
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci. , 18 November 2022
Sec. Quaternary Science, Geomorphology and Paleoenvironment
Volume 10 - 2022 | https://doi.org/10.3389/feart.2022.974582
This article is part of the Research Topic Living on the Edge - Interdisciplinary Perspectives on Coastal and Marine Ecosystems in Human Prehistory View all 12 articles
Klasies River is a key site in understanding the behaviour of coastal foragers in the southern Cape of South Africa. Faunal remains from Klasies River, recovered from an almost 70,000 year sequence over the Late Pleistocene, are a valuable dataset in investigating subsistence and occupational dynamics, yet few taphonomic studies have been conducted on the fauna from Klasies River. Here, the first taphonomic analyses of faunal remains from the Deacon and Wurz excavation is presented. The aim of this paper is to link occupational patterns during the Late Pleistocene at Klasies River with coastal ecology using taxonomic data from previous studies, and recently analysed taphonomic data. Taphonomic analyses of samples from the MSA II Lower, the Howiesons Poort and the MSA III periods were used to examine occupational intensity based on proportions of anthropogenic and zoogenic bone surface modification, trampling marks, transverse fractures and faunal density. Declining foraging efficiency or subsistence intensification was also investigated using indicators for expanded diet breadth (e.g., small fauna exploitation and increases in juveniles), more intensive marrow extraction and evidence for greater foraging distances. Herbivore functional types and dietary preferences were examined using taxonomic data and these show three main ecological phases at Klasies River: the MSA I; the upper and lower MSA II; and the HP and MSA III phases. Taphonomic data show increased occupational intensity in the MSA II Lower, with subsistence intensification also more evident here than other layers. Trampling data suggests that occupational intensity was greater in the earlier Howiesons Poort than later phase with little evidence of declining foraging efficiency then. The data indicate that, while humans contributed significantly to the MSA III assemblage, carnivores were the main accumulators here suggesting lower occupational intensity in this phase. The results of this study shows a possible link between increased occupational intensity and declining foraging efficiency at Klasies River but larger taphonomic samples are needed to explore this relationship further.
Examining how humans have systematically exploited coastal environments is an important means of understanding human behaviour during the Late Pleistocene (Parkington, 2001, 2003; Stringer, 2000; Joordens et al., 2009; Marean, 2010; Kyriacou et al., 2014; Jerardino, 2016a; Klein and Bird, 2016; Will et al., 2016, 2019; Niespolo et al., 2021). Given their rich resources and productive environments, coastal regions have probably always been desirable destinations for human settlements. Current population densities of coastal and near-coastal regions, for example, are significantly higher than that of other regions (Small and Nicholls, 2003). Their proximity to the shore is probably an important reason why coastal rockshelters are crucial repositories of Stone Age archaeology. Prehistoric human settlements at coastal locations were generally more intensely occupied when shorelines were closer (Wilkins et al., 2017; Reynard, 2022) suggesting that marine resource were key to settlement strategies (Loftus et al., 2019; Jerardino, 2021). Coastal habitats contain highly nutritious food resources. Shellfish and fish are high in vitamin B12, omega-3 fatty acids and iron (Öhrvik et al., 2012; Kyriacou et al., 2014) with seals providing valuable fats. Shellfish would have likely been the first coastal resource to be systematically exploited with evidence of its exploitation documented at c. 164,000 years ago (ka) at Pinnacle Point in the southern Cape of South Africa (Marean et al., 2007). Yet, while the focus is often on marine fauna, terrestrial mammals are vital food resources in coastal adaptation strategies (Marean et al., 2014). The southern Cape is a good example of how the coastal landscape and these various food resources were utilised by Middle Stone Age (MSA) people.
It has been suggested that the Cape Floristic Region (CFR) of the southern Cape—particularly its food resources—played an important role in human evolution in the later Pleistocene (Jerardino and Marean, 2010; Parkington, 2010; Marean, 2010, Faith, 2011; Marean et al., 2014; but see Brink 2016 and Wurz et al., 2018 on the important role of the interior of southern Africa). Besides highly nutritious marine resources along the coast, the CFR is rich in geophytes, which both modern and ancient people have exploited as a reliable source of high-quality carbohydrates (Marean, 2010; De Vynck J. C. et al., 2016a; Botha et al., 2019). The topography of the southern Cape is important in human development through the MSA. The region sits at the edge of the Agulhas Bank, a broad, shallow continental shelf off the coast of southern Africa. During glacial periods, marine regressions exposed a vast landmass referred to as either the Southern Coastal Plain (Compton, 2011) or the Palaeo-Agulhas Plain (Marean et al., 2020). This exposed landscape may have been up to c. 73,000 km2 during extreme glacial periods (Fisher et al., 2010; Marean et al., 2014) and would have had a significant impact on regional ecology. Marine regression would have opened up herbivore migratory routes and habitats (Marean et al., 2014). Productive floral environments and large terrestrial herbivores such as wildebeest (Connochaetes gnou), hartebeest (Alcelaphus buselaphus) and buffalo (Syncerus spp.) (Cowling et al., 2020; Reynard, 2021) demonstrate the diversity of food resources in this region, especially during marine regressions (Helm et al., 2020; Venter et al., 2020).
The southern Cape is also considered a critical region in our understanding of technological innovation and cognitive complexity (Deacon, 1989; Henshilwood et al., 2002, 2004; Marean et al., 2007, 2014; Wadley, 2015; Way et al., 2022; but see Wilkins et al., 2021 on innovation in the interior). Heat-treatment in the manufacture of lithic tools may be evident at the c. 164 ka layers at Pinnacle Point (Brown et al., 2009) and the ability to produce ‘paint’ from ochre is apparent in the 100 ka layers at Blombos Cave (Henshilwood et al., 2011). The recovery of shell-beads and engraved ochre from Blombos Cave suggests symbolically-mediated behaviour before 70 ka (Henshilwood et al., 2002, 2004). The Howiesons Poort (HP) techno-complex is especially significant in that it is associated with engraved ostrich eggshell, microlithics, complex hafting and increased social connectivity (Texier et al., 2010; Henshilwood et al., 2014; Way et al., 2022). The backed lithic artefacts of the HP suggest significant advancements in hunting strategies with the possible presence of bow-hunting by Marine Isotope Stage (MIS) 4 (Wurz and Lombard, 2007; Lombard and Phillipson, 2010).
Demographic pressure and its role in innovation is an important focus of current research (Mackay et al., 2014; Sealy, 2016; Archer, 2021; Wadley, 2021; Reynard, 2022). Many researchers suggest strong links between population expansions and the transmissibility of technological and cultural innovation (Ash and Gallup, 2007; Powell et al., 2009; Mackay et al., 2014). As populations expand, the argument goes, the chances of a novel idea or tool spreading to neighbouring populations increases (Henrich, 2004; Powell et al., 2009; Richerson et al., 2009). An important question is whether coastal environments had significant effects on demographic dynamics in the southern Cape. Changes in occupational intensity at coastal sites could be a useful means of inferring demographic trends, which may help us understand larger-scale settlement dynamics. In this regard, local-scale data from sites with long chronological sequence are especially valuable. Klasies River Main site (KRM) is an important site in documenting human behaviour in the MSA with a near-continuous Late Pleistocene sequence encompassing almost 70,000 years. The faunal remains from the site have had a significant impact on our understanding of bone taphonomy and hominin subsistence behaviour (Binford, 1984; Blumenschine, 1986; Marean and Kim, 1998; Pickering et al., 2003). An abundance of fauna was recovered from the original Singer and Wymer (1982) excavations and analysed in a seminal paper by Klein (1976). However, the significance of its implications for subsistence has been contentious (Binford, 1984; Thackeray, 1990; Bartram and Marean, 1999; Klein et al., 1999; Outram, 2000), with some suggesting that the sampling methods used to recover the fauna had biased any meaningful interpretations of the assemblage (Turner, 1989; Bartram and Marean, 1999; Marean et al., 2004. See discussion in Van Pletzen-Vos et al., 2019). Excavations by H.J. Deacon in the 1980s and 1990s used more modern methods where all faunal material was retained. His excavations sampled sections from the Singer and Wymer cuttings in a finer resolution incorporating natural stratigraphy (Deacon and Geleijnse, 1988). Beginning in 2014, excavations by Wurz and colleagues (2018) followed on from the protocols of the Deacon excavations. These excavation are still ongoing. It is from these two excavations that the analyses in this paper are based.
To explore coastal occupational intensity at a site like KRM, it is useful to examine both the taphonomic data and the environment of the site. Faunal remains offer a good opportunity to investigate both. Combining taxonomic and taphonomic analyses is useful in unpacking human subsistence behaviour (e.g., Faith J. T. 2013a), especially at sites with extensive chronological sequences such as KRM. Taphonomic analyses are particularly critical in understanding subsistence patterns, site formation and occupational intensity (Lyman, 1987, 2010; Reynard, 2022). Only two taphonomic studies (incorporating bone surface modification assessments) were undertaken on the faunal material from KRM (Binford, 1984; Milo, 1998). Both these studies, however, were conducted on the contentiously excavated Singer and Wymer material. Here, I present taphonomic analyses on fauna from the Deacon and Wurz excavations. Although preliminary, this is the first taphonomic study of either of these collections. The aim of this paper is to use these taphonomic data to investigate occupational intensity and to explore its relationship with the environment at KRM from c. 110 ka to c. 55 ka. This will be done by integrating previous palaeoecological analyses of the Deacon fauna with the recent taphonomic analyses of the Deacon and Wurz excavations.
In this study, zooarchaeological data are used to assess occupational intensity. Here, occupational intensity is defined as the size of the groups that occupied a site, the frequency of visits, and the duration of stay at a site (Munro, 2004; Haaland et al., 2021). It has been shown that various taphonomic indicators are a valuable means of investigating occupational intensity (Reynard and Henshilwood, 2018; Reynard, 2022). In addition to occupational intensity, declining foraging efficiency or subsistence intensification is also investigated. Occupational intensity can be linked to the over-exploitation of resources, with dietary diversification an important indicator of subsistence intensification (Stiner and Kuhn, 2006; Lupo et al., 2013). Here, I treat intensification and occupational intensity as two separate criteria. However, it is assumed that evidence of intensification at the site could inform on more intensive human occupations, and it is expected that there would be close links between more intensively occupied layers and intensification (sensu Boserup, 1965). The focus of this paper is not subsistence behaviour (future studies will focus on subsistence strategies through the sequence), rather, the taphonomic data presented here is used to document and explore shifts in occupational patterns and to assess whether these relate to environmental conditions.
The main objective of this study is to use taphonomic data to infer occupational intensity at KRM, and to compare these to taxonomic data as a proxy for the environment of the region. In assessing occupational intensity at KRM, the following criteria are used:
While measuring increased occupations at a site is complex (Haaland et al., 2021), taphonomic data may be a good proxy (Reynard, 2022). The relative proportions of anthropogenic versus zoogenic bone surface modifications (BSM) is often used to infer whether humans or carnivores were the dominant bone collector (e.g., Brain, 1981; Blumenschine, 1988), and these may be a useful means of assessing how frequently or intensively a site was occupied. Higher frequencies of tooth-marked bone may therefore reflect more intense carnivore activity, signifying lower human occupations in those periods (Thompson et al., 2017). It then follows that anthropogenic marks may be useful measures of higher occupations. In comparing taphonomic to micro-morphological and geoarchaeological data, Reynard (2022) showed that higher frequencies of anthropogenic marks such as percussion and cut marks correlate well with increased occupations along the southern Cape coast. This is not to suggest that higher occupational intensity is intrinsically linked to more intensive butchery events at a site, but rather that it is likely that increased site-use activity would result in a greater probability of faunal remains being modified or damaged by people. Other taphonomic modifications such as burnt bone and trampling marks are also useful indicators of occupation since these are closely linked to anthropogenic activities in rockshelters (Marean, 2010; Reynard and Henshilwood, 2018).
Higher frequencies of transverse fractures may be a useful indicator of more intensive occupations at archaeological sites. It has been shown that higher frequencies of transverse fractures in MSA assemblages along the southern Cape coast correlate well with more intense occupations (Reynard, 2022). It is likely that the relationship between transverse breakage and occupational intensity may be the result of the accumulated weight of occupants within enclosed rockshelters. However, geomorphic and site formation processes such as rock falls may also account for higher frequencies of transverse fractures. An increase in transverse breaks has been linked to sediment compaction (Villa and Mahieu, 1991).
Artefact, lithic and bone density is often used to inform on occupational intensity (e.g., Shiffer, 1987; Mitchell, 1993; Marean 2010; Reynard et al., 2016). However, volumetric density may be affected by sediment accumulation rates (Jerardino, 1995; Jerardino, 2016b) and faunal density may also be influences by carnivore activity at a site. That said, faunal density may be a useful proxy for greater occupational intensity in southern Cape MSA sites (Reynard, 2022).
Another objective of this study is to investigate evidence for intensification at KRM. Subsistence intensification can be defined as the extraction of increased energy from food resources at the expense of foraging efficiency (Munro 2009: 141). The diet breadth model predicts that low-ranked prey are added to the diet as the abundance of high-ranked prey declines (MacArthur and Pianka, 1966; Dusseldorp, 2012). Various constraints and costs aside, prey is usually ranked according to body size, with body size a proxy for caloric value. Intensification—or declining foraging efficiency—is associated with a broadening of diet breadth and more intensive—and costly—carcass processing. Initially, intensification entailed increases in economic productivity linked to population expansions (Boserup, 1965) and was later used to explain declining foraging efficiency at the onset of domestication (Flannery, 1969). Intensification has been widely used to contextualise MSA subsistence strategies and has been linked to occupational intensity and demographic pressure (Stiner et al., 1999; Munro, 2009; Steele and Klein, 2009; Clark, 2011; Dusseldorp, 2012; Dusseldorp and Langejans, 2013; Reynard and Henshilwood, 2017). Yet environmental-induced stress may be an equally significant factor in intensification (Winterhalder and Smith, 2000; Clark, 2011; Morgan, 2015). It is important to reiterate that, here, intensification is not assumed to be intrinsically linked to greater occupational intensity, but rather the objective is to examine whether any relationship between them exists. I follow Reynard and Henshilwood (2017) and use the following three indicators of intensification:
Under the diet breadth model, increases in lower-ranked, smaller prey would indicate declining foraging efficiency given the higher search and handling costs of hunting small fauna (Dusseldorp, 2012). Small fauna associated with the southern Cape region during the MSA includes hyrax (Procavia capensis), Cape dune molerats (Bathyergus suillus), size 1 bovids and shellfish. Juveniles would be considered lower ranked prey because they have less meat and fat than adults so increase in juveniles may also reflect a broadening diet breadth (Munro, 2004; Clark, 2011). Fat is a particularly important criterion in prey selection (Brink, 1997) and with juveniles particularly lacking fat, they were unlikely to have been targeted by hunters. Thus, while seasonality of foraging expeditions may affect juvenile abundance, Speth and Clark (2006, p. 15) argue that, given their low-rank as a source of lipids, seasonal fluctuations in the abundance of juveniles should have little effect on prey choices.
Marrow extraction from high-utility long bones is common at Stone Age sites. During periods of resource stress, it is assumed that more marrow and meat-scarce (low-utility) elements would be exploited (Binford, 1978; Morin, 2007). It is expected that elements with little marrow such as phalanges, pelves and mandibles would more likely be processed with more evidence of percussion marks on them. Because carpal and tarsal bones are mostly dense cancellous bone with virtually no marrow fat (Marean 1991), these were excluded.
Under the central place forager prey choice model, it is expected that foraging is more likely to have occurred closer to base-camp, and that increases in foraging distances implies a decline in hunting efficiency (Cannon, 2003). The patch choice model also predicts that foragers exploit productive regions (or ‘patches’)—usually close to home—and only move to another area once the productivity of the patch has dropped below a specific threshold (Winterhalder, 2001). Intensification may be evident when foraging distances increase (Faith, 2007; Clark, 2011). Ethnographic studies suggest that foragers are more likely to transport high meat-yielding post-cranial bones than skulls back to base-camp (O'Connell et al., 1988; Monahan, 1998). Statistical models by Schoville and Otárola-Castillo (2014) show that skull abundance is a particularly good proxy for transport distances, with skulls for large ungulates decreasing significantly as distances to kill-sites increase.
Klasies River Main site (KRM) is on the Tsitsikamma coast in the southern Cape, and consists of two caves, 1 and 2, and two shallow overhangs, caves 1A and 1B. Together, these contain more than 21 m of deposits (Wurz et al., 2018). The faunal remains examined in this paper were recovered from caves 1 and 1A (Figure 1). The lowermost deposits in the Witness Baulk in cave 1, and squares AA43–Z44—the overlap of cave 1 and 1A—are associated with MSA I artefacts (also termed MSA 2a) (Volman, 1984) and Klasies River (Wurz, 2002) techno-complexes) and dated to MIS 5e (Wurz, 2002). Layers overlying the MSA I deposits are associated with MSA II artefacts (MSA 2b (Volman, 1984) or Mossel Bay (Wurz, 2002)) and are dated to c. 101 ka at the base and c. 77 ka at the top (Vogel, 2001). The MSA II deposits are divided into MSA II Upper and Lower (Wurz, 2002). U-Th dates from the base of MSA II Lower indicates it formed prior to MIS 5c (c. 100 ka) (Wurz et al., 2018). Recently, U-Th dating of flowstone on tufa that fell into layer HHH in the MSA II Lower deposits obtained an age of 126.0 ± 1.5 ka (Wurz et al., 2018). MSA I deposits lie almost a metre below this layer, likely representing several thousand years of occupations (cf. Deacon and Geleijnse, 1988). This suggests that the MSA I is probably significantly earlier than previously described, and may date to early MIS 5e or the MIS 5e/MIS 6 transition. The approximately 2 m thick HP and 1 m thick MSA III (post-HP) layers occur in the overlying Upper member of cave 1A. The HP currently dates to c. 65 ka and the MSA III to c. 57ka (Wurz, 2002; Grine et al., 2017). A more detailed discussion of the site chronology is in Morrissey et al., (2022).
Two key sources of data are used in this study. The first is taxonomic data from Van Pletzen’s analysis of the Deacon excavations (Van Pletzen, 2000; Van Pletzen-Vos et al., 2019). The second is taphonomic data from a sample of fauna analysed from the Deacon and Wurz excavations.
The faunal remains used to infer palaeoenvironments are from samples excavated by Hilary Deacon and colleagues between 1984 and 1995. Taxa were identified by Brink and Van Pletzen-Vos using the South African National Museums comparative faunal collections in Bloemfontein (Van Pletzen-Vos et al., 2019). Only mammals the size of, or larger than, the Cape dune molerat were analysed. The number of identified specimens (NISP) was used to quantify specimens following Klein and Cruz-Uribe (1984). Bovidae (bovids) not assigned to a Linnaean Family were categorised to size classes originally based on Klein (1976), but here are referred to using Brain’s (1974) nomenclature. Ungulate dietary preferences (i.e., grazers, browsers and mixed-feeders) and taxonomic diversity data are taken from Reynard and Wurz (2020). All Raphicerus species were identified as Cape grysbok (Raphicerus melanotis) (Table 3; Klein, 1976; Van Pletzen-Vos et al., 2019) but to account for the possibility of the inclusion of some steenbok (R. campestris) in the assemblage, I use Raphicerus when referring to the Cape grysbok. Large mammal herbivores—defined here as bovid size class (Bov) 1 and larger—were assigned herbivore functional types and categorised into five groups based on Hempson et al. (2015) (Table 1). These herbivore groups encompass habitat characteristics of species which are closely linked to environmental conditions and regional ecology (Hempson et al., 2015).

TABLE 1. Herbivore functional groups at Klasies River based on Hempson et al. (2015).
Taphonomic data are from faunal samples from the Deacon and Wurz excavations. While the taxonomic data cover the entire Late Pleistocene sequence of KRM, taphonomic data are from samples from three periods at KRM: the MSA II Lower, the HP and the MSA III. The faunal remains taphonomically analysed from the MSA II Lower are from the lower Witness Baulk layers of cave 1 from the Wurz excavations (Table 2). The HP taphonomic samples are from the E50 and J51 units and MSA III sample is also from the E50 unit from the Deacon excavations of cave 1A.
In the taphonomic sample, indeterminate mammal specimens that could only be identified to element (e.g., cranial, rib and vertebral remains) were assigned to ‘small’, ‘medium’ or ‘large’ indeterminate mammal size classes. Small mammal is comparable to size 1 bovids, medium mammals to size 2 and 3 bovids, and large mammals to size 4 and 5 bovids. Specimens referred to as ‘identified small mammals’ are non-ruminant size 1 animals such as hyrax, Cape dune molerat and lagomorphs that could be identified to species.
All taxonomically identified specimens, piece-plotted specimens and specimens >2 cm were taphonomically analysed. Types of surface modification element, skeletal portion, side, and state of epiphyseal fusion were recorded for all specimens. Bone surface modifications (BSM) including cut, percussion and carnivore tooth marks, gastric acid etching, gnaw marks, weathering and rootlet etching were recorded following standard criteria (Behrensmeyer, 1978; Blumenschine and Selvaggio, 1988; Fisher, 1995; Blumenschine et al., 1996) using a Nikon binocular light microscope (10–4×0 magnification) under oblique, unidirectional lighting. Burning was reported for carbonised (mostly black) and calcined (mostly grey or white) specimens (Stiner et al., 1995). Trampling marks include lines and pits defined by Reynard (2014) on un-burnt specimens (Reynard and Henshilwood, 2018). The complete sample reported here includes all specimens identified to element, specimens >2 cm and unidentified long bone fragments (Reynard et al., 2014). A sub-set taphonomic sample was restricted to long bone mid-shafts following Thompson et al. (2017). These were used to assess whether humans or carnivores were the dominant accumulator of the assemblage by noting the proportion of percussion marks (PM) to tooth marks (TM) calculated as PM/(PM+TM) following Thompson et al. 2017. Values more than 50% suggest anthropogenically accumulated assemblages.
Transverse fracture analyses were based on fracture outlines for long bone mid-shafts (Villa and Mahieu, 1991). Because of small samples, all size classes were combined to assess fracture patterns. The circumference of long bone shafts was also noted under the assumption that more compete long bone shafts (i.e., those with more than half the circumference retained) are more indicative of carnivore activity (Bunn, 1983; Villa and Mahieu, 1991; Marean et al., 1992). More ‘splintered’ fragments was assumed to reflect human processing. Faunal density was measured using previously recorded excavation volumetric data from Thackeray (1988) and NISP data from Van Pletzen (2000). Density based on weight used MSA III and HP data from Pearson (2021) and MSA II Lower data from Lap (2020). Because small fauna may have also been collected by carnivores and raptors, only taxa with taphonomic evidence of human accumulation are considered when examining small mammal exploitation. The abundance of percussion marks on phalanges, pelves and mandibles were used to assess more intensive marrow extraction.
The proportion of skull versus long bone remains was used to investigate transport decisions. Only high-survival elements (i.e., high-density elements comprising the skull and long bones) are included in this study to limit the influence of bone density-mediated attrition (cf. Faith and Thompson, 2018). Here, skull remains include the crania, mandible and horn cores. Long bones include the forelimb (humerus and radius), the hindlimb (femur and tibia) and the distal limbs (metacarpal, metatarsal and metapodium). By using high survival elements, there was no need to ‘normalise’ the NISP of these elements. Because skull bones and long bones are paired (cf. Clark, 2013), raw NISP values can be used (the exception is metapodia, which was divided by two).
Taphonomic data between and within layers were grouped using Principal Component Analyses (PCA; PAST® free software; Hammer et al., 2001). A PCA incorporating anthropogenic and zoogenic BSM on small, medium and large mammal size classes was used to assess the impact of human and carnivore activity in the MSA II Lower, the HP and the MSA III. Data in PCA were rescaled to percentages. Hereafter, faunal remains from the Singer and Wymer excavations are referred to as the S-W sample, those from the Deacon excavations as the D sample and the Wurz excavations as the W sample. The faunal remains taphonomically analysed from both the D and W samples are referred to as the taphonomic sample.
Previous analyses of the D sample show that a broad range of taxa was recovered from KRM (Table 3). Identified small mammals are generally the most abundant (40.1% of identified species; NISP=612), followed by seals (30.1%; NISP=460), which are also the most common taxa in the S-W sample. As a percentage of identified taxa, seal remains are particularly common in the upper MSA II (39% of species in this period; NISP=110) and HP layers (57%; NISP=62), dropping significantly from the HP to the MSA III (χ2=80.747; df=1; p< 0.001). Raphicerus (6.6% of all identified species; NISP=101), eland (5.2%; NISP=80) and African buffalo (2.8%; NISP=43) are the most common bovids. Both the MSA III and MSA II Lower yield substantial numbers of identified small mammal remains. Raphicerus is a common fynbos species and relatively prevalent throughout the sequence. To compare the ratio of fynbos to grasslands at KRM, the proportion of Raphicerus (from the total number of identified species in each phase) was compared to the proportion of grazers throughout the KRM sequence. There is a significant inverse relationship between the frequencies of Raphicerus and grazers (rs = -0.9; p= 0.0374).
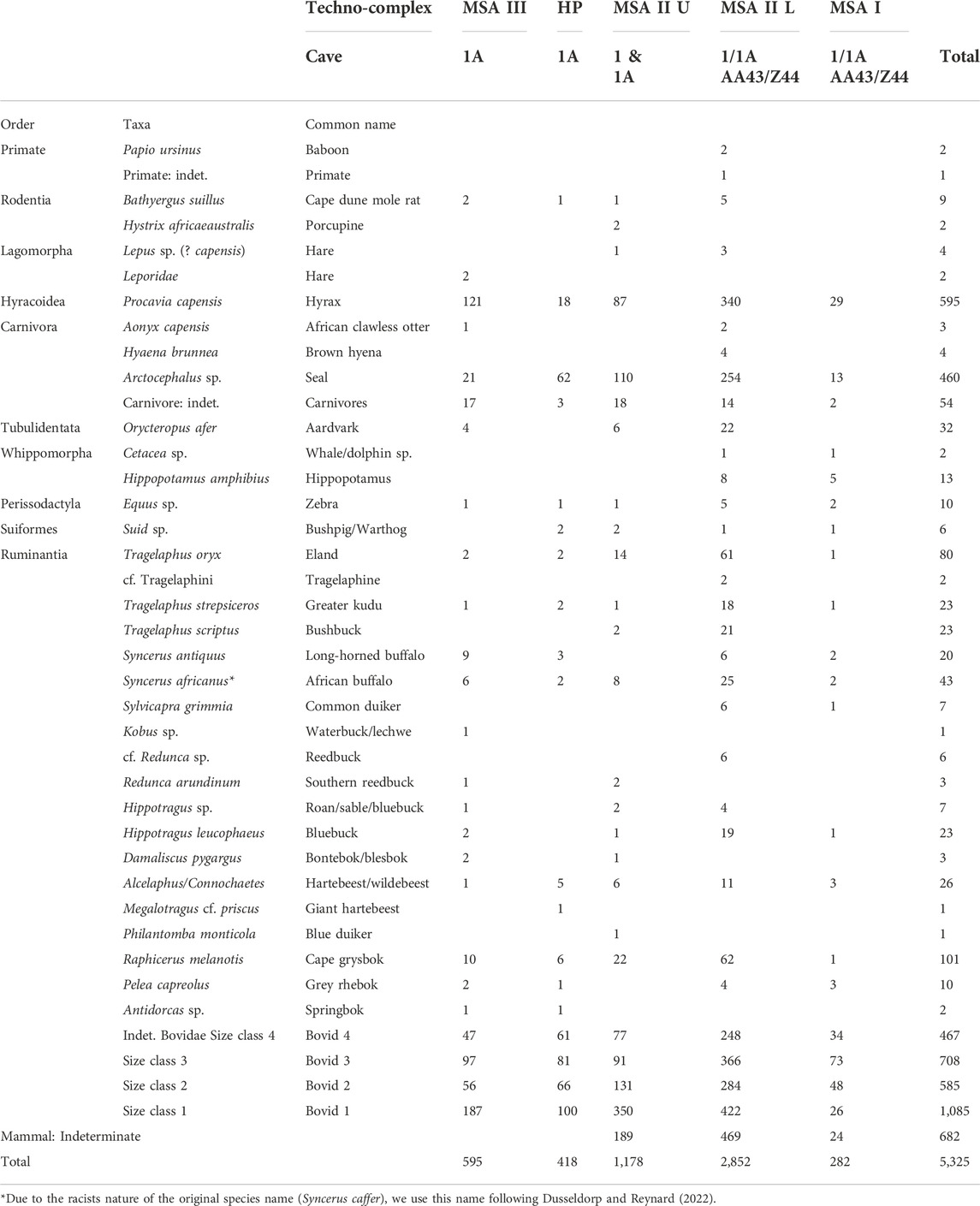
TABLE 3. Number of identified specimens (NISP) in cave 1 and 1A at Klasies River. Data from Reynard and Wurz (2020). Taxonomic names based on Skinner and Chimimba (2005).
The various herbivore categories show that the MSA I was significantly different to the other periods. In terms of ungulate dietary preferences, grazers are proportionally higher in the MSA I (68.2%; NISP=15) than in any other phase (Figure 2A). There is a significant decrease in grazer proportions from the MSA I to the MSA II Lower (Table 4). While grazers are proportionally quite prevalent in the MSA III (60%; NISP=24) and HP (50%; NISP=12), there is no significant difference in grazer abundance between the HP and MSA III (Table 4). Grazers are not significantly different to mixed-browsers between these layers (χ2= 0.610; df=1; p=0.435). Herbivore types are generally not significantly different through the sequence (Figure 2B). Again, the exception here is the MSA I layer where a chi-squared test shows a significant difference between this layer and the lower MSA II (Table 4). A hierarchical cluster analysis using the UPGMA algorithm in the PAST4 package (Hammer et al., 2001) indicates three groupings for herbivore functional types (Figure 3). Group 1 encompasses the MSA I, the upper and lower MSA II layers encompass group 2 and group 3 includes the HP and MSA III.
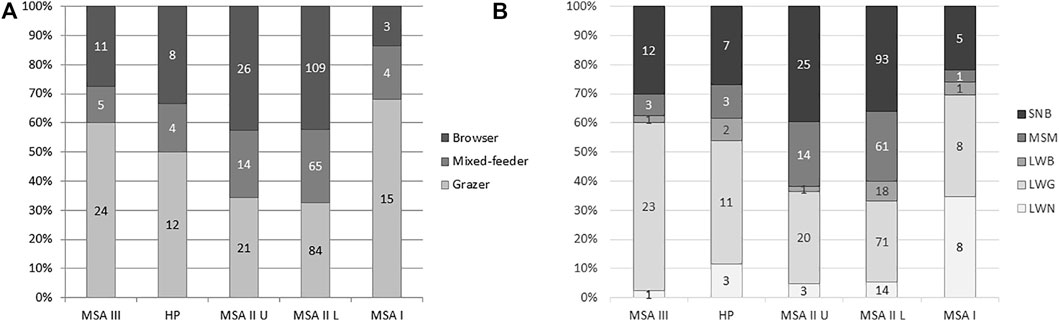
FIGURE 2. (A) Ungulate dietary preference proportions at Klasies River. (B) Herbivore functional type proportions based on Hempson et al. (2015). SNB = small non-social browser, MSM = medium-sized social mixed-diets, LWB = large water dependent browsers, LWG = large-medium water dependent grazers, LWN = large water dependent non-ruminants. Number of specimens in columns.
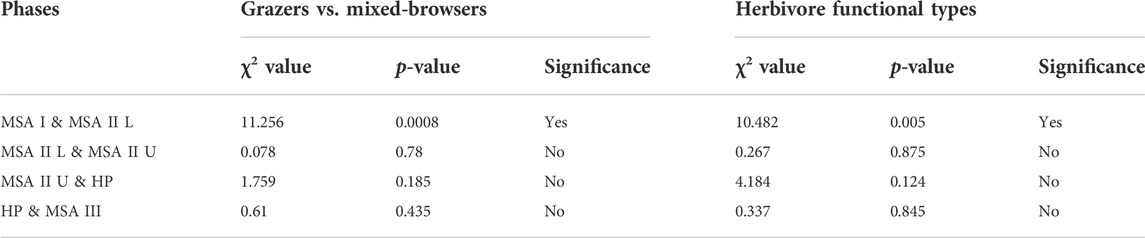
TABLE 4. Chi-squared tests of significance for ungulate categories at Klasies River. ‘Mixed-browsers’ are mixed-and intermediate feeders and browsers. Herbivore functional types based on Hempson et al. (2015).
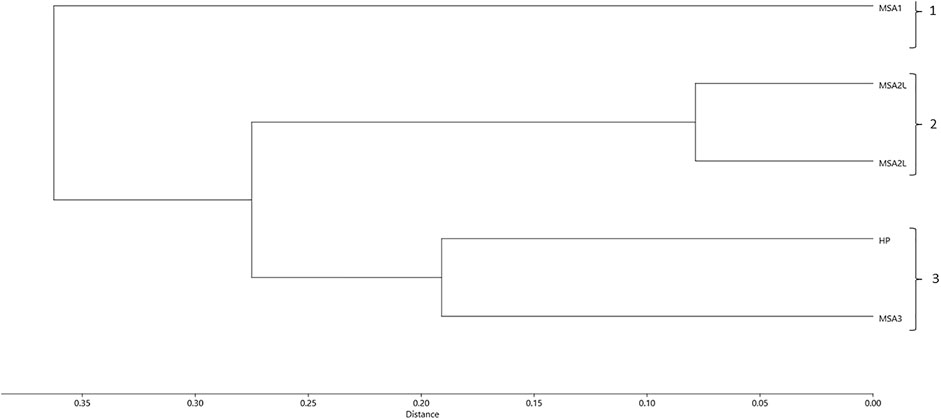
FIGURE 3. Cluster analysis of herbivore functional types based on Hempson et al. (2015) at Klasies River.
Skull and long bone profiles were constructed using the taphonomy sample with only patterns from the lower MSA II, the HP and MSA III presented. Skull abundance patterns in the MSA II Lower layers are notably different to the MSA III and HP (Figure 4). Here, skulls are more prevalent as animal size decreases and are significantly less common among large compared to medium mammals (χ2=14.076; df=1; p=0.0002). Skulls are less common in the HP (Figure 4) but more common in larger mammals. In the MSA III sample, skulls are substantially more common in larger mammals. In both the HP and MSA III, skulls become more prevalent as animal size increases.
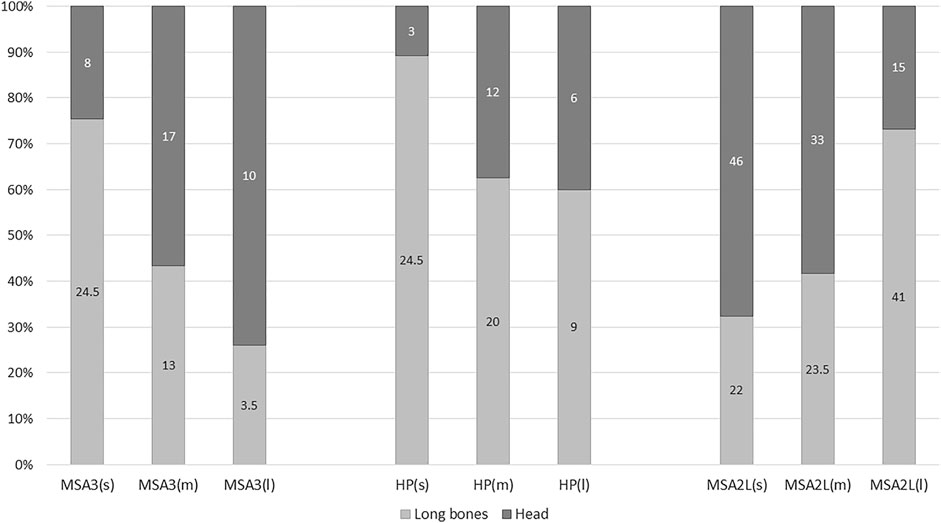
FIGURE 4. Head versus long bone distribution patterns for small (s), medium (m) and large mammals (l) at Klasies River. Number of specimens in columns.
The majority of assessable specimens have fused ends and could be considered adult (79.9%; n=444) with 20.1% (n=112) displaying unfused or recently-fused ends and considered juvenile (Figure 5). No juveniles were recovered from the MSA III, while 16.7% (n=39) of the HP sample are juvenile and 83.3% (n=194) are adult. The MSA II Lower sample contains significantly more juvenile specimens than the HP (χ2=34.114; df=1; p<0.0001) with 43.2% (n=73) classed as juvenile and 56.8% (n=96) considered adult. There is no significant difference in adults and juveniles in the MSA II Lower between small versus medium mammals (χ2=0.188; df=1; p=0.664) but a weakly significant difference between medium versus large mammals (χ2=3.611; df=1; p=0.057).
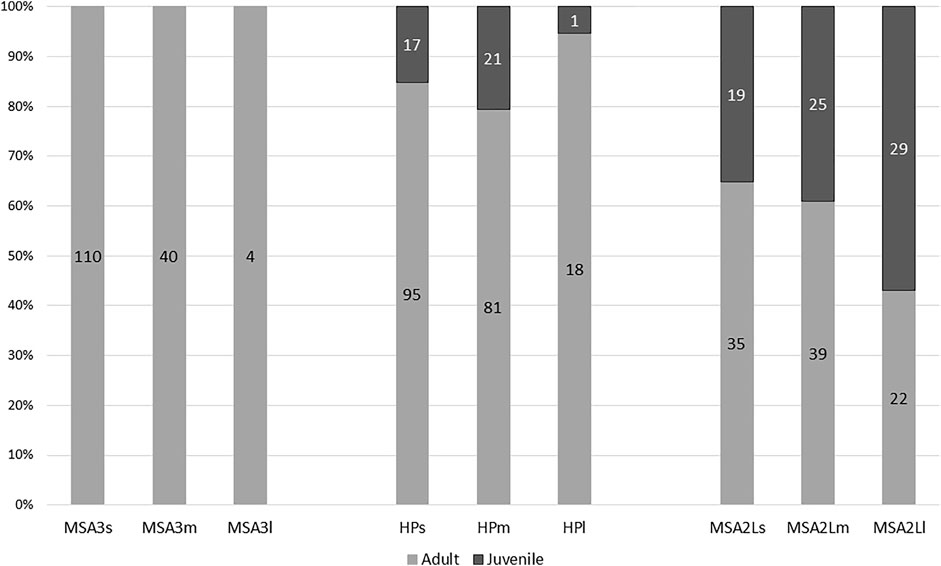
FIGURE 5. Mortality profiles for small (s), medium (m) and large mammals (l) at Klasies River. Number of specimens in columns.
Proportions of percussion versus tooth marks show that values for medium and large mammals were generally over 50% and likely anthropogenically accumulated (Table 5). The exception is large mammals in MSA II Lower (48.8%). Small mammals all suggest carnivore contributions since none are over 50%. The small mammal values from the MSA III is especially low (7.7%) indicating very little human contribution. Transverse fractures are relatively low with the highest values from the MSA II Lower layers (19.3%; n=56) and the lowest from MSA III (7.9%; n=14) (Table 5). Faunal density is significantly higher in MSA II Lower (789 NISP/m3; 159.1 kg/m3) compared to the MSA III (366 NISP/m3; 9.54 kg/m3) and HP (402 NISP/m3; 9.56 kg/m3) phases (Table 6). Trampling modification is more common in the HP phase (17.8%; n=411) and the least common in MSA III (4.4%; n=47) (Table 7). Tooth marks are the most prevalent BSMs in all phases at KRM (14.8%; n=778) with percussion marks also common (10.8%; n=569).
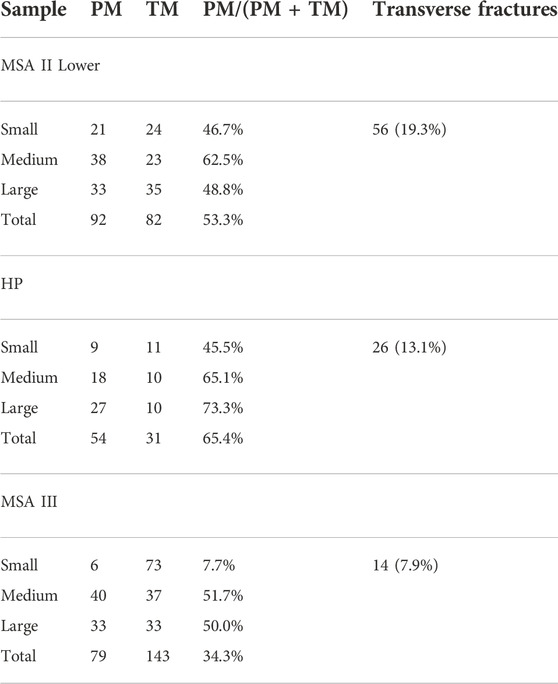
TABLE 5. The proportion of percussion (PM) versus tooth marks (TM) at Klasies River based on Thompson et al. 2017. Values above 50% indicate anthropogenically accumulated assemblages.
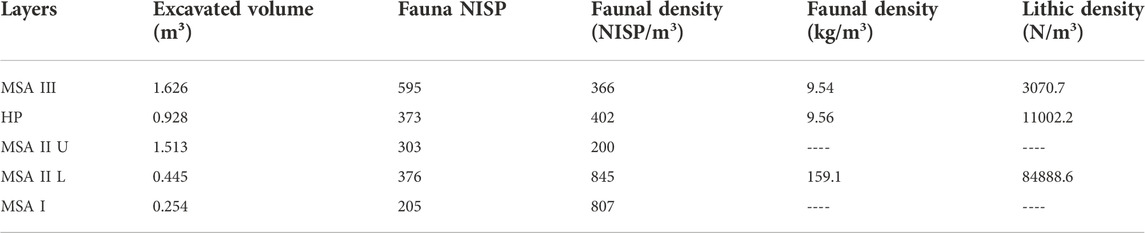
TABLE 6. Faunal and lithic density at Klasies River. Excavated volume of deposit from Thackeray (1988). Numbers of identified specimens (NISP) of fauna from Van Pletzen (2000). Faunal density data for weight (kg) based on data from Lap (2020) and Pearson (2021). Lithic density for MSA III and HP from Wurz (2000) using Thackeray’s (1988) excavated volumes. Lithic density for MSA II Lower from Brenner et al. (2022).
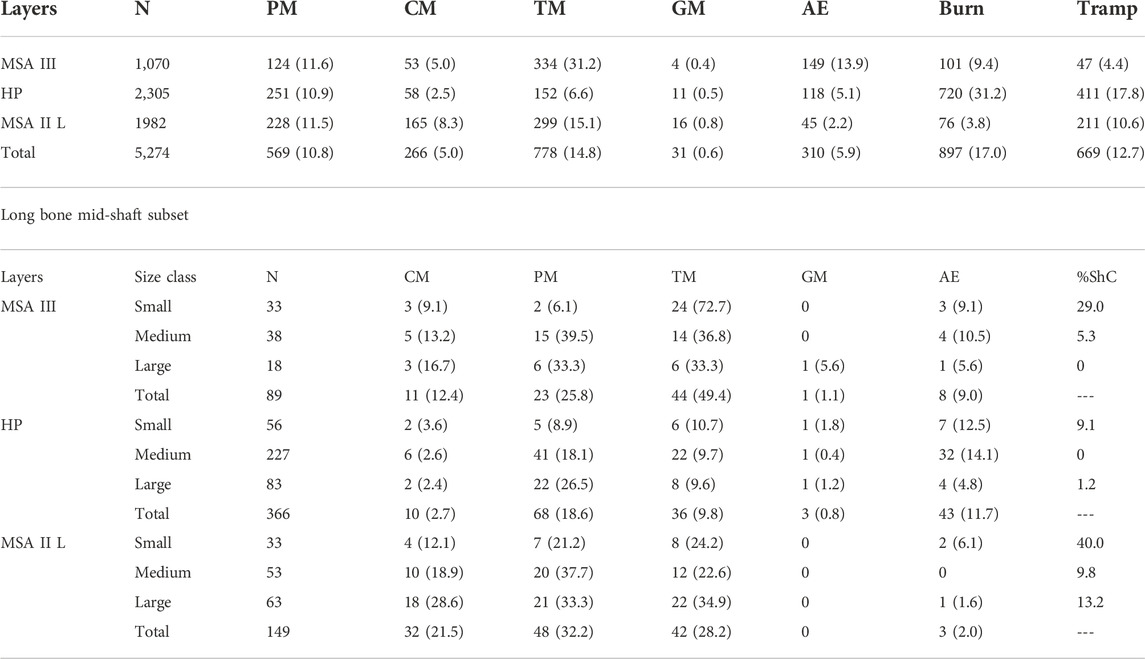
TABLE 7. Bone surface modification at Klasies River. Percentages in brackets. PM = percussion marks. CM = cut marks. TM = tooth marks. GM = gnaw marks. AE = acid etching. Burn = burnt bone. Tramp = trampling marks. %ShC = percentage of circumferentially complete long bone shafts.
No taphonomic analyses were conducted on remains in these layers but a study by Ezmeiro, 2022 on the faunal remains from the MSA I layers in cave 1B shows that burning was relatively common (13%; n= 2,520). No percussion marks were reported, and cut marks (0.3%; n=65) and tooth marks (0.02%; n=5) were rare.
Tooth marks are the most prevalent BSM in the MSA II Lower (15.1%; n=299) although percussion marks (11.5%; n=228) are also common (Table 7). Cut marks (8.3%; n=165) are significantly more common here than in the HP (χ2=72.916; df=1; p<0.0001). Despite the high frequency of tooth marks, acid etched bones (2.2%; n=45) are significantly less common in this phase compared to the HP (χ2=23.647; df=1; p<0.0001). Data from the long bone mid-shaft subset show that frequencies of tooth marks (35%; n=22) are almost on par with percussion marks (33%; n=21) in large mammals (Table 7). Cut marks (29%; n=18) are also common on large mammals and percussion marks are especially prevalent in medium mammals (38%; n=20). While tooth marks are common in all size classes (28.2%; n=42), percussion marks are slightly more prevalent (32.2%; n=48) (Table 5). Long bone shaft are less splintered in this phase, with 13% of large (n=7) and 40% of small mammal specimens (n=12) retaining a near-complete circumferences (Table 7).
In the HP, burning is the most dominant BSM (31.2%; n=720) and substantially higher here than in the other periods (Table 7). Percussion marks are also prevalent (10.9%; n=251). Tooth marks (6.6%; n=152) are significantly less common in this phases compared to the MSA II Lower (χ2=81.627; df=1; p<0.0001). Cut marks are not especially common (2.5%; n=58) but still within the norm for Stone Age assemblages (cf. Bunn, 1991). Trampling marks are significantly more prevalent in this phase compared to the MSA II Lower (χ2=44.352; df=1; p<0.0001). A breakdown of trampling marks per layer shows that trampling is significantly more prevalent in the early HP (layers CP6, CP9 and CPx4) than the later HP (CP5 and YS4) (Figure 6). The long bone subset shows that about 10% of small (n=6), medium (n=22) and large mammal (n=8) specimens display tooth marks (Table 7). Percussion marks are more prevalent on large (27%; n=22) rather than small mammal (9%; n=5) long bones, with relatively low cut mark frequencies for all size classes. Very few long bones retain near-complete circumferences with only one large mammal (1.2%) and no medium sized specimens.
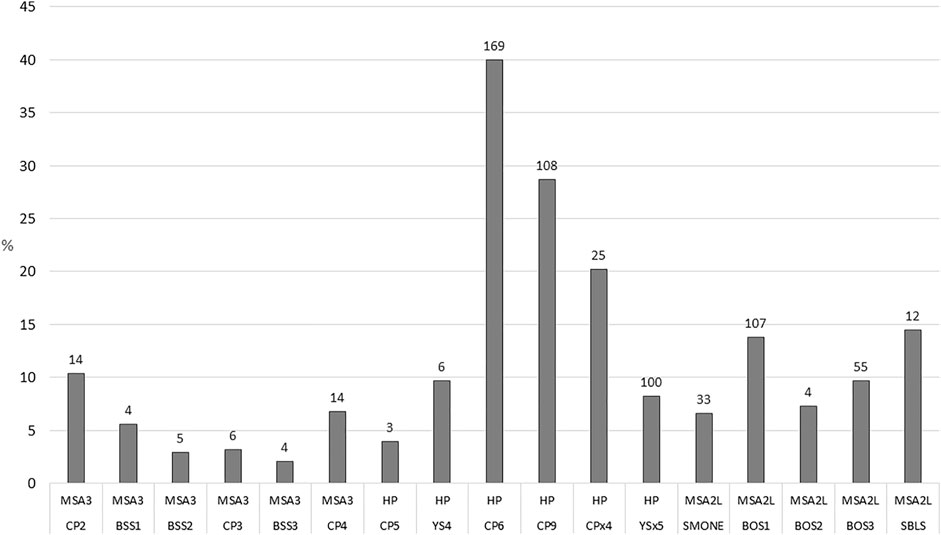
FIGURE 6. Frequencies of trampled bone in the MSA III, Howiesons Poort (HP) and MSA II Lower (L) phases per layer at Klasies River. Number of specimens above columns.
Evidence of carnivore activity such as tooth marks are especially prevalent in this phase (31.2%; n=334) and substantially higher than in other layers (Tables 5, 7). The frequency of gastric acid etching (13.9%; n=149) is also significantly higher here than in the HP (χ2=77.783; df=1; p<0.0001). A fair number of bones display percussion (11.6%; n=124) and cut marks (5%; n=53), and burning (9.4%; n=101). Regarding the long bone sunset, most small mammal bones display tooth marks (73%; n=24) with few percussion marks (6%; n=2). Compared to other size classes, more small mammals long bone retain near-complete circumferences (29%; n=9) (Table 7). Medium (40%; n=15) and large mammals (33%; n=6) generally have relatively high frequencies of percussion marks, with similar frequencies of tooth marks on medium (37%; n=14) and large mammals (33%; n=6). Only two long bones of medium mammals (5.3%) and no large mammal have relatively completed circumferences (Table 7).
To examine occupational patterns using anthropogenic (cut and percussion marks) and zoogenic (tooth marks and acid etching) BSM, a PCA was conducted incorporating small, medium and large mammals from the three periods under analysis (Figure 7). Components 1 and 2 explain 63% and 31% of the variance of the PCA, respectively. Positive values on component 1 and 2 for medium and large mammals from MSA III, and large mammals from MSA II Lower group these classes in the first quadrant. HP mammal classes are tightly bunched in the third quadrant with small mammals from MSA III isolated with significantly higher values on component 1.
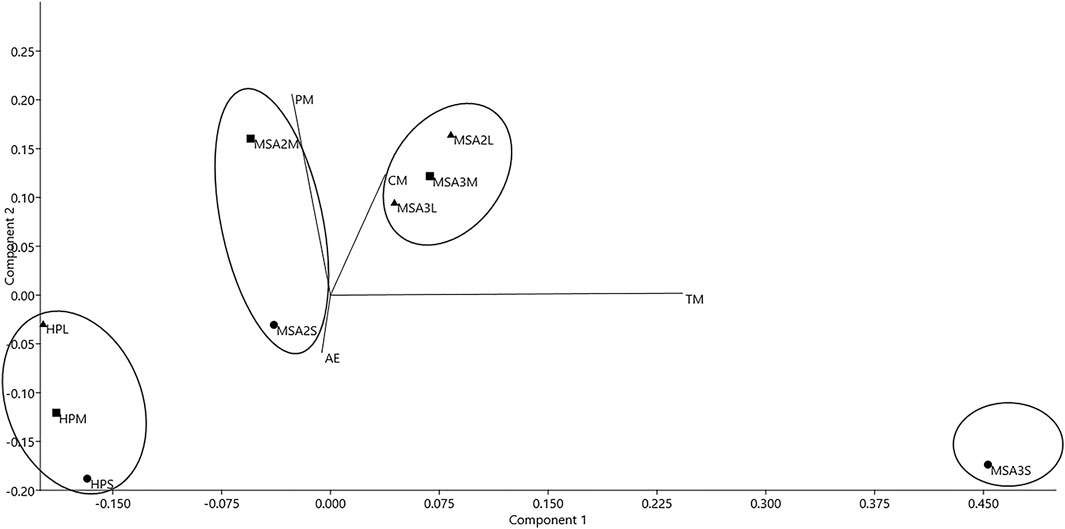
FIGURE 7. Principal component analysis of anthropogenic and zoogenic bone surface modification for small (s), medium (m) and large (l) mammal size class at Klasies River. Triangles are large mammals, squares are medium-sized mammals, and circles are small mammals. PM = percussion marks. CM = cut marks. TM = tooth marks. AE = acid etching.
Small mammals are more common in the MSA II Lower and MSA III but, as can be expected, few small mammals display anthropogenic marks (Table 8). Over 40% of size 1 mammals in the MSA II Lower layers are burnt (n=85), with percussion marks (10%; n=21) and anthropogenic marks (17.1%; n=36) relatively common in this phase. Anthropogenic marks are significantly more common on size 1 mammals in the MSA II Lower than in the HP layers (17.1%; n=36; χ2=5.214; df=1; p=0.022). Regarding identified small fauna, nine specimens (24.3%) in the MSA II Lower show evidence of burning with a cut mark visible of one hyrax specimen (2.7%). Half of the porcupine specimens (50%; n=5) and three rodent specimens (30%) are burnt. Percussion marks do not occur on any of these remains with no evidence of anthropogenic modification on Cape dune mole rate and hare specimens. Low utility element processing frequencies are highest in this phase (10.2%; n=6) although not significantly higher than those from the HP (χ2 =2.003; df=2; p=0.157) (Table 8).
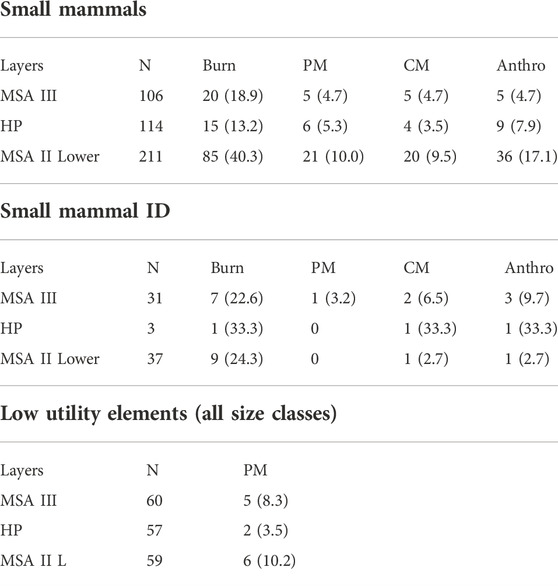
TABLE 8. Anthropogenic marks on small mammal remains and low utility elements. Burn = burnt bone, PM = percussion marks, CM = cut marks, Anthro = anthropogenic marks (cut and/or percussion marks per specimen). ‘Small mammals’ are all specimens identified to size 1 and smaller. ‘Small mammal ID’ are identified small mammals such as hyrax, Cape dune mole rat and porcupine. Low utility elements = phalanges, innominate and mandibles.
In the HP, 13% (n=15) of size 1 mammals are burnt with 8% (n=9) displaying anthropogenic marks (Table 8). Only three identified small mammals were noted in the sample with one burnt and one displaying cut marks. For all size classes in the HP, only two specimens display percussion marks (4%) on low utility specimens. In the MSA III, 19% (n=20) of size 1 mammals display burning modification. For identified small mammals (all of these identified as hyrax), 22.6% (n=7) are burnt. Few of these specimens show cut or percussion marks (8.5%; n=9) with only two cut marks (6.5%) and one percussion mark (3.2%) recorded (Table 8). Across all size classes in MSA III, there is no significant difference between anthropogenic marks on low utility bones (8%; n=5) and high utility bones (20%; n=11) (χ2 =3.261; df=1; p=0.071).
Generally, the region surrounding KRM had a fairly stable environment over the Late Pleistocene (Klein, 1976; Reynard and Wurz, 2020). However, the data presented here show evidence of environmental changes that may have had significant effects on regional ecology. Dietary categories and functional herbivore groups suggest three relatively distinct habitat clusters through the KRM sequence (Figure 3).
The first phase (group 1) corresponds to the MSA I with faunal data indicating a particularly significant difference between the MSA I and MSA II environments. Ungulate diversity indices are significantly higher in the MSA I compared to other phases with significantly more grazers here compared to the MSA II (Reynard and Wurz, 2020). Micromammal data also suggest that the MSA I was associated with a mosaic of grassland, fynbos and forested environs. Grassland-indicator species such as Soricidae and xeric four striped grass rats are relatively more common in the MSA I than MSA II Lower implying an MSA I environment dryer than the MSA II Lower (Nel et al., 2018). Analyses of lithic data also indicate that MSA I lithic end-products are generally significantly different to those in the preceding MSA II lower layers (Wurz et al., 2003).
The second ecological phase (group 2) comprises the MSA II layers. This is the largest recovered sample at KRM encompassing a palimpsest of deposits likely corresponding to MIS 5d-b. Here, browsers are the most common taxa suggesting a more closed environment (Figure 2B) corresponding to browse-dominated environs at the c. 100 ka layers at other MSA sites such as Blombos and Ysterfontein (Avery et al., 2008; Badenhorst et al., 2016; Niespolo et al., 2021). Ungulate diversity indices, such as species richness and Fisher’s alpha, are lowest in this phase (Reynard and Wurz, 2020). This may be linked to the MSA II being dominated by browse, with a wetter environment than currently and a mean annual precipitation above 750 mm/year (Cowling et al., 2020). The southern African vlei rat (Otomys irroratus), a species often occurring in Afromontane forests, also increases significantly from MSA I to MSA II (Nel et al., 2018). Wetter, more closed environs are often associated with lower herbivore biomass (Olff et al., 2002; Faith J. T., 2013b), which could explain the lower ungulate diversity. Lithic data also indicate that the MSA II layers are closely aligned, and both these phases show more connection with each other than the MSA I (Wurz et al., 2003).
The third phase (group 3) encompasses the HP and MSA III in the upper layers. The HP and MSA III are usually associated with MIS 4 and 3, respectively (Reynard and Wurz, 2020). MIS 4 is a glacial period while the MIS 3 is variable interstadial phase linked to cooler periods with warmer oscillations (EPICA Community Members, 2004). The increase in grazer abundance in the HP from the browser dominated MSA II layers reflects a noticeable shift in terrestrial environment, likely linked to the advent of MIS 4. Other sites dated to MIS 4 such as Klipdrift Shelter and Die Kelders also show a significant increase in grazing herbivores (Klein and Cruz-Uribe, 2000; Reynard et al., 2016). The prevalence of long-horned (‘giant’) buffalo is indicative of the persistence of open, grassland-dominated environments in the MSA III. Klein (1976) also noted the presence of black wildebeest in this layer—some of the few examples of this species in the southern Cape at this time, which aligns with a more open environment. This is supported by the dominance of grass-indicator micromammals such as golden moles (Amblysomus hottentotus) (Nel et al., 2018).
Changes in the coastal environment may be linked to fluctuating shorelines off KRM. The continued presence of marine taxa such as shellfish and seal throughout the KRM sequence indicates that the site remained relatively close to the shoreline through much of the Late Pleistocene. However, the effects of glacial/interglacial-induced marine regressions combined with local offshore bathymetry meant that the site may have been over 30 km away from the coast during intense glacial periods (Dor, 2017). KRM would have been part of the eastern edge of the Palaeo-Agulhas Plain during glacial phases. This vast coastal plain would have encompassed diverse habitats with a narrow band of the shoreline dominated by fynbos vegetation, and more grasslands further inland (Marean et al., 2020). The significantly inverse correlation between the proportions of Raphicerus and grazers suggests a dominance of fynbos-indicator species when shorelines were close, with grassland-indicator species more prevalent during regression phases (Compton, 2011; Cowling et al., 2020). Reynard and Wurz (2020) have previously argued that, given new U-Th dates of tufa in the MSA II Lower deposits, the basal layers of MSA I may well date to the transition between MIS 5e and 6. Large herbivore data from multiple analyses of cave 1, 1A and 1B show a significant abundance of grazers in this period, concordant with MIS 6 sites such Pinnacle Point 30 (Klein, 1976; Rector and Reed, 2010; Van Pletzen-Vos et al., 2019; Ezmeiro, 2022). However, molluscan data indicate that this phase was likely deposited during an interglacial period (Langejans et al., 2017; Loftus et al., 2017). Furthermore, the D sample from MSA I is quite small so it is important not to overestimate statistically significant trends in this phase. Refined dates from these layers are therefore critical to establish the provenance of these chronologies.
Shellfish data are a particularly useful means of inferring coastal environments such as shore topography and sea surface temperatures (SST) (Kilburn and Rippey, 1982). KRM is located within the Algoa Marine Province, a marine biogeographical zone influenced by the relatively warm Agulhas current flowing south from the Indian Ocean (Kilburn and Tankard, 1975; Sink et al., 2005). Brown mussels (Perna perna) are the most prevalent species at the site (Thackeray, 1988). Virtually all the large shellfish specimens identified in the MSA I layers require a rocky shore environment, and are indicative of relatively warm SSTs (Langejans et al., 2017). This would be consistent with a closer shoreline and interglacial period associated with MIS 5e. Although slightly cooler than the MSA I, SSTs may not have varied significantly throughout MSA II (Loftus et al., 2017). Using Thackeray’s (1988) data, Loftus et al. (2017) argue that the relative stability of SST during MIS 5 at KRM suggests that these layers were generally deposited during interstadial periods (but see Brenner et al., 2022; Wurz et al., 2022). This may be associated with a short-lived sea-level stillstand at c.108 ka that is possibly linked to extensive dune deposition at c. 110 ka (Cawthra et al., 2018). More recent data from Brenner et al. (2022) show that, while Perna perna still dominate (%MNI= 39), alikreukel (Turbo sarmaticus) become almost equally common in the MSA II Lower (%MNI=29). Wurz et al. (2022) also indicate that opercula and the sizes of alikreukel are noticeably smaller in the MSA II Lower layers. Taken together, this could suggests a change in coastal ecology or foraging strategies in this phase. Shellfish density appears to drop significantly from the MSA II layers to the HP, suggesting a relatively distant shoreline from the site during MIS 4. Interestingly, species linked to warm waters are slightly more common in this period than in the preceding MSA II deposits—seemingly inconsistent with expected cooler, glacial MIS 4 conditions (Langejans et al., 2012, 2017). Compared to other layers, more shellfish from the MSA III prefer a sandy/muddy environment and indicator species suggest cooler SST (Langejans et al., 2017). This conforms to extensive marine regressions at this time.
Unlike shellfish, seal remains are a more complex proxy for shoreline distance since their occurrence along the coast would be linked to seal ecology, taphonomy and human transport decisions (Marean, 1986; Dusseldorp and Langejans, 2013). Most of the recovered seal remains are from the earliest HP layers, which may have been closer to the coast than the later periods (Klein, 1976; Reynard and Wurz, 2020). The abundance of seal remains in the HP are in stark contrast to the lack of shellfish in most HP layers. Seals also make up between 34% and 10% of identified remains from the various phases in the S-W sample (Marean, 1986). In the S-W sample, seal mortality profiles show a mixture of juvenile and adults suggesting that young seals were not necessary targeted at this time (Klein and Cruz-Uribe, 1996). However, in the taphonomic sample, almost 40% of the remains in the HP (36.8%; n= 16) and three out of the five specimens measurable in the MSA II Lower are juveniles (60%), with no juveniles identified in MSA III deposits from. This does imply that seasonality may have been a factor in coastal visits at KRM but research on seal seasonality is still ongoing (Richardson et al., in prep.).
The prevalence of species such as reedbuck, otter, Kobus spp. and hippo shows that wetlands would have been a prominent aspect of the Late Pleistocene habitat near KRM. Studies of the shoreline bathymetry shows undersea terraces and a prominent palaeo dune cordon off the coast of KRM (Van Andel, 1989; De Wet and Compton, 2021). This would have likely resulted in lagoons beyond the mouth of the Klasies River when sea levels regressed (Singer and Wymer, 1982; Van Andel, 1989). Wetland-linked species are proportionally the most common in the MSA I phase which suggest the presence of lagoons, vleis or floodplains at that time. Wetland-linked species are also common in the MSA III. The HP and MSA III also yield more sandy-beach and lagoon molluscan species (Langejans et al., 2017) which suggest that wetlands and/or lagoons played in important role in the ecology of the region then. The data suggest that wetland formations were more prevalent when shorelines receded (which again pose interesting questions about the nature of the glacial/interglacial positioning of the MSA I phase). One scenario is that the undersea terraces and dune cordon off the coast of KRM would have functioned as natural breakwater barriers aiding the formation of lagoons and attracting wetland-linked fauna apparent in these layers.
The taphonomic analyses reveal some interesting occupational trends. Tooth marks on bone are substantial throughout the sequence, showing that carnivore activity was always common at the site and that KRM was never continuously occupied by people. It is possible that the pervasiveness of carnivore activity, in especially the MSA III and MSA II Lower, may have obscured anthropogenic marks in these layers, but the fact that anthropogenic marks are higher in these layers suggests otherwise. Human/carnivore accumulation patterns indicate that human accumulation was more prevalent in the MSA II and HP, with carnivore ravaging a dominant feature of the MSA III (Table 5). Faunal density, anthropogenic BSM and transverse fracture patterns indicate higher occupational intensity during the MSA II Lower (although density values may have been affected by sediment compaction and depositional rates (Jerardino, 2016b)). The HP appears to be the phase least affected by carnivore activity suggesting more intensified human occupations in the HP layers analysed (Table 5). Other taphonomic proxies for occupational intensity such as trampling marks and burning (Reynard 2022) are relatively common in the MSA II Lower and exceptionally abundant in the HP (Table 7; Figure 6). By the MSA III, these marks become less common. Generally then, the HP—and particularly the MSA II Lower—show evidence for the most intensely occupied phases.
Interestingly, BSM associated with butchery are not as prevalent in the HP compared to other layers. Burnt bone is abundant in the HP and while burning often damages the cortical surfaces of bone making it problematic to identify BSM, cut marks (1.5%; n=19) and percussion marks (4.8%; n=60) are even less common in the unburnt sample. The lack of butchery marks may signify a change in subsistence behaviour then which will be discussed later. It must be noted that studies of modern Central African foragers show that butchery marks are not associated with intensification (Lupo et al., 2013), so cut mark data may be problematic in this regard. The MSA III displays a different taphonomic signature to the other layers. Small fauna are very prevalent in this layer but over 70% display tooth marks. Given the abundance of carnivore marks and gastric-etched bone, the MSA III was likely a low occupational phase. The site was probably often used as a carnivore or raptor den.
Although occupational intensity appears high in the MSA II Lower, there is also good evidence for significant carnivore contributions to this assemblage. This is the only period where large mammal human/carnivore proportions are below 50% (Table 5). The shaft circumference of large mammals are also significantly more complete in the MSA II (Table 7). This could mean that large carnivores were using the site as a den at this time; however, acid-etched bone is not common in this phase (Table 7). Micro-morphological analyses also show that occupational hiatuses occurred in this phase (Wurz et al., 2022), which may be apparent as periods of carnivore activity. It is likely then that, during these hiatuses in the MSA II, carnivores may have ravaged the remains of large herbivores. Binford (1984) interpreted the prevalence of tooth marks at KRM as evidence that scavenging was the dominant subsistence strategy. Yet carnivore ravaging of discarded bone is a common form of bone attrition at MSA sites (Marean et al., 1992). A more detailed discussion of subsistence behaviour in the MSA II is outside the scope of this paper and will be addressed in a forthcoming study (Reynard et al., in prep.). Suffice to say that the disparate patterns of large herbivore skeletal patterns between the MSA II and upper layers may indicate differential subsistence patterns between the earlier and later KRM sequence.
Evidence for subsistence intensification at KRM reveals some interesting patterns. Outside of the MSA III (where most small mammals were likely accumulated by carnivores or raptors), identified small mammals are not particularly common. Indeed, in the HP they are rare, making up less than 5% of the assemblage. The MSA II Lower yields a moderate amount of hyrax and small mammals, but relatively few of these species show evidence of human processing. Size 1 bovid processing is significantly higher in the MSA II Lower compared to the MSA III and HP (Table 8). Small bovids are not especially common in the MSA II Lower (Table 3) so higher frequencies of processed bone are probably not a by-product of larger sample sizes. Therefore this does suggest that, to a certain extent, people focused more on size 1 bovids in this phase.
Other evidence of intensification also occurs in the MSA II Lower. Juveniles are significantly more common here and low-ranked elements such as phalanges and pelves are significantly more likely to have been processed. The lack of large mammal crania suggests increasing transport distances or extensive foraging ranges during the MSA II Lower. In fact, while human-processed small fauna and low-utility element frequencies are low throughout the sequence, they appear to pulse in the MSA II Lower. High shellfish densities and the occurrence of fish and bird remains also suggest an expanded diet breadth for Klasies people then (Von den Driesch, 2004; Van Niekerk, 2011; Reynard et al., in prep.). Wurz et al. (2022) reports smaller sizes of Turbo sarmaticus opercula from the MSA II Lower layers which is often used as evidence for more intensive shellfish harvesting as large species become more depleted (Steele and Klein, 2009). Furthermore, micro-morphological data show intensive shell middening in the MSA II (Wurz, et al., 2022) indicating more intensified site-use activities at this time. Overall, the taphonomic and faunal data show more evidence of intensified resource extraction in the MSA II Lower than in other layers.
An important question is whether these data signify decline foraging efficiency or changing environmental conditions. While shellfish exploitation was clearly a significant subsistence strategy during the MSA II, by far the dominant species were Turbo and Perna, both high-ranked mollusc that could be accessed relatively easily (Langejans et al., 2012). If over-exploitation was a factor then we would expect more lower-ranked shellfish dominating the molluscan assemblage. However, Perna is a relatively low-ranked species and only rises in rank because it was mass collected and mass processed (Langejans et al., 2012). The abundance of both Turbo and Perna over lower-ranked species may be because these species are more likely to be transported over greater distances than other shellfish (Dusseldorp and Langejans, 2013).
Likewise, given its ubiquity in the region, we should see more evidence of the exploitation of small fauna such hyrax in the sample. However, any evidence of human processing on small fauna would be rare (Armstrong, 2016) since tools are not needed to process these taxa (Henshilwood, 1997). In any case, the sample from which hyrax were taphonomically analysed is significantly smaller than the larger D sample, so it is possible further analyses of larger samples could reveal more evidence of small fauna exploitation. Interestingly, while ungulate diversity is generally low, overall taxonomic diversity is quite high in the MSA II Lower with richness (NTAXA) and the richness index (NTAXA/logNISP) especially high (Reynard and Wurz, 2020). This suggests that as encounter rates with large herbivores decreased, the focus may have shifted to small game and non-ungulates. Dusseldorp and Langejans (2015) propose that small nocturnal animals began to be targeted in MIS 5 in the southern Cape, which, they argue, could be indicative of trapping and snaring at this time. Blue duiker (Philantomba monticola) are extraordinarily abundant in the c. 71 and c. 77 ka layers at Sibudu Cave—accounting for over 40% of the faunal assemblage in some layers—which suggests that remote capture may have been used in their procurement in the MIS 5 period there (Clark, 2019). This may explain the abundance of size 1 bovid processing in the MSA II Lower, with technology playing an important role in prey selection. Size 1 bovids are also generally associated with bushier habitats so the combined impact of environmental conditions and technology may have had a significant effect on prey selection.
If this indeed represents intensification, the question remains as to whether demographic pressure during MIS 5 may have contributed to this possible decline in foraging efficiency during MSA II. Bayesian skyline plots by Rito et al. (2019), p. 3 show no significant shifts in population expansions in southern Africa until between c. 20 and 15 ka. The demographic shift during the terminal Pleistocene may be linked to more evidence of intensification in the LSA compared to the MSA (Klein and Cruz-Uribe, 1996; Klein, 2000; Steele and Klein, 2009). The systematic, more intense resource extraction apparent in the Holocene may differ from the patterns seen during the MSA II at KRM because intensification in the LSA may have been associated with large-scale, regional demographic expansion. For example, the significantly smaller shellfish and tortoise sizes in the LSA compared to the MSA layers at sites that transcend both periods such as KRM, Blombos Cave and Die Kelders has been argued to suggest more intensive harvesting by LSA people (Henshilwood et al., 2001; Steele and Klein, 2009). In contrast, evidence of intensification during MSA II Lower may be linked to either environmental-induced stress, small-scale local demographic changes, or a combination of both. Generally, the relatively small sample sizes makes it challenging to unpack these taphonomic patterns. More taphonomic analyses of comparative samples, particularly from the MSA Upper and the MSA I, are needed to explore these issues further.
Trampling and burning data suggests that occupations may have been relatively intense in the HP at KRM, yet there is little indication of intensification here. While small bovids may have been targeted in the MSA II, this does not appear to be the case in the HP. Small mammals and size 1 bovids seemed to be a focus of prey selection at other HP sites such as Sibudu, Klipdrift Shelter and Die Kelders (Klein and Cruz-Uribe, 2000; Clark, 2017; Reynard and Henshilwood, 2017). Yet, in the HP at KRM, small mammals are relatively rare, and size 1 bovids are not particularly common. This is all the more surprising since there is good evidence that bow hunting occurred in the HP at KRM (Bradfield et al., 2020). The cognitive capacity associated with bow hunting (knowledge of remote capture), and the technology (elastic twine and high-tensile string), are closely linked to trapping and snaring, so it is reasonable to assume that people at that time would have had the know-how and ability to construct snares (Wadley, 2010). It may be that foraging strategies may not have been affected by resource depression here, with little need for a broadening of diet breadth. The low frequencies of anthropogenic marks during the HP may, in fact, reflect this decrease in hunting pressure which may be linked to changing settlement patterns at this time. Other studies have shown some evidence of increased social connectivity during the HP (Douze et al., 2018; Way et al., 2022) possibly linked to demographic expansion (Reynard et al., 2016; Rito et al., 2019; Archer, 2021), so the lack of evidence of intensification may suggest that declining foraging efficiency in the HP and occupational intensity are not necessary linked.
It is also possible that the relatively scant evidence of faunal processing during the HP may be linked to increased plant consumption. The Cape south coast is rich in geophytes and plants with underground storage organs, which would have been a key source of carbohydrates (De Vynck J. C. et al., 2016b). Micro-morphological analyses show that carbohydrate-rich plants were cooked at KRM at c. 120 ka and again in the HP (Larbey et al., 2019). Deacon (1993, p. 89) argued that the numerous small, circular hearths at KRM may contain burnt plant residues, and suggested that people in the Late Pleistocene may have ‘farmed’ geophytes by burning fields. It is possible that this type of strategy may have been more effective in grassier periods such as during the HP.
Non-faunal data such as lithics may also highlight occupational and mobility trends at KRM. Lithic density has been used to indicate increased occupational intensity (Shiffer, 1987; Deacon, 1984; Brenner et al., 2022), and the abundance of ‘non-local’ raw material is sometimes used to infer greater foraging ranges (e.g., Singer and Wymer, 1982). Barton and Riel-Salvatore (Barton et al., 1999; Riel-Salvatore and Barton, 2004; Barton and Riel-Salvatore, 2014) have proposed that higher frequencies of retouched lithics may signify more resource stress since retouch would conserve limited lithic resources by extending the life of a stone tool (Riel-Salvatore and Barton, 2004). Correlations between retouch and lithic density, they argue, may be associated with residential or logistical mobility (Barton and Riel-Salvatore, 2014).
Lithic data generally show similar patterns to the faunal data. Both lithic and faunal density is highest in the MSA II Lower (Table 6). Retouch is relatively rare at KRM with only 0.9% (n=22) of pieces retouched in the MSA II Lower. This is comparable to Blombos Cave and Pinnacle Point 13B where the overall percentages of retouch there are generally between 0.2 and 2% (Henshilwood et al., 2001; Thompson and Marean, 2008; Reynard and Henshilwood, 2018). Frequencies of retouch in the HP at KRM (2.6%; n=61) are similar to those at other HP deposits such as Klipdrift Shelter (Henshilwood et al., 2014). Informal retouch is generally slightly higher in the MSA II layers, with 13% (n=311) of pieces in the MSA II Lower notched, compared to 0.5% (n=11) and 0.9% (n=7) in the HP and MSA III, respectively. The relative abundance of notched lithics in the MSA II Lower suggests that tools were often re-used and may imply more lithic scarcity at this time. Non-local raw material (i.e., silcrete) is significantly more common in the HP compared to other periods at KRM (Wurz, 2000). However, silcrete was likely sourced from nearby beach cobbles and local bedrock which were probably relatively close to the site during marine regressions (Van Andel, 1989; Minichillo, 2006), so its prevalence may be linked to the manufacture of microlithic and backed tools in general (Will and Mackay, 2017). Overall, the lithic data support other research (e.g., Douze et al., 2018; Way et al., 2022) suggesting more extensive foraging ranges during the HP.
Occupational intensity may be linked to the regional environment. Size 1 bovids such as Raphicerus are ubiquitous in fynbos habitats and a key reason these bovid were targeted in the MSA II Lower may relate the predominance of fynbos vegetation then. However, small bovids were not only selected. As with all phases at KRM—and most MSA fauna (e.g., Thompson, 2010; Reynard and Henshilwood, 2019)—evidence of anthropogenic processing is more common on larger herbivores and, being high-ranked, these were preferentially targeted. Nevertheless, it again highlights the complexity of disentangling demographic and environmental factors in assessing resource intensification. This is not to say that hunting pressure was not a factor in increased small bovid exploitation here. Other data also show an increase in extraction of food resources at this time, and there is good evidence of higher occupations during the MSA II Lower.
The upper members (the HP and MSA III) display quite different occupational histories. The HP shows some conflicting occupational signals. On the one hand, the lack of anthropogenically-marked bone, the relatively low faunal densities and little-to-no evidence of over-hunting does not correspond with intense occupations. On the other hand, the high frequencies of burnt and trampled bone, and the relatively low incidence of tooth marks suggest that human activities were relatively intense in the HP. Other evidence such as the abundance of hearths (likely linked to high frequencies of burnt bone), high artefact densities and the sheer scale of HP anthropogenic deposits also imply more intense occupations (Wurz, 1997; Deacon and Wurz, 2005). Yet, the HP sequence has an extensive age range (Feathers, 2002; Jacobs and Roberts, 2017) consist of alternating dark, highly carbonised layers—representing higher occupational intensity—and light, sandy geogenic strata, probably reflecting less intense occupations (Singer and Wymer, 1982; Deacon and Geleijnse, 1988). The HP taphonomy sample reflects a time-averaged palimpsest and, in all likelihood, includes intensively occupied periods interspersed with occupational hiatuses (Achieng, 2019).
There is good evidence the MSA III was a less intense occupational phase. Besides the taphonomic data presented here, Singer and Wymer (1982, p. 21) observed that Layers 1 to 9—corresponding to MSA III—consists of mostly ‘sandy scree’, with ‘laminated ash’ occurring in the lower layers with some carbonised partings occurring throughout. This suggests that the MSA III was probably more intensely occupied in the earlier periods indicating gradually decreasing occupational intensity from the HP through the MSA III. It must also be noted that data from these phases are combined palimpsests made up of multiple, often thin, layers very likely representing variable occupational events. Evidence of increased occupational intensity in any phase is a time-averaging of numerous occupations over thousands of years, and does not necessary correspond to larger populations or increased demographic density. It may relate to more frequent occupational events or changes in mobility patterns (Fisher et al., 2010; Haaland et al., 2021). Furthermore, given that cave 1 and 1A had dissimilar taphonomic histories (Wurz et al., 2022), it can also be expected that site formation processes played a significant role in these deposits. Variations in depositional rates, formation events and water and aeolian processes may have affected the more exposed deposits in cave 1A (MSA III and HP), and the more sheltered assemblage in cave 1 (MSA II) differently (Morrissey et al., 2022). Given its importance and the fact that these issues exceed the scope of this paper, site formation processes will be addressed in a forthcoming study.
Although the HP and MSA III reflect different taphonomic signatures, they appear to share similar habitats (Figures 2, 3). Both periods seem to be marked by major marine regressions. In fact, the differences in skeletal part profiles between these layers and the MSA II may be linked to transport decisions resulting from shifting shorelines. Furthermore, more intensive occupations are often linked to a site’s proximity to the coast (Gravel-Miguel et al., 2022; Reynard, 2022), which may be the case at KRM. More intense occupations are evident in the early HP, for example, when the coast was likely closer, while the later HP shows more evidence of occupational hiatuses. This may explain the conflicting taphonomic signals for occupations in this phase since the palimpsests of deposits would reflect evidence of both more and less intense occupations. By all accounts, the shoreline during the MSA III phase was never close, hence its association with less intense occupations.
Occupational intensity could have been impacted by changing environmental conditions and available resources. The eastern regions of the southern Cape near KRM probably would not have been as affected by marine regressions as the more central regions near Blombos (Van Andel, 1989; Cawthra et al., 2020). Nevertheless, shifting land availability resulting from stadial/interstadial fluctuations would have had a destabilising effect on occupations at KRM as population densities change and the ecology of the area is disrupted (Marean et al., 2020). Even if the most significant effects of the Palaeo-Algulhas Plain contractions were further to the west, this would have still had a substantial impact on human landscape use and herbivore communities further east near KRM (Compton, 2011; Reynard and Wurz, 2020). Another factor could be changing precipitation. Processional forcing of rainfall regimes may be an important driver of environmental change in the south-eastern Cape (Partridge et al., 1997; Dupont et al., 2022) and this may have affected subsistence strategies and resource availability. The smaller shellfish and shifting species abundance reported by Wurz et al. (2022) may not reflect periods of anthropogenic intensification, but may rather point to environmental instability at this time. Any declining foraging efficiency during the MSA II Lower phase may be linked to environmentally-induced resource stress. In contrast, marine transgressions and more land availability during the MSA III and HP may correspond to lower population densities, with less evidence of intensification or increases in diet breadth. This is not to suggest that foraging groups were isolated and restricted within the southern coastal plain, rather that fluctuating shorelines, disrupted herbivore migrations and/or changing rainfall regimes together with an expected focus of occupations at the coast, may explain lower occupational intensity during the later HP and MSA III at KRM.
In this paper, taxonomic and taphonomic data were used to explore trends in occupational intensity during the Late Pleistocene at KRM. This study is an example of how zooarchaeological analyses incorporating taphonomic data can be used to examine the links between settlement patterns and the environment at long-sequence sites. Ungulate dietary preference and functional herbivore groups suggest three relatively distinct habitat clusters through the KRM sequence: the MSA I, the MSA II, and the HP and MSA III. Marine resources remain important at KRM throughout the sequence but shellfish contribution appears to diminish from the MSA II to the HP, probably linked to marine regressions. The MSA I shows a significantly different ecological pattern to the other layers. This may be linked to glacial/interglacial changes but it could also be a result of the small sample of specimens in the Deacon sample.
This is the first study to report on taphonomic data from the Deacon assemblage, and from the HP and MSA III periods at KRM. Various taphonomic indicators was used to explore occupational intensity and subsistence intensification in the MSA II Lower, the HP and the MSA III at KRM. Similar to the environmental data, the taphonomy suggest different taphonomic histories between the MSA II, and the HP and MSA III. While humans were the dominant bone accumulator in most layers, carnivore activity was common in all phases indicating that human occupations were never continuous and suggesting abundant occupational hiatuses in the sequence. This is especially evident in the MSA III where most small mammals were likely accumulated by non-humans. Faunal density, increased frequencies of transverse fractures and BSM data indicate that the MSA II Lower was the most intensely occupied period, with trampling marks and burning also suggesting intensive occupations in the early HP. This appears to be supported by other, non-faunal data such as lithic density and micro-morphological analyses (Wurz et al., 2022). Density, bone fracture pattern and BSM data show less intense occupations in the MSA III.
Subsistence intensification is more apparent in the MSA II Lower. There is more evidence of the exploitation of low-ranked fauna in this period. For example, size 1 mammals are significantly more processed in this phase than in any other, and mortality data indicate significantly more juveniles here than the other layers. Low-ranked elements such as phalanges, pelves and mandibles show more evidence of marrow extraction here as well. Large mammal cranial remains are significantly rarer in this phase, suggesting more extensive foraging ranges at this time. It is important to note that intensification in the Late Pleistocene was probably not as systematic and extensive as later periods of intensification in the LSA. Declining foraging efficiency in the MSA II Lower may be linked to changing environmental conditions and/or fluctuating available land. That said, the fact that intensive occupations and declining foraging efficiency are both prevalent in the MSA II Lower suggests that these two phenomena may be linked.
Lower occupational intensity during the later HP and MSA III appears to be associated with marine regressions during MIS 4 and 3. The data presented here and other studies (e.g., Villa et al., 2010; Pearson, 2021; Wurz et al., 2022) show that the transition from the HP to MSA III was not as abrupt as the combined data may suggest with a more gradual drop in occupational events through time, possibly as coastlines regressed. While changing depositional rates and other site formation processes linked to environmental change may have contributed to this, it is fair to assume that shifting occupational intensities can be associated with fluctuating population densities as shorelines migrate. This is supported by other research that indicates that occupations in coastal regions are more intense as shorelines become closer (e.g., Reynard, 2022; Gravel-Miguel et al., 2022). In the southern Cape, there may be a particularly close relationship between occupational intensity and marine regressions, and it is reasonable to suggest that contracting landscapes could have resulted in increased population densities during glacial periods. In contrast, marine transgression may have led to an increase in available land, lower population densities and less resource stress.
This study shows that, while occupational intensity may be associated with declining foraging efficiency in the MSA II Lower, this is not necessarily the case in all periods at KRM, or at different sites. The early HP at KRM, for example, although displaying good evidence of increased occupational intensity shows no indication of intensification. However, more data is needed to explore these factors. It is important to note that these are relative, site-specific datasets, and the evidence presented here of declining foraging efficiency in the MSA II is only in relation to the MSA III and HP at KRM. This study only reports on preliminary taphonomic analyses. Larger samples need to be taphonomically analysed and the MSA I in particular should be further studied.
The raw data supporting the conclusion of this article will be made available by the authors on request, without undue reservation.
The author confirms being the sole contributor of this work and has approved it for publication.
JR is funded by a South African Department of Science and Technology (DST) National Research Foundation (NRF), Thuthuka grant (grant # 129689). Any opinion, finding, conclusion or recommendation expressed in this article is that of the author, and the NRF does not accept any liability in this regard.
I thank Sarah Wurz, Amy Lap, Alexandra Pearson and Pam Achieng for permission to use their data. I am grateful to Manuel Will for inviting me to contribute to the Living on the Edge volume. I also thank the anonymous reviewers whose insightful comments improved this paper significantly.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GLD declared a past collaboration with the author to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achieng, P. (2019). A taphonomic analysis of the large ungulate mammal fauna from the Howiesons Poort sequence at Klasies River site. University of the Witwatersrand. Unpublished Masters Dissertation.
Archer, W. (2021). Carrying capacity, population density and the later Pleistocene expression of backed artefact manufacturing traditions in Africa. Phil. Trans. R. Soc. B 376 (1816), 20190716. doi:10.1098/rstb.2019.0716
Armstrong, A. (2016). Small mammal utilization by Middle Stone Age humans at Die Kelders cave 1 and Pinnacle Point site 5-6, western Cape Province, South Africa. J. Hum. Evol. 101, 17–44.
Ash, J., and Gallup, G. G. (2007). Paleoclimatic variation and brain expansion during human evolution. Hum. Nat. 18 (2), 109–124. doi:10.1007/s12110-007-9015-z
Avery, G., Halkett, D., Orton, J., Steele, T., Tusenius, M., and Klein, R. (2008). The Ysterfontein 1 Middle Stone Age rock shelter and the evolution of coastal foraging. S. Afri. Archaeol. Soc. Goodwin S. 10, 66–89.
Badenhorst, S., Van Niekerk, K. L., and Henshilwood, C. S. (2016). Large mammal remains from the 100 ka middle stone age layers of Blombos cave, South Africa. S. Afri. Archaeol. Bull. 71, 46–52.
Barton, C. M., and Riel-Salvatore, J. (2014). The formation of lithic assemblages. J. Archaeol. Sci. 46, 334–352. doi:10.1016/j.jas.2014.03.031
Barton, M., Bernabeu, J., Emili Aura, J., and Garcia, O. (1999). Land-use dynamics and socioeconomic change: An example from the Polop Alto Valley. Am. Antiq. 64, 609–634. doi:10.2307/2694208
Bartram, L. E., and Marean, C. W. (1999). Explaining the “Klasies pattern”: Kua ethnoarchaeology, the Die Kelders middle stone age archaeofauna, long bone fragmentation and carnivore ravaging. J. Archaeol. Sci. 26 (1), 9–29. doi:10.1006/jasc.1998.0291
Behrensmeyer, A. K. (1978). Taphonomic and ecological information from bone weathering. Paleobiol. 4, 150–162.
Blumenschine, R. J. (1988). An experimental model of the timing of hominid and carnivore influence on archaeological bone assemblages. J. Archaeol. Sci. 15 (5), 483–502. doi:10.1016/0305-4403(88)90078-7
Blumenschine, R. J. (1986). Carcass consumption sequences and the archaeological distinction of scavenging and hunting. J. Hum. Evol. 15, 639–659. doi:10.1016/s0047-2484(86)80002-1
Blumenschine, R. J., Marean, C. W., and Capaldo, S. D. (1996). Blind tests of inter-analyst correspondence and accuracy in the identification of cut marks, percussion marks, and carnivore tooth marks on bone surfaces. J. Archaeol. Sci. 23, 493–507. doi:10.1006/jasc.1996.0047
Blumenschine, R. J., and Selvaggio, M. M. (1988). Percussion marks on bone surfaces as a new diagnostic of hominid behaviour. Nature 333, 763–765. doi:10.1038/333763a0
Botha, M. S., Cowling, R. M., Esler, K. J., De Vynck, J., and Potts, A. J. (2019). Have humans living within the Greater Cape Floristic Region used the same plant species through time? S. Afr. J. Bot. 122, 11–20. doi:10.1016/j.sajb.2019.01.013
Bradfield, J., Lombard, M., Reynard, J., and Wurz, S. (2020). Further evidence for bow hunting and its implications more than 60 000 years ago: Results of a use-trace analysis of the bone point from Klasies River Main site, South Africa. Quat. Sci. Rev. 236, 106295. doi:10.1016/j.quascirev.2020.106295
Brain, C. K. (1974). Some suggested procedures in the analysis of bone accumulations from southern African Quaternary sites. Ann. Transvaal Mus. 29 (1), 1–8.
Brain, C. K. (1981). The hunters or the hunted? An introduction to african cave taphonomy. Chicago: University of Chicago Press.
Brenner, M. J., Ryano, K. P., and Wurz, S. (2022). Coastal adaptation at Klasies River main site during MIS 5c-d (93, 000–110, 000 years ago) from a southern Cape perspective. J. Isl. Coast. Archaeol. 17 (2), 218–245. doi:10.1080/15564894.2020.1774444
Brink, J. (2016). “Faunal evidence for mid- and late Quaternary environmental change in southern Africa,” in Quaternary environmental change in southern Africa: Physical and human Dimensions. Editors J. Knight, and S. Grab (Cambridge: Cambridge University Press), 284–305.
Brink, J. W. (1997). Fat content in leg bones of Bison bison, and applications to archaeology. J. Archaeol. Sci. 24, 259–274. doi:10.1006/jasc.1996.0109
Brown, K. S., Marean, C. W., Herries, A. I., Jacobs, Z., Tribolo, C., Braun, D., et al. (2009). Fire as an engineering tool of early modern humans. Science 325 (5942), 859–862. doi:10.1126/science.1175028
Bunn, H. T. (1991). A taphonomic perspective on the archaeology of human origins. Annu. Rev. Anthropol. 20 (1), 433–467. doi:10.1146/annurev.an.20.100191.002245
Bunn, H. T. (1983). “Comparative analysis of modern bone assemblages from a San hunter-gatherer camp in the Kalahari Desert, Botswana, and from a spotted hyena den near Nairobi, Kenya,” in Animals and archaeology: 1. Hunters and their prey. Editors J. Clutton-Brock, and C. Grigson (Oxford, 163, 143–148. BAR Internat. S.
Cannon, M. D. (2003). A model of central place forager prey choice and an application to faunal remains from the Mimbres Valley, New Mexico. J. Anthropol. Archaeol. 22 (1), 1–25. doi:10.1016/s0278-4165(03)00002-3
Cawthra, H. C., Anderson, R. J., De Vynck, J. C., Jacobs, Z., Jerardino, A., Kyriacou, K., et al. (2020). Migration of Pleistocene shorelines across the palaeo-Agulhas Plain: Evidence from dated sub-bottom profiles and archaeological shellfish assemblages. Quat. Sci. Rev. 235, 106107. doi:10.1016/j.quascirev.2019.106107
Cawthra, H. C., Jacobs, Z., Compton, J. S., Fisher, E. C., Karkanas, P., and Marean, C. W. (2018). Depositional and sea-level history from MIS 6 (Termination II) to MIS 3 on the southern continental shelf of South Africa. Quat. Sci. Rev. 181, 156–172. doi:10.1016/j.quascirev.2017.12.002
Clark, J. L. (2013). “Exploring the relationship between climate change and the decline of the Howieson’s Poort at Sibudu cave (south Africa),” in Zooarchaeology and modern human origins. Editors J. D. Speth, and J. L. Clark (Dordrecht: Springer), 9–18.
Clark, J. L. (2011). The evolution of human culture during the later Pleistocene: Using fauna to test models on the emergence and nature of “modern” human behavior. J. Anthropol. Archaeol. 30, 273–291. doi:10.1016/j.jaa.2011.04.002
Clark, J. L. (2017). The howieson's Poort fauna from Sibudu cave: Documenting continuity and change within middle stone age industries. J. Hum. Evol. 107, 49–70. doi:10.1016/j.jhevol.2017.03.002
Clark, J. L. (2019). The still Bay and pre-still Bay fauna from Sibudu cave: Taphonomic and taxonomic analysis of the macromammal remains from the Wadley excavations. J. Paleolit. Archaeol. 2, 26–73. doi:10.1007/s41982-019-0021-6
Compton, J. S. (2011). Pleistocene sea-level fluctuations and human evolution on the southern coastal plain of South Africa. Quat. Sci. Rev. 30, 506–527. doi:10.1016/j.quascirev.2010.12.012
Cowling, R. M., Potts, A. J., Franklin, J., Midgley, G. F., Engelbrecht, F., and Marean, C. W. (2020). Describing a drowned Pleistocene ecosystem: Last glacial Maximum vegetation reconstruction of the palaeo-Agulhas Plain. Quat. Sci. Rev. 235, 105866. doi:10.1016/j.quascirev.2019.105866
De Vynck, J. C., Cowling, R. M., Potts, A. J., and Marean, C. W. (2016b). Seasonal availability of edible underground and aboveground carbohydrate resources to human foragers on the Cape south coast, South Africa. PeerJ 4, e1679. doi:10.7717/peerj.1679
De Vynck, J. C., Van Wyk, B. E., and Cowling, R. M. (2016a). Indigenous edible plant use by contemporary Khoe-San descendants of South Africa's Cape South Coast. S. Afr. J. Bot. 102, 60–69. doi:10.1016/j.sajb.2015.09.002
De Wet, W. M., and Compton, J. S. (2021). Bathymetry of the South African continental shelf. Geo-Mar. Lett. 41 (3), 40–19. doi:10.1007/s00367-021-00701-y
Deacon, H. J., and Geleijnse, V. B. (1988). The stratigraphy and sedimentology of the main site sequence, Klasies River, South Africa. South Afr. Archaeol. Bull. 43, 5–14. doi:10.2307/3887608
Deacon, H. J. (1989). “Late Pleistocene palaeoecology and archaeology in the southern cape, south Africa;” in The human revolution: Behavioural and biological perspectives on the origins of modern humans. 547–564.
Deacon, H. J. (1993). Planting an idea: An archaeology of stone age gatherers in south Africa. South Afr. Archaeol. Bull. 48, 86–93. doi:10.2307/3888947
Deacon, H. J., and Wurz, S. (2005). “A late Pleistocene archive of life at the coast, Klasies river,” in African archaeology: A critical introduction. Editor A. Stahl (Oxford, Blackwell, 213–228.
Deacon, J. (1984). The later stone age of southernmost Africa, 213. Oxford: British Archaeological Reports International Series.
Dor, G. (2017). Using GIS to model the changing Land-Ocean Interface for the southern coast of South Africa associated with the fluctuations in Sea level through an Important Period of human occupation between 190-40ka. Johannesburg: University of the Witwatersrand. Unpublished Honours report.
Douze, K., Delagnes, A., Wurz, S., and Henshilwood, C. S. (2018). The Howiesons Poort lithic sequence of Klipdrift shelter, southern cape, south Africa. PloS One 13 (11), e0206238. doi:10.1371/journal.pone.0206238
Dupont, L. M., Zhao, X., Charles, C., Faith, J. T., and Braun, D. (2022). Continuous vegetation record of the greater cape floristic region (south Africa) covering the past 300 000 years (IODP U1479). Clim. Past. 18, 1–21. doi:10.5194/cp-18-1-2022
Dusseldorp, G. L., and Langejans, G. H. J. (2013). Carry that weight: Coastal foraging and transport of marine resources during the South African middle stone age. S. Afri. Humanit. 25, 105–135.
Dusseldorp, G. L., and Langejans, G. H. J. (2015). ‘Trapping the past’? Hunting for remote capture techniques and planned coastal exploitation during MIS 5 at Blombos cave and Klasies river, south Africa. APL 45, 15–27.
Dusseldorp, G. L., and Reynard, J. P. (2022). “Faunal exploitation strategies during the later Pleistocene in southern Africa,” in Oxford research Encyclopedia of Anthropology. doi:10.1093/acrefore/9780190854584.013.559
Dusseldorp, G. L. (2012). Studying prehistoric hunting proficiency: Applying optimal foraging theory to the middle palaeolithic and middle stone age. Quat. Int. 252, 3–15. doi:10.1016/j.quaint.2011.04.024
EPICA Community Members (2004). Eight glacial cycles from an Antarctic ice core. Nature 429, 623–628. doi:10.1038/nature02599
Ezmeiro, J. (2022). Faunal exploitation and the palaeoenvironment during the middle stone age at Klasies cave 1B. Johannesburg: University of the Witwatersrand. Unpublished Masters Dissertation.
Faith, J. T. (2007). Changes in reindeer body part representation at Grotte XVI, Dordogne, France. J. Archaeol. Sci. 34, 2003–2011. doi:10.1016/j.jas.2007.01.014
Faith, J. T. (2013a). Taphonomic and paleoecological change in the large mammal sequence from Boomplaas Cave, Western Cape, South Africa. J. Hum. Evol. 65, 715–730. doi:10.1016/j.jhevol.2013.09.001
Faith, J. T., and Thompson, J. C. (2018). “Low-survival skeletal elements tracks attrition, not carcass transport behavior in Quaternary large mammal assemblages,” in Zooarchaeology in Practice: Case studies in methodology and interpretation in archaeofaunal analysis. Editors C. M. Giovas, and M. J. LeFebvre (Cham, Switzerland: Springer), 109–126.
Faith, J. T. (2011). Ungulate community richness, grazer extinctions, and human subsistence behavior in southern Africa's Cape Floral Region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 306, 219–227. doi:10.1016/j.palaeo.2011.04.025
Faith, J. T. (2013b). Ungulate diversity and precipitation history since the last glacial Maximum in the western cape, south Africa. Quat. Sci. Rev. 68, 191–199. doi:10.1016/j.quascirev.2013.02.016
Feathers, J. K. (2002). Luminescence dating in less than ideal conditions: Case studies from Klasies river main site and Duinefontein, south Africa. J. Archaeol. Sci. 29, 177–194. doi:10.1006/jasc.2001.0685
Fisher, E. C., Bar-Matthews, M., Jerardino, A., and Marean, C. W. (2010). Middle and Late Pleistocene paleoscape modeling along the southern coast of South Africa. Quat. Sci. Rev. 29, 1382–1398. doi:10.1016/j.quascirev.2010.01.015
Fisher, J. W. (1995). Bone surface modifications in zooarchaeology. J. Archaeol. Method Theory 2, 7–68. doi:10.1007/bf02228434
Flannery, K. V. (1969). “Origins and ecological effects of early domestication in Iran and the Near East,” in The Domestication and Exploitation of Plants and Animals. Editors P. J. Ucko, and G. W. Dimbleby (Chicago: Aldine), 73–100.
Gravel-Miguel, C., De Vynck, J., Wren, C. D., Murray, J. K., and Marean, C. W. (2022). Were prehistoric coastal sites more intensively occupied than inland sites? Using an agent-based model to understand the intensity of prehistoric coastal occupation, and what it means for studies on the evolution of the coastal adaptation. Quat. Int. 638/639, 148–158. doi:10.1016/j.quaint.2022.02.003
Grine, F. E., Wurz, S., and Marean, C. W. (2017). The Middle Stone Age human fossil record from Klasies River main site. J. Hum. Evol. 103, 53–78. doi:10.1016/j.jhevol.2016.12.001
Haaland, M. M., Miller, C. E., Unhammer, O. F., Reynard, J. P., van Niekerk, K. L., Ligouis, B., et al. (2021). Geoarchaeological investigation of occupation deposits in Blombos Cave in South Africa indicate changes in site use and settlement dynamics in the southern Cape during MIS 5b-4. Quat. Res. 100, 170–223. doi:10.1017/qua.2020.75
Hammer, Ø., Harper, D. A., and Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4 (1), 9.
Helm, C. W., Cawthra, H. C., Cowling, R. M., De Vynck, J. C., Lockley, M. G., Marean, C. W., et al. (2020). Pleistocene vertebrate tracksites on the Cape south coast of South Africa and their potential palaeoecological implications. Quat. Sci. Rev. 235, 105857. doi:10.1016/j.quascirev.2019.07.039
Hempson, G. P., Archibald, S., and Bond, W. J. (2015). A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350 (6264), 1056–1061. doi:10.1126/science.aac7978
Henrich, J. (2004). Demography and cultural evolution: How adaptive cultural processes can produce maladaptive losses – the tasmanian case. Am. Antiq. 69 (2), 197–214. doi:10.2307/4128416
Henshilwood, C. S. (1997). Identifying the collector: Evidence for human processing of the Cape dune mole-rat, Bathyergus suillus, from Blombos Cave, southern Cape, South Africa. J. Archaeol. Sci. 24 (7), 659–662.
Henshilwood, C., d’Errico, F., Vanhaeren, M., van Niekerk, K., and Jacobs, Z. (2004). Middle stone age shell beads from south Africa. Science 304, 404. doi:10.1126/science.1095905
Henshilwood, C. S., d’Errico, F., Van Niekerk, K. L., Coquinot, Y., Jacobs, Z., Lauritzen, S. E., et al. (2011). A 100, 000-year-old ochre-processing workshop at Blombos cave, south Africa. Science 334 (6053), 219–222. doi:10.1126/science.1211535
Henshilwood, C. S., d’Errico, F., Yates, R., Jacobs, Z., Tribolo, C., Duller, G. A. T., et al. (2002). Emergence of modern human behavior: Middle stone age engravings from south Africa. Science 295, 1278–1280. doi:10.1126/science.1067575
Henshilwood, C. S., Sealy, J. C., Yates, R., Cruz-Uribe, K., Goldberg, P., Grine, F. E., et al. (2001). Blombos cave, southern cape, south Africa: Preliminary report on the 1992–1999 excavations of the middle stone age levels. J. Archaeol. Sci. 28 (4), 421–448. doi:10.1006/jasc.2000.0638
Henshilwood, C. S., van Niekerk, K. L., Wurz, S., Delagnes, A., Armitage, S. J., Rifkin, R. F., et al. (2014). Klipdrift shelter, southern cape, south Africa: Preliminary report on the Howiesons Poort layers. J. Archaeol. Sci. 45, 284–303. doi:10.1016/j.jas.2014.01.033
Jacobs, Z., and Roberts, R. G. (2017). Single-grain OSL chronologies for the still Bay and Howiesons Poort industries and the transition between them: Further analyses and statistical modelling. J. Hum. Evol. 107, 1–13. doi:10.1016/j.jhevol.2017.02.004
Jerardino, A. (2021). Coastal foraging and transgressive sea levels during the terminal Pleistocene: Insights from the central west coast of South Africa. J. Anthropol. Archaeol. 64, 101351. doi:10.1016/j.jaa.2021.101351
Jerardino, A., and Marean, C. W. (2010). Shellfish gathering, marine paleoecology and modern human behavior: Perspectives from cave PP13B, Pinnacle point, south Africa. J. Hum. Evol. 59, 412–424. doi:10.1016/j.jhevol.2010.07.003
Jerardino, A. (2016a). On the origins and significance of Pleistocene coastal resource use in southern Africa with particular reference to shellfish gathering. J. Anthropol. Archaeol. 41, 213–230. doi:10.1016/j.jaa.2016.01.001
Jerardino, A. (2016b). Shell density as proxy for reconstructing prehistoric aquatic resource exploitation, perspectives from southern Africa. J. Archaeol. Sci. Rep. 6, 637–644. doi:10.1016/j.jasrep.2015.06.005
Jerardino, A. (1995). The problem with density values in archaeological analysis: A case study from tortoise cave, western cape, south Africa. South Afr. Archaeol. Bull. 50, 21–27. doi:10.2307/3889271
Joordens, J. C. A., Wesselingh, F. P., de Vos, J., Vonhof, H. B., and Kroon, D. (2009). Relevance of aquatic environments for hominins: A case study from Trinil (java, Indonesia). J. Hum. Evol. 57, 656–671. doi:10.1016/j.jhevol.2009.06.003
Kilburn, R., and Rippey, E. (1982). Sea shells of southern Africa. Johannesburg: Macmillan South Africa.
Kilburn, R., and Tankard, A. J. (1975). Pleistocene molluscs from the west and south coasts of the Cape Province, South Africa. Ann. S. Afri. Mus. 67, 183–226.
Klein, R. G. (2000). Archeology and the evolution of human behavior. Evol. Anthropol. 9, 17–36. doi:10.1002/(sici)1520-6505(2000)9:1<17::aid-evan3>3.0.co;2-a
Klein, R. G., and Bird, D. W. (2016). Shellfishing and human evolution. J. Anthropol. Archaeol. 44, 198–205. doi:10.1016/j.jaa.2016.07.008
Klein, R. G., and Cruz-Uribe, K. (1996). Exploitation of large bovids and seals at middle and later stone age sites in south Africa. J. Hum. Evol. 31, 315–334. doi:10.1006/jhev.1996.0064
Klein, R. G., and Cruz-Uribe, K. (2000). Middle and later stone age large mammal and tortoise remains from Die Kelders cave 1, western cape Province, south Africa. J. Hum. Evol. 38, 169–195. doi:10.1006/jhev.1999.0355
Klein, R. G., Cruz-Uribe, K., and Milo, R. G. (1999). Skeletal part representation in archaeofaunas: Comments on “explaining the ‘Klasies pattern’: Kua ethnoarchaeology, the Die Kelders middle stone age archaeofauna, long bone fragmentation and carnivore ravaging” by Bartram and marean. J. Archaeol. Sci. 26 (9), 1225–1234.
Klein, R. G., and Cruz-Uribe, K. (1984). The analyses of animal bones from archaeological sites. Chicago: University of Chicago Press.
Klein, R. G. (1976). The mammalian fauna of the Klasies river mouth sites, southern cape Province, south Africa. S. Afri. Archaeol. Bull. 32, 14–27.
Kyriacou, K., Parkington, J. E., Marais, A. D., and Braun, D. R. (2014). Nutrition, modernity and the archaeological record: Coastal resources and nutrition among middle stone age hunter-gatherers on the western cape coast of south Africa. J. Hum. Evol. 77, 64–73. doi:10.1016/j.jhevol.2014.02.024
Langejans, G. H., Dusseldorp, G. L., and Thackeray, J. F. (2017). Pleistocene molluscs from Klasies river (south Africa): Reconstructing the local coastal environment. Quat. Int. 427, 59–84. doi:10.1016/j.quaint.2016.01.013
Langejans, G. H. J., van Niekerk, K. L., Dusseldorp, G. L., and Thackeray, J. F. (2012). Middle stone age shellfish exploitation: Potential indications for mass collecting and resource intensification at Blombos cave and Klasies river, south Africa. Quat. Int. 270, 80–94. doi:10.1016/j.quaint.2011.09.003
Lap, A. (2020). Bones for Thought: A taphonomic study of the faunal remains from early MIS 5 at Klasies river main site. University of the Witwatersrand. Unpublished Masters Dissertation.
Larbey, C., Mentzer, S. M., Ligouis, B., Wurz, S., and Jones, M. K. (2019). Cooked starchy food in hearths ca. 120 kya and 65 kya (MIS 5e and MIS 4) from Klasies River Cave, South Africa. J. Hum. Evol. 131, 210–227. doi:10.1016/j.jhevol.2019.03.015
Loftus, E., Lee-Thorp, J., Leng, M., Marean, C., and Sealy, J. (2019). Seasonal scheduling of shellfish collection in the middle and later stone ages of southern Africa. J. Hum. Evol. 128, 1–16. doi:10.1016/j.jhevol.2018.12.009
Loftus, E., Sealy, J., Leng, M. J., and Lee-Thorp, J. A. (2017). A late Quaternary record of seasonal sea surface temperatures off southern Africa. Quat. Sci. Rev. 171, 73–84. doi:10.1016/j.quascirev.2017.07.003
Lombard, M., and Phillipson, L. (2010). Indications of bow and stone-tipped arrow use 64 000 years ago in KwaZulu-Natal, South Africa. Antiquity 84 (325), 635–648. doi:10.1017/s0003598x00100134
Lupo, K. D., Fancher, J. M., and Schmitt, D. N. (2013). The taphonomy of resource intensification: Zooarchaeological implications of resource scarcity among Bofi and Aka forest foragers. J. Archaeol. Method Theory 20, 420–447. doi:10.1007/s10816-012-9159-y
Lyman, R. L. (1987). Archaeofaunas and butchery studies: A taphonomic perspective. J. Archaeol. Method Theory 10, 249–337.
Lyman, R. L. (2010). What taphonomy is, what it isn’t, and why taphonomists should care about the difference. J. Taph. 8 (1), 1–16.
MacArthur, R. H., and Pianka, E. R. (1966). On optimal use of a patchy environment. Am. Nat. 100, 603–609. doi:10.1086/282454
Mackay, A., Stewart, B. A., and Chase, B. M. (2014). Coalescence and fragmentation in the late Pleistocene archaeology of southernmost Africa. J. Hum. Evol. 72, 26–51. doi:10.1016/j.jhevol.2014.03.003
Marean, C. W., Bar-Matthews, M., Bernatchez, J., Fisher, E., Goldberg, P., Herries, A. I., et al. (2007). Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature 449, 905–908. doi:10.1038/nature06204
Marean, C. W., and Binford, L. R. (1986). On the seal remains from Klasies river mouth: An evaluation of Binford's interpretations. Curr. Anthropol. 27, 365–368. doi:10.1086/203449
Marean, C. W., Cawthra, H. C., Cowling, R. M., Esler, K. J., Fisher, E., Milewski, A., et al. (2014). “Stone age people in a changing South African greater cape floristic region,” in Fynbos: Ecology, evolution and conservation of a Megadiverse region. Editors N. Allsopp, J. F. Colville, and G. A. Verboom (London: Oxford University Press), 164–199.
Marean, C. W., Cowling, R. M., and Franklin, J. (2020). The palaeo-Agulhas Plain: Temporal and spatial variation in an extraordinary extinct ecosystem of the Pleistocene of the cape floristic region. Quat. Sci. Rev. 235, 106161. doi:10.1016/j.quascirev.2019.106161
Marean, C. W., Domínguez-Rodrigo, M., and Pickering, T. R. (2004). Skeletal element equifinality in zooarchaeology begins with method: The evolution and status of the “shaft critique”. J. Taph. 2, 69–98.
Marean, C. W., and Kim, S. Y. (1998). Mousterian large-mammal remains from Kobeh Cave behavioral implications for Neanderthals and early modern humans. Curr. Anthropol. 39 (S1), S79–S114. doi:10.1086/204691
Marean, C. W. (1991). Measuring the postdepositional destruction of bone in archaeological assemblages. J. Archaeol. Sci. 18, 677–694. doi:10.1016/0305-4403(91)90029-o
Marean, C. W. (2010). Pinnacle point cave 13B (western cape Province, south Africa) in context: The cape floral kingdom, shellfish, and modern human origins. J. Hum. Evol. 59, 425–443. doi:10.1016/j.jhevol.2010.07.011
Marean, C. W., Spencer, L. M., Blumenschine, R. J., and Capaldo, S. (1992). Captive hyaena bone choice and destruction, the Schlepp effect and olduvai archaeofaunas. J. Archaeol. Sci. 19, 101–121. doi:10.1016/0305-4403(92)90009-r
Milo, R. G. (1998). Evidence for hominid predation at Klasies River Mouth, South Africa, and its implications for the behaviour of early modern humans. J. Archaeol. Sci. 25, 99–133.
Minichillo, T. (2006). Raw material use and behavioral modernity: Howiesons Poort lithic foraging strategies. J. Hum. Evol. 50, 359–364.
Mitchell, P. J. (1993). The archaeology of Tloutle rockshelter, Maseru District, Lesotho. J. Nat. Mus. Bloem. 9, 77–132.
Monahan, C. M. (1998). The Hadza carcass transport debate revisited and its archaeological implications. J. Archaeol. Sci. 25 (5), 405–424.
Morgan, C. (2015). Is it intensification yet? Current archaeological perspectives on the evolution of hunter-gatherer economies. J. Archaeol. Res. 23, 163–213. doi:10.1007/s10814-014-9079-3
Morin, E. (2007). Fat composition and Nunamiut decision-making: a new look at the marrow and bone grease indices. J. Archaeol. Sci. 34 (1), 69–82.
Morrissey, P., Mentzer, S. M., and Wurz, S. (2022). A critical review of the stratigraphic context of the MSA I and II at Klasies river main site, south Africa. J. Paleolit. Archaeol. 5 (1), 5–47. doi:10.1007/s41982-022-00110-2
Munro, N. D. (2004). Zooarchaeological measures of hunting pressure and occupation intensity in the natufian: Implications for agricultural origins. Curr. Anthropol. 45 (S4), S5–S34. doi:10.1086/422084
Munro, N. (2009). “Epipaleolithic subsistence intensification in the southern levant: The faunal evidence,” in The evolution of human diets: Integrating Approaches to the study of paleolithic diets. Editors J.-J. Hublin, and M. P. Richards (New York: Springer), 141–155.
Nel, T. H., Wurz, S., and Henshilwood, C. S. (2018). Small mammals from marine Isotope stage 5 at Klasies river, south Africa – reconstructing the local palaeoenvironment. Quat. Int. 471, 6–20. doi:10.1016/j.quaint.2017.08.074
Niespolo, E. M., Sharp, W. D., Avery, G., and Dawson, T. E. (2021). Early, intensive marine resource exploitation by Middle Stone Age humans at Ysterfontein 1 rockshelter, South Africa. Proc. Natl. Acad. Sci. U. S. A. 118 (16), e2020042118. doi:10.1073/pnas.2020042118
O'Connell, J. F., Hawkes, K., and Jones, N. B. (1988). Hadza scavenging: Implications for Plio/Pleistocene hominid subsistence. Curr. Anthropol. 29 (2), 356-363.
Öhrvik, V., Malmborg, A., Mattisson, I., Wretling, S., and Åstrand, C. (2012). Fish, shellfish and fish productsanalysis of nutrients. Livsmedels verket. Sweden: National food agency.
Olff, H., Ritchie, M. E., and Prins, H. H. T. (2002). Global environmental controls of diversity in large herbivores. Nature 415, 901–904. doi:10.1038/415901a
Outram, A. K. (2000). “Hunting meat and scavenging marrow? A seasonal explanation of middle stone age subsistence strategies at Klasies river mouth, southern cape Province, south Africa,” in Animals bones, human societies. Editor P. Rowley-Conwy (Oxford: Oxbow Books), 20–27.
Parkington, J. (2010). “Coastal diet, encephalization, and innovative behaviours in the late Middle Stone Age of southern Africa,” in Human brain evolution: The influence of freshwater and marine food resources. Editors S. C. Cunnane, and K. M. Steward (Hoboken, New Jersey: Wiley-Blackwell), 189–202.
Parkington, J. E. (2003). Middens and moderns: Shellfishing and the middle stone age of the western cape, south Africa. S. Afri. J. Sci. 99, 243–247.
Parkington, J. E. (2001). “Milestones: The impact of systematic exploitation of marine foods on human evolution,” in Humanity from african naissance to coming millenia. Editors P. V. Tobias, M. A. Raath, J. Moggi-Cechi, and G. A. Doyle (Florence: Firenze University Press), 327–336.
Partridge, T. C., Demenocal, P. B., Lorentz, S. A., Paiker, M. J., and Vogel, J. C. (1997). Orbital forcing of climate over South Africa: A 200, 000-year rainfall record from the Pretoria saltpan. Quat. Sci. Rev. 16 (10), 1125–1133. doi:10.1016/s0277-3791(97)00005-x
Pearson, A. (2021). A zooarchaeological analysis of faunal remains from the middle stone age III and Howiesons Poort layers at Klasies river, eastern cape, South Africa. Unpublished Masters Dissertation, University of the Witwatersrand.
Pickering, T. R., Marean, C. W., and Dominguez-Rodrigo, M. (2003). Importance of limb bone shaft fragments in zooarchaeology: A response to “on in situ attrition and vertebrate body part profiles” (2002) by M.C. Stiner. J. Archaeol. Sci. 30, 1469–1482. doi:10.1016/s0305-4403(03)00042-6
Powell, A., Shennan, S., and Thomas, M. G. (2009). Late Pleistocene demography and the appearance of modern human behavior. Science 324 (5932), 1298–1301. doi:10.1126/science.1170165
Rector, A. L., and Reed, K. E. (2010). Middle and late Pleistocene faunas of Pinnacle Point and their paleoecological implications. J. Hum. Evol. 59, 340–357. doi:10.1016/j.jhevol.2010.07.002
Reynard, J. P., Discamps, E., Wurz, S., Badenhorst, S., van Niekerk, K. L., and Henshilwood, C. S. (2016). Occupational intensity and environmental changes during the Howiesons Poort at Klipdrift shelter, southern cape, south Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 449, 349–364. doi:10.1016/j.palaeo.2016.02.035
Reynard, J. P., and Henshilwood, C. S. (2019). Environment versus behaviour: Zooarchaeological and taphonomic analyses of fauna from the still Bay layers at Blombos cave, south Africa. Quat. Int. 500, 159–171. doi:10.1016/j.quaint.2018.10.040
Reynard, J. P., and Henshilwood, C. S. (2017). Subsistence strategies during the late Pleistocene in the southern cape of south Africa: Comparing the still Bay of Blombos cave with the Howiesons Poort of Klipdrift shelter. J. Hum. Evol. 108, 110–130. doi:10.1016/j.jhevol.2017.04.003
Reynard, J. P., and Henshilwood, C. S. (2018). Using trampling modification to infer occupational intensity during the still Bay at Blombos cave, southern cape, south Africa. Afr. Archaeol. Rev. 35 (1), 1–19. doi:10.1007/s10437-018-9286-2
Reynard, J. P. (2021). Paradise lost: Large mammal remains as a proxy for environmental change from MIS 6 to the Holocene in southern Africa. South Afr. J. Geol. 124, 1055–1072. doi:10.25131/sajg.124.0057
Reynard, J. P. (2022). Tracking occupational intensity using archaeo-faunal data: Case studies from the Late Pleistocene in the southern Cape of South Africa. J. Archaeol. Method Theory 29, 214–250. doi:10.1007/s10816-021-09513-x
Reynard, J. P. (2014). Trampling in coastal sites: An experimental study on the effects of shell on bone in coastal sediment. Quat. Int. 330, 156–170. doi:10.1016/j.quaint.2013.11.007
Reynard, J. P., Henshilwood, C. S., and Badenhorst, S. (2014). Inferring animal size from the unidentified long bones from the Middle Stone Age layers at Blombos Cave, South Africa. Ann. DNMNH. 4 (1), 9–25.
Reynard, J. P., and Wurz, S. (2020). The palaeoecology of Klasies River, South Africa: An analysis of the large mammal remains from the 1984–1995 excavations of Cave 1 and 1A. Quat. Sci. Rev. 237, 106301. doi:10.1016/j.quascirev.2020.106301
Richerson, P. J., Boyd, R., and Bettinger, R. L. (2009). Cultural innovations and demographic change. Hum. Biol. 81 (2-3), 211–235. doi:10.3378/027.081.0306
Riel-Salvatore, J., and Barton, C. M. (2004). Late Pleistocene technology, economic behavior, and land-use dynamics in southern Italy. Am. Antiq. 69, 257–274. doi:10.2307/4128419
Rito, T., Vieira, D., Silva, M., Conde-Sousa, E., Pereira, L., Mellars, P., et al. (2019). A dispersal of Homo sapiens from southern to eastern Africa immediately preceded the out-of-Africa migration. Sci. Rep. 9 (1), 1-10.
Schoville, B. J., and Otárola-Castillo, E. (2014). A model of hunter-gatherer skeletal element transport: The effect of prey body size, carriers, and distance. J. Hum. Evol. 73, 1–14.
Schiffer, M. B. (1987). Formation processes of the archaeological record. Albuquerque: University of New Mexico Press.
Sealy, J. (2016). “Cultural change, demography, and the archaeology of the last 100 kyr in southern Africa,” in Africa from MIS 6-2. Editors S. Jones, and B. Steward (Dordrecht: Springer), 65–75.
Singer, R., and Wymer, J. (1982). The middle stone age at Klasies river mouth in South Africa. Chicago: Chicago University Press.
Sink, K. J., Branch, G. M., and Harris, J. M. (2005). Biogeographic patterns in rocky intertidal communities in KwaZulu-Natal, South Africa. Afr. J. Mar. Sci. 27, 81–96. doi:10.2989/18142320509504070
Skinner, J. D., and Chimimba, C. T. (2005). The mammals of the southern African sub-region. Cambridge: Cambridge University Press.
Small, C., and Nicholls, R. J. (2003). A global analysis of human settlement in coastal zones. J. Coast. Res. 19, 584–599.
Speth, J. D., and Clark, J. L. (2006). Hunting and overhunting in the levantine late middle palaeolithic. Before Farming 3, 1–42. doi:10.3828/bfarm.2006.3.1
Steele, T., and Klein, R. G. (2009). “Late Pleistocene subsistence strategies and resource intensification in Africa,” in The evolution of hominin diets: Integrating Approaches to the study of palaeolithic subsistence. Editors J.-J. Hublin, and M. P. Richards (New York: Springer), 113–126.
Stiner, M. C., and Kuhn, S. L. (2006). Changes in the ‘connectedness’ and resilience of Paleolithic societies in Mediterranean ecosystems. Hum. Ecol. 34 (5), 693–712. doi:10.1007/s10745-006-9041-1
Stiner, M. C., Kuhn, S. L., Weiner, S., and Bar-Yosef, O. (1995). Differential burning, recrystallization, and fragmentation of archaeological bone. J. Archaeol. Sci. 22, 223–237. doi:10.1006/jasc.1995.0024
Stiner, M. C., Munro, N. D., Surovell, T. A., Tchernov, E., and Bar-Yosef, O. (1999). Paleolithic population growth pulses evidenced by small animal exploitation. Science 283, 190–194. doi:10.1126/science.283.5399.190
Texier, P. J., Porraz, G., Parkington, J., Rigaud, J. P., Poggenpoel, C., Miller, C., et al. (2010). A Howiesons Poort tradition of engraving ostrich eggshell containers dated to 60, 000 years ago at Diepkloof Rock Shelter, South Africa. Proc. Natl. Acad. Sci. U. S. A. 107 (14), 6180–6185. doi:10.1073/pnas.0913047107
Thackeray, J. F. (1990). Carnivore activity at Klasies river mouth: A response to Binford. Palaeontol. Afr. 27, 101–109.
Thackeray, J. F. (1988). Molluscan fauna from Klasies river, south Africa. South Afr. Archaeol. Bull. 43, 27–32. doi:10.2307/3887610
Thompson, E., and Marean, C. W. (2008). The Mossel Bay lithic variant: 120 years of middle stone age research from cape st blaize cave to Pinnacle point. S. Afri. Archaeol. Soc. Goodwin S. 10, 90–104.
Thompson, J. C., Faith, J. T., Cleghorn, N., and Hodgkins, J. (2017). Identifying the accumulator: Making the most of bone surface modification data. J. Archaeol. Sci. 85, 105–113. doi:10.1016/j.jas.2017.06.013
Thompson, J. C. (2010). Taphonomic analysis of the middle stone age faunal assemblage from Pinnacle point cave 13B, western cape, south Africa. J. Hum. Evol. 59, 321–339. doi:10.1016/j.jhevol.2010.07.004
Van Andel, T. H. (1989). Late Pleistocene sea levels and the human exploitation of the shore and shelf of southern South Africa. J. Field Archaeol. 16, 133–155. doi:10.2307/529887
Van Niekerk, K. L. (2011). Marine fish exploitation during the middle and later stone age of South Africa. Town: University of Cape. Unpublished PhD Thesis.
Van Pletzen, L. (2000). The large mammal fauna from Klasies river. Stellenbosch: University of Stellenbosch. Unpublished Masters dissertation.
Van Pletzen-Vos, L., Brink, J., Reynard, J. P., and Wurz, S. (2019). Revisiting Klasies river: A report on the large mammal remains from the Deacon excavations of Klasies river main site, south Africa. S. Afri. Archaeol. Bull. 74, 127–137.
Venter, J. A., Brooke, C. F., Marean, C. W., Fritz, H., and Helm, C. W. (2020). Large mammals of the Palaeo-Agulhas Plain showed resilience to extreme climate change but vulnerability to modern human impacts. Quat. Sci. Rev. 235, 106050. doi:10.1016/j.quascirev.2019.106050
Villa, P., and Mahieu, E. (1991). Breakage patterns of human long bones. J. Hum. Evol. 21, 27–48. doi:10.1016/0047-2484(91)90034-s
Villa, P., Soriano, S., Teyssandier, N., and Wurz, S. (2010). The Howiesons Poort and MSA III at Klasies river main site, cave 1A. J. Archaeol. Sci. 37 (3), 630–655. doi:10.1016/j.jas.2009.10.028
Vogel, J. C. (2001). “Radiometric dates for the middle stone age in south Africa,” in Humanity from african naissance to coming millennia-colloquia in human Biology and Palaeoanthropology. Editors P. V. Tobias, M. A. Raath, J. Maggi-Cecchi, and G. A. Doyle (Johannesburg: Wits University Press), 261–268.
Volman, T. P. (1984). “Early prehistory of southern Africa,” in Southern african prehistory and palaeoenvironments: 169–220. Editor R. G. Klein (Rotterdam: Balkema).
Von den Driesch, A. (2004). The Middle Stone Age fish fauna from the Klasies river main site, South Africa. Anthropozoologica 39, 33–59.
Wadley, L. (2010). Were snares and traps used in the middle stone age and does it matter? A review and case study from Sibudu, south Africa. J. Hum. Evol. 58, 179–192. doi:10.1016/j.jhevol.2009.10.004
Wadley, L. (2015). Those marvellous millennia: The Middle Stone Age of southern Africa. Azania 50 (2), 155–226.
Wadley, L. (2021). What stimulated rapid, cumulative innovation after 100, 000 years ago? J. Archaeol. Method Theory 28 (1), 120–141. doi:10.1007/s10816-020-09499-y
Way, A. M., Paloma de la Peña, P., de la Peña, E., and Wadley, L. (2022). Howiesons Poort backed artifacts provide evidence for social connectivity across southern Africa during the Final Pleistocene. Sci. Rep. 12, 9227. doi:10.1038/s41598-022-12677-5
Wilkins, J., Brown, K. S., Oestmo, S., Pereira, T., Ranhorn, K. L., Schoville, B. J., et al. (2017). Lithic technological responses to late Pleistocene glacial cycling at Pinnacle point site 5-6, south Africa. PloS One 12 (3), 0174051. doi:10.1371/journal.pone.0174051
Wilkins, J., Schoville, B. J., Pickering, R., Gliganic, L., Collins, B., Brown, K. S., et al. (2021). Innovative Homo sapiens behaviours 105, 000 years ago in a wetter Kalahari. Nature 592 (7853), 248–252. doi:10.1038/s41586-021-03419-0
Will, M., Kandel, A. W., and Conard, N. J. (2019). Midden or molehill: The role of coastal adaptations in human evolution and dispersal. J. World Prehist. 32 (1), 33–72. doi:10.1007/s10963-018-09127-4
Will, M., Kandel, A. W., Kyriacou, K., and Conard, N. J. (2016). An evolutionary perspective on coastal adaptations by modern humans during the Middle Stone Age of Africa. Quat. Int. 404, 68–86. doi:10.1016/j.quaint.2015.10.021
Will, M., and Mackay, A. (2017). What factors govern the procurement and use of silcrete during the Stone Age of South Africa? J. Archaeol. Sci. Rep. 15, 630–645. doi:10.1016/j.jasrep.2016.11.046
Winterhalder, B., and Smith, E. A. (2000). Analyzing adaptive strategies: Human behavioral ecology at twenty-five. Evol. Anthropol. 9 (2), 51–72. doi:10.1002/(sici)1520-6505(2000)9:2<51::aid-evan1>3.0.co;2-7
Winterhalder, B. (2001). “The behavioural ecology of hunter-gatherers,” in Hunter-gatherers: An interdisciplinary perspective. Editors C. Panter-Brick, R. H. Layton, and P. Rowley-Conwy (Cambridge: Cambridge University Press), 12–38.
Wurz, S., Bentsen, S. E., Reynard, J., Van Pletzen-Vos, L., Brenner, M., Mentzer, S., et al. (2018). Connections, culture and environments around 100 000 years ago at Klasies River main site. Quat. Int. 495, 102–115. doi:10.1016/j.quaint.2018.03.039
Wurz, S., Le Roux, N. J., Gardner, S., and Deacon, H. J. (2003). Discriminating between the end products of the earlier Middle Stone Age sub-stages at Klasies River using biplot methodology. J. Archaeol. Sci. 30, 1107–1126. doi:10.1016/s0305-4403(03)00009-8
Wurz, S., and Lombard, M. (2007). 70 000-year-old geometric backed tools from the Howiesons Poort at Klasies river, south Africa: Were they used for hunting? S. Afri. Humanit. 19 (1), 1–16.
Wurz, S., Pickering, R., and Mentzer, S. (2022). U-Th dating, taphonomy and taxonomy of shell middens at Klasies River main site indicate stable and systematic coastal exploitation by MIS 5c-d. Front. Earth Sci., 1–25. doi:10.3389/feart.2022.1001370
Wurz, S. (1997). The Howiesons Poort at Klasies river: From artefacts to cognition. Stellenbosch: Unpublished Master Dissertation, University of Stellenbosch.
Wurz, S. (2000). The middle stone age at Klasies river, South Africa. Stellenbosch: Stellenbosch University. Unpublished PhD thesis.
Keywords: Middle Stone Age, zooarchaeology, taphonomy, occupational intensity, Southern Cape, South Africa, Late Pleistocene, Coastal palaeoenvironments
Citation: Reynard JP (2022) Human occupational intensity and palaeoecology at Klasies River from MIS 5–3: Preliminary taphonomic analyses of faunal remains from the Deacon and Wurz excavations. Front. Earth Sci. 10:974582. doi: 10.3389/feart.2022.974582
Received: 21 June 2022; Accepted: 20 October 2022;
Published: 18 November 2022.
Edited by:
Manuel Will, University of Tübingen, GermanyReviewed by:
Tyler Faith, The University of Utah, United StatesCopyright © 2022 Reynard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jerome P. Reynard, amVyb21lLnJleW5hcmRAd2l0cy5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.