
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 29 May 2017
Sec. Behavioral and Evolutionary Ecology
Volume 5 - 2017 | https://doi.org/10.3389/fevo.2017.00053
This article is part of the Research Topic Behavioural and Ecological Consequences of Urban Life in Birds View all 31 articles
As an extension of the classic life history theory, the recently highlighted pace-of-life syndrome hypothesis predicts the coevolution of behavioral, physiological and life-history traits. For instance, bolder and shyer individuals do not only differ in personality profiles, but also in neuro-endocrinology and breeding patterns. While theory predicts that bolder (i.e., proactive), more aggressive individuals should colonize more rapidly urbanized habitats than shyer (i.e., reactive), less aggressive individuals, it is also predicted that across generations, adaptive selection processes could favor shyer individuals that are more sensitive to novel environmental cues. Here we compared two personality traits (handling aggression, exploration score in a novel environment), one physiological trait related to stress response (breath rate) and four breeding traits (lay date, clutch size, hatching success and fledging success) in a rural and an urban study population of Mediterranean great tits Parus major. Mixed models revealed strong phenotypic divergence between forest and city in most traits explored, in particular in personality, whereby urban great tits were more reactive to stress and faster explorers compared to rural birds (yet not more aggressive). Urban birds also laid smaller broods earlier in spring compared to their rural conspecifics, and city broods resulted in lower hatching success yet interestingly fledging success was similar. Nest-box centered measures of anthropogenic (artificial light, pedestrians, and cars) perturbation and resource abundance allowed us to go beyond the classical forest/city comparison by exploring the phenotypic variation across an urbanization gradient. This revealed that high urbanization in nest-box surroundings was associated overall with earlier breeding and smaller clutches, but also with faster breath rate, although these trends showed strong annual variation. Ongoing rapid urbanization and non-random gene flow between rural and urban great tits could both contribute to the high prevalence of bold breeders in the city. Our study suggests the existence of urban and rural great tit ecotypes with different pace-of-life, but also a finer-scale divergence along the degree of urbanization within the city. Future studies are required to determine whether this phenotypic variation at different spatiotemporal scales is adaptive and whether it has a genetic basis or results from phenotypic plasticity.
Understanding the mechanisms by which organisms adapt to spatiotemporal environmental heterogeneity remains a fundamental goal in evolutionary ecology. It is tightly linked to another major goal in the field, namely understanding the maintenance of the wide phenotypic (and genetic) variation we observe in nature. As an extension of the classic life history theory (Roff, 1992, 2002; Stearns, 1992), the recently highlighted pace-of-life syndrome (POLS) hypothesis (Ricklefs and Wikelski, 2002; Martin et al., 2006; Réale et al., 2010), predicts the coevolution of life histories with a suite of ecologically relevant co-varying traits in metabolism, immunology, and behavior. The POLS hypothesis provides testable predictions concerning the association between the slow-fast life history continuum and behavioral, physiological, and immunological traits. In short, the pace-of-life continuum differentiates between, on the one hand slow individuals with slow life-histories (e.g., high survival, delayed reproduction), reactive behaviors (e.g., shyness, low aggressiveness, and low activity) and low metabolism, and on the other hand fast individuals with fast life-histories, proactive behaviors and high metabolism (Réale et al., 2010). Although the POLS concept has attracted paramount attention in behavior ecology and eco-physiology, empirical support for the POLS hypothesis is currently mixed (Hille and Cooper, 2015), perhaps because POLS theory still lacks a conceptual framework to predict which ecological conditions will favor which syndromes. Most notably, many studies in the last decade have tested adaptive explanations for the evolution of repeatable individual differences in behavior (aka personality) (Wolf et al., 2007) and a number of theoretical studies have attempted to predict the conditions favoring the evolution of personalities (Dingemanse and Wolf, 2010).
The urban matrix and its progression across rural landscapes represent for many organisms a drastic and very rapid change in environmental conditions compared to the natural habitats where they have evolved (Sih et al., 2011). These urban conditions such as novel social interactions, pollution, or altered plant-animal interactions, induce novel selection pressures requiring adaptive changes from native organisms (Atwell et al., 2012). Moreover, the urban matrix is often highly heterogeneous, offering little connectivity between favorable habitats (Lindenmayer and Fischer, 2006). This radical environmental shift causing the fragmentation, isolation and degradation of natural habitats represents an acute global biodiversity conservation issue (e.g., McKinney, 2002; Aronson et al., 2014). However, urban development can also be viewed as a unique opportunity to investigate eco-evolutionary processes in a rapidly changing environment (Alberti, 2015; McDonnell and Hahs, 2015). Indeed, cities have recently been highlighted as hotspots of contemporary evolution, where rates of phenotypic changes are greater in urbanized areas compared to natural but also to non-urban anthropogenic habitats (Hendry et al., 2008; Alberti et al., 2017). In the context of the evolution of pace-of-life syndromes as outlined above, urban landscapes can be viewed as an ideal setting to test whether animals display differential phenotypes for physiological, life history and behavioral traits across the urban/natural landscape, but also within the heterogeneous urban matrix.
It is widely recognized that urbanization can act as an ecological filter favoring certain species that are more urban tolerant (Williams et al., 2009; Silva et al., 2016), thus leading to differences in species compositions across urbanization gradients (e.g., for avian communities, Sol et al., 2014; Clucas and Marzluff, 2015). For instance, in such inter-specific context, the “cognitive buffer hypothesis” predicts that larger brained animals have higher adaptive abilities in novel and challenging environmental conditions such as urban habitats (Sol et al., 2005). Since individuals within a species can show strong persistent variation in a large set of traits related to adaptation, a similar process of filtering can be predicted to occur between conspecifics, whereby individuals with particular phenotypes will be favored in the urban landscape. Although there are presently no formal theoretical predictions on POLS in urban environments, the general expectation is that urban habitats will be more favorable to fast living individuals (Evans et al., 2010; Atwell et al., 2012) which are often higher dispersers hence more likely colonizers (Cote et al., 2010). The ornithological literature already offers many examples of adjustments in urban birds compared to rural conspecifics that are concordant with this expectation (see recent review in Marzluff, 2017). Urban birds are usually more aggressive (e.g., Minias, 2015; Davies and Sewall, 2016), bolder (e.g., Ducatez et al., 2017), can be approached more closely by humans (e.g., Lin et al., 2012; Møller et al., 2015), are less neophobic (e.g., Tryjanowski et al., 2016), have higher levels of disturbance tolerance (Lowry et al., 2013) and reduced levels of physiological stress response (e.g., Partecke et al., 2006; Minias, 2015). Other empirical comparisons between urban and rural birds have also suggested shifts in breeding strategies: urban birds usually have earlier laying dates, smaller clutch sizes, lower reproductive success (e.g., Peach et al., 2008; review in Chamberlain et al., 2009), and higher adult survival rates (e.g., Rebolo-Ifran et al., 2015). Hence overall, the empirical literature so far seems to suggest that urban birds tend to have slow life histories yet fast behavioral syndromes (Figure 1 in Réale et al., 2010). This discrepancy could result from the lack of integrative study exploring all types of traits simultaneously in the same urban context, but it could also be indicative of the emergence of novel covariances between life history and behavioral traits. In order to test whether urban birds occupy a particular position on the POLS continuum, we now need multivariate studies that investigate breeding, physiological and behavioral traits in concert. Also, the heterogeneity of the urban matrix providing a variety of environments from forested parks to industrial areas could be exploited in order to go beyond the urban/rural dichotomy and test whether the fast-slow continuum matches the urbanization gradient.
Using longitudinal data across 6 years from a great tit Parus major population monitoring in the city of Montpellier (France) and in a nearby rural oak forest (La Rouvière), we address in this study the following main questions:
(i) Do great tits show divergent urban versus forest ecotypes for personality, physiological, and breeding traits?
(ii) Do these traits display gradual phenotypic variation across an urbanization gradient?
(iii) Is the phenotypic variation observed in these traits concordant with the pace-of-life syndrome hypothesis, whereby individuals in more constraining and novel environments will have faster life histories, will be bolder and more susceptible to acute stress?
The comparative approaches between urban and forest birds and across the urban gradient include two personality traits (handling aggression, exploration score in a novel environment), one physiological trait related to stress response (breath rate under constraint) and four breeding traits (laying date, clutch size, hatching success, and fledging success).
Breeding great tits Parus major were monitored and captured in nest-boxes in two main study areas in the south of France: a rural oak forest and an urban environment (Figure S1). First, captures and measures were done in the La Rouvière forest dominated by downy oaks (Quercus humilis), located 20 km North-West of the city of Montpellier. Great tits (and blue tits) have been monitored in this forest since 1991 with weekly visits to all nest-boxes and all individuals marked with unique leg rings (see details on monitoring in following paragraph and in Doutrelant et al., 2000; Blondel et al., 2006). Second, captures were also done in nest-boxes within the city of Montpellier (area 56.88 km2, human population ca 272,000 in 2013, source: Wikipedia), where a similar breeding monitoring is performed in areas containing both parks and streets (Demeyrier et al., 2016). The monitoring in the city of Montpellier started in 2011, providing information on brood-specific life history (exact lay date, clutch size, hatching, and fledgling success) yet capture of breeding parents started in 2013 and behavioral assays (see below) in 2014. In this study we used datasets of varying sizes depending on the variables studied, with data collected between 2011 and 2016. Number of nest-boxes fluctuated over the years because of theft and changes in entrance-hole size. The number of nest-boxes with large entrance holes (32 mm) allowing great tits to enter and breed, fluctuated between 51 (in 2011) and 92 (in 2016) in La Rouvière forest, and between 163 (in 2012) and 183 (in 2013) in the city of Montpellier.
Monitoring of broods started before nest construction and ended after the last nestling had fledged. Weekly nest-box field visits to each brood resulted in the observation of the exact date when the first egg was laid (lay date, March 1st = 1), the number of eggs laid (clutch size), the number of hatchlings, and the number of fledglings (nests were visited at 15 days, and then 21 days after hatching to check fledging). In order to compare with previous studies (e.g., Vaugoyeau et al., 2016), only data collected on first broods were considered in the analyses. Parents were captured and measured when their nestlings were 9–15 days old and uniquely identified with metal rings provided by the CRBPO (Centre de Recherches sur la Biologie des Populations d'Oiseaux, Museum National d'Histoire Naturelle, Paris). Nestlings were also uniquely ringed and measured at 14–15 days old.
As previously described in Demeyrier et al. (2016), four features of the urban matrix were measured within a 50 m radius around each nest-box: vegetation cover, car traffic, pedestrian count, and intensity of artificial light. Demeyrier et al. (2016) provide all the details on how these measures were performed, but also how they were analyzed in a principal component analysis (PCA, see Figure 4 in Demeyrier et al., 2016). From this PCA, we extracted the two major axes: PC1 reflecting habitat naturalness and hereafter abbreviated “naturalness” (positively related to vegetation cover and negatively correlated with car traffic and artificial lights, explaining 56.6% of the variance), and PC2 reflecting the pedestrian frequency, hereafter abbreviated “pedestrian” (explaining 16.7% of the variance in the urban environmental features).
The behavioral trials were performed in both La Rouvière forest and Montpellier in 2014, 2015, and 2016. Great tit parents were caught in their breeding nest-boxes while feeding their 9–15 days old nestlings. A captured bird was kept for around 20 min in total, during which time it was submitted to the following sequence of events: capture in the nest-box, handling aggression scoring, resting phase in cloth bag for 5 min, measure of breath rate during handling, resting phase of 2 min in small acclimatization compartment adjacent but separated from the main open-field cage (Figure 1), exploratory scoring in open-field trial during 4 min, ringing (if not already ringed), morphological measurements (body mass, tarsus, tail and wing lengths), and blood sampling.
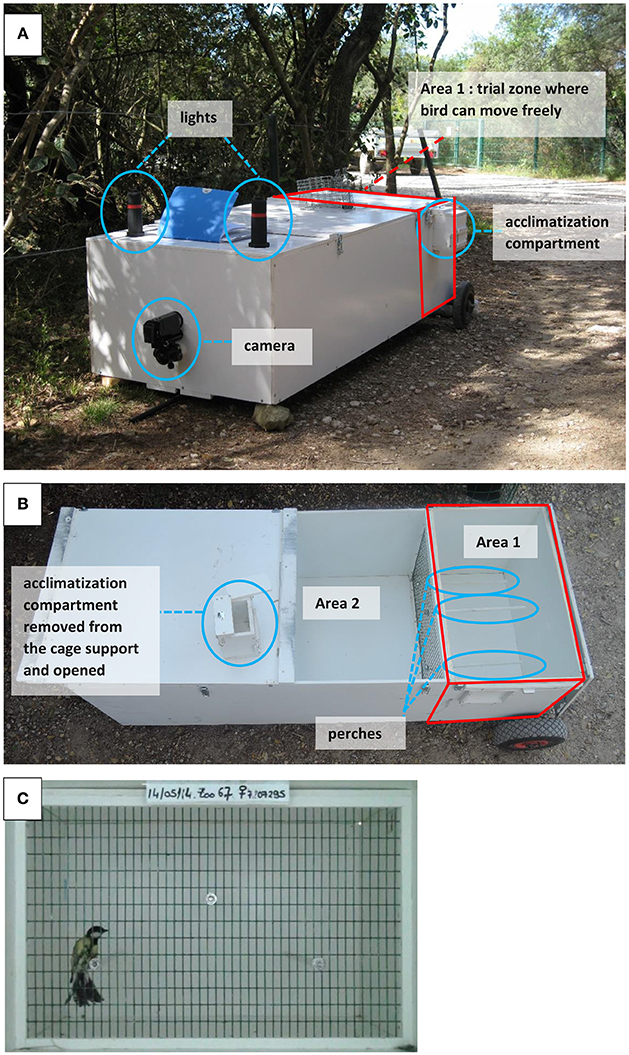
Figure 1. Pictures of the experimental open-field cage for the novel environment test. (A) View of the operational cage at the zoo of Lunaret (Montpellier). (B) View from above to see the division between area 1 of the cage where the bird is released during the trial and area 2 (the rest of the cage) which serves as an empty compartment to control for distance with the camera and light conditions. (C) Picture of the movie view during a trial.
First, immediately after its capture, a bird was tested for handling aggression by the capturer. The handler held the bird high up in the legs with one hand and nagged the bird with one finger from his/her other hand following a standardized procedure where the finger approaches the bird but does not touch it. After ca. 15 s of test and observation, the handler attributed a handling aggression score (HA, following the standardized criteria list in Table S4 of Dubuc-Messier et al., 2016, see also Brommer and Kluen, 2012; Class et al., 2014), ranging from 0 (completely unreactive bird) to 3 (bird aiming for the handler's finger all the time, spreading its wings and tail), with 0.5 increments. Following this first behavioral assay, the bird was placed in a bag for 5 min and brought to the open-field apparatus which was less than 5 min walking distance from the nest-box. Second, right after it was removed from the cloth bag, the bird was held still on its back by the handler who counted twice in a row the number of breaths during 30 s (breaths were visually expressed via fast movements of the chest). Handling breath rate (HBR) was the number of breaths per minute, averaged over the two measures (Carere and van Oers, 2004; Fucikova et al., 2009; Brommer and Kluen, 2012; Kluen et al., 2014). HBR has been shown in other tit populations to be positively related to stress response (Carere and van Oers, 2004). Third, a “novel environment test” was performed in a unique experimental open-field cage built following the exact set up and dimensions described in Stuber et al. (2013) (Figure 1). This kind of set-up has been classically used to measure exploration behavior in great tits (e.g., Stuber et al., 2013) and blue tits (e.g., Mutzel et al., 2013; Dubuc-Messier et al., 2016), and provides exploratory scores that are repeatable and well correlated to those performed in the laboratory (e.g., in Dutch and German great tits, Dingemanse et al., 2002; Stuber et al., 2013). Right after the HBR measure, the bird was placed in a double door small compartment on the side of the open-field cage for acclimatization during 2 min with no visual cue other than the small cage of dimensions 11 × 12 × 11 cm. Following this resting time, the bird was released through a sliding door into the exploration cage of 61 × 39 × 40 cm dimensions, where it was filmed during 4 min by a camcorder (CH4 Sony Handycam) fixed 110 cm away from the wired side of the open-field cage. The bird was free to move around the cage and/or to settle on three perches. The light was standardized since the open-field cage was placed in a wider cage of dimension 61 × 150 × 40 cm, lighted by two led lights. Following previous studies on great tits, the total number of flights and hops during the open-field trial was used as a proxy of exploratory behavior (e.g. Dingemanse et al., 2002). This number was square root transformed (to minimize deviation from normality and following Nicolaus et al., 2016) and hereafter called exploration score. VD analyzed all videos using the software JWatcher (Blumstein et al., 2006) and scored this behavior.
All statistical analyses were run with the software R (version 3.3.2, R Core Team, 2016). We used several datasets to restrict the limitations of missing data in the various variables. In a first step of the analyses, we compared breeding, personality and physiological traits between the forest and the urban habitats using two-sided t-tests on the entire dataset available (considering the data from both known and unknown parents), hence not restricting to observations where the identity of one parent was known. For this and all other analyses, we removed estimations of number of hatchlings and number of fledglings for all broods that had been (experimentally) manipulated after the egg-laying stage, which explains smaller sample sizes for these two variables compared to lay date and clutch size.
The first step in the statistical analysis compared population means using a dataset where many female identities were unknown (since parents were not captured during the first 2 years of monitoring) providing an idea of results that can be obtained without comprehensive monitoring and individual ringing.
In a second step, we more formally tested for differences between forest and urban birds with univariate linear mixed models (LMM, package lme4, Bates et al., 2015) which included habitat type (forest versus city) as a fixed effect and female identity as a random effect. All LMMs initially included as fixed effects habitat (forest versus urban), year, a habitat × year interaction and other trait-specific fixed effects (see below). The fixed effect model was selected in a backward stepwise procedure starting with a model including all variables. The significance of each fixed effect was tested using likelihood-ratio tests (Bates et al., 2015). While all behavioral and physiological traits showed no significant correlations (Tables 1C,D), the three reproductive performance traits were highly correlated (Tables 1A,B and Table S1). Hence clutch size was included as a fixed effect in brood size models (hereafter called hatching success), and brood size was included as a fixed effect in number of fledglings models (hereafter called fledgling success). For handling aggression and breath rate, the initial full model included fixed effects for sex (and sex × habitat interaction), age, individual life-long capture rank (from 1 to 3, for first to third capture of the same individual) and ambient temperature, and observer/manipulator identity as a random effect. For exploration score, we included sex (and sex × habitat interaction), age, test rank (from 1 to 3), and temperature as fixed effects. As our factor of interest, habitat was kept in all models, even if not significant, to provide the effect size and statistics associated with its fit in the model. Since these LMMs included a female random effect, the dataset used in this second step included breeding parameters that had been recorded for broods where the female was captured only, hence the number of observations was reduced compared to the first dataset.
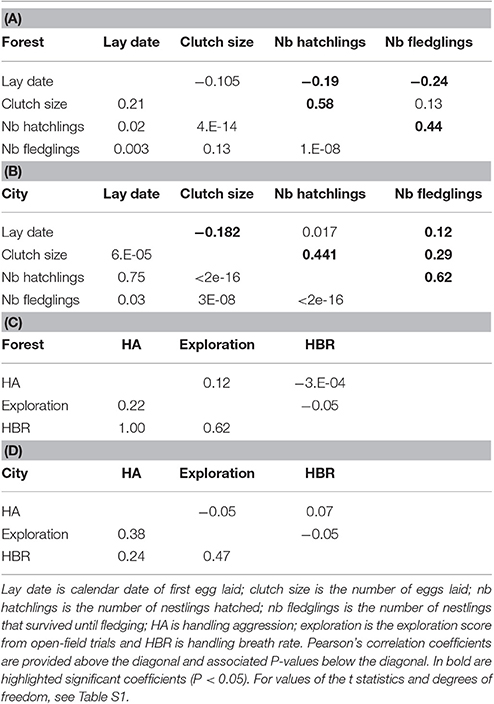
Table 1. Phenotypic correlations (above the diagonal) among (A,B) breeding traits (in forest and city) and (C,D) behavior and physiological traits (in forest and city), and their associated P-values (below the diagonal).
Based on the best models retained in this step 2, we estimated an adjusted repeatability with the package rptR (Nakagawa and Schielzeth, 2010) for all focal traits. We estimated overall repeatabilities across the whole dataset but also sex-specific and habitat-specific repeatabilities.
In a third and final step, we used only data collected in the city of Montpellier to contrast the measured traits against the two urbanization gradients revealed by the PCA of environmental features in the city: habitat naturalness, abbreviated “naturalness” (PC1) and pedestrian frequency, abbreviated “pedestrian” (PC2). Model selection was similar to that in step 2 (with similar fixed and random effects structure), apart from the use of naturalness and pedestrian instead of the habitat variable which previously was used to compare forest and city. As previously, naturalness was kept in all models since it was our main variable of interest.
Sample sizes are indicated in Table 2 (for step 1), Table 3 (for step 2), and Table 4 (for step 3). While models for phenology and reproductive success were female-specific, models on behavior and physiology pooled data on both sexes, with balanced sampling across sexes since 47–48% of observations were on males (detailed sex-specific sample sizes are in Table S3).
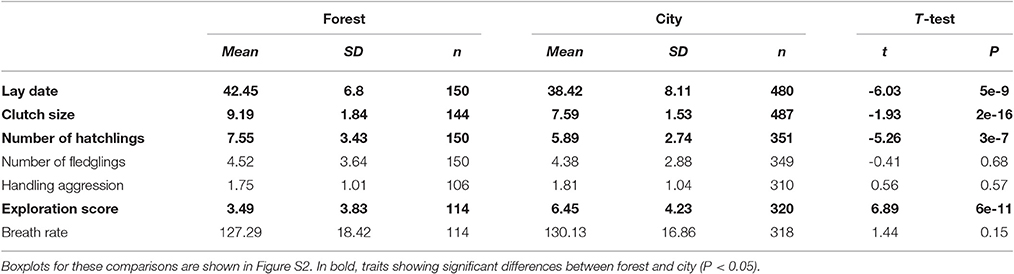
Table 2. Comparison of mean traits between forest and city great tits based on the full monitoring dataset (hence including broods with unknown parents).
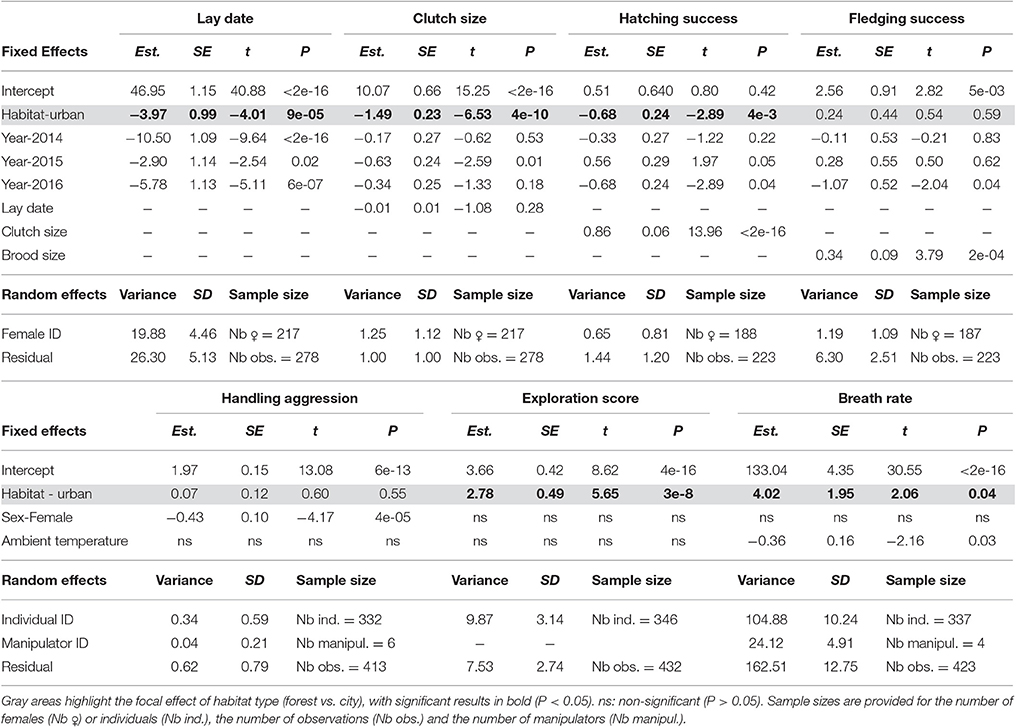
Table 3. Contrasting phenology, reproductive success, behavior, and physiology between forest and city birds (Habitat) using linear mixed models with female/individual identity and manipulator identity (for handling aggression and breath rate) as random effects.
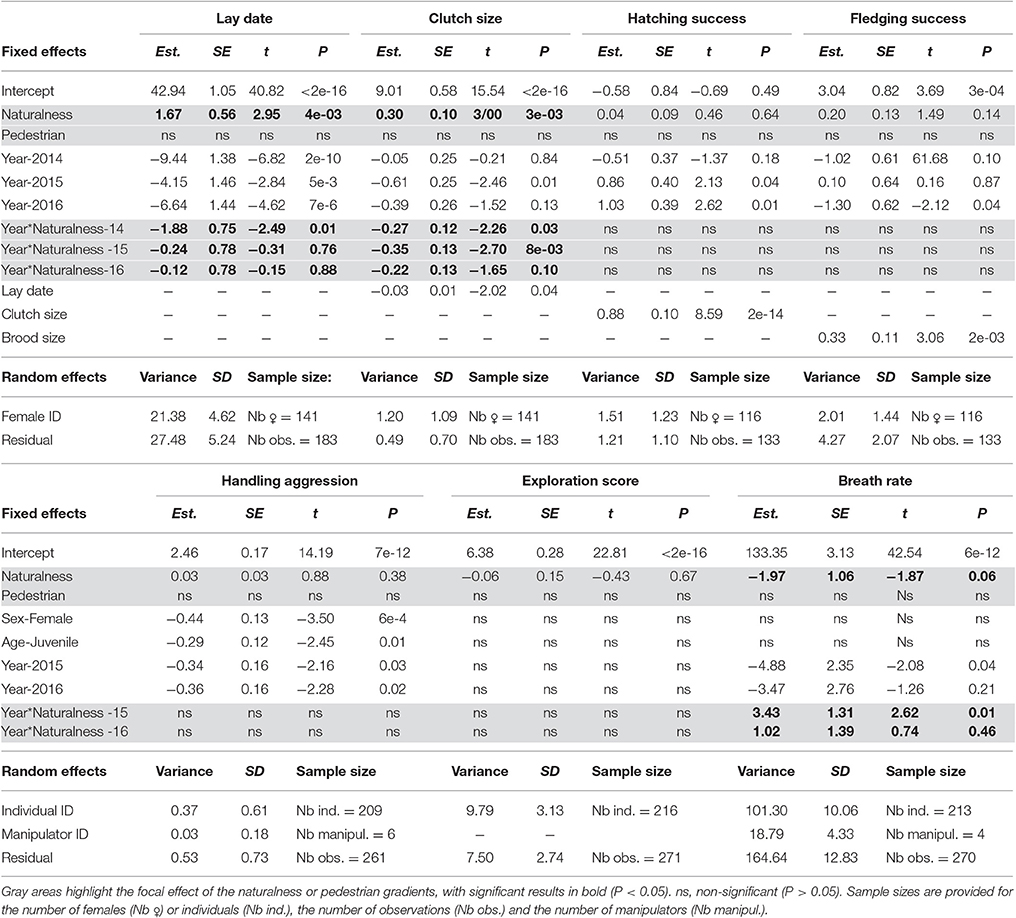
Table 4. Contrasting phenology, reproductive success, behavior, and physiology along urban gradients of habitat naturalness (Naturalness) and pedestrian frequency (Pedestrian) using linear mixed models with female/individual identity and manipulator identity (for handling aggression and breath rate) as random effects.
Comparison of mean traits between forest and city using the largest dataset (2011–2016) without controlling for the identity of individuals showed strong differences in mean lay dates, clutch sizes, number of hatchlings, and exploration score, but no significant differences in number of fledglings, handling aggression, and breath rate during constraint (Table 2, Figure S2).
An interesting result from this investigation on all monitoring data collected (after removing experimental broods) was a lower percentage of complete brood failure (i.e., no fledgling) in the city: 31% of complete brood failure in the forest versus 21% in the city (t = −2.167, df = 253.82, P = 0.03). Unfortunately, this data could not be analyzed using Generalized Linear Mixed Models with individual identity as random effect since most failed broods did not allow parent capture and identification. In the latter dataset used in the LMM for breeding traits, brood failure was 32% in the forest vs. 29% in the city. Unfortunately, the large majority of these failures were of unknown cause, but we can hypothesize predation on a parent, catastrophic climatic event or abandonment by parents because of high levels of stress and parental care. Future research should investigate whether some of these drivers of brood failure differ between the forest and city environments.
Hence at first glance when comparing mean traits without controlling for fixed effects that may be important or the non-independence of data collected on the same individuals, city birds laid earlier, and smaller first clutches, with decreased hatching success yet decreased occurrence of complete brood failure, and they did not fledge a lower number of offspring (see statistical details in Table 1). They also presented significantly higher exploration activity as well as slightly higher handling aggression and breath rate, although not significantly so.
When accounting in step 2 for the non-independence of data collected on the same individuals, restricting to records from known individuals (2013–2016, sample sizes in Table 3), and controlling for fixed effects, LMMs confirmed strong differences between forest and city great tits in their lay date, clutch size, hatching success (number of hatchlings when controlling for clutch size) (Table 3 and Figure 2), and interestingly still no significant difference between forest and city birds in their fledging success (number of fledglings controlling for number of hatchlings, Table 3 and Figure 2). Note that this non-significant result remained when modeling number of fledglings without controlling for the strong positive correlation with number of hatchlings, that is when comparing absolute fledgling number (Table S2). As in step 1, step 2 confirmed that city birds laid earlier and smaller first clutches, with decreased hatching success (Table 3). Urban great tits also displayed faster exploration scores and faster breath rates, but did not differ from their forest conspecifics in handling aggression (see statistical details in Table 3 and Figure 2).
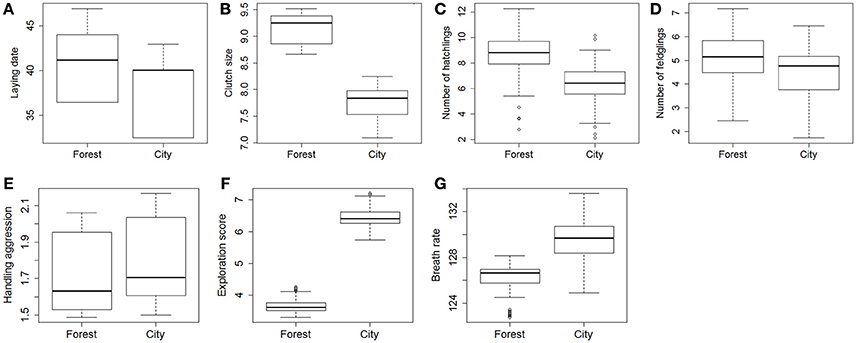
Figure 2. Predicted contrasts in breeding [A, lay date; B, clutch size; C, number of hatchlings (from a model controlling for clutch size), D, number of fledglings (controlling for number of hatchlings)], behavior (E, handling aggression; F, exploration score) and physiological traits (G, breath rate) between great tits from the forest of La Rouvière and great tits from the city of Montpellier. The graphs present the boxplot of predicted values from models detailed in Table 3. For lay date, 1 = 1st March. Breath rate is in number of breaths/min.
Both estimations of reproductive success were not repeatable among females (adjusted repeatabilities of 0.31 (SE = 0.14) for hatching success and 0.16 (SE = 0.14) for fledgling success, P = 1, Nakagawa and Schielzeth, 2010). All other traits were significantly repeatable with adjusted repeatabilities of 0.43 (SE = 0.10, P = 2.3e-5) for lay date, 0.55 (SE = 0.08, P = 4.8e-3) for clutch size, 0.34 (SE = 0.09, P = 2.6e-4) for handling aggression, 0.55 (SE = 0.07, P = 5.2e-7) for exploration score and 0.35 (SE = 0.08, P = 8.6e-6) for breath rate during constraint. While sex- and habitat-specific repeatabilities were based on small sample sizes, they suggested that behavioral and physiological traits might be more repeatable in females than in males and more repeatable in forest than urban habitats (see Table S3). In contrast, laying date and clutch size were repeatable in urban birds but not in forest birds (where again, sample sizes were small, Table S3).
The step 3 analysis along the gradients of naturalness and pedestrian pressure showed no influence of pedestrian frequency on any of the focal traits (Table 4). Three out of the seven traits studied showed variation across the naturalness gradient: in more urbanized areas great tit females laid earlier and smaller broods and birds had generally (see below) faster breath rates during handling (i.e., similar trends as found when contrasting urban and forest birds). In all three cases however, the strength (and even direction in the case of breath rate) of the urbanization effect showed significant variation across years (Table 4, Figure 3): for example, later laying in more natural habitats was true for 3 years out of four (Figure 3A) and breath rate showed inconsistent changes along the urban gradient across the 3 years of study (Figure 3C).
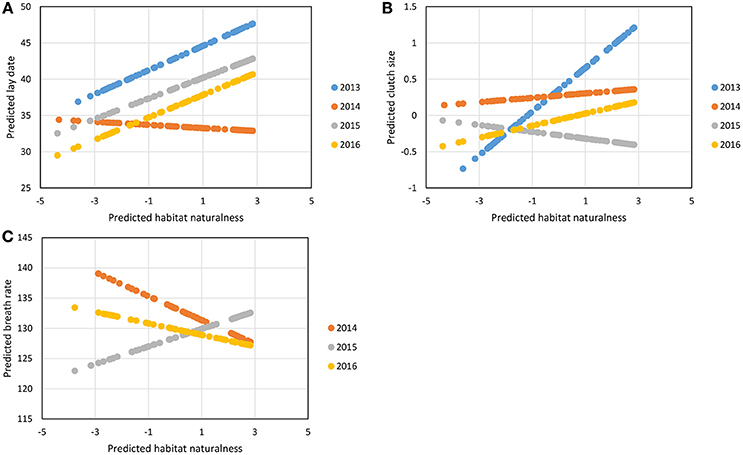
Figure 3. Predicted variation in great tit lay date (A), clutch size (B), and breath rate (C) along the naturalness gradient in the city of Montpellier, for each year of data sampling, from the best linear mixed models, presented in Table 4. For lay date, 1 = 1st March. Breath rate is in number of breaths/min.
Based on 6 years of great tit monitoring in an oak forest and the nearby city of Montpellier in southern France, our study reveals strong phenotypic divergence for a suite of breeding, behavioral and physiological traits, both when comparing forest and city birds, but also for some of the traits, within the urban gradient. We discuss below how these results fit with the pace-of-life syndrome hypothesis, and what processes could be involved in these divergences.
We confirm here a commonly found breeding divergence, which is that city birds breed earlier and produce smaller clutches, and even more so in more urbanized areas of the city (Tables 3, 4, Figures 2A,B and 3A,B). Such urban effects on the timing of breeding and clutch size have been demonstrated before in great tits (e.g., Dhondt et al., 1984; Wawrzyniak et al., 2015) and many other avian species (see review in Marzluff, 2017). Note however that in a recent comparative analysis of forest and urban passerines at a macro-geographic scale (including 138 great tit populations), Vaugoyeau et al. (2016) found that average laying date was not correlated with the degree of urbanization, while average clutch size decreased with urbanization in collared (Ficedula albicollis) and pied flycatchers (Ficedula hypoleuca) but not in great tits. Later laying and smaller clutches in cities have been attributed to the urban heat island effect, as well as the artificial lights and the lack of adequate resources (i.e., arthropods) during the breeding season (Chamberlain et al., 2009; Kempenaers et al., 2010).
Hatching success was lower in urban broods, yet this did not lead to lower fledging success (Table 3, Figure 2D) nor to lower fledgling numbers (Table S2). Within the city, fledging success varied significantly across years but again was not significantly lower in more urbanized areas. It is possible that the slightly lower fledgling productivity in urban broods (Table 2, Table S2) was not significant in our 6 years study because of insufficient power. However, it remains that while the other breeding traits show strong significant differences between forest and city, it did not translate in a similarly strong divergence for fledgling success. This result contrasts with previous findings in great tits (Bailly et al., 2016) and with the common assessment that the urban environment induces strong constraints on avian reproduction (Gil and Brumm, 2014). It raises the very interesting question of whether urban birds might display adaptive breeding strategies that differ from birds in the forest, yet do not lead to lower reproductive success (see further discussion on this possibility, below). Reduced clutch size would hence be the result of an adaptive process rather than the expression of energetic constraints. Overall in terms of POLS, the breeding data suggested that city birds displayed one element pointing to slower life histories (smaller clutches) yet did not differ consistently from forest birds in their reproductive output.
Our results on personality traits and breath rate were more consistent with the prediction of faster behavioral syndromes in urban compared to forest habitats. Handling aggression was not significantly influenced by the urban habitat, yet city birds were faster explorers with generally faster breath rate under constraint (Table 3). Breath rate also varied across the urban gradient, yet with important annual variation (Table 4, Figure 3C). Previous rural vs. urban avian comparisons for these traits found: lower short-term stress response in urban European blackbirds (Partecke et al., 2006), reduced physiological stress and faster exploratory behavior in urban dark-eyed juncos Junco hyemalis (Atwell et al., 2012; Abolins-Abols et al., 2016), higher territorial aggression in urban song sparrows Melospiza melodia (Evans et al., 2010), urban Australian magpies Gymnorhina tibicen (Cilento and Jones, 1999), urban Eurasian coot Fulica atra (Minias, 2015), and urban noisy miners Manorina melanocephala (Lowry et al., 2011), and finally higher breath rates in urban great tits during handling (Torne-Noguera et al., 2014, but see Senar et al. from this issue).
Mean breath rate during handling for Montpellier great tits was similar to that found in the study by Torne-Noguera et al. (2014, i.e., around 130 breaths/min) yet it was only around 2% higher than breath rate in forest birds, compared to a 14% difference in Barcelona (Torne-Noguera et al., 2014). Our singular analysis along the urban gradient revealed strong annual variation in the breath rate response to urbanization level (Figure 3C), which could partly explain the discrepancies between previous studies (Partecke et al., 2006; Torne-Noguera et al., 2014; Senar et al. this special issue). While heart rate and breath rate are highly related (see e.g., Dubuc-Messier et al., 2016), their interpretation is complicated by the fact that they reflect both sympathetic and parasympathetic reactivity (e.g., Koolhaas et al., 1999), and that responses to long term or acute stresses differ (Abolins-Abols et al., 2016). In rodents more aggressive and active individuals have a strong sympathetic response yet a low parasympathetic reactivity to acute stress, which translates into increased heart rate response (Koolhaas et al., 1997). Similarly, domestic hens with active coping styles (high-feather peckers) have faster heart rates during manual restraint (Korte et al., 1999). Hence overall, in the context of POLS, higher breath rate and faster exploration scores in urban great tits are suggestive of a faster pace-of-life.
While we have demonstrated in this study that forest and city great tits display strong phenotypic differences for breeding parameters, behavioral traits and physiological reaction to stress, two fundamental questions remain unanswered. The first question is whether these physiological differences emanate from underlying genetic differences, rather than plasticity. This would suggest that urban birds form a genetically distinct ecotype compared to forest birds, possibly because of a process of local adaptation. The second, related question, is whether the phenotypic differences we have described here result from an adaptive process since both plasticity and genetic processes (such as drift) can result in maladaptive responses.
As regards the first issue of a plastic vs. genetic origin in the phenotypic differences, a genetic difference can occur only if the focal traits are heritable, and in the case of restricted gene flow (Lenormand, 2002). An interesting point to make here is that in our study populations, hatching and fledging success were not repeatable, and there is very little evidence for heritability of reproductive success in the literature (review in Postma, 2014). All other focal traits studied here were repeatable and they were previously shown heritable, in great tits or in closely related avian species (for lay date and clutch size see review in Postma, 2014, for aggression and exploration behavior see review in Dingemanse and Dochtermann, 2014, for breath rate see Class et al., 2014). This overview on heritability suggests that a rapid evolution of the focal traits is possible except for hatching and fledging success. Recent results in population genomics also provide useful knowledge on the neutral and adaptive genomic divergence of Montpellier great tits at both scales studied here (forest vs. city and urban gradient). Using a RAD sequencing analysis of nearly 50,000 SNPs in 140 great tits, Perrier and colleagues (Perrier et al. in review) show that the neutral genetic structure is weakly impacted by urbanization, with an average Fst between La Rouvière forest and Montpellier city of 0.009 and an Fst of 0.012 between the most and least urbanized sites within the city of Montpellier. While all Fst were significant, their low value suggests that gene flow is common along the urban gradient and between forest and city. However, it could well be that non-random gene flow, e.g., via individuals matching their habitat choice to their phenotype (Edelaar et al., 2008; Edelaar and Bolnick, 2012) reinforces local adaptation. In fact it has been suggested that urbanization should favor individuals with high behavioral plasticity (Lowry et al., 2013). Our preliminary results on habitat-specific repeatability (Table S3) show lower repeatability in the behavior and physiology of urban compared to forest birds which could be indicative of higher plasticity, although this is not true for breeding traits. An interesting avenue would hence be to focus on plasticity as a trait per se, evaluating how/whether it is heritable and selected for in the urban landscape and across the range of traits studied here (e.g., following the approach of Nussey et al., 2005 for plasticity of timing of breeding). We also envisage in the future a common garden study to experimentally test whether birds from more or less urbanized areas yet raised in aviaries display genetically based differences in the traits studied here.
Addressing the second question to find out whether the phenotypic variation described here is adaptive or not will require more long term data (including survival and reproductive data) to compare the direction and force of selection acting on the focal traits across the habitats (Isaksson, 2015). It is commonly assumed that the novelty of the urban environmental features induces new and strong selection pressures providing the potential for rapid trait change (Markó et al., 2013; Alberti, 2015; Hendry et al., 2017). However, direct empirical measures of urban natural selection remain scarce (but see e.g., the emblematic peppered moth case, Cook and Saccheri, 2013). New genomic resources offer a powerful way to bypass the data consuming classical selection analyses by testing for genome-wide signatures of selection associated with urbanization, even in non-model species (Harris et al., 2015). While at first hand, breeding parameters show decreased clutch size and hatching success in city great tits compared to their forest conspecifics (Table 3), a very important result from our study is that these city birds do not have lower fledgling rate or success (Table 3 and Table S2). This stability of reproductive performance in the nest despite strong differences in breeding traits, suggests that city birds might have evolved an adapted breeding strategy in response to urbanization with smaller clutches and broods to adjust to the lack of food for nestlings in the urban habitat (Chamberlain et al., 2009). A comparison of morphological traits in our focal populations also points to possibly adaptive changes since urban great tits are smaller and lighter than forest birds yet they do not differ in body condition (Demeyrier et al. in prep.).
Previous studies have shown enhanced annual survival in urban adult birds, in particular because of higher resource availability over winter (Rebolo-Ifran et al., 2015; Marzluff et al., 2016). In the context of the pace-of-life theory, such decreased probability of mortality would be indicative of a slower pace-of-life (Réale et al., 2010). However, new predation pressures in the city are also thought to affect avian survival, especially nestlings', and overall rural/urban survival comparisons are still scarce and show no consistent pattern (Chamberlain et al., 2009). With only 3 years of breeding captures, our study lacked power to conduct capture-mark-recapture analyses, yet it is most definitely our objective to pursue the long term research and compare survival rates across the urbanization gradient in the future. In the same vein, a longer monitoring will enable a comparison of recruitment success across urbanization, providing a complementary perspective to our results on fledgling success. Survival data will also be crucial to understand selection pressures acting on urban great tits, and to compare them with selection in more natural habitats.
While the present analysis focused on first broods following classical approaches in the literature, considering second broods in the study of forest and urban reproductive strategies could reveal further divergences. Between 2011 and 2016, 11.6% of Montpellier urban females laid second broods vs. 4.5% in La Rouvière forest (along with 20.6 and 23.1% of replacement broods). In our study sites in the city of Montpellier, Pinus spp. and the evergreen oak Quercus ilex are dominant tree species. In these breeding habitats, later breeding could be favored because the food required to rear nestlings peaks later in the season (Blondel et al., 2006). Also, in such habitats, second clutches can be typically larger than first broods, while the reverse is true in deciduous habitats such as found in La Rouvière forest (Lambrechts et al., 2008). Hence a complementary analysis involving both first and second broods might confirm that city birds have adopted a different yet adaptive breeding strategy.
Finally, the colonization of an urban landscape by an avian species is likely to be a long process, possibly leading to a succession of adaptive phenotypes. In line with the general finding that individuals that are superior colonizers are inferior competitors (Yu and Wilson, 2001), it has been suggested that while a proactive fast lifestyle would be favored during the colonization stage of urban habitats (Sol et al., 2013), the higher sensitivity of reactive slow individuals to the environmental conditions might favor them on the long run when facing ecological novelty (Robertson et al., 2013). In the case of such a sequence of colonization, findings regarding the pace-of-life of birds adapted to the urban habitat will depend a. on how advanced the colonization process is, and b. on how much the urban population is isolated from surrounding rural populations. Although Montpellier is a millenary city where great tits have a long history of presence, it is still undergoing a rapid urbanization process, which could explain the high prevalence of bold birds in the most urbanized areas. Also, recent genetic analyses based on genome-wide RAD-sequences have revealed that Montpellier and La Rouvière great tits show significant but low genetic differentiation (Perrier et al. in review). Uncovering the direction of gene flow between city and forest, and testing for a source-sink dynamic would reveal whether the city population could be regularly supplied with new, possibly bold, individuals.
Overall, our study shows strong phenotypic divergence between forest and urban great tits, but also across the urbanization gradient. Behavioral and physiological traits suggested that urban birds were on a faster end of the pace-of-life continuum than their forest conspecifics, yet breeding data did not support this conclusion. Further investigations on the plastic vs. genetic origin of the differences revealed here, along with the selection forces on the focal trait, will improve our understanding of how urban life shapes great tit pace-of-life in the future.
Capture and ringing was performed under individual ringing permits delivered by the CRBPO and all the protocols involved, including the behavioral assays described below, were approved by the Animal Care and Use Committee Languedoc Roussillon (CEEA-LR-12066) as well as by Regional Institutions (bylaw issued by the Prefecture of Montpellier number 2012167-0003).
Conceived study: AC, VD, ML, AG. Collected data: AC, VD, ML, SP, AG. Analyzed videos: VD. Statistical analyses: AC. Wrote the manuscript: AC. Improved the manuscript: VD, ML, AG. Contributed funding and materials: AC, AG.
This research was supported by a recurrent annual funding from the OSU-OREME and by the European Research Council (Starting grant ERC-2013-StG-337365-SHE to AC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past collaboration with one of the authors AG and states that the process nevertheless met the standards of a fair and objective review.
We thank the many participants to the field monitoring, the City of Montpellier (Direction Paysage et Biodiversité) and the Zoo de Lunaret for logistic help. We thank Aude Caizergues, Arnaud Béchet, Gabrielle Dubuc Messier, and Denis Réale for useful discussions.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2017.00053/full#supplementary-material
Abolins-Abols, M., Hope, S. F., and Ketterson, E. D. (2016). Effect of acute stressor on reproductive behavior differs between urban and rural birds. Ecol. Evol. 6, 6546–6555. doi: 10.1002/ece3.2347
Alberti, M. (2015). Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. doi: 10.1016/j.tree.2014.11.007
Alberti, M., Marzluff, J., and Hunt, V. M. (2017). Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160029. doi: 10.1098/rstb.2016.0029
Aronson, M. F. J., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 281, 20133330. doi: 10.1098/rspb.2013.3330
Atwell, J. W., Cardoso, G. C., Whittaker, D. J., Campbell-Nelson, S., Robertson, K. W., and Ketterson, E. D. (2012). Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav. Ecol. 23, 960–969. doi: 10.1093/beheco/ars059
Bailly, J., Scheifler, R., Berthe, S., Clement-Demange, V. A., Leblond, M., Pasteur, B., et al. (2016). From eggs to fledging: negative impact of urban habitat on reproduction in two tit species. J. Ornithol. 157, 377–392. doi: 10.1007/s10336-015-1293-3
Bates, D., Machler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Blondel, J., Thomas, D. W., Charmantier, A., Perret, P., Bourgault, P., and Lambrechts, M. M. (2006). A thirty-year study of phenotypic and genetic variation of blue tits in Mediterranean habitat mosaics. Bioscience 56, 661–673. doi: 10.1641/0006-3568(2006)56[661:ATSOPA]2.0.CO;2
Blumstein, D. T., Evans, C. S., and Daniel, J. C. (2006). JWATCHER. Available online at: http://www.jwatcher.ucla.edu/
Brommer, J. E., and Kluen, E. (2012). Exploring the genetics of nestling personality traits in a wild passerine bird: testing the phenotypic gambit. Ecol. Evol. 2, 3032–3044. doi: 10.1002/ece3.412
Carere, C., and van Oers, K. (2004). Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol. Behav. 82, 905–912. doi: 10.1016/j.physbeh.2004.07.009
Chamberlain, D. E., Cannon, A. R., Toms, M. P., Leech, D. I., Hatchwell, B. J., and Gaston, K. J. (2009). Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151, 1–18. doi: 10.1111/j.1474-919X.2008.00899.x
Cilento, N. J., and Jones, D. N. (1999). Aggression by Australian magpies Gymnorhina tibicen toward human intruders. Emu 99, 85–90. doi: 10.1071/mu99011
Class, B., Kluen, E., and Brommer, J. E. (2014). Evolutionary quantitative genetics of behavioral responses to handling in a wild passerine. Ecol. Evol. 4, 427–440. doi: 10.1002/ece3.945
Clucas, B., and Marzluff, J. M. (2015). A cross-continental look at the patterns of avian species diversity and composition across an urbanisation gradient. Wildl. Res. 42, 554–562. doi: 10.1071/wr15007
Cook, L. M., and Saccheri, I. J. (2013). The peppered moth and industrial melanism: evolution of a natural selection case study. Heredity 110, 207–212. doi: 10.1038/hdy.2012.92
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 4065–4076. doi: 10.1098/rstb.2010.0176
Davies, S., and Sewall, K. B. (2016). Agonistic urban birds: elevated territorial aggression of urban song sparrows is individually consistent within a breeding period. Biol. Lett. 12:20160315. doi: 10.1098/rsbl.2016.0315
Demeyrier, V., Lambrechts, M. M., Perret, P., and Gregoire, A. (2016). Experimental demonstration of an ecological trap for a wild bird in a human-transformed environment. Anim. Behav. 118, 181–190. doi: 10.1016/j.anbehav.2016.06.007
Dhondt, A. A., Eyckerman, R., Moermans, R., and Huble, J. (1984). Habitat and laying date of great and blue tit Parus major and Parus caeruleus. Ibis 126, 388–397. doi: 10.1111/j.1474-919X.1984.tb00260.x
Dingemanse, N. J., Both, C., Drent, P. J., Van Oers, K., and Van Noordwijk, A. J. (2002). Repeatability and heritability of exploratory behaviour in great tits from the wild. Anim. Behav. 64, 929–938. doi: 10.1006/anbe.2002.2006
Dingemanse, N. J., and Dochtermann, N. A. (2014). “Individual behaviour: behavioural ecology meets quantitative genetics,” in Quantitative Genetics in the Wild, eds A. Charmantier, D. Garant, and L. E. B. Kruuk (Oxford: Oxford University Press), 54–67.
Dingemanse, N. J., and Wolf, M. (2010). Recent models for adaptive personality differences: a review. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3947–3958. doi: 10.1098/rstb.2010.0221
Doutrelant, C., Leitao, A., Otter, K., and Lambrechts, M. M. (2000). Effect of blue tit song syntax on great tit territorial responsiveness - an experimental test of the character shift hypothesis. Behav. Ecol. Sociobiol. 48, 119–124. doi: 10.1007/s002650000220
Dubuc-Messier, G., Réale, D., Perret, P., and Charmantier, A. (2016). Environmental heterogeneity and population differences in blue tits personality traits. Behav. Ecol. 28, 448–459. doi: 10.1093/beheco/arw148
Ducatez, S., Audet, J.-N., Rodriguez, J. R., Kayello, L., and Lefebvre, L. (2017). Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds. Anim. Cogn. 20, 33–42. doi: 10.1007/s10071-016-1007-0.
Edelaar, P., and Bolnick, D. I. (2012). Non-random gene flow: an underappreciated force in evolution and ecology. Trends Ecol. Evol. 27, 659–665. doi: 10.1016/j.tree.2012.07.009
Edelaar, P., Siepielski, A. M., and Clobert, J. (2008). Matching habitat choice causes directed gene flow: a neglected dimension in evolution and ecology. Evolution 62, 2462–2472. doi: 10.1111/j.1558-5646.2008.00459.x
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595. doi: 10.1111/j.1439-0310.2010.01771.x
Fucikova, E., Drent, P. J., Smits, N., and van Oers, K. (2009). Handling stress as a measurement of personality in great tit nestlings (Parus major). Ethology 115, 366–374. doi: 10.1111/j.1439-0310.2009.01618.x
Harris, S. E., O'Neill, R. J., and Munshi-South, J. (2015). Transcriptome resources for the white-footed mouse (Peromyscus leucopus): new genomic tools for investigating ecologically divergent urban and rural populations. Mol. Ecol. Resour. 15, 382–394. doi: 10.1111/1755-0998.12301
Hendry, A. P., Farrugia, T. J., and Kinnison, M. T. (2008). Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. doi: 10.1111/j.1365-294X.2007.03428.x
Hendry, A. P., Gotanda, K. M., and Svensson, E. I. (2017). Human influences on evolution, and the ecological and societal consequences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160028. doi: 10.1098/rstb.2016.0028
Hille, S. M., and Cooper, C. B. (2015). Elevational trends in life histories: revising the pace-of-life framework. Biol. Rev. 90, 204–213. doi: 10.1111/brv.12106
Isaksson, C. (2015). Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct. Ecol. 29, 913–923. doi: 10.1111/1365-2435.12477
Kempenaers, B., Borgstrom, P., Loes, P., Schlicht, E., and Valcu, M. (2010). Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. doi: 10.1016/j.cub.2010.08.028
Kluen, E., Siitari, H., and Brommer, J. E. (2014). Testing for between individual correlations of personality and physiological traits in a wild bird. Behav. Ecol. Sociobiol. 68, 205–213. doi: 10.1007/s00265-013-1635-1
Koolhaas, J. M., De Boer, S. F., De Ruiter, A. J. H., Meerlo, P., and Sgoifo, A. (1997). Social stress in rats and mice. Acta Physiol. Scand. 161, 69–72.
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., et al. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. doi: 10.1016/s0149-7634(99)00026-3
Korte, S. M., Ruesink, W., and Blokhuis, H. J. (1999). Heart rate variability during manual restraint in chicks from high- and low-feather pecking lines of laying hens. Physiol. Behav. 65, 649–652.
Lambrechts, M., Rieux, A., Galan, M., Cartan-Son, M., Perret, P., and Blondel, J. (2008). Double-brooded great tits (Parus major) in Mediterranean oak habitats: do first broods always perform better than second broods? Russ. J. Ecol. 39, 516–522. doi: 10.1134/s1067413608070084
Lenormand, T. (2002). Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189. doi: 10.1016/S0169-5347(02)02497-7
Lin, T., Coppack, T., Lin, Q.-x., Kulemeyer, C., Schmidt, A., Behm, H., et al. (2012). Does avian flight initiation distance indicate tolerance towards urban disturbance? Ecol. Indic. 15, 30–35. doi: 10.1016/j.ecolind.2011.09.018
Lindenmayer, D. B., and Fischer, J. (2006). Habitat Fragmentation and Landscape Change. An Ecological and Conservation Synthesis. Washington, DC: Island Press.
Lowry, H., Lill, A., and Wong, B. B. M. (2011). Tolerance of auditory disturbance by an avian urban adapter, the noisy miner. Ethology 117, 490–497. doi: 10.1111/j.1439-0310.2011.01902.x
Lowry, H., Lill, A., and Wong, B. B. M. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Markó, G., Azcárate, M., Hegyi, G., Herceg, G., Laczi, M., Nagy, G., et al. (2013). Behavioural responses to handling stress in the Great Tit: within-individual consistency and the effect of age, sex and body condition. Ornis Hung. 21, 12–25. doi: 10.2478/orhu-2013-0012
Martin, L. B., Hasselquist, D., and Wikelski, M. (2006). Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575. doi: 10.1007/s00442-005-0314-y
Marzluff, J. M. (2017). A decadal review of urban ornithology and a prospectus for the future. Ibis 159, 1–13. doi: 10.1111/ibi.12430
Marzluff, J. M., Clucas, B., Oleyar, M. D., and DeLap, J. (2016). The causal response of avian communities to suburban development: a quasi-experimental, longitudinal study. Urban Ecosystems 19, 1597–1621. doi: 10.1007/s11252-015-0483-3
McDonnell, M. J., and Hahs, A. K. (2015). “Adaptation and adaptedness of organisms to urban environments,” in Annual Review of Ecology, Evolution, and Systematics, Vol. 46, ed D. J. Futuyma (Palo Alto, CA: Annual Reviews), 261–280.
McKinney, M. L. (2002). Urbanization, biodiversity, and conservation. Bioscience 52, 883–890. doi: 10.1641/0006-3568(2002)052[0883:ubac]2.0.co;2
Minias, P. (2015). Successful colonization of a novel urban environment is associated with an urban behavioural syndrome in a reed-nesting waterbird. Ethology 121, 1178–1190. doi: 10.1111/eth.12433
Møller, A. P., Tryjanowski, P., Diaz, M., Kwiecinski, Z., Indykiewicz, P., Mitrus, C., et al. (2015). Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behav. Ecol. 26, 861–865. doi: 10.1093/beheco/arv024
Mutzel, A., Dingemanse, N. J., Araya-Ajoy, Y. G., and Kempenaers, B. (2013). Parental provisioning behaviour plays a key role in linking personality with reproductive success. Philos. Trans. R. Soc. Lond. B Biol. Sci. 280:20131019. doi: 10.1098/rspb.2013.1019
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. Camb. Philos. Soc. 85, 935–956. doi: 10.1111/j.1469-185X.2010.00141.x
Nicolaus, M., Piault, R., Ubels, R., Tinbergen, J. M., and Dingemanse, N. J. (2016). The correlation between coloration and exploration behaviour varies across hierarchical levels in a wild passerine bird. J. Evol. Biol. 29, 1780–1792. doi: 10.1111/jeb.12907
Nussey, D. H., Postma, E., Gienapp, P., and Visser, M. E. (2005). Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. doi: 10.1126/science.1117004
Partecke, J., Schwabl, I., and Gwinner, E. (2006). Stress and the city: urbanization and its effects on the stress physiology in European Blackbirds. Ecology 87, 1945–1952. doi: 10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2
Peach, W. J., Vincent, K. E., Fowler, J. A., and Grice, P. V. (2008). Reproductive success of house sparrows along an urban gradient. Anim. Conserv. 11, 493–503. doi: 10.1111/j.1469-1795.2008.00209.x
Postma, E. (2014). “Four decades of estimating heritabilities in wild vertebrate populations: improved methods, more data, better estimates?,” in Quantitative Genetics in the Wild, eds A. Charmantier, D. Garant, and L. E. B. Kruuk (Oxford: Oxford University Press), 16–33.
Réale, D., Garant, D., Humphries, M. M., Bergeron, P., Careau, V., and Montiglio, P. O. (2010). Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 4051–4063. doi: 10.1098/rstb.2010.0208
Rebolo-Ifran, N., Carrete, M., Sanz-Aguilar, A., Rodriguez-Martinez, S., Cabezas, S., Marchant, T. A., et al. (2015). Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci. Rep. 5:13723. doi: 10.1038/srep13723
Ricklefs, R. E., and Wikelski, M. (2002). The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468. doi: 10.1016/s0169-5347(02)02578-8
Robertson, B. A., Rehage, J. S., and Sih, A. (2013). Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560. doi: 10.1016/j.tree.2013.04.004
Roff, D. A. (1992). The Evolution of Life Histories: Theory and Analysis. New York, NY: Chapman and Hall.
Sih, A., Ferrari, M. C. O., and Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. doi: 10.1111/j.1752-4571.2010.00166.x
Silva, C. P., Sepulveda, R. D., and Barbosa, O. (2016). Nonrandom filtering effect on birds: species and guilds response to urbanization. Ecol. Evol. 6, 3711–3720. doi: 10.1002/ece3.2144
Sol, D., Duncan, R. P., Blackburn, T. M., Cassey, P., and Lefebvre, L. (2005). Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. U.S.A. 102, 5460–5465. doi: 10.1073/pnas.0408145102
Sol, D., Gonzalez-Lagos, C., Moreira, D., Maspons, J., and Lapiedra, O. (2014). Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 17, 942–950. doi: 10.1111/ele.12297
Sol, D., Lapiedra, O., and Gonzalez-Lagos, C. (2013). Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. doi: 10.1016/j.anbehav.2013.01.023
Stuber, E. F., Araya-Ajoy, Y. G., Mathot, K. J., Mutzel, A., Nicolaus, M., Wijmenga, J. J., et al. (2013). Slow explorers take less risk: a problem of sampling bias in ecological studies. Behav. Ecol. 24, 1092–1098. doi: 10.1093/beheco/art035
Torne-Noguera, A., Pagani-Nunez, E., and Senar, J. C. (2014). Great Tit (Parus major) breath rate in response to handling stress: urban and forest birds differ. J. Ornithol. 155, 315–318. doi: 10.1007/s10336-013-1025-5
Tryjanowski, P., Moller, A. P., Morelli, F., Biadun, W., Brauze, T., Ciach, M., et al. (2016). Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 6:28575. doi: 10.1038/srep28575
Vaugoyeau, M., Adriaensen, F., Artemyev, A., Banbura, J., Barba, E., Biard, C., et al. (2016). Interspecific variation in the relationship between clutch size, laying date and intensity of urbanization in four species of hole-nesting birds. Ecol. Evol. 6, 5907–5920. doi: 10.1002/ece3.2335
Wawrzyniak, J., Kalinski, A., Gladalski, M., Banbura, M., Markowski, M., Skwarska, J., et al. (2015). Long-term variation in laying date and clutch size of the great tit Parus major in central Poland: a comparison between urban parkland and deciduous forest. Ardeola 62, 311–322. doi: 10.13157/arla.62.2.2015.311
Williams, N. S. G., Schwartz, M. W., Vesk, P. A., McCarthy, M. A., Hahs, A. K., Clemants, S. E., et al. (2009). A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 97, 4–9. doi: 10.1111/j.1365-2745.2008.01460.x
Wolf, M., van Doorn, G. S., Leimar, O., and Weissing, F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. doi: 10.1038/nature05835
Keywords: urban pace-of-life, great tit, personality traits, breath rate, breeding performance, urbanization gradient
Citation: Charmantier A, Demeyrier V, Lambrechts M, Perret S and Grégoire A (2017) Urbanization Is Associated with Divergence in Pace-of-Life in Great Tits. Front. Ecol. Evol. 5:53. doi: 10.3389/fevo.2017.00053
Received: 01 March 2017; Accepted: 10 May 2017;
Published: 29 May 2017.
Edited by:
Caroline Isaksson, Lund University, SwedenReviewed by:
Hanne Lovlie, Linköping University, SwedenCopyright © 2017 Charmantier, Demeyrier, Lambrechts, Perret and Grégoire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne Charmantier, YW5uZS5jaGFybWFudGllckBjZWZlLmNucnMuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.