- 1Instituto de Investigación en Recursos Cinegéticos, Consejo Superior de Investigaciones Científicas, Universidad de Castilla La Mancha, Ciudad Real, Spain
- 2Departamento de Ecología Evolutiva, Museo Nacional de Ciencias Naturales – Consejo Superior de Investigaciones Científicas, Madrid, Spain
Environmental conditions during early life may shape phenotype in adulthood. Early adverse conditions may increase the oxidative stress in adults, which could affect their reproductive output and survival. It has also been hypothesized that the larger the reproductive investment, the higher the oxidative stress. We tested this and the potential influence of early oxidative stress on how individuals respond to a reproductive stimulation. The synthesis of the antioxidant glutathione was inhibited in captive zebra finches (Taeniopygia guttata) during growth. In adulthood, the expression of a carotenoid-based sexual signal, bill redness, increased in both sexes, with females also being heavier than controls. The social context of control and glutathione-inhibited males was then manipulated to stimulate precopulatory reproductive investments. Males were individually caged in front of a female or another male. We predicted that males enduring lower early antioxidant levels and placed close to a female should pay the highest cost of a hypothetical increase in bill redness in terms of oxidative damage. However, early conditions only influenced the male's phenotype via their partners. Males caged with females showed increases in circulating pigment (carotenoid) levels, but only when females endured early low antioxidant values. This was probably related to the higher attractiveness of these females. Nevertheless, the bill redness of males did not differ during the social manipulation. Moreover, males facing females from any early condition group showed lower oxidative damage levels in plasma lipids. This result agrees with some findings in rodents, also in captivity. However, the effect may be due to increased triglyceride levels and body mass in males not facing females, as variation in these traits explained oxidative damage variability. The importance of considering housing conditions and life history when interpreting oxidative stress-related trade-offs is highlighted.
Introduction
Environmental conditions experienced during early development may exert strong effects on adult phenotype thanks to developmental plasticity (West-Eberhard, 2003). It is also known that the earlier the age at which the environmental conditions affect the organism, the stronger and longer-lasting the effects on the adult phenotype (Metcalfe and Monaghan, 2001; Rinaudo and Wang, 2012). These early development effects may influence a large number of traits in adults, including morphology, physiology and behavior (e.g., Spencer et al., 2003; Naguib and Nemitz, 2007; Rinaudo and Wang, 2012). Among physiological modifications, it has been shown that adverse early conditions may increase oxidative stress in adulthood (e.g., Luo et al., 2006; Tarry-Adkins et al., 2009; Metcalfe and Alonso-Alvarez, 2010).
Oxidative stress is usually defined as the imbalance between the production of pro-oxidative compounds by cell metabolism and immune responses and the state of the antioxidant machinery (Halliwell and Gutteridge, 2007). Oxidative stress has attracted the attention of biomedicine due to its relationship with important human diseases (e.g., cancer), and accelerated senescence in different organisms (Kirkwood and Austad, 2000; Halliwell and Gutteridge, 2007; Yan, 2014).
Early development conditions may influence survival prospects, but also the reproductive investment (e.g., Lindstrom, 1999; Vincenzi et al., 2013; Zedrosser et al., 2013). Reproduction is usually interpreted as a resource (energy, nutrients, time)-demanding process that implies a cost for the individual in terms of a decline in subsequent fecundity or survival (Reznick, 1992; Stearns, 1992, 2011). It is often postulated that individuals of iteroparous species face a trade-off between investing those limiting resources to self-maintenance vs. current reproduction (Stearns, 1992; Alonso-Alvarez and Velando, 2012). Oxidative stress may participate in that trade-off. However, oxidative stress could merely be involved in reproduction by increasing cell metabolism, generating higher levels of pro-oxidants as byproducts and not implying any limiting resource (see Alonso-Alvarez et al., 2004a). Nonetheless, dietary antioxidants (e.g., some vitamins and carotenoids) could additionally act as limiting resources. Thus, a trade-off in the investment of antioxidants in protecting the organism (the soma) vs. in defending reproductive components such as gametes (e.g., sperm protection) or embryos (e.g., in the egg yolk) can also be suggested (see Blount et al., 2000; Velando et al., 2008; Pike et al., 2009).
The cited trade-offs may lead to an oxidative cost of reproduction (Salmon et al., 2001; Alonso-Alvarez et al., 2004a). The existence of that cost has, however, been questioned due to the inconsistent results obtained by the relatively few studies experimentally manipulating the whole (or large part) of the reproductive investment (reviewed in Stier et al., 2012; Metcalfe and Monaghan, 2013; Speakman and Garratt, 2014). One of the criticisms is the scarcity of studies testing the product of oxidative imbalance; that is, the oxidative damage to the main biomolecules (e.g., lipids; Metcalfe and Monaghan, 2013). We should, in any event, consider the more abundant literature on the role of oxidative stress in the production of secondary sexual traits, which are particular components of reproductive investment.
Secondary sexual traits should be costly when they evolve as signals of individual quality. According to the handicap principle, individuals can only express secondary sexual traits at an intensity matching their phenotypic (and/or genetic) quality as a consequence of an inherent cost in the expression of the traits, a fact that would assure their reliability as signals of individual condition (i.e., the handicap theory, Zahavi, 1975; Grafen, 1990). In this context and as in other reproductive trade-offs, a conflict between investing antioxidants in the production of sexual signals and self-maintenance was proposed (Møller et al., 2000; reviewed in Metcalfe and Alonso-Alvarez, 2010). This trade-off is mostly based on the fact that certain pigments used in many yellow-to-red secondary sexual traits (i.e., carotenoids) as well as other antioxidants used in protecting them from oxidative bleaching (e.g., vitamin A, C, and E) are only obtained through the diet, and are perhaps scarce in food and act as antioxidants (Møller et al., 2000; Hartley and Kennedy, 2004; Pérez-Rodríguez, 2009; but see also Hill and Johnson, 2012). Similarly, melanins in sexual signals have also been related to oxidative stress. In this case, the synthesis of yellowish-to-reddish melanins (pheomelanins) depends on the availability of dietary sulfur-containing amino acids, which are also required for synthesizing an important intracellular antioxidant (i.e., glutathione; Galván and Alonso-Alvarez, 2009; Galvan and Solano, 2009). In contrast, a certain low level of glutathione is required to trigger the synthesis of gray-to-black melanins (eumelanins), an adequate availability of alternative antioxidants being necessary to compensate for such a low glutathione concentration (Galván and Alonso-Alvarez, 2008).
The studies experimentally testing the cost of the whole of the reproductive investment in terms of oxidative damage (cited in Stier et al., 2012; Metcalfe and Monaghan, 2013; Speakman and Garratt, 2014) have not explored how early development conditions influence the outcome. This is an important point as the lack of consistency among studies may in part come from differences in the life history trajectory of individuals (i.e., life history trade-offs; Stearns, 1992). Again, however, studies on sexual signaling provide valuable results in this context. Thus, three experimental works have tested the impact of early conditions on the oxidative cost of sexual signaling. Alonso-Alvarez and Galván (2011) showed that red-legged partridges (Alectoris rufa) experimentally exposed to a free radical generator (diquat) during development showed lower levels of lipid oxidative damage in adulthood, but also produced paler red bills and eye rings (also Galván and Alonso-Alvarez, 2009). In contrast, Orledge et al. (2012) did not detect effects on both adult oxidative damage and sexual signaling in ring-necked pheasants (Phasianus colchicus) that received a dietary antioxidant supply during growth. Finally, Romero-Haro and Alonso-Alvarez (2015) found that adult zebra finches (Taeniopygia guttata) whose levels of glutathione were experimentally decreased during development produced redder bills, but endured higher oxidative damage.
In the present study, we tested the hypothesis that early development conditions influence the way in which individuals afford the oxidative cost of reproduction. We used a captive population of zebra finches that was also studied in Romero-Haro and Alonso-Alvarez (2015). In this bird species, the color of the bill is generated by carotenoid pigments (McGraw and Toomey, 2010), and bill color intensity plays a role in mate choice and reproductive decisions (Simons et al., 2012a, and references therein). As mentioned above, the effects of partially inhibiting the synthesis of glutathione on a large sample of birds (N = 409) during their first days of life was studied, describing that these birds produced redder bills in adulthood, with females also being heavier than controls (i.e., the cited Romero-Haro and Alonso-Alvarez, 2015 study). Such as commented, the same birds also showed higher levels of oxidative damage in circulating lipids. We hypothesized that these findings could reveal a higher investment in early reproduction (advanced sexual signaling) at the cost of oxidative stress because the ultimate consequences of oxidative stress would be paid later in life, leading to accelerated senescence (the Harman, 1956 free radical theory; see also an updated version in Sohal and Orr, 2012).
Here, we manipulated the social environment of a large subsample (n = 262) of those adult birds described in Romero-Haro and Alonso-Alvarez (2015), testing the consequences on the color, morphology and oxidative stress level of males (n = 144). We first verified that the morphological and color differences found in the whole sample (i.e., Romero-Haro and Alonso-Alvarez, 2015) remained in the subsample. Each male was then caged in front of another male or a female. The two birds in each cage were separated by a grille that allowed visual and acoustic contact. On the basis of a previous experiment on the same species (i.e., Gautier et al., 2008), we predicted that males housed in front of a female should experience pre-copulatory reproductive costs compared to males housed with same-sex birds. We accordingly assume that reproductive costs should be circumscribed to the production of primary (e.g., Mantei et al., 2008) or secondary (i.e., Gautier et al., 2008) sexual traits, but not to copulation, nest building, nestling feeding, protection, etc. We predict that males housed in front of females should invest more in bill coloration (see Gautier et al., 2008) and, therefore, circulate more carotenoid pigments in the blood. The same birds should experience higher levels of oxidative damage due to allocation of antioxidants to the mechanisms involved in color generation, and lose more body mass due to higher energy demands compared to males housed with males. We also predict that males that supported lower antioxidant levels during early life could in some way be constrained to afford these investments as a direct result of oxidative damage in adulthood (see Romero-Haro and Alonso-Alvarez, 2015) and/or as a derived cost of antioxidant compensatory mechanisms (e.g., Costantini et al., 2010), in both cases perhaps leading to body mass loss during the experiment. Alternatively, the same individuals could invest more in sexual signaling to obtain short-term fitness returns, potentially at the cost of higher oxidative damage and/or body mass loss (e.g., Lee et al., 2014). Furthermore, since the bill color in zebra finches also reveals female fecundity (Simons et al., 2012a), males housed with redder females (i.e., those enduring low glutathione levels in early life) should increase their reproductive investment to improve their fitness.
Materials and Methods
Experimental Design
The study was performed in indoor aviaries located at Dehesa Galiana experimental facilities (Ciudad Real, Spain). Eighty randomly formed zebra finch pairs were placed in breeding cages (0.6 × 0.4 × 0.4 m) with a nestbox (11 × 13 × 13 cm), receiving water and food (a commercial mix of seeds; KIKI, Callosa de Segura, Spain) ad libitum. Temperature (mean 22 ± 1°C) and light-dark daily cycles (16L: 8D) were controlled. The pairs bred over a 5-month period. Twelve pairs did not breed during the experiment, 22 pairs reproduced once, 22 pairs reproduced twice, 21 three times and three pairs produced four broods. Reproduction was monitored every 2 days.
The chronogram is shown in Figure 1. The early environment of the birds was manipulated when nestlings reached a minimum body mass of 3 g (3 days old approximately; mean ± SE: 4.82 g ± 0.03). Half of the nestlings in a brood were randomly assigned to a treatment receiving DL-buthionine-S,R-sulfoximine (BSO; Sigma, ref. B2640) diluted in sterilized serum (n = 206) and the other half (n = 203) received sterilized serum only (controls). BSO is a specific inhibitor of glutathione synthesis that selectively blocks the activity of the enzyme glutamate cysteine ligase, GCL (also γ-glutamylcysteine synthetase, GCS; Griffith, 1982), the first enzyme in the glutathione biosynthesis pathway (Wu et al., 2004). Details are fully described in Romero-Haro and Alonso-Alvarez (2015). Briefly, we randomly allocated a treatment to the heaviest chick in a brood and then successively alternated the treatment category among its siblings (e.g., control, BSO, control, BSO). The BSO dose was previously calculated from a pilot experiment that was, in turn, based on a previous work in great tit (Parus major) nestlings (Galván and Alonso-Alvarez, 2008). The pilot study involved 10 breeding zebra finch pairs and 26 nestlings. Total glutathione (tGSH) in red blood cells (RBCs) was determined in 14 days-old pilot nestlings (below). The dose producing a moderate but significant decrease in tGSH was chosen (i.e., 50 mg BSO per mL of serum). In the definitive experiment, a volume of 0.06 mL of the solution or serum only was subcutaneously injected in the back every 2 days from 6 to 12 days old (i.e., four injections; see also Galván and Alonso-Alvarez, 2008). Thus, BSO chicks received a total of 12 mg BSO. A blood sample was collected for each bird 8 days after the start of the injection (14 days old).
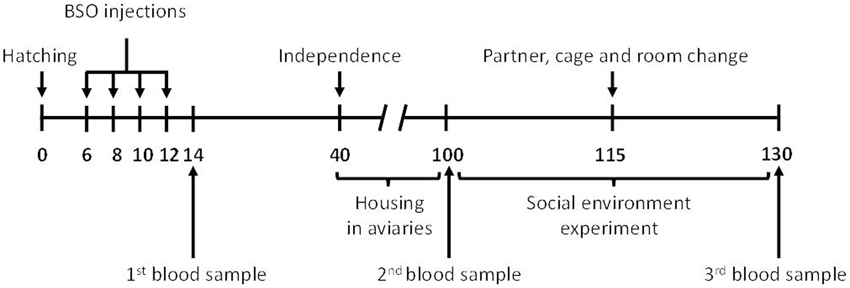
Figure 1. Chronogram of the experiment. The figures are days of life from hatching. Birds were always housed in cages except during the period from independence to the start of the social manipulation. BSO, buthionine sulfoximine, an inhibitor of antioxidant (glutathione) synthesis.
Males and females were separately housed in different rooms (2.80 × 3 × 2.50 m each one) when they reached approximately 40 days of age (mean ± SE: 39 days ± 0.12), i.e., an age at which all birds were independent (Zann, 1996; Alonso-Alvarez et al., 2006). These indoor aviaries were also under controlled temperatures (mean 22 ± 1°C), received food and water ad libitum, and were not acoustically isolated.
Birds were then placed in cages when they reached about 100 days of age (mean ± SE: 97.8 days ± 0.84). The cages (60 cm width, 28 cm long, and 27 cm high) were symmetrically divided by a grille producing two identical compartments that included water and food (see above) ad libitum. One bird was placed on each side of the cage, with birds in the same cage being in visual contact. Some cages included one male and one female (mixed social environment), and other cages included two males only. This was the first time in life that a male was in visual contact with a genetically unrelated (i.e., not the mother or sister) female. The two birds placed in the same cage for manipulating social context were genetically unrelated (i.e., they were not siblings and did not share parents). The males were randomly assigned to one of the two social environments. The early condition treatments (BSO or control) were balanced between males in different social environments (see Results).
Thirty-six cages were distributed in four rooms (2.80 × 3 × 2.50 m each one). Two rooms included mixed social environment cages (12 cages each), and the other two rooms included males only. The two types of rooms were situated in parallel and alternated in the space (i.e., mixed, male-only, mixed and male-only environments). Each room housed 12 males, the total number of birds in each social environment differing due to females only. The cages in each aviary were hung on two opposing walls, half of them in each wall. Hence, in addition to the bird on the other side of its cage, each male was able to see another six males on the other side of the room, that is, independent of the type of environment. Alternatively, if we had equalized the total number of birds (males or females) per aviary (e.g., 24 birds per room), males in the male-only environment would have been exposed to twice as many males as mixed social environment males. In that case, the effect of female presence could have not been distinguished from the effect of different male densities (e.g., different levels of aggressive display).
All birds were exchanged among cages and rooms 15 days after being placed in the cages, with the social environment maintained for each bird, but not the identity of the partners. The early environment treatment of the partner was maintained. These changes were made to reduce habituation to the partner and a potential influence of the room. The experiment was replicated three times including 48 males in each replicate (n = 144 males). These males were a randomly chosen subsample (76 control and 68 BSO males) of the birds described above (N = 208 males). Seven females died during the course of the experiment apparently due to natural causes (see Results), and were substituted with females maintaining the same early treatment category. In total, the females used as partners for the experiment were also a random part (n = 118; 59 control and 59 BSO females) of the whole of the sample (N = 191). Another 10 birds died earlier (before 14 days old) and could not be molecularly sexed or used for the coupling experiment (Romero-Haro and Alonso-Alvarez, 2015).
All the individuals used were weighed just before being placed in the cages and again at the end of the study (30 days after; Figure 1). The tarsus length was measured at the start of this period. Similarly, a blood sample from the jugular vein and color measurements (below) were taken for each bird at the time of placement in the cages and 30 days later. The blood samples were stored at 4°C in Eppendorf tubes after extraction, and centrifuged at 5.000 g within 4 h. The plasma was then removed and the buffy coat discarded by pipetting. Plasma and erythrocyte (RBC) fractions were separately stored at −80°C. Birds were released to outdoor aviaries at the end of the study to be studied over their lifetime.
The present study was approved by the Bioethical Committee of Consejo Superior de Investigaciones Científicas (CSIC, Spain) and Animal Experimentation Ethical Committee of the Universidad de Castilla La Mancha (UCLM, Albacete, Spain; approval number: 1201-08) as well as supervised by the veterinary staff of the Instituto de Investigación en Recursos Cinegéticos (IREC, Spain).
Color Measurements
A digital picture of the upper surface of the upper mandible was obtained by placing the bird in a decubitus prone position. A Nikon D3100 (objective: Nikkor DX 18–55 mm) camera was always used, and high-resolution pictures (3456 × 2304 pixels) were obtained. The focus and diaphragm of the camera were fixed (i.e., the automatic functions were not used). All the images were obtained under standardized light conditions, by placing the camera at the same position and distance from the bird using a reproduction base including lights (Repro Base RB260 2 × 11 W 6000°K; Kaiser Fototechnik, Buchen). The birds were always positioned on a gray standard background to avoid reflections, and a standard red chip was placed close to the bird's head (both by Kodak, NY). Pictures from some individuals could not be analyzed due lack of focus or poor bird position (initial measure: 17 males and 7 females; final measure 15 males and 7 females). Adobe Photoshop CS3 was used for image measurements. All the measures were performed by a technician blind to bird identity. Mean RGB values for redness analyses were obtained per duplicate in a subsample, and were shown to be repeatable [r = 0.91, P = 0.001, n = 68; by following Lessells and Boag (1987), here and thereafter]. The hue values were calculated from RGB data by using the algorithm provided in Foley and Van Dam (1984). Lower hue values denoted redder colors.
Plumage Traits
One picture of the breast was also obtained for adults by placing the animal in a decubitus supine position. The same camera and light conditions were used. The area of the black bib was only measured in males, as females do not exhibit a conspicuous bib (Zann, 1996). Pictures from three individuals could not be analyzed due to poor bird position. Adobe Photoshop CS3 was used for area measurements. Two variables of the bib were measured: the homogeneous black zone only (selected manually), and the total area including the horizontal black stripes (using the magic wand tool in Adobe with a fixed tolerance). In addition, one picture of both flanks was obtained for male adult birds only (it is also a sexually dimorphic trait) by placing the animal in a lateral position and raising the wing. The same camera and light conditions were used. The number of white spots on each flank was counted and the average from both wings was calculated. All measures were taken by a technician blind to bird identity.
Glutathione Quantification
Glutathione was quantified following Griffith's (1980) method with modifications. Briefly, the blood pellet in the tube was thawed and immediately diluted (1:10 w/v) and homogenized in a stock buffer (0.01M PBS and 0.02M EDTA), working on ice to avoid oxidation. Three working solutions were created in the same stock buffer as follows: 0.3 mM NADPH (solution I), 6 mM DTNB (solution II), and 50 units of glutathione reductase mL-1 (solution III). An aliquot (250 μL) of homogenate of blood cells was vortexed with 250 μL phosphate buffer and 0.5 mL of diluted trichloroacetic acid (10% in H2O) three times, for 5 s each time, within a 15-min period. In the meantime, samples were protected from light and refrigerated to prevent oxidation. The mixture was then centrifuged (1125 g for 15 min at 6°C), and the supernatant removed. Subsequent steps were performed in an automated spectrophotometer (A25-Autoanalyzer, Biosystems, Barcelona, Spain). Solutions I and II were mixed at a ratio of 7:1 v/v, respectively. One-hundred and sixty μL of this new mixture was automatically added to 40 μL of sample (i.e., supernatant) in a cuvette. Then, 20 μL of solution III was added after 15 s, and the absorbance at 405 nm was monitored after 30 and 60 s. The change in absorbance was used to determine total glutathione (tGSH) levels by comparing the output with the results from a standard curve generated by serial dilution of glutathione from 1 mM to 0.031 mM. Results are given in μmol per gram of pellet. Previous studies in other bird species (i.e., Alonso-Alvarez et al., 2010) reported that tGSH levels assessed twice are highly repeatable (r = 0.89 and 0.92, respectively, n = 25).
Plasma Total Carotenoids
Plasma aliquots (5 μL) were diluted in ethanol (1:10) in 0.6 mL tubes. Tubes were vortexed for 3 min. The flocculent protein was precipitated by centrifuging at 1500 g for 10 min at 4°C. The absorbance of the supernatant was determined at 446 nm in a Biotek microplate reader (PowerWave XS2, Bio-Tek Instruments Inc., Winooski, VT). A standard curve of lutein (Sigma) was used for quantifying total carotenoids (Romero-Haro and Alonso-Alvarez, 2014). A subsample assessed twice produced high repeatabilities (r = 0.87, P < 0.001, n = 26).
Oxidative Damage in Blood Lipids
The protocol described in Agarwal and Chase (2002) with modifications by Nussey et al. (2009) was followed to assess the amount of a product of lipid peroxidation named malondialdehyde (MDA; Halliwell and Gutteridge, 2007) by means of HPLC. A standard curve for calibration was prepared using a 1,1,3,3-tetraethoxypropane (TEP) stock solution (5 μM in 40% ethanol) serially diluted using 40% ethanol. Fifteen μL of a butylated hydroxytoluene (BHT) solution (0.05% w/v in 95% ethanol), 120 μL phosphoric acid solution (0.44 M) and 30 μL thiobarbituric acid (TBA) solution (42 mM) were added to 15 μL of plasma or standards. Plasma and standards were capped and vortexed for 5 s. The standards and samples were then heated at 100°C for 1 h in a dry bath incubator to allow formation of MDA-TBA adducts. The reaction was stopped by placing samples and standards on ice for 5 min. Subsequently, n-butanol was added to each tube to extract the MDA-TBA complex. Seventy-five μL of n-butanol were added to plasma samples and their standards. Subsequently, tubes were vortexed for 60 s and centrifuged at 18,000 G at 4°C for 3 min. Fifty μL of the plasma and standards of the upper (n-butanol) phase were moved to HPLC vials, which were immediately saturated with N2 to avoid oxidation. Samples and standards were injected into an Agilent 1100 Series HPLC system (Agilent Technologies, Waldbronn, Germany) fitted with a fluorescence detector set and a 5 μm ODS-2 C-18 4.0 × 250 mm column maintained at 37°C. The mobile phase was MeOH:KH2PO4 (50 mM) (40:60 v/v), running isocratically for 10 min at a flow rate of 1 mL min-1. Data were collected at 515 nm (excitation) and 553 nm (emission). A subsample of plasma samples assessed twice produced very high intra-session (r = 0.97, P <0.001, n = 20) and inter-session (r = 0.98, P <0.001, n = 20) repeatabilities.
Erythrocyte Resistance to Oxidative Stress in Adults
The resistance to oxidative stress in adult birds was assessed by measuring the time needed to hemolyse 50% of the erythrocytes exposed to a controlled free radical attack (full description in Romero-Haro and Alonso-Alvarez, 2015). The principle of this in vitro test is to submit whole blood to thermo-controlled free radical aggression by adding 2,2-azobis-(aminodinopropane) hydrochloride (AAPH), measuring the decrease in optical density of the solution. The larger the time required to hemolyze 50% of RBC, the stronger the resistance to oxidative stress.
Plasma Antioxidants
A technique often called Total Antioxidant Status (TAS) was assessed to estimate the availability of non-enzymatic antioxidants. Since the idea that this measure assesses all the antioxidants is questionable, the term “total” was avoided, and hence, we will only use a generic “Plasma Antioxidants” (PLAOX). The procedure is based on Miller et al. (1993) modified by Cohen et al. (2005; see also Romero-Haro and Alonso-Alvarez, 2015).
Plasma Triglycerides and Uric Acid Levels
The glycerol phosphate oxidase/peroxidase method and the uricase/peroxidase method were used for measuring triglyceride and uric acid levels, respectively, by means of commercial kits (Biosystems, Barcelona, Spain). The levels of each parameter were assessed in 5 μl of plasma using the same Bio-Tek microplate reader (see above) fixed at 505 and 520 nm, respectively. Repeatabilities in 45 samples assessed twice were high (triglycerides: r = 0.94, P <0.001; uric acid: r = 0.99, P < 0.001).
Statistical Analyses
Generalized linear mixed models were used to analyze the influence of the early development treatment on the appearance (color and morphological variables) of the birds (both males and females) at the start of the social environment manipulation. These initial analyses (first two subsections of Results) were different from those presented in Romero-Haro and Alonso-Alvarez (2015) as they were performed in a subsample of those birds. To test for these effects, the MIXED procedure in SAS software (9.3 v.) was used (SAS Institute; Littell et al., 2006). The sex of the bird as well as the early development group (BSO-treated or control) were tested as fixed factors, also testing their interaction. Since some parents of the birds produced more than one brood, the identity of the brood where the focus male was born nested into the identity of the cage was included as a random term.
Similar models were used when testing the influence of the early development and adult social environments on the focus male. Here, both the GLIMMIX and MIXED procedures in SAS software were used. The sex of the partner as well as the early development treatment of the focus male and the partner (BSO vs. control) were added as three fixed factors, testing all their interactions. Again, the identity of the brood where the focus male was born nested into the identity of the cage was included as a random term. All the P-values for random factors in this study ranged from 0.001 to 0.798. To test mortality among focus males, the binomial distribution and logit link function were done by the GLIMMIX procedure, testing the three fixed factors and interactions. The other variables were analyzed using the MIXED procedure as they were normally distributed. In the case of models testing blood variables, the laboratory session was always included as an additional random factor. Initial and final samples of the same individual were always analyzed in the same laboratory session. The replicate of the experiment (three blocks) was added as an additional random factor. In the case of the body mass and blood variables, the effect of the social environment experiment and other factors and interactions were tested on the change (dependent variable) calculated by subtracting initial from final values (before and after the manipulation). At the start of that social context manipulation, the values of physiological variables (i.e., tGSH in erythrocytes, plasma carotenoids, MDA and triglycerides) did not differ between the different treatments (all p > 0.11), with interactions between them also being non-significant (all p > 0.10). The body mass change was calculated as the proportion of change with regard to initial values (body mass gain). Alternatively, mixed models testing the final value as the dependent variable and the initial value as a covariate in order to control for initial variability were also used, providing virtually identical results (Supplementary Information, Tables S1–S3). Furthermore, repeated-measures mixed models including the sampling time as a two-level fixed factor (final vs. initial measure) and testing its interaction with treatments were also analyzed, which required the inclusion of the identity of the bird as another random factor (always P < 0.020). This procedure again obtained similar results (Table S4) and allowed testing of whether the difference between the final and initial values was significantly different from zero within each treatment. Here, LSD post-hoc tests for these last within-treatment comparisons are also provided when needed. Nonetheless, for simplicity, only the results obtained from testing the change as the dependent variable (see above) are presented.
With regard to the covariates, in all the models the number of previous reproductive events of the parents, hatching order, hatching date (also as a squared term to control for quadratic relationships), brood size and age at the start of the social environment experiment were tested to control for confounding effects. Alternatives avoiding these covariates were also tested, and main p-values reported. The tarsus length was added in models testing size-corrected body mass (i.e., body condition). In the case of the bill hue, the hue of the reference chip was always non-significant (all p > 0.15) in models analyzing effects on the hue at the start and at the end of the experiment. Therefore, it was not tested as a covariate when analyzing the change in hue values. In the case of the black bib, the tarsus length and body mass at the time of the photograph as well as the area of the reference chip (Kodak; see above) were all covariates for controlling body size and subtle focus variability, respectively. The change in plasma uric acid and triglyceride levels were also added as covariates to control for the confounding influence of the recent food intake on the model testing the change in PLAOX and plasma MDA, respectively (see Costantini, 2011; Romero-Haro and Alonso-Alvarez, 2014; see also Table S1).
All the models were calculated from saturated ones, removing variables sequentially by a traditional backward stepwise procedure to reach the best-fitted final model including only terms at p < 0.10. The Akaike Information Criterion (AIC) and the forward stepwise procedure were also checked, reporting similar results. The variance components (VC) structure of covariance was used as it always reported the lowest AIC. Effect sizes (Cohen's d-values) were reported for significant comparisons. The hue values were log-transformed to meet the normality requirements. We reported all the terms in the model when factors and/or interactions showed p < 0.100. When non-significant, the tests of the factors and interactions are reported at the last step of the backward process, i.e., just before being removed. The reported means and standard errors (SE) are least square means and SEs obtained from the best-fitted model. Degrees of freedom were calculated with the Satterthwaite option in SAS (Littell et al., 2006). Blood volume was not sufficient to perform all the analytical techniques in every individual, which may affect sample size in some comparisons. LSD post-hoc tests were used for pair-wise comparisons.
Results
To determine whether the findings described in Romero-Haro and Alonso-Alvarez (2015) could be extrapolated to the subset of birds used in the social context manipulation, the differences in tGSH levels at 14 days old, and bill color, plumage traits, body mass and size in adulthood between control birds and those early treated with BSO are tested and described in the following two subsections.
Effectiveness of the BSO Manipulation on Early Glutathione Values
The BSO treatment effectively reduced tGSH values at the nestling age (14 days) in the sample of birds used in the social manipulation experiment [F(1, 179) = 30.73, p < 0.001; mean ± SE: control: 4.77 ± 0.13 μmol/g; BSO-treated birds: 4.16 ± 0.13 μmol/g; Cohen's d = 0.70]. The sex of the bird and its interaction with the treatment were excluded as non-significant terms (both p > 0.12). No covariate exerted any significant influence (all p > 0.20), except the age at the first injection [F(1,243) = 9.99, p = 0.002; slope ± SE: −0.148 ± 0.047].
Differences in the Outward Appearance of Adult Birds
Among the birds used in the social context manipulation, the BSO treatment during early life influenced the bill hue of both sexes [F(1, 169) = 4.51, p = 0.035; Cohen's d = 0.28], with BSO-treated individuals showing redder bills (lower hue) than controls at the start of the experiment (Figure 2A). The effect was independent of the sex [F(1, 3.39) = 72.29, p < 0.001; males: 0.979° ± 0.015, females: 1.163° ± 0.016] and sampling age [F(1, 110) = 30.87, p < 0.001; slope ± SE: −0.002 ± 0.001]. The sex x BSO-treatment interaction was clearly non-significant (p = 0.915). The BSO treatment was also significant when the covariate was removed (p = 0.046).
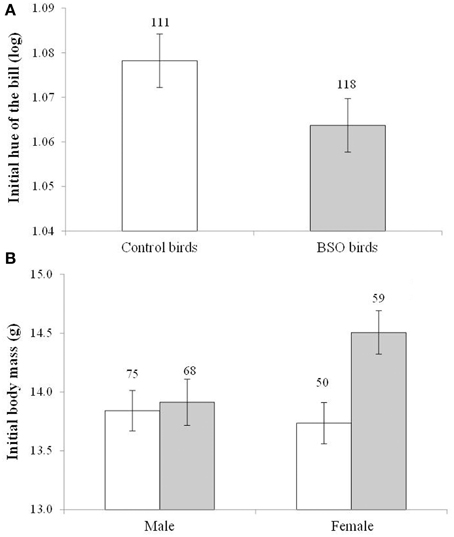
Figure 2. Differences in bill color (A) and body mass (B) in both male and female zebra finches at the start of the social context manipulation. Empty bars show data from control birds and full bars show data from birds that supported low antioxidant (glutathione) levels (BSO-treated birds) during growth. A lower hue indicates a redder color. Sample sizes are shown over the bars. Least squared means ± se from the model. Hue values were controlled for the sex of the bird. Other covariates in the model are described in Results.
Among males, the black area of the bib did not differ between BSO treatments [F(1, 120) = 0.77, p = 0.381]. When the area of the bib included the black stripes the same result was attained [F(1, 108) = 1.02, p = 0.313]. Similarly, the mean number of white flank spots did not differ between treatments [F(1, 104) = 0.06, p = 0.801].
The initial body mass showed a significant interaction between the BSO treatment and sex [F(1, 166) = 6.70, p = 0.010; Figure 2B]. BSO-treated females were heavier than control females (p = 0.003; d = 0.58), whereas males did not differ (p = 0.530). On the other hand, among BSO birds, females were heavier than males (p < 0.001; d = 0.71), whereas no sex-related difference was observed among controls (p = 0.720). The hatching order of each bird was also retained in the model [F(1, 154) = 15.76, p < 0.001; slope ± SE: 0.227 ± 0.057]. The interaction remained significant when the covariate was removed (p = 0.020).
The results on body mass were similar when controlling for body size (i.e., tarsus length) variability (usually body condition). A significant interaction between BSO treatment and sex was again detected [F(1, 184) = 8.85, p = 0.003; tarsus length: F(1, 246) = 158.8, p < 0.001; slope ± SE: 1.262 ± 0.100; brood size: F(1, 105) = 3.16, p = 0.077, slope ± SE: 0.142 ± 0.080]. BSO females showed a better condition than control females (p = 0.005; means ± SE: BSO: 14.52 ± 0.146 g, n = 59; control: 13.99 ± 0.156 g, n = 50; d = 0.55), whereas males did not differ (p = 0.170). On the other hand, among BSO birds, females were in better condition than males (p < 0.001, means ± SE: BSO males: 13.73 ± 0.137 g, n = 68, females see above; d = 0.80), whereas no sex-related difference was observed among controls (p = 0.791). The interaction was also significant when removing the brood size covariate (p = 0.005). Finally, tarsus length did not show any difference between BSO and control birds [BSO × sex interaction: F(1, 19) = 0.20, p = 0.653; BSO: F(1, 171) = 0.61, p = 0.434].
Mortality During the Social Context Experiment
Some birds died during the social manipulation (males: 4.8%; females: 5%). No significant difference in mortality among treatments was detected in males (all P > 0.21; control focus males: 2/76, 2.6%, BSO focus males: 5/68, 7.4%; male with female partner: 4/74, 5.4%, male with male partner: 3/70, 4.3%; male with control partner: 5/75, 6.7%, male with BSO partner: 2/69, 2.9%), or among interactions (all P > 0.75). The same was found for females [F(1, 39) = 0.68, P = 0.416; control: 4/59, 6.8%, BSO: 2/59, 3.4%].
Effects of Early Conditions and Type of Partner on Focus Males
In the case of the change in hue of the bill, only the early development treatment showed a trend toward significance [F(1, 109) = 3.78, p = 0.054; d = 0.32]. This was due to a higher increase in redness (i.e., a stronger decline in hue) among controls (−1.798° ± 0.476, n = 61) compared to BSO-treated males (−1.413° ± 0.479, n = 53). The only other term remaining in the model was the sampling age at the start of the social context manipulation [F(1, 111) = 10.86, p < 0.001; slope ± SE: 0.031 ± 0.009; when it is removed the treatment effect shows: p = 0.076]. This effect was however due to the initial difference described above, with BSO-treated birds being redder at the beginning of the study (when the initial hue value is added as a covariate the experimental effect fades: p > 0.12; see also Figure 3A). Other factors and interactions were non-significant (p > 0.10). Accordingly, bill redness of BSO and control males at the end of the study did not significantly differ [i.e., F(1, 93.5) = 1.14, p = 0.288 for a model testing final values without controlling for initial levels; see also Table S1].
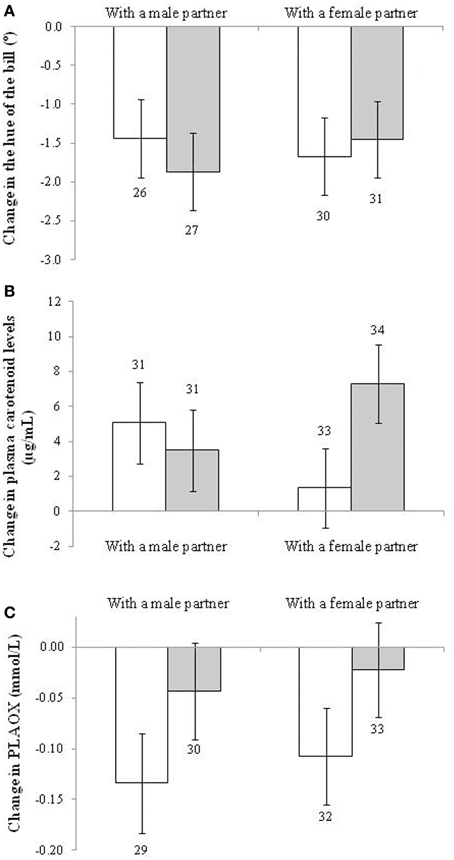
Figure 3. Change in (A) the hue of the bill (the lower the increase, the redder the trait becomes), (B) plasma carotenoid, and (C) hydrosoluble antioxidant (PLAOX) levels of males depending on the sex and early development treatment of their partners. Empty and full bars show data from males housed with control and BSO-treated partners, respectively. Sample sizes over the bars. Least squared means ± se from the model. See description of the models in Results.
The change in plasma levels of carotenoid pigments, however, showed a significant interaction between the sex and BSO treatment of the partner [F(1, 122) = 4.63, p = 0.033; Figure 3B]. Males coupled with BSO females showed a higher increase in pigment levels compared to males coupled with a control female (p = 0.016; d = 0.60), other pair-wise comparisons being non-significant (p > 0.12). Other terms in the model were removed at p > 0.12. The only exceptions were each factor separately (i.e., sex and BSO treatment both showing p > 0.90) and the brood size of the bird [F(1, 121) = 5.78, p = 0.018; slope ± SE: 0.195 ± 0.081]. The interaction remained significant when the brood size covariate was removed from the model (p = 0.029). The increase from the initial to final levels (Figure 3B) was only significant in the case of males housed with control males or with BSO females (both p < 0.01; see also Table S4 for the repeated-measures mixed model).
With regard to other blood antioxidants, no factor or interaction exerted a significant effect on the change in RBC tGSH or erythrocyte resistance to oxidative stress (all p > 0.12). However, PLAOX showed a trend to a significant effect of the early development treatment of the partner [F(1, 119) = 3.40, p = 0.064; d = 0.34; uric acid covariate: F(1, 121) = 120.1, p < 0.001; slope ± SE: 0.053 ± 0.005]. Males housed with control birds decreased plasma antioxidant values (post-hoc from Table S4: p < 0.001), whereas males housed with BSO birds did not (Figure 3C; p = 0.37). The result was independent of the partner sex. No other term was retained (all p > 0.10). The effect of the partner treatment became significant when assessed by alternative models (i.e., Tables S1, S4).
In the case of lipid peroxidation, the sex of the partner showed a significant effect [F(1, 133) = 3.93, p = 0.049; d = 0.34] on plasma MDA change. This was due to the fact that the changes in lipid peroxidation levels were in opposite directions between treatments. Males coupled with other males showed positive values, whereas males housed with females showed negative levels (Figure 4A). Nonetheless, when testing the difference between final and initial values within each treatment (see model in Table S4), the post-hoc tests were non-significant (p = 0.110 and 0.230, respectively). Other factors, interactions and covariates were removed (all p > 0.10).
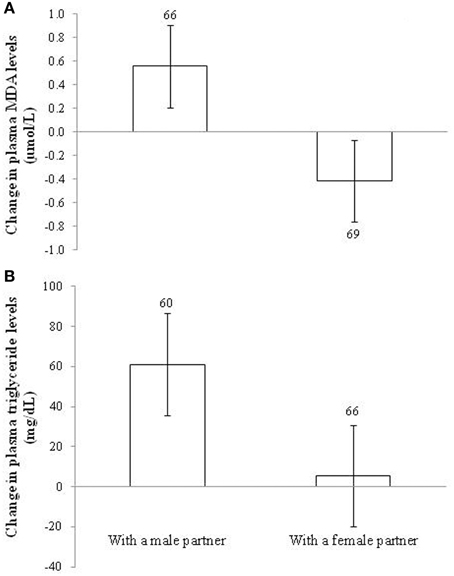
Figure 4. Change in (A) plasma levels of lipid peroxidation (malondialdehydes; MDA) and (B) plasma triglyceride levels in male zebra finches housed with a male or a female partner. Sample sizes are shown over the bars. Least squared means ± se from the model. See description of models in Results.
The effect of the sex of the partner on the change in MDA levels was statistically explained by the change in plasma triglyceride levels. First, the sex of the partner affected the change in circulating triglycerides [F(1, 119) = 9.39, p = 0.003; d = 0.55] with the same sign (Figure 4B). The brood size of the focus male was retained in the model [F(1, 123) = 4.46, p = 0.037; slope ± SE: 17.64 ± 8.36], but the main effect remained (p = 0.004) when it was removed. The difference from zero (Figure 4B) was significant in males housed with males (p < 0.001), but not in males with females (p = 0.715; Table S4). Second, when the change in MDA values was controlled for the change in triglyceride levels [F(1, 105) = 17.57, p < 0.001; slope ± SE: 0.009 ± 0.002], the sex of the partner became non-significant [F(1, 122) = 1.47, p = 0.227; see also Table S1].
In the case of body mass variability, the model showed an interaction between the BSO treatment of both the focus male and its partner [F(1, 129) = 8.51, p = 0.004; Figure 5A]. The focus BSO males increased body weight (about 5%) when caged with control birds compared to BSO males with BSO partners (p = 0.007; d = 0.70). Also, BSO males coupled with a control bird gained more body mass compared to control males with a control partner (p = 0.020; d = 0.58). Control males coupled with a BSO bird also tended to gain more body mass than BSO males coupled with a BSO bird (p = 0.078; d = 0.44). Other pair-wise comparisons reported p > 0.18. In terms of differences from zero within each treatment, males housed with birds of different early treatment groups showed significant increases (both p < 0.018), whereas those whose early treatment matched did not (both p > 0.30; also Table S4).
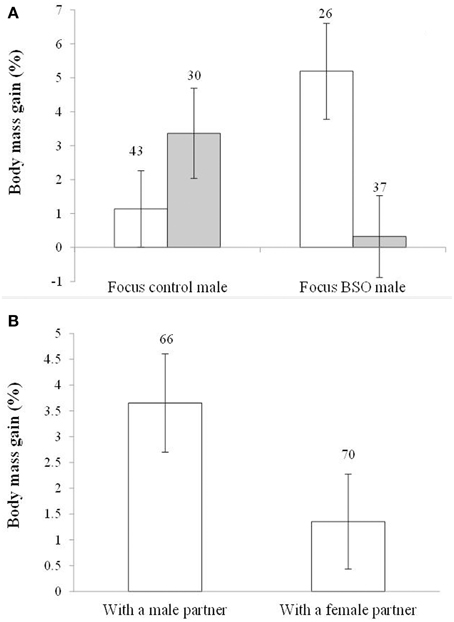
Figure 5. Body mass gain depending on (A) the early development treatment of the focus male and partner and (B) the sex of the partner. Empty and full bars in (A) show data from males housed with control and BSO-treated partners, respectively. Sample sizes are shown over the bars. Least squared means ± se from the models. See other terms in the model in Results.
In the last model, the sex of the partner also showed a trend toward significance [F(1, 129) = 3.70, p = 0.056; d = 0.39]. Males coupled with other males gained more body mass than males coupled with females (Figure 5B). The hatching date was retained in the model [F(1, 129) = 17.62, p < 0.001; slope ± SE: −0.066 ± 0.016]. The significance of the interaction (above) and the sex of the partner did not change when the hatching date covariate was removed (p = 0.007 and 0.053, respectively). The difference from zero (see Figure 5B) was significant in males housed with males (p < 0.001), but not in those paired with females (p = 0.103; Table S4). Variability in body mass changes would be independent of body size because tarsus length was removed from the models at p > 0.30.
Discussion
Our main hypothesis that the early environment should affect the oxidative cost of pre-copulatory reproductive investments was not supported by the whole of the results. Early antioxidant conditions of the focus male did not directly affect the output of the social context, although it did exert indirect effects by way of its partner: (1) males housed in front of a female increased circulating carotenoid pigments, but only when that female endured lower glutathione levels during early development; and (2) the body mass gain of the focus male was lower when both males and partners belonged to the same early development treatment. Independent of early conditions, and in spite of the cited changes in circulating pigments, the social context in adulthood did not affect bill color. Nevertheless, males facing females unexpectedly showed lower plasma lipid peroxidation compared to males caged in front of other males. This result was statistically explained by the change in the level of circulating plasma lipids (triglycerides), which paralleled the change in lipid peroxidation levels.
As mentioned, our analyses did not detect significant changes in bill color other than the disappearance of the initial difference due to early BSO exposure (also Romero-Haro and Alonso-Alvarez, 2015). That result may be a mere consequence of a regression to the mean effect (e.g., Kelly and Price, 2005). In that case, initial values converge to a central (here higher) level (note that hue values in Figure 3A are inversely related to redness). The fact that increased color in all birds could have been due to a lack of exercise (see also body mass gain Figure 5). Birds were previously housed in large rooms and then transferred to cages mostly preventing flying. High carotenoid availability in food improves flying capacity in the zebra finch (Blount and Matheson, 2006). This may suggest that birds housed in cages may not have required carotenoids for flying, and were thus able to re-allocate them to signaling functions, ultimately reducing variability among groups and probably diluting the initial differences.
The lack of a significant effect of the sex of the partner contrasts with a very similar experiment performed by Gautier et al. (2008), also in zebra finches. That study reported higher bill redness in males housed with females. Several explanations can be argued. First, the two studies may have differed in statistical power. Nevertheless, Gautier et al. (2008) was only able to detect large size effects (at power 80% and 0.05 alpha-threshold; Cohen, 1992), whereas our experiment was able to detect medium effects (i.e., 96 and 144 males, respectively). Second, in our study the partners were exchanged after 15 days to avoid habituation and favor sexual stimulation. This could have generated some social stress, constraining the allocation of antioxidants (carotenoids) to sexual signals. Mate separation induces a rapid rise in blood corticosterone levels (i.e., the physiological stress hormone in birds; Remage-Healey et al., 2003) in zebra finches, high corticoid levels also being associated with high oxidative stress in many vertebrates (reviewed in Costantini et al., 2011). Nonetheless, Remage-Healey et al. (2003) also showed that corticosterone recovered initial values 24 h after reunion. We also know that bill redness in male zebra finches increases after only 3 days when blood testosterone levels are artificially increased (Ardia et al., 2010). Therefore, even if partner separation transiently constrained color changes, the 15-days period after the partner exchange should have been sufficient to reveal consistent color modifications. Finally, we cannot discard that zebra finch populations could genetically differ in phenotypic plasticity (e.g., Price, 2006; Nussey et al., 2007; Porlier et al., 2012), with our males being unable to quickly respond to female presence in terms of coloration.
The absence of increased investment in bill color may prevent us from addressing the hypothesis that early conditions affected the oxidative cost of sexual signaling in adulthood. However, males housed with females increased the circulating levels of carotenoid pigments, although only in the case of BSO-treated females. If male carotenoid-based coloration did not change, what was the end use of carotenoids mobilized in blood in that treatment? At the start of the social experiment BSO-treated females were redder and heavier (larger size-corrected body mass) than control females. These differences in outward appearance could have stimulated male endocrinology, increasing blood testosterone levels (e.g., Pinxten et al., 2003). High testosterone levels favor any type of sexual signaling (e.g., Fusani, 2008) but have also been associated with immunodeficiency (reviewed in Peters, 2007) and low resistance to oxidative stress (Alonso-Alvarez et al., 2007). In that case, the immune-stimulant (Chew and Park, 2004) and antioxidant properties (Simons et al., 2012b) of carotenoids could have been required for combating such effects as well as any other demand related to the production of any primary or secondary sexual trait. Among primary sexual traits, it has been shown that carotenoids can be allocated to avian sperm (Rowe et al., 2012) probably to protect spermatozoids from oxidative insults (Helfenstein et al., 2010). Secondary sexual traits other than bill color could also have required carotenoids. Traits needing high oxygen consumption could have demanded antioxidant mobilization (proposed by Metcalfe and Alonso-Alvarez, 2010), including carotenoids. Accordingly, in European starlings (Sturnus vulgaris) high carotenoid availability increased song rates (Van Hout et al., 2011).
In any event, if higher carotenoid levels in males housed with BSO females indicates higher antioxidant demands, this should have been reflected in PLAOX levels, erythrocyte resistance to free radicals or in our measure of oxidative damage, but this was not the case. The decline in plasma antioxidant levels during the experiment (Figure 3C) was not significant in males housed with BSO female partners, which may support the idea. However, the same was found in the case of males housed with BSO males. We have not an explanation for this last result. In the case of MDA, only the effect of the female presence (i.e., not the early development treatment of that female) was significant. We must, however, note that oxidative damage was assessed on circulating lipids only, whereas other molecules (e.g., proteins, DNA) and tissues (e.g., earth, liver and brain) could have been affected. Nonetheless, we must also consider that the study was performed in a species with low body mass for blood sampling, and that birds used here are currently being monitored throughout their entire lifetime, which precluded the analysis of internal tissue other than blood.
The fact that males housed with a female showed lower lipid peroxidation (MDA) in plasma than males paired with other males contradicts the hypothesis that reproduction (here as pre-copulatory reproductive investments) is costly in terms of oxidative stress. However, the finding agrees with recent experiments in different rodent species also under captivity conditions (references in Speakman and Garratt, 2014). These last studies reported that animals prevented from reproducing have higher MDA levels than animals allowed to reproduce, and parents whose litters were enlarged showed lower MDA levels compared to parents with experimentally reduced litters (Speakman and Garratt, 2014). Nonetheless, some studies also showed that the effect can vary depending on the specific tissue on which the analysis was performed (e.g., Garratt et al., 2012; see also Speakman and Garratt, 2014). In any event, the findings in rodents mostly contradict the hypothesis. We must remember that the idea was initially supported by studies showing that an extrinsic source of free radicals have a stronger effect on the longevity of reproductive compared to non-reproducing fruit flies (Salmon et al., 2001; Wang et al., 2001). The results in rodents and our study also contradict other experiments in birds showing that reproduction impairs the capacity of erythrocytes to avoid hemolysis when faced with an in vitro free radical attack (Alonso-Alvarez et al., 2004b; Losdat et al., 2011; Christe et al., 2012). Although this last technique might partially serve to estimate the degree of oxidative damage in the erythrocyte membrane (greater damage favoring hemolysis), and although the technique has also been linked to zebra finch mortality (Alonso-Alvarez et al., 2006), it should not be strictly defined as a measure of oxidative damage (Halliwell and Gutteridge, 2007) and, hence, must be interpreted with caution. On the other hand, lower oxidative damage in reproductive zebra finches may reveal a hormetic (compensatory) response (e.g., Costantini et al., 2010), which could perhaps not be afforded in other more natural (and perhaps harsher) environmental conditions. However, PLAOX or erythrocyte resistance to oxidative stress did not show an increase (compensation) in the same birds. The information obtained from other variables should be considered.
Male zebra finches paired with a female not only showed lower MDA values compared to males housed with other males, but also a lower increase in the level of circulating triglycerides. Furthermore, the difference in MDA values became non-significant when corrected for triglyceride variability. Could the lower oxidative damage of males housed with females thus be considered an artifact? We have previously shown that plasma triglyceride concentration positively correlates with plasma MDA values in zebra finches (Romero-Haro and Alonso-Alvarez, 2014). We suggested that recent lipid intake or mobilization from storage sites (mainly the liver in birds) influences plasma MDA variability (Romero-Haro and Alonso-Alvarez, 2014). The idea has, nonetheless, been broadly documented by biomedicine. Thus, for instance, it is very well established that pregnant women endure higher serum levels of lipid peroxidation when compared to non-pregnant women (Little et al., 1999; Ozkan et al., 2012), and that this is accompanied by an increase in serum lipids (e.g., Toescu et al., 2002; Sarandöl et al., 2004). We highlighted the importance of assessing the covariance between MDA and triglyceride concentrations to interpret the lipid oxidative damage measurements (Romero-Haro and Alonso-Alvarez, 2014; Pérez-Rodríguez et al., in press). The proportion of oxidized lipids (triglyceride-corrected MDA) could have a different functional significance than the absolute concentration of oxidized lipids (uncorrected MDA). In the last case, we should consider that, although MDA has been considered an end-point molecule in the lipid peroxidation cascade (Halliwell and Gutteridge, 2007), it has also been shown that MDA molecules form adducts with proteins, promoting inflammatory responses and even mutations leading to different diseases in humans (Tuma, 2002; Del Rio et al., 2005; Weismann et al., 2011). Accordingly, high uncorrected MDA values during human pregnancy are considered a potential problem because it can lead to vascular diseases, with the question of hyperlipemia being considered as a mere risk factor (Toescu et al., 2002; Agarwal et al., 2012). In contrast, corrected values may reveal the proportion of damage among lipids present everywhere in the organism, mobilized to blood or absorbed from the diet, its functional meaning being less clear. In some ways, it reveals the damage in the past, whereas absolute MDA values indicate the potential for future damage.
However, why did males partnered with other males show increased circulating lipids? The answer may arise from differences in energy balance associated with sexual behavior. Unfortunately, behavior was not recorded. Nonetheless, body mass variability may shed some light on this. A trend toward significant lower body mass gain in males caged in front of a female compared to males paired with other males was found. Similar to triglycerides, when body mass gain is controlled for as a covariate in the model testing MDA change, the factor sex of the partner also became non-significant (p = 0.103), with body mass gain being positively related to MDA change [F(1, 124) = 19.25, p < 0.001; slope ± SE: 14.28 ± 3.25]. This suggests that decreased MDA values in males housed with females were at least partially due to birds eating less and/or consuming more energy from body stores, reducing both body mass and blood triglyceride values (e.g., Jenni-Eiermann et al., 2002). The question can also be interpreted in the opposite direction, as males caged with other males may be less engaged in social interactions allowing an increase in body mass and lipid storage. The results also suggest caution when interpreting the oxidative cost of reproduction by testing blood lipid peroxidation under the particular limitations associated with captivity (Speakman and Garratt, 2014 and references therein). For instance, we do not know how more room for flying may have interacted with the treatments, but we know that flying efforts in zebra finches require carotenoids (Blount and Matheson, 2006) and increase the levels of a proxy of plasma oxidative damage (i.e., hydroperoxides; Costantini et al., 2012). In other words, the result of a trade-off between reproduction and self-maintenance should change under harsher environmental conditions (Braendle et al., 2011). We must note that only two experimental studies (i.e., Losdat et al., 2011; Christe et al., 2012) have tested the oxidative cost of reproduction under free-living conditions, though some correlational work has been carried out (reviewed in Stier et al., 2012; Metcalfe and Monaghan, 2013; Speakman and Garratt, 2014).
Intriguingly, body mass changes also revealed that the same early development treatment in both members of a couple led to lower body mass gain in the focus male (Figure 5A). The result was independent of the sex of the partner. The explanation could be related to the social status of birds and the involvement of some signal. Two individuals of similar quality in visual contact for the first time should produce a similar status signal, and consequently, be engaged in a higher rate of behavioral interactions to establish hierarchy (i.e., the status signaling hypothesis: Rohwer, 1975; e.g., Chaine et al., 2011). A similar level of signal expression would not allow them to avoid a physical conflict. This situation would prevent a gain in body mass. Accordingly, the cited hypothesis assumes that the signals revealing dominance and/or fighting ability in any intra- or inter-sexual context reduce the costs of those interactions with a predictable outcome (Maynard Smith and Harper, 2003; Searcy and Nowicki, 2005). Animals can avoid an escalated contest by modulating their investment in the status signal. For instance, males can increase signal intensity when detecting low quality rivals (e.g., Dijkstra et al., 2007), but in turn reduce signaling to avoid conflicts when facing more powerful opponents (e.g., Candolin, 1999). Thus, males faced with individuals of similar status should change their signaling level to reduce the costs derived from a higher conflict level (here, reduced body mass gain). The type of signal that was involved is however unclear. The bill redness of zebra finches has not been linked to aggressiveness in any sex (i.e., Bolund et al., 2007). We cannot discard differences between BSO and control birds in other traits such as the song or some aggressive displays. In any event, the result suggests that early conditions influence the output of social contests in adulthood, although apparently independently of oxidative stress because the same interaction (i.e., Figure 5A) was not detected in terms of lipid peroxidation.
In summary, the results as a whole suggest that reduced levels of intracellular antioxidants (glutathione) during early development had only a weak influence on how males endure trade-offs related to the adult social environment and reproduction, at least under our particular housing conditions and sampling periods. The experiment, however, revealed that the outward appearance and probably behavior of BSO-treated partners influenced the way in which males managed carotenoids and body mass reserves, suggesting that early oxidative conditions may exert a sort of indirect phenotypic effect (i.e., Hamilton and Ligocki, 2012). Theoretical models predict that the indirect effects of interacting phenotypes influence the rate and direction of selection (Cheverud, 2003; Bijma and Wade, 2008). The potential relevance of such an influence is, in any case, beyond the scope of this study. On the other hand, our experiment also reveals that interaction with a female can favor the health of the male. This apparently contradictory finding should be interpreted with caution considering not only the captivity conditions, but the life history trajectory of individuals. The costs associated with any strategy to reduce oxidative damage at the first steps of reproduction (e.g., reducing lipid stores) could be paid at subsequent stages. We may also hypothesize that selection could have favored reduced oxidative damage in certain (precopulatory) reproductive phases as a way to endure future challenges. To consider these perspectives when testing the oxidative cost of reproduction is imperative for future studies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to María Ester Ferrero, Laura Ramírez Rodríguez, Esther García de Blas, and Rafael Mateo for helping during laboratory analyses and blood sampling. Fernando Dueñas also assisted in bird maintenance. We would like to thank the two anonymous reviewers for their suggestions and comments and to Sarah Young for her English revision. AR was funded by a Formación de Personal de Investigación grant (BES-2010-035013; Ministerio de Economía y Competitividad, MINECO, Spanish Government). Financial support was obtained from the projects CGL-2009-10883-C02-02 and CGL2012-40229-C02-01 (MINECO, Spain).
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fevo.2015.00035/abstract
References
Agarwal, A., Aponte-Mellado, A., Premkumar, B. J., Shaman, A., and Gupta, S. (2012). The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 10:49. doi: 10.1186/1477-7827-10-49
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Agarwal, R., and Chase, S. D. (2002). Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J. Chromatogr. B 775, 121–126. doi: 10.1016/S1570-0232(02)00273-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C., Bertrand, S., Devevey, G., Gaillard, M., Prost, J., Faivre, B., et al. (2004a). An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am. Nat. 164, 651–659. doi: 10.1086/424971
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C., Bertrand, S., Devevey, G., Prost, J., Faivre, B., and Sorci, G. (2004b). Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 7, 363–368. doi: 10.1111/j.1461-0248.2004.00594.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C., Bertrand, S., and Sorci, G. (2007). Sex-specific transgenerational effects of early developmental conditions in a passerine. Biol. J. Linn. Soc. 91, 469–474. doi: 10.1111/j.1095-8312.2007.00811.x
Alonso-Alvarez, C., and Galván, I. (2011). Free radical exposure creates paler carotenoid-based ornaments: a possible interaction in the expression of black and red traits. PLoS ONE 6:e19403. doi: 10.1371/journal.pone.0019403
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C., Pérez-Rodríguez, L., García, J. T., Viñuela, J., and Mateo, R. (2010). Age and breeding effort as sources of individual variability in oxidative stress markers in a bird species. Physiol. Biochem. Zool. 83, 110–118. doi: 10.1086/605395
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C. S., Bertrand, G., Devevey, J., Prost, B., Faivre, O., and Chastel, G. S. (2006). An experimental manipulation of life-history trajectories and resistance to oxidative stress. Evolution (N.Y). 60, 1913–1924. doi: 10.1554/05-644.1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alonso-Alvarez, C., and Velando, A. (2012). “Benefits and costs of parental care,” in The evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kolliker (Oxford: Oxford University Press), 40–61.
Ardia, D. R., Broughton, D. R., and Gleicher, M. J. (2010). Short-term exposure to testosterone propionate leads to rapid bill color and dominance changes in zebra finches. Horm. Behav. 58, 526–532. doi: 10.1016/j.yhbeh.2010.04.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bijma, P., and Wade, M. J. (2008). The joint effects of kin, multilevel selection and indirect genetic effects on response to genetic selection. J. Evol. Biol. 21, 1175–1188. doi: 10.1111/j.1420-9101.2008.01550.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Blount, J. D., Houston, D. C., and Møller, A. P. (2000). Why egg yolk is yellow. Trends Ecol. Evol. 15, 47–49. doi: 10.1016/S0169-5347(99)01774-7
Blount, J. D., and Matheson, S. M. (2006). Effects of carotenoid supply on escape flight responses in zebra finches, Taeniopygia guttata. Anim. Behav. 72, 595–601. doi: 10.1016/j.anbehav.2005.11.014
Bolund, E., Schielzeth, H., and Forstmeier, W. (2007). Intrasexual competition in zebra finches, the role of beak colour and body size. Anim. Behav. 74, 715–724. doi: 10.1016/j.anbehav.2006.10.032
Braendle, C., Heyland, A., and Flatt, T. (2011). “Integrating mechanistic and evolutionary analysis of life history variation,” in Mechanisms of Life History Evolution, eds T. Flatt and A. Heyland (Oxford: Oxford University Press), 3–10.
Candolin, U. (1999). The relationship between signal quality and physical condition: is sexual signalling honest in the three-spined stickleback? Anim. Behav. 58, 1261–1267. doi: 10.1006/anbe.1999.1259
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chaine, A. S., Tjernell, K. A., Shizuka, D., and Lyon, B. E. (2011). Sparrows use multiple status signals in winter social flocks. Anim. Behav. 81, 447–453. doi: 10.1016/j.anbehav.2010.11.016
Cheverud, J. M. (2003). Evolution in a genetically heritable social environment. Proc. Natl. Acad. Sci. U.S.A. 100, 4357–4359. doi: 10.1073/pnas.0931311100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chew, B. P., and Park, J. S. (2004). Carotenoid action on the immune response. J. Nutr. 134, 257–261.
Christe, P., Glaizot, O., Strepparava, N., Devevey, G., and Fumagalli, L. (2012). Twofold cost of reproduction: an increase in parental effort leads to higher malarial parasitaemia and to a decrease in resistance to oxidative stress. Proc. R. Soc. B Biol. Sci. 279, 1142–1149. doi: 10.1098/rspb.2011.1546
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cohen, A., Klasing, K., and Ricklefs, R. (2007). Measuring circulating antioxidants in wild birds. Comp. Biochem. Physiol. B 147, 110–121. doi: 10.1016/j.cbpb.2006.12.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cohen, J. (1992). A power primer. Psychol. Bull. 112, 155–159. doi: 10.1037/0033-2909.112.1.155
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Costantini, D. (2011). On the measurement of circulating antioxidant capacity and the nightmare of uric acid. Methods Ecol. Evol. 2, 321–325. doi: 10.1111/j.2041-210X.2010.00080.x
Costantini, D., Marasco, V., and Møller, A. P. (2011). A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B 181, 447–456. doi: 10.1007/s00360-011-0566-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Costantini, D., Metcalfe, N. B., and Monaghan, P. (2010). Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435–1447. doi: 10.1111/j.1461-0248.2010.01531.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Costantini, D., Mirzai, N., and Metcalfe, N. B. (2012). An automated system to control and manipulate the flight activity of captive birds. Behav. Ecol. Sociobiol. 66, 1195–1199. doi: 10.1007/s00265-012-1362-z
Del Rio, D., Stewart, A. J., and Pellegrini, N. (2005). A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15, 316–328. doi: 10.1016/j.numecd.2005.05.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dijkstra, P. D., Hekman, R., Schulz, R. W., and Groothuis, T. G. G. (2007). Social stimulation, nuptial colouration, androgens and immunocompetence in a sexual dimorphic cichlid fish. Behav. Ecol. Sociobiol. 61, 599–609. doi: 10.1007/s00265-006-0289-7
Foley, J. D., and Van Dam, A. (1984). Fundamentals of Interactive Computer Graphics. Reading, MA: Addison-Wesley.
Fusani, L. (2008). Testosterone control of male courtship in birds. Horm. Behav. 54, 227–223. doi: 10.1016/j.yhbeh.2008.04.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Galván, I., and Alonso-Alvarez, C. (2008). An intracellular antioxidant determines the expression of a melanin-based signal in a bird. PLoS ONE 3:e3335. doi: 10.1371/journal.pone.0003335
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Galván, I., and Alonso-Alvarez, C. (2009). The expression of melanin-based plumage is separately modulated by exogenous oxidative stress and a melanocortin. Proc. Biol. Sci. 276, 3089–3097. doi: 10.1098/rspb.2009.0774
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Galvan, I., and Solano, F. (2009). The evolution of eu- and pheomelanic traits may respond to an economy of pigments related to environmental oxidative stress. Pigment Cell Melanoma Res. 22, 339–342. doi: 10.1111/j.1755-148X.2009.00559.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Garratt, M., McArdle, F., Stockley, P., Vasilaki, A., Beynon, R. J., Jackson, M. J., et al. (2012). Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct. Ecol. 26, 423–433. doi: 10.1111/j.1365-2435.2011.01952.x
Gautier, P., Barroca, M., Bertrand, S., Eraud, C., Gaillard, M., Hamman, M., et al. (2008). The presence of females modulates the expression of a carotenoid-based sexual signal. Behav. Ecol. Sociobiol. 62, 1159–1166. doi: 10.1007/s00265-008-0544-1
Grafen, A. (1990). Biological signals as handicaps. J. Theor. Biol. 144, 517–546. doi: 10.1016/S0022-5193(05)80088-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Griffith, O. W. (1980). Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinilpiridine. Anal. Biochem. 106, 207–212. doi: 10.1016/0003-2697(80)90139-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Griffith, O. W. (1982). Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J. Biol. Chem. 257, 13704–13712.
Halliwell, B., and Gutteridge, J. M. C. (2007). Free Radicals in Biology and Medicine. Oxford: Oxford University Press.
Hamilton, I. M., and Ligocki, I. Y. (2012). The extended personality: indirect effects of behavioural syndromes on the behaviour of others in a group-living cichlid. Anim. Behav. 84, 659–664. doi: 10.1016/j.anbehav.2012.06.022
Harman, D. (1956). Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 2, 298–300. doi: 10.1093/geronj/11.3.298
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hartley, R. C., and Kennedy, M. W. (2004). Are carotenoids a red herring in sexual display? Trends Ecol. Evol. 19, 353–354. doi: 10.1016/j.tree.2004.04.002
Helfenstein, F., Losdat, S., Møller, A. P., Blount, J. D., and Richner, H. (2010). Sperm of colourful males are better protected against oxidative stress. Ecol. Lett. 13, 213–222. doi: 10.1111/j.1461-0248.2009.01419.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hill, G. E., and Johnson, J. D. (2012). The vitamin A–redox hypothesis: a biochemical basis for honest signaling via carotenoid pigmentation. Am. Nat. 180, E127–E150. doi: 10.1086/667861
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jenni-Eiermann, S., Jenni, L., Kvist, A., Lindström, Å., Piersma, T., and Visser, G. H. (2002). Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J. Exp. Biol. 205, 2453–2460.
Kelly, C., and Price, T. D. (2005). Correcting for regression to the mean in behavior and ecology. Am. Nat. 166, 700–707. doi: 10.1086/497402
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kirkwood, T. B. L., and Austad, S. N. (2000). Why do we age? Nature 408, 233–238. doi: 10.1038/35041682
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, W.-S., Metcalfe, N. B., Réale, D., and Peres-Neto, P. R. (2014). Early growth trajectories affect sexual responsiveness. Proc. Biol. Sci. 281:2899. doi: 10.1098/rspb.2013.2899
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lessells, C. M., and Boag, P. T. (1987). Unrepeatable repeatabilities—A common mistake. Auk 104, 116–121. doi: 10.2307/4087240
Lindstrom, J. (1999). Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. doi: 10.1016/S0169-5347(99)01639-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Littell, R. C., Milliken, G. A., Stroup, W. W., Wolfinger, R. D., and Schabenberger, O. (2006). SAS System for Mixed Models. Cary, NC: SAS Institute.
Little, R. E., Sc, D., Gladen, B. C., and Ph, D. (1999). Levels of lipid peroxides in uncomplicated pregnancy: a review of the literature. Reprod. Toxicol. 13, 347–352. doi: 10.1016/S0890-6238(99)00033-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Losdat, S., Helfenstein, F., Gaude, B., and Richner, H. (2011). Reproductive effort transiently reduces antioxidant capacity in a wild bird. Behav. Ecol. 22, 1218–1226. doi: 10.1093/beheco/arr116
Luo, Z. C., Fraser, W. D., Julien, P., Deal, C. L., Audibert, F., Smith, G. N., et al. (2006). Tracing the origins of “fetal origins” of adult diseases: programming by oxidative stress? Med. Hypotheses 66, 38–44. doi: 10.1016/j.mehy.2005.08.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mantei, K. E., Ramakrishnan, S., Sharp, P. J., and Buntin, J. D. (2008). Courtship interactions stimulate rapid changes in GnRH synthesis in male ring doves. Horm. Behav. 54, 669–675. doi: 10.1016/j.yhbeh.2008.07.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McGraw, K. J., and Toomey, M. B. (2010). Carotenoid accumulation in the tissues of zebra finches: predictors of integumentary pigmentation and implications for carotenoid allocation strategies. Physiol. Biochem. Zool. 83, 97–109. doi: 10.1086/648396
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Metcalfe, N. B., and Alonso-Alvarez, C. (2010). Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. doi: 10.1111/j.1365-2435.2010.01750.x
Metcalfe, N. B., and Monaghan, P. (2001). Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. doi: 10.1016/S0169-5347(01)02124-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Metcalfe, N. B., and Monaghan, P. (2013). Does reproduction cause oxidative stress? An open question. Trends Ecol. Evol. 28, 347–350. doi: 10.1016/j.tree.2013.01.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miller, N. J., Rice-Evans, C. A., Davies, M. J., Gopinathan, V., and Milner, A. (1993). A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 84, 407–412.
Møller, A. P., Biard, C., Blount, J. D., Houston, D. C., Ninni, P., Saino, N., et al. (2000). Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 11, 137–159.
Naguib, M., and Nemitz, A. (2007). Living with the past: nutritional stress in juvenile males has immediate effects on their plumage ornaments and on adult attractiveness in zebra finches. PLoS ONE 2:e901. doi: 10.1371/journal.pone.0000901
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nussey, D. H., Kruuk, L. E. B., Morris, A., and Clutton-Brock, T. H. (2007). Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 17, R1000–R1001. doi: 10.1016/j.cub.2007.10.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nussey, D. H., Pemberton, J. M., Pilkington, J. G., and Blount, J. D. (2009). Life history correlates of oxidative damage in a freeliving mammal population. Funct. Ecol. 23, 809–817. doi: 10.1111/j.1365-2435.2009.01555.x
Orledge, J. M., Blount, J. D., Hoodless, A. N., Pike, T. W., and Royle, N. J. (2012). Synergistic effects of supplementation of dietary antioxidants during growth on adult phenotype in ring-necked pheasants, Phasianus colchicus. Funct. Ecol. 26, 254–264. doi: 10.1111/j.1365-2435.2011.01932.x
Ozkan, Y., Yardim-Akaydin, S., Erdem, A., and Simşek, B. (2012). Variability of total thiol compounds, oxidative and nitrosative stress in uncomplicated pregnant women and nonpregnant women. Arch. Gynecol. Obstet. 285, 1319–1324. doi: 10.1007/s00404-011-2150-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pérez-Rodríguez, L. (2009). Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. Bioessays 31, 1116–1126. doi: 10.1002/bies.200900070
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pérez-Rodríguez, L., Romero-Haro, A., Sternalski, A., Muriel, J., Mougeot, F., Gil, D., et al. (in press). Measuring oxidative stress: the confounding effect of lipid concentration in measures of lipid peroxidation. Physiol. Biochem. Zool. 88. doi: 10.1086/680688
Peters, A. (2007). Testosterone and carotenoids: an integrated view of trade-offs between immunity and sexual signalling. Bioessays 29, 427–430. doi: 10.1002/bies.20563
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pike, T., Blount, J., Lindström, J., and Metcalfe, N. B. (2009). Dietary carotenoid availability, sexual signalling and functional fertility in sticklebacks. Biol. Lett. 191–193. doi: 10.1098/rsbl.2009.0815
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pinxten, R., de Ridder, E., and Eens, M. (2003). Female presence affects male behavior and testosterone levels in the European starling (Sturnus vulgaris). Horm. Behav. 44, 103–109. doi: 10.1016/S0018-506X(03)00120-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Porlier, M., Charmantier, A., Bourgault, P., Perret, P., Blondel, J., and Garant, D. (2012). Variation in phenotypic plasticity and selection patterns in blue tit breeding time: between- and within-population comparisons. J. Anim. Ecol. 81, 1041–1051. doi: 10.1111/j.1365-2656.2012.01996.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Price, T. D. (2006). Phenotypic plasticity, sexual selection and the evolution of colour patterns. J. Exp. Biol. 209, 2368–2376. doi: 10.1242/jeb.02183
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Remage-Healey, L., Adkins-Regan, E., and Romero, L. M. (2003). Behavioral and adrenocortical responses to mate separation and reunion in the zebra finch. Horm. Behav. 43, 108–114. doi: 10.1016/S0018-506X(02)00012-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reznick, D. (1992). Measuring the costs of reproduction. Tree 7, 1990–1993. doi: 10.1016/0169-5347(92)90150-A
Rinaudo, P., and Wang, E. (2012). Fetal programming and metabolic syndrome. Annu. Rev. Physiol. 74, 107–130. doi: 10.1146/annurev-physiol-020911-153245
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rohwer, S. (1975). Social significance of avian winter plumage variability. Evolution (N.Y.). 29, 593–610. doi: 10.2307/2407071
Romero-Haro, A. A., and Alonso-Alvarez, C. (2014). Covariation in oxidative stress markers in the blood of nestling and adult birds. Physiol. Biochem. Zool. 87, 353–362. doi: 10.1086/674432
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Romero-Haro, A. A., and Alonso-Alvarez, C. (2015). The level of an intracellular antioxidant during development determines the adult phenotype in a bird species: a potential organizer role for glutathione. Am. Nat. 185, 390–405. doi: 10.1086/679613
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rowe, M., Tourville, E. A., and McGraw, K. J. (2012). Carotenoids in bird testes: links to body carotenoid supplies, plumage coloration, body mass and testes mass in house finches (Carpodacus mexicanus). Comp. Biochem. Physiol. B 163, 285–291. doi: 10.1016/j.cbpb.2012.06.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Salmon, A. B., Marx, D. B., and Harshman, L. G. (2001). A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution (N.Y). 55, 1600–1608. doi: 10.1554/0014-3820(2001)055[1600:ACORID]2.0.CO;2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sarandöl, E., Dirican, M., and Serdar, Z. (2004). Oxidizability of apolipoprotein B-containing lipoproteins, levels of lipid peroxidation products and antioxidants in normal pregnancy. Arch. Gynecol. Obstet. 270, 157–160. doi: 10.1007/s00404-003-0524-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Searcy, W. A., and Nowicki, S. (2005). The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Oxford: Princeton University Press.
Simons, M. J. P., Briga, M., Koetsier, E., Folkertsma, R., Wubs, M. D., Dijkstra, C., et al. (2012a). Bill redness is positively associated with reproduction and survival in male and female zebra finches. PLoS ONE 7:e40721. doi: 10.1371/journal.pone.0040721
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Simons, M. J. P., Cohen, A. A., and Verhulst, S. (2012b). What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds-A meta-analysis. PLoS ONE 7:e43088. doi: 10.1371/journal.pone.0043088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sohal, R. S., and Orr, W. C. (2012). The redox stress hypothesis of aging. Free Radic. Biol. Med. 52, 539–555. doi: 10.1016/j.freeradbiomed.2011.10.445
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Speakman, J. R., and Garratt, M. (2014). Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. Bioessays 36, 93–106. doi: 10.1002/bies.201300108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spencer, K., Buchanan, K., Goldsmith, A., and Catchpole, C. (2003). Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Horm. Behav. 44, 132–139. doi: 10.1016/S0018-506X(03)00124-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stearns, S. C. (2011). “Does impressive progress on understanding mechanisms advance life history theory?” in Mechanisms of Life History Evolution, eds T. Flatt and A. Heyland (Oxford: Oxford University Press), 365–674.
Stier, A., Reichert, S., Massemin, S., Bize, P., and Criscuolo, F. F. (2012). Constraint and cost of oxidative stress on reproduction: correlative evidence in laboratory mice and review of the literature. Front. Zool. 9:37. doi: 10.1186/1742-9994-9-37
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tarry-Adkins, J. L., Chen, J. H., Smith, N. S., Jones, R. H., Cherif, H., and Ozanne, S. E. (2009). Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528. doi: 10.1096/fj.08-122796
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Toescu, V., Nuttall, S. L., Martin, U., Kendall, M. J., and Dunne, F. (2002). Oxidative stress and normal pregnancy. Clin. Endocrinol. 44, 609–613. doi: 10.1046/j.1365-2265.2002.01638.x
Tuma, D. (2002). Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic. Biol. Med. 32, 303–308. doi: 10.1016/S0891-5849(01)00742-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Hout, A. J.-M., Eens, M., and Pinxten, R. (2011). Carotenoid supplementation positively affects the expression of a non-visual sexual signal. PLoS ONE 6:e16326. doi: 10.1371/journal.pone.0016326
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Velando, A., Torres, R., and Alonso-Alvarez, C. (2008). Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. Bioessays 30, 1212–1219. doi: 10.1002/bies.20838
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vincenzi, S., Hatch, S., Mangel, M., and Kitaysky, A. (2013). Food availability affects onset of reproduction in a long-lived seabird. Proc. R. Soc. B Biol. Sci. 280:20130554. doi: 10.1098/rspb.2013.0554
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Y., Salmon, A. B., and Harshman, L. G. (2001). A cost of reproduction: oxidative stress susceptibility is associated with increased egg production in Drosophila melanogaster. Exp. Gerontol. 36, 1349–1359. doi: 10.1016/S0531-5565(01)00095-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Weismann, D., Hartvigsen, K., Lauer, N., Bennett, K. L., Scholl, H. P., Charbel Issa, P., et al. (2011). Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81. doi: 10.1038/nature10449
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
West-Eberhard, M. J. (2003). Developmental Plasticity and Evolution. New York, NY: Oxford University Press.
Wu, G., Fang, Y., Yang, S., Lupton, J. R., and Turner, N. D. (2004). Glutathione metabolism and its implications for health. J. Nutrition 134, 489–492.
Yan, L.-J. (2014). Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2, 165–169. doi: 10.1016/j.redox.2014.01.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zahavi, A. (1975). Mate selection—A selection for a handicap. J. Theor. Biol. 53, 205–214. doi: 10.1016/0022-5193(75)90111-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zann, R. A. (1996). The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford University Press.
Zedrosser, A., Pelletier, F., Bischof, R., Festa-Bianchet, M., and Swenson, J. E. (2013). Determinants of lifetime reproduction in female brown bears: early body mass, longevity, and hunting regulations. Ecology 94, 231–240. doi: 10.1890/12-0229.1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: early development conditions, oxidative stress, cost of reproduction, sexual signaling, carotenoids, social context, status signaling theory
Citation: Romero-Haro AA, Canelo T and Alonso-Alvarez C (2015) Early development conditions and the oxidative cost of social context in adulthood: an experimental study in birds. Front. Ecol. Evol. 3:35. doi: 10.3389/fevo.2015.00035
Received: 16 December 2014; Accepted: 17 March 2015;
Published: 01 April 2015.
Edited by:
Patrick S. Fitze, University of Lausanne, SwitzerlandReviewed by:
Michael Garratt, University of Michigan, USAFabrice Helfenstein, University of Neuchâtel, Switzerland
Copyright © 2015 Romero-Haro, Canelo and Alonso-Alvarez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos Alonso-Alvarez, Departamento de Ecología Evolutiva, Museo Nacional de Ciencias Naturales – Consejo Superior de Investigaciones Científicas, C/José Gutiérrez 13, Abascal 2, 28006 Madrid, Spain carlos.alonso@csic.es
 Ana A. Romero-Haro
Ana A. Romero-Haro Tara Canelo
Tara Canelo Carlos Alonso-Alvarez
Carlos Alonso-Alvarez