- Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Perth, WA, Australia
Failing to test multiple or non-standard variables in studies that investigate the effects of habitat fragmentation on plant populations may limit the detection of unexpected causative relationships. Here, we investigated the impacts of habitat fragmentation on the pollination, reproduction, mating system and progeny performance of Eucalyptus wandoo, a foundation tree that is bird and insect pollinated with a mixed-mating system. We explored a range of possible causative mechanisms, including soil properties that are likely to be altered in the agricultural matrix of a landscape that has naturally nutrient-poor soils and secondary soil salinization caused by the removal of native vegetation. We found very strong negative relationships between soil salinity and fruit production, thus providing some of the first evidence for the effects of salinity on reproduction in remnant plant populations. Additionally, we found unexpectedly higher rates of seedling survival in linear populations, most likely driven by increased soil P content from adjacent cereal cropping. Higher rates of seed germination in small populations were related to both higher pollen immigration and greater nutrient availability. Trees in small populations had unexpectedly much higher levels of pollination than in large populations, but they produced fewer seeds per fruit and outcrossing rates did not vary consistently with fragmentation. These results are consistent with small populations having much higher insect abundances but also increased rates of self-pollination, combined with seed abortion mechanisms that are common in the Myrtaceae. This study highlights the need to better understand and mitigate sub-lethal effects of secondary soil salinity in plants growing in agricultural remnants, and indicates that soil properties may play an important role in influencing seed quality.
Introduction
In the past two decades many studies have investigated the ecological and genetic processes affecting plant population viability and species persistence in fragmented habitats (for reviews see Hobbs and Yates, 2003; Aguilar et al., 2006, 2008). Together, these studies show that habitat fragmentation generally has a negative impact on plant pollination and reproduction, driving mating systems toward increased inbreeding (Coates et al., 2007; Eckert et al., 2010) with attendant declines in seed production, but variable impacts on progeny fitness (Hobbs and Yates, 2003; Aguilar et al., 2006, 2008). However, most empirical studies to date have typically not reflected landscape and biological complexity in the range of variables studied or in the breadth of their sampling design.
To address the need for a greater understanding of the influences of interacting landscape and biological variables, some recent studies have taken a broader approach to assessing plant responses to fragmentation. These studies found important but unexpected causative relationships, such as the effect of remnant shape on pollen diversity, that may be overlooked by failing to test multiple or non-standard variables (Breed et al., 2012; Llorens et al., 2012, 2013). They also emphasize the need to separate the consequences of different processes (Bunnell, 1999), to integrate abiotic and biotic interactions into the same study (Ouborg et al., 2006), and to treat different landscape parameters as independent variables (Fahrig, 2003; Llorens et al., 2012).
Landscape fragmentation and its associated modification has obvious impacts on fragment and landscape parameters, but can also influence landscape fluxes, such as hydrology and nutrients that are associated with soils. These in turn affect individual fragments and plant populations (Hobbs and Yates, 2003), yet their impacts are rarely considered in studies of plant reproduction (but see Lamont et al., 1994; Llorens et al., 2013). The biodiverse Mediterranean-type climate woodlands and shrublands of the Southwest Australian Floristic Region (SWAFR) provide an excellent opportunity to investigate the impact of both fragmentation and land-use change, as they were extensively cleared for agriculture during the 20th C. The remaining very fragmented vegetation has provided the context for several investigations that have revealed various impacts of altered population parameters, such as size, shape and isolation, on several species (Byrne et al., 2007; Krauss et al., 2007; Yates et al., 2007a,b; Llorens et al., 2012, 2013). However, there has been little investigation of the soil composition of native vegetation fragments, which is likely to have been greatly altered in many areas due to the effects of dryland salinity and the use of fertilizers in this agricultural landscape.
Both increased soil salinity and changed soil nutrient composition are two key factors related to soil health that are considered likely to have a major impact on plant species persistence. The replacement of deep-rooted native plants with annual crops has also led to alterations in landscape hydrology, resulting in secondary soil salinity in lower parts of the landscape (Cramer and Hobbs, 2002). Evidence from crop plants indicates that high soil salinity typically reduces growth and negatively affects reproduction (Kozlowski, 1997), leading us to predict that native plants within salt-affected populations might experience similar sub-lethal effects. The widespread use of fertilizers for cereal cropping is likely to have significantly altered the soil nutrient composition of adjacent remnant vegetation, with initial findings showing that altered soil nutrients in this landscape may be impacting reproduction (Lamont et al., 1994; Llorens et al., 2013) and progeny performance (Llorens et al., 2013) in some plants.
Eucalypt species dominate Australian woodlands with many formerly widespread and abundant tree species now restricted to fragments of native vegetation through much of their range. Here, we investigated the impacts of habitat fragmentation and soil properties on Eucalyptus wandoo Blakely (Myrtaceae), a dominant tree species found across the landscape that has undergone significant recent habitat fragmentation and associated landscape changes through much of its range. Wandoo woodlands tend to occur low in the landscape on valley slopes and floors and therefore may be exposed to the effects of secondary salinity, which in some areas has led to mortality of wandoo trees (Cramer et al., 2004). Salinity-related mortality of wandoo appears to occur as a local ecosystem collapse in response to the breaching of critical salinity thresholds, often following extreme events, rather than as the result of a gradual decline in tree health with increasing salinity (Cramer et al., 2004). Two studies found no relationship between secondary soil salinity and the crown health of live E. wandoo trees (Cramer et al., 2004; Brouwers et al., 2013), but they did not investigate other soil properties or aspects of population ecology such as reproduction.
Eucalyptus wandoo trees are potentially very long-lived, are visited by a suite of generalist insect and bird pollinators and have a mixed mating system (Byrne et al., 2008). Small E. wandoo population fragments show high rates of pollen immigration with up to 65% of pollen sourced from populations at least 1 km away (Byrne et al., 2008), but pollen immigration and some tolerance of inbreeding may not prevent detrimental effects of fragmentation on reproduction, as was shown in studies of the bird-pollinated Calothamnus quadrifidus (Byrne et al., 2007; Yates et al., 2007b). The fragmentation of Australian woodlands has caused major changes to pollinator communities, including the loss of some bird species from small remnants (Ford et al., 2001; Major et al., 2001) and the introduction of the European honeybee Apis mellifera, potentially reducing pollination, outcrossing and reproductive success.
Mating system studies have shown that increased within-plant foraging and reduced local mate availability appears to affect pollen quality in fragmented populations of many eucalypts and other Myrtaceae, resulting in reduced seed set (Krauss et al., 2007; Yates et al., 2007b; Gauli et al., 2014), while late-acting self-incompatibility mechanisms may have prevented outcrossing rates from being significantly affected by fragmentation in many species (Ottewell et al., 2009; Gauli et al., 2014; Breed et al., 2015), but not all (Butcher et al., 2005; Mimura et al., 2009; Breed et al., 2015). Despite changes in pollen quality, the level of pollination and pollinator abundance was unchanged in Calothamnus quadrifidus (Yates et al., 2007a), while honeybees have increased (González-Varo et al., 2009) or decreased (Hingston et al., 2004) pollination rates in some species of Myrtaceae. In addition, progeny performance measured as germination and seedling fitness among eucalypts is often unaffected by fragmentation (Krauss et al., 2007; Yates et al., 2007b; Breed et al., 2015; but see Burrows, 2000; Breed et al., 2012).
To evaluate the influence of a broad range of factors, including those not commonly investigated, such as soil properties, we undertook a study of potential effects of fragmentation on the pollination, reproduction, mating system and progeny performance of E. wandoo. We aimed to reveal likely causative mechanisms by independently testing multiple variables as surrogates of fragmentation, as well as a range of potentially important soil properties likely to be greatly altered in fragmented populations. We predicted that for E. wandoo: (i) soil properties would affect plant reproduction; (ii) reproduction would be lower for trees in highly fragmented populations; (iii) trees would show similar rates of pollination regardless of degree of population fragmentation; (iv) the realized mating system would not be affected by fragmentation due to self-incompatibility mechanisms and inter-population pollen flow; and (v) progeny performance would be unaffected by fragmentation. We also assessed the genetic diversity of the populations to indicate the likely diversity of potential mates and to provide baseline data for future comparison.
Materials and Methods
Study Species and Sites
Eucalyptus wandoo trees occur across broad, undulating valleys on sandy or loamy soils over laterite or granite and grow to 25 m in height. Trees produce masses of protandrous white-cream flowers from December to May but often flower asynchronously. Flowers require animal pollination to set fruit (Griffin et al., 1987), which is a serotinous woody capsule. Eucalypt seeds typically have limited dispersal. Two subspecies of E. wandoo are recognized, but only the more common E. wandoo subsp. wandoo is found in the study area and is hereafter referred to as E. wandoo.
We conducted the study in the Dongolocking catchment, approximately 250 km south-east of Perth, Western Australia (Figure 1). The area previously consisted of complex mosaics of woodland, heath and shrublands, and E. wandoo was a key component of the extensive but patchy woodlands. Clearing for agriculture occurred from the early 20th C to the 1980s and approximately 18% of the original vegetation remains. Eucalyptus wandoo trees are distributed among a variety of landscape contexts, including isolated paddock trees, linear remnants along road verges and rail lines, and remnant patches of varying size on private land, in conservation reserves or in other public reserves.
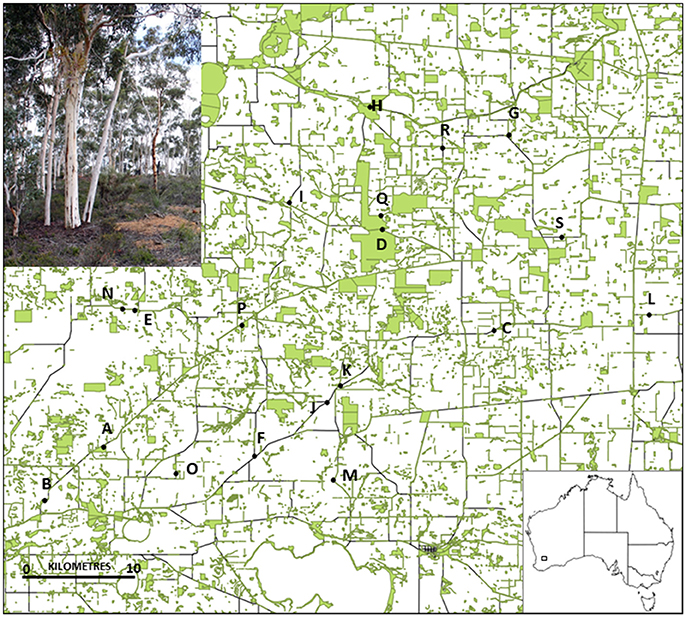
Figure 1. Location of the 19 Eucalyptus wandoo study populations in south-western Australia, and (inset photo) an intact E. wandoo remnant. Remnant vegetation (shown in green) was surveyed for E. wandoo within the 50 × 50 km study area.
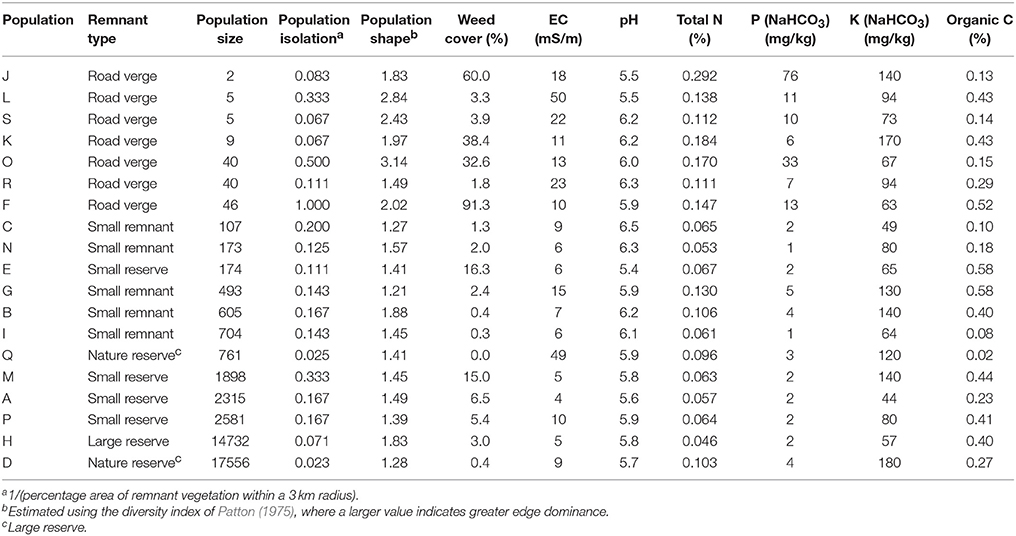
We surveyed a 50 × 50 km area for habitat fragments containing E. wandoo, and selected 19 populations that covered a wide range of population sizes and remnant characteristics (Table 1). We determined the size of small populations by direct count and of large populations by multiplying plant densities estimated using the T-square sampling method (Krebs, 1999) with area of population occupancy determined using aerial photography in ArcView 3.2. Population isolation was defined as the absence of habitat surrounding a fragment (Fahrig, 2003) and was estimated by taking the inverse of the percentage area of remnant vegetation within a 3 km radius of each population, calculated in ArcView. We estimated population shape using the diversity index of Patton (1975), which measures the complexity of population shape compared to a circular shape of the same size. Shape was calculated as Shape = P/(2√(Aπ)), where P was the total population perimeter (measured in ArcView) and A the population area. A larger value indicates a more complex shape with greater edge dominance, which in this landscape often equates to greater linearity. Using linear regression in STATISTICA 7.1 (StatSoft), we identified a significant negative linear relationship between log-transformed values for population size and population shape (r2 = 0.265, P = 0.024), but not between other combinations of fragmentation variables.
We estimated site disturbance for each population by measuring soil chemical properties and weed cover. Within each population, eight 1 × 1 m quadrats were nested within a larger 10 x 10 m quadrat, and from each smaller quadrat we obtained an estimate of weed cover and collected samples from the top 10 cm of soil. Bulked soil samples were analyzed for soil pH (H2O), soil salinity (electrical conductivity in water, EC), organic carbon (C), total nitrogen (N), NaHCO3-extractable phosphorus (P) and NaHCO3-extractable potassium (K). Soil analyses were performed by the Western Australian Chemistry Centre using methods described by McArthur (1991).
Pollination, Reproductive Effort, and Reproductive Output
For 12 populations, we selected ten trees spread throughout the population on which to measure pollination and flower, fruit and seed production. In very small fragments, all reproductive individuals were used. Logistical issues prevented us from measuring reproduction in the remaining seven populations. In each of 2 years (y1 and y2), we collected from around the canopy of each tree 10 mature fruits that resulted from peak flowering. In the laboratory, we counted the number of intact, mature seeds in each fruit and weighed a random selection of 20 seeds per plant from y1. At peak flowering and the following peak fruiting in y2 and y3, we counted the numbers of flowers and fruits on each of three widely-spaced tagged branches. For some trees, the loss of flagging tape to ants led to the selection of new branches for fruit counts in y3.
At peak flowering in y2 we collected 10 flowers from around the canopy of each tree and preserved them in 90% ethanol. Preserved flowers were dissected and five styles per plant were hydrated through an ethanol series (at least 10 min for each of 70% ethanol, 30% ethanol and two changes of dH2O), softened in 0.8 N NaOH overnight at 60°C, soaked in aniline blue for 10 min in the dark, and mounted on a slide in 80% glycerol. The number of pollen tubes at the base of each style was counted in ultraviolet light under a microscope.
Seed Germination, Seedling Growth, and Mortality
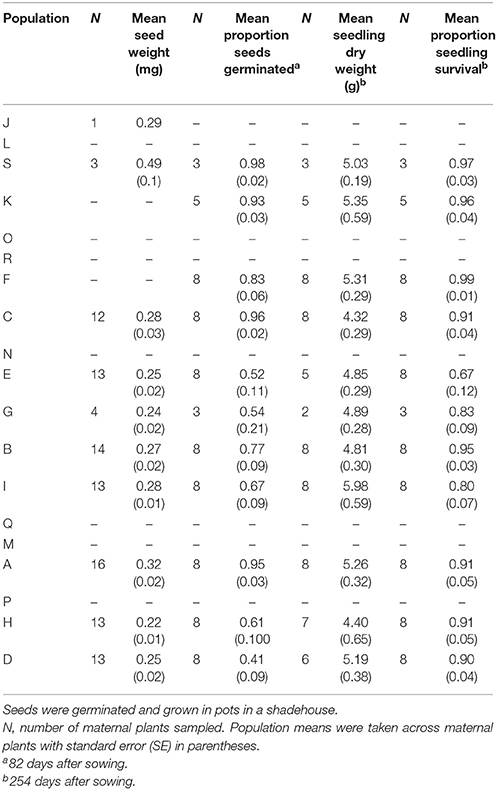
We used seeds produced in y1 to conduct glasshouse experiments on seed germination and seedling growth for the 11 populations for which sufficient seeds were available (Population L did not produce any seeds). Thirty seeds from each of up to eight maternal plants were sown into 200 mm diameter black plastic pots containing a standard commercial potting mix (Richgrow Professional) with a 2 cm layer of Richgrow Seed Raising Mix on top. For each maternal plant, the 30 seeds were evenly spread across two pots. The pots were watered to field capacity daily and their position was regularly randomized within the shade house. We recorded seedling emergence for 12 weeks, after which seedlings were thinned to the five tallest seedlings per pot. Seedlings were grown for a further 24 weeks, at which time we recorded seedling survival, harvested remaining seedlings at the root/shoot node and measured the weight of oven-dried shoots.
Mating System
We used seeds produced in y1 to investigate the mating system in 6–15 plants from six populations. We germinated up to 25 seeds per plant on moistened filter paper at 15°C and used seedlings with recently-emerged radicles for isozyme electrophoresis with the Helena Laboratory cellulose acetate plate electrophoresis system. Sample preparation and isozyme methods are described by Coates (1988). We assayed five enzyme systems: aspartate aminotransferase [AAT, Enzyme Commission (E.C.) number 2.6.1.1], alcohol dehydrogenase (ADH, E.C. 1.1.1.1), malate dehydrogenase (MDH, E.C. 1.1.1.37), phosphoglucose isomerase (PGI, E.C. 5.3.1.9) and phosphoglucomutase (PGM, E.C. 5.4.22). These produced seven polymorphic loci suitable for estimating mating system parameters (AAT-1, AAT-2, AAT-3, ADH-1, MDH-1, PGI-2 and PGM-1).
Genetic Diversity
We assessed genetic diversity and structure for the 19 study populations to provide background information on the population genetic characteristics of E. wandoo. We collected fresh leaves from up to 30 plants spread throughout each population, and sampled all adult plants in the smallest populations. Leaves were stored at −80°C and genomic DNA was extracted and genotyped using the co-dominant Restriction Fragment Length Polymorphism (RFLP) technique as per Byrne et al. (1998). The DNA was digested with either Bg1II or EcoRV, Southern blotted and hybridized with 16 RFLP probes (c135, c170, c238, c299, c333, c395, c411, g59, g86, g95, g99, g154, g195, g233, g243, g250) developed for eucalypts (Byrne et al., 1998). Probes were selected that detected a single locus, were spread across the genome, were not closely linked and were not biased according to polymorphism (Byrne et al., 1998). Probe plasmids were amplified through PCR and labeled with 32P using the random priming method. Banding patterns were interpreted following a Mendelian multi-allelic model and scored in order of decreasing fragment size.
Statistical Analyses
For each pollination, reproduction and progeny performance measure, we calculated the mean value for each maternal plant and from these we calculated population means. We calculated fruit:flower ratios for each plant in y2. Seedling survival was expressed as the proportion of plants that survived from thinning to the end of the experiment and seed germination was the proportion of seeds that germinated. We used paired t-tests in STATISTICA to determine whether tree means for the number of flowers or fruits per branch or the number of seeds per fruit varied between sampling years. Variables were transformed where necessary to achieve approximate linearity, constant variance and normality of residuals. We assessed whether each reproductive and progeny performance measure varied among populations using generalized linear models with population as a fixed factor.
Mating system parameters were estimated using the mixed and correlated matings models implemented in MLTR 3.4 (Ritland, 2002). For each population, we estimated the multilocus outcrossing rate (tm), the difference between multilocus and single- locus estimates of outcrossing (tm− ts), which provides a measure of biparental inbreeding, and the multilocus correlation of outcrossed paternity (rp), with standard errors based on 500 bootstraps with re-sampling among maternal plants. Pollen and ovule gene frequencies were estimated separately using the expectation-maximization (EM) method, and the Newton–Raphson method or the EM method was used to calculate mating system parameters. Family-level estimates for tm, tm− ts and rp were also calculated separately for each maternal plant using the method of Ritland (2002).
We estimated genetic diversity as the proportion of loci that were polymorphic (PL), mean number of alleles per locus (Na), mean number of private alleles per locus (Np) and unbiased expected heterozygosity (He) using GENALEX 6.5 (Peakall and Smouse, 2012). To standardize for sample size variation, we estimated the number of alleles (Ar) and the number of private alleles (Apr) per locus using rarefaction in HP-RARE 1.0 (Kalinowski, 2005). Wright's fixation index Fis was estimated according to Weir and Cockerham (1984) in GENEPOP 4.2 (Rousset, 2008) and exact tests were used to test for heterozygote deficiency within populations. Global genetic differentiation among populations was estimated using FST and Dest in GENALEX, with significance determined using 9999 permutations. We tested for isolation by distance (IBD) among populations using Mantel tests of log(FST/(1 − FST)) against log(geographic distance+1) in GENALEX with 9999 permutations, with pairwise FST calculated in GENEPOP.
To assess whether habitat fragmentation variables (population size, isolation and shape) may explain variation in reproductive, progeny performance or site disturbance measures or in genetic diversity or mating system parameters, we performed hierarchical partitioning using hier.part (Walsh and Mac Nally, 2013) in R 2.15.3 (R Development Core Team, 2013). As soil chemical properties can affect plant reproduction, we also used hierarchical partitioning to assess whether the six soil parameters measured could explain variation in flower, fruit or seed production. The predictor variables were ranked by their independent explanatory power (I) for each response variable using r2 as the goodness-of-fit measure. The statistical significance of each I was determined by randomizing the data matrix 2,000 times; observed I-values that exceeded the 95th percentile were considered significant (Mac Nally, 2002). The results were expressed as Z-scores, with significance at P ≤ 0.05 when Z ≥ 1.65. Significant relationships were further described using linear regression in STATISTICA. Where different predictor variables appeared to both contribute equally to variation in flower, fruit or seed production we carried out multiple regressions to determine which predictor variable was having the most influence.
To further investigate the biological basis for variation in progeny performance measures, we performed hierarchical partitioning on maternal plant means or values across all populations. We assessed the relative contributions made by what we assumed to be the most likely predictor variables to variation in maternal plant means of progeny performance measures, and described significant relationships using linear regression. We also used linear regression on maternal plant data to investigate whether pollen tube quantity may influence tm and whether there is a relationship between tm and the mean number of seeds per fruit.
Results
Soil Nutrients, Salinity, and Weed Cover
The recorded values of soil chemical properties (Table 1) were within the ranges described for sandy soils in Western Australia (McArthur, 1991). Hierarchical partitioning revealed that significant independent contributions were made by population size to among-population variation in EC and total N, by population size and population shape to bicarbonate extractable P, and by population isolation to weed cover (Tables 2, 3A). Linear regressions revealed that population size was negatively related to EC (r2 = 0.30, P = 0.015), N (r2 = 0.49, P < 0.001) and P (r2 = 0.46, P = 0.001), P was higher in more edge-dominated populations (r2 = 0.39, P = 0.004) and more isolated populations had higher weed cover (r2 = 0.21, P = 0.058).
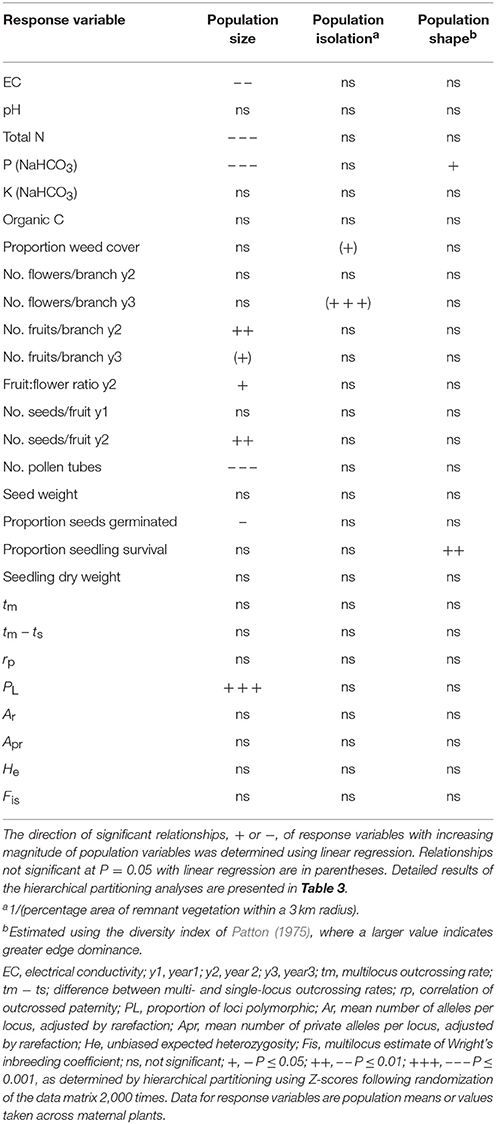
Table 2. Summary of the independent contributions of population size, isolation, and shape to variation in measures of soil chemical properties, weed cover, reproduction, progeny performance, mating system and genetic diversity for 19 populations of Eucalyptus wandoo, determined by hierarchical partitioning.
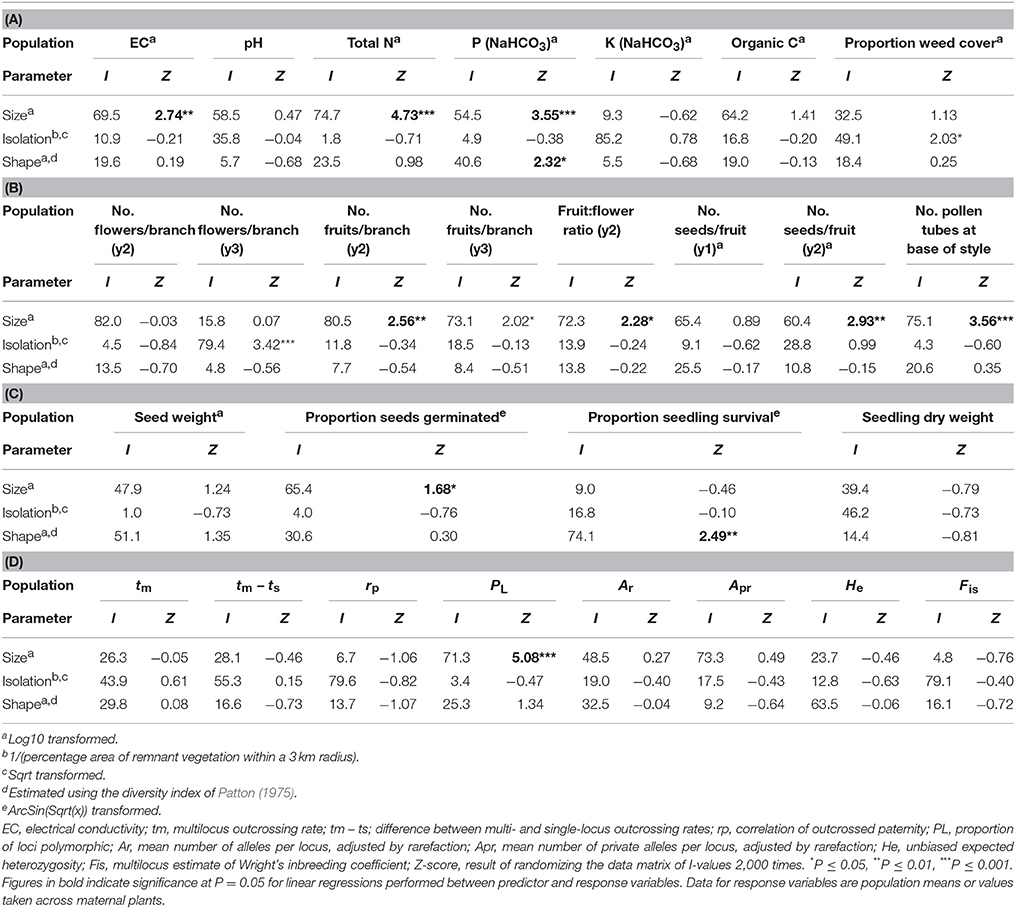
Table 3. The percentage independent contribution (I) of population size, isolation and shape to variation in measures of (A) soil chemical properties and weed cover, (B) reproduction, (C) progeny performance and (D) the mating system and genetic diversity for 19 populations of Eucalyptus wandoo, determined by hierarchical partitioning.
Independent contributions were made by P and N to variation in weed cover (Tables 4, 5). Weed cover increased with increasing P and N (P: r2 = 0.28, P = 0.020; N: r2 = 0.18, P = 0.069).
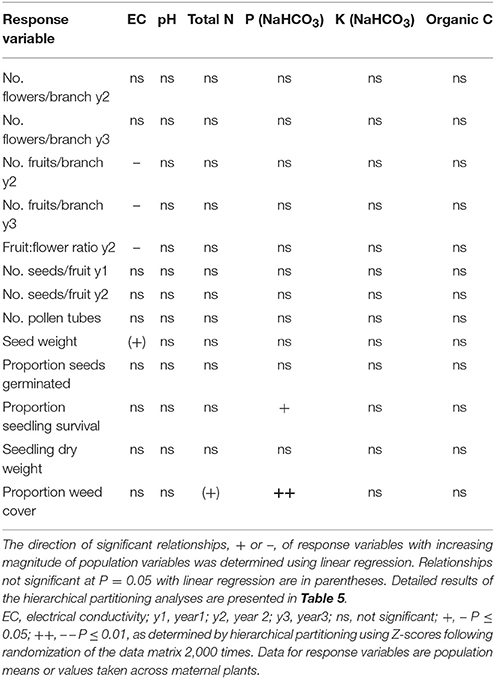
Table 4. Summary of the independent contributions of soil chemical properties to variation in measures of reproduction, progeny performance and weed cover for 19 populations of Eucalyptus wandoo, determined by hierarchical partitioning.
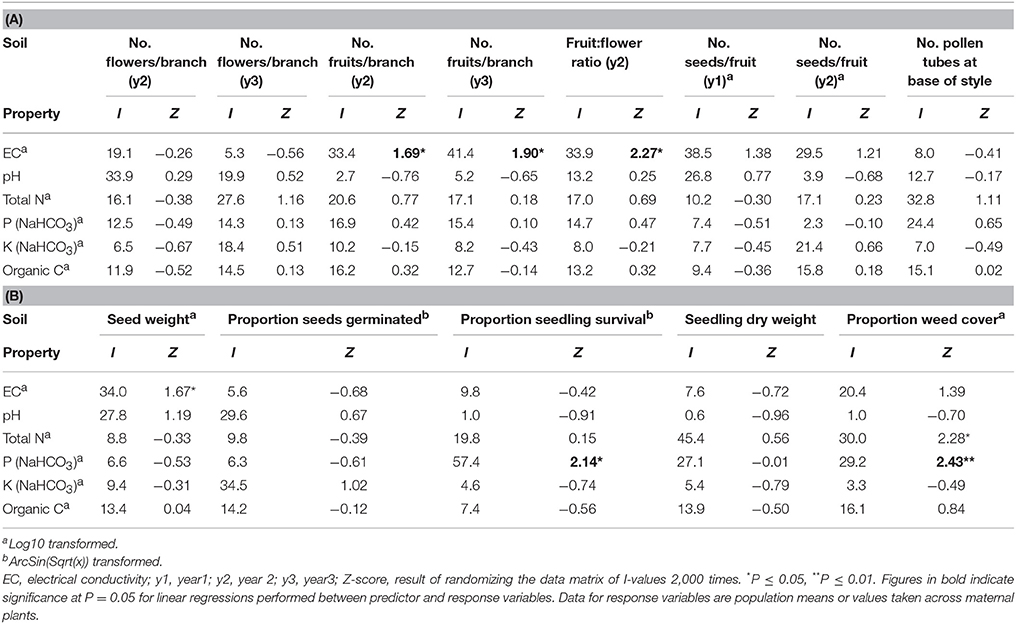
Table 5. The percentage independent contribution (I) of soil chemical properties to (A) reproduction and (B) progeny performance and weed cover for 19 populations of Eucalyptus wandoo, determined by hierarchical partitioning.
Reproductive Output
The number of flowers per branch (t98 = 4.48, P < 0.001) and the number of fruits per branch (t98 = 6.91, P < 0.001) varied significantly between years but the mean number of seeds per fruit did not (t96 = −1.89, P = 0.062). We found significant differences among populations (P < 0.05) for all reproductive variables. Trees in the very small population L did not produce any fruits or seeds. Population means for reproductive variables are given in Table 6.
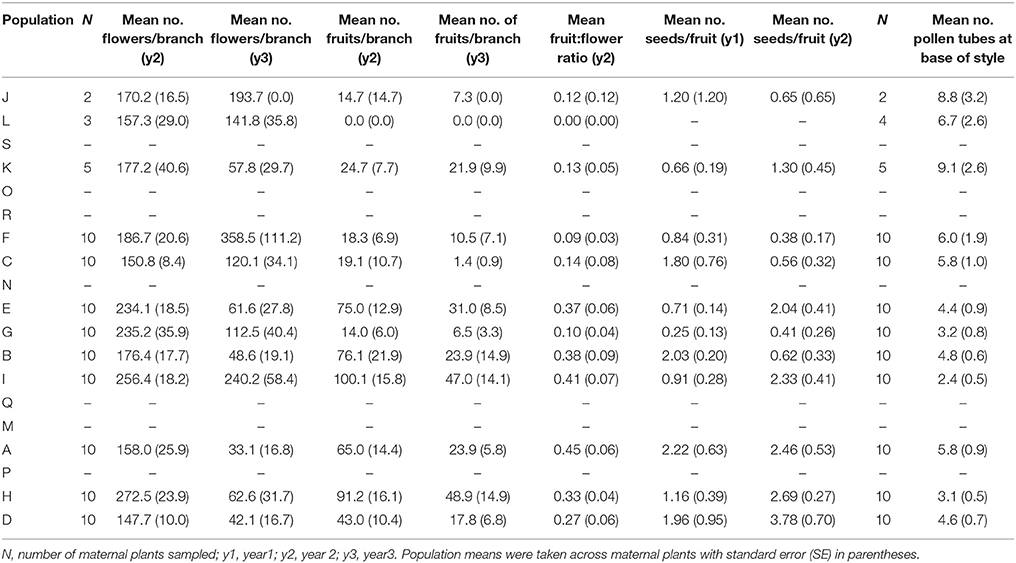
Table 6. The mean number of flowers, fruits, seeds and pollen tubes for 12 populations of Eucalyptus wandoo.
Population size made a significant independent contribution to variation in three reproductive measures. Trees in larger populations had more fruits per branch (y2: r2 = 0.43, P = 0.020; y3: r2 = 0.33, P = 0.052), higher fruit:flower ratios (r2 = 0.45, P = 0.017) and more seeds per fruit in y2 (r2 = 0.57, P = 0.005; Tables 2, 3B; Figures 2A–E). Population isolation made a significant independent contribution to the number of flowers per branch in y3, but this was due to a single outlying value.
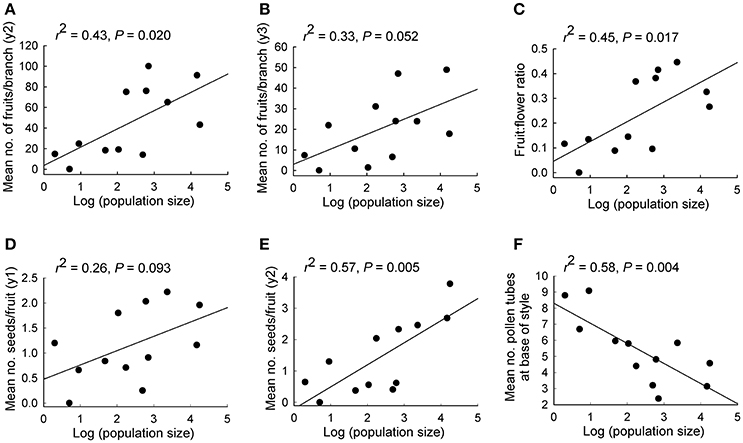
Figure 2. Relationships between population size and various reproductive measures for 12 populations of Eucalyptus wandoo. Regression lines are presented with r2- and P-values. (A,B) the mean number of fruits per branch in each of two years, (C) fruit:flower ratio, (D,E) mean number of seeds per fruit in each of two years, (F) mean number of pollen tubes at the base of the style.
Significant independent contributions were made by EC to among-population variation in fruit production for the two sampling years and to fruit:flower ratio (Tables 4, 5). As EC increased, the number of fruits per branch (y2: r2 = 0.64, P = 0.002; y3: r2 = 0.51, P = 0.009) and fruit:flower ratio (r2 = 0.74, P < 0.001) decreased strongly (Figure 3).
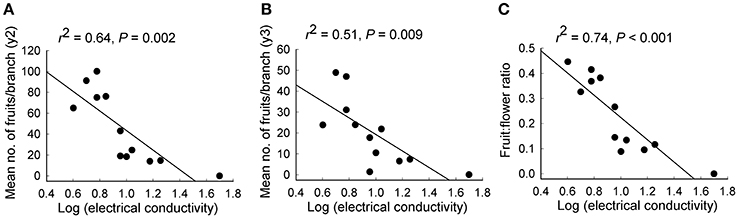
Figure 3. Relationships between soil electrical conductivity and (A,B) the mean number of fruits per branch in each of 2 years and (C) fruit:flower ratio for 12 populations of Eucalyptus wandoo. Regression lines are presented with r2- and P-values.
Although both population size and EC made significant independent contributions to variation in fruit production and fruit:flower ratio, multiple regression revealed that EC rather than population size was the largest contributor to the number of fruits per branch in both y2 (EC: P = 0.035; population size: P = 0.471) and y3 (EC: P = 0.083; population size: P = 0.651) and to fruit:flower ratio (EC: P = 0.009; population size: P = 0.499).
Pollination and the Mating System
We found significant differences among populations (P < 0.05) for the number of pollen tubes at the base of the style (Table 6). Population size made a significant independent contribution to variation in the number of pollen tubes at the base of the style. Trees in larger populations had fewer pollen tubes at the base of the style (r2 = 0.58, P = 0.004) (Tables 2, 3B; Figure 2F). Soil chemical properties did not contribute significantly to variation in the number of pollen tubes (Tables 4, 5A).
Outcrossing rates were moderate to high in all populations, with tm ranging from 0.690 to 0.907, and all were significantly less than one (Table 7). However, family-level tm estimates were quite variable and ranged from 0.202 to 1.197. The differences between multilocus and mean single locus estimates of outcrossing were all positive, suggesting some biparental inbreeding, but were significantly greater than zero for only one population. The correlations of outcrossed paternity (rp) were moderate and significantly greater than zero for five populations, and ranged from 0.084 to 0.213. None of the population variables (size, shape or isolation) made significant independent contributions to any of the mating system parameters (Tables 2, 3D).
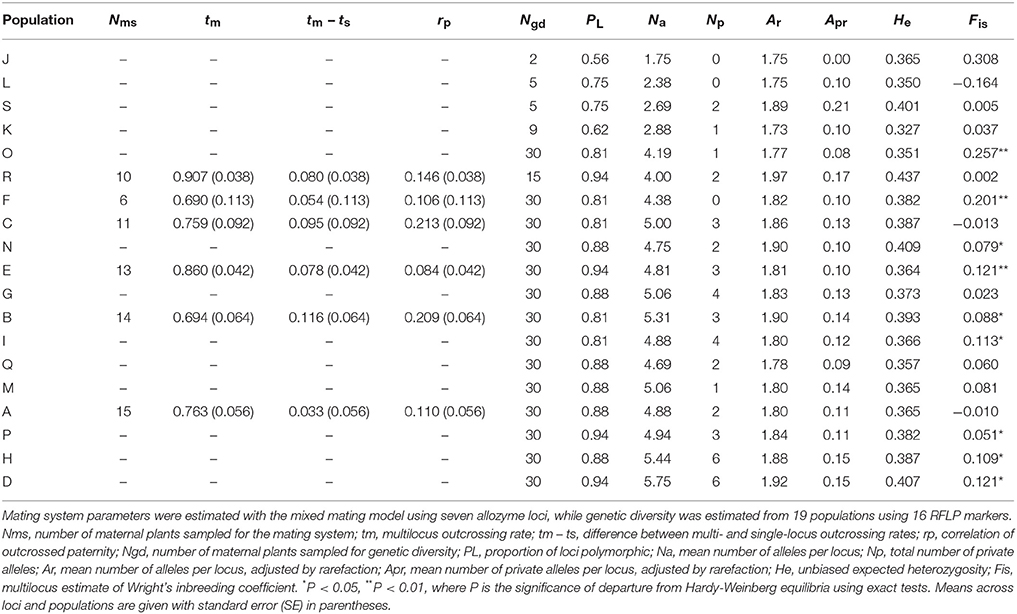
Table 7. Mating system and genetic diversity parameters estimated for Eucalyptus wandoo plants from up to 19 populations.
Linear regressions revealed that the multilocus outcrossing rate (tm) was significantly lower both for plants with more pollen tubes at the base of the style (r2 = 0.14, P = 0.032; Figure 4A) when a single outlying plant with no pollen tubes was removed, and for plants that produced more seeds per fruit in y1 (r2 = 0.15, P = 0.020; Figure 4B).
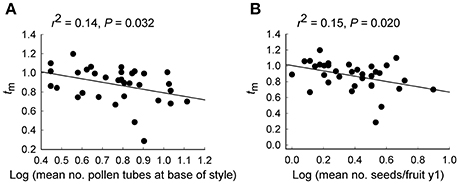
Figure 4. Relationship of the multilocus outcrossing rate (tm) with (A) the number of pollen tubes at the base of the style and (B) the number of seeds per fruit, for 35 maternal Eucalyptus wandoo plants. Regression lines are presented with r2- and P-values.
Progeny Performance
We found significant differences among populations in seed weight [F(9, 92) = 3.82, P < 0.001], the proportion of seeds that germinated [F(10, 64) = 7.07, P < 0.001] and the proportion of surviving seedlings [F(10, 64) = 2.05, P = 0.042], but not in seedling dry weight [F(10, 57) = 1.31, P = 0.245]. Mean seed germination ranged between 41 and 98%, with the lowest germination recorded in the largest population. Mean seedling survival ranged from 80 to 99% for all but one population that had 67% survival (Table 8).
Population size contributed significantly to variation in seed germination, with smaller populations showing higher germination rates (r2 = 0.40, P = 0.036; Tables 2, 3C; Figure 5A). Population shape contributed significantly to variation in seedling survival, with higher seedling survival recorded for plants in more edge-dominated populations (r2 = 0.46, P = 0.023; Tables 2, 3C; Figure 5B).
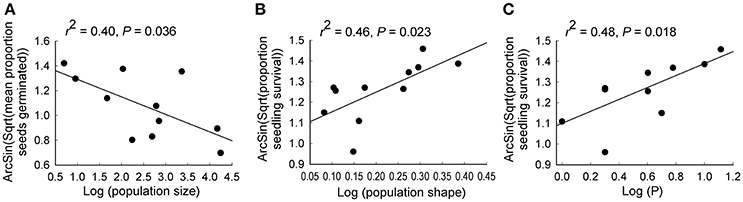
Figure 5. Relationships between (A) population size and seedling survival, (B) population shape and seed germination and (C) bicarbonate extractable P and seedling survival for 11 populations of Eucalyptus wandoo. Regression lines are presented with r2- and P-values.
Significant independent contributions were made by EC to among-population variation in seed weight, and by P to variation in seedling survival (Tables 4, 5B). The linear relationship of EC with seed weight was not significant (r2 = 0.23, P = 0.156), but P was positively related to seedling survival (r2 = 0.48, P = 0.018; Figure 5C).
Hierarchical partitioning conducted at the level of maternal plants revealed that mean seed weight made a significant independent contribution to variation in seed germination (Table 9). Trees that produced heavier seeds recorded higher germination rates (r2 = 0.15, P = 0.002). Neither of the tested mating system variables (tm and rp) contributed significantly to variation in seed weight, seedling survival or seedling dry weight (Table 9).
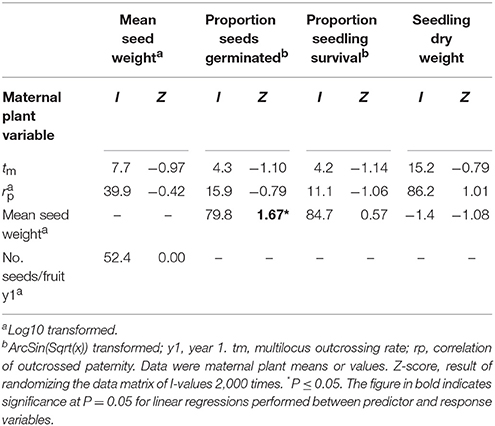
Table 9. The percentage independent contribution (I) of each of two mating system and two reproductive variables to variation in measures of progeny performance for maternal Eucalyptus wandoo plants, determined by hierarchical partitioning.
Genetic Diversity and Structure
Details of genetic diversity for populations are given in Table 7. Genetic diversity was moderate and similar to levels recorded for other eucalypts (Byrne, 2008). Population values for PL ranged from 0.56 to 0.94, Na from 1.75 to 5.75 and He from 0.327 to 0.437. Most populations contained private alleles. Significant heterozygote deficiencies were recorded for nine populations ranging from small to the largest populations.
Population size contributed significantly to variation in the proportion of loci that were polymorphic (Tables 2, 3D), with greater polymorphism recorded in larger populations (r2 = 0.53, P < 0.001), but this relationship was not observed when the four very small populations (N ≤ 9) were removed. None of the population variables (size, shape or isolation) made significant independent contributions to other genetic diversity parameters.
We found low but significant genetic differentiation among populations (overall FST = 0.033 ± 0.003, Dest = 0.008 ± 0.003; P = 0.001). After removing the three smallest populations (N ≤ 5), there was no significant IBD (r16 = 0.041, P = 0.320).
Discussion
Our study shows that investigation of a broad range of factors can reveal unexpected and unconventional relationships regarding impacts of habitat fragmentation on plant species persistence. As expected, we found that Eucalyptus wandoo trees in small populations tended to produce fewer fruit and seeds, and population selfing rates did not vary consistently with fragmentation. We also observed a very strong negative relationship between soil salinity and fruit production. However, we found some unexpected results with trees in small populations having much higher levels of pollination and seed germination, while trees in more linear populations had higher rates of seedling survival. Furthermore, increased soil phosphorous in small populations actually increased progeny performance despite the significantly reduced seed and fruit production. Our results indicate that it is critical to separate the different processes operating in this fragmented landscape in relation to progeny performance and reduced reproductive output. While increased soil salinity appears to be the major factor driving reduced fruit production, small population processes, associated with the Allee effect and inbreeding, are the key causes of reduced seed set.
Reproductive Output and Soil Salinity
Altered reproductive output in fragmented plant populations is typically attributed to biotic factors, such as pollination, mate limitation and outcrossing, while the potential role of abiotic environmental conditions is often overlooked (Saunders et al., 1991; Hobbs and Yates, 2003; Aguilar et al., 2006). Thus, in small populations the Allee effect and inbreeding are considered to be the primary contributors to reduced reproductive output. However, our data indicate that increased soil salinity, rather than biotic processes associated with small population size, is likely to be making a significantly greater contribution to reduced reproductive output in the form of reduced fruit production observed in trees from smaller E. wandoo populations. The number of fruits per branch and fruit:flower ratio were both much more strongly correlated with soil salinity than with population size. Additionally, the relationship of the number of fruits per branch with salinity was consistent across 2 years while the relationship with population size was not. Although soil salinity tended to be higher in small populations (<40 trees), which were all found on road verges, it was also high in some of the larger, non-verge remnants.
Increased salinity can reduce fruit production in crop trees (Kozlowski, 1997; García-Sánchez et al., 2003), with apparent mechanisms including ion toxicity, osmotic stress, induction of nutrient deficiency and limitation of water uptake (Al-Yassin, 2005). However, there have been few attempts to determine if a similar effect exists for plants in remnant vegetation. A study of another Myrtaceous shrub in the same landscape, Calothamnus quadrifidus, showed no relationship between soil salinity and seed or fruit set (Yates et al., 2007b), but salinity levels were lower and much less variable than in our study, as C. quadrifdus occurs in higher parts of the landscape where salinity is likely to be lower. A study of Quercus robur in Europe similarly showed no relationship between salinity and fruit weight, but all study sites were highly saline (Uhl and Wölfling, 2015). Our study sites were not visibly affected by secondary salinization, yet fruit production was nearly an order of magnitude lower in E. wandoo populations with higher soil salinity, suggesting that even relatively mild secondary salinization may reduce long-term population viability.
Reproductive Output and Small Population Processes
While reproductive output expressed as fruit production is strongly influenced by salinity, seed set in E. wandoo was strongly correlated with population size in one of 2 years, with large populations producing up to 10 times as many seeds per capsule as small populations. A reduction in seed set is one of the most consistently-observed impacts of habitat fragmentation on plants, and is usually attributed to reduced levels of pollination or outcrossing (Cunningham, 2000; Aguilar et al., 2006).
Despite the reduction in seed set, pollination rate did not decline with decreasing population size in E. wandoo, and we found no relationship between seed set and soil properties. The most likely explanation for the observed pattern is that trees in small populations receive lower quality pollen through higher proportional transfer of self-pollen. Although we found no decline in effective outcrossing rate, the likelihood of increased selfing in small populations is consistent with evidence from other Eucalyptus species that incomplete pre- or post-zygotic ovule abortion mechanisms typically lead to reduced seed set when faced with increased self-pollination (Griffin et al., 1987; Ellis and Sedgley, 1992; Kennington and James, 1997; Pound et al., 2003; Krauss et al., 2007; Gauli et al., 2014). In addition, the very high rates of pollen immigration into small E. wandoo populations (Byrne et al., 2008) appear insufficient to mitigate the impact of high selfing rates on seed set. Indeed, the preferential elimination of selfed seeds means that gene flow measured through viable seeds (Byrne et al., 2008) is likely to mask the proportion of pollen deposition in small populations that is from local trees.
Pollination and the Mating System
The much higher rates of pollination observed in the smallest E. wandoo populations may be due both to increased pollinator abundance and to more intensive pollinator foraging. Small and isolated populations can act as stepping stones in a fragmented landscape and attract pollinator visits, leading to high rates of pollen immigration (White et al., 2002; Bacles et al., 2005; Byrne et al., 2007, 2008; Ottewell et al., 2009), but levels of pollination are nevertheless usually either unchanged (Yates et al., 2007a), reduced or erratic (Aizen and Feinsinger, 1994; Ottewell et al., 2009). A similar effect may have occurred for insect pollinators within E. wandoo populations, as nutrient enrichment and enhanced productivity at edges appears to have led to greater species diversity and abundance of some canopy-dwelling insects in highly fragmented E. wandoo woodland remnants (Majer et al., 2000). In contrast, the bird-pollinated SWAFR endemic Calothamnus quadrifidus showed similar numbers of honeyeater visits and pollen tubes regardless of population size (Yates et al., 2007a), but experienced high rates of pollen immigration into small populations (Byrne et al., 2007). Within highly fragmented E. wandoo populations, pollen immigration has been shown to be substantial (Byrne et al., 2008) and locally-foraging insects may effect most local pollinations while birds may be responsible for most pollen immigration.
According to optimal foraging theory, pollinators may concentrate their foraging effort among fewer trees as population size decreases, leading to increased within-plant foraging effort (e.g., Yates et al., 2007a). This is supported by lower within-population outcrossing in several small E. wandoo populations (Byrne et al., 2008), and by the negative relationship of effective outcrossing rates with pollen tube abundance in comparisons among maternal plants. Combined with asynchronous mass-flowering that exacerbates reduced mate availability, this should lead to self-pollination being more prevalent in small populations.
Outcrossing rates for E. wandoo were comparable with those generally observed in widespread eucalypts (Byrne, 2008). The wide variation observed among and within populations may reflect individual variation in the strength of seed abortion mechanisms (McGowen et al., 2010) as well as variation in flowering intensity and asynchrony. We found no obvious relationship between effective outcrossing rates estimated in seed progeny and population fragmentation variables, consistent with many other studies in eucalypts (e.g., Ottewell et al., 2009; Breed et al., 2012, 2015). This is most likely due to the post-zygotic seed selection mechanisms in eucalypts that preferentially eliminate selfed progeny, with the surviving seeds that are used to estimate mating system parameters largely the result of outcrossing (see Kennington and James, 1997).
The presence of biparental inbreeding in all populations suggests that limited seed dispersal may result in the geographic proximity of half-sibs. We did not detect any relationship between fragmentation and either correlated paternity or biparental inbreeding, in contrast to some studies where this has been observed (Yates et al., 2007a; Mimura et al., 2009; Breed et al., 2015).
Progeny Performance
Most plant species appear to display either negative (Nason and Hamrick, 1997; Kéry et al., 2000; Breed et al., 2012; Llorens et al., 2013) or no (Costin et al., 2001; Yates et al., 2007b; Breed et al., 2015) impact of habitat fragmentation on different aspects of progeny performance. In contrast to those studies, we identified evidence in E. wandoo for enhancement of both seedling survival and seed germination with increasing fragmentation. These results appear to be highly unusual, although a study of seedlings in E. salmonophloia found larger basal stem diameters in fragmented populations than in large, unfragmented populations (Krauss et al., 2007).
Survival of E. wandoo seedlings was greater in populations with a higher shape index and was positively correlated with soil P content. In edge-dominated remnants, most trees are close to the agricultural boundary and hence have access to elevated levels of soil P and N. Supplemental addition of these nutrients typically increases shoot growth in eucalypts (Grove, 1988; Kirschbaum et al., 1992) and may also allow mature trees to better provision seeds in these nutrient-poor soils. Seeds produced in high-P environments may contain more P, which can increase offspring vigor (Marco and De Marco, 1990; Hanley and Fenner, 1997). In contrast, seedling survival for the SWAFR shrub Banksia sphaerocarpa declined as populations became more edge-dominated, a result that also appeared to be partly mediated by increased nutrient availability but via different mechanisms (Llorens et al., 2013). The influence of population shape on progeny fitness clearly warrants further investigation.
The absence of fragmentation effects on germination rates has typically been attributed to high levels of pollen immigration and/or self-incompatibility mechanisms eliminating inbred seeds (Krauss et al., 2007; Yates et al., 2007b). A combination of these is likely to have led to the observed strong trend for greater germination rates in seeds collected from smaller E. wandoo populations. Pollen immigration rates in these small populations are typically higher than local outcrossing rates (Byrne et al., 2008), while the reverse is likely to occur in large populations due to their much higher local mate availability (e.g., White et al., 2002; Bacles et al., 2005). Several studies have found immigrant pollen to produce fitter seeds than local outcross pollen due to heterosis (Dudash, 1990; Heliyanto et al., 2006; Bermingham and Brody, 2011). It is also possible that enhanced soil availability of N and P in small populations may have influenced germination rates via increased seed nutrient content.
Conservation Implications
Higher pollination rates and improved progeny performance in highly fragmented populations of E. wandoo suggest that pollinator services have been somewhat resilient to fragmentation and that soil nutrient content may play an important but poorly-understood role in influencing seed quality. Small patches and linear remnants form an integral part of a landscape-scale network of interconnected populations (Byrne et al., 2008) and our study confirms that apart from the smallest populations (<10 trees), they should form a vital component of seed sourcing strategies for restoration. Nevertheless, substantially reduced seed production may limit the use of small populations as seed sources and compromise future recruitment success.
While we have focussed here on seed production and seedling performance over the years of this study, E. wandoo is a long-lived tree (>150 years) and we are unable to assess lifetime reproductive fitness. However, we noted no recent recruitment in any populations. This is common in many remnant eucalypt woodlands in the Western Australian wheatbelt (Yates et al., 1994). The reasons for this are many fold and include changes in natural disturbance regimes which initiate episodic recruitment in tree populations (Yates et al., 1994; Parsons and Gosper, 2011), and changes in the environments of woodland remnants which have a negative impact on seedling establishment (Yates et al., 2000a). We suggest that the declines in reproductive performance for trees like E. wandoo are another critical factor affecting the long-term viability of small remnant woodlands in the region.
The dramatic decline in fruit production in some sites indicates the need to better understand sub-lethal effects of secondary soil salinity in plants growing in agricultural remnants. Attempting to mitigate rising salinity levels in remnants with the greatest conservation value may improve reproductive output in E. wandoo, a species that is a key component of remnant woodlands in this fragmented landscape. Additional active conservation management will be required to enable future recruitment into fragmented populations, as natural recruitment is unlikely to occur in these agricultural landscapes (Yates and Hobbs, 1997; Yates et al., 2000b). Tree decline is also occurring on the inland semi-arid margin of the range of E. wandoo, consistent with contraction under current changing climates (Dalmaris et al., 2015). This is likely to be one of a growing number of species that are currently under stress from fragmentation but may be further threatened by climate change.
Author Contributions
TL, CY, MB, and DC designed the research; all authors carried out the research; TL, CY, CE, JS, MB, and DC analyzed the data; and all authors contributed to the preparation and revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by Land and Water Australia, CSIRO National Research Collections Australia and the Western Australian Department of Biodiversity, Conservation and Attractions.
References
Aguilar, R., Ashworth, L., Galetto, L., and Aizen, M. A. (2006). Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol. Lett. 9, 968–980. doi: 10.1111/j.1461-0248.2006.00927.x
Aguilar, R., Quesada, M., Ashworth, L., Herrerias-Diego, Y., and Lobo, J. (2008). Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Mol. Ecol. 17, 5177–5188. doi: 10.1111/j.1365-294X.2008.03971.x
Aizen, M. A., and Feinsinger, P. (1994). Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology 75, 330–351. doi: 10.2307/1939538
Bacles, C. F. E., Burczyk, J., Lowe, A. J., and Ennos, R. A. (2005). Historical and contemporary mating patterns in remnant populations of the forest tree Fraxinus excelsior L. Evolution 59, 979–990. doi: 10.1554/04-653
Bermingham, L. H., and Brody, A. K. (2011). Pollen source affects female reproductive success and early offspring traits in the rare endemic plant Polemonium vanbruntiae (Polemoniaceae). Plant Spec. Biol. 26, 244–253. doi: 10.1111/j.1442-1984.2011.00326.x
Breed, M. F., Marklund, M. H., Ottewell, K. M., Gardner, M. G., Harris, J. B. C., and Lowe, A. J. (2012). Pollen diversity matters: revealing the neglected effect of pollen diversity on fitness in fragmented landscapes. Mol. Ecol. 21, 5955–5968. doi: 10.1111/mec.12056
Breed, M. F., Ottewell, K. M., Gardner, M. G., Marklund, M. H., Dormontt, E. E., and Lowe, A. J. (2015). Mating patterns and pollinator mobility are critical traits in forest fragmentation genetics. Heredity (Edinb). 115, 108–114. doi: 10.1038/hdy.2013.48
Brouwers, N. C., Mercer, J., Lyons, T., Poot, P., Veneklaas, E., and Hardy, G. (2013). Climate and landscape drivers of tree decline in a Mediterranean ecoregion. Ecol. Evol. 3, 67–79. doi: 10.1002/ece3.437
Bunnell, F. L. (1999). “Foreword. Let's kill a panchreston: giving fragmentation meaning,” in Forest Fragmentation: Wildlife and Management Implications, eds J. A. Rochelle, L. A. Lehmann, and J. Wisniewsi (Leiden: Brill), vii-xiii.
Burrows, G. E. (2000). Seed production in woodland and isolated trees of Eucalyptus melliodora (yellow box, Myrtaceae) in the South Western Slopes of New South Wales. Aust. J. Bot. 48, 681–685. doi: 10.1071/BT99058
Butcher, P. A., Skinner, A. K., and Gardiner, C. A. (2005). Increased inbreeding and inter-species gene flow in remnant populations of the rare Eucalyptus benthamii. Conserv. Genet. 6, 213–226. doi: 10.1007/s10592-004-7830-x
Byrne, M. (2008). “Phylogeny, diversity and evolution of eucalypts,” in Plant Genome: Biodiversity and Evolution, 1E: Phanerogams – Angiosperm, eds A.K. Sharma and A. Sharma (Enfield: Science Publishers), 303–346.
Byrne, M., Elliott, C. P., Yates, C., and Coates, D. J. (2007). Extensive pollen dispersal in a bird-pollinated shrub, Calothamnus quadrifidus, in a fragmented landscape. Mol. Ecol. 16, 1303–1316. doi: 10.1111/j.1365-294X.2006.03204.x
Byrne, M., Elliott, C. P., Yates, C. J., and Coates, D. J. (2008). Maintenance of high pollen dispersal in Eucalyptus wandoo, a dominant tree of the fragmented agricultural region in Western Australia. Conserv. Genet. 9, 97–105. doi: 10.1007/s10592-007-9311-5
Byrne, M., Parrish, T. L., and Moran, G. F. (1998). Nuclear RFLP diversity in Eucalyptus nitens. Heredity (Edinb). 81, 225–233. doi: 10.1046/j.1365-2540.1998.00386.x
Coates, D. J. (1988). Genetic diversity and population genetic structure in rare Chittering grass wattle, Acacia anomala Court. Aust. J. Bot. 36, 273–286. doi: 10.1071/BT9880273
Coates, D. J., Sampson, J. F., and Yates, C. J. (2007). Plant mating systems and assessing population persistence in fragmented landscapes. Aust. J. Bot. 55, 239–249. doi: 10.1071/BT06142
Costin, B. J., Morgan, J. W., and Young, A. G. (2001). Reproductive success does not decline in fragmented populations of Leucochrysum albicans subsp. albicans var. tricolor (Asteraceae). Biol. Conser. 98, 273–284. doi: 10.1016/S0006-3207(00)00165-8
Cramer, V. A., and Hobbs, R. J. (2002). Ecological consequences of altered hydrological regimes in fragmented ecosystems in southern Australia: impacts and possible management responses. Austral Ecol. 27, 546–564. doi: 10.1046/j.1442-9993.2002.01215.x
Cramer, V. A., Hobbs, R. J., Atkins, L., and Hodgson, G. (2004). The influence of local elevation on soil properties and tree health in remnant eucalypt woodlands affected by secondary salinity. Plant Soil 265, 175–188. doi: 10.1007/s11104-005-0889-4
Cunningham, S. A. (2000). Depressed pollination in habitat fragments causes low fruit set. Philos. Trans. R. Soc. Lond. B Biol. Sci. 267, 1149–1152. doi: 10.1098/rspb.2000.1121
Dalmaris, E., Ramalho, C. E., Poot, P., Veneklaas, E. J., and Byrne, M. (2015). A climate change context for the decline of a foundation tree species in south-western Australia: insights from phylogeography and species distribution modelling. Ann. Bot. 116, 941–952. doi: 10.1093/aob/mcv044
Dudash, M. R. (1990). Relative fitness of selfed and outcrossed progeny in a self-compatible, protandrous species, Sabatia angularis L. (Gentianaceae): a comparison in three environments. Evolution 44, 1129–1139. doi: 10.1111/j.1558-5646.1990.tb05220.x
Eckert, C. G., Kalisz, S., Geber, M. A., Sargent, R., Elle, E., Cheptou, P.-O., et al. (2010). Plant mating systems in a changing world. Trends Ecol. Evol. (Amst). 25, 35–43. doi: 10.1016/j.tree.2009.06.013
Ellis, M. F., and Sedgley, M. (1992). Floral morphology and breeding system of three species of Eucalyptus, section Bisectaria (Myrtaceae). Aust. J. Bot. 40, 249–262. doi: 10.1071/BT9920249
Fahrig, L. (2003). Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Syst. 34, 487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419
Ford, H. A., Barrett, G. W., Saunders, D. A., and Recher, H. F. (2001). Why have birds in the woodlands of Southern Australia declined? Biol. Conserv. 97, 71–88. doi: 10.1016/S0006-3207(00)00101-4
García-Sánchez, M., Carvajal, M., Porras, I., Botía, P., and Martínez, V. (2003). Effects of salinity and rate of irrigation on yield, fruit quality and mineral composition of ‘Fino 49’ lemon. Eur. J. Agron. 19, 427–437. doi: 10.1016/S1161-0301(02)00138-7
Gauli, A., Vaillancourt, R. E., Steane, D. A., Bailey, T. G., and Potts, B. M. (2014). Effect of forest fragmentation and altitude on the mating system of Eucalyptus pauciflora (Myrtaceae). Aust. J. Bot. 61, 622–632. doi: 10.1071/BT13259
González-Varo, J. P., Arroyo, J., and Aparicio, A. (2009). Effects of fragmentation on pollinator assemblage, pollen limitation and seed production of Mediterranean myrtle (Myrtus communis). Biol. Conserv. 142, 1058–1065. doi: 10.1016/j.biocon.2009.01.017
Griffin, A. R., Moran, G. F., and Fripp, Y. J. (1987). Preferential outcrossing in Eucalyptus regnans F. Muell. Aust. J. Bot. 35, 465–475. doi: 10.1071/BT9870465
Grove, T. S. (1988). Growth responses of trees and understorey to applied nitrogen and phosphorus in karri (Eucalyptus diversicolor) forest. Forest Ecol. Manag. 23, 87–103. doi: 10.1016/0378-1127(88)90076-X
Hanley, M. E., and Fenner, M. (1997). Seedling growth of four fire-following Mediterranean plant species deprived of single mineral nutrients. Funct. Ecol. 11, 398–405. doi: 10.1046/j.1365-2435.1997.00104.x
Heliyanto, B., Krauss, S. L., Lambers, H., Cawthray, G. R., and Veneklaas, E. J. (2006). Increased ecological amplitude through heterosis following wide outcrossing in Banksia ilicifolia R.Br. (Proteaceae). J. Evolution Biol. 19, 1327–1338. doi: 10.1111/j.1420-9101.2005.01067.x
Hingston, A. B., Potts, B. M., and McQuillan, P. B. (2004). The swift parrot Lathamus discolor (Psittacidae), social bees (Apidae), and native insects as pollinators of Eucalyptus globulus ssp. globulus (Myrtaceae) Aust. J. Bot. 52, 371–379. doi: 10.1071/BT03018
Hobbs, R. J., and Yates, C. J. (2003). Impacts of ecosystem fragmentation on plant populations: generalising the idiosyncratic. Aust. J. Bot. 51, 471–488. doi: 10.1071/BT03037
Kalinowski, S. T. (2005). HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol. Ecol. Notes 5, 187–189. doi: 10.1111/j.1471-8286.2004.00845.x
Kennington, W. J., and James, S. H. (1997). The effect of small population size on the mating system of a rare clonal mallee, Eucalyptus argutifolia (Myrtaceae). Heredity (Edinb). 78, 252–260. doi: 10.1038/hdy.1997.39
Kéry, M., Matthies, D., and Spillman, H.-H. (2000). Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea. J. Ecol. 88, 17–30. doi: 10.1046/j.1365-2745.2000.00422.x
Kirschbaum, M. U. F., Bellingham, D. W., and Cromer, R. N. (1992). Growth analysis of the effect of phosphorus nutrition on seedings of Eucalyptus grandis. Aust. J. Plant Physiol. 19, 55–66. doi: 10.1071/PP9920055
Kozlowski, T. T. (1997). Responses of woody plants to flooding and salinity. Tree Physiol. Monogr. doi: 10.1093/treephys/17.7.490
Krauss, S. L., Hermanutz, L., Hopper, S. D., and Coates, D. J. (2007). Population-size effects on seeds and seedlings from fragmented eucalypt populations: implications for seed sourcing for ecological restoration. Aust. J. Bot. 55, 390–399. doi: 10.1071/BT06141
Lamont, B. B., Rees, R. G., Witkowski, E. T. F., and Whitten, V. A. (1994). Comparative size, fecundity and ecophysiology of roadside plants of Banksia hookeriana. J. Appl. Ecol. 31, 137–144. doi: 10.2307/2404606
Llorens, T. M., Byrne, M., Yates, C. J., Nistelberger, H. M., and Coates, D. J. (2012). Evaluating the influence of different aspects of habitat fragmentation on mating patterns and pollen dispersal in the bird-pollinated Banksia sphaerocarpa var. caesia. Mol. Ecol. 21, 314–328. doi: 10.1111/j.1365-294X.2011.05396.x
Llorens, T. M., Yates, C. J., Byrne, M., Nistelberger, H. M., Williams, M. R., and Coates, D. J. (2013). Complex interactions between remnant shape and the mating system strongly influence reproductive output and progeny performance in fragmented populations of a bird-pollinated shrub. Biol. Conserv. 164, 129–139. doi: 10.1016/j.biocon.2013.05.011
Mac Nally, R. (2002). Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodivers. Conserv. 11, 1397–1401. doi: 10.1023/A:1016250716679
Major, R. E., Christie, F. J., and Gowing, G. (2001). Influence of remnant and landscape attributes on Australian woodland bird communities. Biol. Conserv. 102, 47–66. doi: 10.1016/S0006-3207(01)00090-8
Majer, J. D., Recher, H., and Keals, N. (2000). “Canopy arthropod faunas in fragmented agricultural landscapes,” in Temperate Eucalypt Woodlands in Australia: Biology, Conservation, Management and Restoration, eds R. J. Hobbs and C. J. Yates (Chipping Norton: Surrey Beatty & Sons), 235–247.
Marco, D. G. D., and De Marco, D. G. (1990). Effect of seed weight, and seed phosphorus and nitrogen concentrations on the early growth of wheat seedlings. Aust. J. Exp. Agr. 30, 545–549. doi: 10.1071/EA9900545
McArthur, W. M. (1991). Reference Soils of South-Western Australia. Perth, WA: Department of Agriculture.
McGowen, M. H., Vaillancourt, R. E., Pilbeam, D. J., and Potts, B. M. (2010). Sources of variation in self-incompatibility in the Australian forest tree, Eucalyptus globulus. Ann. Bot. 105, 737–745. doi: 10.1093/aob/mcq036
Mimura, M., Barbour, R. C., Potts, B. M., Vaillancourt, R. E., and Watanabe, K. N. (2009). Comparison of contemporary mating patterns in continuous and fragmented Eucalyptus globulus native forests. Mol. Ecol. 18, 4180–4192. doi: 10.1111/j.1365-294X.2009.04350.x
Nason, J. D., and Hamrick, J. L. (1997). Reproductive and genetic consequences of forest fragmentation: two case studies of neotropical canopy trees. J. Hered. 88, 264–276. doi: 10.1093/oxfordjournals.jhered.a023104
Ottewell, K. M., Donnellan, S. C., Lowe, A. J., and Paton, D. C. (2009). Predicting reproductive success of insect- versus bird-pollinated scattered trees in agricultural landscapes. Biol. Conserv. 142, 888–898. doi: 10.1016/j.biocon.2008.12.019
Ouborg, N. J., Vergeer, P., and Mix, C. (2006). The rough edges of the conservation genetics paradigm for plants. J. Ecol. 94, 1233–1248. doi: 10.1111/j.1365-2745.2006.01167.x
Parsons, B. C., and Gosper, C. R. (2011). Contemporary fire regimes in a fragmented and an unfragmented landscape: implications for vegetation structure and persistence of the fire-sensitive malleefowl. Int. J. Wildl. Fire. 20, 184–194. doi: 10.1071/WF09099
Patton, D. R. (1975). A diversity index for quantifying habitat “edge”. Wildl. Soc. Bull. 3, 171–173.
Peakall, R., and Smouse, P. E. (2012). GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics 28, 2537–2539. doi: 10.1093/bioinformatics/bts460
Pound, L. M., Wallwork, M. A. B., Potts, B. M., and Sedgley, M. (2003). Pollen tube growth and early ovule development following self- and cross-pollination in Eucalyptus nitens. Sex. Plant Reprod. 16, 59–69. doi: 10.1007/s00497-003-0175-7
Ritland, K. (2002). Extensions of models for the estimation of mating systems using n independent loci. Heredity (Edinb). 88, 221–228. doi: 10.1038/sj.hdy.6800029
R Development Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rousset, F. (2008). Genepop'007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 8, 103–106. doi: 10.1111/j.1471-8286.2007.01931.x
Saunders, D. A., Hobbs, R. J., and Margules, C. R. (1991). Biological consequences of ecosystem fragmentation: a review. Conserv. Biol. 5, 18–32. doi: 10.1111/j.1523-1739.1991.tb00384.x
Uhl, B., and Wölfling, M. (2015). Anthropogenic influences on the condition of Pinus pinea L. and Quercus robur L. in Pineta san Vitale (Ravenna, Italy). J. Mediterr. Ecol. 13, 5–12.
Walsh, C., and Mac Nally, R. (2013). Package hier.part: Hierarchical Partitioning, version 1.0-4. Available online at: https://cran.r-project.org/web/packages/hier.part
Weir, B. S., and Cockerham, C. C. (1984). Estimating F -statistics for the analysis of population structure. Evolution 38, 1358–1370.
White, G. M., Boshier, D. H., and Powell, W. (2002). Increased pollen flow counteracts fragmentation in a tropical dry forest: an example from Swietenia humilis Zuccarini. Proc. Natl. Acad. Sci. U.S.A. 99, 2038–2042. doi: 10.1073/pnas.042649999
Yates, C. J., Hobbs, R. J., and Bell, R. W. (1994). Landscape-scale disturbances and regeneration in semi-arid woodlands of south-western Australia. Pac. Cons. Biol. 1, 214–221. doi: 10.1071/PC940214
Yates, C. J., and Hobbs, R. J. (1997). Temperate eucalypt woodlands: a review of their status, processes threatening their persistence and techniques for restoration. Aust. J. of Bot. 45, 949–973. doi: 10.1071/BT96091
Yates, C. J., Norton, D. A., and Hobbs, R. J. (2000a). Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: implications for restoration. Aust. Ecol. 25, 26–47. doi: 10.1046/j.1442-9993.2000.01030.x
Yates, C. J., Hobbs, R. J., and Atkins, L. (2000b). Establishment of perennial shrub and tree species in degraded Eucalyptus salmonophloia (salmon gum) remnant woodlands: effects of restoration treatments. Rest. Ecol. 8, 135–143. doi: 10.1046/j.1526-100x.2000.80020.x
Yates, C. J., Coates, D. J., Elliott, C. P., and Byrne, M. (2007a). Composition of the pollinator community, pollination and the mating system for a shrub in fragments of species rich kwongan in south-west Western Australia. Biodivers. Conserv. 16, 1379–1395. doi: 10.1007/s10531-006-6736-y
Keywords: habitat fragmentation, reproduction, mating system, progeny performance, soil salinity, population size, linear populations
Citation: Llorens TM, Yates CJ, Byrne M, Elliott CP, Sampson J, Fairman R, Macdonald B and Coates DJ (2018) Altered Soil Properties Inhibit Fruit Set but Increase Progeny Performance for a Foundation Tree in a Highly Fragmented Landscape. Front. Ecol. Evol. 6:39. doi: 10.3389/fevo.2018.00039
Received: 10 November 2017; Accepted: 26 March 2018;
Published: 12 April 2018.
Edited by:
James Guy Castley, Griffith University, AustraliaReviewed by:
John Morgan, La Trobe University, AustraliaGrant Wesley Wardell-Johnson, Curtin University, Australia
Copyright © 2018 Llorens, Yates, Byrne, Elliott, Sampson, Fairman, Macdonald and Coates. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David J. Coates, ZGF2ZS5jb2F0ZXNAZGJjYS53YS5nb3YuYXU=
 Tanya M. Llorens
Tanya M. Llorens Margaret Byrne
Margaret Byrne David J. Coates
David J. Coates