- 1Centre for Healthcare Science Research, Manchester Metropolitan University, Manchester, UK
- 2Department of Anesthesia, Boston Children's Hospital, Boston, MA, USA
- 3Department of Biomedical Sciences, Tufts University Cummings School of Veterinary Medicine, North Grafton, MA, USA
- 4Department of Infectious Disease and Global Health, Tufts University Cummings School of Veterinary Medicine, North Grafton, MA, USA
A social signal transduction theory of depression has been proposed that states that exposure to social adversity alters the immune response and these changes mediate symptoms of depression such as anhedonia and impairments in social behavior. The exposure of maternal rats to the chronic social stress (CSS) of a male intruder depresses maternal care and impairs social behavior in the F1 and F2 offspring of these dams. The objective of the present study was to characterize basal peripheral levels of several immune factors and related hormone levels in the adult F2 offspring of CSS exposed dams and assess whether changes in these factors are associated with previously reported deficits in allogrooming behavior. CSS decreased acid glycoprotein (α1AGP) and intercellular adhesion molecule-1 (ICAM-1) in F2 females, and increased granulocyte macrophage-colony stimulating factor (GM-CSF) in F2 males. There were also sex dependent changes in IL-18, tissue inhibitors of metalloproteinases 1 (TIMP-1), and vascular endothelial growth factor (VEGF). Progesterone was decreased and alpha melanocyte stimulating hormone (α-MSH) was increased in F2 males, and brain-derived neurotrophic factor (BDNF) was decreased in F2 females. Changes in α1AGP, GM-CSF, progesterone, and α-MSH were correlated with decreased allogrooming in the F2 offspring of stressed dams. These results support the hypothesis that transgenerational social stress affects both the immune system and social behavior, and also support previous studies on the adverse effects of early life stress on immune functioning and stress associated immunological disorders, including the increasing prevalence of asthma. The immune system may represent an important transgenerational etiological factor in disorders which involve social and/or early life stress associated changes in social behavior, such as depression, anxiety, and autism, as well as comorbid immune disorders. Future studies involving immune and/or endocrine assessments and manipulations will address specific questions of function and causation, and may identify novel preventative measures and treatments for the growing number of immune mediated disorders.
Introduction
A social signal transduction theory of depression has been proposed that states that exposure to social adversity, especially during early life, alters immune responses, and these changes mediate depression symptoms such as anhedonia and impaired social behavior (Slavich and Irwin, 2014). Cytokine mediated effects have been specifically implicated in depression and anxiety associated alterations in social behavior (Dantzer et al., 2008). Stressors during the perinatal period can induce persistent changes in the immune system and offspring behavior (Bilbo and Schwarz, 2012). For example, maternal immune activation induces adverse changes in offspring social behavior (Hodes et al., 2014; Machado et al., 2015). Exposure to early life stress also alters the systemic immune response and behavior (O'Mahony et al., 2009, 2011) and there is recent evidence that inflammatory factors (IL-1 and IL-6) increase vulnerability to stress related disorders and are associated with impairments in social behavior (Hodes et al., 2014; Wood et al., 2014). Social defeat, a robust social stressor often used in rodent studies, increases cytokine secretion (Powell et al., 2009), deficient maternal care alters immune associated gene expression (Cole et al., 2012), and it is postulated that inflammation may mediate the adverse effects of early life stress on mental health (Danese et al., 2007; Carpenter et al., 2010).
Exposure to early life stress, both prenatal and neonatal, can induce robust, behaviorally relevant changes in brain development (Howerton and Bale, 2012; Bale, 2015), and it has been suggested that these effects may be immune mediated (Howerton and Bale, 2012). Neonatal endotoxin treatment affects the development of the stress response and may increase susceptibility to stress related disorders (Shanks et al., 1995) and several additional studies have supported the hypothesis that the adverse effects of early life adversity on neural plasticity are mediated by inflammation (Musaelyan et al., 2014). The chronic social stress (CSS) model of postpartum depression and anxiety (Figure 1) induces substantial changes in the maternal behavior of F0 rat dams exposed to chronic male intruder stress (interaction with a novel male intruder during days 2–16 of lactation; Nephew and Bridges, 2011; Carini and Nephew, 2013; Carini et al., 2013; Murgatroyd et al., 2015b) and also disrupts maternal care in F1 dams (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013; Murgatroyd et al., 2015a) and social behavior in juvenile and adult F2 offspring (Babb et al., 2014). For the F1 and F2 offspring of stressed dams, the effects of CSS on social behavior may be mediated by early life exposure to depressed F0 maternal care and/or the male intruder stressor (F1 offspring) or depressed F1 maternal care (F2 offspring). The social behavior of both male and female F2 offspring of CSS exposed dams is disrupted, with a decrease in allogrooming during adult social interactions (Babb et al., 2014), supporting the use of the CSS model to study the transgenerational effects of stress on the etiology of disorders that involve maladaptive changes to social behavior, such as depression, anxiety, and autism.
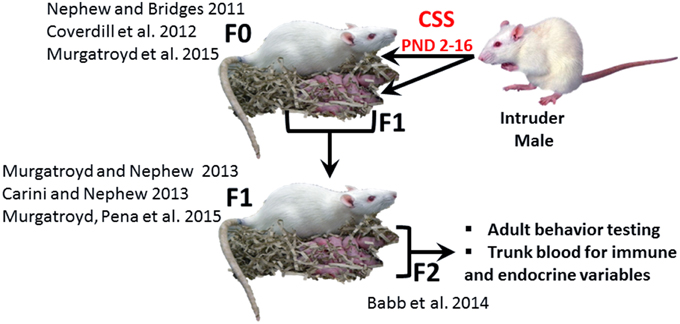
Figure 1. The CSS model of postpartum depression and anxiety. F0 dams are exposed to novel male intruder stress for 1 h/day during lactation days 2–15. This social stress is early life stressor for the F1 generation. Both the F0 and F1 dams exhibit depressed maternal care and increased maternal anxiety, and the depressed maternal care of the F1 dams is an early life stressor for the F2 generation. The current study focused on male and female F2 adults. (F0: Nephew and Bridges, 2011; Coverdill et al., 2012; Murgatroyd et al., 2015b; F1: Carini and Nephew, 2013; Murgatroyd and Nephew, 2013; Murgatroyd et al., 2015a; F2: Babb et al., 2014).
The objective of the present study was to characterize basal peripheral levels of immune factors and related hormone levels in the adult F2 offspring of CSS exposed dams and assess whether changes in these factors are associated with previously reported alterations in social behavior. The F2 generation was chosen for this study due to the observation of significant social deficits (most notably allogrooming) in these animals (Babb et al., 2014), similar to recent work on IL-6 and social behavior in rodent studies of resilience to social stress (Hodes et al., 2014). Due to the novelty of this research topic in the context of the CSS model, we targeted a broad panel of immune factors as well as progesterone, brain derived neurotrophic factor (BDNF), and alpha melanocyte stimulating hormone (α-MSH) which comprise interacting networks that are not fully understood (Lipton and Catania, 1997; Luger and Brzoska, 2007; Tait et al., 2008; Calabrese et al., 2014). The specific immune factors and hormones were chosen based on a review of applicable rodent and clinical literature, previous published data from the CSS model, and discussions with several basic and clinical immunologists. Given the evidence implicating exposure to social stress and immune factors in altered social behavior, it was hypothesized that basal immune factors, progesterone, BDNF, and α-MSH levels would be altered in CSS F2 offspring and that these changes would be associated with decreased social behavior.
Methods
Animals
Sprague-Dawley rats in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. Food and water were provided ad libitum, and light cycle was 12L/12D, with lights on at 0700. All F0 dams were purchased as adults (Charles River, Wilmington, MA), mated with breeder males, and housed in groups of three until the day prior to parturition. Litters were culled to five males and five females on the day of parturition. The F0 sample sizes were 11 control dams and 13 CSS dams.
CSS Model
See Figure 1 for a visual overview of the CSS model. The F0 CSS dams were subjected to a CSS protocol from postnatal days (PND) 2 to 16 as reported previously (Nephew and Bridges, 2011; Carini et al., 2013). This procedure consisted of placing a similarly sized (220–300 g) novel Sprague Dawley male intruder into a lactating female's home cage (10.5″W × 19″D × 8″H) for 1 h from PND 2 to 16. The F1 pups were left in the cage during the intruder presentation. Control dams were not exposed to the CSS protocol; they were only tested for maternal care and maternal aggression on PND days 2, 9, and 16 to allow for behavioral comparisons with the CSS exposed animals. Behavioral, endocrine, and neural gene expression data from these F0 dams has been published (Murgatroyd et al., 2015b).
F1 and F2 Offspring
F1 CSS females were the offspring of F0 dams exposed to CSS. F1 control females were the offspring of the F0 control dams who were not exposed to CSS. Thus, the differences in the F1 generations were presence (F1 CSS) or absence (F1 control) of attenuated maternal care and conflict between F0 dams and the male intruders during age 2–16 days. After day 16, the F1 CSS and F1 controls were treated identically. After weaning all F1 pups on day 23, the female offspring from the 12 control and 12 CSS dams were housed in groups of four until 70 days of age when two from each litter were mated with six proven breeder males from Charles River (24 F1 females for the control and CSS groups). Behavioral and endocrine data from those F1 females have been previously reported (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013; Murgatroyd et al., 2015a).
Total F2 pup number and litter weights were recorded on the day of parturition, and litters were then culled to five females and five males. The control F2 and CSS F2 animals were treated identically throughout the study; the only difference between the two groups was the attenuated maternal care (including deficits in pup grooming, nursing, and milk intake by pups) and increased restlessness and anxiety-related behavior (nesting, self-grooming, locomotor activity) expressed by the CSS F1 dams toward the F2 offspring. To summarize, the early life stress experience of the F2 generation consisted of exposure to attenuated maternal care from the F1 dams. The final F2 adult sample sizes were 10 for the control groups and 13 for the CSS groups, and there were no treatment differences in litter size or number or bodyweights at the juvenile, or adult stage, (all p > 0.2). Juveniles were euthanized at 42 days old, and adults were euthanized at 72 days.
Adult F2 Social Behavior Testing
The experimental rat was removed from the home cage and placed in a clean breeding cage (16 × 20 × 8 min.) for 10 min to allow for locomotor acclimation to the novel environment. An empty clear plastic mouse cage covered with a plastic mesh top was then placed in the breeding cage for 10 min, and a randomly selected same sex novel rat from the same treatment group was placed under the cage top to test for social approach for 10 min. At the end of the 10 min of social approach recording, the mouse cage top was removed, and the focal and novel animals were allowed to interact for 10 min. Behaviors scored for social approach consisted of time spent near and distant from the novel rat (area next to the mouse top was divided into two sections), time spent on top of the mouse cage top, olfactory investigation of the novel rat through the mesh of the mouse cage cover, self grooming, and total social approach (the sum of time near novel rat, on top of mouse cage top, and olfactory investigation). Adult social behaviors scored consisted of rostral and caudal investigation, lateral contact, dorsal contact, tail grabbing, allogrooming, self grooming, locomotor activity, aggression, and total social contact (the sum of investigation, contact, tail grabbing, and allogrooming). The social behavior data from the animals in this study have been previously reported in Hormones and Behavior (Babb et al., 2014), and only the novel cytokine data and cytokine/allogrooming correlations will be presented in the current study. During the social interaction test, adult F2 males and females spent more time investigating a novel conspecific and less time in direct social interaction, as assessed by time spent allogrooming (Babb et al., 2014). The current decrease in F2 adult allogrooming is supported by decreased pup grooming in F2 dams which were littermates of the animals in the present study (unpublished data). All experimental animals were euthanized within 3 min of entering the animal room between 0800 and 1000 the day following social behavior testing, and trunk blood was collected for the analysis of basal immune and hormone levels.
Immune and Endocrine Assays
An assay panel of pro- and anti-inflammatory cytokines (CXCL3, CXCL2, GM-CSF, sICAM-1, IFNγ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-13, IL-18, L-Selectin, TIMP-1, TNFα, VEGF) from R&D Systems (R&D Systems, UK) was conducted on a Luminex 200 Bio-Plex Platform. Immediately prior to the initiation of the study, the Bio-Plex platform underwent a complete on-site maintenance cycle. Samples were thawed directly on the day of analysis. Working wash solutions and protein standards were prepared within 1 h of beginning the assay by reconstituting the standard in assay diluent and performing serial dilutions according to manufacturer specifications. A magnetic plate washer was utilized during the plate washing stages. Following processing, protein concentrations were calculated and analyzed with the xPONENT software (Luminex, v.3.1.871). Additional cytokines and endocrine targets, IgE, CRP, α1AGP, BDNF, progesterone, and α-MSH were measured by individual ELISAs (R&D Systems, U.S.). Samples were run in duplicate in an individual assay to eliminate interassay variation, and intraassay variability was of 3–7%.
Statistics
Basal cytokine and hormone levels were analyzed with two-way ANOVA (treatment and sex), which were followed with individual two-tailed t-tests within each sex (t-tests were not corrected for multiple comparisons). Pearson correlations were used to test for significant cytokine and hormone behavioral associations on the combined male and female data from the control and CSS F2 groups, as well as separate tests on combined control and CSS data from each sex in targets where there was an effect of CSS.
Results
The assay values for CXCL2, IFNγ, IL-1β, IL-2, IL-4, IL-10, IL-13, and TNFα fell below the standard curve of the Luminex ELISA. There were no significant differences in the values for IgE, CRP, CXCL3, IL-1α, or L-Selectin (all p > 0.1). There was a significant effect of sex on α1AGP levels [F(1, 45) = 5.0, p = 0.03] and a significant interaction between sex and treatment [F(1, 45) = 6.1, p = 0.03, Figure 2A]. α1AGP levels were lower in CSS F2 females compared to control F2 females (p = 0.03, Figure 2A). There was an overall effect of treatment on soluble ICAM-1, with lower levels in CSS F2 animals [F(1, 45) = 5.0, p = 0.03, Figure 2B]. ICAM-1 levels were decreased in CSS F2 females compared to controls (p = 0.02, Figure 2B). There were significant effects of sex [F(1, 45) = 24.8, p < 0.01] and treatment (F(1, 45) = 7.1, p = 0.01] on GM-CSF, with levels being generally lower in males but higher in CSS F2 males compared to control F2 males (p = 0.02, Figure 2C). IL-18 [F(1, 45) = 10.3, p < 0.01, Figure 2D] and TIMP-1 [F(1, 45) = 14.9, p < 0.01, Figure 2E] levels were higher in F2 males compared to F2 females, where levels of VEGF were lower in F2 males [F(1, 45) = 32.0, p < 0.01, Figure 2F]. There was also a non-significant trend for increased levels of IL-6 in the CSS adult F2 females [2023.2 ± 324.2 vs. 1223.1 ± 246.0, F(1, 22) = 3.9, p = 0.06].
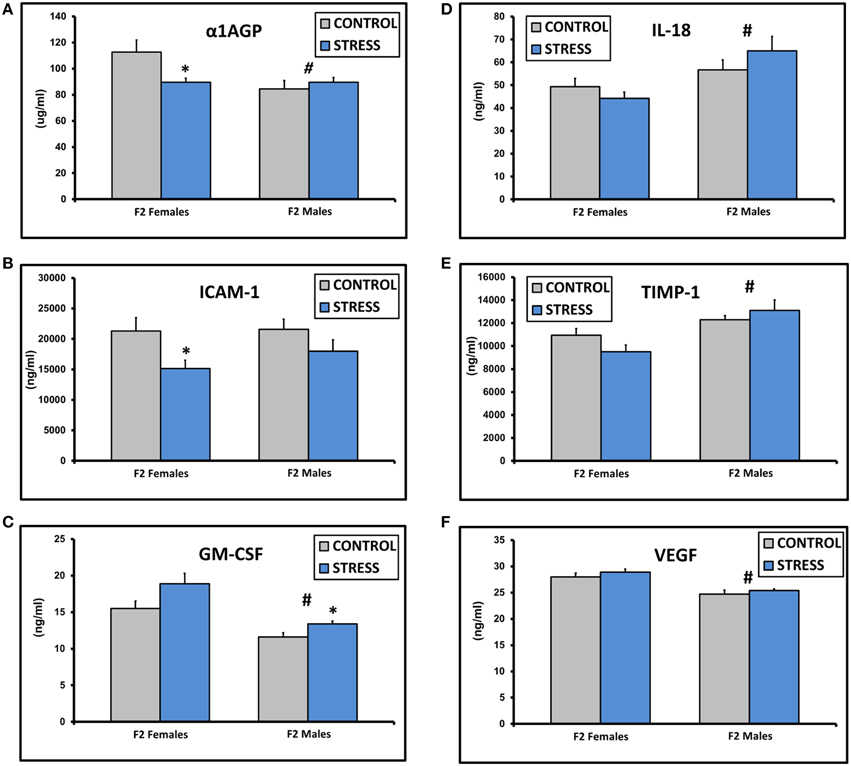
Figure 2. Mean ± SEM basal plasma levels of α-1AGP (A), ICAM-1 (B), GM-CSF (C), IL-18 (D), TIMP-1 (E), and VEGF (F) in the adult F2 male and female offspring of control dams and dams exposed to chronic social stress during lactation. *Denotes significant effect of CSS treatment, #denotes significant effect of sex (p < 0.05).
Progesterone levels were decreased in males [F(1, 45) = 75.5, p < 0.01], and levels in CSS F2 males (97.0 ± 10.8) were lower compared to control F2 males (129.4 ± 12.1, p < 0.05, Figure 3A). BDNF levels were higher in males [F(1, 45) = 8.6, p < 0.01], and CSS F2 females had lower BDNF levels than control F2 females (p = 0.03, Figure 3B). α-MSH levels were higher in CSS F2 males compared to control F2 males (p < 0.01, Figure 3C).
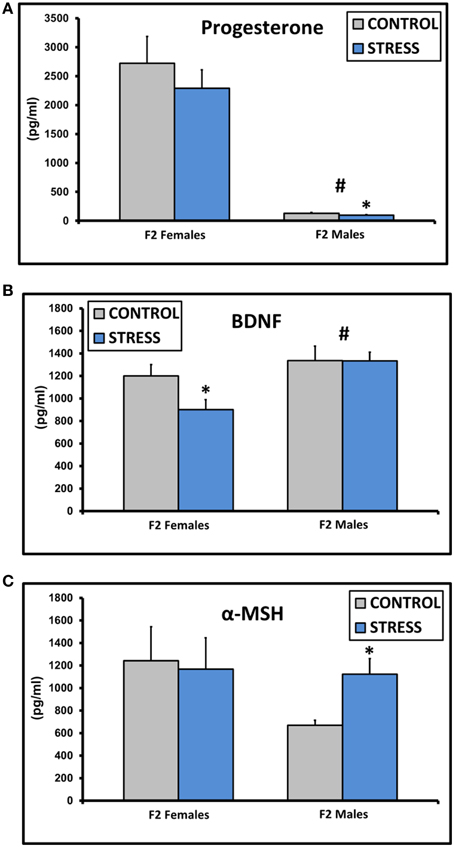
Figure 3. Mean ± SEM basal plasma levels of progesterone (A), BDNF (B), and α-MSH (C) in the adult F2 male and female offspring of control dams and dams exposed to chronic social stress during lactation. *Denotes significant effect of CSS treatment, #denotes significant effect of sex (p < 0.05).
α1AGP levels were positively correlated with allogrooming in all groups combined as well as specifically in female control and CSS F2 animals (Table 1). GM-CSF and α-MSH were negatively associated with allogrooming in males, and progesterone was positively associated with allogrooming in all groups combined (Table 1).
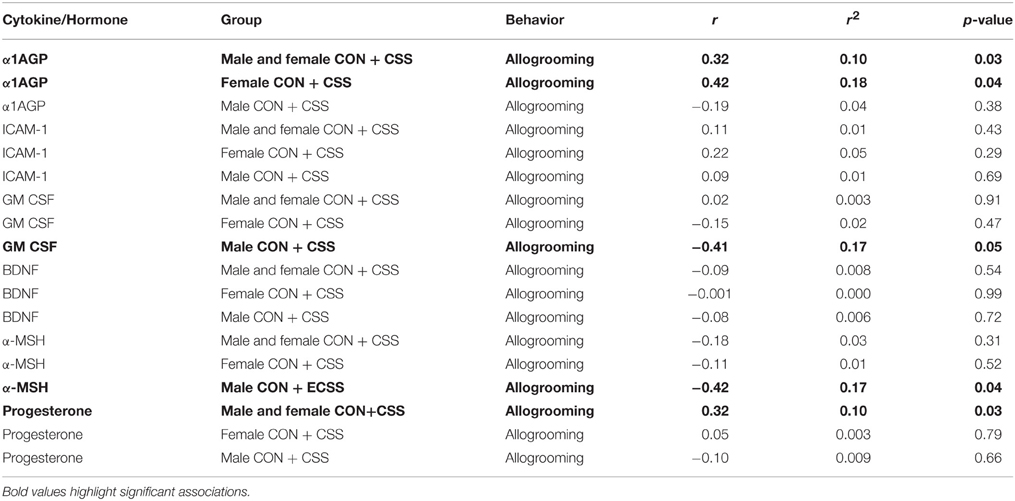
Table 1. Pearson Correlation coefficient and p-values for immune targets and hormones and allogrooming behavior during a social interaction test for factors significantly affected by chronic social stress in adult male and female F2 offspring of control (CON) and chronic social stress (CSS) dams.
Discussion
Recent interest in the role of the immune system in behavior and the pathophysiology of stress associated psychiatric disorders stimulated the present investigation of the effects of CSS on peripheral immune factors and related hormones in the F2 offspring of CSS exposed dams. There is growing interest in the interactions between the immune system and neurohormonal signaling, and relevant behavioral models are needed to determine the mechanisms of these interactions in the context of disease pathology. The CSS paradigm had transgenerational sex specific effects in the F2 generation on basal α1AGP, ICAM-1, GM-CSF, progesterone, BDNF, and α-MSH. In contrast, while there were overall sex differences in IL-18, TIMP-1, and VEGF, these factors were not affected by CSS. Changes in α1AGP, GM-CSF, progesterone, and α-MSH were associated with allogrooming in a social interaction test. While it is possible that the social behavior testing had an effect on the immune factor and hormone levels collected the next day, the data can be considered basal in terms of the lack of any acute behavioral or immune (LPS, infection, etc.) challenge prior to collection. The basal state of the samples is supported by previously published F2 adult corticosterone and prolactin levels from the animals in the current study (Atkinson and Waddell, 1997; Babb et al., 2014). The data support the hypothesis that social stress has extensive sex specific transgenerational effects on the immune and endocrine systems which are associated with changes in social behavior. Given the design of the CSS paradigm, it is unknown if these effects are due to early life stress exposure of the F1 dams, early life stress exposure of the F2 animals, or a combination. It is postulated that the changes in immune factors, hormones, and social behavior may be the result of additive epigenetic influences in successive generations (Lyall et al., 2014).
Alpha 1 acid glycoprotein (α1AGP), a major acute phase reactant secreted into the plasma by the liver, is believed to act as an anti-inflammatory and immunomodulatory agent with increased plasma levels occurring during tissue injury, inflammation and infection (Fournier et al., 2000). In male rats, acute stress such as tail shock elevates α1AGP (Deak et al., 1997). In humans, α1AGP levels are associated with exposure to psychological stress (Maes et al., 1997) and depression (Nieto et al., 2000). The current data show decreased α1AGP in CSS F2 females, and this attenuation is associated with a related decrease in allogrooming that is driven by the effects of CSS in females. These data indicate that general disruptions in the inflammatory pathway due to early life stress, in either direction, may be associated with behavioral effects. In support of this hypothesis, the direction of changes in immune factors has been linked to coping strategy in rodent models, where passive coping is associated with pro-inflammatory processes, and active coping and resistance to stress related pathology is associated with inflammatory suppression (Finnell et al., 2015; Wood et al., 2015). Given the available behavioral data from the CSS F2 adult animals, it is difficult to speculate on their stress coping strategy or resistance to stress related pathology. It is also possible that there are sex specific α1AGP responses to stress in rats, which is supported by the present data.
A member of the immunoglobulin superfamily of adhesion receptors, intercellular adhesion molecule-1 (ICAM-1) is involved the generation of the immune response and is a primary marker of immune activation (Boyd et al., 1988), and plasma concentration of ICAM-1 are a biomarker of infection and prognosis in patients with various chronic inflammation-related diseases (de Pablo et al., 2013). Similar to α1AGP, ICAM-1 levels were decreased in CSS F2 females. This decrease in both pro and anti-inflammatory factors suggests that CSS has a general suppressive effect on the production of immune factors. While there are not many studies on the effects of early life stress specifically on ICAM-1, basal cell carcinoma patients that were maltreated by their parents exhibited poor ICAM-1 responses (Fagundes et al., 2012). A lack of significant association with allogrooming indicates that it may not mediate allogrooming in the CSS paradigm. Alternative roles for the CSS induced change in ICAM-1 include the regulation of inflammatory responses to disease related immune challenges, and ongoing CSS studies are investigating the effects of CSS on responses to immune challenges.
Granulocyte macrophage-colony stimulating factor (GM-CSF) is a hematopoietic growth factor with pro-inflammatory functions and mediates the adverse effects of social stress on inflammatory pathways (Powell et al., 2013). This cytokine may mediate global changes in inflammatory responses in stressed animals, as exposure to repeated social stress prior to allergen inhalation worsens airway inflammation in rodents, including levels of GM-CSF (Bailey et al., 2009). In addition, resident intruder stress and anhedonia have recently been associated with elevated levels of GM-CSF in the locus coeruleus (Finnell et al., 2015), and the current elevated basal levels in CSS F2 males could represent a transgenerational behavioral effect of a similar social stress through GM-CSF given that the F2 animals were not directly exposed to CSS. Children with autism spectrum disorder (ASD) exhibit a trend for elevated GM-CSF compared to healthy controls (Ashwood et al., 2011), social deficits are reported in ASD and in the CSS F2 animals (Babb et al., 2014), and early life stress in mothers is associated with an increased risk for autism in offspring (Roberts et al., 2013, 2014). Both animal and human studies of GM-CSF indicate that it may be involved in the adverse behavioral effects of social stress, but it is unknown if changes in this cytokine mediate changes in F2 social behavior or are an inflammatory indicator of exposure to social stress.
The matrix metalloproteinases and their inhibitors (tissue inhibitors of metalloproteinases, TIMP) mediate the remodeling of the pericellular environment and have been postulated to regulate methamphetamine induced sensitization and reward through modulation of extracellular dopamine (Mizoguchi et al., 2008). While we did not see effects of CSS on basal levels of TIMP-1, increased levels in males may reflect sex-dependent responses to stress and/or reward. There was also a sex difference in basal VEGF, and while most of the focus on stress, depression, and neuroplasticity has been directed toward BDNF, VEGF is also a potent mediator of neuroplasticity (Duman and Monteggia, 2006; Pittenger and Duman, 2007). It is possible that there were effects of the CSS on basal VEGF in the F2 animals at earlier life history stages (infant, juvenile) that mediated alterations in neural development only during those periods, or that there were changes in central VEGF that were not reflected in peripheral levels.
Behavioral research on progesterone has focused more on parental behavior than adult social behavior, but progesterone levels in marmosets have been associated with social grooming patterns, similar to the present findings (Azevedo et al., 2001). In addition, perinatal progesterone treatment in males has significant effects on adult social behavior (Hull et al., 1980). Immune studies of progesterone indicate that it inhibits both central and peripheral inflammation (Tait et al., 2008; Giannoni et al., 2011; Lei et al., 2014), and it is postulated that the decrease in CSS F2 male progesterone mediates the increase in GM-CSF and the decrease in social behavior. Based on earlier reports of the inhibitory actions of progesterone on maternal care (Bridges et al., 1978) and increased paternal behavior in male progesterone receptor KO mice and male mice treated with a progesterone antagonist (Schneider et al., 2003), future studies should investigate the effects of CSS on progesterone, the immune system, and maternal and paternal care.
The increased risk for psychiatric illness following exposure to early life stress may be mediated by enduring sex dependent changes in BDNF (Cirulli et al., 2009a,b). In mice, social deprivation, which may be similar to the depressed maternal care experienced by CSS F2 rats when young, decreases BDNF levels in the brain and increases anxiety (Berry et al., 2012). Exposure to low levels of maternal care in childhood is associated with increased DNA methylation of the BDNF and OXTR genes (Unternaehrer et al., 2015), and physical neglect and child abuse are associated with decreased plasma BDNF (Grassi-Oliveira et al., 2008; Elzinga et al., 2011). While studies of depression support the hypothesis that increases in inflammation result in decreased BDNF and attenuated neuroplasticity (Calabrese et al., 2014), we report decreases in both peripheral BDNF and inflammatory factors (although there is a trend for elevated IL-6 in CSS F2 females). In addition, although CSS decreased basal BDNF in F2 females, this change was not correlated with the decrease in allogrooming, suggesting that the difference in peripheral BDNF does not directly mediate the change in allogrooming. It may be that peripheral levels of BDNF do not reflect central levels that are more directly involved in mediating neuroplasticity and regulating behavior.
Alpha melanocyte stimulating hormone (α-MSH) mediates the immune response through the downregulation of pro-inflammatory cytokines, immunomodulatory cytokines, and costimulatory molecules (Lipton and Catania, 1997; Luger et al., 2003; Luger and Brzoska, 2007). This peptide, acting through MC4 receptors, is also involved in the etiology of depression and anxiety in animal models (Kokare et al., 2008, 2010; Liu et al., 2013). The stress of social isolation decreases social interaction and increases immobility in the forced swim test and treatment with HS014, a selective MC4 antagonist, attenuates depression symptoms (Kokare et al., 2010). Furthermore, HS014 has prophylactic actions on the adverse behavioral and neural effects of stress on depression and anxiety behaviors (Serova et al., 2013, 2014; Sabban et al., 2014). The elevated basal levels of α-MSH in the F2 males following transgenerational exposure to CSS may be adaptive in an inflammatory context by preventing excessive inflammatory responses, yet they may also increase susceptibility to stress induced depression and anxiety and disrupt social behavior.
In conclusion, the present study reports on several transgenerational treatment and sex dependent changes in the immune and hormonal profiles of the adult F2 offspring of dams exposed to CSS. Changes in α1AGP, GM-CSF, progesterone, and α-MSH are associated with transgenerational social stress induced decreases in allogrooming. These results support the hypothesis that transgenerational social stress affects both the immune system and social behavior, and also support previous studies on the adverse effects of early life stress on immune functioning and stress associated immunological disorders, including the increasing prevalence of asthma. The immune system may represent an important transgenerational etiological factor in disorders which involve social and/or early life stress associated changes in social behavior, such as depression, anxiety, and autism, as well as comorbid immune disorders. Future studies involving immune and/or endocrine assessments and manipulations will address specific questions of function and causation and may identify novel preventative measures and treatments for the growing number of immune mediated disorders.
Author Contributions
BN, GB, CM, and JB contributed to the design of the study. All authors contributed to data collection, analysis, and development of the manuscript.
Funding
The work in this study was supported by NIH/NICHD Award R00 HD056643 and a Tufts CTSI Award UL1 RR025752 to BN, and a Tufts University Collaborates Award to BN and GB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the Tufts University Cummings School of Veterinary Medicine Laboratory Animal Medicine Services for providing exceptional care for the study animals and cooperative logistical support for the experiments.
References
Ashwood, P., Krakowiak, P., Hertz-Picciotto, I., Hansen, R., Pessah, I., and Van de Water, J. (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 25, 40–45. doi: 10.1016/j.bbi.2010.08.003
Atkinson, H. C., and Waddell, B. J. (1997). Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology 138, 3842–3848. doi: 10.1210/en.138.9.3842
Azevedo, C. V. M., Camillo, C. S., Xavier, C. A., Moreira, L. F. S., Cordeiro de Sousa, M. B., and Marques, N. (2001). Grooming circadian rhythmicity, progesterone levels and partner preference of the reproductive pair of a captive common marmoset (Callithrix jacchus) family group during pregnancy and after parturition. Biol. Rhythm Res. 32, 145–157. doi: 10.1076/brhm.32.2.145.1354
Babb, J. A., Carini, L. M., Spears, S. L., and Nephew, B. C. (2014). Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm. Behav. 65, 386–393. doi: 10.1016/j.yhbeh.2014.03.005
Bailey, M. T., Kierstein, S., Sharma, S., Spaits, M., Kinsey, S. G., Tliba, O., et al. (2009). Social stress enhances allergen-induced airway inflammation in mice and inhibits corticosteroid responsiveness of cytokine production. J. Immunol. 182, 7888–7896. doi: 10.4049/jimmunol.0800891
Bale, T. L. (2015). Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 16, 332–344. doi: 10.1038/nrn3818
Berry, A., Bellisario, V., Capoccia, S., Tirassa, P., Calza, A., Alleva, E., et al. (2012). Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology 37, 762–772. doi: 10.1016/j.psyneuen.2011.09.007
Bilbo, S. D., and Schwarz, J. M. (2012). The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 33, 267–286. doi: 10.1016/j.yfrne.2012.08.006
Boyd, A. W., Wawryk, S. O., Burns, G. F., and Fecondo, J. V. (1988). Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc. Natl. Acad. Sci. U.S.A. 85, 3095–3099. doi: 10.1073/pnas.85.9.3095
Bridges, R. S., Rosenblatt, J. S., and Feder, H. H. (1978). Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation after progesterone withdrawal and inhibition by progesterone maintenance. Endocrinology 102, 258–267. doi: 10.1210/endo-102-1-258
Calabrese, F., Rossetti, A. C., Racagni, G., Gass, P., Riva, M. A., and Molteni, R. (2014). Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 8:430. doi: 10.3389/fncel.2014.00430
Carini, L. M., Murgatroyd, C. A., and Nephew, B. C. (2013). Using chronic social stress to model postpartum depression in lactating rodents. J. Vis. Exp. e50324. doi: 10.3791/50324
Carini, L. M., and Nephew, B. C. (2013). Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Horm. Behav. 64, 634–641. doi: 10.1016/j.yhbeh.2013.08.011
Carpenter, L. L., Gawuga, C. E., Tyrka, A. R., Lee, J. K., Anderson, G. M., and Price, L. H. (2010). Association between Plasma IL-6 Response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 35, 2617–2623. doi: 10.1038/npp.2010.159
Cirulli, F., Francia, N., Berry, A., Aloe, L., Alleva, E., and Suomi, S. J. (2009a). Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 33, 573–585. doi: 10.1016/j.neubiorev.2008.09.001
Cirulli, F., Francia, N., Branchi, I., Antonucci, M. T., Aloe, L., Suomi, S. J., et al. (2009b). Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology 34, 173–180. doi: 10.1016/j.psyneuen.2008.08.020
Cole, S. W., Conti, G., Arevalo, J. M. G., Ruggiero, A. M., Heckman, J. J., and Suomi, S. J. (2012). Transcriptional modulation of the developing immune system by early life social adversity. Proc. Natl. Acad. Sci. U.S.A. 109, 20578–20583. doi: 10.1073/pnas.1218253109
Coverdill, A. J., McCarthy, M., Bridges, R. S., and Nephew, B. C. (2012). Effects of chronic central arginine vasopressin (AVP) on maternal behavior in chronically stressed rat dams. Brain Sci. 2, 589–604. doi: 10.3390/brainsci2040589
Danese, A., Pariante, C. M., Caspi, A., Taylor, A., and Poulton, R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U.S.A. 104, 1319–1324. doi: 10.1073/pnas.0610362104
Dantzer, R., O'Connor, J. C., Freund, G. G., Johnson, R. W., and Kelley, K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. doi: 10.1038/nrn2297
Deak, T., Meriwether, J. L., Fleshner, M., Spencer, R. L., Abouhamze, A., Moldawer, L. L., et al. (1997). Evidence that brief stress may induce the acute phase response in rats. Am. J. Physiol. 273, R1998–R2004.
de Pablo, R., Monserrat, J., Reyes, E., Díaz, D., Rodriguez-Zapata, M., de la Hera, A., et al. (2013). Circulating sICAM-1 and sE-Selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur. J. Intern. Med. 24, 132–138. doi: 10.1016/j.ejim.2012.10.009
Duman, R. S., and Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127. doi: 10.1016/j.biopsych.2006.02.013
Elzinga, B. M., Molendijk, M. L., Oude Voshaar, R. C., Bus, A. A. B., Prickaerts, J., and Spinhoven, P. (2011). The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF val66met. Psychopharmacol 214, 319–328. doi: 10.1007/s00213-010-1961-1
Fagundes, C. P., Glaser, R., Johnson, S. L., Andridge, R. R., Yang, E. V., Di Gregorio, M. P., et al. (2012). Basal cell carcinoma: stressful life events and the tumor environment. Arch. Gen. Psychiatry 69, 618–626. doi: 10.1001/archgenpsychiatry.2011.1535
Finnell, J., Lombard, C., Singh, N., Nagarkatti, M., Nagarkatti, P., Wood, C., et al. (2015). Protective effects of resveratrol on social stress-induced neuroinflammation and depressive-like behavior. FASEB J. 29. Available online at: http://www.fasebj.org/content/29/1_Supplement/770.5.short
Fournier, T., Medjoubi, -N. N., and Porquet, D. (2000). Alpha-1-acid glycoprotein. Biochim. Biophys. Acta. 1482, 157–171. doi: 10.1016/S0167-4838(00)00153-9
Giannoni, E., Guignard, L., Knaup Reymond, M., Perreau, M., Roth-Kleiner, M., Calandra, T., et al. (2011). Estradiol and progesterone strongly inhibit the innate immune response of mononuclear cells in newborns. Infect. Immun. 79, 2690–2698. doi: 10.1128/IAI.00076-11
Grassi-Oliveira, R., Stein, L. M., Lopes, R. P., Teixeira, A. L., and Bauer, M. E. (2008). Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression—a preliminary report. Biol. Psychiatry 64, 281–285. doi: 10.1016/j.biopsych.2008.02.023
Hodes, G. E., Pfau, M. L., Leboeuf, M., Golden, S. A., Christoffel, D. J., Bregman, D., et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. U.S.A. 111, 16136–16141. doi: 10.1073/pnas.1415191111
Howerton, C. L., and Bale, T. L. (2012). Prenatal programing: at the intersection of maternal stress and immune activation. Horm. Behav. 62, 237–242. doi: 10.1016/j.yhbeh.2012.03.007
Hull, E. M., Franz, J. R., Snyder, A. M., and Ken Nishita, J. (1980). Perinatal progesterone and learning, social and reproductive behavior in rats. Physiol. Behav. 24, 251–256. doi: 10.1016/0031-9384(80)90082-7
Kokare, D. M., Dandekar, M. P., Singru, P. S., Gupta, G. L., and Subhedar, N. K. (2010). Involvement of α-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 58, 1009–1018. doi: 10.1016/j.neuropharm.2010.01.006
Kokare, D. M., Singru, P. S., Dandekar, M. P., Chopde, C. T., and Subhedar, N. K. (2008). Involvement of alpha-melanocyte stimulating hormone (α-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res. 24, 53–67. doi: 10.1016/j.brainres.2008.03.064
Lei, B., Mace, B., Dawson, H. N., Warner, D. S., Laskowitz, D. T., and James, M. L. (2014). Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS ONE 9:e103969. doi: 10.1371/journal.pone.0103969
Lipton, J. M., and Catania, A. (1997). Anti-inflammatory actions of the neuroimmunomodulator α-MSH. Immunol. Today 18, 140–145. doi: 10.1016/S0167-5699(97)01009-8
Liu, J., Garza, J. C., Li, W., and Lu, X.-Y. (2013). Melanocortin-4 receptor in the medial amygdala regulates emotional stress-induced anxiety-like behaviour, anorexia and corticosterone secretion. Int. J. Neuropsychopharmacol. 16, 105–120. doi: 10.1017/S146114571100174X
Luger, T. A., and Brzoska, T. (2007). α-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann. Rheum. Dis. 66, iii52–iii55. doi: 10.1136/ard.2007.079780
Luger, T. A., Scholzen, T. E., Brzoska, T., and Böhm, M. (2003). New insights into the functions of α-MSH and related peptides in the immune system. Ann. N.Y. Acad. Sci. 994, 133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x
Lyall, K., Constantino, J. N., Weisskopf, M. G., Roberts, A. L., Ascherio, A., and Santangelo, S. L. (2014). Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry 71, 936–942. doi: 10.1001/jamapsychiatry.2014.476
Machado, C. J., Whitaker, A. M., Smith, S. E. P., Patterson, P. H., and Bauman, M. D. (2015). Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiatry 77, 823–832. doi: 10.1016/j.biopsych.2014.07.035
Maes, M., Hendriks, D., van Gastel, A., Demedts, P., Wauters, A., Neels, H., et al. (1997). Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology 22, 397–409. doi: 10.1016/S0306-4530(97)00042-5
Mizoguchi, H., Yamada, K., and Nabeshima, T. (2008). Neuropsychotoxicity of abused drugs: < br>involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J. Pharmacol. Sci. 106, 9–14. doi: 10.1254/jphs.FM0070139
Murgatroyd, C. A., and Nephew, B. C. (2013). Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38, 219–228. doi: 10.1016/j.psyneuen.2012.05.020
Murgatroyd, C. A., Peña, C. J., Podda, G., Nestler, E. J., and Nephew, B. C. (2015a). Early life social stress induced changes in depression and anxiety associated neural pathways which are correlated with impaired maternal care. Neuropeptides 52, 103–111. doi: 10.1016/j.npep.2015.05.002
Murgatroyd, C. A., Taliefar, M., Bradburn, S., Carini, L. M., Babb, J. A., and Nephew, B. C. (2015b). Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression. Behav. Pharmacol. 26, 642–653. doi: 10.1097/FBP.0000000000000147
Musaelyan, K., Egeland, M., Fernandes, C., Pariante, C. M., Zunszain, P. A., and Thuret, S. (2014). Modulation of adult hippocampal neurogenesis by early-life environmental challenges triggering immune activation. Neural Plast. 2014:194396. doi: 10.1155/2014/194396. Available online at: http://www.hindawi.com/journals/np/2014/194396/cta/
Nephew, B. C., and Bridges, R. S. (2011). Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14, 677–684. doi: 10.3109/10253890.2011.605487
Nieto, E., Vieta, E., Alvarez, L., Torra, M., Colom, F., and Gastó, C. (2000). Alpha-1-acid glycoprotein in major depressive disorder: relationships to severity, response to treatment and imipramine plasma levels. J. Affect. Disord. 59, 159–164. doi: 10.1016/S0165-0327(99)00145-7
O'Mahony, S. M., Hyland, N. P., Dinan, T. G., and Cryan, J. F. (2011). Maternal separation as a model of brain–gut axis dysfunction. Psychopharmacology 214, 71–88. doi: 10.1007/s00213-010-2010-9
O'Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A.-M., Quigley, E. M. M., et al. (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263–267. doi: 10.1016/j.biopsych.2008.06.026
Pittenger, C., and Duman, R. S. (2007). Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33, 88–109. doi: 10.1038/sj.npp.1301574
Powell, N. D., Bailey, M. T., Mays, J. W., Stiner-Jones, L. M., Hanke, M. L., Padgett, D. A., et al. (2009). Repeated social defeat activates dendritic cells and enhances Toll-like receptor dependent cytokine secretion. Brain Behav. Immun. 23, 225–231. doi: 10.1016/j.bbi.2008.09.010
Powell, N. D., Sloan, E. K., Bailey, M. T., Arevalo, J. M. G., Miller, G. E., Chen, E., et al. (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. U.S.A. 110, 16574–16579. doi: 10.1073/pnas.1310655110
Roberts, A. L., Koenen, K. C., Lyall, K., Ascherio, A., and Weisskopf, M. G. (2014). Women's posttraumatic stress symptoms and autism spectrum disorder in their children. Res. Autism Spectr. Disord. 8, 608–616. doi: 10.1016/j.rasd.2014.02.004
Roberts, A. L., Lyall, K., Rich-Edwards, J. W., Ascherio, A., and Weisskopf, M. G. (2013). Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry 70, 508–515. doi: 10.1001/jamapsychiatry.2013.447
Sabban, E. L., Serova, L. I., Alaluf, L. G., Laukova, M., and Peddu, C. (2014). Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav. Brain Res. 24, 00841–00849. doi: 10.1016/j.bbr.2014.12.038
Schneider, J. S., Stone, M. K., Wynne-Edwards, K. E., Horton, T. H., Lydon, J., O'Malley, B., et al. (2003). Progesterone receptors mediate male aggression toward infants. Proc. Natl. Acad. Sci. U.S.A. 100, 2951–2956. doi: 10.1073/pnas.0130100100
Serova, L. I., Laukova, M., Alaluf, L. G., and Sabban, E. L. (2013). Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav. Brain Res. 250, 139–147. doi: 10.1016/j.bbr.2013.05.006
Serova, L. I., Laukova, M., Alaluf, L. G., and Sabban, E. L. (2014). Blockage of melanocortin-4 receptors by intranasal HS014 attenuates single prolonged stress-triggered changes in several brain regions. J. Neurochem. 131, 825–835. doi: 10.1111/jnc.12847
Shanks, N., Larocque, S., and Meaney, M. (1995). Neonatal endotoxin exposure alters the development of the hypothalamic- pituitary-adrenal axis: early illness and later responsivity to stress. J. Neurosci. 15, 376–384.
Slavich, G. M., and Irwin, M. R. (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815. doi: 10.1037/a0035302
Tait, A. S., Butts, C. L., and Sternberg, E. M. (2008). The role of glucocorticoids and progestins in inflammatory, autoimmune, and infectious disease. J. Leukoc. Biol. 84, 924–931. doi: 10.1189/jlb.0208104
Unternaehrer, E., Meyer, A. H., Burkhardt, S. C. A., Dempster, E., Staehli, S., Theill, N., et al. (2015). Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress 18, 451–461. doi: 10.3109/10253890.2015.1038992
Wood, S. K., Wood, C. S., Lombard, C. M., Lee, C. S., Zhang, X. Y., Finnell, J. E., et al. (2014). Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol. Psychiatry 25, 00891–00899. doi: 10.1016/j.biopsych.2014.10.026
Keywords: social stress, social behavior, immune system, transgenerational effects, Inflammation, BDNF, progesterone, cytokines
Citation: Murgatroyd CA, Babb JA, Bradburn S, Carini LM, Beamer GL and Nephew BC (2016) Transgenerational Social Stress, Immune Factors, Hormones, and Social Behavior. Front. Ecol. Evol. 3:149. doi: 10.3389/fevo.2015.00149
Received: 14 September 2015; Accepted: 14 December 2015;
Published: 12 January 2016.
Edited by:
Hans A. Hofmann, The University of Texas at Austin, USAReviewed by:
Ross Gillette, The University of Texas at Austin, USABrian Dias, Emory University, USA
Copyright © 2016 Murgatroyd, Babb, Bradburn, Carini, Beamer and Nephew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin C. Nephew, YmNuZXBoZXdAYW9sLmNvbQ==
 Christopher A. Murgatroyd
Christopher A. Murgatroyd Jessica A. Babb
Jessica A. Babb Steven Bradburn
Steven Bradburn Lindsay M. Carini3
Lindsay M. Carini3 Benjamin C. Nephew
Benjamin C. Nephew