- 1Wild Urban Evolution and Ecology Lab, Centre of New Technologies, University of Warsaw, Warsaw, Poland
- 2Museum and Institute of Zoology, Polish Academy of Sciences, Warsaw, Poland
- 3Laboratoire Évolution et Diversité Biologique UMR 5174, Université Toulouse III Paul Sabatier, Toulouse, France
Environmental conditions are key drivers of life-history evolution, and the urban environment is an extreme form of land-use readily inhabited by avian wildlife, whose life-history variation in such altered environment is still poorly understood. Recently, the study of environmental variables associated with urban living—which include shifts in temperature, light, noise or food availability—has attracted increased attention. Another environmental axis that sets the urban space at odds relative to natural habitats is high human abundance, yet very little is known about its effect on avian fitness. We developed a protocol to quantify human presence by performing repeated counts of humans on the ground within a 15 m radius of nestboxes monitored in two centrally-located study areas of a European capital city. In parallel, a GIS-based approach was used to infer nestbox distance to the nearest path and road. Multiple counts of human presence around each nestbox yielded moderate to high repeatabilities (0.6 ≤ r ≤ 0.8) while requiring considerable resources time- and people- wise. In contrast, GIS-based estimates of nestbox distance to paths and roads were time efficient and generated highly repeatable results. The effects of (i) human presence around each nestbox, (ii) nestbox distance to the nearest path and (iii) nestbox distance to the nearest road were tested on reproductive traits of blue tits Cyanistes caeruleus and great tits Parus major breeding in two urban sites. Human presence did not influence blue tit or great tit life-history traits and reproductive success, suggesting reproductive habituation to humans in an urban landscape. In contrast, nestbox distance to roads shortened incubation time in great tits while nestbox distance to paths increased incubation time in blue tits. Moreover, blue tit offspring 2 weeks after hatching were lighter closer to roads. Our study confirms the reliability of a field protocol capturing human presence around multiple fixed locations that can be easily implemented in either urban or rural landscapes. At the same time, it appears that when applied to two urban sites where habituation to humans might have occurred, it is infrastructural networks rather than human presence per se that played a greater role in tit reproductive trait variation.
Introduction
Humans are a keystone species in an urban habitat, modifying and shaping original ecosystem structures with often drastic consequences for their functionalities (Rees, 1997). Urban space is a habitat that attracts humans: while 54% of the world human population already resides in urban settlements, it is likely to increase to 66% by 2050 (United Nations, 2014). Consequently, 0.5% of the planet's land area has become urban space (Schneider et al., 2009), a value that is expected to grow several-fold in the next decades (Seto et al., 2011).
Urban growth is one of the major causes of natural habitat loss and fragmentation at a global scale (Grimm et al., 2008) as it modifies extensively the original landscapes (Shochat et al., 2006), leading to an overall rearrangement of habitats and animal communities (McDonnell and Pickett, 1990; Chamberlain et al., 2009). Urban areas are characterised by impervious surfaces including buildings and infrastructural networks, but also modified greenspaces; they encapsulate a multitude of ecological niches at a local scale, set in a multidimensional space of modified biotic and abiotic elements and processes (Sprau et al., 2016). Unavoidable interactions between urban wildlife and humans are thus increasing in prevalence due to growing urban sprawl taking over rural areas (Ditchkoff et al., 2006; Grimm et al., 2008). Birds are a convenient model to study the effects of urbanization on wildlife, due to their conspicuousness and ubiquity in both cities and rural sites. Moreover, much knowledge on bird biology is readily available from long-term studies carried out in the wild, thus offering a valuable background to infer change occurring in an urban environment (Savill et al., 2011).
A large number of previous studies have reported changes in bird ecology triggered by the urban environment (reviewed in Chace and Walsh, 2006; Gil and Brumm, 2013); these pointed out how this distinctive habitat may be defined by a multitude of environmental factors which rarely act in isolation. Overall, even though cities and towns are generally characterised by altered climatic profiles (increased minimum temperatures, Marzluff, 2001), predator communities (Churcher and Lawton, 1987) and food resources (i.e., supplementary feeding by humans; Fuller et al., 2008; Robb et al., 2008), the latter is emerging as a paramount in terms of its influence on habitat quality (Solonen, 2001; Robb et al., 2008; Chamberlain et al., 2009).
Urban-driven abiotic factors are also known to impact avian ecology and breeding biology, such as noise, light and chemical pollution (Slabbekoorn and Ripmeester, 2008; Dominoni et al., 2013). Thus, bird species were shown to decline in terms of richness, density and abundance due to noise pollution (Stone, 2000; Bayne et al., 2008; Mockford and Marshall, 2009). At the population level, noise pollution was found to induce an increased pitch in birds or a shift in their singing activity to the night time, avoiding noisy periods (Slabbekoorn and Peet, 2003; Kirschel et al., 2009; Nemeth and Brumm, 2009). Artificial night lighting—light pollution—may attract many birds during their nocturnal migration: harsh consequences for such important detours are an increased number of predation events and/or collisions with artificial structures in cities (van de Laar, 2007, reviewed in Erritzoe et al., 2003), as well as a reduction in energy storage or delayed arrival at wintering or breeding areas (Seress and Liker, 2015). Light pollution can also affect bird behaviour by advancing avian singing time at dawn and dusk (Da Silva et al., 2014). Finally, the increased exposure to environmental toxic chemicals—chemical pollution—can affect birds' physiology and phenotype directly but also indirectly because of habitat alterations or parasites spread (Morrison, 1986; Eeva et al., 1994; Fry, 1995; Isaksson, 2015).
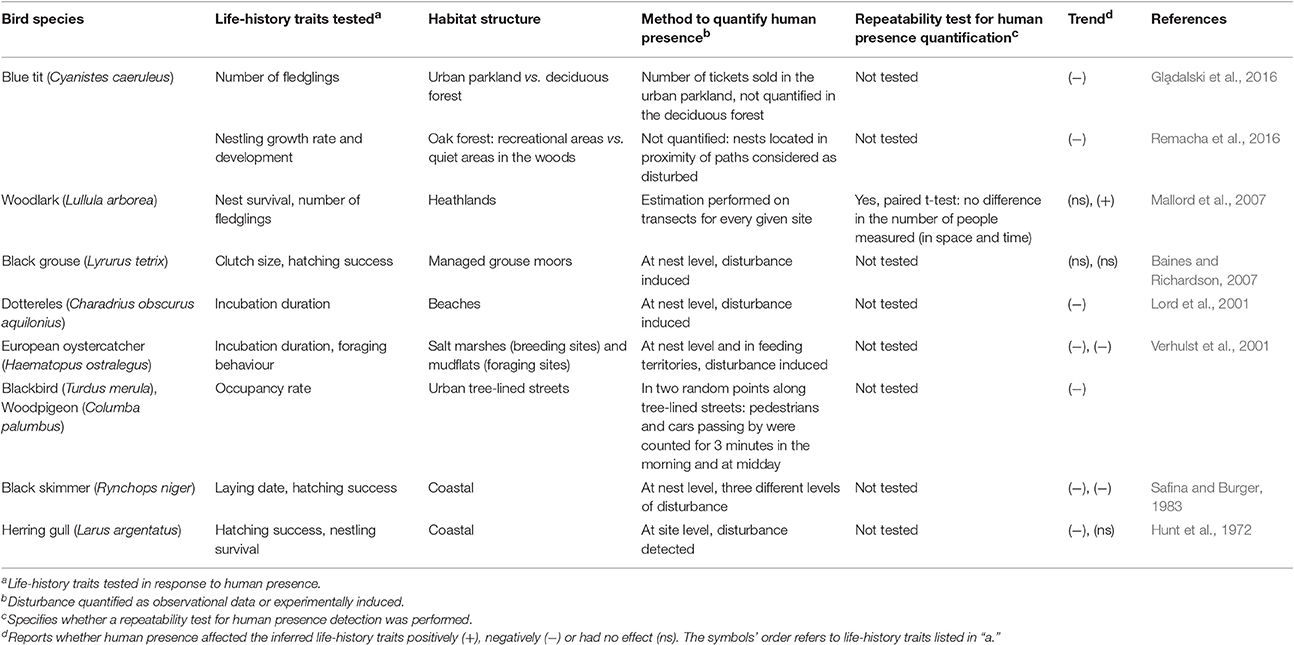
While the effect of a large range of human triggered, biotic and abiotic variables specific to urban landscapes have been tested on avian reproductive traits, knowledge on the “direct” effect of human presence on avian reproduction is much more limited. Humans (often deterministically defined as “human disturbance”) are known to trigger various responses in terms of changes in animal distribution, demography and population size (as reviewed in Gill, 2007). Earlier studies often tested the effect of human disturbance on bird species by inducing artificially different levels of noise, but without explicitly linking noise treatment levels to real life distribution of visitors on the ground (Lord et al., 2001; Verhulst et al., 2001; Baines and Richardson, 2007). Moreover, the direct effect of human disturbance at the individual level has largely focused on behavioural responses, where flush distance (Fernández-Juricic and Tellería, 2000; Miller et al., 2001; Tarjuelo et al., 2015), feeding patterns (Fernández-Juricic and Tellería, 2000) or physiological responses (e.g., increased corticosterone levels; Fowler, 1999; Walker et al., 2006; Ellenberg et al., 2007; Almasi et al., 2015) were measured. However, knowledge on the direct effect of human presence on individual fitness in an urban habitat is limited, as variation in life-history traits and reproductive success caused by human presence has been largely investigated in non-urban habitats and largely in non-cavity nesting bird species (Table 1). While we may expect that birds breeding in urbanised habitats are continuously exposed to humans, thus habituated to their often not-threatening activities, no evidence in terms of life-history trait variations for such specific stressor was reported in cities. Even though many studies underlined how avian populations breeding in habitat characterised by frequent human disturbance, might often reduce their flee distance as a result of habituation (Metcalf et al., 2000; Rodriguez-Prieto et al., 2009; Clucas and Marzluff, 2012), it is also known that the presence of humans near the nest might ordinarily trigger strong behavioural responses in parental behaviour while feeding their young—for example, by alarm calling and avoiding entering nests for a certain time (Müller et al., 2006).
Human presence at the site and at the nest level is not often easy to monitor due to its inherent property of being variable in space and time. It can depend on season and weather conditions (sunny vs. rainy days; Gla̧dalski et al., 2016), days of the week (weekends vs. working days; Remacha et al., 2016) or hours of the day (morning peak hour vs. afternoon). It is thus important that such variability is taken into account when quantifying human presence and its possible disturbance to wildlife. Moreover, and in order to detect the potential biological effect of human presence on avian reproduction, protocols for capturing human disturbance need to be repeatable—an information that is only rarely reported in studies of human disturbance on wildlife (Table 1).
This study quantifies the biological effect of humans on great tit (Parus major) and blue tit (Cyanistes caeruleus) life-history traits and reproductive success, characterised at the nestbox level and estimated in two contrasted yet centrally located urban study sites. Great tits and blue tits are two well-studied songbird species that breed in nestboxes in a large range of environments, from undisturbed rural areas to the chaos of urban settlements. The two study sites presented in this study—an urban park and a cemetery—offer the opportunity to compare two different urban habitat structures whose distributions of visitors vary gradually from a regularly visited site during specific opening hours (cemetery) to frequently visited without specific rules (urban park). Here, the effects of human presence—quantified with an easily implemented and repeatable protocol capturing human presence on the ground and at the nestbox level—is inferred and contrasted with the effects of nestbox distance to the nearest path and road, which are infrastructural networks acting as key sources of human presence in urban areas.
Methods
Study Sites
This study was conducted in 2016 as part of a new long-term research project whose main aim is to study the effect of cities on the genotype and phenotype of two wild passerine species—great tits P. major and blue tits C. caeruleus. 196 Schwegler woodcrete nestboxes (type 1b with an entrance hole of 32 mm and suitable for both great tits and blue tits) were erected in two study sites—91 in a cemetery and 105 in an urban park (Pole Mokotowskie), located in the city centre of Warsaw, Poland. Warsaw (52°14′N, 21°1′E) is the largest city of Poland, and ranks as the 9th most populous capital city in the European Union with a population of over 1.7 million people (Eurostat, 2017). While both study sites are centrally located, the two areas are defined by different habitat structures, whose spatial location and actual surface use leads wildlife to face contrasted environmental pressures, especially in terms of human presence. Nestboxes were laid out in a grid, with an inter-nestbox distance of c. 50 m, an average south-east orientation (N = 196, mean = 106.7 degrees, SE = 5.6), and fixed at a height ranging from 2.5 to 3.0 m above ground level.
The urban park Pole Mokotowskie (52°12′N, 20°59′E) covers c. 65 hectares. Despite its current design, the park lays on a surface initially used as an airport until 1945. Pole Mokotowskie is now a typical managed green space framed by four large roads, where grass, flower beds and trees alternate to generate covered and open areas. Due to both size and central location, this urban public park plays an important role for city dwellers both as recreational area and a bike-friendly commuting space.
The Jewish Cemetery (52°14′N, 20°58′E), with its area of 34 hectares, is one of the largest Jewish necropolis in the world and still serves as burial place today. Established in 1806, the cemetery was largely neglected and abandoned during the German invasion. Due to a strongly reduced number of visitors after the II World War, only a small portion of the site was regularly visited, leading to a general overgrowth of trees and shrubs in the rest of the area. Because of selective forces mainly driven by important historical events, the Jewish cemetery is now formed by a naturally regenerating habitat. It is distinguished by a particular landscape of moss-covered tombstones in a large wild urban forest mainly composed by a mixture of both native and exotic tree species; oaks (Quercus spp.), silver birch (Betula pendula), Norway maples (Acer platanoides), elms (Ulmus spp.) and black locust (Robinia spp.) are the most common tree species in the site.
Human abundance and space use is highly contrasted in the two study sites: human presence in the cemetery is heterogeneous (many areas have few or hardly no visitors), and it is limited in terms of opening hours to daytime and outside of religious holidays. Cemetery visitors often arrive in groups and remain on marked paths. In contrast, the urban park is an open site where one can roam freely, and is visited by numerous commuting urban dwellers (pedestrians or cyclists) or those interested in recreational activities.
Life-History Data
Starting from mid-March, each nestbox was visited at weekly intervals to record the date of the first egg laid. The following breeding variables were compiled: nest occupancy (a nestbox was considered occupied if at least one egg was laid by a blue tit or great tit; empty nestboxes and those occupied by other species were considered as not occupied in our analyses), egg laying date (lay date recorded as day 1 = 1st of April), standardised lay date (standardised for each site and species), clutch size, incubation duration (defined as the number of days between the last egg laid to the first egg hatched), hatching success (number of hatchlings/clutch size), fledging success (number of fledglings/number of hatchlings) and chick mass 15 days after hatching (hatching day = day 1). Birds were ringed at day 15 using standard-numbered metal rings supplied by the Polish Bird Ringing Centre (Museum and Institute of Zoology, Polish Academy of Sciences). Nestboxes were checked c. 25 days after hatching to establish fledging success. Key summary statistics of reproductive success for each study site are presented in Supplementary Material Table 1.
Ethics Statement
Research was carried out under permit from the Regional Directorate for Environmental Protection in Warsaw, Poland.
Quantifying Human Presence
The possible disturbance to great tit and blue tit reproductive biology caused by human presence was quantified in both sites and for each nestbox by measuring: (i) the number of humans and dogs present around each nestbox in a 15 m radius, (ii) nestbox distance from the nearest path and (iii) nestbox distance from the nearest road.
Human Presence Protocol
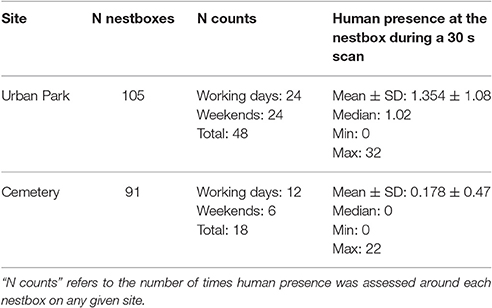
The ground-based data collection for human presence was performed from March until July 2016 on specific days throughout the season and aimed to capture human and dog presence at each nestbox. Each site was split in two paths grouping a similar number of nestboxes to minimize the time between the start and the end of each count. During each trial, two fieldworkers per site were counting for 30 s all humans (i.e., bikers and pedestrians) and dogs within a 15 m radius of each nestbox in the two study sites, following two assigned paths (i.e., track 1 and track 2, see maps in Supplementary Material Figure 1). In order to guarantee that all counts at each nestbox were performed at similar timeframes during the respective counts, the tracks' directions followed by the two fieldworkers during the human presence protocol were switched at each trial. The time needed to perform counts of human presence around each nestbox in the field did not vary significantly between sites: fieldworkers spent on average 83.2 min in the cemetery (SE = 1.75, N = 36) and 80.15 min in the urban park (SE = 1.45, N = 96) on their assigned path. To capture variation in human presence that can be influenced by human activities, and which might depend on time of day and working vs. weekend day, counts in the urban park were replicated across 2 working days (Mondays and Fridays) and 2 days during the weekend (Saturdays and Sundays). In each of these days, counts of human presence for each nestbox were performed 4 times throughout the day (at 8:00 a.m., 12:00 p.m., 3:00 p.m., and 6:00 p.m. for the urban park). Due to restricted opening hours, the cemetery counts were performed twice daily, and were restricted to Mondays, Fridays, and Sundays (Table 3). Dogs represented only 6% of the total number of humans and dogs (N = 7,586) recorded in a 15-m radius to each nestbox, we therefore use for simplicity the term “human presence” to refer to any human (pedestrian or biker) or dog presence around any given nestbox.
Distances from Paths and Roads
We recorded the spatial location of nestboxes using a GPS Garmin Map 64s and all the nestboxes coordinates were downloaded using the open source Software DNRGPS Minnesota. All nestbox distances to the closest path and road were calculated using the free and Open Source Geographic Information System Quantum GIS 2.8.2 “Wien,” (version released on the 9th of May 2015), with a default projection of WGS84-Geographic Coordinate System. Built on the Plugin OpenLayers, the OpenStreetMap has been used as base map to measure all distances with the Distance Matrix tool of the software. Specifically, the distances were measured in meters from the nestbox point to the middle of both the closest path and road. Since the measurements recorded with the Distance Matrix tool may change in precision due to the zoom function manually selected by the observer at each record, the repeatability of the process was tested comparing the measurements taken in two different days by the same person (M.C.).
Statistical Analyses
Repeatability
The repeatability of human presence around each nestbox measured on the ground as well as the repeatability of distance measurement of every nestbox to the closest path and road computed in qGIS was calculated using the “rptR-package” in R following Stoffel et al. (2017). This package allowed for repeatability estimation using a generalised linear model. Specifically, the repeatability test to infer human presence on the ground was performed for four different temporal combinations (early vs. late in the day, between working days, weekend vs. working days, early vs. late in the season) within which the total number of visitors around each nestbox was inferred and the nestbox ID was fitted as random effect in the model. The same method was extended to verify the reliability of the GIS-based approach, where the same observer (M.C.) measured nestbox distances to roads and paths in two separate days. Finally, correlation tests were performed to quantify the strength of association between human presence and distance from the closest path and from the closest road within sites.
Statistical Analyses
Statistical analyses were implemented using the computing environment R (version 3.3.1, released on the 21st of June 2016). To test the effects of human presence and the effects of distance to paths and roads on blue tit and great tit life-history traits and reproductive success, we used the glm function of Generalised Linear Models (GLM) in R. All interactions between human presence index, distance from paths and roads and site were tested; however, these were found non-significant and consequently removed from the final models. The following traits were inferred, tested for blue tits and great tits in separate analyses: egg laying date, clutch size and incubation time were analysed with a general linear model with normal error distribution. Occupancy rate, hatching success (i.e., the number of hatchlings on clutch size) and fledging success (i.e., the number of fledglings on the number of hatchlings in nests with at least one hatched offspring) were tested with a quasi-binomial distribution to control for over-dispersion. Human presence and nestbox distance to paths and roads were fitted as a continuous variable in all models while the site was fitted as a fixed effect with two levels corresponding to the two study areas in blue tits: the cemetary was excluded as predictor in great tits due to the low sample size recorded in the site (N = 2). Egg laying date (standardised for site and species) was fitted in all models with the exception of two models where occupancy rate and lay date were fitted as response variables. Finally, we used a linear mixed model (lme function in R) to test the effects of human presence and the effects of nestbox distance to paths and roads on individual nestling mass at day 15 (response variable), where nestbox ID was fitted as random effect to control for non-independence of nestlings.
Results
Repeatability of Human Presence on the Ground and With a GIS-Based Approach
Human presence recorded in a 15 meters radius around each nestbox during a 30 second long scan varied from 0 to 33 detected humans, and was on average 7.6 times higher in the urban park than in the cemetery (Figures 1A,B, Table 2). Interpolated maps illustrating average human presence for each nestbox (Figure 1) show that the flux of people varied in terms of intensity and scatter between and within sites: while people were heterogeneously distributed and most abundant in the southern areas of the urban park (Figure 1C), visitors in the cemetery were usually detected while walking on the main path near the entrance gate (Figure 1D). Due to renovation works in the cemetery, workers were systematically detected in the North-Eastern part of the site during the trials.
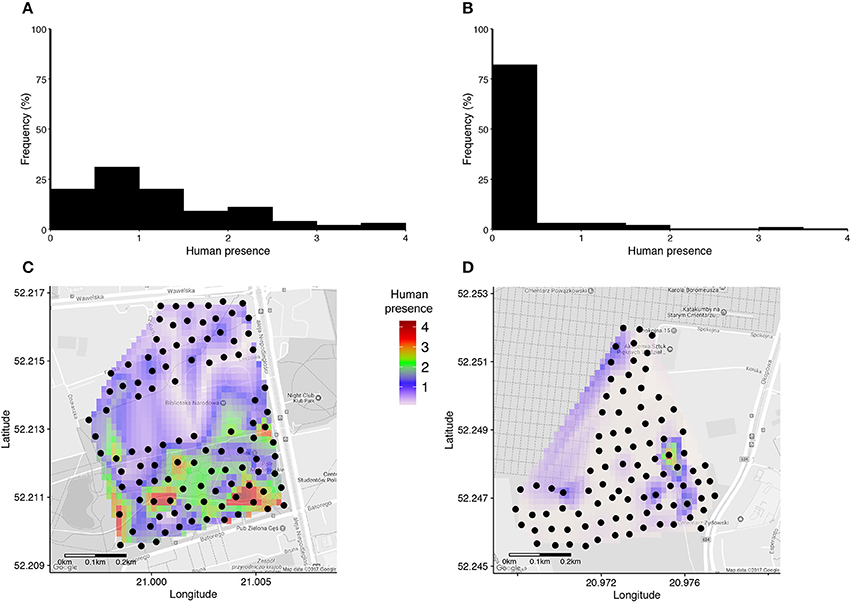
Figure 1. (A,B) Histograms of average human presence around each nestbox in the urban park (A) and in the cemetery (B). Average human presence was calculated as the total number of humans and dogs in a 15 m radius to each nestbox standardised by the number of counts performed within each site (i.e., N = 48 in A, N = 18 in B). (C,D) Interpolated maps of average human presence around each nestbox (black dots) are indicated for the urban park (C) and the cemetery (D), respectively.
The repeatability of human presence, measured on the ground for each site separately, was high in the urban park, reaching values between 0.808 and 0.840 and overlapping confidence intervals across the four temporal combinations tested (Table 3). The repeatability of human presence in the cemetery was lower, with values ranging between 0.205 and 0.563, which is likely to be the consequence of both reduced human presence and fewer counts (Table 3).
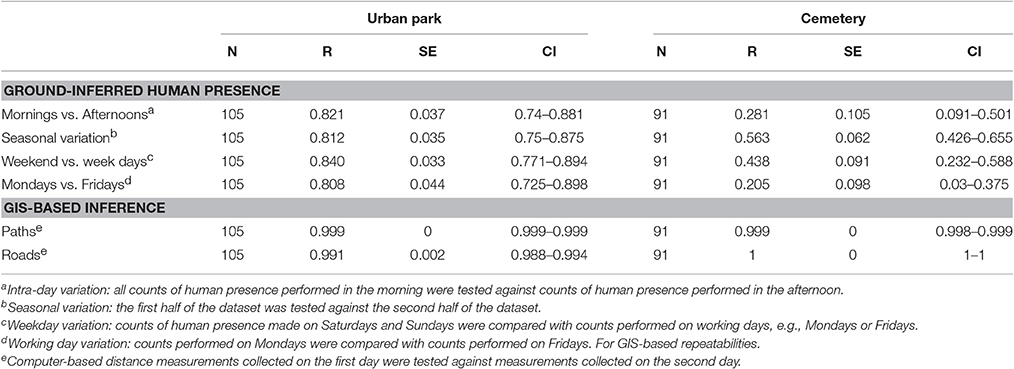
Table 3. Repeatability of the human presence protocol and GIS-based approaches, with standard errors (SE) and 95% confidence intervals (CI) reported on their original scale).
Distances from paths and roads at each nestbox, estimated using a computer-based GIS approach, showed near-perfect measurement repeatability, which ranged between 0.991 and 1 (Table 3). Additional tests were carried out to determine the strength and direction of any possible linear relationship between human presence (human abundance detected at each nestbox) and distances from paths and roads (obtained via GIS-based approaches). Correlations were tested at a within site levels and were all found non-significant but for the exception of the urban park where there was a weak yet significant negative correlation between the number of people around any given nestbox and its distance to the nearest path (Table 4).
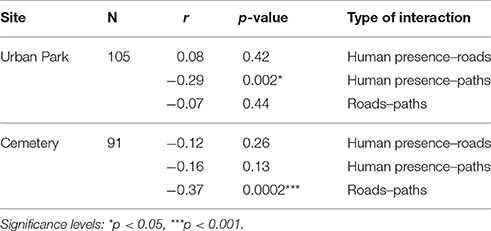
Table 4. Pearson's correlations and significance tests investigating collinearity between human presence and distances to infrastructural facilities.
Effects of Human Presence, Distance to Paths and Roads on Life-History Traits and Reproductive Success
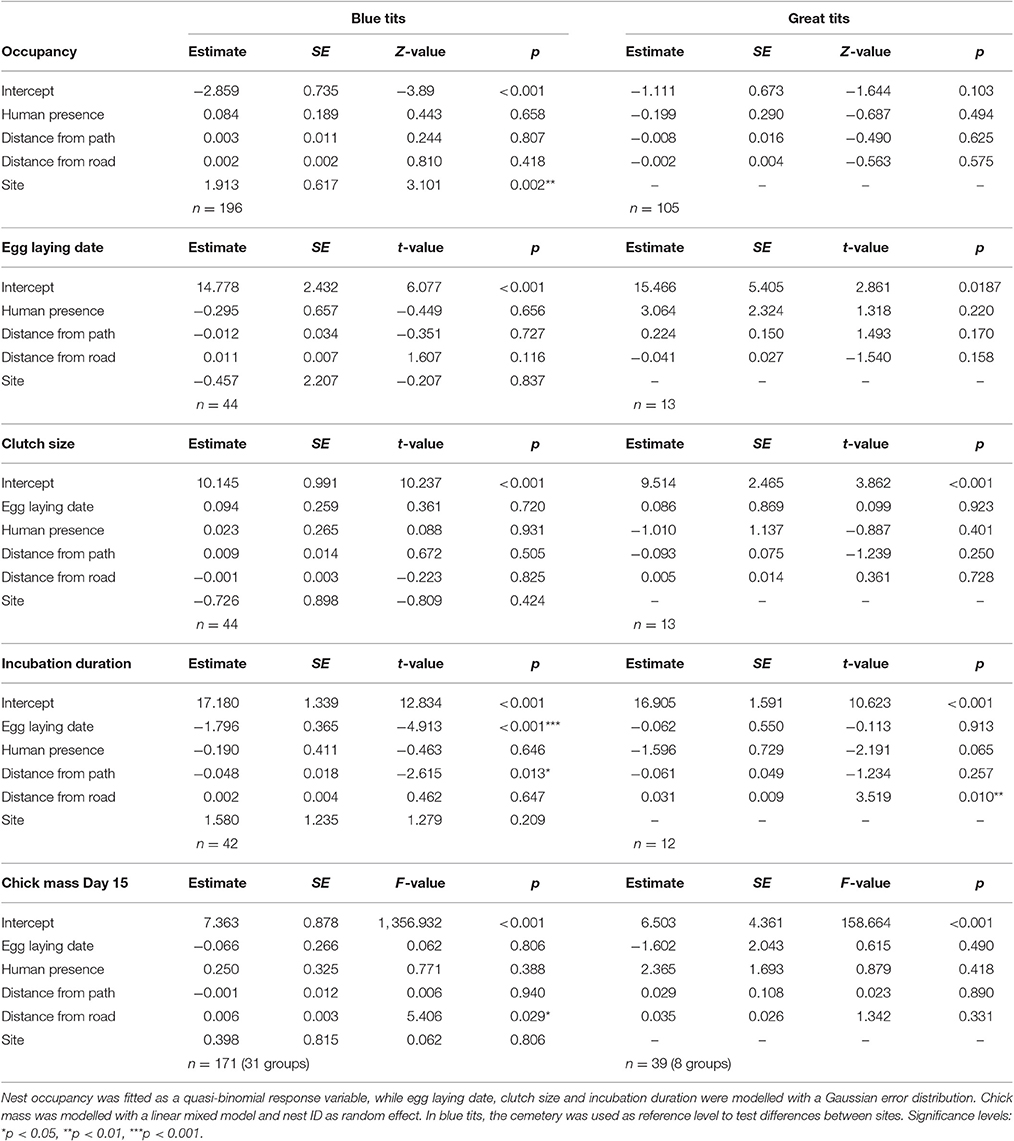
Human presence estimated in a 15 m radius around each nestbox did not influence any of the investigated blue tit or great tit life-history and reproductive traits (Table 5). In contrast, in blue tits incubation duration was significantly longer closer to paths while chick mass 15 days after hatching was significantly lower closer to roads. In great tits, incubation duration was significantly shorter closer to roads (Table 5). No effect of human presence, distance to paths and roads was detected on hatching and fledging success (Supplementary Material Table 2).
Discussion
This study is one of the very few testing the biological effect of human presence on great tit and blue tit life-history and reproductive success in an urban environment (see also Table 1). To the extent of our knowledge, this is the first study that takes into consideration both the intensity of human presence at the nestbox level and its repeatability. The nestbox-based protocol of human presence quantification, as well as the heterogeneous human distribution in space reported in this study that occurred both at a within-site and between-site levels (Figure 1), allows for the testing of human disturbance at the finest biological level—the breeding event itself.
Available reports testing the effects of human presence were usually carried out in natural and semi-natural areas, and the methods applied in quantifying such dynamics varied among studies. Human presence was often induced experimentally (Lord et al., 2001; Verhulst et al., 2001; Baines and Richardson, 2007), and the relationship to a natural distribution of these variables is not always explicit. In addition, while most of the previous studies were conducted in colonial waterbirds far from an urban context (see review by Carney and Sydeman, 1999) only a few studies testing the effect of human presence were carried out in urban settings (Fernández-Juricic, 2000; Fernández-Juricic and Tellería, 2000).
The protocol for capturing human presence on the ground required considerable effort in terms of time and number of observers relative to a computer-based GIS approach. Repeatability tests were performed for several temporal combinations that could detect whether human presence is consistent (or not) between times of the day (mornings vs. afternoons), between working days (Mondays vs. Fridays), between working days and weekends, and finally testing whether human presence is stable at the nestbox level throughout the season. The 4 types of combinations tested generated highly equivalent repeatabilities (0.81 < r < 0.84) with overlapping confidence intervals, overall revealing that human presence in the urban park is temporally stable. In the cemetery site in contrast, we detected lower intra-day and intra-week repeatabilities (Table 3), yet a satisfactory repeatability of r = 0.56 when human presence was compared early vs. late in the breeding season. The lower repeatability of human presence recorded in the cemetery is likely to be the result of fewer counts combined with a low and irregular distribution of humans within the study site (Figure 1). Thus, our study reveals that the repeatability of human presence was considerably larger in the site with higher human presence (the urban park) and with a greater heterogeneity in human presence between nestboxes. In the urban park where human presence was high, the high repeatability of ground-based counts (r > 0.8) confirmed the reliability of our protocol to accurately capture human presence, thereby revealing a stable yet heterogeneous human distribution in both urban sites.
Further work is required to test (i) the extent to which human presence can be captured with high repeatability in a gradient of urbanization with contrasted human presence at the nestbox and site level (indeed, repeatability values for human presence in the cemetery, characterised by considerably lower human presence, were also lower) and (ii) to define a minimum number of counts that would reduce ground effort while securing satisfactory repeatability (r ≥ 0.6) of human presence at the level of individual breeding events (Corsini et al., in preparation). Human preferences in terms of space use were particularly marked in the urban park, which was also the site with the highest number of visitors (Figures 1A,C). Indeed, the pattern of space use within this area was associated with the presence of recreational facilities, mostly located in the southern part of the study site. The GIS-based approach, in contrast, was a considerably less-time consuming method compared to the protocol for detecting human presence on the ground (involving only one observer and 2 days of measurements), and proved to be highly repeatable (r ≥ 0.991). Importantly, the easily-computed GIS-based approach for measuring nestbox-level distances to paths and roads provided an alternative approach to capture human presence on the ground, and was found to have a much greater influence on tit life-history trait variation than human presence per se: while no direct effect of human presence was detected in great and blue tit life-history, incubation time in blue tits and great tits, as well as blue tit offspring mass 2 weeks after hatching were associated with distance to infrastructural facilities such as paths and roads.
Policy regulations of a given area are likely to play an important role in shaping the dynamics of people flow within sites. While in both sites visitors can roam freely, paths remain the major communication channels throughout both study sites. However, human presence correlated with distance to paths only in the urban park, which may be caused by a larger detection power of such associations due to a larger number of visitors overall. Our results are thus in line with the findings of Remacha et al. (2016), who reported that distance to paths is a reliable index of human disturbance. In the case of roads, there is no evidence that this infrastructure is correlated with distribution of human presence detected on the ground, thereby suggesting that the presence of such infrastructural facilities at the site level is not always an important source of human presence, although it can impact reproduction through independent means, as explained below.
In this study, variation in blue tit or great tit life-history traits and reproductive success was not explained by human presence estimated using the ground-based protocol, suggesting that human presence does not affect birds' survival or life-history traits in the long-term. In contrast with our findings, Remacha et al. (2016) showed that blue tit nestlings from forested areas used as a recreational site that hatched on days with increased activity of visitors (weekends and other non-working days) were found to grow slower and fledge with lower body mass; this difference may be due to differences in birds' environmental backgrounds: in a more semi-natural context, where human presence is higher during the breeding season, it is possible that birds are more sensitive to human disturbance. Birds inhabiting rural realities already showed a higher physiological and behavioural stress response than their urban counterparts, often revealed through a higher flight-initiation distance (McGiffin et al., 2013; Abolins-Abols et al., 2016). On the other hand, birds inhabiting urban parks are almost continuously exposed to humans—so despite the fact that each nestbox can be consistently exposed to high or low human presence, birds may become habituated through their daily experience of humans while foraging. Studies of the effect of human presence in other bird species reveal mixed results. Similarly to our findings, in a wild population of black grouse located in a recreational area, the presence of visitors did not affect productivity in terms of clutch size, hatching success and breeding success (Baines and Richardson, 2007). In the woodlark, on the other hand, productivity was related to the level of human disturbance, although in the opposite direction than expected, with birds breeding in more disturbed areas raising more fledglings (Mallord et al., 2007). Human presence was also found to extend the duration of incubation period in the dotterel and the European oystercatcher (Lord et al., 2001; Verhulst et al., 2001), while recreational activities had a negative-marginal effect on incubation behaviour in American oystercatchers (McGowan and Simons, 2006).
These results suggest that behavioural responses are usually context-dependent and related to the trade-offs experienced by individuals: indeed, it is easier to expect habituation in birds that are used to human presence, which is a common scenario in an urban park, while birds in rural environments may react to human presence adversely (Remacha et al., 2016). Furthermore, the impact of human disturbance is likely to change among avian species or species with variable ecological niches: ground and shrub nesting birds, for example, are likely to be more sensitive to human disturbance. The same pattern was reported for ground-foraging species (Rodgers and Smith, 1995; Gill, 2007). In addition, while there is an often-held assumption that human presence triggers changes in behaviour [like flush responses, reduction in incubation duration or changes in foraging behaviour (Lord et al., 2001; Verhulst et al., 2001; Thiel et al., 2007)], which in turn may be translated into reduced fitness, those fitness consequences are in fact rarely tested (Gill et al., 2001). Here, we did not find any evidence that human presence affects the life-history or reproductive success of birds in an urban context, and suggest that structural properties of the environment, such as paths and roads, overrides the weak or non-existent effect of human presence in cities.
In contrast to a lack of effect caused by human presence, nestbox distance to paths and roads influenced incubation duration and chick mass on day 15 after hatching. A larger dataset (in terms of study sites and years of study) is likely to be required to detect finer scale effects of the effect of human presence or distance to paths and roads on tit reproductive trait variation. Roads have already been shown to have a strong impact on fitness in birds (e.g., Halfwerk et al., 2011; Dietz et al., 2013; but see review by Kociolek et al., 2011). Their negative effect may arise through noise pollution leading to disruption in communication, for example in the context of mate attraction, predator avoidance or parent-offspring communication (Slabbekoorn and Ripmeester, 2008; Francis, 2015). Thus, communication breakdown caused by noise may at least partly be responsible for lower body mass in nestlings located closer to roads. A more direct mechanism associating proximity to roads with lower offspring body mass is the fact that a greater proportion of impervious surface close to the nestbox automatically results in lower levels of biomass and resulting food availability for our focal species (but see the opposite effect in Florida scrub jays Morgan et al., 2010). Moreover, roads are associated with many biotic and abiotic changes, for example: increased mortality in birds due to direct collisions with vehicles, but also the general alteration of physical and chemical properties in these novel environments (Reijnen et al., 1995; Erritzoe et al., 2003; but see review by Trombulak and Frissell, 2000).
The fact that blue tits significantly increased their incubation time closer to paths (while great tits show an equivalent yet non-significant trend in the same direction) independently of human presence is surprising and suggests that paths act as a disruptor to incubation that may be independent of human presence. Indeed, the correlation between human presence and distance from paths was significant but weak (r = −0.29, p = 0.002, Table 4), suggesting that due to the frequency of off-trail events, the presence of visitors in the urban park is not strictly connected with infrastructural networks. However, previous studies reported that habituation to walkers is common in several species, especially when people remain on marked paths: however, this level of habituation may change during the breeding season, whenever off-trail events occur (Nisbet, 2000). In addition, it is possible that the mere presence of humans in proximity of a nest can induce increased alertness in both adults affecting parental care (while feeding the nestlings or, as in this case, during incubation). In contrast, incubation duration was significantly shorter in great tits breeding in proximity to paved roads, which also coincides with c. 0.5°C increase every 100 m closer to the largest road neighbouring the urban park (daily averages over 4 months starting on the 25th of March; Corsini, unpublished data).
In conclusion, we confirm that the protocol for quantifying human presence developed in this study proved to be highly repeatable in a site with high intra-site variance of human presence, thereby confirming its usefulness to other studies testing the effects of human presence around fixed point locations in avian and non-avian species alike. At the same time, our results suggest that urban great tits and blue tits are habituated to human presence, a keystone species in the urban environment, and generally perceive humans as harmless stimuli (Lowry et al., 2013). While the process of habituation is known to reduce stress responses in avian species (Walker et al., 2006), fitness consequences due to a long-term exposure to human generated stressors may be complex, especially in an urban context where several environmental variables other than human presence interact. Finally, in the context of urban study sites, it is mostly infrastructural networks rather than human presence per se that played a greater role in tit reproductive trait variation.
Author Contributions
MS designed the study; MC, AD, and MS collected the data; MC, PM, and MS performed the analyses, MC prepared a first draft of the manuscript; MC, AD, PM, and MS edited and approved the final version of the manuscript.
Funding
This study was financed with a Sonata Bis grant 2014/14/E/NZ8/00386 from the National Science Centre, Poland.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Tomasz Mazgajski, Jerzy Bańbura and Anne Charmantier for constructive discussions on urban ecology. We also thank Karol Kobiałka, Marta Celej, Justyna Kubacka and Fatima Hayatli for help in the field. We are grateful to ground managers for enabling research on their sites.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2017.00082/full#supplementary-material
References
Abolins-Abols, M., Hope, S. F., and Ketterson, E. D. (2016). Effect of acute stressor on reproductive behavior differs between urban and rural birds. Ecol. Evol. 6, 6546–6555. doi: 10.1002/ece3.2347
Almasi, B., Béziers, P., Roulin, A., and Jenni, L. (2015). Agricultural land use and human presence around breeding sites increase stress-hormone levels and decrease body mass in barn owl nestlings. Oecologia 179, 89–101. doi: 10.1007/s00442-015-3318-2
Baines, D., and Richardson, M. (2007). An experimental assessment of the potential effects of human disturbance on Black Grouse Tetrao tetrix in the North Pennines, England. Ibis 149, 56–64. doi: 10.1111/j.1474-919X.2007.00638.x
Bayne, E. M., Habib, L., and Boutin, S. (2008). Impacts of chronic anthropogenic noise from energy–sector activity on abundance of songbirds in the boreal forest. Conserv. Biol. 22, 1186–1193. doi: 10.1111/j.1523-1739.2008.00973.x
Carney, K. M., and Sydeman, W. J. (1999). A review of human disturbance effects on nesting colonial waterbirds. Waterbirds 22, 68–79. doi: 10.2307/1521995
Chace, J. F., and Walsh, J. J. (2006). Urban effects on native avifauna: a review. Landsc. Urban Plan. 74, 46–69. doi: 10.1016/j.landurbplan.2004.08.007
Chamberlain, D. E., Cannon, A. R., Toms, M. P., Leech, D. I., Hatchwell, B. J., and Gaston, K. J. (2009). Avian productivity in urban landscapes: a review and meta–analysis. Ibis 151, 1–18. doi: 10.1111/j.1474-919X.2008.00899.x
Churcher, P. B., and Lawton, J. H. (1987). Predation by domestic cats in an English village. J. Zool. 212, 439–455. doi: 10.1111/j.1469-7998.1987.tb02915.x
Clucas, B., and Marzluff, J. M. (2012). Attitudes and actions toward birds in urban areas: human cultural differences influence bird behavior. Auk 129, 8–16. doi: 10.1525/auk.2011.11121
Da Silva, A., Samplonius, J. M., Schlicht, E., Valcu, M., and Kempenaers, B. (2014). Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 25, 1037–1047. doi: 10.1093/beheco/aru103
Dietz, M. S., Murdock, C. C., Romero, L. M., Ozgul, A., and Foufopoulos, J. (2013). Distance to a road is associated with reproductive success and physiological stress response in a migratory landbird. Wilson J. Ornithol. 125, 50–61. doi: 10.1676/11-201.1
Ditchkoff, S. S., Saalfeld, S. T., and Gibson, C. J. (2006). Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst. 9, 5–12. doi: 10.1007/s11252-006-3262-3
Dominoni, D., Quetting, M., and Partecke, J. (2013). Artificial light at night advances avian reproductive physiology. Proc. R. Soc. Lond. B Biol. Sci. 280:20123017. doi: 10.1098/rspb.2012.3017
Eeva, T., Lehikoinen, E., and Nurmi, J. (1994). Effects of ectoparasites on breeding success of great tits (Parus major) and pied flycatchers (Ficedula hypoleuca) in an air pollution gradient. Can. J. Zool. 72, 624–635. doi: 10.1139/z94-085
Ellenberg, U., Setiawan, A. N., Cree, A., Houston, D. M., and Seddon, P. J. (2007). Elevated hormonal stress response and reduced reproductive output in Yellow-eyed penguins exposed to unregulated tourism. Gen. Comp. Endocrinol. 152, 54–63. doi: 10.1016/j.ygcen.2007.02.022
Erritzoe, J., Mazgajski, T. D., and, Rejt, L. (2003). Bird casualties on European roads – a review. Acta Ornithol. 38, 77–93. doi: 10.3161/068.038.0204
Eurostat, I. (2017). Population on 1 January by Age Groups and Sex- Functional Urban Areas. Home-Eurostat. Available online at: http://ec.europa.eu/eurostat/
Fernández-Juricic, E. (2000). Avifaunal use of wooded streets in an urban landscape. Conserv. Biol. 14, 513–521. doi: 10.1046/j.1523-1739.2000.98600.x
Fernández-Juricic, E., and Tellería, J. L. (2000). Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merula in urban parks in Madrid, Spain. Bird Study 47, 13–21. doi: 10.1080/00063650009461156
Fowler, G. S. (1999). Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biol. Conserv. 90, 143–149. doi: 10.1016/S0006-3207(99)00026-9
Francis, C. D. (2015). Vocal traits and diet explain avian sensitivities to anthropogenic noise. Glob. Chang. Biol. 21, 1809–1820. doi: 10.1111/gcb.12862
Fry, D. M. (1995). Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Perspect. 103:165. doi: 10.1289/ehp.95103s7165
Fuller, R. A., Warren, P. H., Armsworth, P. R., Barbosa, O., and Gaston, K. J. (2008). Garden bird feeding predicts the structure of urban avian assemblages. Divers. Distrib. 14, 131–137. doi: 10.1111/j.1472-4642.2007.00439.x
Gil, D., and Brumm, H. (eds.). (2013). Avian Urban Ecology: Behavioural and Physiological Adaptations. Oxford: Oxford University Press.
Gill, J. A. (2007). Approaches to measuring the effects of human disturbance on birds. Ibis 149, 9–14. doi: 10.1111/j.1474-919X.2007.00642.x
Gill, J. A., Norris, K., and Sutherland, W. J. (2001). Why behavioural responses may not reflect the population consequences of human disturbance. Biol. Conserv. 97, 265–268. doi: 10.1016/S0006-3207(00)00002-1
Gla̧dalski, M., Bańbura, M., Kaliński, A., Markowski, M., Skwarska, J., Wawrzyniak, J., et al. (2016). Effects of human-related disturbance on breeding success of urban and non-urban blue tits (Cyanistes caeruleus). Urban Ecosys. 19, 1–10. doi: 10.1007/s11252-016-0543-3
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J., Bai, X., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Halfwerk, W., Holleman, L. J., Lessells, C. K., and Slabbekoorn, H. (2011). Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 48, 210–219. doi: 10.1111/j.1365-2664.2010.01914.x
Hunt, G. L. Jr. (1972). Influence of food distribution and human disturbance on the reproductive success of herring gulls. Ecology 53, 1051–1061. doi: 10.2307/1935417
Isaksson, C. (2015). Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 29, 913–923. doi: 10.1111/1365-2435.12477
Kirschel, A. N., Blumstein, D. T., Cohen, R. E., Buermann, W., Smith, T. B., and Slabbekoorn, H. (2009). Birdsong tuned to the environment: green hylia song varies with elevation, tree cover, and noise. Behav. Ecol. 20, 1089–1095. doi: 10.1093/beheco/arp101
Kociolek, A. V., Clevenger, A. P., St. Clair, C. C., and Proppe, D. S. (2011). Effects of road networks on bird populations. Conserv. Biol. 25, 241–249. doi: 10.1111/j.1523-1739.2010.01635.x
Lord, A., Waas, J. R., Innes, J., and Whittingham, M. J. (2001). Effects of human approaches to nests of northern New Zealand dotterels. Biol. Conserv. 98, 233–240. doi: 10.1016/S0006-3207(00)00158-0
Lowry, H., Lill, A., and Wong, B. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Mallord, J. W., Dolman, P. M., Brown, A. F., and Sutherland, W. J. (2007). Linking recreational disturbance to population size in a ground–nesting passerine. J. Appl. Ecol. 44, 185–195. doi: 10.1111/j.1365-2664.2006.01242.x
Marzluff, J. M. (2001). “Worldwide urbanization and its effects on birds,” in Avian Ecology and Conservation in An Urbanizing World, eds J. M. Marzluff, R. Bowman, and R. Donnelly (New York, NY: Springer), 19–47.
McDonnell, M. J., and Pickett, S. T. (1990). Ecosystem structure and function along urban–rural gradients: an unexploited opportunity for ecology. Ecology 71, 1232–1237. doi: 10.2307/1938259
McGiffin, A., Lill, A., Beckman, J., and Johnstone, C. P. (2013). Tolerance of human approaches by Common Mynas along an urban–rural gradient. Emu 113, 154–160. doi: 10.1071/MU12107
McGowan, C. P., and Simons, T. R. (2006). Effects of human recreation on the incubation behavior of American Oystercatchers. Wilson J. Ornithol. 118, 485–493. doi: 10.1676/05-084.1
Metcalf, B. M., Davies, S. J. J. F., and Ladd, P. G. (2000). Adaptation of behaviour by two bird species as a result of habituation to humans. Aus. Bird Watch. 18, 306–312.
Miller, S. G., Knight, R. L., and Miller, C. K. (2001). Wildlife responses to pedestrians and dogs. Wildl. Soc. Bull. 29, 124–132.
Mockford, E. J., and Marshall, R. C. (2009). Effects of urban noise on song and response behaviour in great tits. Proc. R. Soc. Lond. B Biol. Sci. 276, 1669. doi: 10.1098/rspb.2009.0586
Morgan, G. M., Boughton, R. K., Rensel, M. A., and Schoech, S. J. (2010). Road effects on food availability and energetic intake in Florida scrub-jays (Aphelocoma coerulescens). Auk 127, 581–589. doi: 10.1525/auk.2010.09033
Morrison, M. L. (1986). “Bird populations as indicators of environmental change,” in Current Ornithology, ed R. F. Johnston (New York, NY: Springer), 429–451.
Müller, C., Jenni-Eiermann, S., Blondel, J., Perret, P., Caro, S. P., Lambrechts, M., et al. (2006). Effect of human presence and handling on circulating corticosterone levels in breeding blue tits (Parus caeruleus). Gen. Comp. Endocrinol. 148, 163–171. doi: 10.1016/j.ygcen.2006.02.012
Nemeth, E., and Brumm, H. (2009). Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641. doi: 10.1016/j.anbehav.2009.06.016
Nisbet, I. C. (2000). Disturbance, habituation, and management of waterbird colonies. Waterbirds 23, 312–332.
Rees, W. E. (1997). Urban ecosystems: the human dimension. Urban Ecosys. 1, 63–75. doi: 10.1023/A:1014380105620
Reijnen, R., Foppen, R., Braak, C. T., and Thissen, J. (1995). The effects of car traffic on breeding bird populations in woodland. III. Reduction of density in relation to the proximity of main roads. J. Appl. Ecol. 32, 187–202. doi: 10.2307/2404428
Remacha, C., Delgado, J. A., Bulaic, M., and Pérez-Tris, J. (2016). Human disturbance during early life impairs nestling growth in birds inhabiting a nature recreation area. PLoS ONE 11:e0166748. doi: 10.1371/journal.pone.0166748
Robb, G. N., McDonald, R. A., Chamberlain, D. E., and Bearhop, S. (2008). Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. doi: 10.1890/060152
Rodgers, J. A., and Smith, H. T. (1995). Set–back distances to protect nesting bird colonies from human disturbance in Florida. Conserv. Biol. 9, 89–99. doi: 10.1046/j.1523-1739.1995.09010089.x
Rodriguez-Prieto, I., Fernández-Juricic, E., Martín, J., and Regis, Y. (2009). Antipredator behavior in blackbirds: habituation complements risk allocation. Behav. Ecol. 20, 371–377. doi: 10.1093/beheco/arn151
Safina, C., and Burger, J. (1983). Effects of human disturbance on reproductive success in the Black Skimmer. Condor 85, 164–171. doi: 10.2307/1367250
Savill, P., Perrins, C., Fisher, N., and Kirby, K. (2011). Wytham Woods: Oxford's Ecological Laboratory. Oxford: Oxford University Press.
Schneider, A., Friedl, M. A., and Potere, D. (2009). A new map of global urban extent from MODIS satellite data. Environ. Res. Lett. 4:044003. doi: 10.1088/1748-9326/4/4/044003
Seress, G., and Liker, A. (2015). Habitat urbanization and its effects on birds. Acta Zool. Acad. Sci. Hungar. 61, 373–408. doi: 10.17109/AZH.61.4.373.2015
Seto, K. C., Fragkias, M., Güneralp, B., and Reilly, M. K. (2011). A meta-analysis of global urban land expansion. PLoS ONE 6:e23777. doi: 10.1371/journal.pone.0023777
Shochat, E., Warren, P. S., Faeth, S. H., McIntyre, N. E., and Hope, D. (2006). From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. (Amst). 21, 186–191. doi: 10.1016/j.tree.2005.11.019
Slabbekoorn, H., and Peet, M. (2003). Ecology: birds sing at a higher pitch in urban noise. Nature 424, 267–267. doi: 10.1038/424267a
Slabbekoorn, H., and Ripmeester, E. A. P. (2008). Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83. doi: 10.1111/j.1365-294X.2007.03487.x
Solonen, T. (2001). Breeding ofthe Great Tit and Blue Tit in urban and rural habitats in southern Finland. Ornis Fennica 78, 49–60.
Sprau, P., Mouchet, A., and Dingemanse, N. J. (2016). Multidimensional environmental predictors of variation in avian forest and city life histories. Behav. Ecol. 28, 59–68. doi: 10.1093/beheco/arw130
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. doi: 10.1111/2041-210X.12797. [Epub ahead of print].
Stone, E. (2000). Separating the noise from the noise: a finding in support of the “Niche hypothesis,” that birds are influenced by human-induced noise in natural habitats. Anthrozoös 13, 225–231. doi: 10.2752/089279300786999680
Tarjuelo, R., Barja, I., Morales, M. B., Traba, J., Benítez-López, A., Casas, F., et al. (2015). Effects of human activity on physiological and behavioral responses of an endangered steppe bird. Behav. Ecol. 26, 828–838. doi: 10.1093/beheco/arv016
Thiel, D., Menoni, E., Brenot, J. F., and Jenni, L. (2007). Effects of recreation and hunting on flushing distance of capercaillie. J. Wildl. Manag. 71, 1784–1792. doi: 10.2193/2006-268
Trombulak, S. C., and Frissell, C. A. (2000). Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 14, 18–30. doi: 10.1046/j.1523-1739.2000.99084.x
United Nations, Department of Economic and Social Affairs, Population Division. (2014). World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352).
van de Laar, I. N. G. F. J. T. (2007). Green light to birds. Investigation into the Effect of Bird-Friendly Lighting. Report NAM locatie L15-FA-1. Nederlandse Aardolie Maatschappij, Assen.
Verhulst, S., Oosterbeek, K., and Ens, B. J. (2001). Experimental evidence for effects of human disturbance on foraging and parental care in oystercatchers. Biol. Conserv. 101, 375–380. doi: 10.1016/S0006-3207(01)00084-2
Keywords: human disturbance, habituation, Parus major, Cyanistes caeruleus, life-history traits, paths, roads, incubation
Citation: Corsini M, Dubiec A, Marrot P and Szulkin M (2017) Humans and Tits in the City: Quantifying the Effects of Human Presence on Great Tit and Blue Tit Reproductive Trait Variation. Front. Ecol. Evol. 5:82. doi: 10.3389/fevo.2017.00082
Received: 10 February 2017; Accepted: 11 July 2017;
Published: 09 August 2017.
Edited by:
Amanda D. Rodewald, Cornell University, United StatesReviewed by:
Carolina Remacha, Complutense University of Madrid, SpainDavide M. Dominoni, Netherlands Institute of Ecology (NIOO-KNAW), Netherlands
Copyright © 2017 Corsini, Dubiec, Marrot and Szulkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michela Corsini, bWljaGVsYS5jb3JzaW5pQGNlbnQudXcuZWR1LnBs
 Michela Corsini
Michela Corsini Anna Dubiec
Anna Dubiec Pascal Marrot3
Pascal Marrot3 Marta Szulkin
Marta Szulkin