
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 20 July 2016
Sec. Behavioral and Evolutionary Ecology
Volume 4 - 2016 | https://doi.org/10.3389/fevo.2016.00083
This article is part of the Research Topic The Importance of Olfaction in Intra- and Interspecific Communication View all 7 articles
The complexity of social signals is thought to depend on the complexity of social systems, but evidence from wild animals is scarce. Here, we investigated the chemical information provided by individual male greater sac-winged bats (Saccopteryx bilineata), a small, long-lived neotropical bat species with a harem-polygynous mating system. We analyzed the chemical fingerprints of wing-sac liquids that are displayed by males in front of females. Specifically, we tested if fingerprints of 45 males included information about age (adult, juvenile), year of sampling, and distance between colonies. We confirmed age-specific differences in male-specific substances, but show furthermore that chemical fingerprints correlate with year of collection and distance between colonies. Thus, the wing-sac chemistry of male S. bilineata conveys a multitude of information, which can potentially be used by conspecifics, especially by females to assess the status of potential mates. Our study provides evidence for a multidimensionality of chemical information in a free-ranging mammal with high social complexity.
Mammalian chemical signals are often complex and encode multiple information for intraspecific communication (Burger, 2005; Setchell et al., 2010; delBarco-Trillo et al., 2012; Stoffel et al., 2015). This is especially true for long-lived species that form stable social groups, where signals are shaped by a variety of selective forces (Gorman and Trowbridge, 1989; Voigt et al., 2008). Besides chemical signatures that encode the identity of individuals, chemical signals may also convey information about the reproductive status (Marler, 1961), social status (Gosling and Roberts, 2001), genetic variability, and relatedness (Boulet et al., 2009; Stoffel et al., 2015), as well as about the current health of an individual, which can be used by potential mates to assess the quality of a partner (Penn and Potts, 1998; Wyatt, 2003). While the complexities of chemical signals in mammals are usually well-studied in laboratory model-species, information on the potential multidimensionality of chemicals used by free-ranging mammals is scarce.
The greater sac-winged bat (Saccopteryx bilineata) is a small neotropical bat of the family Emballonuridae with a harem-polygynous mating system (Bradbury and Vehrencamp, 1977). Colonies of up to 50 members are organized in harems consisting of one territorial male and several females (Bradbury and Emmons, 1974). Males possess wing sacs in their front wing membrane, which they clean daily (Voigt et al., 2005) and actively refill with odoriferous secretions from various body regions during a stereotypic time consuming 2-stage process (Voigt and von Helversen, 1999). This “perfume blending” results in a composite odor that males display mostly during hover flights in front of females, but also when roosting next to a colony member or in agonistic male-male encounters. As females are larger than males and forced copulation is unlikely (Heckel et al., 1999), these displays are thought to play an important role in female mate choice (Voigt and Schwarzenberger, 2008). Thus, the presence, relative abundance or combination of specific compounds in the chemical fingerprints of male wing-sacs may reveal information that is important for females in order to select an optimal mate.
The odorous liquid of male S. bilineata consists mainly of aromatic compounds, fatty acids and terpenoids (Caspers et al., 2008). Nine of these substances are male-specific, i.e., do not occur in females and juveniles (Caspers et al., 2008, 2011; Voigt et al., 2008). Some substances are known to be species-specific and do not occur in S. leptura, a sister species of S. bilineata (Caspers et al., 2009). The concentrations of some fatty acids in the odor of S. bilineata are higher during the mating season than during the non-mating season (Caspers et al., 2008). Furthermore, males of the same colony produce different, non-overlapping concentrations of C15H24O2, indicating that there might be a signature of individual identity (Caspers et al., 2008). In short, previous studies suggested that the chemical fingerprint of male S. bilineata contains information about the sex, reproductive status as well as individual identity. In S. bilineata, unlike many other mammals, females rather than males disperse from their natal colony once they reach the age of maturity, thus most males are related within a colony (Nagy et al., 2007, 2013) and therefore geographic distance between males may correlated with genetic distance. Potentially, females can use information about the origin of a male to avoid mating with relatives and thus further reduce the risk of inbreeding.
To assess the multidimensionality of chemical information, and thus the complexity of social signals, we here aimed at analysing complete individual odor profiles in a large set of males over several years which originated from different colonies. So far, studies have focussed mainly on male-specific substances, and sample sizes were quite limited (e.g., N = 7 in Caspers et al., 2008; N = 8 in Caspers et al., 2009; N = 32 in Caspers et al., 2011). One aim of this study was to reproduce the result of a former study and to analyse whether sexual maturity is encoded in the chemical fingerprints of male greater sac-winged bats. Moreover, as we sampled males from different colonies, we asked whether the chemical fingerprint of males hold information about geographic distance.
Samples were collected during the mating season of S. bilineata in November and December 2009, 2010, and 2011 in the vicinity of La Selva Biological Station (10°25′N; 84°00′W, 37 m a.s.l., Province Heredia, Costa Rica) administered by the Organization of Tropical Studies. Samples were collected with the permission of the Costa Rican Authorities (162-2009-SINAC, 137-2010-SINAC and163-2011-SINAC). We captured bats at dawn between 0500 and 0600 h using monofilament nylon mist nets (2.5 m height, Ecotone, Gdynia, Poland) when bats returned to their roost. As part of a long-term study, all bats have been marked individually with colored plastic bands (AC Hughes LTD., Middlesex, UK, size XCL), most of them in the colony in which they were born and in the year in which they were born.
We categorized bats that were born in the year of capture as juveniles. For bats of unknown date of birth, we assessed the age by the color and structure of the wing sacs (Tannenbaum, 1975). Wing sacs were wiped out with a piece of cotton of ~5 mg that had previously been washed with dichloromethane (99.9%) to remove potential contaminating substances. We stored the samples in a Teflon-capped glass vial (2 ml RotilaboH, Karlsruhe, Germany) at −80°C until analysis. After sample collection, we released all bats at the site of capture.
Distances among capture locations were calculated using the digital map of La Selva biological reserve and the graphics program “GIMP.” In total, we captured bats at six locations with distances between around 100 and 3300 m (mean 1960 m; Table 1).
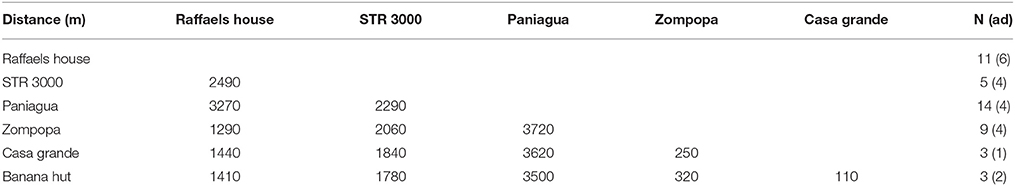
Table 1. Distance between colonies (in m) with number of sampled bats (N) on each location and the number of adult bats (in brackets).
All samples were prepared for analysis and analyzed at Bielefeld University. Prior to chemical bouquet extraction, we added 100 μl of dichloromethane (DCM) to each sample. Samples were then extracted by squeezing out the cotton dapper using a blunt point syringe (100 μl, ILS, Stützerbach, Germany). We transferred each extract into a glass vial (2 ml, Rotilabo®, Karlsruhe, Germany) that was equipped with a 100 μl inlet (Rotilabo®, Karlsruhe, Germany). The extracts were concentrated by evaporation to ~5 μl before analysis. These samples were analyzed by gas chromatography (GC) equipped with a VF-5 ms capillary column (30 m × 0.25 mm ID, DF 0.25, 10 m guard column, Varian Inc., Lake Forest, California, USA) linked to a quadruple mass spectrometer (MS; Focus GC-DSQ MS system, Thermo Electron S.p.A., Rodano, Italy) in electron ionization mode at 70 eV. One μl of each sample was injected into a deactivated glasswool-packed liner at an inlet temperature of 220°C and processed in a split 10 mode with 20 ml/min split flow. Helium was used as carrier gas and its flow rate was held at 1 ml/min. The GC temperature started at 60°C initial time of 3 min, followed by a 10°C/min rate to a final temperature of 280°C, which was kept for 20 min. Male specific substances were determined by spectra of authentic samples (Caspers et al., 2009, 2011). To compare the composition of wing sac chemical fingerprints, we used all substances that were absent in the cotton blank samples and were found at least in two greater sac-winged bat samples. In total, we found 131 different putative substances, of which 31 substances were found only once and thus excluded from the analysis. Former studies on wing-sac chemical composition in male greater sac winged bats used a subset of substances only, including the male specific substances [indole, anthranilic acid, pyrocoll, indol-3-carbaldehyde, 2,6,10-trimethyl-3-oxo-6,10-dodecadienolide, C15H24O2 (not yet identified), thryptanthrin, and cholesterol]. To compare the composition of wing sac fingerprints, we calculated the relative contribution (%) of peak area of each substance, including the male specific substances, to the total peak area of all substances. This procedure guaranteed that all samples were comparable on a similar scale, since the total amount of wing sac composition differed among individuals and among samples of the same individual.
We compared the chemical composition by computing a similarity matrix based on the Bray-Curtis-Similarity Index and analyzed potential differences between a priori defined groups (factor: ID, year, age) using a non-parametric analysis of similarities (ANOSIM) with 999 permutations. The ANOSIM is a permutation test that allows to statistically determine whether samples within a priori defined groups are more similar on average than samples between groups. To test whether differences of individual fingerprints can be explained by geographic distance, i.e., whether individuals that are living closer together have more similar wing sac chemistry, we run a Mantel-Test-Type correlation using the RELATE procedure including only adult sac-winged bats. Four bats were not captured close to a colony and thus were not included in the analysis here. Thereby the correlation coefficient of two matrices is calculated in a permutation procedure. Statistical analyses were performed on the square root transformed data, using PRIMER 6.1.12 (Primer-236 E 2000 Ltd., Plymouth, UK). The significance level was set to α = 0.05 and we used two-tailed tests. We visualized our data using non-metric scaling plots (nMDS). The nMDS plot gives a two dimensional representation of the multidimensional matrix of pair-wise similarities. The closer two symbols appear on that plot the more similar the two samples are in their chemical composition.
We analyzed in total 56 samples of 45 different individuals. Seven individuals were sampled more than once. The analysis of similarities showed that chemical samples of the same individual were on average more similar to each other than between different individuals (square root transformed data, ANOSIM: Global R = 0.35, p = 0.024). Therefore, we averaged the samples of the same individual in order to avoid pseudo-replication.
Chemical fingerprints of wing-sac contents differed significantly between years (Global R = 0.231, p = 0.001; N 2009 = 20; N 2010 = 20; N 2011 = 5). To account for year differences, we added year as a factor to the following analysis. In addition, wing sac chemistry of males differed significantly between the two status groups, i.e., juvenile and adult males (Two-way ANOSIM, year: Global R = 0.24; p = 0.002; status: Global R = 0.153; p = 0.018, Figure 1; 2009: N juveniles = 8; N adults = 11; 2010: N juveniles = 10; N adults = 9; 2011: N juveniles = 3; N adults = 2).
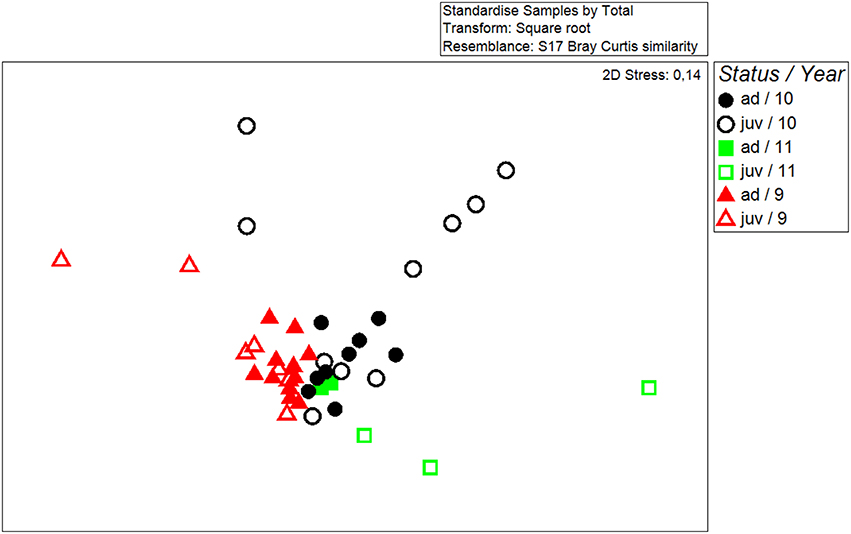
Figure 1. Non-metric multidimensional scaling plot showing the similarity in chemical fingerprints of all analyzed samples. The axes are arbitrary and dimensionless. The closer two symbol appear on the plot the more similar the two fingerprints were. Samples were collected in three different years [2009 (9), 2010 (10), and 2011 (11)] and from males of two different status groups (adult = ad, juvenile = juv).
When considering only male-specific substances, we could not find any differences between years (two way ANOSIM, year: Global R = −0.061; p = 0.82), however samples of juveniles and adults differed in the composition of the male-specific substances (two-way ANOSIM, status: Global R = 0.159; p = 0.008). When investigating the composition of the wing sac chemistry without any known male-specific substances, samples differed significantly among years (two-way ANOSIM, year: Global R = 0.504; p = 0.001), but not between juveniles and adults (two-way ANOSIM, status: Global R = 0.056; p = 0.19), indicating that status, i.e., sexual maturity of males, is encoded in the male-specific substances.
In a second step, we analyzed whether the composition of adult male wing sac chemistry correlated with the geographic distance of colonies. Based on a mantel type permutation test (RELATE), we could not find a correlation between the overall composition of wing-sac chemistry and geographic distance (RELATE: Spearman Rank Correlation; Rho = 0.01; p = 0.41). Furthermore, there was no correlation between similarity in chemical composition and geographic distance, when repeating the analysis using a subset with the male-specific substances only (RELATE: Spearman Rank Correlation; Rho = 0.064; p = 0.23). However, there was a significant correlation between the similarity of chemical composition and geographic distance when using the subset of substances, i.e., without male-specific substances (RELATE: Spearman Rank Correlation; Rho 0.167; p = 0.04).
Analyses of chemical fingerprints of male greater sac-winged bats revealed differences between adult and juvenile males. Changes in chemical profiles during maturation have so far been shown in laboratory rats (Rattus norvegicus; Osada et al., 2009), but also in other mammals such as otters (Lutra lutra; Kean et al., 2011), and black rhinoceros (Diceros bicornis; Linklater et al., 2013). Our result is in line with these studies, but also confirms previous findings that male-specific substances vary between juvenile and adult male S. bilineata (Caspers et al., 2011). When male-specific substances were removed from the analysis, no difference could be detected between the chemical fingerprints of juvenile and adult males. Thus, the male-specific substances are a reliable indicator for the status of sexual maturity of an individual and may be used by females to assess whether a potential mate has reached sexual maturity and therewith an adult phenotype.
When ignoring male-specific substances in the analysis, overall chemical profiles differed among years, whereas male-specific substances alone did not show this differentiation. Differences in chemical profiles among years may be caused by changes in environmental conditions such as temperature, humidity, diet composition, and/or potential differences in the rates of pathogen infection rate (Zala et al., 2004; Kavaliers et al., 2005). Furthermore, the bacterial diversity in wing sacs may change across year, potentially altering certain components of the odor (Voigt et al., 2005). Male-specific substances potentially impose a reliable signal for females, independent of environmental conditions, since they did not vary among years.
Besides annual variations in fingerprints, we found that differences in chemical profiles increased with the geographic distance between colonies. Males from colonies that were spatially more separated showed lower similarities in their chemical profiles than males from colonies that were close-by. Potentially, these differences may be caused by local variation of bacterial diversity. In fact, microbial species found in the wing sacs of male S. bilineata vary greatly among individuals and may contribute to individual differences in odor profiles (Voigt et al., 2005). Differences in chemical fingerprints among individuals from two different colonies have for example previously been found in fur seals (Stoffel et al., 2015) and in Bechstein bats (Safi and Kerth, 2003), but correlations of differences in chemical profiles with geographic distance are so far unknown in free-ranging mammals. However, signal variation correlating with geographic distance exist in other signals, for example, in bird songs, such as those of the greenish warbler (Phylloscopus trochiloides; Irwin et al., 2008) and in chemical communication, such as in female sex pheromones in moths (Groot et al., 2009). In S. bilineata, pups learn a vocal group signature depending on their natal colony, which is hypothesized to facilitate female inbreeding avoidance (Knörnschild et al., 2012). While acoustic differences may be learned during ontogeny, chemical differences among male S. bilineata from different colonies may be caused either by environmental variation or via greater genetic distance between individuals of different colonies. As females and not males are dispersing from their natal colony in S. bilineata, males within a colony are usually related (Nagy et al., 2007, 2013). Thus, females may use the chemical fingerprint of males from their natal colony as a proxy to assess the genetic distance to males of another colony, and thereby further reduce the risk of inbreeding, even if encountering a male from the same natal colony that, unlike other males, dispersed.
In addition to the complex information that is contained in the individual chemical signature, greater sac-winged bats are also known to communicate acoustically (Knörnschild and von Helversen, 2008; Knörnschild et al., 2012) and potentially also visually (Caspers, 2008), as they roost in well-lit territories (Bradbury and Emmons, 1974). The various sensory modalities used and the complexity of communication represents a good example of the social complexity hypothesis, which hypothesizes that complex social systems should have evolved a complex communication system (Freeberg et al., 2012).
In conclusion we showed that the wing-sac chemistry of male greater sac-winged bats is a potentially complex multidimensional cue, encoding information about individual identity and age as well as geographic distance, which could be of great importance for female mate choice.
KS, CV, and BC designed the experiment, KS collected the data, KS, BC, and CM analyzed the data, KS, CV, CM, and BC wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by funds from the Leibniz Institute for Zoo and Wildlife Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Boulet, M., Charpentier, M. J., and Drea, C. M. (2009). Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9:281. doi: 10.1186/1471-2148-9-281
Bradbury, J., and Vehrencamp, S. (1977). Social organization and foraging in emballonurid bats. Behav. Ecol. Sociobiol. 2, 1–17. doi: 10.1007/BF00299284
Bradbury, J. W., and Emmons, L. H. (1974). Social organization of some Trinidad bats. Z. Tierpsychol. 36, 137–183. doi: 10.1111/j.1439-0310.1974.tb02130.x
Burger, B. V. (2005). “Mammalian semiochemicals,” in The Chemistry of Pheromones and other Semiochemicals II, ed S. Schulz (Berlin; Heidelberg: Springer), 231–278.
Caspers, B. (2008). Olfactory Communication in the Greater sac-winged Bat, Saccopteryx bilineata. Ph.D. Thesis, Humboldt University, Berlin.
Caspers, B. A., Schroeder, F. C., Franke, S., Streich, W. J., and Voigt, C. C. (2009). Odour-based species recognition in two sympatric species of sac-winged bats (Saccopteryx bilineata, S. leptura): combining chemical analyses, behavioural observations and odour preference tests. Behav. Ecol. Sociobiol. 63, 741–749. doi: 10.1007/s00265-009-0708-7
Caspers, B. A., Schroeder, F. C., Franke, S., and Voigt, C. C. (2011). Scents of adolescence: the maturation of the olfactory phenotype in a free-ranging mammal. PLoS ONE 6:e21162. doi: 10.1371/journal.pone.0021162
Caspers, B., Franke, S., and Voigt, C. C. (2008). “The wing-sac odour of male greater sac-winged bats Saccopteryx bilineata (Emballonuridae) as a composite trait: seasonal and individual differences,” in Chemical Signals in Vertebrates 11, eds J. L. Hurst, R. J. Beynon, S. C. Roberts, and T. D. Wyatt (New York, NY: Springer), 151–160.
delBarco-Trillo, J., Sacha, C., Dubay, G., and Drea, C. (2012). Eulemur, me lemur: the evolution of scent-signal complexity in a primate clade. Philoso. Trans. R. Soc. Lond. B Biol. Sci. 367, 1909–1922. doi: 10.1098/rstb.2011.0225
Freeberg, T. M., Dunbar, R. I., and Ord, T. J. (2012). Social complexity as a proximate and ultimate factor in communicative complexity. Philos. Trans. R. Soc. London B Biol. Sci. 367, 1785–1801. doi: 10.1098/rstb.2011.0213
Gorman, M. L., and Trowbridge, B. J. (1989). “The role of odor in the social lives of carnivores,” in Carnivore Behavior, Ecology, and Evolution, ed J. L. Gittleman (New York, NY: Springer), 57–88.
Gosling, L. M., and Roberts, S. C. (2001). Scent-marking by male mammals: cheat-proof signals to competitors and mates. Adv. Study Behav. 30, 169–217. doi: 10.1016/S0065-3454(01)80007-3
Groot, A. T., Inglis, O., Bowdridge, S., Santangelo, R. G., Blanco, C., López, J. D. Jr., et al. (2009). Geographic and temporal variation in moth chemical communication. Evolution 63, 1987–2003. doi: 10.1111/j.1558-5646.2009.00702.x
Heckel, G., Voigt, C. C., Mayer, F., and Von Helversen, O. (1999). Extra-harem paternity in the white-lined bat Saccopteryx bilineata (Emballonuridae). Behaviour 136, 1173–1185. doi: 10.1163/156853999501829
Irwin, D., Thimgan, M., and Irwin, J. (2008). Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): a strong role for stochasticity in signal evolution? J. Evol. Biol. 21, 435–448. doi: 10.1111/j.1420-9101.2007.01499.x
Kavaliers, M., Choleris, E., and Pfaff, D. W. (2005). Recognition and avoidance of the odors of parasitized conspecifics and predators: differential genomic correlates. Neurosci. Biobehav. Rev. 29, 1347–1359. doi: 10.1016/j.neubiorev.2005.04.011
Kean, E. F., Müller, C. T., and Chadwick, E. A. (2011). Otter scent signals age, sex, and reproductive status. Chem. Senses 36, 555–564. doi: 10.1093/chemse/bjr025
Knörnschild, M., Nagy, M., Metz, M., Mayer, F., and von Helversen, O. (2012). Learned vocal group signatures in the polygynous bat Saccopteryx bilineata. Anim. Behav. 84, 761–769. doi: 10.1016/j.anbehav.2012.06.029
Knörnschild, M., and von Helversen, O. (2008). Nonmutual vocal mother–pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009. doi: 10.1016/j.anbehav.2008.05.018
Linklater, W. L., Mayer, K., and Swaisgood, R. R. (2013). Chemical signals of age, sex and identity in black rhinoceros. Anim. Behav. 85, 671–677. doi: 10.1016/j.anbehav.2012.12.034
Marler, P. (1961). The logical analysis of animal communication. J. Theor. Biol. 1, 295–317. doi: 10.1016/0022-5193(61)90032-7
Nagy, M., Günther, L., Knörnschild, M., and Mayer, F. (2013). Female-biased dispersal in a bat with a female-defence mating strategy. Mol. Ecol. 22, 1733–1745. doi: 10.1111/mec.12202
Nagy, M., Heckel, G., Voigt, C. C., and Mayer, F. (2007). Female-biased dispersal and patrilocal kin groups in a mammal with resource-defence polygyny. Proc. R. Soc. London B Biol. Sci. 274, 3019–3025. doi: 10.1098/rspb.2007.1008
Osada, K., Kashiwayanagi, M., and Izumi, H. (2009). Profiles of volatiles in male rat urine: the effect of puberty on the female attraction. Chem. Senses 34, 713–721. doi: 10.1093/chemse/bjp058
Penn, D., and Potts, W. K. (1998). Chemical signals and parasite-mediated sexual selection. Trends Ecol. Evol. 13, 391–396. doi: 10.1016/S0169-5347(98)01473-6
Safi, K., and Kerth, G. (2003). Secretions of the interaural gland contain information about individuality and colony membership in the Bechstein's bat. Anim. Behav. 65, 363–369. doi: 10.1006/anbe.2003.2067
Setchell, J. M., Vaglio, S., Moggi-Cecchi, J., Boscaro, F., Calamai, L., and Knapp, L. A. (2010). Chemical composition of scent-gland secretions in an old world monkey (Mandrillus sphinx): influence of sex, male status, and individual identity. Chem. Senses. 35, 205–220. doi: 10.1093/chemse/bjp105
Stoffel, M. A., Caspers, B. A., Forcada, J., Giannakara, A., Baier, M., Eberhart-Phillips, L., et al. (2015). Chemical fingerprints encode mother–offspring similarity, colony membership, relatedness, and genetic quality in fur seals. Proc. Natl. Acad. Sci. U.S.A. 112, E5005–E5012. doi: 10.1073/pnas.1506076112
Tannenbaum, B. R. (1975). Reproductive Strategies in the White-Lined Bat. Ph.D. Cornell University, New York, NY.
Voigt, C. C., Behr, O., Caspers, B., von Helversen, O., Knörnschild, M., Mayer, F., et al. (2008). Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J. Mammal. 89, 1401–1410. doi: 10.1644/08-MAMM-S-060.1
Voigt, C. C., Caspers, B., and Speck, S. (2005). Bats, bacteria, and bat smell: sex-specific diversity of microbes in a sexually selected scent organ. J. Mammal. 86, 745–749. doi: 10.1644/1545-1542(2005)086[0745:BBABSS]2.0.CO;2
Voigt, C. C., and Schwarzenberger, F. (2008). Reproductive endocrinology of a small tropical bat (female Saccopteryx bilineata; Emballonuridae) monitored by fecal hormone metabolites. J. Mammal. 89, 50–57. doi: 10.1644/06-MAMM-A-432.1
Voigt, C. C., and von Helversen, O. (1999). Storage and display of odour by male Saccopteryx bilineata (Chiroptera, Emballonuridae). Behav. Ecol. Sociobiol. 47, 29–40. doi: 10.1007/s002650050646
Wyatt, T. D. (2003). Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge, UK: Cambridge University Press.
Keywords: chemical fingerprints, mammalian semiochemicals, sexual selection and mate choice, Saccopteryx bilineata, greater sac-winged bat, ANOSIM
Citation: Schneeberger K, Voigt CC, Müller C and Caspers BA (2016) Multidimensionality of Chemical Information in Male Greater Sac-Winged Bats (Saccopteryx bilineata). Front. Ecol. Evol. 4:83. doi: 10.3389/fevo.2016.00083
Received: 30 April 2016; Accepted: 01 July 2016;
Published: 20 July 2016.
Edited by:
Luisa Amo, Spanish National Research Council, SpainReviewed by:
Ximena J. Nelson, University of Canterbury, New ZealandCopyright © 2016 Schneeberger, Voigt, Müller and Caspers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara A. Caspers, YmFyYmFyYS5jYXNwZXJzQHVuaS1iaWVsZWZlbGQuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.