- 1School of Earth System Science, Tianjin University, Tianjin, China
- 2College of Resources and Environment, Xingtai University, Xingtai, Hebei, China
- 3Dip.to di Scienze del Suolo, Della Pianta e Degli Alimenti, Università degli Studi di Bari “Aldo Moro”, Bari, Italy
- 4CNR - Istituto per la Scienza e Tecnologia dei Plasmi (ISTP) - Sede di Bari Via Amendola, Bari, Italy
- 5Università degli Studi di Torino, Dipartimento di Chimica, Torino, Italy
- 6Centro Interdipartimentale NatRisk, Grugliasco, Italy
Introduction
Dark matter, also known as hidden/missing mass or nonluminous matter, is a component of the universe that spans 90 orders of magnitude in mass, ranging from ultralight bosons (often referred to as “fuzzy dark matter” (Hui et al., 2017), to massive primordial black holes (Bertone and Tait, 2018). These concepts have raised renewed interest following the detection of gravitational waves by the Laser Interferometer Gravitational-Wave Observatory (LIGO) and Virgo, which originated from the merging of black holes several tens of times more massive than the Sun (Bertone and Tait, 2018; Bird et al., 2016; Clesse and García-Bellido, 2017), which respond to gravity, and remains invisible to light (Hecht, 2016). Biogeochemical scientists have to tackle a similarly puzzling issue with dark dissolved organic matter (DDOM) in surface waters (Cai et al., 2024; Hu et al., 2023). Recently, Cai et al. (2024) using ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS), identified 9,141 dark DOM molecules that exhibited high-molecular-weight (HMW) and greater diversity than the classical DOM subset, analyzing 38 DOM extracts covering the continuum of the Yangtze River-to-ocean, whereas undetected peaks and bacterial nodes were considered to represent DDOM (Cai et al., 2024; Hu et al., 2023). Notably, the HMW DDOM fraction was found to increase along this river-to-ocean continuum (Bird et al., 2016). Other studies have shown that only 8.7% and 9.6% of the 50,942 and 48,392 m/z peaks of DOM measured by FT-ICR MS in, respectively, sediments and waters of worldwide rivers, could be assigned to identifiable molecular formulae (Toyoda, 2020; Goldman, 2020). However, these undetected peaks derived from DOM remain elusive, primarily due to lack of reference spectra available in current databases (da Silva et al., 2015).
Many of the mentioned studies, however, have not taken into account the authentic sources of DOM, specifically allochthonous (terrestrial) and autochthonous (aquatic) sources, along with their optical and chemical characteristics. This oversight may lead to misconceptions regarding the authenticity of DDOM. In particular, which fractions or components of DOM should be prioritized for consideration as DDOM candidates, and what are the key fundamental questions regarding DDOM in the biosphere that remain unresolved?
Source characteristics of DOM and their relevance as dark DOM
In general, allochthonous DOM detected in natural waters is primarily derived from soil containing decaying terrestrial plant materials (Senesi and Loffredo, 1999; Piccolo, 2002), and is then partially transported to surface waters through surface runoff and groundwater leaching (Catalán et al., 2016; Zark and Dittmar, 2018; Yi et al., 2021; Mostofa et al., 2019). Allochthonous DOM is predominantly composed of humic substances (HS), which include humic acids (HA), fulvic acids (FA), and protein-like substances (PLS) (Figure 1A–C) (Gao et al., 2018; Mohinuzzaman et al., 2020; Tadini et al., 2018; Yang et al., 2024).
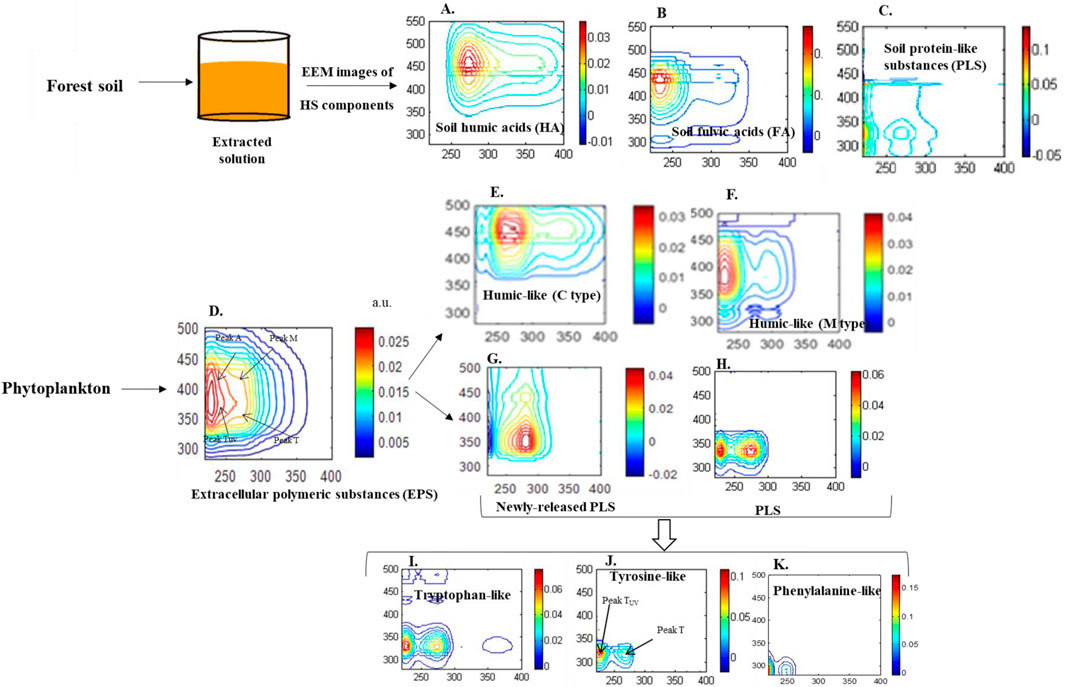
Figure 1. Fluorescence (excitation-emission matrix, EEM) spectra of terrestrial humic substances [humic acids-HA (A), fulvic acids-FA (B) and protein-like substances-PLS (C)] extracted from a forest soil, and of extracellular polymeric substances (EPS, (D)) from phytoplankton and their subsequently released autochthonous humic-like substances (C-type, (E) and M-type, (F) and protein-like substances (G, H), which then generate their individual components, i.e., tryptophan-like substances (I), tyrosine-like substances (J) and phenylalanine-like substances (K) in water.
In contrast, autochthonous DOM in water originates from planktonic photosynthetic organisms (e.g., phytoplankton) via photo/microbial respiration processes (Guidi et al., 2016; Shammi et al., 2017; Yang et al., 2021; Flemming et al., 2016), and is primarily exported as extracellular polymeric substances (EPS) (Figure 1D) (Shammi et al., 2017; Yang et al., 2021; Flemming et al., 2016). The EPS subsequently release various components of DOM, including autochthonous humic-like substances (C and M types), carbohydrates, lipids, and protein-like substances, which encompass a range of amino acids, including tryptophan-like, tyrosine-like, and phenylalanine-like substances (Figures 1E–G) (Shammi et al., 2017; Yang et al., 2021; Adav et al., 2008; Parlanti et al., 2000; Wei and Jin, 2022). Importantly, all soil FA and PLS fractions, as well as autochthonous DOM exhibit recognized solubility across all pH conditions, and they are highly degradable and undergo modifications when passing from inland to marine waters (Catalán et al., 2016; Zark and Dittmar, 2018; Mostofa et al., 2019; Shammi et al., 2017; Yang et al., 2021; Smith et al., 2017; Zhang et al., 2009; Mostofa et al., 2007; Moran et al., 2000). In contrast, HA possess a macromolecular/supramolecular structure that is chemically, and microbially recalcitrant. They exhibit multifunctional properties, including polyfunctionality, polyelectrolyticy, size polydispersity, physical heterogeneity, and structural lability (Senesi and Loffredo, 1999; Piccolo, 2002; Tadini et al., 2018; Schulten and Schnitzer, 1993; Steelink, 2002; Sutton and Sposito, 2005). These characteristics are primarily responsible for the remarkable ability of HA to form organo-mineral complexes, which contribute to the stabilization of organic C (Hemingway et al., 2019; Moore et al., 2023; Zhang et al., 2023) and serve as essential constituents in the continuous supply of nutrients for plant and microorganism growth (Senesi and Loffredo, 1999; Piccolo, 2002; Sutton and Sposito, 2005; Garciá et al., 2016; de Melo et al., 2016; Wang et al., 2022; Tiwari et al., 2023).
The solubility and/or insolubility characteristics of HA are significant features in their behavior (Senesi and Loffredo, 1999; Piccolo, 2002; Yang et al., 2024). In principle, a decrease in the solution pH enhances the intramolecular forces (IF) of HA by increasing the protonation of their functional groups. This, in turn, reduces their electron-donating capacity in aqueous solutions (Yang et al., 2024). Specifically, as acidity increases, the net IF become predominant, leading to enhanced intramolecular interactions among various functional groups through hydrogen bonding. This interaction can render some fractions or functional groups of HA undetectable (Yang et al., 2024). Ultimately, all functional groups associate, resulting in the precipitation of HA from the solution (Yang et al., 2024). For instance, as pH decreases, the concentration of alkali-extracted HA (dissolved in a 0.1 M NaOH solution at pH ∼13.0) gradually diminishes, with a fraction precipitating at pH 6.0, while the remaining HA fractions completely precipitate at pH 1.0. The corresponding pH-dependent changes in fluorescence (excitation-emission matrix, EEM) spectra and their peaks (C and A) are illustrated in Figure 2 (Yang et al., 2024). The alkali-extracted, complexed state dissolved organic carbon (DOCCS) is estimated to decrease by approximately 39.1%–46.4% at pH 6 and by 48.1%–53.8% at pH 1. This process is accompanied by a reduction in the intensity of the HA fluorescence peak C by approximately 29.7%–47.0% at pH 6, with a complete disappearance at pH 1-2 (Figure 2) (Yang et al., 2024).
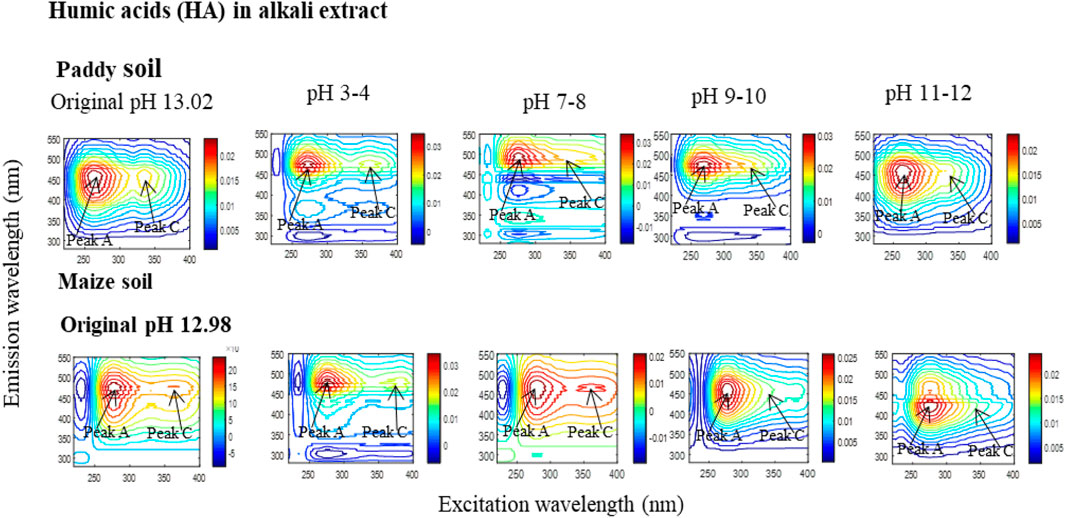
Figure 2. Fluorescence (excitation-emission matrix, EEM) spectra of complexed state (CS) humic acids (HACS), alkali extracted (Ae) from a paddy and a maize soil, and identified by applying EEM-PARAFAC modeling to the original solution before and after pH adjustment. The modifications of the fluorescence peaks (C and A) maxima are indicated with an arrow in the corresponding EEM images.
Similarly, the water extracted, labile state DOC (DOCLS) decreases by approximately 48.3%–49.2% at pH 6, and completely disappears at pH 1-2 (Figure 2) (Yang et al., 2024). These results suggest that as pH decreases, the TOC analyzer is unable to detect certain fractions of HA as DOC because these fractions remain in an insoluble state. Consequently, a pH-dependent disappearance of HA from the solution occurs. This missing fraction of DOM/HA likely consists of high-molecular-weight (HMW) DOM, where intramolecular interactions among functional groups impede their identification (Yang et al., 2024). In essence, the pH-dependent behavior of soil HA involves aggregation and precipitation processes that are also influenced by the pH and salinity of seawater. The supersaturation of coastal seawater composition can lead to the settling and storage of HA fractions in the form of organo-mineral complexes, e.g., Fe-(oxy)hydroxide minerals, at coastal seawater sites (Hemingway et al., 2019; Moore et al., 2023; Zhang et al., 2023). Therefore, the pH-dependent soil HA fractions could be recognized as DDOM.
Discussion
In essence, the solid-phase extraction (SPE) method used by Cai et al. (2024) is unable to recover a significant portion of the hydrophilic DOC fraction, achieving only 3–28% recovery (Grasset et al., 2023). Consequently, major DOM fractions, such as pH-dependent soil HA, are selectively excluded from the subsequent mass spectrometry analysis following SPE (Grasset et al., 2023). In particular, the SPE-based DOM extracts collected along the river-to-ocean continuum exhibit an increasing abundance of HMW components (Cai et al., 2024), which may be associated with the rise of autochthonous protein-like and carbohydrate matter, rather than the decline of terrestrial pH-dependent soil HA fractions throughout the river-to-ocean continuum (Figure 3) (Catalán et al., 2016; Mostofa et al., 2019; Mostofa et al., 2013).
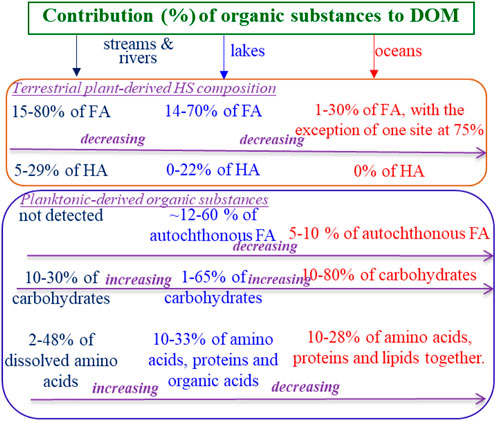
Figure 3. A schematic diagram illustrating a decrease in allochthonous humic substances (HS), including humic acids (HA) and fulvic acids (FA), which primarily originate from decaying terrestrial plant materials as reviewed in Ref. 42. This decrease is accompanied by a corresponding increase in autochthonous FA, carbohydrates, and proteinaceous matter, which primarily originates from the planktonic community.
Notably, HA release sequentially degraded organic molecules that were originally bound to HA through photochemical and microbial processes (Mostofa et al., 2013; Amador et al., 1989). It is highly likely that these SPE-based DOM components are optically active (Mostofa et al., 2019; Zhang et al., 2009; Grasset et al., 2023; Mostofa et al., 2013). Differently, the pH-dependent soil HA are optically inactive in terms of fluorescence intensity, which diminishes with decreasing pH and are not detectable through TOC analysis (Yang et al., 2024). The discussion above suggests that the currently proposed DDOM is not substantiated by the considerations of DOM sources.
Finally, the pH-dependent soil/terrestrial HA, tentatively classified as a DDOM fraction, are prevalent in important soil and sediment environments due to their long-term C stabilization and accumulation through organo-mineral complexes (Hemingway et al., 2019; Moore et al., 2023). This process, in turn, contributes to soil stability and health, promoting sustainable agricultural productivity, as well as providing living habitats for various organisms. Furthermore, a portion of pH-dependent terrestrial HA might be one of the key DOM contributors to the long-term C stability in oceanic environments (Catalán et al., 2016). Additionally, a fraction of pH-dependent soil HA could be recognized as DDOM and would also be optically inactive. Undoubtedly, pH-dependent soil HA cannot be extracted by SPE-based methanol solvents due to their insoluble macromolecular and supramolecular nature (Senesi and Loffredo, 1999; Piccolo, 2002; Yang et al., 2024), leaving them uncharacterized at the molecular level. Therefore, a substantial fraction of pH-dependent soil HA, continuously produced from photosynthetically active terrestrial plants, might be classified as DDOM, i.e., dark matter in the biosphere. This soil HA fraction fundamentally plays a crucial role in soil’s structural framework, serving as a primary building block of the soil matrix across the Earth’s crust and facilitating C stabilization by forming organo-mineral complexes. Lastly, the connections between DDOM and the significance of sensitivity analysis in the monitoring and management of natural water resources (Errico et al., 2019; Pirone et al., 2024; Lama and Chirico, 2020) should be the focus of further studies.
Author contributions
KM: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing–original draft. JY: Formal Analysis, Methodology, Software, Validation, Writing–review and editing. XY: Formal Analysis, Investigation, Methodology, Validation, Writing–review and editing. MM: Formal Analysis, Investigation, Methodology, Validation, Writing–review and editing. C-QL: Resources, Validation, Writing–review and editing. NS: Validation, Writing–original draft, Resources. GS: Validation, Writing–review and editing. DV: Validation, Writing–review and editing. S-LL: Funding acquisition, Validation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (41925002, U1612441 and 42230509).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adav, S. S., Lee, D. J., and Tay, J. H. (2008). Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 42, 1644–1650. doi:10.1016/j.watres.2007.10.013
Amador, J. A., Alexander, M., and Zika, R. G. (1989). Sequential photochemical and microbial degradation of organic molecules bound to humic acid. Appl. Environ. Microbiol. 55, 2843–2849. doi:10.1128/aem.55.11.2843-2849.1989
Bertone, G., and Tait, T. M. P. (2018). A new era in the search for dark matter. Nature 562, 51–56. doi:10.1038/s41586-018-0542-z
Bird, S., Cholis, I., Muñoz, J. B., Ali-Haïmoud, Y., Kamionkowski, M., Kovetz, E. D., et al. (2016). Did LIGO detect dark matter? Phys. Rev. Lett. 116, 201301. doi:10.1103/physrevlett.116.201301
Cai, R., Yao, P., Yi, Y., Merder, J., Li, P., and He, D. (2024). The hunt for chemical dark matter across a River-to-Ocean continuum. Environ. Sci. Technol. 58, 11988–11997. doi:10.1021/acs.est.4c00648
Catalán, N., Marcé, R., Kothawala, D. N., and Tranvik, L. J. (2016). Organic carbon decomposition rates controlled by water retention time across inland waters. Nat. Geosci. 9, 501–504. doi:10.1038/ngeo2720
Clesse, S., and García-Bellido, J. (2017). Detecting the gravitational wave background from primordial black hole dark matter. Phys. Dark Universe 18, 105–114. doi:10.1016/j.dark.2017.10.001
da Silva, R. R., Dorrestein, P. C., and Quinn, R. A. (2015). “Illuminating the dark matter in metabolomics,” Proc. Natl. Acad. Sci. U. S. A. 112. 12549–12550. doi:10.1073/pnas.1516878112
de Melo, B. A. G., Motta, F. L., and Santana, M. H. A. (2016). Humic acids: structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 62, 967–974. doi:10.1016/j.msec.2015.12.001
Errico, A., Lama, G. F. C., Francalanci, S., Chirico, G. B., Solari, L., and Preti, F. (2019). “Validation of global flow resistance models in two experimental drainage channels covered by Phragmites australis (common reed),” in Proceedings of the 38th IAHR world congress-water connecting the world, 1313–1321. doi:10.3850/38WC092019-1215
Flemming, H.-C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A., and Kjelleberg, S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. doi:10.1038/nrmicro.2016.94
Gao, J., Lv, J., Wu, H., Dai, Y., and Nasir, M. (2018). Impacts of wheat straw addition on dissolved organic matter characteristics in cadmium-contaminated soils: insights from fluorescence spectroscopy and environmental implications. Chemosphere 193, 1027–1035. doi:10.1016/j.chemosphere.2017.11.112
Garciá, A. C., de Souza, L. G. A., Pereira, M. G., Castro, R. N., García-Mina, J. M., Zonta, E., et al. (2016). Structure-Property-Function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 6, 20798. doi:10.1038/srep20798
Goldman, A. E. (2020). “WHONDRS summer 2019 sampling campaign: global river corridor sediment FTICR-MS, dissolved organic carbon, aerobic respiration, elemental composition, grain size, total nitrogen and organic carbon content,” in Bacterial abundance, and stable isotopes (v8). doi:10.15485/1729719
Grasset, C., Groeneveld, M., Tranvik, L. J., Robertson, L. P., and Hawkes, J. A. (2023). Hydrophilic species are the most biodegradable components of freshwater dissolved organic matter. Environ. Sci. Technol. 57, 13463–13472. doi:10.1021/acs.est.3c02175
Guidi, L., Chaffron, S., Bittner, L., Eveillard, D., Larhlimi, A., Roux, S., et al. (2016). Plankton networks driving carbon export in the oligotrophic ocean. Nature 532, 465–470. doi:10.1038/nature16942
Hemingway, J. D., Rothman, D. H., Grant, K. E., Rosengard, S. Z., Eglinton, T. I., Derry, L. A., et al. (2019). Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570 (570), 228–231. doi:10.1038/s41586-019-1280-6
Hu, A., Meng, F., Tanentzap, A. J., Jang, K. S., and Wang, J. (2023). Dark matter enhances interactions within both microbes and dissolved organic matter under global change. Environ. Sci. Technol. 57, 761–769. doi:10.1021/acs.est.2c05052
Hui, L., Ostriker, J. P., Tremaine, S., and Witten, E. (2017). Ultralight scalars as cosmological dark matter. Phys. Rev. D. 95, 043541. doi:10.1103/physrevd.95.043541
Lama, G. F. C., and Chirico, G. B. (2020). “Effects of reed beds management on the hydrodynamic behaviour of vegetated open channels,” in Proceedings of the 2020 IEEE international workshop on metrology for agriculture and forestry (MetroAgriFor) (Trento, Italy), 149–154. doi:10.1109/MetroAgriFor50201.2020.9277622
Mohinuzzaman, M., Yuan, J., Yang, X., Senesi, N., Li, S. L., Ellam, R. M., et al. (2020). Insights into solubility of soil humic substances and their fluorescence characterisation in three characteristic soils. Sci. Total Environ. 720, 137395–137438. doi:10.1016/j.scitotenv.2020.137395
Moore, O. W., Curti, L., Woulds, C., Bradley, J. A., Babakhani, P., Mills, B. J. W., et al. (2023). Long-term organic carbon preservation enhanced by iron and manganese. Nature 621, 312–317. doi:10.1038/s41586-023-06325-9
Moran, M. A., Sheldon, W. M., and Zepp, R. G. (2000). Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol. Oceanogr. 45, 1254–1264. doi:10.4319/lo.2000.45.6.1254
Mostofa, K. M. G., Jie, Y., Sakugawa, H., and Liu, C. Q. (2019). Equal treatment of different EEM data on PARAFAC modeling produces artifact fluorescent components that have misleading biogeochemical consequences. Environ. Sci. Technol. 53, 561–563. doi:10.1021/acs.est.8b06647
Mostofa, K. M. G., Yoshioka, T., Konohira, E., and Tanoue, E. (2007). Photodegradation of fluorescent dissolved organic matter in river waters. Geochem J. 41, 323–331. doi:10.2343/geochemj.41.323
Mostofa, K. M. G., Yoshioka, T., Mottaleb, M. A., and Vione, D. (2013). Photobiogeochemistry of organic matter: principles and practices in water environments. Berlin Heidelberg: Springer.
Parlanti, E., Wörz, K., Geoffroy, L., and Lamotte, M. (2000). Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem 31, 1765–1781. doi:10.1016/s0146-6380(00)00124-8
Piccolo, A. (2002). The supramolecular structure of humic substances: a novel understanding of humus chemistry and implications in soil science. Adv. Agron. 75, 57–134. doi:10.1016/s0065-2113(02)75003-7
Pirone, D., Cimorelli, L., and Pianese, D. (2024). The effect of flood-mitigation reservoir configuration on peak-discharge reduction during preliminary design. J. Hydrology Regional Stud. 52, 101676. doi:10.1016/j.ejrh.2024.101676
Schulten, H.-R., and Schnitzer, M. (1993). A state of the art structural concept for humic substances. Naturwissenschaften 80, 29–30. doi:10.1007/bf01139754
Senesi, N., and Loffredo, E. (1999). The chemistry of soil organic matter in soil physical chemistry. 2nd ed.
Shammi, M., Pan, X., Mostofa, K. M. G., Zhang, D., and Liu, C. Q. (2017). Photo-flocculation of microbial mat extracellular polymeric substances and their transformation into transparent exopolymer particles: chemical and spectroscopic evidences. Sci. Rep. 7, 9074–9112. doi:10.1038/s41598-017-09066-8
Smith, H. J., Foster, R. A., McKnight, D., Lisle, J., Littmann, S., Kuypers, M. M. M., et al. (2017). Microbial formation of labile organic carbon in Antarctic glacial environments. Nat. Geosci. 10, 356–359. doi:10.1038/ngeo2925
Steelink, C. (2002). Peer reviewed: investigating humic acids in soils. Anal. Chem. 74, 326 A–333 A. doi:10.1021/ac022040m
Sutton, R., and Sposito, G. (2005). Molecular structure in soil humic substances: the new view. Environ. Sci. Technol. 39, 9009–9015. doi:10.1021/es050778q
Tadini, A. M., Nicolodelli, G., Senesi, G. S., Ishida, D. A., Montes, C. R., Lucas, Y., et al. (2018). Soil organic matter in podzol horizons of the Amazon region: humification, recalcitrance, and dating. Sci. Total Environ. 613–614, 160–167. doi:10.1016/j.scitotenv.2017.09.068
Tiwari, C., Ramanathan, A., Bauddh, K., and Korstad, J. (2023). Humic substances: structure, function and benefits for agroecosystems—a review. Pedosphere 33, 237–249. doi:10.1016/j.pedsph.2022.07.008
Toyoda, J. G. (2020). WHONDRS summer 2019 sampling campaign: global river corridor surface water FTICR-MS, NPOC, TN, anions, stable isotopes, bacterial abundance, and. Dissolved Inorg. Carbon (v6). doi:10.15485/1603775
Wang, C., Cheng, T., Tang, S., Zhang, D., and Pan, X. (2022). Chemical structure and nanomechanics relevant electrochemistry of solid-phase humic acid along a typical forest-river-paddy landscape section in eastern China and its environmental implications. Sci. Total Environ. 838, 156147. doi:10.1016/j.scitotenv.2022.156147
Wei, J., and Jin, F. (2022). Illuminating bacterial behaviors with optogenetics. Curr. Opin. Solid State Mater Sci. 26, 101023. doi:10.1016/j.cossms.2022.101023
Yang, X., Yuan, J., Yue, F. J., Li, S. L., Wang, B., Mohinuzzaman, M., et al. (2021). New insights into mechanisms of sunlight- and dark-mediated high-temperature accelerated diurnal production-degradation of fluorescent DOM in lake waters. Sci. Total Environ. 760, 143377. doi:10.1016/j.scitotenv.2020.143377
Yang, X., Zhang, J., Mostofa, K. M. G., Mohinuzzaman, M., Teng, H. H., Senesi, N., et al. (2024). Solubility characteristics of soil humic substances as a function of pH. Biogeosciences Interact. Discuss. after Accept. Rev. doi:10.5194/egusphere-2023-2994
Yi, Y., Zhong, J., Bao, H., Mostofa, K. M., Xu, S., Xiao, H. Y., et al. (2021). The impacts of reservoirs on the sources and transport of riverine organic carbon in the karst area: a multi-tracer study. Water Res. 194, 116933. doi:10.1016/j.watres.2021.116933
Zark, M., and Dittmar, T. (2018). Universal molecular structures in natural dissolved organic matter. Nat. Commun. 9, 3178–8. doi:10.1038/s41467-018-05665-9
Zhang, J., Mostofa, K. M. G., Yang, X., Mohinuzzaman, M., Liu, C. Q., Senesi, N., et al. (2023). Isolation of dissolved organic matter from aqueous solution by precipitation with FeCl3: mechanisms and significance in environmental perspectives. Sci. Rep. 13 (13), 4531–4615. doi:10.1038/s41598-023-31831-1
Keywords: dark matter, dark dissolved organic matter, soil humic acids, autochthonous humiclike substances, soil, surface water
Citation: Mostofa KMG, Yuan J, Yang X, Mohinuzzaman M, Liu C-Q, Senesi N, Senesi GS, Vione D and Li S-L (2025) The potential nature of “dark” dissolved organic matter in the biosphere. Front. Earth Sci. 13:1517025. doi: 10.3389/feart.2025.1517025
Received: 25 October 2024; Accepted: 18 March 2025;
Published: 26 March 2025.
Edited by:
Alexandra Gogou, Hellenic Centre for Marine Research (HCMR), GreeceReviewed by:
Giuseppe Francesco Cesare Lama, University of Naples Federico II, ItalyCopyright © 2025 Mostofa, Yuan, Yang, Mohinuzzaman, Liu, Senesi, Senesi, Vione and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khan M. G. Mostofa, bW9zdG9mYUB0anUuZWR1LmNu
 Khan M. G. Mostofa
Khan M. G. Mostofa Jie Yuan2
Jie Yuan2 Xuemei Yang
Xuemei Yang Mohammad Mohinuzzaman
Mohammad Mohinuzzaman Nicola Senesi
Nicola Senesi Giorgio S. Senesi
Giorgio S. Senesi Davide Vione
Davide Vione Si-Liang Li
Si-Liang Li