
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol., 25 March 2015
Sec. Interdisciplinary Climate Studies
Volume 3 - 2015 | https://doi.org/10.3389/fevo.2015.00029
This article is part of the Research TopicClimate Change and Marine Top PredatorsView all 15 articles
Cod, harp seal and minke whale are the main top predators in the Barents Sea ecosystem. In the last decade, the abundance of cod has increased considerably, and is at a record high level. In spite of this, the growth and condition of cod has remained rather stable, although some decrease is seen in size at age of large, mature cod. During the same period, the abundance of harp seals has declined whereas the minke whale stock has been at a stable level. The body condition (blubber thickness) of these two mammal stocks has, however, decreased, with the strongest decrease observed for harp seals. A possible hypothesis for explaining this is that cod outperform the marine mammal stocks in the competition for food. The main advantages for cod are most likely larger availability of food (mainly capelin) during winter-spring than for marine mammals, as well as a wider range of prey species being available to cod than to marine mammals. Harp seals are more dependent on prey items found close to the ice edge than the other two predator stocks are, which could partly explain why the performance of harp seals is worse than that of the two other main top predators in the area.
The abundance of high trophic level or apex predators such as marine mammals and large piscivorous fish have varied substantially worldwide over the past five decades (e.g., Pauly et al., 1998; Myers and Worm, 2003; Estes et al., 2011). Current trends in harvested fish populations appear to be diverging among regions; stabilizing in some regions but continued to decline in others (Worm and Branch, 2012). As for marine mammals they are either protected or harvested at very low and sustainable levels. In the North Atlantic there has been an increasing trend in whale species such as fin whales and humpback whales (Haug et al., 2011), while there are examples of both increasing (harp seals; ICES, 2013a; Øigård et al., 2014a) and decreasing (hooded seals; Øigård et al., 2014b) seal stocks. Although the ecological consequences of such predator changes vary among ecosystems (e.g., due to functional diversity of top predators) several studies point out that cascading effects through the entire food web is a likely outcome (Pauly et al., 1998; Frank et al., 2005). Understanding the role of high trophic predators and how they respond to changes in the resource availability is important to predict future functioning of ecosystems.
The Barents Sea ecosystem (BSE) is a shallow (average depth 220 m, depth range 20–500 m) high latitude shelf sea that supports major fisheries and where the productivity is high but vary considerably between years (e.g., Wassmann et al., 2006) that supports major fisheries. The BSE has been displaying decadal shifts in species abundance and trophic control the past four decades (e.g., Wassmann et al., 2006; Johannesen et al., 2012) where climate change, particularly increasing temperatures and effects on sea ice cover and distribution, appears to have noticeable effects on the distribution and abundance of species (Wassmann et al., 2006; Dalpadado et al., 2012). There has been a gradual shift toward more Atlantic species the past decade (Fossheim et al., under revision), most likely as a result of warming and reduced ice coverage.
The BSE is an important feeding area for several migratory and resident apex predators (Dolgov et al., 2011). The three most important are cod Gadus morhua, harp seals Pagophilus groenlandicus and common minke whales Balaenoptera acutorostrata (Bogstad et al., 2000; Wassmann et al., 2006; Dolgov et al., 2011). There are several other piscivorous fish and marine mammal species that reside in the Barents Sea (see e.g., Dolgov et al., 2011; Haug et al., 2011), but we have decided to not take them into consideration in this paper as their impact on fish stocks probably is much less conspicuous than that of cod, harp seal and minke whale due to their stock sizes and diet compositions.
The Northeast Arctic cod is by far the most abundant, and hence, conspicuous piscivorous /planktivorous fish species in the Barents Sea ecosystem, with a total biomass of about 3 million tons at present (ICES, 2014a). The biology of this stock is described in detail e.g., by Yaragina et al. (2011). The abundance and spatial distribution of this stock has increased considerably in recent years, both due to a combination of favorable climatic conditions and good management (Kjesbu et al., 2014). Cod is also the most important commercial valuable fish stock in the area. Similar to cod, harp seals and minke whales exploit several trophic levels in Arctic systems, and because of their large body size, high metabolic demands and abundance, they are thought to have an important top-down effect on the structure and function of the food web (see Bowen, 1997; Bogstad et al., 2000; Folkow et al., 2000; Nilssen et al., 2000; Wassmann et al., 2006; Kovacs et al., 2009). They have also been target species for important hunting activities for a long period of time (Øien et al., 1987; Skaug et al., 2007; Haug et al., 2011).
Food-limitation, which is one of the most common density dependent factors in nature, appears to have affected both the harp seal and whale populations but hardly the cod population. The growth and body condition of cod have remained relatively unchanged the past decade despite a major increase in abundance whereas the body condition of harp seals (Øigård et al., 2013) and, to some extent also, minke whales (Hiroko Solvang, Institute of Marine Research, Norway, pers. comm.) has declined in the same period despite stable or declining stock sizes.
The purpose of the paper is to explore possible mechanisms why cod, harp seal and minke whale have reacted differently and discuss the short and long-term outcome of the competition. Will the cod outperform the sea mammals in the struggle for food?
The temperature in the Kola section (monthly observations from a transect from 70°30′ to 72°30′ N along 33°30′ E, depth range 0–200 m) in the southern Barents Sea is often used as an indicator of the oceanographic conditions in the area (Tereshchenko, 1996, www.pinro.ru). The temperature has been at a historic high level in the last decade (Figure 1). The ice coverage in the Barents Sea (Special Sensor Microwave/Imager (SSM/I) passive microwave remote sensing data from the National Snow and Ice Data Centre, USA (http://www.nsidc.org); Cavalieri et al., 1996; Meier et al., 2006) has also decreased considerably (Figure 2; McBride et al., 2014), and the Barents Sea was for instance completely free of ice in early autumn in 2013 (Figure 3).
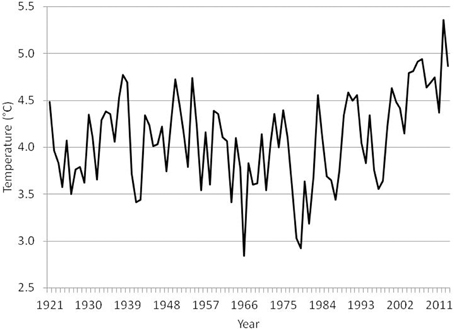
Figure 1. Temperature (°C) in Kola section (0–200 m) during the period 1921–2013 (annual averages of monthly observations).
Figure 4 shows the development in abundance of the main fish prey stocks in the Barents Sea (also including shrimp Pandalus borealis—the main commercial shellfish stock in the area). The total abundance of these prey items has been relatively stable over the last decade.
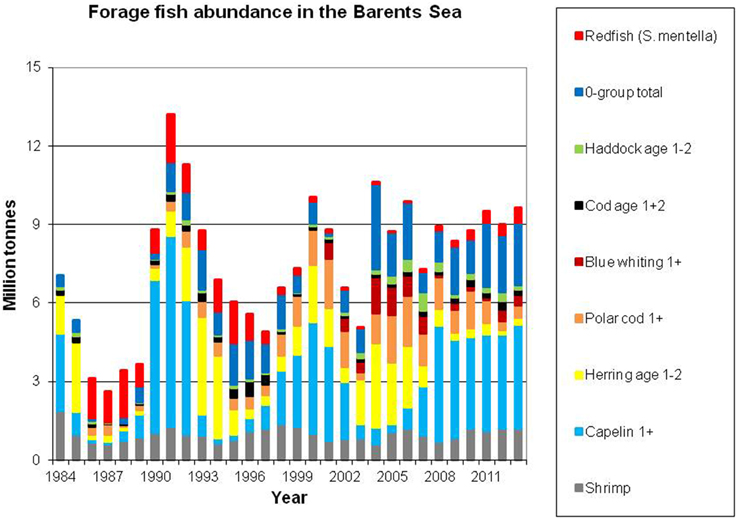
Figure 4. Abundance of main fish prey stocks, as well as shrimp. Data sources: Capelin: acoustic estimates in September, age 1+ (ICES, 2014a); Herring: VPA biomass estimates of age 1 and 2 herring (ICES, 2014b) using constant weights at age (10 g for age 1 and 44 g for age 2); Polar cod and blue whiting: acoustic estimates in September, age 1+ (Prokhorova, 2013); 0–group: estimates of biomass of cod, haddock, herring and capelin, corrected for catch efficiency (ICES, 2014a); Cod and haddock age 1+2: VPA biomass estimates of age 1–2 (ICES, 2014a) Redfish (Sebastes mentella): survey biomass of 5–24 cm fish (bottom trawl swept area), scaled to adjust for catchability and vertical distribution (ICES, 2014a) Shrimp: biomass (bottom trawl swept area, ICES, 2013b), scaled to adjust for catchability).
Capelin (Mallotus villosus) is regarded as the most important forage fish species in the Barents Sea despite major decadal fluctuation in stock size (Figure 4). In periods, this stock completely dominates the pelagic fish biomass; the biomass has historically been estimated to exceed seven million tons. However, on three occasions during the last 30 years, each spanning about 5 years, the stock dwindled and reached a level two orders of magnitude lower (Gjøsæter, 1998; Gjøsæter et al., 2009). The spatial distribution and size of the capelin stock has been monitored since the early 1970s (acoustic surveys). In cold years the capelin stock is normally distributed to the north of the polar front during autumn, and in periods it has extended to the ice edge. In recent warm years, when the ice edge is north of the Barents Sea, capelin have been observed both north of the shelf edge i.e., north of the Svalbard and Franz Josef peninsulas at 81°N (Ingvaldsen and Gjøsæter, 2013), and also in the northern Kara Sea (ICES, 2014a; Figure 5). Ingvaldsen and Gjøsæter (2013) suggested that this north- and eastward expansion of the capelin feeding area could be attributed to both density dependent and environmental factors because there has been an eastward shift in water masses and plankton fields due to ocean warming.
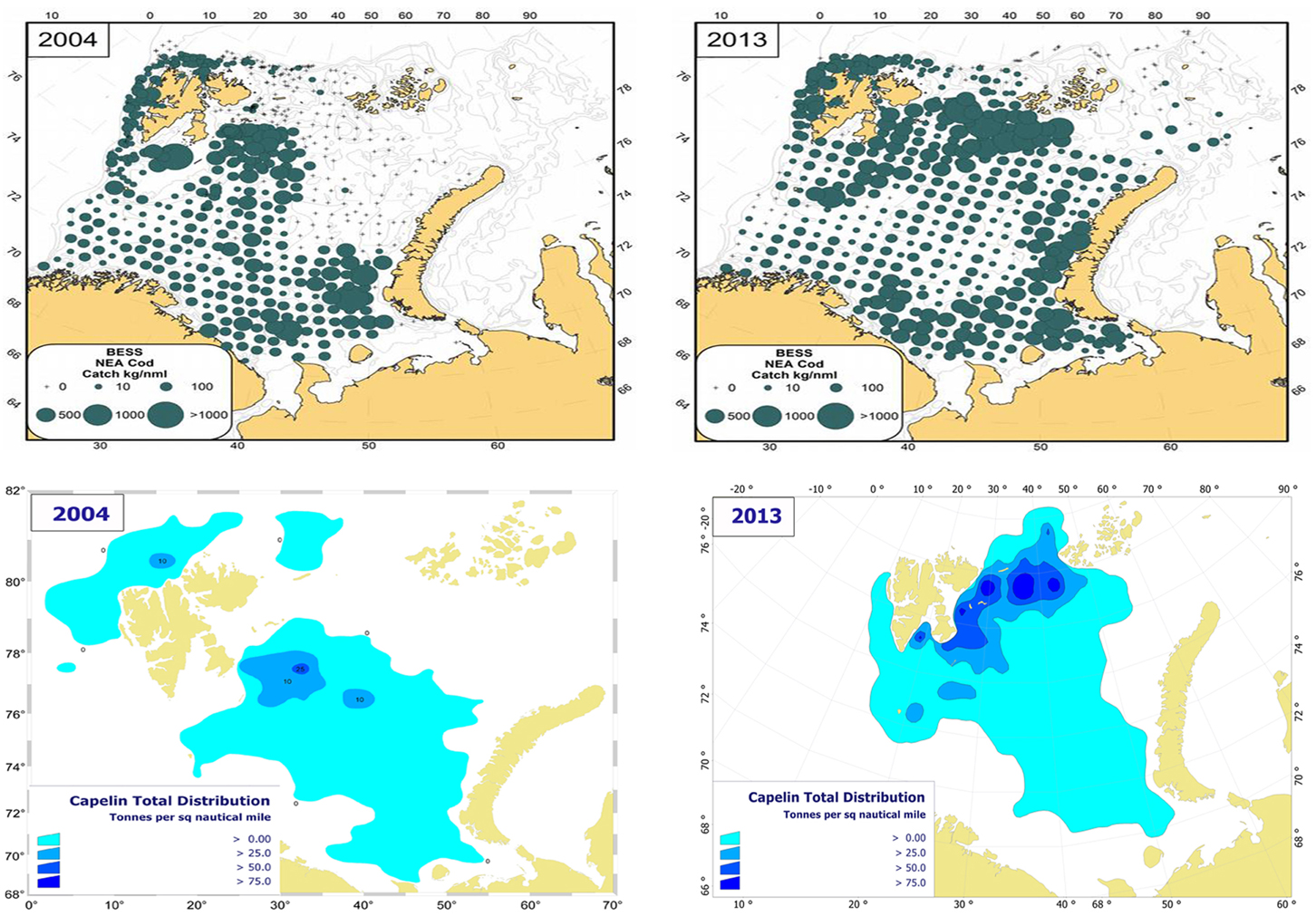
Figure 5. Spatial distribution of cod (upper panels) and capelin (lower panels) in August-September 2004 and 2013, from the Norwegian-Russian ecosystem survey. Cod: bottom trawl catches (kg/nm), capelin: acoustic estimates (tons/nm2).
Polar cod (Boreogadus saida) is another forage fish species inhabiting the Barents Sea and the adjacent areas north and east of the Barents Sea. The size and distribution of the stock has been monitored since 1986, but since an unknown part of the stock is distributed outside the survey area, it is unknown how well the acoustic indices reflect the true stock size. Hop and Gjøsæter (2013) compared the distribution and ecological role of polar cod and capelin, and found that polar cod is distributed in colder water than capelin and therefore further to the north and east. The observed distribution of polar cod has been more static than that of capelin, but it should be noted that the northern and eastern border of the polar cod distribution is poorly defined. In recent years, the abundance of polar cod in the Barents Sea has shown a serious decline, perhaps as a result of loss of habitat (ice). Polar cod is known to be more associated with ice than is capelin, both during the juvenile stage, when drifting sea ice mostly serves as habitat, and during the adult stage, when ice associated fauna constitutes a part of the food base (Hop and Gjøsæter, 2013).
Among fish prey, juvenile herring (Clupea harengus) and blue whiting (Micromesistius poutassou) may also play important roles as forage fish in periods (Figure 4). They reside in the Barents Sea 2–3 years and then join the adult stocks found in the Norwegian Sea prior to maturation. These species mainly occupy the southern (herring) and south-western (blue whiting) parts of the Barents Sea.
The deepwater redfish (Sebastes mentella) played an important role as food for cod during the 1980s and beginning of the 1990s when the abundance of preferred prey was low and the abundance of redfish was high. The dietary importance of redfish has been low the past two decades, when the stock size was heavily reduced (ICES, 2014a). Deepwater shrimp (Pandalus borealis) is also important food for cod, and have constituted a much more stable proportion of the diet during the study period compared with the other food items. The amount of shrimp has increased in recent years but their distribution area has shifted eastwards. It is uncertain whether this is a result of higher temperatures in the near-bottom layers.
The youngest age group (0-group) of various fish species might be considered as another “stock” of forage fish. The young stages of cod, haddock (Melanogrammus aeglefinus), polar cod, capelin and herring, as well as numerous other fish species in the Barents Sea, inhabit the upper 50 m of the water column the first 6 month of life (summer and fall). They are distributed over most of the Barents Sea but the highest densities are normally found in the central part of the sea (Eriksen et al., 2012). The abundance of this “stock” has not changed much the past decade (Eriksen et al., 2012). Also, juvenile cod are eaten by adult cod as well as harp seals and minke whales, and this food source has been abundant and increasing during the last decade.
Macrozooplankton, mainly pelagic amphipods and krill are important prey for all these three predator species. The production and standing stocks of zooplankton is more difficult to monitor than fish and sea mammals, and there are probably spatial shifts and geographical variation that are difficult to trace but may be important when considering the importance of these prey groups. The total abundance of amphipods has shown a decreasing trend over the last couple of decades (Figure 8B in Dalpadado et al., 2012). Krill abundance estimated from the Russian autumn (October-December) survey (Figure 3 in Zhukova et al., 2009) is at present at or slightly above the long-term mean (McBride et al., 2014), and krill abundance estimates from the joint ecosystem survey (Prokhorova, 2013) also indicate that krill abundance in the last decade has been above average.
The Northeast Arctic Cod (NEA cod) is a large, mainly demersal, fish that may attain sizes of 1.5 m and 40 kg and an age of 20 years. The annual growth rate is about 10 cm until maturation thereafter the growth slows down. Average age and size at maturation is about 7 years and 70 cm. The NEA cod spawns along the west and northwest coasts of Norway; the Lofoten area is the most important spawning area. The fish larvae are transported to the Barents Sea by the currents and enter the south-western Barents Sea. They spend their first years mainly in the southern and central parts of the Barents Sea. The adult individuals have a more northern distribution in the Barents Sea. They perform seasonal spawning and feeding migrations during early spring and summer, respectively.
Although cod is considered a bottom-dwelling fish, acoustic observations and analysis of data storage tags (e. g., Heffernan et al., 2004), as well as analysis of stomach content (e.g., Bogstad and Mehl, 1997), have shown that cod frequently use pelagic habitats to feed on pelagic prey. The abundance of large cod (age 7+) has increased much since 2000 whereas the abundance of medium-sized (age 3–6) increased strongly until 2009 and then declined to levels observed prior to 2000 (Figure 6; ICES, 2014a). The peak in abundance of age 3–6 cod around 2009 is due to the very abundant 2004 and 2005 year classes. During the last decade, the geographical distribution of cod has expanded north- and eastwards both in autumn (Figure 5) and winter (Johansen et al., 2013; Mehl et al., 2013).
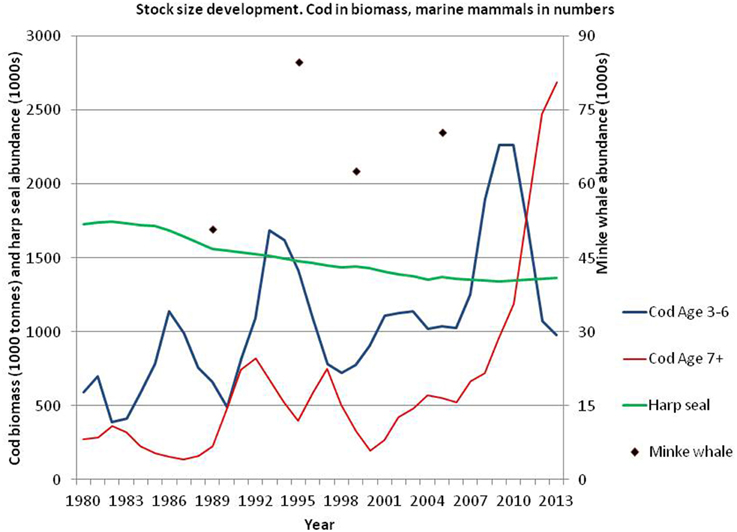
Figure 6. Biomass (1000 tons) of age 3–6 and 7+ Northeast Arctic cod (ICES, 2014a) as well as abundance (1000s) of harp seals (Øigård et al., 2014a) and minke whales (Bøthun et al., 2009).
Cod is regarded as a generalist feeder, particularly on a population level, with a diverse food base including pelagic and demersal fish, shrimp, epi-benthos and zooplankton. Age groups 1 and 2 feed mainly on crustaceans (Figure 7). Capelin is the main prey item for age groups 3+ (Figure 8), however, large cod (age 7+) is far more piscivorous, feeding on cod, haddock and long rough dab (Hippoglossoides platessoides), compared with smaller cod (age 3–6) (Figure 9). Details about the feeding of cod in the Barents Sea can be found in Dolgov et al. (2011).
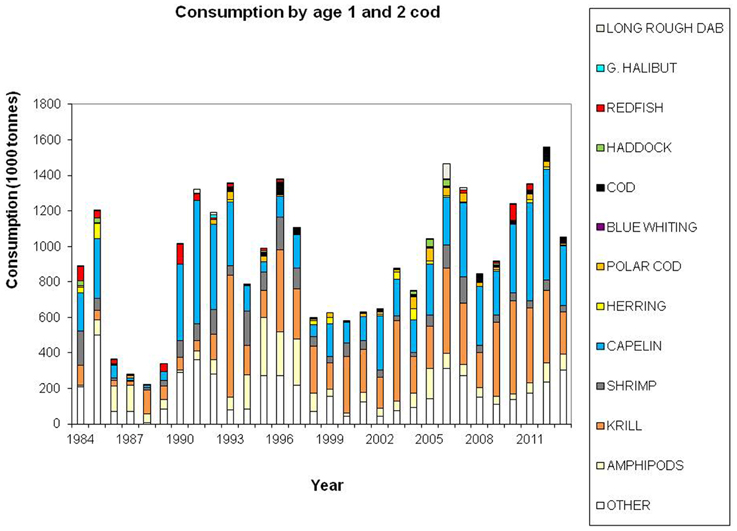
Figure 7. Total consumption by age 1–2 cod based on calculations presented in ICES (2014a), using the method described by Bogstad and Mehl (1997). These calculations are based on stomach content data and a model for gastric evacuation rate, and the consumption is calculated by cod age group (1–11+), half-year and three areas in the Barents Sea.
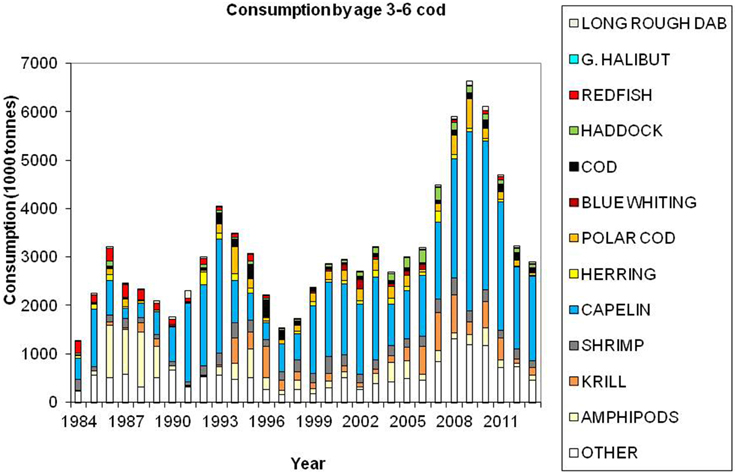
Figure 8. Total consumption by age 3–6 cod based on calculations presented in ICES (2014a), using the method described by Bogstad and Mehl (1997). These calculations are based on stomach content data and a model for gastric evacuation rate, and the consumption is calculated by cod age group (1–11+), half-year and three areas in the Barents Sea.
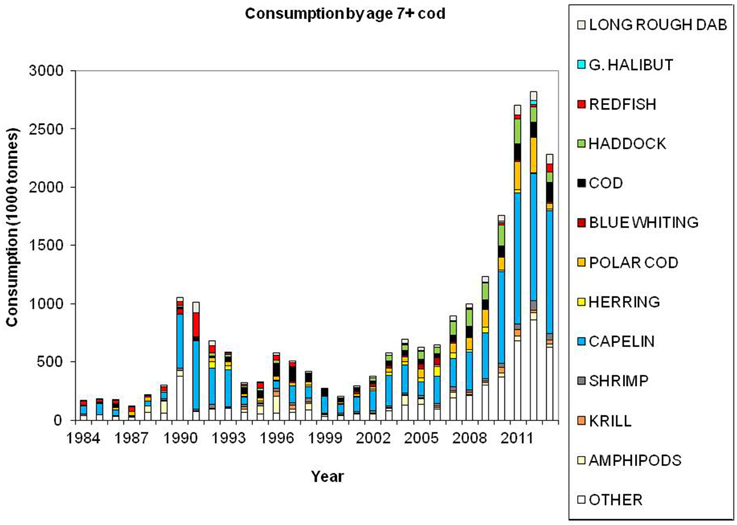
Figure 9. Total consumption by age 7+ cod based on calculations presented in ICES (2014a), using the method described by Bogstad and Mehl (1997). These calculations are based on stomach content data and a model for gastric evacuation rate, and the consumption is calculated by cod age group (1–11+), half-year and three areas in the Barents Sea.
Harp seals are highly migratory animals with a generalist foraging behavior. The two stocks inhabiting the Barents Sea, whelp and molt on the pack ice off the east coast of Greenland (the Greenland Sea or West Ice stock), and in the White Sea and south-eastern Barents Sea (the Barents Sea or East Ice stock) (Lavigne and Kovacs, 1988; Sergeant, 1991). After the molt the Barents Sea stock disperse in small herds to feed, primarily around Svalbard and in the northern Barents Sea (Figure 10). The southward movement of the seals toward the breeding areas in the White Sea begins in November-December (Haug et al., 1994; Nordøy et al., 2008). Telemetry studies suggest that a major fraction of the West Ice stock forage in the northern Barents Sea during summer and autumn (Haug et al., 1994; Folkow et al., 2004; Nordøy et al., 2008). These data also show that there is major individual variation in diving behavior on a diurnal and seasonal time scale (Folkow et al., 2004; Nordøy et al., 2008). Seals tend to dive deeper during winter and daytime compared with the summer and night time. Further, harp seals appears to dive more frequently during early summer (June-July), when they have a low body condition and reside close to the pack ice, compared with other periods of the year. As the sea ice retreats the seals spend more time in the water, perhaps as a result of longer traveling distances between the ice-edge and the feeding areas (Nordøy et al., 2008). In fact, the East Ice seals were observed to spend at least 53% of the year in areas with less than 40% ice coverage (Nordøy et al., 2008).
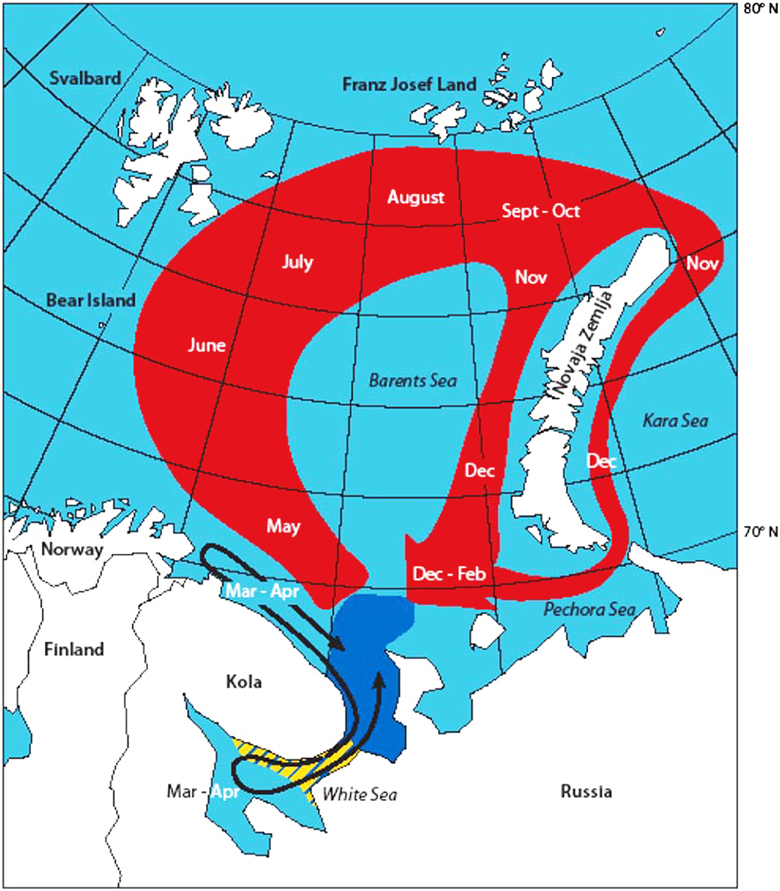
Figure 10. Suggested gross annual migrations of the Barents Sea harp seal population between breeding in the White Sea in February-March and molting in May (black arrow), and between molting and breeding the following year (red area). The approximate extension of the breeding area is indicated as a yellow area, while the approximate extension of the molting area is indicated with dark blue. From Nordøy et al. (2008).
Similar to cod, Barents Sea harp seals is regarded as generalist predators, at least on a population level, primarily feeding on the most available prey in the area and by doing so their diets vary much in both time and space (e.g., Nilssen et al., 1995a,b; Lindstrøm et al., 2013). Harp seals can potentially eat large prey but studies shows that they primarily feed on small specimens (10–25 cm) of gadoids such as polar cod, haddock and cod (e.g., Lindstrøm et al., 2013). The bulk of the harp seal diet is comprised of relatively few species, in particular capelin, polar cod, herring, krill Thysanoessa spp. and the pelagic amphipod Themisto libellula (Nilssen et al., 2000; Lindstrøm et al., 2013). The crustaceans appear to be of particular importance as food for harp seals during summer and autumn when they are feeding in the northern parts of the Barents Sea (July-October). As the ice cover expands southwards in late autumn and winter, the southward migrating seals appear to switch from crustaceans to fish (particularly capelin and polar cod) (Nilssen et al., 1995a; Lindstrøm et al., 1998). In the southernmost areas of the Barents Sea, where the seals occur during winter and early spring, herring may be an important forage fish (Nilssen et al., 1995b; Lindstrøm et al., 1998).
Nilssen et al. (2000) estimated the total food consumption by 2.2 million (c. 700 000 more than today) harp seals in the Barents Sea using a bioenergetics model. The annual food consumption was estimated to 2.69–3.96 million tons of biomass or more specifically 1.22 million tons crustaceans, 808,000 tons capelin, 605,000 tons polar cod, 212,000 tons herring and a mix of gadoids and other more Arctic fishes of ca. 500,000 tons. A small capelin stock (as in 1993–1996) made harp seals switch diet; the consumption of other fish species, in particular polar cod, other gadoids and herring increased.
The seals appear to target primarily the most lipid-rich prey during summer: krill, followed by other crustaceans and polar cod (see Grahl-Nielsen et al., 2011). Availability of high-energetic food in the northern areas in spring and summer presumably provide the energetic advantage necessary to account for the long migrations of harp seals from their more southerly located winter distributions.
There is a regular seasonal pattern of deposition of energy reserves as fat in the subcutaneous blubber layer (see Iverson, 2002). Barents Sea harp seals (see Nilssen et al., 1997) are generally thin in spring and early summer (May—June). Their condition improves over the summer, and the seals are very fat by September—October. The energy stores built up during the summer and autumn are maintained until February, but then the seals become thinner as the stores of blubber decrease rapidly during the breeding and molting period (March–June).
The migration of minke whales into Norwegian waters, including the Barents Sea, starts in early spring (see Haug et al., 2011). In September-October a southward migration has been observed, however, old catch statistics reveal that minke whales have been caught in Norwegian waters nearly all year around, indicating that not all animals leave the northern areas in winter.
Minke whales have been the target species of Norwegian small-type whaling. Abundance estimates have been provided through dedicated sighting surveys, and the most recent estimate for the Barents Sea area is 81,400 (cv 0.23) (Skaug et al., 2004; Haug et al., 2011). Their general distribution and migration pattern is illustrated in Figure 11.
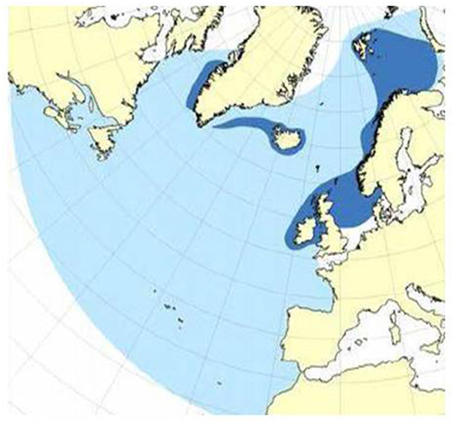
Figure 11. General migration pattern of minke whales in the Barents Sea and adjacent areas. Summer feeding area (dark blue) and total distribution area (light blue).
By combining data on energy requirements, diet composition, and stock size, Folkow et al. (2000) estimated the total annual consumption of various prey species by minke whales during their assumed feeding period (mid-April to mid-October) in northeast Atlantic waters. In the period 1992–1995, a stock of 85,000 minke whales consumed more than 1.8 million tons (95% CI: 1.4–2.1 million tons) of prey per year in coastal waters off northern Norway, in the Barents Sea and around Spitsbergen: 602,000 tons of krill, 633,000 tons of herring, 142,000 tons of capelin, 256,000 tons of cod, 128,000 tons of haddock and 54,500 tons of other fish species, including saithe (Pollachius virens) and sandeel (Ammodytes spp.).
Minke whales have a very flexible foraging behavior and are normally able to switch among species without compromising the body condition. As a result their diets vary much in time (year and season) and space due to spatio-temporal variation in prey availability (see Figure 12). The whales exploit a variety of species and sizes of fish and crustaceans (Haug et al., 2002), however they appear to selectively forage on capelin, herring and occasionally krill (Lindstrøm and Haug, 2001). In extreme events such as in 1995–1996, when the abundance of capelin and herring was low simultaneously, the minke whales had to switch to krill and gadoid fish (cod and haddock) and as a result their body condition declined.
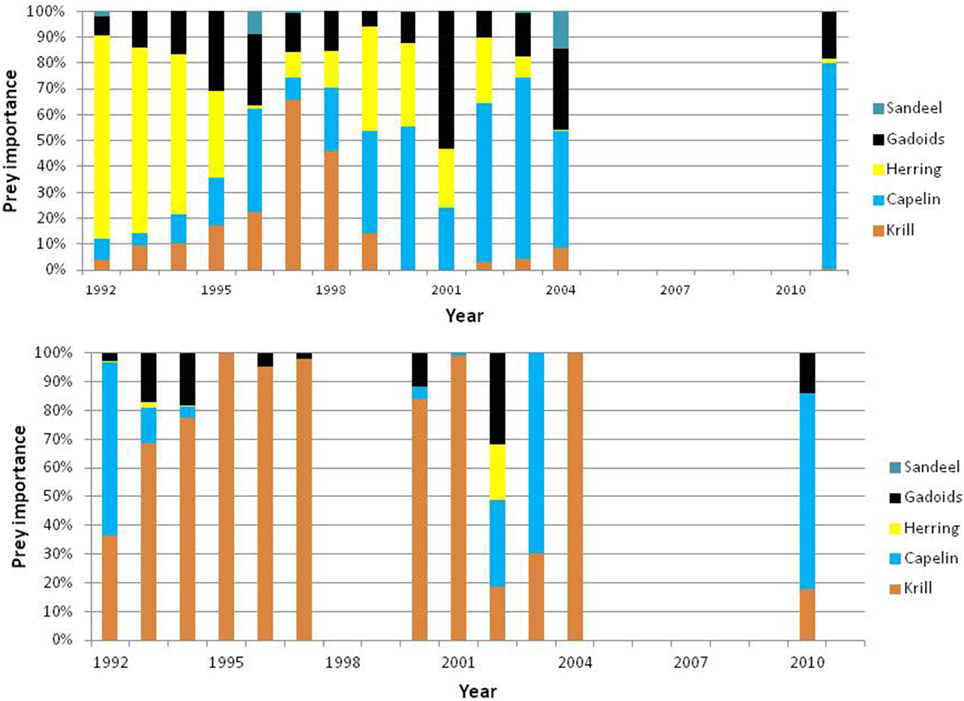
Figure 12. Biomass-based diet composition of minke whales sampled in the northern (North) and southern (South) Barents Sea in the periods 1992–2004 and 2010–2011. Based on data from Haug et al. (2002), Windsland et al. (2007) and Meier et al. (in press).
More recent studies (2000–2004 and 2010–2011) confirmed previous findings of significant differences in diet composition between areas and some differences between years (Windsland et al., 2007; Meier et al., in press). The importance of krill in the Barents Sea increased with latitude and dominated the Spitsbergen diet. Capelin dominated the diet around Bear Island and contributed considerable to the diet along the coast of northern Norway. In the latter area, herring and haddock were also a great part of the diet. The considerable size range of consumed prey (0.2—78 cm) confirms previous findings that minke whales are not particularly size selective (Windsland et al., 2007). The size of prey seems to be determined by the availability of different size classes, rather than selectivity by the whales (Lindstrøm and Haug, 2001; Windsland et al., 2007). The age composition of the capelin consumed by the whales varied in space. It was mainly 3 or 4 years old capelin (i.e., mature, see Gjøsæter, 1998) in the southern Barents Sea, while capelin eaten north of the spawning grounds, around Spitsbergen and Bear Island, were considerably smaller with only ca. 50% being in the size range of mature fish (i. e., > 14 cm). The majority of herring eaten in the southern Barents Sea were below 20 cm (≤2 years old), whereas haddock taken in this area had a wide size distribution (from 5 to 65 cm) including both juvenile and large adult individuals, although both length and age analysis showed that the majority were smaller haddock.
In 2003—2013 the minke whale distribution was surveyed in August-September, along with the distribution of their main prey species, in joint Norwegian-Russian ecosystem surveys (e.g., Michalsen et al., 2013). During the first part of this period (2003–2007), the majority of minke whales were feeding in the northern Barents Sea, north of the polar front in association with high zooplankton (krill) concentrations in the productive marginal ice zone (Skern-Mauritzen et al., 2011). They were also predominantly north of the capelin, further indicating that capelin was not the major forage species, at least not in these years with low capelin abundances. Only a small fraction of the minke whales foraged in the southern Barents Sea, in areas inhabited by blue whiting and herring. Hence, there is likely a shift in whale distributions and diet from early summer, when diet samples are collected during the minke whale harvest, to late summer and early autumn when most whales feed in the north. Variations in diets between periods of the year were also demonstrated during dedicated ecological studies of minke whales in the Barents Sea in 1993–1994 (Haug et al., 1996).
The per capita consumption and growth of younger cod has been stable in recent years, while for older cod, this has decreased slightly in the last couple of years (ICES, 2014a, Figure 13). The condition factors show the same development (Figure 14). Although the condition factor of older fish has been slightly below the long-term mean in the last years, it is still well above the lowest levels experienced during the first capelin collapse in the late 1980s.
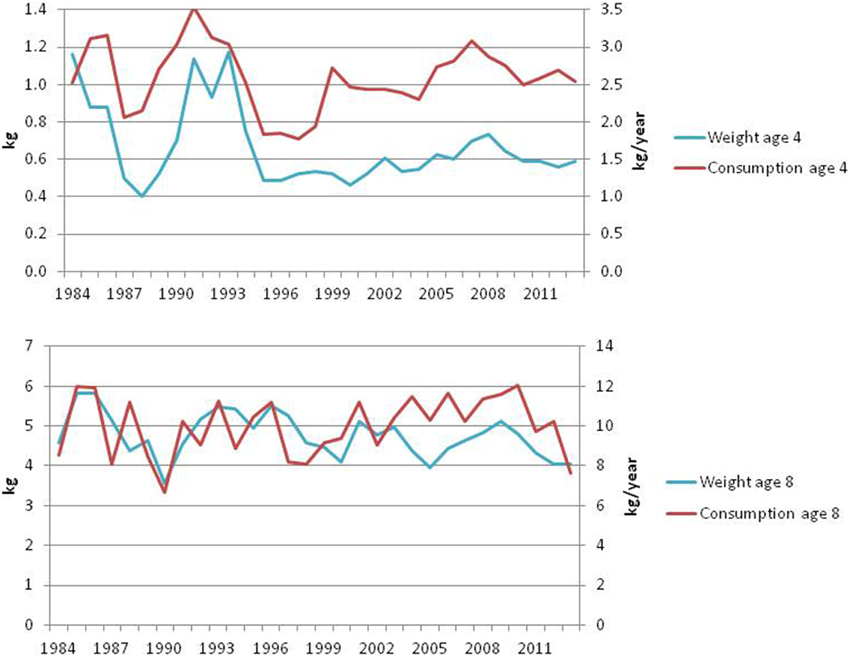
Figure 13. Annual weight at age (kg) and per capita consumption (kg/year) for medium (age 4) and large (age 8) cod (ICES, 2014a).
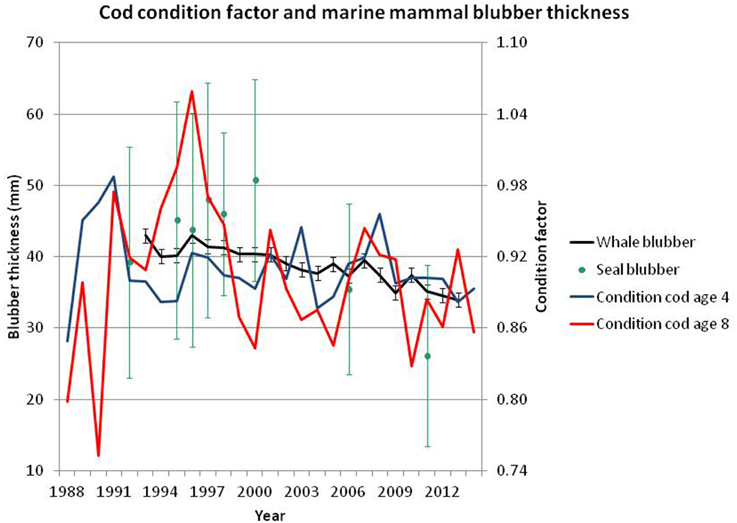
Figure 14. Blubber thickness (mm, with 5–95% confidence intervals) for harp seal and minke whale (left axis) and condition factor (100000 × weight (kg)/L3 (cm)) (right axis) for age 4 and 8 cod. Range of annual sample sizes (individuals measured): Harp seals 75–310, minke whales 216–635. The condition factor for age 4 cod is calculated from the winter survey (ICES, 2014a, Tables A5, A6), and for age 8 it is calculated from the Lofoten survey (ICES, 2014a, Tables A7, A8).
Harp seal body condition, estimated from samples taken during spring in 1992–2011, exhibited a slow increase from 1992 to 2001, where after a significant decrease to a minimum in 2011 has occurred (Øigård et al., 2013, Figure 14).
Similar to other baleen whales, minke whales improve their body condition by increasing their fat deposits during the feeding period, and thereby store energy reserves for wintering and breeding at lower latitudes when their feeding activity is assumed to be low (Næss et al., 1998). Comparison of minke whales body condition in years of high and low abundances of immature herring showed that minke whales in the southern Barents Sea, particularly immatures and adult females, were in significantly better condition in years when the abundance of immature herring was high (Haug et al., 2002). A time series of blubber measurements, made dorsally right behind the blowhole as described in Haug et al. (2002), taken under commercial whaling in the period 1992–2013 shows a significant negative trend over the period with particular low values in 2013 (see Figure 14).
How a stock develops over time depends on survival, feeding, and reproduction, and the population dynamics is a result of how these factors vary throughout the life-cycle and during the year. We here present the results in terms of total stock status (abundance, condition etc.) and try to relate that to processes (competition, predation etc.) going on in different seasons and for different life stages of the stocks under consideration.
A more recent study on harp seals body condition indicated that high abundance of krill impacted the seal condition positively, emphasizing the ecological significance of krill as key food for harp seals during summer (Øigård et al., 2013). High abundances of capelin, polar cod and cod, however, had a negative impact on the seal condition. Indirect effects such as competition between harp seals and prey for shared resources such as krill may have a negative effect on the condition with subsequent implications for the breeding success. Longer migration routes with increased energy expenditure, as a result of less ice, between the breeding/molting areas and feeding areas along the ice edge may certainly also have contributed to the reduced recent harp seal body condition.
The variation in blubber thickness within and between years is much more conspicuous for harp seals than for minke whales. This is to some extent expected given that minke whales are much larger animals and, as such, are expected to have less blubber thickness relative to their size (due to smaller surface-volume ratio). Also, the large-scale migratory behavior of minke whales may bias the blubber thickness toward less variability because lean minke whales may not be able to perform long-distance migrations or may migrate out of the Barents Sea due to bad feeding conditions thereby being under-represented in the catches. In contrast, harp seals are present in the Barents Sea all year round.
The distributions of cod, particularly medium and large individuals, and minke whales overlap to various degree during the year. The most intensive spatial overlap between the two predators occurs during summer and autumn in the central and northern parts of the Barents Sea. Given our dietary knowledge of these predators they compete (directly) for krill and capelin in these periods (Haug et al., 2002; Johannesen et al., 2012). A recent study in which the intra- and interspecific competition among top-predators (cod, minke whale and sea birds) was analyzed concludes that minke whales and cod compete for food and that their diets depend on the abundance of herring and capelin, respectively (Durant et al., 2014). That study further suggests that the diet overlap among these predators generally increase with changes in herring and krill abundances. Unfortunately, the harp seals were not included in that due to lack of synoptic (space and time) diet data. Harp seals are assumed to be distributed in open waters along and at moderate distances from the ice edge in the period June-October (Nordøy et al., 2008) and as such one would expect some degree of spatial overlap between harp seals and the two other predators (cod and minke whale), however, there is little empirical evidence to support that. It should be emphasized, however, that predators may still compete for food despite lack of spatio-temporal overlap between the predators as long as their diets overlap and they feed on the same stocks of prey. No direct diet comparisons between harp seals and cod have been conducted earlier but they share some preferred prey such as capelin, krill, amphipods and polar cod and as such there may be competition among them. In fact, a recent study (Øigård et al., 2013) suggests competition between harp seals and for example cod for shared resources, such as krill. Medium and large cod have similar geographical distributions during summer/autumn, although the areas close to the northern and north-eastern boundary of the cod distribution are dominated by large cod. Predators of different size and feeding behavior probably also react differently to variable prey density; a possible reason why small/medium cod do better could be that they can utilize low densities of zooplankton which are not attractive for larger predators.
Spatial overlap between these predators also occurs during other parts of the year. Medium-sized cod and harp seal overlap to some extent close to coast of Norway/Russia in March/April when harp seals migrate back from the White Sea. During this period they feed on spawning capelin. It is also likely that there is overlap in distribution between minke whales and large (mature) cod in spring in the south-western Barents Sea when both are migrating northwards. However, the food abundance in that area at that time is likely to be fairly low.
If several stocks are found in the same geographical area, the main factors in their competition for prey are their vertical distribution relative to that of the prey, as well as their prey type preferences and ability (mobility, mouth opening etc.) to catch prey of different species and sizes.
To properly understand predatory interactions we need to understand the predator's potential predation space in 3D. Some benthic organisms are, however, available for cod, but not for seals or whales. In contrast to cod there is a trade-off between energy use and predation for sea mammals because they have to pay an energetic price for diving. Diet studies and diving experiments (Blix and Folkow, 1995; Haug et al., 2002) indicate that minke whales primarily feed in the upper 100 m of the water column. Measurements of dive duration have indicated that minke whales usually stay submerged in dives lasting between 4 and 6 min (Folkow and Blix, 1993). Harp seals use the entire water column but are obviously short-duration divers that normally stay submerged for less than 10 min which would leave very little time at depth if the animal chose to dive as deep as 300 m (Nordøy et al., 2008). It should be emphasized that there were few animals included in the mentioned study and the seals displayed major individual as well as spatio-temporal variation in dive behavior most likely due to spatio-temporal variability in prey availability. Nevertheless, more than 50% of all dives were shallower than 100 m. Diving behavior of both minke whales and harp seals seems consistent with animals primarily exploiting schooling fish or high concentrations of zooplankton.
The hunting pressure on harp seals decreased substantially in all areas around 1980 (ICES, 2013a) and as a result the populations increased in size. However, Barents Sea/White Sea harp seals has faced severe problems with a recent dramatic pup production decrease following a slow increase of the population from the mid-1970s (Skaug et al., 2007) to a current level of approximately 1.4 million individuals (ICES, 2013a). Problems with ice retreat seem to affect the Barents Sea/White Sea population in the same manner as observed in the Northwest Atlantic (Stenson and Hammill, 2014). Barents Sea / White Sea harp seals were observed to exhibit poorer body condition in recent years than 10–15 years ago by Øigård et al. (2013) who also observed possible links between the abundance of several distributional overlapping and food-competing fish species (cod, polar cod, capelin) and seal body condition (the more fish, the poorer seal condition). Similar body condition data are not available for the Greenland Sea harp seal population, but it is known that the two Northeast Atlantic harp seal populations share the same feeding grounds in the northern Barents Sea during their most intensive feeding period from July to November (Folkow et al., 2004; Nordøy et al., 2008).
Apparently, it may look as if the harp seal is paying a price of having a big cod stock; the body condition has declined much the past decade (Øigård et al., 2013). Similar observations has been made in common minke whales where there is a negative trend in body condition over the period 1992–2013, with particular low values in 2013 (Hiroko Kato Solvang, Institute of Marine Research, Norway, pers. comm.). While the stocks of fish in the Barents Sea, cod in particular, are at record high levels now (ICES, 2014a), the situation is the opposite in the Northwest Atlantic. For example the cod has been almost completely absent, due to the fisheries, the past two decades (Link et al., 2009; Hutchings and Rangeley, 2010). Furthermore, in 1991 there was a major reduction in capelin biomass in the Newfoundland—Labrador shelf area, and to date this stock has not recovered (DFO, 2010). Concomitant increases in the abundance of Northwest Atlantic harp seals has prompted modeling enquiries to assess whether these events were related (Buren et al., 2014): Cod biomass dynamics were best explained by a combination of fisheries removals and capelin availability, whereas seal consumption was found not to be an important driver of the cod stock. Buren et al. (2014) did, however, not assess if the currently generally low pressure on common prey species by cod might have some beneficial effects on harp seal growth and condition in the area. Long term fluctuations in body condition have been observed in Northwest Atlantic harp seals where adult seals were in poorer condition in the early 1990s (after the collapse of capelin and with cod on its way down, Buren et al., 2014) than in the mid 1980s before the fish stocks declined (Chabot et al., 1996).
Cod cannibalism decrease with prey size and medium sized cod (above 50 cm) are rarely exposed to cannibalism (Yaragina et al., 2009). All the major predator groups (medium/large cod, harp seal, and minke whale) may prey upon small fish. Only minke whales and some large cod appear to prey on larger fish above ca. 30 cm.
General performance of stocks will depend on the conditions they are exposed to throughout the year and throughout their distribution area. In the summer-autumn feeding period, it is not obvious that the food abundance and distribution of predators and prey has changed in a way that would favor cod, in particular medium sized cod, compared to harp seals and minke whales. The recent decline of polar cod in the Barents Sea (Prokhorova, 2013) is, however, more likely to affect the harp seals more than the other stocks due to the dietary importance of polar cod. Also medium-sized cod has been able to feed on capelin during the spawning migration each year, and the abundance of such cod has decreased toward an average level during the last couple of years. The abundance of large cod has increased so strongly during the last years that some reduction in individual growth rate is to be expected. The reduction in the herring stock during the last years (ICES, 2013b) would affect the minke whale stock considerably, as minke whales overlap considerably with young and, to a lesser degree, adult herring in the Barents Sea (Lindstrøm et al., 2002). Cod also feed on herring during cod spawning migration (Michalsen et al., 2008), but this is a fairly small part of the total cod consumption and a decline in adult herring abundance would thus have a minor direct impact on cod.
Macro-zooplankton is seemingly playing a key role in the diet of harp seals and minke whales. In periods of low capelin abundance, minke whales increased the predation on cod and krill whereas harp seals switched to amphipods. In recent years, the capelin stock has been of medium or large size, and zooplankton has not played any role in cod feeding. There is a negative relationship between the amount of capelin and macrozooplankton (Dalpadado and Skjoldal, 1996) explained as a direct effect of variable grazing pressure from capelin. This mechanism may also affect the competition among cod, profiting on a large capelin stock and the sea mammals, profiting on a high abundance of zooplankton. This may partly explain the negative relationship between seal condition and amount of capelin referred to above.
The general retraction of the ice edge northwards during the last decade may have opened up new areas both for primary and secondary producers, and there are indications from the ecosystem surveys (Michalsen et al., 2013) that macrozooplankton is much more abundant in the marginal northern areas than centrally in the Barents Sea. It is difficult to infer from these observations how that would affect the feeding situations for the predatory stocks. The lack of zooplankton in central areas may be caused by down grazing since the capelin stock has moved through this area on the feeding migration northwards. Whether the sea mammals may benefit from increased plankton stocks in the north, depends on whether the capelin halt the northward migration before they reach the northern limit of zooplankton distribution, that is, whether there are rich feeding areas for the mammals north of the capelin feeding area. Observations from the ecosystem surveys (Michalsen et al., 2013) suggest that the minke whales may have found such areas to feed in Skern-Mauritzen et al. (2011). However, few seal observations have been made during these surveys, probably because the seals are dependent on ice and have moved north of the area observed during these surveys. If the zooplankton rich areas do not extend to the ice edge, the harp seal may suffer food shortages more than the whales do, that can feed where the food is available, disregarding the distance to the ice edge. The fact that blubber thickness has decreased more among seals than among minke whales (Figure 14) indicates that such mechanisms may have been present in recent years.
Given the recent average recruitment we expect that the cod stock will decline from the high level presently observed and this may result in improved individual growth, as the growth rate of a cod cohort is dependent on the abundance of the cohort itself as well as adjacent cohorts (Kovalev and Yaragina, 2009). The harp seal stock is also likely to continue to decrease due to recent poor recruitment, and the available per capita food may increase and improve the conditions for the seals. The Norwegian spring spawning herring stock has suffered from low recruitment for several years, and historically, such periods of poor recruitment have alternated with single or a few very strong year classes (ICES, 2013b). New strong herring year classes may occur any time, and this could have a significant negative effect on capelin recruitment and thus possible cascading effects on the ecosystem (see e.g., Hjermann et al., 2010). A decreasing capelin stock will probably (but not necessarily—see Gjøsæter et al., 2009) affect the cod growth and condition negatively. Krill is observed to be a major component in the diets of both harp seals (Lindstrøm et al., 2013) and minke whales (Haug et al., 1996, 2002), particularly during summer. Increased krill biomass, as a result of relaxed top-down control by capelin, is therefore likely to be more beneficial to the sea mammals than the cod. However, if juvenile herring does not replace capelin it is possible that minke whales may experience the same conditions as in 1995–1996 when they had to switch to krill and gadoids.
Increasing water temperatures and reduction or loss of sea ice may have major effects on the energy flux in the system (e.g., Wassmann et al., 2006; Post et al., 2013). Model simulations suggests that reduced sea ice weakens the stratification and hence the phenology of the lower trophic levels. Weaker and more variable water stratification is assumed to result in a higher pelagic production and a weaker benthic-pelagic coupling (Wassmann et al., 2008). If these simulations are correct, we might expect a lower benthic production which is likely to affect cod more than the mammal predators since cod feed on the benthic community (Johannesen et al., 2012). On the other hand earlier and prolonged seasonal ice-melt may cause increased mismatches between phytoplankton blooms and zooplankton production (e.g., Søreide et al., 2010) and as a result lower pelagic production. The ecological consequence of more light and ocean freshening, which have antagonistic effects on phytoplankton production, is poorly understood (Wassmann et al., 2011). A more recent study (Ardyna et al., 2014) suggests that loss of sea ice triggers late autumn phytoplankton blooms but which of the predators that will benefit most from this is difficult to say, however, changes in the distribution and length of the production period is likely to affect the migration patterns of both cod and the marine mammal species.
In case of a continued warming, predator and prey stocks are likely to move further north—but cod is not likely to move into deep Polar Ocean. Although minke whales are shelf feeders they do feed in deep sea areas and as such they will have no problem of feeding successfully in the polar ocean and, to a lesser extent, the same applies for harp seals. However, if the ice conditions in the White Sea, the main breeding area for Barents Sea harp seals, deteriorate, the seals may have to change to other breeding areas and this could affect their migration pattern considerably. Cod migration patterns could also change considerably if new spawning areas such as the Bear Island area or the Novaya Zemlya coast appear. A radical shift of breeding/spawning areas represents a profound change to the whole life history of a stock, since this will affect the drift pattern of larvae (fish) and feeding area of the young (seals) in an unpredictable way.
A gradual shift in species composition toward more Atlantic dominated zooplankton species which has been observed the past decade (Dalpadado et al., 2012) implies that fatty arctic ice-associated prey such as amphipods is being replaced by less fatty Atlantic species (Wassmann et al., 2006; Falk-Petersen et al., 2007). The outcome of this change is difficult to predict but, in general, less energy-rich food will allow the predators to build up less energy during the feeding season which, in turn, will affect their ability to undertake long migrations to spawning/breeding areas and to develop gonads or suckle their young. Increased water temperature will speed up all the processes in the ecosystem due to increased metabolism with the exception of sea mammals that will use less energy because they are thermoregulated animals. As a result the energy turnover will increase and this will most likely result in a more variable ecosystem.
There have been several modeling attempts using dynamics multispecies models (e. g., Bogstad et al., 1997; Schweder et al., 2000; Lindstrøm et al., 2009); “what-if” scenarios (20–100 years into the future) has been run with respect to harp seal, minke whale, cod, capelin and herring. Model comparisons are difficult to make and conclusions are difficult to draw due to differences in model structure and assumptions. For example, in Gadget and MULTSPEC, a larger whale population resulted in higher stock levels of capelin and slightly reduced cod and herring stocks; the rise in predation on capelin as a result of a larger whale stock was more than compensated for by reduced predation pressure on capelin from cod and herring (Bogstad et al., 1997; Lindstrøm et al., 2009). In contrast, increased whale numbers in the Scenario Barents Sea model (Schweder et al., 1998) resulted in a reduced capelin stock. Thus the indirect effect of whales on capelin through the food web was dominant in the MULTSPEC and Gadget models, while in the Scenario Barents Sea model the direct effect of whales preying on capelin were dominant. The difference may be due both to the functional forms as well as to parameter values used in the models. Of these three models, only the Gadget model for the Barents Sea is still operative.
The multispecies models mentioned above have all focused on top-down effects (predation mortalities induced by the top predators). Less attention has been given to modeling bottom-up effects (effects of varying food abundance on predator population dynamics), but our present study indicates that such effects also are important. The same model frameworks could still be used.
The results from such studies should in any case be treated with great caution. One reason is that in those models migration and thus geographical overlap is at present not affected by climatic conditions or stock size. That kind of effects could, however, be included in such models (see Howell and Filin, 2014 for an example with a cod-capelin model), and could possibly improve the ability of such models to give realistic future scenarios. Appropriate modeling of the direct and indirect impact of marine mammals on commercial species is also very important, as seen from the difference between results from different models described above.
There are a variety of factors influencing the competition between cod, harp seals and minke whales in the Barents Sea. Their performance is affected both by their food consumption in the main feeding season in summer-autumn, when they compete for food, as well as their feeding during the rest of the year. The main advantages for cod are likely: (1) Larger availability of food (mainly capelin) during winter-spring than for marine mammals. This particularly affects immature cod, which has being doing better than mature cod, and (2) A wider range of prey species available to cod than to marine mammals, due to the cod's larger vertical range. This may more than outweigh the wider size range of prey available to marine mammals. Harp seals are more dependent on prey items found close to the ice edge than the other two predator stocks are, and thus it makes sense that the performance of harp seals is worse than that of the two other top predators in the area.
Data on Barents Sea cod diets and condition are collected routinely every year, while collection of similar data from harp seals and minke whales are done much more fragmentarily. For a more proper and quantitative comparison of the relation between cod and the mammals in the Barents Sea, diet and condition data from the mammals must be collected in such a way that a time series similar to the one on cod becomes available.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Randi Ingvaldsen, IMR, for making Figures 2, 3, Jaime Alvarez, IMR for making Figure 5, Karen Gjertsen, IMR, for making Figure 11, and Hiroko Kato Solvang, IMR, for providing data on blubber thickness in minke whale. We also thank two referees for their constructive comments.
Ardyna, M., Babin, B., Gosselin, M., Devred, E., Rainville, L., and Tremblay, J. E. (2014). Recent Arctic Ocean sea ice loss triggers novel fall phytoplankton blooms. Geophys. Res. Lett. 41, 6207–6212. doi: 10.1002/2014GL061047
Blix, A. S., and Folkow, L. (1995). Daily energy-expenditure in free-living minke whales. Acta Physiol. Scand. 153, 61–66. doi: 10.1111/j.1748-1716.1995.tb09834.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bogstad, B., Haug, T., and Mehl, S. (2000). Who eats whom in the Barents Sea? NAMMCO Sci. Publ. 2, 65–80. doi: 10.7557/3.2975
Bogstad, B., Hiis Hauge, K., and Ulltang, Ø. (1997). MULTSPEC – a multi-species model for fish and marine mammals in the Barents Sea. J. Northw. Atl. Fish. Sci. 22, 317–341. doi: 10.2960/J.v22.a23
Bogstad, B., and Mehl, S. (1997). “Interactions between Atlantic Cod (Gadus morhua) and its Prey Species in the Barents Sea,” in Proceedings of the International Symposium on the Role of Forage Fishes in Marine Ecosystems (Fairbanks, AK: Alaska Sea Grant College Program Report No. 97–01, University of Alaska Fairbanks), 591–615.
Bøthun, G., Skaug, H. J., and Øien, N. (2009). Abundance of Minke Whales in the Northeast Atlantic Based on Survey Data Over the Period 2002-2007. International Whaling Commission SC/61/RMP 2, 13.
Bowen, W. D. (1997). Role of marine mammals in aquatic ecosystems. Mar. Ecol. Prog. Ser. 158, 267–274. doi: 10.3354/meps158267
Buren, A. D., Koen-Alonso, M., and Stenson, G. B. (2014). The role of harp seals, fisheries and food availability in driving the dynamics of northern cod. Mar. Ecol. Prog. Ser. 511, 265–284. doi: 10.3354/meps10897
Cavalieri, D. J., Parkinson, C. L., Gloersen, P., and Zwally, H. J. (1996). Sea Ice Concentrations From Nimbus-7 SMMR and DMSP SSM/ISSMI PassiveMicrowave Data, January 1979 to August 2011, Natl. Boulder, CO: Snow and Ice Data Cent.
Chabot, D., Stenson, G. B., and Cadigan, N. G. (1996). Short- and long term fluctuations in the size and condition of harp seal (Phoca groenlandica) in the Northwest Atlantic. NAFO Sci. Counc. Stud. 26, 15–32.
Dalpadado, P., Ingvaldsen, R. B., Stige, L. C., Bogstad, B., Knutsen, T., Ottersen, G., et al. (2012). Climate effects on the Barents Sea ecosystem dynamics. ICES J. Mar. Sci. 69, 1303–1316. doi: 10.1093/icesjms/fss063
Dalpadado, P., and Skjoldal, H. R. (1996). Abundance, maturity and growth of the krill species Thysanoessa inermis and T. longicaudata in the Barents Sea. Mar. Ecol. Prog. Ser. 144, 175–183. doi: 10.3354/meps144175
DFO. (2010). “Assessment of capelin in SA2+Div. 3KL in 2010,” in Canadian Scientific Advisory Section, Scientific Advisory Report 2010/090 (St. John's, NL).
Dolgov, A. V., Orlova, E. L., Johannesen, E., and Bogstad, B. (2011). “8.4 Piscivorous fish,” in The Barents Sea. Ecosystem, Resources, Management. Half a Century of Russian-Norwegian Cooperation, eds T. Jakobsen and V. K. Ozhigin (Trondheim: Tapir Academic Press), 466–484.
Durant, J., Skern-Mauritzen, M., Krasnov, Y. V., Nikolaeva, N. G., Lindstrøm, U., and Dolgov, A. V. (2014). Temporal dynamics of top predators interactions in the Barents Sea. PLoS ONE 9:e110933. doi: 10.1371/journal.pone.0110933
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Eriksen, E., Ingvaldsen, R., Stiansen, J. E., and Johansen, G. O. (2012). Thermal habitat for 0-group fish in the Barents Sea; how climate variability impacts their density, length, and geographic distribution. ICES J. Mar. Sci. 69, 870–879. doi: 10.1093/icesjms/fsr21
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., Bond, W. J., et al. (2011). Trophic downgrading of Planet Earth. Science 333, 301–306. doi: 10.1126/science.1205106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Falk-Petersen, S., Timofeev, S., Pavlov, V., and Sargent, J. R. (2007). “Climate variability and the effect on arctic food chains,” in Arctic-Alpine Ecosystems and People in a Changing Environment, eds J. B. Ørbæk, R. Kallenmborn, I. Tombre, E. Hegseth, S. Falk-Petersen, and A. H. Hoel (Berlin;Heidelberg; New York, NY: Springer), 147–161.
Folkow, L. P., and Blix, A. S. (1993). Daily changes in surfacing rates of minke whales (Balaenoptera acutorostrata) in Norwegian waters. Rep. int. Whal. Commn. 43, 311–314.
Folkow, L. P., Haug, T., Nilssen, K. T., and Nordøy, E. S. (2000). Estimated food consumption of minke whales (Balaenoptera acutorostrata) in northeast Atlantic waters in 1992-1995. NAMMCO Sci. Publ. 2, 65–80. doi: 10.7557/3.2972
Folkow, L. P., Nordøy, E. S., and Blix, A. S. (2004). Distribution and diving behaviour of harp seals Pagophilus groenlandica from the Greenland Sea stock. Polar Biol. 27, 281–298. doi: 10.1007/s00300-004-0591-7
Frank, K., Petrie, B., Choi, J. S., and Leggett, W. C. (2005). Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623. doi: 10.1126/science.1113075
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gjøsæter, H. (1998). The population biology and exploitation of capelin (Mallotus villosus) in the Barents Sea. Sarsia 83, 453–496. doi: 10.1080/00364827.1998.10420445
Gjøsæter, H., Bogstad, B., and Tjelmeland, S. (2009). Ecosystem effects of three capelin stock collapses in the Barents Sea. Mar. Biol. Res. 5, 40–53. doi: 10.1080/17451000802454866
Grahl-Nielsen, O., Haug, T., Lindstrøm, U., and Nilssen, K. T. (2011). Fatty acids in harp seal blubber do not necessarily reflect their diet. Mar. Ecol. Prog. Ser. 426, 263–276. doi: 10.3354/meps09011
Haug, T., Bjørge, A., Øien, N., and Ziryanov, S. V. (2011). “7. Marine mammals,” in The Barents Sea; Ecosystem, Resources, Management; Half a Century of Russian-Norwegian Cooperation, eds T. Jakobsen and V. K. Ozhigin (Trondheim: Tapir Academic Press), 395–430.
Haug, T., Lindstrøm, U., and Nilssen, K. T. (2002). Variations in minke whale Balaenoptera acutorostrata diet and body condition in response to ecosystem changes in the Barents Sea. Sarsia 87, 409–422. doi: 10.1080/0036482021000155715
Haug, T., Lindstrøm, U., Nilssen, K. T., Røttingen, I., and Skaug, H. J. (1996). Diet and food availability for northeast Atlantic minke whales, Balaenoptera acutorostrata. Rep. int. Whal. Commn. 46, 371–382.
Haug, T., Nilssen, K. T., Øien, N., and Potelov, V. (1994). Seasonal distribution of harp seals (Phoca groenlandica) in the Barents Sea. Polar Res. 13, 161–172. doi: 10.3402/polar.v13i2.6690
Heffernan, O., Righton, D., and Michalsen, K. (2004). Use of data storage tags to quantify vertical movements of cod: effects on acoustic measures. ICES J. Mar. Sci. 61, 1062–1070. doi: 10.1016/j.icesjms.2004.07.003
Hjermann, D. Ø., Bogstad, B., Dingsør, G. E., Gjøsæter, H., Ottersen, G., Eikeset, A. M., et al. (2010). Trophic interactions affecting a key ecosystem component: a multi-stage analysis of the recruitment of the Barents Sea capelin. Can. J. Fish. Aquat. Sci. 67, 1363–1375. doi: 10.1139/F10-064
Hop, H., and Gjøsæter, H. (2013). Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar. Biol. Res. 9, 878–894. doi: 10.1080/17451000.2013.775458
Howell, D., and Filin, A. A. (2014). Modelling the likely impacts of climate-driven changes in cod-capelin overlap in the Barents Sea. ICES J. Mar. Sci. 71, 72–80. doi: 10.1093/icesjms/fst172
Hutchings, J. A., and Rangeley, R. W. (2010). Correlates of recovery for Canadian Atlantic cod (Gadus morhua). Can. J. Zool. 89, 386–400. doi: 10.1139/z11-022
ICES. (International Council for the Exploration of the Sea). (2013a). Report of the ICES Working Group On Harp And Hooded Seals. Murmansk,: ICES CM 2013/ACOM: 20, 55.
ICES (International Council for the Exploration of the Sea). (2013b). Report of the NAFO/ICES Pandalus Working Group. Dartmouth, NS: ICES CM 2013/ACOM:14, 70.
ICES (International Council for the Exploration of the Sea). (2014a). Report of the Arctic Fisheries Working Group. Lisbon: ICES CM. 2014/ACOM:05, 656.
ICES (International Council for the Exploration of the Sea). (2014b). Report of the Working Group on Widely Distributed Stocks (WGWIDE). Copenhagen: ICES CM 2014/ACOM:15, 938.
Ingvaldsen, R., and Gjøsæter, H. (2013). Responses in spatial distribution of Barents Sea capelin to changes in stock size, ocean temperature and ice cover. Mar. Biol. Res. 9, 867–877. doi: 10.1080/17451000.2013.775450
Iverson, S. (2002). “Blubber.” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (San Diego, CA: Academic Press), 107–112.
Johannesen, E., Lindstrøm, U., Michalsen, K., Skern-Mauritzen, M., Fauchald, P., Bogstad, B., et al. (2012). Feeding in a heterogeneous environment: spatial dynamics in summer foraging Barents Sea cod. Mar. Ecol. Prog. Ser. 458, 181–197. doi: 10.3354/meps09818
Johansen, G. O., Johannesen, E., Michalsen, K., Aglen, A., and Fotland, Å. (2013). Seasonal variation in geographic distribution of NEA cod - survey coverage in a warmer Barents Sea. Mar. Biol. Res. 9, 908–919. doi: 10.1080/17451000.2013.775456
Kjesbu, O. S., Bogstad, B., Devine, J. A., Gjøsæter, H., Howell, D., Ingvaldsen, R., et al. (2014). Synergies between climate and management for Atlantic cod fisheries at high latitudes. Proc. Natl. Acad. Sci. U.S.A. 111, 3478–3483. doi: 10.1073/pnas.1316342111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kovacs, K., Haug, T., and Lydersen, C. (2009). “Marine mammals,” in Ecosystem Barents Sea, eds E. Sakshaug, T. Henriksen, and K. M. Kovacs (Trondheim: Tapir Academic Press), 453–395.
Kovalev, Y. A., and Yaragina, N. A. (2009). The effects of population density on the rate of growth, maturation and productivity of the stock of the Northeast Arctic cod. J. Ichthyol. 49, 56–65. doi: 10.1134/S003294520901007X
Lavigne, D., and Kovacs, K. M. (eds.). (1988). Harps and Hoods, Ice-Breeding Seals in the Northwest Atlantic. Waterloo, ON: University of Waterloo Press.
Lindstrøm, U., Harbitz, A., Haug, T., and Nilssen, K. T. (1998). Do harp seals Phoca groenlandica exhibit particular prey preferences? ICES J. Mar. Sci. 55, 941–953. doi: 10.1006/jmsc.1998.0367
Lindstrøm, U., and Haug, T. (2001). Feeding strategy and prey selectivity in common minke whales (Balaenoptera acutorostrata) foraging in Southern Barents Sea during early summer. J. Cetacean Res. Manage. 3, 239–249.
Lindstrøm, U., Haug, T., and Røttingen, I. (2002). Predation on herring, Clupea harengus, by minke whales, Balaenoptera acutorostrata, in the Barents Sea. ICES J. Mar. Sci. 59, 58–70. doi: 10.1006/jmsc.2001.1135
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lindstrøm, U., Nilssen, K. T., Pettersen, L. M. S., and Haug, T. (2013). Harp seal foraging behaviour during summer around Svalbard in the northern Barents Sea: diet composition and selection of prey. Polar Biol. 36, 305–320. doi: 10.1007/s00300-012-1260-x
Lindstrøm, U., Smout, S., Howell, D., and Bogstad, B. (2009). Modelling multispecies interactions in the Barents Sea ecosystem with special emphasis on minke whales, cod, herring and capelin. Deep Sea Res. II Topol. Stud. Oceanogr. 56, 2068–2079. doi: 10.1016/j.dsr2.2008.11.017
Link, J. S., Bogstad, B., Sparholt, H., and Lilly, G. R. (2009). Trophic role of Atlantic cod in the ecosystem. Fish Fish. 10, 58–87. doi: 10.1111/j.1467-2979.2008.00295.x
McBride, M. M., Filin, A., Titov, O., and Stiansen, J. E. (2014). “IMR/PINRO update of the ‘Joint Norwegian-Russian environmental status report on the Barents Sea Ecosystem’ giving the current situation for climate, phytoplankton, zooplankton, fish, and fisheries during 2012-13,” in IMR/PINRO Joint Report Series 1-2014 (Bergen), 64.
Mehl, S., Aglen, A., Alexandrov, D. I., Bogstad, B., Dingsør, G. E., Gjøsæter, H., et al. (2013). “Fish investigations in the Barents Sea winter 2007-2012,” in IMR/PINRO Joint Report Series 1-2013, 97.
Meier, S., Falk-Petersen, S., Gade-Sørensen, L. A., Greenacre, M., Haug, T., and Lindstrøm, U. (in press). Fatty acids in common minke whale (Balaenoptera acutorostrata) blubber reflect the feeding area and food selection, but also high endogenous metabolism. Mar. Biol. Res.
Meier, W. N., Stroeve, J. C., and Fetterer, F. (2006). Whither Arctic sea ice? A clear signal of decline regionally, seasonally and extending beyond the satellite record. Ann. Glaciol. 46, 428–434. doi: 10.3189/172756407782871170
Michalsen, K., Dalpadado, P., Eriksen, E., Gjøsæter, H., Ingvaldsen, R. B., Johannesen, E., et al. (2013). Marine living resources of the Barents Sea – Ecosystem understanding and monitoring in a climate change perspective. Mar. Biol. Res. 9, 932–947. doi: 10.1080/17451000.2013.775459
Michalsen, K., Johannesen, E., and Bogstad, B. (2008). Feeding of mature cod (Gadus morhua L.) at the spawning grounds in Lofoten. ICES J. Mar. Sci. 65, 571–580. doi: 10.1093/icesjms/fsn019
Myers, R., and Worm, B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283. doi: 10.1038/nature01610
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Næss, A., Haug, T., and Nilssen, E. M. (1998). Seasonal variation in body condition and muscular lipid contents in northeast Atlantic minke whales Balaenoptera acutorostrata. Sarsia 83, 211–218.
Nilssen, K. T., Haug, T., Grotnes, P. E., and Potelov, V. A. (1997). Seasonal variation in body condition of adult Barents Sea harp seals (Phoca groenlandica). J. Northw. Atl. Fish. Sci. 22, 17–25. doi: 10.2960/J.v22.a1
Nilssen, K. T., Haug, T., Potelov, V., Stasenkov, V. A., and Timoshenko, Y. K. (1995b). Food habits of harp seals (Phoca groenlandica) during lactation and moult in March-May in the southern Barents Sea and White Sea. ICES J. Mar. Sci. 52, 33–41. doi: 10.1016/1054-3139(95)80013-1
Nilssen, K. T., Haug, T., Potelov, V., and Timoshenko, Y. K. (1995a). Food habits and food availability of harp seals (Phoca groenlandica) during early summer and autumn in the northern Barents Sea. Polar Biol. 15, 485–493. doi: 10.1007/BF00237462
Nilssen, K. T., Pedersen, O.-P., Folkow, L., and Haug, T. (2000). Food consumption estimates of Barents Sea harp seals. NAMMCO Sci. Publ. 2, 9–28. doi: 10.7557/3.2968
Nordøy, E. S., Folkow, L. P., Potelov, V., Prischemikhin, V., and Blix, A. S. (2008). Seasonal distribution and dive behaviour of harp seals (Pagophilus groenlandicus) of the White Sea – Barents Sea stock. Polar Biol. 31, 1119–1135. doi: 10.1007/s00300-008-0453-9
Øien, N., Jørgensen, T., and Øritsland, T. (1987). A stock assessment for northeast Atlantic minke whales. Rep. Int. Whal. Commn. 37, 225–236.
Øigård, T. A., Haug, T., and Nilssen, K. T. (2014a). From pup production to quotas: Current status of harp seals in the Greenland Sea. ICES J. Mar. Sci. 71, 537–545. doi: 10.1093/icesjms/fst155
Øigård, T. A., Haug, T., and Nilssen, K. T. (2014b). Current status of hooded seals in the Greenland Sea. Victims of climate change and predation? Biol. Conserv. 172, 29–36. doi: 10.1016/j.biocon.2014.02.007
Øigård, T. A., Lindstrøm, U., Haug, T., Nilssen, K. T., and Smout, S. (2013). Functional relationship between harp seal body condition and available prey in the Barents Sea. Mar. Ecol. Prog. Ser. 484, 287–301. doi: 10.3354/meps10272
Pauly, D., Christensen, V., Dalsgaard, J., Froese, R., and Torres, F. C. Jr. (1998). Fishing down marine food webs. Science 279, 860–863. doi: 10.1126/science.279.5352.860
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Post, E., Bhatt, U. S., Bitz, C. M., Brodie, J. F., Fulton, T. L., Hebblewhite, M., et al. (2013). Ecological consequence of Sea-Ice decline. Science 341, 519–524. doi: 10.1126/science.1235225
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prokhorova, T. (ed.). (2013). “Survey report from the joint Norwegian/Russian ecosystem survey in the Barents Sea August-October 2013,” in IMR/PINRO Joint Report Series, No. 4/2013. ISSN 1502-8828 (Bergen), 131.
Schweder, T., Hagen, G. S., and Hatlebakk, E. (1998). On the effect of cod and herring fisheries of retuning the Revised Management Procedure for minke whaling in the Greater Barents Sea. Fish. Res. 37, 77–95. doi: 10.1016/S0165-7836(98)00128-3
Schweder, T., Hagen, G. S., and Hatlebakk, E. (2000). Direct and indirect effects of minke whale abundance on cod and herring fisheries: a scenario experiment for the Barents Sea. NAMMCO Sci. Publ. 2, 120–133. doi: 10.7557/3.2976
Skaug, H. J., Frimannslund, L., and Øien, N. I. (2007). Historical population assessment of Barents Sea harp seals (Pagophilus groenlandicus). ICES J. Mar. Sci. 64, 1356–1365. doi: 10.1093/icesjms/fsm118
Skaug, H. J., Øien, N., Schweder, T., and Bøthun, G. (2004). Abundance of minke whales (Balaenoptera acutorostrata) in the Northeastern Atlantic. Can. J. Fish. Aquat. Sci. 61, 870–886. doi: 10.1139/f04-020
Skern-Mauritzen, M., Johannesen, E., Bjørge, A., and Øien, N. (2011). Baleen whale distributions and prey associations in the Barents Sea. Mar. Ecol. Prog. Ser. 426, 289–301. doi: 10.3354/09027
Søreide, J. E., Leu, E., Graeve, M., and Falk-Pedersen, S. (2010). Timing of food, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Change Biol. 16, 3154–3163.
Stenson, G. B., and Hammill, M. O. (2014). Can ice breeding seals adapt to habitat loss in a time of climate change? ICES J. Mar. Sci. 71, 1977–1986. doi: 10.1093/icesjms/fsu074
Tereshchenko, V. V. (1996). “Seasonal and year-to-year variations of temperature and salinity along the Kola meridian transect,” in ICES CM 1996/C:11 24 (Copenhagen).
Wassmann, P., Carroll, J., and Bellerby, R. G. J. (2008). Carbon flux and ecosystem feedback in the northern Barents Sea in an era of climate change: an introduction. Deep Sea Res. II 55, 2143–2153. doi: 10.1016/j.dsr2.2008.05.025
Wassmann, P., Duarte, C. M., Agusti, S., and Sejr, M. K. (2011). Footprints of climate change in the Arctic marine ecosystem. Glob. Change Biol. 17, 1235–1249. doi: 10.1111/j.1365-2486.2010.02311.x
Wassmann, P., Reigstad, M., Haug, T., Rudels, B., Carroll, M. L., Hop, H., et al. (2006). Food web and carbon flux in the Barents Sea. Progr. Oceanogr. 71, 232–287. doi: 10.1016/j.pocean.2006.10.003
Windsland, K., Lindstrøm, U., Nilssen, K. T., and Haug, T. (2007). Relative abundance and size composition of prey in the common minke whale diet in selected areas of the northeast Atlantic during 2000-04. J. Cetacean Res. Manage. 9, 167–178.
Worm, B., and Branch, T. A. (2012). The future of fish. Trends Ecol. Evol. 27, 594–599. doi: 10.1016/j.tree.2012.07.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yaragina, N. A., Aglen, A., and Sokolov, K. M. (2011). “Cod,” in The Barents Sea, Ecosystem, Resources, Management. Half a Century of Russian–Norwegian cooperation, eds T. Jakobsen and V. K. Ozhigin (Trondheim: Tapir academic press), 225–270.
Yaragina, N. A., Bogstad, B., and Kovalev, Y. A. (2009). Variability in cannibalism in Northeast Arctic cod (Gadus morhua) during the period 1947–2006. Mar. Biol. Res. 5, 75–85. doi: 10.1080/17451000802512739
Keywords: cod, harp seal, minke whale, Barents Sea, competition, top predators
Citation: Bogstad B, Gjøsæter H, Haug T and Lindstrøm U (2015) A review of the battle for food in the Barents Sea: cod vs. marine mammals. Front. Ecol. Evol. 3:29. doi: 10.3389/fevo.2015.00029
Received: 31 October 2014; Accepted: 06 March 2015;
Published: 25 March 2015.
Edited by:
Morten Frederiksen, Aarhus University, DenmarkReviewed by:
Karin Charlotta Harding, Gothenburg University, SwedenCopyright © 2015 Bogstad, Gjøsæter, Haug and Lindstrøm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bjarte Bogstad, Institute of Marine Research, Demersal Fish Division, PO Box 1870 Nordnes, N-5817 Bergen, NorwayYmphcnRlQGltci5ubw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.