- 1Abteilung für Verhaltensneurobiologie, Max-Planck-Institut für Ornithologie, Seewiesen, Germany
- 2Coucal Project, Chimala, Tanzania
- 3Department of Zoology, University of Dar es Salaam, Dar es Salaam, Tanzania
The period of parental care can be a demanding life-history stage because parents need to find sufficient resources to feed themselves and their offspring. Often, this is reflected by elevated baseline levels of glucocorticoids—hormones that regulate metabolism and energy allocation. During 10 breeding seasons, we studied plasma corticosterone (the major avian glucocorticoid) concentrations as a physiological correlate of parental expenditure in two closely related coucal species with fundamentally different mating systems: the sex-role reversed black coucal (Centropus grillii) with female competition and male-only care and the socially monogamous and biparental white-browed coucal (C. superciliosus). The two species live in the same habitat and share a similar breeding biology. However, female black coucals aggressively defend a territory and produce many eggs for their various male partners, and male black coucals feed their offspring much more frequently and rest less often than female and male white-browed coucals. These differences were reflected in baseline and stress-induced concentrations of corticosterone: male black coucals had higher baseline and stress-induced corticosterone concentrations when they were feeding young than outside a feeding context, and also the concentrations of female black coucals were higher during the main period of breeding when they defended territories and produced multiple clutches. In contrast, baseline and stress-induced concentrations of corticosterone in female and male white-browed coucals did not differ between periods when they were feeding young and periods without dependent offspring. Paradoxically, on an individual basis feeding effort was negatively related to baseline corticosterone in male black coucals and female white-browed coucals. In conclusion, corticosterone concentrations of coucals reflected differences in competition and parental roles and support the notion that a switch from biparental to uniparental care and an increase in mate competition may come at a physiological and energetic cost.
Introduction
Typically, the period of parental care is a demanding life-history stage. During this time, parents need to find enough food to sustain not only themselves but also their offspring. Physiologically, such increasing demands are often reflected by increased levels of baseline glucocorticoids, steroid hormones that play a paramount role in energy metabolism and resource allocation (Harvey et al., 1984; Lea et al., 1992; Landys et al., 2006; Crespi et al., 2013). Glucocorticoids mediate different levels of homeostasis that are required to tune critical internal variables to existing environmental conditions and life-history demands (Landys et al., 2006). These different homeostatic levels can be regarded as different allostatic states, characterized by changes in the level of energy demands (Goymann and Wingfield, 2004; Landys et al., 2006). Typically, baseline corticosterone—the major glucocorticoid of birds and many other vertebrates—is elevated during the parental phase (reviewed by Romero, 2002) reflecting the increased energetic demands (and allostatic state) of parenting. As a consequence, corticosterone has frequently been considered a physiological correlate of parental resource allocation to offspring care (= parental expenditure sensu Smiseth et al., 2012; see e.g., Love et al., 2004; Kern et al., 2005; Angelier et al., 2007; Groscolas et al., 2008; Bonier et al., 2009b; Kitaysky et al., 2010; Ouyang et al., 2011; Crossin et al., 2012; Ouyang et al., 2013a; Villavicencio et al., 2014). If baseline corticosterone concentrations reflect parental expenditure, then individuals with a lower parental expenditure should express lower levels of the hormone than individuals with higher parental expenditure. For example, brood manipulation in tree swallows (Tachycineta bicolor) affected baseline corticosterone concentrations: females with enlarged broods showed a larger increase in baseline corticosterone between the incubation and the nestling phase than females with normal or reduced broods (Bonier et al., 2011, but see Kern et al., 2007, for a study on pied flycatchers where brood manipulation apparently had no effect on baseline corticosterone).
Severe stressors typically trigger a glucocorticoid stress response resulting in elevated levels of stress-induced corticosterone that may induce an emergency life-history stage (Wingfield et al., 1998). This emergency life-history stage redirects an individual's behavior toward immediate survival and terminates non-essential processes such as reproduction. For example, severe weather events during the breeding season can lead to brood abandonment (Wingfield et al., 1998; Krause et al., 2016b). The induction of an emergency life-history stage may be suppressed in bird species breeding in severe environments with short breeding seasons, such as deserts or the Arctic (Wingfield et al., 1992, 1995; Krause et al., 2016a). Suppressing the magnitude of the glucocorticoid stress response may reduce the likelihood of brood abandonment, which may be adaptive in extreme environments because of the very limited opportunities to initiate a second breeding attempt within the same season. Comparative data indicate that the stress responses of Arctic shorebirds are reduced in the sex that provides most parental care, rendering further support for this hypothesis (O'Reilly and Wingfield, 2001). Similar mechanisms may operate in short-lived species that are unlikely to survive until the next breeding season (Wingfield et al., 1995). In more benign environments with longer breeding seasons, and in longer-lived birds, a suppression of the glucocorticoid stress-response during breeding is not expected (Romero and Wingfield, 2016).
Here, we compared baseline and stress-induced concentrations of corticosterone in parenting and non-parenting black coucals (C. grillii) and white-browed coucals (C. superciliosus). Coucals are Old-World non-parasitic cuckoos (Payne, 2005; Erritzoe et al., 2012) and most of them live in socially monogamous pairs in which both partners provide parental care. Typically, female coucals are slightly larger than males and the scarce information that is available on this taxon suggests that males contribute slightly more to incubation and offspring care than females (Andersson, 1995; Maurer, 2008). Black coucals and white-browed coucals have been described as sister clades (Irwin, 1985, but see Sorenson and Payne, 2005 for a different view) and represent the taxon's two extremes with regard to differences in sexual size dimorphism, mating system, and parental care.
The black coucal is exceptional and exhibits the taxon's largest reversed sexual dimorphism (females are ca. 70% larger than males). It operates a classically polyandrous mating system with reversed sex roles: Females sing, defend large territories, and form polyandrous groups with up to five males whom they provide with individual clutches. Male black coucals incubate their respective clutches and raise the young all by themselves (Vernon, 1971; Goymann et al., 2004, 2005, 2015; Geberzahn et al., 2009, 2010). In contrast to the black coucal, the white-browed coucal is the least sexually dimorphic of all 26 coucal species with females being only ca. 13% larger than males, and the one with the greatest similarity in sex roles (Van Someren, 1956; Rowan, 1983; Andersson, 1995; Goymann et al., 2015, 2016). Both partners sing and often duet (Brumm and Goymann, 2017), jointly defend a territory, and form socially monogamous pairs with both partners contributing to offspring care equally (Goymann et al., 2016). At our study site in the Usangu plains, Tanzania, we have the unusual situation that both species co-occur in the same habitat. Here, both species feed on the same prey, and breed during a similar time of the year, often in close proximity to one another. Black coucals are migrants and hence start breeding slightly later than white-browed coucals, which stay in the area year-round (Goymann et al., 2015). Further comparative details on the life-history, breeding biology, and parental behavior of the two species can be found in the studies by Goymann et al. (2015, 2016).
Favorable environmental conditions with a high abundance of food (and a mild climate) have been suggested to play a key-role in enabling a single bird parent to successfully raise offspring and free the other parent to seek additional mating partners (Erckmann, 1983; Andersson, 1995). However, when feeding dependent offspring, individual male black coucals have a four-fold higher feeding rate than individual white-browed coucals and, in between feeding bouts, rest only 1/3rd of the time white-browed coucals do (Goymann et al., 2016). These data suggest a substantial difference in time and energy costs of offspring care between male black coucals and pairs of white-browed coucals. Female black coucals are very active during the whole breeding season: they frequently sing to attract males and negotiate territorial borders with neighboring or newly arriving females, patrol their territories, and chase after males or competing females (Goymann et al., 2004, 2008; Geberzahn et al., 2009, 2010). Further, female black coucals build nests and lay several clutches of typically 4 eggs for their various male partners. Nest predation in black coucals is particularly high during the laying and incubation stage (32%) and because females are very secretive not all nests can be found in time. Thus, a conservative estimate of the number of nests a female produces during one season is eight, but in reality may be much higher (Goymann et al., 2015). Thus, during breeding, the energetic demands of female black coucals are most likely high. In contrast, white-browed coucals typically produce only 2 (or sometimes 3) clutches of typically 4 eggs (nest predation during the egg-stage is only 18% and all nests of a pair can be found, because parents are less secretive about nest-building; Goymann et al., 2015). Thus, the energetic demands for clutch production are likely to be substantially lower in white-browed coucals.
Here, we asked whether the large differences in parental care between the two coucal species are reflected by differences in baseline corticosterone concentrations. We expected baseline corticosterone concentrations to be higher during the phase when coucals feed dependent offspring than during the preceding mating (and incubation) stage. Further, we expected a larger difference between these two stages in male black coucals, because of their higher feeding effort compared to white-browed coucals. For female black coucals we expected baseline corticosterone concentrations to be lower during the pre-breeding period, when song activity is still low and when they have not yet started to lay clutches, than during the breeding period, when they sing frequently, defend their territories, and continuously lay clutches for their various males. On the individual level, we expected that birds with a higher feeding rate should express higher levels of baseline corticosterone. Finally, compared to Arctic birds the breeding season of coucals is long and severe weather events that constitute an energetic challenge for the animals are rare. Thus, in contrast to Arctic or desert birds and in line with predictions for birds living in more benign environments (Romero and Wingfield, 2016), we did not expect a suppression of the corticosterone stress-response in the parental sex.
Materials and Methods
We studied black coucals and white-browed coucals in partially flooded grassland of the Usangu wetland (8°41′S 34°5′E; 1000 m above sea level) in Mbeya region, Tanzania, during the breeding seasons of 2005 (Jan. 23–May 27), 2006 (Jan. 15–Apr. 25), 2008 (Feb. 10–March 13), 2010 (Feb. 20–April 25), 2011 (Feb. 18–June 6), 2012 (Dec. 18–June 18), 2013 (Jan. 25–June 29), 2014 (Jan. 15–June 29), 2015 (Feb. 3–June 27), and 2016 (Feb. 10–June 30). Further details regarding the study site can be found in the work by Goymann et al. (2015, 2016).
Coucals build dome-shaped grass nests that are either well-hidden in dense high grass (black coucal) or in an acacia or other thorny shrub (white-browed coucal). The egg-laying period of migratory black coucals is <3 months, typically between February and April. White-browed coucals are residents and start egg-laying slightly earlier, typically in January, and they continue to lay eggs until April. The modal clutch size and number of nestlings in both species is four. Each year we monitor ca. 20–40 nests per species. Incubation typically lasts 15 days in black coucals and 16 days in white-browed coucals, and their nestlings leave the nest typically after ca. 13 days (black coucals) and 14 days (white-browed coucals). The parents continue to feed the young for 2–3 more weeks after they have left the nest. Further details on the comparative life history and breeding biology of black coucals and white-browed coucals can be found in Goymann et al. (2015, 2016).
All coucals were caught with mist nets. During the nestling feeding phase, coucals were caught while approaching or after leaving the nest, with a mist net placed 5–20 m away from the nest. Immediately upon capture (within 3 min of hitting the net: mean ± sd: 01:50 ± 00:53 min) we took a small blood sample (<50 μl) from the wing vein for corticosterone analysis. 30–35 min after the initial capture we took a second blood sample (<50 μl) for the measurement of stress-induced levels of corticosterone. In between, the birds were measured, banded, and placed in a cotton bird bag. All birds were banded with a numbered aluminum and two colored plastic rings for individual identification. Amongst other biometric parameters, we measured the right tarsus (to the nearest 0.1 mm) and body mass (to the nearest g), and from these measures calculated a scaled body mass index following Peig and Green (2010). The scaling exponent (bsma) and the mean tarsus length necessary for this analysis were calculated from the measurements of all of the coucals that we ever caught and for each species and sex separately (159 female and 118 male black coucals, 48 female, and 79 male white-browed coucals). Most birds were equipped with a Holohil BD-2 radio-transmitter (<2 g; Holohil Systems Ltd., Carp, Ontario, Canada) fitted with a Rappole-Tipton harness (Rappole and Tipton, 1991) to ease individual identification and relocation of individuals (see Goymann et al. (2015) for more details on capture, measurement, and tagging).
To determine parental feeding rates, we conducted focal observations of nests (typically of 60 min duration) using binoculars from a distance of ca. 40–100 m, either sitting on the roof of a car, on top of a ladder, or on a mud bank. We identified the individual coming to the nest and we only considered nest visits as feeding visit, when the arriving bird carried food in its bill. Our final data set included 131 observations (158 h) of 31 black coucal males, 78 observations (101 h) of 19 white-browed coucal females, and 50 observations (51 h) of 14 white-browed coucal males. These data represent the subset of previously published feeding rate observations (Goymann et al., 2016) for which corticosterone concentrations of the feeding parents were available. To avoid a potential influence of capture stress on feeding rates, we did not include feeding observations during the day an individual was captured and sampled. The comparison between corticosterone concentrations and feeding rates assumed that a single point measure of corticosterone was representative for the period during which parental care behavior was recorded. All procedures were approved by the respective governmental authorities of Tanzania, i.e., the Tanzania Wildlife Research Institute (TAWIRI) and the Tanzanian Commission for Science and Technology (COSTECH).
Hormone Analysis
Blood samples were stored in a thermos on ice until we returned from the field each day, when the plasma was separated from cells by centrifugation (Hettich EBA 3S, 10 min, ca. 2,000 rpm) and frozen in liquid nitrogen. Plasma samples were transported from Tanzania to Germany on dry ice and then stored at −80°C until measurement.
Corticosterone samples were extracted and measured by radioimmunoassay (RIA, following Goymann et al., 2006). The mean extraction efficiency for plasma corticosterone was 88.4%. Standard curves and sample concentrations were calculated with Immunofit 3.0 (Beckman Inc. Fullerton, CA), using a four parameter logistic curve fit and corrected for individual recoveries. The lower detection limits of the standard curves was determined as the first value outside the 95% confidence intervals for the zero standard (Bmax) and ranged between 31 and 66 pg/ml. All sample concentrations fell within the detectable range of the assays. Intra-assay coefficients of variation were 5.2% (1.9–12.4%, N = 10), the inter-assay coefficient of variation of all assays was 6.7%.
Statistical Analysis
All statistical analyses were conducted with R, version 3.2.2 (R Core Development Team, Vienna 2015). First, we used a linear mixed model (R package lme4; Bates et al., 2015) comparing baseline corticosterone concentrations of individuals of the two coucal species that were either caught and sampled while they were feeding nestlings or outside a nestling feeding context (mating or incubation stage). Because female black coucals did not feed offspring, data from females could be only included for a non-feeding context, which corresponds to defending a territory, mating, and egg-laying (i.e., behaviors that female black coucals do simultaneously throughout the breeding season). Species, sex, and feeding context as well as the interactions of these variables were included as fixed factors. Because female black coucals only contributed data to a non-nestling feeding context, the resulting model matrix was rank-deficient, i.e., the interaction between sex and feeding context and species and feeding context could only be modeled for male black coucals and male and female white-browed coucals. To control for potential increases of corticosterone between capture and blood sampling we included the time lag between capture and sampling as a control (z-transformed fixed effect covariate). Further, we included the year of sampling as a random effect. Stress-induced corticosterone concentrations were analyzed in a similar way, with species, sex and feeding context, as well as the interactions of these variables as fixed factors, and year of sampling as a random effect. Because it happened only rarely, that two partners were caught in the same breeding stage we assumed that the data of individuals belonging to a pair or polyandrous group were statistically independent.
For female black coucals we conducted additional mixed model analyses in which we compared baseline and stress-induced concentrations of corticosterone between females caught very early during the breeding season (i.e., during the pre-breeding period when they had arrived on the breeding grounds, but did not sing much, and had not begun nesting) with females during the breeding season, when song activity was high and females were providing males with nests and eggs. For the baseline corticosterone data we again included time between capture and sampling as a covariate, and in both analyses we included year as a random effect.
For the subset of individual black coucals and white-browed coucals that were sampled while feeding offspring and for which we had information about individual feeding rates we compared baseline corticosterone concentrations and mean feeding rates using linear models implemented in the R package lme4 (Bates et al., 2015). The analysis was conducted for each species and sex separately. We controlled for the time lag between capture and sampling and the mean brood mass of all observations (as a combined measure for the number and the age of the nestlings in relation to feeding rates), and we also included the scaled body mass index as a measure of adult body condition as a covariate. Prior to testing we checked whether mean brood mass and mean feeding rate were correlated, which was not the case for any of the three tests. All covariates were z-transformed to allow an unbiased estimate of the slope between feeding rates and baseline corticosterone concentrations. Please note that the corticosterone concentrations represent point measures, whereas the mean feeding rate represents an interval measure collected over several days.
We used a Poisson mixed model to estimate whether the number of fledglings was related to baseline corticosterone concentrations. Following the suggestions by Korner-Nievergelt et al. (2015) we controlled for overdispersion by including individual ID as a random factor. In all analyses, we only included baseline corticosterone data from individuals that were sampled within 3 min after capture.
Model residuals were examined using graphical methods (i.e., qq plots of residuals and random effects, fitted values vs. residuals) for homogeneity of variance, violation of normality assumptions, or other departures from model assumptions and model fit (Korner-Nievergelt et al., 2015). For inferences from the models we obtained Bayesian parameter estimates and their 95% credible intervals using bsim of the R package arm (Gelman et al., 2014) with an uninformed prior distribution (Korner-Nievergelt et al., 2015). The Bayesian approach is the only method that allows the drawing of exact inferences while avoiding the difficulties of determining the degrees of freedom in mixed model analyses (Bolker et al., 2009). Further, Bayesian methods refer to the probability of a hypothesis given the data. Biologically meaningful differences between groups can be assessed by comparing the ranges of the 95% credible intervals between groups or a regression slope. This 95% credible interval provides an estimate for the mean with a probability of 0.95. If the credible interval of one group does not overlap with the mean estimate of another group, the groups can be assumed to differ from each other. Similarly, if the 95% credible interval of the slope in a regression does not include zero it can be assumed that there is a meaningful relationship between the continuous predictor and the dependent variable (Korner-Nievergelt et al., 2015). If not indicated otherwise, data are presented as individual data points in combination with posterior means and their respective 95% credible intervals (in text and tables reported within squared brackets). We also provide the posterior probability P(β) of the likelihood that the parameter estimates are larger than zero, with values of P(β) close to zero or one indicating statistically meaningful effects. A P(β) of zero indicates a negative effect with a mean effect size and its 95% credible interval being negative (smaller than zero), and a P(β) of one indicates a positive effect with a mean effect size and its 95% credible interval being positive (larger than zero). Finally, we provide measures of the goodness-of-fit of the models (i.e., how much of the variance they explain) by reporting R2-values for linear models, or the respective marginal and conditional R2-values for mixed models following Nakagawa and Schielzeth (2013). The marginal R2-value represents the variation explained by the fixed effects of a mixed model, whereas the conditional R2-value reflects the combined variation of fixed and random effects.
Results
Baseline Corticosterone Concentrations in Relation to Species and Breeding Stage
Male black coucals with nestlings expressed higher baseline concentrations of corticosterone than males without nestlings (Figure 1A, comparing white and gray bars). During the breeding period female black coucals had higher levels of baseline corticosterone than males with and without nestlings (Figure 1A). Baseline corticosterone concentrations of female and male white-browed coucals did not differ between periods with and without nestlings (Figure 1A).
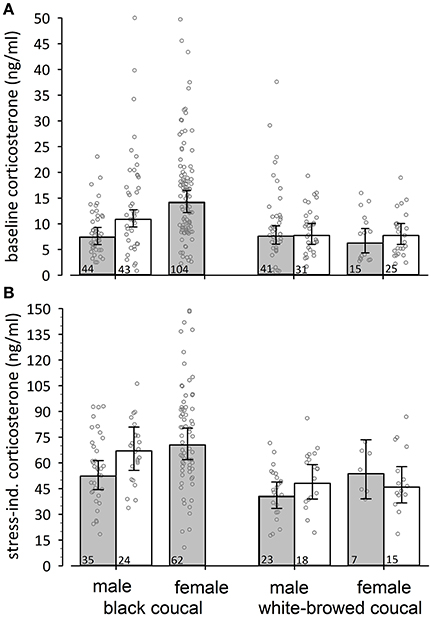
Figure 1. Back-transformed posterior means (± 95% credible intervals) of (A) baseline and (B) stress-induced corticosterone concentrations of black coucals, and white-browed coucals outside (gray bars) and within (white bars) a parental context. If the 95% credible intervals of one group do not overlap with the mean estimate of another group one can assume a meaningful difference. Thus, male black coucals expressed higher baselines in a feeding context than outside a feeding context, whereas the baseline concentrations of female and male white-browed coucals did not differ between contexts. Female black coucals did not provision offspring and thus can only presented outside a feeding context. Their levels were higher than those of conspecific males and higher than those of white-browed coucals. Male black coucals also expressed higher levels of stress-induced corticosterone when they were feeding nestlings than outside a nestling-feeding context. In female and male white-browed coucals, stress-induced corticosterone did not differ between contexts. Breeding female black coucals expressed similar stress-induced concentrations as conspecific males in a nestling-feeding contest. Numbers inside bars refer to sample sizes, and jittered dots represent individual data points.
Differences in baseline corticosterone may be species-specific, and thus a comparison among species may provide limited information regarding differences in allostatic states. With this limitation in mind, we looked at among-species differences in baseline corticosterone concentrations. Baseline corticosterone concentrations were substantially higher in male black coucals with nestlings compared to male and female white browed coucals in the same breeding stage (Figure 1A, comparing white bars and their 95% credible intervals among species). Outside a parenting context, the levels of male black coucals and female and male white-browed coucals were similar (Figure 1A, comparing gray bars and their 95% credible intervals among species). Baseline corticosterone concentrations of female black coucals during the breeding season were higher than those of female and male white-browed coucals, regardless whether white-browed coucals were feeding nestlings or not (Figure 1A; Table 1; marginal R2 = 0.19, conditional R2 = 0.22).
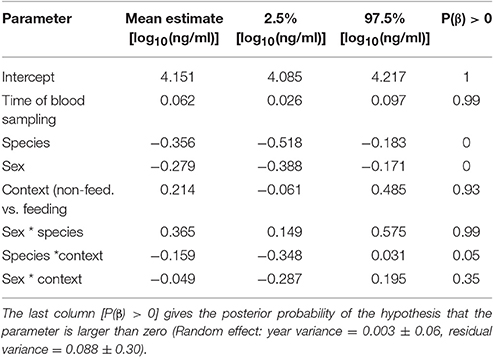
Table 1. Estimates of effect sizes and the 95% credible intervals of the posterior distribution of baseline corticosterone concentrations.
To further explore state-dependent differences in baseline corticosterone concentrations of female black coucals we compared baseline corticosterone concentrations of females during the pre-breeding period (= little singing activity, no egg-laying) with those of females during the breeding period (= high singing activity, egg-laying): female black coucals expressed lower baseline concentrations of corticosterone during the pre- breeding period than during the breeding period (Figure 2; fixed effects: time between capture and sampling: 0.055 [0.002–0.110], P(β) = 0.98; stage: −0.319 [−0.470 to −0.170]. P(β) = 0; random effect: year variance = 0.002 ± 0.05, residual variance = 0.084 ± 0.29; marginal R2 = 0.15, conditional R2 = 0.17). A comparison of the 95% credible intervals of pre-breeding female black coucals (Figure 2) with those of non-parenting male black coucals and male and female white-browed coucals (Figure 1A) shows that during the pre-breeding season, when female black coucals have not yet started to intensely compete for territories and did not yet produce clutches, their baseline corticosterone concentrations are similar to those of male black coucals that do not feed nestlings, and female and male white-browed coucals, regardless of their stage.
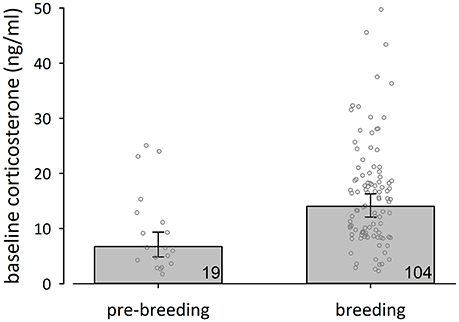
Figure 2. Back-transformed posterior means (± 95% credible intervals) of baseline corticosterone concentrations of female black coucals were lower during the pre-breeding than during the breeding period. Numbers inside bars refer to sample sizes, and jittered dots represent individual data points.
Stress-Induced Corticosterone Concentrations in Relation to Species and Parental Stage
Male black coucals with nestlings expressed higher levels of stress-induced corticosterone concentrations than males sampled before the nestling stage (Figure 1B). Female black coucals had higher stress-induced corticosterone concentrations than non-feeding male black coucals, but their levels were similar to those of males feeding nestlings (Figure 1B). Female and male white-browed coucals expressed similar levels of corticosterone within and outside a nestling-feeding context (Figure 1B).
Among species, stress-induced corticosterone concentrations were higher in male black coucals with nestlings than in male and female white-browed coucals in the same breeding stage (Figure 1B, white bars only). Before the nestling stage, stress-induced concentrations of male black coucals were higher than those of male, but not female white-browed coucals. Female black coucals in the breeding stage expressed higher stress-induced corticosterone concentrations than non-feeding male and female white-browed coucals, and than feeding male white-browed coucals. Sample size for stress-induced corticosterone in feeding female white-browed coucals was limited and showed large variation. This resulted in a large 95% credible interval and a low power to distinguish their levels from those of all other groups (Figure 1B, gray bars only; see Table 2 for overall model parameters; marginal R2 = 0.19, conditional R2 = 0.27).
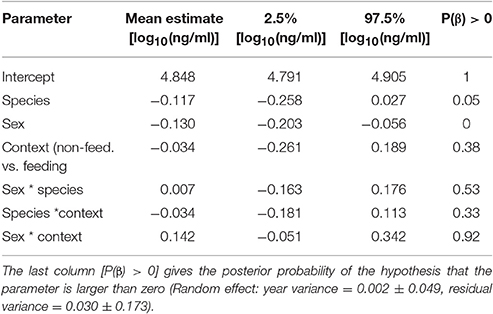
Table 2. Estimates of effect sizes and the 95% credible intervals of the posterior distribution of stress-induced corticosterone concentrations.
Stress-induced corticosterone concentrations of female black coucals did not differ between the pre-breeding (69.2 [58.7–82.0] ng/ml) and the breeding season (70.8 [47.4–104.7] ng/ml). Unlike all previous tests, most of the variance was explained by differences between years (fixed effect: stage: 0.005 [−0.162 to 0.173], P(β) = 0.52; random effect: year variance = 0.004 ± 0.07, residual variance = 0.040 ± 0.200; marginal R2 < 0.001, conditional R2 = 0.10).
Baseline Corticosterone Concentrations in Relation to Feeding Rates and Number of Fledglings
Male black coucals with a higher mean feeding rate expressed lower baseline corticosterone concentrations than males with a lower mean feeding rate (Figure 3; log10 slope: −0.11 [−0.17 to −0.05]; P(β) = 0). Again, baseline corticosterone was positively related to the time delay between capture and blood sampling (log10 slope: 0.13 [0.02–0.24]; P(β) = 0.99), but the mean brood mass (log10 slope: −0.006 [−0.101 to 0.089]; P(β) = 0.44) and the scaled body mass index (log10 slope: −0.002 [−0.013 to 0.010]; P(β) = 0.38) did not explain much of the variation in baseline corticosterone concentrations (N = 29, adjusted R2 = 0.44).
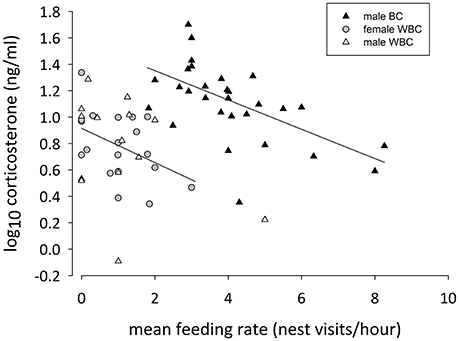
Figure 3. Mean feeding rate and baseline corticosterone concentrations of feeding male black coucals (black triangles), male (white triangles), and female white browed coucals (gray circles). The regression lines refer to male black coucals and female white-browed coucals, only. For male white-browed coucals, there was no relationship between mean feeding rates and baseline corticosterone.
Also in female white-browed coucals, baseline corticosterone concentrations were marginally lower in individuals with a higher mean feeding rate (Figure 3; log10 slope: −0.12 [−0.28 to 0.01]; P(β) = 0.03). In this case the time between capture and sampling (log10 slope: 0.08 [−0.04 to 0.20], P(β) = 0.90) did not strongly influence the result. Also the mean brood mass (log10 slope: 0.002 [−0.135 to 0.014]; P(β) = 0.52) and the scaled body mass index (log10 slope: 0.002 [−0.010 to 0.014]; P(β) = 0.63) did not explain much of the variation (N = 19, adjusted R2 = 0.23).
In male white-browed coucals the credible intervals for the slope of the mean feeding rate clearly included zero (Figure 3; log10 slope: −0.06 [−0.26 to 0.13]; P(β) = 0.25) suggesting no relationship between baseline corticosterone and mean feeding rate in males. Also, the time between capture and sampling did not influence baseline corticosterone in male white-browed coucals (log10 slope: −0.29 [−0.68 to 0.11]; P(β) = 0.07), and the mean brood mass also did not affect the result (log10 slope: 0.09 [−0.19 to 0.37]; P(β) = 0.76). However, the scaled body mass index showed a slight negative relationship with baseline corticosterone (log10 slope: −0.024 [−0.044 to −0.002]; P(β) = 0.02), suggesting that feeding males in better condition expressed slightly lower baseline concentrations of corticosterone (N = 14, adjusted R2 = 0.38). However, with a sample size of only 14 individuals and 4 factors to control for, this result should be considered with caution.
Baseline corticosterone concentrations of parenting male black coucals (N = 29), parenting female white-browed coucals (N = 19), and parenting male white-browed coucals (N = 14) were unrelated to the number of fledglings (male black coucals: number of fledglings = −0.007 [−0.031 to 0.016], P(β) = 0.26; time between sampling and bleeding: slope = 0.16 [−0.10 to 0.43], P(β) = 0.89; marginal and conditional R2 = 0.02; female white-browed coucals: number of fledglings = 0.02 [−0.05 to 0.08], P(β) = 0.68; time between sampling and bleeding: slope = 0.06 [−0.27 to 0.39], P(β) = 0.64; marginal and conditional R2 = 0.01; male white-browed coucals: number of fledglings = 0.01 [−0.05 to 0.08], P(β) = 0.65; time between sampling and bleeding: slope = −0.01 [−0.38 to 0.36], P(β) = 0.46; marginal R2 = 0.004 and conditional R2 = 0.05). Because of the limited sample size the results on white-browed coucals should be considered with caution, though.
Discussion
We compared parental expenditure and basal and stress-induced corticosterone concentrations of two coucal species: the sex-role reversed and uniparental black coucal and the socially monogamous and biparental white-browed coucal. The high mating and egg-laying effort of female black coucals and the large parental investment of male black coucals were reflected in their baseline corticosterone profiles. Baseline corticosterone levels of female black coucals were higher during the breeding period, when territorial activities and egg-laying peaked, than during the pre-breeding period, when they sang little and did not yet provide clutches for their mates. Similarly, baseline (and stress-induced) corticosterone concentrations of parenting male black coucals were higher than those of males that did not feed dependent offspring. In contrast baseline and stress-induced corticosterone levels did not differ between non-parenting and parenting white-browed coucals regardless of sex. Among species, baseline corticosterone concentrations of breeding female black coucals and parenting male black coucals were higher than baseline corticosterone concentrations of parenting white-browed coucals. Unexpectedly, though, baseline corticosterone concentrations were negatively related to feeding rates in male black coucals and female white-browed coucals: individuals with lower feeding rates expressed higher baseline levels of corticosterone than individuals with higher feeding rates.
If baseline corticosterone can be considered a physiological correlate of parental expenditure and reflects allostatic state (Landys et al., 2006), then the corticosterone levels of parenting male black coucals that were about one third higher than those of males without dependent offspring suggest an elevated allostatic state during parenting (Landys et al., 2006). A similar pattern exists for example in starlings (Love et al., 2004). Hence, the rich food resources of the Usangu may allow for uniparental care in male black coucals. But being a single parent may come with an energetic cost, because male black coucals work at least four times harder than individual white-browed coucals, who can exploit similar food resources as a pair (Goymann et al., 2016). In contrast to male black coucals, the similarity of baseline corticosterone in white-browed coucals with and without dependent offspring indicates that they did not enter a higher allostatic state when feeding nestlings. Hence, the corticosterone data support behavioral observations which suggest that parental care does not require substantially higher energetic effort in this species: feeding rates in white-browed coucals are low and in-between their rare feeding visits white-browed coucals typically spend substantially more time perching on acacia bushes or grasses than male black coucals (Goymann et al., 2016).
During the breeding season, female black coucals expressed levels of baseline corticosterone that were even higher than those of parenting males. Female levels likely reflect the high energetic needs related to the effort females put in acquiring mates, fending off competing females, and producing eggs for the various clutches of their male partners (Goymann et al., 2004, 2008, 2015; Geberzahn et al., 2009, 2010). A comparison of females during the pre-breeding period—when they sang little and had not started producing clutches—with the time when breeding activities were in full swing, supports this notion. In summary, behavioral data and baseline corticosterone profiles of female and male black coucals suggest that members of this species have a substantially higher energy expenditure during breeding than white-browed coucals.
Bókony et al. (2009) found in a comparative study that baseline levels of corticosterone during the parental phase were higher in short-lived bird species (that place higher importance on current reproduction) than in longer-lived species with a higher value for future reproductive events. We rarely re-sighted black coucals during more than one breeding season: so far only 3 males and 1 female out of 93 males and 120 females ringed between 2005 and 2015 have been re-sighted during subsequent seasons, and only two of the 253 nestlings ringed during the same period had been recovered in subsequent seasons. This may either indicate low between-year survival or—because black coucals migrate—low site fidelity between years. In contrast, we re-sighted ~30% of adult white-browed coucals that were ringed in the previous season, and regularly find birds that were ringed as nestlings in previous years (Goymann, unpublished data). Hence, between-year survival may be lower in black coucals than in white-browed coucals, but because the species differ in their migratory habits we cannot distinguish between survival and site-fidelity. The higher baseline corticosterone concentrations in black coucals would be consistent with the predictions substantiated by Bókony et al. (2009).
On an individual level, higher levels of baseline corticosterone were—unexpectedly—associated with a lower feeding rate in male black coucals and female white-browed coucals. This result is in contrast to previous findings in other birds, such as common murres (Uria algae), Adelie penguins (Pygoscelis adeliae), Macaroni penguins (Eudyptes chrysolophus), mourning doves (Zenaida macroura), and house sparrows (Passer domesticus), in which individuals that fed at a higher rate also expressed higher levels of baseline corticosterone (Angelier et al., 2008; Doody et al., 2008; Miller et al., 2009; Crossin et al., 2012; Ouyang et al., 2013b). In black redstarts (Phoenicurus ochruros), feeding rates and baseline corticosterone concentrations were unrelated (Villavicencio et al., 2014). Even though the majority of species shows a positive relationship between feeding rate and baseline corticosterone, the pattern does not seem to be consistent. A recent evaluation of the corticosterone fitness hypothesis, which predicts that lower levels of baseline corticosterone is associated with higher fitness, found that roughly 50% of all published studies support the hypothesis, whereas the remainder of published study finds no relationship between corticosterone and fitness, or the opposite pattern (Bonier et al., 2009a). Life-history factors and environmental conditions may influence the relationship between corticosterone concentrations, feeding rates and fitness (Bonier et al., 2009a; Crossin et al., 2016). An inherent problem of comparisons between hormone levels and life-history parameters is the time scale of measurements: hormones typically represent a one point-in-time measure that is then related to a fitness measure which represents an integrated measure over a longer period of time. It is questionable, though, if a one-time hormone measure is representative of the hormonal status of an individual over a longer period of time, which would be relevant for the integrated life-history context.
At first sight, it appears paradoxical that in male black coucals parental care should lead to higher baseline corticosterone concentrations on the population level, whereas on the individual level higher parental expenditure corresponded with lower baseline corticosterone concentrations. However, such paradoxical relationships are not so uncommon and have been, for example, modeled in a famous study by Van Noordwijk and De Jong (1986). On the population level, an elevated baseline concentration during parental care may reflect the higher metabolic or energetic needs of this life-history stage. On the individual level, however, there may be other factors that further influence the already elevated baseline, and these modifying factors could even result in a negative relationship between baseline corticosterone and feeding rates. For example, individuals in poor condition may express higher levels of corticosterone in combination with lower feeding rates. Our measure for condition (i.e., the scaled body mass index) did not predict baseline corticosterone concentrations in male black coucals or female white-browed coucals, but in male white-browed coucals, individuals with a lower condition expressed higher levels of baseline corticosterone. The feeding rate of male white-browed coucals, however, was unrelated to baseline corticosterone. Because the sample size was low, this result should be considered with caution, though.
Alternatively, individuals in poor quality territories may need to work harder to feed their offspring. Thus, higher levels of baseline corticosterone in combination with lower feeding rates may reflect the increased work-load required in such poor territories: owners of such territories may feed their offspring less frequently simply because food is harder to find. However, given the overall high food abundance of the Usangu plains during the rainy season we consider this explanation unlikely.
Previous studies on songbirds and seabirds found that parents with higher levels of baseline corticosterone typically had lower nesting success, supporting the idea that elevated baseline concentrations of corticosterone reflect the condition of the parents and/or environmental quality (Love et al., 2004; Angelier et al., 2007; Groscolas et al., 2008; Bonier et al., 2009b; Kitaysky et al., 2010; Ouyang et al., 2011, 2013b; Crossin et al., 2012). The main factors driving nesting success in these studies was food availability, often in combination with cold weather, and the effect of these variables on the condition of the parents. In contrast to these northern temperate or Arctic species, food supply, and weather condition showed little variation at our study site and we did not find a relationship between nesting success and baseline corticosterone concentrations. The almost exclusive factor determining nesting success in coucals accounting for roughly 99% of all nest-losses is predation (Goymann et al., 2015). Because nest predators are omnipresent in coucal habitat, nest predation represents an unpredictable event unlikely to be related to baseline corticosterone concentrations. This is unlike the situation described by Scheuerlein et al. (2001), where feeding male African stonechats (Saxicola torquata axillaris) expressed higher baseline corticosterone levels when they shared a territory with a fiscal shrike (Lanius collaris), which regularly preys on nestling and adult stonechats. Such ecological differences between study sites and species highlight the importance of considering life history and the ecological context of studies when interpreting hormone data (see Crossin et al., 2016, for a recent discussion of this topic). Our data clearly show a negative relationship between feeding rates and baseline corticosterone concentrations of male black coucals and female white-browed coucals, but we currently lack an explanation as to whether internal (condition) or external factors (environment) or a combination of both factors are responsible for this curious finding.
Stress-induced concentrations of corticosterone in coucals were related to parental expenditure only in male black coucals, but not in white-browed coucals. Parenting male black coucals expressed higher stress-induced corticosterone concentrations than males sampled outside a parenting context. This finding is in contrast to those of Arctic shorebirds, in which stress-induced corticosterone concentrations seemed to be suppressed during parental care, in particular in the sex that provides the majority of care (O'Reilly and Wingfield, 2001). Compared to Arctic birds or desert birds with breeding seasons that allow for only one reproductive attempt, the breeding seasons of coucals are long and food is highly abundant. Further, with the exception of occasional heavy rains, severe and unpredictable weather conditions are absent. In one breeding season, a male black coucal can raise 2–3 clutches and re-nesting after losing a nest to a predator is also common (Goymann et al., 2015). Hence, the environmental conditions that have shaped the stress-response in tropical seasonal breeders, such as the two coucal species, are likely to be quite different from those shaping the stress responses of Arctic or desert birds. The suppression of the stress response in the parent that provides the majority of parental care may be a common phenomenon in Arctic or desert birds with short breeding seasons, but predictions derived for such habitats most likely cannot be transferred to other, more benign environments.
In conclusion, the current study showed that sex-role reversal and uniparental care in black coucals was associated with elevated baseline and stress-induced corticosterone concentrations of males during the parental phase and of females during the main breeding period. In contrast, the “laid-back” style of parenting in biparental white-browed coucals did not come with elevated baseline corticosterone concentrations. Rich food resources have been identified as a necessary condition for the evolution of sex-role reversal in coucals, birds, and terrestrial vertebrates in general (Andersson, 1995, 2005; Goymann et al., 2015). Although two-species comparisons allows only for limited conclusions (Garland and Adolph, 1994) the results from this study support the idea that a switch from biparental to uniparental care may come at the physiological cost of elevated baseline corticosterone concentrations that most likely reflect a higher metabolic expenditure. This is especially so because by studying the two species at the same time and in the same habitat, we were able to “control” for many other confounding environmental factors. The rich food resources in the habitat may compensate for the larger energetic needs of uniparental care and female competition in black coucals, and they probably allow biparental white-browed coucals to live at a more relaxed pace-of-life.
Author Contributions
WG conceived of, designed, and coordinated the study, carried out field work, conducted the statistical analysis, and drafted the manuscript. MT conducted the hormone analysis, FU helped obtaining the necessary permits and participated in field work. MT and FU gave intellectual input during the study design and while drafting the manuscript. All authors gave final approval for publication.
Funding
This project was funded by grants from the DFG (Go985/2) and the National Geographic Society (GEFNE92-13) to WG, who acknowledges further support from Manfred Gahr (Max-Planck-Gesellschaft).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Musa Makomba, Christina Muck, Ingrid Schwabl, Ignas Safari, Beate Apfelbeck, Emmanuel Ayoub, Raimund Barth, Makubi R. Joseph, Poyo Makomba, and Andrea Wittenzellner for assistance in the field, Liz and Neil Baker and Susanna Joos for logistical support, and Henrik Brumm for reading a previous draft of the manuscript. We also thank the Tanzania Wildlife Research Institute (TAWIRI) and the Tanzanian Commission for Science and Technology (COSTECH) for permissions to work in Tanzania, and the Kapunga Rice Irrigation Project and Merere family (Utengule Ward) for support and allowing us to work on their property. The authors declare that they do not have a conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2017.00015/full#supplementary-material
References
Andersson, M. (1995). Evolution of reversed sex roles, sexual size dimorphism, and mating system in coucals (Centropodidae, Aves). Biol. J. Linnean Soc. 54, 173–181. doi: 10.1111/j.1095-8312.1995.tb01030.x
Andersson, M. (2005). Evolution of classical polyandry: three steps to female emancipation. Ethology 111, 1–23. doi: 10.1111/j.1439-0310.2004.01057.x
Angelier, F., Bost, C.-A., Giraudeau, M., Bouteloup, G., Dano, S., and Chastel, O. (2008). Corticosterone and foraging behavior in a diving seabird: the Adélie penguin, Pygoscelis adeliae. Gen. Comp. Endocrinol. 156, 134–144. doi: 10.1016/j.ygcen.2007.12.001
Angelier, F., Weimerskirch, H., Dano, S., and Chastel, O. (2007). Age, experience and reproductive performance in a long-lived bird: a hormonal perspective. Behav. Ecol. Sociobiol. 61, 611–621. doi: 10.1007/s00265-006-0290-1
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bókony, V., Lendvai, Á., Liker, A., Angelier, F., Wingfield, J. C., and Chastel, O. (2009). Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598. doi: 10.1086/597610
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Bonier, F., Martin, P. R., Moore, I. T., and Wingfield, J. C. (2009a). Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. doi: 10.1016/j.tree.2009.04.013
Bonier, F., Moore, I. T., and Robertson, R. J. (2011). The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol. Lett. 7, 944–946. doi: 10.1098/rsbl.2011.0391
Bonier, F., Moore, I. T., Martin, P. R., and Robertson, R. J. (2009b). The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 163, 208–213. doi: 10.1016/j.ygcen.2008.12.013
Brumm, H., and Goymann, W. (2017). On the natural history of duetting in white-browed coucals: sex and body-size dependent differences in a collective vocal display. J. Ornithol. doi: 10.1007/s10336-016-1429-0
Crespi, E. J., Williams, T. D., Jessop, T. S., and Delehanty, B. (2013). Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct. Ecol. 27, 93–106. doi: 10.1111/1365-2435.12009
Crossin, G. T., Love, O. P., Cooke, S. J., and Williams, T. D. (2016). Glucocorticoid manipulations in free-living animals: considerations of dose delivery, life-history context and reproductive state. Funct. Ecol. 30, 116–125. doi: 10.1111/1365-2435.12482
Crossin, G. T., Trathan, P. N., Phillips, R. A., Gorman, K. B., Dawson, A., Sakamoto, K. Q., et al. (2012). Corticosterone predicts foraging behavior and parental care in Macaroni penguins. Am. Nat. 180, E31–E41. doi: 10.1086/666001
Doody, L. M., Wilhelm, S. I., McKay, D. W., Walsh, C. J., and Storey, A. E. (2008). The effects of variable foraging conditions on common murre (Uria aalge) corticosterone concentrations and parental provisioning. Horm. Behav. 53, 140–148. doi: 10.1016/j.yhbeh.2007.09.009
Erckmann, W. J. (1983). “The evolution of polyandry in shorebirds: an evaluation of hypotheses,” in Social Behavior of Female Vertebrates, ed S. K. Wasser (New York, NY: Academic Press), 113–168.
Erritzoe, J., Mann, C. F., Brammer, F. P., and Fuller, R. A. (2012). Cuckoos of the World. London: Christopher Helm.
Garland, T., and Adolph, S. C. (1994). Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol. Zool. 67, 797–828. doi: 10.1086/physzool.67.4.30163866
Geberzahn, N., Goymann, W., and Ten Cate, C. (2010). Threat signaling in female song—evidence from playbacks in a sex-role reversed bird species. Behav. Ecol. 21, 1147–1155. doi: 10.1093/beheco/arq122
Geberzahn, N., Goymann, W., Muck, C., and Ten Cate, C. (2009). Females alter their song when challenged in a sex-role reversed bird species. Behav. Ecol. Sociobiol. 64, 193–204. doi: 10.1007/s00265-009-0836-0
Gelman, A., Su, Y.-S., Yajima, M., Hill, J., Pittau, M. G., Kerman, J., et al. (2014). Data Analysis Using Regression and Multilevel/Hierarchical Models. (Vienna: CRAN R repository: CRAN).
Goymann, W., and Wingfield, J. C. (2004). Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. doi: 10.1016/j.anbehav.2003.08.007
Goymann, W., Kempenaers, B., and Wingfield, J. (2005). Breeding biology, sexually dimorphic development and nestling testosterone concentrations of the classically polyandrous African black coucal, Centropus grillii. J. Ornithol. 146, 314–324. doi: 10.1007/s10336-005-0004-x
Goymann, W., Makomba, M., Urasa, F., and Schwabl, I. (2015). Social monogamy vs. polyandry: ecological factors associated with sex roles in two closely related birds within the same habitat. J. Evol. Biol. 28, 1335–1353. doi: 10.1111/jeb.12657
Goymann, W., Safari, I., Muck, C., and Schwabl, I. (2016). Sex roles, parental care and offspring growth in two contrasting coucal species. R. Soc. Open Sci. 3:160463. doi: 10.1098/rsos.160463
Goymann, W., Trappschuh, M., Jensen, W., and Schwabl, I. (2006). Low ambient temperature increases food intake and dropping production, leading to incorrect estimates of hormone metabolite concentrations in European stonechats. Horm. Behav. 49, 644–653. doi: 10.1016/j.yhbeh.2005.12.006
Goymann, W., Wittenzellner, A., and Wingfield, J. C. (2004). Competing females and caring males. Polyandry and sex-role reversal in African black coucals, Centropus grillii. Ethology 110, 807–823. doi: 10.1111/j.1439-0310.2004.01015.x
Goymann, W., Wittenzellner, A., Schwabl, I., and Makomba, M. (2008). Progesterone modulates aggression in sex-role reversed African black coucals. Proc, R. Soc. B 275, 1053–1060. doi: 10.1098/rspb.2007.1707
Groscolas, R., Lacroix, A., and Robin, J.-P. (2008). Spontaneous egg or chick abandonment in energy-depleted king penguins: a role for corticosterone and prolactin. Horm. Behav. 53, 51–60. doi: 10.1016/j.yhbeh.2007.08.010
Harvey, S., Phillips, J. G., Res, A., and Hall, T. R. (1984). Stress and adrenal function. J. Exp. Zool. 232, 633–646. doi: 10.1002/jez.1402320332
Irwin, M. P. S. (1985). Interrelationships among African species of Centropus (Cuculidae). Ostrich 56, 132–134. doi: 10.1080/00306525.1985.9639581
Kern, M. D., Bacon, W., Long, D., and Cowie, R. J. (2007). Blood metabolite levels in normal and handicapped pied flycatchers rearing broods of different sizes. Comp. Biochem. Physiol. Mol. Integr. Physiol. 147, 70–76. doi: 10.1016/j.cbpa.2006.11.006
Kern, M., Bacon, W., Long, D., and Cowie, R. J. (2005). Blood metabolite and corticosterone levels in breeding adult pied flycatchers. Condor 107, 665–677. doi: 10.1650/0010-5422(2005)107[0665:BMACLI]2.0.CO;2
Kitaysky, A. S., Piatt, J. F., Hatch, S. A., Kitaiskaia, E. V., Benowitz-Fredericks, Z. M., Shultz, M. T., et al. (2010). Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct. Ecol. 24, 625–637. doi: 10.1111/j.1365-2435.2009.01679.x
Korner-Nievergelt, F., Roth, T., Von Felten, S., and Guélat, J. (2015). Bayesian Data Analysis in Ecology Using Linear Models with R, BUGS, and Stan. Amsterdam: Academic Press.
Krause, J. S., Pérez, J. H., Chmura, H. E., Meddle, S. L., Hunt, K. E., Gough, L., et al. (2016a). The stress response is attenuated during inclement weather in parental, but not in pre-parental, Lapland longspurs (Calcarius lapponicus) breeding in the Low Arctic. Horm. Behav. 83, 68–74. doi: 10.1016/j.yhbeh.2016.05.018
Krause, J. S., Pérez, J. H., Chmura, H. E., Sweet, S. K., Meddle, S. L., Hunt, K. E., et al. (2016b). The effect of extreme spring weather on body condition and stress physiology in Lapland longspurs and white-crowned sparrows breeding in the Arctic. Gen. Comp. Endocrinol. 237, 10–18. doi: 10.1016/j.ygcen.2016.07.015
Landys, M. M., Ramenofsky, M., and Wingfield, J. C. (2006). Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149. doi: 10.1016/j.ygcen.2006.02.013
Lea, R. W., Klandorf, H., Harvey, S., and Hall, T. R. (1992). Thyroid and adrenal function in the ring dove (Streptopelia risoria) during food deprivation and a breeding cycle. Gen. Comp. Endocrinol. 86, 138–146. doi: 10.1016/0016-6480(92)90135-7
Love, O. P., Breuner, C. W., Vezina, F., and Williams, T. D. (2004). Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 46, 59–65. doi: 10.1016/j.yhbeh.2004.02.001
Maurer, G. (2008). Who cares? Males provide most parental care in a monogamous nesting cuckoo. Ethology 114, 540–547. doi: 10.1111/j.1439-0310.2008.01498.x
Miller, D. A., Vleck, C. M., and Otis, D. L. (2009). Individual variation in baseline and stress-induced corticosterone and prolactin levels predicts parental effort by nesting mourning doves. Horm. Behav. 56, 457–464. doi: 10.1016/j.yhbeh.2009.08.001
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
O'Reilly, K. M., and Wingfield, J. C. (2001). Ecological factors underlying the adrenocortical response to capture stress in Arctic-breeding shorebirds. Gen. Comp. Endocrinol. 124, 1–11. doi: 10.1006/gcen.2001.7676
Ouyang, J. Q., Muturi, M., Quetting, M., and Hau, M. (2013a). Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Horm. Behav. 63, 776–781. doi: 10.1016/j.yhbeh.2013.03.002
Ouyang, J. Q., Sharp, P. J., Dawson, A., Quetting, M., and Hau, M. (2011). Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R. Soc. B Biol. Sci. 278, 2537–2545. doi: 10.1098/rspb.2010.2490
Ouyang, J. Q., Sharp, P., Quetting, M., and Hau, M. (2013b). Endocrine phenotype, reproductive success and survival in the great tit, Parus major. J. Evol. Biol. 26, 1988–1998. doi: 10.1111/jeb.12202
Payne, R. B. (2005). “Centropodinae,” in The Cuckoos, ed R. B. Payne. (Oxford: Oxford University Press), 208–262.
Peig, J., and Green, A. J. (2010). The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct. Ecol. 24, 1323–1332. doi: 10.1111/j.1365-2435.2010.01751.x
Rappole, J. H., and Tipton, A. R. (1991). New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62, 335–337.
Romero, L. M. (2002). Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24. doi: 10.1016/S0016-6480(02)00064-3
Romero, L. M., and Wingfield, J. C. (2016). “Modulation of the adrenocortical response to stress,” in Tempests, Poxes, Predators, and People. Stress in Wild Animals and How They Cope, eds L. M. Romero and J. C. Wingfield (New York, NY: Oxford University Press), 397–445.
Rowan, M. K. (1983). The Doves, Parrots, Louries and Cuckoos of Southern Africa. Cape Town: David Philip Publisher.
Scheuerlein, A., Van't Hof, T. J., and Gwinner, E. (2001). Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris). Proc. R. Soc. Lond. B 268, 1575–1582. doi: 10.1098/rspb.2001.1691
Smiseth, P. T., Kölliker, M., and Royle, N. J. (2012). “What is parental care?” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 1–17.
Sorenson, M. D., and Payne, R. B. (2005). “A molecular genetic analysis of cuckoo phylogeny,” in The Cuckoos, ed R. B. Payne (Oxford: Oxford University Press), 68–94.
Van Noordwijk, A. J., and De Jong, G. (1986). Acquisition and allocation of resources. Their influence on variation in life history tactics. Am. Nat. 128, 137–142. doi: 10.1086/284547
Vernon, C. J. (1971). Notes on the biology of the black coucal. Ostrich 42, 242–258. doi: 10.1080/00306525.1971.9634415
Villavicencio, C. P., Apfelbeck, B., and Goymann, W. (2014). Parental care, loss of paternity and circulating levels of testosterone and corticosterone in a socially monogamous song bird. Front. Zool. 11:11. doi: 10.1186/1742-9994-11-11
Wingfield, J. C., Maney, D. L., Breuner, C. W., Jacobs, J. D., Lynn, S., Ramenofsky, M., et al. (1998). Ecological bases of hormone-behavior interactions - the emergency life-history stage. Am. Zool. 38, 191–206. doi: 10.1093/icb/38.1.191
Wingfield, J. C., O'reilly, K. M., and Astheimer, L. B. (1995). Modulation of the adrenocortical responses to acute stress in arctic birds: a possible ecological basis. Am. Zool. 35, 285–294. doi: 10.1093/icb/35.3.285
Keywords: centropus, classical polyandry, glucocorticoids, mating system, parental care, sex-role reversal, social monogamy
Citation: Goymann W, Trappschuh M and Urasa F (2017) Corticosterone Concentrations Reflect Parental Expenditure in Contrasting Mating Systems of Two Coucal Species. Front. Ecol. Evol. 5:15. doi: 10.3389/fevo.2017.00015
Received: 28 December 2016; Accepted: 28 February 2017;
Published: 15 March 2017.
Edited by:
Carlos Alonso Alvarez, Consejo Superior de Investigaciones Científicas, SpainReviewed by:
Lynn Marie Siefferman, Appalachian State University, USALiesbeth De Neve, Ghent University, Belgium
Copyright © 2017 Goymann, Trappschuh and Urasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolfgang Goymann, Z295bWFubkBvcm4ubXBnLmRl
 Wolfgang Goymann
Wolfgang Goymann Monika Trappschuh1
Monika Trappschuh1