
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci., 24 August 2023
Sec. Quaternary Science, Geomorphology and Paleoenvironment
Volume 11 - 2023 | https://doi.org/10.3389/feart.2023.1148299
Coastal societies have lived at the seaward edge of the Atacama Desert since at least 12,000 years ago. Kelp forest ecosystems provide evidence for important subsistence activity along the entire Chilean coast through fishing and gathering. Despite their importance, especially in hyperarid contexts with limited plant abundance, there is scarce evidence of kelp in archaeological contexts, hampering the study of kelp use in the past. In the present study, we use the presence of small marine invertebrates, inhabitants of stipes and holdfasts of macroalgae, as proxies that indicate past kelp presence. We analyze samples of three species of snails (Tegula atra, Tegula tridentata, and Diloma nigerrima) and one limpet (Scurria scurra) from nine archaeological sites dated between 7,000 and 500 cal years before present located around the area of Taltal (25°Lat S). Modern samples of these species were collected to reconstruct the size of fragmented archaeological shells and subsequently estimate the size of harvested kelps. Through this approach, we estimated the size and relative abundance of kelp used by coastal groups that inhabited the southern part of the Atacama Desert for around 6,500 years. Our results are a contribution to the scarce information on the presence and use of kelp in the prehistory of the Americas and contribute to comparative perspectives with other areas of the world where the use of kelp by humans in the past has already been explored.
Algae have been important for people since ancient times. The kelp highway hypothesis for early human peopling routes into the American continents is one example (Erlandson et al., 2007). The marine route goes along the productive coastal ecosystems provided by giant Kelp (Macroystis pyrifera) forests; the use of kelp environments by early humans is evidenced in the profuse nearshore resources present in late Pleistocene–early Holocene sites along the Eastern Pacific shores. In present times, kelp, and algae in general, are important resources exploited worldwide for the cosmetic, pharmaceutical, and food industries (Steneck et al., 2002; Nirmal-Kumar et al., 2009; Araos, 2015; Araos et al., 2018; Pérez-Lloréns, 2019; Cárcamo and Gelcich, 2020). Chile is among the top exporters of brown algae worldwide, which are harvested from wild subtidal and intertidal kelp populations (Tellier et al., 2011; Lotze et al., 2019; FAO, 2020). These kelp populations support a diverse and productive rocky shore ecosystem (Docman et al., 2017) dominated by gastropods, crustaceans, sea otters (nutria/chungungo), and neritic fishes (Vásquez and Santelices, 1984; Vega, 2016; Salazar et al., 2018). The elevated primary productivity observed along the coast is fueled by the semi-permanent coastal upwelling regime along western South America, which brings cold, nutrient-rich waters to the benthic zone (Chavez and Messié, 2009).
Considering the great abundance and distribution of algae and their variety of uses, their scarcity in archaeological contexts raises questions about their use and relevance in past human societies. Preservation and strategies of sampling and recovery may be affecting the representativeness of algae in archaeological records. Humidity, temperature, and acidity of sediments hinder the conservation of organic remains, which quickly decompose in humid and aerobic environments (Mooney, 2021). Nevertheless, the direct archaeological evidence of algae reported in Chile indicates that people have harvested and used seaweed in different places and times since at least 14,000 years ago (e.g., Uhle, 1922; Ramírez and Quevedo, 2000; Vidal et al., 2004; Dillehay et al., 2008).
Ethnographic and historical accounts mention the process of extraction, drying, and use of certain species of algae along the coast of Chile (Borie et al., 2006; Araos et al., 2018). Chroniclers of the 17th century noticed the use of algae by Mapuche communities in the southern zone for food, fertilizer, dyes for textiles, ceremonial elements, and for exchange with communities that inhabited the valley and mountains (Ovalle 1,646, Pineda and Bascuñan 1,676, Molina 1,776 in Borie et al., 2006). On the northern coast, travelers in the 19th century mentioned the trade of algae between the coast of Cobija (22°33′S) and the southern Bolivian highlands.
Most of the direct evidence of alga use in the archaeological record of Chile comes from the northern coast (Supplementary Table S1). One site is Arica (18°S), where remains of Cochayuyo (Durvillaea incurvata) were found as part of a tomb offering, without a known date (Uhle, 1922). Two other records come from Pisagua (19°S); one corresponds to five tied pieces of Lessonia berteroana found in a burial from the Punta Pichalo site, with dates between 800 and 1,300 years before present (BP) (Ramírez and Quevedo, 2000); and the second comes from the sites of Pisagua N and Pisagua B and consists of packed leaves of Macrocystis pyrifera inside rock structures and areas of food processing dated between 960 and 1,470 years before Christ (BC) (Vidal et al., 2004). South of Iquique, evidence of packed leaves of Macrocystis spp. and Lessonia sp. are recorded at Punta Patache archaeological sites (20°S) in different domestic and funerary deposits with Middle to Late Holocene dates (Moragas and Mendez-Quiros, 2022). The single case from southern Chile comes from the Monte Verde site (43°S), in deposits dated around 14,000 cal years BP. Nine species of seaweed, among them the brown macroalgae Durvillaea antarctica and M. pyrifera, were identified and interpreted as direct evidence of algae used as medicine and food (Dillehay et al., 2008).
A few other archaeological studies mention the presence of algae but without species identification (Supplementary Table S1). One comes from the coast of Arica and describes a package of algae tied with red vegetable fibers at the La Capilla site. This cave was dated between 1,300 and 2,500 years BC, and the package of algae is interpreted as part of an ornament in a ritual context (Muñoz, 2014). The use of kelp as raw material for fuel and construction is also suggested from sites dated between 6,000 and 4,000 years cal BP along the coast of Antofagasta (23°S). Seaweed ash is interpreted to be the main material used to seal floors inside Caleta Huelén-type rock structures (Núñez et al., 1974; Núñez, 1976; Llagostera, 1989; Hernández, 2019; Power et al., 2022). Finally, despite the importance that kelp may have had in humans’ diets, as a source of minerals and vitamins, studies on isotopic analysis and dietary patterns around the Atacama Desert show strong reliance on marine resources but are not able to differentiate among fish, mollusks, or kelp (Andrade et al., 2015; Santana-Sagredo et al., 2021).
Despite the possible problems of algae preservation in archaeological contexts, the examples presented above show their relevance and diversity of use and function. It is necessary to develop methodologies to identify and analyze archaeological evidence of kelp, to explore research questions about their importance in human history, the characteristics of kelp forest ecosystems during the last millennia, and the effect of environmental and human factors on their use and abundance.
Questions about the presence, abundance, and use of kelp forests in the past have been raised by several researchers around the world. One of the approaches has been the use of archaeo-malacological species as indirect evidence for the presence of algae. Rowland (1977) in New Zealand, interprets the presence of small gastropods of the species Maurea punctulate as having arrived at the site attached to kelp. In Italy, small gastropods of the species Jujubinum exasperatus have also been interpreted as an indirect indicator of alga extraction and deposition in archaeological sites (Colonese and Wilkens, 2005). Finally, in Baja California, Mexico, and the Californian coast of the United States, Ainis et al. (2014); Ainis et al. (2019) conducted studies on small archaeo-malacological fauna, which, due to their small size, are believed not to be collected for food purposes. One type of these small-sized mollusks corresponds to species associated with algae and seagrasses (Ainis et al., 2014).
Although there are several mentions of the link between small gastropods or limpets and algae in Chilean archaeological contexts (e.g., Borie et al., 2006; Castro et al., 2016; Power and Salazar, 2020; Power et al., 2022), there is no methodological approach explicitly applied to explore this link. Among the rich set of shellfish species identified in shell-midden archaeological sites along the northern coast of Chile (e.g., Santoro et al., 2017), several species of small size may be directly associated with kelp. The patellogastropod S. scurra lives exclusively on holdfast cavities and stipes of L. berteroana and Lessonia spicata throughout the benthic phase of its life cycle (Figure 2). Juveniles (±5 mm basal width) inhabit the internal cavities of Lessonia spp. Holdfasts, while larger individuals use stipe scars (Vega et al., 2016). Several individuals of Scurria scurra can be found on holdfasts but only one per stipe (Muñoz and Santelices, 1989; Oróstica et al., 2014; Vega et al., 2016). This herbivore-kelp association is highly specialized and has been used as an indicator of the ecological integrity of Lessonia species. Studies in Chile have recorded the abundance and size structure of S. scurra in plants of L. berteroana, comparing places with differential human pressure (Muñoz and Santelices, 1989; Vega, 2016). The results showed lower abundance and smaller S. scurra in areas without regulation on kelp extraction, proposing S. scurra as an ecological indicator easy to observe on kelp stipes and holdfasts, but also on kelp drying areas on the shore, after algae have been taken to the market.
Several snails of the family Trochidae, like T. atra, T. tridentata, Tegula quadricostata, Tegula luctulosa, and Diloma nigerrima, are herbivores that inhabit and feed on different macroalgae (Veliz and Vásquez, 2000). Tegula atra and Tegula tridentata are found in the intertidal zone associated with Lessonia spp. and in the subtidal associated with holdfasts of Lessonia trabeculata and Durvillaea sp. (Veliz and Vásquez, 2000; Pinochet et al., 2018). Diloma nigerrima is found in intertidal rock pools and on holdfasts of L. berteroana throughout its entire life cycle (Veliz and Vásquez, 2000; Zagal and Hermosilla, 2007) and can be found in kelp drying areas together with T. atra and S. scurra. Tegula atra can reach up to 70 mm in diameter and inhabit intertidal and shallow subtidal zones (Veliz and Vásquez, 2000). This makes it easy to collect them for food, which is shown by their abundance in several archaeological sites (e.g., Olguín, 2013; Olguín et al., 2015). In contrast, D. nigerrima and T. tridentata are small snails with a maximum diameter of 25 mm and 16 mm, respectively (Veliz and Vásquez, 2000; Zagal and Hermosilla, 2007), which make them less “attractive” as food and, therefore, less common in archaeological sites. Unlike S. scurra, the relationship between kelp and T. atra is not specialized since adults may be attached to rocks (Veliz and Vásquez, 2000). An exception is during its juvenile stage when T. atra are not larger than 20 mm and inhabit the holdfasts of brown algae (Cancino and Santelices, 1984; Veliz and Vásquez, 2000; Pinochet et al., 2018).
Considering the above, we will use the presence of S. scurra, T. atra, T. tridentata, and D. nigerrima as a proxy of harvesting/use of algae in archaeological sites located along the coast of Taltal, with dates between 7,000 and 500 years cal BP (Figure 1). Our study attempts to advance our understanding of the use of algae by prehistoric coastal inhabitants through the study of small-size mollusk shells from archaeological contexts and present-time kelp drying areas.
The Taltal area (25° S), located on the southern coast of the Atacama Desert (Figure 1), has a lack of permanent freshwater courses and a hyperarid climate, which translates into sparse terrestrial flora and fauna (Latorre et al., 2005). The paucity of terrestrial resources is in stark contrast with the Humboldt Current ecosystem, where intense wind-driven coastal upwellings supply plentiful nutrients to primary producers, including benthic algae, and fuel productive food webs from the intertidal to the pelagic zone (Montecino and Lange, 2009). Consequently, the study area maintains a large macroalgal industry based on the extraction or collection of kelp species such as Lessonia spp. and Macrocystis pyrifera (Araos et al., 2018; Esper, 2022).
Hunter-gatherer groups and fishers have inhabited the coastal strip of the study area for approximately 12,000 BP (Salazar et al., 2018). The sustained use of the coastal zone by humans has generated numerous archaeological sites that point to a way of life specialized in the use of marine resources (e.g., Castelleti, 2007; Olguín, 2013; Andrade et al., 2014; Salazar et al., 2015; Rebolledo et al., 2016). Shell midden archaeological sites contain a diverse assemblage of gastropods characteristic of kelp-dominated rocky shorelines, such as keyhole limpets, chitons, and muricid snails (Olguín, 2013; Olguín et al., 2015; Salazar et al., 2015; Power and Salazar, 2020).
Archaeo-malacological samples come from nine archaeological sites: five open-air sites, three rocky shelters, and one ferrous oxide mine, all distributed along a strip of approximately 100 km of coastline and located at different distances from the current coastline (30 m–300 m) (Figure 1).
Zapatero is the farthest north shell midden site of the study area, approximately 64 km north of Taltal and no more than 30 m from the current coastline. The sample from this open-air site comes from one column sample (0.20 m3 of excavated volume (EV)) and three excavation units (1.18 m3 EV). Rockshelter Paposo Norte 9 is approximately 4 km south of Zapatero, 200 mt from the coast, and shell samples come from one column sample (0.26 m3 EV) and two excavation units (2.10 m3 EV). Rockshelter site Alero Bandurrias 3 is 33 km south of Zapatero and 200 m from the shore; shells analyzed come from a single excavation unit (0.6 m3 EV). Alero 224-A is also a rockshelter, situated 12 km north of Taltal and around 300 m from the coast; shells from this site come from one column sample (0.13 m3 EV). The open-air mine San Ramón 15 is 3.5 km northeast of Taltal, 1.5 km from the current coastline, and approximately 125 m.a.s.l.; shells analyzed from this site come from one excavation unit called Mine-1 (48 m3). Morro Colorado and Punta Morada are open-air shell midden sites located 2 km north of Taltal and less than 20 mts from the shore. Shells from Morro Colorado and Punta Morada come from single excavated units (0.56 m3 and 0.60 m3 EV). Finally, the two open-air shell midden sites, Plaza de Indios Norte and Hornos de Cal, are south of the city of Taltal. The first is 5 km south and 100 mt from the shore, and the second is 15 km south and 50 mts from the rocky shore. Samples from both sites come from one excavation unit, one of 0.50 m3 EV (Plaza de Indios) and the second of 2.15 m3 EV (Hornos de Cal) (Salazar et al., 2011; Salazar et al., 2015; Salazar et al., 2018).
The age of sampled sites fluctuates between ca. 7,000 and 500 years cal BP (Salazar et al., 2015); malacological samples were obtained from archaeological excavations done on the sites during several research projects (Fondecyt 1,110,196 and 1151203). All shells of S. scurra, T. tridentata, and D. nigerrima were used for analysis. In the case of T. atra, only shells up to 20 mm were selected to guarantee that only juvenile specimens, which inhabit the holdfasts of brown algae, were included in the study.
Complete and fragmented shells at least 50% complete were selected for analysis. To estimate the size of the incomplete individuals, a linear regression equation was established using complete shells of S. scurra, T. atra, and D. nigerrima from modern samples collected near the localities of Punta Talca (33°25′S) and Guanaqueros (30°11′S), in the semi-arid region of northern Chile (Table 1). Modern shells were collected from shoreline areas where kelp is dried and accumulated after collection and spanned a broad range of shell sizes. All archaeological and modern shells were measured with a digital caliper considering basal height and width (Figure 2).
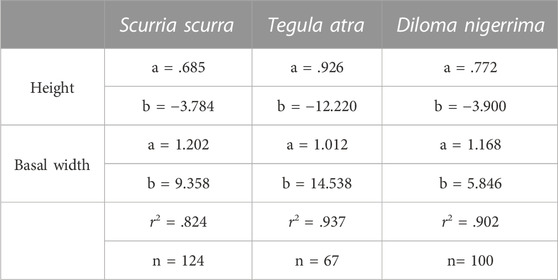
TABLE 1. Slope (a) and intercept (b) for the regression equation calculated from modern shells to estimate the sizes of incomplete archaeological specimens of the different gastropod species used in this study as algal proxies. The coefficient of determination (r2) is highly significant in all cases (p < 0.001). Note: Standard linear regression equation Y = (a*X + b).
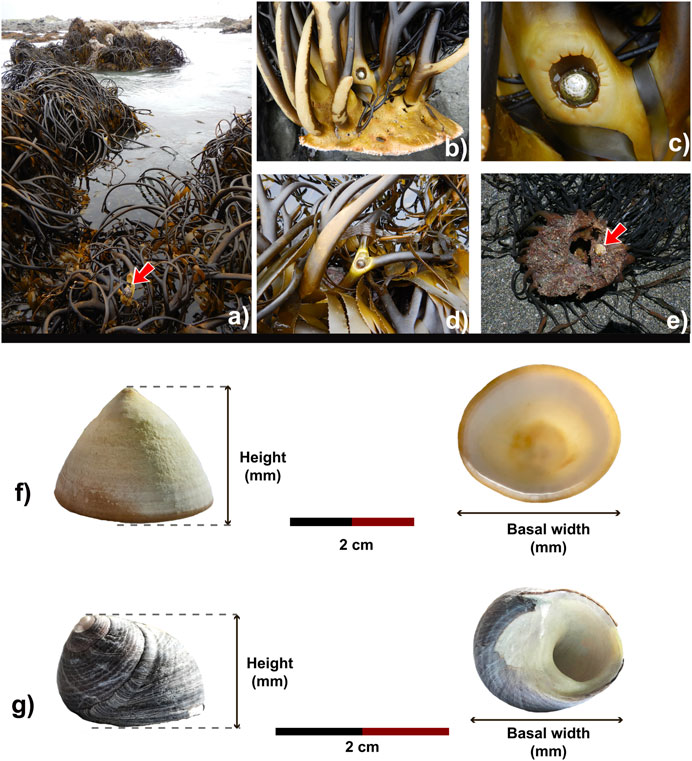
FIGURE 2. (A) Intertidal zone kelp beds. (B–E), S. scurra in algae stipes and adhesion discs. Pictures taken by the authors. Morphometric measurements; for (F) S. scurra; (G) snails of the family Trochidae.
We used the regression equations to reconstruct width or height data for the entire sample of archaeological shells that were missing one of these measurements. This approximation allowed us to estimate the size of the shells and consequently estimate the size of the kelp stipe where they were attached when algae were harvested and transported to the archaeological sites. This may allow us to evaluate the degree of human pressure on kelp forests during prehistoric times (Vega et al., 2016).
Finally, we radiocarbon dated 22 individual shells. This allowed us to determine chronological boundaries to include shells without direct dating. Obtained dates were calibrated (Oxcal v4.4.4 Bronk Ramsey, 2021) using the reservoir effect published by Ortlieb et al. (2011) for the northern coast of Chile and the Marine20 curve (Heaton et al., 2020). In order to evaluate the presence of algae over time, we have divided the four thousand years in which our study is framed into chronological blocks of one thousand years each.
Table 2 Shows the 22 dates obtained for shells from the nine archaeological sites under study. Our dates indicate that the earliest indirect evidence of kelp in the Taltal area appears around 6,600 years cal BP in Morro Colorado and then around 5,580 cal BP in Hornos de Cal, both open-air shell midden sites. The most recent archaeological records come from the rockshelter site Paposo Norte 9, dated around 600 cal BP, and the open-air shell midden Plaza de Indios Norte, with a date of 898 cal BP.
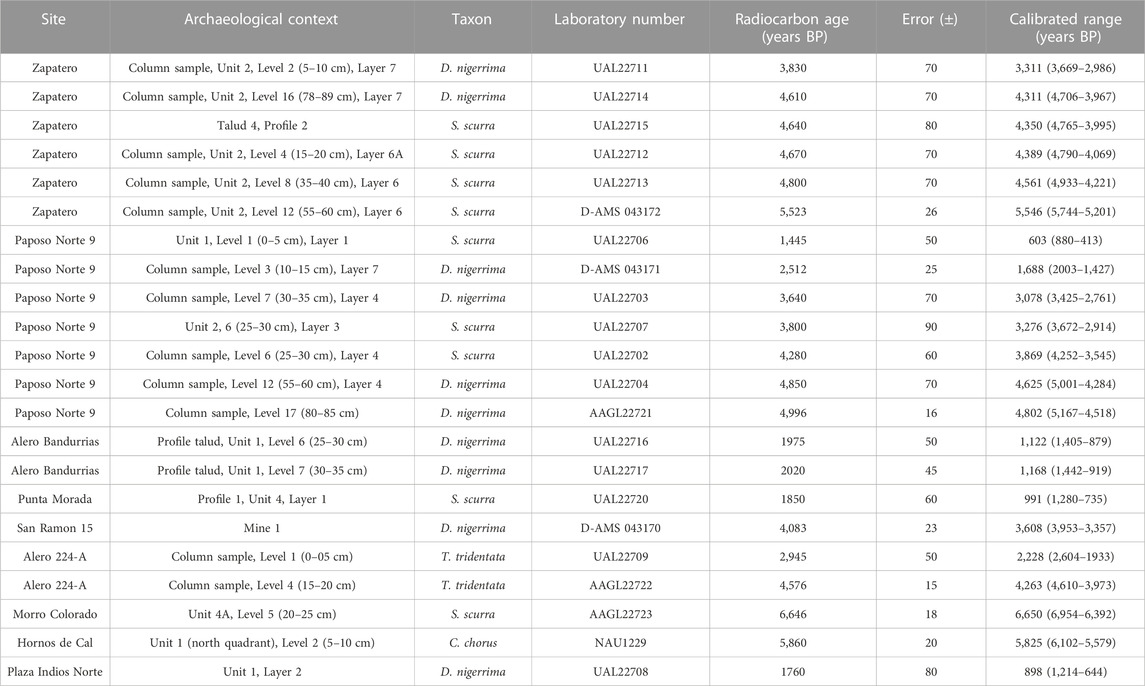
TABLE 2. Radiocarbon dates obtained from shells under study. Obtained dates were calibrated (95% of probability) (Oxcal v4.4.4 Bronk Ramsey, 2021) using the reservoir effect value of 226 ± 98, published by Ortlieb et al. (2011) for the northern coast of Chile and Marine20 curve (Heaton et al., 2020).
Based on the stratigraphic association between dated levels and shells, we were able to situate 85% of our sample in a chronological context (270 of 319 shells) (Table 3; and Supplementary Table S2) and to group data on shell abundance (Table 3) and size (Table 4) in time periods of 1,000 years each. The exception is the period between 5,500 and 6,600 years cal BP, which was extended to fit the date 6,650 obtained from the Morro Colorado site.
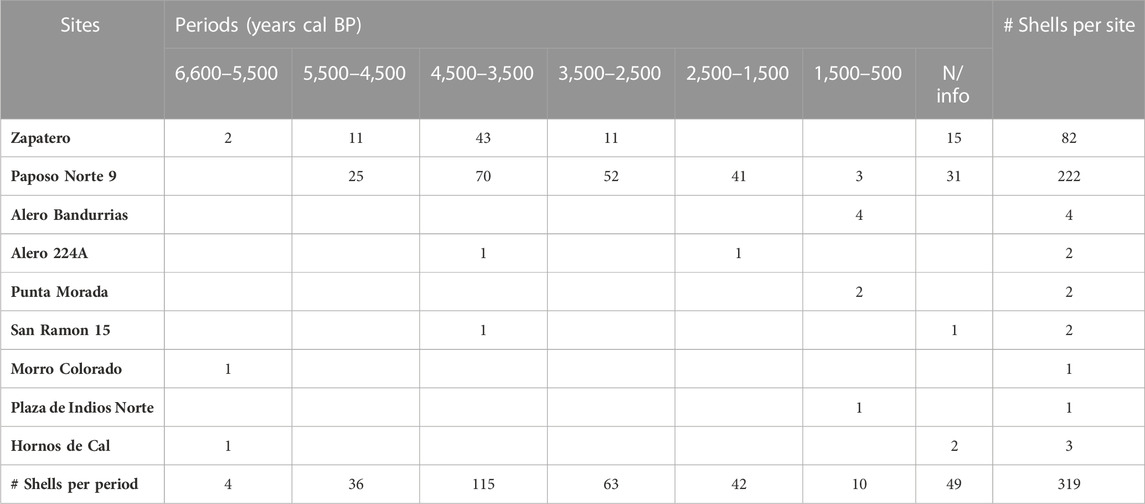
TABLE 3. Abundance (MNI) of proxy shells (Scurria scurra, Tegula atra, Tegula tridentata, and D. nigerrima) according to time periods and archaeological site. Sites sorted by geographic location (north to south).
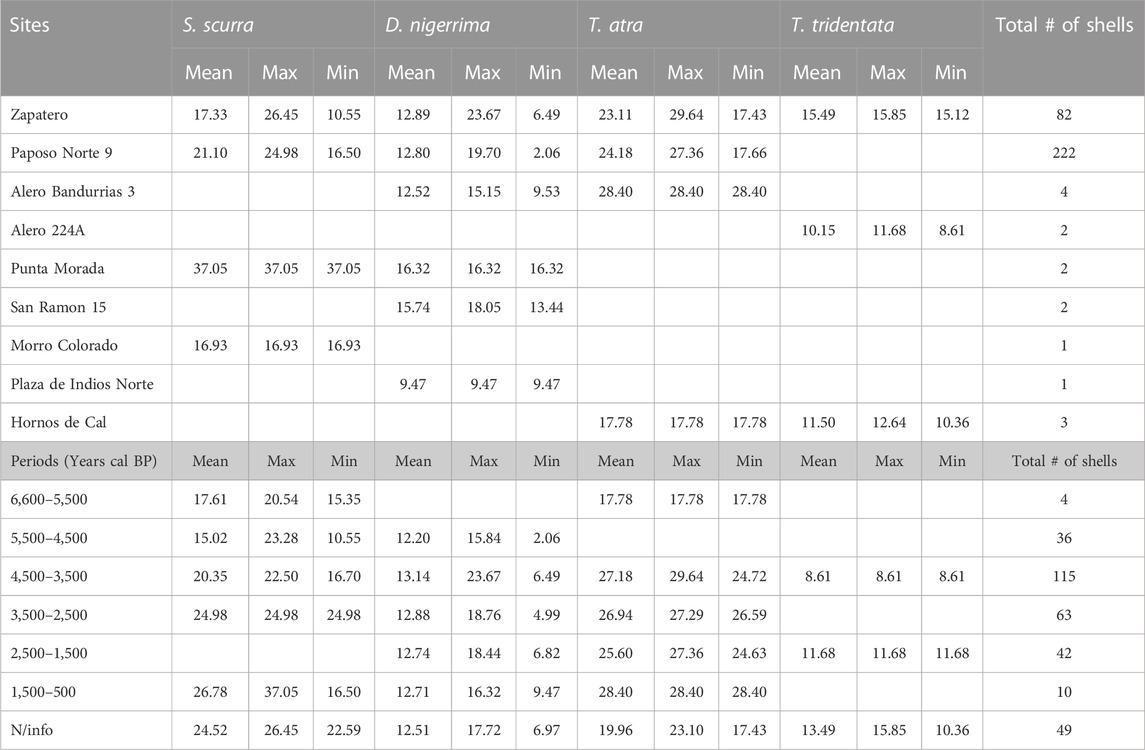
TABLE 4. Basal diameter (mm) of alga proxy species by time period and archaeological site. Sites sorted by geographical location (north to south). Total # of shells refers to the assemblage in each period or site, not to a particular species.
During the time period 6,500–5,500 cal BP, alga proxy shells S. scurra, T. atra, T. tridentata, and D. nigerrima were present in three sites: Zapatero in the north coast of the study area, Morro Colorado in the center, and Hornos de Cal in the south (Table 3; Figure 1). The 5,500–4,500 cal BP time period shows an increase in the relative abundance of shells, but only in two sites located on the north coast. This is followed by a peak during the period 4,500–3,500 cal BP and a subsequent decrease from the period 3,500–2,500 cal years BP onward. The sites Zapatero and Paposo Norte 9, both on the north coast, had the highest numbers of shells (95% of the sample) and dominated the pattern throughout the whole sequence. The distribution was relatively homogenous in the rest of the sites, with few shells of the studied species. Even though the low abundance of shells in these sites could be interpreted as incidental, their presence adds to the ubiquity of the kelp-indicating mollusks in different types of sites, located at different locations, and occupied at different moments in time.
Although distance to the coast is a relevant factor for humans to consider when deciding what to transport to their camps (Bird and Bird, 1997; Bird et al., 2002), it did not seem to influence the composition of our archaeological samples. Sites with a small number of algal proxies are located both near to (Punta Morada, Morro Colorado, Hornos de Cal) and far from (Alero Bandurrias 3, 224A, San Ramon 15) the coast. The function of the site is also a factor that may influence the amount of kelp transported to and used at the location; nevertheless, abundance was similar in open-air shell middens and rockshelters. The highest abundance and most continuous record of algal proxy shells were present in rockshelter Paposo Norte 9, 200 mt away from the shore, and in Zapatero, shell midden less than 100 mt from the shore. The shared characteristic between these two sites is their location on the northern edge of the study area (Table 3; Figure 1).
Based on the regression equations used to reconstruct the width and height of Scurria scurra, Tegula atra, Tegula tridentata, and D. nigerrima shells (Table 1), we were able to estimate the height and basal width (mm) for nine fragmented archaeological shells. Table 4 shows the mean, maximum (max), and minimum (min) basal diameter for the four mollusk species organized by periods and sites. The archaeological assemblage under study includes Scurria scurra shells from 10.5 to 37 mm diameter, D. nigerrima from 2 to 23.6 mm, Tegula atra shells between 17.4 and 29.6 mm, and Tegula tridentata between 8.6 and 15.8 mm (Table 4). The longest mean basal diameter for Scurria scurra was 37 mm in site Punta Morada, and the smallest was 16.9 mm in Morro Colorado. Looking at time periods, the longest mean diameter of Scurria scurra shells was found in the latest period, 1,500–500 cal BP, and the smallest in the period 5,500–4,500 cal BP. For trochid species, the longest mean basal diameters of D. nigerrima were 15.7 and 16.3 mm in site Punta Morada and San Ramón 15; however, mean sizes over time did not show significant differences, with basal lengths between 12 and 13 mm. The mean size of Tegula atra shells was greater than that found for Tegula tridentata, which corresponds to natural interspecies variation (Veliz and Vásquez, 2000). Finally, the largest mean diameter for juvenile Tegula atra was 28.4 mm in Alero Bandurrias and the period 1,500–500 cal BP, while for Tegula tridentata, they were 15.4 mm in Zapatero and 11.6 mm in the period 2,500–1,500 cal BP (Table 4).
Figure 3 shows the size distribution of the 319 archaeological shells analyzed in the study, organized by archaeological site and time period. There was a wide distribution of shell sizes, although most of the shells are grouped around sizes of 4 mm height-8 mm basal width and 10 mm height-18 mm basal width. This applies for size distribution per site and period and, therefore, implies that a basal diameter size range between 8 and 18 mm is characteristic of the area in archeological times. Zapatero and Paposo Norte 9, located on the northern coast of the study area, show greater dispersion of sizes and have the largest individuals among samples. Although shell sizes are widely distributed during all time periods, it is during the later time periods, from 4,500 to 500 years cal BP, that the largest shells appeared, almost exclusively at the Zapatero and Paposo Norte 9 sites.
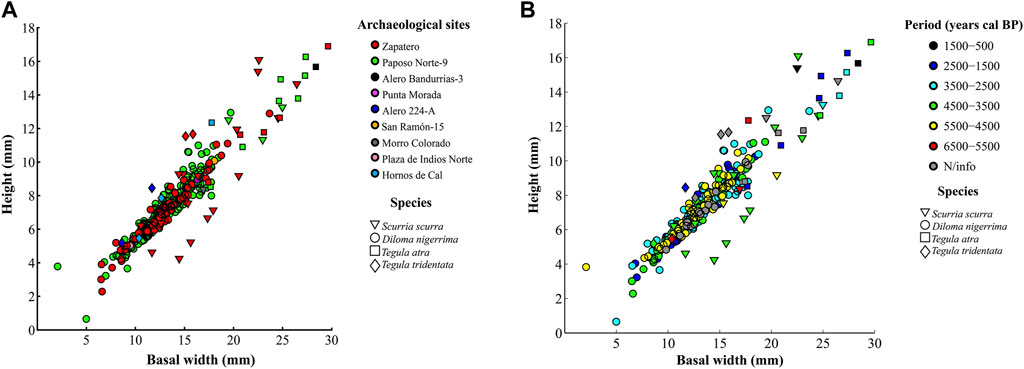
FIGURE 3. Scatter plot of proxy species ‘shell sizes, (A) by species and archaeological site; (B) by species and period.
Finally, taphonomic observations carried out on the shells show a good state of preservation, without evidence of abrasion or exfoliation. This applies to all sites during all time periods (Supplementary Table S1). The same goes for thermal alterations, which suggests that shells were not exposed.
We used non-dietary malacological fauna as a proxy to infer the presence of algae in nine archaeological sites of the coast of Taltal. These species of snails and limpets, inhabiting kelp stipes and holdfasts, arrived at the archaeological sites and endured over time.
Salazar and collaborators (2018) reported the presence of T. atra, Tegula sp., D. nigerrima, and Scurria parasitica in early Holocene sites like Paposo Norte 9 and Alero 224-A. Archaeo-malacological samples reviewed for the present study included shell remains of these sites associated with early dates, but none of these species were found, nor shells of S. scurra. Alga proxy shells were found in samples from these two sites but with dates associated with the 5,500–4,500 cal BP period for Paposo Norte 9 and 4,500–3,500 cal BP for Alero 224-A (Tables 2–4). Despite not having alga proxy shells in our samples, information published by Salazar et al. (2018) suggests that the first evidence of algae in Taltal appears between 12,000 and 11,000 years cal BP. Our analyses show indirect evidence of kelp in Morro Colorado around 6,600 years cal BP and Hornos de Cal around 5,800 years cal BP, both open-air shell middens located on the south coast of the study area (Figure 1; Table 2). This big gap in kelp presence in archaeological sites of Taltal is intriguing but agrees with the sparse record of archaeological sites in the area with only two sites dating between 10,000 and 8,500 cal BP (Salazar et al., 2015; 2018). Later on, the period between 7,500 and 5,500 cal BP was identified (Salazar et al., 2015) as a moment of increased human occupation, with more and larger shell-midden sites near the shore, interpreted as residential locations with evidence of technological diversification. This led to the expectation of higher exploitation of nearshore resources, including kelp, but our samples do not reflect that until a little later (6,600 cal BP). One of the reasons for this may be related to space organization within settlements, with kelp drying areas next to the shore and far from dumping areas near camps (shell middens sampled by archaeologists).
The presence of algae in the archaeological record increased significantly between 5,500 and 3,500 cal BP and was concentrated in Zapatero, an open-air site, and the rockshelter Paposo Norte 9, both located on the north coast of the study area. It is interesting that the greater presence of algae in the Zapatero site between 4,500 and 3,500 years BP coincides with the time of construction of stone structures of the Caleta Huelén 42 type. These structures have been interpreted as containing sealed floors made by the action of burning algae (Núñez et al., 1974). The San Ramón 15 site, a ferrous oxide mine located 3 km from the coast at 125 m. a.s.l., also presents evidence for this period and reveals the transport of algae to this place of specific and distant activities (Salazar et al., 2015). Other examples of coastal and marine resources making their way inland can be found in interior areas of the Atacama Desert, with the presence of dried fish, shell body ornaments such as necklace beads, and utilitarian textile artifacts made also from shells. This is evidence of the circulation of coastal goods far inland from early times to post-Hispanic historical periods (Latorre et al., 2013; Soto and Power, 2013; Castillo et al., 2017; Gallardo, 2017; Soto et al., 2018).
The only sites with evidence of algae between 2,500 and 1,500 cal BP are Paposo Norte 9 and Alero 224-A, located on the north and central coast, with Paposo Norte 9 being the most abundant. Finally, during the last period of the Late Holocene (1,500–500 cal BP), the presence of algae is identified, although in low abundance, in two open-air shell midden type sites and two rockshelters located on the north, center, and south coast.
Kelp abundance and growth in rocky benthic habitats are closely related to seawater temperature, which is a proxy of upwelling intensity, and nutrient supply along eastern boundary upwelling systems (Broitman and Kinlan, 2006; Cavanaugh et al., 2011; Pérez-Matus et al., 2017). Sea surface temperature displayed considerable variability along southern Peru and northern Chile during our study period (Flores et al., 2018; Salvatteci et al., 2019; Flores and Broitman, 2021). Therefore, it is worth noting that the relative abundance of algal proxies (Table 3) is negatively associated, albeit non-significantly, with the long-term temperature variation across the Holocene (Flores and Broitman, 2021, r = −0.625, p = 0.183), with higher concentrations of algal proxies during colder periods (4,500–3,500 cal BP), potentially in association with a higher abundance of kelps. Moreover, the archaeological sites with a higher abundance of algal proxies (Paposo Norte 9 and Zapatero) are located at the northern end of the study area, with currently distinctively cooler waters than sites located southward (Flores et al., 2018 Table 4).
The sizes of complete and reconstructed archaeological shells of S. scurra, D. nigerrima, T. atra, and T. tridentata show the presence of small individuals that inhabit juvenile stipes, located in the periphery of the algae (Figure 2A), holdfasts (Figure 2D), or stipe bifurcations (Figures 2B,C). We may, therefore, suggest that leaves, stipes, and holdfasts of algae were taken to the archaeological sites. The predominance of juvenile individuals in the archaeological sample (shells between 10 and 18 mm basal width) indicates stipes of small diameter, characteristic of areas with constant human collection (Vega et al., 2016). The presence of small shells could indicate the collection of juvenile algae due to the ease of extraction and transport, or human pressure on the kelp bed that prevented S. scurra, D. nigerrima, T. atra, and T. tridentata shells from reaching their adult size.
A total of 291 modern shells of S. scurra, T. atra, and D. nigerrima were measured to calculate linear regression equations and estimate the size of the incomplete archaeological individuals. Even though the modern specimens were collected in kelp drying areas at places of constant kelp harvesting, the sizes of modern S. scurra shells are larger than archaeological ones, with a mean basal width of 27.4 mm, including sizes between 15.7 mm and 41.6 mm, corresponding to juvenile and adult specimens (Table 5). The mean basal width of archaeological S. scurra shells was 18.7 mm, with sizes between 10.5 mm and 37 mm, corresponding exclusively to juveniles (Table 4).
The locations from which we collected modern specimens are areas where fishermen dry algae before transporting them for processing and are adjacent to the rocky intertidal. The sediment left after kelps have been taken shows a high density of S. scurra, D. nigerrima, and Tegula sp., which detached from the collected algae and were deposited on the sand in a radius of approximately 10 m (authors’ personal observations). Compared to the great abundance of mollusks of different species that make up the archaeological shell middens of the coast of Taltal (Olguín, 2013; Olguín et al., 2015; Salazar et al., 2015), the low number of alga proxy shells found in some sites rules out the location of drying areas on or adjacent to the main dumping areas. These are the areas commonly selected for the location of excavation units of column samples.
Because the sites included in this study are at different distances from the coast, their low shell abundance does not seem to be related to the distance between the sites and the coast but is probably related to the decision to use dry or wet kelp in the camps or to the functionality of the shell midden areas sampled by archaeological excavations. If kelp were taken dry to the camps, alga proxy shells would have fallen somewhere else or during transportation. However, if kelp were taken wet and then dried on the sites, the number of expected shells would be high, as observed in Zapatero and Paposo Norte 9, where drying activity was possibly carried out near dumping areas. This suggests that future studies should consider unusually high density of alga proxy shells as a possible indicator of alga drying areas.
Some of the multiple uses of kelp by coastal societies on the Atacama Desert coast imply calcination by exposure to fire. These include fuel for stoves, preparation of floors or construction material (Borie et al., 2006), fuel to smoke fish and seafood, and fuel to create smoke signals to communicate. However, the absence of traces of combustion in the analyzed shells leads us to suggest that algae were not processed with fire but were used fresh for other purposes, such as roofs or floors for huts (Araos et al., 2018) or food (Ainis et al., 2014).
Kelp have been crucial for coastal societies around the whole Pacific rim (Erlandson et al., 2007; Dillehay et al., 2008). Their uses were certainly diverse and associated with different social contexts and meanings (Pérez-Lloréns et al., 2020). This is shown by the presence of cochayuyo in the domestic context of the Monte Verde site during Paleoindian times (Dillehay et al., 2008) and of black huiro ties in funerary contexts at Pisagua for the Tiahuanaco period (Ramírez and Quevedo, 2000). Data from Taltal, on the Atacama Desert coast complement and enrich this record, providing information on the date, distribution, size, and abundance of these snail and limpet shells, which are proxies for kelp presence in archaeological contexts.
Preliminary results presented in this study contribute to the study of the relevance and uses of kelp through millennia, their geographic and chronological distribution in human contexts, and the relationship between kelp forest productivity and human use on the Pacific coast.
The temporal and spatial distributions of alga proxy shells show that coastal groups in the Taltal area have been using this resource for at least 12,000 years. This was reported by Salazar et al. (2018) in rockshelter sites located on the north and central coast of the study area. Almost 5,000 years later, alga proxy shells have been found in open-air shell midden sites, rockshelters, and a mine. Alga use increased around 5,500 cal BP, with a peak between 3,500 and 2,500 years cal BP. This emphasis on kelp collection is observed exclusively at sites on the north coast of the study area, where more intense upwelling conditions favor the growth of kelp forests. The shell sizes at Zapatero and Paposo Norte 9 sites were larger than the rest of the sites; however, none exceeded 40 mm basal width, which suggests that there was a constant use of algae that could reduce their growth cycle due to high pressure on the resource by human populations. Subsequently, during the Late Holocene, around 2,500 cal years BP, the Paposo Norte 9 site remains the coastal settlement with the highest abundance of shell proxies, until the period between 1,500 and 500 years cal BP, where the abundance in the study area decreases considerably, but evidence of algae reappears at sites on the south coast of Taltal.
The present study demonstrates how the use of marine invertebrates as proxies for algae use gives value to small and infrequent fauna generally not included in archaeomalacological analyses and, thus, expands the spectrum of resources considered in the interpretation of the ways of life of the fisher communities of the Pacific coast. The use of these shell proxies also highlights the importance of considering what is not present/conserved in archaeological contexts and the role of indirect evidence under contexts of poor organic preservation.
The development of proper methodologies is crucial to identifying and analyzing archaeological evidence of kelp to explore research questions about their importance in human history, characteristics of kelp forest ecosystems during the last millennia, and the effect of environmental and human factors on their use and abundance.
Future archaeological and ethnographic studies will be necessary to expand interpretations on the use and processing of algae, through systematic surveys and sampling in possible drying areas, as well as through archaeobotanical, sedimentological, and microstratigraphic analyses focused on the detection of kelp remains.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
VA, CF, and BB contributed equally to this manuscript. They conceived the original idea of using small limpets and snails to evaluate presence of algae in the archaeological sites of Taltal. They planned the methodology and analyses, made tables, figures, wrote and edited the manuscript. Additionally, VA obtained modern shells, selected archaeological samples, and performed the measurements over all samples. VA and BB performed the regression analysis. JG and LO, did the original archaeomalacological analyzes of sampled sites. All authors contributed to the article and approved the submitted version.
BB is supported by FONDECYT 1221699, 122534, 123286, and Instituto Milenio de Socio-Ecología Costera (SECOS, ICN 2019-015). CF and VA are supported by Millennium Nucleus UPWELL (NCN19_153).
The authors thank Projects FONDECYT 11200953, 1181300, 1151203, and 1110196; Millennium Nucleus UPWELL NCN19_153, and all their teams. Special thanks to Alonso Vega, Nicole Piaget, Valentina Hernández, Mauricio Oróstica, and Manuel Núñez. We also acknowledge Diego Salazar and the archaeological team of Taltal.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2023.1148299/full#supplementary-material
SUPPLEMENTARY TABLE S1 | Direct archaeological evidence of algae mentioned in the text. Ages are presented in BC or BP in accordance with the information provided in the literature.
SUPPLEMENTARY TABLE S2 | Database used in this study.
Ainis, A. F., Vellanoweth, R. L., Lapeña, Q. G., and Thornber, C. S. (2014). Using non-dietary gastropods in coastal shell middens to infer kelp and seagrass harvesting and paleoenvironmental conditions. J. Archaeol. Sci. 49, 343–360. doi:10.1016/j.jas.2014.05.024
Ainis, A. F., Erlandson, J. M., Gill, K. M., Graham, M. H., and Vellanoweth, R. L. (2019). “The potential use of seaweeds and marine plants by native peoples of alta and Baja California: implications for “marginal” island ecosystems,” in An archaeology of abundance: Reevaluating the marginatliy of California’s Channel Islands. Editors K. M. Gill, M. Fauvelle, and J. M. Erlandson (Gainesville: University of Florida Press), 135–170.
Andrade, P., Salazar, D., Urrea, J., and Castro, V. (2014). Modos de vida de los cazadores-recolectores de la costa arreica del norte grande de Chile: una aproximación bioarqueológica a las poblaciones prehistóricas de Taltal. Chungara. Rev. Antropol. Chil. 46 (3), 467–491. doi:10.4067/S0717-73562014000300010
Andrade, P., Fernandes, R., Codjambassis, K., Urrea, J., Olguín, L., Rebolledo, S., et al. (2015). Subsistence continuity linked to consumption of marine protein in the formative period in the interfluvic coast of northern Chile: re-assessing contacts with agropastoral groups from highlands. Radiocarbon 57 (4), 679–688. doi:10.2458/azu_rc.57.18448
Araos, F., Borie, C., Romo, M., Lira, N., and Duarte, A. (2018). Algas: breves antecedentes etnográficos y arqueológicos. Fogón, Rev. Int. Estud. las Tradic. 1 (2), 40–52.
Araos, F. (2015). Habitando la Orilla": la Recolección de algas en el litoral central de Chile. Espac. Reg. 2 (12), 137–151. doi:10.32735/esp.reg.v2i12.113
Bird, D. W., and Bird, R. L. B. (1997). Contemporary shellfish gathering strategies among the meriam of the torres strait islands, Australia: testing predictions of a central place foraging model. J. Archaeol. Sci. 24 (1), 39–63. doi:10.1006/jasc.1995.0095
Bird, D. W., Richardson, J. L., Veth, P. M., and Barham, A. J. (2002). Explaining shellfish variability in middens on the meriam islands, torres strait, Australia. J. Archaeol. Sci. 29 (5), 457–469. doi:10.1006/jasc.2001.0734
Borie, C., Araos, F., Romo, M., Lira, N., and Duarte, A. (2006). “Potencialidades, usos y evidencias de explotación de algas marinas,” in Actas del XVII Congreso Chileno de Arqueología Chilena (Sociedad Chilena de Arqueología), 1191–1201. Tomo 2. Antecedentes etnográficos y arqueológicos, implicancias y líneas de Investigación.
Broitman, B. R., and Kinlan, B. P. (2006). Spatial scales of benthic and pelagic producer biomass in a coastal upwelling ecosystem. Mar. Ecol. Prog. Ser. 327, 15–25. doi:10.3354/meps327015
Bronk Ramsey, C. (2021). OxCal program, v4.4.4. [Online]. UK: Radiocarbon Accelerator Unit, University of Oxford. Available at: https://c14.arch.ox.ac.uk/oxcal.html (Accessed, 2022).
Cancino, J. M., and Santelices, B. (1984). Importancia ecologica de los discos adhesivos de Lessonia nigrescens Bory (Phaeophyta) en Chile Central. Rev. Chil. Hist. Nat. 57, 23–33.
Cárcamo, S., and Gelcich, S. (2020). Mujeres navegantes y de orilla. Innovación y tradición alimentaria con sabor a mar. Pontificia Universidad Católica de Chile.
Castelleti, J. (2007). Patrón de asentamiento y uso de recursos a través de la secuencia ocupacional prehispana en la costa de Taltal. Master's thesis. Instituto de Investigaciones Arqueológicas y Museo, Universidad Católica del Norte.
Castillo, C., Ballester, B., Calás, E., Labarca, R., and Gallardo, F. (2017). “La ruta de los peces más allá del litoral: sobre el ciclo del pescado seco en el Desierto de Atacama (periodo formativo),” in Monumentos Funerarios de la Costa del Desierto de Atacama. Los Cazadores-Recolectores Marinos y sus Intercambios (500 a.C. - 700 d.C.) Serie monográfica de la Sociedad chilena de Arqueología N°7. Editor F. B. N. Gallardo Ballester Fuenzalida (Santiago de Chile), 55–66.
Castro, V., Aldunate, C., Varela, V., Olguín, L., Andrade, P., García-Albarido, F., et al. (2016). Ocupaciones arcaicas y probables evidencias de navegación temprana en la costa arreica de Antofagasta, Chile. Chungara. Rev. Antropol. Chil. 48, 0–530. doi:10.4067/S0717-73562016005000039
Cavanaugh, K. C., Siegel, D. A., Reed, D. C., and Dennison, P. E. (2011). Environmental controls of giant-kelp biomass in the santa barbara channel, California. Mar. Ecol. Prog. Ser. 429, 1–17. doi:10.3354/meps09141
Chavez, F. P., and Messié, M. (2009). A comparison of Eastern boundary upwelling ecosystems. Prog. Oceanogr. 83, 80–96. doi:10.1016/j.pocean.2009.07.032
Colonese, A. C., and Wilkens, B. (2005). “The malacofauna of the upper paleolithic levels at grottadella serratura (salerno, southern Italy): preliminary data,” in Archaeomalacology: Molluscs in former environments of human behaviour. Editor D. E. Bar-Yosef Mayer (Great Britain: Oxbow Books), 63–70.
Dillehay, T. D., Ramírez, C., Pino, M., Collins, M. B., Rossen, J., and Pino-Navarro, J. D. (2008). Monte Verde: seaweed, food, medicine and the peopling of south America. Science 320, 784–786. doi:10.1126/science.1156533
Docman, F., Araya, M., Hinojosa, I. A., Dorador, C., and Harrod, C. (2017). Habitat coupling writ large: pelagic-derived materials fuel benthivorous macroalgal reef fishes in an upwelling zone. Ecology 98 (9), 2267–2272. doi:10.1002/ecy.1936
Erlandson, J. M., Graham, M. H., Bourque, B. J., Corbett, D., Estes, J. A., and Steneck, R. S. (2007). The kelp highway hypothesis: marine ecology, the coastal migration theory, and the peopling of the Americas. J. Isl. Coast. Archaeol. 2, 161–174. doi:10.1080/15564890701628612
Esper, E. (2022). El impacto de la exportación de huiro negro en las experiencias de recolectores, procesadores y comerciantes de Paposo, región Antofagasta. Taltalia 14, 63–89. doi:10.5281/zenodo.6425252
Flores, C., and Broitman, B. R. (2021). Nearshore paleoceanogaphic conditions through the holocene: shell carbonate from archaeological sites of the Atacama Desert coast. Palaeogeogr. Palaeoclimatol. Palaeoecol. 562, 110090. doi:10.1016/j.palaeo.2020.110090
Flores, C., Gayo, E. M., Salazar, D., and Broitman, B. R. (2018). δ18O of Fissurella maxima as a proxy for reconstructing Early Holocene sea surface temperatures in the coastal Atacama Desert (25° S). Palaeogeogr. Palaeoclimatol. Palaeoecol. 499, 22–34. doi:10.1016/j.palaeo.2018.03.031
Gallardo, F. (2017). “Arqueología de los intercambios recíprocos: costa y oasis del río loa medio e inferior, época formativa (500 cal a.C. - 700 d.C.),” in Monumentos Funerarios de la Costa del Desierto de Atacama. Los Cazadores-Recolectores Marinos y sus Intercambios (500 a.C. - 700 d.C.) Serie monográfica de la Sociedad chilena de Arquoelogía N°7. Editor F. B. N. Gallardo Ballester Fuenzalida (Santiago de Chile).
Heaton, T. J., Köhler, P., Butzin, M., Bard, E., Reimer, R. W., Austin, W. E., et al. (2020). Marine20—The marine radiocarbon age calibration curve (0–55,000 cal BP). Radiocarbon 62 (4), 779–820. doi:10.1017/RDC.2020.68
Hernández, V. (2019). Algas, fuego y cenizas Una aproximación experimental a la preparación de estratos de ceniza en contextos ca 6000-4000 cal AP de la costa arreica. Bachelor's thesis. Universidad de Tarapacá.
Latorre, C., Betancourt, J. L., Rech, J. A., Quade, J., Holmgren, C., Placzek, C., et al. (2005). “Late quatenary history of the Atacama Desert,” in 23°S. Archaeology and environmental history of the southern deserts. Editors M. Smith,, and P. Hesse (Australia: National Museum of Australia Press), 73–90.
Latorre, C., Santoro, C. M., Ugalde, P. C., Gayo, E. M., Osorio, D., Salas-Egaña, C., et al. (2013). Late Pleistocene human occupation of the hyperarid core in the Atacama Desert, northern Chile. Quat. Sci. Rev. 77, 19–30. doi:10.1016/j.quascirev.2013.06.008
Llagostera, A. (1989). “Caza y pesca marítima (9.000 a 1.000 a.C.),” in Culturas de Chile. Prehistoria. Desde sus Orígenes hasta los Albores de la Conquista. Editors J. Hidalgo, V. Schiappacasse, H. Niemeyer, C. Aldunate, and I. Solimano (Santiago de Chile: Editorial Andrés Bello), 57–80.
Lotze, H. K., Milewski, I., Fast, J., Kay, L., and Worm, B. (2019). Ecosystem-based management of seaweed harvesting. Bot. Mar. 62 (5), 395–409. doi:10.1515/bot-2019-0027
Montecino, V., and Lange, C. B. (2009). The Humboldt current system: ecosystem components and processes, fisheries, and sediment studies. Prog. Oceanogr. 83, 65–79. doi:10.1016/j.pocean.2009.07.041
Moragas, C., and Mendez-Quiros, P. (2022). La secuencia cronológica de Punta Patache y la ocupación de la costa arreica del desierto de Atacama (21°S). Estud. Atacameños 68, e5060. doi:10.22199/issn.0718-1043-2022-0029
Mooney, D. E. (2021). Charred fucus-type seaweed in the north atlantic: a survey of finds and potential uses. Environ. Archaeol. 26 (2), 238–250. doi:10.1080/14614103.2018.1558805
Muñoz, M., and Santelices, B. (1989). Determination of the distribution and abundance of the limpet Scurria scurra on the stipes of the kelp Lessonia nigrescens in Central Chile. Mar. Ecol. Prog. Ser. 54, 277–285. doi:10.3354/meps054277
Muñoz, I. (2014). “Ritualidad y memoria de los pescadores de la costa de Arica durante el periodo arcaico tardío: el caso de la cueva de La Capilla,” in Mil años de historia de los constructores de túmulos de los valles desérticos de Arica: Paisaje, monumentos y memoria. Editors I. Muñoz,, and M. Férnandez (Arica, Chile: Ediciones Universidad de Tarapacá), 39–64.
Nirmal-Kumar, J. I., Kumar, R. N., Patell, K., Viyol, S., and Bhoi, R. (2009). Nutrient composition and calorific value of some seaweeds from bet dwarka, west coast of Gujarat, India. Our Nat. 7, 18–25. doi:10.3126/on.v7i1.2565
Núñez, L., Zlatar, V., and Núñez, P. (1974). Caleta huelén-42: una aldea temprana en el norte de Chile (nota preliminar). Hombre Cult. 2, 67–103.
Núñez, L. (1976). Registro regional de fechas radiocarbónicas del norte de Chile. Estud. Atacameños 4, 69–111. doi:10.22199/S07181043.1976.0004.00009
Olguín, L., Flores, C., and Salazar, D. (2015). “Aprovechamiento humano de moluscos marinos en conchales arqueológicos del Holoceno Temprano y Medio (12.000-5.500 años cal AP). Costa meridional del desierto de Atacama, Chile,” in Arqueomalacología. Abordajes metodológicos y casos de estudio en el Cono Sur. Fundación de Historia natural félix de Azara. Editors H. Hammond,, and M. A. Zubimendi First ed (Buenos Aires, Argentina), 13–34.
Olguín, L. (2013). Aprovechamiento de invertebrados marinos en conchales arqueológicos del arcaico medio (6.000 – 4.000 a.C.) en la costa de Taltal: estudios preliminares Taltalia 5-6, 37–53Available at: https://api.nakala.fr/data/11280%2Fd02bef31/0491745a63bb20656d87c919cd784daa3366a1cb.
Oróstica, M., Aguilera, M., Donoso, G., Vásquez, J., and Broitman, B. (2014). Effect of grazing on distribution and recovery of harvested stands of Lessonia berteroana kelp in northern Chile. Mar. Ecol. Prog. Ser. 511, 71–82. doi:10.3354/meps10931
Ortlieb, L., Vargas, G., and Saliège, J. F. (2011). Marine radiocarbon reservoir effect along the northern Chile–southern Peru coast (14–24°S) throughout the Holocene. Quat. Res. 75, 91–103. doi:10.1016/j.yqres.2010.07.018
Pérez-Lloréns, J. L., Mouritsen, O. G., Rhatigan, P., Cornish, M. L., and Critchley, A. T. (2020). Seaweeds in mythology, folklore, poetry, and life. J. Appl. Phycol. 32, 3157–3182. doi:10.1007/s10811-020-02133-0
Pérez-Lloréns, J. L. (2019). Seaweed consumption in the Americas. Gastronomica 19 (4), 49–59. doi:10.1525/gfc.2019.19.4.49
Pérez-Matus, A., Carrasco, S. A., Gelcich, S., Fernandez, M., and Wieters, E. A. (2017). Exploring the effects of fishing pressure and upwelling intensity over subtidal kelp forest communities in Central Chile. Ecosphere 8 (5), e01808. doi:10.1002/ecs2.1808
Pinochet, R., Soto, J. C., Palacios, M., and Oyarzún, S. (2018). Selección dietaria de Tegula atra (Lesson, 1830) como una aproximación de preferencia sobre distintas especies de macroalgas en el sur de Chile. An. Inst. Patagon. (Chile) 46 (3), 51–60. doi:10.4067/S0718-686X2018000300051
Power, X., and Salazar, D. (2020). Estudio intrasitio de un yacimiento arcaico con arquitectura en la costa de Taltal, Desierto de Atacama, Norte de Chile. Chungara. Rev. Antropol. Chil. 52 (2), 0–207. doi:10.4067/S0717-73562020005001201
Power, X., Sitzia, L., Yrarrázaval, S., Salazar, D., Andrade, P., Hernández, V., et al. (2022). Ritual stone-built architecture and shell midden foundation: a semisubterranean structure in hyperarid Atacama Desert coast, northern Chile. Geoarchaeology 37, 198–226. doi:10.1002/gea.21857
Ramírez, M., and Quevedo, S. (2000). Hallazgo de Lessonia nigrescens (Phaeophyceae) en enterrtorio del cementerio Tiahuanaco Atacameño Pisagua (Colección Max Uhle). Bol. del Mus. Nac. Hist. Nat. 49, 99–108Available at: https://publicaciones.mnhn.gob.cl/668/w3-article-64451.html.
Rebolledo, S., Béarez, P., Salazar, D., and Fuentes, F. (2016). Maritime fishing during the Middle holocene in the hyperarid coast of the Atacama Desert. Quat. Int. 391, 3–11. doi:10.1016/j.quaint.2015.09.051
Salazar, D., Jackson, D., Guendon, J. L., Salinas, H., Morata, D., Figueroa, V., et al. (2011). Early evidence (ca. 12,000 BP) for iron oxide mining on the pacific coast of south America Curr. Anthropol. 52, 463–475. doi:10.1086/659426
Salazar, D., Figueroa, V., Andrade, P., Salinas, H., Olguín, L., Power, X., et al. (2015). Cronología y organización económica de las poblaciones arcaicas de la costa de Taltal. Estud. Atacameños 50, 07–46. doi:10.4067/S0718-10432015000100002
Salazar, D., Arenas, C., Andrade, P., Olguín, L., Torres, J., Flores, C., et al. (2018). From the use of space to territorialisation during the early holocene in taltal, coastal Atacama Desert, Chile. Quat. Int. 473, 225–241. doi:10.1016/j.quaint.2017.09.035
Salvatteci, R., Schneider, R. R., Blanz, T., and Mollier-Vogel, E. (2019). Deglacial to Holocene ocean temperatures in the Humboldt Current System as indicated by alkenone paleothermometry. Geophys. Res. Lett. 46 (1), 281–292. doi:10.1029/2018GL080634
Santana-Sagredo, F., Schulting, R. J., Méndez-Quiros, P., Vidal-Elgueta, A., Uribe, M., Loyola, R., et al. (2021). ‘White gold’guano fertilizer drove agricultural intensification in the Atacama Desert from AD 1000. Nat. Plants 7 (2), 152–158. doi:10.1038/s41477-020-00835-4
Santoro, C. M., Gayo, E. M., Carter, C., Standen, V. G., Castro, V., Valenzuela, D., et al. (2017). Loco or no loco? Holocene climatic fluctuations, human demography, and community based management of coastal resources in northern Chile. Front. Earth Sci. 5 (77). doi:10.3389/feart.2017.00077
Soto, C., and Power, X. (2013). Argopecten purpuratus en el contexto de la arqueomalacología de Taltal. Taltalia 5-6, 21–35.
Soto, C., Power, X., and Ballester, B. (2018). Circulación de objetos perforados de concha: aportes para la interpretación de su rol en las relaciones sociales del desierto de Atacama entre los 6000-3500 AP. Bol. del Mus. Chil. Arte Precolomb. 23, 0–69. doi:10.4067/s0718-68942018005000303
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29 (4), 436–459. doi:10.1017/S0376892902000322
Tellier, F., Vega, J. M. A., Broitman, B. R., Vasquez, J. A., Valero, M., and Faugeron, S. (2011). The importance of having two species instead of one in kelp management: the Lessonia nigrescens species complex. Cah. Biol. Mar. 54 (4), 455–465.
Uhle, M. (1922). Fundamentos étnicos y arqueología de Arica y Tacna. Quito, Ecuador: Imprenta de la Universidad Central.
Vásquez, J., and Santelices, B. (1984). Comunidades de macroinvertebrados en discos adhesivos de Lessonia nigrescens bory (phaeophyta) in Chile Central. Rev. Chil. Hist. Nat. 57, 131–154.
Vega, A. J., Asorey, C. M., and Piaget, N. (2016). Asociación Scurria-Lessonia, indicador de integridad ecológica en praderas explotadas de huiro negro Lessonia berteroana (ex L. nigrescens) en el norte de Chile. Rev. Biol. Mar. Oceanogr. 51 (2), 337–345. doi:10.4067/S0718-19572016000200011
Vega, A. J. (2016). Fauna asociada a discos de adhesión del complejo Lessonia nigrescens. ¿Es un indicador de integridad ecológica en praderas explotadas de huiro negro, en el norte de Chile? Lat. Am. J. Aquatic Res. 44 (3), 623–637. doi:10.3856/vol44-issue3-fulltext-21
Veliz, D., and Vásquez, J. (2000). La familia Trochidae (Mollusca: gastropoda) en el norte de Chile: consideraciones ecológicas y taxonómicas. Rev. Chil. Hist. Nat. 73, 757–769. doi:10.4067/S0716-078X2000000400018
Vidal, A., García, M., and Vega, G. (2004). Trabajando con las plantas en la localidad arqueológica de Pisagua. I Región. Bol. Soc. Chil. Arqueol. 37, 49–59. Available at: https://boletin.scha.cl/index.php/boletin/article/view/59.
Keywords: kelp, hunter-gatherers, Atacama Desert coast, archaeo-malacologic proxy, Holocene
Citation: Alcalde V, Flores C, Guardia J, Olguín L and Broitman BR (2023) Marine invertebrates as proxies for early kelp use along the western coast of South America. Front. Earth Sci. 11:1148299. doi: 10.3389/feart.2023.1148299
Received: 19 January 2023; Accepted: 02 August 2023;
Published: 24 August 2023.
Edited by:
David K. Wright, University of Oslo, NorwayReviewed by:
Jon McVey Erlandson, University of Oregon, United StatesCopyright © 2023 Alcalde, Flores, Guardia, Olguín and Broitman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verónica Alcalde, dmVyb25pY2FwYXouYWxjYWxkZUBnbWFpbC5jb20=; Carola Flores, Y2Fyb2xhLmZsb3Jlc2Zlcm5hbmRlekB1YWNoLmNs
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.