
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol., 29 May 2019
Sec. Conservation and Restoration Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00191
This article is part of the Research TopicNorth American Monarch Butterfly Ecology and ConservationView all 35 articles
The Eastern migratory monarch butterfly has declined in recent decades, partly because widespread adoption of herbicide-resistant corn and soybean has nearly eliminated common milkweed from crop fields in the US Midwest. We argue that in addition to milkweed loss, monarch declines were likely exacerbated by shifting disturbance regimes within their summer breeding range. Monarchs prefer to lay eggs on younger, vegetative milkweed stems. They also benefit from enemy-free space, as most eggs and early-instar larvae succumb to predators. Historically, ecological disturbances during the growing season could have provided these conditions. During most of the 19th and 20th centuries, milkweed was abundant in crop fields where manual weeding and mechanical cultivation set milkweed back, but rather than killing it would often stimulate regrowth later in the summer. Before European settlement, large mammals and fires (natural and anthropogenic) perturbed grasslands during the summer and could have had similar effects. However, presently most remaining milkweed stems in the Midwest are in perennial grasslands like roadsides, old-fields, parks, and conservation reserves, which often lack growing season disturbance. As a result, monarchs may be left with limited options for oviposition as the summer progresses and could have lower survival in grasslands where predation pressure is high. Our recent work has shown that targeted disturbance during the growing season produces milkweed stems that are attractive to ovipositing monarchs and harbor fewer arthropod predators. Targeted disturbance in perennial grasslands could improve habitat heterogeneity and phenologic diversity of milkweeds, and should be explored as a monarch conservation strategy.
The Eastern North American migratory population of monarch butterflies is in a decades-long decline and the migratory phenomenon is considered at risk (Brower et al., 2012; Vidal and Rendón-Salinas, 2014). The overwintering population in Mexico is estimated to have declined more than 80% from the 1990s to 2014 (Semmens et al., 2016), and monarchs are under review for listing under the US Endangered Species Act (CBD, 2014). Potential causes of this decline include logging of overwintering habitat in Mexico, increased pathogen loads, lost nectar resources, exposure to insecticides, climate change, and loss of breeding habitat [see reviews by Inamine et al. (2016); Thogmartin et al. (2017a); Malcolm (2018); Stenoien et al. (2018)]. While the relative importance of these factors is a topic of ongoing research, there is increasing evidence that a major contributor to the recent decline is the loss of milkweed host plants from breeding habitat in the US Midwest (Flockhart et al., 2013, 2015; Pleasants and Oberhauser, 2013; Oberhauser et al., 2017; Saunders et al., 2017; Thogmartin et al., 2017a; Stenoien et al., 2018).
The majority of monarchs arriving in Mexico each year for overwintering are born in the Midwest and North Central US, where they lay most of their eggs on common milkweed, Asclepias syriaca L. (Malcolm et al., 1993; Flockhart et al., 2017). This species, which we will hereafter refer to simply as milkweed, is considered an agricultural weed. Until recently, milkweed stems growing in annual crop fields, mostly corn and soybeans, supported more monarch eggs and larvae than stems growing in other habitat types (Oberhauser et al., 2001). However, since the 1990s, more than 90% of corn and soybean production has switched to transgenic herbicide-resistant varieties (USDA Economic Research Service, 2018). Now, fields are sprayed with broad-spectrum herbicides that effectively control milkweed, resulting in a ~40% loss of milkweed stems from midwestern landscapes (Hartzler, 2010; Pleasants, 2016). In response to this loss, recent research has focused on how to rebuild milkweed populations in the US, including calls to restore 1.3–1.6 billion additional stems in the Midwest (Pleasants, 2016; Thogmartin et al., 2017b).
In addition to habitat loss, monarchs have also undergone a habitat shift. Until recent decades, a large proportion of milkweed stems in the Midwest were found in annual crop field interiors. Today, however, remaining milkweeds are predominantly located in perennial grasslands. Thus, a greater proportion of monarchs now rely on grassland habitats. These habitats include ditches, old-fields, pastures, transportation rights-of-way, conservation reserve program (CRP) lands, parks, and reserves (Thogmartin et al., 2017b). Perennial grasslands differ from agricultural fields in multiple respects; understanding these differences and managing in light of them may be key to stabilizing monarch populations.
Crop fields are a distinct type of ecosystem, and the phenologic, nutritional, and chemical characteristics of milkweed growing in crop fields may differ from those in grasslands. Crop fields are nutrient-enriched, may be irrigated, and in the case of corn, become shaded as summer progresses. Before the widespread use of effective herbicides, they also would have been mechanically disturbed during the growing season.
Farmers controlled weeds with hand tools or draft animals until at least the 1930s, after which time tractor-based mechanical cultivation became the norm (Swinton and Van Deynze, 2017). In following decades, each year fields were cultivated until late June or early July, when the soybean canopy closes and corn grows too tall (Curran, 2004; Specht et al., 2012). For soybeans, additional manual control often continued later into the summer (Horlyk, 2013; Eller, 2014). Mechanical control was only moderately effective against milkweed; while aboveground tissue was easy to remove, milkweed's modular growth form made it resilient. Plants tended to survive and send up new shoots following cultivation, and equipment often spread roots to new areas (Bhowmik and Bandeen, 1976). Similarly, many herbicides applied to crop fields beginning in the 1960s killed aboveground milkweed growth but left the roots unscathed (Bhowmik, 1994). Monarchs prefer to lay eggs on very young and vegetative stems (Urquhart, 1987; Bergström et al., 1994), so frequent disturbance could have benefitted monarchs by supplying attractive new milkweed growth for oviposition as summer progressed. These patterns stand in contrast to perennial grasslands, where milkweeds often flower by mid-summer and afterwards can begin to senesce. Therefore, we suspect monarchs relying on milkweeds in perennial grasslands are left with increasingly poor options for oviposition as summer progresses.
As a rule, host plants for herbivorous insects vary in suitability. Conservation managers working toward butterfly recovery often need to differentiate between host plants that are suitable and ones that are not (Thomas et al., 2011). Plant suitability to herbivores can change with phenology: newly grown tissues are often replete with water and N, while older tissues tend to be tougher and lacking in these resources (Thomas and Stoddart, 1980; Scriber and Slansky, 1981; Slansky, 1993; Lim et al., 2007). Consequently, many caterpillars perform better on younger tissues compared to senescent or near-senescent ones (Scriber and Slansky, 1981; Slansky, 1993). This pattern is evident in multiple butterflies of conservation concern that avoid senescing plants or fare poorly on them (e.g., Singer, 1972; Grundel et al., 1998; Lane and Andow, 2003; Haan et al., 2018). In the case of monarchs, while it is clear they prefer to oviposit on younger milkweed stems (Urquhart, 1987; Bergström et al., 1994), we know less about how milkweed senescence affects survival and growth of larvae. This should be an area of future research. Along the same lines, milkweed in cornfields would have been shaded in late summer. While we do not know if ovipositing monarchs favored heavily-shaded milkweeds in cornfields, their caterpillars grow larger on shade-grown stems, which are less-defended and have lower leaf toughness and C:N ratios (Agrawal et al., 2012a).
Finally, nutrient enrichment in crop field soils could provide monarchs with more nutrient-dense milkweed tissues to eat. Monarch growth rates can increase with foliar N concentrations in common milkweed (Tao et al., 2014, but see Schroeder, 1976; Lavoie and Oberhauser, 2004). Nutrient enrichment can also cause milkweeds to produce less toxic cardenolides (Agrawal et al., 2012b), which limit the growth and survival of monarch caterpillars (Rasmann et al., 2009). While recent work in our lab suggests nutrients alone do not drive oviposition patterns (Myers et al., 2019), details of how crop field nutrient enrivonments could influence monarch nutrition and response to cardenolides need further investigation.
Enemy-free space where predation pressure is minimized can be an important component of a species' niche (Jeffries and Lawton, 1984). For monarchs, enemy-free space may be much more limited in perennial grasslands than in crop fields, as predatory arthropods are more diverse and abundant in grasslands (Werling et al., 2014) and predation rates on invertebrates are consistently higher (Werling et al., 2011). Like many Lepidoptera, survival of monarch eggs and first instars is low, with a large fraction of immature monarchs succumbing to predators. For example, in a restored prairie <2% of eggs survived to third instar, with lower survival when plants contained spiders or aphids, the latter of which attract ants and other predators (De Anda and Oberhauser, 2015). Similarly, monarch eggs in an old-field had a 2% survival rate after 7 d, with ants predating eggs and larvae (Prysby, 2004). Finally, Myers et al. (2019) found >80% mortality of monarch eggs over 72 h periods in grasslands, with lower mortality in corn.
Disturbance during the growing season could have historically reduced predation risk to monarchs through multiple mechanisms. First, milkweed stems with aphids attract more predators (Haan and Landis, 2019), so disturbances that remove aphid-infested stems should reduce predators as well. Second, vegetative milkweed stems host lower predator densities than other stages (Haan and Landis, 2019), so disturbances that reset milkweed phenology could also serve to reduce predator abundance on the regenerating stems. Finally, disturbance temporarily simplifies the structure of surrounding vegetation, which could limit habitat suitability for some predators, particularly spiders (Rypstra et al., 1999).
Ecological disturbance may be a key factor determining the quality of breeding habitat for Eastern monarchs, as it potentially provides both phenologically-attractive host plants and enemy-free space. Disturbance during the growing season was a defining characteristic of annual crop fields, but it occurs much less often in most perennial grasslands that monarchs now rely on. Positive recovery efforts for several rare butterfly species have depended on whether managers reinstated historical disturbance regimes (Thomas, 1980; Schultz and Crone, 1998, 2015; Thomas et al., 2009; Dunwiddie et al., 2016; Haddad, 2018). In contrast to many rare butterfly species, monarchs breed in landscapes that have been transformed by humans, and thus may have come to depend on agricultural disturbance regimes during the 20th century. Interestingly, habitat management recommendations for monarchs currently discourage disturbances during the breeding season (MJV [Monarch Joint Venture], 2018).
Multiple studies have documented that as milkweed stems regenerate after fields are mowed, they can support large numbers of monarch eggs and larvae (Marsh, 1888; Borkin, 1982; Fischer et al., 2015; Alcock et al., 2016). Building on these observations, we conducted a field experiment in Michigan to determine if strategically-timed disturbance can enhance monarch habitat in perennial grasslands (Haan and Landis, 2019). Monarchs laid more eggs on milkweeds that regenerated after being cut back compared to those we left undisturbed, and predators took 2–4 weeks to recolonize the regenerating milkweed stems, potentially providing a window of enemy-free space. We believe these results suggest disturbance is an important process influencing monarch habitat suitability in the Midwest, and that some types of habitat could be enhanced with strategically-timed disturbance during the growing season (Figure 1).
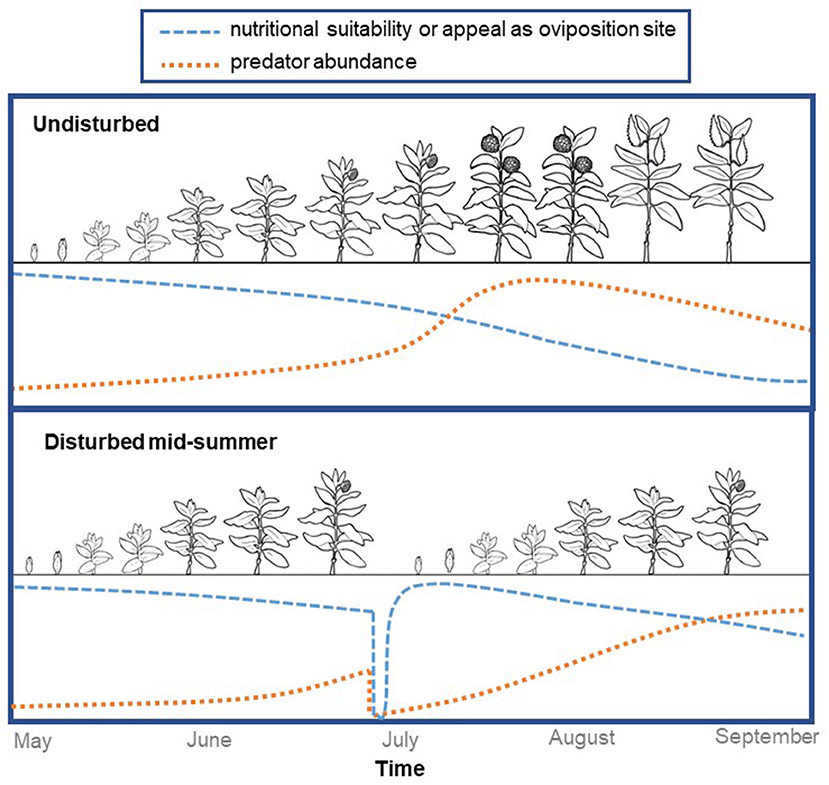
Figure 1. Disturbance resets milkweed phenology, providing new stems in the vegetative stage for monarch oviposition while also reducing predator abundance. Here we show idealized curves representing changes in milkweed's oviposition appeal and/or nutritional quality (blue) and in arthropod predator abundance (orange). In the plot representing undisturbed milkweeds (top) we show predator abundance peaking during the flowering stage because in recent work we found that predators were particularly dense on flowering stems.
Growing season disturbance may have been common before Euro-American settlement of the Midwest, although its historic effects on monarchs are left to speculation. Native Americans farmed corn, at times quite extensively (Riley et al., 1994; Benson et al., 2009), and managed Midwest and Great Plains ecosystems with fire for thousands of years. In contrast to current prairie restoration practices which concentrate burns during spring and fall, evidence suggests fires were historically set almost any time of year, including summer (Bragg, 1982; Higgins, 1986). Similarly, lightning-ignited fires are most common during summer (Komarek, 1964; Bragg, 1982; Higgins, 1984). Milkweed readily regenerates with new stems after summer fire; in Oklahoma, prescribed fire in July produced regenerating milkweeds (Asclepias viridis) which were used by monarchs in late summer and early fall (Baum and Scharber, 2012). Large mammals could have also been an important historical source of disturbance. Bison were ubiquitous in grasslands of the Midwest and Great Plains until the late 19th century and could have produced regenerating milkweed stems through grazing, trampling, or wallows (Knapp et al., 1999). Similarly, prior to humans' arrival in North America, grasslands hosted diverse and abundant megafauna which would have caused a variety of year-round disturbances (Mack and Thompson, 1982; Milchunas et al., 1988).
On the other hand, contemporary monarch ecology could differ markedly from past centuries. Common milkweed may have proliferated in agricultural landscapes precisely because it tolerates mechanical disturbances, and monarchs may have historically relied more heavily on the several other milkweed species native to the Midwest, as these could have been much more abundant before the destruction of North American prairie (Gray et al., 1889). It has also been hypothesized that the migratory phenomenon in its current form is itself anthropogenic; that it only came about because deforestation of the Eastern US in the 19th century caused a population explosion of milkweeds and monarchs as they colonized newly-available habitat (Vane-Wright, 1993).
The idea that milkweed shortage in the Midwest underlies monarch declines has been met with controversy (Davis and Dyer, 2015; Dyer and Forister, 2016; Inamine et al., 2016; Oberhauser et al., 2017; Thogmartin et al., 2017a; Zaya et al., 2017; Stenoien et al., 2018; Boyle et al., 2019). Even the casual observer will notice that milkweed is a common sight outside of crop fields in the Midwest, but that most stems contain no monarch eggs or larvae. It follows that milkweed abundance per se does not limit monarchs. This line of thinking parallels that of Hairston et al. (1960). In their landmark paper they proposed that in contrast to other consumers, herbivores are not generally limited by the availability of food—if they were, the earth would not be covered in such an excess of plants. Therefore, herbivore populations must be limited by something other than plant abundance. This generated two competing, although not mutually-exclusive, hypotheses: first, herbivores could be limited because some plant material is unsuitable, e.g., if some plants are poisonous or nutritionally inferior (Murdoch, 1966). Second, herbivores could be limited by predators. This latter position was the one espoused by Hairston et al., and became part of the basis for the trophic cascade concept.
It is interesting to apply the same logical framework to monarchs and milkweeds. If monarchs were limited by milkweed stem quantity per se, we would expect to find competition for milkweed stems. However, most milkweed stems are not used by monarchs; on undisturbed milkweeds often < 1 monarch egg is found per ten stems (e.g., Fischer et al., 2015; Pitman et al., 2018; Haan and Landis, 2019). If the supposition is that monarchs are limited by milkweed quantity per se, then these simple observations disprove it. However, we believe this apparent discrepancy can be solved by one or both of the following hypotheses, which correspond to the ones generated by Hairston et al. (1960): First, monarchs are limited not by milkweed quantity per se, but rather in terms of the quality and suitability of extant milkweed stems for oviposition and larval feeding. Second, monarch populations are limited by enemies like predators and parasitoids. An important process underpinning both possible mechanisms is disturbance.
If the migratory monarch phenomenon is to persist, we need to design and manage agricultural landscapes with abundant, phenologically-diverse milkweeds and associated windows of enemy-free space. This will require disturbance regimes that are coordinated and carried out at a regional scale. Our purpose here is not to be prescriptive about the type or timing of disturbance, as these are likely to be context-dependent, but practices could include fire, grazing, haying, mowing, or others. In our recent work (Haan and Landis, 2019) we focused on occasional mowing, which is an appropriate grassland management technique for some, although certainly not all, contexts: effects of mowing and hay harvesting on biodiversity can be negative, neutral, or positive depending on timing and technique (Dale et al., 1997; Johst et al., 2005; Roth et al., 2005; Humbert et al., 2009; Cizek et al., 2012). Large areas of perennial grasslands in Midwest landscapes are already mowed for agricultural, safety, and aesthetic reasons. Redirecting or adjusting even a fraction of that annual effort into strategically-timed disturbance of milkweed could create a mosaic of phenologically-diverse milkweed stems and patches of enemy free space for monarchs. We emphasize that we are not advocating for wholesale increase in mowing frequency or extent in the Midwest. Instead, we envision a heterogeneous landscape in which some chronically-disturbed areas are left alone to allow for growth of milkweeds and other plants, while some currently-undisturbed grasslands could be intentionally perturbed mid-season. We believe this would significantly improve the productivity of the current stock of milkweeds, as well as those that are added to the landscape as part of conservation efforts.
In the short-term, more research is needed on how to effectively utilize strategic disturbance for monarch conservation as many questions remain. For example, should we disturb (e.g.) one in three milkweed patches, or a third of each patch? What is the opportunity-cost associated with disturbance, since milkweeds require 1–3 weeks to regenerate? Could disturbance create ecological traps by concentrating oviposition effort in certain areas, increasing natural enemy effectiveness or disease transmission? Will the prevalence of young milkweed tissues in late summer cause butterflies to skip reproductive diapause and fail to migrate south? What are long term effects of repeated disturbance on milkweed persistence? Can we ensure disturbance regimes are compatible with other conservation objectives (e.g., pollinators and grassland nesting birds)? Future work predicting effects of disturbance at the regional level could interface with existing models designed to help us understand where and how to improve monarch habitat (Thogmartin et al., 2017a) and predict the resulting monarch population response (Oberhauser et al., 2017).
In the long term, we also need to consider how to create and maintain heterogeneity in agricultural landscapes to benefit biodiversity and ecosystem services more broadly. Intensified agricultural practices often lead to landscape simplification and loss of biodiversity services (Landis, 2017) but including perennial elements within agricultural landscapes can have many potential benefits. For example, incorporating prairie strips into corn and soybean fields can reduce erosion and nutrient loss while increasing biodiversity (Schulte et al., 2017). Future production of bioenergy crops on marginal soils also could produce multiple benefits for biodiversity and ecosystem services depending on the crops selected (Landis et al., 2018). Perennial polycultures based on prairie systems support a wide array of biodiversity and are highly compatible with monarch conservation (Werling et al., 2014).
In conclusion, we believe recent shifts in disturbance regimes across the Midwest US have caused not just a reduction in milkweed quantity, but also reductions in the suitability of extant plants, and in enemy-free space. While we support calls to introduce more milkweed to grasslands in the Midwest, evidence suggests we should also examine ways to improve productivity of existing milkweeds and reduce pressure from natural enemies. More work is needed to understand how the type, timing, and frequency of disturbance could influence monarchs and their complex interactions with milkweeds and other arthropods. More broadly, habitat manipulations to support monarchs must be integrated into the landscape in ways that support other conservation goals (e.g., pollinators and grassland birds) as well as contribute to soil and water quality and the aesthetic aspects of agricultural landscapes.
NH and DL generated ideas for the manuscript. NH led the writing effort.
This material is based upon work supported in part by USDA National Institute for Food and Agriculture (2017-68004-26323), the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Numbers DE-SC0018409 and DE-FC02-07ER64494, and MSU AgBioResearch.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank A. Myers, S. Hermann, and C. Gratton for helpful discussions on this topic.
Agrawal, A. A., Kearney, E. A., Hastings, A. P., and Ramsey, T. E. (2012a). Attenuation of the jasmonate burst, plant defensive traits and resistance to specialist monarch caterpillars on shaded common milkweed (Asclepias syriaca). J. Chem. Ecol. 38, 893–901. doi: 10.1007/s10886-012-0145-3
Agrawal, A. A., Petschenka, G., Bingham, R. A., Weber, M. G., and Rasmann, S. (2012b). Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New. Phytol. 194, 28–45. doi: 10.1111/j.1469-8137.2011.04049.x
Alcock, J., Brower, L. P., and Williams, E. H. (2016). Monarch butterflies use regenerating milkweeds for reproduction in mowed hayfields in northern Virginia. J. Lepid. Soc. 70, 177–181. doi: 10.18473/107.070.0302
Baum, K. A., and Scharber, W. V. (2012). Fire creates host plant patches for monarch butterflies. Biol Lett. 8, 968–971. doi: 10.1098/rsbl.2012.0550
Benson, L., Pouketat, T. R., and Cook, E. R. (2009). Cahokia's boom and bust in the context of climate change. Am. Antiq. 74, 467–483. doi: 10.1017/S000273160004871X
Bergström, G., Rothschild, M., Groth, I., and Crighton, C. (1994). Oviposition by butterflies on young leaves: investigation of leaf volatiles. Chemoecology 5, 147–158. doi: 10.1007/BF01240599
Bhowmik, P. C. (1994). Biology and control of common milkweed (Asclepias syriaca). Rev. Weed Sci. 6, 227–250.
Bhowmik, P. C., and Bandeen, J. D. (1976). The biology of Canadian weeds: 19. Asclepias syriaca L. Can. J. Plant. Sci. 56, 579–589. doi: 10.4141/cjps76-094
Borkin, S. (1982). Notes on shifting distributing patterns and survival of immature Danaus plexippus (Lepidoptera: Danaidae) on the food plant Asclepias syriaca. Great Lakes Entomol. 15, 199–206.
Boyle, J. H., Dalgleish, H. J., and Puzey, J. R. (2019). Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc. Nat. Acad. Sci. U.S.A. 116, 3006–3011. doi: 10.1073/pnas.1811437116
Bragg, T. B. (1982). Seasonal variations in fuel and fuel consumption by fires in a bluestem prairie. Ecology 63, 7–11. doi: 10.2307/1937024
Brower, L. P., Taylor, O. R., Williams, E. H., Slayback, D. A., Zubieta, R. R., and Ramirez, M. I. (2012). Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect cons. Div. 5, 95–100. doi: 10.1111/j.1752-4598.2011.00142.x
CBD (2014). Petition to Protect the Monarch Butterfly (Danaus plexippus plexippus) Under the Endangered Species Act. Available online at: https://www.biologicaldiversity.org/species/invertebrates/pdfs/Monarch_ESA_Petition.pdf (accessed December 5, 2018)
Cizek, O., Zamecnik, J., Tropek, R., Kocarek, P., and Konvicka, M. (2012). Diversification of mowing regimes increases arthropods diversity in species-poor cultural hay meadows. J. Insect Conserv. 16, 215–226. doi: 10.1007/s10841-011-9407-6
Curran, W. (2004). Weed Management in Organic Cropping Systems. State College, PA: Pennsylvania State University Extension Agronomy Facts sheet 64.
Dale, B. C., Martin, P. A., and Taylor, P. S. (1997). Effects of hay management on grassland songbirds in Saskatchewan. Wildl. Soc. Bull. 25, 616–626.
Davis, A. K., and Dyer, L. A. (2015). Long-term trends in eastern North American monarch butterflies: a collection of studies focusing on spring, summer, and fall dynamics. Ann. Entomol. Soc. Am. 108, 661–663. doi: 10.1093/aesa/sav070
De Anda, A., and Oberhauser, K. S. (2015). “Invertebrate natural enemies and stage-specific mortality rates of monarch eggs and larvae,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser., K. R. Nail., S. Altizer (Ithaca, NY: Cornell University Press), 60–70.
Dunwiddie, P. W., Haan, N. L., Linders, M., Bakker, J. D., Fimbel, C., and Thomas, T. B. (2016). Intertwined fates: opportunities and challenges in the linked recovery of two rare species. Nat. Area J. 36, 207–215. doi: 10.3375/043.036.0214
Dyer, L. A., and Forister, M. L. (2016). Wherefore and whither the modeler: understanding the population dynamics of monarchs whill require integrative and quantitative techniques. Ann. Entomol. Soc. Am. 109, 172–175. doi: 10.1093/aesa/sav160
Eller, D. (2014). Iowa Farmers Bringing Back Walking Beans? Iowa City Press-Citizen. Available online at: https://www.press-citizen.com/story/news/local/2014/07/16/iowa-farmers-bringing-back-walking-beans/12764661/ (accessed July 16, 2014).
Fischer, S. J., Williams, E. H., Brower, L. P., and Palmiotto, P. A. (2015). Enhancing monarch butterfly reproduction by mowing fields of common milkweed. Am. Midl. Nat. 173, 229–240. doi: 10.1674/amid-173-02-229-240.1
Flockhart, D. T., Brower, L. P., Ramirez, M. I., Hobson, K. A., Wassenaar, L. I., Altizer, S., et al. (2017). Regional climate on the breeding grounds predicts variation in the natal origin of monarch butterflies overwintering in Mexico over 38 years. Glob. Change Biol. 23, 2565–2576. doi: 10.1111/gcb.13589
Flockhart, D. T., Pichancourt, J. B., Norris, D. R., and Martin, T. G. (2015). Unravelling the annual cycle in a migratory animal: breeding-season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Flockhart, D. T., Wassenaar, L. I., Martin, T. G., Hobson, K. A., Wunder, M. B., and Norris, D. R. (2013). Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc. R. Soc. Lond. 280:20131087. doi: 10.1098/rspb.2013.1087
Gray, A., Watson, S., and Coulter, J. M. (1889). Manual of the Botany of the Northern United States, Including the District East of the Mississippi and North of North Carolina and Tennessee. NewYork, NY: American Book Company. doi: 10.5962/bhl.title.17542
Grundel, R., Pavlovic, N. B., and Sulzman, C. L. (1998). The effect of canopy cover and seasonal chanange on host plant quality for the endangered Karner blue butterfly (Lycaeides melissa samuelis). Oecologia 114, 243–250. doi: 10.1007/s004420050442
Haan, N. L., Bakker, J. D., Dunwiddie, P. W., and and, Linders, M. J. (2018). Instar-specific effects of host plants on survival of endangered butterfly larvae. Ecol. Ent. 43, 742–753. doi: 10.1111/een.12656
Haan, N. L., and Landis, D. A. (2019). Grassland disturbance increases monarch butterfly oviposition and decreases arthropod predator abundance. Biol. Conserv. 233, 185–192. doi: 10.1016/j.biocon.2019.03.007
Haddad, N. M. (2018). Resurrection and resilience of the rarest butterflies. PLoS Biol. 16:e2003488. doi: 10.1371/journal.pbio.2003488
Hairston, N. E., Smith, F. E., and Slobodkin, L. B. (1960). Community structure, population control, and competition. Am. Nat. 94, 421–425. doi: 10.1086/282146
Hartzler, R. G. (2010). Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Prot 29, 1542–1544. doi: 10.1016/j.cropro.2010.07.018
Higgins, K. F. (1984). Lighning fires in North Dakota grasslands and in pine-savanna lands of South Dakota and Montana. J. Range Manage. 37, 100–103. doi: 10.2307/3898892
Higgins, K. F. (1986). Interpretation and Compendium of Historical Fire Accounts in the Northern Great Plains. Washington, D.C: Research Publication 161, U.S. Fish and Wildlife Service.
Horlyk, E. (2013). No beans about it, whacking weeds a rite of passage for generations of farm kids. Souix City J. Accessed online at: https://siouxcityjournal.com/special-section/local/no-beans-about-it-whacking-weeds-a-rite-of-passage/article_cb5d5bd3-a2f7-58b2-b1de-bd0a74bf268c.html
Humbert, J.-Y., Ghazoul, J., and Walter, T. (2009). Meadow harvesting techniques and their impacts on field fauna. Agric. Ecosyst. Environ. 130, 1–8. doi: 10.1016/j.agee.2008.11.014
Inamine, H., Ellner, S. P., Springer, J. P., and Agrawal, A. A. (2016). Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125, 1081–1091. doi: 10.1111/oik.03196
Jeffries, M. J., and Lawton, J. H. (1984). Enemy free space and the structure of ecological communities. Biol. J. Linn. Soc. Lond. 23, 269–286. doi: 10.1111/j.1095-8312.1984.tb00145.x
Johst, K., Drechsler, M., Thomas, J., and Settele, J. (2005). Influence of mowing on the persistence of two endangered large blue butterfly species. J. Appl. Ecol. 43, 333–342. doi: 10.1111/j.1365-2664.2006.01125.x
Knapp, A. K., Blair, J. M., Briggs, J. M., Collins, S. L., Hartnett, D. C., Johnson, L. C., et al. (1999). The keystone role of bison in North American tallgrass prairie: bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience 76, 39–50. doi: 10.2307/1313492
Komarek, E. V. (1964). “The natural history of lightning,” in Proceedings: Third Annual Tall Timbers Fire Ecology Conference (Tallahassee, FL: Tall Timbers Research Station), 139–183.
Landis, D. A. (2017). Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 18, 1–12. doi: 10.1016/j.baae.2016.07.005
Landis, D. A., Gratton, C., Jackson, R. D., Gross, K. L., Duncan, D. S., Liang, C., et al. (2018). Biomass and biofuel crop effects on biodiversity and ecosystem services in the North Central US. Biomass Bioenergy, 114, 18–29. doi: 10.1016/j.biombioe.2017.02.003
Lane, C. P., and Andow, D. A. (2003). Oak savanna subhabitat variation and the population biology of Lycaeides melissa samuelis (Lepidoptera: Lycaenidae). Ann. Entom. Soc. Am. 96, 799–809. doi: 10.1603/0013-8746(2003)096[0799:OSSVAT]2.0.CO;2
Lavoie, B., and Oberhauser, K. S. (2004). Compensatory feeding in Danaus plexippus (Lepidoptera: Nymphalidae) in response to variation in host plant quality. Environ. Entomol. 33, 1062–1069. doi: 10.1603/0046-225X-33.4.1062
Lim, P., Kim, H., and Nam, H. (2007). Leaf senescence. Ann. Rev. Plant. Biol. 58, 115–136. doi: 10.1146/annurev.arplant.57.032905.105316
Mack, R. N., and Thompson, J. N. (1982). Evolution in steppe with few large hooved mammals. Am. Nat. 119, 757–773. doi: 10.1086/283953
Malcolm, S. B. (2018). Anthropogenic impacts on mortality and population viability of the monarch butterfly. Ann. Rev. Entomol. 63, 277–302. doi: 10.1146/annurev-ento-020117-043241
Malcolm, S. B., Cockrell, B. J., and Brower, L. P. (1993). “Spring recolonization of eastern North America by the monarch butterfly: successive brood or single sweep migration?” in Biology and Conservation of the Monarch Butterfly, eds S. B. Malcolm and M. P. Zalucki (Los Angeles, CA: Natural History Museum of Los Angeles County), 253–267.
Marsh, W. D. (1888). Some observations made in 1887 on Danais archippus, Fabr. Can. Entomol. 20, 45–47. doi: 10.4039/Ent2045-3
Milchunas, D. G., Sala, O. E., and Lauenroth, W. K. (1988). A generalized model of the effects of grazing by large herbivores on grassland community structure. Am. Nat. 132, 87–106. doi: 10.1086/284839
MJV [Monarch Joint Venture] (2018). Available online at: https://monarchjointventure.org/images/uploads/documents/MowingForMonarchs.pdf (accessed December 18, 2018)
Murdoch, W. M. (1966). Community structure, population control, and competition – a critique. Am. Nat. 100, 219–226. doi: 10.1086/282415
Myers, A. T., Bahlai, C. A., and Landis, D. A. (2019). Habitat type influences Danaus plexippus (Lepidoptera: Nymphalidae) oviposition and egg survival on Asclepias syriaca (Gentianales: Apocynaceae). Environ Entomol. doi: 10.1093/ee/nvz046. [Epub ahead of print].
Oberhauser, K. S., Prysby, M. D., Mattila, H. R., Stanley-Horn, D. E., Sears, M. K., Dively, G., et al. (2001). Temporal and spatial overlap between monarch larvae and corn pollen. Proc. Nat. Acad. Sci. U.S.A. 98, 11913–11918. doi: 10.1073/pnas.211234298
Oberhauser, K. S., Wiederholt, R., Diffendorfer, J. E., Semmens, D., Ries, L., Thogmartin, W. E., et al. (2017). A trans-national monarch butterfly population model and implications for regional conservation priorities. Ecol. Entomol. 42, 51–60. doi: 10.1111/een.12351
Pitman, G. M., Flockhart, D. T. T., and Norris, D. R. (2018). Patterns and causes of oviposition in monarch butterflies: implications for milkweed restoration. Biol. Cons. 217, 54–65. doi: 10.1016/j.biocon.2017.10.019
Pleasants, J. (2016). Milkweed restoration in the Midwest for monarch butterfly recovery: estimates of milkweeds lost, milkweeds remaining and milkweeds that must be added to increase the monarch population. Insect Conserv. Diver. 10, 42–53. doi: 10.1111/icad.12198
Pleasants, J. M., and Oberhauser, K. S. (2013). Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Diver. 6, 135–144. doi: 10.1111/j.1752-4598.2012.00196.x
Prysby, M. D. (2004). “Natural enemies and survival of monarch eggs and larvae,” in The Monarch Butterfly: Biology and Conservation, eds K. S. Oberhauser and M. J. Solensky (Ithaca, NY: Cornell University Press), 27–38.
Rasmann, S., Johnson, M. D., and Aagrawal, A. A. (2009). Induced responses to herbivory and jasmonate in three milkweed species. J. Chem. Ecol. 35, 1326–1334. doi: 10.1007/s10886-009-9719-0
Riley, T. J., Walz, G. R., Bareis, C. J., Fortier, A. C., and Parker, K. E. (1994). Accelerator mass spectrometry (AMS) dates confirm early Zea mays in the Missippi River valley. Am. Antiq. 59, 490–498. doi: 10.2307/282461
Roth, A. M., Sample, D. W., Ribic, C. A., Paine, L., Undersander, D. J., and Bartelt, G. A. (2005). Grassland bird response to harvesting switchgrass as a bioenergy crop. Biomass Bioenergy 28, 490–498. doi: 10.1016/j.biombioe.2004.11.001
Rypstra, A. L., Carter, P. E., Balfour, R. A., and Marshall, S. D. (1999). Architectural features of agricultural habitats and their impact on the spider inhabitants. J. Arachnol. 27, 371–377.
Saunders, S. P., Ries, L., Oberhauser, K. S., Thogmartin, W. E., and Zipkin, E. F. (2017). Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography 41, 278–290. doi: 10.1111/ecog.02719
Schroeder, L. A. (1976). Energy, matter and nitrogen utilization by the larvae of the monarch butterfly Danaus plexippus. Oikos 27, 259–264. doi: 10.2307/3543904
Schulte, L. A., Niemi, J., Helmers, M. J., Liebman, M., Arbuckle, J. G., James, D. E., et al. (2017). Prairie strips improve biodiversity and the delivery of multiple ecosystem services from corn-soybean croplands. Proc. Nat. Acad. Sci. U.S.A. 114, 11247–11252. doi: 10.1073/pnas.1620229114
Schultz, C. B., and Crone, E. E. (1998). Burning prairie to restore buttefly habitat: a modeling approach to management tradeoffs for the Fender's blue. Restor. Ecol. 6, 244–252. doi: 10.1046/j.1526-100X.1998.00637.x
Schultz, C. B., and Crone, E. E. (2015). Using ecological theory to develop recovery criteria for an endangered butterfly. J. Appl. Ecol. 52, 1111–1115. doi: 10.1111/1365-2664.12450
Scriber, J. M., and Slansky, F. (1981). The nutritional ecology of immature insects. Ann. Rev. Entomol. 26, 183–211. doi: 10.1146/annurev.en.26.010181.001151
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Singer, M. C. (1972). Complex components of habitat suitability within a butterfly colony. Science 176, 75–77. doi: 10.1126/science.176.4030.75
Slansky, F. (1993). “Nutritional ecology: the fundamental quest for nutrients,” in Caterpillars: Ecological and Evolutionary Constraints on Foraging, eds N. E. Stamp and T. M Casey (NewYork, NY: Chapman and Hall), 29–91.
Specht, J. E., Rees, J. M., Zoubek, G. L., Glewen, K. L., VanDeWalle, B. S., Schneider, J. W., et al. (2012). Soybean Planting Date – When and Why. Lincoln, NE: University of Nebraska Extension, EC145.
Stenoien, C., Nail, K. R., Zalucki, J. M., Parry, H., Oberhauser, K. S., and Zalucki, M. P. (2018). Monarchs in decline: a collateral landscape-level effect of modern agriculture. Insect Sci. 25, 528–541. doi: 10.1111/1744-7917.12404
Swinton, S. M., and Van Deynze, B. (2017). Hoes to herbicides: economics of evolving weed management in the United States. Eur. J. Dev. Res. 29, 560–574. doi: 10.1057/s41287-017-0077-4
Tao, L., Berns, A. R., and Hunter, M. D. (2014). Why does a good thing become too much? Interactions between foliar nutrients and toxins determine performance of an insect herbivore. Funct. Ecol. 28, 190–196. doi: 10.1111/1365-2435.12163
Thogmartin, W., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017b). Restoring monarch butterfly habitat in the Midwestern U.S. “All hands on deck”. Environ. Res. Lett. 12:074005. doi: 10.1088/1748-9326/aa7637
Thogmartin, W. E., Wiederholt, R., Oberhauser, K., Drum, R. G., Diffendorfer, J. E., Altizer, S., et al. (2017a). Monarch butterfly population decline in North America: identifying the threatening processes. R. Soc. Open Sci. 4:170760. doi: 10.1098/rsos.170760
Thomas, H., and Stoddart, J. L. (1980). Leaf senescence. Ann. Rev. Plant Phys. 31, 83–111. doi: 10.1146/annurev.pp.31.060180.000503
Thomas, J. A. (1980). Why did the large blue become extinct in Britain? Oryx 15, 243–247. doi: 10.1017/S0030605300024625
Thomas, J. A., Simcox, D. J., and Clarke, R. T. (2009). Successful conservation of a threatened Maculinea butterfly. Science 325, 80–83. doi: 10.1126/science.1175726
Thomas, J. A., Simcox, D. J., and Hovestadt, T. (2011). Evidence-based conservation of butterflies. J. Insect Conserv. 15, 241–258. doi: 10.1007/s10841-010-9341-z
USDA Economic Research Service (2018). Adoption of Genetically Engineered Crops in the U.S. Avaialble online at: https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us.aspx (accessed March 22, 2019).
Vane-Wright, D. (1993). “The Columbus hypothesis: an explanation for the dramatic 19th century range expansion of the monarch butterfly,” in Biology and Conservation of the Monarch Butterfly, eds S. B. Malcolm and M. P. Zalucki (Los Angeles, CA: Natural History Museum of Los Angeles County), 179–187.
Vidal, O., and Rendón-Salinas, E. (2014). Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol. Conserv. 180, 165–175. doi: 10.1016/j.biocon.2014.09.041
Werling, B. P., Dickson, T. L., Isaacs, R., Gains, H., Gratton, C., Gross, et al. (2014). Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Nat. Acad. Sci. U. S. A. 111, 1652–1657. doi: 10.1073/pnas.1309492111
Werling, B. P., Meehan, T. D., Robertson, B. A., Gratton, C., and Landis, D. A. (2011). Biocontrol potential varies with changes in biofuel-crop plant communities and landscape perenniality. Glob. Change Biol. Bioenergy 3, 347–359. doi: 10.1111/j.1757-1707.2011.01092.x
Keywords: disturbance, predation, monarch butterfly, butterfly conservation, agricultural landscapes
Citation: Haan NL and Landis DA (2019) The Importance of Shifting Disturbance Regimes in Monarch Butterfly Decline and Recovery. Front. Ecol. Evol. 7:191. doi: 10.3389/fevo.2019.00191
Received: 19 December 2018; Accepted: 13 May 2019;
Published: 29 May 2019.
Edited by:
Jay E. Diffendorfer, United States Geological Survey, United StatesReviewed by:
Stephen Baillie Malcolm, Western Michigan University, United StatesCopyright © 2019 Haan and Landis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan L. Haan, aGFhbm5hdGhAbXN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.