
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 15 May 2019
Sec. Agroecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00120
This article is part of the Research Topic Ecosystem Services and Disservices Provided by Plant-Feeding Predatory Arthropods View all 12 articles
Generalist predators bring a complex mix of beneficial and harmful effects to agroecosystems. When these predators feed on herbivorous pests, biological control is improved with the potential to increase crop yield. However, generalists often feed on predators, pollinators, and plants, which might worsen pest outbreaks and reduce fruit set. For example, weaver ants (Oecophylla smaragdina) are major predators of several key, economically-damaging pest insects of tropical fruit and nut crops. Yet the ants also attack other predatory arthropods and important pollinators, while tending trophobiont honeydew producers like mealybugs (Pseudococcidae) that are themselves pests. Finally, ants will supplement their diet with sugars from floral and extrafloral nectaries, a form of plant feeding that presumably carries a physiological cost to the plant. Here, across previously-published experimental studies that compared treatments where ants were present vs. excluded, we summarize the effects of weaver ants on beneficial and pest insects and tree-crop productivity. Our quantitative review revealed nearly ubiquitous benefits of Oecophylla ants for tropical agriculture. Treatments with ants present generally showed lower pest densities and damage from pest insects in the families Coreidae, Miridae, Pentatomidae, and Tephritidae. Pest reduction was seen on cacao (Theobroma cacao), cashew (Anacardium occidentale), and mango (Mangifera indica) trees. The single exception to these pest reductions occurred when ants facilitated the population growth of mealybugs and other honeydew producers. In general, we found that Oecophylla ants provided the valuable ecosystem service of natural pest control to a diversity of tropical tree crops. Despite the potential for the ants to harm other predators or pollinators, evidence for these ecosystem disservices was rare and other beneficial insects co-exist well with this group of ants. Our findings bolster the general finding that ant species that tend herbivores who are not themselves pests can provide broad-reaching benefits to plant productivity. More generally, our findings are consistent with the many cases where non-pest prey bolster densities of polyphagous predators with benefits for biological control despite some degree of plant feeding by the predators.
Biological control agents can be broadly divided into specialists and generalists. Specialists have long been used as successful biological control agents, with part of their effectiveness lying in an ability to quickly reproduce and outnumber the relatively few prey/host species on which they feed (Snyder and Ives, 2001). The disadvantage of these predators' specificity, however, is that they provide little protection against the emergence of pest species other than the relatively narrow range of hosts/prey species that they attack (Symondson et al., 2002). In contrast, generalist predators can combat a suite of pests and this polyphagy allows them to remain in a field as various prey species become more or less common (Offenberg, 2015; Thurman et al., 2017). However, the same diverse feeding habits can lead to harmful attacks on predators and parasitoids (Snyder and Ives, 2001, 2003; Mathews et al., 2011; Ramesh et al., 2016) weakening biological control, or on pollinators (Dukas, 2005; Rodriguez-Girones et al., 2013; Yamasaki et al., 2016), decreasing fruit set (Abdulla et al., 2015, 2017; Anato et al., 2015, 2017). Furthermore, some broadly polyphagous generalists feed on the host plant in addition to arthropod prey (Eubanks and Denno, 1999; Bluthgen and Fiedler, 2002, 2004; Ingegno et al., 2011), and we must consider the potential direct crop damage caused by their plant-feeding.
Arboreal ants (Formicidae) present one class of generalist predators that supplements its diet with plant material. This can occur either when ants feed directly on host plant nectar, or indirectly while tending herbivorous insects that release sugars or other nutrients on which the ants feed (i.e., a trophobiotic relationship is in place) (Hölldobler and Wilson, 1990; Bluthgen and Fiedler, 2002; Bluthgen et al., 2004). An example of this trophobiosis is when aphids (Aphididae) produce a sugary honeydew secretion while feeding on a plant, which the ants can then consume. Stable food resources provided by the plant and/or trophobionts can then support relatively large ant colonies able to effectively antagonize and kill herbivore species that otherwise might damage the plant (Bluthgen and Fiedler, 2002; Davidson et al., 2003). Weaver ants (Oecophylla spp.) are recorded to harvest nectar from their host plant, while also “farming” trophobionts like soft scales (Coccidae) and mealybugs (Pseudococcidae) (Bluthgen and Fiedler, 2002). This is thought to make the ants highly territorial with large resident colonies unlikely to leave a site, and thus likely to heavily impact any herbivores attempting to colonize the host plant (Peng et al., 2002; Offenberg, 2015). In fact, weaver ants are the oldest documented form of biological control with records of Oecophylla smaragdina being conserved for natural pest control in 304 AD China (Way and Khoo, 1992).
Weaver ants have the potential to control agricultural pests across many tropical countries, as Oecophylla smaragdina (Fabricius) is found in Australia, India, and South-East Asia, and Oecophylla longinoda (Latrielle) in Sub-Saharan Africa (Vayssieres et al., 2015; Wetterer, 2017). Oecophylla smaragdina has been recorded to control over 50 pest species in eight different horticultural crops (Peng and Christian, 2004; Offenberg et al., 2013), while in sub-Saharan Africa its congener O. longinoda was observed consuming 48 arthropod species comprising 78.7% of all mango pests (Vayssieres et al., 2015). Previous reviews of Oecophylla spp. as biological control agents suggest that these ants successfully manage pests in some situations (Way and Khoo, 1992; Van Mele, 2008; Offenberg, 2015), with the potential to improve yields, although individual cases suggest harmful effects of the ants on other beneficial arthropods that could counterbalance these benefits (e.g., Delabie, 2001; Bluthgen et al., 2006). In order to determine when ant presence is generally improving crop production in the tropics, we need to carefully consider the relative strengths of these ant's helpful vs. harmful effects.
Here, we conduct a quantitative literature review of the potential for the weaver ants Oecophylla smaragdina and O. longinoda to control a diversity of pests and improve yields across a wide range of tropical tree fruit and nut crops. We first quantify the impact of Oecophylla spp. on crop yield, pest density and damage. We then supplement this analysis with case studies on how Oecophylla spp. feed on their host plant, harvest honeydew producers, and deter some predators, parasitoids and pollinators. Together, this generates a holistic assessment of the costs and benefits of using these ants as biocontrol agents. Insights from the case studies presented here help define the conditions when weaver ants provide ecosystem services vs. disservices. We also seek to provide a blueprint for when other ant species, and generalist biological control agents more broadly, might be expected to improve biological control based on their relationship to their host plant and resident harmful and beneficial arthropods.
We began with a comprehensive literature search using Web of Science and “Oecophylla” as the search term. A total of 356 papers resulted from this search (last conducted March 2018), and all abstracts from these papers were reviewed to find 97 that considered the effects of Oecophylla spp. in agriculture. After reviewing the methods and data of these 97 articles, we identified 34 that (1) experimentally compared ant treatments that included either O. smaragdina or O. longinoda to a no-ant control treatment, and/or (2) reported changes in crop yield (11 of the 34 studies), pest numbers (8 of the 34 studies), and/or pest damage (15 of the 34 studies) under +ant and − ant treatments. These studies came from a range of crop systems with fourteen studies in mango, eight in cashew, four in mahogany, three in cacao, three in citrus, two in coconut palm, only one study each for palm oil and pongamia crops. We had hoped to find data reporting how ant manipulation impacted beneficial insects like predators and pollinators in agroecosystems, but these data were too sparsely studied for analysis. After extracting data from the studies, we then analyzed the pooled results of these papers according to their response variables (crop yield, pest density, or pest damage) by performing sign tests on how frequently ant treatments (ants and no ants) were shown to increase or decrease the aforementioned response variables.
Additional studies located in the original literature search were then reviewed for case studies that investigated how weaver ants interact with their host tree and arthropod community to provide possible mechanistic underpinnings for the patterns seen in the quantitative literature review.
Our quantitative literature review found that the weaver ants O. smaragdina and O. longinoda generally reduced pest density and damage when summed across crop and pest types (Figures 1, 2). Overall, ant presence in trees resulted in higher yields than were seen in trees where ants were experimentally excluded (sign test: 12/13, p < 0.005; Figure 1). There were lower pest densities in 13 out of 16 case studies (sign test: 13/16, p < 0.05; average = −13.94% pests; Figure 2A) and less pest damage in 18 out of 19 case studies (sign test: 18/19, p < 0.0001; average = −10.92%) (Figure 2B). Ants presence correlated with higher yields across five crop types, with average increases of 7.96% in cacao (Theobroma cacao), 55.75% in cashew (Anacardium occidentale), 16.47% in citrus, 49.51% in coconut palm (Cocos nucifera), and 30.37% in mango (Mangifera indica) orchards (Figure 3). Ants reduced pest density and/or damage for herbivores in seven insect families (Chrysomelidae, Coreidae, Curculionidae, Miridae, Pentatomidae, Tephritidae, and Thripidae) (Figures 4A,C). Reductions in pest damage and/or densities were seen in trees with ants compared to those without ants, across all crops for which these data were sufficient to be statistically analyzed, in cacao (Theobroma cacao), cashew (Anacardium occidentale), mango (Mangifera indica), and mahogany (Khaya senegalensis) (Figures 4B,D). These results were also consistent across the two ant species (Supplementary Figure 1).
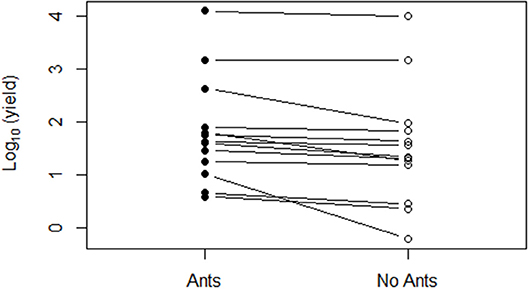
Figure 1. Measurements of the influence of Oecophylla spp. as biological control agents on crop yields (log-transformed) for cacao, cashew, citrus, coconut palm, and mango trees were compiled from 11 studies. Lines compare treatments for each study, indicating how yields increased under ant treatments compared to yields from trees without ants (Vanderplank, 1960; Sporleder and Rapp, 1998; Peng et al., 1999; Ayenor et al., 2007; Dwomoh et al., 2009; Bharti and Silla, 2011; Abdulla et al., 2015, 2017; Anato et al., 2015, 2017; Bisseleua et al., 2017).
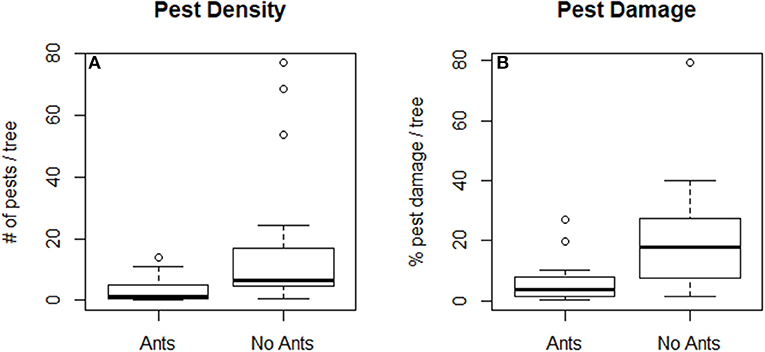
Figure 2. Percentage of pest density (A) and pest damage (B) per tree for several tropical crops for trees with and without Oecophylla spp. ants. Ant treatments had significantly fewer pests and less pest damage than treatments without ants. Outliers are shown as white dots, while the solid black line indicates the median and the box shows the distribution of the dataset compiled from 25 studies (Peng et al., 1995, 1999, 2011, 2012, 2013, 2014; Sporleder and Rapp, 1998; Peng and Christian, 2004, 2005b, 2006, 2007, 2008; Ayenor et al., 2007; Van Mele et al., 2007; Adandonon et al., 2009; Dwomoh et al., 2009; Hosetti and Rudresh, 2012; Olotu et al., 2013; Pierre and Idris, 2013; Abdulla et al., 2015, 2016, 2017; Anato et al., 2015; Diame et al., 2015; Forbes and Northfield, 2017a).
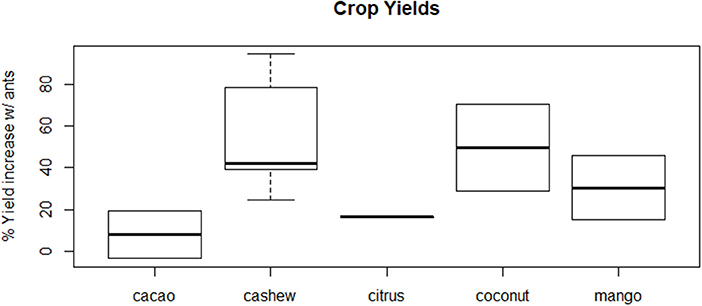
Figure 3. Crop yields were higher when ants (Oecophylla spp.) were present, compared to when they were absent in cacao (Theobroma cacao, average increase = 7.96%; Ayenor et al., 2007; Bisseleua et al., 2017), cashew (Anacardium occidentale, average increase = 55.75%; Dwomoh et al., 2009; Abdulla et al., 2015; Anato et al., 2015, 2017), citrus (average increase = 16.47%; Bharti and Silla, 2011), coconut palm [Cocos nucifera, average increase = 49.51%; (Vanderplank, 1960; Sporleder and Rapp, 1998)], and mango [Mangifera indica, average increase = 30.37%; (Bharti and Silla, 2011; Abdulla et al., 2017)] orchards.
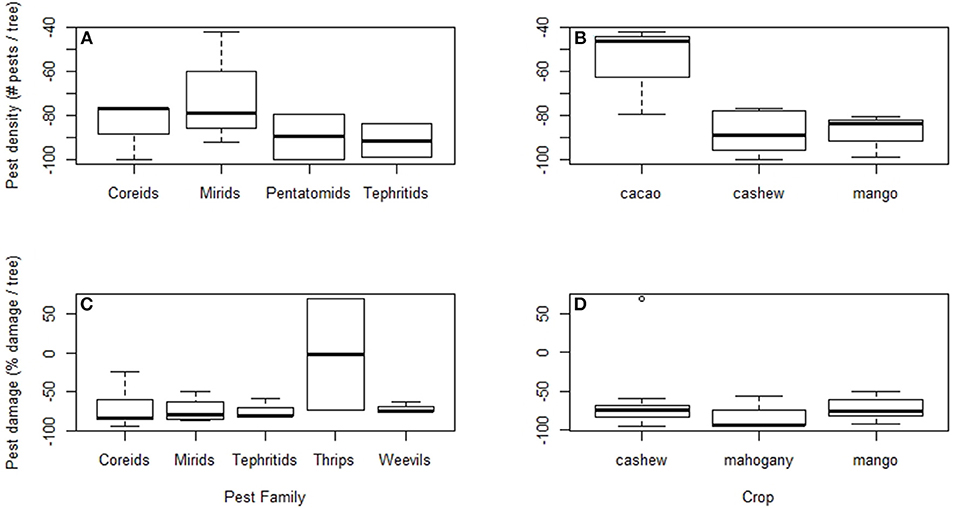
Figure 4. When compared to a control, pest density under ant treatments (Oecophylla spp.) was significantly lower across pest insect families (A) and crop types (B). Similarly, pest damage was significantly reduced under ant treatments across pestiferous insect families (C) and three different crops (D). Reduction in pest damage and density was calculated as a percentage of pest density or damage in ant treatments compared to the control [((Ant-No Ant)/No Ant)*100] (Peng et al., 1995, 2011, 2012, 2014; Sporleder and Rapp, 1998; Peng and Christian, 2006, 2007, 2008; Ayenor et al., 2007; Van Mele et al., 2007; Adandonon et al., 2009; Dwomoh et al., 2009; Abdulla et al., 2015, 2016, 2017; Anato et al., 2015; Diame et al., 2015).
In particular studies, the presence of ants significantly reduced pest densities of leaf-footed bugs (Coreidae; Peng et al., 1999; Dwomoh et al., 2009), mirid bugs (Miridae; Peng et al., 1999; Ayenor et al., 2007; Dwomoh et al., 2009; Forbes and Northfield, 2017a), stink bugs (Pentatomidae; Peng et al., 1999; Hosetti and Rudresh, 2012) and fruit flies (Tephritidae; Van Mele et al., 2007; Adandonon et al., 2009; Migani et al., 2017) (Figure 4A). Particular studies reported reduced pest damage from weevils (Curculionidae; (Peng and Christian, 2007; Peng et al., 2014; Abdulla et al., 2016)), fruit flies (Peng and Christian, 2006; Diame et al., 2015; Abdulla et al., 2017), and mirid bugs (Peng et al., 1995, 2014; Peng and Christian, 2008; Olotu et al., 2013; Abdulla et al., 2015) in the presence of ants (Figure 4C). Most studies focused on heteropteran, weevil, or fruit fly pests, with single studies focusing on density of leaf beetles (Chrysomelidae; average = −46.1%; Forbes and Northfield, 2017a) and thrips (Thripidae; average = −80.74%; Peng and Christian, 2004), and damage by snout moths (Pyralidae; average = −94.86%; Peng et al., 2011, 2014). Interestingly, damage from thrips increased by 68.75% under ant treatments compared to the no ant control treatment in one cashew orchard study (Anato et al., 2015), which contrasts with other cases of reduced thrips density (Peng and Christian, 2004) and damage (Abdulla et al., 2015) by ants in mango and cashew orchards, respectively. In the case where thrips damage increased, overall crop yield increased by as much as 150% under ant treatments compared to the control (Anato et al., 2015), suggesting that increases in pest damage by thrips was still outweighed by overall benefits from Oecophylla spp. being present.
Weaver ants, like many other generalist arthropod predators, fill complex ecological roles in agroecosystems (e.g., Peng et al., 1999, 2014; Van Mele and Cuc, 2000; Peng and Christian, 2004; Pierre and Idris, 2013; Abdulla et al., 2015, 2017; Anato et al., 2017; Forbes and Northfield, 2017a). The ants have the potential to contribute beneficial ecosystem services by feeding on pest insects, but also could provide disservices when they shelter other herbivores with which they form trophobiotic relationships (Offenberg et al., 2013; Forbes and Northfield, 2017a, and/or when the ants prey upon beneficial arthropod predators and pollinators (Gonzalvez et al., 2013; Rodriguez-Girones et al., 2013; Figures 5A,C). Thus, it was perhaps surprising that our quantitative review for studies across studies from Africa, Australia, and Southeast Asia, from a diversity of pest complexes, and a range of tree crop species (Supplementary Table 1) yielded such consistently beneficial effects of Oecyphylla ants (Figures 1–4). These benefits were robust across several pest families and species, the species of tree upon which ants were experimentally manipulated, and which of the two key Oecyphylla species was being considered (Figures 1–4; Supplementary Figure 1). Thus, any concerns about weaver ants sufficiently degrading biological control or pollination to harm crop yields (e.g., Tsuji et al., 2004; Offenberg et al., 2013) appear to be largely rare and unwarranted. Indeed, it appears that any harm weaver ants cause to crops from harvesting homopterans, or deterring predators, parasitoids, and pollinators is outweighed by their benefits in pest reduction and increased yields (Figure 5B). Several lines of evidence suggest that suppression of pests by ants can be as effective as insecticide applications for controlling pests (Figure 5B) (e.g., Peng and Christian, 2005b, 2007, 2008; Dwomoh et al., 2009; Abdulla et al., 2016, 2017), although ants might be most usefully deployed as one aspect of integrated pest management schemes that incorporate a range of tactics (Peng and Christian, 2005a).
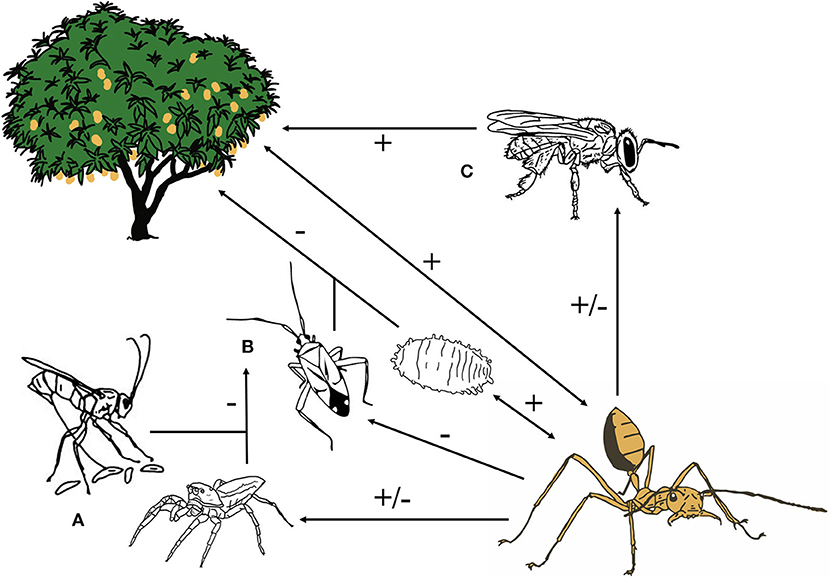
Figure 5. Weaver ants (Oecophylla spp.) interact with predators and parasitoids (A), pests (B), pollinators (C), and a host tree. Overall impacts of Oecophylla spp. on these groups of arthropods and their host tree can be positive, negative, or both where certain groups of predators, parasitoids or pollinators benefit from ant presence, while others are deterred. Information on these interactions was gathered from a global meta-analysis on both O. smaragdina and O. longinoda as biological control agents in mango, cashew, and other tropical crops.
The sole exception to the broader trend of Oecophylla spp. increasing crop yields was found when no profits were gained for a Thai mango orchard after a leafhopper pest, Idioscopus clypealis (Cicadellidae), wiped out the crop (Offenberg et al., 2013). In this study, the weaver ant O. smaragdina was observed protecting and harvesting honeydew from the leafhopper, in effect facilitating the pest's destruction of mango flowers (Offenberg et al., 2013). Additionally, a single study recorded an >150% increase in mealybug (Pseudococcidae) density in an Australian cacao orchard when weaver ants were present (Forbes and Northfield, 2017a. In this case, mealybugs were not considered major cacao pests and their outbreak with ants present was not considered an ecosystem disservice (Forbes and Northfield, 2017a); however, crop yields were not measured and ant-mediated disservices cannot be entirely excluded in this case study. While our quantitative review revealed general benefits for crop yields despite concomitant benefits to some herbivores that the ants tend for honeydew, some caveats are needed. Some honeydew producers can be vectors for plant viruses or other pathogens (e.g., Parrella et al., 2003; Dzahini-Obiatey et al., 2006; Tsai et al., 2010), with the potential to yield infections that harm plants. Because weaver ants tend a wide diversity of potential pathogen vectors this indirect facilitation of plant diseases could be widespread if overlooked; for example, a survey of O. smaragdina in tropical northern Australia revealed that ants were engaged in tropobiotic relationships not only with homopterans such as scale insects (Coccidae, Psuedococcidae, and Margarodidae) and aphids (Aphidae), but also treehoppers (Membracidae), leafhoppers (Cicadellidae), and caterpillars (Lepidoptera: Lycaenidae) (Bluthgen and Fiedler, 2002). Surveys of potential honeydew producers and the diseases they may vector must therefore be closely monitored in weaver ant biocontrol programs.
The literature also includes many instances of weaver ants feeding on predatory insects (e.g., Vayssieres et al., 2015), including deterring predatory mantids (Ramesh et al., 2016), other beneficial ants (Philpott and Armbrecht, 2006), and parasitoid wasps (Mathews et al., 2011; Appiah et al., 2014; Figure 5A). Weaver ants have also been observed deterring and capturing several pollinator species (Tsuji et al., 2004; Gonzalvez et al., 2013; Rodriguez-Girones et al., 2013; Figure 5C). Thus it is perhaps surprising that net ant impacts were overwhelmingly positive for crop yields (Figure 3), rather than ants disrupting biological control through intense intraguild predation (e.g., Snyder and Ives, 2001) or weakening fruit set by deterring pollinators (Tsuji et al., 2004; see also, Huey and Nieh, 2017). Unfortunately, our literature search yielded only a few cases where other predators or pollinators (e.g., Vayssieres et al., 2015) were counted following weaver ant exclusion, preventing us from making a meaningful quantitative analysis of how the ants interact with other arthropod groups. Nonetheless, a careful reading of the literature presents several possible resolutions to this apparent conundrum. First, beneficial insects may represent a relatively small proportion of all prey taken by the ants. For example, in a mango orchard only five of 241 prey species captured by O. longinoda were predators or pollinators (Vayssieres et al., 2015). Second, weaver ants often co-exist with a suite of predators adapted to forage among ants such as the ant-mimicking mantis Euantissa pulchra (Ramesh et al., 2016), jumping spider ant-mimics such as Cosmophasis bitaeniata (Allan and Elgar, 2001), Myrmarachne sp. (Ceccarelli, 2009) and Phintella piatensis (Nelson and Jackson, 2009) and a suite of web-spinning spiders that co-exist with the ants (Forbes and Northfield, 2017b; Figure 5A). A similar situation has been reported for ant-pollinator interactions, where weaver ants were observed capturing and deterring stingless bees (Nomia sp.) on the shrub Melastoma malabathricum but not larger carpenter bees (Xylocopa sp.); because the carpenter bees were the most effective pollinators ants yielded no overall change in pollination efficiency (Gonzalvez et al., 2013; Figure 5C).Altogether then, the apparent potential for widespread negative interactions between ants and beneficial insects may rarely be realized in real world agroecosystems. A few instances have been recorded, however, as parasitism rates of the mealybug Rastrococus iceryoides (Pseudococcidae) decreased 35% (from 86.6 to 51.4%) in the presence vs. absence of tending weaver ants (Tanga et al., 2016). So, it remains possible that there are some cases of severe interference between weaver ants and beneficial insects that remain to be recognized.
While Oecophylla spp. has been observed feeding on floral and extra floral nectaries on their host trees (Rickson and Rickson, 1998; Bluthgen and Fiedler, 2002), along with other plant materials like seeds (Vayssieres et al., 2015), plant resources have never been experimentally manipulated to measure impacts on weaver ant densities and impacts. Nutrient flow from the ants back to their host plant was inferred when Asian weaver ants (O. smaragdina) were fed 15N-labeled glycine and the ant's fecal droplets were absorbed on coffee leaves (Coffea arabica). This in turn led to increased levels of total nitrogen and 15N compared on leaves with than without ants (Pinkalski et al., 2018; see also Pinkalski et al., 2016; Vidkjaer et al., 2016). These direct nutrient exchanges from ants to their host plant are suspected to play a role in improving plant health (Pinkalski et al., 2018). This foliar uptake of ant-provided nutrients has only recently been recorded, but sheds light on the extent of possible mechanisms for nutrient exchange which may support a mutualistic relationship between Oecophylla spp. and a broad range of host trees. In general, there is a need for more work specifically documenting benefits of plant feeding for weaver ants, and vice versa.
In general, ants are abundant, cooperative and polyphagous, two characteristics which emphasize their potential to control pests in agroecosystems around the world (Philpott and Armbrecht, 2006). Red imported fire ants (Solenopsis invicta) were previously recorded to have variable effects on arthropod communities in agroecosystems. However, a study on the relationship between fire ants and the cotton aphid (Aphis gossypii) explained some of this variation as the ants were more likely to forage and deter pests from cotton plants with aphids (Kaplan and Eubanks, 2005). In effect, this ant-homopteran trophobiotic relationship facilitated ant impact on the cotton arthropod community where roughly 27% of herbivore and 54% of predator taxa were adversely effected (Kaplan and Eubanks, 2005). Similarly, Azteca ants harvest scale insects and the ants' presence in coffee plantations has a negative relationship with potential herbivores (Vandermeer et al., 2002). These trophobiotic relationships between ants and honeydew-producing insects may facilitate biological pest suppression (Styrsky and Eubanks, 2007). In a review of ants harvesting trophobionts, this relationship was found to indirectly benefit host plants in the majority of cases as the density of more antagonistic herbivores were reduced (Styrsky and Eubanks, 2007). Interference in pollination from ant-guards also seems relatively rare as plants and their ant guards have evolved different mechanisms to promote plant-ant health. For instance, ant-guards (such as Crematogaster spp.) on Acacia trees are deterred from early flower dehiscence to allow pollinator access (Willmer and Stone, 1997). Additionally, ant guards (Crematogaster spp.) of the myrmecophytic plant Macaranga winkleri are prevented from interfering with pollination as the primary pollinators, thrips (Dolichothrips fialae), produce an ant-repelling acid from their anuses (Yamasaki et al., 2016). Overall, we see ant-plant (Yamasaki et al., 2016) and ant-trophobiont (Kaplan and Eubanks, 2005) nutrient exchanges facilitating ant-defense of plants with novel strategies to avoid conflicts of interest like decreased pollination.
Previous reviews of generalist predators have found that generalists can significantly reduce pest species in agroecosystems (Symondson et al., 2002; Offenberg, 2015). Symondson et al. (2002) found that approximately 75% of case studies showed that pest species were significantly reduced under generalist predator treatments. In order to be successful in biological control programs, generalist predators must maintain a high population density when pest populations decline, be opportunistic in feeding habits in order to maintain that population abundance, and exploit attacks by resurgent pests (Symondson et al., 2002). These characteristics can be found in generalist predators which supplement their diet by feeding on their host plant (Eubanks and Denno, 1999). Big-eyed bugs, Geocoris punctipes (Geocoridae) are omnivorous predators which supplement their predatory diet by feeding on lima bean pods (Eubanks and Denno, 1999). When prey density is low or of poor quality, these predators can be sustained based on the quality of their host plant (Eubanks and Denno, 1999). Overall, host-plant feeding, appears to sustain generalist predators through prey scarcity and improve biological pest suppression.
Ecosystem services are the products of complex interactions and we emphasize the importance of crop, pest, pollinator, and predator context for when these services may be reaped. We also suggest that host-plant feeding, whether directly or indirectly through trophobionts, may be a predictive variable for when generalist predators provide ecosystem services. The classification of host-plant feeding as an ecosystem disservice is also largely context specific as no instances of direct crop damage from generalist predators was found in this review and indirect damage cause by predators via their trophobionts primarily occurs when certain diseases are present (Forbes and Northfield, 2017a). Weaver ants (Oecophylla spp.) have been shown to effectively reduce pest damage and density from a suite of arthropods and increase crop yields around the world. The case-dependency of when their trophobiotic relationships become antagonistic, however, must be further investigated. Further research on the dynamics of nutrient exchanges between ants, trophobionts, and plants, particularly when this nutrient exchange may be critical to pest suppression, remains to be investigated. Additionally, the interactions that Oecophylla spp. have with other beneficial arthropod taxa should be explored for agroecosystems to record the impact of ants on pollination, predation, and parasitism. These insights may then shed light on the context-dependent cases for when ecosystem services from ants and other plant-feeding generalist predators may be most reliably achieved.
JT developed the paper, conducted the quantitative literature review, wrote and revised the body of the text, performed statistical analyses, and created all figures in R and original illustrations using Photoshop. TN helped develop the paper, revised drafts of the manuscript, conducted statistical analyses, and assisted in creating figures in R. WS helped develop the paper and revised drafts of figures and the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
JT was supported by a J. William Fulbright Study/Research Grant during the preparation of this manuscript, and WS was supported by Organic Research and Extension Initiative grant no. 2015-07405 from the USDA National Institute of Food and Agriculture.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00120/full#supplementary-material
Abdulla, N. R., Rwegasira, G. M., Jensen, K. M. V., and Mwatawala, M. W. (2017). Efficacy of African weaver ant, Oecophylla longinoda (Hymenoptera: Formicidae) in reducing losses due to frugivorous fruit flies (Diptera: Tephritidae) in smallholder mango production systems in Eastern Tanzania. Biocontrol Sci. Techn. 27,1205–1219. doi: 10.1080/09583157.2017.1391173
Abdulla, N. R., Rwegasira, G. M., Jensen, K. M. V., Mwatawala, M. W., and Offenberg, J. (2015). Effect of supplementary feeding of Oecophylla longinoda on their abundance and predatory activities against cashew insect pests. Biocontrol Sci. Techn. No. 25, 1333–1345. doi: 10.1080/09583157.2015.1057476
Abdulla, N. R., Rwegasira, G. M., Jensen, K. M. V., Mwatawala, M. W., and Offenberg, J. (2016). Control of mango seed weevils (Sternochetus mangiferae) using the African Weaver Ant (Oecophylla longinoda Latreille) (Hymenoptera: Formicidae). J. Appl. Entomol. 140, 500–506. doi: 10.1111/jen.12260
Adandonon, A., Vayssieres, J. F., Sinzogan, A., and Van Mele, P. (2009). Density of pheromone sources of the weaver ant Oecophylla longinoda affects oviposition behaviour and damage by mango fruit flies (Diptera: Tephritidae). Int. J. Pest. Manage. 55, 285–292. doi: 10.1080/09670870902878418
Allan, R. A., and Elgar, M. A. (2001). Exploitation of the green tree ant, Oecophylla smaragdina, by the salticid spider Cosmophasis bitaeniata. Aust. J. Zool. 49, 129–137. doi: 10.1071/ZO00088
Anato, F. M., Sinzogan, A. A. C., Offenberg, J., Adandonon, A., Wargui, R. B., Deguenon, J. M., et al. (2017). Oecophylla longinoda (Hymenoptera: Formicidae) lead to increased cashew kernel size and kernel quality. J. Econ. Entomol. 110. 1133–1137. doi: 10.1093/jee/tox054
Anato, F. M., Wargui, R. B., Sinzogan, A. A. C., Offenberg, J., Adandonon, A., Vayssieres, J. F., et al. (2015). Reducing losses inflicted by insect pests on cashew, using weaver ants as a biological control agent. Agri. Forest Entomol. 17, 285–291. doi: 10.1111/afe.12105
Appiah, E. F., Ekesi, S., Afreh-Nuamah, K., Obeng-Ofori, D., and Mohamed, S. A. (2014). African weaver ant-produced semiochemicals impact on foraging behavior and parasitism by the Opiine parasitoid, Fopius arisanus on Bactrocera invadens (Diptera: Tephritidae). Biol. Control. 79, 49–57. doi: 10.1016/j.biocontrol.2014.08.004
Ayenor, G. K., Van Huis, A., Obeng-Ofori, D., Padi, B., and Röling, N. G. (2007). Facilitating the use of alternative capsid control methods towards sustainable production of organic cocoa in Ghana. Int. J. Trop. Insect. Sci. 27, 85–94. doi: 10.1017/S1742758407780840
Bharti, H., and Silla, S. (2011). Notes on the life history of Oecophylla smaragdina (Fabricius) and its potential as biological control agent. Halteres 3, 57–64.
Bisseleua, D. H. B., Begoude, D., Tonnang, H., and Vidal, S. (2017). Ant-mediated ecosystem services and disservices on marketable yield in cocoa agroforestry systems. Agric. Ecosyst. Environ. 247, 409–417. doi: 10.1016/j.agee.2017.07.004
Bluthgen, N., and Fiedler, K. (2002). Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an Australian rain forest canopy. J. Anim. Ecol. 71, 793–801. doi: 10.1046/j.1365-2656.2002.00647.x
Bluthgen, N., and Fiedler, K. (2004). Preferences for sugars and amino acids and their conditionality in a diverse nectar-feeding ant community. J. Anim. Ecol. 73, 155–166. doi: 10.1111/j.1365-2656.2004.00789.x
Bluthgen, N., Mezger, D., and Linsenmair, K. E. (2006). Ant-hemipteran trophobioses in a Bornean rainforest –diversity, specificity and monopolization. Insect. Soc. 53, 194–203. doi: 10.1007/s00040-005-0858-1
Bluthgen, N., Stork, N. E., and Fiedler, K. (2004). Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. OIKOS 106, 344–358. doi: 10.1111/j.0030-1299.2004.12687.x
Ceccarelli, F. S. (2009). Ant-mimicking spider, Myrmarachne species (Araneae: Salticidae), distinguishes its model, the green ant, Oecophylla smaragdina, from a sympatric batesian O. smaragdina mimic, Riptortus serripes (Hemiptera: Alydidae). Aust. J. Zool. 57, 305–309. doi: 10.1071/ZO08014
Davidson, D. W., Cook, S. C., Snelling, R. R., and Chua, T. H. (2003). Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972. doi: 10.1126/science.1082074
Delabie, J. H. C. (2001). Trophobiosis between formicidae and hemiptera (Sternorrhyncha and Auchenorrhyncha): an overview. Neotrop. Entomol. 30, 501–516. doi: 10.1590/S1519-566X2001000400001
Diame, L., Grechi, I., Rey, J. Y., Sane, C. A. B., Diatta, P., Vayssieres, J. F., et al. (2015). Influence of Oecophylla longinoda Latreille, 1802 (Hymenoptera: Formicidae) on mango infestation by Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) in relation to Senegalese orchard design and management practices. Afr. Entomol. 23, 294–305. doi: 10.4001/003.023.0207
Dukas, R. (2005). Bumble bee predators reduce pollinator density and plant fitness. Ecology 86, 1401–1406. doi: 10.1890/04-1663
Dwomoh, E. A., Afun, J. V. K., Ackonor, J. B., and Agene, V. N. (2009). Investigations on Oecophylla longinoda (Latreille) (Hymenoptera: Formicidae) as a biocontrol agent in the protection of cashew plantations. Pest. Manag. Sci. 65, 41–46. doi: 10.1002/ps.1642
Dzahini-Obiatey, H., Ameyaw, G. A., and Ollennu, L. A. (2006). Control of cocoa swollen shoot disease by eradicating infected trees in Ghana: a survey of treated and replanted areas. Crop. Prot. 25, 647–652. doi: 10.1016/j.cropro.2005.09.004
Eubanks, M. D., and Denno, R. F. (1999). The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 80, 1253–1266. doi: 10.2307/177072
Forbes, S.J., and Northfield, T. D. (2017b). Increased pollinator habitat enhances cacao fruit set and predator conservation. Ecol. Appl. 27, 887–899. doi: 10.1002/eap.1491
Forbes, S. J., and Northfield, T. D. (2017a). Oecophylla smaragdina ants provide pest control in Australian cacao. Biotropica 49, 328–336. doi: 10.1111/btp.12405
Gonzalvez, F. G., Santamaria, L., Corlett, R. T., and Rodriguez-Girones, M. A. (2013). Flowers attract weaver ants that deter less effective pollinators. J. Ecol. 101, 78–85. doi: 10.1111/1365-2745.12006
Hosetti, B. B., and Rudresh, B. S. (2012). Studies on Oecophylla smaragdina as a bio-control agent against pentatomid bug infesting on Pongamia tree. J. Environ. Biol. 33, 1103–1106.
Huey, S., and Nieh, J. C. (2017). Foraging at a safe distance: crab spider effects on pollinators. Ecol. Entomol. 42, 469–476. doi: 10.1111/een.12406
Ingegno, B. L., Pansa, M. G., and Tavella, L. (2011). Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol. Control. 58, 174–181. doi: 10.1016/j.biocontrol.2011.06.003
Kaplan, I., and Eubanks, M. D. (2005). Aphids alter the community-wide impact of fire ants. Ecology 86, 1640–1649. doi: 10.1890/04-0016
Mathews, C. R., Bottrell, D. G., and Brown, M. W. (2011). Interactions between extrafloral nectaries, ants (Hymenoptera: Formicidae), and other natural enemies affect biological control of Grapolita molesta (Lepidoptera: Tortricidae) on peach (Rosales: Rosaceae). Environ. Entomol. 40, 42–51. doi: 10.1603/EN10161
Migani, V., Ekesi, S., Merkel, K., and Hoffmeister, T. (2017). At Lunch with a killer: the effect of weaver ants on host-parasitoid interactions on mango. PLoS ONE 12:170101. doi: 10.1371/journal.pone.0170101
Nelson, X. J., and Jackson, R. R. (2009). The influence of ants on the mating strategy of a myrmecophilic jumping spider (Araneae, Salticidae). J. Nat. Hist. 43, 713–735. doi: 10.1080/00222930802610469
Offenberg, J. (2015). Ants as tools in sustainable agriculture. J. Appl. Ecol. 52, 1197–1205. doi: 10.1111/1365-2664.12496
Offenberg, J., Cuc, N. T. T., and Wiwatwitaya, D. (2013). The effectiveness of weaver ant (Oecophylla smaragdina) biocontrol in Southeast Asian citrus and mango. Asian Myrmecol. 5, 139–149. doi: 10.20362/am.005015
Olotu, M. I., du Plessis, H., Seguni, Z. S., and Maniania, N. K. (2013). Efficacy of the African weaver ant Oecophylla longinoda (Hymenoptera: Formicidae) in the control of Helopeltis spp. (Hemiptera: Miridae) and Pseudotheraptus wayi (Hemiptera: Coreidae) in cashew crop in Tanzania. Pest Manag. Sci. 69, 911–918. doi: 10.1002/ps.3451
Parrella, G., Gognalons, P., Gebre-Selassie, K., Vovlas, C., and Marchoux, G. (2003). An update of the host range of Tomato Spotted Wilt Virus. J Plant Pathol. 85, 227–264.
Peng, R., Lan, L. P., and Christian, K. (2014). Weaver ant role in cashew orchards in vietnam. J. Econ. Entomol. 107, 1330–1338. doi: 10.1603/EC14039
Peng, R. K., and Christian, K. (2004). The weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), an effective biological control agent of the red-banded thrips, Selenothrips rubrocinctus (Thysanoptera: Thripidae) in mango crops in the Northern Territory of Australia. Int. J. Pest Manage. 50, 107–114. doi: 10.1080/09670870410001658125
Peng, R. K., and Christian, K. (2005a). Integrated pest management in mango orchards in the Northern Territory Australia, using the weaver ant, Oecophylla smaragdina, (Hymenoptera: Formicidae) as a key element. Int. J. Pest Manage. 51, 149–155. doi: 10.1080/09670870500131749
Peng, R. K., and Christian, K. (2005b). The control efficacy of the weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), on the mango leafhopper, Idioscopus nitidulus (Hemiptera: Cicadellidea) in mango orchards in the Northern Territory. Int. J Pest Manage. 51, 297–304. doi: 10.1080/09670870500151689
Peng, R. K., and Christian, K. (2006). Effective control of Jarvis's Fruit Fly, Bactrocera jarvisi (Diptera: Tephritidae), by the weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), in mango orchards in the Northern Territory of Australia. Int. J. Pest Manage. 52, 275–282. doi: 10.1080/09670870600795989
Peng, R. K., and Christian, K. (2007). The effect of the weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae), on the mango seed weevil, Sternochetus mangiferae (Coleoptera: Curculionidae), in mango orchards in the Northern Territory of Australia. Int. J. Pest Manage. 53, 15–24. doi: 10.1080/09670870600968859
Peng, R. K., and Christian, K. (2008). The dimpling bug, Campylomma austrina Malipatil (Hemiptera: Miridae): the damage and its relationship with ants in mango orchards in the Northern Territory of Australia. Int. J. Pest Manage. 54, 173–179. doi: 10.1080/09670870701875243
Peng, R. K., Christian, K., and Gibb, K. (1995). The effect of the Green Ant, Oecophylla smaragdina (Hymenoptera, Formicidae), on insect pests of cashew trees in Australia. B Entomol. Res. 85, 279–284. doi: 10.1017/S0007485300034374
Peng, R. K., Christian, K., and Gibb, K. (1999). The effect of colony isolation of the predacious ant, Oecophylla smaragdina (F.) (Hymenoptera: Formicidae), on protection of cashew plantations from insect pests. Int. J. Pest Manage. 45, 189–194. doi: 10.1080/096708799227789
Peng, R. K., Christian, K., and Gibb, K. (2002). Biological control of Amblypelta spp. (Hemiptera: Coreidae) using Oecophylla smaragdina (Hymenoptera: Formicidae)- progress, prospects and challenges. Acta Hort. 575, 495–502. doi: 10.17660/ActaHortic.2002.575.57
Peng, R. K., Christian, K., and Reilly, D. (2011). The effect of weaver ants Oecophylla smaragdina on the shoot borer Hypsipyla robusta on African mahoganies in Australia. Agric. Forest Entomol. 13, 165–171. doi: 10.1111/j.1461-9563.2010.00514.x
Peng, R. K., Christian, K., and Reilly, D. (2012). Biological control of the fruit-spotting bug Amblypelta lutescens using weaver ants Oecophylla smaragdina on African mahoganies in Australia. Agric. Forest Entomol. 14, 428–433. doi: 10.1111/j.1461-9563.2012.00584.x
Peng, R. K., Christian, K., and Reilly, D. (2013). Using weaver ants Oecophylla smaragdina to control two important pests on African mahogany Khaya senegalensis in the Northern Territory of Australia. Aust. Forestry 76, 76–82. doi: 10.1080/00049158.2013.776938
Philpott, S. M., and Armbrecht, I. (2006). Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol. Entomol. 31, 369–377. doi: 10.1111/j.1365-2311.2006.00793.x
Pierre, E. M., and Idris, A. H. (2013). Studies on the predatory activities of Oecophylla smaragdina (Hymenoptera: Formicidae) on Pteroma pendula (Lepidoptera: Psychidae) in oil palm plantations in Teluk Intan, Perak (Malaysia). Asian Myrmecol. 5, 163–176. doi: 10.20362/am.005017
Pinkalski, C., Damgaard, C., Jensen, K. M. V., Peng, R. K., and Offenberg, J. (2016). Macronutrient exchange between the Asian weaver ant Oecophylla smaragdina and their host plant. Ecosystems 19, 1418–1428. doi: 10.1007/s10021-016-0013-z
Pinkalski, C., Jensen, K. M. V., Damgaard, C., and Offenberg, J. (2018). Foliar uptake of nitrogen from ant faecal droplets: an overlooked service to ant-plants. J. Ecol. 106, 289–295. doi: 10.1111/1365-2745.12841
Ramesh, A., Vijayan, S., Sreedharan, S., Somanathan, H., and Uma, D. (2016). Similar yet different: differential response of a praying mantis to ant-mimicking spiders. Biol. J Linn. Soc. 119, 158–165. doi: 10.1111/bij.12793
Rickson, F. R., and Rickson, M. M. (1998). The cashew nut, Anacardium occidentale (Anacardiaceae), and its perennial association with ants: extrafloral nectary location and the potential for ant defense. Am. J. Bot. 85, 835–849. doi: 10.2307/2446419
Rodriguez-Girones, M. A., Gonzalvez, F. G., Llandres, A. L., Corlett, R. T., and Santamaria, L. (2013). Possible role of weaver ants, Oecophylla smaragdina, in shaping plant-pollinator interactions in South-East Asia. J. Ecol. 101, 1000–1006. doi: 10.1111/1365-2745.12100
Snyder, W. E., and Ives, A. R. (2001). Generalist predators disrupt biological control by a specialist parasitoid. Ecology 82, 705–716. doi: 10.2307/2680190
Snyder, W. E., and Ives, A. R. (2003). Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84, 91–107. doi: 10.1890/0012-9658(2003)084[0091:IBSAGN]2.0.CO;2
Sporleder, M., and Rapp, G. (1998). The effect of Oecophylla longinoda (Latr.) (Hym., Formicidae) on coconut palm productivity with respect to Pseudotheraptus wayi Brown (Hem.,Coreidae) damage in Zanzibar. J Appl. Entomol. 122, 475–481. doi: 10.1111/j.1439-0418.1998.tb01530.x
Styrsky, J. D., and Eubanks, M. D. (2007). Ecological consequences of interactions between ants and honeydew-producing insects. Proc. R. Soc. B. 274, 151–164. doi: 10.1098/rspb.2006.3701
Symondson, W. O., Sunderland, K. D., and Greenstone, M. H. (2002). Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 47, 561–594. doi: 10.1146/annurev.ento.47.091201.145240
Tanga, C. M., Ekesi, S., Govender, P., Nderitu, P. W., and Mohamed, S. A. (2016). Antagonistic interactions between the African weaver ant Oecophylla longinoda and the parasitoid Anagyrus pseudococci potentially limits suppression of the invasive mealybug Rastrococcus iceryoides. Insects 7:010001. doi: 10.3390/insects7010001
Thurman, J. H., Crowder, D. W., and Northfield, T. D. (2017). Biological control agents in the Anthropocene: current risks and future options. Curr. Opin. Insect Sci. 23, 59–64. doi: 10.1016/j.cois.2017.07.006
Tsai, C. W., Rowhani, A., Golino, D. A., Daane, K. M., and Almeida, R. P. (2010). Mealybug transmission of grapevine leafroll viruses: an analysis of virus–vector specificity. Phytopathology 100, 830–834. doi: 10.1094/PHYTO-100-8-0830
Tsuji, K., Hasyim, A., and Harlion, Nakamura, K. (2004). Asian weaver ants, Oecophylla smaragdina, and their repelling of pollinators. Ecol. Res. 19, 669–673. doi: 10.1111/j.1440-1703.2004.00682.x
Van Mele, P. (2008). A historical review of research on the weaver ant Oecophylla in biological control. Agric. Forest Entomol. 10, 13–22. doi: 10.1111/j.1461-9563.2007.00350.x
Van Mele, P., and Cuc, N. T. T. (2000). Evolution and status of Oecophylla smaragdina (Fabricius) as a pest control agent in citrus in the Mekong Delta, Vietnam. Int. J. Pest Manage. 46, 295–301. doi: 10.1080/09670870050206073
Van Mele, P., Vayssieres, J. F., Van Tellingen, E., and Vrolijks, J. (2007). Effects of an African weaver ant, Oecophylla longinoda, in controlling mango fruit flies (Diptera: Tephritidae) in Benin. J. Econ. Entomol. 100, 695–701. doi: 10.1603/0022-0493(2007)100[695:EOAAWA]2.0.CO;2
Vandermeer, J., Perfecto, I., Nunez, G. I., Phillpott, S., and Ballinas, A. G. (2002). Ants (Azteca sp.) as potential biological control agents in shade coffee production in Chiapas, Mexcio. Agroforest. Syst. 56, 271–276. doi: 10.1023/A:1021328820123
Vanderplank, F. L. (1960). The bionomics and ecology of the Red Tree Ant, Oecophylla sp., and its relationship to the coconut bug Pseudotheraptus wayi Brown (Coreidae). J. Anim. Ecol. 29, 15–33. doi: 10.2307/2268
Vayssieres, J. F., Ouagoussounon, I., Adandonon, A., Sinzogan, A., Korie, S., Todjihounde, R., et al. (2015). Seasonal pattern in food gathering of the weaver ant Oecophylla longinoda (Hymenoptera: Formicidae) in mango orchards in Benin. Biocontrol Sci. Techn. 25, 1395–1387. doi: 10.1080/09583157.2015.1048425
Vidkjaer, N. H., Wollenweber, B., Jensen, K. M. V., Ambus, P. L., Offenberg, J., and Fomsgaard, I.S. (2016). Urea in weaver ant feces: quantification and investigation of the uptake and translocation of urea in Coffea arabica. J. Plant. Growth Regul. 35, 803–814. doi: 10.1007/s00344-016-9586-1
Way, M. J., and Khoo, K. C. (1992). Role of ants in pest-management. Annu. Rev. Entomol. 37, 479–503. doi: 10.1146/annurev.en.37.010192.002403
Wetterer, J. K. (2017). Geographic distribution of the weaver ant Oecophylla smaragdina. Asian. Myrmecol. 9:e009004. doi: 10.20362/am.009004
Willmer, P.G., and Stone, G.N. (1997). How aggressive ant-guards assist seed-set in Acacia flowers. Nature 388, 165–167.
Keywords: Oecophylla, biocontrol, yield, generalists, predators, agroecosystems, ants
Citation: Thurman JH, Northfield TD and Snyder WE (2019) Weaver Ants Provide Ecosystem Services to Tropical Tree Crops. Front. Ecol. Evol. 7:120. doi: 10.3389/fevo.2019.00120
Received: 09 August 2018; Accepted: 25 March 2019;
Published: 15 May 2019.
Edited by:
Felix Wackers, Biobest, BelgiumReviewed by:
Neelendra K. Joshi, University of Arkansas, United StatesCopyright © 2019 Thurman, Northfield and Snyder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessa H. Thurman, ai50aHVybWFuQHVxLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.