- Connecticut College, New London, CT, United States
Horseshoe crabs (Limulus polyphemus) have been in decline in Long Island Sound, and recently there has been discussion of whether the state of Connecticut should stop issuing licenses for commercial harvesting. This paper argues that in spite of concerns about the living fossil concept, the fact that the horseshoe crabs are living fossils should count in favor of more stringent protection. The paper distinguishes four different views about the status of the living fossil concept: 1) eliminativism; 2) redefinition; 3) reframing; and 4) conceptual pluralism. Approaches 2–4 all treat the criteria associated with living fossils as picking out distinctive features of evolutionary history. Those distinctive features of evolutionary history link up with conservation values in several ways. More generally, drawing upon relevant work in environmental philosophy, it is argued that evolutionary history is relevant to aesthetic and environmental value. Moreover, eliminativists have trouble rendering intelligible a striking pattern in the recent scientific literature. Researchers undertaking conservation-relevant work frequently highlight the living fossil status of the taxa under study.
1 Introduction
The American horseshoe crab (Limulus polyphemus) appears as “vulnerable to extinction” on the International Union for the Conservation of Nature’s (IUCN) red list.1 In a comprehensive region-by-region assessment, Smith (2017) report that the American horseshoe crab is “vulnerable to local extirpation” (p. 135). Long Island Sound is one place where the horseshoe crab population has been in decline, largely due to overharvesting for use as bait (Beekey and Mattei 2015; Moritz 2022). The Connecticut Department of Energy and Environmental Protection recently issued stricter regulations on horseshoe crab harvesting (Connecticut Department of Energy and Environmental Protection, 2022). The new regulations shift the start date for the opening of the season from May 22 until 3 days after the first new or full Moon in May, among other changes. The rationale for this change is to prevent anyone from harvesting the animals when they come ashore to lay their eggs. The DEEP also reduced the daily harvest limit from 500 to 150 animals. This move fell short of a total ban on harvesting, which is what regional environmental activists and organizations such as the Connecticut Audubon Society had called for (Feral 2022). The Connecticut Audubon Society has taken an interest, in part, because migrating bird species, such as red knots, rely on horseshoe crab eggs as a food source during their stopover on Long Island Sound (Connecticut Audubon Society, 2020). Regionally, this is something of a classic conservation issue that pits some local commercial interests, especially the small number of people who hold commercial fishing licenses for horseshoe crabs in Connecticut, and to a lesser extent the biomedical industry, against advocates for biodiversity conservation. The biomedical industry uses limulus amebocyte lysate from their blood to test biomedical products for contamination by endotoxins (Gorman 2020).
Horseshoe crabs also happen to be a paradigm case of a living fossil taxon (Werth and Shear 2014). The family Limulidae contains four extant species, including L. polyphemus. Limulidae is well represented in the fossil record, with the oldest fossils possibly dating to the Carboniferous some 350 million years ago (mya). Other closely related members of the order Xiphosurida are even older. There are lots of fossils assigned to the family Limulidae from the Mesozoic (roughly 252-66 mya). And as a group, horseshoe crabs exhibit a good deal of morphological stability. They have survived multiple mass extinction events, including the “great dying” at the end of the Permian, some 252 mya. Intuitively, it is tempting to say that there is something very special about horseshoe crabs, that their distinctive evolutionary history should justify prioritizing them for conservation (Turner 2019a). This evolutionary history might provide reasons in support of stricter local conservation policies, such as the stricter harvesting limits recently adopted in Connecticut, or even an outright ban on harvesting in places where their numbers are in decline, such as Long Island Sound.
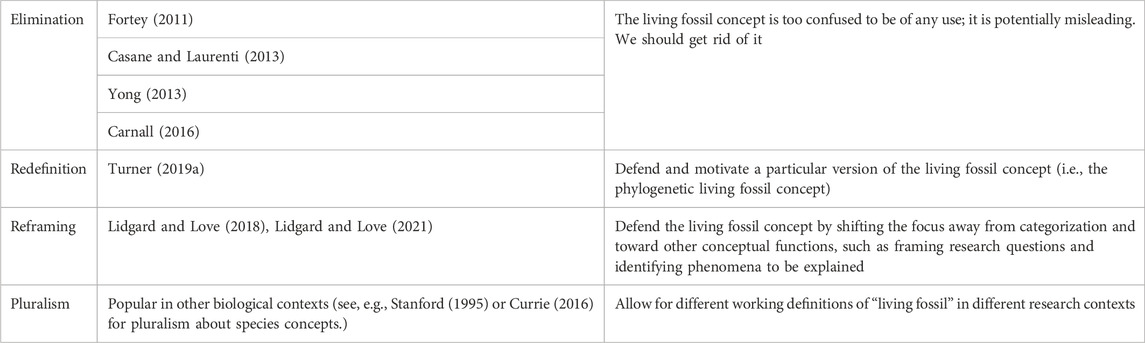
In what follows, we develop this line of argument in greater detail, while attending to recent discussions about the status of the living fossil concept. In the recent literature, there is a range of views regarding that concept’s value to evolutionary theory. A series of studies focusing on particular taxa has revealed some surprising things that seem to question their status as living fossil taxa. For example, cycads experienced a relatively recent evolutionary radiation (Nagalingum et al., 2011). New Zealand’s tuataras turned out to have a high rate of molecular evolutionary change (Hay et al., 2008). The status of coelacanths as living fossils has also been questioned (Casane and Laurenti 2013; Cavin and Guinot 2014). The status of tadpole shrimp has also been challenged (Mathers et al., 2013). Lidgard and Love (2018) observe that there are several different cross-cutting criteria in the literature (see Table 2) and that different researchers sometimes deploy these criteria in ways that generate conflicting verdicts about whether something should count as a living fossil.
To make matters worse, those criteria also exhibit some vagueness. Empirically, different taxa will exhibit different combinations of these features, and in varying degrees. Some researchers worry that the very idea of a living fossil has the potential to mislead, perhaps because it contributes to the mistaken impression that some lineages have not evolved. This confusing situation has generated a range of responses. On the one hand, some have called for retiring the concept of a living fossil altogether (e.g., Eldredge and Stanley, 1984; Fisher, 1984; Fortey 2011; Casane and Laurenti 2013; Yong 2013; Frazier, 2014; Carnall 2016). Lidgard and Love (2018), Lidgard and Love (2021) argue for a rethinking of the concept. According to their view, concepts have functions other than categorizing, and the living fossil concept has some usefulness as a tool for structuring research questions and highlighting phenomena to be explained. Watkins (2021) takes a different approach to sorting through this complexity, by examining what sorts of inferences might (or might not) be supported by a taxon’s living fossil status. Watkins argues that the concept has some epistemic usefulness. Meanwhile, Turner (2019a) has pursued a different sort of project, specifying a particular version of the living fossil concept—phylogenetic living fossils—and arguing that the concept, thus specified, is theoretically well-motivated. Phylogenetic living fossil taxa i) exhibit considerable morphological stability; ii) make a significant contribution to phylogenetic diversity; and iii) contain few extant species. Drawing on the work of others who see phylogenetic diversity as key to defining biodiversity (e.g., Lean and McLaurin 2016; Lean 2017), Turner also argues that the phylogenetic living fossil concept successfully picks out taxa that we should prioritize for conservation. Lidgard and Love (2021) also acknowledge that their view leaves room for conservation values to come into play in research on living fossils.
In this paper, we argue that one of the main reasons for retaining some version of the living fossil concept is normative. If we try to define “living fossil” in terms of some combination of the criteria listed in Table 2, then the living fossil concept will pick out taxa with distinctive evolutionary histories. And distinctiveness of evolutionary history is something that matters in conservation contexts, such as debate about horseshoe crab harvesting regulations. Turner (2019a) makes a version of this argument by defending a particular definition of “living fossil” and showing how that definition links up with aesthetic and environmental values. Here, we show how a more generalized version of that argument would go. Without defending any particular definition of “living fossil,” we suggest that the familiar criteria link up with environmental and conservation values in interesting ways.
In developing this argument, we lean heavily on the notion of a “distinctive evolutionary history.” There might be different ways of understanding what this means. For example, 1) a taxon with a distinctive evolutionary history might have a history that is atypical or highly unusual. Or 2) it might have a historical trajectory that is surprising in some way, given the default expectations of evolutionary theory. Or again, 3) it might just have a history that is very different from its near evolutionary relatives. On any of these accounts, evolutionary distinctiveness will vary in degree. In what follows, we will generally go with interpretation 1) and treat evolutionary distinctiveness as a matter of having an unusual or atypical evolutionary history. This reading dovetails with ideas about rarity, and with the thought that we might value some things more because they are rare (see Russow 1981 for one version of this).
2 The “living fossil concept”: Four projects
Our central claim is that the concept of a living fossil has a normative dimension, because living fossils (given any of the usual approaches to defining the term) have distinctive evolutionary histories that are relevant to debates about conservation. Nevertheless, the status of the living fossil concept is controversial. Table 1 shows a variety of different views that can be found in the current literature.
The relationships among these projects are complex. For example, reframers and redefiners both reject eliminativism, though for different reasons. There is also some tension between the redefinition and reframing projects (Lidgard and Love 2021). The redefinition project presupposes that one of the main things we want a living fossil concept to do is to sort taxa, or to delineate taxa that count as living fossils from those that do not. However, Lidgard and Love challenge the idea that the primary function of scientific concepts must be categorization; they contend that the living fossil concept should “play a role in representing broad investigative domains” (Smith et al., 2009, p. 762). They further elucidate that “the primary role of the (living fossil) concept is to mark out more precisely what requires explanation in a given instance for a particular entity in order to account for morphological and molecular stability or persistence over long periods of evolutionary time” (Lidgard and Love 2018, p. 763; see also Brigandt, 2003; Brigandt and Love 2012). Thus, proponents of reframing argue that both eliminativists and redefiners make the mistake of overemphasizing categorization.
At first glance, the redefinition project might seem committed to a form of conceptual monism. But that first impression is misleading: the redefinition project is compatible with pluralism. A proponent of redefinition, such as Turner (2019a), could argue that their favored version of the living fossil concept is especially useful in some contexts, but that in other research settings it might be appropriate to define ‘living fossil’ in other ways. The reframing project also meshes well with pluralism, though with one possible caveat. Many pluralists in other areas do assume that categorization is a central function of any concept. For example, pluralists about species concepts still think that one of the main jobs of a species concept is to supply grouping criteria, or to sort organisms into species. The reframing project sits uneasily with any version of pluralism that emphasizes categorization.
To complicate matters even further, even eliminativism might come in different forms. Here we are primarily interested in a version of eliminativism that some scientists have espoused. This view, roughly, is that we should stop using the living fossil concept because it is flawed or misleading in some irredeemable way. In other contexts, though, such as the species debate, philosophers have understood eliminativism differently. For example, Ereshefsky (1992) defends “eliminative pluralism” about species. He is, as it were, a pluralist about species concepts. What makes his view eliminativist is that he proposes that we stop talking about “species” in general and talk instead about “phylospecies,” “ecospecies,” “biospecies,” and so on: “Eliminate the term “species” and replace it with a plurality of more accurate terms” (1992, p. 681). One can imagine someone pursuing an analogous project with respect to living fossils. And eliminativism in Ereshefsky’s sense seems compatible with pluralism. The eliminativism we are focusing on, however, is more thoroughgoing. The eliminativists whom we have in mind doubt that there is any version of the living fossil concept that is useful or helpful.
For purposes of this paper, we will not dwell too much on the internal differences between redefiners, reframers, and pluralists. Sorting through those differences would require a wide detour into philosophical questions about the nature and functions of scientific concepts. All who favor retaining the living fossil concept are working with roughly the same set of criteria listed in Table 2 below. They differ only in how they approach that list of criteria. Redefiners pick out a subset of criteria and argue for that subset’s centrality and importance. Pluralists argue that scientists may legitimately use different subsets of criteria for different purposes. And reframers argue that we should shift our emphasis away from using the criteria to sort living fossils from non-living fossils, and use them instead to help guide research and frame questions about evolutionary processes. Importantly, everyone who favors retaining (some version of) the living fossil concept agrees about one thing: the criteria associated with the living fossil concept are picking out significant features of the history of some biological taxa. In this paper, we use this point of agreement to leverage an argument about the normativity of the living fossil concept.
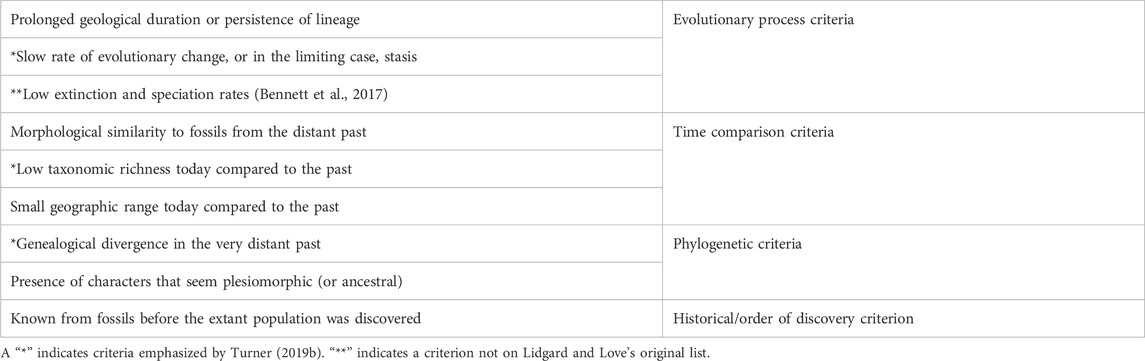
TABLE 2. Criteria for Counting as a Living Fossil, following Lidgard and Love (2018) (p. 761).
Watkins (2021) develops a project that does not fit easily into the categorization of Table 1. Her project might be compatible with redefinition, reframing, or pluralism. She focuses on one criterion that shows up in pretty much any account of living fossils—morphological similarity between extant taxa and extinct taxa from the deep past. Then she asks what this morphological similarity might serve as evidence for. Because morphological similarity does support some biological inferences, Watkins takes this as evidence that the living fossil concept has some epistemic utility.
With these distinctions in place, we’ll now turn to developing our main argument. In the next section, we argue that knowing something’s history is relevant to appreciating its value. This argument draws on more general ideas from environmental philosophy. Then, in Section 4, we apply this general principle concerning history and value to the case of living fossils. There we argue that the “living fossil” concept has normative force. The reason for this is that the criteria in Table 2 refer, whether directly or indirectly, to features that give living fossil taxa distinctive evolutionary histories. And having a distinctive evolutionary history is something we should value in conservation contexts.
3 The significance of historical knowledge to appreciation of values
In 2012, Starbucks abruptly changed the recipe for its Strawberries and Crème Frappucino drink (Fulton 2012). Someone had discovered that the chain was using cochineal as a food coloring, to give the frappucinos, as well as a few other menu items, their distinctive strawberry red color. Cochineal is a dye derived from a tiny scale insect, Dactylopius coccus, that lives on prickly pear cactus. The insects contain carminic acid, which can be extracted by crushing them, and then, with some preparation, used as a dye. Many Starbucks customers—especially vegans—were not happy to learn that the coloring in their strawberry frappucinos came from insects. Although some objected on principled vegan grounds, it seems likely that many people who regularly consume animal products were a bit put off by the thought of drinking something colored by dye derived from crushed bugs. Starbucks ended up replacing the cochineal ingredient with lycopene, a red food coloring derived from tomatoes.
This example illustrates a fairly mundane but important point: learning where your food came from can affect how you experience it. Knowledge of causal history makes a difference to our aesthetic engagement with things. Not only that, it affects our emotional responses, and it bears on our assessments of something’s value. This is reflected in the Starbucks business decision: people would not value—and would not pay for—a drink whose ingredients have a certain sort of causal history. This of course works both ways. People routinely pay more for some food because of what they think about where the food came from. For example, they might pay more for organic or locally grown food. In this and other ordinary contexts, history is closely connected with value. Or to put it a bit more precisely, most people think it is reasonable for knowledge of something’s history to bear on evaluative judgments.
This general point about causal history and value is a familiar one in environmental philosophy. For example, Robert Elliot (1982) used a thought experiment to make the point. Consider two paintings that are qualitatively indistinguishable: say, an original Vermeer, and a perfect forgery painted more recently by a skilled forger. The two paintings have different causal histories, and so we should value them differently. That does not necessarily mean that the forgery has no value; a perfect forgery might itself be a remarkable achievement. The point is that it would be a mistake to value that achievement in the same way that we value the original. Elliot uses this point in the service of an argument about restoration ecology. A restored ecosystem will never have the same value as the original one, because the two have different causal histories. More recently, the same issue has cropped up in discussions of the possible use of biotechnology to create ecological proxies for recently extinct species (Katz 2022; Turner 2022).
Turner (2019b) develops this point about history and value into a more general view that he calls historical cognitivism in aesthetics (see also (Currie 2021) for some critical discussion). Drawing on Allen Carlson’s scientific cognitivism in aesthetics (Carlson, 1977; 1981), he argues that:
Knowledge of living things and natural systems—including knowledge of the history of those things—deepens and enhances our aesthetic engagement with those things, relative to various kinds of naïve engagement (Turner 2019b, p. 20).
According to this view, having some knowledge, and in particular knowledge of causal history, better positions us to appreciate landscapes, natural systems, and living things. This is closely related to the point that something’s value depends in part on its causal history. If that is correct, then knowing something about that causal history better positions us to appreciate an item’s value. To revert to our example at the opening of this section: Imagine three people enjoying strawberry frappucinos, circa 2010. One happens to know that the red color of the beverage is derived from cochineal. The other falsely believes that the drink is red because it has strawberries mixed in. The third has no idea why the drink is red and has not given the issue any thought. Without denying that the third person might really enjoy the drink, historical cognitivism is the view that the first person—the one who understands the history of the ingredients—is best positioned to appreciate and engage with what they are drinking. This view implies that historical scientific knowledge generally has aesthetic payoff. This view also has implications for conservation biology, and for how we think about putative living fossil taxa, such as horseshoe crabs.
The idea that our interest in protecting biological diversity is largely, though not entirely, aesthetic is nothing new (Russow 1981; Sober 1986). If history matters to aesthetic value, and if a central aim of conservation biology is to protect things having aesthetic value, then evolutionary history is relevant to conservation biology. And taxa with especially distinctive evolutionary histories might be especially worth protecting. One thing that we might be doing when we refer to a taxon, such as horseshoe crabs, as a “living fossil” is signaling that it has a distinctive evolutionary history. That, in rough outline, is our argument.
The suggestion that evolutionary history is relevant to conservation biology is not new. One familiar proposal is that conservation biology’s aim should be to protect and promote biodiversity (e.g., Soulé 1985). Just how to clarify what we are aiming to protect is a difficult challenge (Sarkar 2005; MacLaurin and Sterelny 2008). At least one potential approach to this challenge emphasizes phylogenetic diversity (Winter et al., 2013; Lean and McLaurin 2016). Although there are disagreements about how exactly to measure phylogenetic diversity (see, e.g., Faith 1992; Vellend, et al., 2011), the rough idea is that we want to maximize coverage of the phylogenetic tree of life. To a first approximation, a species adds phylogenetic diversity to a system when it has higher evolutionary distinctness, or when it is less closely related to the other species in the system. On any account, degree of phylogenetic distinctness is a historical notion. This is not to suggest that phylogenetic diversity is the only or the best way to think about biodiversity. The point, rather, is a more modest one—namely, that one of the things we might reasonably care about in conservation biology is a distinctively historical feature of biological communities.
Some species add more phylogenetic diversity than others. “Genealogical divergence in the very distant past” is one of the criteria for living fossils that Lidgard and Love (2018) entertain, and it is a criterion that horseshoe crabs meet. Moreover, and anticipating some of the arguments of the next section, making an unusually large contribution to phylogenetic diversity is one way for a species to have a distinctive evolutionary history. The case of horseshoe crabs illustrates this nicely: we have to go back an unusually long way to find their nearest common ancestor with other extant groups—i.e., with spiders and scorpions (Ballesteros, et al., 2022). And that distance of phylogenetic relatedness is one potential ingredient of a distinctive evolutionary history.
In this section, based on the discussion of the role of historical knowledge in aesthetic appreciation (i.e., historical cognitivism), we have argued that causal historical knowledge of a species is relevant to its conservation. In the following section, we will show how most of the criteria for counting something a living fossil end up highlighting its distinctive evolutionary history. Then the two sections will jointly suggest that most definitions of a living fossil have a bearing on conservation biology.
4 Normative dimensions of the living fossil concept
To set the stage for our main argument, it is worth pausing to consider one of the central worries about the notion of “living fossil” expressed by at least one eliminativist. In his popular book, Horseshoe Crabs and Velvet Worms (2011), Richard Fortey worries that the term ‘living fossil’ has pejorative connotations:
“Living fossil” seems to imply a negative judgment somehow, as if the poor old organism was just about tottering along on its last legs, having hardly changed in tune with a changing world, awaiting an inevitable end. A similar misplaced judgmental tone is often applied to dinosaurs (Fortey 2011, p. 18).
Fortey himself does not endorse this negative judgment at all. He argues, rather, that horseshoe crabs are an evolutionary success story. They have survived mass extinction and are now well integrated into ecological systems. Thinking of them as throwbacks or as relics of a distant past might, if anything, lead us to underestimate the importance of protecting them today. Fortey observes, for example, that “millions of birds of many species depend on the horseshoe crabs’ eggs every year,” which is a reason why the Connecticut Audubon Society has taken an interest in harvesting regulations (2011, p. 18). But of course, horseshoe crabs long predated the evolution of birds.
One response to Fortey’s worry is to point out that even if it’s legitimate, it does not support eliminativism. He draws an analogy between the term “living fossil” and the negative associations that sometimes go along with calling something or someone a “dinosaur,” but no one thinks that the occasional pejorative use of “dinosaur” would be a reason for eschewing the term in scientific contexts. Still, although it does not do much to motivate eliminativism, Fortey’s observation highlights something important, which is that concepts such as “living fossil” have normative or evaluative dimensions. What is not clear is why we should think that the valence of the “living fossil” concept has to be negative. Insofar as the “living fossil” concept picks out biological taxa with distinctive evolutionary histories, it might, given plausible assumptions about the relationship between history and aesthetic value, also be picking out taxa that we should care about. Fortey is right about the normativity, but rather than rejecting the term “living fossil” on account of its negative connotations, another approach—and one in the spirit of much of the rest of Fortey’s discussion—might be to retain the term and rethink its normative valence.
Table 2 lists the criteria that Lidgard and Love (2018) associate with the living fossil concept, and which scientists routinely invoke in empirical research on potential living fossil taxa. It reorganizes the familiar criteria into several thematic clusters. Phylogenetic criteria have to do with evolutionary relationships. Time comparison criteria all involve comparisons of the present state of a taxon with its state at various points in the deep past. How similar does it look to its predecessors? Are there fewer species around today? Is its geographic range smaller today? Evolutionary process criteria focus on what we might loosely call the “staying power” of a lineage, as well as rates of evolutionary change, and/or low speciation and extinction rates. And finally, the last criterion focuses not on evolutionary history but on the more recent history of discovery. This table also highlights one feature of Turner’s (2019a) phylogenetic living fossil concept, which is that it includes a time comparison criterion, one process criterion, and one phylogenetic criterion. For present purposes, the more important observation is that nearly all of the criteria in Table 2 are directly historical.
The qualification “nearly all” has mainly to do with the criterion of morphological similarity, which needs a bit more discussion. That criterion is particularly central. For example, Watkins (2021) defines living fossil taxa in terms of the morphological similarity criterion alone, and then explores what sorts of inferences morphological similarity might license. One might think, though, that morphological similarity, taken all by itself, does not necessarily point to any distinctive evolutionary history. Different sorts of evolutionary processes, such as lineage persistence or evolutionary convergence, could in principle generate morphological similarity. However, one source of interest in morphological similarity between an extant species and fossils from the deep past is that it might serve as evidence of distinctive evolutionary history. With this in mind, it might be helpful to distinguish between direct vs. indirect criteria, with respect to evolutionary distinctiveness. Some of the criteria, such as “slow rate of evolutionary change,” are directly indicative of evolutionary distinctiveness. The morphological similarity criterion is only indirectly indicative, but still can (depending on circumstances, and in ways that Watkins 2021 explores) serve as evidence of distinctive evolutionary history.
Recall that one of the problems in the scientific literature is that different researchers sometimes deploy different criteria, yielding different verdicts about what does or does not count as a living fossil. Are cycads, for example, living fossils? That might depend on whether we emphasize the criterion of low taxonomic richness as compared with the past, or the criterion of morphological similarity to fossils from the distant past. Cycads might meet the latter criterion (Wang et al., 2009), but not the former (Nagalingum et al., 2011). However, most of the relevant criteria are historical. Not only that, but taken individually, the criteria pick out aspects of evolutionary history that are distinctive in the sense alluded to earlier—namely, in the sense that they are unusual or atypical. For example, we might be focusing on taxa with unusually low rates of evolutionary change, or on a taxon where the difference between its current geographic range and its range in the deeper past is atypically large. Different combinations of these criteria point to different ways in which evolutionary history can be distinctive. And if evolutionary history is relevant to determinations of value, then it will turn out that any deployment of these criteria will have potential evaluative implications, and potential relevance to conservation biology.
It is possible to apply this point to the case of horseshoe crabs without necessarily committing oneself to the redefinition, the reframing, or the pluralist projects. Whichever of those projects one sympathizes with, it will involve working with some combination of the criteria in Table 2. Horseshoe crabs clearly meet a number of those criteria, such as “genealogical divergence in the very distant past,” and “morphological similarity to fossils from the distant past,” among others. They may not meet all the criteria, such as “small geographic range today compared to the past.” Interestingly, though, the whole point of conservation measures such as harvesting regulations in Long Island Sound is to help make sure that they continue to thrive in all parts of their current geographic range. Conservation failures over time could result in the species meeting that geographic range criterion as well. Whichever of the projects (redefinition, reframing, pluralism) one sympathizes with the most, these criteria give us ways of highlighting horseshoe crabs’ distinctive evolutionary history. Our main suggestion is that this amounts to the same thing as highlighting reasons to protect them.
The different types of criteria in Table 2 link up with different kinds of environmental and aesthetic values. First, we saw earlier that phylogenetic criteria have a very close connection to the notion of phylogenetic diversity, and to the suggestion that phylogenetic diversity is one form of biodiversity that we should value. Some of the other criteria also link up with conservation goals, sometimes in obvious ways, and sometimes less directly. For a more obvious example, the issue of extinction rates is also of central concern in conservation contexts. For a less direct example, morphological disparity may also be something worth caring about in conservation contexts (MacLaurin and Sterelny 2008, Ch. 3). In some cases, living fossil taxa, such as horseshoe crabs, could enhance the disparity of the biological communities to which they belong, simply because their morphology is different from that of their closest evolutionary relatives. The loss of horseshoe crabs from a coastal ecosystem would mean the loss of a body design that’s rather different from anything else present in the system.
Some of the other criteria in Table 2 link up with values in still other ways. In her discussion of why we value “real old things,” Korsmeyer (2016) argues that “genuineness” is an aesthetic property of many old things. Although she focuses mainly on artefacts, her point arguably generalizes to include other things, such as fossils and other traces of the distant past. We appreciate the genuineness of old things because of the ways in which those things place us into contact with an irretrievable past. Visiting a historic home, for example, connects us in a way to the people who built the home, and to the events that transpired there. There is perhaps also a sense in which evolving biological lineages might count as “real old things.” A lineage that has exhibited staying power (“prolonged geological duration or persistence”) might, in a way, place us into connection with the deep past, and that might be something we value.
The historical/order of discovery criterion in Table 2 also deserves some reflection. Perhaps the classic example of a group that was known (at least to western science) from fossils before being discovered is the coelacanth (Weinberg 2001). The wollemi pine is another (Woodford 2000). The epistemic criterion is closely related to the notion of a “Lazarus taxon,” or a species that turns out to have existed long after it was thought to have gone extinct. At first glance, it might be rather difficult to see how this criterion might connect with conservation values. However, there are other fascinating cases where people have held out hope that a small population of a species thought to be extinct may still be “out there” in some under-explored ecological refuge. In some more extreme cases, such as that of the thylacine, amateurs and enthusiasts may seek to find evidence that the species is still hanging on (Jarvis 2018). But in cases of more recent extinctions, it may be an open biological and ecological question whether a small population persists. One interesting case in this connection is the search for a remaining population of the ivory-billed woodpecker (Jackson 2006; Rudkin et al., 2008). Reported sightings over the years have generated controversy, and the US Fish and Wildlife Service is currently conducting a review to determine whether to declare the ivory-billed woodpecker extinct. Living fossil taxa that meet the epistemic criterion have plausibly played a role in boosting many people’s hopes that other species might not really be extinct.
Let us now pause to take stock: We have argued that nearly all of the going criteria associated with the living fossil concept are historical, and that any combination of them will serve to pick out taxa with distinctive evolutionary histories. Not only that, but history is relevant to value: having a distinctive evolutionary history bears on a species’ aesthetic value. We’ve developed this argument in a more general way, by invoking Turner’s (2019b) historical cognitivism in aesthetics. We’ve also shown how to develop this argument in more specific ways, by focusing on specific criteria and how they connect with conservation values, and with Korsmeyer’s (2016) account of the value of “real old things.” Thus, regardless of which project one leans toward—the redefinition, reframing, or pluralist project—the living fossil concept turns out to have a significant normative dimension. And this normative dimension is quite the opposite of the negative connotations that Fortey (2011) was worried about. However we think about the conceptual and definitional issues, part of our interest in living fossils should have to do with conservation.
This result should be congenial to proponents of any of the projects outlined in Section 2, except eliminativism. The argument of this section generalizes Turner’s (2019a) point about the normative dimensions of the phylogenetic living fossil concept. Lidgard and Love also allow that “conservation priorities are a part (though not the whole) of the (living fossil) research program...” (Lidgard and Love 2021, p. 13). Indeed, the one project that is at odds with this argument is eliminativism. One of the functions of the living fossil concept is to serve as a normative tag: whether we favor redefinition, reframing, or pluralism, when we call a taxon a “living fossil,” one thing we are doing is highlighting its distinctive evolutionary history, and that its distinctive history has normative significance.
In ethics, it is common to define “thick concepts” as those having both descriptive and normative dimensions (see e.g., Williams 1985). One important source for subsequent work on thick concepts is Philippa Foot’s (1958) discussion of rudeness. To characterize someone’s behavior as rude is both to describe it and to assess it negatively. Many other concepts in ethics seem to have both empirical/descriptive and evaluative dimensions. For example, virtue concepts such as “courageous” or “generous” have both descriptive and normative content. Shockley (2012) suggests that certain concepts of environmental science, such as “ecological integrity,” could also qualify as thick concepts. Indeed, many concepts from the life and environmental sciences, ranging from ecological stability to biological diversity, would seem to have both descriptive and normative dimensions. Our suggestion here is that the living fossil concept belongs in this larger group of concepts that have both descriptive and evaluative content. In the next section, we argue that appreciating this can help make sense of a striking pattern in recent scientific research.
5 Conservation-focused research on living fossils
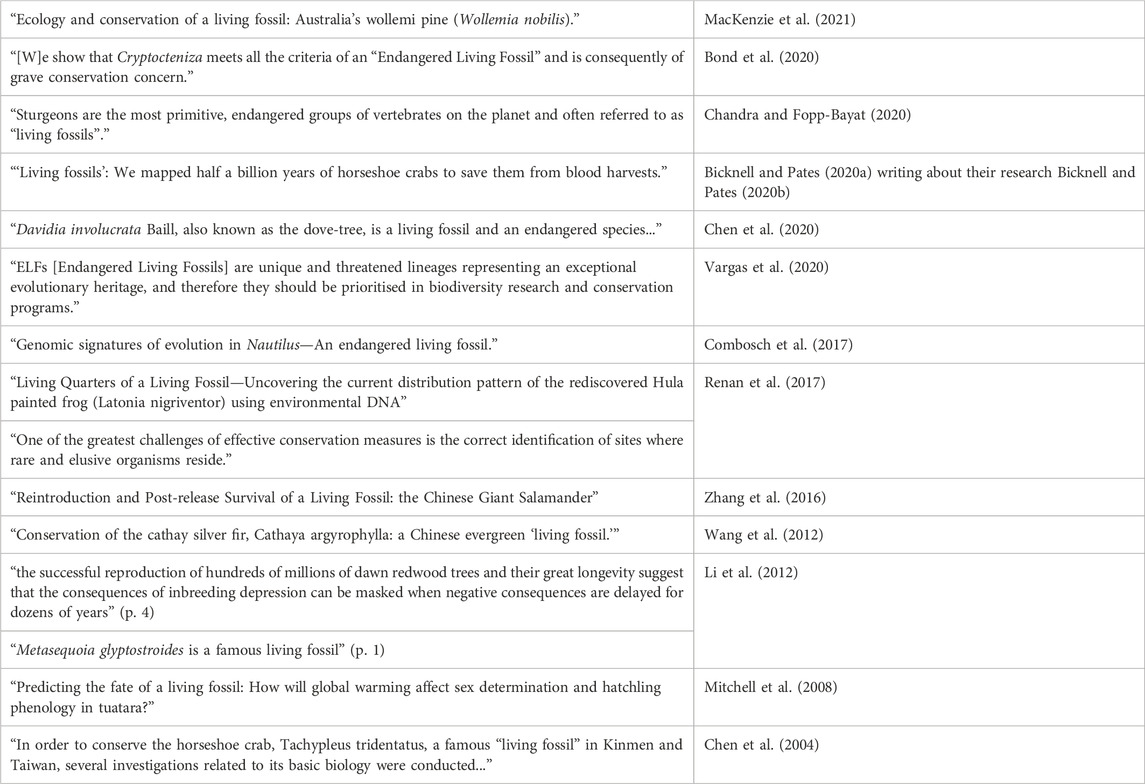
Another way to approach these issues is to note that in the scientific literature, one common practice is to highlight a taxon’s status as a living fossil while also emphasizing the need for conservation efforts. Scientists sometimes frame their research on this or that living fossil taxon as contributing in some way to conservation efforts. Many (though not all) of the traditionally paradigmatic living fossil taxa are threatened by human activities, and thus are foci of conservation interest. To illustrate this pattern in the scientific literature, Table 3 highlights some lines drawn from papers focusing on living fossil taxa.
Table 3 is not intended to be exhaustive, and it leaves out a lot of detail. The goal is simply to highlight a pattern of association between a taxon’s (alleged) living fossil status and its interestingness from a conservation perspective. In some of these cases, it remains controversial whether the taxon in question should be considered a living fossil at all. At best, Table 3 offers a superficial and incomplete look at some of the recent literature. There is, however, a phenomenon to be explained here: Why would researchers highlight a taxon’s (alleged) living fossil status in the context of conservation-relevant research? Our account of the normative dimension of the living fossil concept affords an explanation here. If the various criteria associated with being a living fossil do pick out taxa with distinctive evolutionary histories, and if this historical distinctiveness is relevant to their value, then it makes sense for researchers to highlight a taxon’s living fossil status as a way of motivating conservation-relevant research.
Eliminativists, by contrast, would have trouble making sense of the pattern in Table 3. If, as Fortey (2011) suggests, the term “living fossil” has negative connotations of a species that has outlived its time, then it is hard to see why anyone would want to highlight the living fossil status of taxa in conservation contexts. Similarly, if the worry is that the term is misleading, then it might seem to be especially important not to mislead anyone in conservation contests. In short, if eliminativism is correct, then we would have to reject the pattern of usage in Table 3 as confused or misguided, or else interpret the references to living fossils as a mere rhetorical or attention-getting ploy. Thus, eliminativism forces an uncharitable reading of a good deal of recent scientific research. An eliminativist could, of course, double down here: If the living fossil concept really is hopelessly confused and/or misleading, then perhaps a more critical stance toward this pattern of practice is appropriate. However, the argument developed in Sections 3 and 4 provides a rational reconstruction of this pattern of scientific practice. If the living fossil concept is picking out taxa with distinctive evolutionary histories, and if having a distinctive evolutionary history is something that matters in conservation contexts, then it makes sense for researchers to highlight the living fossil status of the taxa they are focusing on.
Generally speaking, where the question is whether scientists should continue to employ some concept vs. jettisoning it, we can approach that question using a version of cost-benefit analysis. What does (or could) that concept do for us? And what, if any, are the potential downsides of continuing to use it? Eliminativists tend to fixate on the downsides: the living fossil concept, they worry, is potentially misleading to non-specialist audiences. We acknowledge that this is a legitimate worry, and that some might be misled into thinking that living fossil taxa have somehow stopped evolving, but this worry is not one unique to the living fossil concept. Many scientific concepts are potentially misleading. And in this case, the conceptual benefits outweigh any potential downside risks. Whereas Watkins (2021) highlights potential epistemic benefits, and (Lidgard and Love, 2018; Lidgard and Love, 2021) highlight the concept’s usefulness in guiding investigation and structuring research questions, we have argued here that the living fossil concept also has an important normative role to play. Someone who is deeply committed to eliminativism here could read this paper as identifying some unacknowledged intellectual costs that eliminativists must pay.
While the view proposed here helps explain and make intelligible the pattern shown in Table 3, the view also has some limitations that are worth emphasizing. One limitation of our proposal is that it does not offer (and is not intended to offer) specific policy guidance. One question we have not addressed, for example, is how much weight to accord to a taxon’s living fossil status in conservation policy contexts, such as the debate about harvesting regulations for horseshoe crabs in Long Island Sound. We take the above argument to show that the horseshoe crabs’ distinctive evolutionary history—a history that we can flag by calling them “living fossils”—gives us extra reason to protect them. Thus, the argument lends some weight to proposals for more stringent protections, such as a ban on commercial harvesting in Connecticut. However, actual policy decisions involve complex weighting of many different values, including institutional, procedural, political, and environmental values, as well as empirical assessments of threats. Our suggestion here is only that the term “living fossil” can and should signal that certain historical and aesthetic values are in play, and that these might in some cases tip the balance toward conservation.
Our argument has focused on a fairly local case—that of the horseshoe crab (L. polyphemus) population in Long Island Sound. The species is not listed as endangered under the federal Endangered Species Act, and overall, the species is fairly abundant and has a large geographic range. The conservation challenges we have focused on are very local—i.e., how to reverse the decline in abundance in a particular region. One might wonder, then, what this line of argument means for the bigger picture of global biodiversity conservation. Does the argument have any implications for efforts to define ‘biodiversity’ in ways that capture things worth caring about? The project of defining and clarifying the value of biodiversity is a much larger one than we can take on here (See Sarkar 2005; MacLaurin and Sterelny 2008; Newman et al., 2017). Most of the research summarized in Table 3, however, is more localized in nature, focusing on this or that particular taxon. There is perhaps a natural affinity between our approach and efforts to define ‘biodiversity’ in terms of phylogenetic diversity (Lean 2017). However, contribution to phylogenetic diversity is just one of the criteria for living fossils. There is perhaps a better, more general way to state the upshot of the argument we have developed here. Part of our interest in biodiversity is historical. That means that general accounts of biodiversity should allow that one contributor to biodiversity is having a distinctive or unusual evolutionary history.
6 Conclusion
In this paper, we have sought to highlight the connection between the concept of a living fossil, on the one hand, and conservation values on the other. We began by distinguishing three different projects—redefinition, reframing, and pluralism—that are all committed (contra eliminativism) to retaining the living fossil concept. We then argued that on any of these projects, the living fossil concept will serve to pick out taxa having distinctive evolutionary histories, and/or to structure investigation of those histories. The reason for this is that those projects all rely on the criteria compiled in Table 2, and most of those criteria point, whether directly or indirectly, to features of evolutionary history. Next, we argued that causal history is relevant to aesthetic and environmental value. This second conceptual move relies on a historical cognitivist view in environmental aesthetics. Taken together, these two moves mean that the term “living fossil” has normative force, that it signals the presence of historical features that are relevant to conservation. Appreciating the normative dimension of the living fossil concept also helps explain a striking pattern of recent scientific practice: scientists routinely highlight the living fossil status of a taxon in the course of conservation-relevant research. Finally, the normative dimension of the living fossil concept bears on real conservation policy issues, such as the question of whether to prohibit commercial harvesting of horseshoe crabs in Connecticut.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
DT is the primary author. JH contributed research assistance and shared in the philosophical work. DT and JH co-authored the paper together, and the arguments of the paper are the result of their joint work and discussion. DT is responsible for any deficiencies in the argument.
Funding
This work was supported by a Research Matters grant from Connecticut College.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1The IUCN Red List entry for Limulus Polyphemus is available at https://www.iucnredlist.org/species/11987/80159830#green-assessment-information. Last accessed 24 Oct 2022
References
Ballesteros, J. A., Santibanez-Lopez, C. E., and Baker, C. M., (2022). Comprehensive species sampling and sophisticated algorithmic approaches refute the monophyly of arachnida. Mol. Biol. Evol. 39, 21. doi:10.1093/molbev/msac021
Beekey, M. A., and Mattei, J. H. (2015). The mismanagement of Limulus polyphemus in long Island Sound, USAWhat are the characteristics of a population in decline? in Changing global perspectives on horseshoe crab biology, conservation, and management. Editor R. H. Carmichael (Berlin, Germany: Springer).
Bennett, D. J., Sutton, M. D., and Turvey, S. T. (2017). Evolutionarily distinct “living fossils” require both lower speciation and lower extinction rates. Paleobiology 43, 34–48. doi:10.1017/pab.2016.36
Bicknell, R. D. C., and Pates, S. (2020b). Pictorial atlas of fossil and extant horseshoe crabs, with emphasis on Xiphosurida. Front. Earth Sci. 8, 98. doi:10.3389/feart.2020.00098
Bicknell, R. D. C., and Pates, S. (2020a). ‘Living fossils’: We mapped half a billion years of horseshoe crabs to save them from blood harvests. Australia: University of New England.
Bond, J. E., Hamilton, C. A., Godwin, R. L., Ledford, J. M., and Starrett, J. (2020). Phylogeny, evolution, and biogeography of the North American trapdoor spider family Euctenizidae (Araneae: Mygalomorphae) and the discovery of a new ‘endangered living fossil’ along California’s central coast. Insect Syst. Divers. 4, 5. doi:10.1093/isd/ixaa010
Brigandt, I. (2003). Species pluralism does not imply species eliminativism. Philosophy Sci. 70 (5), 1305–1316. doi:10.1086/377409
Brigandt, I., and Love, A. C. (2012). Conceptualizing evolutionary novelty: Moving beyond definitional debates. J. Exp. Zoology B Mol. Dev. Evol. 318, 417–427. doi:10.1002/jez.b.22461
Carlson, A. (1977). Appreciation and the natural environment. J. Aesthet. Art Crit. 37 (3), 267–275. doi:10.2307/430781
Carlson, A. (1981). Nature, aesthetic judgment, and objectivity. J. Aesthet. Art Crit. 40 (1), 15–28. doi:10.1111/1540_6245.jaac40.1.0015
Carnall, M. (2016). Let’s make living fossils extinct. Available at: https://www.theguardian.com/science/2016/jul/06/why-its-time-to-make-living-fossils-extinct (Retrieved Jan 27, 2018).
Casane, D., and Laurenti, P. (2013). Why coelacanths are not ‘living fossils’. BioEssays 35, 332–338. doi:10.1002/bies.201200145
Cavin, L., and Alvarez, N. (2022). Why coelacanths are almost living fossils. Front. Ecol. Evol. 2022, 896111. doi:10.3389/fevo.2022.896111
Cavin, L., and Guinot, G. (2014). Coelacanths as ‘almost living fossils’. Front. Ecol. Evol. 2, 1–5.
Chandra, G., and Fopp-Bayat, D. (2020). Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 13 (1), 119–137. doi:10.1111/raq.12466
Chen, C. P., Yeh, H. Y., and Lin, P. F. (2004). Conservation of the horseshoe crab at kinmen, taiwan: Strategies and practices. Biodivers. Conservation 13, 1889–1904. doi:10.1023/b:bioc.0000035868.11083.84
Chen, Y., Ma, T., Zhang, L., Kang, M., Zhang, Z., Zheng, Z., et al. (2020). Genomic analyses of a “Living Fossil”: The endangered dove-tree. Mol. Ecol. Resour. 20 (3), 756–769. doi:10.1111/1755-0998.13138
Combosch, D. J., Lemer, S., Ward, P. D., Landman, N. H., and Giribet, G. (2017). Genomic signatures of evolution in nautilus—an endangered living fossil. Mol. Ecol. 26, 5923–5938. doi:10.1111/mec.14344
Connecticut Audubon Society (2020). Coalition forms to save the American horseshoe crab. Available at: https://www.ctaudubon.org/2020/06/coalition-forms-to-save-the-american-horseshoe-crab/(Accessed June 11, 2020).
Connecticut Department of Energy and Environmental Protection (2022). Notice to horseshoe crab fishermen. DEEP Marine Fisheries News Available at: https://portal.ct.gov/-/media/DEEP/fishing/NoticesFishermen/N22-22_2022-05-12-Notice-to-Horseshoe-Crab-Fishermen.pdf (Accessed May 12, 2022).
Currie, A. M. (2021). Epistemic engagement, aesthetic value, and scientific practice. Br. J. Philosophy Sci. 2020, 714802. doi:10.1086/714802
Currie, A. M. (2016). The mystery of the Triceratops’s mother: How to be a realist about the species category. Erkenntnis 4, 795–816. doi:10.1007/s10670-015-9769-3
Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. doi:10.1016/0006-3207(92)91201-3
Feral, P. (2022). Opinion: Let’s ban the slaughter of horseshoe crabs. CT Post Available at: https://www.ctpost.com/opinion/article/Opinion-Let-s-ban-the-slaughter-of-horseshoe-16975023.php (Accessed March 02, 2022).
Fisher, D. C. (1984). “The Xiphosurida: Archetypes of bradytely?,” in Living fossils. Editors N. Eldredge, and S. M. Stanley (New York: Springer), 196–213.:
Fortey, R. (2011). Horseshoe crabs and velvet worms: The story of the animals and plants that time has left behind. New York: Random House/Vintage Books.
Gorman, R. (2020). Atlantic horseshoe crabs and endotoxin testing: Perspectives on alternatives, sustainable methods, and the 3Rs (replacement, reduction, and refinement). Front. Mar. Sci. 7, 582132. doi:10.3389/fmars.2020.582132
Hay, J. M., Subramanian, S., Millar, C. D., Mohandesan, E., and Lambert, D. M. (2008). Rapid molecular evolution in a living fossil. Trends Genet. 24 (3), 106–109. doi:10.1016/j.tig.2007.12.002
Isaac, N. J. B., Turvey, S. T., Collen, B., Waterman, C., and Baillie, J. E. M. (2007). Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2, e296. doi:10.1371/journal.pone.0000296
Jackson, J. A. (2006). Ivory-billed woodpecker (Campephilus principalis): Hope, and the interfaces of science, conservation, and politics. Auk 123 (1), 1–15. doi:10.1642/0004-8038(2006)123[0001:iwcpha]2.0.co;2
Jarvis, B. (2018). The obsessive search for the Tasmanian Tiger. Available at: https://www.newyorker.com/magazine/2018/07/02/the-obsessive-search-for-the-tasmanian-tiger (Accessed June 25, 2018).
Katz, E. (2022). Considering de-extinction: Zombie arguments and the walking (and flying and swimming) dead. Ethics, Policy, Environ. 25 (2), 81–103. doi:10.1080/21550085.2022.2071550
Lean, C. H. (2017). Biodiversity realism: Preserving the tree of life. Biol. Philosophy 32, 1083–1103. doi:10.1007/s10539-017-9592-0
Lean, C., and McLaurin, J. (2016). “The value of phylogenetic diversity,” in Biodiversity conservation and phylogenetic systematics. Editors R. Pellens, and Grandcolas P (Berlin, Germany: Springer), 19–30.
Li, Y.-Y., Tsang, E. P. K., Cui, M.-Y., and Chen, X.-Y. (2012). Too early to call it success: An evaluation of the natural regeneration of the endangered Metasequoia glyptostroboides. Biol. Conserv. 150 (1), 1–4. doi:10.1016/j.biocon.2012.02.020
Lidgard, S., and Love, A. C. (2018). Rethinking living fossils. BioScience 68 (10), 760–770. doi:10.1093/biosci/biy084
Lidgard, S., and Love, A. C. (2021). The living fossil concept: Reply to turner. Biol. Philosophy 36 (2), 13–16. doi:10.1007/s10539-021-09789-z
Mackenzie, B. D. E., Clarke, S. W., Zimmer, H. C., Liew, E. C. Y., Phelan, M. T., Offord, C. A., et al. (2021). Ecology and conservation of a living fossil: Australia's wollemi pine (wollemia nobilis). Amsterdam, Netherlands: Elsevier. doi:10.1016/B978-0-12-821139-7.00188-4
MacLaurin, J., and Sterelny, K. (2008). What is Biodiversity? Chicago, IL: University of Chicago Press.
Mathers, T. C., Hammond, R. L., Jenner, R. A., Hänfling, B., and Gómez, A. (2013). Multiple global radiations in tadpole shrimps challenge the concept of “living fossils. PeerJ 1, e62. doi:10.7717/peerj.62
Mitchell, N. J., Kearney, M. R., Nelson, N. J., and Porter, W. P. (2008). Predicting the fate of a living fossil: How will global warming affect sex determination in hatchling phenology in tuatara. Proc. R. Soc. B Biol. Sci. 275, 1648. doi:10.1098/rspb.2008.0438
Moritz, J. (2022). Horseshoe crabs face “ecological extinction” in Long Island Sound, driven by harvest and habitat loss. Available at: https://www.ctinsider.com/hartford/article/Horseshoe-crabs-face-ecological-extinction-17250285.php (Accessed June 18, 2022).
Nagalingum, N. S., Marshall, C. R., Quental, T. B., Rai, H. S., Little, D. P., and Mathews, S. (2011). Recent synchronous radiation of a living fossil. Science 334, 796–799. doi:10.1126/science.1209926
Newman, J. A., Varner, G., and Linquist, S. (2017). Defending biodiversity: Environmental science and ethics. Cambridge: Cambridge University Press.
Renan, S., Gafny, S., Perl, R. G. B., Roll, U., Malka, Y., Vences, M., et al. (2017). Living quarters of a living fossil—uncovering the current distribution pattern of the rediscovered hula painted frog (Latonia nigriventer) using environmental DNA. Mol. Ecol. 26, 6801–6812. doi:10.1111/mec.14420
Rudkin, D. M., Young, G. A., and Nowlan, G. S. (2008). The oldest horseshoe crab: A new xiphosurid from late ordovician konservat-lagerstätten deposits, manitoba, Canada. Palaeontology 51, 1–9. doi:10.1111/j.1475-4983.2007.00746.x
Russow, L.-M. (1981). Why do species matter? Environ. Ethics 3 (2), 101–112. doi:10.5840/enviroethics19813248
Sarkar, S. (2005). Biodiversity and environmental philosophy. Cambridge: Cambridge University Press.
Schopf, T. J. M. (1984). Rates of evolution and the notion of “living fossils. Annu. Rev. Earth Planet. Sci. 12, 245–292. doi:10.1146/annurev.ea.12.050184.001333
Shockley, K. (2012). Thinning the thicket: Concepts, context, and evaluative frameworks. Environ. Ethics 34 (3), 227–246. doi:10.5840/enviroethics201234320
Smith, D. R., Brockmann, H. J., Beekey, M. A., King, T. L., Millard, M. J., and Zaldivar-Rae, J. (2017). Conservation status of the American horseshoe crab (Limulus polyphemus): A regional assessment. Rev. Fish Biol. Fish. 27, 135–175. doi:10.1007/s11160-016-9461-y
Smith, D. R., Millard, M. J., and Carmichael, R. H. (2009). “Comparative status and assessment of Limulus Polyphemus with emphasis on the new england and Delaware bay populations,” in Biology and conservation of horseshoe crabs. Editor J. T. Tancredi (Berlin, Germany: Springer), 361–386.
Sober, E. (1986). “Philosophical problems for environmentalism,” in The preservation of species. Editor B. Norton (Princeton, NJ: Princeton University Press), 173–194.:
Stanford, P. K. (1995). For pluralism and against realism about species. Philosophy Sci. 62 (1), 70–91. doi:10.1086/289840
Turner, D. (2022). Causal history, environmental art, and biotechnologically assisted restoration. Ethics, Policy, Environ. 25 (2), 125–128. doi:10.1080/21550085.2022.2071557
Turner, D. (2019a). In defense of living fossils. Biol. Philosophy 34, 23. doi:10.1007/s10539-019-9678-y
Turner, D. (2019b). Paleoaesthetics and the practice of Paleontology. Cambridge: Cambridge University Press.
Vargas, P., Jimenez-Mejias, P., and Fernandez-Mazuecos, M. (2020). Endangered living fossils (ELFs): Long-term survivors through periods of dramatic climate change. Environ. Exp. Bot. 170, 103892. doi:10.1016/j.envexpbot.2019.103892
Vellend, M., Cornwell, W. K., Magnuson-Ford, K., and Mooers, A. O. (2011). “Measuring phylogenetic biodiversity,” in Biological diversity: Frontiers in measurement and assessment. Editors A. E. MacGurran, and B. J. McGill (Oxford: Oxford University Press), 194–207.:
Wang, H. F., Wang, Z. S., Friedman, C. R., and López-Pujol, J. (2012). “Conservation of the cathay silver fir, cathaya argyrophylla: A Chinese evergreen ‘living fossil’,” in Evergreens: Types, ecology and conservation. Editors D. Bezerra Adriano, and S. Ferreira Tadeu (New York: Nova Science Publishers).
Wang, X., Nan, L., Wang, Y., and Zheng, S. (2009). The discovery of whole-plant fossil cycad from the upper Triassic in Western Liaoning and its significance. Chin. Sci. Bull. S4, 3116–3119. doi:10.1007/s11434-009-0384-z
Watkins, A. (2021). The epistemic value of the living fossils concept. Philosophy Sci. 88 (5), 1221–1233. doi:10.1086/714875
Weinberg, S. (2001). A fish caught in time: The search for the coelacanth. New York: Harper Perennial.
Werth, A. J., and Shear, W. A. (2014). The evolutionary truth about living fossils. Am. Sci. 102, 434–443. doi:10.1511/2014.111.434
Winter, M., Devictor, V., and Schweiger, O. (2013). Phylogenetic diversity and nature conservation: Where are we? Trends Ecol. Evol. Biol. 28 (4), 199–204. doi:10.1016/j.tree.2012.10.015
Woodford, J. (2000). The wollemi pine: The incredible discovery of a living fossil from the age of the dinosaurs. Melbourne, Australia: The Text Publishing Company.
Yong, E. (2013). The falsity of living fossils, the Scientist. Available at: https://www.the-scientist.com/?articles.view/articleNo/34927/title/The-Falsity-of-Living-Fossils/(Accessed May 12, 2013).
Zhang, L., Jiang, W., Wang, Q. J., Zhao, H., Zhang, H. X., Marcec, R. M., et al. (2016). Reintroduction and post-release survival of a living fossil: The Chinese giant salamander. PLoS One 11, e0156715. doi:10.1371/journal.pone.0156715
Fulton, A. (2012). Starbucks ditches bug-based red dye in strawberry drink. National Public Radio Available at: https://www.npr.org/sections/thesalt/2012/04/19/150972539/starbucks-ditches-bug-based-red-dye-in-strawberry-drink. (Accessed 12 March 2012).
Keywords: aesthetic value, conservation, concepts, horseshoe crab (Limulus polyphemus), living fossil, phylogenetic diversity, stasis
Citation: Turner D and Han J (2023) Living fossils and conservation values. Front. Earth Sci. 11:1086066. doi: 10.3389/feart.2023.1086066
Received: 01 November 2022; Accepted: 10 January 2023;
Published: 20 January 2023.
Edited by:
Alan C. Love, University of Minnesota Twin Cities, United StatesReviewed by:
Aja Watkins, Boston University, United StatesAdrian Currie, University of Exeter, United Kingdom
Copyright © 2023 Turner and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Derek Turner, ZGVyZWsudHVybmVyQGNvbm5jb2xsLmVkdQ==
 Derek Turner
Derek Turner Junhyung Han
Junhyung Han