies of waglerin I, a lethal peptide from the venom of Wagler's pit viper, Trimeresurus wagleri. Toxicon 33, 1043–1049. doi: 10.1016/0041-0101(95)00043-L
Segura, Á., Villalta, M., Herrera, M., León, G., Harrison, R., Durfa, N., et al. (2010). Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP®) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon 55, 369–374. doi: 10.1016/j.toxicon.2009.08.010
Seo, Y. H., Park, M. R., and Yoo, S. H. (2014). Development of complex regional pain syndrome after a snake bite: a case report. Korean J. Pain 27, 68–71. doi: 10.3344/kjp.2014.27.1.68
Serrano, S. M. T. (2013). The long road of research on snake venom serine proteinases. Toxicon 62, 19–26. doi: 10.1016/j.toxicon.2012.09.003
Silva, L. C., Pucca, M. B., Pessenda, G., Campos, L. B., Martinez, E. Z., Cerni, F. A., et al. (2018). Discovery of human scFvs that cross-neutralize the toxic effects of B. jararacussu and C. d. terrificus venoms. Acta Trop. 177, 66–73. doi: 10.1016/j.actatropica.2017.09.001
Six, D. A., and Dennis, E. A. (2000). The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta 1488, 1–19. doi: 10.1016/S1388-1981(00)00105-0
Slagboom, J., Kool, J., Harrison, R. A., and Casewell, N. R. (2017). Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 177, 947–959. doi: 10.1111/bjh.14591
Soares, A. M., Mancin, A. C., Cecchini, A. L., Arantes, E. C., Franca, S. C., Gutierrez, J. M., et al. (2001). Effects of chemical modifications of crotoxin B, the phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom, on its enzymatic and pharmacological activities. Int. J. Biochem. Cell Biol. 33, 877–888. doi: 10.1016/S1357-2725(01)00065-6
Sribar, J., Oberckal, J., and Krizaj, I. (2014). Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2: an update. Toxicon 89, 9–16. doi: 10.1016/j.toxicon.2014.06.019
Stocker, K., Fischer, H., and Brogli, M. (1986). Chromogenic assay for the prothrombin activator ecarin from the venom of the saw-scaled viper (Echis carinatus). Toxicon 24, 81–89. doi: 10.1016/0041-0101(86)90168-6
Stocker, K., Hauer, H., Muller, C., and Triplett, D. A. (1994). Isolation and characterization of Textarin, a prothrombin activator from eastern brown snake (Pseudonaja textilis) venom. Toxicon 32, 1227–1236. doi: 10.1016/0041-0101(94)90352-2
Sunagar, K., Jackson, T. N., Undheim, E. A., Ali, S. A., Antunes, A., and Fry, B. G. (2013). Three-fingered RAVERs: rapid accumulation of variations in exposed residues of snake venom toxins. Toxins 5, 2172–2208. doi: 10.3390/toxins5112172
Takeda, S., Takeya, H., and Iwanaga, S. (2012). Snake venom metalloproteinases: strcture, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta-Prot. Proteomics 1824, 164–176. doi: 10.1016/j.bbapap.2011.04.009
Tan, C., Tan, N., Tan, K., and Kwong, K. (2015). Antivenom cross-neutralization of the venoms of Hydrophis schistosus and Hydrophis curtus, two common sea snakes in Malaysian waters. Toxins 7, 572–581. doi: 10.3390/toxins7020572
Tasoulis, T., and Isbister, G. K. (2017). A review and database of snake venom proteomes. Toxins 9:E290. doi: 10.3390/toxins9090290
Teixeira, S. S., Silveira, L. B., Da Silva, F. M., Marchi-Salvador, D. P., Silva, F. P. Jr., Izidoro, L. F., et al. (2011). Molecular characterization of an acidic phospholipase A2 from Bothrops pirajai snake venom: synthetic C-terminal peptide identifies its antiplatelet region. Arch. Toxicol. 85, 1219–1233. doi: 10.1007/s00204-011-0665-6
Torres, A. M., Kini, R. M., Selvanayagam, N., and Kuchel, P. W. (2001). NMR structure of bucandin, a neurotoxin from the venom of the Malayan krait (Bungarus candidus). Biochem. J. 360, 539–548. doi: 10.1042/bj3600539
Triggiani, M., Granata, F., Oriente, A., De Marino, V., Gentile, M., Calabrese, C., et al. (2000). Secretory phospholipases A2 induce β-glucuronidase release and IL-6 production from human lung macrophages. J. Immunol. 164, 4908–4915. doi: 10.4049/jimmunol.164.9.4908
Triplett, D. A., Stocker, K. F., Unger, G. A., and Barna, L. K. (1993). The Textarin/Ecarin ratio: a confirmatory test for lupus anticoagulants. Thromb. Haemost. 70, 925–931. doi: 10.1055/s-0038-1649701
Tsetlin, V. I. (2015). Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 36, 109–123. doi: 10.1016/j.tips.2014.11.003
Urs, N. A, N., Yariswamy, M., Joshi, V., Nataraju, A., Gowda, T. V., et al. (2014). Implications of phytochemicals in snakebite management: present status and future prospective. Toxin Rev. 33, 60–83. doi: 10.3109/15569543.2013.854255
Utkin, Y. N. (2019). Last decade update for three-finger toxins: newly emerging structures and biological activities. World J. Biol. Chem. 10, 17–27. doi: 10.4331/wjbc.v10.i1.17
Vaiyapuri, S., Thiyagarajan, N., Hutchinson, E. G., and Gibbins, J. M. (2012). Sequence and phylogenetic analysis of viper venom serine proteases. Bioinformation 8, 763–772. doi: 10.6026/97320630008563
Verri, W. A. Jr., Cunha, T. M., Parada, C. A., Poole, S., Cunha, F. Q., and Ferreira, S. H. (2006). Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 112, 116–138. doi: 10.1016/j.pharmthera.2006.04.001
Vonk, F. J., Casewell, N. R., Henkel, C. V., Heimberg, A. M., Jansen, H. J., Mccleary, R. J., et al. (2013). The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. U. S. A. 110, 20651–20656. doi: 10.1073/pnas.1314702110
Vonk, F. J., Jackson, K., Doley, R., Madaras, F., Mirtschin, P. J., and Vidal, N. (2011). Snake venom: from fieldwork to the clinic: recent insights into snake biology, together with new technology allowing high-throughput screening of venom, bring new hope for drug discovery. Bioessays 33, 269–279. doi: 10.1002/bies.201000117
Walkinshaw, M. D., Saenger, W., and Maelicke, A. (1980). Three-dimensional structure of the “long” neurotoxin from cobra venom. Proc. Natl. Acad. Sci. U. S. A 77, 2400–2404. doi: 10.1073/pnas.77.5.2400
Waqar, M., and Batool, S. (2015). In silico analysis of binding of neurotoxic venom ligands with acetylcholinesterase for therapeutic use in treatment of Alzheimer's disease. J. Theor. Biol. 372, 107–117. doi: 10.1016/j.jtbi.2015.02.028
Ward, R. J., Chioato, L., De Oliveira, A. H., Ruller, R., and Sa, J. M. (2002). Active-site mutagenesis of a Lys49-phospholipase A2: biological and membrane-disrupting activities in the absence of catalysis. Biochem. J. 362, 89–96. doi: 10.1042/bj3620089
Warrell, D. (1992). The global problem of snake bite: its prevention and treatment. Rec. Adv. Toxinol. Res. 1, 121–153.
Whiteley, G., Casewell, N. R., Pla, D., Quesada-Bernat, S., Logan, R. A. E., Bolton, F. M. S., et al. (2019). Defining the pathogenic threat of envenoming by South African shield-nosed and coral snakes (genus Aspidelaps), and revealing the likely efficacy of available antivenom. J. Proteomics 198, 186–198. doi: 10.1016/j.jprot.2018.09.019
WHO (2018). Neglected Tropical Diseases. Available online at: http://www.who.int/neglected_diseases/en/ (accessed 2019).
Williams, D. J., Gutierrez, J. M., Calvete, J. J., Wuster, W., Ratanabanangkoon, K., Paiva, O., et al. (2011). Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteomics 74, 1735–1767. doi: 10.1016/j.jprot.2011.05.027
Yang, D. C., Deuis, J. R., Dashevsky, D., Dobson, J., Jackson, T. N., Brust, A., et al. (2016). The snake with the scorpion's sting: novel three-finger toxin sodium channel activators from the venom of the long-glanded blue coral snake (Calliophis bivirgatus). Toxins 8:E303. doi: 10.3390/toxins8100303
Yip, J., Shen, Y., Berndt, M. C., and Andrews, R. K. (2005). Primary platelet adhesion receptors. IUBMB Life 57, 103–108. doi: 10.1080/15216540500078962
Zambelli, V. O., Chioato, L., Gutierrez, V. P., Ward, R. J., and Cury, Y. (2017a). Structural determinants of the hyperalgesic activity of myotoxic Lys49-phospholipase A2. J. Venom Anim. Toxins Incl. Trop. Dis. 23:7. doi: 10.1186/s40409-017-0099-6
Zambelli, V. O., Picolo, G., Fernandes, C. A. H., Fontes, M. R. M., and Cury, Y. (2017b). Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 9:406. doi: 10.3390/toxins9120406
Zancolli, G., Calvete, J. J., Cardwell, M. D., Greene, H. W., Hayes, W. K., Hegarty, M. J., et al. (2019). When one phenotype is not enough: divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. Biol. Sci. 286:20182735. doi: 10.1098/rspb.2018.2735
Zelanis, A., Menezes, M. C., Kitano, E. S., Liberato, T., Tashima, A. K., Pinto, A. F., et al. (2016). Proteomic identification of gender molecular markers in Bothrops jararaca venom. J. Proteomics 139, 26–37. doi: 10.1016/j.jprot.2016.02.030
Zelanis, A., and Tashima, A. K. (2014). Unraveling snake venom complexity with ‘omics' approaches: challenges and perspectives. Toxicon 87, 131–134. doi: 10.1016/j.toxicon.2014.05.011
Zhang, C. C., Medzihradszky, K. F., Sanchez, E. E., Basbaum, A. I., and Julius, D. (2017). Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc. Natl. Acad. Sci. U. S. A. 114, E2524–E2532. doi: 10.1073/pnas.1615484114
Zhang, Y. (2015). Why do we study animal toxins? Dongwuxue Yanjiu 36, 183–222. doi: 10.13918/j.issn.2095-8137.2015.4.183
Keywords: snake venoms, multifunctional toxins, pathological mechanisms, evolution, snakebite treatment, pain, hemotoxicity, myotoxicity
Citation: Ferraz CR, Arrahman A, Xie C, Casewell NR, Lewis RJ, Kool J and Cardoso FC (2019) Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 7:218. doi: 10.3389/fevo.2019.00218
Received: 31 March 2019; Accepted: 24 May 2019;
Published: 19 June 2019.
Edited by:
Kartik Sunagar, Indian Institute of Science (IISc), IndiaReviewed by:
Kempaiah Kemparaju, University of Mysore, IndiaStephen P. Mackessy, University of Northern Colorado, United States
Copyright © 2019 Ferraz, Arrahman, Xie, Casewell, Lewis, Kool and Cardoso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernanda C. Cardoso, Zi5jYWxkYXNjYXJkb3NvQHVxLmVkdS5hdQ==
 Camila R. Ferraz1,2
Camila R. Ferraz1,2 Arif Arrahman
Arif Arrahman Chunfang Xie
Chunfang Xie Nicholas R. Casewell
Nicholas R. Casewell Fernanda C. Cardoso
Fernanda C. Cardoso