
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 17 December 2015
Sec. Behavioral and Evolutionary Ecology
Volume 3 - 2015 | https://doi.org/10.3389/fevo.2015.00139
This article is part of the Research Topic Fitness Costs and Benefits of Female Song View all 18 articles
Recent advances have revealed that female birdsong is widespread and multifunctional. Female song was likely ancestral among songbirds and persists in many lineages today. Nevertheless, many species lack female song, and researchers are interested in understanding the selective factors that promote and counter the persistence of this trait. Female song is associated with life-history traits including year-round territoriality, non-migratory behavior, sexual monochromatism, and monogamy. Most studies examining these relationships have looked at clades with a migratory ancestor and have found that gains of migratory behavior are strongly correlated with losses of female song (and duetting). Here, we ask if the reverse pattern exists: in a large clade of songbirds with a migratory ancestor, do losses of migratory behavior correlate with gains of female song and visual signaling traits? We investigated correlations between female song, migration, and dichromatism in 107 species of New World Warblers (Family Parulidae). All of these species are predominantly monogamous and territorial when breeding, 50 (47%) are migratory, 49 (46%) are monochromatic, and 25 (23%) show female song. On a robust genetic phylogeny maximum likelihood methods recover migration and monochromatism as the ancestral state in warblers. Female song is generally not reconstructed as present in any deep nodes of the phylogeny, suggesting that most extant species with female song evolved this trait independently and relatively recently. Gains of female song do not correlate with losses of migration. Losses of dichromatism do correlate with losses of migration. Thus, in this clade, visual signals are associated with sedentary vs. migratory lifestyles, but female acoustic signals are not. Our results show a different pattern from that seen in similar studies and support the hypothesis that losses, but not gains, of female song are driven by life history.
For most of the history of its academic study, song in birds has been considered an almost exclusively male trait (Langmore, 1998; Catchpole and Slater, 2008). Much recent work has corrected this bias and found that while male song is certainly more common in some geographical regions, female song is widespread among songbirds (Odom et al., 2014) and can have important functions in many (if not all) of the same contexts as male song: defense of a territory, mate attraction, maintenance of the pair bond, and mate guarding (Hall, 2004; Slater and Mann, 2004).
In addition to understanding the functions of female song, researchers are interested in the evolutionary history, and ecological correlates of female song (Price, 2009; Odom et al., 2014). Observers have long noted that female song is more common in tropical and sub-tropical areas than it is in temperate regions (Slater and Mann, 2004). Traditionally this pattern is attributed to the fact that resources in tropical regions are divided among more individuals, making them available at relatively low levels year-round and are therefore defended by both members of a territorial pair, leading to a general convergence of sex roles (Morton, 1996).
A recent surge of interest in this topic has supported the idea that female song is associated with life-history traits that are common in tropical areas, including year-round territoriality and/or non-migratory behavior, sexual monochromatism, carotenoid dichromatism, and monogamy (Malacarne et al., 1991; Garamszegi et al., 2007; Benedict, 2008; Price, 2009; Price et al., 2009; Logue and Hall, 2014). In particular, gain of migratory behavior is strongly correlated with loss of female song (including duetting; Price et al., 2009; Logue and Hall, 2014). “Migration” may be best thought of not as one trait, but as an attribute that represents an amalgam of traits (navigational abilities, flight behavior, etc.) that are likely to affect general life history strategies (Zink, 2002). Migratory species tend to be dichromatic (Hamilton, 1961), have higher divorce rates (Jeschke and Kokko, 2008), and have higher rates of extra-pair paternity (Spottiswoode and Møller, 2004) than sedentary species; all of these life history characters might be summed up as factors contributing to divergence in sex roles, and potentially as factors selecting against the presence of female song in migratory groups.
The New World blackbirds (family Icteridae) are the best-studied group in regards to the life history correlates of female song (Hofmann et al., 2008; Price, 2009; Price et al., 2009). Barring the basal meadowlark clade, the ancestor of the rest of the New World blackbirds is inferred to have been a sedentary, monochromatic species with singing females. Many blackbird species retained this sedentary, tropical lifestyle along with monochromatism, and female song. Species that lost female song generally did so in conjunction with gains in migration, losses of bright female plumage, and/or changes in breeding strategy, such as switching from monogamy to polygyny or colonial breeding (Price, 2009). Thus, studies of this group have revealed much about when and why female song is lost in species descended from a sedentary common ancestor with female song. A comprehensive study of male-female duet presence in songbirds also found that losses of female song were correlated with gains of migration, and indicated that losses of female song are much more common than are gains of this trait (Logue and Hall, 2014). This pattern fits well with the result that female song is ancestral in all songbirds (Odom et al., 2014).
While existing evidence suggests that the lack of female song in temperate regions likely represents losses of this trait, it is less clear if gains of female song are common and if they are driven by the same selective forces as losses of female song. Here, we investigate this question using a large clade (107 species) of new world songbirds whose ancestor is inferred to be migratory (Winger et al., 2014). The New World wood-warblers (family Parulidae) are the sister group to the New World blackbirds (Barker et al., 2015). Like blackbirds, warblers are represented by a mix of non-migratory and migratory species as well as monochromatic and dichromatic species, and include members with females reported to sing regularly or rarely. Unlike among blackbirds, the stronghold of warbler diversity is in North America and the base of the warbler phylogeny is dominated by migratory species. Additionally, apart from whether they migrate, nearly all warblers have similar life histories: they are socially monogamous, defend territories during the breeding season, and build cup nests on or near the ground.
Making use of recently published molecular phylogenies resolving the relationships among warblers (Lovette et al., 2010; Barker et al., 2015) we reconstructed the evolution of female song in this group and we tested for correlations among female song, dichromatism, and migratory behavior to investigate the possible relationships among these life history traits. Following the hypothesis that non-migratory, monogamous species with similar sex roles are more likely to express female song (Morton, 1996; Benedict, 2008; Price, 2009), we predicted that gains of female song in warblers would be strongly correlated with losses of migration and losses of dichromatic plumage.
We searched the literature for any reference to female song in each of the 107 warbler species represented in Lovette et al.'s (2010) molecular phylogeny. We used primary literature, field guides, Neotropical Birds Online, the Birds of North America Online, and the Handbook of the Birds of the World (Del Hoyo et al., 2011). Using Web of Science, we searched for the common name, old scientific name, and recently revised scientific name of each species (per Lovette et al., 2010). Given the difficulty of detecting female song and an overrepresentation of North American species in the literature, we attempted to take into account the research effort associated with each species. Accordingly, we scored a species as being “well-researched” if we could find five or more publications where the focal species was being directly observed by a researcher and “poorly researched” if we could not. We used this method because it reflects the bimodal nature of our search results. Searches generally either returned more than five papers focused on a single species, or simply returned publications where a species is mentioned only as part of a list and was never the subject of direct focal observation. Occasionally searches returned one or two brief reports focused on a single species, but these tended not to be focused on song behavior. We searched English-language sources only.
We scored a species as having female song if we found any reference to a singing female, including solitary reports (Appendix 1 in Supplementary Material). Not all species are reported to sing regularly, so we marked a species as being a “rare” singer if accounts described female song as rare or uncommon or if main sources (like the Birds of North America reports) do not mention female song but a report exists for that species. We scored a species as not having female song if species accounts specifically state that females do not sing, or if we scored that species as being well-researched and we found no mention of female song. We scored female song as “unknown” for species in which we could find no report of female song but were also poorly researched.
We looked at photographs and plates of each species to score carotenoid and melanin dichromatism (Appendix 1 Supplementary Material). Melanin and carotenoid pigments are acquired through different modes and thus are thought to carry different signals in birds (Badyaev and Hill, 2000). We counted plumage colors that might be described as black, brown, gray, rufous, cinnamon, or buff as melanin coloration and colors that might be described as reds, oranges, or yellows as carotenoid coloration. Plumage dichromatism was scored on a scale of 0–2 for both melanin and carotenoid plumage characters, with 0 indicating no difference between males and females, 1 indicating a weak difference, and 2 indicating a striking difference between males and females. For example, we scored Geothlypis trichas as carotenoid monochromatic (0) since females and males appear equally brightly yellow but are strikingly melanin dichromatic (2), with females lacking the black mask characteristic of males. We scored only the nominate subspecies for each species. Two raters scored plumage characters for all species. A third rater scored species where there was disagreement, and a consensus score was assigned to each species. This approach is supported by research showing that painted plates and photographs are reliable sources of biologically relevant patterns in birds (Møller and Birkhead, 1994). Like most species of birds, parulid warblers have plumage that reflects UV light, which can result in humans estimating a bird to be monochromatic when it is actually dichromatic (i.e., one sex has a UV patch). However, a study comparing human estimates of dichromatism with spectrophotometer estimates of dichromatism found that, in warblers, human estimates are reliable, indicating both males and females have similar UV reflectance or that species dichromatic in the UV spectrum are also dichromatic in the visible light spectrum (Armenta et al., 2008).
We used the sources described in our literature search methods above to determine whether a species is migratory or sedentary (Appendix 1 in Supplementary Material). We found one species to be described as an altitudinal migrant, the arrowhead warbler, Setophaga pharetra, and two species to be described as partial migrants (not all individuals migrate), the pine warbler, Setophaga pinus, and the painted whitestart, Myioborus pictus. We counted altitudinal or partial migrants as being “migratory” since they are still making seasonal movements which require many (if not all) of the same tools used by a long-distance migrant (i.e., a compass, restlessness, etc.)
We determined the ancestral states of female song, migration, and plumage dimorphism in Parulidae using maximum likelihood (ML; one or two parameter Markov k-state) using the stored models in the ancestral state reconstruction package of Mesquite 3.02 (Maddison and Maddison, 2015) with a recent molecular phylogeny of the wood-warblers (Lovette et al., 2010). A one parameter likelihood model assumes the backward and forward rates of evolution are the same (symmetrical). That is, the rate of gain of a trait is the same as the rate of loss. A two parameter likelihood model has a backward rate different from the forward rate (asymmetrical). A likelihood ratio test was used to determine which likelihood model (symmetrical or asymmetrical) to use. This test compares the one and two parameter reconstructions of the same character on the same tree to determine whether the complex two parameter model fits the data significantly better than the simpler one parameter model. We used an alpha of 0.05 as our cutoff for “significantly better fit.” It should be noted that asymmetrical models can only have two character states, so we did not use an asymmetrical model for the three-state characters carotenoid 2 and melanin 2. We called a particular character state to be likely at a given node if it was reconstructed as being at least 70% likely, i.e., had a proportional likelihood of 0.70 or greater. The proportional likelihood is the probability that a given node was a particular state, calculated by adding the two likelihoods and taking the ratio of one to the total. For maximum likelihood models in general, models with a value closer to 0 are better than models farther away from zero (e.g., a model with log likelihood −20 is better than a model with log likelihood −30). We discuss increases in the prevalence of a state as “gains” and decreases as “losses,” although we did not apply a statistical test to our character state reconstructions to do so as Mesquite does not currently support statistical analysis of character state reconstructions.
Coding scheme can be extremely important when reconstructing character histories (e.g., Thorpe, 1984; Wilkinson, 1995; Strong and Lipscomb, 1999). We therefore used multiple coding schemes to reconstruct character histories in Mesquite in order to evaluate the possible effect of coding strategy on the reconstruction of female song. Coding schemes are listed in Table 1.
Female song coding scheme 1 included three possible character states (song present, song absent, and unknown), while all of the other female song coding schemes included only two possible character states (song present, song absent). All coding schemes were used in ancestral state reconstruction, and coding schemes with only two possible character states were used in analyses of trait correlations. We included female song coding schemes 2, 3, and 4 in order to assess the potential effects of low research effort on some species. We included female song coding scheme 5 because rare female song may have little biological importance. Melanin and carotenoid dichromatism were coded in two ways: a scheme with all three states for use in ancestral state reconstructions and a scheme with two states for use in correlation analyses. Melanin dichromatism was split into none/weak (0/1) and striking (2) since most species had striking dichromatism, while carotenoid dichromatism was split into none (0) and weak/striking (1/2) since only two species had striking carotenoid dichromatism. We then pruned the tree to exclude species with low research effort and compared the pruned reconstruction with the full dataset. In general, the pruned tree was simpler to interpret with fewer equivocal nodes, but the overall pattern of gains and losses were the same, and so we do not report ancestral state reconstructions from pruned trees.
We used Pagel's method (1994) to test for correlated evolution between two characters in the correlation package of Mesquite 3.02. Pagel's method takes phylogeny into account as it looks for correlated changes in character states by testing between an independent and dependent model of character evolution on a tree. Two characters are correlated (or not independent from each other) when the dependent model (assumes traits are changing together with eight parameters) is significantly different from the independent model (assumes traits are not changing together with four parameters). The p-values are generated from parametric bootstrapping of the two models using a likelihood ratio test over many simulations (requiring a minimum of ~1000 simulations). This method requires all characters to be binary and to have no missing data, so we did not run this analysis on female song 1, carotenoid dichromatism 2, or melanin dichromatism 2. Random assignment of female song to species with missing data (song coding scheme 4) and assigning missing data as indicating presence of song (song coding scheme3) yielded very poorly supported trees (log likelihood: −71.67 and −70.77, respectively; all other trees we used had log likelihoods >–58) so these coding schemes were not used in subsequent analyses. We tested for correlated evolution between female song 2, 5, migration, any plumage dimorphism, carotenoid dimorphism, and melanin dimorphism, as well as between migration and overall plumage dimorphism, migration, and carotenoid dimorphism, and migration and melanin dimorphism (3000 simulations, 1000 searches, any effect). To assess the potential effects of missing data we pruned the tree to remove all species with “unknown” female song. We conducted two preliminary correlation analyses between female song 2, 5, and migration using the pruned tree, but these results were not appreciably different from the results using the full tree, so we used the full tree for all subsequent correlations. The patterns of evolution of female song reconstructed using the full tree for female song coding schemes 2 and 5 were not different from reconstructions obtained from the pruned tree.
Of the 107 species included in Lovette et al.'s (2010) phylogeny of the Parulidae 50 (46.7%) species are migratory, 49 (45.7%) species are monochromatic, and 25 (23.3%) species have at least one report of female song. Of these 25 species, 12 are reported to sing regularly, and seven of those 12 species are duetters. Of the seven duetting species, six species are sedentary and monomorphic (only M. pictus is not, see the Appendix in Supplementary Material for the full data set).
We marked 41 (38.3%) species as “unknown” for female song due to poor research effort. Only 19.5% (8) of the poorly researched species were migratory (including two extinct species, Vermivora bachmanii and Leucopeza semperi), while 51.2% (21) of them were monochromatic. Among the 73 relatively well-researched species in the dataset, 34.2% had female song, 36.8% (28) were monochromatic and 57.5% (42) were migratory.
Of the species scored as dichromatic, 17 species were weakly carotenoid dichromatic, two species were strikingly carotenoid dichromatic, 22 species were weakly melanin dichromatic, and 34 species were strongly melanin dichromatic. Two species were weakly dichromatic because the females had carotenoids where the male had none (Setophaga cerulea and Setophaga caerulescens).
Character histories for female song 1, 2, 4, and 5 were reconstructed using the two-parameter model. Female song is generally not reconstructed as present in any deep nodes of the phylogeny, indicating that most extant species with female song evolved this trait independently and relatively recently (Figures 1, 2).
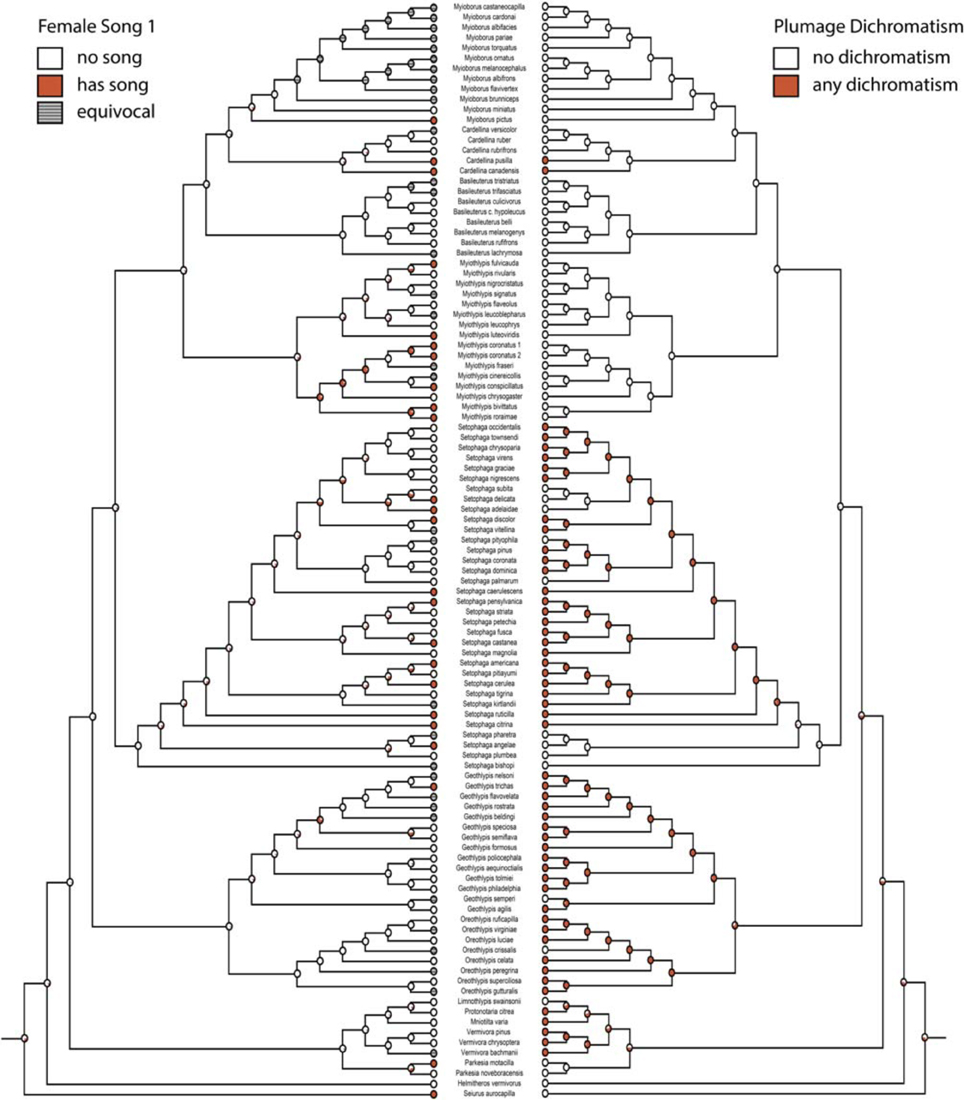
Figure 1. Ancestral state reconstruction with female song 1 (mk2, log likelihood: −45.95) on the left mirrored with any plumage dichromatism (mk1, log likelihood: −56.91) on the right. Red circles have female song or are dichromatic and white circles do not, gray circles are equivocal. Pie charts indicate proportional likelihoods.
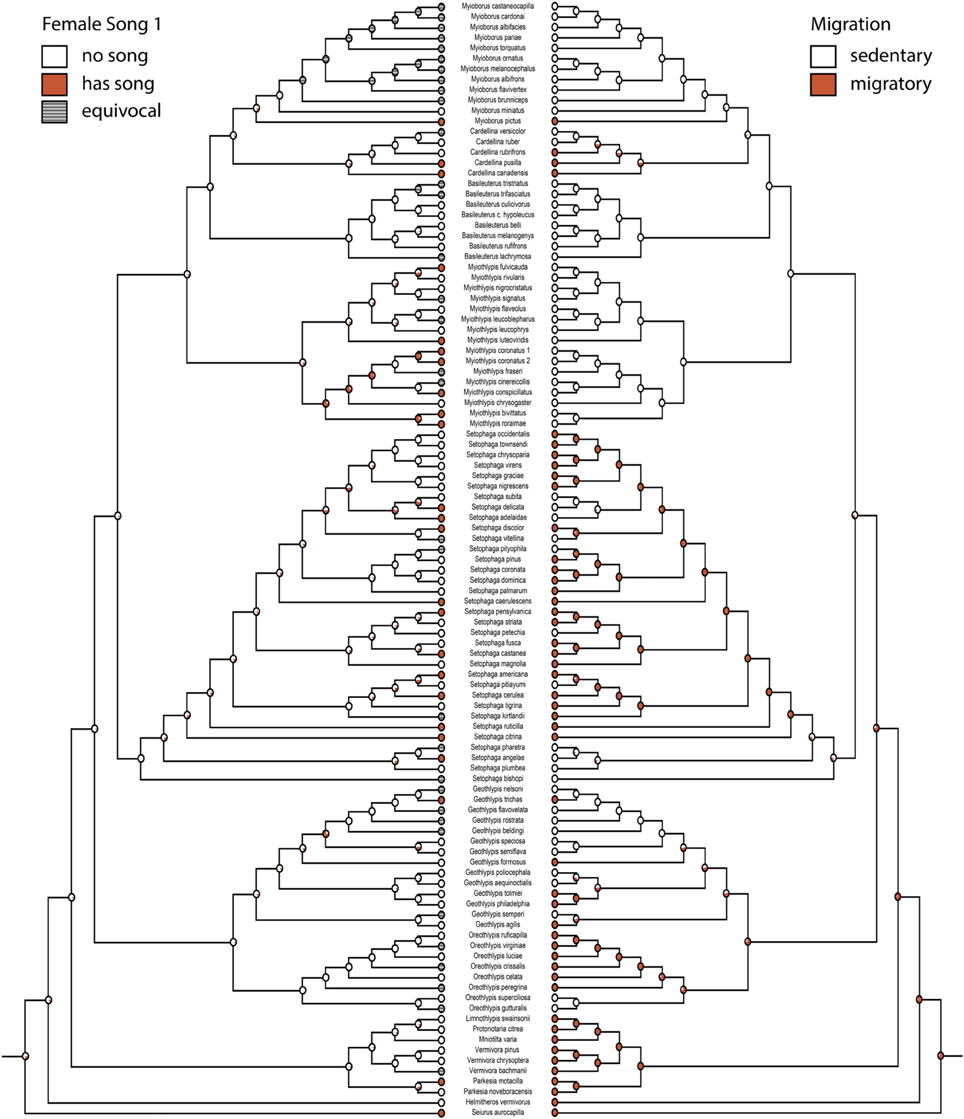
Figure 2. Ancestral state reconstruction for female song 1 (mk2, log likelihood: −45.95) mirrored with migration (mk1, log likelihood: −46.81). Red circles have female song or are migratory, white circles do not. Gray circles are equivocal. Pie charts indicate proportional likelihoods.
If we code female song as unknown in species where there are no explicit reports on the presence or absence of female song (female song 1), we recover many estimated gains of female song and few losses (two-parameter log likelihood: −45.95). The one clade where female song appears to be relatively ancestral is within the genus Myiothlypis. The deepest node (representing the ancestral warbler) has a proportional likelihood of 0.32 that it had female song.
Female song 2 (all missing data coded as “female song absent”) yields an almost identical reconstruction to female song 1, albeit not as strongly supported, with Myiothlypis again containing a relatively ancestral node that may have had female song (two parameter log likelihood: −57.62, proportional likelihood: 0.72). The deepest node has a proportional likelihood of 0.27 that it had female song. Pruning the tree to remove species with unknown song status due to low research effort results in the same overall reconstruction, with all taxa (including Myiothlypis) being largely equivocal for female song (Table 2).
Using female song coding scheme 5 (only common female song) produced a tree with only 12 species with female song, nearly all of which appear homoplasious, and with no deep ancestral female singers (two parameter log likelihood: −38.13). Removing species with low research effort results in a marginally better reconstruction of female song (log likelihood: −36.2; Table 2).
Overall, the likelihood that ancestral nodes had female song increases when we indicate missing data, but the pattern of evolution of female song is fairly resistant to changes in coding scheme using likelihood methods and under nearly every scheme results indicate many gains of song, but a rate of loss that is at least twice as large as the rate of gain (e.g., female song 2 forward rate: 12.9, reverse rate: 42.3). Removing species with missing data does not change the overall pattern, although it does generally improve the likelihood values of the reconstructions (Table 2).
Migration is recovered as the ancestral state in warblers (Figures 2,3). Maximum likelihood strongly supports a migratory ancestor (log likelihood: −36.79, proportional likelihood migratory: 0.98). Monochromatism is also recovered as the ancestral state in warblers (Figures 1, 3, 4; log likelihood: −23.84, proportional likelihood monochromatic: 0.98). Carotenoid dichromatism (weak and strong) appears to have been gained many times with strong carotenoid dichromatism evolving only twice (in Setophaga ruticilla and Setophaga fusca). Melanin dichromatism, on the other hand, is predominately represented by strong differences between males and females with 34 species exhibiting striking dichromatism and 22 species exhibiting weak dichromatism (Figure 4). More dichromatic species were melanin dichromatic than carotenoid dichromatic.
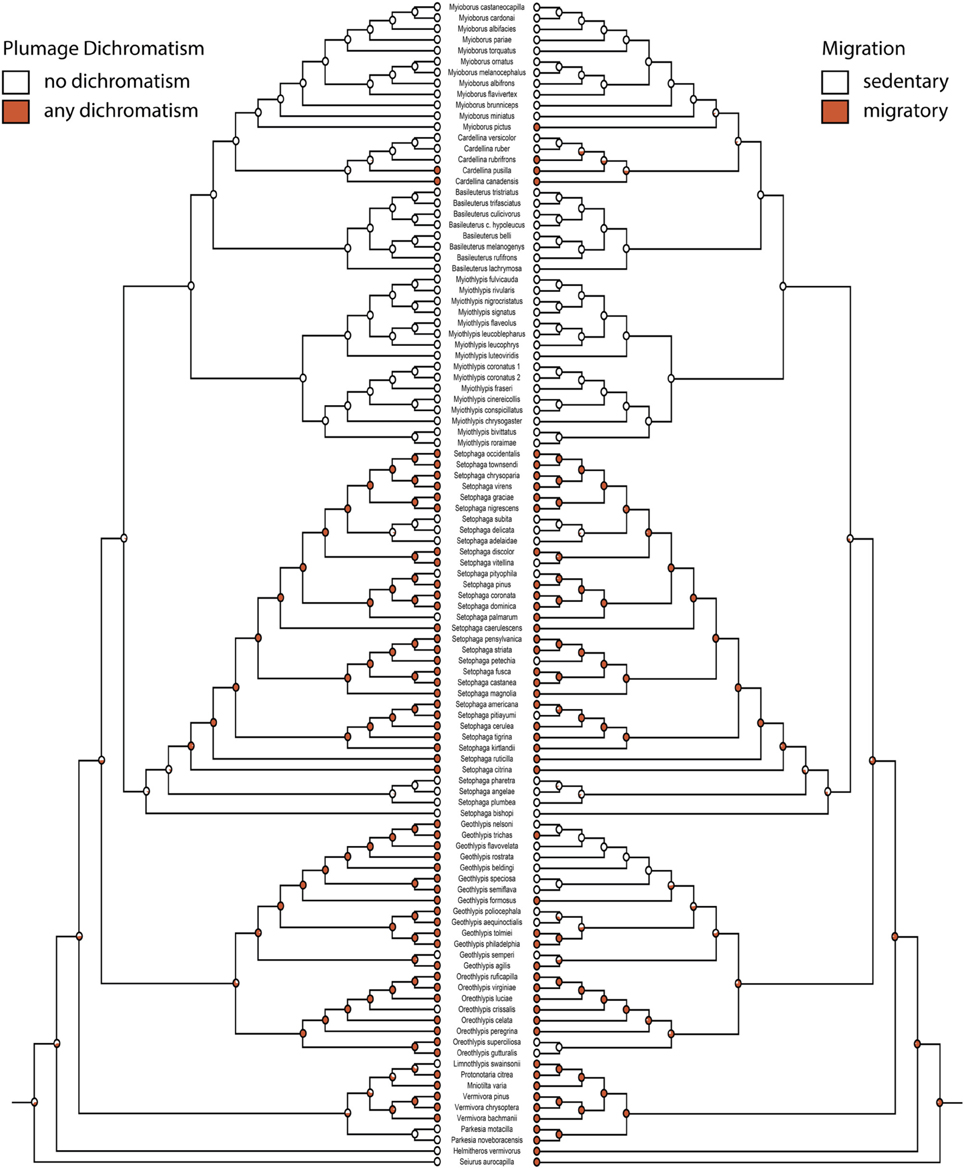
Figure 3. Any plumage dichromatism (mk1, log likelihood: −56.91) on the left mirrored with migration (mk1, log likelihood: −46.81) on the right. Red circles are dichromatic or migratory, white circles are not. Pie charts indicate proportional likelihoods.
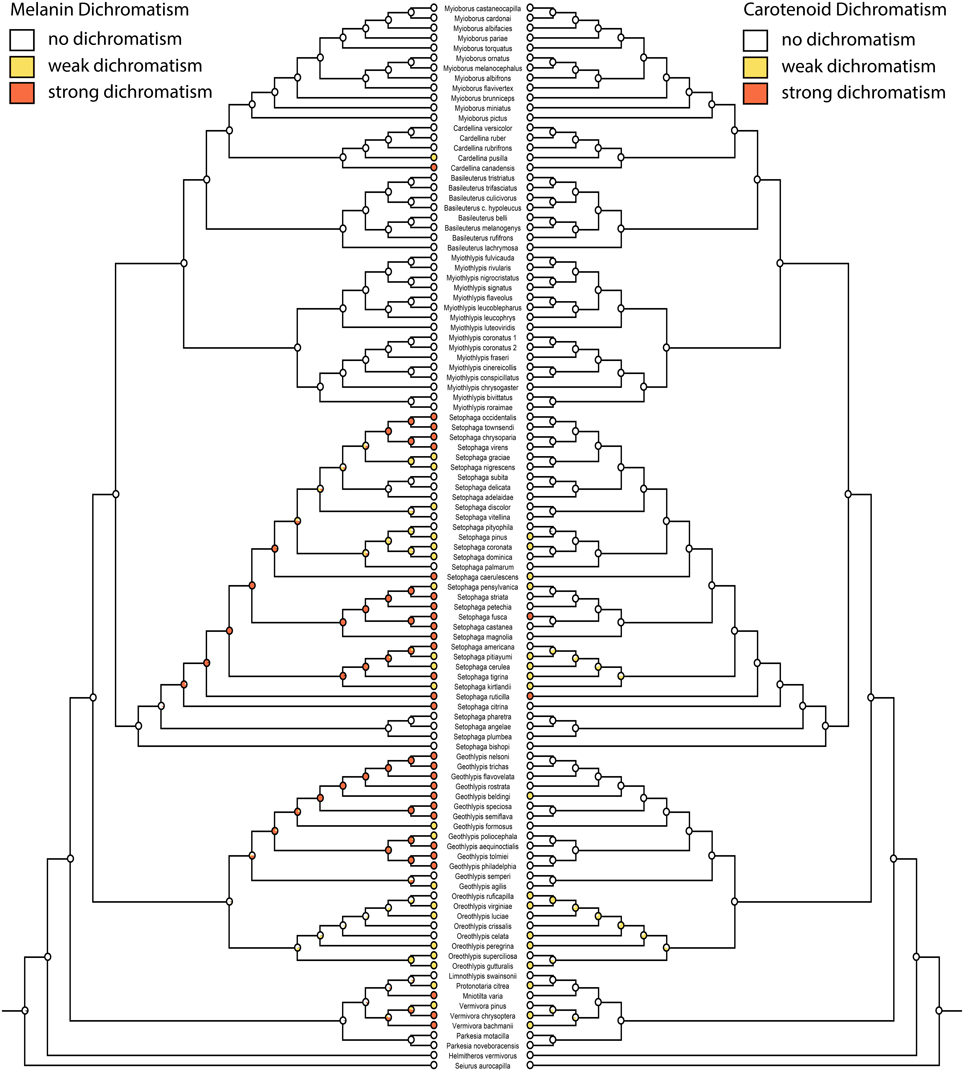
Figure 4. Ancestral state reconstructions of melanin dichromatism 2 (mk1, log likelihood: −86.99) on the left mirrored with carotenoid dichromatism 2 (mk1, log likelihood: −68.94) on the right. White circles are monochromatic, yellow circles weakly dichromatic, and red circles strongly dichromatic.
Most correlation analyses were conducted using song coding scheme 2 (female song coded as either present or absent) since this coding scheme includes all reports of female song and using other coding schemes doesn't change the resulting correlations. Using the full parulid tree (including species with missing data coded as song absent) song coding scheme 2 was not correlated with migration (3000 simulations, p = 0.16, Figure 2), general dichromatism (3000 simulations, p = 0.09, Figure 1), melanin dichromatism 1 (1000 simulations, p = 0.77, Figure 4), or carotenoid dichromatism 1 (1000 simulations, p = 0.98, Figure 4). Removing species with low research effort (i.e., using a pruned tree) did not change whether or not a correlation with female song 2 was significant (migration— p = 0.11, monochromatism— p = 0.19; Table 3). Since removing species with low research effort did not change character correlation results, we used the full tree in all subsequent analyses. Female song 5 (rare reports coded as “absent”) was not correlated with migration (1000 simulations, p = 0.38) or plumage dichromatism (1000 simulations, p = 0.34; Table 3). As our female song 3 and 4 coding schemes are biologically unrealistic and the character reconstructions were poorly supported we did not run correlation analyses for them.
Migration and dichromatism were significantly correlated with each other (3000 simulations, p < 0.001; Figure 3, Table 3). More specifically, migration was significantly correlated with both melanin dichromatism 1 (1000 simulations, p = 0.001) and carotenoid dichromatism 1 (1000 simulations, p = 0.003; Table 3).
The transition rates of the four possible character state combinations in each of three correlations (female song 2 vs. migration, female song 2 vs. any plumage dichromatism, and migration vs. any plumage dichromatism) were compared to assess the relative rates of transition to and from particular states within a given correlation (Figure 5).
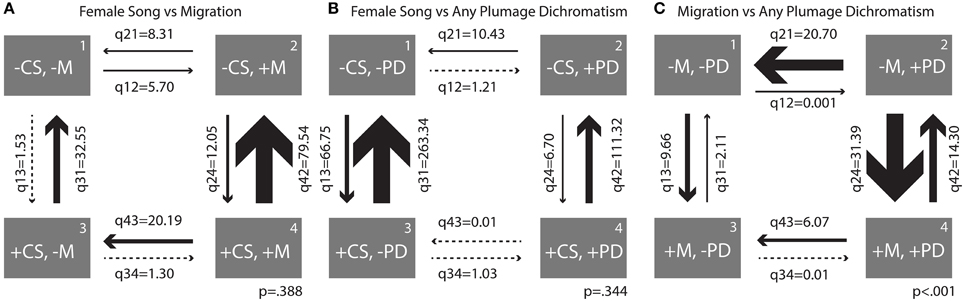
Figure 5. Transition rates derived from correlation analyses between (A) female song 2 and migration, (B) female song 2 and any plumage dichromatism, and (C) migration and any plumage dichromatism. Arrows are scaled to the largest transition rate in each figure.
When comparing female song and migration, the largest transition was overwhelmingly from being migratory and having song to losing song (q42 = 79.54, Figure 5A); this transition occurred about 6.5 times more frequently than the opposite transition (gaining song while migratory). The largest relative rate among two states was switching from being sedentary and having female song to losing female song, with losses occurring 21 times more frequently than gains (Figure 5A).
Similarly, song was lost much more frequently than it was gained when a species is dichromatic (rate of loss ~16.5 times larger than gains) and to a lesser extent when a species is monochromatic (rate of loss ~4 times larger, Figure 5B). Overall transition rates between presence and absence of female song in monochromatic species were larger than in dichromatic species (q13 = 66.75, Figure 5B). Plumage characters seem resistant to change relative to song characters and had low transition rates overall, with the highest being the transition from dichromatic to monochromatic without female song (q21 = 10.43, Figure 5B). Female song was not significantly correlated with migration or plumage dichromatism, so the results of the transition rates between these states should be interpreted with caution.
The largest transition rate of the correlation between migration and any plumage dichromatism was the transition from sedentary to migratory in dimorphic species (q24 = 31.39, Figure 5C), occurring about twice as frequently as the reverse transition (q42 = 14.30, Figure 5C). The next largest transition was from dichromatic to monochromatic in sedentary species (q21 = 20.70, Figure 5C); transitions in the opposite direction (gaining dichromatism while sedentary) almost never happens (q12 = 0.001, Figure 5C).
Female song is uncommon but not rare in Parulidae with 23% of species having at least one report of a singing female. Unexpectedly, given current hypotheses and previously published literature on the distribution of female song in birds (Morton, 1996; Garamszegi et al., 2007), these species are distributed relatively evenly among migratory and sedentary clades and show no associations with sexual monochromatism vs. dichromatism.
It can be argued that female song is rare, aberrant, or biologically unimportant in species for which we found only one or a few reports of female song. Most Parulid species with frequent female song (12 species, 11% of Parulids) are in the large clade consisting of Myiothlypis, Basileuterus, Cardellina, and Myioborus warblers. This group is generally monochromatic and sedentary and thus conforms to current hypotheses on life history correlates associated with female song. Indeed, all of the duetting species occur in this large clade and duetters account for seven of 12 species reported to sing regularly. However, not all species with frequent female song conform to this pattern, and removing species with rare reports does not change character state reconstructions, only somewhat improves the likelihood of the reconstructions, and does not result in significant correlations between female song and either migration or plumage dichromatism.
Overall the ancestral warbler is reconstructed to not have had singing females. This holds true using different coding schemes for the presence or absence of female song to account for differential research effort. Therefore, we think it much more likely that female song has been repeatedly gained in warblers. Even if we restrict our analysis to species well known to sing or duet, there is not just one origin of this behavior but rather multiple independent origins.
There are only two species branching early in parulid evolution that have reports of female song, the ovenbird (Seiurus aurocapilla) and the Louisiana waterthrush (Parkesia motacilla). Female song in both of these species is thought to be rare, and is likely aberrant in the ovenbird, an extremely well-studied species with only one report of a singing female (Hiatt, 1943). When rare reports of female song such as these are considered aberrant behavior, then the ancestor of all New World warblers does not reconstruct with female song. Therefore, we conclude that the ancestral warbler did not have frequent female song and we think it is very likely that it also did not have infrequent female song.
Prior studies reconstructing the ancestral state of female song in New World blackbirds (Icteridae) found song to be equivocal (Price, 2009). This seemed to be driven by the most basal ingroup Icterid clade, the meadowlarks and allies, which do not have female song. Given that Parulidae is the sister taxon to Icteridae, it now seems likely that the common ancestor of these groups did not have female song. However, it is also possible that the ancestor of all Icteridae (and possibly Parulidae) had female song and the loss of female song is derived in the meadowlark clade and also occurred relatively early in warbler evolution.
The only characters strongly correlated with each other in our analyses were migration and plumage dimorphism. This is in accordance with other studies of life history correlates of migration in parulids (Cardoso and Hu, 2011; Simpson et al., 2015) and other birds (Hamilton, 1961; Hofmann et al., 2008; Price, 2009; Price et al., 2009). There are two major (and not necessarily mutually exclusive) hypotheses attempting to explain widespread dichromatism in migratory species. Environmental factors, especially predation, may select for decreased plumage elaboration in migratory females since they are generally not defending territories on the breeding grounds (Badyaev and Hill, 2000). Alternatively, migratory species have less time to pair and breed, so changes in female plumage may be selected for reduced male aggression toward the female and to facilitate rapid pair-bonding (Hamilton, 1961). Generally, female dichromatic warblers are less colorful than males, but are usually not cryptically colored and may overall look very similar to males. Given this pattern, it may be possible that there is selection for reduced male aggression, but not for the cryptic plumage seen in other groups, such as blackbirds, where dichromatism is driven by losses of elaborate carotenoid plumage in females (Hofmann et al., 2008).
In our analyses the ancestral warbler is reconstructed to be a migrant. This is in agreement with a recent study of the Emberizoidea, which reconstructed the ancestral state of all warblers as north temperate breeders that migrate to the Neotropics (Winger et al., 2014). Migration is lost in the more recently derived Neotropical clades (Myiothlypis, Basileuterus, Cardellina, and Myioborus) and only regained twice (in the ancestor of the Cardellina group and in M. pictus). Incidentally, this finding is contrary to the long-held hypothesis that migration in birds evolves in the tropics as an escape from competition pressure (Levey and Stiles, 1992; Rappole, 1995; Rappole and Jones, 2003), particularly given the likely dispersal of the ancestor of the Emberizoidea (including warblers) into north America from Eurasia over Beringia (Barker et al., 2004).
When counting all 25 parulid species with reports of singing females as indeed having common female song, our analysis found that the evolution of female song is not correlated with losses or gains of migration in this group. Given that the ancestral warbler was likely migratory and had no female song, it's possible that female song is simply difficult to re-gain even if female song confers higher fitness in sedentary species. In a study comparing the evolution of duetting and migration, an ancestor that is both a duetter and a migrant was found to be five times more likely to lose duetting than to lose migration (Logue and Hall, 2014). In our study both migrants and non-migrants lose female song at rates 6–20 times higher than they gain it. Indeed, in all of our correlation analyses with female song, losses of this trait are more common than gains, regardless of migratory status or whether species are dichromatic. These patterns, coupled with the trait reconstructions suggest that female song is often gained independently, but is unstable and easily lost. Such losses are not correlated with migratory status or plumage dichromatism.
Sedentary species without female song may be in a fitness “valley” or on a relatively low fitness “peak” (Wright, 1982) that is difficult to get out of. Song was lost early in the evolution of warblers (possibly before they split from the blackbirds), so female song in warblers likely requires the reactivation of genes and physiological processes involved in song production. Females of many species can be induced to sing with the administration of testosterone (Kern and King, 1972; Nottebohm, 1980; Langmore, 1998) indicating the physiological machinery for song is often in place, but early developmental changes associated with testosterone production may be complicated and difficult to turn back on once lost.
For the purposes of understanding the evolution of female song, a characterization of territoriality in this clade might be informative. Species that are territorial year-round or that maintain a pair bond through the winter are exactly the sort of species we would predict to have female song (Benedict, 2008; Price, 2009). While most Neotropical migrants do not seem to be territorial in the winter, some, such as Leiothlypis peregrina (Birds of North America), hold winter territories, and are thus territorial year-round. Unfortunately, few data exist on these traits for most species of warblers, precluding more detailed analysis.
The ancestral warbler is reconstructed as being monochromatic in both separate analyses of carotenoid and melanin dichromatism, and is equivocal when reconstructing any dichromatism. We think it more likely that the ancestor was monochromatic given that extant species near the base of the warbler tree are dichromatic. Existing studies have shown that either monochromatism (Malacarne et al., 1991; Price, 2009) or dichromatism (Garamszegi et al., 2007) may be correlated with female song in birds. Our reconstruction indicates that parulid warblers underwent an early shift to dichromatism, but that gains of female song were not correlated with either losses or gains of melanin, carotenoid, or general dichromatism (Table 3). Female song appears much more labile than plumage characteristics as both transitions toward and away from female song were much larger than changes in plumage state (Figure 5B). Combined with other research, our study adds to the argument that there is no clear pattern of association between the presence of female song and dichromatism, suggesting that these traits may evolve relatively independently (Mason et al., 2014).
Our correlation analyses and character state reconstructions did not distinguish between monochromatic “dull” and monochromatic “ornamented” warblers. A recent study of the evolution of dichromatism in parulid warblers concluded that the common ancestor was monochromatic and bright (Simpson et al., 2015). Overall, our data suggest that dichromatism in this group is driven more by changes in melanin pigmentation than carotenoid pigmentation (often yellow) in females. Only two species were strongly dimorphic in carotenoid pigmentation, while 34 species were strongly dimorphic in melanin pigmentation. Whether the ancestor of warblers was ornamented or not is equivocal and should be the subject of future study.
In contrast with this pattern in parulids, plumage dichromatism in orioles (genus Icterus) is driven specifically by losses of bright plumage (both melanins and carotenoids) in females (Hofmann et al., 2008; Friedman et al., 2009). However, all male orioles are bright and/or saturated, so the ancestral state for male orioles is bright and relatively unchanging; thus it is relatively simple to interpret the directionality of dichromatism gains and losses. Regardless, monochromatic blackbird species tend to have female song and we found a similar (although statistically unsupported) trend in warbler species with common female song.
Among Parulid warblers, female song is not correlated with migratory status, melanin or carotenoid dichromatism, even though migration and plumage dichromatism are correlated with each other. Nearly all species that duet are sedentary and monochromatic, but the presence of dichromatic and migratory species with female song prevent any correlation of losses of song with losses of migration. This result counters our predictions based on similar studies and suggests that gains of female song may evolve due to different selective pressures than losses of female song. In contrast, the correlated evolution of migration and plumage dichromatism may indicate that coloration in this group evolves following many of the same pressures that other species (notably the Icterid blackbirds) face when adopting a migratory or sedentary strategy. Additionally, the different prevalence of female song in these two families may simply reflect the fact that blackbirds gained female song early in their radiation and warblers did not. If gains and losses of female song occur at different rates, then ancestral condition can set clades on very distinct evolutionary trajectories.
NN and LB wrote the paper and scored plumage characters. NN scored song and migration characters and ran analyses in Mesquite.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We'd like to thank Irby Lovette for providing us with the warbler phylogeny.nexus file, Dr. Mitchell McGlaughlin for help and advice about using Mesquite, and Nora Covy for scoring plumage characters.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2015.00139
ML, maximum likelihood.
Armenta, J. K., Dunn, P. O., and Whittingham, L. A. (2008). Quantifying avian sexual dichromatism: a comparison of methods. J. Exp. Biol. 211, 2423–2430. doi: 10.1242/jeb.013094
Badyaev, A. V., and Hill, G. E. (2000). Evolution of sexual dichromatism: contribution of carotenoid- versus melanin-based coloration. Biol. J. Linn. Soc. 69, 153–172. doi: 10.1111/j.1095-8312.2000.tb01196.x
Barker, F. K., Burns, K. J., Klicka, J., Lanyon, S. M., and Lovette, I. J. (2015). New insights into New World biogeography: an integrated view from the phylogeny of blackbirds, cardinals, sparrows, tanagers, warblers, and allies. Auk: Ornithol. Adv. 132, 333–348. doi: 10.1642/AUK-14-110.1
Barker, F. K., Cibois, A., Schikler, P., Feinstein, J., and Cracraft, J. (2004). Phylogeny and diversification of the largest avian radiation. Proc. Natl. Acad. Sci. U.S.A. 101, 11040–11045. doi: 10.1073/pnas.0401892101
Benedict, L. (2008). Occurrence and life history correlates of vocal duetting in North American passerines. J. Avian Biol. 39, 57–65. doi: 10.1111/j.0908-8857.2008.04103.x
Cardoso, G. C., and Hu, Y. (2011). Birdsong performance and the evolution of simple (rather than elaborate) sexual signals. Am. Nat. 178, 679–686. doi: 10.1086/662160
Catchpole, C. K., and Slater, P. J. (2008). Bird Song: Biological Themes and Variations. 2nd Edn. Cambridge: Cambridge University Press.
Del Hoyo, J., Elliott, A., and Christie, D. (2011). “Family parulidae,” in Handbook of the Birds of the World. Vol. 15. Weavers to New World Warblers, ed J. Curson (Lynx Edicions).
Friedman, N. R., Hofmann, C. M., Kondo, B., and Omland, K. E. (2009). Correlated evolution of migration and sexual dichromatism in the New World orioles (Icterus). Evolution 63, 3269–3274. doi: 10.1111/j.1558-5646.2009.00792.x
Garamszegi, L. Z., Pavlova, D. Z., Eens, M., and Møller, A. P. (2007). The evolution of song in female birds in Europe. Behav. Ecol. 18, 86–96. doi: 10.1093/beheco/arl047
Hall, M. L. (2004). A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. (Print). 55, 415–430. doi: 10.1007/s00265-003-0741-x
Hamilton, T. H. (1961). On the functions and causes of sexual dimorphism in breeding plumage characters of North American species of warblers and orioles. Am. Nat. 95, 121–123. doi: 10.1086/282167
Hofmann, C. M., Cronin, T. W., and Omland, K. E. (2008). Evolution of sexual dichromatism. 1: convergent losses of elaborate female coloration in New World orioles (Icterus spp.). Auk 124, 778–789. doi: 10.1525/auk.2008.07112
Jeschke, J. M., and Kokko, H. (2008). Mortality and other determinants of bird divorce rate. Behav. Ecol. Sociobiol. (Print). 63, 1–9. doi: 10.1007/s00265-008-0646-9
Kern, M. D., and King, J. R. (1972). Testosterone-induced singing in female white-crowned sparrows. Condor 74, 204–209. doi: 10.2307/1366289
Langmore, N. E. (1998). Functions of duet and solo songs of female birds. Trends Ecol. Evol. (Amst). 13, 136–140. doi: 10.1016/S0169-5347(97)01241-X
Levey, D. J., and Stiles, F. G. (1992). Evolutionary precursors of long-distance migration: resource availability and movement patterns in Neotropical landbirds. Am. Nat. 140, 447–476. doi: 10.1086/285421
Logue, D. M., and Hall, M. L. (2014). Migration and the evolution of duetting in songbirds. Proc. R. Soc. B 281:20140103. doi: 10.1098/rspb.2014.0103
Lovette, I. J., Pérez-Emán, J. L., Sullivan, J. P., Banks, R. C., Fiorentino, I., Córdoba-Córdoba, S., et al. (2010). A comprehensive multilocus phylogeny for the wood-warblers and a revised classification of the Parulidae (Aves). Mol. Phylogenet. Evol. 57, 753–770. doi: 10.1016/j.ympev.2010.07.018
Maddison, W. P., and Maddison, D. R. (2015). Mesquite: A Modular System for Evolutionary Analysis. Version 3.02. Available online at: http://mesquiteproject.org
Malacarne, G., Cucco, M., and Camanni, S. (1991). Coordinated visual displays and vocal duetting in different ecological situations among Western Palearctic non-passerine birds. Ethol. Ecol. Evol. 3, 207–219. doi: 10.1080/08927014.1991.9525369
Mason, N. A., Shultz, A. J., and Burns, K. J. (2014). Elaborate visual and acoustic signals evolve independently in a large, phenotypically diverse radiation of songbirds. Proc. R. Soc. B 281, 20140967. doi: 10.1098/rspb.2014.0967
Møller, A. P., and Birkhead, T. R. (1994). The evolution of plumage brightness in birds is related to extrapair paternity. Evolution 48, 1089–1100. doi: 10.2307/2410369
Morton, E. S. (1996). “A comparison of vocal behavior among tropical and temperate passerine birds,” in Ecology and Evolution of Acoustic Communication in Birds, eds D. E. Kroodsma and E. H. Miller (Ithaca, NY; London: Comstock Publishing Associates), 258–268.
Nottebohm, F. (1980). Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 189, 429–436. doi: 10.1016/0006-8993(80)90102-X
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5, 3379. doi: 10.1038/ncomms4379
Pagel, M. (1994). Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. B. 255, 37–45. doi: 10.1098/rspb.1994.0006
Price, J. J. (2009). Evolution and life-history correlates of female song in the New World blackbirds. Behav. Ecol. 20, 967–977. doi: 10.1093/beheco/arp085
Price, J. J., Lanyon, S. M., and Omland, K. E. (2009). Losses of female song with changes from tropical to temperate breeding in the New World blackbirds. Proc. R. Soc. B 276, 1971–1980. doi: 10.1098/rspb.2008.1626
Rappole, J. H., and Jones, P. (2003). Evolution of old and new world migration systems. Ardea 90, 525–537.
Simpson, R. K., Johnson, M. A., and Murphy, T. G. (2015). Migration and the evolution of sexual dichromatism: evolutionary loss of female coloration with migration among wood-warblers. Proc. R. Soc. B. 282, 20150375. doi: 10.1098/rspb.2015.0375
Slater, P. J., and Mann, N. I. (2004). Why do the females of many bird species sing in the tropics? J. Avian. Biol. 35, 289–294. doi: 10.1111/j.0908-8857.2004.03392.x
Spottiswoode, C., and Møller, A. P. (2004). Extrapair paternity, migration, and breeding synchrony in birds. Behav. Ecol. 15, 41–57. doi: 10.1093/beheco/arg100
Strong, E. E., and Lipscomb, D. (1999). Character coding and inapplicable data. Cladistics 15, 363–371. doi: 10.1111/j.1096-0031.1999.tb00272.x
Thorpe, R. S. (1984). Coding morphometric characters for constructing distance Wagner networks. Evolution 38, 244–255. doi: 10.2307/2408484
Wilkinson, M. (1995). A comparison of two methods of character construction. Cladistics 11, 297–308. doi: 10.1016/0748-3007(95)90017-9
Winger, B. M., Barker, F. K., and Ree, R. H. (2014). Temperate origins of long-distance seasonal migration in New World songbirds. Proc. Natl. Acad. Sci. U.S.A. 111, 12115–12120. doi: 10.1073/pnas.1405000111
Wright, S. (1982). The shifting balance theory and macroevolution. Ann. Rev. Genet. 16, 1–19. doi: 10.1146/annurev.ge.16.120182.000245
Keywords: female song, dichromatism, migration, correlated evolution, Parulidae
Citation: Najar N and Benedict L (2015) Female Song in New World Wood-Warblers (Parulidae). Front. Ecol. Evol. 3:139. doi: 10.3389/fevo.2015.00139
Received: 02 September 2015; Accepted: 26 November 2015;
Published: 17 December 2015.
Edited by:
Naomi Langmore, Australian National University, AustraliaReviewed by:
Gonçalo C. Cardoso, Universidade do Porto, PortugalCopyright © 2015 Najar and Benedict. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nadje Najar, bmFkamUubmFqYXJAdW5jby5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.