- 1Key Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China
- 2College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
Records of Paleogene arctoids are scarce in Asia, but there are abundant records in Europe and North America. In this study, we report a new arctoid taxon, Lonchocyon qiui gen. et sp. nov., from the late Eocene Baron Sog Formation of the Erlian Basin, Inner Mongolia, China. This is the first report of a relatively complete, large arctoid taxon from the Erlian Basin. The new taxon is characterized by its overall large size; a deep mandible with a marginal process and a rudimentary premasseteric fossa; and much-reduced premolars, including p4, sectorial carnassial m1 with basined talonid, and unreduced m2-3. Lonchocyon exhibits a combination of morphologies present in both amphicyonids and the early ursid hemicyonines. The mandibular force profiles suggest that Lonchocyon could have delivered powerful canine bites while subduing prey, like large felids do today, and it may have occupied a specialized ecological niche as a predator consuming both soft flesh and hard objects.
1 Introduction
Amphicyonidae is a diverse extinct family of Carnivora, with their oldest fossil records in the middle Eocene and their youngest in the late Miocene. Amphicyonidae have been considered as close relatives of Ursidae (Hunt, 1998a; Wang et al., 2005; Rose, 2006). Fossil records of amphicyonids are relatively scarce in Asia compared to the abundant materials from Europe and North America, and most known Asian amphicyonids are from the Miocene, taking Gobicyon (Jiangzuo et al., 2019), Amphicyon, and Cynelos (Jiangzuo et al., 2018) for instance. The only two unequivocal Paleogene amphicyonids are Guangxicyon from the middle Eocene Nadu Formation and Amphicyonidae gen. et sp. indet. from the late Eocene Ergilin Dzo Formation (Zhai et al., 2003; Egi et al., 2009). The early ursid group Hemicyoninae is known to have existed from the early Oligocene to the Miocene of Eurasia and is considered as an ancestor of the ursid group Ursinae (Hunt, 1998b; Rose, 2006; Bonis, 2013). Only a few Paleogene ursids have been reported in Asia, including Cephalogale sp. from the early Oligocene of Saint Jacques and ?Cephalogale sp. from the early Oligocene Hsanda Gol Formation (Wang and Qiu, 2003; Wang et al., 2005).
The Erlian Basin in Inner Mongolia has nearly continuous fossiliferous sedimentary deposits from the late Paleocene to the Oligocene and has been explored extensively and investigated since the third Central Asiatic Expedition (CAE) of the American Museum of Natural History in the early 20th century (Wang et al., 2012; Bai et al., 2018). Based on the nearly continuous Paleogene deposits and their abundance of mammalian fossils, the Eocene mammalian faunas of the Erlian Basin form the basis of the Eocene Asian Land Mammal Ages (ALMA) (Wang et al., 2007; Wang et al., 2019). A number of carnivorous or scavenging mesonychids and creodonts have been reported from the Erlian Basin (Matthew and Granger, 1925a; Matthew and Granger, 1925b; Szalay and Gould, 1966). However, reports of Carnivora from the Erlian Basin are rarer. Apart from a left m1 of Miacis invictus from the Irdin Manha Formation (Matthew and Granger, 1925a) and a left p4 of Miacidae indet. from the Ulan Shireh Formation (Ye, 1983), there are only a few mentions of carnivorans in the fossil faunal lists without further description, such as Carnivora gen. et sp. indet. from the Arshanto Formation (Russell and Zhai, 1987) and cf. “Cynodictis” from the Ulan Shireh Formation (Manning, pers. comm. 1977 cited in an article by Russell and Zhai (1987)). In this study, we report a new genus and species of arctoids from the late Eocene Baron Sog Formation of the Erlian Basin (Figure 1). This new material is not only the first arctoid collected from the Erlian Basin but is also one of the earliest arctoid records from Eurasia.
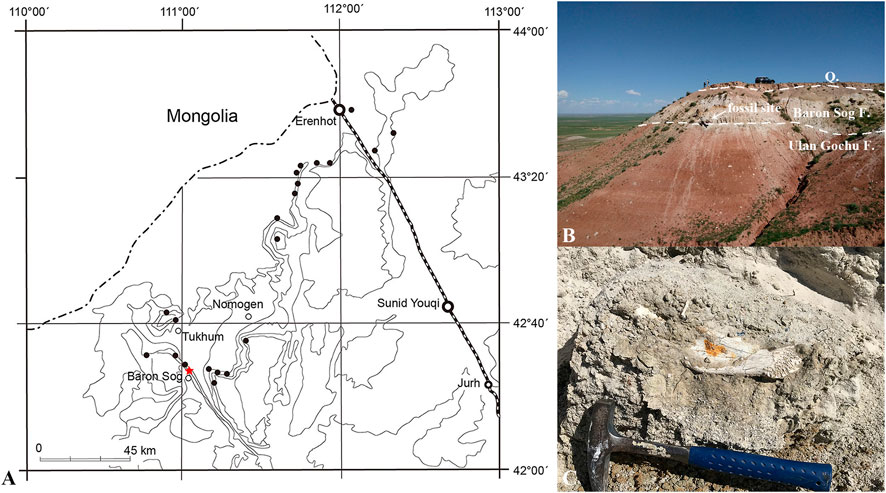
FIGURE 1. Locality bearing Lonchocyon qiui gen. et sp. nov. (IVPP V 28616) from the late Eocene of the Baron Sog Formation, Erlian Basin, Inner Mongolia, China. (A) Topographic map showing the fossil localities in the Erlian Basin with Haerhada marked by a red star, modified from Wang et al. (2012). (B) Outcrop of the fossil site showing the Baron Sog Formation and underlying Ulan Gochu Formation; (C) the lower jaw of IVPP V 28616 in situ.
2 Materials and methods
This fossil specimen (IVPP V 28616, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing) is composed of a left mandible with the canine, p4, m1, root of p3, and alveoli of i1-3, p1-2, and m2-3 (Figures 2, 3). The specimen was collected at the base of the Baron Sog Formation at Haerhada, Baiyin Obo Sumu in the Erlian Basin, Inner Mongolia, China (Wang et al., 2012; Bai et al., 2018) (Figure 1A). The formation was named after the Baron Sog Lamasery and can be easily traced along the northern escarpment of the Baron Sog Mesa (Berkey et al., 1929; Wang et al., 2012). The sediments of the late Eocene Baron Sog Formation are dominated by grayish-white sandstone (Figures 1B, C), bearing Embolotherium andrewsi and Zaisanamynodon brosovi from the Baron Sog Mesa, where the Baron Sog Formation was named (Wang, 2003; Wang et al., 2012; Bai et al., 2018). The Ulan Gochu Formation, underlying the Baron Sog Formation, is dominated by red silty clay and once yielded Amynodontopsis parvidens and Embolotherium grangeri at the Baron Sog Mesa (Wang et al., 2012; Bai et al., 2018). Ardynomys olsoni was also reported from the Ulan Gochu Formation, 4 miles north of the Baron Sog Lamasery (Wang and Meng, 2009). The Shara Murun Formation, which is overlain by the Ulan Gochu Formation and dominated by gray sandstone and sandy clays with varied colors, produced Sharamynodon mongoliensis, Rhinotitan sp., Pachytitan ajax, and Titanodectes minor at the Baron Sog Mesa (Bai et al., 2018). The top of the section is capped by Quaternary sediments that commonly form a weathering layer and cover the upper (most) part of the underlying Baron Sog Formation at the slope in some places (Figure 1B). Some new materials of perissodactyls and artiodactyls have been unearthed from the Baron Sog Formation at Haerhada in our recent fieldwork and are under preparation or study.
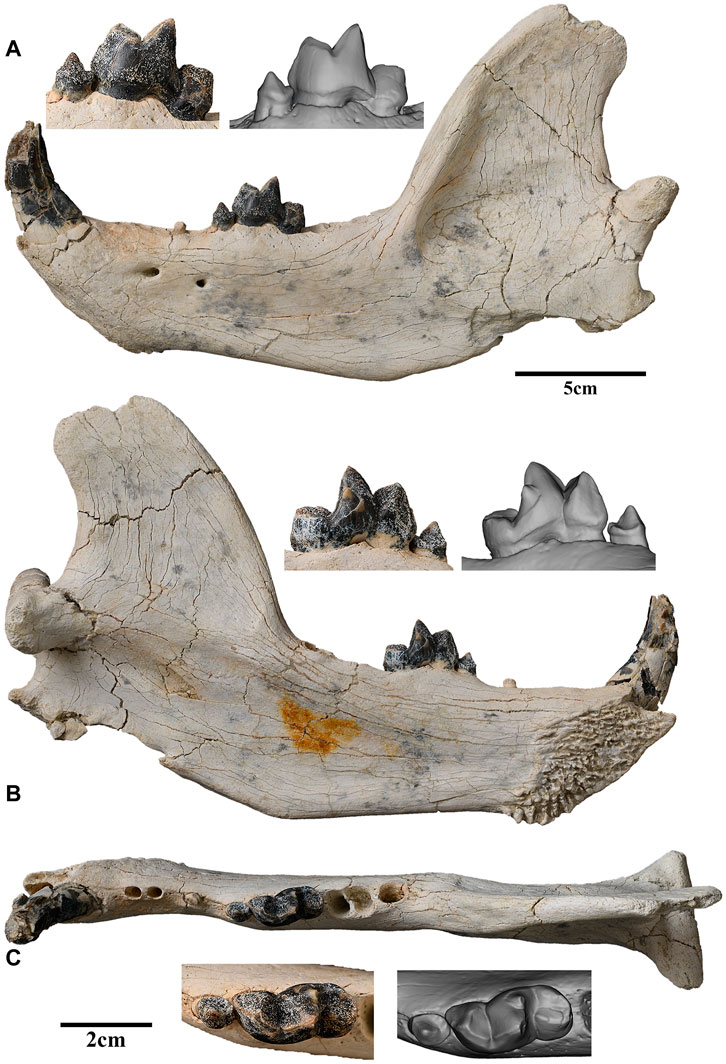
FIGURE 2. Left lower jaw of Lonchocyon qiui gen. et sp. nov. with c, p4, and m1 (IVPP V 28616): (A) lateral view; (B) lingual view; and (C) occlusal view. Scale bar equals 5 cm for the lower jaw and 2 cm for the teeth.
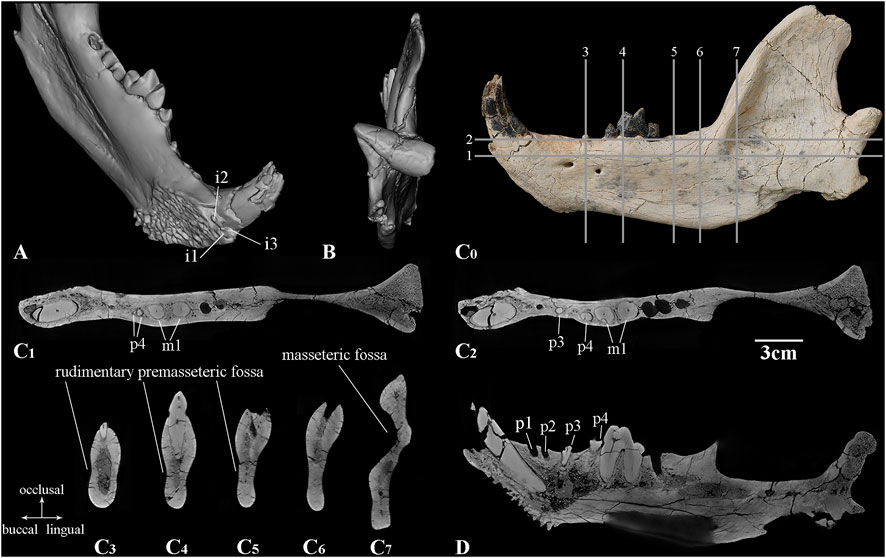
FIGURE 3. CT images of the left lower jaw of Lonchocyon qiui gen. et sp. nov. (IVPP V 28616): (A) dorsolingual view showing the incisors alveoli; (B) posterior view; (C0) lateral view of the lower jaw with the lines showing the positions of horizontal sections in (C1, C2); cross sections in (C3–C7); and (D) sagittal section of the lower jaw. Scale bar equals 3 cm for the CT sections.
We adopt the widely accepted phylogenetic hypothesis that Amphicyonidae consists of five subfamilies, Haplocyoninae, Temnocyoninae, Daphoeninae, Thaumastocyoninae, and Amphicyoninae (Hunt, 1998a; Morales et al., 2019), and belongs to Arctoidea (e.g., Jiangzuo et al., 2018; Morales et al., 2021a), which also comprises Ursidae, Pinnipedia, and Musteloidea; Cephalogalini and Phoberocyonini belong to Hemicyoninae, and the latter is a subfamily of Ursidae (Bonis, 2013).
The methods of dental and mandibular measurements follow Peigné and Heizmann (2003), Yang et al. (2005), and Xia et al. (2005) (Tables 1, 2). The specimen was CT scanned using the GE phoenix v|tome|x m 300/180 KV housed at the Key Laboratory of Vertebrate Evolution and Human Origins of the Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, with a beam energy of 150 kV and flux of 130 μA. The CT data are available in the MorphoSource: https://www.morphosource.org/concern/media/000495496. This published work and the nomenclatural acts it contains have been registered in ZooBank: https://zoobank.org/References/878ad9f1-66de-41be-9384-db339a130560.
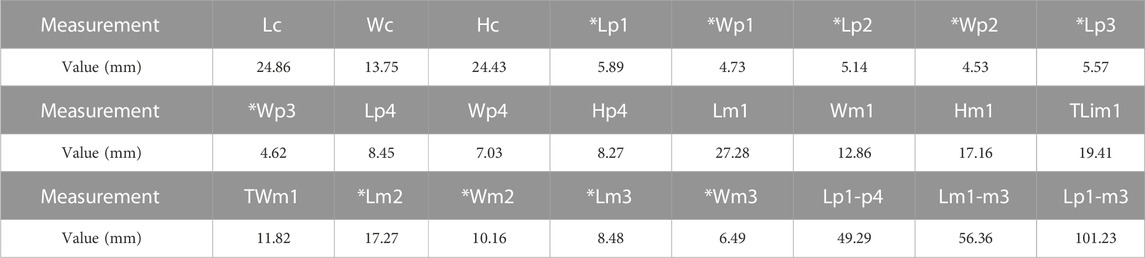
TABLE 1. Measurements of the lower teeth of L. qiui gen. et sp. nov. (IVPP V 28616) (mm). Abbreviations: L, length; W, width; H, height; TLi, trigonid lingual length; TW, talonid width; *, measured from alveoli or root.

TABLE 2. Measurements of the mandible of L. qiui gen. et sp. nov. (IVPP V 28616) (mm). Abbreviations: HVR, height of the vertical ramus (from the apex of the coronoid to the most ventral point of the angular process); MHVR, middle height of the vertical ramus (from the deepest point of the mandibular notch to the most ventral point of the angular process); LM, length of the mandible; MAT, moment arm of the temporalis muscle; Cm3, distance between the condyle and the posterior margin of m3; DMm3, depth of the mandible on the posterior margin of m3; WMm3, width of the mandible on the posterior margin of m3.
We use the beam theory to study the mandibular force profiles of the new specimen. This method is improved and explained in detail by Therrien (2005) in order to estimate the dorsoventral and labiolingual buttress of the mandibles in carnivorans and to reconstruct their feeding behaviors. The data from the new holotype specimens, Ysengrinia tolosana (IVPP FV 0086) and Ursus arctos (IVPP OV 2103), are from first-hand measurements, and other comparative data are derived from Therrien (2005) (Panthera leo, Crocuta crocuta, and Canis lupus), Hunt (2011) (Temnocyon macrogenys, Delotrochanter oryktes, and Borocyon robustum), and Morales et al. (2021a) (Ammitocyon kainos and Magericyon anceps).
3 Systematic paleontology
Order—Carnivora Bowdich, 1821
Infraorder—Arctoidea Flower, 1869
Lonchocyon gen. nov.
Type species—Lonchocyon qiui gen. et sp. nov.
Included species—Only the type species.
Etymology— “Lonch” is the Greek term for “spear”, indicating its spear-like paraconid of the lower carnassial; “cyon” is the Greek word for dog.
Diagnosis—As for the type and only species.
Lonchocyon qiui gen. et sp. nov.
Holotype—IVPP V 28616, a nearly complete left mandible with the canine, p4, m1, root of p3, and alveoli of i1-3, p1-2, and m2-3.
Etymology—Named in honor of Professor Zhan-Xiang Qiu for his great contributions to our knowledge of carnivoran evolution and systematics.
Type locality and horizon—Haerhada, Baiyin Obo Sumu, Siziwang Qi (Banner), Inner Mongolia, China; Baron Sog Formation; the late Eocene.
Diagnosis—Differs from amphicyonids and early ursids by the combination of a large size with a deep mandible, extremely reduced premolars including p4, sectorial lower carnassial (m1) with a spear-like paraconid, a cristid extending from the apex the paraconid to the carnassial notch, a basined talonid, and unreduced molars posterior to the carnassial.
3.1 Description
The left mandible is overall well-preserved with only slight breakage near the incisor region, the dorsal border of the coronoid process, and the angular process. The mandibular symphysis is rough with interdigitating rugosities, which are anteroposteriorly aligned in the dorsal half and more radial on the more rugose posteroventral part. The posterodorsal corner of the symphysis is relatively smooth compared to the remaining dorsal part. The greatest anteroposterior length of the symphysis is ∼63 mm, and the greatest dorsoventral height is ∼46 mm. The long axis of the symphysis forms an angle of ∼45° with the alveolar border, measuring ∼72 mm of its length. The posterior margin of the symphysis is at the anteroposterior level of p3.
The horizontal ramus is very deep dorsoventrally, gradually becoming deeper from p1 to m3, then its ventral border rises into a small, distinct, and medially projecting marginal process, which is for the insertion of the digastric muscle. There are two mandibular foramina: the larger anterior foramen is ventral to the diastema between p2 and p3 and positioned at the dorsoventral level near the dorsal one-third of the mandibular height, and the smaller posterior foramen is ventral to the anterior border of p4 and slightly ventral to the anterior foramen. There is a rudimentary shallow premasseteric fossa anterior to the marginal process and likely extending ventral to m1 (Figure 3C). The masseteric fossa is deep and extensive for the insertion of the middle and deep layers of the masseter muscle, and its anterior part does not reach the level of m3.
The coronoid crest is inclined posteriorly and broadens in its distal half. The mandibular notch is wide and shallow, gently curved from the coronoid process to the condyloid process. The coronoid process is high and broad, overhanging the anterior border of the condyloid process. The condyloid process, positioned level with the apex of the m1 protoconid, is very robust and composed of two parts with the long axis inclined slightly medially (Figure 3B). The lateral half of the condyloid process is roughly conical, bluntly pointed laterally, and buttressed ventrally with the articular facet facing more dorsally than posteriorly. However, its medial half is semi-cylindrical with a truncated medial border that is buttressed anteriorly and with the articular facet facing posteriorly and extended to the ventral side. The articular facets of the condyloid process are convex dorsoventrally and divided by a distinct synovial fossa on the dorsal side. Although the angular process is incomplete, it is projected posteriorly and separated from the marginal process by a wide, shallow indentation. A rough, narrow triangular depression is present along the lateral side of the indentation. The margin of the mandibular foramen is partially cracked, and it is positioned slightly ventral to the condyloid process.
The incisors are not present, but three alveoli are preserved. These incisor alveoli indicate that i1-i2 are much smaller than i3, and the i1 alveolus is compressed strongly lateromedially (Figure 3A). The root of i2 is slightly smaller than that of i1, and it also is situated posteriorly and slightly labially. Therefore, i2 and i1 are aligned nearly longitudinally rather than transversely. The root of i3 is placed mostly labial to that of i1, is roughly triangular in outline, and is prominently larger than i1 and i2. The canine is large, robust, laterally compressed, and nearly erect with an oval outline in cross section, as in many arctoids. The apex of the canine is broken but was likely recurved distally.
The postcanine diastema between the canine and the most anterior alveolus is 9.73 mm in length. Posterior to the postcanine diastema, two closely placed with a 2.28 mm diastema and anteriorly inclined alveoli are interpreted as deriving from a single-rooted p1 and p2 rather than a double-rooted p2 with p1 absent (Figure 3D). Judging from the coalesced double-rooted p4 and the single-rooted p3, it is unlikely that the two alveoli belong to a double-rooted p2 with p1 absent and would contrast with the common characteristics of most carnivorans that premolars are enlarged posteriorly.
p3 has only one broken root preserved, which is separated from p2 and p4 by two diastemas with 8.29 mm and 9.84 mm length, respectively. p4 is complete and much reduced with two nearly coalesced roots (Figures 3C1, C2). The main cuspid of p4 is pointed and sharp with a flat lingual surface and a convex buccal surface. Both the anterior and posterior crests of p4 are straight and distinct, and the former is anterolingually extended, while the latter is posteriorly directed. A cingulid-like basin is present along the lingual and posterior sides of the crown with a swollen shelf on the posterior side. There is no accessory cuspid or cingulid cuspid on p4.
m1 is very large with a high trigonid. The paraconid is spear-like and composed of three facets, which are bordered by three ridges descending from the apex of the paraconid: an anterior cristid is slanted posteriorly and extends slightly lingually down to the base of the crown; a shorter lingual cristid descends to the notch between the paraconid and protoconid on the lingual side; and third, the posterior cristid forms the anterior half of the carnassial blade. The paraconid is composed of a buccal convex face and anterolingual and posterolingual flat facets. The protoconid is the highest cuspid of m1 but is smaller than the paraconid in a buccal view. The preprotocristid extends anterobuccally and forms the posterior half of the carnassial blade. A slightly worn facet is present along the buccal edge of the blade. The notch between the two cristids of the carnassial blade forms a near-right angle in the buccal view. Another blunt, indistinct ridge descends anterolingually from the protoconid to the notch between the paraconid and protoconid on the lingual side, forming a deep V-shaped notch with the lingual cristid of the paraconid. Therefore, a flat anterior face is present between the two cristids of the protoconid. The paraconid and the protoconid are separated by a distinct groove on the occlusal and lingual sides. The enamel of the lingual surface of the metaconid is partially broken. The much smaller metaconid is about half the height of the protoconid (but nearly as high as the paraconid), positioned posterolingually to the protoconid, and does not surpass the protoconid posteriorly in buccal view. The posterior surface of the trigonid is nearly vertical or slightly anteriorly slanted. The talonid is short, but not very low, and slightly narrower than the trigonid. The buccal surface of the talonid is lingually inclined, while the lingual surface of the talonid is vertical. The hypoconid crest is slightly anterolingually extended and lies in the middle of the talonid. The entoconid crest is slightly lower than the hypoconid crest, and the two crests join on the posterior side. Therefore, the talonid forms a loop on the occlusal surface. The boundary between the trigonid and talonid is demarcated by a distinct groove, which is continuous on the occlusal and buccal surfaces. There is no cingulid on m1.
The alveolus of m2 is relatively large and composed of two equal-sized portions, indicating m2 is double-rooted and not reduced. The alveolus of m2 is oriented slightly obliquely rather than perpendicularly to the long axis of the crown. The alveolus of m3 is much smaller than that of m2 and oval in outline, indicating m3 is single rooted. Furthermore, the size of the molars becomes smaller posteriorly from m1 to m3. The long axis of the molar series is slightly anterobuccally extended, whereas that of the premolar series is slightly anterolingually directed. Therefore, the angle between the two axes is about 160°.
3.2 Comparison and discussion
The most conspicuous characteristics of the late Eocene Lonchocyon are the spaced, highly reduced premolars with single-rooted p1-3, and the two fused roots of p4. Among the five subfamilies of Amphicyonidae (i.e., Haplocyoninae, Temnocyoninae, Daphoeninae, Thaumastocyoninae, and Amphicyoninae), Haplocyoninae and Temnocyoninae share a synapomorphy of developed premolars as sister groups (Hunt, 2011), and the North American endemic Daphoeninae has unreduced premolars. By contrast, both Thaumastocyoninae and Amphicyoninae tend to reduce the premolars, but the earliest members of these two subfamilies from the Paleogene still retain the primitive unreduced premolars, unlike the new specimen. For instance, the earliest thaumastocyonine Ysengrinia tolosana from MP 30 and the amphicyonine Cynodictis from the late Eocene have a well-developed p4 with a posterior accessory cuspid and a p3 with two roots, while Cynodictis has a much smaller size than Y. tolosana and the new specimen (Kuss, 1965; Bonis, 1978; Heizmann and Kordikova, 2000; Solé et al., 2021). The amphicyonine Pseudocynopsis and Cynelos from Quercy, France, have reduced, spaced premolars, but p2-4 retains two separate roots (Ginsburg, 1965; 1966; Kuss, 1965). The presence of a single root of p3 has been reported in the Late Miocene amphicyonine Magericyon. However, the latter lacks dp1/p1-p2 and has a double-rooted p4 (Peigné et al., 2008). Ursidae also exhibits a trend toward reduced premolars. However, only Pliocene and extant Ursus could have a single root in p1-3, which is even sometimes absent. Hemicyoninae and early members of Ursinae Ballusia and Ursavus have reduced, simple premolars, but p2-3 is double-rooted and p4 is relatively large (Qiu et al., 1985; Ginsburg and Morales, 1998; Qiu et al., 2014).
The p4 of Lonchocyon is characterized by its rather small size with two fused roots and a small posterior shelf, as well as the lack of a posterior accessory cuspid. The length ratio of p4 to m1 is 0.31 in Lonchocyon (Figure 4A), which is less than the ratio in Magericyon (0.38, 0.42), while the ratios in other genera of amphicyonines (0.44–0.67) and thaumastocyoninae (0.48–0.71) are usually much greater (Solé et al., 2022). The ratio of p4 to m1 length in the ursine Ballusia and Ursavus ranges from 0.44 to 0.61 (Qiu et al., 2014), and the ratio in Ursus minimus ranges from 0.45 to 0.59 (Baryshnikov and Lavrov, 2013). The p4 of Lonchocyon has no posterior accessory cuspid, which is in contrast to the Amphicynodontidae, hemicyonine Cephalogalini, Phoberocyon, and most Amphicyonidae with a distinct posterior accessory cuspid on p4 (Hunt, 1998b; Ginsburg and Morales, 1998). However, the posterior accessory cuspid on p4 also is reduced or even absent in some amphicyonids, such as Guangxicyon (Zhai et al., 2003), the amphicyonine Magericyon (Peigné et al., 2008), and the Pseudarctini, which comprises Pseudarctos, Ictiocyon, and Dehmicyon (Morales et al., 2021b). p4 has lost the posterior accessory cuspid in some derived Ursidae, including Hemicyon, Zaragocyon, Plithocyon, and Ursinae (Ginsburg and Morales, 1995; Ginsburg and Morales, 1998; Hunt, 1998b).
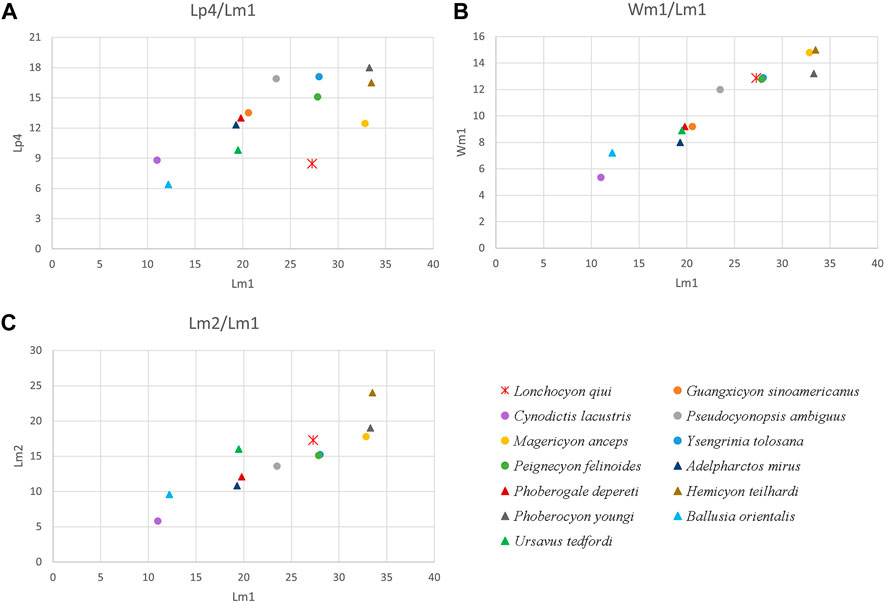
FIGURE 4. Scatter diagram of dental proportions in L. qiui gen. et sp. nov. (IVPP V 28616) and other compared arctoids: (A) length of p4 versus m1; (B) width versus length of m1; (C) length of m2 versus m1.
The m1 of Lonchocyon is characterized by its larger size (length = 27.28 mm) compared to contemporaneous Amphicyonidae and Hemicyoninae and by its large, spear-like paraconid (Figure 4B). The m1 paraconid of Lonchocyon is composed of three faces bordered by a slanted, anterior cristid, a lingual cristid, and a posterior cristid. The lingual cristid is present in some mustelids like extant Gulo gulo. By contrast, the lingual cristid is absent and the lingual surface of the paraconid is somewhat swollen in the compared Paleogene Amphicyonidae and hemicyonine Cephalogalini (Hunt, 1998a; Bonis, 2013). In addition, a faint, blunt ridge descends from the anterolingual surface of the protoconid on m1 of Lonchocyon, forming a triangular anterior surface of the protoconid with the preprotocristid. A similar anterior surface of the protoconid on m1 seems only present in the Oligocene Cephalogalini Adelpharctos, according to Bonis (1971). The metaconid of m1 is moderately reduced, nearly as high as the paraconid, and not retracted, relative to the protoconid in Lonchocyon, which is similar to the development of the m1 metaconid in early amphicyonine Cynodictis palmidens (Bonis, 1978), Pseudocyonopsis (Kuss, 1965), haplocyonine Haplocyon dombroskyi (Bonis, 1966), and thaumastocyonine Y. tolosana (Kuss, 1965). By contrast, the m1 metaconid is either relatively large in the daphoenine Daphoenus, Brachyrhynchocyon (Loomis, 1931; Scott and Jepsen, 1936), and haplocyonine Parhaplocyon (Bonis, 1966) or more reduced or even absent in the daphoenine Daphoenictis (Hunt, 1974), derived temnocyonines (Hunt, 2011), haplocyonine Haplocyon crucians, Haplocyonides (Hürzeler, 1940; Bonis, 1966), some derived amphicyonines like Magericyon (Peigné et al., 2008), and derived thaumastocyoninae (Morales et al., 2019; Morales et al., 2021a). The hemicyonine ursids are characterized usually by a retracted metaconid relative to the protoconid on m1, which is different from Amphicyonidae, Amphicynodontidae, and Lonchocyon. However, the m1 metaconid is not retracted in the hemicyonine cephalogalini Filholictis (Cirot and Bonis, 1992; Bonis, 2013). On the other hand, the m1 metaconid is slightly retracted in the haplocyonine Parhaplocyon (Bonis, 1966) and strongly retracted (but sometimes lost) in Gobicyon (Jiangzuo et al., 2019). The talonid of m1 in Lonchocyon is more similar to early ursids than to amphicyonids in being nearly as wide as the trigonid and forming a shallow basin with the almost equally developed hypoconid crest and entoconid crest joining posteriorly. By contrast, the m1 talonid is usually slightly narrower than the trigonid (sometimes wider; for instance, in Crassidia (Heizmann and Kordikova, 2000)) hypoconid (crest) dominates the talonid with a low entoconid (crest), and the talonid opens posteriorly in amphicyonids (Kuss, 1965; Hunt, 1998a; 2011; Morales et al., 2021b). However, the hemicyonine Cephalogalini differs from Lonchocyon by having a continuous lingual ridge of the talonid gently joining the metaconid without any notch and displaying a relatively wider basin of the talonid with a more buccally placed hypoconid crest (Bonis, 2013).
The ratio of m2 length to m1 length is 0.63 in Lonchocyon (Figure 4C), similar to the ratios in early thaumastocyonine Ysengrini (Crassidia and Ysengrinia) (0.58–0.62) but greater than the more derived Thaumastocyonini (0.37–0.54) (Solé et al., 2022). Furthermore, the talonid of m2 is shorter and narrower than the trigonid in the earliest thaumastocyonine Y. tolosana from the late Oligocene, but the talonid alveolus is slightly wider than the trigonid alveolus in Lonchocyon. Among the three tribes in Amphicyoninae, the ratio of m2 length to m1 length in Lonchocyon is close to those of Amphicyonini (0.63-0.71), Pseudocyon (0.6, 0.64), and Pseudarctini Dehmicyon schlosseria (0.59), greater than that of the Magericyonini Magericyon (0.45, 0.54) and lesser than that of the Pseudarctini Ictiocyon (0.72) and Pseudarctos (0.71) (Morales et al., 2021b; Solé et al., 2022). The early amphicyonine Pseudocyonopsis from the Oligocene to early Miocene has a similar ratio between m2 length to m1 length (0.59, 0.61) to Lonchocyon (Kuss, 1965). Among the Hemicyoninae, the ratio of m2 to m1 length in the Cephalogalini is the variable between 0.51 and 0.71 (Bonis, 2013), and the ratios of the early Hemicyon, H. gargan, and Zaragocyon (0.58–0.61) are similar to that of Lonchocyon (Ginsburg and Morales, 1995; Ginsburg and Morales, 1998).
The lower jaw of Lonchocyon is very large, with a deep horizontal ramus and a distinct marginal process, which are distinguished from relatively slender mandibles without marginal processes in early Amphicyonidae and Hemicyoninae (Scott and Jepsen, 1936; Ginsburg, 1966; Bonis, 2013). Few caniform taxa have a marginal process, except for Ursus, Ailuropoda (Davis, 1964), and some fossil ursids like Ursavus tedfordi (Qiu et al., 2009). The marginal process is the main insertion site for the digastric muscle in Ursus and Ailuropoda (Davis, 1964). This feature may not have many phylogenetic implications with the currently limited sample, but it does suggest that Lonchocyon likely had powerful digastric muscles, as in Ursus and Ailuropoda. In addition, the mandible of Lonchocyon has a shallow depression along the posterior ventral border of the horizontal ramus on the lateral surface, possibly suggesting a rudimentary premasseteric fossa (Figure 3C), which is more distinct in derived hemicyonines, some derived amphicyonids like Gobicyon and Ammitocyon, and some ursines (Frick, 1926; Hunt, 1998b; Jiangzuo et al., 2019; Morales et al., 2021a).
There are only two unequivocal amphicyonids with supporting detailed descriptions from the Eocene of Asia. One is a small indeterminate amphicyonid with a right M2 (for the second upper molar, rather than lower molar) (length: 6.35 mm; width: 8.5 mm) found in the Upper Eocene Ergilin Dzo Formation of Mongolia (Egi et al., 2009). Few comparisons can be made with our new specimen because of the lack of lower dentitions. However, the amphicyonid from the Ergilin Dzo Formation is much smaller than the specimen IVPP V 28616. Another is Guangxicyon sinoamericanus, an aberrant, short-jawed amphicyonid from the middle Eocene Nadu Formation of the Bose Basin, Guangxi Province, southern China (Zhai et al., 2003; Wang et al., 2019 for timescale). Lonchocyon differs from Guangxicyon in having a larger size (Guangxicyon m1 length and width are 20.6 mm and 9.2 mm, respectively), a deeper mandible, a single-rooted p3, a much-reduced p4, an m1 with more trenchant trigonid and a shallow-basined talonid, and a double-rooted m2. However, Guangxicyon is also very aberrant among amphicyonids in terms of its early development of brachygnathy with a single-rooted p2 and m2 instead of losing p1-3 or m2-3.
Only a few Cephalogalini have been discovered, with scarce materials in Paleogene Asia. A left M2 (the second upper molar) of Cephalogale sp. (IVPP V 12429) from the early Oligocene of Saint Jacques (Wang and Qiu, 2003) and a left ramal fragment with m2 of ?Cephalogale sp. (MAE SG.97.5396) from the early Oligocene Hsanda Gol Formation (Wang et al., 2005) are the only reported Asian Paleogene Cephalogalini. Our specimen can make few comparisons with them due to the lack of comparable materials, although the former is evidently larger than these Cephalogale.
In summary, Lonchocyon is unique due to its relatively large size, highly reduced premolars separated by diastemas, and spear-like paraconid on m1 compared to other Paleogene arctoids. The carnassial tooth and lower jaw show a combination of both Amphicyonidae and Hemicyoninae traits, with the trigonid having a reduced metaconid that is not retracted, which is similar to the former and having a shallowly basined talonid of m1 and a rudimentary premasseteric fossa, which are probably allied with both Amphicyonidae and Hemicyoninae. There is no doubt that Lonchocyon represents an early offshoot of Arctoidea, but its phylogenetic relationships among amphicyonids or early ursids remain unclarified since the discovery of additional complete material is pending.
4 Paleobiology
The robust canine, the sectorial trigonid of m1, and the deep mandible of Lonchocyon suggest its hypercarnivorous adaptations. This trend in adaptation evolved independently many times among all subfamilies of the Amphicyonidae, such as the Borocyon of Daphoencyoninae, Haplocyonoides of Haplocyoninae, some Temnocyon species of Temnocyoninae, the Magericyonini of Amphicyoninae, and all genera of Thaumastocyoninae (Hunt, 2011; Morales et al., 2019; Morales et al., 2021b). The shallow talonid basin of m1 indicates the presence of a plesiomorphic pattern in Lonchocyon, but other hypercarnivorous amphicyonids have hypoconid-dominant talonids. The hypercarnivorous trend also evolved in the Phoberocyon–Plithocyon clade of Hemicyoninae, or Phoberocyonini, which is distinguished from other hemicyonines by well-developed carnassials (P4 and m1) (Ginsburg and Morales, 1995). Inferred from the alveoli, the m2 of Lonchocyon is unreduced, which is a plesiomorphic trait, and different from Magericyonini and Thaumastocyoninae with their reduced m2, while the m2 of hypercarnivorous hemicyonines are also unreduced. The function of m2 is mainly crushing in carnivorans. Hypercarnivores tend to develop the shearing rather than crushing functions of their dentitions, at the same time, they often have a reduced m2. Additionally, all premolars are highly reduced including p4 in Lonchocyon, but many hypercarnivorous arctoids have a functional p4 without much reduction, except the Magericyonini of Amphicyoninae. The Magericyonini, consisting of Magericyon and possibly Pseudocyon, have reduced p4 as in Lonchocyon. The similarity between the reduced p4 of Lonchocyon and Magericyonini could be the result of parallel evolution, considering the younger Pseudocyon distributed from MN4 to MN7 and Magericyon distributed from MN9 to MN10. In conclusion, the hypercarnivorous adaptations of Lonchocyon are plesiomorphic and aberrant.
In order to investigate the paleobiology of Lonchocyon in a quantitative way, we use beam theory (Therrien, 2005) to study the mandibular force profiles of the new specimen. Some carnivorans with a similar mandibular length to that of Lonchocyon were selected and include the extinct amphicyonids Y. tolosana, Temnocyon macrogenys, B. robustum, D. oryktes, M. anceps, and A. kainos and the extant carnivorans C. lupus, U. arctos, P. leo, and Crocuta crocuta. We compared the dorsoventral mandibular force profiles (Zx/L), the labiolingual mandibular force profiles (Zy/L), and the relative mandibular force (Zx/Zy) of these carnivorans (Tables 3, 4, 5; Figure 5) to assess the mandibular function of Lonchocyon. Generally, a large Zx/L value indicates the ability to withstand high dorsoventral stresses, and a large Zy/L indicates high labiolingual and torsional stresses (Therrien, 2005).
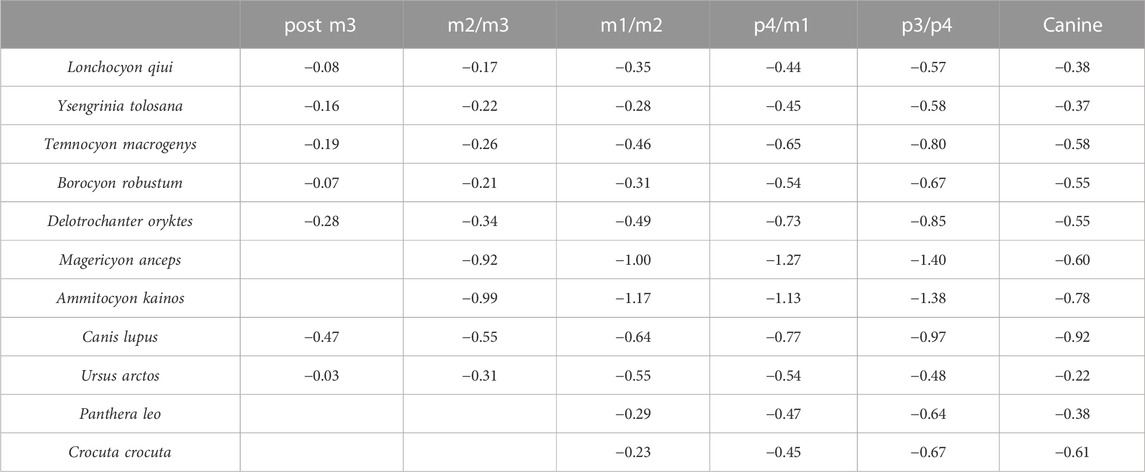
TABLE 3. Dorsoventral mandibular force (logZx/L) values of L. qiui and compared carnivorans along the mandible.
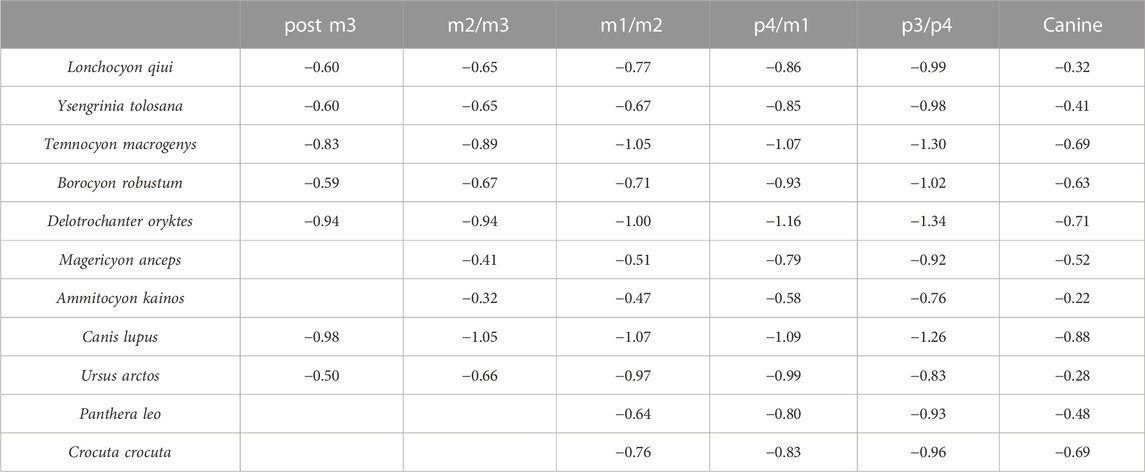
TABLE 4. Labiolingual mandibular force (logZy/L) values of L. qiui and compared carnivorans along the mandible.
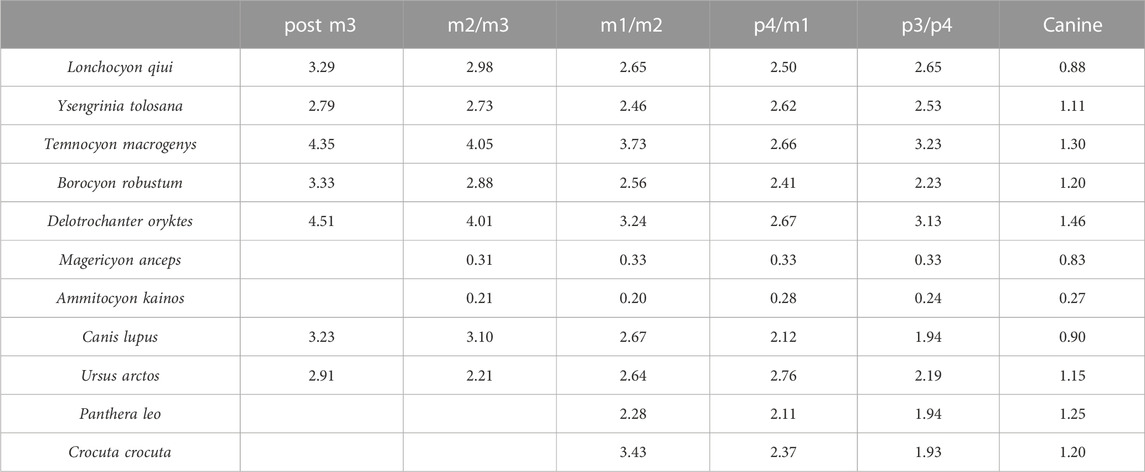
TABLE 5. Relative mandibular force (Zx/Zy) values of L. qiui and compared carnivorans along the mandible.
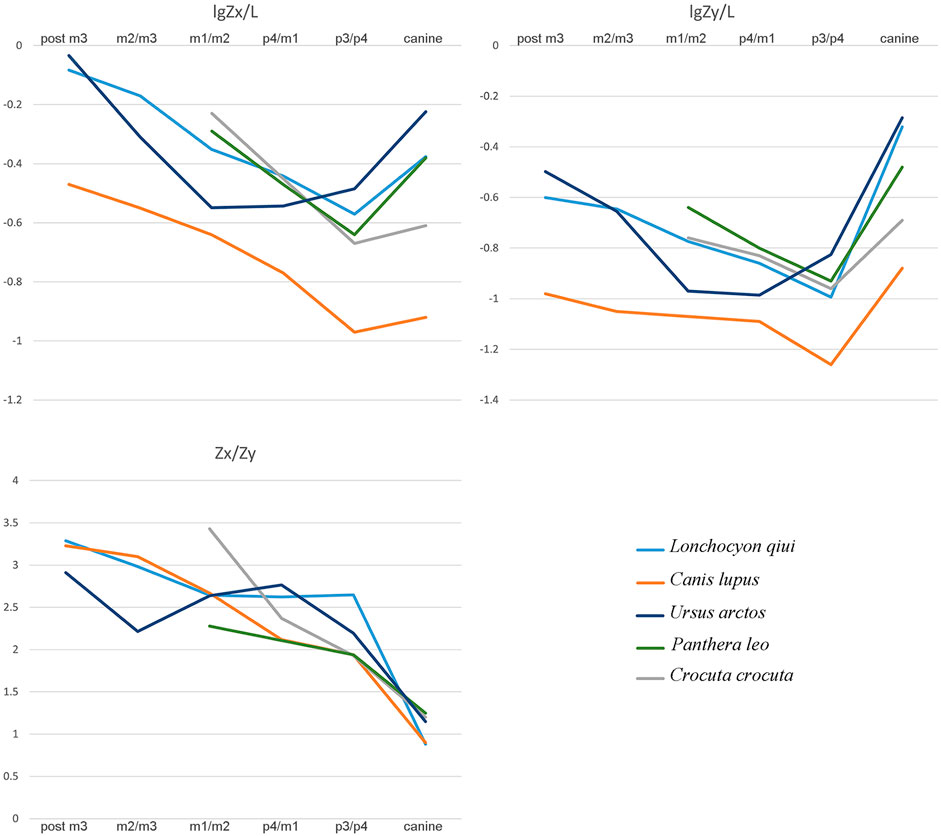
FIGURE 5. Comparison of mandibular force profiles among L. qiui, C. lupus, U. arctos, P. leo, and C. crocuta.
The dorsoventral mandibular force profiles (Zx/L) of Lonchocyon exhibit similar tendencies to canids and other amphicyonids (Table 3). The Zx/L values of Lonchocyon are similar to Y. tolosana and P. leo and are higher than most other amphicyonids compared at the same loci. The Zx/L values of the canine are higher than other taxa except Y. tolosana and U. arctos, reaching nearly the same value as P. leo, which suggests that the new taxon could deliver powerful canine bites to subdue prey as large felids do, rather than the rapid and shallow bites delivered by canids, which always hunt in packs. The Zx/L value after canine rises gradually along the horizontal ramus, which is similar to other carnivorans except U. arctos, with a decline from p3/p4 to m1/m2.
The labiolingual mandibular force profiles (Zy/L) of Lonchocyon also exhibit similarities with canids and other amphicyonids and are lower than the Zx/L values at the same loci (Table 4), as in most carnivorans, except for the value of the canine larger than Zx/L. The Zy/L values of Lonchocyon (except the canine) are similar to those of Y. tolosana, B. robustum, P. leo, and C. crocuta, lower than those of A. kainos and M. anceps and higher than those of other amphicyonids. The Zy/L value of the canine even surpasses P. leo and is lower than those of A. kainos and U. arctos. The extremely large value suggests that the new taxon could withstand huge labiolingual and torsional stresses while restraining prey with its powerful canine bites. The Zy/L values also rise steadily along the horizontal ramus posterior to the canine, similar to other carnivorans, except U. arctos.
In terms of the relative mandibular force (Zx/Zy) (Table 5), Lonchocyon exhibits a distinct difference with other caniforms which is its near plateau from p3/p4 to m1/m2, corresponding to its sectorial carnassial tooth and reduced premolars that the former is mainly used to slice meat with little need for withstanding extra buccolingual or torsional stresses. From the m1/2 boundary to the posterior side of m3, the Zx/Zy values exhibit a steep slope, indicating that the molars posterior to the carnassial possess the ability to crack hard objects as extant canids can, which needs to suffer more buccolingual stresses. In addition, the Zx/Zy value of Lonchocyon is lower than those in other amphicyonids except Y. tolosana and B. robustum.
According to the aforementioned analyses, Lonchocyon possesses a robust mandibular symphysis that would have facilitated the delivery of powerful canine bites while subduing the prey. After the probably functionless premolars and the meat-slicing carnassial, the posterior molars may have had the ability to crush certain hard materials. Specifically, Lonchocyon has a dentition with a combination of both shearing and crushing functions and likely occupied a special ecological niche as a predator consuming both flesh and hard objects. Considering its size, the new taxon likely fed primarily on prey animals of the same size or even larger than itself, as large felids are able to do today. It was also likely to have been a solitary hunter, different from extant canids, which have a pack-hunting lifestyle (Therrien, 2005).
5 Conclusion
Lonchocyon represents a specialized carnivoran from the late Eocene of Asia. The new specimen is the first arctoid discovered in the Erlian Basin and the first late Eocene arctoid from northern China. This large, deep-jaw arctoid has reduced premolars, a sectorial m1 with a cristid from the paraconid to the carnassial notch on the lingual side, an unretracted metaconid, a shallowly basined talonid, and unreduced m2-3. Lonchocyon shows a combination of morphologies present in both amphicyonids and the early ursid hemicyonines and represents an early offshoot of amphicyonids or hemicyonines in Asia. Furthermore, the analyses of the mandibular force profile indicate that Lonchocyon has primary hypercarnivorous characteristics with powerful canine bite, a sectorial carnassial tooth for slicing, and posterior molars that are able to process hard objects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Author contributions
X-YZ wrote the manuscript, analyzed the data, and prepared the figures; BB and Y-QW designed the research and improved and edited the manuscript.
Funding
This study was financially supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000) and the National Natural Science Foundation of China (42272011, 41572021, and 41672014).
Acknowledgments
The authors would like to thank Qian Li, Ran-Cheng Xu, Xiao-Yang Wang, Wei Zhou, Shi-Jie Li, Fu-Qiao Shi, Yan Li, Yong-Xing Wang, Yong-Fu Wang, and Qi Li for their assistance in the fieldwork; Zhan-Xiang Qiu, Zhao-Qun Zhang, Qi-Gao Jiangzuo, and Dan Lu for their helpful discussions; Zhan-Xiang Qiu for providing some important references; Shi-Jie Li for preparation of the specimen; Wei Gao for photography; and Ye-Mao Hou for CT scanning assistance. The authors are grateful to Thomas Stidham for his assistance with the English editing of an early draft of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bai, B., Wang, Y. Q., Li, Q., Wang, H. B., Mao, F. Y., Gong, Y. X., et al. (2018). Biostratigraphy and diversity of Paleogene perissodactyls from the Erlian Basin of inner Mongolia, China. Am. Mus. Novit. 3914, 1–60. doi:10.1206/3914.1
Baryshnikov, G. F., and Lavrov, A. V. (2013). Pliocene bear Ursus minimus Devèze de Chabriol et Bouillet, 1827 (Carnivora, Ursidae) in Russia and Kazakhstan. Russ. J. Theriol. 12, 107–118. doi:10.15298/rusjtheriol.12.2.07
Berkey, C. P., Granger, W., and Morris, F. K. (1929). Additional new formations in the later sediments of Mongolia. Am. Mus. Novit. 385, 1–12.
Bonis, L. D. (1966). Sur l'évolution du genre Haplocyon Schlosser (Carnivora). Bull. Soc. Géol. Fr. S7-VIII, 114–117. doi:10.2113/gssgfbull.S7-VIII.1.114
Bonis, L. de. (1971). Deux nouveaux carnassiers des Phosphorites du Quercy. Ann. Paléontol. 57, 117–127.
Bonis, L. D. (1978). La poche à phosphate de Ste-Neboule (Lot) et sa faune de vertébrés du Ludien supérieur. 12. – fissipèdes (Carnivores). Palaeovertebrata 8, 301–311.
Bonis, L. D. (2013). Ursidae (mammalia, Carnivora) from the late Oligocene of the “phosphorites du Quercy” (France) and a reappraisal of the genus Cephalogale geoffroy, 1862. Geodiversitas 35, 787–814. doi:10.5252/g2013n4a4
Cirot, E., and Bonis, L. de. (1992). Révision du genre Amphicynodon, carnivore de l'Oligocène. Palaeontogr. Abt. A 220, 103–130.
Davis, D. D. (1964). The giant panda: A morphological study of evolutionary mechanisms. Field. (Zool. Mem.) 3, 1–339.
Egi, N., Tsubamoto, T., and Tsogtbaatar, K. (2009). New amphicyonid (mammalia: Carnivora) from the upper Eocene Ergilin Dzo Formation, Mongolia. Paleontol. Res. 13, 245–249. doi:10.2517/1342-8144-13.3.245
Frick, C. (1926). The Hemicyoninae and an American tertiary bear. Bull. Am. Mus. Nat. Hist. 56, 1–119.
Ginsburg, L. (1965). L'"Amphycion" ambiguus des Phosphorites du Quercy. Bull. Mus. Natl. Hist. Nat. 37, 724–730.
Ginsburg, L., and Morales, J. (1998). Les Hemicyoninae (Ursidae, Carnivora, Mammalia) et les formes apparentées du Miocène inférieur et moyen d'Europe occidentale. Ann. Paléontol. 84, 71–123. doi:10.1016/S0753-3969(98)80003-7
Ginsburg, L., and Morales, J. (1995). Zaragocyon daamsi n. gen. sp. nov., Ursidae primitif du Miocène inférieur d'Espagne. Cr. Acad. Sci. Série 2. Sci. Terre Planèt. 321, 811–815.
Heizmann, E. P. J., and Kordikova, E. G. (2000). Zur systematischen Stellung von "Amphicyon" intermedius H. v. Meyer, 1849 (Carnivora, Amphicyonidae). Carolinea 58, 69–82.
Hunt, R. M. (1998a). “Amphicyonidae,” in Tertiary mammals of north America. Editors C. Janis, K. Scott, and L. Jacobs (London: Cambridge University Press), 196–227.
Hunt, R. M. (1974). Daphoenictis, a cat-like carnivore (mammalia, Amphicyonidae) from Oligocene of north America. J. Paleontol. 48, 1030–1047.
Hunt, R. M. (2011). Evolution of large carnivores during the mid-cenozoic of north America: The temnocyonine radiation (mammalia, Amphicyonidae). Bull. Am. Mus. Nat. Hist. 358, 1–153. doi:10.1206/358.1
Hunt, R. M. (1998b). “Ursidae,” in Tertiary mammals of north America. Editors C. Janis, K. Scott, and L. Jacobs (London: Cambridge University Press), 174–195.
Hürzeler, J. (1940). Haplocyonoides nov. gen., ein aberranter Canide aus dem Aquitanien des Hesslers (Mainzer Becken). Eclogae Geol. Helv. 33, 224–229.
Jiangzuo, Q. G., Li, C. X., Zhang, X. X., Wang, S. Q., Ye, J., and Li, Y. (2018). Diversity of Amphicyonidae (Carnivora, mammalia) in the middle Miocene halamagai formation in ulungur river area, xinjiang, northwestern China. Hist. Biol. 32, 187–202. doi:10.1080/08912963.2018.1477142
Jiangzuo, Q. G., Wang, S. Q., Li, C., Sun, D. H., Zhang, X. X., and O'Regan, H. (2019). New material of Gobicyon (Carnivora, Amphicyonidae, Haplocyoninae) from northern China and a review of aktaucyonini evolution. Pap. Palaeontol. 7, 307–327. doi:10.1002/spp2.1283
Kuss, S. E. (1965). Revision der europäischen Amphicyoninae (Canidae, Carnivora, Mammalia) ausschließlich der voroberstampischen Formen. Sitz. Heid. Akad. Wiss. 1, 1–168.
Loomis, F. B. (1931). A new Oligocene dog. Am. J. Sci. s5-22, 100–102. doi:10.2475/ajs.s5-22.128.100
Matthew, W. D., and Granger, W. (1925a). New mammals from the Irdin Manha Eocene of Mongolia. Am. Mus. Novit. 198, 1–10.
Matthew, W. D., and Granger, W. (1925b). New mammals from the shara Murun Eocene of Mongolia. Am. Mus. Novit. 196, 1–12.
Morales, J., Abella, J., Sanisidro, O., and Valenciano, A. (2021a). Ammitocyon kainos gen. et sp. nov., a chimerical amphicyonid (Mammalia, Carnivora) from the late Miocene carnivore traps of Cerro de los Batallones (Madrid, Spain). J. Syst. Palaeontol. 19, 393–415. doi:10.1080/14772019.2021.1910868
Morales, J., Fejfar, O., Heizmann, E., Wagner, J., and Abella, J. (2019). A new Thaumastocyoninae (Amphicyonidae, Carnivora) from the early Miocene of tuchořice, the Czech republic. Foss. Impr. 75, 397–411. doi:10.2478/if-2019-0025
Morales, J., Fejfar, O., Heizmann, E., Wagner, J., Valenciano Vaquero, A., and Abella, J. (2021b). The Amphicyoninae (Amphicyonidae, Carnivora, mammalia) of the early Miocene from tuchořice, the Czech republic. Foss. Impr. 77, 126–144. doi:10.37520/fi.2021.011
Peigné, S., and Heizmann, E. P. (2003). The Amphicyonidae (mammalia: Carnivora from ulm-westtangente (MN2, early Miocene), baden-württemberg, Germany-systematics and ecomorphology. Stuttg. Beitr. Nat. Ser. B. Geol. Paläontol. 54, 21–35.
Peigné, S., Salesa, M. J., Antón, M., and Morales, J. (2008). A new amphicyonine (Carnivora: Amphicyonidae) from the upper Miocene of batallones-1, madrid, Spain. Palaeontology 51, 943–965. doi:10.1111/j.1475-4983.2008.00788.x
Qiu, Z. X., Deng, T., and Wang, B. Y. (2014). A late Miocene Ursavus skull from guanghe, gansu, China. Vert. Palasiat 52, 1–9.
Qiu, Z. X., Deng, T., and Wang, B. Y. (2009). First bear material from dongxiang, gansu ---Addition to the longdan mammalian fauna (2). Vert. Palasiat. 47, 245–264.
Qiu, Z. X., Yan, D. F., and Jia, H. (1985). Dentition of the Ursavus skeleton from shanwang, shandong province. Vert. Palasiat. 23, 264–275.
Rose, K. D. (2006). The Beginning of the age of mammals. Baltimore: The John Hopkins University Press.
Russell, D. E., and Zhai, R. J. (1987). The Paleogene of Asia: Mammals and stratigraphy. Mem. Mus. Natl. Hist. Nat. Série C. Sci. Terre 52, 1–488.
Scott, W. B., and Jepsen, G. L. (1936). The mammalian fauna of the white river Oligocene: Part I. Insectivora and Carnivora. Trans. Am. Philos. Soc. 28, 1–153. doi:10.2307/1005507
Solé, F., Fischer, V., Denayer, J., Speijer, R., Fournier, M., Le Verger, K., et al. (2021). The upper Eocene-Oligocene carnivorous mammals from the Quercy Phosphorites (France) housed in Belgian collections. Geol. Belg. 24, 1–16. doi:10.20341/gb.2020.006
Solé, F., Lesport, J. F., Heitz, A., and Mennecart, B. (2022). A new gigantic carnivore (Carnivora, Amphicyonidae) from the late middle Miocene of France. PeerJ 10, e13457. doi:10.7717/peerj.13457
Szalay, F. S., and Gould, S. J. (1966). Asiatic mesonychidae (mammalia, condylarthra). Bull. Am. Mus. Nat. Hist. 132, 129–173.
Therrien, F. (2005). Mandibular force profiles of extant carnivorans and implications for the feeding behaviour of extinct predators. J. Zool. 267, 249–270. doi:10.1017/S0952836905007430
Wang, B. Y., and Meng, J. (2009). Ardynomys (cylindrontidae, rodentia) from nei mongol, China. Vert. Palasiat. 47, 240–244.
Wang, B. Y. (2003). Oligocene rodents from the nomogen (= nom khong) area of nei mongol, China, and comments on related stratigraphy. Vert. Palasiat. 41, 211–219.
Wang, B. Y., and Qiu, Z. X. (2003). Notes on early Oligocene ursids (Carnivora, mammalia) from Saint Jacques, nei mongol, China. Bull. Am. Mus. Nat. Hist. 22, 116–124. doi:10.1206/0003-0090(2003)279<0116:C>2.0.CO;2
Wang, X. M., McKenna, M. C., and Dashzeveg, D. (2005). Amphicticeps and amphicynodon (Arctoidea, Carnivora) from Hsanda Gol Formation, Central Mongolia and and phylogeny of basal arctoids with comments on zoogeography. Am. Mus. Novit. 3483, 1–57. doi:10.1206/0003-0082(2005)483[0001:AAAACF]2.0.CO;2
Wang, Y. Q., Li, Q., Bai, B., Jin, X., Mao, F. Y., and Meng, J. (2019). Paleogene integrative stratigraphy and timescale of China. Sci. China Earth Sci. 62, 287–309. doi:10.1007/s11430-018-9305-y
Wang, Y. Q., Meng, J., and Jin, X. (2012). Comments on Paleogene localities and stratigraphy in the Erlian Basin, nei mongol, China. Vert. Palasiat. 50, 181–203.
Wang, Y. Q., Meng, J., Ni, X. J., and Li, C. K. (2007). Major events of Paleogene mammal radiation in China. Geol. J. 42, 415–430. doi:10.1002/gj.1083
Xia, L., Yang, Q. S., Feng, Z. J., Quan, G. Q., and Ma, Y. (2005). A guide to the measurement of mammal skull Ⅱ: Perissodactyla, Artiodactyla and Carnivora. Chin. J. Zool. 40, 67–73. doi:10.13859/j.cjz.2005.06.012
Yang, Q. S., Xia, L., Ma, Y., Feng, Z. J., and Quan, Q. G. (2005). A guide to the measurement of mammal skull Ⅰ:Basic measurement. Chin. J. Zool. 40, 50–56. doi:10.13859/j.cjz.2005.03.011
Ye, J. (1983). Mammalian fauna from the late Eocene of ulan Shireh area, inner Mongolia. Vert. Palasiat. 21, 109–118.
Keywords: Erlian Basin, late Eocene, Amphicyonidae, Hemicyoninae, arctoid, mandibular force profile
Citation: Zhang X-Y, Bai B and Wang Y-Q (2023) Bear or bear-dog? An enigmatic arctoid carnivoran from the late Eocene of Asia. Front. Earth Sci. 11:1137891. doi: 10.3389/feart.2023.1137891
Received: 05 January 2023; Accepted: 10 February 2023;
Published: 02 March 2023.
Edited by:
Lucja A. Fostowicz-Frelik, Institute of Paleobiology, Polish Academy of Sciences, PolandReviewed by:
Mieczyslaw Wolsan, Museum and Institute of Zoology, Polish Academy of Sciences, PolandXiaoming Wang, Natural History Museum of Los Angeles County, United States
Copyright © 2023 Zhang, Bai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Bai, YmFpYmluQGl2cHAuYWMuY24=; Yuan-Qing Wang, d2FuZ3l1YW5xaW5nQGl2cHAuYWMuY24=
 Xin-Yue Zhang
Xin-Yue Zhang Bin Bai
Bin Bai Yuan-Qing Wang
Yuan-Qing Wang