- 1Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 2Department of Renewable Resources, University of Alberta, Edmonton, AB, Canada
The mountain pine beetle (MPB; Dendroctonus ponderosae) has recently expanded its range from lodgepole pine forest into the lodgepole × jack pine hybrid zone in central Alberta, within which it has attacked pure jack pine. This study tested the effects of water limitation on tree defense response of mature lodgepole and jack pine (Pinus contorta and Pinus banksiana) trees in the field. Tree defense response was initiated by inoculation of trees with the MPB-associated fungus Grosmannia clavigera and measured through monoterpene emission from tree boles and concentration of defensive compounds in phloem, needles, and necrotic lesion tissues. Lodgepole pine generally emitted higher amounts of monoterpenes than jack pine; particularly from fungal-inoculated trees. Compared to non-inoculated trees, fungal inoculation increased monoterpene emission in both species, whereas water treatment had no effect on monoterpene emission. The phloem of both pine species contains (−)-α-pinene, the precursor of the beetle's aggregation pheromone, however lodgepole pine contains two times as much as jack pine. The concentration of defensive compounds was 70-fold greater in the lesion tissue in jack pine, but only 10-fold in lodgepole pine compared to healthy phloem tissue in each species, respectively. Water-deficit treatment inhibited an increase of L-limonene as response to fungal inoculation in lodgepole pine phloem. The amount of myrcene in jack pine phloem was higher in water-deficit trees compared to ambient trees. Beetles reared in jack pine were not affected by either water or biological treatment, whereas beetles reared in lodgepole pine benefited from fungal inoculation by producing heavier female offspring. Female beetles that emerged from jack pine bolts contained more fat than those that emerged from lodgepole pine, even though lodgepole pine phloem had a higher nitrogen content than jack pine phloem. These results suggest that jack pine chemistry is suitable for MPB pheromone production and aggregation on the host tree.
Introduction
Conifers possess complex defense mechanisms, which can protect them from herbivory (Phillips and Croteau, 1999; Franceschi et al., 2005; Raffa et al., 2005). In response to a stem–invading insect, trees exude resin that provides a physical barrier to prevent further insect damage (Raffa and Berryman, 1983; Phillips and Croteau, 1999; Keeling and Bohlmann, 2006; Raffa et al., 2008). Resin contains a diverse mixture of defensive terpenoid compounds, such as monoterpenes, sesquiterpenes, and diterpenes (Keeling and Bohlmann, 2006). Some herbivores can utilize these compounds for host species selection, and to identify weakened trees that can be easily colonized (Keeling and Bohlmann, 2006). A few bark beetle species are even known to exploit host monoterpenes as precursors for pheromone production to attract mates and to initiate mass attacks that allow them to rapidly overcome tree defense (Wood, 1982; Seybold et al., 2000), ultimately resulting in tree death. Thus, bark beetles are ecologically and economically important disturbance agents in conifer forests (Raffa et al., 2008; Bentz et al., 2010) and due to the significance of chemical defenses to bark beetle biology, differences in chemical characteristics among hosts may be important in determining beetle attack behavior and host susceptibility.
The recent outbreak of the mountain pine beetle (MPB; Dendroctonus ponderosae, Coleoptera: Curculionidae) has killed 18.1 million hectares of mainly lodgepole pine (Pinus contorta) forests in British Columbia alone (https://www.for.gov.bc.ca/hfp/mountain_pine_beetle/facts.htm). In 2002, the outbreak expanded beyond the Rocky Mountains and since then has moved beyond the eastern edge of the lodgepole pine range in north-central Alberta (Safranyik et al., 2010). In this region, forest composition shifts from lodgepole pine to jack pine (Pinus banksiana)-dominated boreal forests through a zone of lodgepole × jack pine hybrids (Moss, 1949; Mirov, 1956). Within this hybrid zone, MPB has attacked both hybrid and pure jack pines (Cullingham et al., 2011).
There is a close relationship between tree secondary metabolites and MPB during host colonization and establishment (Wood, 1982). During the early stages of host location, host volatiles can act as kairomones for flying bark beetles, like β-phellandrene (Miller and Borden, 1990) which is the main monoterpene of lodgepole pine. During the colonization process female MPBs require the host monoterpene α-pinene as a precursor for production of their aggregation pheromone trans-verbenol (Conn et al., 1984; Blomquist et al., 2010). In general Dendroctonus species convert the respective S or R enantiomer of α-pinene into the corresponding enantiomer of trans-verbenol (Byers, 1983, 1989), hence MPB most likely requires (−)-α-pinene to produce the significantly more attractive stereoisomer of its aggregation pheromone: (−)-trans-verbenol (Whitehead et al., 1989), which attracts beetles of both sex. During further host colonization, male beetles produce exo-brevicomin which acts synergistically with trans-verbenol to attract conspecifics to overwhelm tree defenses (Pureswaran et al., 2000). Once the optimal attack density is achieved, males produce the anti-aggregation pheromone frontalin, and both sexes produce the anti-aggregation pheromone verbenone to minimize intraspecific competition (Rudinsky et al., 1974; Ryker and Libbey, 1982; Blomquist et al., 2010). Additionally, some host monoterpenes including myrcene, 3-carene, terpinolene, and α-pinene are known to synergize the response of MPB to its aggregation pheromone (Borden et al., 2008). The blue stain fungi, Grosmannia clavigera and Ophiostoma montium, associated with D. ponderosae help the beetle to deplete tree defenses and kill their host (Reid et al., 1967; Rice et al., 2007). G. clavigera is considered more virulent than O. montium (Yamaoka et al., 1990) and is often used to simulate beetle attack in order to study tree defenses (Reid et al., 1967; Boone et al., 2011).
Outbreaks of phytophagous insects, including MPB and other bark beetles (Dobbertin et al., 2007; Alfaro et al., 2010; Netherer et al., 2015), have been linked to drought (Mattson and Haack, 1987). Drought stress can affect the production of secondary plant metabolites (Herms and Mattson, 1992), and thus might interfere with host defenses. Jack pine may be less influenced by drought conditions than lodgepole pine, since it can sustain growth in relatively dry and nutrient poor soils across its range (Vidacović, 1991). Currently, studies on the defensive response of lodgepole and jack pine under drought conditions are largely lacking. Particularly, an understanding of how variation in host tree defense chemistry affects tree colonization by MPB could be crucial to predict beetle behavior in its expanding range. Hence the objectives of our study are: (1) to develop chemical profiles of volatile organic compounds released from the bole of mature lodgepole and jack pines in the field, as well as profiles of needle and phloem tissue; (2) to determine changes of volatile chemical profiles when exposed to different water (water-deficit vs. ambient) and biological treatments that stimulate tree stress/defense; (3) to evaluate whether the monoterpene content of phloem and needle tissue is affected by any of our treatments; and (4) to assess whether water and biological treatments applied to trees affect MPB brood success in both host species.
Materials and Methods
A field study was conducted in the summer of 2010 to investigate the role of water limitation on chemically mediated interactions between MPB and its historical and novel host, lodgepole and jack pine, respectively. The study was conducted at two sites in Alberta, Canada: a lodgepole pine site located 80 km NW of Hinton (53°45′55.5″ N, 118°22′17.9″ W), and a jack pine site at the Alberta Tree Improvement and Seed Centre east of Smoky Lake (54°05′18.5″ N, 112°14′48.6″ W). Due to the geographic distribution of pine hosts in Alberta, it is not possible to select field sites where both pine species occur naturally. At the lodgepole pine site, 60 mature healthy pine trees with a diameter at breast height (DBH) of 22.0 cm ± 1.63 SD were randomly selected. None of the selected trees contained any signs or symptoms of infection or insect attack. Twenty of the 60 trees were part of a MPB management plot implemented and managed by Alberta Agriculture and Forestry. Three of the 20 trees were baited with a MPB lure (Contech Enterprises Inc., Delta, B.C., Canada) to attract MPB into the area and achieve natural attack in the management plot. At the jack pine site, 40 pine trees with a DBH of 21.9 cm ± 2.35 SD were randomly chosen.
Water Treatments
Thirty and 20 trees were randomly chosen at the lodgepole and jack pine sites, respectively, for inclusion in one of two water treatments that were initiated in the first week of May, 2010. Ambient trees were left under natural conditions, whereas the soil at the base of water-deficit trees was covered with a tarpaulin [size: 12 × 14′ (3.66 × 4.27 m); G. Hjukstrom Limited, Surrey, B.C., Canada] to hold off rain water. Soil water content around each tree was measured using time domain reflectometry (Hillel, 1998). The apparent dielectric constant of the soil was measured with a Tektronix 1502B (Beaverton, Oregon, USA) connected to stainless steel probes of varying length that were put into the soil and related it to its water content using the empirical equation for mineral soils (Robinson et al., 2003). At the jack pine site, soil water content was measured at depths of 30 and 90 cm 1 day, 3 and 9 weeks after biological treatment applications (described below). Due to a broken cable, we were unable to collect soil water content data for all jack pines at the time of harvest. At the lodgepole pine site, soil water content was measured only at a depth of 30 cm, since the ground remained frozen at the depth of 90 cm throughout the summer. Measurements at the lodgepole pine site took place 1 day, 3 and 9 weeks after biological treatment applications. Soil water content was also measured at 12 and 13 months after treatment application around the 20 trees in the management plot.
Biological Treatments
Five weeks after the water treatments were initiated, 15 lodgepole and 10 jack pine trees in both water treatment groups were additionally exposed to one of two biological treatments: a control (no inoculation) or inoculation with G. clavigera. In inoculated trees, eight wounds were made with a cork borer (1 cm diameter) evenly spaced around the bole at DBH located at the cardinal points: N, NE, E, SE, S, SW, W, and NW. Bark plugs removed by the cork borer were kept on ice for phloem monoterpene analysis (described below). A malt extract agar plug with active fungal mycelium was placed into the wound sites with the mycelium facing the sapwood. The inoculation sites were covered with a layer of Parafilm M® (Bemis Flexible Packaging, Oshkosh, WI, USA) and a 15 cm wide strip of fiberglass insect screening. Control trees were simply left unharmed. No mechanical wounding alone treatment was applied, since our previous work showed that it only caused a minor defense response in mature lodgepole pine × jack pine hybrids in Alberta (Lusebrink et al., 2013).
Volatile Collection
Volatile organic compounds released from the bole of the variously treated trees were collected 1 day before, and 1 day, 3 and 6 weeks post biological treatment application. None of the lodgepole pine trees in the MPB management plot were naturally attacked by beetles during the summer of 2010 and therefore were left on site until the summer of 2011. Volatile organic compounds were also collected from the 20 lodgepole pine trees that were left on site over winter 1 year after fungal inoculation. To enable volatile collection, two strips of foam were attached to each experimental tree: one 15 cm above and one 15 cm below breast height (Figure 1). An oven bag (LOOK®, 45 × 55 cm) was cut open and wrapped around the tree covering both pieces of foam and then secured to the tree. A pump (Grab Air Sample Pump, SKC Inc., Pennsylvania, USA) was attached to each tree with Velcro below the foam, and an absorbent tube [Porapak Q (OD 6 mm, length 110 mm; absorbent: front layer 150 mg, back up layer 75 mg; separated by glass wool) SKC Inc.] was inserted into the space covered by the oven bag. Volatiles were collected on the north aspect of each tree for 1 h at a flow rate of 1 L/min. During volatile collection, we recorded temperature, light intensity (HOBO Pendant® Temperature/Light Data Logger, Onset Computer Corp, 470 MacArthur Blvd, Bourne, MA 02532, USA) and humidity at each tree using dataloggers (Temperature and Humidity Data Logger 16540, Climate Doctors, 8505 K Street, Omaha, NE 68127, USA). Porapak Q tubes were extracted with 1 mL of dichloromethane (Sigma-Aldrich, St. Louis, Missouri, USA) spiked with 0.01% (v/v) tridecane (Sigma-Aldrich) as a surrogate standard and subsequently stored at −40°C before GC/MS analysis (Lusebrink et al., 2011).
Tissue Extracts and Lesion Length
After the volatile collection period in 2010, all jack pine trees and 40 of the 60 lodgepole pine trees were felled in mid-August. The 20 lodgepole pine trees left on site over winter were felled in July 2011. The lesion lengths induced by G. clavigera inoculation on each tree that received the biological treatment in the two water treatment groups at each site were measured to the nearest mm and tissue inside the lesion was sampled. Phloem from between the lesions at DBH and needle samples from the mid-crown were also sampled from all felled trees. Tissue samples were directly frozen on dry ice and stored at −40°C in the lab prior to extraction. Tissue was ground in liquid nitrogen, and 100 mg of tissue was transferred to 1.5 mL microcentrifuge tubes. Samples were extracted twice with 0.5 mL dichloromethane and 0.01% tridecane as a surrogate standard. After adding solvent, samples were vortexed for 30 s, sonicated for 10 min, subsequently centrifuged at 13,200 rpm and 0°C for 15 min, and placed in a freezer for at least 2 h to let the pellet freeze. Extracts were transferred individually into an amber GC vial and stored at −40°C before GC/MS analysis.
In addition to lesion length, phloem thickness, the tissue between the outer bark and sapwood, was measured after trees were felled in the field using digital calipers (d = 0.01 mm). A cross section from the base of each harvested tree was transported to the laboratory to determine tree age (see Table 2). We scanned the stem cross sections and analyzed the tree rings with WinDENDRO™ (Regent Instruments Inc., Quebec, Canada).
GC/MS Analysis
Volatile and tissue sample extracts (3 μL) were injected at a split ratio of 20:1 in an Agilent 7890A/5062C Gas Chromatograph/Mass Spectrometer (Agilent Technologies, Santa Clara, California, USA) with a HP-Chiral-20B column (I.D. 0.25 mm, length 30 m; Agilent Technologies), helium carrier gas flow at 1.1 mL/min, temperature 75°C for 15 min, increased to 230°C by 5°C.
Peaks were identified using the following standards: Borneol, pulegone, α-terpinene, γ-terpinene, α-terpineol (Sigma-Aldrich, St. Louis, Missouri, USA), camphor, 3-carene, α-humulene, terpinolene, α- and β-thujone, (−)-α- and β-pinene, (+)-α- and β-pinene, (S)-(−)- and (R)-(+)-limonene, sabinene hydrate, myrcene, camphene, p-cymene (Fluka, Sigma-Aldrich, Buchs, Switzerland), bornyl acetate, cis-ocimene, α-phellandrene (SAFC Supply Solutions, St. Louis, Missouri, USA), β-phellandrene (Glidco Inc., Jacksonville, Florida, USA). Compounds were identified by comparing retention times and mass spectra to those of the standard chemicals. Calibration with these standards allowed for quantification of chemicals in the volatile and tissue samples, as well as the analysis of differences in stereoisomer composition of compounds derived from the differently treated trees.
Elemental and Nutrient Content Analysis
Phloem samples of all experimental trees and lesion samples from trees that received the biological treatment were ground under liquid nitrogen and oven dried for 24 h at 70°C before total N and C analysis at the University of Alberta Biogeochemical Analytical Service Laboratory (University of Alberta, Edmonton, Canada).
Beetle Condition Experiment
To test the hypothesis that water and biological treatment combinations affected beetle fitness, 50 cm bolts from 1.5 m above the ground from all 80 felled pine trees (40 lodgepole pine, 40 jack pine) were transported to the lab and stored in a walk-in growth chamber (22°C, 50% humidity, 16 h light/8 h dark). Both ends of each bolt were covered in paraffin wax to avoid desiccation. Four pairs of live MPB were artificially introduced into each log. Beetles were collected from infested pine trees (harvested at the Eagle fire lookout tower, east of Fox Creek, Alberta; 54°33′23.7″ N, 116°33′57.7″ W). One female beetle was introduced into each of four 1.5 mL microcentrifuge tubes that were glued to each bolt at evenly spaced intervals around the bolt. Once frass excavated by female beetles was visible in the tubes, a male beetle was added to each tube. Beetles were replaced if needed until the introduction of both sexes was successful. All bolts were kept in a growth chamber (22°C, 50% humidity, 16:8 h L:D) for 4–5 weeks to allow for gallery establishment and larval development. At the end of this period, bolts from all treatment combinations were divided into two groups: the bolts of the first group were transferred into a cold room at 4°C for 3 months to emulate winter conditions and then kept in a growth chamber until all beetles emerged and the second group was transferred directly into rearing bins (114 L hinged top tote, 81 × 51.4 × 44.5 cm, Rubbermaid, Mogadore, OH, USA) and remained in the growth chamber without exposure to a cold period. Fresh weight to the nearest 0.01 mg (Mettler Toledo, XS105, Columbus Ohio), size [mm3; cylindrical body = π(pronotum width/2)2 × total body length] and sex of adult beetles that emerged were measured before beetles were killed and stored at −20°C prior to fat extraction. Dead beetles were oven dried for 24 h at 60°C and their dry weight (mg) was determined. The mass of fat (mg) from each beetle and fat content (% of removed dry weight) was determined by fat extraction with petroleum ether (Fisher Scientific, Ottawa, ON, Canada). Each individual oven-dried beetle was transferred into a perforated 0.2 mL microcentrifuge tube and placed into the extraction unit of a 250 mL soxhlet apparatus. Beetle fat was extracted for 8 h before beetles were dried again for 24 h and then weighed. Mass of fat was calculated as the difference in mass before and after fat extraction. After all beetles had emerged, the outer bark was removed and the number and length of each maternal gallery was measured.
Statistical Analyses
All statistical analyses were conducted using SPSS 20.0 for Windows (IBM Corporation, Armonk, NY, USA), unless otherwise stated. Data were checked for assumptions of homogeneity of variance and normality using Levene's and Kolmogorov-Smirnov tests, respectively. Soil water content data was analyzed with a repeated measures ANOVA to compare the difference across water treatments (ambient and water-deficit trees) separately at each of the study sites. The effect of water and biological treatment on total monoterpene emission at the different time points was analyzed with a repeated measures ANOVA, for which the monoterpene data were log(x+1) transformed to meet the assumptions of the analysis. The impact of water and biological treatments on the emission of individual monoterpenes from both tree species separately at each time point was analyzed by canonical redundancy analysis (RDA) with the rdaTest package (Legendre and Durand, 2010) in R (R Development Core Team, 2015). RDA axes were tested for significance by permutations with the vegan package (Oksanen et al., 2010). Explanatory variables included water and biological treatments, DBH and tree age (see Table 2), and temperature, humidity and light intensity data recorded from dataloggers. The quantities of all individual monoterpenes released at each time point were the response variables in each individual model. Factors that influenced lesion length including water and biological treatment and the inoculation position on the tree bole were analyzed separately for each tree species with an ANOVA followed by the Bonferroni post-hoc procedure. A Mann–Whitney U-test was employed to compare lesion length data between inoculated lodgepole pine trees harvested in 2010 and 2011. The amount of each chemical compound in the different tissues was distributed normally among treatment groups, therefore the treatment effect on individual compounds in phloem and needles was analyzed with ANOVAs for each compound tested. The assumption of homogeneity was not met, however, so tissue extract data was pooled when the alpha level for non-significant differences between treatment groups was P > 0.25. Pooled data was analyzed using independent t-tests. Since variances were not equal, t-statistics not assuming homogeneity of variance were computed. For significant interaction terms of heterogeneous data, bootstrapped 95% confidence intervals were computed and reported. The water treatment effect on lesion chemical composition was tested using independent t-tests for each compound tested. The comparison of total carbon and nitrogen content of the phloem and lesion tissue was analyzed with a paired t-test, and the effect of water and biological treatment on carbon and nitrogen tissue content was analyzed with a two-way ANOVA for each tree species. The effect of cold storage (with or without exposure to winter conditions), biological and water treatment on the dependent variables of beetle fresh weight and fat content was analyzed with ANOVAs.
Direct statistical comparisons between lodgepole and jack pine were not conducted, since our experimental design does not allow us to statistically separate a treatment effect from a location effect (see Heffner et al., 1996), as only one field site per species is used. However, it is still important to be aware of possible differences between the tree species that might influence host attractiveness by MPB as it expands its range eastward into the boreal forest and therefore tree species differences are presented in a descriptive manner.
Results
Soil Water Content
In 2010, water-treatment significantly affected soil water content at the lodgepole pine site [repeated measures ANOVA, F(1, 28) = 8.394, P = 0.007], the soil water content was lower around the water-deficit trees than around the ambient trees. No difference in soil water content was observed between the two water tr eatment groups 1 year after biological treatment application and at the time of harvest of lodgepole pine trees in 2011. Within the jack pine site, soil water content was measured at two depths: 30 and 90 cm. Soil water content was significantly lower for the water-deficit trees at both soil depths compared to the ambient trees [repeated measures ANOVA, 30 cm: F(1, 15) = 14.148, P = 0.002; 90 cm: F(1, 15) = 57.483, P < 0.001, Figure 2].
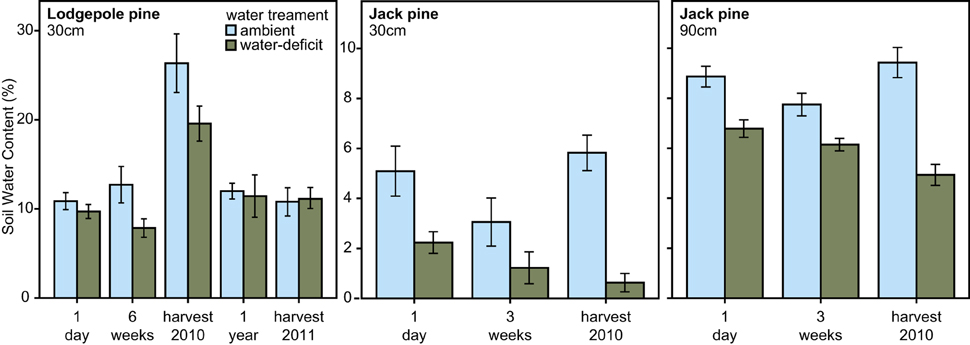
Figure 2. Soil water content (mean ± S.E.) measured at 30 cm soil depth for the lodgepole pine site (P. contorta; repeated measures ANOVA, 2010: P = 0.007; 2011: not significant) and at 30 and 90 cm soil depth for the jack pine site (P. banksiana; repeated measures ANOVA, 30 cm: P = 0.002; 90 cm: P < 0.001) over the time course of the experiment.
Volatile Emission from Stem Section
The following monoterpenes were detected in one or both of the species volatile profiles: (−) and (+)-α-pinene, myrcene, camphene, 3-carene, (−)-β-pinene, cis-ocimene, (S)-(−)- and (R)-(+)-limonene, β-phellandrene, γ-terpinene, terpinolene. The volatile profile of lodgepole pine was dominated by β-phellandrene, followed by 3-carene and (−)-β-pinene (Table 1), whereas, in jack pine the main compound was (+)-α-pinene, followed by 3-carene and (−)-α-pinene. Interestingly, in jack pine one quarter of the trees tested did not emit any 3-carene. Water treatment had no effect on total monoterpene emission in either pine species (Figure 3A). However, fungal inoculation increased volatile emission compared to control trees in both pine species [repeated measures ANOVA, lodgepole pine: F(1, 36) = 147.047, P < 0.001; jack pine: F(1, 36) = 32.563, P < 0.001; Figure 3A]. The RDA triplots illustrate the correlation between explanatory variables and the emission of individual monoterpenes in lodgepole and jack pines (Figure 3B). In both species, the first axis of the RDA was significant (P = 0.001 for both species) and explained 13.86 and 16.07% of the variation in lodgepole and jack pines, respectively. The second axis was not significant (lodgepole pine: P = 0.450, jack pine: P = 0.623) and explained <1% of the variation in the RDA. In lodgepole pine, the emission of (−)-β-pinene, 3-carene, and β-phellandrene was correlated mainly with fungal inoculation and light intensity. In jack pine the emission of (−) and (+)-α-pinene, myrcene, and 3-carene was correlated with fungal inoculation, as well as with temperature and light intensity.
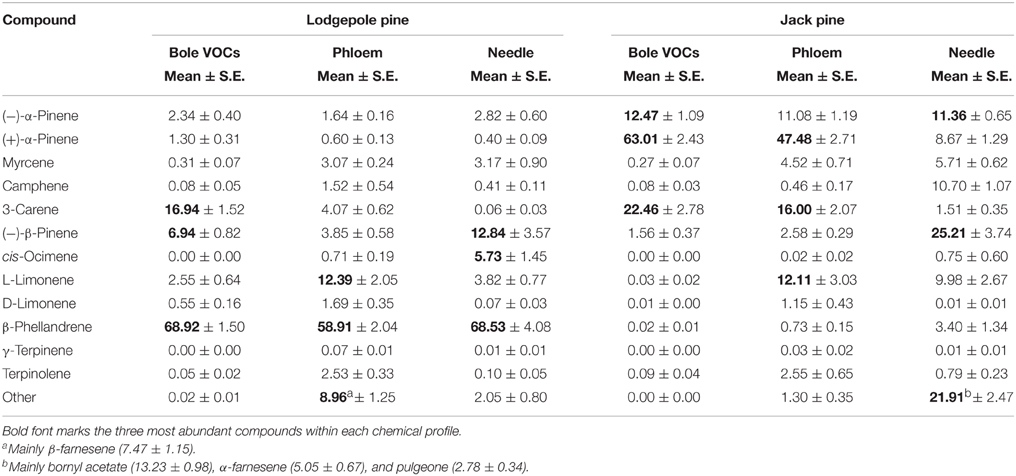
Table 1. Chemical profiles (ratios %) of bole volatiles (VOCs) of all untreated control trees throughout the experiment, phloem from the start and end of the experiment without biological treatment, and needle extracts of control trees from the day of harvest 2010.
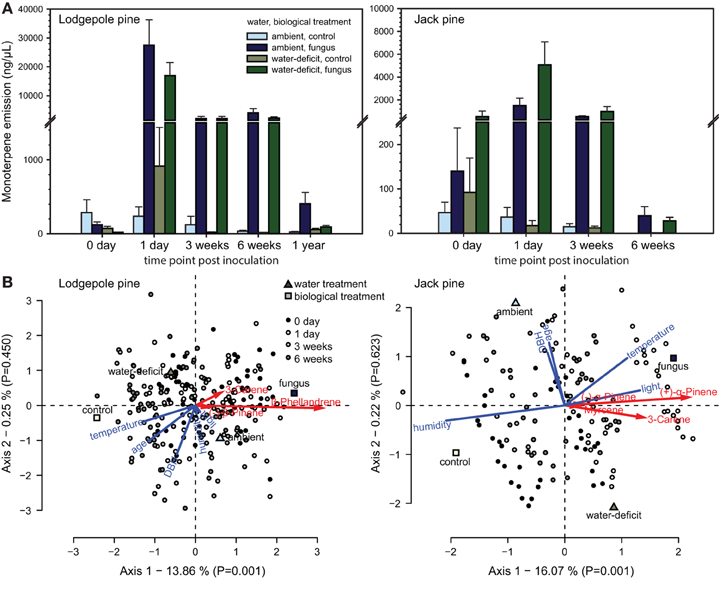
Figure 3. (A) Total monoterpene emission (mean + S.E.) of ambient and water-deficit lodgepole (P. contorta) and jack (P. banksiana) pines before (0 days) and after biological treatment (1 day, 3 and 6 weeks; and 1 year in lodgepole pine) application. Water treatment had no effect, but fungal inoculation increased volatile emission in both pine species (repeated measures ANOVA, lodgepole pine: P < 0.001; jack pine: P < 0.001; (B) Canonical redundancy analysis (RDA) triplots (scaling 2) illustrating the influence of water and biological treatments as well as tree characteristics [age, and diameter at breast height (DBH)] and environmental variables [temperature, humidity, and light intensity (light)] on volatile emission of individual monoterpenes in lodgepole pine (P. contorta) and jack pine (P. banksiana).
Independent of the water treatment, both species' volatile emission increased upon fungal inoculation, which was more pronounced in lodgepole than jack pine. Volatile emission was 6.8-fold higher in lodgepole pine compared to jack pine on day 1, only 2.8-fold higher in week 3, and remained at high levels in week 6 in lodgepole pine, whereas the emission in jack pine was reduced to the amount before treatment application (see Figure 3A).
Phloem, Needle, and Lesion Extracts
Chemical profiles of phloem and foliage are listed in Table 1. Briefly, the major compound emitted from the stem of lodgepole pine trees, β-phellandrene, also dominated the chemical profile of the phloem and needle extracts in that species (Table 1). In jack pine, the main bole volatile (+)-α-pinene is also the key compound in the phloem extract, but it is not as abundant in the needle tissue, which is dominated by (−)-β-pinene and bornyl actetate.
As with volatile emission, a quarter of the jack pine trees sampled did not contain 3-carene in their phloem. The stereoisomer composition of α-pinene, of which (−)-α-pinene is the main MPB aggregation pheromone precursor, differed between control trees of lodgepole and jack pine: lodgepole pines contained more (−)-α-pinene (111.33 ng/mg (±11.31 S.E.) and less (+)-α-pinene (41.57 ng/mg (±3.81 S.E.), than jack pines [(−)-α-pinene: 54.22 ng/mg (±9.30 S.E.); (+)-α-pinene: 195.95 ng/mg (±27.32 S.E.)].
In lodgepole pine phloem sampled in 2010, a significant interaction between the water and biological treatments affected the amount of L-limonene [ANOVA, F(1, 34), P = 0.019]. However, the data did not fulfill the assumption of homogeneity, therefore bootstrapped confidence intervals were computed, which confirmed that within the group of trees under ambient conditions, the phloem of fungal inoculated trees had a higher L-limonene concentration than control trees (ambient, control: mean = 1059, 95% CI [472, 1684], fungus: mean = 6270, 95% CI [2214, 10320]; water-deficit, control: mean = 1334, 95% CI [600, 2145], fungus: mean = 774, 95% CI [360, 1174]; Figure 4). Within the water-deficit trees the biological treatment had no effect on L-limonene concentration.
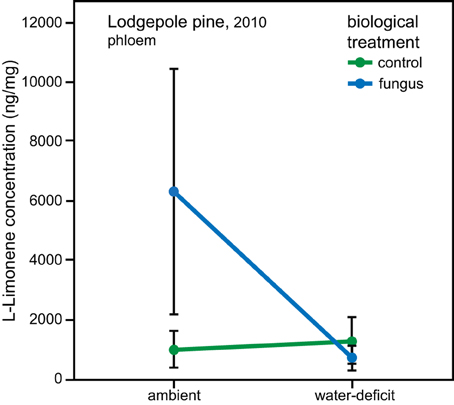
Figure 4. Interaction effect of water and biological treatment on L-limonene concentration in phloem of lodgepole pine (P. contorta). Error bars represent bootstrapped 95% CI, non-overlapping error bars indicate a significant difference.
In jack pine phloem, myrcene was the only compound that showed a response to the water treatment with a higher concentration in water-deficit trees than in trees exposed to ambient weather conditions [t-test, t(21.550) = 2.236, P = 0.036; Figure 5]. In 2011, there was a higher 3-carene concentration in the phloem of water-deficit lodgepole pine trees [t-test, t(12.448) = 2.236, P = 0.049; Figure 5] and a lower β-phellandrene concentration [t-test, t(8) = −2.846, P = 0.022; Figure 5] as compared to ambient control trees.
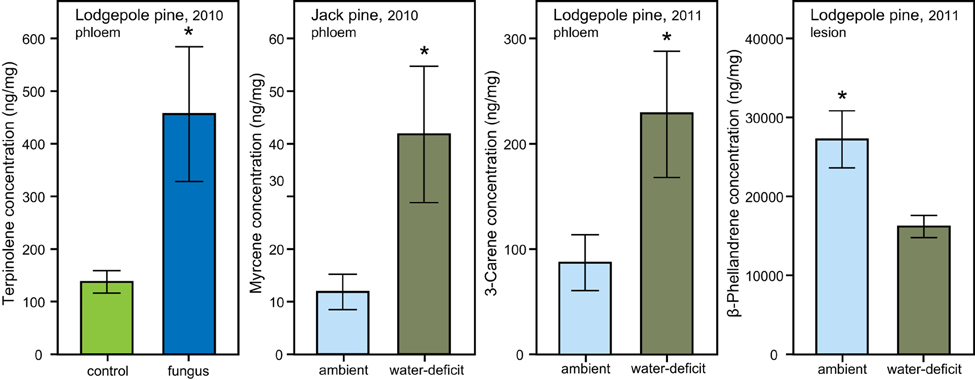
Figure 5. Individual monoterpene concentrations (mean ± S.E.) in phloem and lesion tissue of lodgepole (P. contorta) and jack pine (P. banksiana) influenced by water- and biological treatment. Significant differences are marked with an asterisk (independent t-test, P < 0.05).
Fungal inoculation significantly increased terpinolene in lodgepole pine phloem in 2010 [t-test, t(24.057) = −2.240, P = 0.035; Figure 5]. The biological treatment did not influence the concentration of any other individual monoterpene in lodgepole pine or have any impact on the phloem chemistry of jack pine. However, fungal inoculation increased the monoterpene concentration of the phloem in the lesion area from day 0 to harvest by 9.83-fold in lodgepole pine and by 71.85-fold in jack pine (Table 2).
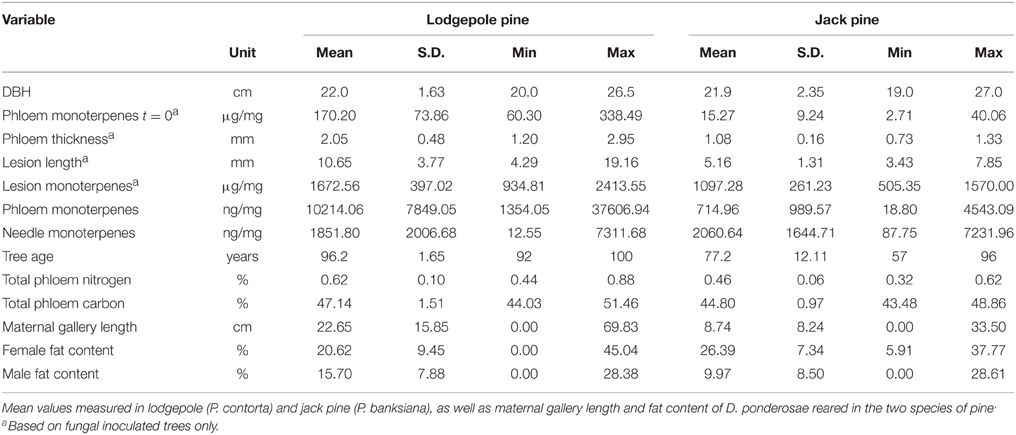
Table 2. Mean measurements of variables measured in experimental trees and for beetles reared in experimental trees.
Total monoterpene concentrations were higher in phloem and lesion tissue of lodgepole pine compared to jack pine (Table 2). The needles of both species contained similar amounts of monoterpenes (Table 2).
Lesion Length upon Fungal Inoculation
Water treatment did not influence lesion length in fungal-inoculated trees in either species in 2010. It did, however, influence lesion length in trees that were harvested in 2011 at the lodgepole pine site, with water-deficit trees having significantly smaller lesions than ambient ones [ANOVA, F(1, 64) = 12.323, P = 0.001; Figure 6]. In lodgepole pine, the location where the inoculum was placed on the stem significantly affected lesion formation. Lesions from south-facing inoculation points were smaller than those placed at the other cardinal directions [ANOVA, ambient: F(7, 72) = 3.652, P = 0.002; water-deficit: F(7, 72) = 3.316, P = 0.004; Figure 6]. This pattern was preserved in the water-deficit trees harvested in 2011. The lesions of inoculated and harvested lodgepole pine trees in 2011 were significantly shorter compared to lesions from the lodgepole pine trees harvested in 2010 (Mann–Whitney U: U = 2678.500, z = −7.340, P < 0.001; Figure 7). Lesions were twice as long in lodgepole pine compared to jack pine (Table 2).
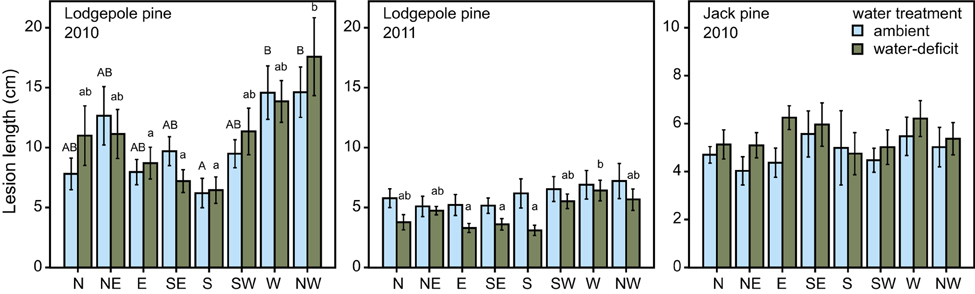
Figure 6. Lesion length (mean ± S.E.) in lodgepole pine (P. contorta) after tree harvest in 2010 and 2011 and in jack pine (P. banksiana) in 2010. Cardinal point of the fungal inoculum significantly influenced lesion length in lodgepole pine. Bars marked with different uppercase letters indicate a statistically significant difference in ambient trees, whereas bars marked with different lowercase letters indicate a statistically significant difference in water-deficit trees (ANOVA, P < 0.05).

Figure 7. Lesions in lodgepole pine: (A) lesion in 2010, (B) lesion in 2011, and (C) close up on lesion in 2011 showing new tissue growth ( ) into the lesion caused by fungal inoculation in 2010.
) into the lesion caused by fungal inoculation in 2010.
Nutrient Content of Phloem
In both pine species, the carbon content of lesion tissue was significantly higher than the carbon in the surrounding phloem [paired t-test: lodgepole pine: t(18) = 27.008, P < 0.001; jack pine: t(19) = 24.124, P < 0.001; Figure 8]. Fungal-inoculated lodgepole pine phloem showed a higher carbon content compared to control trees [ANOVA, F(1, 35) = 5.500, P = 0.025; Figure 8]. The lesions of jack pine exposed to ambient water condition had a higher nitrogen content than water-deficit trees [ANOVA, F(1, 16) = 6.789, P = 0.018]. Phloem total nitrogen concentrations were higher in lodgepole pine than in jack pine (Table 2).
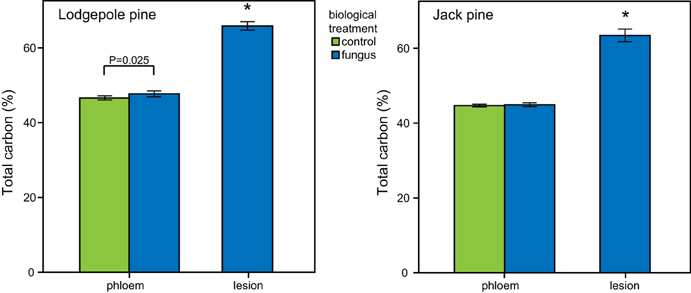
Figure 8. Total carbon content (mean ± CI) in lodgepole (P. contorta) and jack pine (P. banksiana). Lesion tissue contains significantly more total carbon (marked by asterisk, independent t-test, p < 0.05) than phloem in both species. Additionally there is a significant difference in total carbon in lodgepole pine phloem caused by biological treatment (ANOVA, P < 0.05).
Beetle Condition
In lodgepole pine, 146 of 160 beetle-pair introductions were successful, whereas in jack pine only 112 of 160 introductions were successful. Altogether 144 mature adult beetles emerged from lodgepole pine bolts (75 female, 69 males), but only 41 beetles emerged from jack pine bolts (24 female, 17 male). The low number of brood beetles from the jack pine bolts was most likely caused by the presence of saprophytic fungi (e.g., like Phlebiopsis gigantea) which hindered successful colonization in some bolts.
Fresh weight and beetle size are correlated (Pearson correlation; lodgepole pine, female: r = 0.866, P < 0.001, male: r = 0.883, P < 0.001; jack pine, female: r = 0.698, P < 0.001, male: r = 0.828, P < 0.001), therefore the analysis was conducted on just fresh weight. In lodgepole pine, the biological treatment significantly affected female beetle fresh weight [F(1, 60) = 10.151, P = 0.002]. Female beetles had a higher fresh weight when reared in fungal-inoculated trees. The biological treatment influenced male beetles that emerged from lodgepole pine differently depending on water treatment. This interaction was significant for fresh weight [F(1, 59) = 6.730, P = 0.012], as male beetles that emerged from ambient fungal inoculated bolts weighed more than those that emerged from any other treatment combination. In jack pine, neither the water nor the biological treatment or the way bolts were stored had an effect on male beetles which emerged from jack pine bolts. Cold storage increased the fresh weight [F(1, 17) = 14.576, P = 0.001] of female beetles that emerged from jack pine. No treatment affected the fat content of emerging beetles.
Female beetles that were reared in lodgepole pine excavated maternal galleries that were more than double the length of those made by female beetles in jack pine (Table 2). Female beetles which emerged from jack pine bolts contained more fat than female beetles which emerged from lodgepole pine bolts, the opposite relationship occurred in male beetles (Table 2).
Discussion
Chemical Profiles of Stem Volatiles, Needle, and Phloem Tissue and their Importance in MPB Biology
This is the first study to collect bole volatiles from live, mature lodgepole, and jack pine in the field. Earlier studies report bole volatile profiles from cut bolts or tree disks (Pureswaran et al., 2004a,b; Jost et al., 2008; Erbilgin et al., 2014), which might not represent stem volatile emission under natural conditions. In general, lodgepole pine emits higher amounts of volatile monoterpenes than jack pine. Lodgepole pine trees mainly emit β-phellandrene, which is the only known monoterpene to elicit attraction from MPB in the absence of aggregation pheromones (Miller and Borden, 1990). The volatile bole profile of jack pine is dominated by an unequal mixture of α-pinene stereoisomers. Volatiles from the stem of host trees may be important in host-finding and colonization behavior of MPB as conifer-infesting bark beetles disperse at lower stem height (Byers, 2000; Safranyik and Carroll, 2006; Seybold et al., 2006). Our results suggest that lodgepole pine might be more apparent to MPB than jack pine during beetle dispersal. Although debate exists over the role of tree volatiles in primary attraction of MPB (Pureswaran and Borden, 2005), MPB discriminates between hosts and prefers lodgepole pine within mixed pine stands (Raffa et al., 2013), suggesting the role of species-specific chemical profiles in host location by beetles.
In both pine species, the main volatile compound also dominated the respective phloem chemistry, as β-phellandrene and α-pinene were the major constituents of the phloem chemistry of lodgepole pine and jack pine, respectively. β-Phellandrene was also the main constituent of lodgepole pine needle extracts, whereas in jack pine (−)-β-pinene and bornyl acetate were the major compounds of needle tissue. High amounts of bornyl acetate in jack pine needles has been previously reported (Pauly and von Rudloff, 1971; Lapp and von Rudloff, 1982). Bornyl acetate might not be biologically important for MPB, since its antennae do not respond to it (Pureswaran et al., 2004b). The low amount of α-pinene in jack pine needles compared to phloem suggests that foliar chemistry alone is likely not a good predictor of chemically mediated interactions between bark beetles and their host trees.
Phloem chemistry is relevant to chemically mediated bark beetle—host interactions, as some beetles require chemical precursors from the phloem to synthesize aggregation pheromones (Wood, 1982). In particular, female MPB require (−)-α-pinene to produce their aggregation pheromone: (−)-trans-verbenol (Whitehead et al., 1989). In this, and many other studies, α-pinene is the most abundant monoterpene in jack pine phloem, but its enantiomeric composition has rarely been measured. Our results show that jack pine phloem contains only half the amount of (−)-α-pinene per unit of phloem tissue compared to lodgepole pine, which is in accordance with the findings of Clark et al. (2014). Nonetheless, in laboratory experiments, MPB females emit twice the amount of (−)-trans-verbenol when tunneling in jack pine bolts compared to lodgepole pine (Erbilgin et al., 2014).
Effect of Water and Biological Treatment on Volatile Emission
The canonical redundancy analysis reveals that environmental variables (temperature, light intensity, and humidity) influence volatile emission in the two pine species differently. Light intensity at the volatile collection site increases stem volatile emission in both species, but temperature was only positively correlated with volatile emission in jack pine. Humidity was negatively correlated with monoterpene emission in jack pine and played no apparent role in lodgepole pine. Generally, extrinsic factors like mechanical and biotic wounding, light, temperature, and relative humidity can influence volatile emissions from trees (Tingey et al., 1991; Zhang and Schlyter, 2004). Damaging the bark of a pine tree will enhance monoterpene release by liberating resin at the plant surface (Gershenzon and Croteau, 1991). Therefore, it is not surprising that fungal inoculation with G. clavigera increased stem volatile emission in both species. Water treatment had no effect on total monoterpene emission in either pine species. The fact that volatile emission from both pine species relates differently to the measured environmental factors might be due to the thicker outer bark in jack pine, which might add more diffusion resistance.
Effect of Water and Biological Treatment on Tissue Defense Response
Lesions caused by inoculation with G. clavigera in this study are twice as long in lodgepole pine compared to those in jack pine. These findings are in contrast to those of Rice et al. (2007) who show that G. clavigera induce shorter lesions in lodgepole pine (8.2 cm) relative to jack pine (11.5 cm). In the current study, lesion size might be affected by differences in soil water content at the two sites; the lodgepole pine site had higher soil water content than the jack pine site at the time of tree harvest likely due to water release through seasonal thawing. Arango-Velez et al. (2015), however, also found longer lesions in lodgepole pine than jack pine in an arboretum-based study in Alberta, where both pine species occur at the same field site. Although it is commonly assumed short lesions indicate host plant resistance (Raffa and Berryman, 1983; Krokene and Solheim, 1998), assessment of qualitative and quantitative changes in phloem chemistry following fungal inoculation might be more relevant to explain tree resistance against bark beetles (Peterman, 1977; Raffa and Berryman, 1982, 1983; Paine et al., 1997). We found that the concentration of phloem monoterpenes increases 10-fold upon inoculation with G. clavigera, in lodgepole pine and 70-fold within the area of the lesion in the jack pine, suggesting that the localized defense response in jack pine is more efficient and precise compared to lodgepole pine.
Water treatment variably impacts the concentration of some monoterpenes in the phloem of both tree species. In lodgepole pine the water treatment influences the L-limonene response to the biological treatment. L-Limonene concentration remains at a similar level within water-deficit trees when stimulated with G. clavigera, but increases highly after inoculation under ambient conditions. Low limonene concentration is associated with low soil moisture levels in loblolly pine (Pinus taeda) (Gilmore, 1977). Limonene is toxic to Dendroctonus species (Smith, 1965; Coyne and Lott, 1976; Raffa and Berryman, 1983), and increases survivorship of ponderosa pine (Pinus ponderosae) under beetle attack (Smith, 1966, 1975; Sturgeon, 1979). Hence, the water-deficit condition seems to reduce the defense response against bark beetles in lodgepole pine. In this study, fungal inoculation causes an increase of terpinolene concentrations in the phloem of lodgepole pine. Myrcene is the only monoterpene that varies with water treatment in jack pine. Mycerne levels are higher in the phloem of trees from the water-deficit treatment. The MPB has evolved to use the compounds that increase either upon fungal inoculation (terpinolene) or due to water stress (myrcene) as kairomones for host location, since they synergize beetle response to the aggregation pheromone trans-verbenol (Borden et al., 2008).
Mountain Pine Beetle Brood Success
Fungal inoculation of trees influences the fresh weight of female beetles that emerge from lodgepole pine bolts. Females weigh more when reared in fungal-inoculated trees. In a previous study in which beetles were reared in lodgepole × jack hybrid bolts, female beetles benefited from the water-deficit treatment by exhibiting a higher fat content (Lusebrink et al., 2013). The condition of MPB that emerge from jack pine was not influenced by either the water- or biological treatment.
In the current study, jack pine phloem was much thinner than that of lodgepole pine, however the fat content of females was higher in beetles reared in jack pine bolts. Fat content and beetle size are important measures for beetle dispersal capacity, since heavier beetles with higher fat content can disperse further than small beetles (Evenden et al., 2014). Besides dispersal, larger body size and high fat content might positively influence host colonization and reproductive success of MPB. Trees with thick phloem produce larger broods of beetles (Amman and Pace, 1976), with higher absolute fat content (Graf et al., 2012). However, Erbilgin et al. (2014) found that female MPBs that emerge from jack pine weigh more than brood beetles from lodgepole pine, despite shorter maternal galleries, and equal phloem thickness. In this study, maternal galleries in jack pine are also shorter than in lodgepole pine, females reared in jack pine must consume less phloem (thinner phloem and shorter galleries) than those reared in lodgepole pine. Less consumption of nutritious phloem might lead to smaller eggs (Elkin and Reid, 2005), which might result in smaller offspring beetles (McGhehey, 1971), unless jack pine phloem is higher in nutrients.
The performance of wood-feeding insects has been mainly studied in relation to nitrogen, starch and water content (Haack and Slansky, 1987). In the ambrosia beetle (Pityophtorus lautus) adult, reproductive success is positively correlated with phloem nitrogen and total available carbohydrate levels (Kirkendall, 1983). MPBs respond to dietary nitrogen as a result of G. clavigera inoculation through increased body size (Bleiker and Six, 2007). In the current study, total carbon content was the same in both pine species, but lodgepole pine phloem contained more nitrogen than jack pine. Despite the lower nitrogen content in the phloem of jack pine, female brood beetles from jack pine still contain more fat than beetles from lodgepole pine, suggesting that other nutrients might be important for beetle performance, or that the beetles need to spend less energy on detoxifying tree defensive compounds in jack pine.
Conclusion
The phloem of both pine species contains the necessary precursor for MPB's main aggregation pheromone. Water-deficit inhibits an increase in the defensive compound limonene in response to inoculation with G. clavigera in lodgepole pine phloem. Jack pine could be more attractive than lodgepole pine during periods of drought, since the kairomone myrcene, increases due to water-deficit in jack pine. The localized defense response of jack pine is more efficient than that of lodgepole pine. However, the total monoterpene concentrations of lesion and phloem tissue in jack pine remain below those of lodgepole pine. Female brood beetles emerge with high fat content from jack pine bolts despite short maternal galleries. Jack pine might support a higher attack density and its thicker bark could protect the brood from harsh winter temperatures and therefore facilitate further range expansion into the boreal forest of eastern Canada.
Author Contributions
IL, NE, and ME designed the work. IL did the field and lab work, analyzed the data, and wrote the first versions of the manuscript. NE and ME contributed to the interpretation of the work and revised the manuscript critically. IL, NE, and ME have approved the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was funded through grants from the Government of Alberta through Genome Alberta, the Government of British Columbia through Genome BC and Genome Canada in support of the Tria 1 and Tria 2 projects (http://www.thetriaproject.ca) of which ME and NE are co-investigators, and the NSERC Discovery Grant for which NE is a principal investigator.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge Adriana Arango, Kyle Artym, Heather Bird, Janice Cooke, Christina Elliott, Matt Ferguson, Ed Hunt, Mehvash Malik, and Patrick Welch for their valuable help before and during the field season; Miles Dyck for providing us with the TDR equipment; Celia Boone for sharing β-phellandrene. We particularly acknowledge Rick Bonar (West Fraser Mills Ltd.) and Leonard Barnhardt (Alberta Tree Improvement and Seed Centre) for providing us with suitable field sites.
References
Alfaro, R. I., Campbell, E., and Hawkes, B. C. (2010). Historical Frequency, Intensity and Extent of Mountain Pine Beetle Disturbance in British Columbia. Victoria, BC: Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre.
Amman, G. D., and Pace, V. E. (1976). Optimum Egg Gallery Densities for the Mountain Pine Beetle in Relation to Lodgepole Pine Phloem Thickness. Ogden, UT: USDA Forest Service Research Note INT-209.
Arango-Velez, A., El Kayal, W., Copeland, C. C. J., Zaharia, L. I., Lusebrink, I., and Cooke, J. E. K. (2015). Differences in defense responses of Pinus contorta and Pinus banksiana to the mountain pine beetle fungal associate Grosmannia clavigera are affected by water deficit. Plant Cell Environ. doi: 10.1111/pce.12615. [Epub ahead of print].
Bentz, B. J., Régnière, J., Fettig, C. J., Hansen, E. M., Hayes, J. L., Hicke, J. A., et al. (2010). Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience 60, 602–613. doi: 10.1525/bio.2010.60.8.6
Bleiker, K. P., and Six, D. L. (2007). Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics. Environ. Entomol. 36, 1384–1396. doi: 10.1093/ee/36.6.1384
Blomquist, G. J., Figueroa-Teran, R., Aw, M., Song, M., Gorzalski, A., Abbott, N. L., et al. (2010). Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 40, 699–712. doi: 10.1016/j.ibmb.2010.07.013
Boone, C. K., Aukema, B. H., Bohlmann, J., Carroll, A. L., and Raffa, K. F. (2011). Efficacy of tree defense physiology varies with bark beetle population density: a basis for positive feedback in eruptive species. Can. J. For. Res. 41, 1174–1188. doi: 10.1139/x11-041
Borden, J. H., Pureswaran, D. S., and Lafontaine, J. P. (2008). Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). J. Econ. Entomol. 101, 1266–1275. doi: 10.1093/jee/101.4.1266
Byers, J. A. (1983). Bark beetle conversion of a plant compound to a sex-specific inhibitor of pheromone attraction. Science 220, 624–626. doi: 10.1126/science.220.4597.624
Byers, J. A. (1989). Chemical ecology of bark beetles. Experientia 45, 271–283. doi: 10.1007/BF01951813
Byers, J. A. (2000). Wind-aided dispersal of simulated bark beetles flying through forests. Ecol. Modell. 125, 231–243. doi: 10.1016/S0304-3800(99)00187-8
Clark, E. L., Pitt, C., Carroll, A. L., Lindgren, B. S., and Huber, D. P. W. (2014). Comparison of lodgepole and jack pine resin chemistry: implications for range expansion by the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae). PeerJ 2, e240. doi: 10.7717/peerj.240
Conn, J. E., Borden, J. H., Hunt, D. W. A., Holman, J., Whitney, H. S., Spanier, O. J., et al. (1984). Pheromone production by axenically reared Dendroctonus ponderosae and Ips paraconfusus (Coleoptera: Scolytidae). J. Chem. Ecol. 10, 281–290. doi: 10.1007/BF00987856
Coyne, J., and Lott, L. (1976). Toxicity of substances in pine oleoresin to southern pine beetles. J. Ga Entomol. Soc. 11, 301–305.
Cullingham, C. I., Cooke, J. E. K., Dang, S., Davis, C. S., Cooke, B. J., and Coltman, D. W. (2011). Mountain pine beetle host-range expansion threatens the boreal forest. Mol. Ecol. 20, 2157–2171. doi: 10.1111/j.1365-294X.2011.05086.x
Dobbertin, M., Wermelinger, B., Bigler, C., Bürgi, M., Carron, M., Forster, B., et al. (2007). Linking increasing drought stress to scots pine mortality and bark beetle infestations. ScientificWorldJournal 7, 231–239. doi: 10.1100/tsw.2007.58
Elkin, C. M., and Reid, M. L. (2005). Low energy reserves and energy allocation decisions affect reproduction by mountain pine beetles, Dendroctonus ponderosae. Funct. Ecol. 19, 102–109. doi: 10.1111/j.0269-8463.2005.00935.x
Erbilgin, N., Ma, C., Whitehouse, C., Shan, B., Najar, A., and Evenden, M. (2014). Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. New Phytol. 201, 940–950. doi: 10.1111/nph.12573
Evenden, M. L., Whitehouse, C. M., and Sykes, J. (2014). Factors influencing flight capacity of the mountain pine beetle (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 43, 187–196. doi: 10.1603/EN13244
Franceschi, V. R., Krokene, P., Christiansen, E., and Krekling, T. (2005). Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 167, 353–376. doi: 10.1111/j.1469-8137.2005.01436.x
Gershenzon, J., and Croteau, R. (1991). “Terpenoids,” in Herbivores: Their Interactions with Secondary Plant Metabolites, Vol. I, The Chemical Participants, eds G. Rosenthal and M. Berenbaum (San Diego, CA: Academic Press), 165–219.
Gilmore, A. R. (1977). Effects of soil moisture stress on monoterpenes in loblolly pine. J. Chem. Ecol. 3, 667–676. doi: 10.1007/BF00988066
Graf, M., Reid, M. L., Aukema, B. H., and Lindgren, B. S. (2012). Association of tree diameter with body size and lipid content of mountain pine beetles. Can. Entomol. 144, 467–477. doi: 10.4039/tce.2012.38
Haack, R., and Slansky, F. (1987). “Nutritional ecology of wood-feeding Coleoptera, Lepidoptera, and Hymenoptera,” in Nutritional Ecology of Insects, Mites, Spiders, and Related Invertebrates, eds F. Slansky and J. Rodriguez (New York, NY: John Wiley & Sons), 449–486.
Heffner, R. A., Butler, M. J., and Reilly, C. K. (1996). Pseudoreplication Revisited. Ecology 77, 2558–2562. doi: 10.2307/2265754
Herms, D. A., and Mattson, W. J. (1992). The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335. doi: 10.1086/417659
Jost, R. W., Rice, A. V., Langor, D. W., and Boluk, Y. (2008). Monoterpene emissions from lodgepole and jack pine bark inoculated with mountain pine beetle−associated fungi. J. Wood Chem. Technol. 28, 37–46. doi: 10.1080/02773810801916407
Keeling, C. I., and Bohlmann, J. (2006). Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 170, 657–675. doi: 10.1111/j.1469-8137.2006.01716.x
Kirkendall, L. R. (1983). The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool. J. Linn. Soc. 77, 293–352. doi: 10.1111/j.1096-3642.1983.tb00858.x
Krokene, P., and Solheim, H. (1998). Pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 88, 39–44. doi: 10.1094/PHYTO.1998.88.1.39
Lapp, M. S., and von Rudloff, E. (1982). Chemosystematic studies in the genus Pinus. IV. Leaf oil composition and geographic variation in jack pine of eastern North America. Can. J. Bot. 60, 2762–2769. doi: 10.1139/b82-338
Legendre, P., and Durand, S. (2010). Rdatest: Canonical Redundancy Analysis (R Package Version 1.7). Available online at: http://www.bio.umontreal.ca/legendre/
Lusebrink, I., Erbilgin, N., and Evenden, M. L. (2013). The lodgepole x jack pine hybrid zone in Alberta, Canada: a stepping stone for the mountain pine beetle on its journey east across the boreal forest? J. Chem. Ecol. 39, 1209–1220. doi: 10.1007/s10886-013-0334-8
Lusebrink, I., Evenden, M. L., Blanchet, F. G., Cooke, J. E., and Erbilgin, N. (2011). Effect of water stress and fungal inoculation on monoterpene emission from an historical and a new pine host of the mountain pine beetle. J. Chem. Ecol. 37, 1013–1026. doi: 10.1007/s10886-011-0008-3
Mattson, W. J., and Haack, R. A. (1987). The role of drought in outbreaks of plant-eating insects. Bioscience 37, 110–118. doi: 10.2307/1310365
McGhehey, J. H. (1971). Female Size and Egg Production of the Mountain Pine Beetle, Dendroctonus Ponderosae Hopkins. Canadian Forest Service, Northern Forest Research Centre, Information Report NOR-X-9, 18.
Miller, D. R., and Borden, J. H. (1990). β-Phellandrene: kairomone for pine engraver, Ips pini (Say) (Coleoptera: Scolytidae). J. Chem. Ecol. 16, 2519–2531. doi: 10.1007/BF01017475
Mirov, N. T. (1956). Composition of turpentine of lodgepole × jack pine hybrids. Can. J. Bot. 34, 443–457. doi: 10.1139/b56-036
Moss, E. H. (1949). Natural pine hybrids in Alberta. Can. J. Res. 27c, 218–229. doi: 10.1139/cjr49c-018
Netherer, S., Matthews, B., Katzensteiner, K., Blackwell, E., Henschke, P., Hietz, P., et al. (2015). Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 205, 1128–1141. doi: 10.1111/nph.13166
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., O'Hara, R. B., Simpson, G. L., et al. (2010). Vegan: Community Ecology Package. R Package Version 1.17-4. Available online at: http://CRAN.R-project.org/package=vegan
Paine, T. D., Raffa, K. F., and Harrington, T. C. (1997). Interaction among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 42, 179–206. doi: 10.1146/annurev.ento.42.1.179
Pauly, G., and von Rudloff, E. (1971). Chemosystematic studies in the genus Pinus: the leaf oil of Pinus contorta var. latifolia. Can. J. Bot. 49, 1201–1210. doi: 10.1139/b71-168
Peterman, R. M. (1977). Evaluation of fungal inoculation method of determining resistance of lodgepole pine to mountain pine beetle (Coleoptera-Scolytidae) attacks. Can. Entomol. 109, 443–448. doi: 10.4039/Ent109443-3
Phillips, M. A., and Croteau, R. B. (1999). Resin-based defenses in conifers. Trends Plant Sci. 4, 184–190.
Pureswaran, D. S., and Borden, J. H. (2005). Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric. For. Entomol. 7, 219–230. doi: 10.1111/j.1461-9555.2005.00264.x
Pureswaran, D. S., Gries, R., and Borden, J. H. (2004a). Quantitative variation in monoterpenes in four species of conifers. Biochem. Syst. Ecol. 32, 1109–1136. doi: 10.1016/j.bse.2004.04.006
Pureswaran, D. S., Gries, R., and Borden, J. H. (2004b). Antennal responses of four species of tree-killing bark beetles (Coleoptera: Scolytidae) to volatiles collected from beetles, and their host and nonhost conifers. Chemoecology 14, 59–66. doi: 10.1007/s00049-003-0261-1
Pureswaran, D. S., Gries, R., Borden, J. H., and Pierce, J. H. D. (2000). Dynamics of pheromone production and communication in the mountain pine beetle, Dendroctonus ponderosae Hopkins, and the pine engraver, Ips pini (Say) (Coleoptera: Scolytidae). Chemoecology 10, 153–168. doi: 10.1007/P.L.00001818
Raffa, K. F., Aukema, B. H., Bentz, B. J., Carroll, A. L., Hicke, J. A., Turner, M. G., et al. (2008). Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 58, 501–517. doi: 10.1641/B580607
Raffa, K. F., Aukema, B. H., Erbilgin, N., Klepzig, K. D., and Wallin, K. F. (2005). “Chapter IV Interactions among conifer terpenoids and bark beetles across multiple levels of scale: An attempt to understand links between population patterns and physiological processes,” in Recent Advances in Phytochemistry, ed T. R. John (Toronto, ON: Elsevier), 79–118.
Raffa, K. F., and Berryman, A. A. (1982). Physiological differences between lodgepole pines resistant and susceptible to the mountain pine beetle and associated microorganisms. Environ. Entomol. 11, 486–492. doi: 10.1093/ee/11.2.486
Raffa, K. F., and Berryman, A. A. (1983). Physiological aspects of lodgepole pine wound responses to a fungal symbiont of the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. Entomol. 115, 723–734. doi: 10.4039/Ent115723-7
Raffa, K. F., Powell, E. N., and Townsend, P. A. (2013). Temperature-driven range expansion of an irruptive insect heightened by weakly coevolved plant defenses. Proc. Natl. Acad. Sci. U. S. A. 110, 2193–2198. doi: 10.1073/pnas.1216666110
R Development Core Team (2015). R: A language and Environment for Statistical Computing (Version 2.12.0). Vienna: R Foundation for Statistical Computing. Available online at: http://www.Rproject.org
Reid, R. W., Whitney, H. S., and Watson, J. A. (1967). Reactions of lodgepole pine to attack by Dendroctonus ponderosae Hopkins and blue stain fungi. Can. J. Bot. 45, 1115–1126. doi: 10.1139/b67-116
Rice, A. V., Thormann, M. N., and Langor, D. W. (2007). Virulence of, and interactions among, mountain pine beetle associated blue-stain fungi on two pine species and their hybrids in Alberta. Can. J. Bot. 85, 316–323. doi: 10.1139/B07-016
Robinson, D. A., Jones, S. B., Wraith, J. M., Or, D., and Friedman, S. P. (2003). A review of advances in dielectric and electrical conductivity measurement in soils using time domain reflectometry. Vadose Zone J. 2, 444–475. doi: 10.2136/vzj2003.4440
Rudinsky, J. A., Morgan, M. E., Libbey, L. M., and Putnam, T. B. (1974). Antiaggregative-rivalry Pheromone of the mountain pine beetle, and a new arrestant of the southern pine beetle. Environ. Entomol. 3, 90–98. doi: 10.1093/ee/3.1.90
Ryker, L. C., and Libbey, L. M. (1982). Frontalin in the male mountain pine beetle. J. Chem. Ecol. 8, 1399–1409. doi: 10.1007/BF01403103
Safranyik, L., and Carroll, A. L. (2006). “The biology and epidemiology of the mountain pine beetle in lodgepole pine forests,” in The Mountain Pine Beetle: A Synthesis of its Biology, Management and Impacts on Lodgepole Pine, eds L. Safranyik and B. Wilson. (Victoria, BC: Pacific Forestry Centre, Canadian Forest Service, Natural Resources Canada), 3–66.
Safranyik, L., Carroll, A. L., Régnière, J., Langor, D. W., Riel, W. G., Shore, T. L., et al. (2010). Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can. Entomol. 142, 415–442. doi: 10.4039/n08-CPA01
Seybold, S., Huber, D., Lee, J., Graves, A., and Bohlmann, J. (2006). Pine monoterpenes and pine bark beetles: a marriage of convenience for defense and chemical communication. Phytochem. Rev. 5, 143–178. doi: 10.1007/s11101-006-9002-8
Seybold, S. J., Bohlmann, J., and Raffa, K. F. (2000). Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: evolutionary perspective and synthesis. Can. Entomol. 132, 697–753. doi: 10.4039/Ent132697-6
Smith, R. H. (1965). Effect of monoterpene vapors on the western pine beetle. J. Econ. Entomol. 58, 509–510. doi: 10.1093/jee/58.3.509
Smith, R. H. (1966). The monoterpene composition of Pinus ponderosa xylem resin and of Dendroctonus brevicomis pitch tubes. For. Sci. 12, 63–68.
Smith, R. H. (1975). Formula for describing effect of insect and host tree factors on resistance to western pine beetle attack. J. Econ. Entomol. 68, 841–844. doi: 10.1093/jee/68.6.841
Sturgeon, K. B. (1979). Monoterpene variation in ponderosa pine xylem resin related to western pine beetle predation. Evolution 33, 803–814. doi: 10.2307/2407647
Tingey, D. T., Turner, D. P., and Weber, J. A. (1991). “Factors controlling the emissions of monoterpenes and other volatile organic compounds,” in Trace Gas Emissions by Plants, eds T. D. Sharkey, E. A. Holland, and H. A. Mooney (San Diego, CA: Academic Press), 93–119.
Whitehead, A. T., del Scott, T., Schmitz, R. F., and Mori, K. (1989). Electroantennograms by mountain pine beetles, Dendroctonus ponderosae Hopkins, exposed to selected chiral semiochemicals. J. Chem. Ecol. 15, 2089–2099. doi: 10.1007/BF01207440
Wood, D. L. (1982). The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 27, 411–446. doi: 10.1146/annurev.en.27.010182.002211
Yamaoka, Y., Swanson, R. H., and Hiratsuka, Y. (1990). Inoculation of lodgepole pine with four blue-stain fungi associated with mountain pine beetle, monitored by a heat pulse velocity (HPV) instrument. Can. J. For. Res. 20, 31–36. doi: 10.1139/x90-005
Keywords: mountain pine beetle, range expansion, drought, host shift, tree defense
Citation: Lusebrink I, Erbilgin N and Evenden ML (2016) The Effect of Water Limitation on Volatile Emission, Tree Defense Response, and Brood Success of Dendroctonus ponderosae in Two Pine Hosts, Lodgepole, and Jack Pine. Front. Ecol. Evol. 4:2. doi: 10.3389/fevo.2016.00002
Received: 10 October 2015; Accepted: 11 January 2016;
Published: 01 February 2016.
Edited by:
Qing-He Zhang, Sterling International, Inc., USAReviewed by:
Tao Zhao, Royal Institute of Technology, SwedenYigen Chen, University of California, Davis, USA
Copyright © 2016 Lusebrink, Erbilgin and Evenden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inka Lusebrink, aW5rYS5sdXNlYnJpbmtAZ21haWwuY29t
 Inka Lusebrink
Inka Lusebrink Nadir Erbilgin
Nadir Erbilgin Maya L. Evenden
Maya L. Evenden