- 1School of Biological Sciences, University of Tasmania, Hobart, TAS, Australia
- 2Department of Zoology, Edward Grey Institute, University of Oxford, Oxford, UK
- 3Department of Biology, Lund University, Lund, Sweden
Parental care emerges as a result of an increase in the extent of interaction between parents and their offspring. These interactions can provide the foundation for the evolution of a range of complex parental behaviors. Therefore, fundamental to understanding the evolution of parental care is an understanding of the factors that promote this initial increase in parent-offspring association. Here, we used large outdoor enclosures to test how the spatial structure of high-quality habitat affects the occurrence of parent-offspring associations in a social lizard (Liopholis whitii). We found that the extent of parent-offspring association was higher when high-quality habitat was aggregated relative to when it was dispersed. This may be the result of greater competitive exclusion of adults and offspring from high quality crevices sites in the aggregated treatment compared to the dispersed treatment. Associating with parents had significant benefits for offspring growth and body condition but there were no concomitant effects on offspring survival. We did not find costs of parent-offspring association for parents in terms of increased harassment and loss of body condition. We discuss a number of potential mechanisms underlying these results. Regardless of mechanisms, our results suggest that habitat structure may shape the extent of parent-offspring association in L. whitti, and that highly aggregated habitats may set the stage for the diversification of more complex forms of care observed across closely related species.
Introduction
The evolution of parental care is associated with an increase in the level of social interactions between parents and offspring (Clutton-Brock, 1991; Smiseth et al., 2012). The result is a range of complex and diverse parental behaviors (e.g., parental provisioning, care after nutritional dependence) which can have profound effects on offspring development and fitness (Clutton-Brock, 1991; Uller, 2012; Klug and Bonsall, 2014). These characteristics also make parental care a key point in the adaptive radiation of kin relationships and sociality (Queller, 1994; Field and Brace, 2004). Thus, there is enormous interest in understanding the factors responsible for the initial emergence and subsequent diversification of parental care.
The majority of research on the evolution of parental care has focussed on identifying the benefits of parental investment for offspring fitness (reviewed in Clutton-Brock, 1991; Royle et al., 2012). Such benefits may be necessary for parental care to be selected; however, the emergence of parental care will be facilitated, first and foremost, when parents regularly encounter and associate with their offspring (Lion and Van Baalen, 2007). Therefore, conditions that promote increased levels of association between parents and their offspring will be central to facilitating the early emergence of parental care (Wilson, 1975; Lion and Van Baalen, 2007; Davis et al., 2011; Klug et al., 2012). Habitat availability and quality are particularly important in this context. For example, limited availability of suitable habitat can encourage offspring to delay dispersal and remain within the parental home range (Hatchwell and Komdeur, 2000; Covas and Griesser, 2007). If this carries little or no cost to the parents, they may tolerate offspring, resulting in an increased level of parent-offspring association. Where these environmental conditions are recurrent, parent-offspring associations can create a novel selective environment from which more complex forms of parental care, such as parental provisioning, can evolve (e.g., Wong et al., 2013). Indeed, theoretical models have shown that once this initial increase in parent-offspring association emerges parental care can rapidly diversify and increase in complexity (e.g., Gardner and Smiseth, 2011).
Species in which parent-offspring associations are facultative or temporary, such as those exhibited by some insects, fish, amphibians and lizards, provide excellent opportunities for establishing the conditions that promote the early evolution of parental care (Kölliker, 2007; Falk et al., 2014). In lizards, post hatching parent-offspring associations have been demonstrated in at least 60 species (Somma, 2003; While et al., 2014). In most cases these associations are characterized by semi-independent offspring remaining within the parental home range (While et al., 2014). While this level of parent-offspring association is considerably simpler than in many other vertebrates, it is characteristic of what we would expect in the early stages of the evolution of postnatal parental care. Thus, these taxa provide an opportunity to study how selection on more complex forms of parental care initially arise, by examining the factors that influence increased parent-offspring association and the costs and benefits of this association for both parties.
Here we conduct an experimental test of how habitat structure influences parent-offspring associations and the consequences of this for offspring growth and survival in a social lizard species, Liopholis whitii. L. whitii lives in family groups characterized by stable (often life-long) male-female pair bonds and prolonged parent-offspring associations (Chapple and Keogh, 2005, 2006; While et al., 2009a). These prolonged associations involve offspring delaying dispersal and parents tolerating offspring within their core home ranges, sometimes for up to several years. This has two potential benefits to offspring. First, offspring that associate with their parents may gain access to parental resources (i.e., food within a parent's habitat) and hence benefit in terms of increased growth and/or condition (O'Connor and Shine, 2004; but see Langkilde et al., 2007). Second, offspring may gain survival benefits through protection from infanticide (Sinn et al., 2008). Tolerance of offspring within the home range may, however, also have costs to adults. For example, parental body condition may be reduced through sharing resources with their offspring. Parents may also suffer injury and/or reduced body condition through increased harassment from hungry conspecifics.
We manipulated habitat structure by manipulating the distribution of available crevices sites. Rock crevice and burrow sites are a key component of L. whitii's ecology and it has been suggested that the structure and availability of these sites is fundamental in determining the extent of parent-offspring associations in L. whitii and related species (Duffield and Bull, 2002; While et al., 2009a). We created two experimental treatments which differed in the spatial association of crevice sites, a dispersed crevice site treatment whereby available crevice sites were dispersed evenly across the environment and an aggregated crevice site treatment, where available crevice sites were clumped together in a central location. We predicted that the incidence of parent-offspring associations would be lower when suitable crevice sites were clumped, as clumping of high quality habitats should lead to more frequent agonistic encounters over access to high quality habitats between adult lizards from different pairs and thus result in higher costs (and hence a lower incidence) of parents tolerating offspring within their home range and defending these offspring from conspecific aggression.
Methods
Study Species
L. whitii is a medium sized [75−100 mm snout-vent length (SVL)] viviparous skink that occurs throughout south-eastern Australia, including Tasmania (Chapple, 2003; Wilson and Swan, 2003). It occupies a broad range of habitats (including coastal heaths, grasslands, woodlands and dry sclerophyll forests) and altitudes (0−1600 m) (Cogger, 2000; Chapple, 2003; Wilson and Swan, 2003). Typically, L. whitii are closely associated with complex burrow systems under/around rocks and shrubs (Chapple, 2003; Wilson and Swan, 2003) where they typically focus their basking and foraging activities (Greer, 1989). Morphological and life history traits vary geographically in L. whitii (Chapple, 2003). Tasmanian populations are sexually monomorphic, mature at approximately 3 years and have a lifespan of 9−10 years (While et al., 2009b). Reproduction occurs annually, with breeding occurring in the austral spring (September–October), and gestation lasting 3−4 months (While et al., 2007). Parturition occurs in the austral summer (January–February) with litters comprising one to four offspring (most frequently two) born asynchronously, usually over several days (While et al., 2007).
Experimental Protocol
We caught 160 L. whitii (80 males, 80 females) sourced from populations on the east coast of Tasmania (approximately 42°57′ S, 147°88′ E) at the start of the breeding season (September, 2013). Once captured, animals were transported in cool, damp cloth bags back to the University of Tasmania (approximately an hour drive from the populations). At the University, lizards were weighed (±1 mg), measured for SVL and total length (±0.5 mm), indications of previous tail loss recorded, and gender determined via eversion of hemipenes. Each lizard was uniquely toe-clipped to enable individual identification. Lizards were then housed individually in plastic terraria (30 × 60 × 40 cm) kept under a 25 W basking light set to an 8:16 h light/dark cycle with overhead lights set on a 10:14 h light/dark cycle. Each terrarium had a basking rock underneath the basking light, with a wooden shelter at the opposite end of the shelter. Lizards were provided with water and food (Tenebrio larvae and fruit puree mixed with protein powder) ad libitum. Lizards were then moved to our large enclosure facilities at the University of Tasmania's Cambridge Farm facility (16 enclosures, each measuring 8 × 8 m), and assigned to one of two experimental treatments (eight enclosures per experimental treatment).
The enclosures consisted of eight replicates for each of two treatments: (1) a dispersed crevice site treatment, and (2) an aggregated crevice site treatment. The crevice sites within the enclosures were constructed from either a wooden pallet, six hollow concrete bricks (Besser blocks) and sand (representing high quality habitat) or two Besser blocks only (representing low quality habitat). Each treatment had five of these high quality crevice sites and four low quality crevice sites, but the arrangement of crevice sites between treatments differed, with the high quality crevice sites spread apart in the dispersed treatment and aggregated together in the center of enclosure in the aggregated treatment (Figure 1, Supplementary Figure 1). Enclosure treatments were paired, with the order of treatments randomized within each pair. All enclosures were covered by bird netting to prevent predation by birds. While this removes potential sources of mortality for both adult and offspring it is unlikely to influence the costs and benefits of parent-offspring association themselves. Parent-offspring associations are unlikely to function in a predator protection context, given that the majority of predators are significantly larger than an adult L. whitii (e.g., include feral cats, snakes and large birds, such as kookaburras and ravens). Indeed, the main source of mortality for offspring and harassment for parents is conspecifics (Lanham and Bull, 2000; O'Connor and Shine, 2006). The enclosures were stocked with water containers and live insects throughout the duration of the experiment, with these resources distributed evenly throughout the enclosures.
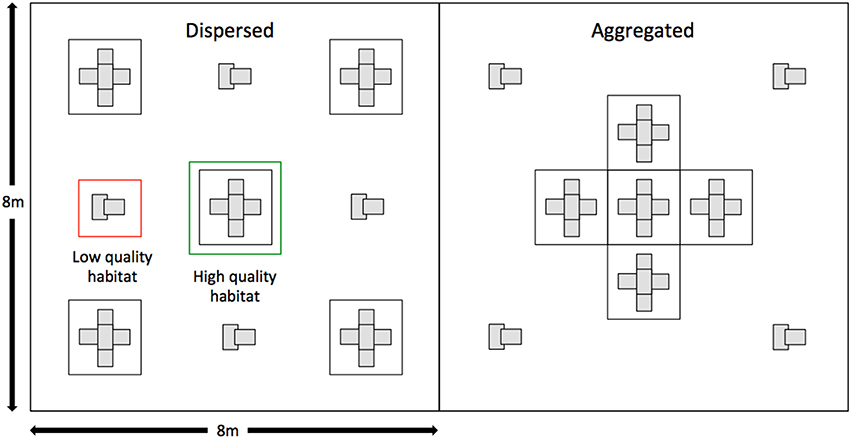
Figure 1. Diagrammatic representation of the two treatments used in the experiment. On the left is the dispersed habitat treatment and on the right is the aggregated treatment. Areas representing high quality and low quality habitat indicated.
Lizards were introduced into enclosures in October 2013. Five females and five males were randomly assigned to each enclosure. This represented a similar, albeit slightly higher, density to that found in natural populations (G. M. While pers. observation). This also resulted in a match between the number of male/female pairs and number of high quality crevice sites in each enclosure. Hence, the treatments differed only in the layout of the high quality crevice sites, which were expected to be preferentially occupied by male/female pairs. Lizards were semi-permanently marked with numbered cloth tape (Tesa, Hamburg, Germany) to enable identification through observation of individuals. From October to December 2013, the lizards were observed up to twice daily, once in the early morning and once in the afternoon, by one experimenter (BH). These time periods were chosen to correspond with the time when lizards are most active and do the majority of their basking before seeking shelter in the middle of the day. The order of observations was rotated so that the starting enclosure differed each observation session, while the order or enclosures observed was consistent. Due to the weather dependence of lizard activity it was not always possible to record observations at set times and in these instances observations were taken opportunistically during the day whenever the weather permitted activity. During observation session, data were collected on the locations of lizards in each enclosure and additional data were taken on any observed interactions between lizards (such as fights or copulations). Observations were made at least 1 m from each enclosure to avoid disturbing lizards, and an observation session ended when all enclosures had been thoroughly observed (typically taking 10 min per enclosure) or when the lizards' activity period finished (i.e., when the weather became too hot or cold). If two observation sessions were taken on the same the day, they were taken at least 4 h apart to reduce spatial autocorrelation of individual locations. In total we collected 2874 observations.
At the start of January 2014 (i.e., at the end of gestation) individuals were captured from the enclosures, brought back to the University, and housed as described above. Upon return to the laboratory individuals were measured for the same traits taken at the start of the breeding season (see above). Female terraria were checked daily for the birth of offspring. Upon birth, the date of birth was recorded and offspring weight [±1 mg (SE)], SVL (±0.5 mm) and total length (±0.5 mm) were measured. Each juvenile was then given a unique toe-clip for identification purposes. In total 67 females were recaptured in January, 37 of which gave birth. This represents 55% of the female population reproducing, which is equivalent to that observed in the natural population where only 68% of females give birth in a given year (While et al., 2009b; see also Chapple, 2003 for the consistency of this pattern across Egernia species). Before release, offspring were marked with a colored bee tag attached with non-toxic glue (Pender Beekeeping Supplies), with five different colors corresponding to a particular mother (Supplementary Figure 2). Position of the tag along the offspring's back identified which member of a litter it was (on the neck = 1st born offspring, on middle of the back = 2nd born offspring, on the pelvis = 3rd born offspring; no litters contained more than three offspring).
All individuals were then re-released into the enclosures from early to late February 2014. All individuals were released at the crevice site within the enclosure that they were most frequently observed (based on the 2874 observational data points; see above). Offspring were released with their mother at their mother's main crevice site. Daily observations were then conducted by two experimenters (TBJ and EB) across both treatments following the same protocols described for the October–December 2013 period. This resulted in a total of 4235 independent observations for all individuals combined over 85 observational sessions.
Lizards were recaptured from late April to early May 2014, and brought back to the University. On average, parents and offspring were in the enclosures for 58.75 ± 0.52 days. The adults were measured for weight (±1 mg), SVL (±0.5 mm), total length (±0.5 mm), toe and tail loss. The juveniles were measured for weight (±1 mg), SVL (±0.5 mm), total length (±0.5 mm) and tail loss. All individuals were then released back into the natural populations from which they came.
Parentage Assignment
All individuals included in the study were genotyped for six microsatellite loci (EST1, EST2, EST4, EST12: Gardner et al., 1999; TruL12, TruL28: Gardner et al., 2008) using standard molecular techniques with DNA extracted from tail tip samples (see While et al., 2009a,b for further details). Paternity was assigned using the computer program CERVUS 3.0 (Marshall et al., 1998) using the following simulation parameters: 10,000 cycles, 95% of candidate parents sampled, 85% loci typed and a genotyping error rate of 1% (calculated in CERVUS from our data). The one known parent option was used with all adult males released into the same enclosure as the mother included as possible fathers. Paternity was assigned to the male with the highest male-female-offspring trio LOD score and the lowest number of mismatches (0 or 1) (e.g., Foerster and Kempenaers, 2004; Chapple and Keogh, 2005). Because there were only five possible fathers for any offspring within an enclosure, paternity could be assigned with high confidence in the majority (>90%) of cases. Seven out of 76 offspring could not be confidently assigned paternity (had ≥2 loci mismatches) and were thus excluded from analyses of father-offspring association.
Home Range Analysis and Assignment of Parent-Offspring Associations
Parent home ranges were constructed using the program ArcView3.3 (ESRI) using a fixed kernel analysis with a least squares cross-validation smoothing parameter (Powell, 2000). Core home range was calculated using 50% isopleths. For L. whitii and related species, this area represents an individual's permanent shelter site from which it basks, feeds and undertakes the majority of its social behavior (e.g., While et al., 2009a). Adults with less than eight observations were excluded from the analysis (n = 20) as home ranges could not be constructed for these individuals. The low number of average sightings of juveniles relative to adults (juveniles = 8 ± 1, adults = 25 ± 1) prevented the assignment of presence or absence of parent-offspring association based on parent-offspring home range overlap. Instead, based on long-term monitoring of a wild population for which home range overlap is available (While et al., 2009a,b) we defined a parent-offspring association when juveniles had 50% or more of their observations within their parent's core home range area (see also While et al., 2009a,b). The average percentage of observations for offspring assigned as being associated with their parents was 73 ± 5 and 72 ± 9% for mothers and fathers, respectively, compared to 8 ± 2 and 3 ± 1% for offspring who were not associated with their parents.
Data Analyses
Data were analyzed using General and Generalized Linear Mixed Models fitted using maximum likelihood implemented in R version 3.0.2 (R development core team 2014), using either the “glmer” (for binary response variables) or “lmer” function (for continuous response variables) under the “lme4” package (Bates et al., 2012). All models used the Laplace approximation to estimate model parameters, as it is considered a more accurate technique than the simpler pseudo quasi-likelihood estimation method (Bolker et al., 2009). Models regarding offspring traits included parental ID as a random factor to account for non-independence arising from litters containing multiple offspring. All models also included enclosure as a random factor to account for differences between enclosures. Because of limited sample size we ran main effects models only and models for maternal and paternal parental-offspring association were run separately. The low incidence of bi-parental parental-offspring association (only 2 cases total) precluded its analysis.
All fixed effects were tested with Wald's χ2 and type III F-tests (Kenward-Rogers approximation for F-tests) obtained with the “car” package (Fox et al., 2014). All models were checked for violation of assumptions. All results are reported as means, with standard errors as the measure of variability.
Parent-Offspring Association
The effect of habitat structure on the extent of parent-offspring association was analyzed by examining the proportion of parents whose offspring remained within their home range, using a Generalized Linear Mixed Model with the binomial family specified. These models included treatment (clumped vs. aggregated treatment) as a fixed factor, parental body condition as a covariate, and enclosure as a random factor. Body condition (as an indicator of an individual's energy stores relative to structural components of the body) was measured by taking mass divided by SVL. This has been suggested as a reliable index of body condition (Green, 2001; Labocha et al., 2014). Analyses of body condition excluded individuals who had lost tails (as tail loss affects mass and therefore estimates of body condition). We then examined the relative occurrence of parent-offspring associations on high vs. low quality habitats between treatments, and the extent to which adults and offspring occupied high vs. low quality habitats between treatments. We assigned individuals as occupying either high or low quality habitat based on the location of their home ranges (for adults) or where 50% or more of their observations occurred (for offspring). Individuals whose home range or majority of observations occurred primarily on grass areas (i.e., neither pallets nor Besser blocks) were excluded from analysis. These analyses were run using Generalized Linear Mixed Model's with treatment as a fixed factor and enclosure as a random factor.
Consequences of Parent-Offspring Association for Offspring
Benefits of parent offspring association for offspring were analyzed in terms of skeletal growth, body condition and survival. Change in SVL between the start and end of the experiment was used to assess offspring skeletal growth (SVL is a common measure of growth for reptiles—e.g., Shine and Charnov, 1992). Analysis of growth used a General Linear Mixed Model with treatment (i.e., aggregated vs. dispersed habitat treatments), and mother-offspring association as fixed factors, the number of days spent in an enclosure as a covariate, and maternal and enclosure ID as random effects. The difference in offspring body condition between treatments at the end of the experiment was analyzed using a Generalized Linear Mixed Model with treatment and mother-offspring association as fixed factors, initial offspring body condition and the number of days spent in an enclosure as covariates, and maternal and enclosure ID as random effects. As there were only 3 cases where offspring associated with their father and we had corresponding measurements for offspring growth and body condition, we ran the above models for mother-offspring association only. Finally, we analyzed differences in offspring survival by running a Generalized Linear Mixed Model on the survival status of offspring (recaptured = survived, not recaptured = dead) at the end of the experiment. These models had mother-offspring association (yes or no), father-offspring association (yes or no) and treatment as fixed factors and parental and enclosure ID as random effects.
Consequences of Parent-Offspring Association for Parents
Increased parent-offspring association may have a number of fitness costs for parents in terms of decreased body condition associated with sharing resources with offspring and increased harassment from other lizards. To test how marks of harassment varied between parents who associated with their own offspring and those who did not and between the two treatments, we used both tail and toe loss as a proxy (loss of tails and toes are key indicators of intraspecific competition in lizards, especially where predation has been eliminated: Norris, 1953; Tinkle, 1967; Vitt et al., 1974). Tail and toe loss were entered as a binary response variable in four separate main effects models (one for each sex and each trait), with parent-offspring association and treatment as fixed factors. Including enclosure ID as a random factor resulted in poor model convergence and was excluded from these models. To test consequences of parent-offspring associations for adult body condition, we ran a General Linear Mixed Model with parent body condition at the end of the experiment as a response variable, parent-offspring association and treatment as fixed factors, initial parental body condition as a covariate, and enclosure ID. as a random factor.
Results
Seventy six offspring were born in the laboratory to 37 mothers (average brood size = 2.05 ± 0.13) and released with their parents into the large outdoor enclosures. Thirty nine of the 76 offspring released into the enclosures were recaptured at the conclusion of the observation sessions in April/May, representing an overall survival of 53%. Survival of adults from release at the start of February until April/May was high, at 95%. Average adult home range size during this period was the same between treatments [aggregated = 7.81 ± 0.72 m2, dispersed = 6.42 ± 1.05 m2; F(1, 13.58) = 1.20, p = 0.29] and between males and females [males = 6.34 ± 0.51 m2, females = 7.89 ± 1.20 m2; F(1, 122.78) = 1.48, p = 0.23]. The extent to which low vs. high quality crevice sites were occupied by adults differed significantly between treatments (χ2 = 21.44, p < 0.01). In the aggregated treatment 14 of the 39 adults who occupied crevice sites occupied high quality sites (36%) compared to 46 of the 54 adults (85%) in the dispersed treatment. For offspring in the aggregated treatment 6 of the 13 (46%) offspring who occupied crevice sites occupied high quality sites compared to 5 out of the 10 offspring (50 %) in the dispersed treatment (χ2 = 1.00, p = 0.32). The remaining adults and offspring established themselves away from the crevice sites in grass patches or the perimeter of the enclosure.
Parent-Offspring Association
Of the 37 females who produced offspring, there was sufficient observational data to establish the extent of mother-offspring association for 34 mothers. Overall, 12 out of these 34 mothers (35%) associated with their offspring. The extent of mother-offspring association differed significantly between treatments (Table 1). Specifically, 9 out of 15 mothers (60%) associated with their offspring in the aggregated treatment compared to 3 out of 19 (16%) in the dispersed treatment. Of the 28 males who sired offspring, there was sufficient observational data to establish the extent of father-offspring association for 25 fathers. Overall, 7 of these 25 fathers (28%) associated with their offspring; 5 out of 12 fathers (43%) in the aggregated treatment and 2 out of 13 (15%) in the dispersed treatment. This difference failed to reach statistical significance (Table 1). There was no effect of a mother's or father's initial body condition on whether or not they associated with their offspring (Table 1). An analysis at the offspring level produced qualitatively similar results, with 35 and 19% of offspring in the aggregated treatment associating with their mother and father, respectively, compared to 9 and 10% in the dispersed treatment. However, these differences were not statistically significant (Mother: χ2 = 2.32, p = 0.13; Father: χ2 = 0.095, p = 0.33). The ratio of parent-offspring associations formed on low vs. high quality crevice sites was higher in the aggregated compared to the dispersed treatment. Only five out of 14 parent-offspring associations (36%) in the aggregated treatment occurred on high quality crevice sites, compared to 4 out of 5 (80%) in the dispersed treatment. This difference, however, was not significant (χ2 = 0.95, p = 0.33).
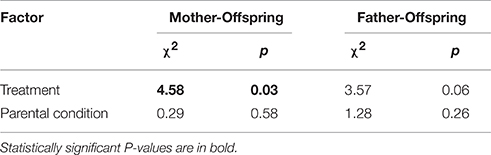
Table 1. Output from models testing for differences in parent-offspring association in Liopholis whitii between treatments and in relation to parental condition.
Consequences of Parent-Offspring Association for Offspring
Sixty nine percent (9/13) of offspring that associated with their mother survived, compared to 62% (25/40) offspring that did not (χ2 = 0.06, p = 0.81). These results were mirrored in the data collected on paternal-offspring association (χ2 = 1.85, p = 0.17). Specifically, 3 out of 7 (43%) offspring that associated with their father survived, and 25 out of 40 (62%) offspring that did not associate with their father survived. Offspring survival did not differ between treatments [aggregated treatment = 57% (20/35), dispersed treatment = 43% (19/41); χ2 = 1.03, p = 0.31].
Mother-offspring association had a significant effect on offspring growth and body condition (Table 2). Offspring that were associated with their mother had increased growth and were in better body condition at the end of the experiment relative to those who were not (Figures 2A,B). There was no significant effect of treatment on either offspring SVL growth or change in body condition (Table 2).
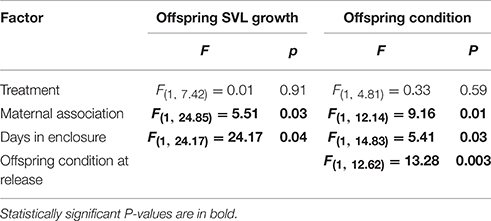
Table 2. Output from models testing for effect of treatment and parent-offspring association on offspring growth and condition in Liopholis whitii.
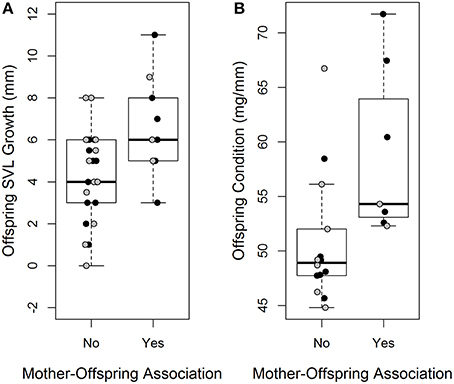
Figure 2. Difference in offspring svl growth (mm) (A) and offspring body condition (mg/mm) (B) between offspring who associated with their mother and those who did not. Black data points indicate offspring from the clumped treatment, gray data points indicate offspring from the dispersed treatment.
Consequences of Parent-Offspring Association for Parents
We found no costs of increased parent-offspring association for mothers or fathers in the form of harassment suffered from conspecifics (e.g., frequency of tail and toe loss did not differ between treatments for mothers or fathers; Table 3). There was no difference in mother or father body condition at the end of the experiment between those parents who did and did not associate with their offspring nor were there any differences between treatments (Table 3).
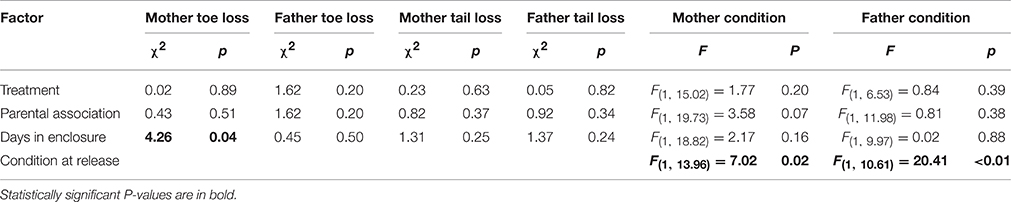
Table 3. Output from models testing for effect of treatment and parent-offspring association on parental condition and harassment in Liopholis whitii.
Discussion
Testing how the structure of the environment influences associations between offspring and their parents is fundamental to our understanding of the origins of parental care (Gardner and Smiseth, 2011; Klug et al., 2012). Here we show that approximately a third of males and females associate with their offspring following birth. This level of parent-offspring association is in accordance with what is observed in the wild, where the number of parents associating with offspring can vary from between 10 and 70% (While et al., 2009b; Botterill-James et al. unpublished data). We further show that the structure of high quality habitat significantly influenced the extent of parent offspring association. This increased parent-offspring association has benefits for offspring growth and body condition, but does not appear to carry a substantial cost for parents. Below, we discuss our results in the context of findings in other species, the mechanisms which may underlie the observed effects of habitat structure on parent-offspring associations, and discuss the broader implications of these findings for the evolutionary origins of more complex forms of parental care.
Habitat structure and availability is an important ecological variable in L. whitii, which has been suggested to influence the social complexity of this and other species of Egernia (Duffield and Bull, 2002; Chapple, 2003; O'Connor and Shine, 2003; While et al., 2009a). Here we experimentally demonstrate that the spatial aggregation of high-quality crevice sites promote parent-offspring association. Specifically, both mothers and fathers were more likely to associate with their offspring when high quality habitat was aggregated compared to when it was dispersed, although that latter result failed to reach statistical significance. These results are consistent with the suggestion that the availability and structure of habitat are key to facilitating the evolution of postnatal parental care by increasing habitat sharing between closely related individuals (Wilson, 1975; Lion and Van Baalen, 2007). This is believed to be fundamental to the formation of family groups across the Egernia (Duffield and Bull, 2002; Chapple, 2003; O'Connor and Shine, 2003; While et al., 2009a), but current empirical evidence for this hypothesis is mixed. For example, manipulation of shelter availability in E. striolata altered adult pair bonding, with more pairs forming when shelter availability was low (Lancaster et al., 2011), whereas Gardner et al. (2007) found no effect of crevice site abundance on social group structure in Egernia stokesii.
Despite a general effect of habitat structure on parent-offspring association the direction of this effect requires some explanation. Specifically, there was a greater level of parent-offspring association when high-quality habitat was aggregated compared to when it was dispersed. This is perhaps counter-intuitive; it might be expected that there would be strong costs to parents from associating with offspring in the aggregated treatment, due to increased harassment from conspecifics relative to the dispersed treatment. However, we found little evidence that parental-offspring association carries costs to either parent. The analysis looking at where adults and offspring settled within enclosures suggests an alternative explanation. Adult, but not offspring, occupation of low vs. high quality habitats differed between treatments; more adults were present on low quality habitats in the aggregated treatment, probably as a result of competitive exclusion from home ranges of dominant individuals. There were also more parent-offspring associations formed on low vs. high quality habitats in the aggregated treatment (although the low statistical power limited the confirmation that this deviated from the null expectation of no difference between habitats). The tight spacing of crevice sites in the aggregated treatment may therefore have facilitated their monopolization by a small proportion of adults while the majority of (more subordinate) adults were forced into the lower quality areas. This would then increase habitat saturation and reduce the overall availability of crevice sites (both of high and low quality) facilitating greater overlap of habitat use between these adults and their offspring, with this overlap then maintained by no/low costs of parent-offspring association for adults. Therefore, enhanced parent-offspring association may be a result of some adults being restricted to low quality habitats where the majority of offspring are residing as opposed to any benefits of delayed dispersal to offspring per se. This supports natural population data on E. saxatilis, where habitats occupied by solitary vs. parentally-associated offspring were similar when measured across a range of habitat quality indicators (Langkilde et al., 2007). Further tests are required to confirm whether the proposed explanation of habitat monopolization, (and forced habitat sharing between ousted parents and their offspring) is the mechanism responsible for the observed pattern of parent-offspring association. This could be achieved by directly manipulating habitat density rather than structure or by altering dominance-subordination hierarchies within enclosures (similar manipulations have been performed, for example, to examine the evolution of sociality in coral fish—Buston, 2004). Additionally, this mechanism could be investigated by observing natural populations and determining the frequency of parent-offspring associations across environments that differ in density, (and hence availability) of suitable habitats, and determining whether less dominant individuals (assessed by behavioral interactions or their location on lower quality crevice sites) more often associate with their offspring. These offer potential avenues for future research.
There were clear benefits for offspring that associated with their mother. Specifically, offspring who resided within their mother's home range grew significantly more and were in significantly better body condition at the end of the experiment than offspring who did not. There are at least two mechanisms that could lead to these benefits. First, parental protection from conspecific harassment may allow offspring to spend more time foraging freely. Such an effect has been demonstrated in the laboratory for the related Egernia saxatilis (O'Connor and Shine, 2004). Second, offspring growth could simply result from a higher resource availability within their parent's relatively high quality habitat compared to what they would encounter if they dispersed (Duffield and Bull, 2002). However, our results suggest no “resource access” benefits, as parent-offspring associations tended to form on low quality habitat sites. Characterization of habitats occupied by offspring associated vs. not associated with their parents, and detailed observational studies that look at how parental presence influences offspring foraging behavior are potential research directions to consolidate our understanding in this system of the mechanisms underlying the positive effect of mother-offspring association on offspring growth and body condition. Alternatively, the increased offspring growth and body condition may be a result of parental effects as opposed to benefits acquired as a result of association per se; however, we did not find that parents in better body condition (as a proxy of parental quality) were more likely to associate with their offspring.
Despite benefits of maternal association for offspring growth, we did not find any benefits for survival. This was surprising given that one of the key hypotheses for the benefits of parent-offspring association in the Egernia lineage of lizards is protection from conspecific infanticide (Langkilde et al., 2007; Sinn et al., 2008). Our results instead suggest that parental tolerance of offspring has the primary function of enabling a safer and more efficient foraging environment, as opposed to direct protection from conspecifics. In support of this conclusion, L. whitii, and other species of Egernia have been shown to tolerate their own, but not unrelated offspring, within their home range (O'Connor and Shine, 2004; While et al., 2009a); if parental-offspring association has low costs, the presence of unrelated offspring may nevertheless negatively affect a parent's own offspring through competition over resources within the parent's habitat. The observation of parental aggression toward unrelated offspring fits this “resources or foraging benefits” hypothesis. If parental-offspring association has benefits for protection from infanticide, we would expect no parental aggression toward unrelated offspring, as this should not increase the risk of infanticide to the parent's genetic offspring, (and may even reduce it, through a dilution effect).
We found no costs to parents of associating with offspring. This was true when costs were measured both in terms of body condition or marks of aggression suffered. This is consistent with studies on reptiles more broadly where the costs associated with the early stages of parental care are often small (Aubret et al., 2005; Huang, 2007; Stahlschmidt et al., 2012). An absence of costs associated with increased parent offspring association may help facilitate the evolution of more complex forms of care because it promotes a kin structure that could favor the expression of more costly behaviors (for example, parental provisioning). However, similar to many other studies, the lack of costs to parents may be because true fitness costs are difficult to detect from a single season analysis (reviewed in Alonso-Alvarez and Velando, 2012), and with low sample size (Graves, 1991). Thus, more data on the long-term consequences of increased parent-offspring association for both parents and offspring is required.
This study has provided evidence for effects of habitat structure on the extent of parental-offspring association within L. whitii. We believe that such a simple increase in parental-offspring association may be characteristic of the early stages of the evolution of complex forms of parental care and group living. When the costs to care are low, parents will tolerate offspring, facilitating prolonged associations between parents and offspring. This enhanced kin association sets the foundation from which more complex care behaviors can emerge. The Egernia lineage show variation between populations and species in the environments they inhabit and the degree to which they associate with offspring (from no care in species, such as L. inornata, to extended family groups with multiple cohorts of offspring cared for in E. cunninghami—reviewed in Chapple, 2003; While et al., 2015). These species therefore offer opportunities to connect within species patterns between ecology and parent-offspring association with the emergence and diversification of more complex forms of parental care across species in the Egernia lineage. Ultimately this will provide a greater understanding of the casual effects of specific ecological conditions on the emergence of parental care more broadly.
Author Contributions
TB, BH, EC, TU, EW, and GW conceived of the research. TB, BH, and EC collected the morphomometric, reproductive and positional data with assistance from EW and GW. TB analyzed the data with assistance from BH and GW. TB and GW wrote the MS with input from all other authors (BH, EC, TU, EW).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Hannah Fogarty, Victoria Russel and Yean Fong Tan for assistance in the field. This research was funded by Australian Research Council grants awarded to GW and TU; DP150102900; DE150100336). TB was supported by a University of Tasmania honors scholarship, BH by a University of Tasmania PhD scholarship, TU was supported by the Royal Society of London and the Knut and Alice Wallenberg Foundations, EW and GW were supported by the Australian Research Council. All work was carried out with approval from the Animal Ethics Committee at the University of Tasmania (Ethics Approval number A13390).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2016.00096
The data associated with this MS can be accessed via the Dryad repository: doi: 10.5061/dryad.41fs7.
References
Alonso-Alvarez, C., and Velando, A. (2012). “Benefits and costs of parental care,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth and M. Kolliker (Oxford: Oxford University Press), 41–61.
Aubret, F., Bonnet, X., Shine, R., and Maumelat, S. (2005). Energy expenditure for parental care may be trivial for brooding pythons, Python regius. Anim. Behav. 69, 1043–1053. doi: 10.1016/j.anbehav.2004.09.008
Bates, M. D., Maechler, M., and Bolker, B. (2012). Package ‘lme4’. Version 0.999375-41: Linear Mixed-Effects Models Using S4 Classes. Available online at: http://cran.r-project.org/web/packages/lme4/lme4.pdf
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Buston, P. M. (2004). Territory inheritance in clownfish. Proc. R. Soc. Lond. B 271, S252–S254. doi: 10.1098/rsbl.2003.0156
Chapple, D. G. (2003). Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol. Monogr. 17, 145–180. doi: 10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2
Chapple, D. G., and Keogh, J. S. (2005). Complex mating system and dispersal patterns in a social lizard, Egernia whitii. Mol. Ecol. 14, 1215–1227. doi: 10.1111/j.1365-294X.2005.02486.x
Chapple, D. G., and Keogh, J. S. (2006). Group structure and stability in social aggregations of white's skink, Egernia whitii. Ethology 112, 247–257. doi: 10.1111/j.1439-0310.2006.01153.x
Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Covas, R., and Griesser, M. (2007). Life history and the evolution of family living in birds. Proc. R. Soc. B Biol. Sci. 274, 1349–1357. doi: 10.1098/rspb.2007.0117
Davis, A. R., Corl, A., Surget-Groba, Y., and Sinervo, B. (2011). Convergent evolution of kin-based sociality in a lizard. Proc. R. Soc. B Biol. Sci. 278, 1507–1514. doi: 10.1098/rspb.2010.1703
Duffield, G., and Bull, M. (2002). Stable social aggregations in an Australian lizard, Egernia stokesii. Naturwissenschaften 89, 424–427. doi: 10.1007/s00114-002-0346-7
Falk, J., Wong, J. W. Y., Kolliker, M., and Meunier, J. (2014). Sibling cooperation in earwig families provides insights into the early evolution of social life. Am. Nat. 183, 547–557. doi: 10.1086/675364
Field, J., and Brace, S. (2004). Pre-social benefits of extended parental care. Nature 428, 650–652. doi: 10.1038/nature02427
Foerster, K., and Kempenaers, B. (2004). Experimentally elevated plasma levels of testosterone do not increase male reproductive success in blue tits. Behav. Ecol. Sociobiol. 56, 482–490. doi: 10.1007/s00265-004-0809-2
Fox, J., Weisberg, S., and Adler, D. (2014). Package ‘car’. Version 2.0-2.5: Companion to Applied Regression. Available online at: http://cran.r-project.org/web/packages/car/car.pdf
Gardner, A., and Smiseth, P. T. (2011). Evolution of parental care driven by mutual reinforcement of parental food provisioning and sibling competition. Proc. R. Soc. B Biol. Sci. 278, 196–203. doi: 10.1098/rspb.2010.1171
Gardner, M. G., Bull, C. M., Fenner, A., Murray, K., and Donnellan, S. C. (2007). Consistent social structure within aggregations of the Australian lizard, Egernia stokesii across seven disconnected rocky outcrops. J. Ethol. 25, 263–270. doi: 10.1007/s10164-006-0022-z
Gardner, M. G., Cooper, S. J. B., Bull, C. M., and Grant, W. N. (1999). Isolation of microsatellite loci from a social lizard, Egernia stokesii, using a modified enrichment procedure. J. Hered. 90, 301–304. doi: 10.1093/jhered/90.2.301
Gardner, M. G., Sanchez, J. J., Dudaniec, R. Y., Rheinberger, L., Smith, A. L., and Saint, K. M. (2008). Tiliqua rugosa microsatellites: isolation via enrichment and characterisation of loci for multiplex PCR in T-rugosa and the endangered T-adelaidensis. Conserv. Genet. 9, 233–237. doi: 10.1007/s10592-007-9316-0
Graves, J. (1991). Comments on the sample sizes used to test the effect of experimental brood enlargement on adult survival. Auk 108, 967–969.
Green, A. J. (2001). Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82, 1473–1483. doi: 10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2
Greer, A. E. (1989). The Biology and Evolution of Australian Lizards. Sydney, NSW: Surrey Beatty and Sons.
Hatchwell, B., and Komdeur, J. (2000). Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 59, 1079–1086. doi: 10.1006/anbe.2000.1394
Huang, W. S. (2007). Costs of egg caring in the skink, Mabuya longicaudata. Ecol. Res. 22, 659–664. doi: 10.1007/s11284-006-0068-y
Klug, H., Alonzo, S., and Bonsall, M. B. (2012). “Theoretical foundations of parental care,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford, UK: Oxford University Press), 21–39.
Klug, H., and Bonsall, M. B. (2014). What are the benefits of parental care? The importance of parental effects on developmental rate. Ecol. Evol. 4, 2330–2351. doi: 10.1002/ece3.1083
Kölliker, M. (2007). Benefits and costs of earwig (Forficula auricularia) family life. Behav. Ecol. Sociobiol. 61, 1489–1497. doi: 10.1007/s00265-007-0381-7
Labocha, M. K., Schutz, H., and Hayes, J. P. (2014). Which body condition index is best? Oikos 123, 111–119. doi: 10.1111/j.1600-0706.2013.00755.x
Lancaster, P., Jessop, T. S., and Stuart-Fox, D. (2011). Testing the independent effects of population and shelter density on behavioural and corticosterone responses of tree skinks. Aust. J. Zool. 58, 295–302. doi: 10.1071/ZO10056
Langkilde, T., O'Connor, D., and Shine, R. (2007). Benefits of parental care: do juvenile lizards obtain better-quality habitat by remaining with their parents? Aust. Ecol. 32, 950–954. doi: 10.1111/j.1442-9993.2007.01783.x
Lanham, E., and Bull, C. (2000). Maternal care and infanticide in the Australian skink, Egernia stokesii. Herpetol. Rev. 31, 151–152.
Lion, S., and Van Baalen, M. (2007). From infanticide to parental care: why spatial structure can help adults be good parents. Am. Nat. 170, 26–46. doi: 10.1086/519462
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M. (1998). Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655. doi: 10.1046/j.1365-294x.1998.00374.x
Norris, K. S. (1953). The ecology of the desert iguana, Dipsosaurus dorsalis. Ecology 34, 265–287. doi: 10.2307/1930895
O'Connor, D., and Shine, R. (2003). Lizards in ‘nuclear families’: a novel reptilian social system in Egernia saxatilis (Scincidae). Mol. Ecol. 12, 743–752. doi: 10.1046/j.1365-294X.2003.01777.x
O'Connor, D. E., and Shine, R. (2004). Parental care protects against infanticide in the lizard Egernia saxatilis (Scincidae). Anim. Behav. 68, 1361–1369. doi: 10.1016/j.anbehav.2004.02.014
O'Connor, D. E., and Shine, R. (2006). Kin discrimination in the social lizard Egernia saxatilis (Scincidae). Behav. Ecol. 17, 206–211. doi: 10.1093/beheco/arj019
Powell, R. A. (2000). “Animal home ranges and territories and home range estimators,” in Research Techniques in Animal Ecology: Controversies and Consequences, eds L. Boitani, and T. Fuller (New York, NY: Columbia University Press), 65–110.
Queller, D. C. (1994). Extended parental care and the origin of eusociality. Proc. R. Soc. B Biol. Sci. 256, 105–111. doi: 10.1098/rspb.1994.0056
Royle, N. J., Pike, T. W., Heeb, P., Richner, H., and Kölliker, M. (2012). Offspring social network structure predicts fitness in families. Proc. R. Soc. Lond. B 279, 4914–4922. doi: 10.1098/rspb.2012.1701
Shine, R., and Charnov, E. L. (1992). Patterns of survival, growth, and maturation in snakes and lizards. Am. Nat. 139, 1257–1269. doi: 10.1086/285385
Sinn, D. L., While, G. M., and Wapstra, E. (2008). Maternal care in a social lizard: links between female aggression and offspring fitness. Anim. Behav. 76, 1249–1257. doi: 10.1016/j.anbehav.2008.06.009
Smiseth, P. T., Kolliker, M., and Royle, N. J. (2012). “What is parental care?,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kolliker (Oxford: Oxford University Press), 1–17.
Somma, L. A. (2003). Parental Behavior in Lepidosaurian and Testudinian Reptiles: A Literature Survey. Malabar: Krieger Publishing Company.
Stahlschmidt, Z. R., Shine, R., and Denardo, D. F. (2012). The consequences of alternative parental care tactics in free-ranging pythons in tropical Australia. Funct. Ecol. 26, 812–821. doi: 10.1111/j.1365-2435.2012.02003.x
Tinkle, D. W. (1967). The life and demography of the side-blotched lizard, Uta stansburiana. Misc. Publ. Mus. Zool. 132, 1–182.
Uller, T. (2012). “Parental effects in development and evolution,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kolliker (Oxford: Oxford University Press), 247–263.
Vitt, L. J., Congdon, J. D., Hulse, A. C., and Platz, J. E. (1974). Territorial aggressive encounters and tail breaks in the lizard Sceloporus magister. Copeia 1974, 990–993. doi: 10.2307/1442608
While, G., Jones, S., and Wapstra, E. (2007). Birthing asynchrony is not a consequence of asynchronous offspring development in a non-avian vertebrate, the Australian skink Egernia whitii. Funct. Ecol. 21, 513–519. doi: 10.1111/j.1365-2435.2007.01272.x
While, G. M., Chapple, D. G., Gardner, M. G., Uller, T., and Whiting, M. J. (2015). Egernia lizards. Curr. Biol. 25, R593–R595. doi: 10.1016/j.cub.2015.02.070
While, G. M., Uller, T., Bordogna, G., and Wapstra, E. (2014). Promiscuity resolves constraints on social mate choice imposed by population viscosity. Mol. Ecol. 23, 721–732. doi: 10.1111/mec.12618
While, G. M., Uller, T., and Wapstra, E. (2009a). Family conflict and the evolution of sociality in reptiles. Behav. Ecol. 20, 245–250. doi: 10.1093/beheco/arp015
While, G. M., Uller, T., and Wapstra, E. (2009b). Within-population variation in social strategies characterize the social and mating system of an Australian lizard, Egernia whitii. Aust. Ecol. 34, 938–949. doi: 10.1111/j.1442-9993.2009.02002.x
Wilson, S., and Swan, G. (2003). A Complete Guide to Reptiles of Australia. Sydney, NSW: Reed New Holland.
Keywords: parental care, parent-offspring association, habitat structure, lizard, social complexity
Citation: Botterill-James T, Halliwell B, Cooper-Scott E, Uller T, Wapstra E and While GM (2016) Habitat Structure Influences Parent-Offspring Association in a Social Lizard. Front. Ecol. Evol. 4:96. doi: 10.3389/fevo.2016.00096
Received: 16 May 2016; Accepted: 22 July 2016;
Published: 11 August 2016.
Edited by:
Marian Yi-ling Wong, University of Wollongong, AustraliaReviewed by:
William Croft Ratcliff, Georgia Institute of Technology, USAJoël Meunier, University of Tours, France
Copyright © 2016 Botterill-James, Halliwell, Cooper-Scott, Uller, Wapstra and While. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geoffrey M. While, Z3doaWxlQHV0YXMuZWR1LmF1
 Thomas Botterill-James
Thomas Botterill-James Ben Halliwell
Ben Halliwell Emily Cooper-Scott1
Emily Cooper-Scott1 Tobias Uller
Tobias Uller Erik Wapstra
Erik Wapstra Geoffrey M. While
Geoffrey M. While