- Biocenter Grindel, Zoological Institute, University of Hamburg, Hamburg, Germany
Individuals of many species across the animal kingdom are found to be less plastic than expected, even in behavioral traits. The existence of consistent behavioral differences between individuals, termed “personality differences”, is puzzling, since plastic behavior is considered ideal to enable animals to adaptively respond to changes in environmental conditions. In order to elucidate which mechanisms are important for the evolution of personality differences, it is crucial to understand which aspects of the environment are important for the development of personality differences. Here, we tested whether physical or social aspects of the environment during development influence individual differentiation (mean level of behavior) using the jumping spider Marpissa muscosa. Furthermore, we assessed whether those behaviors were repeatable, i.e. whether personalities existed. We applied a split-brood design and raised spider siblings in three different environments: a deprived environment with no enrichment, a socially and a physically enriched environment. We focused on exploratory behavior and repeatedly assessed individual behavior in a novel environment and a novel object test. Results show that the environment during development influenced spiders' exploratory tendencies: spiders raised in enriched environments tended to be more exploratory. Most investigated behaviors were repeatable (i.e., personalities existed) across all individuals tested, whereas only few behaviors were also repeatable across individuals that had experienced the same environmental condition. Taken together, our results indicate that external stimuli can influence the development of one aspect of personality, the inter-individual variation (mean level of behavior), in a jumping spider. We also found family by environment interactions on behavioral traits potentially suggesting genetic variation in developmental plasticity.
Introduction
Consistent behavioral differences among individuals of the same population are widespread across various taxa in the animal kingdom (reviewed in e.g., Gosling, 2001; Bell et al., 2009; Kralj-Fišer and Schuett, 2014). This means individuals differ in their mean level of behavior (inter-individual behavioral variation) while being (more or less) consistent in their behavior over time and/or different contexts (intra-individual consistency). The existence of such personality differences is puzzling, given that it would seem sensible for individuals to be plastic and to adjust their behavior adaptively to changes in the environmental conditions (e.g., Sih et al., 2004).
Hypotheses that explain the adaptive value of animal personalities are linked to information use (McElreath and Strimling, 2006; Wolf et al., 2008), life-history (McElreath et al., 2007; Wolf et al., 2007), sexual selection (Schuett et al., 2010), and social interactions (McNamara et al., 2009) amongst others (Mangel, 1991; Dall et al., 2004; Nettle, 2006; Réale et al., 2007; Dingemanse and Wolf, 2010), yet empirical tests of these hypotheses remain scarce (but see e.g., Schuett et al., 2011b; Kralj-Fišer and Schneider, 2012; Nicolaus et al., 2012; Schuett et al., 2015). In order to understand the evolution of personality differences, it is also crucial to elucidate the development of personality differences. There is a general consensus that across species, on average about 30% of inter-individual variation in behavior (e.g., Stirling et al., 2002; van Oers et al., 2005; Quinn et al., 2009; van Oers and Sinn, 2011) and about 50% of personality variation is genetically inherited (Dochtermann et al., 2015), while the remaining variation originates from environmental sources (Buss and Greiling, 1999). In particular, environmental conditions experienced during early life may contribute to the development of personality differences by directing individuals into different life-history strategies and personalities (“early experiential calibration”, Buss and Greiling, 1999; see also Carere et al., 2005). It has been proposed that similar to life-history traits, personality traits can adjust within a genetically predetermined reaction norm (see e.g., Dingemanse et al., 2010; Groothuis and Trillmich, 2011). As for developmental behavioral plasticity in general, the potential for these plastic responses might be restricted to sensitive periods during ontogenesis (e.g., Groothuis and Trillmich, 2011; or “developmental windows”: Luttbeg and Sih, 2010; Faulk and Dolinoy, 2011), since changing an once adopted behavioral phenotype is associated with cost (reviewed in Snell-Rood, 2013). These processes can therefore lead to consistently different phenotypes even with similar genotypes (see Sih et al., 2004; Luttbeg and Sih, 2010) and these differences may be under frequency dependent selection (Lichtenstein and Pruitt, 2015).
To truly understand the evolution of personality differences, we need a comprehensive understanding of the specific environmental aspects shaping the development of personality differences (see Duckworth, 2010; Stamps and Groothuis, 2010). Previous studies have already shown developmental effects on mean behavioral levels such as social interactions (Iba et al., 1995; Arnold and Taborsky, 2010; Ballen et al., 2014; Liebgold, 2014), motor activity (Carducci and Jakob, 2000; Buchsbaum and Morse, 2012), or parental care (Margulis et al., 2005; Branchi et al., 2006). More studies are now desirable that investigate whether behavioral differences induced by developmental effects are consistent and stable, i.e., whether environmental conditions experienced influence animal personalities. Indeed, there is an increasing number of studies focusing on the development of animal personality (e.g., Sinn et al., 2008; Brodin, 2009; Schuett et al., 2011a; Gyuris et al., 2012; Hedrick and Kortet, 2012; Niemelä et al., 2012b; Petelle et al., 2013; Sweeney et al., 2013; Tremmel and Müller, 2013; Guenther et al., 2014; Johnson et al., 2015). To clearly identify underlying processes, experimental studies in which environmental conditions are manipulated are needed. The majority of studies that measured personality development in an experimental setting manipulated either food availability (e.g., Carere et al., 2005; Edenbrow and Croft, 2013), or stress by inducing immune challenge (e.g., Butler et al., 2012; DiRienzo et al., 2015), by increasing antipredator pressure (e.g., Bell and Sih, 2007; Niemelä et al., 2012a; Edenbrow and Croft, 2013), or by preventing access to shelter (Bengston et al., 2014).
Another aspect (potentially overlapping with above mentioned environmental aspects), which might influence the development of personality, is the complexity of the environment itself. Studies on animal intelligence have shown that increasing complexity in the social and/or in the physical environment induces behavioral and neural responses across different taxa (see e.g., Renner and Rosenzweig, 1987; Schrijver et al., 2004; Gonda et al., 2009; Brockmark et al., 2010; Kotrschal et al., 2012). This suggests that an increase in complexity directs animals to develop enhanced cognitive abilities allowing them to cope with increased information. Increased cognitive abilities (i.e., the ability to perceive and compute information) may itself lead to changes in individual behavior and life-history strategies (reviewed e.g., in Mettke-Hofmann, 2014; Trompf and Brown, 2014). Therefore, we assume that exploratory behavior, for example, should be generally positively linked to the amount of information (i.e., the complexity) available in the environment because knowledge of the environment allows behaving adaptively (at least up to a certain point; compare e.g., Niemelä et al., 2013). If, however, information gathering is potentially harmful individuals may show less exploratory tendencies. Such potentially harmful situations might be predation risk or risky interactions with conspecifics. To date, only few studies have investigated the effect of environmental complexity on personality either by increasing the social (Carere et al., 2005; DiRienzo et al., 2012) or the physical complexity (Bolhuis et al., 2005; Fox and Millam, 2007). Also, it remains unclear whether both aspects induce similar or different responses as these two aspects have rarely been manipulated in conjunction (but compare Carere et al., 2005; Bengston et al., 2014). A better understanding of these aspects is essential for elucidating which mechanisms are important for generating and maintaining personality.
In this study we investigated the effects of the social and the environmental complexity as well as genetic effects on the development of personality types using the jumping spider Marpissa muscosa. Jumping spiders are active hunters, have highly developed eyes and are sensitive to multiple aspects of their environment (Foelix, 2011). Therefore, we expect their personality development to be influenced by external stimuli (see for an example Royauté et al., 2014), including environmental complexity. Furthermore, we expect exploratory behavior to be a highly relevant behavior for jumping spiders because, among others, they need to search for prey, shelter, and mates. Carducci and Jakob (2000) showed indeed that jumping spiders reared in a physically enriched environment were on average more exploratory later in life. Here, we also added a social component to compare potential effects of the physical environment with effects of the social enrichment (see above).
We used a split-brood design and raised jumping spider siblings in three different environments: a deprived environment with no enrichment, a socially, and a physically enriched environment. This design allowed us to test for family effects, environmental effects, and their interaction on personality (mean level of behavior; behavioral repeatability within and among treatment groups) and plasticity. We repeatedly measured individual behavior in a novel environment and towards a novel object, and interpreted these as measures of exploratory behavior (see e.g., Réale et al., 2007). We predicted that enrichment, both physical and social, would lead to the development of more exploratory personalities (mean level of exploratory behavior) because information gathering in complex environments should be more advantageous than in less complex or deprived environments. However, we predicted that on average group living spiders might be less exploratory than physically enriched spiders due to the risk of harmful interactions with conspecifics. Even though M. muscosa are not considered social animals, they repeatedly interact with conspecifics in their natural environment (on and beneath the bark of trees). Furthermore, we assessed whether, beside those predicted effects on the mean behavioral level, behavior was also repeatable among and within treatment groups, i.e., whether personalities existed in the investigated traits. Finally, by presenting two different analytical approaches (i.e., analyzing repeatability over the whole data set vs. within each treatment separately) we want to highlight the possibility of obtaining different results when ignoring potential effects of developmental background on behavior. For example, the characteristics of the study area (from which individuals are sampled), such as the area's size, might influence the likelihood to detect personality differences: with increasing area the environmental heterogeneity often increases, too, and with it maybe also the potential of detecting (environmentally-induced) personality differences.
Methods
Rearing Conditions
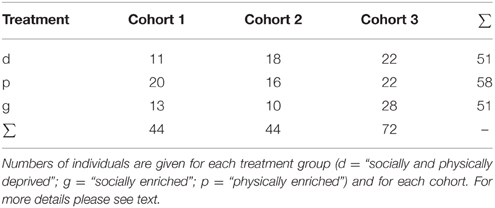
A total of 160 individuals of 14 maternal lines participated in the experiment. These were derived from three different cohorts and were assigned to one of three experimental groups (for details see below and Table 1).
In June–July 2012 we collected in total 18 adult and 17 subadult females and 18 males in northern Germany. Those females, which did not produce eggsacs in captivity (i.e., had probably not yet successfully mated in the field), were mated in the laboratory (by placing the female with a male in a box over night; males were used only once). Females were held solitary in plastic boxes (145 × 110 × 68 mm) enriched with some dry leaves, bark and white tissue paper. For the experiments we used spiderlings derived from the nine females, which were first to produce offspring. Eggsacs were separated from these females 2 weeks after they had been built to prevent any post-hatching maternal effects. After hatching juvenile siblings were assigned to one of three treatments pseudo-randomly (to ensure a balanced number of siblings in all treatments): a “deprived”, a “physically enriched”, or a “socially enriched” treatment.
In all three treatments, spiders were held in translucent plastic boxes with holes that were covered with blue gauze to ensure air circulation. We raised spiders in the “deprived” treatment (treatment: “d”) alone and without visual contact to conspecifics in boxes of 98 × 58 × 35 mm size. The bottom of the box was covered with white tissue paper and a small ball of the same material was included to give the spiders the opportunity to hide. Spiders in the “physically enriched” treatment (treatment: “p”) were raised alone and without visual contact to conspecifics in boxes of 145 × 110 × 68 mm size. These boxes were enriched with both natural and artificial objects [such as bark, Iceland moss (Cetraria islandica), dry leaves, orange colored cords, Lego©bricks, bottle caps]. We increased the degree of enrichment over the weeks until an age of 46 weeks (by which time most spiders had reached maturity) and we altered the arrangement of objects every other week. Also a wooden plateau was included to increase the surface and structure of the box. The bottom of the box was covered with white tissue paper. In the “socially enriched”, group treatment (treatment: “g”) siblings were held together in groups of five to 15 individuals in one box (mean ± SE = 8.1 ± 3.3). The actual number of individuals per group depended on the total clutch size from which the siblings were allocated to the treatments, i.e., only siblings from large clutches reached the maximum size of 15 group members. The size of the box was matched to the actual group size so that on average each spider had a surface area of roughly 222 cm2, which is similar to the area in the deprived treatment. The bottom of the box was covered with white tissue paper and a few paper balls were included to provide cover. In the socially enriched treatment, we separated spiders from their group when they reached subadulthood (at mean ± SE = 44 ± 8.4 weeks) to prevent uncontrolled matings. The new boxes had the same size and content as in treatment “d” but were put in close proximity to facilitate visual contact among conspecifics.
All animals were kept in the same laboratory room under constant conditions with a 17:7 h light:dark regime and temperatures between 22 and 24°C. Humidity was between 30 and 60% in the room (higher in boxes due to regular spraying into boxes). Depending on its age we fed each spider with 3–15 Drosophila spec. per week. Because spiders were held in groups in the social treatment the number of flies consumed by individual spiders might have varied. A total of five cannibalistic acts were observed in four out of twelve social groups. Every other week we monitored the developmental stage of each spider (juvenile, subadult, or mature) by inspecting the reproductive organs. At maturity the pedipalps of males are differentiated and turn dark and the epigyne of females becomes more pronounced and turns dark.
In 2012, we lost 56 of 142 spiderlings through unsuccessful molting or escapes (equally distributed across treatments: unsuccessful molting: GLM, χ2 = 0.745; p = 0.689; escapes: GLM, χ2 = 4.368; p = 0.113). To compensate for the reduction in sample size we also included individuals from family groups in which spiderlings had been raised together in a physically deprived environment for 2 months after hatching within larger groups (11–35 spiderlings per group). We pseudo-randomly assigned these spiders into the three treatments groups as described above. In the following we will refer to the original spiders as “cohort 1”, to the spiders that were included later to compensate for the loss of individuals as “cohort 2”.
In June 2013, we collected additional 23 adult, and presumably mated, females from the field. The offspring of five of those females were used to create cohort 3. These spiderlings were raised in similar ways to cohort 1 with some minor variations: we constantly provided small plastic tubes filled with wet cotton wool to prevent dehydration problems. Secondly, in the first week hatchlings received a sugar water drop in addition to the three flies. Finally, to prevent hatchlings from escaping their boxes (in the deprived treatment) they were held in plastic cylindrical containers (5.5 cm diameter). After 10 weeks they were transferred to the standard boxes described above for the deprived treatment.
Behavioral Tests
We tested all individuals twice each for their behavior in an open field and towards a novel object. In total, we recorded eight different behaviors during these tests of which seven were analyzed (see below; Table 2). Behavioral tests took place in a soundproof room with no windows between 16.07.2013 and 10.08.2013 for cohort 1 and 2 when spiderlings were 51.0 (± 0.85 SD) and 52.2 (± 1.9 SD) weeks of age, respectively. Spiders of cohort 3 were tested between 27.02.2014 and 26.03.2014 aged 35.1 (± 0.97 SD) weeks. All individuals were retested after 7 days to determine behavioral consistency. We tested three individuals simultaneously, if possible one from each (49%) or at least from two (40%) treatments. All spiders were tested in visual isolation from one another.
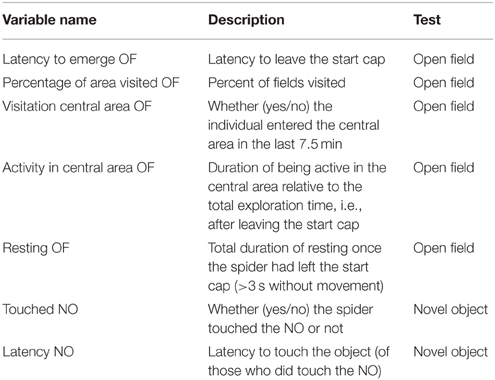
Table 2. Variables recorded from the open field (OF) and novel object (NO) test as measures of exploratory behavior.
The open-field test started after a 30 min acclimatization phase to the test room. In a similar approach to Carducci and Jakob (2000) we divided the arena (a plastic box 145 × 110 × 68 mm) into 30 small quadratic fields (2.80 × 2.90 cm) with a central and an edge area to quantify activity (see Figure 1). Acclimatization started after the spider was put into a white opaque plastic cap (5.5 cm diameter, 1.2 cm high, Figure 1). The cap was half-covered with gray plastic foil to generate cover for the spiders. The rationale was that the cap would function as a safe retreat that the spiders would only leave when motivated to explore the open field. Spiders were given a total of 60 min to climb out of the start cap and to explore the arena. If spiders did not leave the start cap we removed them from analyses for that trial (in the first trial: d: N = 3, g: N = 6, p: N = 4 and in the second trial: d: N = 3, g: N = 2, p: N = 2).
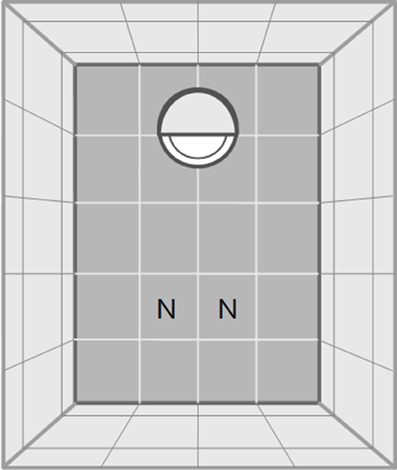
Figure 1. Schematic drawing of the test arena for both, the open field and the novel object test. Dark gray fields indicate ground, light gray fields indicate walls. In one end of the arena a white opaque plastic cap is shown which functioned as start point. The cap was half-covered with gray plastic foil to generate shelter for the spiders. The letters “N” indicate the two possible positions in which the novel object was introduced at the beginning of the novel object test. The drawing is not to scale.
After the open field test we transferred spiders back into the plastic cap, which was covered to prevent spiders from climbing out. A novel object (a greenish wooden barrel: 1.5 cm diameter, 1 cm high) was placed at the opposite end of the arena (Figure 1). After removal of the cover of the cap the spiders were allowed to explore the arena and the novel object for 30 min.
We videotaped the behavior and the experimenter (D.R.) left the room for the duration of the tests. After each test the arenas and novel objects were cleaned with water.
Video Analysis
All video clips were anonymized and randomized by a third person before being analyzed (by D.R.). For the open field test we analyzed the first 7.5 min and at minutes 22.5–30 of each trial (15 min total). The remaining minutes were not included in order to reduce time of analyzing. For the novel object test all 30 min were analyzed.
Data Analyses
All analyses were done using R 3.1.0 (R Core Team, 2014) except calculations using the R package “rptR” (Schielzeth and Nakagawa, 2011) for which we used R 2.15.1 (R Core Team, 2012) because this package was not yet implemented for latest R versions.
In order to explore whether different behavioral variables correlated with one another we ran Spearman rank correlations with data obtained from the first trial. To avoid duplication of results we excluded the total number of fields visited during the open field test (visits and revisits) which correlated strongly with the percentage of total area visited in the open field (“percentage of area visited OF”; rs = 0.606; p < 0.001). All other variable combinations correlated only moderately or less (rs < 0.42) and thus a total of seven variables were included in further analyses (see Table 2). We also ran a principal component analysis to reduce the number of variables. However, sufficient principal components together should account for 90 % of the total variation (Crawley, 2013). In our case this would have meant to use nearly as many components as original variables. We therefore only used the original variables which are easy to interpret and facilitate comparison with other studies.
To assess the influence of our treatments and cohorts on the behavioral level of individuals, we used several GEEs (general estimated equations); GEEs are extensions of GLMs and are a robust way for analyzing correlated data (here: data of individuals from the same family) and especially useful when comparing population averages (Liang and Zeger, 1986; Quinn and Keough, 2002; Zuur et al., 2009; Zhang et al., 2012). We used the R package “geepack” (Halekoh et al., 2006) to estimate the effects of rearing conditions (treatment and cohorts) on the population mean level of the in Table 2 mentioned seven behavioral variables obtained from the first test series. Thus, each individual contributed only one data point for these analyses. To account for potential family effects, we included the ID of the mother as cluster variable. In all models we included the two-way interactions between treatment and cohort and between treatment and sex as explanatory variables, as well as their main effects. We also included the variables “latency to emerge OF” and “latency to emerge NO”, respectively, in the analyses because we wanted to control for differences in the actual duration each individual had spent in the arena outside of the start cap. The “latency to emerge OF” was not included in the analysis of the variable “activity in central area OF” which is a relative estimate. Here, the variation in the time in the arena is already corrected for by different start times. Because many spiders did not touch the novel object (37 of 141) and thus were removed for estimations of the depending variable “latency NO” we excluded the factor “sex” in this analysis as not to overly decrease the sample size (the sex could not be determined for all individuals).
Prior to analysis we excluded missing data so that sample sizes vary for different analyses (see Table 3). If required, variables were transformed using the “powerTransform” function of the R package “car” (Fox and Weisberg, 2011) or adequate error structures were used to meet model assumptions (i.e., binomial error structure for binary data; see Table 3). Maximal models were simplified step-wise by taking each term out in turn, then excluding the least significant term at each step, starting with interactions first, given the removal of a term did not significantly reduce the explanatory power of the model (Crawley, 2002). We tested whether the explanatory power of the simpler model was significantly reduced compared to the more complex model using Wald statistics (Zuur et al., 2009). Model simplification was continued until the minimal model was found, i.e., the model which included only significant explanatory variables (or main effects which were included in significant interactions). P-values and associated test statistics given for non-significant terms come from the time a term dropped out of the model (see Table 3). When the rearing variables (treatment p, d, and g and cohorts 1, 2, and 3) were not included in significant interactions but had significant effects on the response variable, we checked for differences between the levels by merging factor levels (compare Crawley, 2002) and compared the explanatory power of the simpler and more complex model. P-values given come from these comparisons (see Table 4). Please note that we did not adjust p-values for multiple comparisons.
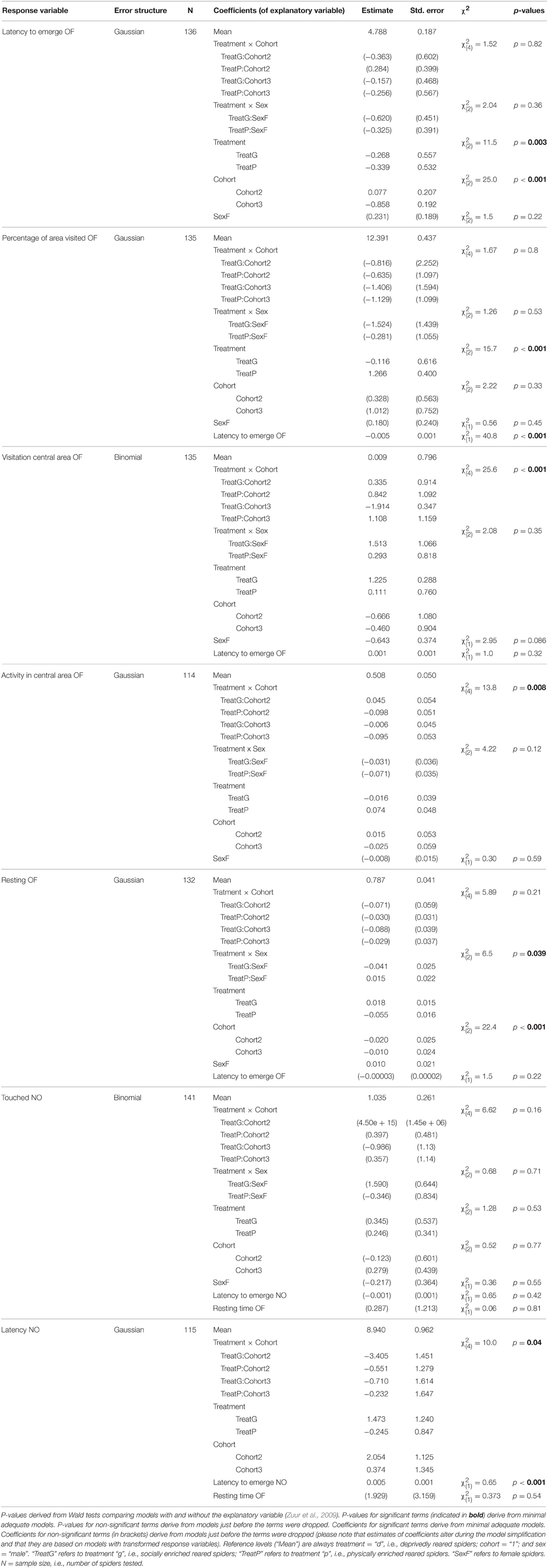
Table 3. Model outputs (GEEs) indicating effects on the mean level behavior shown in an open field test (OF) and a novel object test (NO).
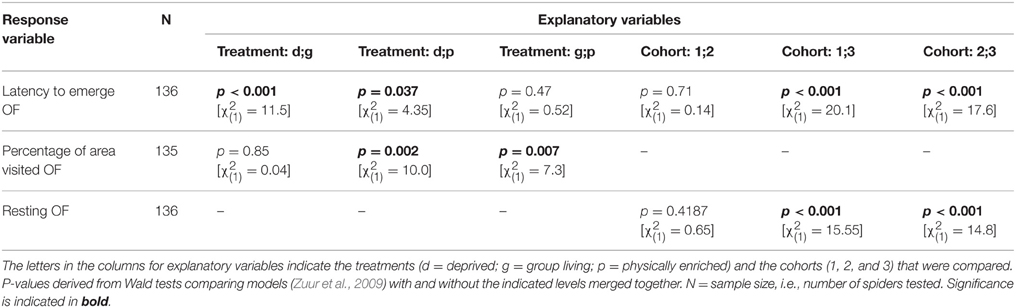
Table 4. Model outputs (GEEs) testing for behavioral differences among treatments or cohorts in an open field test (first trial).
To assess behavioral consistency we estimated behavioral repeatabilities and their 95% confidence intervals from generalized linear mixed effects models using R package “rptR” (with 1000 bootstraps and permutations; Nakagawa and Schielzeth, 2010). If confidence intervals did not include zero, repeatability was regarded as significant. We analyzed repeatability over the whole data set (Table 5) and within each treatment separately (Table 6). As above noted we did not adjust p-values for multiple comparisons. For further details on the specific models used, please see Tables 5, 6.
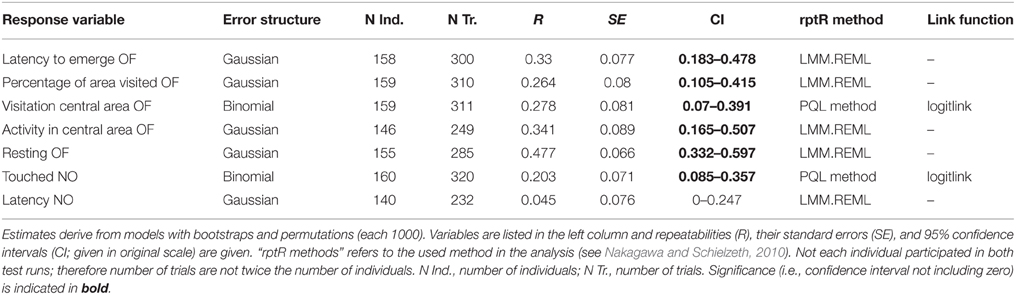
Table 5. Repeatabilities of behavior shown in the open field test (OF) and the novel object test (NO) over all individuals.
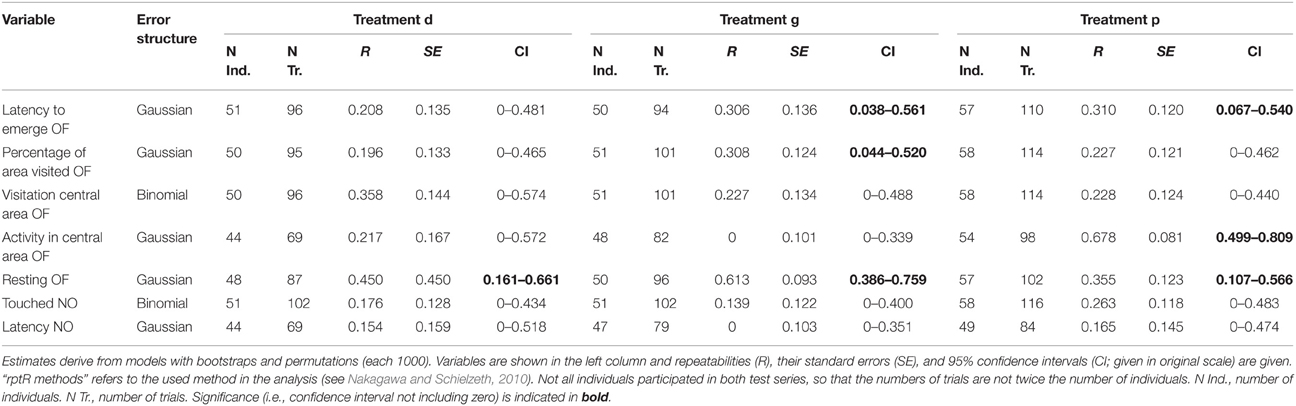
Table 6. Repeatabilities of behavior shown in the open field test (OF) and the novel object test (NO) tested separately for each treatment.
To test whether the degree of behavioral consistencies differed among treatment groups, we would have needed to test whether repeatability differed significantly among treatments. Yet, sample sizes within each treatment were rather low and many behavioral variables were not repeatable within each treatment group (see Table 6). Therefore, we only tested whether the population mean level of behavior differed among treatment groups and whether those behaviors were stable over all individuals (regardless of the environment they had experienced), i.e., whether measured behaviors are personality traits in the species.
In further analyses we investigated genotype by environment interactions. We used the maternal line as a proxy for genotype (but please note that individuals within a family were not genetically identical and that we cannot rule out pre-hatching maternal effects; we therefore use the term “family by environment” interaction). We fitted generalized linear models, GLMs, with our behavioral variables as responses and the interaction between maternal line and treatment as well as their main effects as explanatory variables. We included only families for which we had data from at least two individuals per treatment (total number of individuals per families and test ranged from 10 to 17 across treatments). Only data of the first round of behavioral tests were used in these analyses. In order to meet model assumptions, data were either transformed using the “powerTransform” function of the R package “car” (Fox and Weisberg, 2011) or adequate error structures were used (see above; for details see Table 7). Significance of interactions was tested with likelihood ratio tests comparing the model with and without this interaction (see Crawley, 2002).
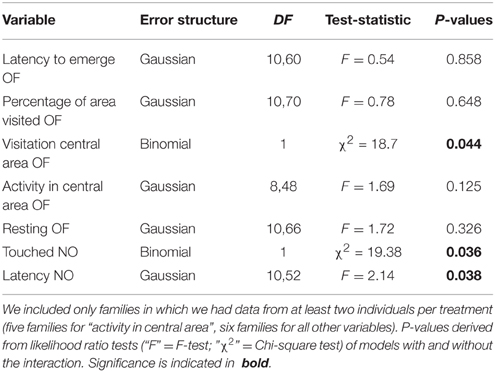
Table 7. Model outputs (GLMs) testing for family × environment interactions fitting an interaction between maternal line and treatment as explanatory variables on behavior shown in the open field test (OF) and the novel object test (NO).
Results
Early Environmental Effects on Inter-Individual Variation in Behavior (Mean Level Differences)
All behavioral variables were affected by the rearing condition with the exception of whether or not spiders touched the novel object (“touched NO”; Table 3). Spiders from the deprived treatment tended to be least exploratory: they needed longer to leave the start cap (“latency to emerge OF”) than spiders from the physically and socially enriched treatments in the open field test (Figure 2A; Table 4). Spiders from the physically enriched treatment visited more percent of the total area (“percentage of area visited OF”) than spiders from the other two treatments (Figure 2B; Table 4). There was a significant effect of treatment on resting duration (“resting OF”) depending on the sex of the individual with males resting less in the deprived and physically enriched treatments but more in the social treatment than females (Table 3). Furthermore, there were treatment effects on the likelihood for entering the central area (“visitation central area OF”), time spent active in the center (“activity in central area OF”), and in the latency to touch the novel object (“latency NO”) but different for the cohorts (cohort x treatment, Table 3). Finally, cohort 3 needed less time to climb out the start cap in the open field tests and rested less than spiders from the other two cohorts (Table 4).
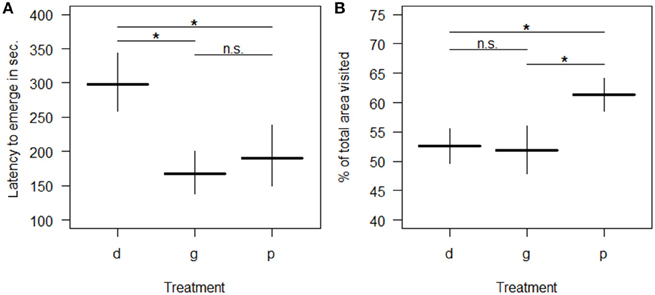
Figure 2. Predicted mean levels (± SE) of behavioral responses in an open field test by spiders raised in one of three different treatment groups (d = “socially and physically deprived”; g = “socially enriched”; p = “physically enriched”). Panel (A) shows the latency to emerge; Panel (B) shows the percentage of area visited. All predictions derive from general estimated equations models (GEEs) after stepwise reduction to minimal adequate model including only significant terms. “n.s.” indicates non-significant (p > 0.05) and “*” significant (p ≤ 0.05) differences between the mean levels of groups.
Repeatability
All behavioral measures were repeatable over time, except the latency to touch the novel object (“latency NO”; Table 5). The significant repeatabilities were moderate (0.203–0.447). However, when analyzed separately for each treatment, few behavioral variables remained significantly repeatable (Table 6): one in the deprived (“resting OF”) and three out of seven in the socially (“resting OF”; “latency to emerge OF”; “percentage of area visited OF”) and in the physically (“resting OF”; “latency to emerge OF”; “activity in central area OF”) enriched treatment. Furthermore, confidence intervals of most repeatability values overlapped greatly among treatment groups.
Family by Environment Interactions
Family by environment interactions were found on those three behavioral variables that were not repeatable in any of the three treatment groups, namely: whether or not spiders entered the central area of the open field, whether they touched the novel object, and the latency to do so (Table 7). The effects were not driven by single families (as seen from visual inspection of interaction plots and model estimates, not shown); yet, the exact patterns of these interactions are beyond the scope of the manuscript.
Discussion
The early environment in which spiders were raised significantly affected their exploratory tendencies (i.e., the population mean level of behavior). All but one behaviors measured were repeatable (at least over the whole study population), hence, we found evidence for personality differences. These findings combined indicate that external stimuli can influence the development of personality traits. We also found evidence for family by environmental interactions on behavioral traits. This means that families differed in their response to environmental conditions and suggests that families differed in their plasticity.
We found differences in the mean level of behaviors in our treatment groups, suggesting that the early environment influenced the development of exploratory behavior in the jumping spiders. In particular, individuals raised in the physically enriched treatment group were more exploratory than their siblings in the deprived treatment. This finding corroborates results from earlier studies on spiders (e.g., Carducci and Jakob, 2000; Buchsbaum and Morse, 2012; Bengston et al., 2014), nematodes (Rose et al., 2005), and vertebrates (e.g., Rosenzweig and Bennett, 1996; van Praag et al., 2000). Exploration, as an information-gathering process, might be more beneficial in an enriched (or generally more complex) than in a deprived (or generally very simple) environment with little to explore. Exploration can be costly (e.g., in terms of increased metabolism, or mortality risk) and thus individuals should not explore if not necessary. We found furthermore a sex-dependent treatment effect on the resting duration with group living males resting more than solitarily reared ones. Sexual size dimorphism is associated with a risk of cannibalism by the larger females (Wilder and Rypstra, 2008; Liedtke, J., personal observation), which may suggest that group living males are less active and thereby reduce encounter rates with females (compare sex-reversed pattern found in mice offspring: Heiming et al., 2009; and Hedrick and Kortet, 2012, for sex-dependent consistency over metamorphosis). Accordingly, a plastic response to the (early) environmental condition that an individual experiences seems sensible. Indeed, external influences particularly during development might have long lasting effects (reviewed in e.g., Snell-Rood, 2013).
The different responses of the three cohorts in our experiment may be an indication for sensitive phases (e.g., Groothuis and Trillmich, 2011) or “developmental-windows” (Luttbeg and Sih, 2010; Faulk and Dolinoy, 2011) within the developmental process of personality differences. The cohorts experienced different experimental conditions: in contrast to spiders from cohort 1 and 3, spiders from the cohort 2 were raised in groups for the first 2 months before they were assigned to the three treatments. Therefore, this cohort received an early social enrichment, regardless of later treatment. Results show that individuals from cohort 2 differed from the other two cohorts in several behaviors. Although it is difficult to explain the direction of these effects, these results indicate that, at least for the social enrichment, environmental conditions encountered in the first 2 months seem to have long lasting effects (permanent environmental effects sensu Dochtermann et al., 2015) on the development of behavioral tendencies. These patterns deserve further attention by follow-up studies in order to understand the proximate mechanisms of these apparently sensitive periods and if such effects can be induced by manipulation of the physical environment as well.
Group living also had positive effects on exploratory behavior in non-social contexts. This is in contrast to previous studies showing no effects of group living on behavior in non-social tests (reviewed in Taborsky et al., 2012). Yet, other studies found impairments of social isolation in multiple aspects of behavior (reviewed in e.g., Ballen et al., 2014). Hence, at least in some species contact to conspecifics can induce stable behavioral differences in other than the social realm. This suggests that early environment conditions can create behavioral differences in a context-general way.
Noteworthy, we found significant family by environment interactions on three of the investigated behavioral variables. This potentially indicates genetic variation for plasticity and suggests that plasticity itself might be under natural selection (Pigliucci, 2005; Dingemanse et al., 2010). Whether higher or lower plasticity is favored might depend on how stable and predictable environmental conditions are over time, with more stable conditions potentially favoring lower plasticity (see e.g., Dingemanse et al., 2010; Snell-Rood, 2013). But please note that we cannot rule out pre-hatching maternal effects in our study. Further studies are required to provide more insights, especially studies in which the paternity is also known.
Five behaviors that were repeatable over the whole population were not repeatable in all subpopulations (i.e., treatment groups) when estimated separately. Also, most confidence intervals of repeatabilities overlapped among treatment groups, suggesting repeatability was not necessarily significantly different among groups. Therefore, the extent of repeatability was likely not induced by the environmental conditions experienced. The pattern, that behaviors were repeatable across all individuals but not within all treatment groups, could potentially arise if between-individual variation in behavior within treatment groups is rather low (compared to between-individual variation across treatments) and/or if within-individual consistency in behavior is low. In both behavioral tests the average response of the deprived group was lower than that of the two enriched treatment groups, thereby leading to mean-level consistency (i.e., consistent differences between the average responses of each group; sensu Stamps and Groothuis, 2010). These consistent differences between treatment groups may explain why we found significant effects when we tested for repeatability over the whole population. The behavioral consistency of individual spiders within each treatment, on the other hand, may have been rather low, so that we found behavioral repeatability in fewer variables when treatments were assessed separately. This may indicate that for these variables, repeatability is mostly an effect of environmental induction by divergently shifting the mean level of each subpopulation (i.e., deprived group toward lower vs. enriched groups toward higher exploratory tendencies). Yet, these interpretations should be viewed with caution, since the absence of repeatable behavior within treatments in the five variables mentioned above could alternatively be an artifact of lower sample sizes within than among treatments. However, sample sizes in each subpopulation were still decent (≥44; see Table 6) indicating that these patterns might be biologically relevant and deserve attention in further studies. For example, studies using samples derived from larger study areas may be more likely to find repeatability even with relative low individual stability because they might include individuals with different environmental backgrounds.
Nevertheless, we also should bear in mind that environmental induction does not necessarily lead to differential consistency but could even lead to the opposite. If individuals have different genotypes they may have different innate levels in behavioral expressions. However, plasticity, i.e., the ability to respond sensitively to the environment, could lead to an approximation of these initial differences according to local conditions. Furthermore, we expect that, with plasticity being costly (see e.g., Dall et al., 2004; Pigliucci, 2005), individuals having an innate behavioral level closer to the local optimum to have an improved fitness (all other things being equal) because they need less modification in their responses. This implies that mean level should be under selection which may explain the differences between families in this study.
Taken together, results found in this study indicate that exploratory tendencies of M. muscosa are influenced by the environmental conditions experienced; families may differ in plasticity and thus provide the raw material for natural selection to act upon; and finally, observed patterns of personality distribution found in the field may be crucially influenced by plastic responses of sensitive systems.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Tomma Dirks, Ralf Mistera, and Angelika Taebel-Hellwig for building home boxes for the animals and Svenja Lund for assistance with spider-maintenance.
References
Arnold, C., and Taborsky, B. (2010). Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim. Behav. 79, 621–630. doi: 10.1016/j.anbehav.2009.12.008
Ballen, C., Shine, R., and Olsson, M. (2014). Effects of early social isolation on the behaviour and performance of juvenile lizards, Chamaeleo calyptratus. Anim. Behav. 88, 1–6. doi: 10.1016/j.anbehav.2013.11.010
Bell, A. M., Hankison, S. J., and Laskowski, K. L. (2009). The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. doi: 10.1016/j.anbehav.2008.12.022
Bell, A. M., and Sih, A. (2007). Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828–834. doi: 10.1111/j.1461-0248.2007.01081.x
Bengston, S. E., Pruitt, J. N., and Riechert, S. E. (2014). Differences in environmental enrichment generate contrasting behavioural syndromes in a basal spider lineage. Anim. Behav. 93, 105–110. doi: 10.1016/j.anbehav.2014.04.022
Bolhuis, J. E., Schouten, W. G. P., Schrama, J. W., and Wiegant, V. M. (2005). Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Appl. Anim. Behav. Sci. 93, 213–228. doi: 10.1016/j.applanim.2005.01.006
Branchi, I., D'Andrea, I., Fiore, M., Di Fausto, V., Aloe, L., and Alleva, E. (2006). Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry 60, 690–696. doi: 10.1016/j.biopsych.2006.01.005
Brockmark, S., Adriaenssens, B., and Johnsson, J. I. (2010). Less is more: density influences the development of behavioural life skills in trout. Proc. R. Soc. B Biol. Sci. 277, 3035–3043. doi: 10.1098/rspb.2010.0561
Brodin, T. (2009). Behavioral syndrome over the boundaries of life-carryovers from larvae to adult damselfly. Behav. Ecol. 20, 30–37. doi: 10.1093/beheco/arn111
Buchsbaum, D., and Morse, D. H. (2012). The effect of experience and rearing environment on the behaviour of crab spiderlings during their first weeks of life. Behaviour 149, 667–683. doi: 10.1163/156853912X649939
Buss, D. M., and Greiling, H. (1999). Adaptive individual differences. J. Pers. 67, 209–243. doi: 10.1111/1467-6494.00053
Butler, M. W., Toomey, M. B., McGraw, K. J., and Rowe, M. (2012). Ontogenetic immune challenges shape adult personality in mallard ducks. Proc. R. Soc. B Biol. Sci. 279, 326–333. doi: 10.1098/rspb.2011.0842
Carducci, J. P., and Jakob, E. M. (2000). Rearing environment affects behaviour of jumping spiders. Anim. Behav. 59, 39–46. doi: 10.1006/anbe.1999.1282
Carere, C., Drent, P. J., Koolhaas, J. M., and Groothuis, T. G. G. (2005). Epigenetic effects on personality traits: early food provisioning and sibling competition. Behaviour 142, 1329–1355. doi: 10.1163/156853905774539328
Crawley, M. J. (2002). Statistical Computing - An Introduction to Data Analysis using S-Plus. Chichester: John Wiley and Sons Ltd.
Dall, S. R. X., Houston, A. I., and McNamara, J. M. (2004). The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739. doi: 10.1111/j.1461-0248.2004.00618.x
Dingemanse, N. J., Kazem, A. J. N., Réale, D., and Wright, J. (2010). Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. doi: 10.1016/j.tree.2009.07.013
Dingemanse, N. J., and Wolf, M. (2010). Recent models for adaptive personality differences: a review. Philos. Trans. R. Soc. B Biol. Sci. 365, 3947–3958. doi: 10.1098/rstb.2010.0221
DiRienzo, N., Niemelä, P. T., Skog, A., Vainikka, A., and Kortet, R. (2015). Juvenile pathogen exposure affects the presence of personality in adult field crickets. Front. Ecol. Evol. 3:36. doi: 10.3389/fevo.2015.00036
DiRienzo, N., Pruitt, J. N., and Hedrick, A. V. (2012). Juvenile exposure to acoustic sexual signals from conspecifics alters growth trajectory and an adult personality trait. Anim. Behav. 84, 861–868. doi: 10.1016/j.anbehav.2012.07.007
Dochtermann, N. A., Schwab, T., and Sih, A. (2015). The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B Biol. Sci. 282, 20142201. doi: 10.1098/rspb.2014.2201
Duckworth, R. A. (2010). Evolution of personality: developmental constraints on behavioral flexibility. Auk 127, 752–758. doi: 10.1525/auk.2010.127.4.752
Edenbrow, M., and Croft, D. P. (2013). Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus. Oikos 122, 667–681. doi: 10.1111/j.1600-0706.2012.20556.x
Faulk, C., and Dolinoy, D. C. (2011). Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics 6, 791–797. doi: 10.4161/epi.6.7.16209
Fox, J., and Weisberg, S. (2011). An {R} Companion to Applied Regression, 2nd Edn. Thousand Oaks, CA.
Fox, R. A., and Millam, J. R. (2007). Novelty and individual differences influence neophobia in orange-winged Amazon parrots (Amazona amazonica). Appl. Anim. Behav. Sci. 104, 107–115. doi: 10.1016/j.applanim.2006.04.033
Gonda, A., Herczeg, G., and Merilä, J. (2009). Habitat-dependent and -independent plastic responses to social environment in the nine-spined stickleback (Pungitius pungitius) brain. Proc. R. Soc. B Biol. Sci. 276, 2085–2092. doi: 10.1098/rspb.2009.0026
Gosling, S. D. (2001). From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127, 45–86. doi: 10.1037/0033-2909.127.1.45
Groothuis, T. G. G., and Trillmich, F. (2011). Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641–655. doi: 10.1002/dev.20574
Guenther, A., Finkemeier, M. A., and Trillmich, F. (2014). The ontogeny of personality in the wild guinea pig. Anim. Behav. 90, 131–139. doi: 10.1016/j.anbehav.2014.01.032
Gyuris, E., Feró, O., and Barta, Z. (2012). Personality traits across ontogeny in firebugs, Pyrrhocoris apterus. Anim. Behav. 84, 103–109. doi: 10.1016/j.anbehav.2012.04.014
Halekoh, U., Hojsgaard, S., and Yan, J. (2006). The R Package geepack for generalized estimating equations. J. Stat. Softw. 15, 1–11. doi: 10.18637/jss.v015.i02
Hedrick, A. V., and Kortet, R. (2012). Sex differences in the repeatability of boldness over metamorphosis. Behav. Ecol. Sociobiol. 66, 407–412. doi: 10.1007/s00265-011-1286-z
Heiming, R. S., Jansen, F., Lewejohann, L., Kaiser, S., Schmitt, A., Lesch, K. P., et al. (2009). Living in a dangerous world: the shaping of behavioral profile by early environment and 5-HTT genotype. Front. Behav. Neurosci. 3:26. doi: 10.3389/neuro.08.026.2009
Iba, M., Nagao, T., and Urano, A. (1995). Effects of population density on growth, behavior and levels of biogenic amines in the cricket, Gryllus bimaculatus. Zool. Sci. 12, 695–702. doi: 10.2108/zsj.12.695
Johnson, J. C., Halpin, R., Stevens, D., Vannan, A., Lam, J., and Bratsch, K. (2015). Individual variation in ballooning dispersal by black widow spiderlings: The effects of family and social rearing. Curr. Zool. 61, 520–528.
Kotrschal, A., Rogell, B., Maklakov, A. A., and Kolm, N. (2012). Sex-specific plasticity in brain morphology depends on social environment of the guppy, Poecilia reticulata. Behav. Ecol. Sociobiol. 66, 1485–1492. doi: 10.1007/s00265-012-1403-7
Kralj-Fišer, S., and Schneider, J. M. (2012). Individual behavioural consistency and plasticity in an urban spider. Anim. Behav. 84, 197–204. doi: 10.1016/j.anbehav.2012.04.032
Kralj-Fišer, S., and Schuett, W. (2014). Studying personality variation in invertebrates: why bother? Anim. Behav. 91, 41–52. doi: 10.1016/j.anbehav.2014.02.016
Liang, K. Y., and Zeger, S. L. (1986). Longitudinal data-analysis using generalized linear-models. Biometrika 73, 13–22. doi: 10.1093/biomet/73.1.13
Lichtenstein, J. L. L., and Pruitt, J. N. (2015). Similar patterns of frequency-dependent selection on animal personalities emerge in three species of social spiders. J. Evol. Biol. 28, 1248–1256. doi: 10.1111/jeb.12651
Liebgold, E. B. (2014). The influence of social environment: behavior of unrelated adults affects future juvenile behaviors. Ethology 120, 388–399. doi: 10.1111/eth.12214
Luttbeg, B., and Sih, A. (2010). Risk, resources and state-dependent adaptive behavioural syndromes. Philos. Trans. R. Soc. B Biol. Sci. 365, 3977–3990. doi: 10.1098/rstb.2010.0207
Mangel, M. (1991). Adaptive walks on behavioral landscapes and the evolution of optimal behavior by natural-selection. Evol. Ecol. 5, 30–39. doi: 10.1007/BF02285243
Margulis, S. W., Nabong, M., Alaks, G., Walsh, A., and Lacy, R. C. (2005). Effects of early experience on subsequent parental behaviour and reproductive success in oldfield mice, Peromyscus polionotus. Anim. Behav. 69, 627–634. doi: 10.1016/j.anbehav.2004.04.021
McElreath, R., Luttbeg, B., Fogarty, S. P., Brodin, T., and Sih, A. (2007). Evolution of animal personalities. Nature 450, E5. doi: 10.1038/nature06326
McElreath, R., and Strimling, P. (2006). How noisy information and individual asymmetries can make ‘personality’ an adaptation: a simple model. Anim. Behav. 72, 1135–1139. doi: 10.1016/j.anbehav.2006.04.001
McNamara, J. M., Stephens, P. A., Dall, S. R. X., and Houston, A. I. (2009). Evolution of trust and trustworthiness: social awareness favours personality differences. Proc. R. Soc. B Biol. Sci. 276, 605–613. doi: 10.1098/rspb.2008.1182
Mettke-Hofmann, C. (2014). Cognitive ecology: ecological factors, life-styles, and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5, 345–360. doi: 10.1002/wcs.1289
Nakagawa, S., and Schielzeth, H. (2010). Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev. 85, 935–956. doi: 10.1111/j.1469-185x.2010.00141.x
Nettle, D. (2006). The evolution of personality variation in humans and other animals. Am. Psychol. 61, 622–631. doi: 10.1037/0003-066X.61.6.622
Nicolaus, M., Tinbergen, J. M., Bouwman, K. M., Michler, S. P. M., Ubels, R., Both, C., et al. (2012). Experimental evidence for adaptive personalities in a wild passerine bird. Proc. R. Soc. B Biol. Sci. 279, 4885–4892. doi: 10.1098/rspb.2012.1936
Niemelä, P. T., DiRienzo, N., and Hedrick, A. V. (2012a). Predator-induced changes in the boldness of naïve field crickets, Gryllus integer, depends on behavioural type. Anim. Behav. 84, 129–135. doi: 10.1016/j.anbehav.2012.04.019
Niemelä, P. T., Vainikka, A., Forsman, J. T., Loukola, O. J., and Kortet, R. (2013). How does variation in the environment and individual cognition explain the existence of consistent behavioral differences? Ecol. Evol. 3, 457–464. doi: 10.1002/ece3.451
Niemelä, P. T., Vainikka, A., Hedrick, A. V., and Kortet, R. (2012b). Integrating behaviour with life history: boldness of the field cricket, Gryllus integer, during ontogeny. Funct. Ecol. 26, 450–456. doi: 10.1111/j.1365-2435.2011.01939.x
Petelle, M. B., McCoy, D. E., Alejandro, V., Martin, J. G. A., and Blumstein, D. T. (2013). Development of boldness and docility in yellow-bellied marmots. Anim. Behav. 86, 1147–1154. doi: 10.1016/j.anbehav.2013.09.016
Pigliucci, M. (2005). Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486. doi: 10.1016/j.tree.2005.06.001
Quinn, G. G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press.
Quinn, J. L., Patrick, S. C., Bouwhuis, S., Wilkin, T. A., and Sheldon, B. C. (2009). Heterogeneous selection on a heritable temperament trait in a variable environment. J. Anim. Ecol. 78, 1203–1215. doi: 10.1111/j.1365-2656.2009.01585.x
R Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., and Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Renner, M. J., and Rosenzweig, M. R. (1987). The golden-mantled ground squirrel (Spermophilus lateralis) as a model for the effects of environmental enrichment in solitary animals. Dev. Psychobiol. 20, 19–24. doi: 10.1002/dev.420200106
Rose, J. K., Sangha, S., Rai, S., Norman, K. R., and Rankin, C. H. (2005). Decreased sensory stimulation reduces behavioral responding, retards development, and alters neuronal connectivity in Caenorhabditis elegans. J. Neurosci. 25, 7159–7168. doi: 10.1523/JNEUROSCI.1833-05.2005
Rosenzweig, M. R., and Bennett, E. L. (1996). Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65. doi: 10.1016/0166-4328(95)00216-2
Royauté, R., Buddle, C. M., and Vincent, C. (2014). Interpopulation variations in behavioral syndromes of a jumping spider from insecticide-treated and insecticide-free orchards. Ethology 120, 127–139. doi: 10.1111/eth.12185
Schielzeth, H., and Nakagawa, S. (2011). rptR: Repeatability for Gaussian and Non-Gaussian Data. R Package Version 0.6.404/r44. ed.
Schrijver, N. C. A., Pallier, P. N., Brown, V. J., and Würbel, H. (2004). Double dissociation of social and environmental stimulation on spatial learning and reversal learning in rats. Behav. Brain Res. 152, 307–314. doi: 10.1016/j.bbr.2003.10.016
Schuett, W., Dall, S. R. X., Baeumer, J., Kloesener, M. H., Nakagawa, S., Beinlich, F., et al. (2011a). “Personality” variation in a clonal insect: the pea aphid, Acyrthosiphon pisum. Dev. Psychobiol. 53, 631–640. doi: 10.1002/dev.20538
Schuett, W., Dall, S. R. X., Kloesener, M. H., Baeumer, J., Beinlich, F., and Eggers, T. (2015). Life-history trade-offs mediate ‘personality’ variation in two colour morphs of the pea aphid, Acyrthosiphon pisum. J. Anim. Ecol. 84, 90–101. doi: 10.1111/1365-2656.12263
Schuett, W., Godin, J.-G. J., and Dall, S. R. X. (2011b). Do female zebra finches, Taeniopygia guttata, choose their mates based on their ‘personality’? Ethology 117, 908–917. doi: 10.1111/j.1439-0310.2011.01945.x
Schuett, W., Tregenza, T., and Dall, S. R. X. (2010). Sexual selection and animal personality. Biol. Rev. 85, 217–246. doi: 10.1111/j.1469-185X.2009.00101.x
Sih, A., Bell, A. M., Johnson, J. C., and Ziemba, R. E. (2004). Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. doi: 10.1086/422893
Sinn, D. L., Gosling, S. D., and Moltschaniwskyj, N. A. (2008). Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 433–442. doi: 10.1016/j.anbehav.2007.05.008
Snell-Rood, E. C. (2013). An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. doi: 10.1016/j.anbehav.2012.12.031
Stamps, J., and Groothuis, T. G. G. (2010). The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301–325. doi: 10.1111/j.1469-185X.2009.00103.x
Stirling, D. G., Réale, D., and Roff, D. A. (2002). Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289. doi: 10.1046/j.1420-9101.2002.00389.x
Sweeney, K., Gadd, R. D. H., Hess, Z. L., McDermott, D. R., MacDonald, L., Cotter, P., et al. (2013). Assessing the effects of rearing environment, natural selection, and developmental stage on the emergence of a behavioral syndrome. Ethology 119, 436–447. doi: 10.1111/eth.12081
Taborsky, B., Arnold, C., Junker, J., and Tschopp, A. (2012). The early social environment affects social competence in a cooperative breeder. Anim. Behav. 83, 1067–1074. doi: 10.1016/j.anbehav.2012.01.037
Tremmel, M., and Müller, C. (2013). Insect personality depends on environmental conditions. Behav. Ecol. 24, 386–392. doi: 10.1093/beheco/ars175
Trompf, L., and Brown, C. (2014). Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim. Behav. 88, 99–106. doi: 10.1016/j.anbehav.2013.11.022
van Oers, K., de Jong, G., Van Noordwijk, A. J., Kempenaers, B., and Drent, P. J. (2005). Contribution of genetics to the study of animal personalities: a review of case studies. Behaviour 142, 1185–1206. doi: 10.1163/156853905774539364
van Oers, K., and Sinn, D. L. (2011). “Toward a basis for the phenotypic gambit: advances in the evolutionary genetics of animal personality,” in From Genes to Animal Behavior: Social Structures, Personalities, Communication by Color, eds M. Inouemurayama, S. Kawamura, and A. Weiss (Tokyo: Springer-Verlag Tokyo), 165–183.
van Praag, H., Kempermann, G., and Gage, F. H. (2000). Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198. doi: 10.1038/35044558
Wilder, S. M., and Rypstra, A. L. (2008). Sexual size dimorphism predicts the frequency of sexual cannibalism within and among species of spiders. Am. Nat. 172, 431–440. doi: 10.1086/589518
Wolf, M., van Doorn, G. S., Leimar, O., and Weissing, F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. doi: 10.1038/nature05835
Wolf, M., van Doorn, G. S., and Weissing, F. J. (2008). Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl. Acad. Sci. U.S.A. 105, 15825–15830. doi: 10.1073/pnas.0805473105
Zhang, H., Yu, Q., Feng, C., Gunzler, D., Wu, P., and Tu, X. M. (2012). A new look at the difference between the GEE and the GLMM when modeling longitudinal count responses. J. Appl. Stat. 39, 2067–2079. doi: 10.1080/02664763.2012.700452
Keywords: animal personality, arachnids, arthropod, behavioral syndromes, exploration, rearing, salticid, temperament
Citation: Liedtke J, Redekop D, Schneider JM and Schuett W (2015) Early Environmental Conditions Shape Personality Types in a Jumping Spider. Front. Ecol. Evol. 3:134. doi: 10.3389/fevo.2015.00134
Received: 06 July 2015; Accepted: 16 November 2015;
Published: 22 December 2015.
Edited by:
Ann Valerie Hedrick, University of California, Davis, USAReviewed by:
Nicholas DiRienzo, University of California, Davis, USARaphaël Royauté, North Dakota State University, USA
Copyright © 2015 Liedtke, Redekop, Schneider and Schuett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wiebke Schuett, d2llYmtlc2NoQGdvb2dsZW1haWwuY29t
 Jannis Liedtke
Jannis Liedtke Daniel Redekop
Daniel Redekop Jutta M. Schneider
Jutta M. Schneider Wiebke Schuett
Wiebke Schuett