
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 08 May 2018
Sec. Behavioral and Evolutionary Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00044
Many territorial animals reduce aggression toward their neighbors once territorial boundaries are established. This relationship is called the dear enemy phenomenon, hypothetically based on a conditional strategy like tit for tat (TFT). However, studies on territorial animals such as male songbirds do not fully support this hypothesis. We tested the TFT hypothesis in females of the territorial cichlid Neolamprologus pulcher, which exhibits dear enemy relationships, under laboratory conditions. Focal fish attacked invading neighbors as frequently as invading strangers, but they immediately stopped attacking the dear neighbor after the neighbor returned to its own territory, whereas they kept attacking strangers even after they stopped invading. These responses of territory owners are consistent with the predictions of TFT theory. Interestingly, fish were likely to remain vigilant toward the neighbor after its invasion and subsequent return to its own territory. This vigilance, while not a required condition for TFT, is consistent with TFT if a betrayer tends to repeat its intrusion.
In defending their territories, animals incur costs such as time, energy, and risk of predation and/or injury during aggressive fighting (e.g., Brown, 1964; Eberhard and Ewald, 1994; Temeles, 1994; Ydenberg et al., 1998). Studies have often documented that once a boundary has been established between adjacent territories, neighbors rarely invade each other's territories, and owners become less aggressive toward their neighbors. This reduction in aggression is known as the dear enemy relationship, and is exhibited by a variety of vertebrates (e.g., Fisher, 1954; Getty, 1989; Temeles, 1994; Ydenberg et al., 1998; Frostman and Sherman, 2004; Briefer et al., 2008). The dear enemy effect is beneficial for participant territory owners because it allows them to reduce territorial defense costs and to spend their energies on other activities that may increase their fitness (e.g., Temeles, 1994; Leiser and Itzkowitz, 1999; Leiser, 2003; Carazo et al., 2007; Briefer et al., 2008). Explanations for the evolution of this relationship have invoked reciprocal altruism based on the tit for tat (TFT) strategy in an iterated Prisoner's Dilemma (Trivers, 1971, 1985; Axelrod and Hamilton, 1981; Getty, 1987), and we do not know alternative mechanical hypotheses for this phenomenon.
Some studies in male songbirds have tested the hypothesis that the dear enemy relationship between territorial neighbors is based on the TFT strategy (e.g., Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009). Researchers expected that the dear enemy relationship between these birds would follow the rules for TFT: (1) avoid intruding when your honest neighbors do not intrude (original expectation from TFT: cooperate if your partner cooperates), (2) retaliate aggressively against your neighbor when the neighbor intrudes (defect or retaliate against your partner if your partner defects), and (3) stop retaliating immediately when the neighbor stops intruding and returns to his own territory (forgive your partner immediately when your partner stops defecting) (Trivers, 1971; Axelrod and Hamilton, 1981; Godard, 1993). Territorial male songbirds studied by those authors followed rules 1 and 2, but not 3, as the territory owners maintained a strong aggressive attitude toward cheating neighbors such as aggression against strangers even after they stopped intruding (Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009). One of the plausible reasons these males do not forgive their cheating neighbors is that such neighbors pose additional and critical risks such as extra-pair copulation (Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009). Extra-pair copulation with an owner's mate directly reduces the owner's fitness, and so the cost of forgiving a defecting neighbor outweighs the benefits of the dear enemy strategy (Olendrf et al., 2004; Akçay et al., 2009). Male songbird territories are multi-functional and contain multiple resources, such as food, nest sites, potential mates, and breeding sites. Thus, rule 3 of the TFT strategy in the dear enemy relationship are difficult to observe in male songbirds.
In the present study, we used female Neolamprologus pulcher, a small territorial cichlid fish found in Lake Tanganyika. These fish exhibit monogamy or haremic polygyny, with up to 15 helper fish inhabiting each shelter nest (crevices or spaces under rocks) (Taborsky and Limberger, 1981; Balshine et al., 1998; Wong and Balshine, 2011). Harem males encompass several females, but females within the same harem defend territories of approximately 50 cm in diameter against each other (Sogawa, unpublished data). The dear enemy relationship has been documented in N. pulcher in aquarium experiments using adult males (Frostman and Sherman, 2004; Kohda et al., 2015) and females (Sogawa et al., 2016). The primary resources in the female territory will presumably be nest sites that they use for sheltering refuge, sleeping, and nesting (Balshine et al., 2001). Their major food source is floating plankton, and is not a defended resource. In female songbirds with haremic polygyny, females are more aggressive toward neighbor females than stranger females (Brunton et al., 2008). This is partly because of the “costs of polygyny”: neighbor females share the harem male's investment in provisioning young, and are more competitive with each other than with strangers (Bensch, 1996, 1997). However, the costs of polygyny will be trivial or not be the case in females of the cichlid N. pulcher: harem males do not provision the young and rarely invest parental care such as defending young or building nests, and females are much less aggressive against neighbors than strangers (Sogawa et al., 2016). Moreover, territory size of female is much smaller than that of males, and thus, N. pulcher females are suitable animals for our experimental study of the dear enemy phenomenon.
Evolutionary stability of TFT requires (1) an indeterminate number of interactions between individuals over a considerably long term, (2) recognition between individual opponents, and (3) conditional retaliation against partners that cheat (Trivers, 1971; Axelrod and Hamilton, 1981; Godard, 1993). Territories of female N. pulcher are stable, and females almost always have interactions with the same neighboring females in the wild (Wong and Balshine, 2011; Sogawa, unpublished data), and they can recognize and distinguish their neighbors from strangers (Kohda et al., 2015; Sogawa et al., 2016). Thus, they fulfill the conditions of rules 1 and 2 for TFT. If conditional retaliation, rule 3, is observed, it will be evidence supporting that the dear enemy relationship in N. pulcher females is based on TFT strategy. Here, we show the results of our experiments and discuss whether reduced aggression to neighbors (dear enemy effect) comes from individuals reducing aggression in response to reduced intrusions (TFT).
Neolamprologus pulcher inhabits the rocky shores of Lake Tanganyika and feeds mainly on plankton in the water column. Males are larger than females [approximately 7 cm and 5–6 cm standard length (SL), respectively]. This fish is monogamous or polygynous with helper fish inhabiting each shelter nest (crevices or spaces under rocks), which is maintained by a female (Taborsky and Limberger, 1981; Balshine et al., 1998; Wong and Balshine, 2011). The territories of haremic males contain several females that are territorial against each other (Sogawa, unpublished data). The dear enemy relationship has been demonstrated in aquarium experiments using adult males (Frostman and Sherman, 2004; Kohda et al., 2015) and females that defend territories that contain sheltering, spawning, and breeding sites (Sogawa et al., 2016).
Experiments were conducted in our laboratory at Osaka City University. The fish for this study were obtained from commercial breeders and stocked in four large stock tanks (180 × 45 × 40 cm3). All fish used in the experiments had been maintained as stock in these tanks for >1 year, and strangers, that had never interacted with a focal individual, were from different tanks. The water in the tanks was well aerated and maintained at a temperature of 26°C. The fish were maintained in a 12:12-h light:dark cycle and fed twice per day with an adequate amount of Tetramin artificial flake food. The experimental tanks were 30 × 18 × 24 (height) cm3 and contained water to a height of 18 cm. The experiments were conducted with adult females with SL of 5.4–5.9 cm. Size-matched females were used in each experiment. A PVC pipe 6 cm in length and 2.5 cm in diameter was fixed on the tank bottom to serve as a shelter for the fish (Figure 1A).
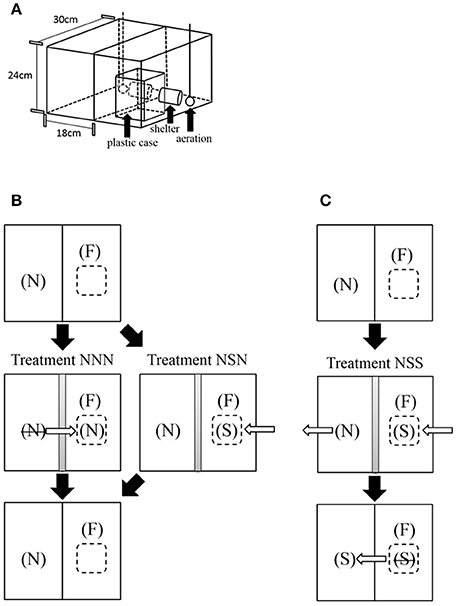
Figure 1. Experimental tanks and experiment procedures. (A) Two experimental tanks were set up side-by-side and a clear plastic case was placed in one tank. The plastic shelter tube and aeration stone are shown. The focal fish was in the tank with the plastic case. (B) Focal fish (F), dear neighbor (N), and stranger (S). Treatment NNN involved N in its tank, N intruding in the center of F's tank (inside the small case), and N returned to its tank. Treatment NSN shows N in its tank, then S in the case in tank F while N remains in its tank. Arrows depict the exchange of the fish. During the presentation of fish in the small case inside F's tank, an opaque sheet is placed between the two tanks. (C) Focal fish (F), dear neighbor (N), and stranger (S). Treatment NSS shows the process with N in its tank, S intruding at the center of F's tank (inside the small case), and S placed in N's tank.
Females of this species make a dear enemy relationship by the fourth day after their first encounter (Sogawa et al., 2016). To make dear neighbors, two tanks were placed adjacent to each other but were separated by an opaque sheet to prevent the female fish subsequently introduced into the tanks from establishing visual contact with each other (Figure 1A). One of the paired tanks, the tank of the focal female, contained a small transparent plastic case [9 × 9 × 12 (height) cm3] into which intruders were placed. Three days after females were introduced into each tank, the sheet between the tanks was removed, enabling them to interact visually. Four days after the initial visual encounter (when they establish a dear enemy relationship), the behaviors of the two fish were video recorded for 5 min using a video camera (Sony, HDR-CX390).
We performed three types of experimental treatments (Figures 1B,C; Table 1). The first type simulated the intrusion of a dear neighbor female into the focal fish territory (tank F), and subsequent return to its territory (Treatment NNN, n = 8), and we monitored the response of the focal fish to the neighbor at the three experimental phases. The second type of experimental treatment, set up as a control, simulated intrusion not by the neighbor but by a stranger (Treatment NSN, n = 8), and we monitored the response of the focal female to the invading stranger and to the dear neighbor before and after the intrusion by the stranger. We predicted that if the dear enemy phenomenon of N. pulcher is based on the TFT strategy, focal females will stop aggression toward the dear neighbor female after it is returned to its own territory in Treatment NNN (Table 1). In Treatment NSN, we predicted the focal female will be highly aggressive to the intruding stranger, but will rarely attack the dear neighbor after attacking the intruding stranger, just as before the intrusion, because the neighbor did not defect against the focal fish (Table 1). If focal females attack the neighbor that returns to its territory after invading (Treatment NNN), the results will be similar to those observed in male songbirds in the wild (Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009).

Table 1. Predictions of focal-female reactions toward fish in neighbor tanks after fish intrusi on in the three treatments NNN, NSN, and NSS.
Detailed procedures of Treatment NNN are as follows: after video recording the interaction between the dear neighbor and focal fish, an opaque sheet was placed between the tanks, and a white bottomless tetra-opaque cover (10 × 10 × 20 cm3) was placed on the small plastic case in tank F, slowly so as to not frighten the focal female. Then the neighbor female was captured in a small hand net and put inside the opened small case, and the ceiling board was closed. Several minutes after the neighbor female became accustomed to the case, the white opaque cover was slowly removed, and the reaction of the focal fish to the neighbor inside the tank was video recoded. After 5 min of video recording, the plastic case was covered with the white opaque cover again, and the neighbor female was captured and returned to her own tank. One minute after the return, when the females' swimming behavior returned to how it was before treatment, the opaque sheet was removed and their social interactions were recorded for 5 min (Figure 1B).
In Treatment NSN, first the social interactions between neighbor and focal fish were video recorded. Then, after the opaque sheet was placed between the tanks, the small plastic case was covered with the white opaque cover and a stranger fish was put in the plastic case (Figure 1B). Several minutes after this fish became accustomed to the case, the white opaque cover was removed. Then, the response of the focal fish to the stranger in the tank was recorded for 5 min. The plastic case was covered with the opaque cover again and the stranger was removed. After 1 min, the white prism cover and opaque sheet were removed, and the focal fish faced her neighbor in the adjacent tank, and the behaviors of the two fish were video recorded for 5 min (Figure 1B). On the bottom of tank F, a line had been drawn 7 cm from the glass beside the neighboring tank, and the duration of time the focal fish spent inside the 7-cm line was recorded from the video recordings of both treatments.
To test whether the focal fish attacked strangers outside of its territory, we monitored the response of the focal fish to a stranger placed in the dear neighbor's territory (third type; Treatment NSS, n = 6; Figure 1C; Table 1). This is control experiment for the treatment NSN, and we predict that focal females attack the stranger after they move to the neighbor tanks. In Treatment NSS, after the opaque sheet was placed between the tanks and the case was covered with the white prism, a stranger fish was put in the plastic case. After the fish became accustomed to the case, the white prism was removed. Then, the response of the focal fish to the stranger in the case was recoded for 5 min. The plastic case was then covered with the prism again, and the stranger was put into the adjacent tank after removal of the dear neighbor. After 1 min, the white cover and opaque sheet were removed and the focal fish faced the stranger that had recently intruded, and the behaviors of the two fish were video recorded for 5 min (Figure 1C).
Treatment NSN was carried out first, and then Treatment NNN was secondarily, using same focal fishes. If treatment NNN was carried out first, the defecting neighbor would strongly influence on focal fishes in Treatment NSN. In Treatment NSS, different focal fish were used.
Response intensity (aggression) was measured by recording the duration of mouth fighting and dashing behaviors exhibited by the focal fish (Stiver et al., 2007; Sopinka et al., 2009) from the video record. Staying time was measured by recording the duration of the time that focal fish stayed within 6 cm of the territorial boundary. All statistical analyses were performed using the R software (R core team). Scedasticity was examined by Bartlett test, and homosedasticity data set was examined by two way ANOVA and heterosedasticity data set was examined by Friedman test. Parametric data sets (Shapiro-Wilk normality test) were analyzed by var. test and homosedasticity data set was analyzed by t-test and heterosedasticity data set was analyzed by Welch paired t-tests.
None of the fish used in this study were killed. Adequate food was consistently provided and the aquaria were maintained in good condition. When disease occurred in the stock tanks, individuals were treated medically (Green-F, Sanei Pharmacy Inc., Japan) to accelerate their recovery. All fish used in the experiments were healthy. Our experiments were conducted in compliance with the Guideline of Animal Welfare of the Japan Ethological Society, and were specially approved by the Animal Care and Use Committee of Osaka City University.
Focal fish increased their aggression against neighbors that intruded into the center of their territory (Friedman, df = 5, P < 0.001; Welch pared t-test, t = 11.11, df = 7, P < 0.0001, n = 8; Figure 2), but decreased their aggression intensity immediately after neighbors stopped intruding and returned to their own territory (Welch pared t-test, t = 9.16, df = 7, P < 0.0001, n = 8; Figure 2). The intensities of aggression against the intrusion did not differ between neighbors and strangers (t-test, t = −1.51, df = 7, P = 0.25, n = 8; Figure 2), and focal fish aggression against neighbor fish was no different before or after intrusion by either strangers or neighbors (t-test, t = 1.25, df = 7, P = 0.17, n = 8; Figure 2).
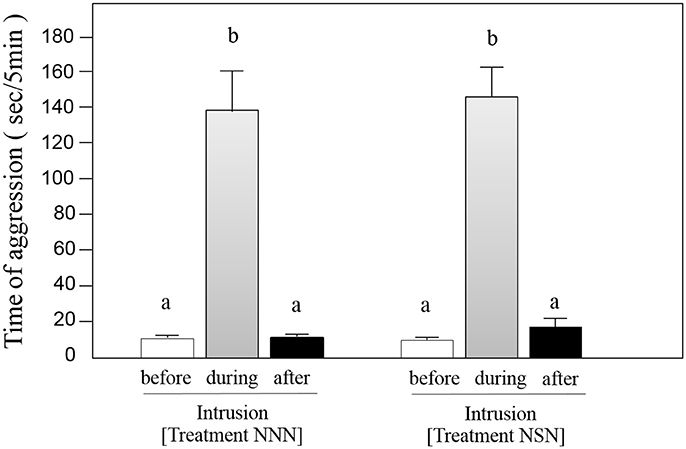
Figure 2. Aggressive responses of focal fish toward exhibited fish in Treatments NNN and NSN. Average duration (± SE, n = 8) of aggressive behavior by the focal fish against a neighbor before (open bar) and after (solid bar) a 5-min intrusion of the neighbor or a stranger (gray bar). The different letters above the bars denote differences at P < 0.001.
The strangers' intrusions did not affect the aggressive behavior of focal fish toward their neighbors (t-test, t = −1.37, df = 7, P = 0.9, n = 8; Figure 2). There was no difference in the amount of time the focal fish stayed on the border side (glass border) of the territory before and after intrusions by strangers (two-way ANOVA, df = 3, P = 0.006, t-test, t = −0.11, df = 7, P = 0.92, n = 8; Figure 3). In contrast, after intrusions by neighbors, the amount of time the focal fish stayed near the territorial borders significantly increased (t-test, t = −8.93, df = 7, P < 0.0001, n = 8; Figure 3). The duration of time the focal fish spent near the boundary after neighbor's intrusion did not differ incrementally by minute over the 5-min observation period (two-way ANOVA, df = 4, P = 0.91). These statistic results did not change after being analyzed by GLMM with fish ID as a random effect (z = −13.08, P < 0.001; NNN, NSN and NSS, z = −8.42, P < 0.001; before, during and after intrusion).
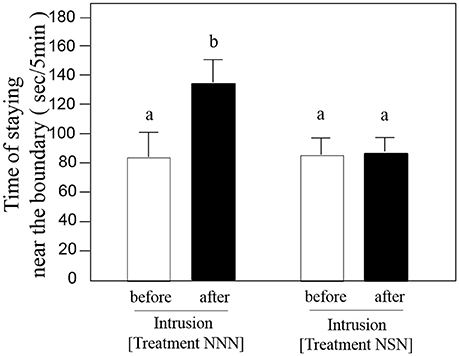
Figure 3. Average duration (± SE, n = 8) of time spent at the boundary of the neighbor's territory by the focal fish before (open bar) and after (solid bar) the 5-min intrusion of the neighbor [Treatment NNN] or a stranger [Treatment NSN]. The different letters above the bars denote differences at P = 0.001.
The aggression intensities of focal fish against strangers did not differ between intrusion in the focal fish's tank and when the strangers were in the dear neighbor's territory (t-test, t = 0.72, df = 5, P = 0.50, n = 6; Figure 4A). The time spent near the territorial borders when the strangers were in the adjacent tank increased significantly (Welch paired t-test, t = −5.43, df = 5, P < 0.002, n = 6; Figure 4B).
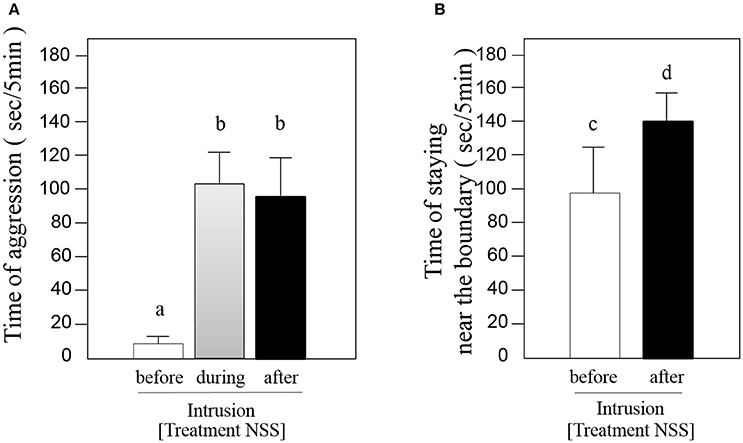
Figure 4. Results of Treatment NSS. (A) Average duration (± SE, n = 6) of aggressive behavior by the focal fish against a dear neighbor (open bar), introduced stranger (gray bar) and the stranger after being shifted into neighbor's territory from the small plastic case (solid bar). (B) Average duration (± SE, n = 6) of time spent at the boundary by the focal fish against dear neighbor (open bar) and the stranger after being shifted into neighbor's territory (solid bar). The different letters above the bars denote differences at P < 0.01.
Our results are consistent with the prediction and support the hypothesis that the dear enemy relationship between N. pulcher females is based on the TFT strategy. The conditions of this TFT strategy are to retaliate or punish a partner if it defects, and to end the retaliation or punishment and tolerate the partner if it stops the defection and acts as a dear neighbor (Trivers, 1971; Axelrod and Hamilton, 1981; Godard, 1993). The strong aggression displayed by our fish against intruding neighbors is suggested to be retaliation or punishment against neighbors. The defecting neighbors can be considered a strong source of threat, and the dear enemy relationship in females of this species is based on the threat-level hypothesis (Temeles, 1994; Sogawa et al., 2016): territorial females truly recognize that the intruders was her dear neighbor. This reaction of the focal fish to defecting neighbors is similar to that observed in territorial songbirds.
There were no differences in the aggression level of our focal fish against neighbors or strangers during their intrusion, which also supports results seen in songbirds (Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009). This suggests that defecting neighbors represent the same threat level as strangers, or even if such neighbors pose a greater threat, the territorial owners could not attack the defecting neighbor more strongly due to physical limitations on aggressive behavior. At this time we cannot distinguish which factor is responsible for the lack of difference in aggression against neighbors and strangers. However, we can at least say that they attacked their cheating neighbors as intensively as they possibly could in 5 min, and it was the same in the case of stranger intruders. As a result, their aggression against a defecting neighbor was much higher than against a non-invading neighbor. Is this retaliation against the cheating neighbors actually adaptive? The benefit of retaliation of N. pulcher females is resource protection, and the costs are mainly injury and predation risk (Heg et al., 2004). In Lake Tanganyika, crevices and spaces under rocks are probably limited resources, and N. pulcher females use them not only as breeding nest sites but also as shelter from predation. Thus, their nests and shelters are highly important resources, and the benefit of protecting them is probably greater than the costs of retaliation.
Our focal fish stopped attacking immediately after their neighbors stopped invading and returned back to their territories. The owner female was likely to forgive the cheating neighbor, and this result is consistent with the prediction of the TFT hypothesis. However, it is inconsistent with studies in male songbirds using playback experiments, wherein males remained aggressive against neighbors that returned to their own territories (Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009), a critical difference. There is one alternative explanation for the aggression reduction in our fish: territorial owners always attack conspecific fish inside their territory, and are always tolerant of all fish outside their territory, without discriminating whether fish are neighbors or strangers. However, the territorial females can distinguish between dear neighbors and strangers immediately after encountering them (Sogawa et al., 2016), and focal fish attacked strangers outside of their territory just as intensively as inside their territory (Figure 4A), but not so with returning neighbors. These results contradict this alternative explanation. Neolamprologus pulcher females probably compete only for their nest/shelter sites; thus we may regard this non-aggressive reaction toward neighbors that stop cheating as forgiveness like.
Interestingly, our focal fish stayed near the territorial boundary for a longer time (1.5 times longer) after a neighbor stopped intruding than before the defection (Treatment NNN), and such boundary-guarding behavior did not occur against the neighbor when a stranger intruded (Treatment NSN) (Figure 3). This difference suggests that the defection of the dear neighbor fish evoked the longer time spent at the boundary by the partner. During these stays near the border, the focal fish erected their vertical fins more frequently than at other times in the boundary zones (Sogawa, personal observation). This suggests that the focal fish was likely to become nervous and remain vigilant toward the defecting neighbor without direct aggression. Vigilance against the cheater is likely to be advantageous, if there is a possibility the defector will be a repeat offender (Temeles, 1994; Akçay et al., 2009; Monclús et al., 2014). Of course, the degree of this risk from the defector will gradually decrease as the defector continues to play good neighbors, and the amount of time spent near the boundary, i.e., vigilance, will decrease, as observed in our experiment (Sogawa, personal observation). This provides but conceivable perspective that the territorial owner watches the defecting neighbor, and is consistent with results of experiments in song sparrows and European rabbits that territorial owners were more aggressive and paid attention to defecting neighbors than before they defect (Akçay et al., 2009; Monclús et al., 2014). This type of vigilance may not be considered a required condition in, but may also not be inconsistent with, the theory of a conditional strategy such as TFT (Axelrod, 1984).
This vigilance displayed by our fish may help explain why rule 3 of TFT was not demonstrated in songbirds (Godard, 1993; Hyman, 2002; Olendrf et al., 2004; Akçay et al., 2009). A bird's aggressive response (i.e., latency time, closest distance, and flying time) to a neighbor after its defection may not always be an indicator of aggression, but could probably signify vigilance. Aggressive behaviors of songbirds in these experiments against invisible (only playback sound) neighbors that stopped invading would not be direct attacks, but indicators of aggressive response, i.e., their vigilance toward an invisible neighbor. Because the territorial owner watches a neighbor that once defected more closely, the strength of their response after intrusion by the neighbor did not decrease. During intrusion by a neighbor or stranger, the strength of their response was high, and this response after stopping intrusion meant they were watching for an invisible intruder intensively. We suggest that if direct interaction between a territorial owner and an intruder could be observed in songbirds, a decrease in aggression against neighbors that stopped defecting would be confirmed.
Nasty neighbors, that are more competitive each other than against non-neighbors (Müller and Manser, 2007), sometimes mask the dear enemy effect (Olendrf et al., 2004; Müller and Manser, 2007; Akçay et al., 2009). Here we consider the possibility of nasty neighbor effect in female N. pulcher because costs of polygyny occur in polygynous songbirds (Bensch, 1997). Bird females in the same territory share parental care of the territorial males such as food provisioning to young and nest defense, and would be nasty neighbors. In contrast, males of this cichlid neither provide food to young nor maintain nests, and thus, the cichlid females mating with polygynous males seem to less experience such costs of polygyny. Polygynous cichlid males that visit multiple females will spend less time inside a mate's territory than monogamous males (Wong et al., 2012), and the frequency of aggression of polygynous males against predators, including female predators, per female territory will be likely lower than monogamous males (Desjardins et al., 2008). As such it will not be rejected the possibility that the females of this cichlid will be nasty neighbors, although the degree of the nastiness will be less that those in bird females mating with polygynous males.
The responses of our fish to their dear neighbors were consistent with the prediction from the TFT hypothesis, and there do not seem to be other alternative explanations. As such, we conclude that the dear enemy phenomenon in female N. pulcher is based on TFT strategy, which is applicable to songbirds. There are many examples of TFT behavioral interactions in humans, and recently, it has been thought that reciprocal altruism largely dependent on a conditional strategy like TFT would be rare in vertebrates aside from humans (e.g., Clutton-Brock, 2009; West et al., 2011). However, reciprocal altruism has been documented in some social vertebrate species, for example, olive baboons (Packer, 1977), vampire bats (Wilkinson, 1984; Carter and Wilkinson, 2013), and sticklebacks (Ward et al., 2002), and thus it is argued that the present claim is merely a lack of examples, and we cannot conclude this behavior is rare (Taborsky, 2013; Carter, 2014). The possibility that the dear enemy relationship, often regarded as being beneficial for both dear neighbors, is a kind of reciprocal altruism based on TFT strategy, would mean that reciprocal altruism is actually a rather common phenomenon in territorial vertebrates, with many strong examples that argue against the conventional idea that observation of reciprocity is largely restricted to humans and great apes (e.g., Clutton-Brock, 2009; West et al., 2011). Since the TFT strategy was first proposed by Trivers (1971), there have been many studies of dear enemy relationships, but it remains uncertain whether reciprocal altruism actually operates through the dear enemy effect, because it is difficult to confirm that the dear enemy phenomenon is based on TFT, and restraint incurs a cost (Koenig, 1988; Wilkinson, 1988). The results of our present study, at least, show that dear enemy of N. pulcher is TFT like.
This study only examined short-term relationships with a single partner and could not focus on long-term benefits and costs. We suggest that in the future, these contentions will be resolved, and many more examples of the reciprocal altruism dependent on TFT strategy other than humans will be found.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was financially supported by KAKENHI grants (nos. 26540070, and 26118511) to MS from the JSPS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the members of the Laboratory of Animal Sociology for their support in the performance of experiments and for fruitful discussions.
Akçay, Ç., Wood, W., Searcy, W., Templeton, C., Campbell, E., and Beecher, M. (2009). Good neighbour, bad neighbour: song sparrows retaliate against aggressive rivals. Animal Behav. 78, 97–102. doi: 10.1016/j.anbehav.2009.03.023
Axelrod, R., and Hamilton, W. D. (1981). The evolution of cooperation. Science 211, 1390–1396. doi: 10.1126/science.7466396
Balshine, S., Neat, C., Reid, H., and Taborsky, M. (1998). Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav. Ecol. 9, 432–438. doi: 10.1093/beheco/9.5.432
Balshine, S., Neat, F., Reid, H., Taborsky, M., and Werner, N. (2001). Correlates of group size in a cooperatively breeding cichlid fish (Neolamprologus pulcher). Behav. Ecol. Sociobiol. 50, 134–140. doi: 10.1007/s002650100343
Bensch, S. (1996). Female mating status and reproductive success in the Great Reed Warbler: is there a potential cost of polygyny that requires compensation. J. Animal Ecol. 65, 283–296. doi: 10.2307/5875
Bensch, S. (1997). The Cost of Polygyny: definitions and applications. J. Avian Biol. 28, 345–352. doi: 10.2307/3676949
Briefer, E., Rybak, F., and Aubin, T. (2008). When to be a dear enemy: flexible acoustic relationships of neighbouring skylarks, Alauda arvensis. Anim. Behav. 76, 1319–1325. doi: 10.1016/j.anbehav.2008.06.017
Brown, J. L. (1964). The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169.
Brunton, D. H., Evans, B., Cope, T., and Ji, W. (2008). A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19, 791–798. doi: 10.1093/beheco/arn027
Carazo, P., Font, E., and Desfilis, E. (2007). Chemosensory assessment of rival competitive ability and scent mark function in a lizard (Podarcis hispanica). Anim. Behav. 74, 895–902. doi: 10.1016/j.anbehav.2007.02.011
Carter, G. (2014). The reciprocity controversy. Anim. Behav. Cogn. 1, 368–386. doi: 10.12966/abc.08.11.2014
Carter, G., and Wilkinson, S. (2013). Food sharing in vampire bats, reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. Lond. B 280:1753. doi: 10.1098/rspb.2012.2573
Clutton-Brock, T. (2009). Cooperation between non-kin in animal societies. Nature 462, 51–57. doi: 10.1038/nature08366
Desjardins, J. K., Fitzpatrick, J. L., Stiver, K. A., Van Der Kraak, G. J., and Balshine, S. (2008). Costs and benefits of polygyny in the cichlid Neolamprologus pulcher. Anim. Behav. 75, 1771–1779. doi: 10.1016/j.anbehav.2007.09.037
Eberhard, J. R., and Ewald, P. W. (1994). Food availability, intrusion pressure and territory size: an experimental study of Anna's hummingbirds (Calypte anna). Behav. Ecol. Sociobiol. 34, 11–18. doi: 10.1007/BF00175453
Fisher, J. (1954). “Evolution and bird sociality,” in Evolution as a Process, ed. J. Huxley, A. C. Hardy and E. B. Ford (London: Allen and Unwin), 71–83.
Frostman, P., and Sherman, T. (2004). Behavioral response to familiar and unfamiliar neighbors in a territorial cichlid, Neolamprologus pulcher. Ichthyol. Res. 51, 283–285. doi: 10.1007/s10228-004-0223-9
Getty, T. (1987). Dear enemies and the prisoner's dilemma: why should territorial neighbors form defensive coalition? Am. Zool. 27, 327–336. doi: 10.1093/icb/27.2.327
Getty, T. (1989). Are dear enemy in a war of attrition? Anim. Behav. 37, 337–339. doi: 10.1016/0003-3472(89)90125-5
Godard, R. (1993). Tit for tat among hooded warblers. Behav. Ecol. Sociobiol. 33, 45–50. doi: 10.1007/BF00164345
Heg, D., Bachar, R., Brouwer, L., and Taborsky, M. (2004). Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. Lond. B 271, 2367–2374. doi: 10.1098/rspb.2004.2855
Hyman, J. (2002). Conditional strategies in territorial defense: do Carolinawrens play tit-for-tat? Behav. Ecol. 13, 664–669. doi: 10.1093/beheco/13.5.664
Koenig, D. (1988). Reciprocal altruism in birds: a critical review. Ethol. Sociobiol. 23, 395–399. doi: 10.1016/0162-3095(88)90014-3
Kohda, M., Jordan, A., Hotta, T., Kosaka, N., Karino, K., Tanaka, H., et al. (2015). Facial recognition in a group-living cichlid fish. PLoS ONE 10:e0142552. doi: 10.1371/journal.pone.0142552
Leiser, K. (2003). When are neighbours ‘dear enemies’ and when are they not? The responses of territorial male variegated pupfish, Cyprinodon variegatus, to neighbours, strangers and heterospecifics. Anim. Behav. 65, 453–462. doi: 10.1006/anbe.2003.2087
Leiser, K., and Itzkowitz, M. (1999). The benefit of the dear enemy recognition in three-contender convict cichlid (Cichlasoma nigrofaseiatum) contests. Behaviour 136, 983–1003. doi: 10.1163/156853999501685
Monclús, R., Saavedra, I., and Miguel, J. (2014). Context-dependent responses to neighbours and strangers in wild European rabbits (Oryctolagus cuniculus). Behav. Process. 106, 17–21. doi: 10.1016/j.beproc.2014.04.004
Müller, C. A., and Manser, M. B. (2007). ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proc. R. Soc. B 274, 959–965. doi: 10.1098/rspb.2006.0222
Olendrf, R., Getty, T., Scribner, K., and Robinson, K. (2004). Male red-winged blackbirds distrust unreliable and sexually attractive neighbors. Proc. R. Soc. Lond. B 271, 1033–1038. doi: 10.1098/rspb.2004.2687
Sogawa, S., Ota, K., and Kohda, M. (2016). A dear enemy relationship in a territorial cichlid: evidence for the threat-level hypothesis. Behaviour 153, 387–400. doi: 10.1163/1568539X-00003351
Sopinka, N. M., Fitzpatrick, J. L., Desjardins, J. K., Stiver, K. A., Marsh-Rollo, S. E., and Balshine, S. (2009). Liver size reveals social status in the African cichlid Neolamprologus pulcher. J. Fish Biol. 75, 1–16. doi: 10.1111/j.1095-8649.2009.02234.x
Stiver, K. A., Desjardins, J. K., Fitzpatrick, J. L., Neff, B., Quin, J. S., and Balshine, S. (2007). Evidence for size and sex-specific dispersal in a cooperatively breeding cichlid fish. Mol. Ecol. 16, 2974–2984. doi: 10.1111/j.1365-294X.2007.03350.x
Taborsky, M. (2013). Social evolution: reciprocity there is. Curr. Biol. 23, 486–488. doi: 10.1016/j.cub.2013.04.041
Taborsky, M., and Limberger, D. (1981). Helpers in fish. Behav. Ecol. Sociobiol. 8, 143–145. doi: 10.1007/BF00300826
Temeles, E. (1994). The role of neighbors in territorial systems: when are they ‘dear enemies’? Anim. Bhav. 47, 339–350. doi: 10.1006/anbe.1994.1047
Trivers, R. (1985). Recognition of neighbor's duets by stripe-backed wrens. Social evolution Campylorhynchus nuchalis. Behaviour 62, 10–34.
Ward, A. J. W., Botham, M. S., Hoare, D. J., James, R., Broom, M., Godin, J.-G. J., et al. (2002). Association patterns and shoal fidelity in the three-spined stickleback. Proc. R. Soc. Lond. B 269, 2451–2455. doi: 10.1098/rspb.2002.2169
West, S. A., El Mouden, C., and Gardner, A. (2011). Sixteen common misconceptions about the evolution of cooperation in humans. Evol. Hum. Behav. 32, 231–262. doi: 10.1016/j.evolhumbehav.2010.08.001
Wilkinson, S. (1984). Reciprocal food sharing in the vampire bat. Nature 308, 181–184. doi: 10.1038/308181a0
Wilkinson, S. (1988). Reciprocal altruism in bats and other mammals. Ethol. Sociobiol. 23, 85–100. doi: 10.1016/0162-3095(88)90015-5
Wong, M. Y. L., Jordan, L. A., Marsh-Rollo, S., St-Cyr, S., Reynolds, J. O., Stiver, K. A., et al. (2012). Mating systems in cooperative breeders: the roles of resource dispersion and conflict mitigation. Behav. Ecol. 23, 521–530. doi: 10.1093/beheco/arr218
Wong, M., and Balshine, S. (2011). The evolution of cooperative breeding in the African cichlid fish, Neolamprologus pulcher. Biol. Rev. 82, 511–530. doi: 10.1111/j.1469-185X.2010.00158.x
Keywords: tit for tat, dear enemy, Neolamprologus pulcher, laboratory experiment, reciprocal altruism, cichlids
Citation: Sogawa S and Kohda M (2018) Tit for Tat in the Dear Enemy Relationship Between Territorial Females of a Cichlid Fish. Front. Ecol. Evol. 6:44. doi: 10.3389/fevo.2018.00044
Received: 29 October 2017; Accepted: 03 April 2018;
Published: 08 May 2018.
Edited by:
Ann Valerie Hedrick, University of California, Davis, United StatesReviewed by:
Marian Yi-ling Wong, University of Wollongong, AustraliaCopyright © 2018 Sogawa and Kohda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masanori Kohda, a29oZGEudGFuZ2FueWlrYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.