- 1Institute of Loess Plateau, Shanxi University, Taiyuan, China
- 2Key Laboratory of Western China’s Environmental Systems (Ministry of Education), College of Earth and Environmental Sciences, Lanzhou University, Lanzhou, China
- 3College of Environmental and Resource Sciences, Shanxi University, Taiyuan, China
- 4Hebei Key Laboratory of Environmental Change and Ecological Construction, College of Resources and Environment Sciences, Hebei Normal University, Shijiazhuang, China
- 5Group of Alpine Paleoecology and Human Adaptation (ALPHA), State Key Laboratory of Tibetan Plateau Earth System, Resources and Environment (TPESRE), Institute of Tibetan Plateau Research, Chinese Academy of Sciences (CAS), Beijing, China
The current lake environmental problem of harmful cyanobacterial blooms cannot be mitigated effectively despite numerous eutrophication control strategies, and climate warming may have been considered as a potential key driver. However, it is still unclear how climate change and associated natural processes influence cyanobacterial development. Here we use a sedimentary pigment record from a remote, alpine, small and eutrophic lake (Lake Mayinghai) on the Chinese Loess Plateau and take the mid-Holocene as an analog to explore the possible influences of temperature, precipitation and terrestrial vegetation on in-lake and catchment processes, algal compositional changes and cyanobacterial development. The pigment data indirectly suggest that a distinctly low β-carotene to chlorophyll a ratio during the mid-Holocene is likely due to an increase in non-nitrogen-fixing colonial coccoid cyanobacteria at the expense of nitrogen-fixing filamentous cyanobacteria. There are two probable synergistic driving mechanisms, of which one is high temperatures and associated increased lake thermal stratification and the other is high inorganic nitrogen supply and resultant increased lake nitrogen to phosphorus ratio. This study provides implications for the potential influences of future climate change on cyanobacterial development under a warmer, wetter and re-forested environment on the Chinese Loess Plateau.
Introduction
It was recognized that recent anthropogenic nutrient pollution drives lake eutrophication and harmful cyanobacterial blooms that threaten water quality and public health (Huisman et al., 2005; Kudela et al., 2015; Hou et al., 2022). However, considerable management strategies to reduce nutrient loading often have not successfully mitigated this environmental problem (Paerl and Otten, 2016; Huisman et al., 2018), and even there is a long debate on whether reducing phosphorus (Schindler et al., 2008, 2016; Paterson et al., 2011) or controlling both phosphorus and nitrogen (Conley et al., 2009; Scott and McCarthy, 2010; Paerl et al., 2016) is an effective method. It was also found that cyanobacterial blooms can occur in oligotrophic lakes (Ewing et al., 2020; Freeman et al., 2020). Climate change and associated in-lake and catchment processes are considered to be drivers of cyanobacterial blooms (Smol, 2019; Reinl et al., 2021). Recent warming-driven prolonged and intensified lake thermal stratification can cause cyanobacterial blooms (Smol, 2019) whereas its associated reduced nutrient suspension is not favorable to cyanobacterial blooms (Reinl et al., 2021). An increase in precipitation can cause high erosive nutrient input and lake eutrophication (Sinha et al., 2017) but can also cause flushing and destratification to inhibit cyanobacterial blooms (Reichwaldt and Ghadouani, 2012). These climatic processes affect cyanobacterial development in synergistic or antagonistic ways. It is unclear what the net effect of climate change is and how climate change interacts with current eutrophication control efforts to influence cyanobacterial blooms (Paerl and Scott, 2010; Lürling et al., 2018; Ho et al., 2019; Paerl and Barnard, 2020). Therefore, to foster lake management in the warmer future, it is essential to improve understanding of the influence of climate change and associated natural processes on cyanobacteria without human impact, and paleolimnology provides a valuable way to investigate the impacts of past climate change on cyanobacterial development under natural conditions.
Although lake properties and catchment landscapes may complicate the processes of climate change and anthropogenic eutrophication on cyanobacterial blooms (Qin et al., 2020; Reinl et al., 2021; Paltsev and Creed, 2022), it was recognized that small and nutrient-rich lakes are more sensitive to increases in temperature while large and oligotrophic lakes are more sensitive to changes in precipitation patterns and associated nutrient supply (Kosten et al., 2012; Rigosi et al., 2014; Reinl et al., 2021; Paltsev and Creed, 2022). Here, a remote, alpine, small and eutrophic lake (Lake Mayinghai) on the Chinese Loess Plateau was investigated to provide implications for predicting the potential influences of future climate warming. This lake is ideal to examine the roles of climate change on lake mixing regimes, natural nutrient dynamics, phytoplankton biomass and community composition. Firstly, alpine lakes on the Chinese Loess Plateau are sensitive to increases in air temperature (Wang et al., 2014; Yan et al., 2020) and associated enhanced lake thermal stratification (Liu et al., 2017; Yan et al., 2022). Secondly, Chinese Loess Plateau lies in the northernmost part of the East Asian summer monsoon domain (Figure 1A), and lakes are sensitive to changes in monsoonal precipitation (Chen et al., 2015; Chen et al., 2021) and associated natural nutrient dynamics (Liu et al., 2017). Thirdly, Chinese Loess Plateau is known as a region of severe soil erosion due to loess loose and collapsible properties, which causes high nutrient delivery into water bodies and even makes the Yellow River crossing the region (Figure 1A) as one of the most nutrient-laden world’s large rivers (Chen et al., 2004; Yu et al., 2010; Fu et al., 2017), and lakes are sensitive to catchment nutrient processes. We use sedimentary pigments, which comprise mainly chlorophylls and carotenoids produced by photosynthetic organisms and can be used as excellent proxies for past algal production and community compositional changes (Leavitt and Hodgson, 2001), and take the mid-Holocene thermal maximum as an analog to explore the influences of temperature, precipitation and terrestrial vegetation on in-lake and catchment processes, algal compositional changes and cyanobacterial development.
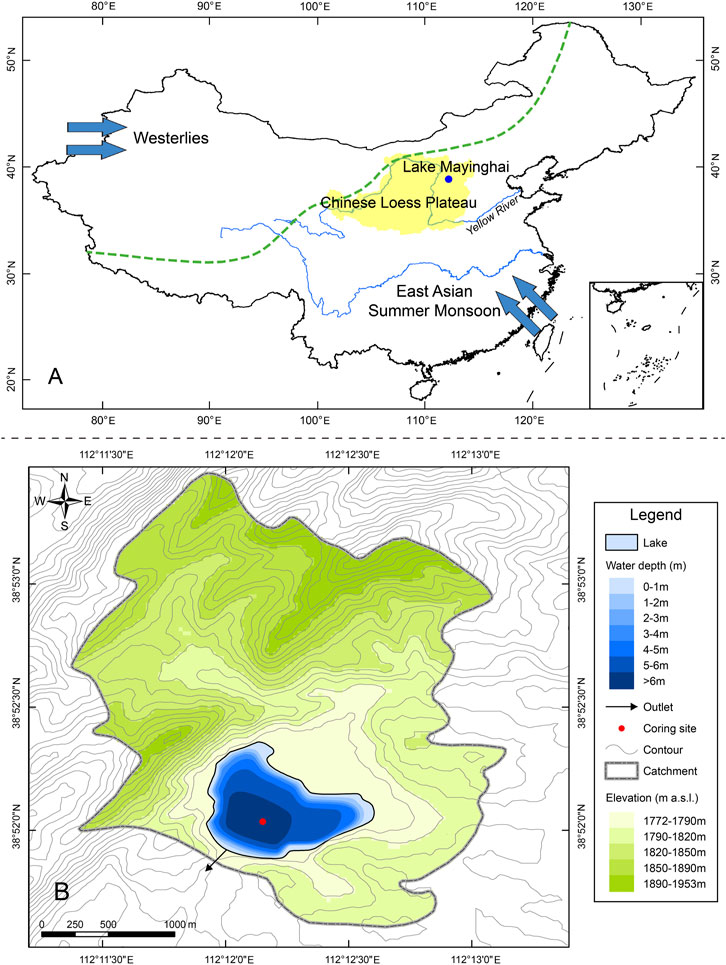
FIGURE 1. The geographical background of Lake Mayinghai. (A) Map of China showing the location of Lake Mayinghai (blue dot) on the Chinese Loess Plateau, which is in the northern East Asian summer monsoon region, with the green dashed line indicating the modern boundary between the East Asian summer monsoon and the westerly climate systems. (B) Map of Lake Mayinghai showing the lake bathymetry with the coring site (red dot) and the 10 m-interval contour topography of its catchment and surrounding area.
Materials and methods
Lake properties
Lake Mayinghai (112°12′9″E, 38°52′2″N, 1772 m a. s. l., Figure 1A) is a remote, alpine, small and eutrophic lake in the northern Lvliang Mountains, Chinese Loess Plateau, northern China. It has a maximum water depth of 6.4 m, a surface area of ca. 0.6 km2 and a catchment area of ca. 6 km2 (Figure 1B). The pelagic lake-water annual average total phosphorus and total nitrogen concentrations are 39 and 708 μg l−1, respectively. The nitrogen to phosphorus ratio is 18, and the lake is currently phosphorus and nitrogen co-limited (Guildford and Hecky, 2000). The lake is influenced by a typical monsoonal climate (i.e., warm, wet summers and cold, dry winters), and the average annual precipitation is 657.3 mm, 70% of which occurs between June and September. The lake is fed primarily by precipitation, with no perennial water inflow, and lake water is lost through evaporation and groundwater outflow. There is no surface outflow today, but it was possible at the southwestern end of the lake (Figure 1B) during previous phases of high lake levels and drained southward into the Fen River, the Yellow River’s second largest tributary. The lake is ice-covered from December to March, overturns from April to May and from August to November, and experiences stratification in June and July (Zhang et al., 2022). Lake algal community composition comprises mainly diatoms (62%), chlorophytes (22%), cyanobacteria (10%) and cryptophytes (3%) (Zhang et al., 2012). Diatoms are dominated by Cyclotella, Aulacoseira, Fragilaria and Navicula sensu lato spp.. Chlorophytes consist mainly of Chlorella, Oocystis, Ankistrodesmus, Ulothrix and Scenedesmus spp.. Cyanobacteria includes Aphanocapsa, Oscillatoria, Phormidium, Lyngbya and Anabaena spp.. The catchment vegetation is mainly shrubs and herbs, together with some mixed broadleaved-coniferous forests (Xu et al., 2017; Huang et al., 2021).
Sediment archives
A 1,490 cm-long core (MYH14B) was retrieved from the deepest part of Lake Mayinghai (Figure 1B) using UWITEC piston coring equipment from the lake ice in January 2014. An age-depth model was developed based on radiocarbon dating of 18 plant macrofossils and two charcoal fragments (see Supplementary Material), which improves the age model published in Cheng et al. (2020). Radiocarbon dates were measured at Beta Analytic Inc. (United States). The calibration of radiocarbon ages into calendar ages is based on OxCal 4.4 software (Bronk Ramsey, 2009) and IntCal 20 calibration curve (Reimer et al., 2020). The classical age-depth modelling with a smoothing spline (smoothing parameter = 0.4) in the software package Clam 2.3.5 (Blaauw, 2010) was selected to generate a smooth curve between dated levels (Trachsel and Telford, 2017) in R platform (R Development Core Team, 2022). The age model shows that this sequence covers approximately the entire Holocene (Figure 2).
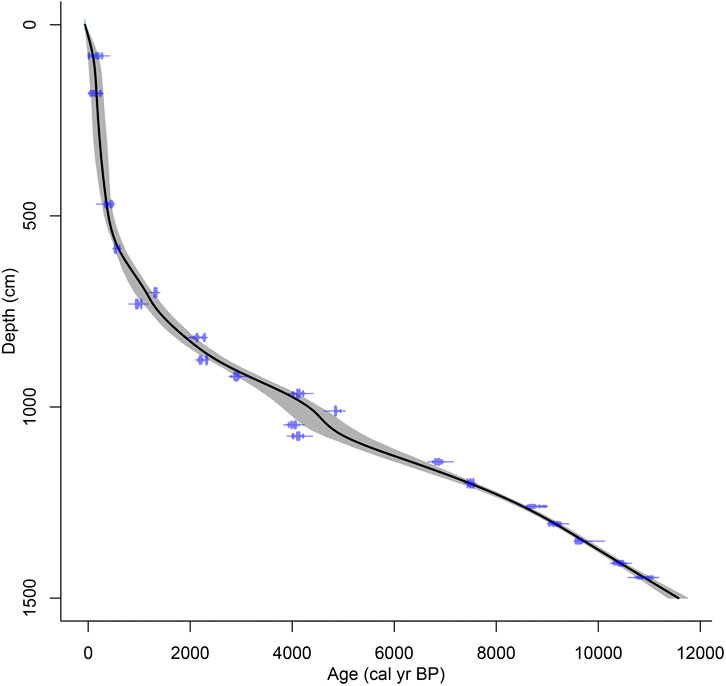
FIGURE 2. Age-depth model of the Lake Mayinghai core MYH14B [improved from Cheng et al. (2020)].
Pigment analysis
Pigment analysis was carried out on 216 samples, taken every 4 cm for the topmost 30 cm and between 700 and 1,490 cm depth, every 8 cm between 600 and 700 cm depth, and every 16 cm between 30 and 600 cm depth due to high sediment accumulation rates in this interval. The age resolution between samples is ca. 10–20 years between 0 and 500 cm depth (ca. 400 cal year BP-present), ca. 30–40 years between 500 and 700 cm depth (ca. 1,100–400 cal year BP), ca. 20–40 years between 700 and 800 cm (ca. 1,800–1,100 cal year BP), ca. 40–80 years between 800 and 1,100 cm (ca. 5,300–1,800 cal year BP), ca. 100–120 years between 1,100 and 1,200 cm (ca. 7,500–5,300 cal year BP), ca. 50–70 years between 1,200 and 1,300 cm (ca. 9,100–7,500 cal year BP) and ca. 50 years between 1,300 and 1,490 cm (ca. 11,400–9,100 cal year BP).
Standard procedures were adopted for chlorophyll and carotenoid pigment analyses (Leavitt and Hodgson, 2001; McGowan, 2013). Pigment analysis was conducted at the University of Nottingham (United Kingdom) following Moorhouse et al. (2014). Pigment samples were frozen at −18°C and freeze-dried shortly before analysis. Approximately 0.2 g sediment was extracted overnight at −4°C in an acetone: methanol: water (80:15:5) solution. Extracts were filtered with a 0.22 μm PTFE filter, dried under N2 gas, re-dissolved in an acetone: ion-pairing reagent: methanol (70:25:5) mixture (ion-pairing reagent is 0.75 g tetra butyl ammonium acetate, 7.7 g ammonium acetate and 100 ml water), and injected into the high-performance liquid chromatography (HPLC) unit. The HPLC system comprised an Agilent 1,200 series quaternary pump, an autosampler, an ODS Hypersil column (205 mm × 4.6 mm; 5 μm particle size), a photo-diode array detector, and the Chemstation software. The separation method was modified from Chen et al. (2001). Solvent A was methanol: 0.5 M ammonium acetate (80:20), solvent B was acetonitrile: water (90:10) and solvent C was ethyl acetate. After injection (100 μl) a gradient program (1 ml min−1) began isocratically with 100% solvent A, ramped to 100% solvent B in 4 min and then to 25% solvent B and 75% solvent C over 34 min, held isocratically for 1 min, returned to 100% solvent A in 4 min, and finally ran isocratically for 9 min. Pigments were identified by comparing spectra and peak retention times with commercial standards (DHI Denmark), and peak areas were calibrated to calculate pigment concentrations. Pigment concentrations are expressed as nanomoles per gram total organic carbon in the sediment (nmol g−1 TOC) (see Supplementary Material). TOC analysis was conducted at Lanzhou University (China). Approximately 1.0 g freeze-dried sediment was treated with 10% HCl to remove total inorganic carbon, rinsed to remove remaining HCl, and then re-dried. Approximately 0.2 g re-dried sediment was wrapped using aluminum foil and measured in the Jena HT 1,300 TOC analyzer. TOC concentration was calculated relative to total dry sediment.
Results and discussion
Mid-Holocene algal compositional changes and cyanobacterial development
Pigment preservation in the Lake Mayinghai sediment record can be qualitatively assessed according to pheophytin a and fucoxanthin concentrations. Pheophytin a is a general, more stable degradation product of chlorophyll a, and its nearly consistent level throughout the sequence (Figure 3B) suggests that a high chlorophyll a to pheophytin a ratio during the mid-Holocene (ca. 7,300–5,450 cal year BP) was driven more by high production than low degradation. Despite the possibly different degradation degrees of pigments, fucoxanthin is an instable pigment and highly prone to degradation, with its final degradation product colorless and undetectable (Reuss and Conley, 2005), its presence throughout the sequence and no significant change during the mid-Holocene (Figure 3I) is an excellent indicator of good pigment preservation. So, degradation could be considered negligible for interpretation of the pigment assemblage data.
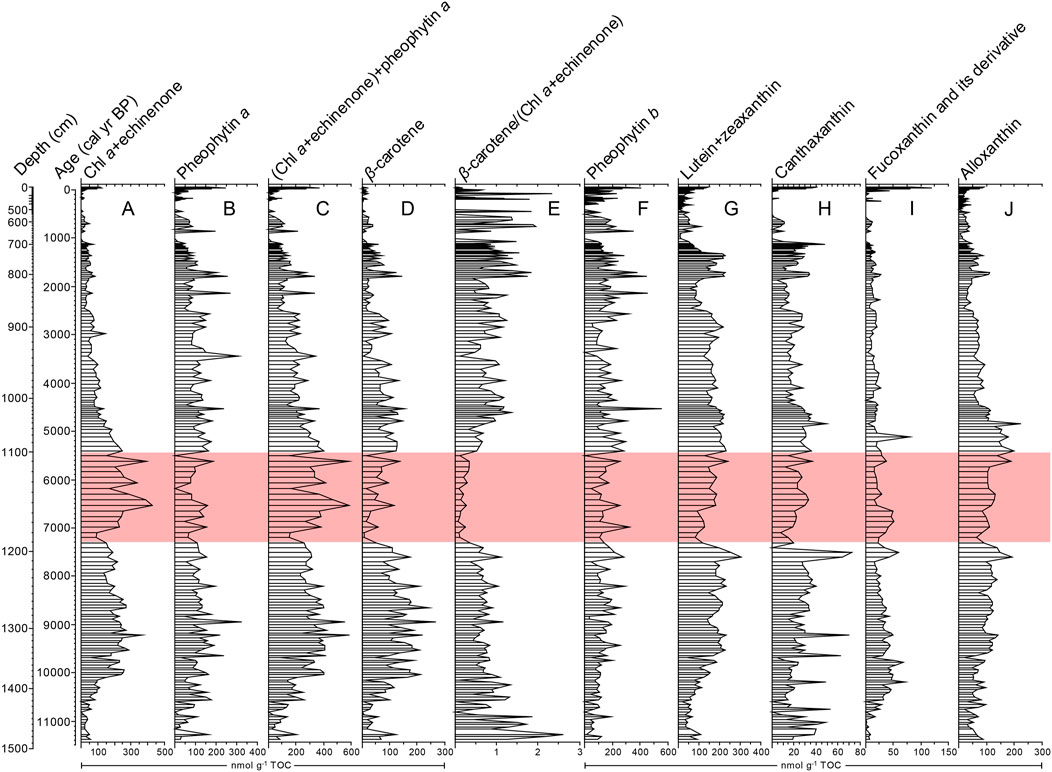
FIGURE 3. Pigments from the Lake Mayinghai core MYH14B. The pink bar indicates the mid-Holocene period with a distinctly low β-carotene to chlorophyll a + echinenone ratio.
It should be emphasized that chlorophyll a and echinenone co-eluted in this study, because their peak areas cannot be separated in the chromatogram, but echinenone was a minor component and we calibrated to chlorophyll a alone. Chlorophyll a is a ubiquitous, dominant pigment in all photosynthetic algae and higher plants (Leavitt and Hodgson, 2001; McGowan, 2013). Echinenone and canthaxanthin are usually minor pigments in filamentous cyanobacteria (Jeffrey et al., 1997; Egeland et al., 2011), and canthaxanthin concentration was generally low and less than 40 nmol g−1 TOC in this sequence, even with a decrease during the mid-Holocene (Figure 3H), suggesting a similar change in echinenone concentration. However, chlorophyll a + echinenone concentrations were much higher (243 nmol g−1 TOC on average) during the mid-Holocene. This confirms that high chlorophyll a + echinenone concentrations can represent high chlorophyll a concentration and indicate high chlorophyll a production during the mid-Holocene (Figure 3A). β-carotene is also derived from all algae and higher plants, but it is dominant in chlorophytes and higher plants and usually minor in other algal groups (Jeffrey et al., 1997; Egeland et al., 2011). Low β-carotene concentration during the mid-Holocene (Figure 3D) is not consistent with high chlorophyll a concentration, and the ratio of β-carotene to chlorophyll a + echinenone is distinctly low during the mid-Holocene (Figure 3E). β-carotene is much more stable than chlorophyll a, and given the primary signal of chlorophyll a for production, high temperature-induced β-carotene degradation during the mid-Holocene can also be negligible. However, high temperature-induced thermal stratification was favorable to pigment preservation. Thus, the low β-carotene to chlorophyll a ratio was attributed to pigment sources rather than preservation qualities. Moreover, given that there was no clear change in terrestrial organic input during the mid-Holocene as indicated by the C/N ratio (Figure 4E), terrestrial inputs of chlorophyll a and β-carotene from higher plants were consistent during the mid-Holocene, so the distinctly low β-carotene to chlorophyll a ratio is indicative of algal compositional changes, and high chlorophyll a concentration indicates high algal production.
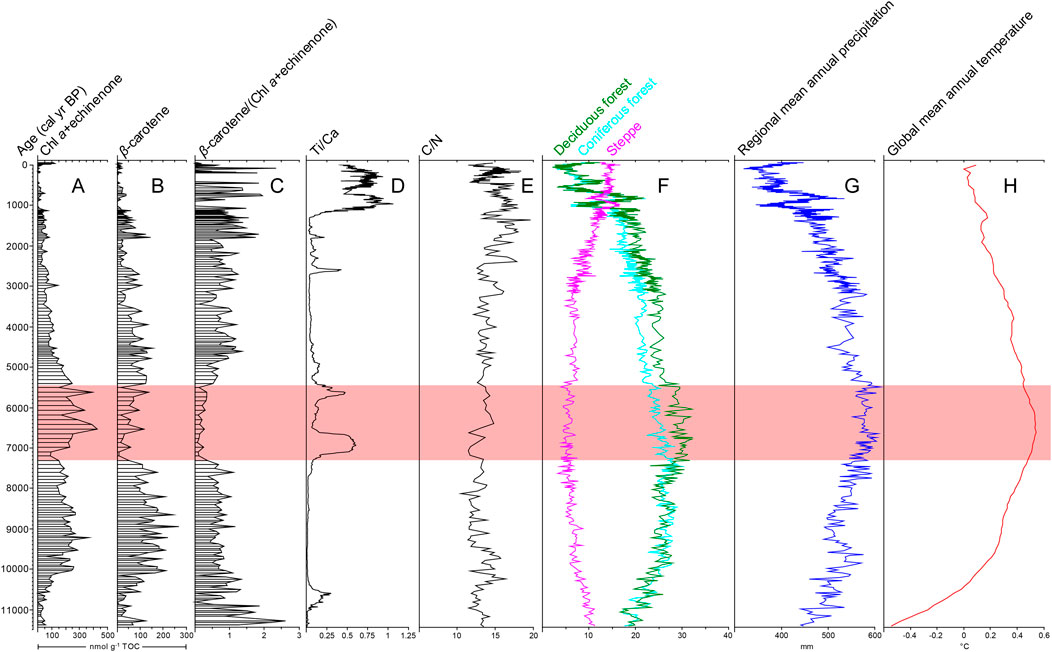
FIGURE 4. Comparison of Holocene pigment data from Lake Mayinghai (A–C) with Ti/Ca (D) (Shen et al., 2018; Cheng et al., 2020) and C/N (E) (Cheng et al., 2020; Li et al., 2020) from the same core, terrestrial vegetation biomes from nearby Lake Gonghai (F) (Xu et al., 2017), mean annual precipitation in northern China (mainly East Asian summer monsoon precipitation) (G) (Chen et al., 2015) and global mean annual temperature (H) (Kaufman et al., 2020).
Based on the difference of main phytoplankton sources between chlorophyll a and β-carotene as described above, the low ratio of β-carotene to chlorophyll a + echinenone is possibly linked to a reduction in chlorophytes and/or an increase in other algal groups. Pheophytin b is a general degradation product of chlorophyll b that is a dominant pigment in chlorophytes and higher plants (Jeffrey et al., 1997; Egeland et al., 2011). Pheophytin b is highly stable (Reuss and Conley, 2005), and its nearly consistent concentration suggests no clear change of chlorophyte production during the mid-Holocene (Figure 3F). Lutein is also a major pigment in chlorophytes and higher plants, and it is known as a biomarker for macrophytes (Bianchi and Finlay, 1990). Despite high precipitation during the mid-Holocene (see below), this lake was hydrologically open with surface outflow modulating the water balance, making an increase in water level and a shift from the dominance of macrophytes to phytoplankton unlikely. This is supported by the aquatic pollen data that show Typhaceae and Myriophyllum were consistently extremely low during the mid-Holocene (Ren et al., 2022). Thus, there was no clear change of macrophytes during this period. Lutein co-eluted with zeaxanthin. Zeaxanthin is usually a major pigment in total cyanobacteria (Egeland et al., 2011). The decrease in lutein + zeaxanthin concentrations suggests that total cyanobacteria might have a decline during the mid-Holocene (Figure 3G), which was synchronous with canthaxanthin, indicating a large contribution of the decrease in filamentous cyanobacteria. Fucoxanthin is a major pigment in diatoms, chrysophytes and synurophytes (Jeffrey et al., 2011). The concentrations of fucoxanthin and its derivative indicate no significant change of the production of siliceous algae during the mid-Holocene (Figure 3I). Alloxanthin is a particular, major pigment in cryptophytes. Its concentration remained consistent during the mid-Holocene (Figure 3J), indicating no change of cryptophyte production. Collectively, given the clear evidence of the nearly consistent production in chlorophytes, siliceous algae and cryptophytes and the decline in the production of filamentous cyanobacteria, it is possible that the mid-Holocene high chlorophyll a concentration and low β-carotene to chlorophyll a ratio could be driven by an increase in colonial coccoid cyanobacteria.
Cyanobacterial response to mid-Holocene climate and environmental changes
Holocene temperature changes have not been reconciled (Marcott et al., 2013; Liu et al., 2014b; Marsicek et al., 2018; Kaufman et al., 2020; Bova et al., 2021; Osman et al., 2021). However, Marcott and colleagues’ temperature reconstruction relies more on northern mid-latitude marine sites (Marcott et al., 2013), Marsicek and colleagues’ reconstruction focuses on northern mid-latitude terrestrial sites (Marsicek et al., 2018), and Bova and colleagues’ reconstruction lays emphasis on tropical marine sites (Bova et al., 2021). Thus, we adopt the temperature reconstruction from the Temperature 12 k database that comprises a large number of both terrestrial and marine sites across the world (Kaufman et al., 2020). This reconstruction exhibits high temperatures during the mid-Holocene (Figure 4H), and confirms the traditional view of mid-Holocene thermal maximum (Shi et al., 1994). Our pigment result of the mid-Holocene low β-carotene to chlorophyll a ratio is associated with the thermal maximum. High temperatures would have promoted cyanobacterial growth directly, because cyanobacteria reach their maximum growth rates at higher temperatures than eukaryotic algae (Paerl, 2014; Huisman et al., 2018) and high temperatures also reduce lake ice cover and prolong their growing-season period (Smol et al., 2005). Meanwhile, warming-induced intensified and prolonged thermal stratification and increased lake stability provided a competitive advantage for the development of cyanobacteria, because they have buoyancy to regulate their positions in the water column (Smol, 2019; Reinl et al., 2021; Sivarajah et al., 2021). Moreover, the currently dominant colonial coccoid Aphanocapsa in Lake Mayinghai (Zhang et al., 2012) and the common bloom-forming colonial coccoid Microcystis, both of which cannot fix nitrogen, are more sensitive to temperature than nitrogen-fixing filamentous cyanobacteria such as Anabeana (Jöhnk et al., 2008; Wu et al., 2010; Rigosi et al., 2014). Meanwhile, colonial coccoid Aphanocapsa and Microcystis have a generally smaller cell volume and higher surface area to volume ratio than filamentous Anabeana (Napiórkowska-Krzebietke and Kobos, 2016), and thus colonial coccoid cyanobacteria have higher buoyancy and benefit more from enhanced thermal stratification (Smol, 2019). So, the increase in non-nitrogen-fixing colonial coccoid cyanobacteria at the expense of filamentous cyanobacteria was likely linked to high temperatures during the mid-Holocene.
Robust pollen-based precipitation reconstruction from Lake Gonghai, ca. 5 km apart from Lake Mayinghai, shows that mean annual precipitation, consisting mainly of the East Asian summer monsoon precipitation, reached the maximum during the mid-Holocene in this region (Figure 4G) (Chen et al., 2015). The East Asian summer monsoon intensity can be represented by the amount of precipitation in northern China (Liu et al., 2014a; Chen et al., 2015), which is located in the northern monsoonal region (Figure 1A), and thus high summer monsoon intensity also occurred during the mid-Holocene. The mid-Holocene low β-carotene to chlorophyll a ratio in Lake Mayinghai is associated with high precipitation and high summer monsoon wind intensity. Winter monsoon wind intensity can be ignored due to the lake ice cover (Zhang et al., 2022). Given the consistent lake level (see above) and the consistent wind-shielding catchment forest (as shown by a consistently low steppe component in Figure 4F), high monsoon wind intensity, which would weaken lake stratification, did not favor cyanobacterial development. High precipitation would have brought more nutrients into the lake through high surface runoff and catchment erosion, favoring lake eutrophication and cyanobacterial development (Sinha et al., 2017). However, this effect can be modulated by catchment landscape properties (Paltsev and Creed, 2022). Although the C/N ratio (Cheng et al., 2020; Li et al., 2020) shows that there was no clear change in terrestrial organic input during the mid-Holocene (Figure 4E), the Ti/Ca ratio, an excellent proxy for terrestrial siliciclastic input (Shen et al., 2018; Cheng et al., 2020), shows that there was fluctuating but generally higher inorganic elemental input during the mid-Holocene (Figure 4D). This may be linked to high organic matter decomposition and high chemical weathering during the process of soil development under optimal climatic conditions. Thus, the intensity of catchment erosion was strong during the mid-Holocene, and high precipitation-induced runoff overrode the hindering effect of dense terrestrial forest cover on catchment erosion. On the other hand, this lake was open with surface outflow modulating the water and nutrient balance. This would induce a low water residence time and a low steady-state concentration of nutrients during the mid-Holocene, which did not favor cyanobacterial development. Collectively, the increase in non-nitrogen-fixing colonial coccoid cyanobacteria may be partially linked to high precipitation-induced inorganic nutrient input during the mid-Holocene, but this cannot explain the decline in filamentous cyanobacteria.
Pollen-based biome reconstruction from nearby Lake Gonghai shows that, although terrestrial forest cover was consistently dense (as shown by a constantly low steppe component in Figure 4F), deciduous forests reached the maximum at the expense of coniferous forests during the mid-Holocene (Figure 4F) under optimal climatic conditions (Xu et al., 2017). The Lake Mayinghai mid-Holocene low β-carotene to chlorophyll a ratio is coeval with maximum deciduous forest cover. As mentioned above, the potential influences of dense forest cover in reducing wind speed (Vautard et al., 2010) and erosion intensity (Fu et al., 2017) were consistent during the mid-Holocene. However, deciduous forests and coniferous forests covering the catchment have different effects on lake trophic state (Klimaszyk and Rzymski, 2011). Deciduous broadleaf forests have higher defoliation, higher leaf litter decomposition, and higher soil carbon and nitrogen concentrations than evergreen coniferous forests (Aerts and Chapin III, 2000; Chen et al., 2016; Zhang et al., 2017). Thus, combined with precipitation-induced high erosion intensity as indicated by the Ti/Ca ratio (Figure 4D), maximum deciduous forests would have provided high inorganic carbon and nitrogen supplies to the lake, and the lake possibly became phosphorus limited during the mid-Holocene. Cyanobacteria evolve CO2-concentrating mechanisms and possess several inorganic carbon uptake systems (Huisman et al., 2018), so inorganic carbon concentration may be not significant. It is widely accepted that, although cyanobacteria can store the nutrients compared to eukaryotic algae (Reinl et al., 2021), both phosphorus and nitrogen are primary nutrient elements for controlling cyanobacterial development (Paerl et al., 2016; Qin et al., 2020) and nitrogen becomes even more important than phosphorus in summer (Ma et al., 2015; Xu et al., 2015). High nitrogen concentration, low phosphorus concentration and high nitrogen to phosphorus ratio are traditionally considered unfavorable to cyanobacterial development (Tilman et al., 1982; Jeppesen et al., 2005; Schindler et al., 2016). However, it is recently found that high nitrogen to phosphorus ratio can shift the cyanobacterial composition from nitrogen-fixing cyanobacteria to non-nitrogen-fixing cyanobacteria (Paerl and Otten, 2016; Huisman et al., 2018). Filamentous Anabaena can fix nitrogen for their long-term survival and thrives under low nitrogen conditions (Chia et al., 2018), whereas non-nitrogen-fixing colonial coccoid Microcystis develops under high nitrogen and low phosphorus environments (Chia et al., 2018; Wan et al., 2019). So, the increase in non-nitrogen-fixing colonial coccoid cyanobacteria at the expense of filamentous cyanobacteria was likely linked to catchment vegetation modulation during the mid-Holocene.
Conclusion
This study uses pigments from a Holocene sediment core of Lake Mayinghai on the Chinese Loess Plateau to explore the influences of mid-Holocene climate and environmental changes on algal compositional changes and cyanobacterial development. A distinctly low β-carotene to chlorophyll a ratio during the mid-Holocene is likely driven by an increase in non-nitrogen-fixing colonial coccoid cyanobacteria. The driving factors are high temperatures, high precipitation and high terrestrial deciduous forest cover during the mid-Holocene. High temperatures may have promoted the development of non-nitrogen-fixing colonial coccoid cyanobacteria in direct (by increasing their growth rates) and/or indirect (by increasing thermal stratification) ways. Dense deciduous forest in the catchment may have provided high inorganic nitrogen supply and thus increased lake nitrogen to phosphorus ratio under the influence of precipitation-induced high erosion intensity. High nitrogen to phosphorus ratio favored non-nitrogen-fixing colonial coccoid cyanobacteria at the expense of nitrogen-fixing filamentous cyanobacteria. These three factors affected cyanobacterial development in a synergistic way during the mid-Holocene on the Chinses Loess Plateau. This study provides implications for the potential influences of future climate change on cyanobacterial development in this region. In the future, when temperatures go up, the East Asian summer monsoon will be intensified, precipitation will increase in this region, and vegetation will be recovered (IPCC, 2021). Future lake management should take into consideration the in-lake processes influenced by higher temperatures and the catchment processes modulated by increased precipitation and terrestrial vegetation.
Data availability statement
The datasets generated for this study can be found in the Supplementary Material.
Author contributions
JL designed this research; XZ performed pigment analysis; XZ, LS, and JZ interpreted the data; XZ, SZ, QX, and JL discussed the driving factors; LS, JZ, and ZS performed subsampling, dating-material collection and TOC analysis; XZ and ZS did the fieldwork and developed the age model; XZ wrote the paper with editorial comments from all co-authors.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 41601194) and the Fundamental Research Program of Shanxi Province, China (grant number 202203021211316).
Acknowledgments
We would especially like to thank Prof. Suzanne McGowan (University of Nottingham, United Kingdom), Prof. John P. Smol (Queen’s University, Canada) and Prof. Fahu Chen (Institute of Tibetan Plateau Research, China) for discussion. We would thank Prof. Xin Wang (Lanzhou University, China) and Haihong Liang (Shanxi Ningwu Luya Mountain Scenic Area, China) for providing help for fieldwork. We also thank Fanyi Li and Bei Cheng (Lanzhou University, China) for providing the C/N data. Two reviewers Prof. BW and Prof. CH are thanked for their detailed and constructive comments, suggestions and improvements on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2022.984420/full#supplementary-material
References
Aerts, R., and Chapin, F. S. (2000). The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 30, 1–67. doi:10.1016/S0065-2504(08)60016-1
Bianchi, T. S., and Findlay, S. (1990). Plant pigments as tracers of emergent and submergent macrophytes from the Hudson River. Can. J. Fish. Aquat. Sci. 47, 492H–494. doi:10.1139/f90-054
Blaauw, M. (2010). Methods and code for ‘classical’ age-modelling of radiocarbon sequences. Quat. Geochronol. 5, 512–518. doi:10.1016/j.quageo.2010.01.002
Bova, S., Rosenthal, Y., Liu, Z., Godad, S. P., and Yan, M. (2021). Seasonal origin of the thermal maxima at the Holocene and the last interglacial. Nature 589, 548–553. doi:10.1038/s41586-020-03155-x
Bronk Ramsey, C. (2009). Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360. doi:10.1017/S0033822200033865
Chen, F., Xu, Q., Chen, J., Birks, H. J. B., Liu, J., Zhang, S., et al. (2015). East Asian summer monsoon precipitation variability since the last deglaciation. Sci. Rep. 5, 11186. doi:10.1038/srep11186
Chen, J., He, D., Zhang, N., and Cui, S. (2004). Characteristics of and human influences on nitrogen contamination in Yellow River system, China. Environ. Monit. Assess. 93, 125–138. doi:10.1023/B:EMAS.0000016796.51583.80
Chen, L., Li, P., and Yang, Y. (2016). Dynamic patterns of nitrogen: Phosphorus ratios in forest soils of China under changing environment. J. Geophys. Res. Biogeosci. 121, 2410–2421. doi:10.1002/2016JG003352
Chen, N., Bianchi, T. S., McKee, B. A., and Bland, J. M. (2001). Historical trends of hypoxia on the Louisiana shelf: Application of pigments as biomarkers. Org. Geochem. 32, 543–561. doi:10.1016/S0146-6380(00)00194-7
Chen, S., Liu, J., Wang, X., Zhao, S., Chen, J., Qiang, M., et al. (2021). Holocene dust storm variations over northern China: Transition from a natural forcing to an anthropogenic forcing. Sci. Bull. 66, 2516–2527. doi:10.1016/j.scib.2021.08.008
Cheng, B., Liu, J., Chen, S., Zhang, Z., Shen, Z., Yan, X., et al. (2020). Impact of abrupt late Holocene monsoon climate change on the status of an alpine lake in North China. J. Geophys. Res. Atmos. 125, e2019JD031877. doi:10.1029/2019JD031877
Chia, M. A., Jankowiak, J. G., Kramer, B. J., Goleski, J. A., Huang, I. S., Zimba, P. V., et al. (2018). Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 74, 67–77. doi:10.1016/j.hal.2018.03.002
Conley, D. J., Paerl, H. W., Howarth, R. W., Boesch, D. F., Seitzinger, S. P., Havens, K. E., et al. (2009). Controlling eutrophication: Nitrogen and phosphorus. Science 323, 1014–1015. doi:10.1126/science.1167755
Egeland, E. S., Garrido, J. L., Clementson, L., Andresen, K., Thomas, C. S., Zapata, M., et al. (2011). “Data sheets aiding identification of phytoplankton carotenoids and chlorophylls,” in Phytoplankton pigments: Characterization, chemotaxonomy and applications in oceanography. Editors S. Roy, C. A. Llewellyn, E. S. Egeland, and G. Johnsen (Cambridge: Cambridge University Press), 675–822.
Ewing, H. A., Weathers, K. C., Cottingham, K. L., Leavitt, P. R., Greer, M. L., Carey, C. C., et al. (2020). “New” cyanobacterial blooms are not new: Two centuries of lake production are related to ice cover and land use. Ecosphere 11, e03170. doi:10.1002/ecs2.3170
Freeman, E. C., Creed, I. F., Jones, B., and Bergström, A. K. (2020). Global changes may be promoting a rise in select cyanobacteria in nutrient-poor northern lakes. Glob. Change Biol. 26, 4966–4987. doi:10.1111/gcb.15189
Fu, B., Wang, S., Liu, Y., Liu, J., Liang, W., and Miao, C. (2017). Hydrogeomorphic ecosystem responses to natural and anthropogenic changes in the Loess Plateau of China. Annu. Rev. Earth Planet. Sci. 45, 223–243. doi:10.1146/annurev-earth-063016-020552
Guildford, S. J., and Hecky, R. E. (2000). Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 45, 1213–1223. doi:10.4319/lo.2000.45.6.1213
Ho, J. C., Michalak, A. M., and Pahlevan, N. (2019). Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 574, 667–670. doi:10.1038/s41586-019-1648-7
Hou, X., Feng, L., Dai, Y., Hu, C., Gibson, L., Tang, J., et al. (2022). Global mapping reveals increase in lacustrine algal blooms over the past decade. Nat. Geosci. 15, 130–134. doi:10.1038/s41561-021-00887-x
Huang, X., Ren, X., Chen, X., Zhang, J., Zhang, X., Shen, Z., et al. (2021). Anthropogenic mountain forest degradation and soil erosion recorded in the sediments of Mayinghai Lake in Northern China. Catena 207, 105597. doi:10.1016/j.catena.2021.105597
Huisman, J., Codd, G. A., Paerl, H. W., Ibelings, B. W., Verspagen, J. M. H., and Visser, P. M. (2018). Cyanobacterial blooms. Nat. Rev. Microbiol. 16, 471–483. doi:10.1038/s41579-018-0040-1
Huisman, J., Matthijs, H. C. P., and Visser, P. M. (2005). Harmful cyanobacteria. Dordrecht: Springer, 1–243.
IPCC (2021). Climate change 2021: The physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press, 1–2391.
Jeffrey, S. W., Mantoura, R. F. C., and Bjørnland, T. (1997). “Data for the identification of 47 key phytoplankton pigments,” in Phytoplankton pigments in oceanography: Guidelines to modern methods. Editors S. W. Jeffrey, R. F. C. Mantoura, and S. W. Wright (Paris: UNESCO Publishing), 447–560.
Jeffrey, S. W., Wright, S. W., and Zapata, M. (2011). "Microalgal classes and their signature pigments," in Phytoplankton pigments: Characterization, chemotaxonomy and applications in oceanography. Editors S. Roy, C. A. Llewellyn, E. S. Egeland, and G. Johnsen (Cambridge: Cambridge University Press), 3–77.
Jeppesen, E., Søndergaard, M., Jensen, J. P., Havens, K. E., Anneville, O., Carvalho, L., et al. (2005). Lake responses to reduced nutrient loading – An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 50, 1747–1771. doi:10.1111/j.1365-2427.2005.01415.x
Jöhnk, K. D., Huisman, J., Sharples, J., Sommeijer, B., Visser, P. M., and Stroom, J. M. (2008). Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Change Biol. 14, 495–512. doi:10.1111/j.1365-2486.2007.01510.x
Kaufman, D., McKay, N., Routson, C., Erb, M., Dätwyler, C., Sommer, P. S., et al. (2020). Holocene global mean surface temperature, a multi-method reconstruction approach. Sci. Data 7, 201. doi:10.1038/s41597-020-0530-7
Klimaszyk, P., and Rzymski, P. (2011). Surface runoff as a factor determining trophic state of midforest lake. Pol. J. Environ. Stud. 20, 1203–1210.
Kosten, S., Huszar, V. L. M., Bécares, E., Costa, L. S., van Donk, E., Hansson, L. A., et al. (2012). Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Change Biol. 18, 118–126. doi:10.1111/j.1365-2486.2011.02488.x
Kudela, R. M., Berdalet, E., Bernard, S., Burford, M., Fernand, L., Lu, S., et al. (2015). Harmful algal blooms. A scientific summary for policy makers. Paris: IOC/UNESCO. 1–20.
Leavitt, P. R., and Hodgson, D. A. (2001). “Sedimentary pigments,” in Tracking environmental change using lake sediments, volume 3: Terrestrial, algal, and siliceous indicators. Editors J. P. Smol, H. J. B. Birks, and W. M. Last (Dordrecht: Kluwer Academic Publishers), 295–325.
Li, F., Liu, J., Chen, G., Kong, L., and Zhang, X. (2020). A rapid late Holocene lake ecosystem shift driven by climate change: Evidence from the first cladoceran record from an alpine lake in northern China. Sci. Bull. 65, 253–256. doi:10.1016/j.scib.2019.12.010
Liu, J., Rühland, K. M., Chen, J., Xu, Y., Chen, S., Chen, Q., et al. (2017). Aerosol-weakened summer monsoons decrease lake fertilization on the Chinese Loess Plateau. Nat. Clim. Change 7, 190–194. doi:10.1038/nclimate3220
Liu, Z., Wen, X., Brady, E. C., Otto-Bliesner, B., Yu, G., Lu, H., et al. (2014a). Chinese cave records and the East Asia summer monsoon. Quat. Sci. Rev. 83, 115–128. doi:10.1016/j.quascirev.2013.10.021
Liu, Z., Zhu, J., Rosenthal, Y., Zhang, X., Otto-Bliesner, B. L., Timmermann, A., et al. (2014b). The Holocene temperature conundrum. Proc. Natl. Acad. Sci. U. S. A. 111, E3501–E3505. doi:10.1073/pnas.1407229111
Lürling, M., Mello, M. M., van Oosterhout, F., de Senerpont Domis, L., and Marinho, M. M. (2018). Response of natural cyanobacteria and algae assemblages to a nutrient pulse and elevated temperature. Front. Microbiol. 9, 1851. doi:10.3389/fmicb.2018.01851
Ma, J., Qin, B., Wu, P., Zhou, J., Niu, C., Deng, J., et al. (2015). Controlling cyanobacterial blooms by managing nutrient ratio and limitation in a large hyper-eutrophic lake: Lake Taihu, China. J. Environ. Sci. 27, 80–86. doi:10.1016/j.jes.2014.05.042
Marcott, S. A., Shakun, J. D., Clark, P. U., and Mix, A. C. (2013). A reconstruction of regional and global temperature for the past 11,300 years. Science 339, 1198–1201. doi:10.1126/science.1228026
Marsicek, J., Shuman, B. N., Bartlein, P. J., Shafer, S. L., and Brewer, S. (2018). Reconciling divergent trends and millennial variations in Holocene temperatures. Nature 554, 92–96. doi:10.1038/nature25464
McGowan, S. (2013). “Pigment studies,” in The encyclopedia of Quaternary science. Editor S. A. Elias (Amsterdam: Elsevier), 326–338.
Moorhouse, H. L., McGowan, S., Jones, M. D., Barker, P., Leavitt, P. R., Brayshaw, S. A., et al. (2014). Contrasting effects of nutrients and climate on algal communities in two lakes in the Windermere catchment since the late 19th century. Freshw. Biol. 59, 2605–2620. doi:10.1111/fwb.12457
Napiórkowska-Krzebietke, A., and Kobos, J. (2016). Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 104, 532–546. doi:10.1016/j.watres.2016.08.016
Osman, M. B., Tierney, J. E., Zhu, J., Tardif, R., Hakim, G. J., King, J., et al. (2021). Globally resolved surface temperatures since the Last Glacial Maximum. Nature 599, 239–244. doi:10.1038/s41586-021-03984-4
Paerl, H. W., and Barnard, M. A. (2020). Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 96, 101845. doi:10.1016/j.hal.2020.101845
Paerl, H. W. (2014). Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 4, 988–1012. doi:10.3390/life4040988
Paerl, H. W., and Otten, T. G. (2016). Duelling ‘CyanoHABs’: Unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-N2-fixing harmful cyanobacteria. Environ. Microbiol. 18, 316–324. doi:10.1111/1462-2920.13035
Paerl, H. W., Scott, J. T., McCarthy, M. J., Newell, S. E., Gardner, W. S., Havens, K. E., et al. (2016). It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 50, 10805–10813. doi:10.1021/acs.est.6b02575
Paerl, H. W., and Scott, J. T. (2010). Throwing fuel on the fire: Synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 44, 7756–7758. doi:10.1021/es102665e
Paltsev, A., and Creed, I. F. (2022). Multi-decadal changes in phytoplankton biomass in northern temperate lakes as seen through the prism of landscape properties. Glob. Change Biol. 28, 2272–2285. doi:10.1111/gcb.16079
Paterson, M. J., Schindler, D. W., Hecky, R. E., Findlay, D. L., and Rondeau, K. J. (2011). Comment: Lake 227 shows clearly that controlling inputs of nitrogen will not reduce or prevent eutrophication of lakes. Limnol. Oceanogr. 56, 1545–1547. doi:10.4319/lo.2011.56.4.1545
Qin, B., Zhou, J., Elser, J. J., Gardner, W. S., Deng, J., and Brookes, J. D. (2020). Water depth underpins the relative roles and fates of nitrogen and phosphorus in lakes. Environ. Sci. Technol. 54, 3191–3198. doi:10.1021/acs.est.9b05858
R Development Core Team, (2022). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Reichwaldt, E. S., and Ghadouani, A. (2012). Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 46, 1372–1393. doi:10.1016/j.watres.2011.11.052
Reimer, P. J., Austin, W. E. N., Bard, E., Bayliss, A., Blackwell, P. G., Bronk Ramsey, C., et al. (2020). The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0-55 cal kBP). Radiocarbon 62, 725–757. doi:10.1017/RDC.2020.41
Reinl, K. L., Brooks, J. D., Carey, C. C., Harris, T. D., Ibelings, B. W., Morales-Williams, A. M., et al. (2021). Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 66, 1846–1859. doi:10.1111/fwb.13791
Ren, X., Huang, X., Huang, C., Wang, T., Shen, Z., Zhang, X., et al. (2022). Effects of human activities on mountain forest in northern China during the middle Holocene. Quat. Sci. Rev. 288, 107580. doi:10.1016/j.quascirev.2022.107580
Reuss, N., and Conley, D. J. (2005). Effects of sediment storage conditions on pigment analyses. Limnol. Oceanogr. Methods 3, 477–487. doi:10.4319/lom.2005.3.477
Rigosi, A., Carey, C. C., Ibelings, B. W., and Brooks, J. D. (2014). The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 59, 99–114. doi:10.4319/lo.2014.59.1.0099
Schindler, D. W., Carpenter, S. R., Chapra, S. C., Hecky, R. E., and Orihel, D. M. (2016). Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 50, 8923–8929. doi:10.1021/acs.est.6b02204
Schindler, D. W., Hecky, R. E., Findlay, D. L., Stainton, M. P., Parker, B. P., Paterson, M. J., et al. (2008). Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. U. S. A. 105, 11254–11258. doi:10.1073/pnas.0805108105
Scott, J. T., and McCarthy, M. J. (2010). Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 55, 1265–1270. doi:10.4319/lo.2010.55.3.1265
Shen, Z., Liu, J., Xie, C., Zhang, X., and Chen, F. (2018). An environmental perturbation at AD 600 and subsequent human impacts recorded by multi-proxy records from the sediments of Lake Mayinghai, North China. Holocene 28, 1870–1880. doi:10.1177/0959683618798159
Shi, Y., Kong, Z., Wang, S., Tang, L., Wang, F., Yao, T., et al. (1994). The climatic fluctuation and important events of Holocene Megathermal in China. Sci. China Ser. B 3, 353–365. doi:10.1360/zb1992-22-12-1300
Sinha, E., Michalak, A. M., and Balaji, V. (2017). Eutrophication will increase during the 21st century as a result of precipitation changes. Science 357, 405–408. doi:10.1126/science.aan2409
Sivarajah, B., Simmatis, B., Favot, E. J., Palmer, M. J., and Smol, J. P. (2021). Eutrophication and climatic changes lead to unprecedented cyanobacterial blooms in a Canadian sub-Arctic landscape. Harmful Algae 105, 102036. doi:10.1016/j.hal.2021.102036
Smol, J. P. (2019). Under the radar: Long-term perspectives on ecological changes in lakes. Proc. R. Soc. B 286, 20190834. doi:10.1098/rspb.2019.0834
Smol, J. P., Wolfe, A. P., Birks, H. J. B., Douglas, M. S. V., Jones, V. V., Korhola, A., et al. (2005). Climate-driven regime shifts in the biological communities of Arctic lakes. Proc. Natl. Acad. Sci. U. S. A. 102, 4397–4402. doi:10.1073/pnas.0500245102
Tilman, D., Kilham, S. S., and Kilham, P. (1982). Phytoplankton community ecology: The role of limiting nutrients. Annu. Rev. Ecol. Syst. 13, 349–372. doi:10.1146/annurev.es.13.110182.002025
Trachsel, M., and Telford, R. J. (2017). All age-depth models are wrong, but are getting better. Holocene 27, 860–869. doi:10.1177/0959683616675939
Vautard, R., Cattiaux, J., Yiou, P., Thépaut, J. N., and Ciais, P. (2010). Northern Hemisphere atmospheric stilling partly attributed to an increase in surface roughness. Nat. Geosci. 3, 756–761. doi:10.1038/ngeo979
Wan, L., Chen, X., Deng, Q., Yang, L., Li, X., Zhang, J., et al. (2019). Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 84, 46–55. doi:10.1016/j.hal.2019.02.007
Wang, Q., Fan, X., and Wang, M. (2014). Recent warming amplification over high elevation regions across the globe. Clim. Dyn. 43, 87–101. doi:10.1007/s00382-013-1889-3
Wu, W., Li, G., Li, D., and Liu, Y. (2010). Temperature may be the dominating factor on the alternant succession of Aphanizomenon flos-aquae and Microcystis aeruginosa in Dianchi Lake. Fresenius Environ. Bull. 19, 846–853.
Xu, H., Paerl, H. W., Qin, B., Zhu, G., Hall, N. S., and Wu, Y. (2015). Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in eutrophic Lake Taihu, China. Environ. Sci. Technol. 49, 1051–1059. doi:10.1021/es503744q
Xu, Q., Chen, F., Zhang, S., Cao, X., Li, J., Li, Y., et al. (2017). Vegetation succession and East Asian summer monsoon changes since the last deglaciation inferred from high-resolution pollen record in Gonghai Lake, Shanxi Province, China. Holocene 27, 835–846. doi:10.1177/0959683616675941
Yan, X., Liu, J., Rühland, K. M., Smol, J. P., and Chen, F. (2022). Climate change as the dominant driver of recent ecological changes in a semi-arid alpine lake from the Chinese Loess Plateau. J. Paleolimnol. 68, 39–57. doi:10.1007/s10933-020-00167-5
Yan, Z., Ding, Y., Zhai, P., Song, L., Cao, L., and Li, Z. (2020). Re-assessing climatic warming in China since 1900. J. Meteorol. Res. 34, 243–251. doi:10.1007/s13351-020-9839-6
Yu, T., Meng, W., Ongley, E., Li, Z., and Chen, J. (2010). Long-term variations and causal factors in nitrogen and phosphorus transport in the Yellow River, China. Estuar. Coast. Shelf Sci. 86, 345–351. doi:10.1016/j.ecss.2009.05.014
Zhang, G., Zhang, P., Peng, S., Chen, Y., and Cao, Y. (2017). The coupling of leaf, litter, and soil nutrients in warm temperate forests in northwestern China. Sci. Rep. 7, 11754. doi:10.1038/s41598-017-12199-5
Zhang, J., Feng, J., Xie, S., Wang, S., and Shihui, W. (2012). Characteristics of phytoplankton community structures in Ningwu subalpine lakes, Shanxi Province. J. Lake Sci. 24, 117–122. (in Chinese). doi:10.18307/2012.0116
Keywords: paleolimnology, pigment, algae, cyanobacteria, mid-Holocene, Lake Mayinghai, Chinese Loess Plateau
Citation: Zhang X, Su L, Zhang J, Shen Z, Zhang S, Xu Q and Liu J (2023) Synergistic warming- and catchment-driven mid-Holocene cyanobacterial development: Pigment evidence from shallow eutrophic Lake Mayinghai on the Chinese Loess Plateau. Front. Earth Sci. 10:984420. doi: 10.3389/feart.2022.984420
Received: 02 July 2022; Accepted: 07 November 2022;
Published: 06 January 2023.
Edited by:
Larisa B. Nazarova, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), GermanyReviewed by:
Changchun Huang, Nanjing Normal University, ChinaBernhard Wehrli, ETH Zürich, Switzerland
Copyright © 2023 Zhang, Su, Zhang, Shen, Zhang, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosen Zhang,emhhbmd4czA4QGdtYWlsLmNvbQ==
 Xiaosen Zhang
Xiaosen Zhang Ling Su1
Ling Su1 Jianbao Liu
Jianbao Liu