- 1Biological Sciences Department, California Polytechnic State University, San Luis Obispo, CA, United States
- 2Animal Behavior Graduate Group, Department of Neurobiology, Physiology, and Behavior, University of California, Davis, Davis, CA, United States
To address the hypothesis that male acoustic sexual advertisement signals, in addition to chemical signals, might be indicators of aggressiveness, we examined the relationship between levels of aggression/dominance status and acoustic sexual advertisement signals in the field cricket Gryllus integer. Males were paired in aggression trials and recorded the night before and night after the trial. This allowed us to test whether aggression is inherently linked to song phenotypes, or whether aggressive interactions cause males to alter their songs. We found that dominant (winning) males signaled with higher energy, amplitude, and power the night after winning an aggressive encounter, but we could not detect any differences before the encounter. Time spent calling and the number of calling bouts were apparently unrelated to aggression, whereas winning males increased their bout lengths after winning, and losing males decreased their bout lengths after losing.
Introduction
Sexual selection theory proposes that the selective sex (usually females) selects mates because of preferences for particular traits in the selected sex (usually males), allowing the traits to spread within a population even when those traits appear maladaptive (Darwin, 1874). Males will benefit from producing these traits if this increases their chances of mating (Fisher, 1930), while females will benefit from selecting mates based upon their preferred traits if the traits communicate some aspect of male quality that benefits the female directly (Price et al., 1993) or can benefit her offspring (Zahavi, 1977; Andersson, 1982; Hamilton and Zuk, 1982). Early signaling theory hypothesized that this link was maintained by “handicap signals:” sexual advertisement signals that are inextricably linked to male quality because they are costly to produce (thereby constraining them to be honest) and therefore can only be produced by high-quality males (Zahavi, 1975; Grafen, 1990). Later, Getty (2006) theorized that high-quality males do not “handicap” themselves by investing larger amounts of energy into their signals, but rather are simply more efficient at converting energy into signals, which reduces the cost of producing a large or extravagant signal. Another hypothesis states that it is not the cost of the signal, but rather the potential cost of cheating that keeps these signals honest (Számadó, 2011). According to this hypothesis, most males are able to produce a high-quality signal indicating, for example, that they are very large, but only large males are able to bear the cost of being challenged by other large males responding to their signals (Számadó, 2011).
One aspect of male quality that may be signaled to females is male aggression level. There are a few possible causes for a relationship between male sexual advertisement signals and aggressiveness. First, females may prefer aggressive males and select for males that signal their aggressiveness. In several species aggression levels are linked to reproductive success because females prefer or are constrained to mate with dominant males (Potter et al., 1976; Berglund and Rosenqvist, 2001; López et al., 2002; Double and Cockburn, 2003). Females that prefer aggressive males benefit directly (via access to better territories) or indirectly (any genetic components of aggression would be passed down to the female's offspring). Males that signal their aggressiveness would be at an advantage because females could select them without witnessing aggressive interactions. This type of signal would be constrained to honesty because genuinely aggressive males could challenge other males that dishonestly signal their aggressiveness, and losing those aggressive interactions would be extremely costly to dishonest signalers. Alternatively, females may simply prefer a trait that correlates with both aggression and acoustic signals. For example, acoustic signaling (Hoback and Wagner, 1997) and aggressive behaviors (Hack, 1995) are energetically costly, and only males in good condition (genetic/aerobic/energetic/body) can invest in acoustic signaling and aggression.
To address the hypothesis that male acoustic signals might be indicators of aggressiveness, we performed an experiment on the connection between levels of aggression and/or dominance status with acoustic sexual advertisement signals in the western stutter-trilling cricket, Gryllus integer. Gryllus integer males employ multi-modal courtship, using both chemical and acoustic signals to attract females. Gryllus integer females prefer the cuticular hydrocarbons of dominant males (Kortet and Hedrick, 2005); thus it may be possible that males are signaling their aggression acoustically as well.
Gryllus integer is an ideal species with which to study the relationship between acoustic signals and aggression, for two reasons. First, male Gryllus integer produce an acoustic signal to attract sexually receptive females. The signal is composed of a repeated series of chirps, strung together into bouts. Bouts are defined as periods of chirping with no interruptions longer than 0.1 s (Hedrick, 1986). Bout length is heritable (Hedrick, 1988), and females prefer males that produce longer bouts (Hedrick, 1986). Second, males that produce acoustic signals with longer bouts attract predators (Walker, 1964; Burk, 1982; Zuk and Kolluru, 1998), parasitoids (Cade, 1975; Wagner, 1996), and competitors (Gerhardt and Huber, 2002; Leonard and Hedrick, 2009; Jang, 2011; McCarthy et al., 2013) in addition to females. Competitor males may engage the signaling males in energetically taxing aggressive interactions (Hack, 1995; Tachon et al., 1999) or intercept and mate with females that are also attracted to the acoustic signals (Cade, 1980). If males attract competitor males via their acoustic signals, the males engage in energetically taxing aggressive behaviors with each other (Hack, 1995; Tachon et al., 1999). Thus, the most successful males will have an acoustic signal that is attractive to females while also maintaining aggression levels sufficient to defeat rival males. The question becomes: are male aggression levels and/or dominance statuses linked to acoustic signals, allowing females to gain more information about males from their songs?
Many studies have examined whether and how animal signals are used in aggressive contexts, but only a few have been able to show conclusively that those signals are communicating honest information about the signaler's aggressiveness (Searcy and Beecher, 2009). For example, researchers have established that low-amplitude song is an aggressive signal in the songbirds that use it (Akçay et al., 2015). In addition, Wagner (1992) discovered that frogs lower the carrier frequency of their calls as an honest signal of fighting ability.
Only a handful of studies have examined the direct relationship between aggressive behaviors and acoustic sexual signaling in crickets, with conflicting results. One study found a link between aggression and some aspects of signal structure and signaling effort (Bertram and Rook, 2012), specifically pulse length, pulses per chirp, chirp length, carrier frequency, and amplitude, while others found no link (Wilson et al., 2009; Fitzsimmons and Bertram, 2013). However, only one of these studies (Bertram and Rook, 2012) measured fine song parameters. Given the paucity of data on this relationship, particularly on fine song parameters, further experiments are required.
Acoustic sexual signals may be linked to aggression in two ways. Aggressive males' songs may be intrinsically different from the songs of less aggressive males, for example if both aggressiveness and signal quality covary with an aspect of male condition. Alternatively, aggressive interactions may alter male songs, such that males up- or down-regulate certain features of their acoustic signals depending upon the outcome of an aggressive interaction, allowing females to detect males that have won fights via their acoustic signals. (Whether these particular features are attractive or preferred by females requires further study).
We conducted an experiment using Gryllus integer males that were paired in aggression trials and recorded them the night before and after the trial. If aggression is linked to song phenotypes, then we predicted that males that displayed high levels of aggression in their aggression trials would have different songs than males that displayed low levels of aggression in their aggression trials, but each individual male's songs would not be significantly different pre- and post-trial. If aggressive interactions cause males to alter their songs, then we predicted that individual male songs would differ pre- and post-trial, depending upon the male's dominance status.
Methods
Specimens
Juvenile Gryllus integer were raised from eggs laid by females caught from the field in Davis, CA in the summer of 2012 and reared in family boxes (32 cm L × 18 cm W × 12 cm H). Juveniles were sorted from family boxes into individual waxed paper cups when they were approximately one-quarter adult size. Crickets were provided with chick starter ad libitum and water. Crickets were kept physically isolated from one another but not acoustically isolated. Two weeks after completion of the adult molt, males (n = 42) were recorded overnight. The following day males were placed into their aggression trials (n = 21 dyads), and then recorded overnight again.
Aggression Trials
The day before aggression trials (pre-trial song recording), males were randomly paired into dyads. Males were marked with a single dot of Wite-OutTM on either the left or right side of the pronotum to allow for individual recognition during the trial. On the day of the trial, males were placed under clear plastic vials at the center of a small arena lined with sand (20 cm L × 20 cm W × 7 cm H) and allowed to acclimate for 2 min. After 2 min, the vials were removed, and males were allowed to interact for 6 min. All aggression trials were recorded using a Canon ZR500 camera on SONY Mini DV cassette tapes, and dominance was determined by watching tape playback. Aggressive behaviors were awarded points using an all-occurrence observation method based on an ethogram from Adamo and Hoy (1995), with more aggressive behaviors worth more points (antennal fencing = 1 point; kick = 2 points; mandible flare, lunge, chase, or bite = 3 points; grapple = 4 points). In 100% of the trials that escalated to grapples (7 of 21 total trials), the male with the higher aggressiveness score also won the most grapples during the trial. Therefore, in each dyad, males with higher aggressiveness scores at the end of the trial were determined to be “dominant” and those with lower scores “subordinate.”
Song Recording
Males were placed inside a small plastic container (16 cm L × 18 cm W × 12 cm H) that was then placed inside another plastic tub (40 cm L × 27.5 cm W × 23 cm H) padded with sound-insulating foam with a closed top to prevent sound transfer between different containers. The containers were inside an acoustic chamber for recording, to reduce ambient noise in recordings. Recordings were made using a SM Pro Audi PR8E enhanced 8 channel preamp, Echo Audiofire12 interface and Behringer Super Cardioid XM 1800S microphones, with a 44,100 Hz sampling rate.
Songs were recorded overnight (~17:30–10:00 the next day) using the computer program Reaper (Cockos, New York, NY, USA). In Reaper, song files were parsed to remove all silence and these smaller files were run through the amplitude detector in Raven Pro, version 1.5 (Cornell lab of Ornithology, Ithaca, NY, USA). We used a Hamming spectrogram view set to 42.7 ms, filtering out frequencies below 1,000 Hz and above 17,000 Hz. Most cricket songs are produced around 5 kHz (ranging between 2 and 8 kHz), so this filter did not affect data collection (Robillard et al., 2013). The amplitude detector settings were: 2,000 Hz, 0.21 s, 0.05 smoothing, which could accurately detect individual bouts. All detections were checked by hand to remove false positives and include false negatives. For each bout, the detector measured the value for various measures of the following parameters: frequency, amplitude, energy, power, and entropy. Table 1 contains definitions of each of these parameters. Note that Raven measures amplitude using a custom unit, “U,” that does not reflect absolute amplitude in dB, but rather the relative amplitude of different sounds. Thus, reported measures should be considered relative to one another and not as absolute amplitude. While amplitude is notoriously difficult to accurately measure, our set up accounts for many of these difficulties by limiting cricket movement to a small area (without tethering the animal to a single location, influencing its behavior) and orienting the microphone above the cricket. Previous work using this set up with males not subjected to behavioral trials or other disruption across three consecutive nights of recording showed significant differences in amplitude between individuals, but could not detect significant differences across nights within individuals (repeatability = 0.514, p = 0.0007, Bunting, unpublished data). In addition to these parameters, the Raven selections allowed us to calculate the total time spent calling, the total number of bouts, and the mean bout length for each night.
Data Analysis
All statistical analysis was performed using general linear models (GLM) in R statistical software package, R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Mixed models were used for all analyses with dyad number as a random effect because aggressive behaviors within a dyad are not independent. Models were compared to one another using Akaike Information Criteria (AIC), and Akaike weights (the probability that one model is a better fit for a particular set of data; Anderson, 2008) were calculated (wi).
To test the hypothesis that dominant and subordinate males have intrinsically different songs, pre-trial values of each song parameter were used as outcome variables. If aggression trial results predicted these outcome variables better than post-trial values of these variables, it would be evidence that aggression and acoustic signals are intrinsically linked, since these recordings took place before any males experienced aggressive interactions. To test the hypothesis that male songs are altered by aggressive interactions, post-trial values of each song parameter were used as outcome variables. If aggression trial results predicted these outcome variables better than pre-trial values of these variables, it would be evidence that aggressive interactions alter acoustic signals. Each model set contained two null models: a standard null model with no covariates, and an experimental null model where the only covariate was the outcome variable on the opposite night (i.e., when the pre-trial value is the outcome variable, the post-trial value is in the experimental null model and vice versa). If the experimental null model carried the most weight in any of the model sets, then that would be evidence that acoustic signals are not covarying or changing with aggression levels and/or experience, but rather are intrinsic to the individual and/or not linked to aggression. It is important to note that these hypotheses are not necessarily mutually exclusive: aggressive experience and intrinsic individual differences may both influence acoustic advertisement signals. However, this model selection process allows us to assess the relative influence of these factors on song phenotype and determine which of these has a larger effect on each song parameter.
All model sets included models with single covariates: aggressiveness score, difference in aggressiveness score (each male's aggressiveness score minus his opponent's score), dominance status (dominant or subordinate), male weight, difference in weight (each male's weight minus his opponent's weight), the number of grapples (fights), and the number of grapples won (wins). Where appropriate, combinations of these covariates were used in larger model sets to determine the effect of interactions between these variables. All models used a Gaussian link function, with the exception of models testing whether each male signaled at least once on either night, which used a binomial link function.
Results
All results are summarized in Table 1 (Pre-trial) and Table 2 (Post-trial), each of which includes all song structures and definitions, the model that carried the most weight in each model set, significance levels, mean estimates, and confidence intervals. It is important to note that negative results could be due to Type II errors, as our sample sizes were small.
Signaling Effort
Pre-trial
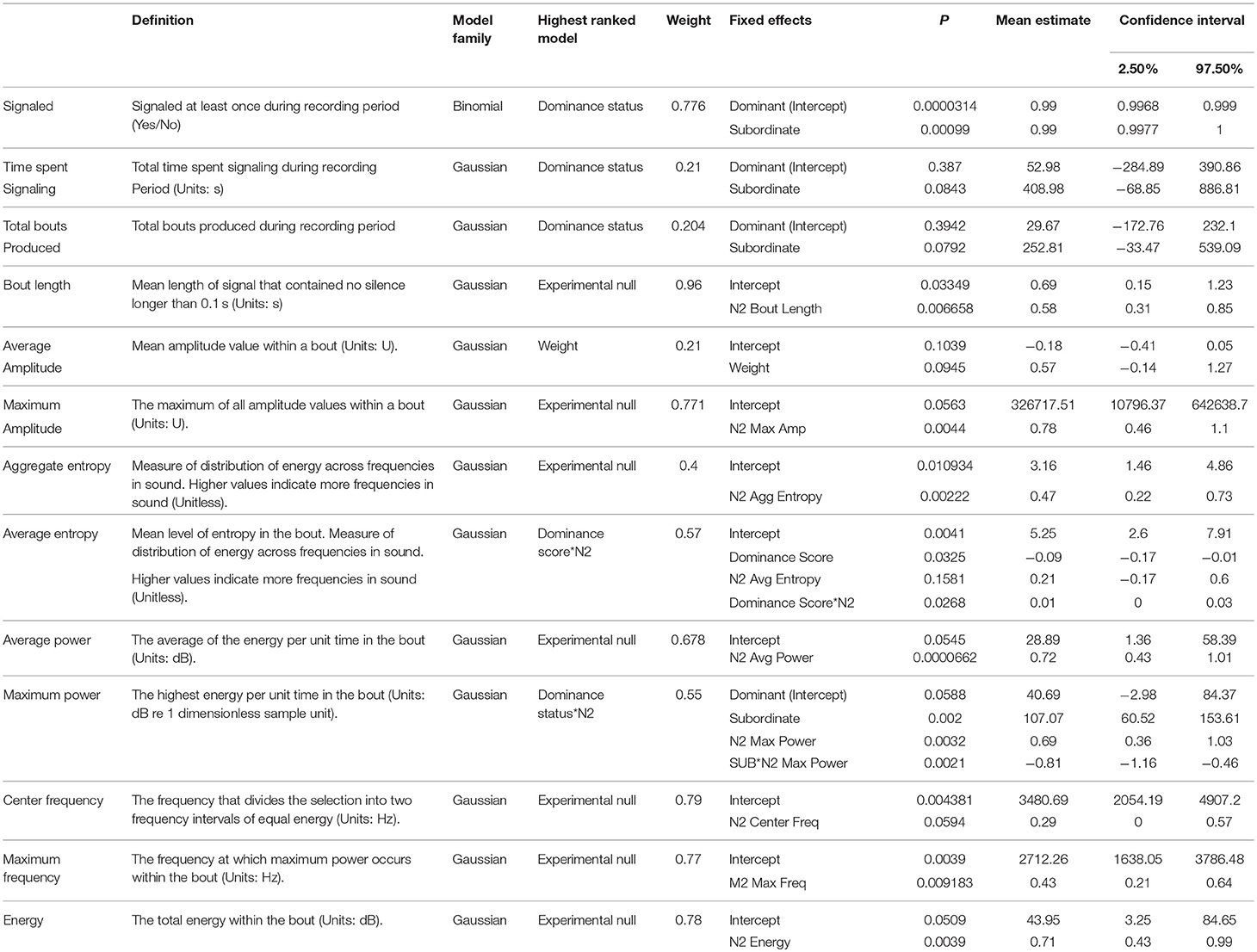
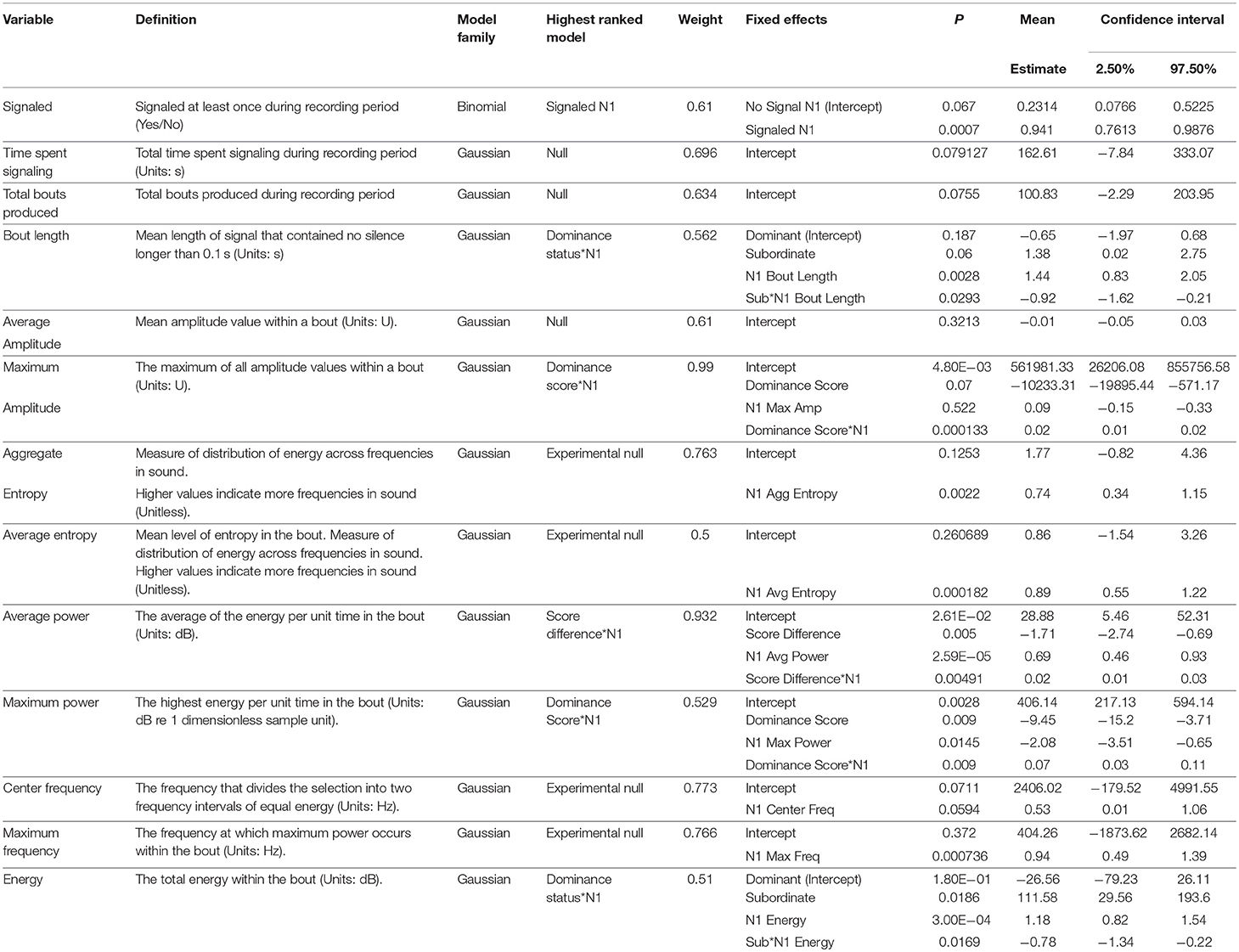
No significant differences were detected between dominant and subordinate males in either the time spent signaling (Figure 1) or the number of bouts they produced pre-trial. The best model in both sets contained dominance status as a covariate, but weight was low (wi = 0.21 for time, wi = 0.20 for bouts) and the effect was not significant. There was also no difference in the bout length produced by dominant or subordinate males (Figure 2), though the mean for dominant males was larger than subordinate males); the experimental null model carried the most weight (wi = 0.80) in that model set, with a significant effect of post-trial bout length. However, dominant males were significantly less likely to signal at least once pre-trial (11/21 dominant males vs. 18/21 subordinate males signaled at least once). The best model contained dominance status as the only covariate and carried a significant proportion of the weight (wi = 0.78), with a significant effect of dominance status.
Figure 1. Dominant males are significantly less likely to signal the night before an aggressive interaction, but there is no difference between dominant and subordinate males after an aggressive interaction.
Figure 2. Dominant males signal with longer bouts than subordinate males. This margin is larger after an aggressive interaction. Graph of mean estimates of model: Bout Length ~ (1|ID) + (1|Pair) + Score Result *Night of Recording.
Post-trial
After the trial, similar to the pre-trial period, there were no significant differences in time spent signaling (Figure 1, null model, wi = 0.70) or the number of bouts produced (null model, wi = 0.63). The pre-trial difference in likelihood to signal at least once over the course of the night was eliminated post-trial (13/21 dominant males and 14/21 subordinate males signaled at least once). There was a significant effect of having signaled pre-trial, and a model containing only pre-trial caller status as a covariate carried the most weight (wi = 0.61). For bout length, a model containing an interaction of dominance status and pre-trial bout length carried the most weight (wi = 0.56), with a statistical trend of dominance status, plus significant effects of pre-trial bout length, and a significant interaction that indicates males increase their bout lengths after an aggressive interaction (Figure 2). While subordinate males also increase their bout lengths after an aggressive interaction, the margin of increase was smaller for subordinate males than dominant males.
Fine Song Parameters
Pre-trial
An experimental model best explained the pre-trial values of two fine song parameters. Dominant males signaled with significantly lower maximum power (Figure 3). The best-supported model contained a significant interaction of dominance status and post-trial values (wi = 0.55). Average entropy was best explained with a model containing an interaction between post-trial entropy and aggressiveness score, indicating that more aggressive males called with higher levels of entropy (wi = 0.57). Pre-trial values for aggregate entropy (wi = 0.40), average power (wi = 0.68), center frequency (wi = 0.79), energy (wi = 0.78), maximum amplitude (wi = 0.77), and maximum frequency (wi = 0.77) were best explained by the experimental null model with significant effects of post-trial values, indicating that these measures do not primarily signal dominance as an intrinsic feature of the songs of highly aggressive males. Average amplitude (Figure 4) was equally well explained by the true null model and model containing only male weight (wi = 0.21). Larger males signaled more loudly, but the relationship was not statistically significant.
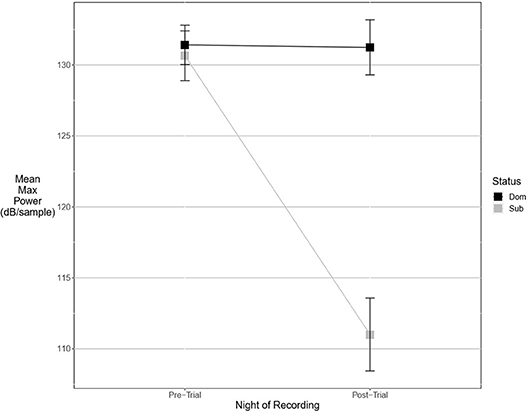
Figure 3. Dominant males signal with higher power only after an aggressive interaction. Graph of mean estimates of model: Power ~ (1|ID) + (1|Pair) + Score Result *Night of Recording.
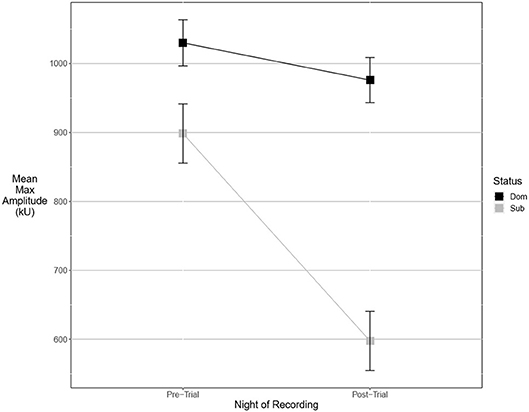
Figure 4. Dominant males signal with higher amplitude than subordinate males. This margin is significant after an aggressive interaction. Graph of mean estimates of model: Amplitude ~ (1|ID) + (1|Pair) + Score Result *Night of Recording.
Post-trial
There were more significant results for post-trial fine song parameters than for pre-trial fine song parameters. A model containing an interaction between pre-trial values and aggressiveness score carried the most weight for model sets explaining maximum amplitude (wi = 0.99) and maximum power (wi = 0.53), indicating that highly aggressive males increased these amplitude measures after aggressive encounters. Males with higher aggressiveness scores signaled with higher amplitude and power after an aggressive encounter but signaled with lower amplitude and power before an aggressive encounter (Figures 3, 4). For maximum power (Figure 3), there were significant effects of aggressiveness score, pre-trial value, and the interaction between the two. For maximum amplitude (Figure 4), there was a near-significant effect of aggressiveness score and a significant interaction between aggressiveness score and pre-trial maximum amplitude, but no significant effect of pre-trial values alone. A model containing an interaction between dominance status and pre-trial song energy carried the most weight (wi = 0.51), with significant effects of pre-trial values and significant interaction, indicating dominant males signaled with higher energy after an aggressive interaction but not before (Figure 5). Males with more positive differences in aggression score (i.e., males that displayed higher numbers of aggressive behaviors relative to their opponent) signaled with higher average power, while the best-supported model likewise contained an interaction between difference in aggression score and pre-trial power (wi = 0.93), with significant effects of pre-trial power and interaction. Post-trial aggregate entropy (wi = 0.76), average entropy (wi = 0.50), center frequency (wi = 0.77), and maximum frequency (wi = 0.77) were once again best explained by the experimental null model, while post-trial average amplitude was best explained with the standard null model (wi = 0.61), with male weight once again positively correlating with amplitude, though not significantly.
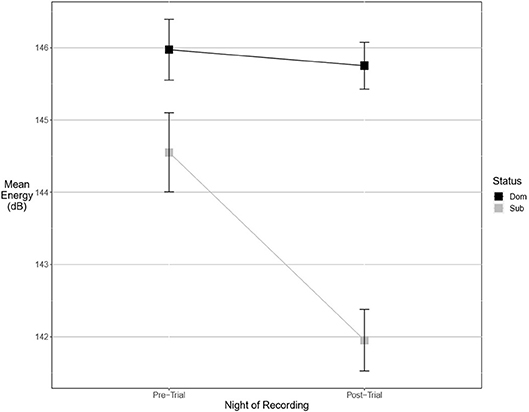
Figure 5. Dominant males signal with higher energy than subordinate males. This margin is significant after an aggressive interaction. Graph of mean estimates of model: Energy ~ (1|ID) + (1|Pair) + Score Result *Night of Recording.
Discussion
We tested two alternative hypotheses for how male acoustic signals were related to aggression: (1) that more aggressive and less aggressive males had intrinsically different signals, or (2) that the signals would change depending upon the result of an aggressive interaction. While these hypotheses are not necessarily mutually exclusive and we found some evidence for both, we found stronger support for the latter hypothesis. A model containing an aggression variable best predicted two pre-trial values of fine song parameters, but best predicted six post-trial values. Dominant males signaled with higher energy, amplitude, and power than subordinate males the night after winning an aggressive encounter but did not before the trial, for this sample of crickets. A model that contained their pre-trial values best predicted nearly all of the fine song parameters measured, even those that changed significantly after aggression trials. This indicates that these measures are highly repeatable within individuals, and the individual's genetic signal phenotype and/or long-lasting early environmental effects limit any effect aggressive experience can have on these measures. Interestingly, aggressive interactions do not apparently change the chemical signals of these crickets: females prefer the scent of dominant males before the males have ever been in a fight (Kortet and Hedrick, 2005).
Although our sample size was relatively low, our results were consistent with previous work indicating that only certain aspects of acoustic signaling effort are significantly linked with aggression in crickets. Wilson et al. (2009) recorded lab-raised Acheta domesticus for four consecutive nights and found no correlation between signaling effort (time spent calling, the number of bouts produced, and bout length) and aggression, although they did not examine how signaling changed with aggressive interactions, and thus only recorded male songs before aggression trials. Bertram and Rook (2012) recorded lab-raised Gryllus assimilis for 14 days and also found no correlation between time spent signaling and aggression. They did, however, find that aggression was correlated with pulse length, pulses per chirp, chirp length, carrier frequency, and amplitude. Fitzsimmons and Bertram (2013) used lab-raised and field-caught Gryllus veletis to test the relationship between acoustic signals and aggression. They examined how song effort differed before and after a fight and found no difference between pre-fight signaling effort and post-fight signaling effort in either dominant or subordinate males (Fitzsimmons and Bertram, 2013).
In our study, the time spent calling and the number of bouts produced were not correlated with aggression, nor did they change significantly after an aggressive interaction. These aspects of male signaling effort may be signaling some aspect of quality—either genetic or current body condition—that does not influence or is uncorrelated with aggressiveness (or influences signaling effort to a much higher degree than aggressiveness). Alternatively, investment in signaling may be subject to energetic trade-offs as it is in Gryllus texensis (Bertram and Warren, 2005), such that increasing signaling effort was not possible for either dominant or subordinate males, particularly after a potentially energetically draining aggressive interaction. Similar to Bertram and Rook (2012), we found a positive relationship between dominance and bout length, but our statistical methods also allowed us to discover an alteration of bout length influenced by aggressive interactions. Dominant males increased their (already longer) bout lengths after winning, while subordinate males decreased their (already shorter) bout lengths after losing. Females prefer males that call with longer bouts in this species (Hedrick, 1986), thus females may be selecting for males that win fights by preferring longer bouts.
There was a significant difference between dominant and subordinate males in whether they signaled the night before the trial (18/21 of subordinate males signaled vs. only 11 of 21 dominant males). This difference disappeared post-trial (14/21 subordinate males vs. 13/21 dominant males), which could indicate, based on these numbers, that dominant males are increasing their signaling, while subordinate males are decreasing their signaling due to the result of the aggressive trial. Subordinate males may reduce their chances of encountering another male by not producing a signal that may attract them (Andersson, 1994; Gerhardt and Huber, 2002; Leonard and Hedrick, 2009; Jang, 2011), while dominant males may not benefit from such a decrease in signaling. However, examination of the raw numbers suggests the effect size is small with two new dominant signalers (out of 21) and four subordinate signalers (out of 21) ceasing signaling after encounters. While our small sample size increases the potential for Type II error and the evidence for a change in probability of signaling post-trial is weak, there is stronger support for the finding that dominant males are less likely to signal acoustically overall.
In our relatively limited sample, aggressive males signaled with higher amplitude, power, and energy than subordinate males in their acoustic signals after an aggressive interaction, but not before. Higher amplitude signals that contain more energy are going to transmit longer distances from the signaler, increasing the number of potential receivers. These high-amplitude signals will potentially attract more females, but also rival males (Gerhardt and Huber, 2002; Leonard and Hedrick, 2009; Jang, 2011; McCarthy et al., 2013). Winners of aggressive contests may perceive the risk of attracting rivals as reduced because of their victory. Alternatively, dominant males may be adjusting their signals because of alterations to their social environment. Patricelli and Krakauer (2010) discovered that male greater sage grouse (Centrocercus urophasianus) that are successful in mating can increase their signal investment when receivers are nearby, whereas males that do not mate successfully are not able to increase investment. Because these crickets were raised in physical isolation, the experimental aggressive interaction represents a dramatically changed social environment and dominant males could be adjusting their signals accordingly, while subordinate males are not able to adjust their signal.
In our analyses, the fine song parameters of average entropy, center frequency, and maximum frequency were not significantly different pre- or post-trial for either subordinate or dominant males. A likely reason these particular measures are not correlated with aggressiveness either pre- or post- trial is that the information contained in these measures may be (relatively) unrelated to aggression. These fine song parameters may be signaling species identification information, which necessarily must be minimally variable between males to avoid hybridization with other closely related species (Jaiswara et al., 2013; Bastian and Jacobs, 2015). Alternatively, the neural mechanisms of females may be tuned to a very narrow frequency distribution, providing no advantage to (and strong selection against) any frequency alterations. In Teleogryllus oceanicus, female auditory neural mechanisms are particularly tuned to a few very specific frequency ranges, one of which includes the mean peak frequency of male T. oceanicus acoustic signals (Pollack and Faulkes, 1998). Changing the frequency of a signal would be disadvantageous if females will not respond to the new frequency.
Models including the number of wins and/or the number of grapples never ranked among the top models for any outcome variable, despite the fact that these data are measures of the most aggressive interactions in this species. This is perhaps because aggression behaviors escalated to grapples in only a small proportion of dyads (7/21), reducing statistical power and increasing the risk of Type II error. Alternatively, it may be evidence that the effect of an aggressive interaction is less dependent upon the level of aggression that occurred within the interaction, but rather on the fact the interaction occurred at all: perhaps the degree of dominance matters less than simply being dominant, even by a small margin. This could explain why a relatively small proportion of dyads escalated to grapples; the cost of the grapple may be too high, if the reward does not proportionately increase.
One open question concerns the duration of the changes we observed in male signals. Because we only recorded males the night before and night after aggressive trials, we are unable to determine whether alterations of male signals are long-lasting, and whether these alterations are of different durations for subordinate vs. dominant males. Moreover, an imperative next step in studies such as this is to assess how these song differences and/or alterations influence female choice. Dominant males increase their bout lengths after an aggressive contest, and longer bout lengths are preferred by females (Hedrick, 1986), but there is less known about female preferences for fine song parameters in this species. Whereas dominant males have higher power, amplitude, and energy in their songs after an aggressive encounter, this does not mean that females are paying attention to those parameters of the signal or making mating decisions based on them. A female choice playback experiment varying those parameters of the song, while keeping other signal features constant, is needed to ascertain whether these changes are independently important in a mating context. Even if females are not using these factors to make mating decisions, these song alterations likely play a role in male-male competition. For example, potential competitors may avoid signals with higher power or amplitude, to reduce damaging aggressive interactions. Though highly consistent within individuals, acoustic signal bout length, power, amplitude, and energy do change due to aggressive experience, indicating that field crickets may be adjusting their signals based upon their social condition. Plasticity in signal phenotype or investment may allow crickets to signal more about themselves than just genetic background or body condition.
Dataset Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
JB and AH contributed conception and design of the study. JB collected the data and performed the statistical analysis. JB wrote the first draft of the manuscript. AH revised the manuscript and prepared it for publication. All authors read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work reported here formed part of a Ph.D. dissertation by JB at the University of California, Davis (Bunting, unpublished data), under the direction of AH and was supported by an award to JB by the Animal Behavior Graduate Group at the University of California, Davis. AH was funded by NSF award IOS-1558069 during the preparation of this manuscript.
References
Adamo, S. A., and Hoy, R. R. (1995). Agonistic behaviour in male and female field crickets, Gryllus bimaculatus, and how behavioural context influences its expression. Anim. Behav. 49, 1491–1501. doi: 10.1016/0003-3472(95)90070-5
Akçay, Ç., Anderson, R. C., Nowicki, S., Beecher, M. D., and Searcy, W. A. (2015). Quiet threats: soft song as an aggressive signal in birds. Anim. Behav. 105, 267–274. doi: 10.1016/j.anbehav.2015.03.009
Anderson, D. R. (2008). Model-Based Inference in the Life Sciences. New York, NY: Springer. doi: 10.1007/978-0-387-74075-1
Andersson, M. (1982). Sexual selection, natural selection and quality advertisement. Biol. J. Linn. Soc. 17, 375–393. doi: 10.1111/j.1095-8312.1982.tb02028.x
Bastian, A., and Jacobs, D. S. (2015). Listening carefully: increased perceptual acuity for species discrimination in multispecies signalling assemblages. Anim. Behav. 101, 141–154. doi: 10.1016/j.anbehav.2014.12.010
Berglund, A., and Rosenqvist, G. (2001). Male pipefish prefer dominant over attractive females. Behav. Ecol. 12, 402–406. doi: 10.1093/beheco/12.4.402
Bertram, S. M., and Rook, V. (2012). Relationship between condition, aggression, signaling, courtship, and egg laying in the field cricket, Gryllus assimilis. Ethology 118, 360–372. doi: 10.1111/j.1439-0310.2011.02019.x
Bertram, S. M., and Warren, P. S. (2005). Trade-offs in signaling components differ with signaling effort. Anim. Behav. 70, 477–484. doi: 10.1016/j.anbehav.2004.09.024
Burk, T. (1982). Evolutionary significance of predation on sexually signaling males. Florida Entomol. 65:90. doi: 10.2307/3494148
Cade, W. (1975). Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. doi: 10.1126/science.190.4221.1312
Cade, W. (1980). Alternative male reproductive behaviors. Florida Entomol. 63, 30–45. doi: 10.2307/3494654
Darwin, C. (1874). The Descent of Man and Selection in Relation to Sex, 2nd Edn. London: John Murray.
Double, M. C., and Cockburn, A. (2003). Subordinate superb fairy-wrens (Malurus cyaneus) parasitize the reproductive success of attractive dominant males. Proc. R. Soc. B Biol. Sci. 270, 379–384. doi: 10.1098/rspb.2002.2261
Fitzsimmons, L. P., and Bertram, S. M. (2013). Signaling effort does not predict aggressiveness in male spring field crickets. Behav. Ecol. Sociobiol. 67, 213–220. doi: 10.1007/s00265-012-1441-1
Gerhardt, H. C., and Huber, F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: The University of Chicago Press.
Getty, T. (2006). Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 21, 83–88. doi: 10.1016/j.tree.2005.10.016
Grafen, A. (1990). Biological signals as handicaps. J. Theor. Biol. 144, 517–546. doi: 10.1016/S0022-5193(05)80088-8
Hack, M. A. (1995). The energetic costs of fighting in the house cricket, Acheta domesticus L. Behav. Ecol. 8, 28–36. doi: 10.1093/beheco/8.1.28
Hamilton, W. D., and Zuk, M. (1982). Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387. doi: 10.1126/science.7123238
Hedrick, A. V. (1986). Female preferences for male calling bout duration in a field cricket. Behav. Ecol. Sociobiol. 19, 73–77. doi: 10.1007/BF00303845
Hedrick, A. V. (1988). Female choice and the heritability of attractive male traits: an empirical study. Am. Nat. 132, 267–276. doi: 10.1086/284849
Hoback, W. W., and Wagner, W. (1997). The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol. Entomol. 22, 286–290. doi: 10.1111/j.1365-3032.1997.tb01170.x
Jaiswara, R., Nandi, D., and Balakrishnan, R. (2013). Examining the effectiveness of discriminant function analysis and cluster analysis in species identification of male field crickets based on their calling songs. PLoS ONE 8:e75930. doi: 10.1371/journal.pone.0075930
Jang, Y. (2011). Male responses to conspecific advertisement signals in the field cricket Gryllus rubens (Orthoptera: Gryllidae). PLoS ONE 6:e16063–e16068. doi: 10.1371/journal.pone.0016063
Kortet, R., and Hedrick, A. (2005). The scent of dominance: female field crickets use odour to predict the outcome of male competition. Behav. Ecol. Sociobiol. 59, 77–83. doi: 10.1007/s00265-005-0011-1
Leonard, A. S., and Hedrick, A. V. (2009). Male and female crickets use different decision rules in response to mating signals. Behav. Ecol. 20, 1175–1184. doi: 10.1093/beheco/arp115
López, P., Muñoz, A., and Martín, J. (2002). Symmetry, male dominance and female mate preferences in the Iberian rock lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 52, 342–347. doi: 10.1007/s00265-002-0514-y
McCarthy, T. M., Keyes, J., and Cade, W. H. (2013). Phonotactic behavior of male field crickets (Gryllus texensis) in response to acoustic calls from conspecific males. J. Insect Behav. 26, 634–648. doi: 10.1007/s10905-013-9375-7
Patricelli, G. L., and Krakauer, A. H. (2010). Tactical allocation of effort among multiple signals in sage grouse: an experiment with a robotic female. Behav. Ecol. 21, 97–106. doi: 10.1093/beheco/arp155
Pollack, G. S., and Faulkes, Z. (1998). Representation of behaviorally relevant sound frequencies by auditory receptors in the cricket Teleogryllus oceanicus. J. Exp. Biol. 201, 155–163.
Potter, D. A., Wrensch, D. L., and Johnston, D. E. (1976). Aggression and mating success in male spider mites. Science 193, 160–161. doi: 10.1126/science.193.4248.160
Price, T., Schluter, D., and Heckman, N. E. (1993). Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 48, 187–211. doi: 10.1111/j.1095-8312.1993.tb00887.x
Robillard, T., Montealegre-Z, F., Desutter-Grandcolas, L., Grandcolas, P., and Robert, D. (2013). Mechanisms of high-frequency song generation in brachypterous crickets and the role of ghost frequencies. J. Exp. Biol. 216, 2001–2011. doi: 10.1242/jeb.083964
Searcy, W. A., and Beecher, M. D. (2009). Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292. doi: 10.1016/j.anbehav.2009.08.011
Számadó, S. (2011). The cost of honesty and the fallacy of the handicap principle. Anim. Behav. 81, 3–10. doi: 10.1016/j.anbehav.2010.08.022
Tachon, G., Murray, A. M., Gray, D. A., and Cade, W. H. (1999). Agonistic displays and the benefits of fighting in the field cricket, Gryllus bimaculatus. J. Insect Behav. 12, 533–543. doi: 10.1023/A:1020970908541
Wagner, W. E. (1992). Deceptive or honest signaling of fighting ability? A test of alternative hypotheses for the function of changes in call dominant frequency by male cricket frogs. Anim. Behav. 44, 449–462.
Wagner, W. E. (1996). Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279–285. doi: 10.1093/beheco/7.3.279
Walker, T. J. (1964). Experimental demonstration of a cat locating orthopteran prey by the prey's calling song. Florida Entomol. 47, 163–165.
Wilson, A. D. M., Whattam, E. M., Bennett, R., Visanuvimol, L., Lauzon, C., and Bertram, S. M. (2009). Behavioral correlations across activity, mating, exploration, aggression, and antipredator contexts in the European house cricket, Acheta domesticus. Behav. Ecol. Sociobiol. 64, 703–715. doi: 10.1007/s00265-009-0888-1
Zahavi, A. (1975). Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214. doi: 10.1016/0022-5193(75)90111-3
Zahavi, A. (1977). The cost of honesty (further remarks on the handicap principle). J. Theor. Biol. 67:603. doi: 10.1016/0022-5193(77)90061-3
Keywords: communication, field cricket, acoustic signal, calling song, aggression, sexual selection, Gryllus integer
Citation: Bunting JE and Hedrick AV (2018) Male Field Cricket Songs Are Altered After Aggressive Interactions. Front. Ecol. Evol. 6:164. doi: 10.3389/fevo.2018.00164
Received: 18 June 2018; Accepted: 28 September 2018;
Published: 18 October 2018.
Edited by:
Varvara Yu. Vedenina, Institute for Information Transmission Problems (RAS), RussiaReviewed by:
Tom Tregenza, University of Exeter, United KingdomDavid Andrew Gray, California State University, Northridge, United States
Copyright © 2018 Bunting and Hedrick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann V. Hedrick, YXZoZWRyaWNrQHVjZGF2aXMuZWR1
 Jamie E. Bunting
Jamie E. Bunting Ann V. Hedrick
Ann V. Hedrick