
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 29 November 2018
Sec. Chemical Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00204
This article is part of the Research Topic Plant Secondary Metabolites in the Fight against Arthropod Pests and Parasites View all 9 articles
 Epameinondas Evergetis1
Epameinondas Evergetis1 Romeo Bellini2
Romeo Bellini2 George Balatsos3
George Balatsos3 Antonios Michaelakis3*
Antonios Michaelakis3* Marco Carrieri2
Marco Carrieri2 Rodolfo Veronesi2
Rodolfo Veronesi2 Dimitrios P. Papachristos3
Dimitrios P. Papachristos3 Arianna Puggioli2
Arianna Puggioli2 Vassiliki-Nafsika Kapsaski-Kanelli1
Vassiliki-Nafsika Kapsaski-Kanelli1 Serkos A. Haroutounian1*
Serkos A. Haroutounian1*Contemporary legislation tends to increase limitation on the use of all synthetic pesticides, promoting bio-pesticides as a safer alternative. Bio-prospecting efforts for bio-pesticides provide results, which rarely reach the industry. Present essay elaborates on our efforts to chart the path from the laboratory bench to field assessment. Eight Mediterranean wild gathered foods provided the essential oils that were assessed as mosquito control agents against the Asian tiger mosquito (Aedes albopictus). Three Lamiaceae essential oils, derived from Satureja thymbra, Origanum onites, and Thymbra spicata presented carvacrol as principal component. All exhibited DEET-like repellent performance and total larvae mortality defining the carvacrol rich essential oil (CREO) as a promising mosquito control agent. A commercial variety of Origanum vulgare ssp. hirtum, was selected as CREO source and subjected to dose-response and eco-toxicity studies. We have found significant larvicidal (LC90 of 58,747 mg/L), and repellent (0.2 μL/cm2) properties, but also severe toxicity (LC90 of 12,806 mg/L) against Macrocyclops albidus. This last figure was the limit for the larvicidal field assessment; while for the repellent evaluation was used double the minimum indication (0.4 μL/cm2). CREO was tested per se as larvicidal agent, and emulsified for both repellent and larvicidal field activity. The emulsified CREO's spatial repellent assessment showed maximum efficacy of 86% in day 1 that gradually declined in the following 2 days (81%, 69%). Both emulsified and crude CREO proved to be efficient larvicidal agents, with crude CREO (3 weeks) overrunning slightly the emulsified (2 weeks) in terms of endurance. Conclusively, CREO in its emulsified form may be considered as a promising mosquito larvicidal and repellent agent, applicable in both precautionary and emergency response measures.
Bio-pesticides emerge as a viable alternative for insect control because of their conformity with organic agriculture limitations and the consumers' trend for natural solutions. Recently published data reveal an average decrease by 78% of insects during the last 24 years (Vogel, 2017). This decline is mainly attributed to the extensive use of pesticides, applied both for agricultural and public health purposes (Vogel, 2017). The impacts from insects decline may be enhanced by the on-going climate change (Pires et al., 2018), and globalization that facilitates the species migration. Among these impacts, our work focused on the expansion of alien invasive species, which translates to significant pressures on public health and biodiversity conservation. Among the numerous invasive invertebrates, mosquitoes have been identified as a major public health threat.
A recent review (Sands et al., 2016) on viral infection outbreaks and pandemics indicated that from the 12 events accounted during the twenty-first century five involved mosquitoes as vectors of Zika and Chikungunya viruses. This fact has pinned mosquito control as a primary target of the World's Health Organization (WHO) in order to mitigate future infectious diseases outbreaks (WHO, 2016). The majority of the first response measures, currently applied in mosquito control, implicate the use of insecticides in combination with personal protection means (Loh and Yap, 1989; Kroeger et al., 2006). The utilization of synthetic insecticides is facing severe restriction because of their adverse effects and resistance development (Morrison et al., 2008). In this context, several alternative mosquito control means have been developed, including the utilization of bacteria such as Bacillus thuringiensis israelensis, Lysinibacillus (Bacillus) sphaericus or sterile male mosquitoes (Lees et al., 2015; Zhang et al., 2015). Most of these methods are oriented to function as preventive and not first response measures. Viral infections outbreak though, do occur and demand the development of effective mosquito control agents for first response application.
Bio-prospecting for natural biocides indicates that Essential Oil (EO) constitute a promising source of bio-pesticides. The consideration of EO as mosquito control agents is advocated through conformity with consumers' demands, but most importantly through their environmental performance. EOs are biodegradable, exhibiting limited ecosystem persistence and bioaccumulation. In addition, EOs have been co-evolved with plant pollinators, therefore encompassing a compatibility that minimizes their adverse effects. As a result EOs have been utilized as active ingredients in commercial products. The EOs of citronella (Kongkaew et al., 2011) and tea tree (Ramadass and Thiagarajan, 2015) are used in repellents directly applicable on human skin, for the production of protective garments and nets (Enayati and Hemingway, 2010), as open space repellents in aromatic candles (Müller et al., 2009), and surface cover sprays (WHO, 2006). Surprisingly, the equally documented larvicidal properties of EOs (Evergetis et al., 2012) remain commercially unexploited. An explanation for this discrepancy is provided by a critical review on mosquito control research (Chellappandian et al., 2018) that proposed the lack of field assessment results as the main handicap for the larvicidal EOs uptake.
The aforementioned constraints and limitations on the development of bio-pesticides were placed in the core of the herein presented study. Between bio-prospecting and field assessment we introduced an intermediate stage aiming to facilitate the up-scaling of the laboratory experiments. The inclusion of this intermediate stage employed three main tasks targeting; the delineation of eco-toxicity; the definitions of the optimum concentration, and formulation. This prioritization enabled resolution of conflicts relating to the selection of the field assessment parcels, and the expected environmental impacts of the bio-pesticides. In this context we present herein field assessment results for mosquito larvicidal and/or repellent agents, which are prerequisites for inclusion in WHO's toolbox against infectious diseases (WHO, 2005). The integrated approach we propose may be perceived as a handy, from cradle to market, roadmap for the development of bio-pesticides. It must be noted, though, that the institutional structure of the team was the most crucial factor for the flawless implementation of the experiments. Specifically, the inclusion of academic, research, and local authorities facilitated the study's progress, enabling the wise selection of experimental parcels, and prompt implementation of field experiments, within the urban grid of Bologna, Italy.
The initial biodiversity sampling was performed through the selection of the appropriate culinary herbs. From 2012 to 2014 two Greek indigenous aromatic herbs and six shrubs were collected from various locations and habitats of Greece: Vitex agnus-castus L. (common: Monk's Pepper), Ruta chalepensis L. (common: Fringed Rue), Origanum onites L. (common: Rígani), Foeniculum vulgare Mill. (common: Fennel), Satureja thymbra L. (common: Savory), Echinophora tenuifolia ssp. sibthorpiana (Guss.) Tutin (common: Cortuk), Salvia fruticosa Mill. (common: Greek Sage), and Thymbra capitata (L.) Cav. (common: Conehead Thyme). Collection details of all herbal taxa are included in Table 1. All taxa were identified through Flora Europaea (Tutin et al., 2013) taxonomical keys and the resulting nomenclature was updated through (The Plant List, 2013). A voucher specimen for each plant sampled is deposited in the herbarium of the Agricultural University of Athens, Athens, Greece.
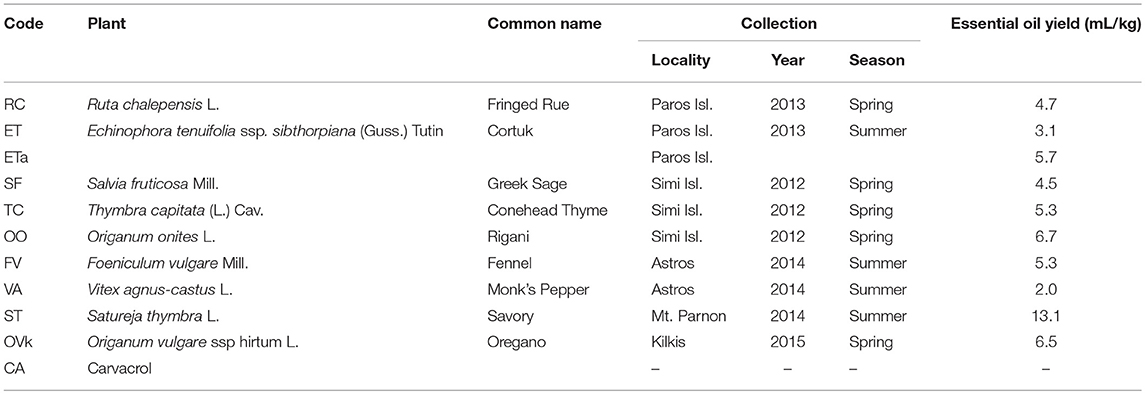
Table 1. Bio prospected taxa; collection location and dates; and essential Oil yield of the herbal material.
All EOs were obtained by hydro-distillation using a modified Clevenger apparatus, according to previously described procedure (Evergetis et al., 2016). In addition the EO from Echinophora tenuifolia ssp. sibthorpiana (Guss.) Tutin, was obtained by acidic hydro-distillation (ETa). The isolation yields of all EOs are included in Table 1. The chemical composition of EOs was determined on a gas chromatographer (GC) coupled to a mass spectrometer (MS) in accordance with a previously described method (Evergetis et al., 2016). Mass spectra were compared with NIST 11 and Willey 275 databases and authentic samples where available.
The adult females of Aedes albopictus used in the bioassays were obtained from a colony maintained in the Benaki Phytopathological Institute (Kifissia, Greece), according to previously described protocols (Giatropoulos et al., 2012).
The assessments of the repellent properties were based on the human landing counts explicitly described in previous studies (Giatropoulos et al., 2012; Govere and Durrheim, 2015). The larval mortality bioassays were carried out according to a modified version of the test method for larval susceptibility recommended by WHO (2005) and also explained in previous studies (Giatropoulos et al., 2012; Govere and Durrheim, 2015).
After the preliminary evaluation, in laboratory scale, of the abovementioned taxa a targeted sampling was performed during 2015 for the determination of a commonly available taxon capable to produce CREO. For this purpose, a commercial variety of the most active EO (Origanum) was selected, namely the Origanum vulgare ssp hirtum. The latter was obtained from Ecopharm Hellas S.A., Greece and produced the oregano EO (OVk) that was used for the performance of dose response, ecotoxicity experiments and field tests. Analytical grade carvacrol (96%) and TWEEN® 20 (97%) were obtained from Sigma Aldrich (Steinheim, Germany). The first was utilized as a reference standard during the second set of bioassays, and the second as emulsifying agent in the field assessments. DEET (N,N-Diethyl-3-methylbenzamide) and dimethyl sulfoxide (DMSO) were also purchased from Sigma-Aldrich.
The adults of Macrocyclops albidus (Copepoda, Cyclopidae) used for the laboratory mortality bioassays were obtained from the rearing unit of the Laboratory of the Medical and Veterinary Entomology Department, Center Agriculture and Environment “G. Nicoli” (Veronesi et al., 2015).
The mortality bioassays against the non-target species M. albidus were carried out in accordance with a modified version of the recommended by WHO test method for larval susceptibility (WHO, 2005). Specifically, for each of the two tested products (oregano EO and carvacrol), different concentrations were tested (solutions of 1, 4, 16, and 64 mg/L) using clean 330 mL glasses filled with 100 mL of each solution. For each dose four replications were tested with 20 adult individuals in each glass. To reduce cannibalism copepods were fed ad libitum with young Ae. albopictus larvae until the day of treatment. The solutions were prepared by transferring the corresponding amounts of each compound to a vial and the solvent was removed. Stock solutions of oregano EO and carvacrol were prepared by using DMSO. Laboratory procedure was adopted to guarantee homogeneous 1% DMSO in the final glasses together with the EOs tested doses. Four glasses, each with 20 copepods were also settled as control with 1% aqueous solution of DMSO. In total 16 glasses with 320 copepods were set up for each testing products while four glasses with 80 copepods were used as a control. The glasses were randomly placed in a climatic chamber maintained at 28 ± 1°C on a 14:10 L:D cycle. At 24 h post-treatment the glasses were examined by visual observations for copepod mortality. In case the mortality registered in the control resulted values >0.0%, the bioassay was repeated.
The field trial was performed in two highly vegetated urban areas, each occupying 50 × 15 m space of a public garden of Bologna, during the second week of July when the Ae. albopictus population achieves its peak of density. The day two and one before the treatment human landing collection (HLC) were conducted by four expert operators using manual aspirators, during the maximum female activity (about 5–7 PM). HLC sessions of 30 min each were implemented within the treated and the control areas. Operators were rotated every day to avoid possible bias. Collected mosquitoes were released back after each session. The tested product was distributed taking care to spray homogeneously both grass and vegetation up to 3 m height, covering completely the test area of 50 × 10 m. The respective control area was sprayed with the same volume of water solution containing only 5% of TWEEN® 20. The treatment was performed on July 12, 2017 at 6.00 PM. Area A was sprayed using a manual rotator Volpi (Casalromano, Mantova, Italy), with 10 L of solution containing 5% TWEEN® 20 and 4% oregano EO, whereas area B was sprayed with the same volume of water solution containing 5% TWEEN® 20.
The trial was conducted in the urban area of Crevalcore (Italy), during the period July 13– August 3, by randomly selecting 15 catch basins colonized by mosquito larvae, from which 5 were treated with 10 ml oregano EO 100%; 5 with 10 mL oregano EO 100% + 10 mL TWEEN®20, diluted in 40 mL water; and 5 left untreated as a control. The mosquito larvae sampling was conducted using an aquarium water net, immediately before the treatment, 48 h post-treatment and 7, 14, 21 days post-treatment. Larvae and pupae were counted in respect their species and age. This test tried to measure the direct larvicidal activity along with the possible repellency on ovipositing females. During each sampling were recorded: time of the day, water and air temperature, and rainfall during the trial.
Data concerning the adult repellency (mosquito landings) and larval toxicity (percentage larval mortality) for the essential oils of each plant were analyzed using Kruskal-Wallis test. When significant differences were detected, Mann-Whitney U-test were carried out for pair-wise comparison (SPSS 21.0). Mosquito and non-target dose response bioassays mortality data were analyzed by probit regression analysis using POLO-PC (LeOra Software POLO-PC, Berkeley, CA, U.S.A.) and SPSS 21.0.
Repellent efficacy was calculated using the formula of Mulla et al. (1971), which takes into account natural changes in the mosquito populations in both treated and untreated areas:
Where C1 is the number of adults collected in the untreated area during pre-treatment; C2 the number of females collected in the untreated area during post-treatment; T1 the mean female density in the treated area during pre-treatment; and T2 the mean female density in the treated area during post-treatment. For the mosquito larvicidal and repellency efficacy in catch basins, the average of larval density was analyzed by ANOVA. The Duncan Test was used as the post ad hoc comparison to identify differences between pairs.
In total 52 phytochemicals were identified, accounting from 94 to 99 % of the 9 EOs studied, and are presented in Table 2, along with the respective percentage of occurrence in each EO. Thirteen phytochemicals exceeded the 10% margin and were considered as major compounds. Among them are included two cyclic monoterpenes: α-phellandrene, and γ-terpinene; Three bicyclic monoterpenes: sabinene, β-pinene, and δ-3-carene; eight oxygenated molecules: the monoterpenes eucalyptol, D-fenchone, 2-nonanone, and 2-undecanone; and the aromatic compounds estragole, carvacrol, p-cymene, and methyl eugenol. These results agree with previous studies regarding the investigated taxa EOs composition. Thus, Fringed Rue is known to contain as major compounds the aliphatic ketones 2-nonanone, and 2-undecanone (Perestrelo et al., 2016); Monk's Pepper has been reported to contain eucalyptol as major compound (Senatore et al., 1996); Conehead Thyme, Rigani, and Savory have been found to contain carvacrol as major compound (Economou et al., 2014); Fennel to contain estragole and D-fenchone as prominent phytochemicals (Rather et al., 2016); Greek Sage to contain β-pinene and eucalyptol as prevailing compounds (Ali et al., 2013); Cortuk to contain methyl eugenol, α-phellandrene, and p-cymene as leading compounds (Baser et al., 1994).
The repellent assessment indicates that four EOs, derived from Greek Sage, Conehead Thyme, Rigani and Savory, showed an efficacy similar to DEET through the achievement of zero landings over 5 min of exposure in the bioassay's course. Another EO derived from Cortuk's conventional distillation presented only 0.13 landings, whereas Cortuk's acidic distillation and Monk's Pepper EOs were also considered highly efficient repellents since their application concluded with 0.88 and 1.25 landings over 5 min of exposure. Finally, Fennel and Fringed Rue EOs presented high to moderate repellence indicated by the respective numbers of 3.5 and 11.13 landings. The repellent bioassay results are presented cumulatively in Table 3.
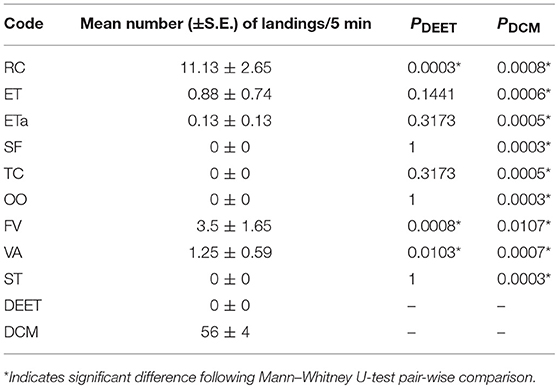
Table 3. Mean number (±S.E.) of Aedes albopictus landings on the uncovered area of the glove in 5 min and comparison with the standard DEET and the control (dichloromethane, DCM) after using different essential oils.
Among the eight taxa assessed for their EOs repellent potentials, Conehead Thyme and Cortuk had never been evaluated before against any insect species. Also, limited results not referring to the target organisms of the present study were noticed for Savory, and Greek Sage repellent activity. Rigani EO presented only one previous assessment as Cx. pipiens repellent that matched present results (Carroll et al., 2017). Finally, Fringed Rue, Monk's Pepper and Fennel EOs, have been extensively studied as repellents against numerous mosquito species, including at least one Aedes species. Fennel EO has been recorded as an efficient repellent against Cx. pipiens (Traboulsi et al., 2005), but has been found of moderate repellence against Ae. aegypti (Kim et al., 2002; Choochote et al., 2007). Monk's Pepper EO was also evaluated as a repellent against Ae. aegypti (Semmler et al., 2009, 2014), and Cx. quinquefasciatus (Semmler et al., 2014) being found in both cases of insignificant efficacy. Fringed Rue EO is the only one tested as a repellent against Ae. albopictus (Ali et al., 2013; Conti et al., 2013), and in both cases, it was found efficient.
Based on our previous studies (Giatropoulos et al., 2012) a discriminate dose of 29 mg/L solution of the material to test was employed for our screening bioassays. The mortality rates of the essential oils tested were arbitrarily classified to “low,” “moderate,” and “very good” for those ranging between 0–50%, 50–80%, and 80–100%, respectively. The EOs larvicidal screening revealed that Conehead Thyme and Rigani were able to achieve 100% Ae. albopictus larval mortality. Along with these two EOs may be categorized also the one retrieved from Savory, which exhibited a larval mortality rate of 96%. The second group of EOs, which presented high toxicity for mosquito larvae, included Fennel with 80% mortality rate, Fringed Rue with 75%, and Cortuk through acidic distillation with 66%. Finally, a third group of EOs, retrieved from Cortuk (conventional distillation), Monk's Pepper, and Greek Sage exhibited moderate to minimal activity with respective mortality rates 44, 14, and 2%. The larvicidal bioassay results are presented cumulatively in Table 4. Cortuk and Conehead Thyme EOs had never been assessed before with respect to arthropod related bioactivities. Conehead Thyme EO biocide potentials are argued through its fungicide and nematicide properties (Saoud et al., 2010), antiparasitic (Machado et al., 2010), and antibacterial (Karampoula et al., 2016) activities, but mostly through its lipoxygenase and acetylcholinesterase inhibition evidence (Carrasco et al., 2016). Greek Sage, Rigani, Monk's Pepper, and Savory EOs present larvicidal activity against various arthropods, but not against Aedes sp. mosquitoes. Specifically Greek Sage has displayed moderate to low activity against Cx. pipiens (Koliopoulos et al., 2010). Rigani EO bioactivities were reviewed by Tepe (Tepe et al., 2016), whom recorded its potentials as Cx. pipiens larvicide. Monk's Pepper was also tested and found of low efficacy against Cx. pipiens larvae (Cetin et al., 2011). A special reference is reserved for Savory EO as control agent against Cx. pipiens biotype molestus (Michaelakis et al., 2007), and Anopheles gambiae (Dellagli et al., 2012).
Fennel and Fringed Rue EOs have resulted efficient larvicidals against Ae. albopictus; Conti et al. (2010), reported a higher toxicity for Fennel EO than that reported here and this may be explained by the EO's different composition. Benelli et al. (2014) in a comparative test of 10 EOs confirmed the herein detected activity of both Fennel and Rue indicating that the last outperformed all other EOs a result consistent with previous dedicated studies of Fringed Rue EO against both the Ae. albopictus (Conti et al., 2013), and Ae. aegypti (Ali et al., 2013).
The repellent efficacies of the carvacrol and Oregano EO are shown in Figures 1, 2 highlighting both as very efficient repellents with activities comparable to DEET. At “high” dose (2 μL/cm2) EOs' repellent activity was “like-DEET” and when tested at “low” dose (1 μL/cm2) the activity was moderate (>20 landings). On the contrary, carvacrol resulted in a 100% repellent (zero landings) in a dose almost 10-fold-lower to DETT (0,026 μL/cm2) (Figure 2).
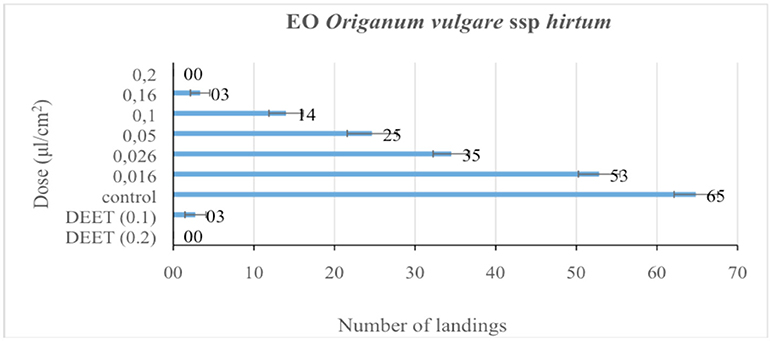
Figure 1. Repellent activity of the oregano EO as mean number of landings (±SE) determined under laboratory conditions. Doses are expressed as μL/cm. DEET was used as the positive control in two doses, 0.1 and 0.2 μL/cm for 5 min. (Means in a column followed by the same letter are not significantly different (P ≥ 0.05), Mann–Whitney U-test).
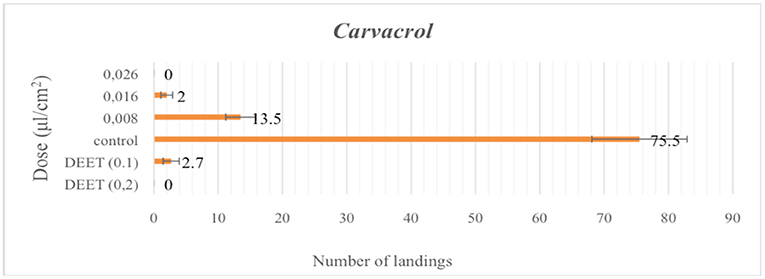
Figure 2. Repellent activity of carvacrol as mean number of landings (±SE) determined under laboratory conditions. Doses are expressed as μL/cm. DEET was used as the positive control in two doses, 0.1 and 0.2 μlcm−2 for 5 min. (Means in a column followed by the same letter are not significantly different (P ≥ 0.05), Mann–Whitney U-test).
These results are in accordance with previous studies establishing the high level of protection exhibited by Carvacrol against Ae. Albopictus, in 5-fold-lower doses to DEET (Giatropoulos et al., 2018) and An. Gambiae (Kröber et al., 2018). Their respective larvicidal bioassays results are summarized in Table 5, verifying that the specific EO displays an average LC90 value of 58.74 (51.60–70.53) mg/L, while the corresponding average LC90 value for pure Carvacrol is 31.08 (25.29–43.15) mg/L.
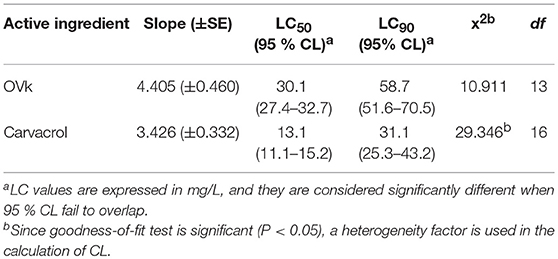
Table 5. LC50 and LC90 values for Origanum vulgare ssp hirtum EO (OVk) and its major component, carvacrol, against third to fourth instar larvae of Ae. albopictus.
The toxicities of Oregano EO and Carvacrol were also determined against the non-target aquatic species Macrocyclops albidus. This species is widely present in European natural water bodies and recently was proposed as a possible biocontrol agent against Ae. albopictus in artificial breeding sites (Mulla et al., 1971). Results indicated (Table 6) that M. albidus adults are highly sensitive to both investigated products, displaying lower LC90 values as compared to those observed for Ae. albopictus. These results clearly indicate that any use of these two agents as larvicidal is not feasible when predator's biocontrol programs are implemented.
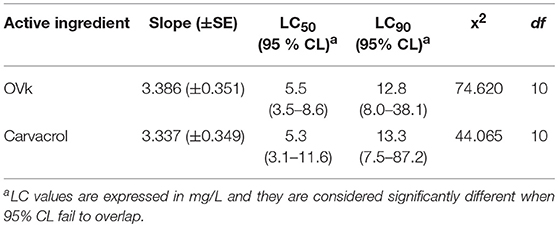
Table 6. LC50 and LC90 values for Origanum vulgare ssp hirtum EO (OVk) and the its major component, carvacrol, against Macrocyclops albidus.
The spatial repellent efficacy of the oregano EO in the form of water-based solution sprayed in a green urban area with high density of Ae. albopictus was also evaluated. In this respect, the capacity of the product to divert Ae. albopictus females out of the treated area was measured at day 1 and 2 up to day 3 after treatment. No adverse effects on the vegetation were observed.
The repellent efficacy measured just after treatment (day 0) was very low (% reduction number female collected in the treated area compared to the control area was 32.5%) and increased to 86.4 % and 81.25 % at day 1 and 2 post-treatment respectively. At day 3 post-treatment the reduction of females was determined as 69.67% (Table 7). The spatial repellency strategy to protect specific target areas from invasive mosquitoes has been recently proposed (Alten et al., 2003; Dame et al., 2014) as a possible mosquito control technology. Due to environmental concern, the spatial repellency approach can be accepted only in cases that products with completely safe profile, such as EOs, will be available.
Finally, a field test was performed in order to evaluate the potency of the oregano EO as both larvicidal and repellent against mosquitoes. The field trial was implemented during the period July 13–August 3 in catch basins, which constitute one of their most common breeding sites and therefore are regularly submitted to larval control. Application of the EO in breeding sites probably results in the simultaneous appearance of the following two main effects: the direct toxicity of the product against the breeding larvae and the repellent activity on the egg-laying females. These two effects cannot be distinguished in our field assessment and are therefore cumulated in the observed data (Figure 3). During the field trials period, the water temperature inside the catch basins was 30.1 ± 1.4°C (minimum 27°C and maximum 33°C), and along the trial period, one rainy event (15.2 mm) was registered at the Sant' Agata Bolognese weather station located 1.7 Km from the study area.
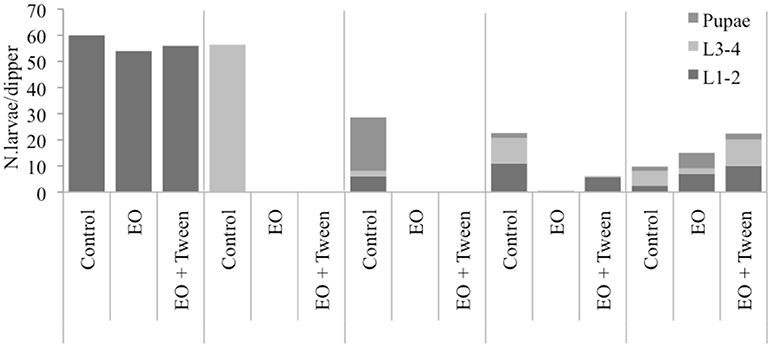
Figure 3. Pre-imaginal mosquito population as sampled in experimental catch basins (Culex pipiens + Aedes albopictus, 22 post treatment days) in Crevalcore, Italy.
In the treated catch basins, the larval mortality (Cx. pipiens & Ae. albopictus) was 100% at days 2 and 7 post-treatment for both products tested (Table 8). At day 14 of post-treatment, the observed mortality was still 100% against Cx. pipiens, for those treated with the oregano EO per se but some Cx. pipiens larvae were detected in the catch basins treated with the emulsified EO. In respect the presence of Ae. albopictus, the presence of some larvae was detected the same day (14 post-treatment) in catch basins treated with both crude and emulsified EO but mortality was still high (Table 8). Finally, at day 21 of post-treatment no significant differences were observed between treated and control catch basins.
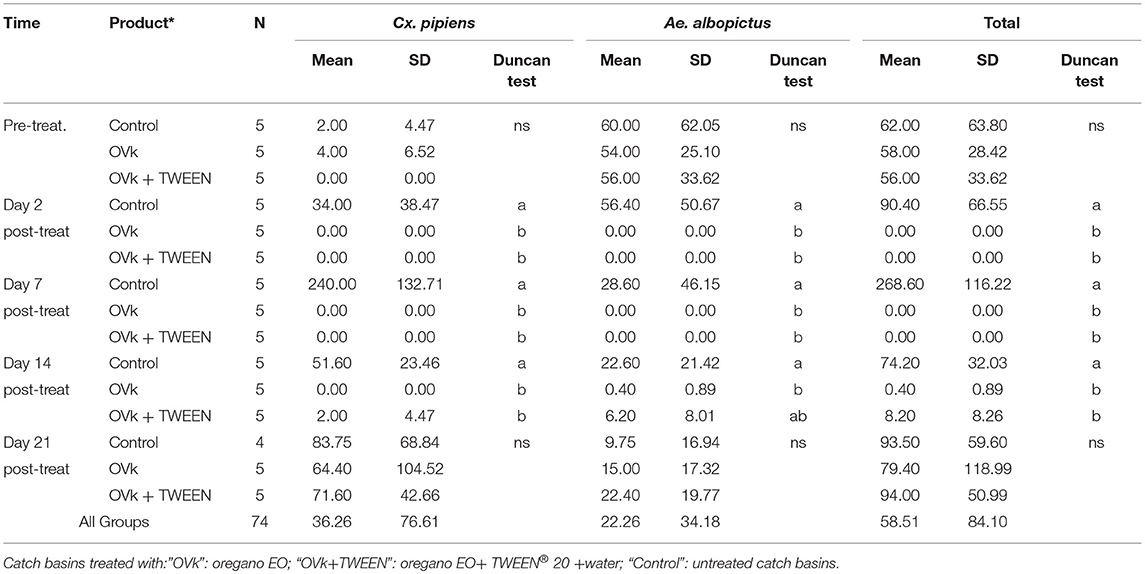
Table 8. Mean number of Culex pipiens and Aedes albopictus larvae (L1-L2-L3-L4) and pupae collected per aquatic net per road drain.
The observed data dynamics advocate the conclusion that the repellent effect of EO on the egg-laying females is not prolonged after 2 weeks. By checking the specific effect of the product against Cx. pipiens and Ae. albopictus it seems that the products have slightly better activity against Cx. pipiens. The emulsifier addition (TWEEN® 20) did not affect the EO's efficacy.
The present study was aiming to develop a streamlined process for the valorization of bio-prospecting results in product development. As subjects of bio-prospecting were defined Mediterranean culinary plants, while as valorization target was selected the Ae. albopictus control. Of increased significance was the transition between experimentation phases that presented three major challenges; first, the identification of a broadly available CREO as indicated by the bio-prospecting results; second, the delineation of the suggested concentration in order to assure efficacy and environmental safety; third, the selection of a CREO formulation that would facilitate field application. Our results present for the first time the repellent properties of Conehead Thyme, Cortuk, Savory, Greek Sage, Monk's Pepper, and Fennel EOs against Ae. albopictus, as well as the larvicidal properties of Cortuk and Conehead Thyme EOs against the same target. The results obtained through field tests indicated that the emulsified CREO might be considered as a potent Ae. albopictus larvicidal and/or repellent agent. In summary, we believe that our approach successfully addressed the identified challenges and represents a methodological example for the exploitation of EOs as mosquito control agents, which may be of interest by relevant industries.
EE was responsible for the initial bioprospecting conceptualization including the collection, identification and documentation of the herbal material; the field assessment formulation composition; and the manuscript drafting. RB was responsible for the field assessment conceptualization and implementation supervision. GB was responsible for the laboratory bioassays including both initial bioprospecting and dose-response experiments. AM was responsible for the overall laboratory experimentation design and implementation supervision of the larvicidal and repellent bioassays. MC was responsible for the implementation of the spatial repellent experimentation. RV was responsible for the implementation of the combined repellent and larvicidal experimentation. DP was responsible for the processing of the raw bioassay results. AP was responsible for the processing of the raw field assessment results and the ecotoxicity bioassay. V-NK was responsible for the essential oils isolation and chemical characterization. SH was responsible for the overall research conceptualization and the manuscript finalization.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research has been co-financed by the European Union (EU Environmental Funding Programme LIFE+ Environment Policy and Governance) along with Greek national funds and Emilia-Romagna Regional funds, through the LIFE CONOPS project (http://www.conops.gr) Development & demonstration of management plans against–the climate change enhanced-invasive mosquitoes in S. Europe (LIFE12 ENV/GR/000466). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also thank the Municipalities of Bologna and Crevalcore for kindly hosting our field trials.
Ali, A., Demirci, B., Kiyan, H. T., Bernier, U. R., Tsikolia, M., Wedge, D. E., et al. (2013). Biting deterrence, repellency, and larvicidal activity of Ruta chalepensis (Sapindales: Rutaceae) essential oil and its major individual constituents against mosquitoes. J. Med. Entomol. 50, 1267–1274. doi: 10.1603/ME12177
Alten, B., Caglar, S. S., Simsek, F. M., Kaynas, S., and Perich, M. J. (2003). Field evaluation of an area repellent system (Thermacell) against Phlebotomus papatasi (Diptera: Psychodidae) and Ochlerotatus caspius (Diptera: Culicidae) in Sanliurfa Province, Turkey. J. Med. Entomol. 40, 930–934. doi: 10.1603/0022-2585-40.6.930
Baser, K. H. C., Erdemgil, F. Z., and Ozek, T. (1994). Essential Oil of Echinophora tenuifolia L. subsp. sibthorpiana (Guss.) Tutin. J. Ess. Oil Res. 6, 399–400. doi: 10.1080/10412905.1994.9698406
Benelli, G., Canale, A, and Conti, B. (2014). Eco-friendly control strategies against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae): repellency and toxic activity of plant essential oils and extracts. PharmacologyOnLine 1, 44–51.
Carrasco, A., Perez, E., Cutillas, A.-B., Martinez-Gutierrez, R., Tomas, V., and Tudela, J. (2016). Origanum vulgare and thymbra capitata essential oils from Spain: determination of aromatic profile and bioactivities. Nat. Prod. Commun. 11, 113–120.
Carroll, J. F., Demirci, B., Kramer, M., Bernier, U. R., Agramonte, N. M., Baser, K. H. C., et al. (2017). Repellency of the Origanum onites L. essential oil and constituents to the lone star tick and yellow fever mosquito. Nat. Prod. Res. 31, 2192–2197. doi: 10.1080/14786419.2017.1280485
Cetin, H., Yanikoglu, A, and Cilek, J. E. (2011). Larvicidal activity of selected plant hydrodistillate extracts against the house mosquito, Culex pipiens, a West Nile virus vector. Parasitol. Res. 108, 943–948. doi: 10.1007/s00436-010-2136-z
Chellappandian, M., Senthil-Nathan, S., Vasantha Srinivasan, P., Ponsankar, A., Thanigaivel, A., Edwin, E., et al. (2018). Botanical essential oils as mosquitocides and repellents against dengue vectors of human disease: phytochemical review. Environ. Int. 113, 214–230. doi: 10.1016/j.envint.2017.12.038
Choochote, W., Chaithong, U., Kamsuk, K., Jitpakdi, A., Tuetun, B., Champakaew, D., et al. (2007). Repellent activity of selected essential oils against Aedes aegypti. Fitoterapia 78, 359–364. doi: 10.1016/j.fitote.2007.02.006
Conti, B., Canale, A., Bertoli, A., Gozzini, F., and Pistelli, L. (2010). Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 107, 1455–1461. doi: 10.1007/s00436-010-2018-4
Conti, B., Leonardi, M., Pistelli, L., Profeti, R., Ouerghemmi, I., and Benelli, G. (2013). Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis L. (Rutaceae) against Aedes albopictus Skuse (Diptera: Culicidae), an arbovirus vector. Parasitol. Res. 112, 991–999. doi: 10.1007/s00436-012-3221-2
Dame, D. A., Meisch, M. V., Lewis, C. N., Kline, D. L., and Clark, G. G. (2014). Field evaluation of four spatial repellent devices against Arkansas rice-land mosquitoes. J. Am. Mosquito Control Assoc. 30, 31–36. doi: 10.2987/13-6379.1
Dellagli, M., Sanna, C., Rubiolo, P., Basilico, N., Colombo, E., Scaltrito, M. M., et al. (2012). Anti-plasmodial and insecticidal activities of the essential oils of aromatic plants growing in the Mediterranean area. Malaria J. 11:219. doi: 10.1186/1475-2875-11-219
Economou, G., Panagopoulos, G., Karamanos, A., Tarantilis, P., Kalivas, D., and Kotoulas, D. (2014). An assessment of the behavior of carvacrol – rich wild Lamiaceae species from the eastern Aegean under cultivation in two different environments. Ind. Crops Prod. 54, 62–69. doi: 10.1016/j.indcrop.2013.12.044
Enayati, A., and Hemingway, J. (2010). Malaria management: past, present, and future. Ann. Rev. Entomol. 55, 569–591. doi: 10.1146/annurev-ento-112408-085423
Evergetis, E., Michaelakis, A., and Haroutounian, S. A. (2012). “Essential oils of Umbelliferae family taxa as potent agents for Mosquito Control,” in Integrated Pest Management and Pest Control, eds M. L. Larramendy, and S. Soloneski (Rijeka: InTech Publishers), 613–637.
Evergetis, E., Michaelakis, A., Papachristos, D. P., Badieritakis, E., Kapsaski-Kanelli, V. N., and Haroutounian, S. A. (2016). Seasonal variation and bioactivity fluctuation of two Juniperus sp. essential oils against Aedes (Stegomyia) albopictus (Skuse 1894). Parasitol. Res. 115, 2175–2183. doi: 10.1007/s00436-016-4959-8
Giatropoulos, A., Kimbaris, A., Michaelakis, A., Papachristos, D. P., Polissiou, M. G., and Emmanouel, N. (2018). Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 117, 1953–1964. doi: 10.1007/s00436-018-5892-9
Giatropoulos, A., Papachristos, D. P., Kimbaris, A., Koliopoulos, G., Polissiou, M. G., Emmanouel, N., et al. (2012). Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 111, 2253–2263. doi: 10.1007/s00436-012-3074-8
Govere, J., and Durrheim, D. (2015). “Techniques for evaluating repellents,” in Insect Repellents: Principles, Methods and Uses, eds Debboun, M., Frances, S. P., and Strickman, D (Boca Raton: CRC Press), 147–159.
Karampoula, F., Giaouris, E., Deschamps, J., Doulgeraki, A. I., Nychas, G. J., and Dubois-Brissonnet, F. (2016). Hydrosol of Thymbra capitata is a highly efficient biocide against Salmonella enterica serovar Typhimurium biofilms. Appl. Environ. Microbiol. 82, 5309–5319. doi: 10.1128/AEM.01351-16
Kim, D., Kim, S., Chang, K., and Ahn, Y. (2002). Repellent activity of constituents identified in Foeniculum vulgare fruit against Aedes aegypti (diptera: Culicidae). J. Agric. Food Chem. 50, 6993–6996. doi: 10.1021/jf020504b
Koliopoulos, G., Pitarokili, D., Kioulos, E., Michaelakis, A., and Tzakou, O. (2010). Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 107, 327–335. doi: 10.1007/s00436-010-1865-3
Kongkaew, C., Sakunrag, I., Chaiyakunapruk, N., and Tawatsin, A. (2011). Effectiveness of citronella preparations in preventing mosquito bites: systematic review of controlled laboratory experimental studies. Trop. Med. Int. Health 16, 802–810. doi: 10.1111/j.1365-3156.2011.02781.x
Kröber, T., Koussis, K., Bourquin, M., Tsitoura, P., Konstantopoulou, M., Awolola, T. S., et al. (2018). Odorant-binding protein-based identification of natural spatial repellents for the African malaria mosquito Anopheles gambiae. Insect Biochem. Mol. Biol. 96, 36–50. doi: 10.1016/j.ibmb.2018.03.008
Kroeger, A., Lenhart, A., Ochoa, M., Villegas, E., Levy, M., Alexander, N., et al. (2006). Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. Brit. Med. J. 332, 1247–1252. doi: 10.1136/bmj.332.7552.1247
Lees, R. S., Gilles, J. R. L., Hendrichs, J., Vreysen, M. J. B., and Bourtzis, K. (2015). Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 10, 156–162. doi: 10.1016/j.cois.2015.05.011
Loh, P. Y., and Yap, H. H. (1989). Laboratory studies on the efficacy and sublethal effects of an insect growth regulator, pyriproxyfen (S-31183) against Aedes aegypti (Linnaeus). Trop. Biomed. 6, 7–12.
Machado, M., Sousa, M. D. C., Salgueiro, L., and Cavaleiro, C. (2010). Effects of essential oils on the growth of Giardia lamblia trophozoites. Nat. Prod. Comm. 5, 137–141.
Michaelakis, A., Theotokatos, S. A., Koliopoulos, G., and Chorianopoulos, N. G. (2007). Essential oils of Satureja species: insecticidal effect on Culex pipiens larvae (Diptera: Culicidae). Molecules 12, 2567–2578. doi: 10.3390/12122567
Morrison, A. C., Zielinski-Gutierrez, E., Scott, T. W., and Rosenberg, R. (2008). Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 5:e68. doi: 10.1371/journal.pmed.0050068
Mulla, M. S., Norland, R. L., Fanara, D. M., Darwazeh, H. A., and McKean, D. W. (1971). Control of chironomid midges in recreational lakes. J. Econ. Entomol. 64, 300–307. doi: 10.1093/jee/64.1.300
Müller, G. C., Junnila, A., Butler, J., Kravchenko, V. D., Revay, E. E., Weiss, R. W., et al. (2009). Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. J. Vector Ecol. 34, 2–8. doi: 10.1111/j.1948-7134.2009.00002.x
Perestrelo, R., Silva, C. L., Rodrigues, F., Caldeira, M., and Câmara, J. S. (2016). A powerful approach to explore the potential of medicinal plants as a natural source of odor and antioxidant compounds. J. Food Sci. Technol. 53, 132–144. doi: 10.1007/s13197-015-2022-x
Pires, M. M., Périco, E., Renner, S., and Sahlén, G. (2018). Predicting the effects of future climate change on the distribution of an endemic damselfly (Odonata, Coenagrionidae) in subtropical South American grasslands. J. Insect Conserv. 22, 309–319. doi: 10.1007/s10841-018-0063-y
Ramadass, M., and Thiagarajan, P. (2015). A review on melaleuca alternifolia (tea tree) oil. Int. J. of Pharma Bio Sci. 6, 655–661.
Rather, M. A., Dar, B. A., Sofi, S. N., Bhat, B. A., and Qurishi, M. A. (2016). Foeniculum vulgare: a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 9, 1574–1583. doi: 10.1016/j.arabjc.2012.04.011
Sands, P., Mundaca-Shah, C., and Djau, V. J. (2016). The neglected dimension of global security- a framework for countering infectious-disease crises. New Engl. J. Med. 374, 1281–1287. doi: 10.1056/NEJMsr1600236
Saoud, I., Hamrouni, L., Hanana, M., Bouzid, S., and Khouja, M. L. (2010). Ethnobotanical and phytopharmacological notes on Coridothymus capitatus (L.) Reichenb. Fil. Phytother. 8, 370–373. doi: 10.1007/s10298-010-0592-3
Semmler, M., Abdel-Ghaffar, F., Al-Rasheid, K., and Mehlhorn, H. (2009). Nature helps: from research to products against blood-sucking arthropods. Parasitol. Res. 105, 1483–1487. doi: 10.1007/s00436-009-1634-3
Semmler, M., Abdel-Ghaffar, F., Schmidt, J., and Mehlhorn, H. (2014). Evaluation of biological and chemical insect repellents and their potential adverse effects. Parasitol. Res. 113, 185–188. doi: 10.1007/s00436-013-3641-7
Senatore, F., Porta, G. D., and Reverchon, E. (1996). Constituents of Vitex agnus-castus L. essential oils. Flavour Fragr. J. 11, 179–182.
Tepe, B., Cakir, A., and Tepe, A. S. (2016). Medicinal uses, phytochemistry, and pharmacology of Origanum onites (L.): a Review. Chem. Biodiv. 13, 504–520. doi: 10.1002/cbdv.201500069
The Plant List (2013). version 1.1. Available online at: http://www.theplantlist.org/
Traboulsi, A. F., El-Haj, S., Tueni, M., Taoubi, K., Nader, N. A., and Mrad, A. (2005). Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 61, 597–604. doi: 10.1002/ps.1017
Tutin, T. G., Heywood, V. H., Burges, N. A., Valentine, D. H., Walters, S. M., and Webb, D. A. (2013). Flora Europaea, 5th Vol. (Cambridge: Cambridge University Press).
Veronesi, R., Carrieri, M., Maccagnani, B., Maini, S., and Bellini, R. (2015). Macrocyclops albidus (Copepoda: Cyclopidae) for the biocontrol of Aedes albopictus and Culex pipiens in Italy. J. Am. Mosquito Contr. Assoc. 31, 32–43. doi: 10.2987/13-6381.1
Vogel, G. (2017). Where have all the insects gone? Science 356, 576–579. doi: 10.1126/science.aal1160
WHO (2005). Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Geneva: World Health Organization, 1–41. Ref: WHO/CDS/WHOPES/GCDPP/2005.11
WHO (2006) Indoor Residual Spraying. Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. Geneva: WHO Position Statement World Health Organization.
WHO (2016). Zika Strategic Response Framework & Joint Operations Plan. New York, NY: World Health Organization, 1–32.
Zhang, D., Zheng, X., Xi, Z., Bourtzis, K., and Gilles, J. R. (2015). Combining the sterile insect technique with the incompatible insect technique: i-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 10:e0121126. doi: 10.1371/journal.pone.0121126
Keywords: carvacrol, mosquito, aedes, larvicidal, repellent, field assessment, essential oil
Citation: Evergetis E, Bellini R, Balatsos G, Michaelakis A, Carrieri M, Veronesi R, Papachristos DP, Puggioli A, Kapsaski-Kanelli V-N and Haroutounian SA (2018) From Bio-Prospecting to Field Assessment: The Case of Carvacrol Rich Essential Oil as a Potent Mosquito Larvicidal and Repellent Agent. Front. Ecol. Evol. 6:204. doi: 10.3389/fevo.2018.00204
Received: 05 September 2018; Accepted: 14 November 2018;
Published: 29 November 2018.
Edited by:
Filippo Maggi, University of Camerino, ItalyReviewed by:
Marcus Carl Stensmyr, Lund University, SwedenCopyright © 2018 Evergetis, Bellini, Balatsos, Michaelakis, Carrieri, Veronesi, Papachristos, Puggioli, Kapsaski-Kanelli and Haroutounian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonios Michaelakis, YS5taWNoYWVsYWtpc0BicGkuZ3I=
Serkos A. Haroutounian, c2VoYXJAYXVhLmdy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.