- Visscher Laboratory, Department of Entomology, University of California, Riverside, Riverside, CA, USA
Numerous activities within honey bee (Apis mellifera L.) colonies rely on feedback loops for organization at the group level. Classic examples of these self-organizing behaviors occur during foraging and swarm nest site selection. The waggle dance provides positive feedback, promoting foraging at a specific location or increased scouting at a potential nest site. Rather less well known than the waggle dance is the stop signal, a short vibration often delivered while butting against a dancing bee. It is currently best understood as a counter to the waggle dance, offering negative feedback toward the advertised foraging location or nest site. When the stop signal is received by a waggle dancer she is more likely to terminate her dance early and retire from the dance floor. Bees that experienced danger or overcrowding at a food source are more likely to perform the stop signal upon their return to the colony, resulting in an inhibition of foraging at that location. During a swarm's nest site selection process, scout bees that visited a different site than the one being advertised are more likely to stop-signal the waggle dancer than are scouts that had visited the same site. Over time, the scout bees build recruitment to a single site until a quorum is reached and the swarm can move to it. The balance between the positive feedback from the waggle dance and the negative feedback from the stop signal allows for a more sensitive adjustment of response from the colony as a unit. Many of the processes associated with the feedback loops organizing a honey bee colony's activities are in striking parallel to other systems, such as intercellular interactions involved in motor neuron function.
Introduction
Honey bees (Apis mellifera L.) employ numerous chemical, tactile, and vibratory communication signals to coordinate their activities. Here, we will focus on a few of the vibratory ones including the well-known waggle dance, which signals the distance and direction of a resource such as a food source or a potential nest site to nestmates (von Frisch, 1967). We will also discuss the tremble dance, which is performed by foragers who have experienced delay in unloading, and recruits more bees to assist in unloading food from incoming foragers (Seeley, 1992). An additional signal, and the focus of this article, is the stop signal. Sometimes referred to as the “brief piping signal,” (e.g., Seeley and Tautz, 2001; Thom et al., 2003) in addition to “peeps” (Esch et al., 1965), “squeaking” (von Frisch, 1967), and “short squeaks” (Kirchner, 1993a), the stop signal is an acoustic signal produced by a bee briefly vibrating her wing muscles (with little wing movement), often while butting her head against another bee (a video of honey bees performing the stop signal can be found in the online Supplementary Material).
Here we review what is known about the stop signal and its uses. The focus will be on exploring the balance among communication signals used by individuals and the resulting adjustment of response by the colony as a unit.
Early Work on the Stop Signal
The first recorded observations of the stop signal did not find clear uses and meanings for it. Esch (1964) observed bees attending waggle dances and noted that they occasionally emitted squeaking sounds, after which they sometimes received food samples from the dancer. Wenner (1962) reported that disturbed bees emit short bursts of sound, similar to the stop signal. von Frisch (1967) also observed it in use by bees interacting with waggle dancers, and agreed with an interpretation by Esch (1964) that it was a begging call for food.
The sounds made by these bees were later identified as vibrations of the comb made by pressing the thorax briefly to it and pulsing the wings (Michelsen et al., 1986), or by a bee butting her head into a dancer and pulsing the wing muscles (Nieh, 1993). Michelsen et al. (1986) described these sounds as typically lasting approximately 100 ms at approximately 380 Hz. The results of Schlegel et al. (2012) averaged 407 Hz for 147 ms. Honey bees also make a similar-sounding acoustic signal known as worker piping, but this can be differentiated from the stop signal by its much longer duration, approximately 602 ms, and a higher and upward sweeping frequency, (451–478 Hz, Schlegel et al., 2012).
The term “stop signal” seems an appropriate name for the signal, since von Frisch (1967) reported that the dancer and surrounding bees are “paralyzed” by the sound. Similarly, Michelsen et al. (1986) found that artificial signals made by vibrating the comb caused bees in the area immediately surrounding the point of vibration to briefly freeze their movements. When Nieh (1993) observed bees on the dance floor and recorded the behavior of individuals before and after sending or receiving the stop signal he found that the sender very seldom receives food (once out of 576 stop signals delivered to waggle dancers), discrediting the idea that the stop signal is a begging call. The most common occurrence after a waggle dancer received the stop signal was to leave the dance floor (Nieh, 1993). Similarly, in a later study Pastor and Seeley (2005) investigated the behavior of waggle dancers and dance followers. They found that dancers that received the stop signal were more likely to stop dancing and they never observed an instance of food exchange between a stop signal sender and receiver. A summary of the roles of the stop signal can be found in Table 1.
Which Bees Produce the Stop Signal?
In an effort to determine which bees within a colony produce the stop signal and which receive it, Nieh (1993) trained foragers from an observation hive to visit an artificial feeding station filled with sugar water and made observations on the bees populating the dance floor. Recently-returned foragers were observed with a video camera and microphone. The study focused on classifying the stop signal senders and receivers, and found that tremble dancers are the most likely individuals to perform the stop signal, although they can occasionally be performed by waggle dancers and dance followers (Nieh, 1993). Waggle dancers and tremble dancers were the most common stop signal receivers, although food exchangers, dance followers, and “other” bees not dancing or observing dances were also targeted (Nieh, 1993).
Pastor and Seeley (2005) revisited the question of which bees send and receive the stop signal after noting that the bees in Nieh's (1993) study may not have been behaving normally due to the large influx of food they were receiving from the feeding station. When they observed a colony that was foraging on naturally-available food resources with no access to a feeder, most of the waggle dance followers that used the stop signal had not previously been tremble dancing (Pastor and Seeley, 2005). Additionally, though Nieh (1993) found that dance followers occasionally use the stop signal on waggle dancers, in Pastor and Seeley's (2005) results the majority of stop signalers were dance followers.
When waggle dancers receive a stop signal they are more likely to leave the dance floor (Nieh, 1993; Pastor and Seeley, 2005) and their average dance length is shorter (Kirchner, 1993b). These factors, combined, likely result in an inhibition of recruitment to that food source and an overall decrease in foraging.
It is possible that this effect was also observed by Wenner (1962), as he described waggle dancers being interrupted in their dances by other bees or abruptly halting their dances, sometimes even in the middle of a waggle run, for unknown reasons. He also mentioned the short sounds made by disturbed bees, which may have been stop signals. Unfortunately, insufficient information was given to determine if these short sounds were stop signals.
What Elicits the Stop Signal?
Aside from its effect of halting waggle dances, the stop signal can also be seen in use by bees not located on the dance floor and received by bees that are not waggle dancers. Thom et al. (2003) observed colonies both when they had access to a sugar water feeding station and when they were foraging under natural conditions.
Stop signaling increased when a feeding station was available (Thom et al., 2003). Most of the stop signaling activity was by tremble dancers, although non-waggle dancing nectar foragers also performed the stop signal (Thom et al., 2003). Tremble dancers that used the stop signal ended up staying in the hive for longer than those that did not use the stop signal (Thom et al., 2003). Foragers that performed the stop signal tended to spend less of their time within the colony on the dance floor, and often continued performing the stop signal outside of the dance floor (Thom et al., 2003). Tremble dancers that performed the stop signal tremble-danced for longer than non-stop signalers and traveled deeper into the hive (Thom et al., 2003). Also, bees that used the stop signal sometimes inspected cells by entering them up to the thorax, which was a behavior not exhibited by non-stop-signaling bees (Thom et al., 2003).
It can be inferred that by inhibiting the waggle dance, the stop signal strengthens the nectar-receiver-recruiting effect of the tremble dance (Figure 1), but this does not account for the bees observed using the stop signal outside of the dance floor. Thom et al. (2003) suggested that the off-dance-floor stop signaling could be an effort to modulate the recruitment of more nectar receivers by lowering potential nectar receivers' response thresholds to the tremble dance.
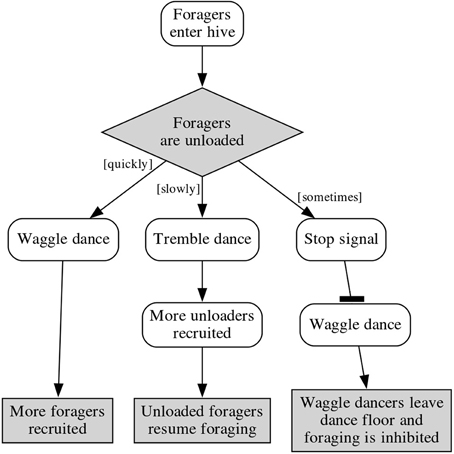
Figure 1. Feedback loops in a foraging colony, showing the effect of the stop signal on waggle dances and forager recruitment.
An interesting finding of this study (Thom et al., 2003) was that many of the tremble dancers that also performed the stop signal began signaling as soon as they entered the colony, i.e., before having an opportunity to be met by a nectar receiver and be influenced by the time-delay cue described by Seeley (1992). Thom et al. (2003) suggested they may have been acting based on their experiences from prior foraging trips or by cues sensed outside of the hive. One source of such cues may have been scramble competition at the feeding station (Thom et al., 2003). This conclusion seems possible, especially since it has been shown that rich food resources such as feeding stations lead to a spike in tremble dancing and stop signaling within the colony (e.g., Nieh, 1993; Pastor and Seeley, 2005). Thom et al. (2003) might have been able to determine the effect of the feeding station on the timing of tremble dancing if they had compared the behavior of the tremble dancers in their colony foraging under natural conditions to that in their colonies that had access to the feeding station. However, they scanned the natural conditions colony for communication signals but did not closely examine the behaviors exhibited by the individual signalers, thus, an effective comparison cannot be made using their results.
Proximate Stimuli that Elicit the Stop Signal
Lau and Nieh (2010) hypothesized that a food source that is declining in quality, for example running out of food or becoming overcrowded, may provoke the use of the stop signal by foragers. Supporting this, they found that as a feeding station grew more crowded, the rate of stop signaling within the colony increased. The foragers that had to wait to access the feeder were not more likely to perform the stop signal themselves, but they were more likely to receive stop signals from other bees that had visited the feeder and also from individuals that had not been observed there. This may be because some bees were not able to access the feeder at all due to overcrowding and returned to the colony without feeding. Thus, feeder crowding probably increases the number of foragers within the nest, thereby increasing the number of opportunities for them to use the stop signal (Lau and Nieh, 2010). Lau and Nieh (2010) conjectured that it is likely that the stop signal is present in the colony at low levels at all times, but does not have a colony-wide effect until some threshold level is reached. Additionally, using the stop signal may enable bees to rapidly adjust foraging efforts in response to shifts in their environment's nectar flow.
The stop signal is also used when foragers experience some form of danger or competition at a food source (Nieh, 2010). Under natural conditions this would likely be a response to being attacked by other bees or a predator while foraging. Nieh (2010) observed fights between conspecifics at a feeding station, and he simulated a predator attack by pinching bees visiting a feeder on the metathoracic femur with forceps. Both the bees that had experienced intraspecific competition and those that were attacked by forceps were more likely to perform the stop signal toward other foragers waggle-dancing for the same food source than they were toward dancers for other locations. Bees that had not had the negative experiences were much less likely to use the stop signal at all (Nieh, 2010).
The intraspecific competition that the bees experienced at the feeding station was probably somewhat artificial. When bees forage on natural food sources such as flowers, these are usually spread across a patchy landscape, individually offer small amounts of food, and are seldom simultaneously visited by more than one bee. In contrast, a feeding station is a very rich food source found only at a single location. Nieh (2010) acknowledged this and suggested that competition at the feeding station may be more similar to the competition experienced when bees rob food from other colonies (though Johnson and Hubbell, 1974, and others, have reported competitive interactions at floral sources.). In a paper modeling a hive-robbing event, Johnson and Nieh (2010) showed the stop signal in use to rapidly shut down robbing by countering the waggle dance. Aside from this model, however, an actual assessment of the signaling that occurs during an actual robbing or dense-flower situation and comparing it to the signaling used while foraging at a feeding station is an area of research that has not yet been explored.
Stop Signals as Cross-Inhibition
Another observed use of the stop signal is during the swarm nest site selection process. When honey bees swarm (reviewed in Visscher, 2007), thousands of workers and the original queen leave the hive and settle in a cluster a short distance away. From there, scout bees depart and search for potential new nest sites that the colony could inhabit. When a scout locates a favorable site, she returns to the swarm and advertises its location using the waggle dance. Over time, multiple sites maybe be advertised by many different dancers, with each group competing to recruit additional scouts to their site. Support for the different sites will wax and wane until a threshold number of scouts, or quorum, is reached at one of the sites, after which recruitment declines, and the swarm can be mobilized to move to its new home. This deadlock avoidance is of key importance to the nest site selection process because unlike during foraging, a decision for a single site must be reached.
The stop signal is used to provide cross inhibition in the form of negative feedback during this decision making process (Seeley et al., 2012). This study made video recordings of waggle dancers on the surfaces of swarms and recorded stop signals performed on the dancers by following bees on video with audio from a microphone held close to dancing bees. The dancers stopped dancing soon after receiving stop signals, and their dances were shorter than those of dancers not receiving stop signals When swarms simultaneously scouted two identical nesting boxes, dancers for either box received more stop signals from bees that had visited and been marked at the other site (contra-signalers) than bees that had visited the same site (ipsi-signalers). After a decision was reached about which nest box to occupy (inferred from the initiation of worker piping, which prepares the swarm for takeoff), the stop signalers no longer selectively targeted dancers advertising the opposing site and dancers received contra- and ipsi- signals equally. Seeley et al. (2012) inferred that negative feedback from the stop signal was provided cross inhibition between the two potential nest sites while the swarm was still making a decision, and that after a decision had been reached it contributed to shutting down waggle dancing. This contributes to having nearly all the swarm's bees at the swarm cluster when it takes off for cross-country flight, which will be guided by the scouts that know the way to the chosen site.
Negative Feedback in Other Social Insect Systems
It is of interest to note that negative feedback is present in other social insect systems. Trail pheromones, which are also used by termites, stingless bees, and social wasps, are used to recruit other individuals to food sources and nest sites (Czaczkes et al., 2015). These can encode complex information as a result of having varying chemical blends, concentrations, and operating synergistically with other factors (Czaczkes et al., 2015). Positive feedback from trail pheromones can cause groups of ants to focus inflexibly on a single food source due to the strong, non-linear response of recruits to the trail, even when other potentially better options exist (e.g., reviewed in Camazine et al., 2001). This effect can be countered by negative feedback from overcrowding at a food source, which results in an equal distribution of foragers across multiple food sources or the quick reallocation of the majority of foragers to a superior food source (Grüter et al., 2012). Negative feedback can also come from encounters with other foragers on a trail, where greater crowding leads to less trail pheromone deposition (Czaczkes et al., 2013), or from repellant trail pheromones used as “no entry” signals marking unrewarding paths (Robinson et al., 2005).
Discussion
Decision-making by groups of animals has received increasing recent attention in part because of recognition of its significance to other systems, in particular complex nervous systems and human engineered systems. Mechanisms of coordination discovered in social insect colonies have provided models for human-engineered systems in computing and robotics, because in both kinds of systems there is a need for reliable, robust decision-making based on simple interactions among components (e.g., Bonabeau and Meyer, 2001; Tsuda et al., 2006) Also, recent discoveries in decision-making mechanisms of vertebrate brains and swarms of honey bees have revealed striking parallels in their mechanisms (Passino et al., 2008; Marshall et al., 2009).
In all such systems, individual units are able to use a relatively small repertoire of behaviors or actions to achieve a complex task as a whole. Each unit, be it an insect, a robot, or a neuron, accumulates evidence until some threshold is reached and a decision can be made. The stop signal reviewed here provides negative feedback that can help modulate achieving that threshold and tune the behavior of honey bee colonies during foraging and swarming. The findings reviewed here suggest that the stop signal has diverse uses and effects. It is quite likely that not all of these have yet been described.
For example, the question of stop signaling during swarming is still not well understood. The results of Seeley et al. (2012) support the idea that cross-inhibition during the decision-making phase by contra-signalers provides negative feedback from scouts that had visited a different nest site. This, however, does not explain the lower-level occurrence of the ipsi-signaling that was also present. In a follow-up study using two nest boxes of differing volumes, much of the stop signaling observed was ipsi-signaling rather than contra-signaling, and some of stop signalers had not visited either nest site (Visscher, Schlegel, and Kietzman, unpublished data). These are puzzling results that beg the immediate questions of what might have been motivating the bees to signal and what the signals' effects were on the decision-making process. There is clearly more to learn about the uses and effects of the stop signal during swarming.
Another not-yet-explored avenue is the idea that tremble dancers that use the stop signal outside of the dance floor may be modulating the recruitment of more nectar receivers by lowering potential nectar receivers' response thresholds to the tremble dance (Thom et al., 2003). This could be tested by assessing whether or not non-dancers that received the stop signal were more likely to become nectar receivers after being contacted by a tremble dancer than individuals that did not. If so it would be a novel use of the stop signal within the context of a foraging colony.
A variety of conditions external to the colony have been explored to determine their effects on the communication signals used by bees, but few have considered the factors within the hive that may influence the bees' communication. We now know that a lack of nectar receivers stimulates tremble dancing, which results in the recruitment of more nectar receivers (Seeley, 1992). We also know that stop signaling inhibits foraging and is also associated with tremble dancing (e.g., Thom et al., 2003). A useful line of research would be to determine what factors, if any, within the hive might help drive bees' decisions to tremble dance or stop signal. For example, decreasing the food storage space available to the bees might be expected to result in an increase in tremble dancing, as the nectar receivers would be unable to store the food brought in by foragers. There would likely also be in an increase in stop signaling as the colony's nectar-handling capacity would be exceeded and foraging would need to be shut down.
While there are unanswered questions about the use of the stop signal, most of what has been discovered fits a picture of the stop signal as a negative-feedback component in recruitment, a sort of anti-waggle dance. The use of such a signal allows the bees to tune their recruitment more accurately and quickly in response to changing conditions, and in a variety of contexts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fevo.2015.00014/abstract
References
Bonabeau, E., and Meyer, C. (2001). Swarm intelligence: a whole new way to think about business. Harv. Bus. Rev. 79, 106–114. Available online at: https://hbr.org/2001/05/swarm-intelligence-a-whole-new-way-to-think-about-business
Camazine, S., Deneubourg, J. L., Franks, N. R., Sneyd, J., and Theraulaz, G. (2001). Self-Organization in Biological Systems. Princeton, NJ: Princeton University Press.
Czaczkes, T. J., Grüter, C., and Ratnieks, F. L. (2013). Negative feedback in ants: crowding results in less trail pheromone deposition. J. R. Soc. Interface 10:20121009. doi: 10.1098/rsif.2012.1009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Czaczkes, T. J., Grüter, C., and Ratnieks, F. L. (2015). Trail pheromones: an integrative view of their role in colony organization. Annu. Rev. Entomol. 60, 581–599. doi: 10.1146/annurev-ento-010814-020627
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Esch, H. E. (1964). Beiträge zum Problem der Entfernungsweisung in den Schwänzeltanzen der Honigbienen. Z. vergl. Physiol. 48, 534–546.
Esch, H., Esch, I., and Kerr, W. E. (1965). Sound: an element common to communication of stingless bees and to dances of the honey bee. Science 149, 320–321. doi: 10.1126/science.149.3681.320
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grüter, C., Schürch, R., Czaczkes, T. J., Taylor, K., Durance, T., Jones, S. M., et al. (2012). Negative feedback enables fast and flexible collective decision-making in ants. PLoS ONE 7:e44501. doi: 10.1371/journal.pone.0044501
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Johnson, B. R., and Nieh, J. C. (2010). Modeling the adaptive role of negative signaling in honey bee intraspecific competition. J. Insect. Behav. 23, 459–471. doi: 10.1007/s10905-010-9229-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Johnson, L. K., and Hubbell, S. P. (1974). Aggression and competition among stingless bees: field studies. Ecology 36, 120–127. doi: 10.2307/1934624
Kirchner, W. (1993a). Acoustical communication in honey bees. Apidologie 24, 297–307. doi: 10.1051/apido:19930309
Kirchner, W. (1993b). Vibrational signals in the tremble dance of the honey bee, Apis mellifera. Behav. Ecol. Sociobiol. 33, 169–172. doi: 10.1007/BF00216597
Lau, C., and Nieh, J. (2010). Honey bee stop-signal production: temporal distribution and effect of feeder crowding. Apidologie 41, 87–95. doi: 10.1051/apido/2009052
Marshall, J. A., Bogacz, R., Dornhaus, A., Planque, R., Kovacs, T., and Franks, N. R. (2009). On optimal decision-making in brains and social insect colonies. J. R. Soc. Interface 6, 1065–1074. doi: 10.1098/rsif.2008.0511
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Michelsen, A., Kirchner, W., and Lindauer, M. (1986). Sound and vibrational signals in the dance language of the honey bee, Apis mellifera. Behav. Ecol. Sociobiol. 18, 207–212. doi: 10.1007/BF00290824
Nieh, J. (1993). The stop signal of the honey bee: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56.
Nieh, J. (2010). A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr. Biol. 20, 310–315. doi: 10.1016/j.cub.2009.12.060
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Passino, K. M., Seeley, T. D., and Visscher, P. K. (2008). Swarm cognition in honey bees. Behav. Ecol. Sociobiol. 62, 401–414. doi: 10.1007/s00265-007-0468-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pastor, K., and Seeley, T. (2005). The brief piping signal of the honey bee: begging call or stop signal? Ethology 8, 775–784. doi: 10.1111/j.1439-0310.2005.01116.x
Robinson, E. J., Jackson, D. E., Holcombe, M., and Ratnieks, F. L. (2005). Insect communication: ‘no entry’ signal in ant foraging. Nature 438, 442. doi: 10.1038/438442a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schlegel, T., Visscher, P. K., and Seeley, T. D. (2012). Beeping and piping: characterization of two mechano-acoustic signals used by honey bees in swarming. Naturwissenschaften 99, 1067–1071. doi: 10.1007/s00114-012-0990-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seeley, T. D. (1992). The tremble dance of the honey bee: messages and meanings. Behav. Ecol. Sociobiol. 31, 375–383. doi: 10.1007/BF00170604
Seeley, T. D., and Tautz, J. (2001). Worker piping in honey bee swarms and its role in preparing for liftoff. J. Comp. Physiol. 187, 667–676. doi: 10.1007/s00359-001-0243-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Seeley, T. D., Visscher, P. K., Schlegel, T., Hogan, P. M., Franks, N. R., and Marshall, J. A. (2012). Stop signals provide cross inhibition in collective decision-making by honey bee swarms. Science 335, 108–111. doi: 10.1126/science.1210361
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thom, C., Gilley, D., and Tautz, J. (2003). Worker piping in honey bees (Apis mellifera): the behavior or piping nectar foragers. Behav. Ecol. Sociobiol. 53, 199–205. doi: 10.1007/s00265-002-0567-y
Tsuda, S., Zauner, K. P., and Gunji, Y. P. (2006). “Robot control: from silicon circuitry to cells,” in Biologically Inspired Approaches to Advanced Information Technology, eds A. J. Ijspeert, T. Masuzawa, and S. Kusumoto (Berlin; Heidelberg: Springer), 20–32.
Visscher, P. K. (2007). Group decision making in nest-site selection among social insects. Annu. Rev. Entomol. 52, 255–275. doi: 10.1146/annurev.ento.51.110104.151025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
von Frisch, K. (1967). The Dance Language and Orientation of Bees. Transl. by L. E. Chadwick. Cambridge, MA: Harvard University Press.
Keywords: honey bee, behavior, decision-making, vibration, sound, negative feedback, recruitment
Citation: Kietzman PM and Visscher PK (2015) The anti-waggle dance: use of the stop signal as negative feedback. Front. Ecol. Evol. 3:14. doi: 10.3389/fevo.2015.00014
Received: 05 December 2014; Accepted: 01 February 2015;
Published online: 17 February 2015.
Edited by:
Roger Schürch, University of Sussex, UKReviewed by:
James C. Nieh, University of California, San Diego, USATomer J. Czaczkes, University of Regensburg, Germany
Copyright © 2015 Kietzman and Visscher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parry M. Kietzman, Department of Entomology, University of California, Riverside, 900 University Ave., Riverside, CA 92521, USA e-mail:YW1hY2QwMDFAdWNyLmVkdQ==
 Parry M. Kietzman
Parry M. Kietzman P. Kirk Visscher
P. Kirk Visscher