
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 14 November 2016
Sec. Behavioral and Evolutionary Ecology
Volume 4 - 2016 | https://doi.org/10.3389/fevo.2016.00127
This article is part of the Research Topic Multiplicity of physiological systems that detect light or are regulated by photic information View all 9 articles
In comparison to complex visual systems, non-directional photoreception—the most primitive form of biological photodetection—has been poorly investigated, although it is essential to many biological processes such as circadian and seasonal rhythms. Here we describe the spatiotemporal expression pattern of the major molecular actors mediating light reception—opsins—localized in the Strongylocentrotus purpuratus larva. In contrast to other zooplanktonic larvae, the echinopluteus lacks photoreceptor cells with observable shading pigments involved in directional visual tasks. Nonetheless, the echinopluteus expresses two distinct classes of opsins: a Go-opsin and a rhabdomeric opsin. The Go-opsin, Sp-opsin3.2, is detectable at early (3 days post fertilization) and four armed pluteus stages (4 days post fertilization) in two cells that flank the apical organ. To rule out the presence of shading pigments involved in directional photoreception, we used electron microscopy to explore the expression domain of Go-opsin Sp-opsin3.2 positive cells. The rhabdomeric opsin Sp-Opsin4 expression is detectable in clusters of cells located around the primary podia at the five-fold ectoderm pentagonal disc stage (day 18–21) and thereafter, thus indicating that Sp-Opsin4 may not be involved in the photoreception mechanism of the larva, but only of the juvenile. We discuss the putative function of the relevant cells in their neural context, and propose a model for understanding simple photodetection in marine larvae.
While the vast majority of studies on animal photoreception have so far focused on directional photoreceptors—systems comprising at least one cell with a photosensitive opsin together with shading pigments that enable it to discriminate the directionality of light—, less is known about non-directional photoreception, the simplest and earliest evolving type of photoreception. Non-directional photoreceptors, which can be difficult to detect due to a lack of visible screening pigments, allow the monitoring of absolute light intensities of the environment. Consequently, they are widely used as an input to the circadian clock system and also for a wide variety of other tasks. For instance, non-directional photoreceptors can be used as a depth gauge, as a warning for harmful levels of UV radiation, for shadow detection, or be involved in the regulation of feeding, movement and reproduction rhythms (Bennett, 1979; Paul and Gwynn-Jones, 2003; Leech et al., 2005; Nilsson, 2009, 2013).
Opsins are G-protein coupled receptors involved in light-perception. Based on their amino acid sequence, they can be divided into four groups: tetraopsin, xenopsin, Gq-opsin, and c-opsin (Ramirez et al., 2016; for other classifications see: Plachetzki et al., 2007; Arendt, 2008; Koyanagi et al., 2008; Porter et al., 2011; Feuda et al., 2012). The presence of opsins provides a clear landmark for localizing putative photoreceptor cells even in the absence of shading pigments and, as a consequence, the localization of opsin-expressing cells is important for finding directional and non-directional photoreceptors.
In echinoderms, efforts to describe photoreceptors have primarily focussed on adult specimens. The phototactic behavior commonly observed in adult sea urchins, in addition to their photosensitive ectoderm associated with an endoskeleton (which could act as shading structure, lens or filter) make them a useful model for studying diffuse photoreception (Raup, 1966; Hendler and Byrne, 1987; Johnsen, 1997; Johnsen and Kier, 1999; Aizenberg et al., 2001). Before the advent of molecular genetics, studies of photoreception in echinoids concentrated on cell morphology and physiology, as well as understanding behavioral responses such as spine movements, tube foot reaction, covering, color change, and more recently visual navigation (Holmes, 1912; Millott, 1953, 1954, 1976; Thornton, 1956; Millot and Yoshida, 1958; Millott and Manly, 1961; Yoshida, 1966; Yoshida et al., 1984; Johnsen, 1994; Blevins and Johnsen, 2004; Yerramilli and Johnsen, 2010). Later, the publication of the sea urchin Strongylocentrotus purpuratus genome lead to the discovery of nine opsins, a number of transcription factors involved in photoreceptor cell differentiation (e.g., irx5, irx6, dlx1/dlx2, rx, ath) and several orthologous genes putatively involved in the phototransduction cascade (e.g., visual G-beta subunit, rhodopsin kinase, arrestin, retinal-binding protein, G-alpha-s subunit, transducin G-gamma-t1, recoverin, G-alpha-q subunit) in this species (Sodergren et al., 2006; D'Aniello et al., 2015). This information has made it possible to use molecular tools to investigate photoreception in echinoids (Burke et al., 2006; Raible et al., 2006). The first biochemical efforts to investigate the mechanisms of photoreception in S. purpuratus have resulted in the localization of the rhabdomeric opsin Sp-Opsin4 in basal (i.e., in the stalk area proximal to the compound plates) and disk (i.e., in the tube feet most apical part) microvillar cells of the adult tube feet (Ullrich-Lüter et al., 2011). Furthermore, a ciliary opsin, Sp-Opsin1 has been immunodetected in cells located in locomotory and buccal tube feet, as well as in the proximal stalk region of tridentate pedicellaria (Ullrich-Lüter et al., 2013), the latter being jawed appendages used against parasites (Coppard et al., 2010). These findings have allowed Ullrich-Lüter et al. (2011) to describe a unique system of photoreception in which the entire sea urchin, using its skeleton as photoreceptor screening device, functions as a “giant eye.” This is also in agreement with previous observations on the photobehaviour of a Diadema species that lead to the suggestion that the shadow produced by the spines on the animal body surface is used for inferring the visual landscape (Woodley, 1982). However, in comparison with the light detection systems of adult echinoids, the photoreception mechanisms of their planktonic larvae have been so far poorly investigated.
While ancestral adult metazoans were likely benthic, it is probable that a pelagic larval stage evolved very early in animal evolution (Jägersten, 1972; Nielsen, 2008). This idea has led many scientists to investigate the directional simple eyespots of marine larvae in search of something resembling a “proto-eye” (Smith, 1935; Thorson, 1964; Brandenburger et al., 1973; Marsden, 1984; Pires and Woollacott, 1997; Leys and Degnan, 2001; Nordstrom et al., 2003; Jékely et al., 2008; Gühmann et al., 2015). Such simple eyespots or ocelli constitute class II photoreceptors (photoreceptor cells associated with shading pigments) in accordance with the classification of Nilsson (2013). To our knowledge, only few cases of non-directional (class I) photoreceptors have been documented in marine zooplanktonic larvae (Arendt et al., 2004; Passamaneck, 2011; Vöcking et al., 2015). In these cases, and in contrast to what we can observe in the echinopluteus, the larvae studied possess eyespots, thus making it more difficult to study class I photoreception in an independent way.
To better elucidate the origins of animal vision, an event that most probably happened in the Precambrian marine environment, the study of larvae with class I photoreception is essential. In this paper we identify a Go based photoreceptor system in a zooplanktonic larva of the deuterostome lineage that potentially lacks directional photoreceptors. To localize the putative photoreceptor cells of the larva at early and late developmental stages, we analyzed the expression of the opsins Sp-opsin3.2 (Go) and Sp-Opsin4 (rhabdomeric) using whole mount in situ hybridization and immunohistochemistry, respectively. Further, the presence of shading pigments in the vicinity of the encountered Go-opsin based photoreceptor cells was ruled out by exploring both the apical organ as well as the basal area of the anterolateral arms by using a transmission electron microscopy (TEM) approach. The putative role of these photoreceptor cells in non-directional photoreception of the pluteus is discussed.
In order to characterize the presence of putative photoreceptor cells in the sea urchin larva we first consulted the transcriptomic expression of S. purpuratus opsins. After analyzing the publicly available RNAseq data coming from a survey of 10 embryonic stages (Tu et al., 2014) we concluded that, of the nine genes encoding opsins found in the genome, the Go opsin Sp-opsin3.2 (SPU027633) and the echinopsin Sp-opsin2 (SPU003451) are the only opsin genes expressed at significant levels. Starting from the late gastrula stage (48 h), these two genes show expression levels reaching the value of about 100 transcripts per embryo at the early pluteus stage (72 h), when neurons start to differentiate (for gene expression profiling see Supplementary Figure 1). Next, successful amplification of the Go opsin Sp-opsin3.2 was carried out, and the corresponding antisense riboprobe was used to localize the cells of interest. Unfortunately, various attempts in the amplification with different set of primers of the “echinopsin” Sp-opsin2 did not give any result.
Here, both RNA fluorescence (Figures 1A–C; 3 days post fertilization: dpf larvae, early four armed larvae) and chromogenic (Figures 1D–F; 4dpf, four armed larvae) whole mount in situ hybridization (WMISH) revealed that the Go opsin Sp-opsin3.2 is expressed in two cells arranged bilaterally adjacent the apical organ—i.e., a portion of the epithelium that form the oral hood that is considered to act as central nervous system of the larva (Byrne et al., 2007)—and at the base of the left and right anterolateral arms (for a schematic view of the 4 armed pluteus in which we included the terminology used in this work, see Figure 2). The expression of this gene in such a small number of cells is consistent with the above mentioned low levels of expression observed from the transcriptomic data. In order to identify the position of these Sp-opsin3.2 positive cells with respect to the ciliary band (the distinct thickening of ciliated epidermis that outlines the oral field and traces the edges of the four larval arms), cilia were labeled by immunohistochemistry with anti-acetylated α-tubulin after Sp-opsin3.2 WMISH. As shown by both in situ techniques (Figure 1), the main body of these cells appear to be located just orally to the thick epidermal band of the ciliated cells (see Figure 2 for schematic representation). The Sp-opsin3.2 positive cells are suggestive of the presence of a photoreception system in the sea urchin larvae.
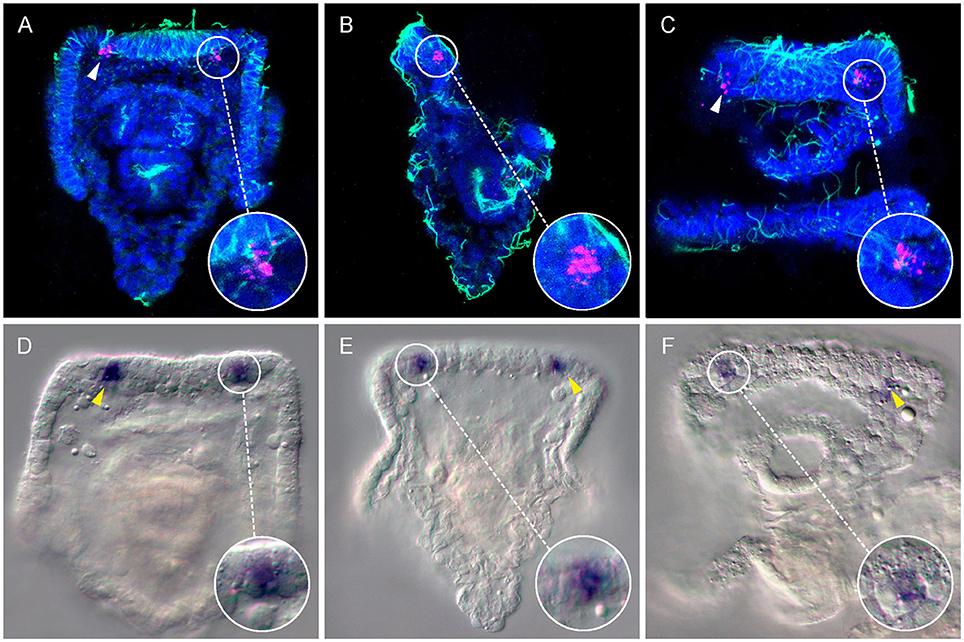
Figure 1. Expression of the Go-opsin Sp-opsin 3.2 in early plutei. A couple of Sp-opsin 3.2 bilateral symmetrical cells were detected at cellular resolution between the base of the anterolateral arms and the apical organ of echinopluteus by means of fluorescent (A–C, 3dpf) and chromogenic (D–F, 4dpf) in situ hybridizations. (A–C) Confocal-micrographs; Sp-opsin3.2 in situ hybridization (magenta) was coupled with acetylated α-tubulin immunohistochemistry (green); nuclei were counterstained with DAPI (blue). (A) Abanal view; (B) right-lateral view; (C) mouth view. (D–F) Light-micrographs. (D,E) abanal view; (F) mouth view. Arrow heads indicate Sp-opsin3.2 positive cells.
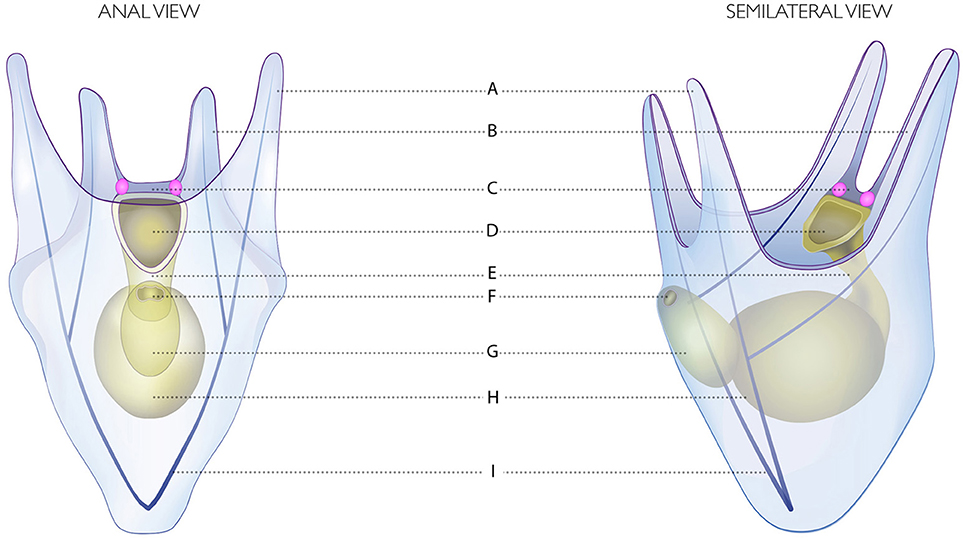
Figure 2. Drawing of the four-armed pluteus of S. purpuratus. (A) Postoral arms. (B) Anterolateral arms. (C) Apical organ. (D) Mouth. (E) Esophagus. (F) Anus. (G) Intestine. (H) Stomach. (I) Skeletal rods. The Go Sp-opsin3.2 opsin positive cells are represented in pink.
A key difference between visual and non-visual photoreception system is the presence of shading structures, generally in the form of pigment cells, in proximity of light perceiving cells. Therefore, S. purpuratus larvae were observed under the light microscope at 4, 6, and 8 arm stages to detect for the presence of observable pigments that can be organized to act as shading for the described Sp-opsin3.2 positive cells. The only pigmented cells found in the vicinity of these cells were the granulated pigment cells, a particular population of red colored cells of dendritic morphology and immune role that are distributed all over the body (Ho et al., 2016). We therefore decided to explore the presence of screening pigments in the vicinities of the Go Sp-opsin3.2 opsin-positive cells by means of TEM.
Shading pigments involved in directional photoreception, which can be located both in the opsin positive cells or adjacently, are easily recognized in TEM as a group of black-solid dots in the cytoplasm (e.g., Marshall and Hodgson, 1990; Leys and Degnan, 2001). For our TEM analysis, three larvae were fixed, and transversal sections of 50–70 nm were made in the apical region in search of shading pigments (Figure 3A). Of them, micrographs corresponding to different sections of the apical organ (Figures 3D,E) and the bases of the left (Figures 3B,C) and right (Figures 3F,G) anterolateral arms were selected. Interestingly, none of the cells embedded in the apical organ nor in the area of the ciliary band exhibited observable shading pigments. In particular, the regions of the ectoderm in between the apical organ and either left or right anterolateral arms (encircled in Figures 3A,C,F) where the Sp-opsin3.2 positive cells are located (see also schematics of Figure 2) are void of shading pigment granules. These findings suggest that the Sp-opsin3.2 positive cells are not involved in directional photoreception. Serial multiplex immunogold labeling experiments are needed to better characterize the morphology of the encountered Sp-opsin3.2 positive cells.
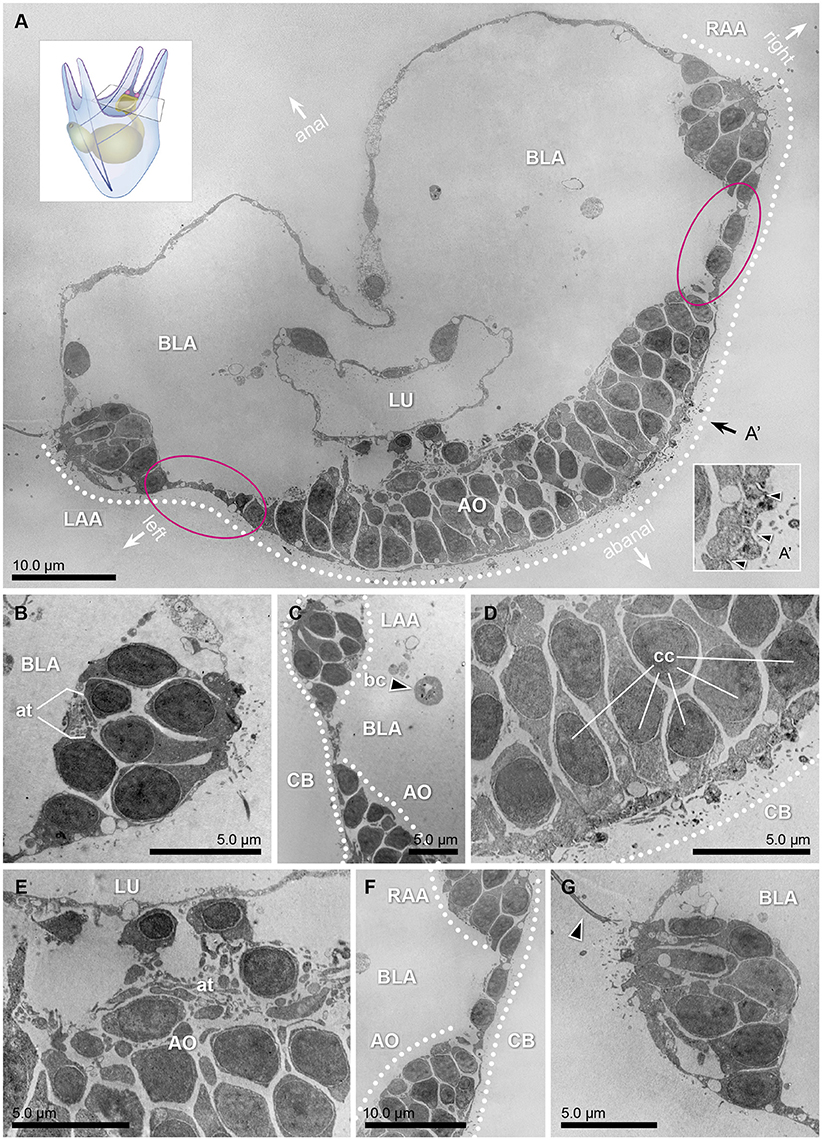
Figure 3. Transmission electron micrographs of a 3dpf (early 4 armed) pluteus, different sections of three specimens at the level of the apical region. (A) Collage of 324 transversal micrographs showing a panoramic view of the abanal half of the larvae (apical region). On it, the bases of the left and right anterolateral arms (LAA and RAA, respectively), as well as the lumen (LU) of the gut, surrounded by the blastocoel (BLA), the ciliary band (CB, dotted line), and the apical organ (AO) are shown. The stippled line corresponds to the ectodermal region in which the ciliary band is located. A representation of the whole 4 armed pluteus larva and cutting area can be seen in the lower right corner. (A') Detail of the cross sectional profile of the motile cilia (arrow heads) that compose the ciliary band. The orientation of the animal is defined by the axes: anal, abanal, left and right. (B) Transversal section of the base of the anterolateral arm, left side. On it, the axon tract (at) that connects this area with the nervous system can be distinguished. (C) Transversal section of the region that connects the left anterolateral arm (LAA) with the apical organ (AO). Pigmented cells cannot be detected in any of the cells flanking the apical organ, where the Go-opsin Sp-opsin3.2 was detected. The black arrow head points to a blastocoelar cell (bc). (D,E) Detail micrographs of the apical organ, an area considered as the central system of the animal, rich on ciliated cells (cc) and axon tracts (at). (F) Transversal section of the region that connects the right anterolateral arm (RAA), with the apical organ (AO). (G) Transversal section of the base of the anterolateral arm, right side. The arrow head points one of the cilia of the region.
Due to limitations of WMISH efficiency on late developmental stages and the availability of a specific antibody against the sea urchin rhabdomeric opsin Sp-Opsin4 (Ullrich-Lüter et al., 2011), we decided to use an immunohistochemical approach to explore the opsin toolkit of the premetamorphic larva. During late larval development (second week of development and thereafter), a portion of the coelom and the overlying ectoderm get in contact and form the imaginal adult rudiment (Smith et al., 2008; Heyland and Hodin, 2014; for a schematic view see drawings in Figure 4). This rudiment represents the presumptive juvenile that grows from the left side of the larva (for a schematic, see Figure 4A). In order to analyze the spatiotemporal expression of the rhabdomeric opsin Sp-Opsin4, we tested its presence in time series of 3, 4, 5, 6, and 7 days (4 armed pluteus), 16d (6 armed pluteus, contact flattened stage), 17d (6 armed pluteus, 5-fold mesoderm stage), 18d (8 armed pluteus, 5-fold ectoderm stage), 19d (8 armed pluteus, primary podia stage), 21d (8 armed pluteus, primary podia-folded stage), and 23d (8 armed pluteus, primary podia-touching stage) post fertilization (for staging of the echinopluteus see also Smith et al., 2008; Heyland and Hodin, 2014). These experiments suggested the absence of expression of the rhabdomeric opsin Sp-Opsin4 prior to the tube feet formation in any part of the larva. No protein expression was found either in sensu stricto larval structures until the 5-fold mesoderm stages (17dpf; Figures 4B,B′) with our method. Larvae started to exhibit Sp-Opsin4 positivity in conspicuous clusters of cells on the vestibular floor at pentagonal disc stage that would give rise to the tube feet disc during 5-fold ectoderm stage, a stage in which the ectoderm and the primordia of the five podia begin to push through the floor of the vestibular ectoderm (day 18; Figures 4C,C′). At this point, the interior of the 5 incipient podia are spherical in shape or shorter than wide. We also detected Sp-Opsin4 positive cells later on, in the tube feet disc during advanced rudiment stage, when the primary podia are taller than they are wide, but the podia are not yet folding in toward one another (day 20–21; Figures 4D,D′). At tube-foot protrusion stage (day 21–45), Sp-Opsin4 positive cells were detected both in disc (Figure 4E) and basal (Figure 4F) photoreceptors of the tube feet. These data indicate that the rhabdomeric opsin Sp-opsin4 may not regulate the photoreception mechanism of the larva, but only of the juvenile, where it appears to be involved in negative phototaxis (Ullrich-Lüter et al., 2011). For a schematic view on the different rudimental stages, see Figures 4B′–F′.
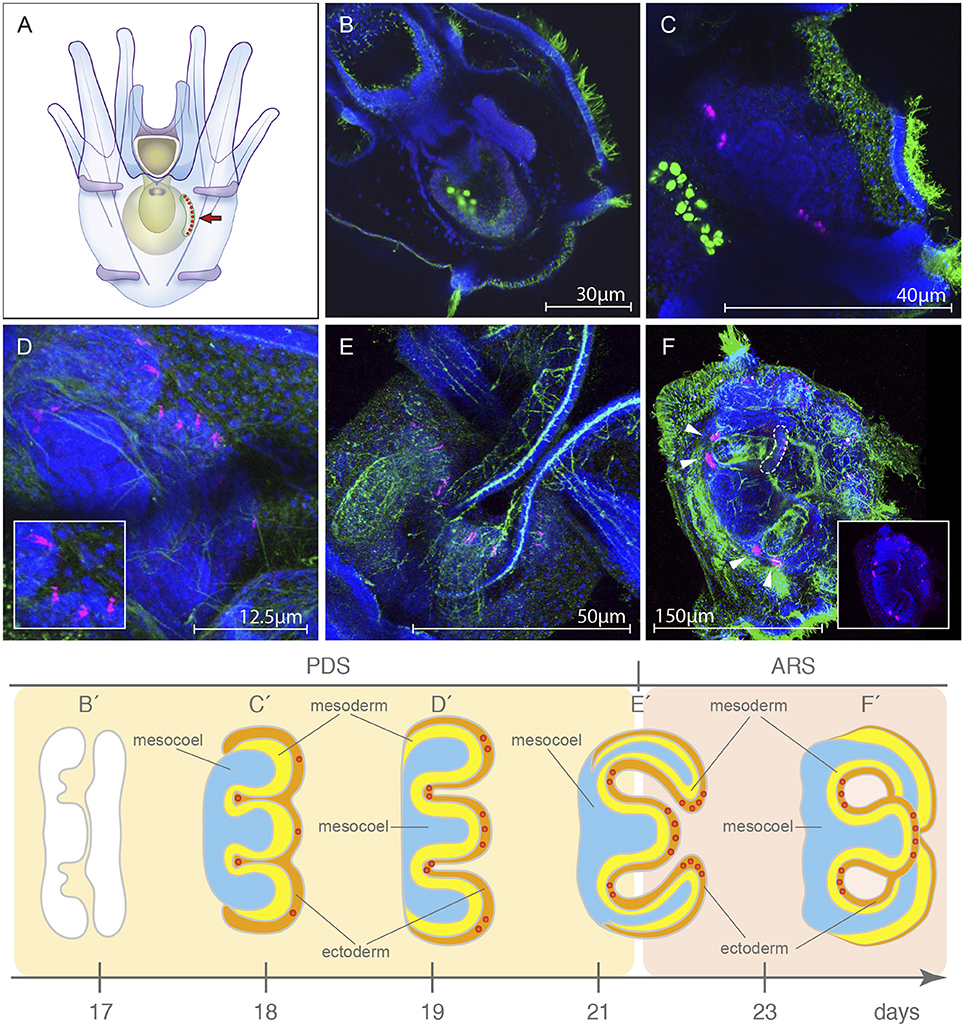
Figure 4. Localization of the rhabdomeric opsin Sp-opsin4 in the developing tube feet of the presumptive juvenile. (A) Schematic of the 8 armed pluteus stage, anal view, in which the area of growing of the rudiment (arrow) is shown. (B,B′) Sp-opsin4 was not detected in adult plate at 5-fold ectoderm stage (day 17; pentagonal disc stage, PDS). (C,C′) During the 5-folding of the ectoderm (day 18, PDS), the rudiment of the larva starts to exhibit Sp-opsin4 positivity in clusters of conspicuous cells at the presumptive basal tube feet. (D,D′) At primary podia stage (day 19, PDS), the presumptive disc tube feet of the vestibular floor are positive for Sp-opsin4. (E,E′) Sp-opsin4 photoreceptor cells are visible in the tube feet disc of the folded primary podia (day 21; transition between the PDS and the advanced rudiment stage, ARS), both in disc and basal photoreceptor cells of the tube feet. (F,F′) Sp-opsin4 positive cells were detected at tube-foot protrusion stage (day 23-45, ARS). Stages redrawn from Heyland and Hodin (2014). PDS and ARS stages are named following the nomenclature proposed by Smith et al. (2008). The red dots in (C′-F′) represent the Sp-opsin4 positive cells and can be used as a landmark to locate and orient rudiment in (C–F). Confocal micrographs color code: Sp-opsin4 in magenta; acetylated α-tubulin (B–E) and 1E11 (F) in green; DAPI in blue. The bright green staining in the stomach of the larvae shown in (B,C) is due to autofluorescence of the ingested microalgae.
Our findings show that, at least, two opsin classes are expressed in Strongylocentrotus purpuratus prior to metamorphosis: first the Go opsin Sp-opsin3.2 in the apical region of the larva at 3 and 4 dpf (4 armed pluteus), and then the rhabdomeric opsin Sp-Opsin4 in the tube feet of the presumptive juvenile (day 29 and thereafter, 8 armed pluteus). The different opsin classes in the sea urchin may serve different needs to integrate light information depending on the life stage, where the pelagic larva and the benthic adult face very different challenges.
Of the opsin-positive cells encountered, just the two Sp-opsin3.2 positive-cells localized in the flanks of the apical organ can be considered part of the sensu-stricto larval tissues. In our study, no rhabdomeric opsins have been found in larval structures. Because the aim of this study is to improve our understanding of photoreception in marine larvae, the rhabdomeric opsin Sp-opsin4, which is expressed in presumptive juvenile tissues, will not be further discussed. For an account on the possible role of Sp-opsin4 positive photoreceptor cells in mediating negative phototaxis of sea urchin juveniles (see Ullrich-Lüter et al., 2011).
Phylogenetic analyses indicate the presence of at least seven opsins in the last common ancestor of Bilateria (Ramirez et al., 2016), thus suggesting that light reception had many roles very early in animal evolution. These opsins, together with present-day animal opsins, have been classified into four groups: (i) tetraopsins (Go-opsins, RGR/retinochrome opsins and neuropsins), (ii) xenopsins, (iii) Gq-opsins (including canonical and non-canonical r-opsins as well as “chaopsins”), and (iv) c-opsins, i.e., canonical c-opsins and bathyopsins (Ramirez et al., 2016). While the canonical c- and r-opsin groups have been extensively studied, little is known about the Go opsin group included in the tetraopsin clade (Gühmann et al., 2015).
In support of the ancient origin of the Go opsins, cells expressing this class of opsins have been localized in diverse animal clades, thereby suggesting the presence of this opsin group before the protostome-deuterostome split. Examples of Go-opsins are found in the ciliary cells of the eyes of the adult scallop Patinopecten yesooensis (Kojima et al., 1997), in the gastrula of the brachiopod Terebratalia transversa (Passamaneck and Martindale, 2013), in the rhabdomeric adult eye of the polychaete Platynereis dumerilii (Gühmann et al., 2015) as well as in the photoreceptor system here described. In the amphioxus Branchiostoma belcheri, a Go-opsin has been demonstrated by an in vitro analysis (Koyanagi et al., 2002) but, to our best knowledge, this is the first report in which the spatial expression of a Go-opsin has been described in a deuterostome larva.
In marine invertebrates, the expression of opsins in non-visual photoreceptors has been documented in the apical organ of planktonic larvae of protostome and deuterostome lineages (e.g., Arendt et al., 2004 and herein). A shared feature of these apical organs—regions specified by conserved developmental patterning mechanisms (Marlow et al., 2014)—is the presence of multiple sensory cells connected to the nervous system, which regulates ciliary beating and the vertical position of the animal in the water column (Tosches et al., 2014). In vertebrates, a population of non-directional retinal ganglion cells (the intrinsically photosensitive photoreceptive retinal ganglion cells: ipRGCs), are critical in relaying light information to the brain in order to control circadian photo-entrainment, pupillary light reflex, and sleep (Provencio et al., 1998; Schmidt et al., 2011).
Our discovery of non-directional photoreceptors in the pluteus of S. purpuratus suggests that these cells may also have a role in controlling the vertical position of the larva in the water column, which may be used for monitoring the time of day or the depth (Nilsson, 2013). This adjustment is likely to be achieved by modulating the length and frequency of ciliary arrests, as proposed for this and other marine larvae (e.g., Wada et al., 1997; Maldonado et al., 2003; Braubach et al., 2006; Jékely et al., 2008). The use of non-directional photoreceptors in vertebrates for tasks such as the regulation of nocturnal-diurnal behaviors (Provencio et al., 1998; Schmidt et al., 2011) could represent the retention of such chronobiological role.
The absence of shading pigments in the region where the Sp-Opsin3.2 is expressed strongly suggests that these opsin-positive cells lack directional sensitivity, but whether this represents a plesiomorphic character or a secondary loss is not immediately clear. Directional photoreception for phototaxis, with shading pigment near the site of opsin expression, is believed to have evolved from non-directional photoreception where screening pigment is not needed (Nilsson, 2009, 2013). Directional photoreceptors are typically bilaterally paired organs (Brandenburger et al., 1973; Arendt and Wittbrodt, 2001; Braun et al., 2015), whereas non-directional photoreceptors are often un-paired median structures (Mano and Fukada, 2007; Van Gelder, 2008). Our finding of paired non-directional photoreceptors represents an interesting intermediate.
The bilateral arrangement of photoreceptor cells is typically associated with helical swimming behaviors in the pluteus and other marine invertebrate larvae (Lacalli et al., 1990; reviewed in Jékely, 2009). Bilaterally paired photoreceptors may seem redundant for non-directional photoreception, and without shading pigment they do not have the directionality required for phototaxis.
It is possible that the shading pigment associated with these opsin-positive cells might have been lost during evolution to increase transparency or reduce energy expenditure. The lack of shading pigments may have been favored by selection to allow a better camouflage against predators (Nilsson, 1996). Consequently, the bilateral arrangement of these opsin positive cells may be primitive, and the lack of screening pigment a consequence of an adaptive transition from a directional to a non-directional role. Alternatively, it is possible that the pluteus have retained the non-directional photoreceptors of “Urbilateria,” an ancestor that may have had both directional and non-directional photoreceptors (Arendt and Wittbrodt, 2001). The bilateral arrangement of these non-directional photoreceptors would have been the result of developmental constrains associated with the bilateral symmetry, or maybe profitable for increasing the robustness and sensitivity of the photoreceptor system.
To better understand when a possible switch occurred (i.e., whether Go-opsins originally mediated a non-directional task in the dipleurula larvae of the Ambulacraria stem group, or if an association with screening pigments was lost secondarily in the Echinodermata crown group) a further comparison of the photoreceptor systems of different dipleurula-type larvae is required.
The fact that the two bilateral photoreceptors connected to the apical organ of the pluteus larva use a Go-opsin, while r-opsins are present in similar structures of nearly all other larvae, results remarkable. One possible explanation to why putative homologous paired photoreceptors express distinct opsins in different Bilateria clades could be that Urbilateria had bilaterally paired photoreceptors with r-opsin, c-opsins and Go-opsins serving different functions (Feuda et al., 2012; Ramirez et al., 2016). This variety of functions can be ascribed to the need of different spectral or temporal properties, as well as to different roles in the transducing the light signal. Losses would then account for the fact that echinoid larvae seem to have only a Go-opsin, most other protostomes only a r-opsin, and vertebrates c- and r-opsins. Cell duplication and subsequent specialization must also be assumed for vertebrates.
The most plausible role of the Go photoreceptors described in this study is the regulation of vertical movement of the larva during photoperiodic transitions (Jékely et al., 2008; Mason and Cohen, 2012). Such a unimodal system could resemble the earliest photoreceptor mechanism in the first marine larvae. If this is the case, study of this system could provide clues as how the first planktonic animals perceived light cues. It remains possible that other opsins are present at the same larval stage that have not been identified.
Because the main locomotory organ of the pluteus is the ciliary band, it would be informative to know whether the Sp-Opsin3.2 positive cells are connected to the ciliary band via the nervous system, which has been described as “a network of cells that span the blastocoel and connect nearly all parts of the larva” (Ryberg and Lundgren, 1977). Previous studies of the nervous system of the pluteus of Strongylocentrotus droebrachiensis (Burke, 1978), a closely related species, report the presence of serotonergic neurons in the area of the apical organ, located between the cells homologous to the Go-opsin expressing cells of S. purpuratus. This serotonergic system is suggested to be involved in the regulation of the ciliary band activity in the pluteus (Gustafson et al., 1972; Burke, 1978; Yaguchi and Katow, 2003) and also in many other marine larvae (e.g., Mackie et al., 1969; Beiras and Widdows, 1995; Pires and Woollacott, 1997; Kuang and Goldberg, 2001). The topology of the SpOpsin3.2 expressing cells in the proximity of serotonergic neurons lead us to hypothesize that Go expressing cells may be involved in locomotory control, probably in the activation or excitation of the ciliary band to position the animal in the upper photic zone. Knockout experiments of this opsin coupled with behavioral experiments could be used to test this hypothesis.
Adult S. purpuratus were obtained from San Diego Bay at 25–30 m in depth (San Diego, CA, USA) and housed in 12°C circulating seawater aquaria at the Stazione Zoologica Anton Dohrn, Italy. Spawning was induced by intracoelomic injection of 0.5M KCl. Embryos/larvae were cultured in Mediterranean filtered seawater (mesh pore size: 0.2 mm) diluted in de-ionized water (final salinity: 32.5‰) and kept at 15°C on a 12/12 h light/dark cycle. From 3 days onwards, larvae were fed with a mixed diet of Isochrysis galbana [~2,000 cells mL−1] and Rhodomonas sp. [~2,000 cells mL−1]. All larval cultures were maintained at a decreasing with age concentration from 5 to 1 pluteus mL−1, mixed by gentle rotary stirring and washed every other day. Larval washes were made by inverted filtration (mesh size: 100 μM).
Contig sequence for Sp-opsin3.2 was identified in the genome (ref. code: SPU027633) and transcriptome (ref. code: WHL22.338995) data sets. A 1,175 bp transcript was amplified by PCR with the cloning primers Sp-opsin3.2-F (5′-CCACTCATTTCGTGCGGATT-3′) and Sp-opsin3.2-R (5′-CTCTAGTGATGACGGGCGAT-3′) from cDNA prepared with a Bio-Rad iScript synthesis kit, ligated into pGEMT-easy vector (Promega), and transformed into Top10 chemically competent E. coli (Invitrogen). Clone fragments were verified by Sanger sequencing prior to riboprobe generation. DIG-labeled antisense and sense (negative control) RNA probes were generated from plasmid DNA with T7- and SP6-RNA polymerases (Roche) respectively, and purify with mini Quick Spim Columns (Roche).
Strongylocentrotus purpuratus larvae were collected at early pluteus stage (3dpf), fixed overnight at 4°C in 4% paraformaldehyde/0.1M MOPS pH 7, 0.5M NaCl, washed thoroughly in MOPS buffer, and stored in 70% ethanol until use. Whole mount fluorescent in situ hybridization (FISH) was performed as described in Andrikou et al. (2013). Immunohistochemistry coupled to WMISH was performed by incubating the larvae with anti-acetylated α-tubulin antibody (Sigma-Aldrich T6793, St Louis, MO, USA) in a dilution 1:250 together with the anti-DIG antibody; the secondary antibody was a goat anti-mouse IgG-Alexa 488 (Invitrogen, CA, USA) diluted 1: 1000.
Four-armed S. purpuratus larvae were fixed at 4dpf as explained above. Single probe chromogenic in situ hybridization on whole mount fixed embryos was performed as previously described by Ransick (2004) with the following changes: (i) all washes were carried out in TBST (0.2M Tris pH 7.5, 0.15M NaCl, 0.1% Tween-20); (ii) hybridization was performed over-night at 60°C; (iii) 1X SSC and 0.1X SSC washes were performed at 60°C; (iv) Anti-Digoxigenin-AP, Fab fragments (Roche) were diluted 1: 2000.
Larvae were fixed in 4% paraformaldehyde in PBS pH 7.4 containing 0.5M NaCl for 30 min at room temperature. Late 6 and 8 armed larvae (days 14–23) were post-treated 2 min with pure cold MetOH in order to partially remove membrane lipids and facilitate antibody penetration. After five 5 min rinses in phosphate buffered saline (PBS), samples were washed thoroughly in PBS/0.1% Tween-20 (PBST). Following incubations were carried out on an orbital shaker. The first blocking step was performed with 4% heat-inactivated Normal Goat Serum (NGS) in PBST for 1 h, prior to incubating specimens with primary antibodies anti-Sp-opsin4 1:50 [1.21 mg mL−1] (Ullrich-Lüter et al., 2011), anti-1E11 1:100 [~10.00 mg mL−1] (monoclonal antibody that recognize S. purpuratus synaptotagmin B and is used as “pan-neural” marker; Nakajima et al., 2004), and anti-acetylated α-tubulin (Sigma T6793) 1:250—in PBST overnight at 4°C. After five washes in PBST, a second blocking step was performed as described above prior to incubating specimens with secondary antibodies—goat anti-rabbit IgG-Alexa 488 and goat anti-mouse IgG-Alexa 647—diluted 1: 1,000 in blocking buffer (4% NGS in PBST) at 4°C overnight. All specimens were washed thoroughly in PBS and then counterstained with DAPI (1 μg/mL in PBS) for nuclear labeling. For Sp-opsin4 antibodies, controls were carried out using their respective rabbit pre-immune sera. For commercial antibodies, control experiments were run in parallel by omitting primary antibodies.
Strongylocentrotus purpuratus plutei were first fixed in modified Karnovsky solution (2.5% glutaraldehyde, 2% paraformaldehyde, and 3% sucrose in 0.1 M phosphate buffer pH 7.4 containing 0.5M NaCl) for 1 h at room temperature. After several rinses in PBS, samples were post fixed in 1% osmium tetraoxide in distilled water 1 h at 7°C, and dehydrated in a series of ethanol (30/50/70/96/100) and infiltrated and embedded in EPON (Agar 100). Samples were kept at 60°C for 48 h to allow polymerization. Thin sections (50–70 nm) were cut with a diamond knife with a Leica EM UC7 ultramicrotome and mounted on pioloform coated copper grids.
Light microscopic images were taken using a Zeiss M1 Axio Imager microscope. Confocal acquisition was performed on a Zeiss LSM 510 Meta confocal microscope. TEM acquisitions were performed on a 120 kV JEOL 1400 plus microscope with a bottom mounted CMOS camera. Figure plates were made with Illustrator CS6 (Adobe). Brightness/contrast and color balance adjustments were always applied to the whole image and not to parts.
AVG and MIA conceptualized and designed the study. AVG grew the larvae, did the fluorescence in situ hybridization of Sp-opsin3.2 coupled with immunohistochemistry, performed the immunohistochemistry assays of Sp-opsin4, supervised the transmission electron microscopy assays done with the assistance of the EM personnel from the Microscopy Facility (Department of Biology, Lund University), and wrote the manuscript with the assistance of DN, MIA, and PO. LP did the molecular cloning and chromogenic in situ hybridization of Sp-opsin3.2. All authors approved the final text.
AVG is supported by the Marie Curie ITN “Neptune” (grant number: 317172, FP7 PEOPLE Work Programme of the European Commission, PI: MIA). LP was supported by HFSP research grant RGY0082/2010 to PO.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Santiago Valero-Medranda (Spain) for the scientific illustrations presented all over the article. The authors would also thank John Kirwan and Emily Baird (Lund University, Sweden) for critical reading and commenting the manuscript. We acknowledge Ola Gustafsson and Carina Rasmussen, from the Microscopy Facility at the Department of Biology (Lund University, Sweden) for their help in preparing and processing the electron microscopy samples, Patrick Leahy (Kerchoff Marine Station, Caltech, Corona del Mar, CA, USA) for collecting S. purpuratus adults, and Davide Caramiello (Stazione Zoologica Anton Dohrn, Italy) for caring of adult urchins and culturing of microalgae.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2016.00127/full#supplementary-material
Supplementary Figure 1. Graph of the quantitative transcriptome data for Sp-opsin 3.2 and Sp-opsin 2 (http://www.echinobase.org:3838/quantdev/).
Aizenberg, J., Tkachenko, A., Weiner, S., Addadi, L., and Hendler, G. (2001). Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature 412, 819–822. doi: 10.1038/35090573
Andrikou, C., Iovene, E., Rizzo, F., Oliveri, P., and Arnone, M. I. (2013). Myogenesis in the sea urchin embryo: the molecular fingerprint of the myoblast precursors. EvoDevo 4:33. doi: 10.1186/2041-9139-4-33
Arendt, D. (2008). The evolution of cell types in animals: emerging principles from molecular studies. Nat. Rev. Genet. 9, 868–882. doi: 10.1038/nrg2416
Arendt, D., Tessmar-Raible, K., Snyman, H., Dorresteijn, A. W., and Wittbrodt, J. (2004). Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306, 869–871. doi: 10.1126/science.1099955
Arendt, D., and Wittbrodt, J. (2001). Reconstructing the eyes of Urbilateria. Philos. Trans. R. Soc. B Biol. Sci. 356, 1545–1563. doi: 10.1098/rstb.2001.0971
Beiras, R., and Widdows, J. (1995). Effect of the neurotransmitters dopamine, serotonin and norepinephrine on the ciliary activity of mussel (Mytilus edulis) larvae. Mar. Biol. 122, 597–603. doi: 10.1007/BF00350681
Bennett, M. F. (1979). “Extraocular Light Receptors and Circadian Rhythms,” in Comparative Physiology and Evolution of Vision in Invertebrates Handbook of Sensory Physiology. (Berlin, Heidelberg: Springer Berlin Heidelberg), 641–663.
Blevins, E., and Johnsen, S. (2004). Spatial vision in the echinoid genus Echinometra. J. Exp. Biol. 207, 4249–4253. doi: 10.1242/jeb.01286
Brandenburger, J. L., Woolacott, R. M., and Eakin, R. M. (1973). Fine structure of eyespots in tornarian larvae (Phylum: Hemichordata). Z. Zellforsch. Mikrosk. Anat. 142, 89–102. doi: 10.1007/BF00306706
Braubach, O. R., Dickinson, A. J. G., Evans, C. C. E., and Croll, R. P. (2006). Neural control of the velum in larvae of the gastropod, Ilyanassa obsoleta. J. Exp. Biol. 209, 4676–4689. doi: 10.1242/jeb.02556
Braun, K., Kaul-Strehlow, S., Ullrich-Lüter, E., and Stach, T. (2015). Structure and ultrastructure of eyes of tornaria larvae of Glossobalanus marginatus. Organ. Divers. Evol. 15, 423–428. doi: 10.1007/s13127-015-0206-x
Burke, R. D. (1978). Structure of nervous-system of pluteus larva of Strongylocentrotus-Purpuratus. Cell Tissue Res. 191, 233–247. doi: 10.1007/BF00222422
Burke, R. D., Angerer, L. M., Elphick, M. R., Humphrey, G. W., Yaguchi, S., Kiyama, T., et al. (2006). A genomic view of the sea urchin nervous system. Dev. Biol. 300, 434–460. doi: 10.1016/j.ydbio.2006.08.007
Byrne, M., Nakajima, Y., Chee, F. C., and Burke, R. D. (2007). Apical organs in echinoderm larvae: insights into larval evolution in the Ambulacraria. Evol. Dev. 9, 432–445. doi: 10.1111/j.1525-142X.2007.00189.x
Coppard, S. E., Kroh, A., and Smith, A. B. (2010). The evolution of pedicellariae in echinoids: an arms race against pests and parasites. Acta Zool. 93, 125–148. doi: 10.1111/j.1463-6395.2010.00487.x
D'Aniello, S., Delroisse, J., Valero-Gracia, A., Lowe, E. K., Byrne, M., Cannon, J. T., et al. (2015). Opsin evolution in the Ambulacraria. Mar. Genomics 24(Pt 2), 177–183. doi: 10.1016/j.margen.2015.10.001
Feuda, R., Hamilton, S. C., McInerney, J. O., and Pisani, D. (2012). Metazoan opsin evolution reveals a simple route to animal vision. Proc. Natl. Acad. Sci. U.S.A. 109, 18868–18872. doi: 10.1073/pnas.1204609109
Gühmann, M., Jia, H., Randel, N., Verasztó, C., Bezares-Calderón, L. A., Michiels, N. K., et al. (2015). Spectral tuning of phototaxis by a Go-opsin in the rhabdomeric eyes of platynereis. Curr. Biol. 25, 2265–2271. doi: 10.1016/j.cub.2015.07.017
Gustafson, T., Lundgren, B., and Treufeldt, R. (1972). Serotonin and contractile activity in the echinopluteus. A study of the cellular basis of larval behaviour. Exp. Cell Res. 72, 115–139. doi: 10.1016/0014-4827(72)90573-3
Hendler, G., and Byrne, M. (1987). Fine structure of the dorsal arm plate of Ophiocoma wendti: evidence for a photoreceptor system (Echinodermata, Ophiuroidea). Zoomorphology 107, 261–272. doi: 10.1007/BF00312172
Heyland, A., and Hodin, J. (2014). A detailed staging scheme for late larval development in Strongylocentrotus purpuratus focused on readily-visible juvenile structures within the rudiment. BMC Dev. Biol. 14:22. doi: 10.1186/1471-213X-14-22
Ho, E. C., Buckley, K. M., Schrankel, C. S., Schuh, N. W., Hibino, T., Solek, C. M., et al. (2016). Perturbation of gut bacteria induces a coordinated cellular immune response in the purple sea urchin larva. Immunol. Cell Biol. 94, 861–874. doi: 10.1038/icb.2016.51
Holmes, S. J. (1912). Phototaxis in the sea urchin Arbacia punctulata. J. Anim. Behav. 2, 126–136. doi: 10.1037/h0076037
Jékely, G. (2009). Evolution of phototaxis. Philos. Trans. R. Soc. B Biol. Sci. 364, 2795–2808. doi: 10.1098/rstb.2009.0072
Jékely, G., Colombelli, J., Hausen, H., Guy, K., Stelzer, E., Nédélec, F., et al. (2008). Mechanism of phototaxis in marine zooplankton. Nature 456, 395–399. doi: 10.1038/nature07590
Johnsen, S. (1994). Extraocular sensitivity to polarized light in an echinoderm. J. Exp. Biol. 195, 281–291.
Johnsen, S. (1997). Identification and localization of a possible rhodopsin in the echinoderms Asterias forbesi (Asteroidea) and Ophioderma brevispinum (Ophiuroidea). Biol. Bull. 193, 97–105. doi: 10.2307/1542739
Johnsen, S., and Kier, W. M. (1999). Shade-seeking behaviour under polarized light by the brittlestar Ophioderma Brevispinum (Echinodermata: Ophiuroidea). J. Mar. Biol. Assoc. 79, 761–763. doi: 10.1017/S0025315498000940
Kojima, D., Terakita, A., Ishikawa, T., Tsukahara, Y., Maeda, A., and Shichida, Y. (1997). A novel Go-mediated phototransduction cascade in scallop visual cells. J. Biol. Chem. 272, 22979–22982. doi: 10.1074/jbc.272.37.22979
Koyanagi, M., Takano, K., Tsukamoto, H., Ohtsu, K., Tokunaga, F., and Terakita, A. (2008). Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc. Natl. Acad. Sci. U.S.A. 105, 15576–15580. doi: 10.1073/pnas.0806215105
Koyanagi, M., Terakita, A., Kubokawa, K., and Shichida, Y. (2002). Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 531, 525–528. doi: 10.1016/S0014-5793(02)03616-5
Kuang, S. H., and Goldberg, J. I. (2001). Laser ablation reveals regulation of ciliary activity by serotonergic neurons in molluscan embryos. J. Neurobiol. 47, 1–15. doi: 10.1002/neu.1011
Lacalli, T. C., Gilmour, T., and West, J. E. (1990). Ciliary band innervation in the bipinnaria larva of Piaster ochraceus. Philos. Trans. R. Soc. B Biol. Sci. 371–390. doi: 10.1098/rstb.1990.0206
Leech, D. M., Padeletti, A., and Williamson, C. E. (2005). Zooplankton behavioral responses to solar UV radiation vary within and among lakes. J. Plankton Res. 27, 461–471. doi: 10.1093/plankt/fbi020
Leys, S. P., and Degnan, B. M. (2001). Cytological basis of photoresponsive behavior in a sponge larva. Biol. Bull. 201, 323–338. doi: 10.2307/1543611
Mackie, G. O., Spencer, A. N., and Strathmann, R. (1969). Electrical activity associated with ciliary reversal in an echinoderm larva. Nature 223, 1384–1385. doi: 10.1038/2231384a0
Maldonado, M., Durfort, M., McCarthy, D. A., and Young, C. M. (2003). The cellular basis of photobehavior in the tufted parenchymella larva of demosponges. Mar. Biol. 143, 427–441. doi: 10.1007/s00227-003-1100-1
Mano, H., and Fukada, Y. (2007). A median third eye: pineal gland retraces evolution of vertebrate photoreceptive organs. Photochem. Photobiol. 83, 11–18. doi: 10.1562/2006-02-24-IR-813
Marlow, H., Tosches, M. A., Tomer, R., Steinmetz, P. R., Lauri, A., Larsson, T., et al. (2014). Larval body patterning and apical organs are conserved in animal Evolution 12, 1–17. doi: 10.1186/1741-7007-12-7
Marsden, J. R. (1984). Swimming in response to light by larvae of the tropical serpulid Spirobranchus giganteus. Mar. Biol. 83, 13–16. doi: 10.1007/BF00393081
Marshall, D. J., and Hodgson, A. N. (1990). Structure of the cephalic tentacles of some species of prosobranch limpet (Patellidae and Fissurellidae). J. Molluscan Stud. 56, 415–424. doi: 10.1093/mollus/56.3.415
Mason, B. M., and Cohen, J. H. (2012). Long-Wavelength photosensitivity in coral planula larvae. Biol. Bull. 222, 88–92. doi: 10.1086/BBLv222n2p88
Millott, N. (1954). Sensitivity to light and the reactions to changes in light intensity of the echinoid Diadema antillarum Philippi. Philos. Trans. R. Soc. B Biol. Sci. 238, 187–220. doi: 10.1098/rstb.1954.0009
Millott, N., and Manly, B. M. (1961). The iridophores of the echinoid Diadema antillarum. J. Cell Sci. s3–102, 181–194.
Millot, N., and Yoshida, M. (1958). The Photosensitivity of the sea echinoid Diadema antillarum Phillipi: responses to increases in light intensity. Proc. Zool. Soc. Lond. 133, 67–71.
Nakajima, Y., Kaneko, H., Murray, G., and Burke, R. D. (2004). Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, 95–104. doi: 10.1111/j.1525-142X.2004.04011.x
Nielsen, C. (2008). Six major steps in animal evolution: are we derived sponge larvae? Evol. Dev. 10, 241–257. doi: 10.1111/j.1525-142X.2008.00231.x
Nilsson, D. E. (1996). Eye ancestry: old genes for new eyes. Curr. Biol. 6, 39–42. doi: 10.1016/S0960-9822(02)00417-7
Nilsson, D. E. (2009). The evolution of eyes and visually guided behaviour. Philos. Trans. R. Soc. B Biol. Sci. 364, 2833–2847. doi: 10.1098/rstb.2009.0083
Nilsson, D. E. (2013). Eye evolution and its functional basis. Vis. Neurosci. 30, 5–20. doi: 10.1017/S0952523813000035
Nordström, K., Wallén, R., Seymour, J., and Nilsson, D. (2003). A simple visual system without neurons in jellyfish larvae. Proc. R. Soc. B Biol. Sci. 270, 2349–2354. doi: 10.1098/rspb.2003.2504
Passamaneck, Y. J. (2011). Ciliary photoreceptors in the cerebral eyes of a protostome larva. Evodevo 2:6. doi: 10.1186/2041-9139-2-6
Passamaneck, Y. J., and Martindale, M. Q. (2013). Evidence for a phototransduction cascade in an early brachiopod embryo. Integr. Comp. Biol. 53, 17–26. doi: 10.1093/icb/ict037
Paul, N. D., and Gwynn-Jones, D. (2003). Ecological roles of solar UV radiation: towards an integrated approach. Trends Ecol. Evol. 18, 48–55. doi: 10.1016/S0169-5347(02)00014-9
Pires, A., and Woollacott, R. M. (1997). Serotonin and dopamine have opposite effects on phototaxis in larvae of the bryozoan Bugula neritina. Biol. Bull. 192, 399–409. doi: 10.2307/1542749
Plachetzki, D. C., Degnan, B. M., and Oakley, T. H. (2007). The origins of novel protein interactions during animal opsin evolution. PLoS ONE 2:e1054. doi: 10.1371/journal.pone.0001054
Porter, M. L., Blasic, J. R., Bok, M. J., Cameron, E. G., Pringle, T., Cronin, T. W., et al. (2011). Shedding new light on opsin evolution. Proc. R. Soc. B Biol. Sci. 279, 3–14. doi: 10.1098/rspb.2011.1819
Provencio, I., Jiang, G., De Grip, W. J., Hayes, W. P., and Rollag, M. D. (1998). Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. U.S.A. 95, 340–345. doi: 10.1073/pnas.95.1.340
Raible, F., Tessmar-Raible, K., Arboleda, E., Kaller, T., Bork, P., Arendt, D., et al. (2006). Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 300, 461–475. doi: 10.1016/j.ydbio.2006.08.070
Ramirez, M. D., Pairett, A. N., Pankey, M. S., Serb, J. M., Speiser, D. I., Swafford, A. J., et al. (2016). The last common ancestor of bilaterian animals possessed at least 7 opsins. bioRxiv 052902. doi: 10.1101/052902
Ransick, A. (2004). Detection of mRNA by in situ hybridization and RT-PCR. Methods Cell Biol. 74, 601–620. doi: 10.1016/S0091-679X(04)74024-8
Raup, D. M. (1966). “The endoskeleton,” in Physiology of Echinodermata, ed R. A. Boolootian (New York, NY: Interscience), 379–395.
Ryberg, E., and Lundgren, B. O. (1977). Extra-ectodermal strands in the ciliated bands of the echinopluteus. Dev. Growth Differ. 19, 299–308. doi: 10.1111/j.1440-169X.1977.00299.x
Schmidt, T. M., Chen, S.-K., and Hattar, S. (2011). Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580. doi: 10.1016/j.tins.2011.07.001
Smith, F. (1935). The development of Patella vulgata. Philos. Trans. R. Soc. B Biol. Sci. 225, 95–125. doi: 10.1098/rstb.1935.0008
Smith, M. M., Cruz Smith, L., Cameron, R. A., and Urry, L. A. (2008). The larval stages of the sea urchin, Strongylocentrotus purpuratus. J. Morphol. 269, 713–733. doi: 10.1002/jmor.10618
Sodergren, E., Weinstock, G. M., Davidson, E. H., Cameron, R. A., Gibbs, R. A., Angerer, R. C., et al. (2006). The Genome of the Sea Urchin Strongylocentrotus purpuratus. Science 314, 941–952. doi: 10.1126/science.1133609
Thornton, I. W. B. (1956). Diurnal migrations of the echinoid Diadema setosum (Leske). Br. J. Anim. Behav. 4, 143–146. doi: 10.1016/S0950-5601(56)80108-1
Thorson, G. (1964). Light as an ecological factor in the dispersal and settlement of larvae of marine bottom invertebrates. Ophelia. 1, 167–208. doi: 10.1080/00785326.1964.10416277
Tosches, M. A., Bucher, D., Vopalensky, P., and Arendt, D. (2014). Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 159, 46–57. doi: 10.1016/j.cell.2014.07.042
Tu, Q., Cameron, R. A., and Davidson, E. H. (2014). Quantitative developmental transcriptomes of the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 385, 160–167. doi: 10.1016/j.ydbio.2013.11.019
Ullrich-Lüter, E. M., D'Aniello, S., and Arnone, M. I. (2013). C-opsin expressing photoreceptors in echinoderms. Integr. Comp. Biol. 53, 27–38. doi: 10.1093/icb/ict050
Ullrich-Lüter, E. M., Dupont, S., Arboleda, E., Hausen, H., and Arnone, M. I. (2011). Unique system of photoreceptors in sea urchin tube feet. Proc. Natl. Acad. Sci. U.S.A. 108, 8367–8372. doi: 10.1073/pnas.1018495108
Van Gelder, R. N. (2008). Non-visual photoreception: sensing light without sight. Curr. Biol. 18, R38–R39. doi: 10.1016/j.cub.2007.11.027
Vöcking, O., Kourtesis, I., and Hausen, H. (2015). Posterior eyespots in larval chitons have a molecular identity similar to anterior cerebral eyes in other bilaterians. Evodevo 6, 1. doi: 10.1186/s13227-015-0036-0
Wada, Y., Mogami, Y., and Baba, S. (1997). Modification of ciliary beating in sea urchin larvae induced by neurotransmitters: beat-plane rotation and control of frequency fluctuation. J. Exp. Biol. 200, 9–18.
Woodley, J. D. (1982). “Photosensitivity in Diadema antillarum: Does it show scototaxis?” in The International Echinoderm Conference, Tampa Bay September 14–17, 1981, ed J. M. Lawrence (Rotterdam: AA Balkema), 61.
Yaguchi, S., and Katow, H. (2003). Expression oftryptophan 5-hydroxylase gene during sea urchin neurogenesis and role of serotonergic nervous system in larval behavior. J. Comp. Neurol. 466, 219–229. doi: 10.1002/cne.10865
Yerramilli, D., and Johnsen, S. (2010). Spatial vision in the purple sea urchin Strongylocentrotus purpuratus (Echinoidea). J. Exp. Biol. 213, 249–255. doi: 10.1242/jeb.033159
Keywords: eye evolution, Go-opsin, invertebrate larvae, r-opsin, sea urchin, zooplankton
Citation: Valero-Gracia A, Petrone L, Oliveri P, Nilsson D-E and Arnone MI (2016) Non-directional Photoreceptors in the Pluteus of Strongylocentrotus purpuratus. Front. Ecol. Evol. 4:127. doi: 10.3389/fevo.2016.00127
Received: 07 August 2016; Accepted: 18 October 2016;
Published: 14 November 2016.
Edited by:
Wayne Iwan Lee Davies, University of Western Australia, AustraliaReviewed by:
Karen Carleton, University of Maryland, College Park, USACopyright © 2016 Valero-Gracia, Petrone, Oliveri, Nilsson and Arnone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria I. Arnone, bWlhcm5vbmVAc3puLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.