- Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway
Seminal fluids are known to have a variety of effects on rival sperm, but in externally fertilizing species it is still unclear what effects seminal fluid can induce under sperm competition. We recorded sperm activity from natural ejaculates (including own seminal fluid) of an external fertilizer, the Arctic charr (Salvelinus alpinus), after activation either in water (the natural medium for milt dilution), in a dilution of water and own seminal fluid or in a dilution of water and seminal fluid of a foreign male. When activation occurred in own or foreign dilutions of seminal fluids, sperm maintained higher velocities than when activated in water only. Yet, velocity did not differ depending on whether sperm was activated in own or foreign seminal fluid solutions. More important, approximately 25% fewer sperm cells were initially activated in own seminal fluid than in foreign seminal fluid or water, indicating that activation is under close control of own seminal fluid only. Our results document that the presence of foreign seminal fluid under sperm competition do not have apparent effect on sperm velocity. Yet, the large inhibitory effect on initial activation of sperm cells seen in own, but not in foreign dilutions of seminal fluids (and water) suggests an individual specific recognition mechanism exerted by something in the seminal fluid on own, but not foreign sperm cells. The importance of this extrasomatic sperm recognition for the outcome of sperm competitions is discussed.
Introduction
Species experiencing sperm competition, i.e., competition between ejaculates of two or more males for the fertilization of a limited number of ova (Parker, 1970) may be selected to adjust reproductive behavior, morphology, physiology, and ejaculate characteristics according to the likelihood and intensity of such competition (Møller and Birkhead, 1989; Parker, 1990; Simmons, 2001; Parker and Pizzari, 2010). An important focus for the bulk of studies addressing evolutionary effects of sperm competition has been on adjustments of sperm numbers, sperm motilities, and velocities, an observational bias that may partly have been fuelled by the development of appropriate tools for sperm observations, i.e., digital sperm trackers. Yet, a recent shift to also include the effects of the ejaculates' non-sperm component, which may represent a substantial portion of the ejaculate investment, has produced research showing a wide variety of effects from seminal fluids and its components on both own and competitor ejaculates including prolonged survival of own sperm and, although controversial, incapacitation of rival sperm (for reviews, see: Poiani, 2006; Simmons and Fitzpatrick, 2012; Perry et al., 2013).
Under sperm competition, sperm cells, and seminal fluid of several males may be mixed either in a female controlled environment or, for external fertilizers, in an external environment not entirely under female control. The current knowledge on the composition and effects of seminal fluids stems mainly from studies of vertebrates (i.e., mammals) and invertebrates (i.e., insects) with internal fertilization (Poiani, 2006). Here ejaculates interact in a complex but closed environment where females also can enforce cryptic influence over the outcome of sperm competitions (Eberhard, 1996). Consequently, effects and compositional adaptations of seminal fluid chemistry in internal fertilizers may not only be influenced by potential rivals in sperm competition but also by the selective environment brought upon the ejaculate by the female in her reproductive tract. Moreover, the functions of adjustments in seminal fluid compositions in males may also be tailored to manipulate female physiology and behavior, including her propensity to remate (Chapman et al., 1995, 2003; Tram and Wolfner, 1999). In sum, the adaptive landscape for the seminal fluids of species with internal fertilization is quite complex (see, e.g., Holman, 2009), making these species difficult models for studies on the specific effects of seminal fluids on sperm competitions. By comparison, external fertilizing species represent models with a relatively uniform environment for fertilizations, they often show high intensity of sperm competition and their reproduction is often easily mimicked in vitro.
Although seminal fluid compositions have been studied in external fertilizers (Hwang and Idler, 1969; Kruger et al., 1984; Gallis et al., 1991; Lahnsteiner et al., 1996; Toth et al., 1997; Alavi et al., 2004), there exist only one study among such species focusing on how seminal fluids may influence sperm characteristics under sperm competition. The Grass goby (Zosterisessor ophiocephalus) adopts both guarding and sneaker reproductive tactics, and seminal fluids from sneaker males have a detrimental effect on the performance of the guarding male's sperm. By contrast, the performance of sneaker male's sperm is improved by the presence of seminal fluids from guarding males, and these tactic specific effects of seminal fluids are also mirrored in fertilization success under sperm competition (Locatello et al., 2013). As sperm velocity of male Grass goby is unaffected by seminal fluids from males employing the same tactic, the proximate mechanisms behind this rival-tactic effect of seminal fluids seems not based on self/non-self recognition systems but rather on a change in sperm quality coupled with a compositional change of the seminal fluid (Locatello et al., 2013).
The Arctic charr (Salvelinus alpinus) is an external fertilizer with flexible reproductive strategies (Liljedal and Folstad, 2003) where social status can easily be identified among free-living male charr during the approximately 1-month long and intensely competitive spawning season where both males and females mate multiply (Sigurjonsdottir and Gunnarsson, 1989; Sørum et al., 2011; http://naturweb.uit.no/amb/evolution/). As the spawning area provides no physical protection for the spawning pair, males often spawn in competition over the released eggs from one female (Sigurjonsdottir and Gunnarsson, 1989; Figenschou et al., 2004; Sørum et al., 2011). That is, in our studied population 76.5% of the ejaculates experience sperm competition and up to 9 males have been observed to ejaculate during one spawning event (Sørum et al., 2011). Moreover, the mean number of males releasing milt in each competition is 2.6 (Sørum et al., 2011). At this intensity of sperm competition, i.e., when approximately two males compete, allocation of resources to sperm production should be most intense (Parker et al., 1996) rendering our system ideal for studies on adaptations to sperm competition. Thus, sperm competition in charr is intense, and subordinate males experience a higher risk of sperm competition than dominant males as they often spawn out of synchrony with the female and also further away from the released eggs (Sørum et al., 2011). In order to compensate for this disadvantages the subordinate males increase investments in sperm production and sperm velocity compared to dominants (Liljedal and Folstad, 2003; Rudolfsen et al., 2006; Serrano et al., 2006; Haugland et al., 2008), traits that are important for fertilization success under sperm competition in charr (Liljedal et al., 2008; Egeland et al., 2015).
In salmonids sperm activation mechanisms is osmotic, that is, spermatozoa motility is initiated by a gradual decrease in potassium concentration when the seminal fluid is gradually diluted in fresh water (Morisawa et al., 1983; rewieved by Dzyuba and Cosson, 2014). As sperm cells in charr are only able to swim half the circumference of the egg (Kime et al., 2001, own observations), the concentration of seminal fluid should reach the dilution initiating activation of spermatozoa when spermatozoa are in close proximity of the egg (i.e., less than 0.5 cm from the egg). Yet, given a general inhibitory effect of potassium concentration on spermatozoa activity, the initiation of spermatozoa activity might be influenced by the number of competitive ejaculates. That is, if many males are involved in sperm competition the concentration of seminal fluid, and potassium, might be high in the proximity of the eggs when a male release his ejaculate, whereas if none or few males are involved in sperm competition the concentration of seminal fluid (and potassium) might be low. Thus, the site-specific initiation of sperm motility close to the eggs might be very complex given a general inhibitory effect of seminal fluids (Morisawa et al., 1983; Dzyuba and Cosson, 2014) coupled with an unpredictable number of competitive ejaculates (Sørum et al., 2011).
Here we mimic sperm competition between subordinate charr males, i.e., those males most often involved in sperm competition. We used a paired-male experimental design and recorded the sperm velocity and motility in ejaculates of different males activated in a solution of water and seminal fluid from a foreign male, and, as a control, in a solution of water and own seminal fluid. Additionally, in order to estimate differences between sperm motility and sperm velocity in seminal fluid and water, we also recorded sperm motility when milt was activated in water only. If seminal fluid has a general effect on velocity and motility (Morisawa et al., 1983; Dzyuba and Cosson, 2014), we would expect that motility and velocity is similar in the two solutions of seminal fluid and that sperm activity in the two seminal fluid solutions differ from that of water.
Materials and Methods
Fish Sampling and Collecting Sperm
During three consecutive nights in mid September 2005, 20 mature male Arctic charr from a naturally interacting spawning population were caught with a gill net in Fjellfrøsvatn, Troms, Northern Norway [see Figenschou et al. (2004) for details on spawning grounds]. To reduce the risk of harm during capture, the net was emptied continuously. Mesh size of the net was chosen to selectively capture the smaller subordinate males that heavily outnumber the few (1–2) substantially larger dominant males at a spawning ground. After sampling, the fish were stored in large collecting cages in the lake. The morning following each catch, individuals where anesthetized (10–12 ml of benzocain per 10.l of water) and fork length (nose to caudal cleft) was measured to the nearest mm (mean length = 25.3 cm, SD ± 1.9 cm). Thereafter, they were stripped for all available milt (mean milt volume = 0.49 ml, SD ± 0.24 ml) by applying a gentle bilateral pressure to the abdominal cavity toward the genital pore after carefully drying the fish to avoid activation of the milt by water. One skilled person stripped all fish. The milt was stored dark in sealed tubes at approximate lake temperature in the laboratory. Within 2 h after sampling, video-recordings of the sperm movements under the different treatments were made from each individual's ejaculate. When mimicking sperm competition each milt sample was paired with a milt sample from another male of similar size (average size difference = 1.7 cm, SD ± 1.1 cm).
Velocity and Motility Measurements
Before video recordings started, seminal fluid from all the individuals was extracted by centrifuging a subsample of 100 μl of homogenized milt from each male at 10,000 r.p.m. for 10 min. Afterwards, 10 μl of the supernatant seminal fluid was carefully pipetted and added to 80 μl of water. This high dilution, which is similar to that used by Locatello et al. (2013), was chosen because seminal fluid released by spawning males is rapidly diluted by water under natural reproduction (see http://naturweb.uit.no/amb/evolution/). Swimming behavior of sperm cells in this dilution of seminal fluid did not appear to be different to that of sperm cells activated in pure water (own observations).
After placing an aliquot of less than 0.12 μl of the untreated ejaculate (i.e., embedded in own seminal fluid at unmanipulated concentration) on a standard counting chamber (Leja products), the sperm cells were activated by three different solutions: (i) water, (ii) a dilution of water and own seminal fluid, and (iii) a dilution of water and seminal fluid from a foreign male (all 4.5 μl). In order to avoid effects of time elapsed from sperm sampling until treatment, we randomly selected the sequence of the three treatments used for activating the sperm cells. Video recordings of activated sperm were made using a Sony CCD black and white video camera (XC-ST50CE PAL) at 50 Hz vertical frequency, mounted on an external negative phase-contrast microscope (Olympus CH30) with a 10-x objective. The recordings were stored on DV tapes and analyzed with computer assisted sperm analysis (CASA), an objective tool for examining sperm motility in fish (Kime et al., 1996, 2001; Elofsson et al., 2003). The recordings were later analyzed using a HTM-CEROS sperm tracker (CEROS version 12, Hamilton Thorne Research, Beverly, MA, USA). The image analyzer was set as follows: frame 50 Hz, no. of frames 25, minimum contrast 10 and minimum cell size 5 pixels. Although several motility parameters where assessed, we used velocity of the average point-to-point track followed by the cell (VCL) in the analysis as sperm cells did not have a gradient of ovarian fluid or an ova for orientation and where thus not suspected to show a linear movement. To remove the effect of drift and Brownian movement, cells having an average path velocity <10 μm/s and a straight line velocity <20 μm/s were considered as static and were not included in the motility analysis. Sperm movement was recorded from the time of activation (marked on the video tape with a sound) until movement ceased. That is, at 10 s intervals from 10 s after activation to 60 or 90 s after activation. The percentages of motile cells were also recorded for each individual at each time interval after activation in the three experimental solutions (see Rudolfsen et al., 2005; Haugland et al., 2008 for more details).
Statistics
We examined the sperm velocity (VCL) and the percentage of motile cells using R software version 3.0.2 and the nlme library (R Developmental Core and Team, 2003). A linear mixed model with repeated measurement was performed to test for differences in sperm velocity or differences in percentage of motile sperm cells in the three different treatments from 5 consecutive measurements through a time span of 50 s after sperm activation (i.e., at 10, 20, 30, 40, and 50 s after activation). These measurements allowed us to examine the change in sperm behavior over time. Time (since activation) and treatment (i.e., whether sperm was activated in water, in water dilutions of own seminal fluid or water dilutions of a seminal fluid from a foreign male) were applied as fixed effects, whereas males were considered as random effect [see Crawley (2005) for further details]. In order to improve model fit and meet the assumption of normality we log-transformed sperm velocity values while percentage of motile cells was arcsine transformed. A quadratic term of time was included in the model to improve model fit. The model for the percentage of motile sperm had improved model fit when time since activation was entered as a factor instead of as a continuous predictor (Tables 1, 2 consequently differ from Tables 3, 4). A likelihood ratio test with maximum-likelihood estimation was used to test the significance of the interaction term (statistics not given). All models were checked for normality distributions with the plot function in R.
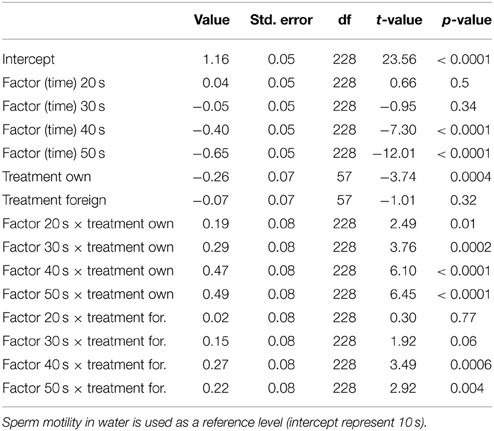
Table 3. Results from our mixed model of treatment effects on sperm motility over the 50 s post activation.
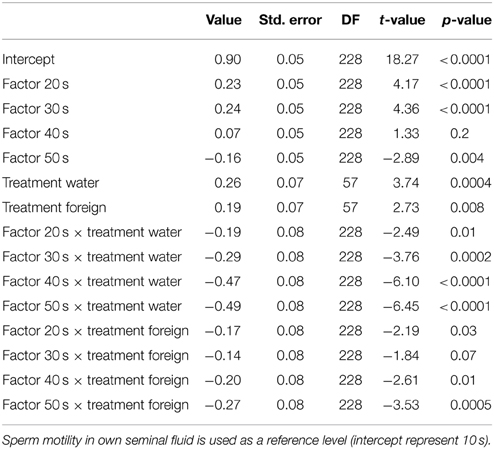
Table 4. Relationships from our mixed model on treatment and sperm motility through the 50 s post activation.
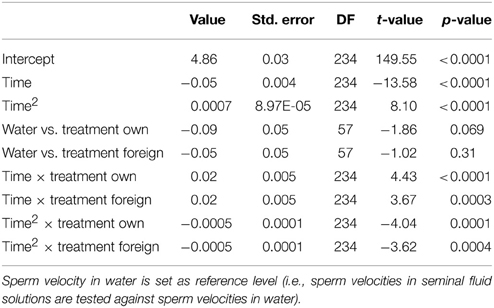
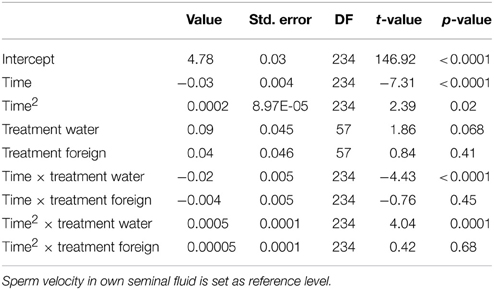
We ran several models for both the sperm velocity and the percentage of motile sperm with different reference levels for the treatments. This allowed us to examine the relative difference between treatments. In our first model we used water as a reference level to examine the effect of seminal fluids, and in later models we used own seminal fluid as reference level to evaluate differences between own and foreign seminal fluids. Additionally, for sperm velocity examinations we ran the model after subtracting 10 s from the time values so that the intercept becomes our first observation. We present the coefficients of the fixed effect and the test statistics in Tables 1–4. The significance and the corresponding F-statistics of the terms were obtained using ANOVA and the statistics are given in the text. Significance levels were set to 0.05.
Results
There was a significant decline in sperm velocity over time [ANOVA, F(1, 234) = 1107.6, p < 0.0001, Figure 1]. Yet, more important, there was a treatment effect on sperm velocity. That is, variance in sperm velocity between the three treatments (i.e., when activated in water, when activated in the water dilution including own seminal fluid and when activated in the dilution including seminal fluid of a foreign male) was larger than within treatments [F(2, 57) = 6.0, p = 0.004]. A further examination of the interaction term between the squared time and treatment showed that the decrease in sperm velocity differed between treatments [ANOVA, F(2, 234) = 9.9, p < 0.0001]. This difference is caused by a lover sperm velocity in water than in seminal fluids 30 and 40 s after activation (Figure 1). There was, however, no difference in sperm velocities depending on whether the dilution was of own or foreign seminal fluid (Figure 1).
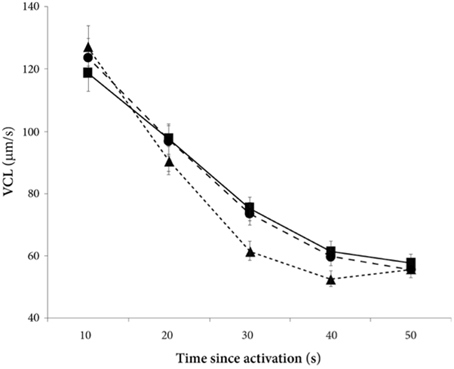
Figure 1. Average sperm velocity (VCL) decreases in seminal fluid from a foreign male (∙), own seminal fluid (■), and water (▴). Sperm activated in water show a different velocity than sperm activated in seminal fluid. Yet, sperm velocity does not depend on whether it is activated in own or foreign water diluted seminal fluid. Vertical bars denote 95% confidence intervals (see Tables 1, 2 for details).
There was a significant decrease in the percentage of motile cells as time elapsed from activation [ANOVA, F(4, 228) = 94.9, p < 0.0001, Figure 2]. However, there was no main treatment effect on percentage of sperm cells activated [F(2, 57) = 0.8, p = 0.47]. Yet, there was a significant interaction effect between percentage of motile cells and time since activation depended on whether the sperm swam in water, in a dilution of own seminal fluid or in a seminal fluid dilution from a foreign male [F(8, 228) = 7.5, p < 0.0001]. That is, the percentage of motile cells is significantly lower when activated in own seminal fluid dilutions than when activated in foreign seminal fluid dilutions (and in water) 10 s after sperm activation (Tables 3, 4, Figure 2). On the other hand, the percentage of motile sperm does not differ between ejaculates activated in foreign seminal fluid and water 10 s post activation. There are smaller difference between the treatments at 20 and 30 s post activation, but later (at 40 and 50 s) we see that the decrease in the percentage of motile sperm is larger in water than in foreign seminal fluid. Additionally, the decrease in sperm motility activated in own seminal fluid is lower than in both water and foreign seminal fluids at 50 s post activation (Table 4, Figure 2).
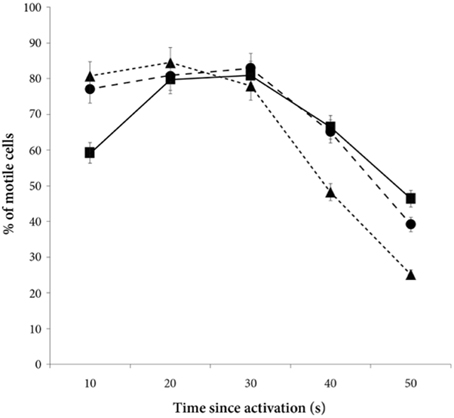
Figure 2. The percentage of motile cells decreases with time since activation in seminal fluid from a foreign male (∙), own seminal fluid (■), and water (▴). Yet, when activated in water or water diluted foreign seminal fluid, the percentage of motile cells is initially higher than when activated in water diluted own seminal fluid. Thus, increased concentration of own seminal fluid has an individual specific negative effect on activation of sperm cells. Vertical bars denote 95% confidence intervals.
Discussion
We document effects of seminal fluid on velocity and motility parameters of sperm cells in charr. Although there is no difference between foreign and own seminal fluid on sperm velocity, sperm of unmanipulated ejaculates activated in seminal fluid dilutions maintain higher velocity through the activity period than sperm activated in water only. Additionally, the initial (i.e., 10 s after activation) number of motile cells is higher when activated in a solution of foreign seminal fluid than when activated in own seminal fluid, yet this initial effect of foreign seminal fluid on sperm motility is not different from that of water. Thus, addition of own seminal fluid has an individual specific negative effect on own, but not foreign sperm activation. Moreover, the numbers of sperm cells active late in the activity period are fewer in water solutions compared to those in solutions of seminal fluids.
Sperm velocity is known to decrease with time after activation especially in external fertilizers, and this is particularly the case within the salmonids, known for their brief fertilizations window (Vladic and Järvi, 1997). Velocity reductions can mechanistically be explained from the rapid depletion of ATP stores following an activation that ensure rapid initial acceleration and high initial swimming speed (Burness et al., 2004). Accordingly, initial sperm velocity is documented to be an important determinant of the fertilization success also in Arctic charr (Liljedal et al., 2008; Egeland et al., 2015). Yet, although initial sperm velocity is high in all of the activation media used in our study, it is maintained at higher levels in the two seminal fluid solutions than in water. Seminal fluids of external fertilizers are known to promote sperm velocity, and this is also documented in species where individuals employ different reproductive tactics (Locatello et al., 2013). Our sample, from one spawning ground where usually one to two large dominant individuals try to monopolize the incoming females (own observations), consist of small individuals restricted to subordinate reproductive strategies. Although small individuals may take dominant positions under experimental conditions, and also make appropriate adjustments of sperm traits according to such position, they are never observed to dominate at the lek. Moreover, as we also size matched “competitors” it is highly unlikely that we, in our experiment, include mixtures of seminal fluids and ejaculates from individuals with different reproductive strategies. Consequently, our results do not differ from those seen in the Grass goby, where seminal fluids from males employing the same mating tactic had no effect on sperm velocity of the focal male (Locatello et al., 2013). Thus, also in charr, with its highly flexible reproductive strategies, seminal fluids from subordinate males may, in mixture, promote sperm velocity indiscriminately among subordinates compared to that of water.
The effect of seminal fluid on the initial activation of sperm cells, on the other hand, seems to be discriminant. It promotes motility of sperm cells of foreign males ejaculate, but, although the effect is very large and different from that of own seminal fluid, it is not different from that of water. The most parsimonious explanation for this finding is that components in own seminal fluid inhibits initial motility if not diluted either in water, or, more puzzling, in a solution including seminal fluid from another male. The latter suggest that the inhibitor of sperm motility in own seminal fluid may be of an individual-specific nature. Although previous studies have shown a large variety of effects from seminal fluids on sperm motility, also many general inhibitory effects [reviewed in Poiani (2006) and in Simmons and Fitzpatrick (2012)], we are unaware of descriptions of individual-specific inhibitory mechanisms that reduce own sperm motility. Self/non-self recognition processes with negative effects on rival sperm from seminal fluids have been documented in polyandrous social insects were females store sperm for extensive periods (den Boer et al., 2010). Yet, in these cases recognition is targeted toward foreign sperm and not, as in the present case, own sperm. As our finding, from a species where the interaction between ejaculates is brief, document an inhibitory effect on own sperm it might be speculated that the recognition mechanism involves an immunological identification of own sperm cells from components in own, but not foreign seminal fluid. It should also be noted that in the examined population of charr, the average time elapsing from the synchronized spawning between the dominant male and the female to the first subordinate male ejaculate is approximately 0.7 s, and that the latest observed spawning after the release of gametes from the spawning pair is 1.9 s (Sørum et al., 2011). Thus, our observation of an approximately 25% decrease in the percentage of activated sperm cells when diluted in a solution including own seminal fluid 10 s after activation, begs the question of what happens within the first few seconds when fertilization most likely occur (Vladic and Järvi, 1997).
It should be noted that when the dominant male spawn in synchrony with the female, sperm and eggs are ejected out into the cracks of the gravel behind them before the up to 8 males release competing ejaculates in a spawning cascade lasting less than 2 s (Sørum et al., 2011). The ejected distance of an ejaculate is approximately 10–30 cm, while the sperm's swimming distance after activation is about 0.5 cm (own observations; Billard and Cosson, 1992). That is, only a very short part of the total traveling distance of the sperm is caused by the sperm's own propulsion. Consequently, activation of sperm swimming need to be precisely adjusted to the water dilutions optimal for three-dimentional “carpet-bombing” of the eggs. Under this scenario the start-up of self-propulsion by the sperm should only be influenced by the concentration of own seminal fluid in water and unaffected by the concentration of seminal fluids from a seemingly uncontrollable number of competitive ejaculates. These environmental constraints may have promoted the evolution of the extrasomatic inhibitory effects of own seminal fluid on own sperm activation, not seen when exposed to foreign seminal fluids.
Moreover, seminal fluids are also known to enhance sperm longevity (Hwang and Idler, 1969; Kruger et al., 1984; Gallis et al., 1991; Lahnsteiner et al., 1996; Toth et al., 1997; Alavi et al., 2004). Thus, higher concentrations of seminal fluid in our seminal fluid solutions may increase sperm longevity compared to our water dilutions and thus explain the higher motility seen in seminal fluid solutions than in water solutions 40 and 50 s after activation. Whether these late observations have implications for fertilization success under natural spawning is questionable, yet the mere observation of activity in sperm cells this long after activation hints to some kind of biological relevance.
In sum, with the exception of decreasing own initial motility, all dilutions of seminal fluids had effects comparable to, or better than, those of water on both of our two measured parameters—percentage of sperm activated and sperm velocity. These positive effects found in an external fertilizer with flexible reproductive roles resonates well with what is previously documented in both external fertilizers and in internal fertilizing species (Poiani, 2006; Simmons and Fitzpatrick, 2012; Locatello et al., 2013). Yet, the large individual-specific inhibition of own initial motility only seen when ejaculates are activated in dilutions of own seminal fluid, seem unparalleled by previous studies. The finding is puzzling as initial sperm motility is a very powerful predictor for fertilization success under the commonly occurring sperm competitions in charr (Liljedal et al., 2008; Egeland et al., 2015). Moreover, what are the proximate biochemical mechanisms behind this extrasomatic control; what are the seminal fluids effect on sperm when subordinate individuals engage in sperm competitions with dominants and how is the seminal fluid's control over sperm modulated by the ovarian fluid?
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Nigel Yoccoz helped with statistics and Lars Figenschou, Thomas Haugland, and Bjørnar Strøm showed a great mixture of professionalism and great humor during exhaustive fieldwork. Three anonymous referees guided us through the last stage.
References
Alavi, S. M. H., Cosson, J., Karami, M., Abdolhay, H., and Amiri, B. M. (2004). Chemical composition and osmolality of seminal fluid of Acipenser persicus; their physiological relationship with sperm motility. Aquac. Res. 35, 1238–1243. doi: 10.1111/j.1365-2109.2004.01132.x
Billard, R., and Cosson, M. P. (1992). Some problems related to the assessment of sperm motility in fresh-water fish. J. Exp. Zool. 261, 122–131. doi: 10.1002/jez.1402610203
Burness, G., Casselman, S., Schulte-Hostedde, A., Moyes, C., and Montgomerie, R. (2004). Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav. Ecol. Sociobiol. 56, 65–70. doi: 10.1007/s00265-003-0752-7
Chapman, T., Arnqvist, G., Bangham, J., and Rowe, L. (2003). Sexual conflict. Trends Ecol. Evol. 18, 41–47. doi: 10.1016/S0169-5347(02)00004-6
Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F., and Partridge, L. (1995). Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244. doi: 10.1038/373241a0
Crawley, M. J. (2005). Statistics: An Introduction Using, R.J.W. Sons. London: Imperial College of London.
den Boer, S. P. A., Baer, B., and Boomsma, J. J. (2010). Seminal fluid mediates ejaculate competition in social insects. Science 327, 1506–1509. doi: 10.1126/science.1184709
Dzyuba, V., and Cosson, J. (2014). Motility of fish spermatozoa: from external signaling to flagella response. Reprod. Biol. 14, 165–175. doi: 10.1016/j.repbio.2013.12.005
Eberhard, W. G. (1996). Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton University Press.
Egeland, T., Rudolfsen, G., Nordeide, J. T., and Folstad, I. (2015). On the relative effect of spawning asynchrony, sperm quantity and sperm quality on paternity under sperm competition. Front. Ecol. Evol. 3:77. doi: 10.3389/fevo.2015.00077
Elofsson, H., McAllister, B. G., Kime, D. E., Mayer, I., and Borg, B. (2003). Long lasting stickleback sperm; is ovarian fluid a key to success in fresh water? J. Fish Biol. 63, 240–253. doi: 10.1046/j.1095-8649.2003.00153.x
Figenschou, L., Folstad, I., and Liljedal, S. (2004). Lek fidelity of male Arctic charr. Can. J. Zool. 82, 1278–1284. doi: 10.1139/z04-106
Gallis, J. L., Fedrigo, E., Jatteau, P., Banpunt, E., and Billard, R. (1991). “Siberian sturgeon spermatozoa: effects of dilution, pH, osmotic presure, sodium and potassium ions on motility,” in Acipenser, ed P. Williot (Antony: Cemagref Publication), 143–151.
Haugland, T., Rudolfsen, G., Figenschou, L., and Folstad, I. (2008). Sperm velocity & its relation to social status in Arctic charr (Salvelinus alpinus). Anim. Reprod. Sci. 115, 231–237. doi: 10.1016/j.anireprosci.2008.11.004
Holman, L. (2009). Drosophila melanogaster seminal fluid can protect the sperm of other males. Funct. Ecol. 23, 180–186. doi: 10.1111/j.1365-2435.2008.01509.x
Hwang, P. C., and Idler, D. R. (1969). A study of major cations osmotic pressure and pH in seminal components of Atlantic salmon. J. Fish. Res. Board Canada 26, 413–419. doi: 10.1139/f69-040
Kime, D. E., Ebrahimi, M., Nysten, K., Roelants, I., Rurangwa, E., Moore, H. D. M., et al. (1996). Use of computer assisted sperm analysis (CASA) for monitoring the effects of pollution on sperm quality of fish; Application to the effects of heavy metals. Aquat. Toxicol. 36, 223–237. doi: 10.1016/S0166-445X(96)00806-5
Kime, D. E., Van Look, K. J., McAllister, B. G., Huyskens, G., Rurangwa, E., and Ollevier, F. (2001). Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 130, 425–433. doi: 10.1016/S1532-0456(01)00270-8
Kruger, J. C. D., Smit, G. L., Vanvuren, J. H. J., and Ferreira, J. T. (1984). Some chemical and physical characteristics of the semen of Cyprinus-carpio L and Oreochromis-Mossambicus (Peters). J. Fish Biol. 24, 263–272. doi: 10.1111/j.1095-8649.1984.tb04797.x
Lahnsteiner, F., Berger, B., Weismann, T., and Patzner, R. A. (1996). Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism. Fish Physiol. Biochem. 15, 167–179. doi: 10.1007/BF01875596
Liljedal, S., and Folstad, I. (2003). Milt quality, parasites, and immune function in dominant and subordinate Arctic charr. Can. J. Zool. 81, 221–227. doi: 10.1139/z02-244
Liljedal, S., Rudolfsen, G., and Folstad, I. (2008). Factors predicting male fertilization success in an external fertilizer. Behav. Ecol. Sociobiol. 62, 1805–1811. doi: 10.1007/s00265-008-0609-1
Locatello, L., Poli, F., and Rasotto, M. B. (2013). Tactic-specific differences in seminal fluid influence sperm performance. Proc. R. Soc. B 280:22891 doi: 10.1098/rspb.2012.2891
Møller, A. P., and Birkhead, T. R. (1989). Copulation behavior in mammals—Evidence that sperm competition is widespread. Biol. J. Linn. Soc. 38, 119–131. doi: 10.1111/j.1095-8312.1989.tb01569.x
Morisawa, M., Suzuki, K., and Morisawa, S. (1983). Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J. Exp. Biol. 107, 105–113.
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in insects. Biol. Rev. Camb. Philos. Soc. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Parker, G. A. (1990). Sperm competition games—Raffles and roles. Proc. R. Soc. London Se. B Biol. Sci. 242, 120–126. doi: 10.1098/rspb.1990.0114
Parker, G. A., Ball, M. A., Stockley, P., and Gage, M. J. G. (1996). Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. B Biol. Sci. 263, 1291–1297. doi: 10.1098/rspb.1996.0189
Parker, G. A., and Pizzari, T. (2010). Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. doi: 10.1111/j.1469-185x.2010.00140.x
Perry, J. C., Sirot, L., and Wigby, S. (2013). The seminal symphony: how to compose an ejaculate. Trends Ecol. Evol. 28, 414–422. doi: 10.1016/j.tree.2013.03.005
Poiani, A. (2006). Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 60, 289–310. doi: 10.1007/s00265-006-0178-0
R Developmental Core and Team. (2003). R: A Language and Environment for Statistical Computing. Viena: R Foundation for Statistical Computing.
Rudolfsen, G., Figenschou, L., Folstad, I., Nordeide, J. T., and Søreng, E. (2005). Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). J. Evol. Biol. 18, 172–179. doi: 10.1111/j.1420-9101.2004.00778.x
Rudolfsen, G., Figenschou, L., Folstad, I., Tveiten, H., and Figenschou, M. (2006). Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. London Ser. B Biol. Sci. 273, 325–332. doi: 10.1098/rspb.2005.3305
Serrano, J. V., Folstad, I., Rudolfsen, G., and Figenschou, L. (2006). Do the fastest sperm cells swim faster in subordinate than in dominant males? Can. J. Zool. 84, 1019–1024. doi: 10.1139/Z06-097
Sigurjonsdottir, H., and Gunnarsson, K. (1989). Alternative Mating Tactics of Arctic Charr, Salvelinus-Alpinus, in Thingvallavatn, Iceland. Environ. Biol. Fishes 26, 159–176. doi: 10.1007/BF00004814
Simmons, L. W. (2001). Sperm Competition and Its Evolutionary Consequences in the Insects. Monographs in Behavioural Ecology. Princeton, NJ: Princeton University Press.
Simmons, L. W., and Fitzpatrick, J. L. (2012). Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. doi: 10.1530/REP-12-0285
Sørum, V., Figenschou, L., Rudolfsen, G., and Folstad, I. (2011). Spawning behaviour of Arctic charr (Salvelinus alpinus): risk of sperm competition and timing of milt release for sneaker and dominant males. Behaviour 148, 1157–1172. doi: 10.1163/000579511X596615
Toth, G. P., Ciereszko, A., Christ, S. A., and Dabrowski, K. (1997). Objective analysis of sperm motility in the lake sturgeon, Acipenser fulvescens: activation and inhibition conditions. Aquaculture 154, 337–348. doi: 10.1016/S0044-8486(97)00066-5
Tram, U., and Wolfner, M. F. (1999). Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics 153, 837–844.
Keywords: sperm competition, sexual selection, sperm motility, ejaculate, Arctic charr
Citation: Rudolfsen G, Serrano JV and Folstad I (2015) Own, but not foreign seminal fluid inhibits sperm activation in a vertebrate with external fertilization. Front. Ecol. Evol. 3:92. doi: 10.3389/fevo.2015.00092
Received: 13 March 2015; Accepted: 20 July 2015;
Published: 03 August 2015.
Edited by:
Peter Schausberger, University of Natural Resources and Life Sciences Vienna, AustriaReviewed by:
Trevor Pitcher, University of Windsor, CanadaShawn Garner, University of Western Ontario, Canada
Copyright © 2015 Rudolfsen, Serrano and Folstad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivar Folstad, Department of Arctic and Marine Biology, University of Tromsø, Dramsveien 201, 9037 Tromsø, Norway,aXZhci5mb2xzdGFkQHVpdC5ubw==
 Geir Rudolfsen
Geir Rudolfsen Ivar Folstad
Ivar Folstad