
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 19 May 2017
Sec. Chemical Ecology
Volume 5 - 2017 | https://doi.org/10.3389/fevo.2017.00049
This article is part of the Research Topic IC-E3 - Insect Chemical Ecology, Ethology and Evolution View all 15 articles
Plants produce chemical defense compounds to resist herbivore attack either by repelling the herbivores or attracting natural enemies of the herbivores. We have previously shown that volatile compounds from cotton released in response to herbivory by conspecifics reduce oviposition in cotton leafworm moth Spodoptera littoralis. It remained, however, unclear whether herbivore-induced changes also affect moth pre-mating and mating behaviors. In this study we examined the effect of herbivore-induced changes in cotton on reproductive behaviors i.e., female calling, male attraction and investment, and mating behavior in S. littoralis. We found a reduction in the number of females calling i.e., females releasing pheromone, in the presence of cotton plants damaged by larvae of S. littoralis compared to undamaged plants. Females also spent significantly less time calling and showed a delay in calling in the presence of damaged plants. Furthermore, males exhibited significantly delayed activation and reduced attraction toward female sex pheromone in the presence of damaged plants. We also found that mating success and the number of matings were significantly reduced in the presence of damaged plants whereas male investment i.e., spermatophore weight, was not affected. Thus, our study provides evidence that herbivory by conspecifics on host plants affect pre-mating and mating behaviors in an insect herbivore.
In phytophagous insects, plant cues are crucial for finding a suitable plant for feeding and oviposition (Bruce, 2015). In addition, cues from plants have also been found to influence sexual communication and can be used to distinguish suitable from unsuitable host plants and can affect mate-finding and mating behavior of insect herbivores (McNeil and Delisle, 1989; Deisig et al., 2012; Félix et al., 2013). Female moths exhibit calling behavior to attract males for mating by releasing sex pheromone compounds (Shorey, 1973; Wertheim et al., 2004). It has been shown that external stimuli may have a large effect on sex pheromone production and calling behavior in female moths (Landolt and Phillips, 1997; Bendera et al., 2015). For instance, synthesis, and production of sex pheromones in females is initiated and enhanced in the presence of host plants suitable for feeding, mating, and oviposition (Raina et al., 1992; Groot and Visser, 2001) whereas non-host plants may produce inverse effects (Zhang and Schlyter, 2004; Sadek and Anderson, 2007). This shows that plant quality as food for the progeny can be important for female calling and thus, attraction of males. Compounds from host plants have also been shown to enhance the attraction of males toward female sex pheromones (Landolt and Phillips, 1997; Schmera and Guerin, 2012).
Host plants undergoing developmental changes, as well as plants under stress, can produce secondary compounds that indicate reduced plant quality (Bukovinszky et al., 2012; Scala et al., 2013; Schuman and Baldwin, 2016). In many plants, damage by herbivores induces a change in both the emission of volatile compounds (Pierik et al., 2014) and the occurrence of non-volatile compounds in the plant tissue (Howe and Schaller, 2008). These compounds can influence the behavior of insect herbivores during site selection for feeding, mating and oviposition (Karban and Thaler, 1999; Dicke and Baldwin, 2010; Reisenman et al., 2010; Knolhoff and Heckel, 2014). It is well established that herbivore damage induces changes in host plant quality by triggering the synthesis of secondary chemical compounds (Agrawal, 2001; Howe and Jender, 2008). Such herbivore-induced chemical compounds are known to increase the resistance of damaged host plants either by repelling the herbivores during host plant choice for feeding and oviposition or by attracting predators and parasitoids of the herbivores (Arimura et al., 2009; Barbosa et al., 2009; Hare et al., 2011; Gols et al., 2012; Allmann et al., 2013; Zakir et al., 2013b; Heil, 2014). Whereas, in some species these compounds have been shown to increase susceptibility of the damaged host plants i.e., lead to increased attraction of conspecifics (Kalberer et al., 2001; Magalhães et al., 2012).
The effect of herbivore-induced chemical changes on host plant quality has been studied in insect herbivores, particularly in Lepidoptera, either through oviposition behavior or through the performance of the offspring (Karban and Baldwin, 1997; Agrawal, 2001; Howe and Jender, 2008). For instance, mated female moths of Heliothis virescens and Manduca sexta avoided ovipositing on herbivore-damaged tobacco plants under laboratory and field conditions (De Moraes et al., 2001; Kessler and Baldwin, 2001). Thus, the effect of herbivore-induced chemical changes on host plant quality has been well studied from a post-mating perspective i.e., oviposition decisions in adult females or through the performance of the offspring (De Moraes et al., 2001; Kessler and Baldwin, 2001; Agrawal, 2005; McCormick et al., 2017). However, less effort has been dedicated to investigate the effect of herbivore-induced changes in host plant quality on pre-mating behaviors.
The Egyptian cotton leafworm Spodoptera littoralis is a generalist herbivore (Brown and Dewhurst, 1975). In North Africa and the Middle East it is a pest species on cotton plants, which produce a suite of volatile and non-volatile chemical compounds in response to herbivore damage (Paré and Tumlinson, 1997; Bezemer et al., 2003; Schmidt et al., 2009). Behavioral studies have shown that adult female S. littoralis moths avoid ovipositing on cotton plants that have been damaged by conspecific larvae (Anderson and Alborn, 1999) and that herbivore-induced chemical compounds reduced the development of 3rd instar S. littoralis larvae (Alborn et al., 1996). Herbivore-damaged cotton plants also produce volatiles (McCall et al., 1994; Röse and Tumlinson, 2005) that can reduce further herbivore attack on damaged plants (Paré and Tumlinson, 1997) and has been shown to provide resistance to neighboring undamaged plants against oviposition by S. littoralis moths (Zakir et al., 2013b). Thus, it was demonstrated that mated females responded to herbivore-induced plant volatiles during oviposition, but the effect of these volatiles on pre-oviposition behaviors i.e., mate-finding, mating, and calling behaviors, has not been investigated.
The aim of this study is to investigate the effects of herbivore induction in cotton plants on pre-mating behavior in females, males, and their interaction during mating. We hypothesize that both male and female S. littoralis are able to detect the chemical changes after herbivore damage and adjust their behaviors accordingly. We aim to study whether herbivore-induced changes in cotton plants affect: (1) the calling behavior in virgin S. littoralis females (2) the mate-finding, mating behavior and male investment during mating, i.e., spermatophore transfer, in male moths, and (3) mating frequency and time of mating.
The S. littoralis moths were obtained from a culture established in 2007 from moths collected in the Alexandria region in Egypt. Insect material collected in the field has been added yearly. The insects were reared on a potato based semi-synthetic diet (Hinks and Byers, 1976) under 25 ± 2°C, 65 ± 5% RH and a L16:D8 photoperiod in the laboratory. Male and female insects were sexed in the pupal stage and kept in separate boxes. Adult moths were kept individually in small (200 ml) boxes and were provided with honey solution (10%) until used in the experiments.
Cotton seeds (G. hirsutum L., var. Delta pineland 90) were soaked overnight in water and planted individually in pots (Ø = 14 cm) filled with soil and grown in a greenhouse at 25 ± 2°C and 65 ± 5% RH. Artificial light (Philips, SON-T, 400 W), positioned 1–2 m above the plants was provided in addition to natural light. In all experiments, cotton plants with 5–6 true leaves were used.
Individual damaged or undamaged cotton plants were placed inside multiple Plexiglas cages (40 × 40 × 80 cm) maintained in a ventilated climate chamber at 25 ± 2°C, 65 ± 5% RH and L16:D8 photoperiod (Figure 1). The Plexiglas cage were ventilated through finer meshes at the top and from the side wall of the cage and the cages were arranged in the experiemental room to prevent interference between damaged and undamaged plants. Plants were damaged by releasing three 3rd-instar larvae of S. littoralis on the second true leaf of the plant and 48 h old ongoing damaged plants were used in all experiments to ensure the emission of volatiles in response to herbivore damage (Loughrin et al., 1994; McCall et al., 1994). To get ongoing damage, larvae were caged on the leaf using a mesh (0.2 × 0.2 mm) enclosing the leaf. The mesh prevented physical and visual contact between larvae and adult moths. Observations were made according to the procedure described by Sadek and Anderson (2007) and Zhao et al. (2009). A newly emerged female was transferred to the large Plexiglass cages with the experimental plants at the start of the scotophase and observations under red light were made daily during the complete scotophase at a 15 min interval. At the end of each scotophase, the moths were transferred to small plastic boxes and were given 10% sugar solution for feeding and were kept in a ventilated climate chamber until the next scotophase. The calling behavior of individual moths was recorded during four consecutive nights. In general, duration of time when an adult female moth release pheromone during the scotophase is termed as “calling behavior” and during this behavior; females do not walk, their wings are lifted from the abdomen and the ovipositor is exposed and easily visible. Repeats of calling behavior per night is termed as “calling pattern” and this has been used to measure the calling behavior of a moth during one scotophase and so on. In total, calling behaviors of 25 females from five different generations were observed, testing the moths with each of the damaged or undamaged cotton plants.
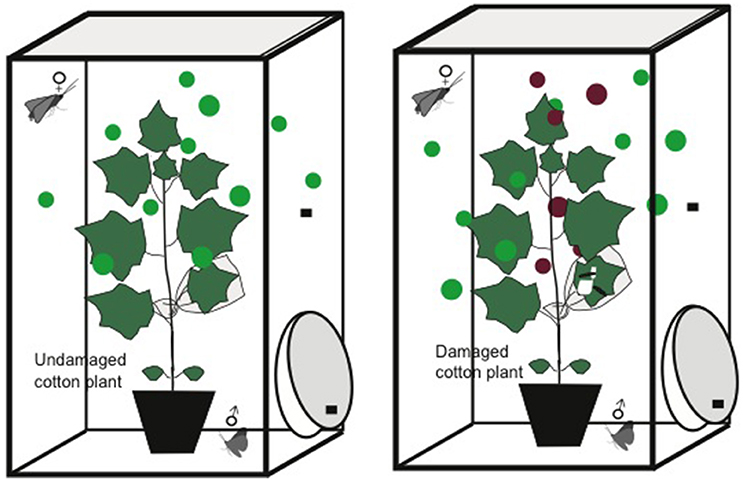
Figure 1. Mating set-up: A herbivore-damaged or undamaged cotton plant was placed inside a Plexiglas cage and moths were introduced. Each cage had openings at the top, with a moveable lid, and one of the side walls from which the plants and insects could be transferred.
The attraction behavior of male S. littoralis moths toward female sex pheromone was tested in two-choice wind tunnel (200 × 90 × 60 cm) experiments, in the presence of plants as background odors (Thöming et al., 2013). The air flow (25 cm/s) through the tunnel, was cleaned by passing it through activated charcoal filters (Camfil, Torsa, Sweden). The tunnel was illuminated from an overhead light at 5 lx and the experimental room was kept at 23 ± 2°C and 40–60% RH. A pair of cotton plants, one undamaged and one damaged plant (as described above in calling behavior), were positioned side by side ≈15 cm apart from each other. On the downwind side of each plant two identical filter paper discs (0.5 × 1 cm) containing female pheromone gland extract, one female gland equivalent, were positioned at 15 cm away from the plants and 30 cm above the floor. The gland extractions were made by following the protocol described by Anderson et al. (2007). For the experiments, 2-day old males (N = 32) were placed singly in glass tubes (12.5 × 2.5 cm) and transferred to the wind tunnel room before the onset of the scotophase. The moths were tested during the third to fifth hour of the scotophase. A single tube was deposited at the downwind end of the tunnel 30 cm from the floor and ≈180 cm from the pheromone source. The number of males attracted to the pheromone sources in front of either the damaged or the undamaged plant was recorded. A moth was given 5 min from activation, when the male starts to move after contact with the pheromone plume and walks toward the upwind end of the release tube, to reach the pheromone source. The filter paper with pheromone gland extract (one female equivalent) was exchanged every 12 min with a newly prepared one and the positions of the two plant treatments were reversed after five replications.
Undamaged and ongoing damaged cotton plants were placed singly inside each of the Plexiglas cages as described above in “calling behavior” and the cages were kept in an acclimatized and ventilated chamber at 25 ± 2°C, 65 ± 5% RH and L16:D8 photoperiod. Observations of mating were made during the scotophase for 2 h per replicate at an interval of 5 min. In each replication, 5 female and 3 male moths of respective ages were released in the cages for mating. More females were used to make sure that female calling frequency would not limit male mating behavior. From each of the four moth ages observed (1–4 days old), 40 females and 24 males were observed, and thus, in total 160 females and 96 males were successfully observed from all ages. Female moths were released on the upper leaves of the caged plants. Thereafter, individual male moths were released into cages with damaged or undamaged plants and were observed thoroughly until successful mating started and the mated males were discarded every time, as male moths has been reported to mate once per night (Sadek, 2001). Mating success, onset and duration of mating, and spermatophore weight were recorded. To retrieve spermatophores, soon after mating females were frozen at −20°C and were dissected carefully under a stereo microscope. Spermatophores were weighed on an analytical scale.
A 2 × 2 chi-square test was used to analyze the data obtained from Plexiglass cages on mating success, onset and duration of mating, male activation, mating place selection, and weight of spermatophores. Whereas, male attraction toward female pheromone blend being released in the presence of either damaged or undamaged cotton plants in wind tunnel was analyzed by using Wilcoxon signed rank test for paired differences. Normality and homoscedasticity of the residuals were checked graphically. All statistical analyses were done using GraphPad Prism v. 5.0a. The level of significance was selected as P < 0.05, 0.01, or 0.001. Microsoft Office Excel 2008 and Adobe Illustrator CS4 was used for calculations and graphical representation of the data.
Over a four night experimental period, a higher percentage of female moths (96%) showed calling behavior in the presence of undamaged cotton plants compared to females kept with damaged cotton plants (76%) (χ2 = 4.65, df = 1, P < 0.05). Calling patterns per night showed that fewer females called during the first (χ2 = 8.96, df = 1, P < 0.01), second (χ2 = 7.83, df = 1, P < 0.01), third (χ2 = 6.54, df = 1, P < 0.05) and fourth night (χ2 = 5.26, df = 1, P < 0.05) on damaged plants, compared to females on undamaged plants (Figure 2). During four nights of calling, females exhibited a delay in calling during the first (χ2 = 9.86, df = 1, P < 0.01), second (χ2 = 8.73, df = 1, P < 0.01) and third (χ2 = 5.64, df = 1, P < 0.05), and fourth night (χ2 = 4.28, df = 1, P < 0.05) in the presence of damaged cotton plants, whereas the females kept with undamaged cotton plants commenced calling much earlier in the scotophase and the overall delay in calling under damaged conditions was significant (χ2 = 29.46, df = 1, P < 0.001, Figure 3).
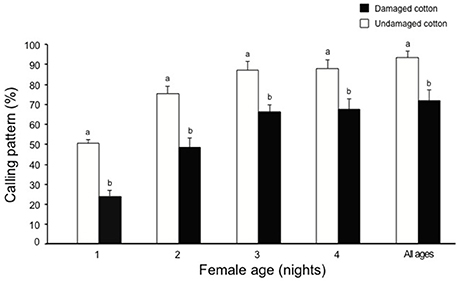
Figure 2. The proportion of S. littoralis females exhibiting calling pattern in the presence of either damaged cotton plants (black bars) or undamaged cotton plants (white bars) for four consecutive nights. Standard errors are represented on each bar. Chi square test was used for statistical analysis. Different letters within the bars of each age show significant effect and the level of significance was selected as P < 0.05, or 0.01 or 0.001.
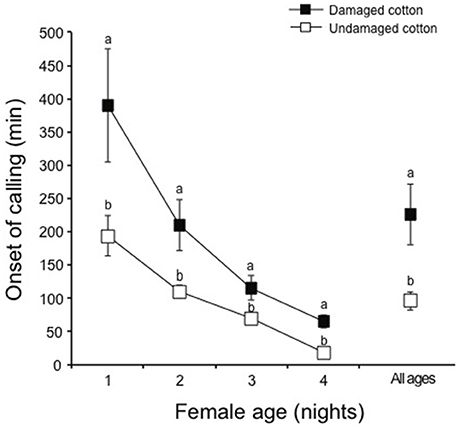
Figure 3. The proportion of S. littoralis females exhibiting onset time of calling in the presence of either damaged cotton plants (black boxes) or undamaged cotton plants (white boxes) for four consecutive nights. Standard errors are represented on each box. Chi square test was used for statistical analysis. Different letters within the bars of each age show significant effect and the level of significance was selected as P < 0.05, or 0.01 or 0.001.
In wind tunnel experiments, 28 males (87%) were, in this two-choice assay, attracted to the female sex pheromone having background odors from undamaged plants and 4 males (13%) to damaged plants (Wilcoxon signed rank test for paired differences; N = 32, P < 0.001, Figure 4).
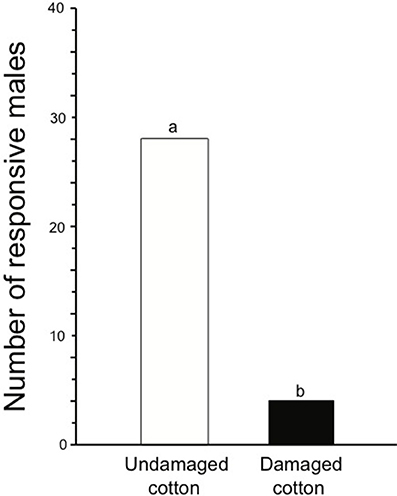
Figure 4. Number of males of S. littoralis attracted toward female pheromone gland extracts in the wind tunnel having damaged (black bar) or undamaged (white bar) cotton plants as background plant volatile source in a two-choice assay. Wilcoxon signed rank test for paired differences was used for statistical analysis. Different letters are used to show significant effect (P < 0.05, or 0.01 or 0.001).
During all scotophases, a higher percentage of male moths (80%) showed activation behavior in the presence of undamaged cotton plants compared to males kept with damaged cotton plants (56%) (χ2 = 4.85, df = 1, P < 0.05). A higher percentage of 1- and 2-day-old male moths showed activation in the presence of undamaged cotton plants, compared to damaged cotton plants (1-d-old males: χ2 = 3.92, df = 1, P < 0.05; 2-d-old males: χ2 = 4.90, df = 1, P < 0.05), whereas, no difference in activation was observed for 3- and 4-day old males (3-d-old males: χ2 = 0.48, df = 1, P > 0.05; 4-d-old males: χ2 = 0.54, df = 1, P > 0.05, Figure 5A). In the presence of undamaged cotton plants, overall mating success was significantly higher (64%) than in the presence of damaged cotton plants (40%) (χ2 = 4.95, df = 1, P < 0.05), and for 1- to 3-d-old males (1-d-old males: χ2 = 4.54, df = 1, P < 0.05; 2-d-old males: χ2 = 4.90, df = 1, P < 0.05; 3-d-old males: χ2 = 4.00, df = 1, P < 0.05). In 4-d-old males, the difference in mating on damaged and undamaged plants was not significant (χ2 = 0.98, df = 1, P > 0.05, Figure 5B). Insects mated significantly earlier in the presence of undamaged cotton plants compared to damaged plants (χ2 = 12.85, df = 1, P < 0.05, Figure 5C). This difference was significant for 1- to 3-d old moths (1-d-old females: χ2 = 8.18, df = 1, P < 0.01; 2-d-old females: χ2 = 12.86, df = 1, P < 0.001; 3-d-old females: χ2 = 4.26, df = 1, P < 0.05), but the difference was not significant with 4-d-old females and males. For spermatophore weight no overall difference between the two plant types (χ2 = 0.40, df = 1, P > 0.05) or at any of the ages tested (1-d-old males: χ2 = 0.63, df = 1, P > 0.05; 2-d-old males: χ2 = 0.65, df = 1, P > 0.05; 3-d-old males: χ2 = 0.48, df = 1, P > 0.05; 4-d-old males: χ2 = 0.56, df = 1, P > 0.05) was observed (Figure 6). Similarly, no differences in mating duration were observed on an average of (χ2 = 0.79, df = 1, P > 0.05) or at any of the specific ages tested (1-d-old males: χ2 = 0.53, df = 1, P > 0.05; 2-d-old males: χ2 = 0.75, df = 1, P > 0.05; 3-d-old males: χ2 = 0.58, df = 1, P > 0.05; 4-d-old males: χ2 = 0.67, df = 1, P > 0.05) among the pairs mated in the presence of either damaged or undamaged plants (Figure 7).
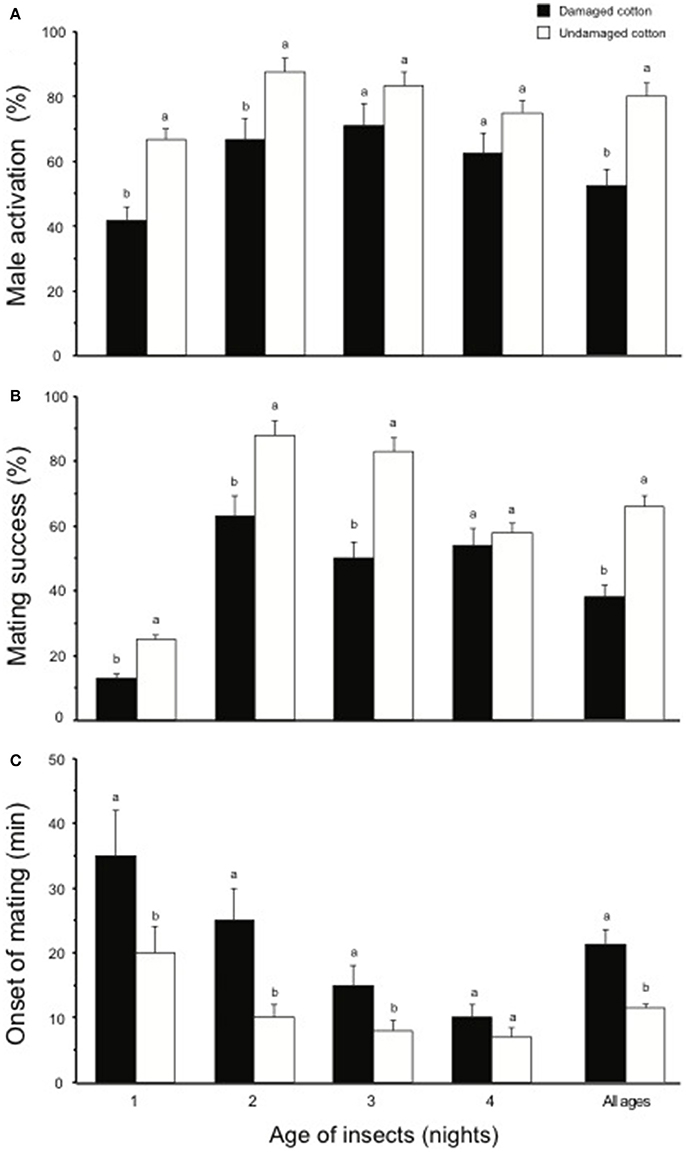
Figure 5. Percentage of male activation (A), mating success (B), and mean onset time of mating (C) in S. littoralis in the presence of damaged and undamaged cotton plants. Male and female moths were allowed to mate in the presence of either damaged cotton plants (black bars) or undamaged cotton plants (white bars), over a period of four consecutive nights. Standard errors are represented on each bar. Chi square test was used for statistical analysis. Different letters within the bars of each age show significant effect (P < 0.05, or 0.01 or 0.001).
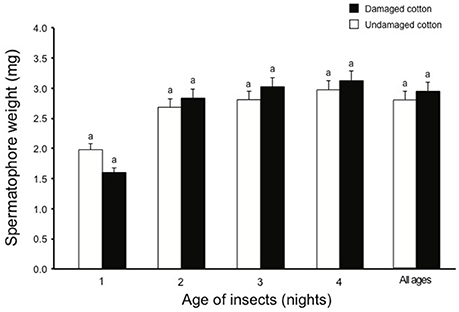
Figure 6. Mean spermatophore weight in S. littoralis moths when allowed to mate in the presence of damaged (black bars) and undamaged (white bars) cotton plants, separately. Standard errors are represented on each bar. Chi square test was used for statistical analysis. Different letters within bars of each age show significant effect (P < 0.05, or 0.01 or 0.001).
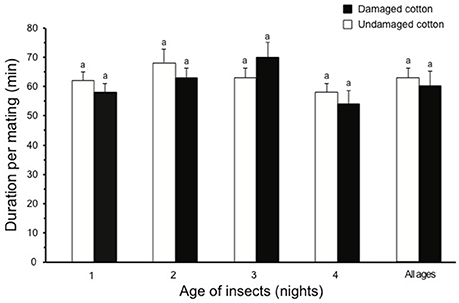
Figure 7. Duration per mating in S. littoralis when allowed to mate in the presence of damaged (black bars) and undamaged (white bars) cotton plants, separately. Standard errors are represented on each bar. Chi square test was used for statistical analysis. Different letters within bars of each age show significant effect (P < 0.05, or 0.01 or 0.001).
This study shows that herbivore-induced volatiles from cotton can affect pre-copulatory and mating behavior of Spodoptera littoralis. The induced changes in damaged host plants influenced mating behavior through modulation of female calling behavior, male attraction to females, and male and female behaviors during mating. The impact of herbivore damage on pre-copulatory and mating behavior in this study, parallels the effect of herbivore-damaged plants on oviposition behavior of mated female S. littoralis (Zakir et al., 2013a). In this previous study, we found that herbivore-induced volatiles from cotton plants damaged by conspecific larvae reduced oviposition on the damaged plants, but also provided associational resistance to neighboring undamaged plants. These results collectively show that the induced changes after herbivore attack on cotton affects several different behaviors connected to reproduction both before and after mating in S. littoralis.
The herbivore-damaged changes in cotton plants can be used by both females and males of S. littoralis to evaluate the quality of the plants before and during mating i.e., for males to orientate toward the plant where a female is calling, for females to select a plant where mating occurs as well as for both sexes during mating. In S. littoralis the female normally starts oviposition shortly after mating (personal observation) and it is, thus, important for both males and females that the plant chosen for mating is suitable for the progeny. The induced changes in cotton can have large effects directly on the physiological development of larvae as they have been shown to reduce growth and prolong development of larvae of Spodoptera species (Alborn et al., 1996; Anderson and Alborn, 1999; Bezemer et al., 2003). In addition to food quality, competition for food resources may also be important in connection to induced changes and affect survival and performance of the progeny. In cotton, damage induction can increase larval migration and their exposure to biotic and abiotic threats (Anderson et al., 2011). It can also increase the attraction of competitors of other species toward common host plant for feeding that may reduce the available food resources (Magalhães et al., 2012). Furthermore, the induced changes can also affect larval survival indirectly as they are involved in the attraction of natural enemies (Röse et al., 1998; Paré and Tumlinson, 1999; Gouinguené et al., 2005; Gols et al., 2012). The ability to respond to induced changes in cotton and to avoid already damaged plants could thus, have a large impact on the fitness of both male and female S. littoralis, as larval development and survival is highly affected by these changes.
Phytophagous insects, including moths, have been found to utilize chemical cues from plants to adjust their mating behavior and investment in reproduction (Zuk and Kolluru, 1998; Reddy and Guerrero, 2004; Zhang and Schlyter, 2004). In female moths of S. littoralis, the presence of a host plant, Ricinus communis, enhanced the time and duration of calling, while the presence of the non-host plant Adhatoda vasica has been observed to reduce both the time and duration of calling (Sadek and Anderson, 2007). Field observations also show that suitable host plants stimulate oviposition and enhance reproductive success in S. littoralis (Sadek, 2001) whereas the presence of non-host plants negatively affected the mating success (Sadek and Anderson, 2007). The delay in mating and reduced mating success on non-host plants was suggested to be due to modulation in calling behavior in females, rather than a modified male response. Under damaged conditions, the delay in onset time of female calling can reduce the time available both for mating and female calling in late night periods, potentially resulting in fitness costs associated with less mating success (Andersson and Simmons, 2006; Groot et al., 2010). For example, male moths of Trichoplusia ni showed increased attraction and mating with normal females compared to the females with suppressed pheromone release (Zhu et al., 1997). It has also been shown in other moth species that reduction in mating occurs after modifying female calling behavior (Zhao et al., 2008, 2009). However, in the present study we show that not only female behavior is affected, but also male behavior connected to mating.
We found that male S. littoralis in the wind tunnel are more attracted toward pheromone having an undamaged host plant as a background compared to an herbivore-damaged host plant. In addition, male S. littoralis moths have also been shown to discriminate between females on different host plants and showed a clear preference hierarchy between the host plants (Thöming et al., 2013; Proffit et al., 2015). These results show that the plant odor background is important for male attraction to female sex pheromone in S. littoralis. In other moths, the presence of host plants has been shown to increase sex pheromone production (Landolt et al., 1994; Witzgall et al., 2008). Furthermore, there is ample evidence that the presence of host plant volatiles synergizes the male response to female sex pheromone in moths (Landolt and Phillips, 1997; Tasin et al., 2007; Varela et al., 2011; Schmera and Guerin, 2012). In a wind tunnel study, it has been reported that in male codling moth, Cydia pomonella, the time of attraction toward female pheromone is reduced when host plant odors were presented in the background (Schmera and Guerin, 2012). On the other hand, direct inhibition of male attraction has been found in the context of non-host plant volatile background (Barbosa et al., 2009; Andersson et al., 2011; Jactel et al., 2011; Schiebe et al., 2011; Tasin et al., 2011). The reduced mating on damaged cotton plants would be affected by reduction in male attraction both directly, through the direct interference of damage-induced plant volatile compounds with male moth attraction to pheromone; and indirectly, by modulating the calling behavior of female moths.
However, no difference between undamaged and damaged plants was seen on the last day of the experiment, day 4, for male activation and mating success and onset. In the onset of mating, it is seen that matings occur very fast in both experiemnts. It is possble that with increasing age it is most important for both males and females to find a mating partner and they are less affected by other factors, such as plant quality. In addition, it seems that there is no difference in mating success between the two treaments, and that they both are on the same level as has been seen for damaged plants on earlier days.
The effects on reproductive behavior of both males and females are correlated with the herbivore-induced production of specific plant compounds in cotton both locally; at the site of damage, and systemically; distal to damaged parts (Loughrin et al., 1994; McCall et al., 1994; Bezemer et al., 2004; Röse and Tumlinson, 2005). During the pre-copulatory behaviors, the moths are exposed first to volatile (McCall et al., 1994; Loughrin et al., 1995) and after landing, to non-volatile induced compounds (Alborn et al., 1996; Bezemer et al., 2004). A de novo synthesis and release of volatile compounds specific to herbivore feeding has been demonstrated from damaged cotton, showing that these compounds are good signals from a distance to indicate plant quality (Paré and Tumlinson, 1997). For example, the herbivore-induced volatile compound DMNT [(E)-4,8-dimethyl-1,3,7-nonatriene], has been found to suppress attraction to other attractive volatiles emitted from intact host plants as well as toward sex pheromones (Zakir et al., 2013b; Hatano et al., 2015). We also know that S. littoralis females can detect many herbivore-induced compounds from cotton and that responses to plant odors and sex pheromone interact in neurons in the antennal lobe (Jönsson and Anderson, 1999; Anton et al., 2007).
The presence of damaged cotton plants did not affect mating duration and male investment. The lack of response during mating may indicate that the onset of mating may have affected the sensitivity of the response to plant compounds and female sex pheromone. Mating-induced behavioral switch toward plants at different physiological stages i.e., floral vs. vegetative stage, have been shown in female S. littoralis during selection for feeding and oviposition sites (Saveer et al., 2012). Mating-induced changes in the sensitivity of male moths to sex pheromones has also been reported in other species (Anton et al., 2007; Anderson and Anton, 2014). For instance, in the moth Agrotis ipsilon, where males exhibited reduced sensitivity to the female sex pheromone both at behavioral and central nervous system levels soon after mating and that the neural responses to plant volatiles were different in virgin and mated male moths (Barrozo et al., 2010, 2011; Deisig et al., 2012; Groot et al., 2016). It is also possible that during mating, the behavior follows a specific sequence, and that this could be due to physiological constraints that reduce the plasticity of mating duration and male investment (Bernays, 2001; Kelly and Jennions, 2011). Thus, it seems that plant quality affects decisions before mating, but once mating has started the most important factor is to ensure that the mating is successful.
Both sexes of S. littoralis moths negatively respond to herbivore-damaged volatiles, which indicate reduced plant quality and competition for resources, both during pre- and post-copulatory behaviors. The induced volatiles are important cues used to evaluate intra-specific variation in plant quality and can be important for the fitness of an insect as it affects behavioral decisions during host plant selection in several ways. Further studies are needed to investigate how the herbivore-induced host plant chemicals affect natural selection through their influence on reproductive success (Andersson and Simmons, 2006; Groot et al., 2016).
AZ, PW, BH, and PA designed the experiments; AZ, MK conducted the experiments; AZ and MK did the statistical analyses of the data; AZ, PW, BH, and PA wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Elisabeth Marling for her help with the rearing. We also thank Dr. William Walker for comments to the manuscrpt. The study was supported by the Linnaeus initiative “Insect Chemical Ecology, Ethology, and Evolution” IC-E3 (Formas, SLU), The Swedish Research Council, VR and the Higher Education Commission (HEC) of Pakistan.
Agrawal, A. A. (2001). Ecology - Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326. doi: 10.1126/science.1060701
Agrawal, A. A. (2005). Future directions in the study of induced plant responses to herbivory. Entomol. Exp. Appl. 115, 97–105. doi: 10.1111/j.1570-7458.2005.00294.x
Alborn, H. T., Rose, U. S. R., and McAuslane, H. J. (1996). Systemic induction of feeding deterrents in cotton plants by feeding of Spodoptera spp. larvae. J. Chem. Ecol. 22, 919–932. doi: 10.1007/BF02029945
Allmann, S., Späthe, A., Bisch-Knaden, S., Kallenbach, M., Reinecke, A., Sachse, S., et al. (2013). Feeding-induced rearrangement of green leaf volatiles reduces moth oviposition. Elife 2:e00421. doi: 10.7554/eLife.00421
Anderson, P., and Alborn, H. (1999). Effects on oviposition behaviour and larval development of Spodoptera littoralis by herbivore-induced changes in cotton plants. Entomol. Exp. Appl. 92, 45–51. doi: 10.1046/j.1570-7458.1999.00523.x
Anderson, P., and Anton, S. (2014). Experience-based modulation of behavioral responses to plant volatiles and other sensory cues in insect herbivores. Plant. Cell Environ. 37, 1826–1835. doi: 10.1111/pce.12342
Anderson, P., Hansson, B. S., Nilsson, U., Han, Q., Sjoholm, M., Skals, N., et al. (2007). Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 32, 483–491.
Anderson, P., Sadek, M. M., and Wäckers, F. L. (2011). Root herbivory affects oviposition and feeding behavior of a foliar herbivore. Behav. Ecol. 22, 1272–1277. doi: 10.1093/beheco/arr124
Andersson, M. N., Binyameen, M., Sadek, M. M., and Schlyter, F. (2011). Attraction modulated by spacing of pheromone components and anti-attractants in a bark beetle and a moth. J. Chem. Ecol. 37, 899–911. doi: 10.1007/s10886-011-9995-3
Andersson, M., and Simmons, L. W. (2006). Sexual selection and mate choice. Trends Ecol. Evol. 21:296. doi: 10.1016/j.tree.2006.03.015
Anton, S., Dufour, M. C., and Gadenne, C. (2007). Plasticity of olfactory-guided behaviour and its neurobiological basis: lessons from moths and locusts. Entomol. Exp. Appl. 123, 1–11. doi: 10.1111/j.1570-7458.2007.00516.x
Arimura, G., Matsui, K., and Takabayashi, J. (2009). Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 50, 911–923. doi: 10.1093/pcp/pcp030
Barbosa, P., Hines, J., Kaplan, I., Martinson, H., Szczepaniec, A., and Szendrei, Z. (2009). Associational resistance and associational susceptibility: having right or wrong neighbors. Annu. Rev. Ecol. Evol. Syst. 40, 1–20. doi: 10.1146/annurev.ecolsys.110308.120242
Barrozo, R. B., Gadenne, C., and Anton, S. (2010). Switching attraction to inhibition: mating-induced reversed role of sex pheromone in an insect. J. Exp. Biol. 213, 2933–2939. doi: 10.1242/jeb.043430
Barrozo, R. B., Jarriault, D., Deisig, N., Gemeno, C., Monsempes, C., Lucas, P., et al. (2011). Mating-induced differential coding of plant odour and sex pheromone in a male moth. Eur. J. Neurosci. 33, 1841–1850. doi: 10.1111/j.1460-9568.2011.07678.x
Bendera, M., Ekesi, S., Ndung'u, M., Srinivasan, R., and Torto, B. (2015). A major host plant volatile, 1-octen-3-ol, contributes to mating in the legume pod borer, Maruca vitrata (Fabricius) (Lepidoptera: Crambidae). Sci. Nat. 102:47. doi: 10.1007/s00114-015-1297-0
Bernays, E. A. (2001). Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 46, 703–727. doi: 10.1146/annurev.ento.46.1.703
Bezemer, T. M., Wagenaar, R., Van Dam, N. M., Van Der Putten, W. H., and Wackers, F. L. (2004). Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30, 53–67. doi: 10.1023/B:JOEC.0000013182.50662.2a
Bezemer, T. M., Wagenaar, R., Van Dam, N. M., and Wackers, F. L. (2003). Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562. doi: 10.1034/j.1600-0706.2003.12424.x
Brown, E. S., and Dewhurst, C. F. (1975). Genus spodoptera (Lepidoptera, Noctuidae) in Africa and Near East. Bull. Entomol. Res. 65, 221–262. doi: 10.1017/S0007485300005939
Bruce, T. J. A. (2015). Interplay between insects and plants: dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 66, 455–465. doi: 10.1093/jxb/eru391
Bukovinszky, T., Gols, R., Smid, H. M., Kiss, G. B., Dicke, M., and Harvey, J. A. (2012). Consequences of constitutive and induced variation in the host's food plant quality for parasitoid larval development. J. Insect Physiol. 58, 367–375. doi: 10.1016/j.jinsphys.2011.12.017
Deisig, N., Kropf, J., Vitecek, S., Pevergne, D., Rouyar, A., Sandoz, J. C., et al. (2012). Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 7:e33159. doi: 10.1371/journal.pone.0033159
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. (2001). Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580. doi: 10.1038/35069058
Dicke, M., and Baldwin, I. T. (2010). The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help.” Trends Plant Sci. 15, 167–175. doi: 10.1016/j.tplants.2009.12.002
Félix, A.-E., Smaïl, T., and Frérot, B. (2013). Host plants and reproductive behaviour in the African maize stemborer, Busseola fusca (Fuller 1901) (Lepidoptera: Noctuidae). Ann. Société Entomol. Fr. 49, 68–72. doi: 10.1080/00379271.2013.767513
Gols, R., Veenemans, C., Potting, R. P. J., Smid, H. M., Dicke, M., Harvey, J. A., et al. (2012). Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim. Behav. 83, 1231–1242. doi: 10.1016/j.anbehav.2012.02.015
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. (2005). Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31, 1023–1038. doi: 10.1007/s10886-005-4245-1
Groot, A. T., Classen, A., Staudacher, H., Schal, C., and Heckel, D. G. (2010). Phenotypic plasticity in sexual communication signal of a noctuid moth. J. Evol. Biol. 23, 2731–2738. doi: 10.1111/j.1420-9101.2010.02124.x
Groot, A. T., Dekker, T., and Heckel, D. G. (2016). The genetic basis of pheromone evolution in moths. Annu. Rev. Entomol. 61, 99–117. doi: 10.1146/annurev-ento-010715-023638
Groot, A. T., and Visser, J. H. (2001). Influence of host plants on sexual communication in the herbivorous bug Lygocoris pabulinus. Chemoecology 11, 161–166. doi: 10.1007/PL00001847
Hare, J. D., Sun, J. J., Daniel Hare, J., and Sun, J. J. (2011). Production of herbivore-induced plant volatiles is constrained seasonally in the field but predation on herbivores is not. J. Chem. Ecol. 37, 430–442. doi: 10.1007/s10886-011-9944-1
Hatano, E., Saveer, A. M., Borrero-Echeverry, F., Strauch, M., Zakir, A., Bengtsson, M., et al. (2015). A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways. BMC Biol. 13:75. doi: 10.1186/s12915-015-0188-3
Heil, M. (2014). Herbivore-induced plant volatiles: targets, perception and unanswered questions. J. Physiol. 204, 297–306. doi: 10.1111/nph.12977
Hinks, C. F., and Byers, J. R. (1976). Biosystematics of genus Euxoa (Lepidoptera-Noctuidae). 5. Rearing procedures, and life-cycles of 36 species. Can. Entomol. 108, 1345–1357.
Howe, G. A., and Jender, G. (2008). Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66. doi: 10.1146/annurev.arplant.59.032607.092825
Howe, G. A., and Schaller, A. (2008). “Direct defenses in plants and their induction by wounding and insect herbivores,” in Induced Plant Resistance to Herbivory, ed A. Schaller (Netherlands: Springer), 7–30.
Jactel, H., Birgersson, G., Andersson, S., and Schlyter, F. (2011). Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 166, 703–711. doi: 10.1007/s00442-011-1918-z
Jönsson, M., and Anderson, P. (1999). Electrophysiological response to herbivore-induced host plant volatiles in the moth Spodoptera littoralis. Physiol. Entomol. 24, 377–385. doi: 10.1046/j.1365-3032.1999.00154.x
Kalberer, N. M., Turlings, T. C. J., and Rahier, M. (2001). Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants. J. Chem. Ecol. 27, 647–661. doi: 10.1023/A:1010389500009
Karban, R., and Baldwin, I. T. (1997). Induced Responses to Herbivory. (Chicago, IL: The University of Chicago Press), 60637.
Karban, R., and Thaler, J. S. (1999). Plant phase change and resistance to herbivory. Ecology 80, 510–517. doi: 10.1890/0012-9658(1999)080[0510:PPCART]2.0.CO;2
Kelly, C. D., and Jennions, M. D. (2011). Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884. doi: 10.1111/j.1469-185X.2011.00175.x
Kessler, A., and Baldwin, I. T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144. doi: 10.1126/science.291.5511.2141
Knolhoff, L. M., and Heckel, D. G. (2014). Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 59, 263–278. doi: 10.1146/annurev-ento-011613-161945
Landolt, P. J., Heath, R. R., Millar, J. G., Davishernandez, K. M., Dueben, B. D., and Ward, K. E. (1994). Effects of host-plant, Gossypium-Hirsutum L, on sexual attraction of cabbage-looper moths, trichoplusia-ni (Hubner) (Lepidoptera, Noctuidae). J. Chem. Ecol. 20, 2959–2974. doi: 10.1007/BF02098402
Landolt, P. J., and Phillips, T. W. (1997). Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 42, 371–391. doi: 10.1146/annurev.ento.42.1.371
Loughrin, J. H., Manukian, A., Heath, R. R., Turlings, T. C. J., and Tumlinson, J. H. (1994). Diurnal cycle of emission of induced volatile terpenoids herbivore-injured cotton plants. Proc. Natl. Acad. Sci. U.S.A. 91, 11836–11840. doi: 10.1073/pnas.91.25.11836
Loughrin, J. H., Potter, D. A., and Hamilton-Kemp, T. R. (1995). Volatile compounds induced by herbivory act as aggregation kairomones for the Japanese beetle (Popillia japonica Newman). J. Chem. Ecol. 21, 1457–1467. doi: 10.1007/BF02035145
Magalhães, D. M., Borges, M., Laumann, R. A., Sujii, E. R., Mayon, P., Caulfield, J. C., et al. (2012). Semiochemicals from herbivory induced cotton plants enhance the foraging behavior of the cotton boll weevil, Anthonomus grandis. J. Chem. Ecol. 38, 1528–1538. doi: 10.1007/s10886-012-0216-5
McCall, P. J., Turlings, T. C. J., Loughrin, J., Proveaux, A. T., and Tumlinson, J. H. (1994). Herbivore-induced volatile emissions from cotton (Gossypium hirsutum L) seedlings. J. Chem. Ecol. 20, 3039–3050. doi: 10.1007/BF02033709
McCormick, A. C., Heyer, J., Sims, J. W., Mescher, M. C., and De Moraes, C. M. (2017). Exploring the effects of plant odors, from tree species of differing host quality, on the response of lymantria dispar males to female sex pheromones. J. Chem. Ecol. 43, 243–253. doi: 10.1007/s10886-017-0825-0
McNeil, J. N., and Delisle, J. (1989). Are host plants important in pheromone-mediated mating systems of lepidoptera? Experientia 45, 236–240. doi: 10.1007/BF01951809
Paré, P. W., and Tumlinson, J. H. (1997). De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 114, 1161–1167. doi: 10.1104/pp.114.4.1161
Paré, P. W., and Tumlinson, J. H. (1999). Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–331. doi: 10.1104/pp.121.2.325
Pierik, R., Ballaré, C. L., and Dicke, M. (2014). Ecology of plant volatiles: taking a plant community perspective. Plant Cell Environ. 37, 1845–1853. doi: 10.1111/pce.12330
Proffit, M., Khallaf, M. A., Carrasco, D., Larsson, M. C., and Anderson, P. (2015). “Do you remember the first time?” Host plant preference in a moth is modulated by experiences during larval feeding and adult mating. Ecol. Lett. 18, 365–374. doi: 10.1111/ele.12419
Raina, A. K., Kingan, T. G., and Mattoo, A. K. (1992). Chemical signals from host plant and sexual behaviour in a moth. Science 255, 592–593. doi: 10.1126/science.255.5044.592
Reddy, G. V. P., and Guerrero, A. (2004). Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 9, 253–261. doi: 10.1016/j.tplants.2004.03.009
Reisenman, C. E., Riffell, J. A., Bernays, E. A., and Hildebrand, J. G. (2010). Antagonistic effects of floral scent in an insect-plant interaction. Proc. R. Soc. B Biol. Sci. 277, 2371–2379. doi: 10.1098/rspb.2010.0163
Röse, U. S. R., Lewis, W. J., and Tumlinson, J. H. (1998). Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J. Chem. Ecol. 24, 303–319. doi: 10.1023/A:1022584409323
Röse, U. S. R., and Tumlinson, J. H. (2005). Systemic induction of volatile release in cotton: how specific is the signal to herbivory? Planta 222, 327–335. doi: 10.1007/s00425-005-1528-2
Sadek, M. M. (2001). Polyandry in field-collected Spodoptera littoralis moths and laboratory assessment of the effects of male mating history. Entomol. Exp. Appl. 98, 165–172. doi: 10.1046/j.1570-7458.2001.00771.x
Sadek, M. M., and Anderson, P. (2007). Modulation of reproductive behaviour of Spodoptera littoralis by host and non-host plant leaves. Basic Appl. Ecol. 8, 444–452. doi: 10.1016/j.baae.2006.08.001
Saveer, A. M., Kromann, S. H., Birgersson, G., Bengtsson, M., Lindblom, T., Balkenius, A., et al. (2012). Floral to green: mating switches moth olfactory coding and preference. Proc. R. Soc. B Biol. Sci. 279, 2314–2322. doi: 10.1098/rspb.2011.2710
Scala, A., Allmann, S., Mirabella, R., Haring, M. A., and Schuurink, R. C. (2013). Green leaf volatiles: a plant's multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 14, 17781–17811. doi: 10.3390/ijms140917781
Schiebe, C., Blazenec, M., Jakus, R., Unelius, C. R., and Schlyter, F. (2011). Semiochemical diversity diverts bark beetle attacks from Norway spruce edges. J. Appl. Entomol. 135, 726–737. doi: 10.1111/j.1439-0418.2011.01624.x
Schmera, D., and Guerin, P. M. (2012). Plant volatile compounds shorten reaction time and enhance attraction of the codling moth (Cydia pomonella) to codlemone. Pest Manag. Sci. 68, 454–461. doi: 10.1002/ps.2292
Schmidt, L., Schurr, U., and Rose, U. S. R. (2009). Local and systemic effects of two herbivores with different feeding mechanisms on primary metabolism of cotton leaves. Plant Cell Environ. 32, 893–903. doi: 10.1111/j.1365-3040.2009.01969.x
Schuman, M. C., and Baldwin, I. T. (2016). The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 61, 373–394. doi: 10.1146/annurev-ento-010715-023851
Shorey, H. H. (1973). Behavioral responses to insect pheromones. Annu. Rev. Entomol. 18, 349–380. doi: 10.1146/annurev.en.18.010173.002025
Tasin, M., Bäckman, A.-C., Coracini, M., Casado, D., Ioriatti, C., and Witzgall, P. (2007). Synergism and redundancy in a plant volatile blend attracting grapevine moth females. Phytochemistry 68, 203–209. doi: 10.1016/j.phytochem.2006.10.015
Tasin, M., Lucchi, A., Ioriatti, C., Mraihi, M., De cristofaro, A., Boger, Z., et al. (2011). Oviposition response of the moth Lobesia botrana to sensory cues from a host plant. Chem. Senses 36, 633–639. doi: 10.1093/chemse/bjr027
Thöming, G., Larsson, M. C., Hansson, B. S., and Anderson, P. (2013). Comparison of plant preference hierarchies of male and female moths and the impact of larval rearing hosts. Ecology 94, 1744–1752. doi: 10.1890/12-0907.1
Varela, N., Avilla, J., Anton, S., and Gemeno, C. (2011). Synergism of pheromone and host-plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 141, 114–122. doi: 10.1111/j.1570-7458.2011.01171.x
Wertheim, B., van Baalen, E.-J. A., Dicke, M., and Vet, L. E. M. (2004). Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu. Rev. Entomol. 50, 321–346. doi: 10.1146/annurev.ento.49.061802.123329
Witzgall, P., Stelinski, L., Gut, L., and Thomson, D. (2008). Codling moth management and chemical ecology. Annu. Rev. Entomol. 53, 503–522. doi: 10.1146/annurev.ento.53.103106.093323
Zakir, A., Bengtsson, M., Sadek, M. M., Hansson, B. S., Witzgall, P., and Anderson, P. (2013a). Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 216, 3257–3263. doi: 10.1242/jeb.083188
Zakir, A., Sadek, M. M., Bengtsson, M., Hansson, B. S., Witzgall, P., and Anderson, P. (2013b). Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J. Ecol. 101, 410–417. doi: 10.1111/1365-2745.12041
Zhang, Q. H., and Schlyter, F. (2004). Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. For. Entomol. 6, 1–19. doi: 10.1111/j.1461-9555.2004.00202.x
Zhao, X. C., Wu, K. M., liang, G. M., and Guo, Y. Y. (2009). Modified female calling behaviour in Cry1Ac-resistant Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 65, 353–357. doi: 10.1002/ps.1697
Zhao, X. C., Wu, K. M., Liang, G. M., and Guo, Y. Y. (2008). Altered mating behaviour in a Cry1Ac-resistant strain of Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 132, 360–365. doi: 10.1111/j.1439-0418.2008.01268.x
Zhu, J., Chastain, B. B., Spohn, B. G., and Haynes, K. F. (1997). Assortative mating in two pheromone strains of the cabbage looper moth, Trichoplusia ni. J. Insect Behav. 10, 805–817. doi: 10.1023/B:JOIR.0000010414.28494.9a
Keywords: herbivory, Spodoptera littoralis, reproductive behavior, repellency, pheromonal communication, plant resistance
Citation: Zakir A, Khallaf MA, Hansson BS, Witzgall P and Anderson P (2017) Herbivore-Induced Changes in Cotton Modulates Reproductive Behavior in the Moth Spodoptera littoralis. Front. Ecol. Evol. 5:49. doi: 10.3389/fevo.2017.00049
Received: 14 February 2017; Accepted: 04 May 2017;
Published: 19 May 2017.
Edited by:
Stefano Colazza, University of Palermo, ItalyReviewed by:
Michael Rostás, Lincoln University, New ZealandCopyright © 2017 Zakir, Khallaf, Hansson, Witzgall and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Zakir, emFraXJhbGlAY2lpdHZlaGFyaS5lZHUucGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.