- Behaviour and Conservation, Institute of Natural and Mathematical Sciences, Massey University, Auckland, New Zealand
Bird song is commonly regarded as a male trait that has evolved through sexual selection. However, recent research has prompted a re-evaluation of this view by demonstrating that female song is an ancestral and phylogenetically widespread trait. Species with female song provide opportunities to study selective pressures and mechanisms specific to females within the wider context of social competition. We investigated the relationship between reproductive success and female song performance in the New Zealand bellbird (Anthornis melanura), a passerine resident year round in New Zealand temperate forests. We monitored breeding behavior and song over 3 years on Tiritiri Matangi Island. Female bellbirds contributed significantly more toward parental care than males (solely incubating young and provisioning chicks at more than twice the rate of males). Female song rate in the vicinity of the nest was higher than that of males during incubation and chick-rearing stages but similar during early-nesting and post-breeding stages. Using GLMs, we found that female song rates during both incubation and chick-rearing stages strongly predicted the number of fledged chicks. However, male song rate and male and female chick provisioning rates had no effect on fledging success. Two measures of female song complexity (number of syllable types and the number of transitions between different syllable types) were also good predictors of breeding success (GLM on PC scores). In contrast, song duration, the total number of syllables, and the number of “stutter” syllables per song were not correlated with fledging success. It is unclear why male song rate was not associated with reproductive success and we speculate that extra-pair paternity might play a role. While we have previously demonstrated that female bellbird song is important in intrasexual interactions, we clearly demonstrate here that female song predicts reproductive success. These results, with others, highlight the need for a change in how we view the significance of female secondary sexual traits; traits long underestimated due to a focus on male song.
Introduction
Darwin (1871) described bird song as a male trait that has evolved through sexual selection. Since then, research has focussed on temperate, northern hemisphere passerines where song has been seen as a male activity, functioning in territoriality, and mate attraction (Catchpole, 1982; Searcy and Andersson, 1986; Kroodsma and Byers, 1991). However, as the number of worldwide studies of songbirds has increased, the phenomenon of complex, territorial female song has been found to be more widespread than first thought (Arcese et al., 1988; Langmore, 1998; Riebel, 2003; Riebel et al., 2005; Brunton and Li, 2006; Garamszegi et al., 2007; Price et al., 2008, 2009; Geberzahn et al., 2009). Indeed, recent research has prompted a re-evaluation of the assumption that song is a male trait by demonstrating that female song is an ancestral and phylogenetically widespread trait in the oscines (Odom et al., 2014; Price, 2015). These findings raise the issue of why female song may have been lost in some species and the possibility of sex differences in not just the benefits of singing but also in the costs (Kleindorfer et al., 2016).
Male song has been extensively studied and there is evidence that measures of male song quality and performance are related to mating success (Searcy and Marler, 1981; Catchpole, 1987; Lampe and Espmark, 1994; Reid et al., 2004) and reproductive success (Catchpole, 1986; Reid et al., 2005; Nemeth et al., 2012; Taff et al., 2012; Woodgate et al., 2012). Researchers have also found links between male song performance and genetic benefits to females (Hasselquist et al., 1996). It has also been suggested that male song can act as a proxy for age (Botero et al., 2009; Kipper and Kiefer, 2010; Nemeth et al., 2012). However, not all studies of male song have found a relationship with reproductive success (Hiebert et al., 1989; Rivera-Gutierrez et al., 2010; Soma and Garamszegi, 2011), highlighting that plumage and other behavioral traits may play a more significant role in demonstrating male quality.
In contrast to male song, female song performance and structure is a long-neglected field (Langmore, 1998; Riebel, 2003; Riebel et al., 2005). Nonetheless, in some species, females are prolific singers (e.g., Cooney and Cockburn, 1995; Pavlova et al., 2005; Brunton and Li, 2006; Soma and Garamszegi, 2015). In several species where both sexes sing, the responses of territory holders may be sex specific, with same sex interactions being typical (Langmore and Davies, 1997; Langmore, 1998; Brunton et al., 2008b; Kleindorfer et al., 2013; Odom et al., 2015). In general however, the functions of female song are not well understood but have been suggested to parallel those associated with male song (Langmore, 1998), particularly in regard to social competition for resources other than a mate i.e., territorial defense (West-Eberhard, 1983; Illes, 2015). Sexual selection is most commonly defined as a process functioning via intrasexual competition for mates and mating opportunities (Clutton-Brock, 2007). Consequently, traits such as female song that may increase the competitive ability of individuals and be important in female-female competition are not included within this narrow definition of sexual selection. However, female-female competition is covered by the broader approach of social selection (West-Eberhard, 1979, 1983; Amundsen, 2000; LeBas, 2006; Kraaijeveld et al., 2007; Rosvall, 2011; Tobias et al., 2011). In species where females contribute more to rearing offspring than males, female-female competition is arguably highest during breeding activities and in dense populations. If characteristics of female song reflect competitive ability, then we predict an association between female song rate and reproductive success and a positive association between aspects of song performance and female fitness. To our knowledge, just one study has examined female song rates and fitness (Cain et al., 2015), and it found a positive relationship between defense responses to song playbacks during territory establishment and the probability of successful nesting. No study has yet examined how female song complexity and song rate during breeding relates to reproductive success.
Our study uses observational data on patterns of female and male song rates, female song complexity, and amount of parental care to examine the association between these variables and reproductive success (a proxy for fitness). We focused on female song in the vicinity of the nest and spontaneous song rates and song complexity in the New Zealand bellbird (here-after referred to as bellbird); an endemic honeyeater with well-developed female territorial song. For bellbirds, intrasexual aggression is an important component of social interaction for both sexes (Brunton and Li, 2006) and previous playback experiments show that females respond aggressively to female song playbacks that simulate territorial intrusions (Brunton et al., 2008a), particularly to playbacks of their female neighbors (Brunton et al., 2008b). Singing and counter-singing by females occurs frequently throughout the breeding season and females often sing upon leaving the nest after an incubation bout (Li, 2002). We used observations of song and parental investment, and structural analysis of song of individual females to test the hypothesis that female song rates and complex female songs are associated with reproductive success. We suggest that female song performance during the breeding season is crucial in competitive social interactions associated with the defense of resources (including male parental investment), which are essential for breeding activities. Therefore, we predicted a positive relationship between female song rate and/or song structure and reproductive success.
Methods and Materials
Study Area and Species Background
Our study was conducted on Tiritiri Matangi Island (Tiri), a 220 ha island in the Hauraki Gulf, 28 km north of Auckland (Lat. 36°60′S, Long. 174.89°E). The bellbird population on Tiri is high-density, with over 10 birds/ha (Baillie and Brunton, 2011; Baillie et al., 2014), and has existed on the island for at least 100 years. Tiri is a wildlife sanctuary with open access to the public and currently has no introduced mammalian predators. The island has been extensively replanted over the last 20 years and a series of avian and reptilian species have been reintroduced for conservation reasons (Rimmer, 2004). Tiri is typical of most northern New Zealand forest ecosystems with year round fruiting and flowering of plant species providing ephemeral, scattered patches of abundant food resources (Gravatt, 1970). Consequently the social behavior and breeding chronology of the avian species and particularly honeyeater species is closely linked to the spatial and temporal patterns of plant breeding phenology (Craig and Douglas, 1986).
Bellbirds are sexually dimorphic in plumage and song (Heather and Robertson, 2000) and easily distinguished in the field (females are approximately 20% smaller and paler gray-green than males, and have a white cheek stripe). Relatively little is known about bellbird breeding biology (Kendrick, 1994; Brunton and Li, 2006; Massaro et al., 2008), although we do know that bellbirds are socially monogamous and that both sexes defend territories and/or resources throughout the year, but particularly during the breeding season (Craig and Douglas, 1986; Kendrick, 1994). The breeding cycle of bellbirds begins in late August and extends until January (Anderson and Craig, 2003). Clutch sizes range from 2 to 5 eggs and several clutches are laid each season. Nest-building activities (2–3 weeks) and incubation (2 weeks) are solely by females, whereas nest-based chick rearing is by both sexes and lasts until chicks fledge at 14–20 days of age. Chick provisioning by both parents can extend past fledging from the nest (Anderson and Craig, 2003). Although only the females incubate the eggs, males feed their mates during this period (Kendrick, 1994).
Bellbird Song
The singing modes of female and male bellbirds differ. Males frequently sing prolonged bouts during dawn chorus and engage in synchronized male chorusing at food sources (Li, 2002). At other times, males sing discrete songs and countersing with other males (Li, 2002). Females sing discrete songs with intervals of at least 3 s between songs (Li, 2002). Although female singing is rarely synchronized, counter-singing by nearby females occurs frequently and females often sing on leaving the nest after an incubation bout (e.g., Li, 2002). Although the number of song types produced by male and female bellbirds is similar (approximately 10 for the Tiri population), this variety is produced in different ways (Brunton and Li, 2006). Females produce song types that have the same basic structure but use different combinations and repetitions of syllables (e.g., ABC, AABC, ABCDE, AABCD, etc.). In contrast, each male song types are structurally distinct and may contain song type-specific syllables. Because of this difference between males and females in song type structure, and the low rate of singing by males in the vicinity of the nest, we were not able to obtain a large enough sample of male song and could not include measures of male song complexity. We based all syllable and song type definitions on previous bellbird studies (Li, 2002; Brunton and Li, 2006).
Data Collection
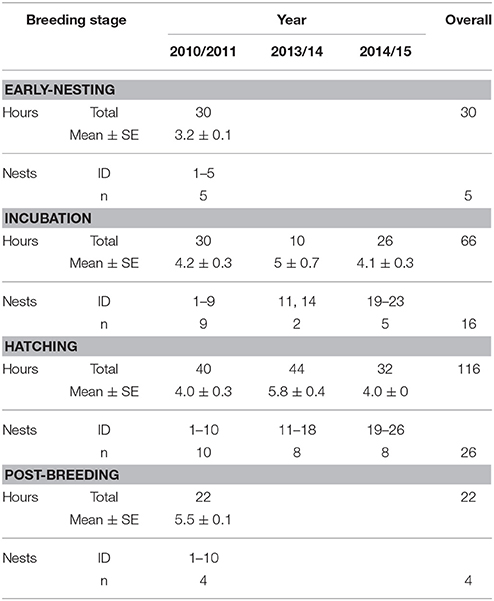
We collected the data used in this study as part of a larger study of bellbird behavioral ecology that has been running since 2007. The breeding season is during the austral summer, from October to late January. We monitored 26 nests (all first clutches of the season) by color banded males and females on Tiri over three breeding seasons 2010/2011, 2013/14, and 2014/2015 for a total of 216 h (x = 8.6 ± 0.2 h/nest; Table 1). We checked nests regularly (every 2–4 days) to determine clutch size, and hatching and fledging success. We captured adults using mist-nets or specially modified catching cages containing sugar-water feeders (each cage had multiple trap door entrances that we released manually when a target bird entered the cage). As part of our regular banding protocol we measured weight (g), wing length (mm), tarsus length (mm), tail length (mm), head-bill length (mm), body fat (0–3), and molt score (presence/absence), then we applied color-bands and collected a blood sample (all handling, banding, and blood sampling was performed under permits from the New Zealand Department of Conservation and the Massey University Animal Ethics Committee).
We compiled two independent datasets to examine female song and reproductive success. The first dataset involved direct observations of banded females and males in the vicinity of the nest to determine female and male song rates and behaviors associated with nesting activities (see below). Where possible, observations of song rates were also conducted 2 weeks prior to nesting and 2 weeks post-breeding. The second dataset used opportunistic audio recordings of color-banded females collected during breeding attempts and with known outcomes, to investigate the relationship between song structure and reproductive success.
Observations of Song Rate (Songs per Hour) and Nesting Behavior
Nests were observed continuously for durations of 1 h from clear vantage points at a distance of 5–10 m using binoculars. All activities within 15 m of the nest were generally visible. Incubation and chick-rearing stages were analyzed separately, and only nests with at least 3 h (max 8 h) of observation in a given stage were included (Table 1). Nests were not observed twice in the same day, observations started from approximately 8 a.m., and time of day was randomized. Observations for each nest spanned the entire nesting cycle but were not done systematically with regard to development stage. Behaviors recorded during 1 h observation periods for males and females included song bouts, and three parental investment behaviors: male and female chick provisioning, and male feeding the incubating female. All rates are reported per hour (averaged per nest per stage). Only nests that successfully hatched (but not necessarily fledged) at least one chick were included in the analysis of song rates and nesting behavior. Due to small sample sizes during early-nesting and post-breeding stages, overall comparisons of male and female song rates across breeding stages were compared using Wilcoxon signed-rank tests.
Female Song Recordings
We made song recordings of color-banded females using a Marantz PMD661 sound recorder and a Sennheiser ME66 K6 shotgun microphone (frequency response 50–22,500 Hz ± 2.5 dB). The microphone was hand-held using a Rycote shock-mount with a pistol grip. Songs were analyzed using spectrograms and waveforms generated in Raven Pro 1.5 (Cornell Laboratory of Ornithology, Ithaca, NY). Spectrograms were created using the Hann algorithm (Filter bandwidth 500 Hz, size 256 samples, and time gird overlap 50%). Five song measures (Figure 1) were taken from each of three songs per female and an average for each female was calculated. Three of the measures represented aspects of duration: song length (s ± 0.01), total number of syllables, and the number of “stutter” syllables (“stutter” syllables are short syllables that distinctively start and end female songs; Brunton and Li, 2006). The other two measures were proxies for song complexity: number of syllable types, and the number of transitions between syllable types.
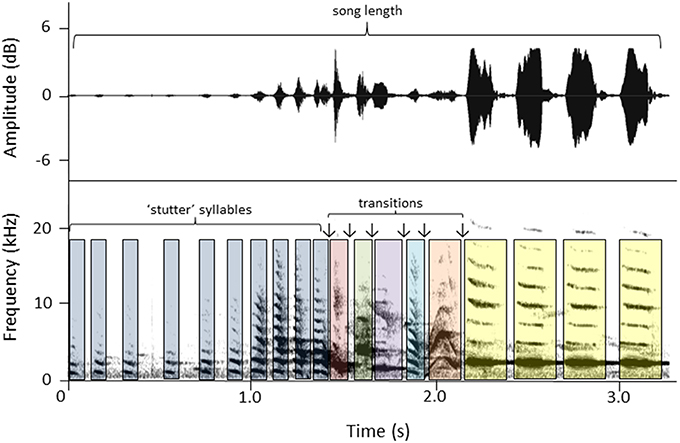
Figure 1. An example of a female song (waveform above, spectrogram below) and the song parameters calculated for each song: song length (s), total number of syllables, syllable types (colors represent different type), number of transitions between syllable types (↓), and number of stutter syllables. Measures for this song example are: # syllables = 19, song length = 3.19 s, # syllable types = 7, # stutter syllables = 10, # syllable transitions = 6.
Statistical Analysis
We investigated the effects of female and male song rate and parental investment on reproductive success (number of chicks fledged) using Poisson regression with a log-link function. Average hourly song rates per nest per stage were calculated and included in the models. Incubation and chick-rearing stages were analyzed separately, with female song rate, male song rate and the rate of male feeding the incubating female as predictors during the incubation stage, and female song rate, male song rate, female chick provisioning rate, and male chick provisioning rate as predictors during the chick-rearing stage. Males only feed females during the incubation stage. Analysis showed that during the incubation stage, female song rate, and male feeding rate were correlated (Figure 2A) and so a reduced model was fitted with male feeding rate removed. For the chick-rearing stage, there was a correlation between female song rate and male provisioning rate (Figure 2B), and so again a reduced model was fitted with male provisioning rate removed. There were no significant interactions in either analysis and so interactions were excluded from the models.
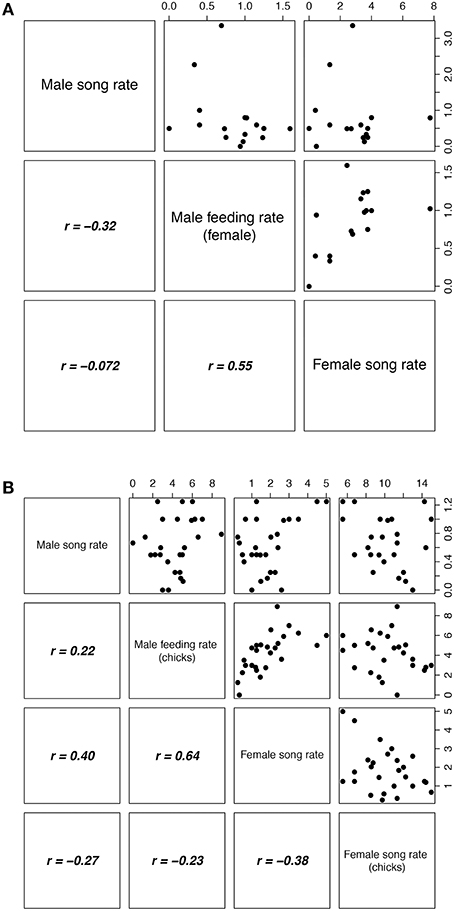
Figure 2. Correlation matrix for behavioral parameters measured during (A) incubation and (B) chick-rearing stages. The four behavioral measures are on the diagonal, the upper triangle shows scatter plots, and the lower triangle shows the respective Pearson's r-values. All values were measured as frequency per hour. Dataset based on means per nest (incubation, 16 nests; chick-rearing, 26 nests).
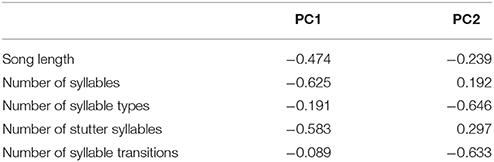
The effect of each of the song measures on nesting success (1) or failure (0) was examined using a Mann-Whitney U test. The relationship between female song structure and reproductive success (number of chicks fledged) was also investigated using Poisson regression with log-link function. However, the five song structure measures (song length, total number of syllables, number of syllable types, transitions between syllable types, and number of stutter syllables) were not independent and so a principle components analysis (PCA) was performed to reduce dimensionality of the data and generate independent predictor variables. Principle components one and two accounted for approximately 80% of the variation in the data, approximately evenly distributed between the two. Principle component loadings (Table 2) indicated that PC1 was composed mainly of total number of syllables and stutters per song, while PC2 was composed of the number of syllables types and the number of transitions per song. The principle component scores for PC1 and PC2 were then used as predictors in the Poisson regression. All analyzes were performed using the programming environment R (R Core Team, 2015). Where reported, means are presented with standard errors (± SE) and a significance level of α = 0.05 was used for all statistical tests.
Results
Female Song Rates and Reproductive Success
Song rates (per hour) on nesting territories varied with breeding stage and were not significantly different between males and females during early nesting and immediately post-breeding (Wilcoxon signed-rank test, W = 0; Figure 3). In contrast, during incubation and chick rearing stages female song rate was significantly higher than male song rate (Wilcoxon signed-rank test, W = 7 and 8, respectively, P < 0.01; Figure 3).
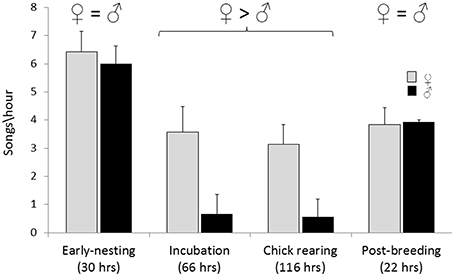
Figure 3. Spontaneous song rate (songs per hour) by males and females on nesting territories (< 15 m from the nest site) based on 234 h of observations of 26 nests. Wilcoxon signed-rank tests were used to compare song rates of males and female within each breeding stage. For early-nesting and post-breeding stages the differences were non-significant, and for incubation and chick-rearing stages female song rates were significantly greater than male song rates, W = 0, P < 0.05.
Observations of 16 nests during incubation (66 h) and 26 nests (the 16 incubation nests plus 10 additional nests) during chick-rearing (116 h) showed that female bellbirds contribute significantly more parental care than males (solely incubating young and provisioning chicks at more than twice the rate of males; paired t-test: t = 8.22, df = 26, P < 0.0001; Figure 4). Female song rate during both incubation and chick-rearing was a significant predictor of the number of chicks fledged (Table 3; Figure 5), but male song rate was not (Table 3). Female chick provisioning was not a significant effect in our model (Table 3).
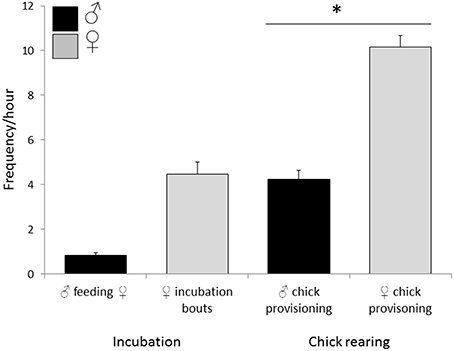
Figure 4. Relative parental investment (PI) by male and female bellbirds. During incubation PI included the male feeding incubating female and female incubation bouts (16 nests, 66 h). During chick-rearing PI included male and female provisioning chicks (26 nests, 116 h). Female chick provisioning rate was significantly greater than male chick-provisioning frequency (paired t-test: t = 8.22, df = 25, P < 0.0001, indicated by *). Only nests that hatched at least one chick were included in the analysis.
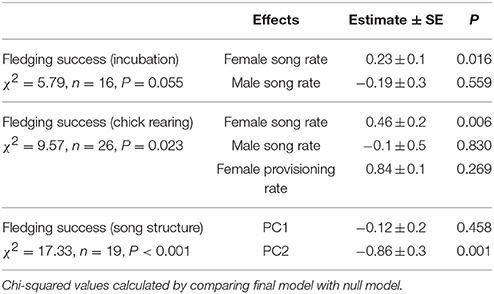
Table 3. Results of generalized linear models relating fledging success to song and provisioning rates, and song structure.
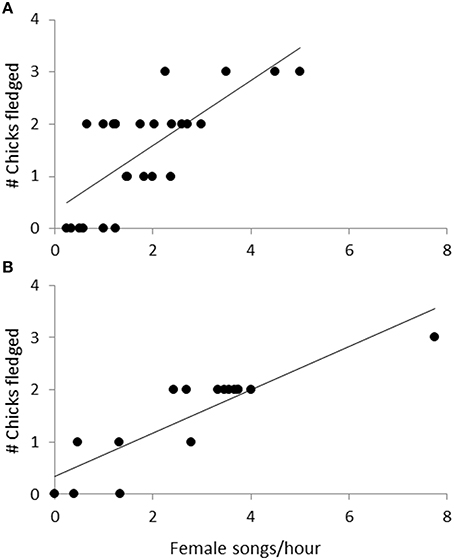
Figure 5. The relationship between the number of chicks fledged and female song rate during (A) incubation (n = 16, 66 observation hours), and (B) chick-rearing (n = 26, 116 observation hours). The line is from a regression.
Female Song Structure and Reproductive Success
Fifty-seven high quality recordings (low noise to signal ratio) of single songs by 19 females (three songs per female), where nesting outcome was known, were included in the analysis. We first examined breeding success as a binary response (failed vs. one or more fledglings) for the five song structure measures, and found that only song complexity (number of syllable types and number of transitions between syllable types) were significantly different (Mann-Whitney U-tests: U = 4, P < 0.05, and tests U = 18, P < 0.05, respectively; Figure 6). We then included fledgling number (0–3) and applied our GLM model using PCA scores from the five song measures and found that PC2 was a predictor of the number of chicks fledged (Table 3) and PC1 was not (Table 3). The number of different syllable types and the number of transitions between different syllable types were both associated with PC2 (Table 2; Figure 7). Hence, the more syllable types and syllable transitions in a female's song, the greater number of chick fledged (Figure 8). The duration of the song, the total number of syllables, and the number of introductory stutter syllables did not predict nesting success.
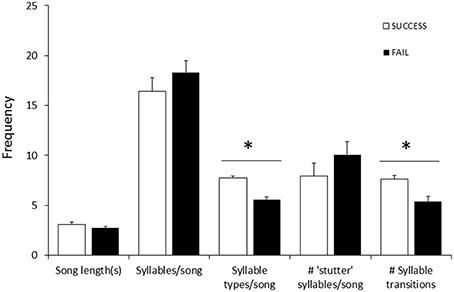
Figure 6. Differences in female song measures (song length, number of syllables, number of syllable types, number of transitions between syllable types, and number of “stutter” syllables; averaged using three songs per female) between nests that successfully fledged chicks (□, n = 10) and nests where eggs hatched but no chicks fledged (■, n = 9). Data included nests from 2013/14 (n = 9) and 2014/15 (n = 10), all by different females. There was no difference between years in the proportion of successful or failed nests (2013: 5 successful, 4 failed; 2014: 4 successful, 5 failed). Significant differences (Mann-Whitney U-test) are indicated (*).
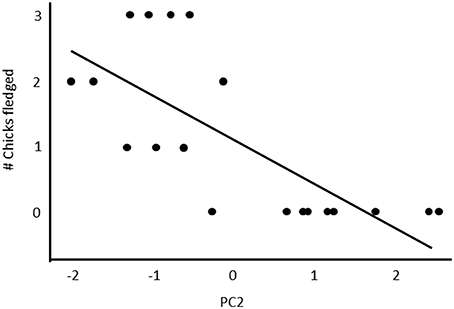
Figure 7. The relationship between the PC2 scores of a PCA analysis and reproductive success. Table 2 indicates the loadings of each of the five song parameters on PC2.
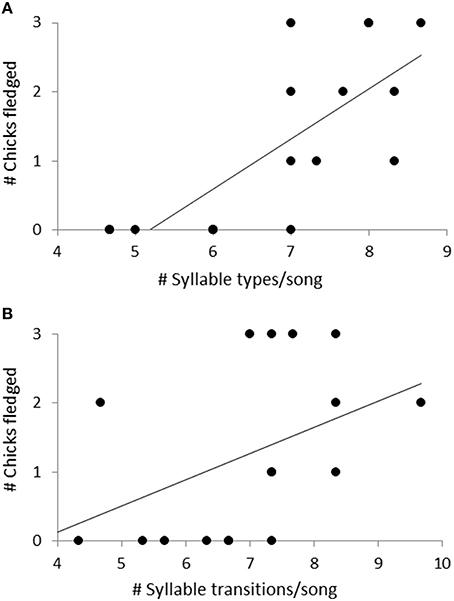
Figure 8. The relationship between the number of chicks fledged and (A) the number of syllable types per song, and (B) the number of transitions between syllable types per song. Linear regression lines show direction of the relationship. These two parameters were associated with the PC2 scores of a PCA analysis looking at the effect of five song parameters on female reproductive success.
Discussion
We examined the relationship between reproductive success and measures of song structure in females and found that spontaneous song rate during breeding positively correlated with reproductive success and that measures of female song complexity (number of different syllable types and number of transitions between different syllable types) predicted greater breeding success in bellbirds. This contrasts somewhat with other studies where female territorial defense behavior in response to a simulated female intruder (but not spontaneous song), predicts reproductive success (Cain et al., 2015), and where higher rates of female song at the nest increases predation risk (Kleindorfer et al., 2016).
It is known that female song in bellbirds has an important role in female competition (Brunton et al., 2008b) and here we found that higher female song rate during both incubation and chick-rearing were positively correlated with male provisioning rate (Figure 2). In northern cardinals (Cardinalis cardinalis), males were more likely to come to the nest when the female sang in the vicinity of the nest than when she did not (Halkin, 1997). In contrast, Kleindorfer et al. (2016) found in superb fairy wrens that female song in the nest incurs a significant cost in terms of increased risk of predation and they suggest this may provide a strong selective pressure for reduced female song during breeding. In our study, without manipulating female song rate or male provisioning rate, it is not possible to infer a causal relationship between these factors and chick fledging success. We cannot of course exclude the possibility of other correlated factors, for example, if female song rate is an honest signal of quality (Gil and Gahr, 2002), males may preferentially invest more in females with higher song rates. Furthermore, higher female song rates may secure better access to resources needed for chick-rearing; a possibility supported by our previous playback experiments that found high levels of aggression by territorial females to song playbacks of female neighbors (Brunton et al., 2008b). The lack of a significant effect of chick-provisioning rates and reproductive success was unexpected and is in part due to the strong effect of female song rate, and in part due to our imprecise measure of feeding rate: we were unable to determine quantity or quality of food being delivered to the chicks. Future experimental studies will be needed to test whether females may be increasing fledging success by soliciting higher male attentiveness at the nest.
For males, the literature provides mixed results for the relationship between song performance measures (repertoire, rate, and complexity) and fitness. Positive relationships between repertoire size and annual reproductive success have been demonstrated in great reed warblers, Arcocephalus arundinaceus (Catchpole, 1986; Hasselquist et al., 1996), song sparrows, Melospiza melodia (Hiebert et al., 1989; Reid et al., 2005), European starlings, (Eens et al., 1991), willow warblers, Phylloscopus trochilus (Gil and Slater, 2000), and zebra finches, Taniopygia guttata (Woodgate et al., 2012). In contrast, song is often not a reliable indicator of male quality and reproductive success [e.g., in great reed warblers (Forstmeier and Leisler, 2004), collared flycatchers, Ficedula albicollis, (Garamszegi et al., 2007), and rock sparrows, Petronia petronia, (Nemeth et al., 2012)]. The variation found in empirical studies of the relationship between male song quality and reproductive success, even within a single species, highlights the multimodal nature of sexually selected signals. Although varying song qualities may influence male song function and mating success, other traits such as plumage and behavior may provide stronger and/or alternative mechanisms for mate attraction, resulting in the absence of a link between song quality and reproductive success (Tobias et al., 2012).
Female Song and Social Selection
Because territorial song is so well-entrenched in the literature as a sexually selected male trait involved in competitive processes, many questions remain unanswered as to the nature of selection for song and other ornaments in females (Langmore, 1998; Riebel et al., 2005; Tobias et al., 2012). What are the selective drivers of female song performance and why should female song matter? In year-round territorial species such as bellbirds, where predators are limited, access to resources is likely to be the main predictor of reproductive success. Our study population is located on a conservation island where the main predators are native avian species (the diurnal, open country hunting Australasian harrier, Circus approximans, and the nocturnal morepork, Ninox novaseelandiae). The bellbird population is dense and likely to be at carrying capacity; therefore we expect that resources probably constrain reproduction and survival. Under such competitive conditions, aggressive social interactions are important and frequent. In general for birds, female reproductive success is not constrained by the number of mates, but by access to resources and parental investment (Bateman, 1948; Trivers, 1972).
If female song is linked to reproductive success but not via classical sexual selection (male attraction), then social selection may provide an alternative hypothesis (i.e., females use song and other competitive indicators to compete for ecological resources). Hence, female territorial song and traits involved in female competition may fit better within the concept of social selection (West-Eberhard, 1979, 1983; Amundsen, 2000; LeBas, 2006; Kraaijeveld et al., 2007; Rosvall, 2011; Tobias et al., 2011). Social selection (defined in West-Eberhard, 1979) focuses on the role of social interactions as drivers of selection, regardless of whether the interaction is sexual or non-sexual (Lyon and Montgomerie, 2012; Tobias et al., 2012). Although still not widely embraced and somewhat controversial, social selection predicts that the strength of social interactions may determine how aggressive traits such as vocalizations, coloration, weapons, and ornaments correlate with measures of reproductive success (McComb et al., 1994; Friedman et al., 2009; Cornwallis and Uller, 2010; Santana et al., 2012; Tobias et al., 2012; Morales et al., 2014).
Our study and several studies of superb fairy wrens (Kleindorfer et al., 2013, 2016; Cain et al., 2015) examine female song and reproductive success in free-living populations. In both bellbirds and superb fairy wrens, females sing complex songs year round at rates comparable to males and in the context of competition between females (Cooney and Cockburn, 1995; Brunton and Li, 2006; Brunton et al., 2008b; Kleindorfer et al., 2013). Aspects of the life history of these two species differ: superb fairy wrens are insectivores and cooperative breeders, whereas bellbirds are primarily nectarivores and exhibit social monogamy (Baillie et al., 2014). However, both species exhibit female dispersal and are non-migratory (Cockburn et al., 2008; Baillie et al., 2014). For these and other species with female song, it is likely that female song traits may convey information about competitive ability such as body condition and age could therefore influence the outcome of non-sexual social interactions and ultimately breeding success and survival.
It is unclear why male song rate does not predict the number of chicks fledged. We were unable to collect a sufficient number of male songs to be able to analyse male song structure and it is possible that song complexity rather than rate may be important in male song performance. Alternatively, part of the answer may be extra-pair paternity. Cope (2008) found high levels of extra-pair paternity in the Tiri bellbird populations and Wells et al. (2015) found high extra-pair paternity rates in a closely related New Zealand honeyeater, the tui (Prosthemadera novaeseelandiae). Taff et al. (2012) found that male plumage features were associated with within-pair mating success while song consistency was associated with extra-pair paternity in the common yellowthroat (Geothlypis trichas). Male song rates and song structure need to be explored further in order to understand how they relate to both mating success and parental investment in bellbirds.
Although our study was observational and we used a single measure of reproductive success, we found that female song rate and structure were good predictors of breeding success. Indeed, because the birds used in the behavioral observation dataset were independent of those used to analyze song structure, our findings are strengthened as analyzes of both datasets showed relationships between female song and breeding success.
The relationships reported here between female song structure, song rate, and reproductive success are based on correlational data. Future work should focus on experimental evidence to confirm the function of song performance in either increasing male provisioning or in social competition. Female song playbacks, clutch size manipulations, and genetic hereditability studies may help us tease apart correlative factors. In addition, how other phenotypic characteristics (e.g., size, body condition, and plumage), female age, and mate quality influence female song structure and rate needs to be explored.
Conclusions
Although this study is among the first to find a relationship between female song and reproductive success, there is a growing literature on the subject of female song and many species with territorial female song have yet to be studied. We used two simple approaches, observations of song rates and measures of song complexity to measure female song quality in relation to breeding success. We found the rate of spontaneous song by females during both incubation and the chick-rearing period was a good predictor for the number of chicks fledged. This was not the case for males. We also found that our two measures of female song complexity were positively correlated with higher numbers of fledglings. Overall our findings support the hypothesis that female song may be a signal of competitive ability (Cain and Ketterson, 2012; Tobias et al., 2012; Stockley and Campbell, 2013; Cain and Rosvall, 2014) but further work is required to experimentally test this relationship and to examine mechanisms (Cain and Ketterson, 2012). It is anticipated that as more studies examining the relationship between female song traits and reproductive success and survival are published, the extent of the connection between female song traits and female fitness will be revealed.
Author Contributions
DB designed the study, developed the methodology, performed aspects of the analysis, and wrote the manuscript. MR collected the data, and assisted development of the methodology and managed the interns. AH performed the analyses and wrote sections of the paper.
Funding
DB and MR were supported by a Marsden Fund Grant (13-MAU-004) from the Royal Society of New Zealand.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank X. Sleeuenhoek, K. Schor, and B. Maste, N. Keehnen, P. Pruisscher for help with fieldwork, and the University of Applied Science in Agriculture, Food Technology, Environmental and Animal Sciences, Leeuwarden, for facilitating the stay of these interns in New Zealand. We would like to thank M. Hall and N. Langmore for the invitation to contribute to this topic, Frontiers in Ecology and Evolution for hosting this special issue, and Behaviour 2015 conference for hosting the symposia. Logistical support was provided by the Institute of Natural and Mathematical Sciences of Massey University, the Department of Conservation, and the Supporters of Tiritiri Matangi Island. We are especially grateful to the DOC rangers present on Tiri during this study. We thank B. Evans for help with collating sound data and K. Rainey, J. Dale, and A. Martin for help with reviewing and editing the manuscript.
References
Amundsen, T. (2000). “Female ornaments: genetically correlated or sexually selected?,” in Signalling and Signal Design in Animal Communication, eds Y. Espmark, T. Amundsen, and G. Rosenqvist (Trondheim: Tapir Academic Press), 133–154.
Anderson, S. H., and Craig, J. L. (2003). Breeding biology of bellbirds (Anthornis melanura). Notornis 50, 75–82.
Arcese, P., Stoddard, P. K., and Hiebert, S. M. (1988). The form and function of song in female song sparrows. Condor 90, 44–50.
Baillie, S. M., and Brunton, D. H. (2011). Diversity, distribution and biogeographical origins of Plasmodium parasites from the New Zealand bellbird (Anthornis melanura). Parasitology 138, 1843–1851. doi: 10.1017/S0031182011001491
Baillie, S. M., Ritchie, P. A., and Brunton, D. H. (2014). Population genetic connectivity of an endemic New Zealand passerine after large-scale local extirpations: a model of re-colonization potential. Ibis 156, 826–839. doi: 10.1111/ibi.12182
Bateman, A. J. (1948). Intra-sexual selection in Drosophila. Heredity 2, 349–368. doi: 10.1038/hdy.1948.21
Botero, C. A., Rossman, R. J., Caro, L. M., Stenzler, L. M., Lovette, I. J., de Kort, S. R., et al. (2009). Syllable type consistency is related to age, social status and reproductive success in the tropical mockingbird. Anim. Behav. 77, 701–706. doi: 10.1016/j.anbehav.2008.11.020
Brunton, D. H., Evans, B., Cope, T., and Ji, W. (2008b). A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19, 791–798. doi: 10.1093/beheco/arn027
Brunton, D. H., Evans, B. A., and Ji, W. (2008a). Assessing natural dispersal of New Zealand bellbirds using song type and song playbacks. NZ J. Ecol. 32, 147–154.
Brunton, D. H., and Li, X. (2006). The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J. Ethol. 24, 17–25. doi: 10.1007/s10164-005-0155-5
Cain, K., and Ketterson, E. (2012). Competitive females are successful females; phenotype, mechanism, and selection in a common songbird. Behav. Ecol. Sociobiol. 66, 241–252. doi: 10.1007/s00265-011-1272-5
Cain, K. E., Cockburn, A., and Langmore, N. (2015). Female song rates in response to simulated intruder are positively related to reproductive success. Front. Ecol. Evol. 3:119. doi: 10.3389/fevo.2015.00119
Cain, K. E., and Rosvall, K. A. (2014). Next steps for understanding the selective relevance of female-female competition. Front. Ecol. Evol. 2:32. doi: 10.3389/fevo.2014.00032
Catchpole, C. K. (1982). “The evolution of bird sounds in relation to mating and spacing behavior,” in Acoustic Communication in Birds, eds D. Kroodsma and E. Miller (New York, NY: Academic Press), 297–319.
Catchpole, C. K. (1986). Song repertoires and reproductive success in the great reed warbler Acrocephalus arundinaceus. Behav. Ecol. Sociobiol. 19, 439–445. doi: 10.1007/BF00300547
Catchpole, C. K. (1987). Bird song, sexual selection and female choice. Trends Ecol. Evol. 2, 94–97. doi: 10.1016/0169-5347(87)90165-0
Clutton-Brock, T. (2007). Sexual selection in males and females. Science 318, 1882–1885. doi: 10.1126/science.1133311
Cockburn, A., Sims, R. A., Osmond, H. L., Green, D. J., Double, M. C., and Mulder, R. A. (2008). Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438. doi: 10.1111/j.1365-2656.2007.01351.x
Cooney, R., and Cockburn, A. (1995). Territorial defence is the major function of female song in the superb fairy-wren, Malurus cyaneus. Anim. Behav. 49, 1635–1647. doi: 10.1016/0003-3472(95)90086-1
Cope, T. (2008). The Behavioural Ecology and Mating System of the Bellbird (Anthornis melanura.), MSc thesis (Auckland: Massey University).
Cornwallis, C. K., and Uller, T. (2010). Towards an evolutionary ecology of sexual traits. Trends Ecol. Evol. 25, 145–152. doi: 10.1016/j.tree.2009.09.008
Craig, J. L., and Douglas, M. E. (1986). Resource distribution, aggressive asymmetries and variable access to resources in the nectar feeding bellbird. Behav. Ecol. Sociobiol. 18, 231–240. doi: 10.1007/BF00290827
Darwin, C. (1871). The Descent of Amn, and Selection in Realtion to Sex. London: John Murray. doi: 10.5962/bhl.title.110063
Eens, M., Pinxten, R., and Verheyen, R. F. (1991). Male song as a cue for mate choice in the European starling. Behavior 116, 210–238. doi: 10.1163/156853991X00049
Forstmeier, W., and Leisler, B. (2004). Repertoire size, sexual selection, and offspring viability in the great reed warbler: changing patterns in space and time. Behav. Ecol. 15, 555–563. doi: 10.1093/beheco/arh051
Friedman, N. R., Hofmann, C. M., Kondo, B., and Omland, K. E. (2009). Correlated evolution of migration and sexual dichromatism in the New World orioles (Icterus). Evol. 63, 3269–3274. doi: 10.1111/j.1558-5646.2009.00792.x
Garamszegi, L. Z., Pavlova, D. Z., Eens, M., and Møller, A. P. (2007). The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96. doi: 10.1093/beheco/arl047
Geberzahn, N., Goymann, W., Muck, C., and ten Cate, C. (2009). Females alter their song when challenged in a sex-role reversed bird species. Behav. Ecol. Sociobiol. 64, 193–204. doi: 10.1007/s00265-009-0836-0
Gil, D., and Gahr, M. (2002). The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141. doi: 10.1016/S0169-5347(02)02410-2
Gil, D., and Slater, P. J. (2000). Song organisation and singing patterns of the willow warbler, Phylloscopus trochilus. Behav. 137, 759–782. doi: 10.1163/156853900502330
Gravatt, D. J. (1970). Honeyeater movements and the flowering cycle of vegetation in Little Barrier Island. Notornis 17, 96–101.
Halkin, S. L. (1997). Nest-vicinity song exchanges may coordinate biparental care of northern cardinals. Anim. Behav. 54, 189–198. doi: 10.1006/anbe.1996.0415
Hasselquist, D., Bensch, S., and von Schantz, T. (1996). Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature 381, 229–232. doi: 10.1038/381229a0
Heather, B. D., and Robertson, H. A. (2000). The Field Guide to the Birds of New Zealand. New York, NY: Viking Books.
Hiebert, S. M., Stoddard, P. K., and Arcese, P. (1989). Repertoire size, territory acquisition and reproductive success in the song sparrow. Anim. Behav. 37, 266–273. doi: 10.1016/0003-3472(89)90115-2
Illes, A. E. (2015). Context of female bias in song repertoire size, singing effort, and singing independence in a cooperatively breeding songbird. Behav. Ecol. Sociobiol. 69, 139–150. doi: 10.1007/s00265-014-1827-3
Kipper, S., and Kiefer, S. (2010). Age-related changes in birds' singing styles: on fresh tunes and fading voices? Adv. Study Behav. 41, 77–118. doi: 10.1016/S0065-3454(10)41003-7
Kleindorfer, S., Evans, C., and Mahr, K. (2016). Female in-nest chatter song increases predation. Biol. Lett. 12:20150513. doi: 10.1098/rsbl.2015.0513
Kleindorfer, S., Evans, C., Mihailova, M., Colombelli-Négrel, D., Hoi, H., Griggio, M., et al. (2013). When subspecies matter: resident Superb Fairy-wrens (Malurus cyaneus) distinguish the sex and subspecies of intruding birds. Emu 113, 259–269. doi: 10.1071/MU12066
Kraaijeveld, K., Kraaijeveld-Smit, F. J. L., and Komdeur, J. (2007). The evolution of mutual ornamentation. Anim. Behav. 74, 657–677. doi: 10.1016/j.anbehav.2006.12.027
Kroodsma, D. E., and Byers, B. E. (1991). The Function(s) of Bird Song. Amer. Zool. 31, 318–328. doi: 10.1093/icb/31.2.318
Lampe, H. M., and Espmark, Y. O. (1994). Song structure reflects male quality in pied flycatchers, Ficedula hypoleuca. Anim. Behav. 47, 869–876. doi: 10.1006/anbe.1994.1118
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. 13, 136–140. doi: 10.1016/S0169-5347(97)01241-X
Langmore, N. E., and Davies, N. B. (1997). Female dunnocks use vocalizations to compete for males. Anim. Behav. 53, 881–890. doi: 10.1006/anbe.1996.0306
LeBas, N. R. (2006). Female finery is not for males. Trends Ecol. Evol. 21, 170–173. doi: 10.1016/j.tree.2006.01.007
Li, X. (2002). The Structure and Function of Female Song in Bellbird (Anthornis melanura), MSc thesis (Auckland: University of Auckland).
Lyon, B. E., and Montgomerie, R. (2012). Sexual selection is a form of social selection. Philo. Trans. R. Soc. Lond. B 367, 2266–2273. doi: 10.1098/rstb.2012.0012
Massaro, M., Starling-Windhof, A., Briskie, J. V., and Martin, T. E. (2008). Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird. PLoS ONE 3:e2331. doi: 10.1371/journal.pone.0002331
McComb, K., Packer, C., and Pusey, A. (1994). Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim. Behav. 47, 379–387.
Morales, J., Gordo, O., Lobato, E., Ippi, S., Martinez-De La Puente, J., Tomás, G., et al. (2014). Female-female competition is influenced by forehead patch expression in pied flycatcher females. Behav. Ecol. Sociobiol. 68, 1195–1204. doi: 10.1007/s00265-014-1730-y
Nemeth, E., Kempenaers, B., Matessi, G., and Brumm, H. (2012). Rock sparrow song reflects male age and reproductive success. PLoS ONE 7:e43259. doi: 10.1371/journal.pone.0043259
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5:3379. doi: 10.1038/ncomms4379
Odom, K. J., Omland, K. E., and Price, J. J. (2015). Differentiating the evolution of female song and male–female duets in the New World blackbirds: can tropical natural history traits explain duet evolution? Evolution 69, 839–847. doi: 10.1111/evo.12588
Pavlova, D., Pinxten, R., and Eens, M. (2005). Female song in european starlings: sex differences, complexity, and composition. Condor 107, 559–569. doi: 10.1650/0010-5422(2005)107[0559:FSIESS]2.0.CO;2
Price, J. J. (2015). Rethinking our assumptions about the evolution of bird song and other sexually dimorphic signals. Front. Ecol. Evol. 3:40. doi: 10.3389/fevo.2015.00040
Price, J. J., Lanyon, S. M., and Omland, K. E. (2009). Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc. R. Soc. B 276, 1971–1980. doi: 10.1098/rspb.2008.1626
Price, J. J., Yunes-Jimenez, L., Osorio-Beristain, M., Omland, K. E., and Murphy, T. E. (2008). Sex-role reversal in song? Females sing more frequently than males in the Streak-backed Oriole. Condor 110, 387–392. doi: 10.1525/cond.2008.8430
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Reid, J. M., Arcese, P., Cassidy, A. L., Hiebert, S. M., Smith, J. N., Stoddard, P. K., et al. (2004). Song repertoire size predicts initial mating success in male song sparrows, Melospiza melodia. Anim. Behav. 68, 1055–1063. doi: 10.1016/j.anbehav.2004.07.003
Reid, J. M., Arcese, P., Cassidy, A. L., Hiebert, S. M., Smith, J. N., Stoddard, P. K., et al. (2005). Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia). Am. Nat. 165, 299–310. doi: 10.1086/428299
Riebel, K. (2003). The “mute” sex revisited: vocal production and perception learning in female songbirds. Adv. Study Behav. 33, 49–86. doi: 10.1016/S0065-3454(03)33002-5
Riebel, K., Hall, M. L., and Langmore, N. E. (2005). Female songbirds still struggling to be heard. Trends Ecol. Evol. 20, 419–420. doi: 10.1016/j.tree.2005.04.024
Rivera-Gutierrez, H. F., Pinxten, R., and Eens, M. (2010). Multiple signals for multiple messages: great tit, Parus major, song signals age and survival. Anim. Behav. 80, 451–459. doi: 10.1016/j.anbehav.2010.06.002
Rosvall, K. A. (2011). Intrasexual selection on females: evidence for sexual selection? Behav. Ecol. 22, 1131–1140. doi: 10.1093/beheco/arr106
Santana, S. E., Alfaro, J. L., and Alfaro, M. E. (2012). Adaptive evolution of facial color patterns in Neotropical primates. Proc. R. Soc. B 279, 2204–2211. doi: 10.1098/rspb.2011.2326
Searcy, W. A., and Andersson, M. (1986). Sexual selection and the evolution of song. Ann. Rev. Ecol. Sys. 17, 507–533. doi: 10.1146/annurev.es.17.110186.002451
Searcy, W. A., and Marler, P. (1981). A test for responsiveness to song structure and programming in female sparrows. Science, 213, 926–928. doi: 10.1126/science.213.4510.926
Soma, M., and Garamszegi, L. (2011). Rethinking birdsong evolution: meta-analysis of the relationship between song complexity and reproductive success. Behav. Ecol. 22, 363–371. doi: 10.1093/beheco/arq219
Soma, M. G., and Garamszegi, L. Z. (2015). Evolution of courtship display in Estrildid finches: dance in relation to female song and plumage ornamentation. Front. Ecol. Evol. 3:4. doi: 10.3389/fevo.2015.00004
Stockley, P., and Campbell, A. (2013). Female competition and aggression: interdisciplinary perspectives. Phil. Trans. R. Soc. B 368:20130073. doi: 10.1098/rstb.2013.0073
Taff, C. C., Steinberger, D., Clark, C., Belinsky, K., Sacks, H., Freeman-Gallant, C. R., et al. (2012). Multimodal sexual selection in a warbler: plumage and song are related to different fitness components. Anim. Behav. 84, 813–821. doi: 10.1016/j.anbehav.2012.07.002
Tobias, J. A., Gamarra-Toledo, V., Garcia-Olaechea, D., Pulgarin, P. C., and Seddon, N. (2011). Year-round resource defence and the evolution of male and female song in suboscine birds: social armaments are mutual ornaments. J. Evol. Biol. 24, 2118–2138. doi: 10.1111/j.1420-9101.2011.02345.x
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. B 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Trivers, R. L. (1972). “Parental investment and sexual selection,” in Sexual Selection and the Descent of Man 1871–1971, ed B. Campbell (Chicago, IL: Aldine), 136–179.
Wells, S. J., Ji, W., Dale, J., Jones, B., and Gleeson, D. (2015). Male size predicts extrapair paternity in a socially monogamous bird with extreme sexual size dimorphism. Behav. Ecol. 26, 200–206. doi: 10.1093/beheco/aru173
West-Eberhard, M. J. (1979). Sexual selection, social competition, and evolution. Proc. Am. Philos. Soc. 123, 222–234.
West-Eberhard, M. J. (1983). Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183. doi: 10.1086/413215
Keywords: female song, parental investment, reproductive success, social selection, song rate, song structure, song performance, female-female competition
Citation: Brunton DH, Roper MM and Harmer AMT (2016) Female Song Rate and Structure Predict Reproductive Success in a Socially Monogamous Bird. Front. Ecol. Evol. 4:13. doi: 10.3389/fevo.2016.00013
Received: 06 November 2015; Accepted: 09 February 2016;
Published: 01 March 2016.
Edited by:
Carlos Alonso Alvarez, Consejo Superior de Investigaciones Cientificas, SpainReviewed by:
Sharon A. Gill, Western Michigan University, USASonia Kleindorfer, Flinders University, Australia
Copyright © 2016 Brunton, Roper and Harmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dianne H. Brunton, ZC5oLmJydW50b25AbWFzc2V5LmFjLm56
 Dianne H. Brunton
Dianne H. Brunton Michelle M. Roper
Michelle M. Roper Aaron M. T. Harmer
Aaron M. T. Harmer