
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 25 October 2017
Sec. Urban Ecology
Volume 5 - 2017 | https://doi.org/10.3389/fevo.2017.00132
This article is part of the Research Topic Integrating Transport Infrastructures with Living Landscapes View all 16 articles
Ants provide a common dispersal vector for a variety of plants in many environments through a process known as myrmecochory. The efficacy of this dispersal mechanism can largely determine the ability of species to track changes in habitat availability caused by ongoing land-use and associated disturbances, and can be critical for population gene flow and persistence. Field studies were conducted in a typical fragmented agricultural landscape in southern NSW, Australia, to investigate the extent to which dispersal services by ants are influenced by anthropogenic disturbances associated with roadwork activities (i.e., soil disturbance as the result of grading of roads). Observational experiments were performed in road segments that were divided into disturbed and non-disturbed zones, where Acacia pycnantha seeds were offered at multiple bait stations and monitored. For combined species, the mean dispersal distance recorded in the disturbed zone (12.2 m) was almost double that recorded in the non-disturbed zone (5.4 m) for all roadside sites. Our findings show that myrmecochory is an unevenly diffuse mutualism, where few ant species contributed to much of the dispersal of seeds. Iridomyrmex purpureus was responsible for all seed dispersal distances >17 m, where a maximum of 120 m in disturbed, vs. 69 m in non-disturbed zones, was recorded. Rhytidoponera metallica and Melophorus bruneus were important seed dispersers in non-disturbed and disturbed zones, respectively. In general, large bodied ants tended to move more seeds to longer distances in disturbed zones, as opposed to non-disturbed zones, where smaller bodied species carried out a greater percentage of short distance dispersals (<1 m). We also recorded secondary dispersal events from nests by I. purpureus, a phenomenon previously not quantified. Infrequent, long distance dispersal to suitable sites may be highly important for seedling recruitment in disturbed or modified habitats in otherwise highly fragmented rural environments.
For many plants, seed dispersal mechanisms following the end of the reproductive cycle are critical for the establishment of future offspring (Wang and Smith, 2002; Vander Wall and Longland, 2004). Plants use various strategies to disperse their propagules into new habitats, such as by wind, water, vertebrate or ants (van der Pijl, 1982; Nathan and Muller-Landau, 2000). Dispersal distances can range from < 1 m (i.e., ants) to > 100 km (i.e., wind) (Corlett, 2009; Thomson et al., 2010). However, the limited dispersal distances provided by some dispersal agents (i.e., ballistic and/or ant-dispersal) may restrain the extent to which plants may colonize new habitats. As a result, recruitment limitations can occur, particularly in habitats subjected to frequent disturbance activities (i.e., natural and/or anthropogenic). This issue can affect population growth and persistence, unless other forms of infrequent long dispersal events occur (Cain et al., 2000; Nathan et al., 2008).
Seed dispersal by ants (myrmecochory) is a globally significant driver of plant diversity and population dynamics (Lengyel et al., 2010). Myrmecochorous plants dominate communities in many habitats, in terms of both species richness and abundance (Berg, 1975; Beattie, 1985; Lengyel et al., 2009). There are numerous ant species that remove and transport seeds, however most dispersal events are performed by a few key removers (e.g., Andersen, 1988; Gove et al., 2007; Zelikova and Breed, 2008). Ants generally disperse seeds only short distances to their nests where they remove the elaiosome to feed to their larvae (Culver and Beattie, 1978; Hughes and Westoby, 1992; Rowles and O'Dowd, 2009; Gómez and Espadaler, 2013). However, infrequent long distance dispersal events can also occur by ants (Andersen, 1988). Maximum recorded seed dispersal distances are 77 m (Davidson and Morton, 1981) and 180 m (Whitney, 2002); both recorded in Australia.
The average dispersal distance, and the nature of the seed dispersal curve, play a crucial role in the rate of colonization of propagules to new sites (Andersen, 1988; Portnoy and Willson, 1993). Recent studies have also highlighted that myrmecochory can be a multiphase process, where after an initial transport to a nest, seeds may then be discarded away from the nest via a secondary dispersal process (Beaumont et al., 2012). Ants may remove discarded seeds to nearby rubbish heaps (Berg, 1975) or be relocated further distances away from nest entrances (Hughes and Westoby, 1992; Lubertazzi et al., 2010; Canner et al., 2012). On most occasions, the elaiosome of the discarded seed is removed (Hughes and Westoby, 1992; Canner et al., 2012), which assists with breaking seed dormancy and influence subsequent germination success of species (Pacini, 1990; Lobstein and Rockwood, 1993).
Infrequent long-distance dispersal events by ants, and seed discarding behavior from ant nests, can have various implications for the fate and distribution of seeds. The extent of this dispersal service is strongly tied to the composition and behavior of the ant species involved, and influenced by prevailing disturbance regimes (e.g., Beaumont et al., 2012). Disturbances can lead to increased dispersal distances, possibly due to simplification of the foraging landscape for ants (Parr et al., 2007). In many habitats, increased anthropogenic activity poses a threat for many species, where understanding the factors that influence ant-plant interactions, such as seed dispersal into new environments, is critical for conservation management (Thomson et al., 2010; Sorrells and Warren, 2011).
We investigated seed dispersal processes by ants in relation to soil disturbances. We undertook investigations in roadside environments in southern NSW, Australia, which provide refuge for endangered woodland communities and associated species (Benson, 1991; Schabel and Eldridge, 2001; Spooner and Lunt, 2004). These novel environments are maintained by anthropogenic activities, such as grading of the road surface and adjacent boundary, which affect existing vegetation along roads, by removing plant biomass and creating a bare soil surface. This process can influence seed dispersal, recruitment and overall plant persistence, depending on their life-history traits (Lugo and Gucinski, 2000; Gelbard and Belnap, 2003; Spooner, 2005). The main objectives of the study were: (i) to investigate the influence of soil disturbance on seed dispersal distances, (ii) identify the relative contribution of individual ant species to this process, and (iii) determine the extent of potential secondary seed dispersal performed by ants.
This study is part of a larger research project investigating seed dispersal processes by ants in roadsides and the detailed description of the study area and site selection can be found in a preceding paper (Palfi et al., 2017). In summary, field work was carried out in the Lockhart Shire, a rural local government area located in southern NSW, Australia. The region has a cool temperate climate, with mean annual rainfall ranging from 450 to 600 mm, and altitude ranging from 200 to 450 m. Topography consists of low undulating hills and flat riverine plains, with sporadic granite and porphyry outcrops (Lockhart Shire Council, 2013). Much of the area is arable farmland dominated by cropping and grazing farm systems, subdivided by a network of minor and major roads. As much of the landscape is cleared or highly fragmented, these roads often harbor the last vestiges of “intact” remnant woodlands, grasslands, and other ecosystems (Lunt and Bennett, 2000).
The Lockhart study area contains a large network of roads (~1,600 km), where minor roads represent almost half of the total road network (750 km; Spooner et al., 2004). Minor roads are maintained by human soil disturbance regimes which although deleterious to many plants, can be advantageous to others, depending on their life history and dispersal traits (Lugo and Gucinski, 2000; Gelbard and Belnap, 2003; Spooner, 2005). In previous studies, historical roadwork activities were shown to facilitate the recruitment and persistence of roadside plant populations (Spooner et al., 2004), and promote some Acacia species (Spooner, 2005). Acacias are important and widespread myrmecochorous genera in Australia and elsewhere (Berg, 1975). As such, roadside environments provide an ideal context to study the impact of soil disturbance on mutualistic interactions such as myrmecochory. The research was confined to this area to ensure that a consistent approach to local government road management was applied across the study area.
A stratified random sampling approach was used to select 24 roadside sites located in the Lockhart Shire council area, southern NSW, where common Acacia populations were known to occur (Bull, 1997; Spooner, 2005). Each roadside site can be subdivided into two parts: (1) the disturbed zone adjacent to the road surface (1–5 m from the road edge), which is frequently disturbed (1–2 times per year) by mechanical soil grading operations to clear all above ground vegetation and top 1 cm of soil, adjacent to the road drainage line (hereafter the disturbed zone); and (2) the undisturbed road verge beyond the disturbed zone where intact vegetation occurs, where no direct effects of grading were visible (i.e., hereafter referred to as the non-disturbed zone). Furthermore, as roadside environments occur at varying widths, which in turn influence habitat and disturbance conditions (Spooner and Lunt, 2004), sites were stratified into two groups: narrow (3–14 m) or wide (15–60 m) roadsides (for disturbed and non-disturbed zones combined).
To assess ant dispersal distances, “Cafeteria” style experiments were carried out. Seed depots of ~9 × 9 cm in size were placed at 5 m intervals along two 25 m transects, located parallel to each other in both the disturbed and non-disturbed zones of the selected roadside site. These transects were separated longitudinally by a gap width of 10–20 m (minimum 10 m in case of narrow road verges). At each depot, 10 seeds of Acacia pycnantha Benth. were placed on the ground. Seeds were collected prior to commencing the study during the seed ripening period in 2013 (November-December). The research was carried out during the summer months of December 2013 to March 2014, where two observational periods were appointed: the morning session consisted from 8:00 to 12:00, and the afternoon session from 16:00 to 20:00, time periods comprising highest ant activities.
A seed dispersal event was considered when ants removed seeds at least 5 cm away from the edge of the seed depot, and subsequently, these ants were followed until a destination point was reached. Dispersal distances were measured from the respective seed depot to a given nest (or drop point) using a 50 m tape measure. The fate of dropped seeds (if they were further removed or not) was not monitored, however the dispersal distance of such seeds was also recorded. As Iridomyrmex purpureus were common and dispersed seeds often great distances, we did not directly measure every dispersal event performed by this species to its termination point. Rather, the first I. purpureus individual transporting a seed from the bait station was followed until it reached a particular nest. I. purpureus nests are large and conspicuous, where individual nests are normally situated sufficiently far from each other. Following individuals heading toward the same nest site were then only monitored for ~5 m from the bait station, until it could be assumed it would complete the journey to this nest (and this distance recorded). Previous trial experiments proved that a large percent (90%) of I. purpureus individuals that carried the offered seeds beyond 5 m of the seed bait stations would reach their nests without dropping them. This method was developed in order to maximize the number of observations of ants at the bait stations during the survey period (4 h per session).
All other dispersal events by other ant species were monitored to their final destination, and the distance recorded. If individual ants could not be identified during field work, they were followed until a drop point or ant nest and were captured and placed into vials filled with 70% ethanol for later identification. Collected individuals were later identified in lab condition using field guides or expert advice (Andersen pers. comm. 2014).
To investigate secondary seed dispersal events, we focussed on Iridomyrmex purpureus nests, which were abundant in the sampled roadsides. We randomly selected a total of 34 independent I. purpureus nests (disturbed n = 14; non-disturbed n = 20) at the combined roadside sites. Acacia pycnantha seeds were again used to perform these observations, which were collected during the ripening period of 2014 November (prior to the relocation experiment in December 2014). Observations were conducted in the morning period between 9.00 and 12.00 h when most ant activity occurs. As I. purpureus builds large and conspicuous nests which have numerous ant entrances, we randomly placed a total of 15 individual seeds/nests ~5 cm from different nest entrances. Elaiosomes were previously detached of seeds to stimulate seed manipulation behavior of ants that often induces seed discarding behavior from ant nests (e.g., Martins et al., 2006). Seeds were placed individually to a random entrance and if an ant displaced the seed the distance was recorded.
To compare the mean dispersal distances between disturbed and non-disturbed zones and roadside width we used mixed models (factorial ANOVA) where zone and width were entered as fixed factors, and sites as random factor. To ensure equal weights to each station rather than number of seeds, we first calculated the average dispersal distance for each depot then calculated the average for each zone and site to get the site average distance. Data was log-transformed if the assumption of normality was not met. As I. purpureus contributed to all dispersal distances >17 m, we used the same statistical configuration to test for differences in mean dispersal distance with data for I. purpureus omitted.
Differences in dispersal distances between zones and widths were investigated for ant species with sufficient dispersal measurements, or more precisely when a species was present in both zones of a site (at least 10 sites). Due to the paired nature of the study design, sites were not included unless dispersal distances were recorded in both zones. Paired T-test or Wilcoxon Signed Rank test was used if the data were normally or not normally distributed, respectively. Descriptive statistics were used to describe differences in mean dispersal distances achieved by respective species in differing zones and roadside widths. A frequency histogram of dispersal distances was constructed to generate a dispersal curve, by calculating the relative frequencies of each zone within each site, then a single composite histogram was aggregated by giving equal weight to each site. By doing this we were able to take site variability into account where the number of dispersal events greatly varied due to the patchy nature of ant activities (Nelson, 2014). The Kolmogorov-Smirnov test was used to test whether the two distributions differed between the zones.
In order to ascertain differences in secondary dispersal distances by I. purpureus between disturbed and non-disturbed zones, we calculated the average relocation distance for each I. purpureus nest and used a paired Student's t-test with Satterthwaite's correction to compare relocation distances between disturbed and non-disturbed zones. All statistical tests were carried out using R statistical software (R Development Core Team, 2013).
Overall, mean seed dispersal distances were significantly influenced by soil disturbance (zone) (F = 4.9, df = 1.22, p = 0.03) and roadside width (F = 6.1, df = 1.22, p = 0.02) (Figure 1). For combined species, the mean dispersal distance recorded in the disturbed zone was almost double that recorded in the non-disturbed zone (12.2 m ± 3.71 SE vs. 5.4 m ± 1.10 SE, respectively) for all roadside widths combined, while wide roadsides had longer dispersal distances than narrow roadsides. Nevertheless, narrow roadsides had both the longest (14.5 m ± 7.2 SE) and shortest (2.9 m ± 0.81 SE) mean dispersal distances in the disturbed and non-disturbed zones, respectively, while there was no statistical difference in dispersal distances between disturbed and non-disturbed zones in wide roadsides (Figure 1).
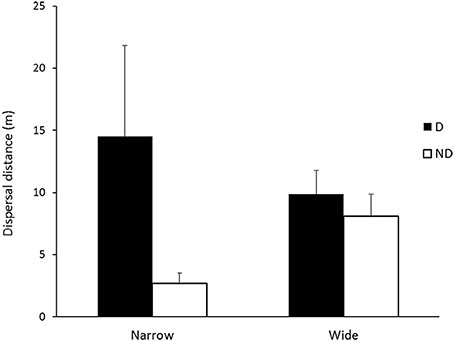
Figure 1. Mean dispersal distances (m) carried out by seed-dispersing ant species in disturbed (D) and non-disturbed (ND) zones in narrow (n = 579 in D, n = 376 in ND) and wide roadsides (n = 442 in D, n = 553 in ND) in the Lockhart Shire, southern NSW. Error bars represent standard error of the mean.
The main seed dispersing ant genera observed were Iridomyrmex (43% of total dispersal events), Rhytidoponera (37%), and Melophorus (10%) (Table 1). Overall, 19 ant species contributed to the dispersal of seeds in roadside environments, with I. purpureus and R. metallica dominating the seed dispersal activities with 31 and 28%, respectively (data combined across sites and zones). When mean dispersal distances for combined species were re-analyzed by omitting I. purpureus, there were no significant differences in mean distances between road disturbance zone or road verge width (F = 2.33, df = 1.22; p > 0.05).
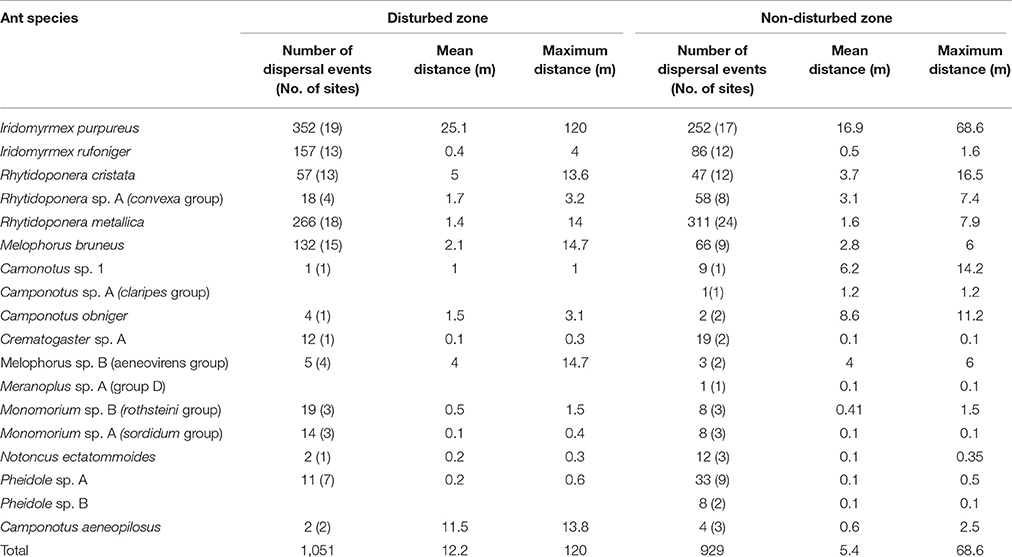
Table 1. Number of seed dispersal events, number of sites (n = 24) each species occurred at, and mean and maximum dispersal distances, recorded for ant species in disturbed and non-disturbed roadside sites in southern NSW, Lockhart Shire.
There were no significant differences in the mean dispersal distances of individual species between disturbed and non-disturbed zones, roadsides of different width or the interaction effect (p > 0.05) However, there were trends in mean dispersal distances of species according to either disturbance zone and/or roadside width (Figures 2, 3, Table 1). Iridomyrmex purpureus dispersed the most seeds over the greatest distances in the disturbed zone (mean = 25.1 m, maximum = 120 m; Table 1). Their average dispersal distances were considerably greater than any other species' (Figure 2 vs. Figure 3), and reflect the overall pattern for combined species, where the mean seed dispersal distance was greatest in disturbed zone of roadsides (Figure 3).
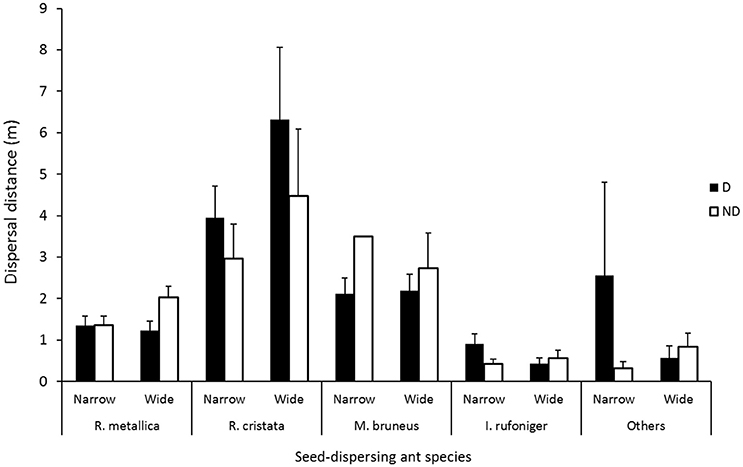
Figure 2. Mean dispersal distances for individual ant species (I. purpureus excluded) in disturbed (D; n = 669) and non-disturbed (ND; n = 677) zones in narrow and wide roadsides. Others = all other remaining species combined (see Table 1). Error bars represent standard error of the mean.
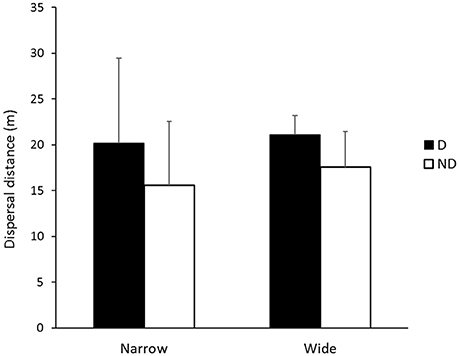
Figure 3. Mean dispersal distances (m) for Iridomyrmex purpureus in disturbed (D; n = 352) and non-disturbed (ND; n = 252) zones in narrow and wide roadside environments. Error bars represent standard error of the mean.
The mean dispersal distance for Rhytidoponera cristata for combined sites was 4.40 m ± 0.64 SE, and was greater in disturbed zones for both wide and narrow roadsides (Figure 2, Table 1). In contrast, the mean dispersal distance for Melophorus bruneus was longer in the non-disturbed zone of both wide and narrow roadsides (Figure 2). Rhytidoponera metallica also followed this pattern for wide roadsides. Mean dispersal distances for Iridomyrmex rufoniger were much lower than any other species' (mean 0.60 ± 0.09 m). Some “other” species showed slight preferences for disturbance zone and/or roadside width, where the long mean dispersal distance in the disturbed zone of narrow roadsides was due to activities of Camponotus species (Figure 2).
A high frequency (64%) of recorded seed dispersal events was <3 m, where a further 20% of dispersals were carried out at distances ranging 10–40 m (Figure 4). The tail end of the dispersal curve shows small frequencies of long distance dispersal events up to 120 m in the disturbed zone. In contrast, the maximum dispersal distance recorded in the non-disturbed zone was 70 m (Figure 4). The relative high frequency of seed dispersal distances <3 m was generally similar in both disturbed and non-disturbed zones, however dispersal events >5 m tended to occur more frequently in the disturbed zone of roadside environments (Figure 4). Nevertheless, there were no significant differences in the frequency of seed dispersal events between the zones or roadside widths (p > 0.05). The maximum distances recorded in this study were by I. purpureus: 69 m in non-disturbed and 120 m in disturbed zones.
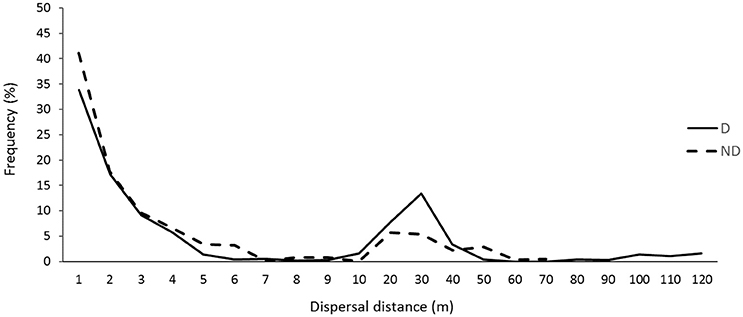
Figure 4. Overall seed dispersal curve across all seed-dispersing ants. Seed dispersal distances are grouped in distance categories (m) in disturbed (D; n = 1,021) and non-disturbed (ND; n = 929) zones of roadside environments in the Lockhart Shire, southern NSW.
There was a significant difference in the secondary dispersal distances I. purpureus carried out between disturbed and non-disturbed zones (t = 4.12, df = 20.73, p < 0.001; Figure 5).
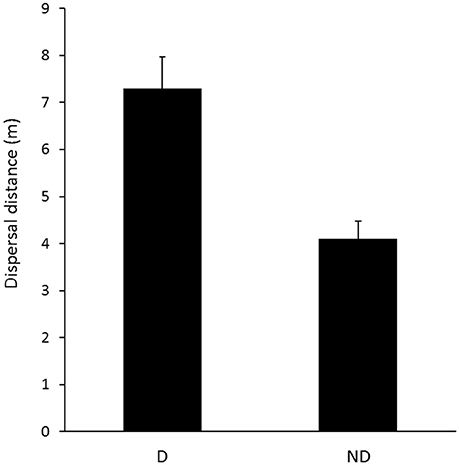
Figure 5. Mean secondary dispersal distances performed by I. purpureus colonies between disturbed (D; n = 111) and non-disturbed (ND; n = 169) zones in the Lockhart Shire. Error bars represent standard error of the mean.
The ant genera responsible for most seed dispersals were Iridomyrmex, Rhytidoponera and Melophorus. Other studies have also found that these genera are the most important dispersers involved in myrmecochory throughout Australia (Berg, 1975; Andersen and Morrison, 1998; Beaumont et al., 2012). In this study, a total of 18 species were recorded dispersing seeds, with Iridomyrmex purpureus and Rhytidoponera metallica being associated with the most seed dispersals (31 and 28% respectively), the former with maximum dispersal distances. Melophorus bruneus carried out a substantial amount of seed dispersal events as well (10%). Pheidole is also a well-known seed taking genus (Beaumont et al., 2012), however it was not a prominent disperser in our study. In general, our findings are consistent with the assertion that myrmecochory is an unevenly diffuse mutualism (Gove et al., 2007), that is, few ant species are responsible for the majority of seed dispersal.
Soil disturbance had a significant effect on seed dispersal distances for combined species, where mean distances were greater in disturbed as compared to non-disturbed zones, and the difference was more pronounced in narrow roadsides. This finding can be largely explained by results for I. purpureus (see below) which strongly influenced our overall findings, as with data for I. purpureus excluded, mean dispersal distances resemble those of other myrmecochorous studies (as reviewed by Gómez and Espadaler, 2013). In a recent review of myrmecochory, Gómez and Espadaler (2013) found a global mean ant dispersal distance of 1.99 m, with greater mean dispersal distances in the Southern Hemisphere (3.71 m). We recorded dispersal distances much greater than this average (mean 5.4 m in non-disturbed, 12.2 m in disturbed zones with data for I. purpureus included) in roadside environments.
I. purpureus was largely responsible for all seed dispersal distances longer than 17 m (mean = 25.1 m, maximum = 120 m; Table 1), with longer distances in the disturbed zone, which greatly influenced overall results. Such distances have been recorded in similar studies, but in more arid environments. Whitney (2002) recorded exceptionally long distances (180 m) based on direct observations; and more recently, Pascov et al. (2015) recorded a distance of 417 m using microsatellite markers and parentage assignments for seeds found in ant nest middens. Both studies included measurements on species from the Iridomyrmex genus in arid environments. This is the first study providing distances to such a degree outside of the Australian arid zone, which suggests that infrequent long dispersal distances may not necessarily be restricted to this environment.
The composition of ant assemblages at any given habitat is known to influence seed dispersal services by ants (Zelikova and Breed, 2008; Beaumont et al., 2013). A previous study along roadsides found a largely similar composition of seed-dispersing ant species between disturbed and non-disturbed zones (Palfi et al., 2017), which can largely explain the lack of significant differences in mean dispersal distances for individual species between zones (Figure 2). Furthermore, the patchy nature of ant activities during the experiment resulted in the exclusion of various species from statistical analyses, therefore forming generalizations in terms of soil disturbance effects on individual species is difficult. Parr et al. (2007) also found no effects of burning on mean dispersal distances at the species level; only in the case of an Iridomyrmex species. Likewise, Andersen and Morrison (1998) found that Iridomyrmex contributed to overall longer mean and maximum distances in relation to disturbance from mining activities.
We found that a somewhat greater percentage of short distance dispersals (<1 m) occurred in non-disturbed vs. disturbed zones, though this difference was not significant (Figure 4), where the activities of R. metallica were primarily important. Despite mean dispersal distances of R. metallica being generally short (1.5 m), this species is considered as a keystone seed disperser throughout Australia (Gove et al., 2007), pointing to other factors than dispersal distance as important features of the seed dispersal mutualism (i.e., rate of seed removal, burial of seeds; Hughes and Westoby, 1992; Lubertazzi et al., 2010; Palfi, unpubl. data). Other smaller bodied species (Monomorium and Pheidole) also contributed to the high frequencies of short distance dispersals in both zones. Severely disturbed sites are often coupled with low dispersal distances due to the predominance of small bodied ant species (Pudlo et al., 1980; Andersen and Morrison, 1998; Leal et al., 2013).
Body size has been demonstrated to be a good proxy for assessing likely dispersal distances a species may be able to perform (Ness et al., 2004), and so does their foraging behavior in search of food resources (e.g., Lubertazzi et al., 2010). Therefore, dispersal distance is largely a function of disperser identity (Andersen, 1988; Gove et al., 2007). Given the predominance of large bodied species we recorded, particularly in disturbed zones, and commensurate large distances they dispersed seeds, suggests that the soil disturbance regime imposed in our study is infrequent, or an intensity of which these ants can still effectively nest and forage within.
The distribution and density of ant nests can greatly determine the shape of the dispersal curve at any particular site (Andersen, 1988). The overall pattern of the seed dispersal curve with a long tail of infrequent but exceptionally long dispersal distances, suggests low nest densities (Green, 1983). Under such circumstances, the foraging effectiveness of dispersers becomes very important for persistence of plant communities in such environments.
Where I. purpureus were present, nests were discovered in both the disturbed and non-disturbed zones. Seed dispersal distances by I. purpureus were strongly influenced by the spatial location of nests, as individual I. purpureus foragers returned to the same nest, or on few occasions to multiple nests (Palfi, pers. obs.; van Wilgenburg and Elgar, 2007). This assertion is supported by our seed dispersal distance data (Figure 4), where high dispersal frequencies at 30–40 m reflect the observed nest distribution patterns. Second, field observations revealed that I. purpureus used the graded soil surface as a “runway” to access resources at great distances in both the disturbed and non-disturbed areas.
Complex vegetation structure is known to reduce the foraging speed and discovery of new resources by ants (Gibb and Parr, 2010), therefore this form of soil disturbance appears to confer advantages for Iridomyrmex to access resources in roadside habitats. Undoubtedly, open areas, such as those prevalent in roadsides, may confer additional advantages to I. purpureus in terms of suitable warmth, and open conditions for nesting (Greenslade, 1976). It has been suggested that the simplified and obstacle-free habitat conditions which exist post-disturbance, especially from an ant point of view, provide conditions commensurate for effective seed dispersal, especially by larger bodied ants (Davidson and Morton, 1981; Parr et al., 2007).
The secondary seed dispersal activities we recorded for I. purpureus may provide additional services for plants, providing the seed remains viable after handling by ants. Whitney (2002) analyzed the density of discarded seeds from Iridomyrmex nests (sometimes up to 25 m), and recorded a high seed viability ratio (40%). Furthermore, middens of Iridomyrmex nests were reported to provide environmental conditions suitable for seedling growth, and facilitate range expansion of plant species in northern Australia (Bebawi and Campbell, 2004). Other species can also discard seeds from their nests (Beaumont et al., 2013), sometimes with the elaiosomes remaining intact on discarded seeds. The potential benefits of multi stages dispersal processes may be particularly important for plants existing in fragmented environments where the maintenance of habitat connectivity may be crucial for overall population persistence.
As many landscapes have been cleared for agricultural purposes in Australia and elsewhere, roadside environments constitute an important landscape element by providing structural and functional connectivity for many plants and animals (e.g., Bennett, 1990; Spooner, 2015). Nevertheless, disturbances often occur in such environments, which can both demote or promote species, depending on the frequency and intensity of given disturbance regimes (e.g., Forman and Alexander, 1998).
In the study, soil disturbances seem to provide ideal nesting and foraging habitat for certain ant species (e.g., Iridomyrmex spp.) to thrive in roadside environments. As a result, increased range expansion for myrmecochorous plants could occur through greater dispersal distances provided by such ants along roads. The adaptability of residing ant species to soil disturbances, and their competitive interactions, will influence the success of this mutualism. For example, the seed dispersal and nesting activity of Melophorus and Iridomyrmex in soil disturbed zones show the adaptability of certain ant species to prevailing habitat conditions in roadside.
Infrequent, long distance dispersal to suitable sites, may be highly important for seedling recruitment in disturbed or modified habitats (Giladi, 2006; He et al., 2009). The extent to which various seed disperser ant species contribute to plant population recruitment and structures requires further investigation of patterns of seedlings establishment in relation to nest sites.
This study was carried out in accordance with AAC Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and Charles Sturt University Animal Care and Ethics Committee policy. This project was exempt from approval requirements because it was deemed “research with limited impact” (category 4).
ZP, PS, and WR conceived and designed the field experiments. ZP performed the experiments. ZP and WR analyzed the data. ZP, PS, and WR wrote the manuscript.
Funding to ZP was provided by a postgraduate scholarship from Charles Sturt University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FA and handling Editor declared their shared affiliation.
We are grateful to Levente Palfi for field work assistance and we thank Alan N. Andersen for guidance with the identification of ant species. We are also grateful to two reviewers who provided helpful suggestions to greatly improve the manuscript.
Andersen, A. N. (1988). Dispersal distance as a benefit of myrmecochory. Oecologia 75, 507–511. doi: 10.1007/BF00776412
Andersen, A. N., and Morrison, S. C. (1998). Myrmecochory in Australia's seasonal tropics: effects of disturbance on distance dispersal. Austral. Ecol. 23, 483–491. doi: 10.1111/j.1442-9993.1998.tb00756.x
Beattie, A. J. (1985). The Evolutionary Ecology of Ant-Plant Mutualisms. Cambridge: Cambridge University Press.
Beaumont, K. P., Mackay, D. A., and Whalen, M. A. (2012). The effects of prescribed burning on epigaeic ant communities in eucalypt forest of South Australia. For. Ecol. Manag. 271, 147–157. doi: 10.1016/j.foreco.2012.02.007
Beaumont, K. P., Mackay, D. A., and Whalen, M. A. (2013). Multiphase myrmecochory: the roles of different ant species and effects of fire. Oecologia 172, 791–803. doi: 10.1007/s00442-012-2534-2
Bebawi, F. F., and Campbell, S. D. (2004). Interactions between meat ants (Iridomyrmex spadius) and bellyache bush (Jatropha gossypiifolia). Aust. J. Exp. Agric. 44, 1157–1164. doi: 10.1071/EA03194
Bennett, A. F. (1990). Habitat corridors and the conservation of small mammals in a fragmented forest environment. Landsc. Ecol. 4, 109–122. doi: 10.1007/BF00132855
Benson, J. (1991). The effect of 200 years of European settlement on the vegetation and flora of New South Wales. Cunninghamia 2, 343–370.
Berg, R. (1975). Myrmecochorous plants in Australia and their dispersal by ants. Aust. J. Bot. 23, 475–508. doi: 10.1071/BT9750475
Bull, L. (1997). Lockhart Shire Roadside Vegetation Survey and Recommendations. Lockhart: Lockhart Shire Council, NSW Australia.
Cain, M. L., Milligan, B. G., and Strand, A. E. (2000). Long-distance seed dispersal in plant populations. Am. J. Bot. 87, 1217–1227. doi: 10.2307/2656714
Canner, J. E., Dunn, R. R., Giladi, I., and Gross, K. (2012). Redispersal of seeds by a keystone ant augments the spread of common wildflowers. Acta. Oecol. 40, 31–39. doi: 10.1016/j.actao.2012.02.004
Corlett, R. T. (2009). Seed dispersal distances and plant migration potential in tropical East Asia. Biotropica 41, 592–598. doi: 10.1111/j.1744-7429.2009.00503.x
Culver, D. C., and Beattie, A. J. (1978). Myrmecochory in viola: dynamics of seed-ant interactions in some west virginia species. J. Ecol. 66, 53–72. doi: 10.2307/2259181
Davidson, D. W., and Morton, S. R. (1981). Myrmecochory in some plants (F. chenopodiaceae) of the Australian arid zone. Oecologia 50, 357–366. doi: 10.1007/BF00344976
Forman, R. T. T., and Alexander, L. E. (1998). Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 29, 207–231. doi: 10.1146/annurev.ecolsys.29.1.207
Gelbard, J. L., and Belnap, J. (2003). Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv. Biol. 17, 420–432. doi: 10.1046/j.1523-1739.2003.01408.x
Gibb, H., and Parr, C. L. (2010). How does habitat complexity affect ant foraging success? A test using functional measures on three continents. Oecologia 164, 1061–1073. doi: 10.1007/s00442-010-1703-4
Giladi, I. (2006). Choosing benefits or partners: a review of the evidence for the evolution of myrmecochory. Oikos 112, 481–492. doi: 10.1111/j.0030-1299.2006.14258.x
Gómez, C., and Espadaler, X. (2013). An update of the world survey of myrmecochorous dispersal distances. Ecography 36, 001–009. doi: 10.1111/j.1600-0587.2013.00289.x
Gove, A. D., Majer, J. D., and Dunn, R. R. (2007). A keystone ant species promotes seed dispersal in a diffuse mutualism. Oecologia 153, 687–697. doi: 10.1007/s00442-007-0756-5
Green, D. S. (1983). The efficacy of dispersal in relation to safe site density. Oecologia 56, 356–358. doi: 10.1007/BF00379712
Greenslade, P. J. M. (1976). Meat ant Iridmoyrmex purpureus (Hymenoptera: Formicidae) as a dominant member ant communities. Aust. J. Entomol. 15, 237–240. doi: 10.1111/j.1440-6055.1976.tb01700.x
He, T., Lamont, B. B., Krauss, S. L., Enright, N. J., Miller, B. P., and Gove, A. D. (2009). Ants cannot account for interpopulation dispersal of the arillate pea Daviesia triflora. New Phytol. 181, 725–733. doi: 10.1111/j.1469-8137.2008.02686.x
Hughes, L., and Westoby, M. (1992). Fate of seeds adapted for dispersal by ants in Australian sclerophyll vegetation. Ecology 73, 1285–1299. doi: 10.2307/1940676
Leal, L. C., Andersen, A. N., and Leal, I. R. (2013). Anthropogenic disturbance reduces seed-dispersal services for myrmecochorous plants in the Brazilian Caatinga. Oecologia 174, 173–181. doi: 10.1007/s00442-013-2740-6
Lengyel, S., Gove, A. D., Latimer, A. M., Majer, J. D., and Dunn, R. R. (2009). Ants sow the seeds of global diversification in flowering plants. PLoS ONE 4:e5480. doi: 10.1371/journal.pone.0005480
Lengyel, S., Gove, A. D., Latimer, A. M., Majer, J. D., and Dunn, R. R. (2010). Convergent evolution of seed dispersal by ants, and phylogeny and biogeography in flowering plants: a global survey. Perspect. Plant. Ecol. Evol. Syst. 12, 43–55. doi: 10.1016/j.ppees.2009.08.001
Lobstein, M. B., and Rockwood, L. L. (1993). Influence of elaiosome removal on germination in five ant-dispesred plant species. Va. J. Sci. 44, 59–72.
Lockhart Shire Council (2013). State of the Environment Report 2013. http://www.lockhart.nsw.gov.au/f.ashx/State-of-Environment-12-13.pdf (Accessed 20 Feb 2017).
Lubertazzi, D., Aliberti Lubertazzi, M. A., McCoy, N., Gove, A. D., Majer, J. D., and Dunn, R. R. (2010). The ecology of a keystone seed disperser, the ant Rhytidoponera violacea. J. Insect Sci. 10, 1–15. doi: 10.1673/031.010.14118
Lugo, A. E., and Gucinski, H. (2000). Function, effects, and management of forest roads. For. Ecol. Manag. 133, 249–262. doi: 10.1016/S0378-1127(99)00237-6
Lunt, I. D., and Bennett, A. F. (2000). “Temperate woodlands in Victoria: distribution, composition and conservation,” in Temperate Eucalypt Woodlands in Australia: Biology, Conservation, Management and Restoration, eds R. J. Hobbs and C. J. Yates (Chipping Norton, NSW: Surrey Beatty and Sons), 17–31.
Martins, V. F., Guimarães, P. R. Jr., Silva, R. R. D., and Semir, J. (2006). Secondary seed dispersal by ants of Ricinus communis (Euphorbiaceae) in the Atlantic forest in southeastern Brazil: influence on seed germination. Sociobiology 47, 265–274.
Nathan, R., and Muller-Landau, H. C. (2000). Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends. Ecol. Evol. 15, 278–285. doi: 10.1016/S0169-5347(00)01874-7
Nathan, R., Schurr, F. M., Spiegel, O., Steinitz, O., Trakhtenbrot, A., and Tsoar, A. (2008). Mechanisms of long-distance seed dispersal. Trends. Ecol. Evol. 23, 638–647.4 doi: 10.1016/j.tree.2008.08.003
Nelson, G. A. (2014). Cluster Sampling: a pervasive, yet little recognized survey design in fisheries research. Trans. Am. Fish. Soc. 143, 926–938. doi: 10.1080/00028487.2014.901252
Ness, J. H., Bronstein, J. L., Andersen, A. N., and Holland, J. N. (2004). Ant body size predicts dispersal distance of ant-adapted seeds: implications of small-ant invasions. Ecology 85, 1244–1250. doi: 10.1890/03-0364
Pacini, E. (1990). Mercurialis annua L. (Euphorbiaceae) seed interactions with the ant Messor structor (Latr.), Hymenoptera: Formicidae. Acta Bot. Neerl. 39, 253–262. doi: 10.1111/j.1438-8677.1990.tb01395.x
Palfi, Z., Spooner, P. G., and Robinson, W. (2017). Soil disturbance effects on the composition of seed-dispersing ants in roadside environments. Oecologia 183, 493–503. doi: 10.1007/s00442-016-3767-2
Parr, C. L., Andersen, A. N., Chastagnol, C., and Duffaud, C. (2007). Savanna fires increase rates and distances of seed dispersal by ants. Oecologia 151, 33–41. doi: 10.1007/s00442-006-0570-5
Pascov, C. M., Nevill, P. G., Elliott, C. P., Majer, J. D., Anthony, J. M., and Krauss, S. L. (2015). The critical role of ants in the extensive dispersal of Acacia seeds revealed by genetic parentage assignment. Oecologia 179, 1123–1134. doi: 10.1007/s00442-015-3400-9
Portnoy, S., and Willson, M. F. (1993). Seed dispersal curves: behavior of the tail of the distribution. Evol. Ecol. 7, 25–44. doi: 10.1007/BF01237733
Pudlo, R. J., Beattie, A. J., and Culver, D. C. (1980). Population consequences of changes in an ant-seed mutualism in Sanguinaria canadensis. Oecologia 46, 32–37. doi: 10.1007/BF00346962
R Development Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation.
Rowles, A. D., and O'Dowd, D. J. (2009). New mutualism for old: indirect disruption and direct facilitation of seed dispersal following Argentine ant invasion. Oecologia 158, 709–716. doi: 10.1007/s00442-008-1171-2
Schabel, J., and Eldridge, D. J. (2001). A Comparison of Roadside and Paddock Vegetation in the Box Woodlands of Eastern Australia. Sydney, NSW: University of NSW.
Sorrells, J., and Warren, R. J. II. (2011). Ant-dispersed herb colonization lags behind forest re-establishment. J. Torrey. Bot. Soc. 138, 77–84. doi: 10.3159/10-RA-037.1
Spooner, P. G. (2005). Response of Acacia species to disturbance by roadworks in roadside environments in southern New South Wales, Australia. Biol. Cons. 122, 231–242. doi: 10.1016/j.biocon.2004.07.012
Spooner, P. G. (2015). Minor rural road networks: values, challenges, and opportunities for biodiversity conservation. Nat. Conserv. 11, 129–142. doi: 10.3897/natureconservation.11.4434
Spooner, P. G., and Lunt, I. D. (2004). The influence of land-use history on roadside conservation values in an Australian agricultural landscape. Aust. J. Bot. 52, 445–458. doi: 10.1071/BT04008
Spooner, P. G., Lunt, I. D., Briggs, S. V., and Freudenberger, D. (2004). Effects of soil disturbance from roadworks on roadside shrubs in a fragmented agricultural landscape. Biol. Cons. 117, 393–406. doi: 10.1016/j.biocon.2003.08.003
Thomson, F. J., Moles, A. T., Auld, T. D., Ramp, D., Ren, S., and Kingsford, R. T. (2010). Chasing the unknown: predicting seed dispersal mechanisms from plant traits. J. Ecol. 98, 1310–1318. doi: 10.1111/j.1365-2745.2010.01724.x
van der Pijl, L. (1982). Principles of Dispersal in Higher Plants. New York NY: Springer-Verlag Berlin Heidelberg.
Vander Wall, S. B., and Longland, W. S. (2004). Diplochory: are two seed dispersers better than one? Trends. Ecol. Evol. 19, 155–161. doi: 10.1016/j.tree.2003.12.004
van Wilgenburg, E., and Elgar, M. A. (2007). Colony structure and spatial distribution of food resources in the polydomous meat ant Iridomyrmex purpureus. Insectes Soc. 54, 5–10. doi: 10.1007/s00040-007-0903-3
Wang, B. C., and Smith, T. B. (2002). Closing the seed dispersal loop. Trends. Ecol. Evol. 17, 379–385. doi: 10.1016/S0169-5347(02)02541-7
Whitney, K. D. (2002). Dispersal for distance? Acacia ligulata seeds and meat ants Iridomyrmex viridiaeneus. Austral Ecol. 27, 589–595. doi: 10.1046/j.1442-9993.2002.01216.x
Keywords: acacia, habitat connectivity, myrmecochory, road ecology, soil disturbance
Citation: Palfi Z, Spooner PG and Robinson W (2017) Seed Dispersal Distances by Ants Increase in Response to Anthropogenic Disturbances in Australian Roadside Environments. Front. Ecol. Evol. 5:132. doi: 10.3389/fevo.2017.00132
Received: 13 March 2017; Accepted: 10 October 2017;
Published: 25 October 2017.
Edited by:
Diane E. Pataki, University of Utah, United StatesReviewed by:
Frederick R. Adler, University of Utah, United StatesCopyright © 2017 Palfi, Spooner and Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter G. Spooner, cHNwb29uZXJAY3N1LmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.