- 1Institute of Molecular Medicine, National Tsing Hua University, Hsinchu, Taiwan
- 2Institute of Systems Neuroscience, National Tsing Hua University, Hsinchu, Taiwan
- 3Department of Life Science, National Tsing Hua University, Hsinchu, Taiwan
Cephalopods use a diverse range of body patterns for visual communication. Each pattern is composed of several distinct chromatic components that are under neural control and are expressed dynamically. In the oval squid Sepioteuthis lessoniana, males use distinct body patterns to interact with females and other males at the spawning site. To systematically examine their visual signals during reproductive behavior, an ethogram of 27 body pattern components produced by S. lessoniana was observed in both the wild and captivity; these were then characterized. Five behaviors were commonly seen among these reproductively active squids, namely parallel swimming, male guarding, male-male fighting, male-parallel mating, and male-upturn mating. Each behavior was found to be composed of the expression in a temporal sequence of different chromatic components. By analyzing the dynamic body patterning time series associated with each behavior, it was found that a certain subset of components was expressed simultaneously or sequentially in response to conspecifics. Importantly, the results not only revealed that each behavior is composed of multiple chromatic components, but the findings also showed that each component is often associated with multiple behaviors. To gain insight into the visual communication associated with each behavior in terms of the body patterning's key components, the co-expression frequencies of two or more components at any moment in time were calculated in order to assess uniqueness when distinguishing one behavior from another. This approach identified the minimum set of key components that, when expressed together, represents an unequivocal visual communication signal. While the interpretation of the signal and the associated response of the receiver during visual communication are difficult to determine, the concept of the component assembly is similar to a typical language within which individual words often have multiple meanings, but when they appeared together with other words, the message becomes unequivocal. The present study thus demonstrates that dynamic body pattering, by expressing unique sets of key components acutely, is an efficient way of communicating behavioral information between oval squids.
Introduction
Animal communication is commonly defined as the transfer of information from one or a group of animals (the sender or senders) to another one or more animals (the receiver or receivers) that alters the current or future behavioral states of the receivers (Manning and Dawkins, 2012; Stegmann, 2013). When the information from the sender changes the behavior of a receiver, the information can be considered to be a “signal” (Witzany, 2014). The theory predicts that for a signal to be maintained in the population, both the sender and receiver needs to obtain a benefit from the other via the interaction (Smith and Harper, 2003). Many modes make up visual signaling and these include gestures, facial expression, gaze following, color change, and bioluminescent communication (Bradbury and Vehrencamp, 1998). Among these modes, color change is highly dynamic in cephalopods because they have a neurally controlled chromatophore system that is able to change the apparent color, opacity, and reflectiveness of their skin (Messenger, 2001; Mäthger et al., 2009).
Visual communication in cephalopods involves sophisticated displays that are characterized by many visually appealing body patterns (Moynihan and Rodaniche, 1982; Hanlon, 1988; Hanlon et al., 1994, 1999; Hanlon and Messenger, 1996; Jantzen and Havenhand, 2003a,b; Caldwell et al., 2015; Scheel et al., 2016). The body pattern of a cephalopod consists of a combination of chromatic, textural, postural, and locomotor components, and a subset of these components may be combined together at any given time to create a specific and different body pattern (Packard and Sanders, 1969, 1971; Packard and Hochberg, 1977; Hanlon, 1982; Roper and Hochberg, 1988; Hanlon et al., 1994, 1999; Hanlon and Messenger, 1996). Most importantly, these components can be thought of, not only as morphological units of the body, but also as physiological units within the brain (Packard, 1982). Among these components, the chromatic components are probably the most conspicuous and diverse. Thus, studying the dynamics of chromatophore expression by the skin during visual communication should help to reveal the principles behind visual signaling by cephalopods.
Cephalopods use multiple mating strategies during reproduction. Many studies have suggested that most cephalopods utilize polyandry, which is where a female mates with multiple males during the spawning season (Hanlon and Messenger, 1996; Hanlon et al., 2005). Previous studies have shown that females not only mate with large size males in a group (the consorts) but also mate with other smaller males (the sneakers) using distinct types of courtship behavior (Hanlon et al., 1997, 2002, 2005; Jantzen and Havenhand, 2003b; Naud et al., 2004, 2016; Iwata et al., 2005; Wada et al., 2005; Iwata and Sakurai, 2007; Huffard et al., 2008; Sato et al., 2014). These results suggest that males of different sizes may adopt different strategies in order to gain access to females and that females may during the same process choose different males in order to maximize their reproduction success. In addition to the male-female interactions, agonistic behavior among males often seems to be involved in these reproductive interactions. Cephalopods are highly visual animals and they communicate regularly through dynamic body patterning, thus it seems likely that visual communication between males and females during courtship behavior and between males and males during agonistic behavior may determine the mating strategies and success of these individuals (Hanlon and Messenger, 1996; Jantzen and Havenhand, 2003a; Scheel et al., 2016). Thus, interpreting this communication system is fundamental to understanding the processes involved in sexual selection among these species.
Sepioteuthis squid species are common in shallow waters (Jereb and Roper, 2005). Their diverse reproductive behaviors as well as their wide variety of body patterning ethograms have been studied in detail in two species, Sepioteuthis sepioidea (Moynihan and Rodaniche, 1982; Byrne et al., 2003) and Sepioteuthis australis (Jantzen and Havenhand, 2003a,b). However, the oval squid Sepioteuthis lessoniana which is widely distributed in Indo-West Pacific waters (Jereb and Roper, 2005) has not been systematically examined and neither have their dynamic body patterns during reproductive behavior been determined. Similar to other cephalopods, it has been shown that S. lessoniana also undertake diverse mating behaviors and show a wide variety of body patterns (Wada et al., 2005). In the present study, we aimed to (1) examine whether different reproductive behaviors are associated with specific body patterns, (2) determine whether some chromatic components are co-expressed for particular behaviors, and (3) assess whether certain components are specific to a particular behavior or are used in multiple contexts. An ethogram of the chromatic components displayed by S. lessoniana during reproductive interactions was characterized, and the expression in a temporal sequence of the different chromatic components was analyzed to reveal the underlying basis of the visual signaling used by this cephalopod for communication (Martin et al., 1993).
Materials and Methods
Animals
In order to observe the mating behavior of S. lessoniana in the field, a spawning ground in the northeast coast of Taiwan (N 25.070886, E 121.925620) was created by placing several bunches of bamboo on the sea floor at a depth of 18 m with the aim of attracting squids. The habitat was open water with sand substrates, and the visual background was typical green coastal water. The bamboo branches and leaves provide a suitable surface for females to lay eggs and act as a shelter for any hatchlings. The spawning ground successfully attracted dozens of squids. Observations were made during scuba dives from July to September in 2011 and the mating behaviors were recorded by either a surveillance camera (GoPro Hero 2) or a hand held video camera (SONY DSC-HX9V). The videos were taken mostly from the side above the squid, so the body patterns on the dorsal and lateral sides of the squids can be seen simultaneously in most cases. Due to the large number of squids at this site, the identity and size of individual animals were difficult to determine. Thus, the sample size reflects the number of times that each behavior was observed from multiple squids, not the exact number of squids that displayed each behavior. Since most squids were in an intense reproductive mode, their behaviors were not affected significantly by the presence of divers. Although the social interactions among many squids at the spawning ground were complex, the recorded behaviors were sufficiently representative in their reproductive contexts. A total of 111 min of footage captured over 11 dives was used during the present study. In addition to the above video surveillance, additional behavior footage (100 min) was obtained from the Public Television Service (PTS) of Taiwan, who had made a film to document the spawning ground and reproductive behavior of S. lessoniana during 2007.
To observe the body pattern of squids more closely and in a more controlled environment, two adult male squids (mantel length = 19.2 and 20.1 cm) and female squids (mantel length = 18.6 and 27.8 cm) were collected by fishermen in Keelung area, Taiwan, and these were then reared in a large indoor aquarium (5 × 5 × 1.5 m depth) at the Marine Research Station, Academia Sinica, near I-Lan, Taiwan from March 2015 to May 2015. The aquarium was supplied with running seawater, and a bunch of bamboos was placed on the bottom as spawning substrate. The aquarium was lit by natural light and overhead fluorescent bulbs, and the visual background was plain walls of light blue color. The mating behaviors of a pair of male and female (mantle length = 19.2 cm and 27.8 cm, respectively) were recorded by a surveillance camera (GoPro Hero 2) on 14 April 2015. Although there were two other squids in the aquarium during this focal sampling, the recorded mating behaviors were largely unaffected by these bystanders. A total of 37 minutes of high resolution video footage was captured during this exercise and this was used during the present study to complement the field videos.
Analysis of the Dynamic Body Patterns
Although the body patterns of cephalopods include a range of different components, chromatic, textual, postural, and locomotor (Hanlon and Messenger, 1996), in order to simplify the data analysis and focus on the most diverse component, only the chromatic component that make up the dynamic body patterning of S. lessoniana was categorized and analyzed during the present study. It should be emphasized here that the so called “chromatic components” do not mean that they are colorful in appearance, rather they are used to describe the basic units constituting the body coloration. By carefully examining the videos collected both in the field and during the laboratory studies, 27 chromatic components were identified. Note that all the components observed in the field can be seen in the laboratory.
An ethogram is a catalog or inventory of behaviors or actions exhibited by an animal (Manning and Dawkins, 2012). Ethograms are often hierarchical and have functional inferences. To create an ethogram of the dynamic body patterning of S. lessoniana during reproductive behavior, the videos were manually analyzed second-by-second to characterize the presence of each chromatic component; this data was used to create, using Pot Player Portable software, a time series. While this was a labor intensive process, such a dataset is able to represent the complete expression in temporal sequence of these chromatic components and forms an inventory of the actions exhibited by S. lessoniana during reproductive behavior, that is it forms an ethogram.
To facilitate the reconstruction and visualization of the body patterns as co-expression of different chromatic components on the squid skin, individual components was created and superimposed. Using the “layer” function in Adobe Photoshop, it is possible for various components to be overlayed and this then generates a composite image of an oval squid; this image represents a specific set of expressed chromatic component and forms a snapshot of a given moment during the animal's dynamic body patterning behavior. If one renders all composite images sequentially, this is essentially forms a playback of the squid's body patterning along the time axis. A similar aproach has been used to describe the body patterns of the Caribbean reef squid S. sepioidea (Byrne et al., 2003).
To further analyze the timing and frequency of each component expressed during the various reproductive behaviors, and to allow examination of the interactions between the different components in various behavioral contexts, each video clip was segmented and specific behaviors identified. Only behavior segments lasting for more than 4 s were considered. To compare component expression during the same category of behavior, all the segmented datasets were normalized such that the start and the stop of the behavior was set as 0 and 100%, respectively. The occurrence frequency of each component in a behavior was calculated as the number of video segments in which the component appeared, which was divided by the total number of video segments available for that behavior. In addition, the co-occurrence frequency of components was computed as the co-expression time of two or more components, which was divided by the total expression time of these involved components during each video segment.
Results
Oval Squids Exhibit Various Different Behaviors that Are Associated with Distinct Body Patterns
After carefully viewing all of the video sequences collected from the field and laboratory, the reproductive behaviors of S. lessoniana could be categorized into five distinct categories, namely parallel swimming, male guarding, male-male fighting, male-parallel mating, and male-upturn mating (Table 1 and Figure 1; also see the Supplementary Movies 1–5). It is very apparent that the males and females or the winners and losers showed distinct body patterns during these reproductive behaviors (Figure 1).
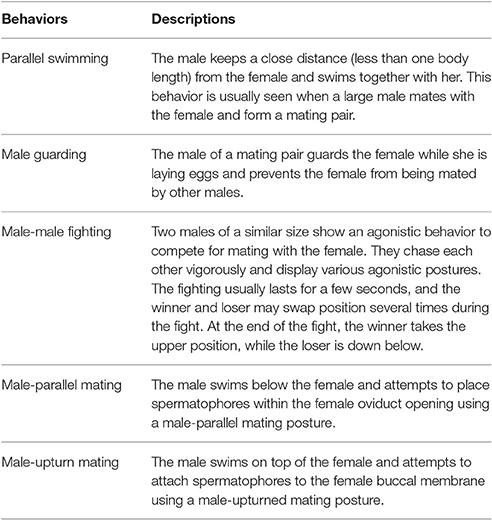
Table 1. Five distinct reproductive behaviors in the oval squid Sepioteuthis lessoniana (see the Supplementary Movies 1–5 for these behaviors).

Figure 1. Five behaviors of adult oval squid Sepioteuthis lessoniana were observed during the spawning season. (A) Parallel swimming. (B) Male guarding. (C) Male-male fighting. (D) Male-parallel mating. (E) Male-upturn mating. M, male; F, female; W, winner; L, loser. See the Supplementary Movies 1–5 for these behaviors.
Chromatic Components and Their Dynamic Expression Form a Series of Different Body Patterns during Reproductive Behaviors
After the systematic examination of the various different body patterns observed during reproductive behavior, 27 distinct chromatic components were identified (Figure 2). Among these components, 11 (MB, WDS, DDS, DPVM, MMS, DA, DAS, DT, SE, DFS, and SF) have been described in S. australis (Jantzen and Havenhand, 2003a) and Loligo vulgaris (Hanlon et al., 1994), and 16 (LDML, LDMS, LUB, MUB, DUB, MM, DADM, DLS, MHA, DTA, DH, MF, DFM, WFS, FMS, and ES) are newly described for S. lessoniana in northeastern Taiwan. These components were identified based on their unique and repeated expression during the videos. Among them, LDML are only seen in matured male squids, while LDMS are unique to matured females. To distinguish overall skin brightness, LUB, MUB, and DUB have been assigned based on the squid's skin tone. The light, medium, and dark were determined subjectively depending on their body intensity relative to background and other nearby squids. There are five distinct dorsal mantle components (MB, MM, DADM, WDS, and DDS) and three unique mantle components that are only able to be seen from a side view (DPVM, MMS, and DLS). In addition, there are seven specific components that affect the arms and/or head (MHA, DTA, DA, DAS, DT, DH, and SE). Finally, there are seven components that are uniquely expressed on the fin (MF, DFM, WFS, DFS, SF, FMS, and ES).
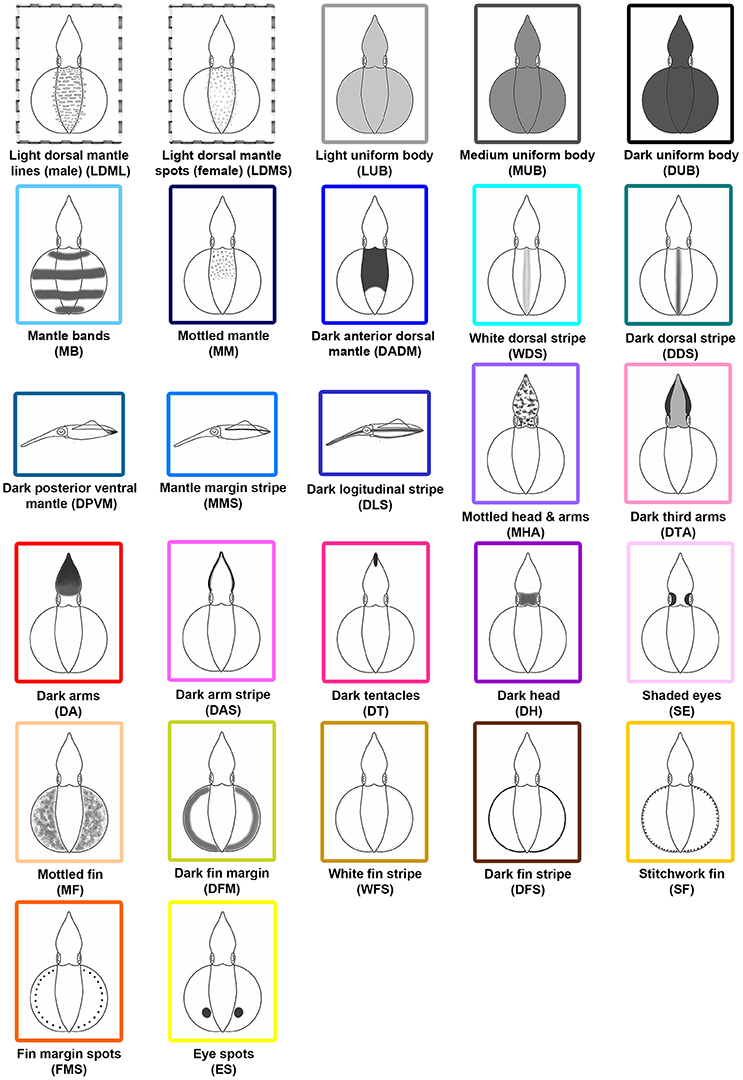
Figure 2. Schematic representations of the 27 chromatic body pattern components observed with adult oval squids Sepioteuthis lessoniana. Among these 27 chromatic components, 11 (MB, WDS, DDS, DPVM, MMS, DA, DAS, DT, SE, DFS, and SF) have been identified in Sepioteuthis australis and Loligo vulgaris, and 16 (LDML, LDMS, LUB, MUB, DUB, MM, DADM, DLS, MHA, DTA, DH, MF, DFM, WFS, FMS, and ES) are newly described for S. lessoniana in northeastern Taiwan. Abbreviated names are parenthesized.
An ethogram of the dynamic body patterns that occur during reproductive behavior among S. lessoniana was then created based on the presence or absence of each chromatic component over a time series of data. The time sequence of different chromatic components expressed by a single pair of squids in a spawning ground was shown in Figure 3 as an example. This inventory of actions exhibited by the squids serves as a basis for the quantitative analysis of their dynamic body patterning that is used for visual communication during the reproductive behavior in S. lessoniana.
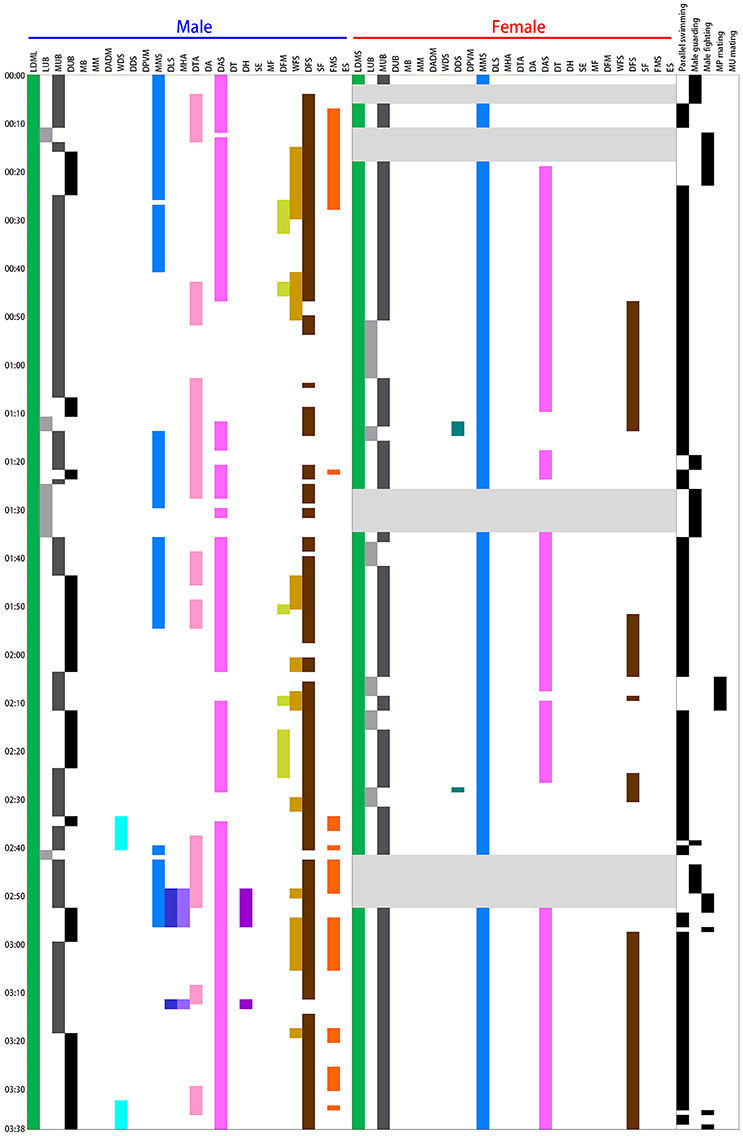
Figure 3. Time sequence of the various chromatic components expressed during different behaviors by a single pair of oval squid Sepioteuthis lessoniana observed in spawning season. Each bar represents the time duration of a single chromatic component expression by the male and female during the 3 min 38 s recording period. There were 15 components expressed by the male squid and 7 by the female. Each line on the right indicates the appearance of individual behaviors during the recording period. This example includes four of the five distinct behavioral categories (Table 1), namely parallel swimming, male guarding, male-male fighting, and male-parallel mating.
Co-expression of Certain Chromatic Components Represents Unique Visual Signals When Placed in a Behavioral Context
In the composite image that forms a snapshot of any moment in the dynamic body patterning behavior, only the components that had an occurrence frequency above 50% for each behavior were included in the analysis. Since both parallel swimming and male guarding behaviors did not show any significant dynamic changes in skin coloration, only one composiste image was created to illustrate each of these two unique body patterns (Figure 4). During parallel swimming behavior, in addition to the presence of the male and female specific components (LDML and LDMS), which were there at all times, males (n = 22) showed a subset of components with that occurred at a range of different frequencies (MMS, DAS, SE, DFS, and WFS). In addition to the above, during the same behavior, females (n = 22) showed another subset of components (MMS, DAS, DH, and DFS). Similarly, during the male guarding behavior, the male squids (n = 23) showed another different subset of components at various frequencies (MMS, DA, DAS, DH, SE, DFS, WFS, and FMS).
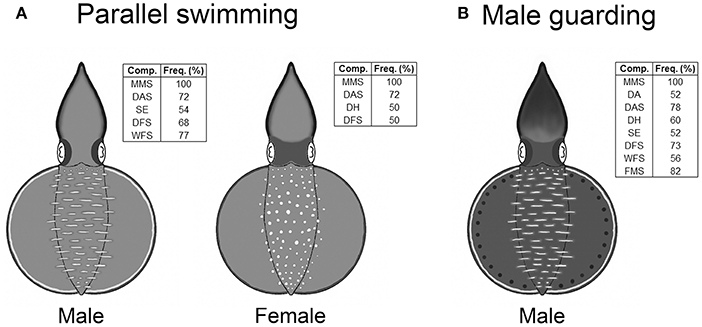
Figure 4. Oval squid Sepioteuthis lessoniana display unique body patterns during parallel swimming and guarding behaviors. (A) The body patterns of parallel swimming consist of MMS (mantle margin stripe), DAS (dark arm stripe), SE (shaded eyes), DFS (dark fin stripe), and WFS (white fin stripe) for males, and MMS, DAS, DH (dark head), and DFS for females. (B) The body pattern of male guarding consist of MMS, DA (dark arms), DAS, DH, SE, DFS, WFS, and FMS (fin margin spots). The inset shows the chromic components with over 50% occurrence for all recorded behaviors.
For the three other behaviors (male-male fighting, male-parallel mating, and male-upturn mating), the body patterns were highly dynamic and a number of different components were present at various different times and for various different durations. Thus, time series of the average expression level of the individual components and their corresponding composite images at a number of specific time points were created to emphasize the dynamic nature of body patterning during these intraspecific visual communication events. During male-male fighting behavior (n = 4), it is apparent that the winner squids showed more chromatic components and a darker body coloration than the loser squids while fighting (Figure 5). Specifically, the winners showed LDML, WDS, MMS, MHA, DTA, DA, DAS, DH, SE, DFM, WFS, DFS, SF, and FMS, namely 14 components, whereas the losers only showed LDML, MMS, MHA, DAS, SE, WFS, DFS, SF, and FMS, namely 9 components. The presence of these additional five components (WDS, DTA, DA, DH, and DFM) could be used to distinguish the winner and the loser, which suggests that some components may be more important than others to the signaling of the fight outcome (see the Supplementary Movie 6 for the switch of the winner and the loser during an agonistic fighting). The body pattern diagrams in Figure 5 contain an overlay of all expressed components that occur at frequencies above 50% at specific time points. A playback of the patternings of a pair of squid bodies along the time axis after rendering to make all composite images sequential, can be viewed as part of the Supplemenatry information (Supplementary Movie 7).
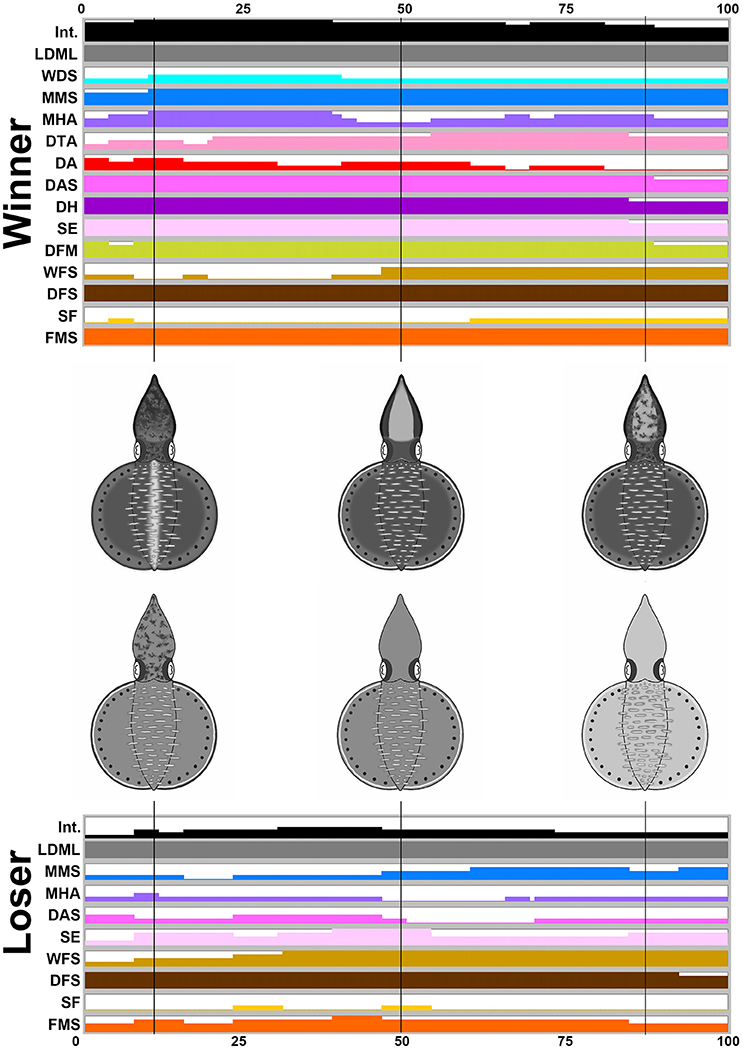
Figure 5. The winner shows more chromatic components and a darker body coloration than the loser during fighting behavior. The time sequence of the average expression level of individual chromatic components recorded from the winner and the loser oval squids during male-male fighting. Time zero on the horizontal axis indicates the start of the fighting behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of chromatic components with over 50% occurrence during the recorded behavior for the winner (top) and the loser (bottom). The intensity (Int) on the first line represents the average strength of the uniform body coloration during fighting behavior. LDML, light dorsal mantle lines; WDS, white dorsal stripe; MMS, mantle margin stripe; MHA, mottled head and arms; DTA, dark third arms; DA, dark arms; DAS, dark arm stripe; DH, dark head; SE, shaded eyes; DFM, dark fin margin; WFS, white fin stripe; DFS, dark fin stripe; SF, stitchwork fin; FMS, fin margin spots. See the Supplementary Movie 7 for this behavior.
During the male-parallel mating behavior (n = 17), when the female squid displayed the “rejection” signal (all cases in the recorded behavior), there is a tendency to increase the expression level of the components and to darken the body skin tone (Figure 6). Note that when the female accepted the male-parallel mating posture, the female body pattern was less conspicuous (observed occasionally and not included in the present study). When the male squid gives up the parallel mating attempt, the female decreased the expression level of components and the skin coloration becomes lighter. In addition, overall, the body pattern of the male was relatively pale when compared with the female. Specifically, the male showed four components, LDML, MMS, SE, and DFS, whereas the female showed eight components, LDMS, DADM, WDS, DA, DH, SE, DFS, and FMS. Except for LDML and LDMS which are sex specific components and MMS, which can be seen only from the side view of the male, there were five additional componnets (DADM, WDS, DA, DH, and FMS) that were present only in the female. This suggests that certain components may function as a “rejection” signal by the female, which is communicated to the male to indicate mating preference. A movie to illustrate the male-parallel mating behavior in action that renders all composite images sequentially is provided as part of the Supplemenatry information (Supplementary Movie 8).
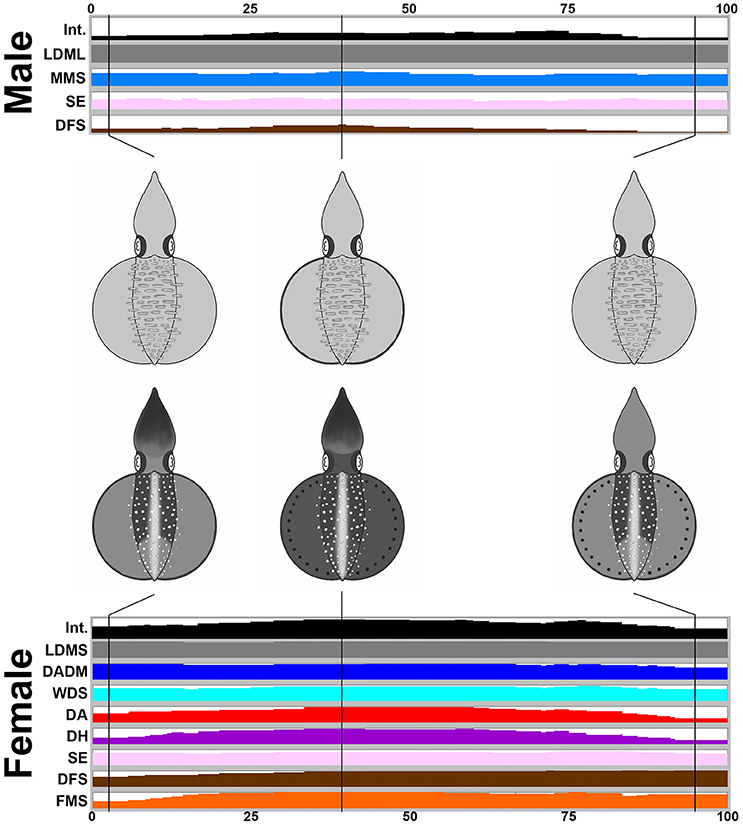
Figure 6. The female shows more chromatic components and a darker body coloration than the male when giving a rejection signal during a male-parallel mating attempt. The time sequence of the average expression level of individual chromatic components recorded for the male and the female oval squids during the male-parallel mating. Time zero on the horizontal axis indicates the start of the mating behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of chromatic components with over 50% occurrence during the recorded behavior for the male (top) and the female (bottom). The intensity (Int) on the first line represents the average strength of the uniform body coloration during the male-parallel mating behavior. LDML, light dorsal mantle lines; MMS, mantle margin stripe; SE, shaded eyes; DFS, dark fin stripe; LDMS, light dorsal mantle spots; DADM, dark anterior dorsal mantle; WDS; white dorsal stripe; DA, dark arms; DH, dark head; FMS, fin margin spots. See the Supplementary Movie 8 for this behavior.
By way of contrast, during male-upturn mating behavior (n = 10), the male squid showed more components and a darker body tone when it attempted to mate with the female using the male-upturn posture (Figure 7). Interestingly, when the male successfully mated with the female (all cases in the recorded behavior), its skin color became lighter and displayed a prominent ES (eye spots) component; this contrasted with the female where only darker body patterns were present during the final stage of the mating process. Specifically, the male showed 10 components, LDML, DDS, MMS, DTA, DAS, DT, DH, SE, DFM, and ES, while the female only showed four components, LDMS, MM, DFS, and FMS. Except for LDML and LDMS, which are sex specific components, the male displayed a subset of components that are completely different to those of the female. This suggests that the male may signal to the female using a distinct body pattern during a male-upturn mating attempt and in turn the female seems to respond to the male with a unique body pattern. To illustare the dynamics of body patetrning during this behavior, another movie that renders all composite images sequentially is provided in the Supplemenatry information (Supplementary Movie 9).
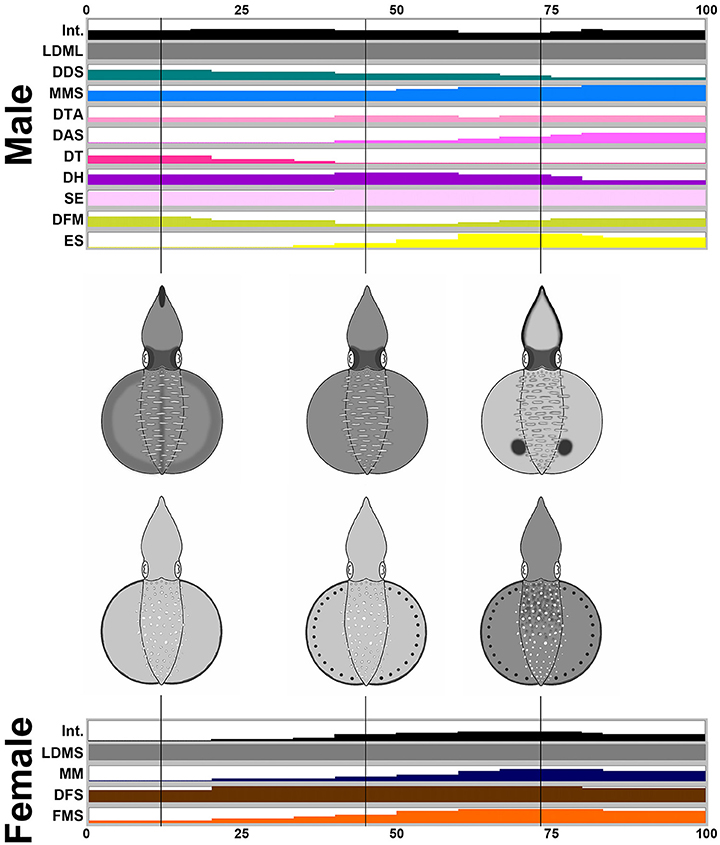
Figure 7. The male shows more chromatic components and greater dynamic body coloration than the female as an attempting signal during male-upturn mating. Time sequence of the average expression level of the individual chromatic components recorded from the male and the female oval squids during male-upturn mating. Time zero on the horizontal axis indicates the start of the mating behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of the chromatic components with over 50% occurrence during the recorded behavior for the male (top) and the female (bottom). The intensity (Int) on the first line represents the average strength of the uniform body coloration during the male-upturn mating behavior. LDML, light dorsal mantle lines; DDS, dark dorsal stripe; MMS, mantle margin stripe; DTA, dark third arms; DAS, dark arm stripe; DT, dark tentacle; DH, dark head; SE, shaded eyes; DFM, dark fin margin; ES, eye spots; LDMS, light dorsal mantle spots; MM, mottled mantle; DFS, dark fin stripe; FMS, fin margin spots. See the Supplementary Movie 9 for this behavior.
Individual Components Are Involved in Multiple Behaviors and Are Differentially Co-expressing with Other Components
To systematicaly examine the involvement of these individual components in the various different behaviors, we have analyzed the occurrence frequencies of all 27 components observed during the five behaviors in terms of different contexts (male and female) or different outcomes (winner and loser). Except during male guarding behavior, which involves only a single individual, the other four behaviors inevitably involve interactions between two individuals. Thus, there are 9 behavioral modes in total. It is evident from this analysis that each behavior is composed of multiple chromatic components and each component is often involved in multiple behaviors (Figure 8). For example, FMS appeared frequently (occurring frequency above 50%) during male guarding, male-male fighting (both winner and loser), male-parallel mating (only female), and male-upturn mating (only female). By way of contrast, ES was only observed with male squids during male-upturn mating behavior. This suggests that, while some components are expressed more often than others and during many types of behavior, nevertheless, each behavior consists of a unique combination of different components.
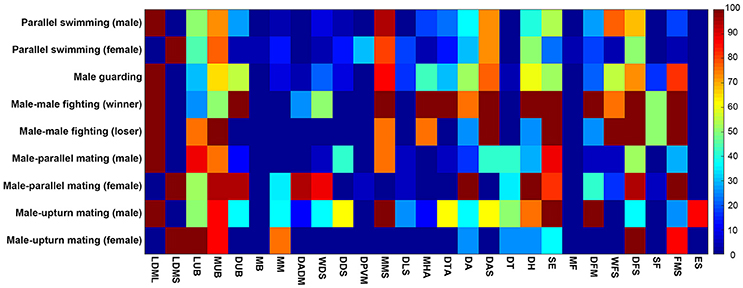
Figure 8. Each behavior is composed of multiple chromatic components and each component is often involved in multiple behaviors. The occurrence frequencies of the 27 chromatic components observed during the 9 modes of the five behaviors of different context (male and female) and with different outcome (winner and loser) are shown and color coded in a matrix. It is apparent that some components are expressed more often than others during many of the behaviors, but each behavior consists of a unique combination of different components.
To further examine whether certain component combination are more common during one behavior than another, the co-expression frequency of various components was arranged in the descending order (Figure 9). Note that five components were excluded in this analysis as they are present either in all behaviors of one sex (LDML and LDMS) or mutually exclusive in all behaviors (LUB, MUB, and DUB). It is apparent that single component and two to three component combinations are dominant in all behaviors. Nevertheless, four to five component combinations can be observed more often during some behaviors than others. Most importantly, the descending rate of these co-expression frequencies is much slower for male-male fighting (winner) and for male-parallel mating (female) than for other behavioral modes. This suggests that more chromatic components are required to express strong visual signals during these behavioral contexts, such as the “dominant” signal for the winner squid and the “rejection” signal by the rejecting female. In contrast, the descending rate of these co-expression frequencies was much steeper for male-parallel mating (male) and male-upturn mating (female) than for other behavioral modes. This suggests that as few as one to two components are sufficient to express the visual signals required during these behavioral contexts, perhaps because these body patterns are less important to determining the behavioral outcome.
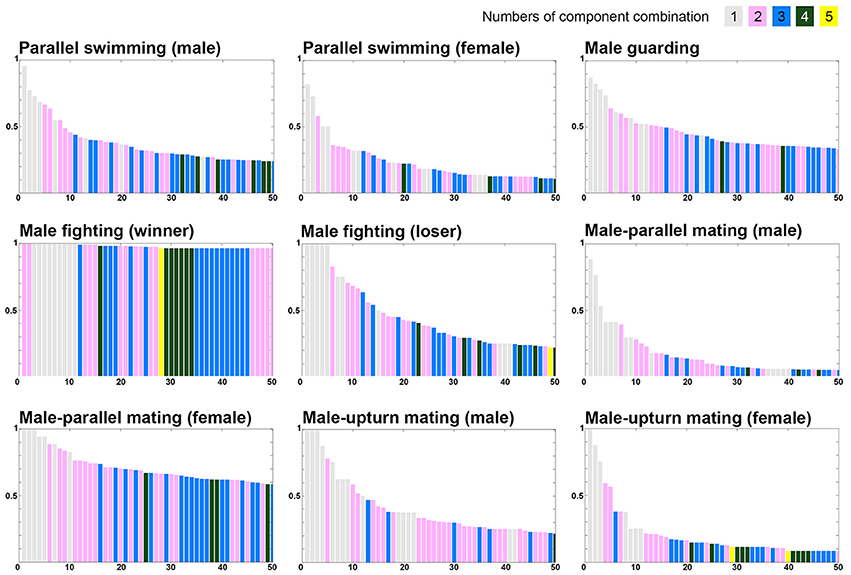
Figure 9. The different numbers and combinations of chromatic components that constitute the unique behaviors. The various combinations of chromatic components were ranked based on the occurrence percentage observed during each behavior and this reveals that combinations of as many as four to five components is common during some behavioral modes. Note the display presented in each panel is in a descending order of component combinations when the occurrence percentages are equal. Five components were excluded in this analysis as they are present either in all behaviors of one sex (LDML and LDMS) or mutually exclusive in all behaviors (LUB, MUB, and DUB).
Since each component is often involved in multiple behaviors (Figure 8) and each behavior includes various combinations of different components (Figure 9), it is clearly impossible for squids to distinguish one behavior from the others just based on the expression of only one “key” component, except for ES during male-upturn mating (male). To characterize the minimum set of components needed for a given behavior that is distinct from all other behaviors, the co-occurrence frequency of any component with other components was used to assess the ease of discrimination of the behaviors (Figure 10). Note that five components were excluded in this analysis as they are present either in all behaviors of one sex (LDML and LDMS) or mutually exclusive in all behaviors (LUB, MUB, and DUB). In other words, one can simply ask the following: is the co-expression of two components better than the expression of either of these components alone for distinguishing the different behaviors? If the answer is positive, then one can ask the following: is the co-expression of three components better than expression of any combination of two components for distinguishing the different behaviors? In principle, by reiterating the same step several times, one should be able to find the minimum set of key components that is unique for each behavioral mode. For example, the FMS appeared frequently (occurring frequency above 50%) during male guarding, male-male fighting (both winner and loser), male-parallel mating (female), and male-upturn mating (female). However, when FMS is combined with DFM, MHA, or DTA, the 50% co-occurrence criterion resulted in a unique behavioral mode, the male-male fighting (winner). Similarly, when the FMS is combined with the WFS, the 50% co-occurrence level resulted in another unique behavioral mode, the male-male fighting (loser). On the other hand, when FMS was combined with DH and SE, the co-expression of this pair of component specified both male-male fighting (winner) and male-parallel mating (female). In contrast, when FMS was combined with DFS, all five possible behavioral modes had co-occurring frequencies above or equal 50%. This indicates that co-expression of three or more components is necessary to distinguish some behaviors. Although only one example is shown here, this approach represents an effective way of deducing the minimum set of key components for any given behavior that is distinct from all other behaviors.
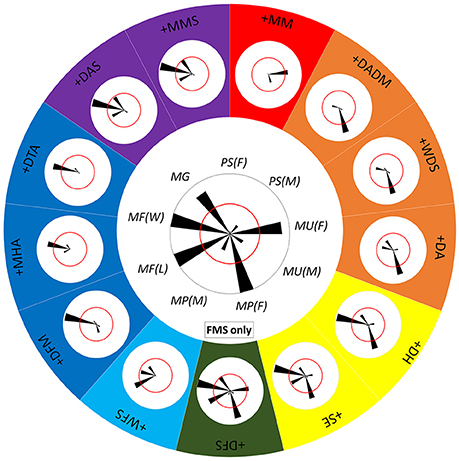
Figure 10. Distinct combinations of two chromatic components constitute different behaviors. The central polar plot shows the occurrence frequency of FMS (fin margin spots) in each of the 9 behavioral modes. The length of each wedge represents the occurrence frequency and the angle of the wedge depicts each behavioral mode. The red circle indicates 50% occurrence during the recorded behavior. When the FMS is combined with a second component, this combination leads to either one or more separate behavioral modes. The polar plots around the large circle show the co-occurrence of FMS and each of the other 13 components. Different colors represent the different individual or mixed behavioral modes. For example, when the FMS is combined with the WFS (white fin stripe), the 50% co-occurrence level results in a unique behavioral mode, male-male fighting (loser), or MF(L). Note that five components were excluded in this analysis as they are present either in all behaviors of one sex (LDML and LDMS) or mutually exclusive in all behaviors (LUB, MUB, and DUB).
Discussion
Analysis of the Temporal Structure of the Squid's Ethogram Reveals the Presence of Key Visual Signals during Different Behavioral Contexts
In addition to classifying unique behaviors, some ethograms also indicate the frequency of their occurrence and the probability that one behavior follows another. In the present study, we analyzed the dynamic structure of this squid's ethogram as a temporal sequence of various combinations of distinct chromatic components across different behavioral modes (Figure 3). Previous research has documented the frequency, prevalence, and duration of body pattern components during reproductive behaviors of a number of different squid species (Hanlon et al., 1994, 1999; DiMarco and Hanlon, 1997; Jantzen and Havenhand, 2003a,b). However, there has been no systematic analysis of these multi-dimensional time-series datasets beyond simple descriptive statistics. By expressing the occurrence frequencies of the 27 chromatic components observed across the 9 modes of five behaviors of S. lessoniana in relation to different contexts (male and female) and outcomes (winner and loser) as a matrix, we have demonstrated that each behavior is composed of multiple chromatic components and that each component is often involved in multiple behaviors (Figure 8). Although some components are expressed more often than others during many types of behavior, each behavior has a unique combination of different components. This observation suggests that combining different components within the body patterning is an effective way of expressing distinct visual signals in different behavioral contexts (Figure 10). However, it should be noted that some components may make the squids more conspicuous than other components, or have other functions in different scenarios. For example, the ES (eye spots) is prominent in the males after they successfully mated with the females using the male-upturn posture (Figure 7). The ES is also known in other cephalopod species as a deimatic display (Langridge et al., 2007; Wood et al., 2008). This suggests that the ES has multiple functions in body patterning. Similarly, the DUB (dark uniform body) and DFM (dark fin margin) are likely involved in deimatic behaviors. Furthermore, the MB (mantle bands) and other components may also function in camouflage behaviors (Staudinger et al., 2011). Nevertheless, these observations emphasize that individual components in various combinations have distinct functions in different behavioral contexts.
By using the co-occurrence frequency to quantify the relationships between the expression events for the various components, we were able to show that different numbers and compositions of chromatic components are associated with a given behavior (Figure 9). Compared with the analysis of the duration of different body component combinations during the reproductive behavior of the squid S. australis (Jantzen and Havenhand, 2003a), the co-occurrence frequency used in the present study emphasizes those components that are short in duration but are expressed in most of the cases. Furthermore, by ranking the various combinations of components based on their co-occurrence frequency for each behavior, we were able to demonstrate that some behavioral modes involve many more combinations of components than others (Figure 9). This suggests that, when an individual squid is actively sending signals to another squid, the body pattern tends to recruit more components, whereas when the animal is passively reacting to another squid, the body pattern is composed of fewer components.
Visual Signals Determine the Behavioral Outcome during Squid's Reproductive Interaction
Squids are polyandrous, in which a female mates with multiple males during a spawning season (Jantzen and Havenhand, 2003b; Iwata et al., 2005; Wada et al., 2005). For S. lessoniana, alternative male mating behaviors have been found to depend on the relative body size of the squid. In a captive study, it has been reported that larger males guard their partners from other males and perform male-parallel mating during the egg-laying period of the paired females, whereas smaller subordinate males used male-upturn mating as an alternative tactic outside the female egg-laying period (Wada et al., 2005). These results suggest that males of different sizes may adopt different strategies in order to gain access to females, and females may choose different males in order to maximize their reproduction success. In addition, it has been observed that male squids showed agonistic behavior and fight for the possession of the female during reproductive interactions (Moynihan and Rodaniche, 1982; Jantzen and Havenhand, 2003b). Cephalopods are highly visual animals and they communicate through dynamic body patterning, thus it is likely that visual communication between males and females during courtship behaviors, as well as between different males during agonistic behavior, may determine their mating strategies (Hanlon et al., 1997, 2002, 2005; Jantzen and Havenhand, 2003a,b; Naud et al., 2004, 2016; Iwata et al., 2005; Wada et al., 2005; Huffard et al., 2008; Buresch et al., 2009; Scheel et al., 2016).
In the present study, the reproductive behaviors of S. lessoniana were categorized into five distinct categories, namely parallel swimming, male guarding, male-male fighting, male-parallel mating, and male-upturn mating. It was found that male squids compete for the upper position to dominate the fight during male-male fighting behavior and that the squid in the upper position show more chromatic components and a darker body coloration (Figure 5). It should be noted that the winner and the loser of the male-male fighting were assigned by their positions relative to each other in the present study. It may seem that the fight is predetermined and the body patterns shown by the squids during the fight are unimportant. However, this is not always the case, because the squids may switch the positions during a fight, so that the winner may become the loser and the loser may become the winner (see the Supplementary Movie 6 for such a switch). In this situation, the body patterns maintained by the squids throughout the fight may be considered as a visual signal to declare the winning status and keep the loser from fighting back. Thus, the winner and loser body patterns shown in Figure 5 have a predictive function of their eventual fighting outcomes. Furthermore, female squids display specific body patterns as the rejection signal to the male squids during the male-parallel mating attempt (Figure 6). Similarly, male squids also express unique body patterns before they use the male-upturn mating strategy to try to sneak the female squids (Figure 7). These observations support the hypothesis that distinct body patterns are effective visual signals and determine the behavioral outcomes during squid reproductive interactions.
Similar evidence has been found for other species. For example, it has been reported that the squid Loligo plei display specific body patterns in order to threaten their opponents during male-male fighting behavior (DiMarco and Hanlon, 1997). It has also been found that a dominant octopus shows dark body patterns in order to threaten their subordinates (Scheel et al., 2016). In addition, it has been demonstrated that female mature squids show bright stripes on the dorsal mantle, similar to the bright white testis of adult males, which is indicative of female choice (avoidance of mating) during courtship behavior (DeMartini et al., 2013). All these findings suggest that the dynamic expression of chromatic components by cephalopods facilitates visual communication between these animals and helps determine their behavioral outcomes.
Dynamic Body Patterning of Squids Functions as a Form of Animal Communication
By characterizing the minimum set of chromatic components that forms one behavior that is distinct from all other behaviors using the component co-occurrence frequency analysis, we were able to systematically examine the interrelationship between the individual components that are involved in different behaviors (Figure 10). This type of analysis is akin to the linguistics analysis of human language, particularly grammar, which is a system of rules that governs the form of the utterances within a given language. In addition to phonology (how sounds or gestures function together), this also includes morphology (the formation and composition of words), and syntax (the formation and composition of phrases and sentences from words) (Akmajian et al., 2010). The findings from our analysis that each component is often involved in multiple behaviors (Figure 8) and each behavior includes various combinations of different components (Figure 9) seems to suggest to us that each component is like a word (phonographic languages like English) or a character (ideographic languages like Chinese) in a sentence and when multiple words/characters are combined together in a right order this forms an unique sentence (syntax) that conveys an unequivocal message. Although the component co-occurrence frequency in the present study does not emphasize the temporal order of component expression, which is important to the syntax of language, the analysis of the temporal structure of the ethogram suggests that the time sequence of various component expression is critical to the sending of clear signals to the receivers and to determining the behavioral outcomes during reproductive interactions (Figures 5–7). In animal communication, bird vocalization (or birdsong) is a well-known example of communicating information to others using an auditory signal. Birdsongs are usually longer and more complex than simple calls, and they are associated with courtship and mating behaviors (Marcus, 2006; Pepperberg, 2009). There are some parallels between birdsong and human speech and language, such as both have recursion and core structural properties (Bolhuis and Everaert, 2013). However, the dynamic body patterns used by S. lessoniana to communicate information to others, because they form a visual signal, may not be as complex as birdsongs and are certainly much simpler than human language. In addition, active signaling during communication requires interpreting the signal and making the correspondent response, but it was difficult to determine in our analyses. While the visual systems of cephalopods have been extensively studied (Messenger, 1991), particularly the visual perception mechanisms that regulate rapid adaptive camouflage (Chiao et al., 2015), how the squids perceive body patterns of the other squids and choose appropriate body patterns in response to the visual signals is largely unknown. Thus, the analogy of squid body pattern as a form of language may be speculative here. Nevertheless, the present study demonstrates that dynamic body pattering, by expressing unique sets of key components acutely, is an efficient way of communicating by S. lessoniana in a behavioral context.
Ethics Statement
The study was using oval squids, a group of invertebrates, which is exempt from the Institutional Animal Care and Use Committee in Taiwan.
Author Contributions
CL conceived, designed, carried out the work, and drafted the manuscript. YT analyzed data and helped draft the manuscript. CC helped plan experiments, interpreted data, and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Drs. Hong Young Yan and Cho-Fat Hui at the Marine Research Station, Academia Sinica for the support and providing the experimental tanks. We also thank Mr. Chih-Chiang Lee for the assistance with the care of animals. In addition, we thank the dive master Mr. Dao-Ren Guo, the film director Mr. Chin-Yuan Ke, and the Public Television Service (PTS) of Taiwan for providing us with the invaluable video footage of S. lessoniana at their spawning ground. In addition, we appreciate Dr. Wen-Sung Chung for discussing the body patterns of squids with us. This study was supported by the Ministry of Science and Technology of Taiwan NSC-102-2628-B-007-001-MY3 (to CC).
Supplementary Material
Supplementary Movie 1. Parallel swimming. The male keeps a close distance from the female and swims together with her. This behavior is usually seen when a large male mates with the female and form a mating pair. https://youtu.be/VPy0dtYNOls.
Supplementary Movie 2. Male guarding. The male of a mating pair guards the female while she is laying eggs and prevents the female from being mated by other males. https://youtu.be/V1_9OKvoBUI.
Supplementary Movie 3. Male-male fighting. Two males of a similar size show an agonistic behavior to compete for mating with the female. The fighting usually lasts for a few seconds, and the winner and loser may swap position several times during the fight. At the end of the fight, the winner takes the upper position, while the loser is down below. This footage was recorded by Mr. Dao-Ren Guo for the Public Television Service of Taiwan. https://youtu.be/RaGBgrskHiE.
Supplementary Movie 4. Male-parallel mating. The male swims below the female and attempts to place spermatophores within the female oviduct opening using a male-parallel mating posture. The male is rejected by the female in this movie. https://youtu.be/giUk9pLl5Vg.
Supplementary Movie 5. Male-upturn mating. The male swims on top of the female and attempts to attach spermatophores to the female buccal membrane using a male-upturned mating posture. https://youtu.be/LoUXfat54JA.
Supplementary Movie 6. Body pattern switching of two males during male-male fighting. This movie shows dynamic body patterning of two male oval squids Sepioteuthis lessoniana during an agonistic fight. The video on the mid-right is the original footage of this fighting behavior, which is played at 0.5X speed. The time sequence of the occurrence of individual chromatic components recorded from these two males (M1 and M2) are shown at the top and bottom screens. The body pattern diagrams on the mid-left illustrate the overlay of chromatic components at any moment in time during fighting for M1 and M2 squids. The movie is rendered by playing back all composite images along the time axis at 1 sec interval. The male squids compete for the upper position to dominate the fight during the interaction. Thus, “Up” indicates the winner and “Down” indicates the loser. Note that there is a switch of the winner and the loser during this agonistic fighting (M1: winner first; M2: loser first). The intensity (Int) on the first line represents the three levels of the uniform body coloration (light, medium, and dark) during fighting behavior. LDML, light dorsal mantle lines; MMS, mantle margin stripe; MHA, mottled head and arms; DTA, dark third arms; DAS, dark arm stripe; DT, dark tentacles; DH, dark head; SE, shaded eyes; DFM, dark fin margin; WFS, white fin stripe; DFS, dark fin stripe; SF, stitchwork fin; FMS, fin margin spots; DADM, dark anterior dorsal mantle; WDS, white dorsal stripe. The position on the last line represents the two positions of squids (up and down) during the fight. https://youtu.be/CbG45visegM.
Supplementary Movie 7. Dynamic body patterning during male-male fighting. The time sequence of the average expression level of individual chromatic components recorded from the winner and the loser oval squids Sepioteuthis lessoniana during male-male fighting. Time zero on the horizontal axis indicates the start of the fighting behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of chromatic components with over 50% occurrence during the recorded behavior for the winner (top) and the loser (bottom). The movie is rendered by playing back all composite images along the time axis for every other time point (50 frames in total). It is apparent that the winner shows more chromatic components and a darker body coloration than the loser during fighting behavior. The intensity (Int) on the first line represents the average strength of the uniform body coloration during fighting behavior. LDML, light dorsal mantle lines; WDS, white dorsal stripe; MMS, mantle margin stripe; MHA, mottled head and arms; DTA, dark third arms; DA, dark arms; DAS, dark arm stripe; DH, dark head; SE, shaded eyes; DFM, dark fin margin; WFS, white fin stripe; DFS, dark fin stripe; SF, stitchwork fin; FMS, fin margin spots. https://youtu.be/d4eMYj8pcoM.
Supplementary Movie 8. Dynamic body patterning during male-parallel mating. The time sequence of the average expression level of individual chromatic components recorded for the male and the female oval squids Sepioteuthis lessoniana during the male-parallel mating. Time zero on the horizontal axis indicates the start of the mating behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of chromatic components with over 50% occurrence during the recorded behavior for the male (top) and the female (bottom). The movie is rendered by playing back all composite images along the time axis for every other time point (50 frames in total). It is apparent that the female shows more chromatic components and a darker body coloration than the male when giving a rejection signal during a male-parallel mating attempt. The intensity (Int) on the first line represents the average strength of the uniform body coloration during the male-parallel mating behavior. LDML, light dorsal mantle lines; MMS, mantle margin stripe; SE, shaded eyes; DFS, dark fin stripe; LDMS, light dorsal mantle spots; DADM, dark anterior dorsal mantle; WDS; white dorsal stripe; DA, dark arms; DH, dark head; FMS, fin margin spots. https://youtu.be/T1b9UDggezs.
Supplementary Movie 9. Dynamic body patterning during male-upturn mating. The time sequence of the average expression level of the individual chromatic components recorded from the male and the female oval squids Sepioteuthis lessoniana during male-upturn mating. Time zero on the horizontal axis indicates the start of the mating behavior, and time 100 indicates the end of the behavior. The body pattern diagrams in the middle illustrate the overlay of the chromatic components with over 50% occurrence during the recorded behavior for the male (top) and the female (bottom). The movie is rendered by playing back all composite images along the time axis for every other time point (50 frames in total). It is apparent that the male shows more chromatic components and greater dynamic body coloration than the female as an attempting signal during male-upturn mating. The intensity (Int) on the first line represents the average strength of the uniform body coloration during the male-upturn mating behavior. LDML, light dorsal mantle lines; DDS, dark dorsal stripe; MMS, mantle margin stripe; DTA, dark third arms; DAS, dark arm stripe; DT, dark tentacle; DH, dark head; SE, shaded eyes; DFM, dark fin margin; ES, eye spots; LDMS, light dorsal mantle spots; MM, mottled mantle; DFS, dark fin stripe; FMS, fin margin spots. https://youtu.be/yYEGquJtkxU.
References
Akmajian, A., Demers, R. A., Farmer, A. K., and Harnish, R. M. (2010). Linguistics: An Introduction to Language and Communication. Cambridge, MA: MIT Press.
Bolhuis, J. J., and Everaert, M. (2013). Birdsong, Speech, and Language: Exploring the Evolution of Mind and Brain. Cambridge, MA: MIT press.
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of Animal Communication. Sunderland, MA: Sinauer Associates.
Buresch, K. C., Maxwell, M. R., Cox, M. R., and Hanlon, R. T. (2009). Temporal dynamics of mating and paternity in the squid Loligo pealeii. Mar. Ecol. Prog. Ser. 387, 197–203. doi: 10.3354/meps08052
Byrne, R. A., Griebel, U., Wood, J. B., and Mather, J. A. (2003). “Squid say it with skin: a graphic model for skin displays in Caribbean reef squid (Sepioteuthis sepioidae),” in Coleoid Cephalopods through Time, Vol. 3, eds K. Warnke, H. Keupp, and S. Boletzky (Berlin: Berliner Paläobiol Abh), 29–35.
Caldwell, R. L., Ross, R., Rodaniche, A., and Huffard, C. L. (2015). Behavior and body patterns of the larger pacific striped octopus. PLoS ONE 10:e0134152. doi: 10.1371/journal.pone.0134152
Chiao, C. C., Chubb, C., and Hanlon, R. T. (2015). A review of visual perception mechanisms that regulate rapid adaptive camouflage in cuttlefish. J. Comp. Physiol. Neuroethol. Sens. Neural Behav. Physiol. 201, 933–945. doi: 10.1007/s00359-015-0988-5
DeMartini, D. G., Ghoshal, A., Pandolfi, E., Weaver, A. T., Baum, M., and Morse, D. E. (2013). Dynamic biophotonics: female squid exhibit sexually dimorphic tunable leucophores and iridocytes. J. Exp. Biol. 216, 3733–3741. doi: 10.1242/jeb.090415
DiMarco, F. P., and Hanlon, R. T. (1997). Agonistic behavior in the squid Loligo paelei (Loliginidae, Teuthoidea): fighting tactics and the effects of size and resource value. Ethology 103, 89–108. doi: 10.1111/j.1439-0310.1997.tb00010.x
Hanlon, R. T. (1982). The functional organization of chromatophores and iridescent cells in the body patterning of Loligo plei (Cephalopoda, Myopsida). Malacologia 23, 89–119.
Hanlon, R. T. (1988). Behavioral and body patterning characters useful in taxonomy and field identification of cephalopods. Malacologia 29, 247–265.
Hanlon, R. T., and Messenger, J. B. (1996). Cephalopod Behaviour. Cambridge, UK: Cambridge University Press.
Hanlon, R. T., Maxwell, M. R., and Shashar, N. (1997). Behavioral dynamics that would lead to multiple paternity within egg capsules of the squid Loligo pealei. Biol. Bull. 193, 212–214. doi: 10.1086/BBLv193n2p212
Hanlon, R. T., Maxwell, M. R., Shashar, N., Loew, E. R., and Boyle, K. L. (1999). An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 197, 49–62. doi: 10.2307/1542996
Hanlon, R. T., Naud, M. J., Shaw, P. W., and Havenhand, J. N. (2005). Behavioural ecology: transient sexual mimicry leads to fertilization. Nature 433, 212. doi: 10.1038/433212a
Hanlon, R. T., Smale, M. J., and Sauer, W. H. H. (1994). An ethogram of body patterning behavior in the squid Loligo vulgaris reynaudii on spawning grounds in South Africa. Biol. Bull. 187, 363–372. doi: 10.2307/1542293
Hanlon, R. T., Smale, M. J., and Sauer, W. H. H. (2002). The mating system of the squid Loligo vulgaris reynaudii (Cephalopoda, Mollusca) off South Africa: fighting, guarding, sneaking, mating and egg laying behavior. Bull. Mar. Sci. 71, 331–345.
Huffard, C. L., Caldwell, R. L., and Boneka, F. (2008). Mating behavior of Abdopus aculeatus (d'Orbigny 1834) (Cephalopoda: Octopodidae) in the wild. Mar. Biol. 154, 353–362. doi: 10.1007/s00227-008-0930-2
Iwata, Y., and Sakurai, Y. (2007). Threshold dimorphism in ejaculate characteristics in the squid Loligo bleekeri. Mar. Ecol. Prog. Ser. 345, 141–146. doi: 10.3354/meps06971
Iwata, Y., Munehara, H., and Sakurai, Y. (2005). Dependence of paternity rates on alternative reproductive behaviors in the squid Loligo bleekeri. Mar. Ecol. Prog. Ser. 298, 219–228. doi: 10.3354/meps298219
Jantzen, T. M., and Havenhand, J. N. (2003a). Reproductive behavior in the squid Sepioteuthis australis from South Australia: ethogram of reproductive body patterns. Biol. Bull. 204, 290–304. doi: 10.2307/1543600
Jantzen, T. M., and Havenhand, J. N. (2003b). Reproductive behavior in the squid Sepioteuthis australis from South Australia: interactions on the spawning grounds. Biol. Bull. 204, 305–317. doi: 10.2307/1543601
Jereb, P., and Roper, C. F. E. (2005). Cephalopods of the World: an Annotated and Illustrated Catalogue of Cephalopod Species Known to Date. Rome: Food and Agriculture Organization of the United Nations.
Langridge, K. V., Broom, M., and Osorio, D. (2007). Selective signalling by cuttlefish to predators. Curr. Biol. 17, R1044–R1045. doi: 10.1016/j.cub.2007.10.028
Manning, A., and Dawkins, M. S. (2012). An Introduction to Animal Behaviour. Cambridge, UK: Cambridge University Press.
Martin, P., Bateson, P. P. G., and Bateson, P. (1993). Measuring Behaviour: An Introductory Guide. Cambridge, UK: Cambridge University Press.
Mäthger, L. M., Denton, E. J., Marshall, N. J., and Hanlon, R. T. (2009). Mechanisms and behavioural functions of structural coloration in cephalopods. J. R. Soc. Interface 6, S149–S163. doi: 10.1098/rsif.2008.0366.focus
Messenger, J. B. (1991). “Photoreception and vision in molluscs,” in Vision and Visual Dysfunction. Vol. 2: Evolution of the Eye and Visual System, eds J. R. Cronly-Dillon and R. L. Gregory (London: Macmillan Press), 364–397.
Messenger, J. B. (2001). Cephalopod chromatophores: neurobiology and natural history. Biol. Rev. 76, 473–528. doi: 10.1017/S1464793101005772
Moynihan, M., and Rodaniche, A. F. (1982). The behavior and natural history of the caribbean reef squid Sepioteuthis sepioidea with a consideration of social, signal, and defensive patterns for difficult and dangerous environments. Fortschr. Verhaltensforsch. 25, 9–150.
Naud, M. J., Hanlon, R. T., Hall, K. C., Shaw, P. W., and Havenhand, J. N. (2004). Behavioural and genetic assessment of reproductive success in a spawning aggregation of the Australian giant cuttlefish, Sepia apama. Anim. Behav. 67, 1043–1050. doi: 10.1016/j.anbehav.2003.10.005
Naud, M. J., Sauer, W. H., McKeown, N. J., and Shaw, P. W. (2016). Multiple mating, paternity and complex fertilisation patterns in the chokka squid Loligo reynaudi. PLoS ONE 11:e0146995. doi: 10.1371/journal.pone.0146995
Packard, A. (1982). Morphogenesis of chromatophore patterns in cephalopods: are morphological and physiological ‘units’ the same? Malacologia 23, 193–201.
Packard, A., and Hochberg, F. G. (1977). “Skin patterning in octopus and other genera,” in Symposia of the Zoological Society of London, Vol. 38 (London), 191–231.
Packard, A., and Sanders, G. D. (1971). Body patterns of Octopus vulgaris and maturation of the response to disturbance. Anim. Behav. 19, 780–790. doi: 10.1016/S0003-3472(71)80181-1
Pepperberg, I. M. (2009). The Alex Studies: Cognitive and Communicative Abilities of Grey Parrots. Cambridge, MA: Harvard University Press.
Roper, C. F. E., and Hochberg, F. G. (1988). Behavior and systematics of cephalopods from Lizard Island, Australia, based on color and body patterns. Malacologia 29, 153–194.
Sato, N., Kasugai, T., and Munehara, H. (2014). Female pygmy squid cryptically favour small males and fast copulation as observed by removal of spermatangia. Evol. Biol. 41, 221–228. doi: 10.1007/s11692-013-9261-4
Scheel, D., Godfrey-Smith, P., and Lawrence, M. (2016). Signal use by octopuses in agonistic interactions. Curr. Biol. 26, 377–382. doi: 10.1016/j.cub.2015.12.033
Staudinger, M. D., Hanlon, R. T., and Juanes, F. (2011). Primary and secondary defences of squid to cruising and ambush fish predators: variable tactics and their survival value. Anim. Behav. 81, 585–594. doi: 10.1016/j.anbehav.2010.12.002
Stegmann, U. (2013). Animal Communication Theory: Information and Influence. Cambridge, UK: Cambridge University Press.
Wada, T., Takegaki, T., Mori, T., and Natsukari, Y. (2005). Alternative male mating behaviors dependent on relative body size in captive oval squid Sepioteuthis lessoniana (Cephalopoda, Loliginidae). Zool. Sci. 22, 645–651. doi: 10.2108/zsj.22.645
Witzany, G. (ed.). (2014). “Why biocommunication of animals?,” in Biocommunication of Animals (Dordrecht: Springer Netherlands), 1–6.
Keywords: chromatic component, body pattern, ethogram, mating behavior, visual communication, cephalopods
Citation: Lin C-Y, Tsai Y-C and Chiao C-C (2017) Quantitative Analysis of Dynamic Body Patterning Reveals the Grammar of Visual Signals during the Reproductive Behavior of the Oval Squid Sepioteuthis lessoniana. Front. Ecol. Evol. 5:30. doi: 10.3389/fevo.2017.00030
Received: 24 December 2016; Accepted: 29 March 2017;
Published: 18 April 2017.
Edited by:
Martin Stevens, University of Exeter, UKReviewed by:
Nadav Shashar, Ben-Gurion University of the Negev, Eilat Campus, IsraelJennifer Kelley, University of Western Australia, Australia
Karen Cheney, The University of Queensland, Australia
Copyright © 2017 Lin, Tsai and Chiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan-Chin Chiao, Y2NjaGlhb0BsaWZlLm50aHUuZWR1LnR3
†Yueh-Chun Tsai orcid.org/0000-0003-3069-3322
‡These authors have contributed equally to this work.
 Chun-Yen Lin
Chun-Yen Lin Yueh-Chun Tsai
Yueh-Chun Tsai Chuan-Chin Chiao
Chuan-Chin Chiao