- 1State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing, China
- 2School of Agriculture, Yangtze University, Jingzhou, China
- 3Center for Medical, Agricultural and Veterinary Entomology, United States Department of Agriculture - Agricultural Research Service, Gainesville, FL, USA
Solenopsis geminata group (Hymenoptera: Formicidae) encompasses ant species commonly called fire ants because of their painful sting. The many physiological effects of the venom are caused by 2-methyl-6-alkyl and/or alkenylpiperidine alkaloids. The variation in piperidine alkaloid structures has useful taxonomic characters. The most well studied Solenopsis species is Solenopsis invicta, which was accidentally imported into the USA in the 1930s from South America. It quickly spread throughout the southern USA and is now a major invasive pest ant in the USA and in other parts of the world. Interestingly, the invasive S. invicta has largely displaced a native USA fire ant, S. geminata, from the southern USA. We explore the possibility that differences in venom chemistry could be correlated with this displacement. The cis and trans alkaloids from body extracts of workers and alate queens of S. geminata were separated by silica gel chromatography, identified, and quantitated by GC-MS analysis. Both workers and alate queens produce primarily cis- and trans-2-methyl-6-n-undecylpiperidines, as well as other minor alkaloid components. Imported fire ant, S. invicta, alate queens produce the same alkaloids as S. geminata alate queens, but in contrast S. invicta workers produce piperidine alkaloids with longer side chains, which are purported to be physiologically more effective. These results are discussed in relation to the evolutionary progression of fire ant venom alkaloids and displacement of S. geminata by S. invicta in the USA.
Introduction
Ants of the genus Solenopsis (Hymenoptera: Formicidae) are known as fire ants because of their painful stings (Vinson and Greenberg, 1986; Blum, 1992). The sting is used for colony defense, prey capture, and antimicrobial action (Blum, 1985). Solenopsis venom is characterized by the presence of up to 95% water-insoluble alkaloids and a small amount of protein (Baer et al., 1979; Jones et al., 1982). The venom sac is the source of large amounts of venom alkaloids (MacConnell et al., 1971). The venoms are composed mainly of 2-methyl-6-alkyl or alkenyl piperidines known as the solenopsins which are responsible for the toxic properties of the venom (MacConnell et al., 1970, 1971). These piperidine alkaloids found in Solenopsis fire ants differ in the lengths of their alkyl/alkenyl chains, which can have 9, 11, 13, 15, or 17 carbon atoms (Brand et al., 1972; MacConnell et al., 1974; Blum et al., 1992). The two alkyl substituent groups can be either on same side of the piperidine ring as cis-solenopsin or on different side of the piperidine ring as trans-solenopsin. Carbon numbers have been widely used to represent these solenopsins (Brand et al., 1972). For example, cis C11 denotes the alkaloid with an 11-carbon side chain cis to the ring methyl group; cis C11:1 denotes the alkaloid with one double bond in the 11-carbon side chain cis to the ring methyl group. The absolute configurations of the cis- and trans-solenopsins were determined to be (2R,6S) and (2R,6R), respectively (Leclercq et al., 1994).
The mix of solenopsins, their cis or trans configuration, and relative proportions, vary both among castes and within Solenopsis species (Brand et al., 1972, 1973a; MacConnell et al., 1976). The venoms of workers of Solenopsis richteri Forel and Solenopsis invicta Buren contain mainly trans solenopsins with C13 and C15 side chains whereas these compounds in the venoms of alate queens of these species are essentially lacking (Brand et al., 1973b). In the venoms of workers of Solenopsis xyloni McCook and S. geminata (F.), only cis and trans C11 components are abundant (Brand et al., 1973b). The difficulty of classical taxonomy in S. geminata and Solenopsis saevissima complexes has been recognized for a long time because of the morphological similarity of many Solenopsis species in these complexes (Creighton, 1930; Buren, 1972; Trager, 1991). The species-specific pattern of venom alkaloids, a biochemical taxonomic character, may be useful in sorting out this very complex taxonomic problem (MacConnell et al., 1976). Indeed, venom alkaloid and cuticular hydrocarbon chemistry has been reported as useful taxonomic tool for differentiating Solenopsis species (Vander Meer, 1986; Vander Meer and Lofgren, 1988; Dall'Aglio-Holvorcema et al., 2009).
The tropical fire ant, S. geminata, is native to coastal areas from the southern United States to Central America and northern South America, and has been introduced into both tropical Asia and west Africa (Trager, 1991; Wetterer, 2011). This species co-occurs extensively with the distantly related fire ant S. saevissima along Brazil's Atlantic coast (Ross et al., 2010), but has been displaced from much of its range in the United States by the introduced congener, S. invicta (Porter et al., 1988; Porter, 1992; Wojcik, 1994). Compared with S. invicta, the chemistry of S. geminata has not been extensively studied. Venom alkaloids of S. geminata workers and alate queens were first determined by Brand et al. (1972, 1973b), and confirmed by Cruz-Lopez et al. (2001), as primarily (2R,6S)-2-methyl-6-n-undecylpiperidine (cis-C11) and (2R,6R)-2-methyl-6-n-undecylpiperidine (trans-C11). Furthermore, cis-2-methyl-6-n-nonylpiperidine (cis-C9) was found to be a minor component in the venom of alate queens of S. geminata (MacConnell et al., 1974). In a series of previous studies (Chen and Fadamiro, 2009a,b; Chen et al., 2010b, 2012; Yu et al., 2014), we developed a method to purify venom alkaloids from the imported fire ants using conventional silica gel column chromatography. Two new alkaloid series, 2,6-dialkyl-Δ1,2-piperideines and 2,6-dialkyl-Δ1,6-piperideines were identified. Qualitative analysis demonstrated that alate queens from S. richteri, S. invicta, and hybrid S. richteri × S. invicta share similar piperideine venom alkaloid profiles. It was thus of interest to investigate whether the S. geminata populations would have similar or different piperideine compositions or produce other minor alkaloid components. In the present study, we conducted both qualitative and quantitative analyses of the venom alkaloid chemistry of workers and alate queens in pooled samples of S. geminata.
Materials and Methods
Insects
Five colonies of the tropical fire ant, S. geminata, were collected in late March, 2014 in Alachua County, Florida, USA where S. geminata continued to persist with the red imported fire ant S. invicta (Tschinkel, 1988; Porter, 1992). Two of these colonies were a red form of S. geminata collected from the west side of Gainesville along Parker Road while other three colonies were a dark form obtained east of Gainesville near Boulware Springs. The alate queens of the dark form had distinctly smaller heads than those of the red form. Each colony was separated from the soil and reared in the laboratory with 1.5 M sugar water and domestic crickets (Acheta domesticus L.) (Gavilanez-Slone and Porter, 2013). All colonies that had been in the lab for 3 months prior to alkaloid extraction contained both workers and alate queens. There were 1200–1600 workers per gram of worker ants from both red and dark forms. The mean weight of alate queens from red and dark forms were 13.40 ± 4.51 mg and 6.92 ± 0.24 mg, respectively.
Chemicals
HPLC grade n-hexane (CNW Technologies GmbH, Düsseldorf, Germany) was used for extraction and purification of the venom alkaloids. Analytical reagent grade acetone and triethylamine (Beijing Chemical Reagent, Beijing, China) were redistilled before use. Silica gel (300–400 mesh, Qingdao Marine Chemical Factory, Qingdao, China) was used for column chromatography. Racemic cis-2-methyl-6-undecylpiperidine (cis-C11) was synthesized by reduction of corresponding pyridine with hydrogen and palladium/carbon catalyst (Pianaro et al., 2012), and used as external standard for quantitative analysis.
Sample Preparation
Alkaloids were extracted and isolated from fire ant samples as previously reported (Chen et al., 2012; Yu et al., 2014). For each colony, ant workers, or alate queens (1 g) were killed by freezing and extracted with hexane (enough hexane to cover ant bodies, ~3.5 mL) for 24 h. The whole body extraction proved to adequately represent the gland extractions (data not shown) and have been widely used for fire ant venom alkaloid analysis (For example, Deslippe and Guo, 2000; Eliyahu et al., 2011). The extract was dried over anhydrous sodium sulfate and concentrated to 0.5 mL under a mild stream of N2, and then loaded onto a 17 mm (o.d.) chromatography column (20 g silica gel). The column was eluted with hexane containing 2% triethylamine and stepwise increasing amounts of acetone (hexane/acetone ratio ranging from 50:1 to 10:1) to obtain the alkaloids. The chemistry of each collection (ca. 2 mL) was analyzed by gas chromatography (GC) on an Agilent 7890A GC equipped with a flame ionization detector (FID) and a 30 m × 0.25 mm i.d., 0.25 μm, HP-5ms capillary column (Agilent Technologies). Nitrogen was used as carrier gas at a flow rate of 2 mL/min. The injection port and detector temperatures were set at 270 and 280°C, respectively. The GC oven was programmed at a rate of 15°C/min from 90 to 270°C, with 2 min initial time and 16 min final holding time. The collections were pooled based on changes observed in the GC chromatograms of each collection to obtain two major alkaloid fractions corresponding to cis and trans alkaloids. The two alkaloid fractions for each colony were concentrated to a volume of 4 mL for GC-MS analyses.
GC-MS Analysis
GC-MS analyses of alkaloid fractions were performed on an Agilent 7890A GC coupled to a 5975C mass selective detector, with an HP-5ms capillary column as descried above. Helium was used as carrier gas at a flow rate of 1 mL/min. Injections (1 μL) were made in the splitless mode at an injector temperature of 230°C. The GC oven temperature was programmed from 90 (isothermal for 1 min) to 160°C at 10°C/min, then to 250°C at 3°C/min, and held for 2 min. Total run time was 40 min. The transfer line temperature was set at 250°C. Mass spectra were obtained using electron impact (EI, 70 eV). The chemical identities of alkaloids were determined by analysis of their mass spectra and by comparison of diagnostic ion fragments with published results (Chen and Fadamiro, 2009a,b; Chen et al., 2010b, 2012).
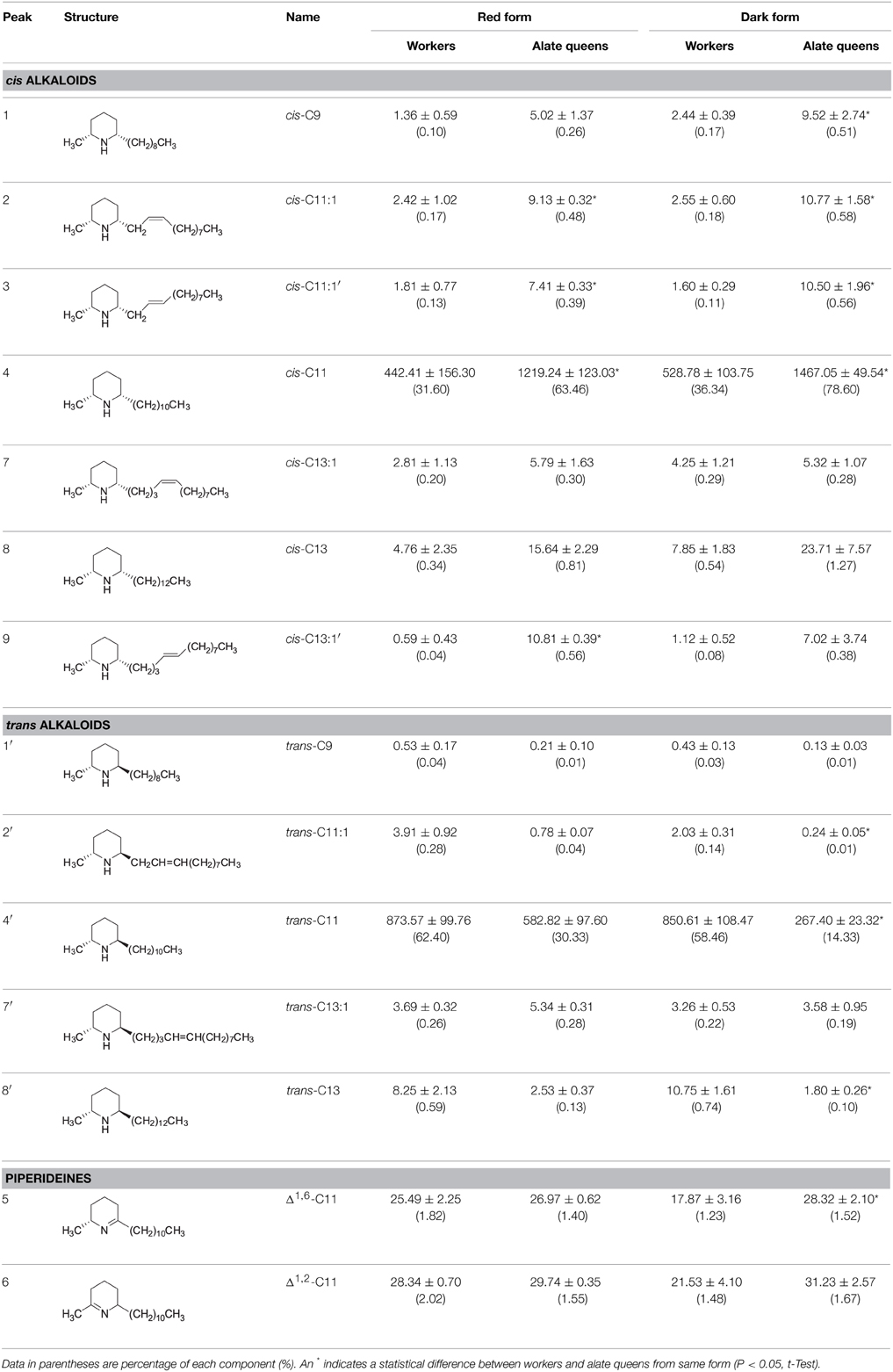
A stock solution of standard compound, synthetic racemic cis-C11 (2000 ng/μL), was prepared and then diluted to a series of concentrations ranging from 1.95 to 1000 ng/μL. All dilutions were transferred to the GC autosampler, and 1 μL of each dilution was used for GC-MS analysis under same conditions as described above. A standard curve was calculated by linear regression analysis. The concentrations of the identified venom alkaloids in the cis and trans alkaloid fractions were calculated against the standard curve. The obtained concentration for a given alkaloid component was multiplied by the final volume of the alkaloid fraction, 4 mL, and then divided by the weight of ants used for extraction to give exact amount of the alkaloid component per 1 g of ants. The absolute abundances of the alkaloid components were analyzed using PROC TTEST procedure (P<0.05) to establish significant difference between workers and alate queens (SAS Institute, 2004).
Results
GC Patterns of the cis and Trans Alkaloid Fractions of Workers and Alate Queens
As previously reported (Chen and Fadamiro, 2009a,b; Chen et al., 2010b, 2012), the cis and trans alkaloid fractions from whole body extracts of workers and alate queens were readily separated through silica gel chromatography. The GC profiles of the cis alkaloid fraction and the trans alkaloid fraction from workers and alate queens were very similar. Only one major peak was detected in each alkaloid fraction (Figure 1). No key qualitative differences were observed between workers and alate queens.
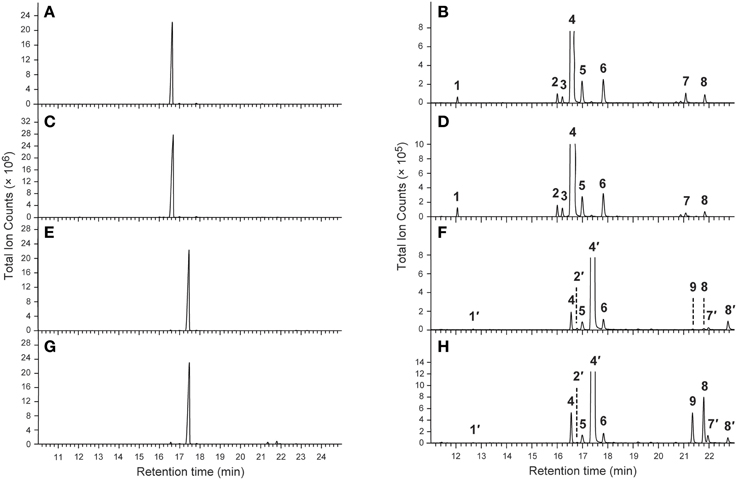
Figure 1. Typical GC-MS chromatograms of the cis- and trans-alkaloid fractions. (A,B) The cis alkaloid fraction of workers; (C,D) The cis alkaloid fraction of alate queens; (E,F) The trans alkaloid fraction of workers; (G,H) The trans alkaloid fraction of alate queens.
Identification of Alkaloids from Workers and Alate Queens
The chemical identities of major peaks in these two alkaloid fractions were readily determined by comparison with published characteristic peaks of fire ant venom alkaloids. Major peak 4 in the cis alkaloid fraction is cis-C11 and peak 4′ in the trans alkaloid fraction is trans-C11, having exactly same mass spectra with the characteristic base peak ion m/z 98 and parent ion [M+] 252. The chemical identities of the minor peaks (Figure 1 and Table 1) were determined by comparing mass spectra and retention times with those already characterized from extracts of workers and alate queens of S. richteri, S. invicta and their hybrid (Chen and Fadamiro, 2009a,b; Chen et al., 2010b, 2012).
In the cis alkaloid fractions, minor peaks 1, 2, 7, 8 were cis-C9, cis-C11:1, cis-C13:1, cis-C13, respectively, having the characteristic base peak ion m/z 98. The configuration of the side chain double bond in the piperidines (dehydrosolenopsins A, B, C, D) is always Z (cis) (MacConnell et al., 1971); therefore, we assign the cis configuration to the double bond in the cis-C11:1 (2) and cis-C13:1 (7) side chains. The mass spectrum of peak 3 was almost identical to that of peak 2. Considering that this peak eluted right after peak 2, we tentatively identified 3 (cis-C11:1′) as a stereoisomer of 2 (cis-C11:1) with a possible trans double bond on the alkyl side chain. We also proposed that the absolute configuration of 3 was the same as 2 (2R,6S) (Table 1). The mass spectra of minor peaks 5 and 6 were identical to 2,6-dialkylpiperideines found generally in Solenopsis fire ants. The important mass peaks at m/z 96, 111 in 5 indicated an N–C6 double bond, whereas ions at m/z 96, 97, 110 in 6 indicated an N–C2 double bond on the piperideine ring. Therefore, 5 and 6 were identified as 2-methyl-6-n-undecyl-Δ1,6-piperideine and 2-methyl-6-n-undecyl-Δ1,2-piperideine, respectively. Because piperideines were proposed to function as precursors for the syntheses of fire ant alkaloids (Leclercq et al., 1996; Chen and Fadamiro, 2009a) and the chirality of 2-CH3 was always found to be R in Solenopsis fire ants (Pianaro et al., 2012), we inferred absolute configuration of Δ1,6-piperideine (5) to be (2R)-CH3. However, both (6R)- and (6S)-C11H23 configurations are possible for Δ1,2-piperideine (6).
In the trans alkaloid fraction, minor peaks 1′, 2′, 7′, 8′ were trans-C9, trans-C11:1, trans-C13:1, trans-C13, respectively. The mass spectrum and retention time of peak 4 in the trans alkaloid fraction were identical to those of peak 4 in the cis alkaloid fraction, suggesting that they were same compound. In the same manner, peaks 5, 6, 8 in the trans alkaloid fraction were identical to those in the cis alkaloid fraction. These cis alkaloids and piperideines retained in silica gel column due to strong absorption by silica gel were eluted with hexane containing increased amount of acetone along with trans alkaloids. The mass spectrum of peak 9 in the trans alkaloid fraction was almost identical to that of peak 7 in the cis alkaloid fraction. We tentatively identified 9 (cis-C13:1′) as stereoisomer of 7 (cis-C13:1) with a trans double bond on the alkyl side chain.
Quantitative Analysis of Alkaloids
The external standard method was used to quantitate the separated piperidine and piperideine alkaloids. A calibration curve was constructed with log-transformed data, log (A) = 1.1681log (C) + 5.3575 (r2 = 0.9797, P<0.001), where A is the peak area, C is the concentration of the standard compound (ng/μL). The obtained calibration curve was used to quantitate alkaloidal components in all ant samples (Table 1).
Over 90% of the venom alkaloids from workers and alate queens are composed of cis-C11 and trans-C11 (Table 1). In red and dark form workers, the total percentages of these two major components were 94.00 and 94.80%, respectively. Similarly, in red and dark form alate queens, the total percentages of these two major components were 93.79 and 92.93%, respectively. Apparently, cis-C11 was the only dominant component in the cis alkaloid fraction, while trans-C11 was the only major component in the trans alkaloid fraction, irrespective of ant form. Furthermore, the amount of cis-C11 from alate queens (1219.24 ± 123.03 μg/g ant in red form; 1467.05 ± 49.54 μg/g ant in dark form) was significantly greater than that from workers (442.41 ± 156.30 μg/g ant in red form; 528.78 ± 103.75 μg/g ant in dark form). On the contrary, although the amount of trans-C11 from alate queens (582.82 ± 97.60 μg/g ant in red form; 267.40 ± 23.32 μg/g ant in dark form) was lower than that from workers (873.57 ± 99.76 μg/g ant in red form; 850.61 ± 108.47 μg/g ant in dark form), significant difference in the amount of trans-C11 was only detected for dark form (t = 2.08, P = 0.1726 for red form; t = 5.26, P = 0.0063 for dark form). The amounts of two piperideines, Δ1,6-C11 and Δ1,2-C11, were quite low ranging from 17.87 ± 3.16 to 31.23 ± 2.57 μg/g ant in workers and alate queens. The amounts of other minor components, cis-C9, cis-C11:1, cis-C13:1, cis-C13, trans-C9, trans-C11:1, trans-C13:1, and trans-C13, were all under 24 μg per 1 g of ant. In general, the alate queens had more cis alkaloid components and less trans alkaloid components than workers, with only one exception, trans-C13:1, even though the differences were not always statistically significant.
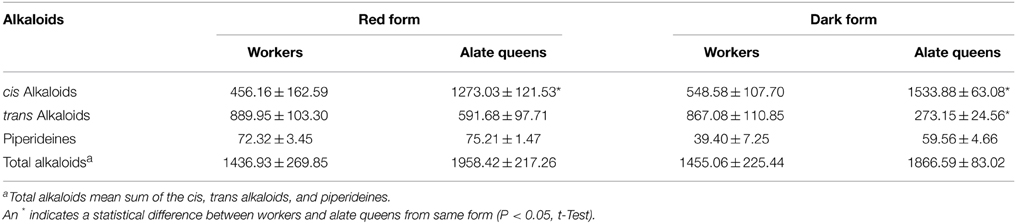
We further calculated the total amount of alkaloids depending on structure of piperidine ring (Table 2). The trend of difference in total amount of alkaloids of different alkaloid type between workers and alate queens was similar to the trend concluded from data in Table 1. The alate queens had higher amount of cis alkaloids and lower amount of trans alkaloids than workers. There was no significant difference in total amount of piperideines between workers and alate queens (df = 2, t = 0.77, P = 0.5226 for red form; df = 4, t = 2.34, P = 0.0795 for dark form). Furthermore, the total amount of alkaloid components in alate queens was relatively higher, but not significantly, than that in workers. The total amount of alkaloids produced by 1 g of ant workers was about 1.4 mg, and the total amount of alkaloids produced by 1 g of ant queens was about 1.9 mg.
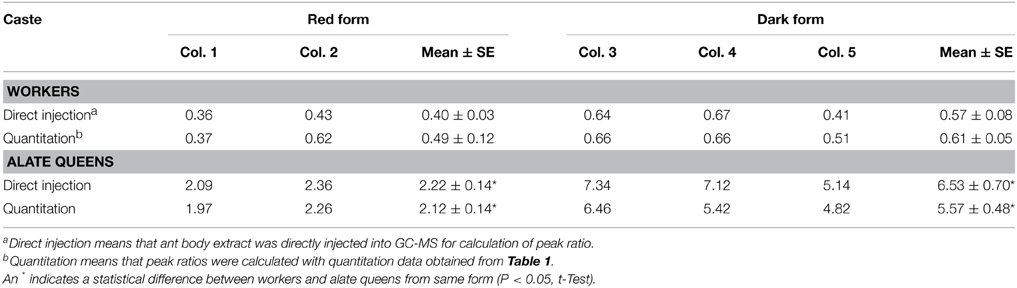
Moreover, we calculated the ratio of cis-C11 to trans-C11 for workers and alate queens (Table 3). To obtain more accurate values of the cis-C11 to trans-C11 ratio, 1 μL of diluted ant body extracts was subjected to GC-MS analysis prior to silica gel chromatography. Areas of GC peaks were used to calculate the cis-C11 to trans-C11 ratio. The ratios of cis-C11 to trans-C11 in alate queens were significantly greater than in workers for both ant forms. A similar difference between alate queens and workers was found in the ratios of cis-C11 to trans-C11 obtained from quantitation data. We also found that there was no significant deference in the ratios of cis-C11 to trans-C11 of workers between red form and dark form (df = 3, t = 1.60, P = 0.2069 for “direct injection,” df = 3, t = 1.01, P = 0.3850 for “quantitation,” t-test). However, the ratio of cis-C11 to trans-C11 in alate queens of dark form was significantly greater than that of red form (df = 3, t = 4.74, P = 0.0178 for “direct injection,” df = 3, t = 5.50, P = 0.0118 for “quantitation,” t-test).
Discussion
The results of this study confirm that both workers and alate queens of S. geminata produce prominently cis-C11 and trans-C11 piperidine alkaloids as previously reported (Brand et al., 1972, 1973b; MacConnell et al., 1976). We also identified some minor components as cis-C9, cis-C11:1, cis-C13:1, cis-C13, trans-C9, trans-C11:1, trans-C13:1, trans-C13, Δ1,6-C11, and Δ1,2-C11. An earlier report (Brand et al., 1972) only showed minor components, cis-C13:1 and cis-C13 in the venom of S. geminata workers. With better analytical instrumentation and methods, we clearly showed the presence of both cis and trans stereoisomers of C9, C11:1, C13:1, and C13 in workers and alate queens.
In the present study, we report for the first time the presence of cis- and trans-C9 as minor components in S. geminata workers. Both cis- and trans-C9 have been reported from the venom of alate queens of S. geminata, S. richteri and hybrid S. richteri × S. invicta (MacConnell et al., 1974; Chen et al., 2012), from the venom of workers of Solenopsis (Diplohoptrum) species collected from Puerto Rico (Jones et al., 1982, 1996; Blum et al., 1985), and of S. conjurata Wheeler collected from Costa Rica (Jones et al., 1984). Chen and Fadamiro (2009a) also reported the detection of cis-C9 in the venom of S. richteri workers. cis-C11:1, but not trans-C11:1, has been previously reported in alate queens of S. richteri, S. invicta, and their hybrid (Chen et al., 2012). The cis and trans stereoisomers of C13:1 and C13 are major components in worker venom of S. richteri, S. invicta, and their hybrid (Chen and Fadamiro, 2009a,b; Chen et al., 2010b).
Brand et al. (1972) first reported the identification of piperideine Δ1,2-C11 from S. xyloni venom. Recently, a number of studies have confirmed the presence of two series of piperideines including Δ1,6-C11 and Δ1,2-C11 in workers and alate queens of S. richteri, S. invicta, and their hybrid (Chen and Fadamiro, 2009a,b; Chen et al., 2009, 2010a,b, 2012). Piperideine Δ1,2-C11 has been hypothesized to act as an intermediate in the interconversion of cis and trans ring isomers (Brand et al., 1972). Later, the piperideines, Δ1,6-C11 and Δ1,2-C11, were proposed to function as precursors for the biosynthesis of fire ant alkaloids, cis- and trans-C11 (Leclercq et al., 1996; Chen and Fadamiro, 2009a). The detected piperideines could be the result of the extraction process, which would halt the normal biosynthetic progression and trap piperideine biosynthesis intermediates. As both cis- and trans-C11 are major components in the venoms of workers and alate queens of S. geminata, it is likely that enantioselective enzymes are present in this species which can reduce Δ1,6-C11 into both (2R,6S)-cis-C11 and (2R,6R)-trans-C11, and Δ1,2-C11 mainly into (2R,6S)-cis-C11 and partially into (2R,6R)-trans-C11. The presence of both Δ1,6- and Δ1,2-piperideines further supports the hypothesis that these piperideines are biosynthetic precursors for fire ant alkaloids. However, it is unlikely that already produced piperidines would undergo cis and trans ring interconversion through piperideine intermediates.
The total amount of alkaloids produced by 1 g of S. geminata workers was about 1.4 mg. However, 1 g of the imported Solenopsis ant workers produce 8–10 mg alkaloids (Yu et al., 2014), meaning that the imported fire ant workers can produce 5–6 times more alkaloids than S. geminata workers. It follows that the significantly higher production of total alkaloids in S. invicta would increase their fitness and competitive capability. Therefore, the much lower production of alkaloids in S. geminata workers may be a factor in the displacement of this species in its native range by introduced S. invicta. However, a previous study has demonstrated that the amounts of major venom alkaloid components in laboratory-maintained S. invicta colonies decreased 3–6 times in 3 months (Liu et al., 2015). Because the S. geminata colonies used for alkaloid extraction in the present study were maintained in the laboratory for several months, it is possible that total amounts of alkaloids produced by freshly collected S. geminata colonies are quantitatively similar to those produced by freshly collected S. invicta colonies.
The ratios of cis-C11 to trans-C11 in workers of two forms of S. geminata ranged from 0.36 to 0.67, indicating that trans-C11 was more dominant than cis-C11 in workers. In this study, we presented data calculated from 1 g of pooled ant samples. Brand et al. (1973a) analyzed venoms milked from 10 individuals with 1 μL capillary tube and estimated the ratio of cis-C11 to trans-C11 by a comparison of the two peak heights. For workers the ratio ranged from 0.71 to 1.98, for major workers 2.89–7.21, and for alate queens from 2.53 to 3.90. The venoms of two other Solenopsis species native to the United States, Solenopsis aurea Wheeler, and S. xyloni, consisted mainly of cis-C11 and trans-C11 as well. The ratio of cis-C11 to trans-C11 in workers of these two species was about 4:1 (Blum et al., 1973; Brand et al., 1973b; MacConnell et al., 1976). This ratio matched that of the equilibrium mixture formed during their chemical synthesis through Δ1,6-C11 or Δ1,2-C11 piperideines in the laboratory (Hill and Yuri, 1977; Jefford and Wang, 1993). While this concurrence is interesting and supports piperideine involvement in piperidine biosynthesis, we realize that a ratio based on thermodynamic equilibrium in a chemical reaction can be readily modified by enzymatic reactions during biosynthesis, thus resulting in the variety of cis/trans C11 ratios reported for Solenopsis species.
Chemical characteristics of Solenopsis piperidine alkaloids have been used as an indicator of fire ant evolution. The primitive venom alkaloid profile would consist of primarily C11 with the cis isomer dominating and the trans isomer at a much lower level (Brand et al., 1973b). This relatively simple venom was followed by a biosynthetic change in the cis- and trans-C11 ratio to a thermodynamically highly unfavorable decrease in cis and an increase in trans isomers, and then by further addition of abundant trans homologs with longer saturated and unsaturated side chains. Thus, the venoms of S. richteri and S. invicta (Brand et al., 1973b; Brand, 1978; Chen and Fadamiro, 2009b) are composed of primarily trans isomers and side chains greater than C11. On this basis, S. aurea, S. xyloni, and S. geminata would be classified as the more primitive species. The venom of Solenopsis eduardi Forel (a close relative of S. geminata) collected from Colombia consisted of more than 98% trans-C11, and about 1% of cis-C11 (MacConnell et al., 1976), which might represent a major shift from cis-C11 to trans-C11 alkaloids. It is likely that Δ1,6- and Δ1,2-piperideines, as biosynthetic precursors, play an important role in the evolutionary progression.
Although alate queens produced slightly more alkaloids than workers, there were no qualitative differences between the venoms of workers and of alate queens. The alate queens of S. geminata were found to have a significantly higher ratio of cis-C11 to trans-C11. The reported ratio of cis-C11 to trans-C11 in alate queens of S. geminata, 2.53–3.90 (Brand et al., 1973a), was slightly higher than our reported ratio for the red form and lower than that of the dark form. Even though peak height, instead of peak area, was used for calculation of the ratio in the previous report (Brand et al., 1973a), the actual ratio of cis-C11 to trans-C11 in alate queens may not be much different. Therefore, it can be suspected that the dark form of S. geminata has not been studied previously. The cis-C11 to trans-C11 ratio in alate queens of S. xyloni ranged from 12.37 to 20.75 (Brand et al., 1973a,b), which was much higher than that in alate queens of S. geminata, suggesting that S. xyloni is evolutionarily more primitive than S. geminata. The alate queens produce significantly higher amount of cis-C11, but much lower amount of trans-C11 than workers, suggesting that these same alkaloids may play distinct roles in alate queens and workers. Workers inject venom directly into other animals for defense and predation, or spray it throughout the nest environment, presumably for protection against microbial pathogens (Chen, 2007). The proportion of cis piperidines was found to gradually increase in the venom of queens as they became fully reproductive (Eliyahu et al., 2011). Queens apply their venoms over eggs as they are laid, presumably for protecting eggs from entomopathogenic fungi (Vander Meer and Morel, 1995; Tschinkel, 2006). These alkaloids in queens may not necessarily function as defensive compounds as in workers (Blum et al., 1958; Javors et al., 1993), but conceivably as queen pheromones. Venom alkaloids on the surface of eggs may play a role in advertising the presence and fertility status of queens (Vander Meer and Morel, 1995; Eliyahu et al., 2011). The workers are aggressive defenders against nest disturbance; however, queens move away from disturbances and seldom attempt to sting. The difference in venom alkaloid chemistry could account for these different behavior patterns of alate queens and workers. With this behavioral difference there would be a strong positive selection pressure for the production of more effective venom in the workers, but probably little selection pressure for more potent venom in the queens (Brand et al., 1973b). Therefore, the workers and queens in the more primitive species as S. xyloni and S. geminata have roughly the same venom composition, and the increasing differences in venom chemistry between workers and queens in newer species as S. richteri and S. invicta indicate evolutionary progression.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Jie-Yu Chen and Xiang-Cui Song (Yangtze University) for technical assistance. This work was supported by National Basic Research Program of China (973 Program, Grant No. 2012CB114105), and National Natural Science Foundation of China (Grant No. 30970402).
References
Baer, H., Liu, T.-Y., Anderson, M. C., Blum, M., Schmid, W. H., and James, F. J. (1979). Protein components of fire ant venom (Solenopsis invicta). Toxicon 17, 397–405. doi: 10.1016/0041-0101(79)90267-8
Blum, M. S. (1985). “Alkaloidal ant venoms: chemistry and biological activities,” in Bioregulators for Pest Control, eds P. A. Hedin, H. G. Cutler, B. D. Hammock, J. J. Menn, D. E. Moreland, and J. R. Plimmer (Washington, DC: American Chemical Society), 393–408. doi: 10.1021/bk-1985-0276.ch028
Blum, M. S. (1992). Ant venoms: chemical and pharmacological properties. Toxin Rev. 11, 115–164. doi: 10.3109/15569549209033107
Blum, M. S., Brand, J. M., Duffield, R. M., and Snelling, R. R. (1973). Chemistry of the venom of Solenopsis aurea (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 66, 702. doi: 10.1093/aesa/66.3.702
Blum, M. S., Fales, H. M., Leadbetter, G., Leonhardt, B. A., and Duffield, R. M. (1992). A new dialkylpiperidine in the venom of the fire ant Solenopsis invicta. J. Nat. Toxins 1, 57–63.
Blum, M. S., Jones, T. H., Lloyd, H. A., Fales, H. M., Snelling, R. R., Lubin, Y., et al. (1985). Poison gland products of Solenopsis and Monomorium species. J. Entomol. Sci. 20, 254–257.
Blum, M. S., Walder, J. R., and Callahan, P. S. (1958). Chemical, insecticidal, and antibiotic properties of fire ant venom. Science 128, 307–308. doi: 10.1126/science.128.3319.306-a
Brand, J. M. (1978). Fire ant venom alkaloids: their contribution to chemosystematics and biochemical evolution. Biochem. Syst. Ecol. 6, 337–340. doi: 10.1016/0305-1978(78)90055-8
Brand, J. M., Blum, M. S., and Barlin, M. R. (1973a). Fire ant venoms: intraspecific and interspecific variation among castes and individuals. Toxicon 11, 325–331. doi: 10.1016/0041-0101(73)90029-9
Brand, J. M., Blum, M. S., Fales, H. M., and MacConnell, J. G. (1972). Fire ant venoms: comparative analyses of alkaloidal components. Toxicon 10, 259–271. doi: 10.1016/0041-0101(72)90011-6
Brand, J. M., Blum, M. S., and Ross, H. H. (1973b). Biochemical evolution in fire ant venoms. Insect Biochem. 3, 45–51. doi: 10.1016/0020-1790(73)90017-6
Buren, W. F. (1972). Revisionary studies on the taxonomy of the imported fire ants. J. Ga. Entomol. Soc. 7, 1–26.
Chen, J. (2007). Qualitative analysis of red imported fire ant nests constructed in silica gel. J. Chem. Ecol. 33, 631–642. doi: 10.1007/s10886-006-9249-y
Chen, J., Cantrell, C. L., Shang, H.-W., and Rojas, M. G. (2009). Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: Formicidae). J. Agric. Food Chem. 57, 3128–3133. doi: 10.1021/jf803561y
Chen, J., Shang, H., and Jin, X. (2010a). Interspecific variation of △1,6-piperideines in imported fire ants. Toxicon 55, 1181–1187. doi: 10.1016/j.toxicon.2010.01.009
Chen, L., and Fadamiro, H. Y. (2009a). Re-investigation of venom chemistry in Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 53, 463–478. doi: 10.1016/j.toxicon.2008.12.019
Chen, L., and Fadamiro, H. Y. (2009b). Re-investigation of venom chemistry in Solenopsis fire ants. II. Identification of novel alkaloids in S. invicta. Toxicon 53, 479–486. doi: 10.1016/j.toxicon.2009.01.016
Chen, L., Hu, Q.-B., and Fadamiro, H. Y. (2010b). Reduction of venom alkaloids in Solenopsis richteri × Solenopsis invicta hybrid: an attempt to identify new alkaloidal components. J. Agric. Food Chem. 58, 11534–11542. doi: 10.1021/jf103402f
Chen, L., Lu, Y.-Y., Hu, Q.-B., and Fadamiro, H. Y. (2012). Similarity in venom alkaloid chemistry of alate queens of imported fire ants: implication for hybridization between Solenopsis richteri and S. invicta in the Southern United States. Chem. Biodivers. 9, 702–713. doi: 10.1002/cbdv.201100109
Creighton, W. S. (1930). The New World species of the genus Solenopsis (Hymenop. Formicidae). Proc. Am. Acad. Arts Sci. 66, 39–151. doi: 10.2307/20026320
Cruz-Lopez, L., Rojas, J. C., De La Cruz-Cordero, R., and Morgan, E. D. (2001). Behavioral and chemical analysis of venom gland secretion of queens of the ant Solenopsis geminata. J. Chem. Ecol. 27, 2437–2445. doi: 10.1023/A:1013671330253
Dall'Aglio-Holvorcema, C. G., Benson, W. W., Gilbert, L. E., Trager, J. C., and Trigo, J. R. (2009). Chemical tools to distinguish the fire ant species Solenopsis invicta and S. saevissima (Formicidae: Myrmicinae) in Southeast Brazil. Biochem. Syst. Ecol. 37, 442–451. doi: 10.1016/j.bse.2009.05.017
Deslippe, R. J., and Guo, Y. (2000). Venom alkaloids of fire ants in relation to worker size and age. Toxicon 38, 223–232. doi: 10.1016/S0041-0101(99)00147-6
Eliyahu, D., Ross, K., Haight, K., Keller, L., and Liebig, J. (2011). Venom alkaloid and cuticular hydrocarbon profiles are associated with social organization, queen fertility status, and queen genotype in the fire ant Solenopsis invicta. J. Chem. Ecol. 37, 1242–1254. doi: 10.1007/s10886-011-0037-y
Gavilanez-Slone, J., and Porter, S. D. (2013). Colony growth of two species of Solenopsis fire ants (Hymenoptera: Formicidae) reared with crickets and beef liver. Fla. Entomol. 96, 1482–1488. doi: 10.1653/024.096.0428
Hill, R. K., and Yuri, T. (1977). An approach to natural 2-alkyl-6-methylpiperidines via N-acyllactam rearrangement. Tetrahedron 33, 1569–1571. doi: 10.1016/0040-4020(77)80162-2
Javors, M. A., Zhou, W., Maas, J. W., Han, S., and Keenan, R. W. (1993). Effects of fire ant venom alkaloids on platelet and neutrophil function. Life Sci. 53, 1105–1112. doi: 10.1016/0024-3205(93)90546-F
Jefford, C. W., and Wang, J. B. (1993). An enantiospecific synthesis of solenopsin A. Tetrahedron Lett. 34, 2911–2914. doi: 10.1016/S0040-4039(00)60479-3
Jones, T. H., Blum, M. S., and Fales, H. M. (1982). Ant venom alkaloids from Solenopsis and Monomorium species: recent developments. Tetrahedron 38, 1949–1958. doi: 10.1016/0040-4020(82)80044-6
Jones, T. H., Highet, R. J., Blum, M. S., and Fales, H. M. (1984). (5Z,9Z)-3-alkyl-5-methylindolizidines from Solenopsis (Diplorhoptrum) species. J. Chem. Ecol. 10, 1233–1249. doi: 10.1007/BF00988551
Jones, T. H., Torres, J. A., Spande, T. F., Garraffo, H. M., Blum, M. S., and Snelling, R. R. (1996). Chemistry of venom alkaloids in some Solenopsis (Diplorhoptrum) species from Puerto Rico. J. Chem. Ecol. 22, 1221–1236. doi: 10.1007/BF02266962
Leclercq, S., Braekman, J. C., Daloze, D., Pasteels, J. M., and Vander Meer, R. K. (1996). Biosynthesis of the solenopsins, venom alkaloids of the fire ants. Naturwissenschaften 83, 222–225. doi: 10.1007/BF01143328
Leclercq, S., Thirionet, I., Broeders, F., Daloze, D., Vander Meer, R. K., and Braekman, J. C. (1994). Absolute configuration of the solenopsins, venom alkaloids of the fire ants. Tetrahedron 50, 8465–8478. doi: 10.1016/S0040-4020(01)85567-8
Liu, X.-F., Chen, L., and Li, J.-K. (2015). Dynamic analysis of venomous alkaloids in workers of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae) maintained in the laboratory. Acta Entomol. Sin. 58, 22–27. doi: 10.16380/j.kcxb.2015.01.003
MacConnell, J. G., Blum, M. S., Buren, W. F., Williams, R. N., and Fales, H. M. (1976). Fire ant venoms: chemotaxonomic correlations with alkaloidal compositions. Toxicon 14, 69–78. doi: 10.1016/0041-0101(76)90122-7
MacConnell, J. G., Blum, M. S., and Fales, H. M. (1970). Alkaloid from fire ant venom: identification and synthesis. Science 168, 840–841. doi: 10.1126/science.168.3933.840
MacConnell, J. G., Blum, M. S., and Fales, H. M. (1971). The chemistry of fire ant venom. Tetrahedron 27, 1129–1139. doi: 10.1016/S0040-4020(01)90860-9
MacConnell, J. G., Williams, R. N., Brand, J. M., and Blum, M. S. (1974). New alkaloids in the venoms of fire ants. Ann. Entomol. Soc. Am. 67, 134–135. doi: 10.1093/aesa/67.1.134
Pianaro, A., Fox, E. G. P., Bueno, O. C., and Marsaioli, A. J. (2012). Rapid configuration analysis of the solenopsins. Tetrahedron 23, 635–642. doi: 10.1016/j.tetasy.2012.05.005
Porter, S. D. (1992). Frequency and distribution of polygyne fire ants (Hymenoptera: Formicidae) in Florida. Fla. Entomol. 75, 248–256. doi: 10.2307/3495627
Porter, S. D., van Eimeren, B., and Gilbert, L. E. (1988). Invasion of red imported fire ants (Hymenoptera: Formicidae): microgeography of competitive replacement. Ann. Entomol. Soc. Am. 81, 913–918. doi: 10.1093/aesa/81.6.913
Ross, K. G., Gotzek, D., Ascunce, M. S., and Shoemaker, D. D. (2010). Species delimitation: a case study in a problematic ant taxon. Syst. Biol. 59, 162–184. doi: 10.1093/sysbio/syp089
Trager, J. C. (1991). A revision of the fire ants, Solenopsis geminata group (Hymenoptera: Formicidae: Myrmicinae). J. N.Y. Entomol. Soc. 99, 141–198.
Tschinkel, W. R. (1988). Distribution of the fire ants Solenopsis invicta and S. geminata (Hymenoptera: Formicidae) in northern Florida in relation to habitat and disturbance. Ann. Entomol. Soc. Am. 81, 76–81. doi: 10.1093/aesa/81.1.76
Vander Meer, R. K. (1986). “Chemical taxonomy as a tool for separating Solenopsis spp.,” in Fire Ants and Leaf Cutting Ants: Biology and Management, eds C. S. Lofgren and R. K. Vander Meer (Boulder, CO: Westview Press), 316–326.
Vander Meer, R. K., and Lofgren, C. S. (1988). Use of chemical characters in defining populations of fire ants, Solenopsis saevissima complex, (Hymenoptera: Formicidae). Fla. Entomol. 71, 323–332. doi: 10.2307/3495440
Vander Meer, R. K., and Morel, L. (1995). Ant queens deposit pheromones and antimicrobial agents on eggs. Naturwissenschaften 82, 93–95. doi: 10.1007/BF01140150
Vinson, S. B., and Greenberg, L. (1986). “The biology, physiology, and ecology of imported fire ants,” in Economic Impact and Control of Social Insects, ed S. B. Vinson (New York, NY: Praeger Publishers), 193–226.
Wetterer, J. K. (2011). Worldwide spread of the tropical fire ant, Solenopsis geminata (Hymenoptera: Formicidae). Myrmecol. News 14, 21–35.
Wojcik, D. P. (1994). “Impact of the red imported fire ant on native ant species in Florida,” in Exotic Ants: Biology, Impact, and Control of Introduced Species, ed D. F. Williams (San Francisco, CA: Westview), 269–281.
Keywords: Solenopsis geminata, fire ant, piperidine, GC-MS, isomer
Citation: Shi Q-H, Hu L, Wang W-K, Vander Meer RK, Porter SD and Chen L (2015) Workers and alate queens of Solenopsis geminata share qualitatively similar but quantitatively different venom alkaloid chemistry. Front. Ecol. Evol. 3:76. doi: 10.3389/fevo.2015.00076
Received: 29 April 2015; Accepted: 29 June 2015;
Published: 22 July 2015.
Edited by:
Qing-He Zhang, Sterling International, Inc., USAReviewed by:
Dong-Hwan Choe, University of California, Riverside, USAGiovanni Benelli, University of Pisa, Italy
Copyright © 2015 Shi, Hu, Wang, Vander Meer, Porter and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen, State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, 1-5 West Beichen Road, Chaoyang District, Beijing 100101, China,Y2hlbmxpQGlvei5hYy5jbg==
†These authors have contributed equally to this work.
 Qun-Hui Shi
Qun-Hui Shi Lin Hu
Lin Hu Wen-Kai Wang
Wen-Kai Wang Robert K. Vander Meer
Robert K. Vander Meer Sanford D. Porter3
Sanford D. Porter3 Li Chen
Li Chen