
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci. , 23 January 2023
Sec. Geochemistry
Volume 10 - 2022 | https://doi.org/10.3389/feart.2022.1056881
This article is part of the Research Topic Tracing Earth Surface Processes Using Novel Isotopic Approaches View all 10 articles
Metal stable isotopic composition of dental enamel is a novel proxy for reconstructing human dietary structure. Magnesium is the second most prevalent element in teeth only after calcium. Significant isotopic fractionation of Mg isotopes during biological processes implies its great advantages in reconstructing human recipes. To evaluate the potential of the Mg isotopic composition of dental enamel in learning the human dietary structure, elemental and Mg isotopic analyses were performed on the modern human teeth from regions in northern and southern China with various dietary characteristics. Our findings reveal that southern Chinese teeth enamel has higher Mg contents and heavier Mg isotopic compositions (-0.69‰ for SN and -0.66‰ for Hangzhou) than those of their northern counterparts (−1.27‰ for Weinan and −1.33‰ for Puyang). Such discrepancy cannot be attributed to the provenance heterogeneity or individual metabolic processes. Instead, the correlations between cereal-based dietary patterns and the δ26Mg in dental enamel demonstrate that the structure of the staple diet is more responsible for the Mg isotopic signatures. Moreover, heavier Mg isotopic compositions have been observed in dental enamel of individuals with higher rice and lower wheat in the diet, indicating that Mg isotopes are a promising tracer for rebuilding individual or population plant-based dietary structures as well as distinguishing more specific species within C3 plants. These findings suggest that Mg isotopes in teeth enamel have the great potential to better identify the food composition and constrain the diet structure of ancient humans.
Diet is a fundamental approach to comprehending human culture, including productivity, economic status, and society’s cultural characteristics (Lambert et al., 1984; Ling, 2010). Furthermore, it has been proposed that the evolution of early humans is accompanied by a shift in nutritional structure (Ungar et al., 2006). As a result, reconstructing the ancient human diet could provide key information about human evolution and paleoecology.
Over the past few decades, stable isotopes have been frequently employed to rebuild diet and trophic structures in ancient food webs (Kiple and Ornelas, 2000). Most proxies rely on stable isotope ratios of light elements (e.g., carbon, nitrogen, oxygen, and sulfur) preserved in fossilized teeth and bones (Schoeninger, 2010). These traditional isotope ratio studies, however, have many limitations. For example, although C isotopes can distinguish C3 plants from C4 plants, no additional information is available within C3 (C4) plant groups (Katzenberg and Waters-Rist, 2018). In addition, traditional isotope ratios analysis relies on organic matter fractions (e.g., collagen), which are difficult to be preserved in human tissues older than 10 ka due to the diagenesis, restricting the application of this method to ancient human remains.
The recent development of Multi-Collector Inductively Coupled Plasma Mass Spectrometry (MC-ICP-MS) allows the precise measurement of metal isotope ratios with low concentrations in human organs or tissues, such as Mg isotopes (Teng, 2017). Magnesium is an essential element that plays an irreplaceable role in both plants and animals. It is the center of chlorophyll in plants and vital to photosynthesis (Black et al., 2006). Due to comparable ionic radii and chemical behavior, Mg2+ typically substitutes Ca2+ into the apatite structure in the tissues of animals (bones and teeth). There is about 25 g Mg in an adult human body, which is involved in more than 600 enzymatic reactions and regulates cellular metabolic processes (Elin, 1987; Nadler and Rude, 1995; Vormann, 2003; De Baaij et al., 2015). In addition, Mg isotopes are usually significantly fractionated during plant and animal physiological activities (Black et al., 2008; Bolou-Bi et al., 2010; Teng, 2017; Wang et al., 2020; Wrobel et al., 2020). Recent research on plants manifested that different species of plants have distinguishable Mg isotopic compositions, and the light Mg isotopes are progressively enriched from the roots to the stems (Black et al., 2006; Black et al., 2007; Black et al., 2008; Bolou-Bi et al., 2010; Wang et al., 2020). Studies on terrestrial vertebrates in the same ecosystem demonstrated that the Mg isotopic compositions of animals feeding on C3 plants were lighter than that of animals grazing on C4 plants and that the δ26Mg values of hard tissues became gradually higher as trophic levels increased (Martin et al., 2014; Martin et al., 2015b). Thus, Mg isotopes may have the potential to trace ancient human recipes.
To date, however, few studies have reported the Mg isotopic composition of human tissues. A recent experiment has displayed that teeth enamel is the most reliable material within all the tissues for dietary and physiological reconstruction because of its dense structure (Weber et al., 2021). Nevertheless, the relationship between the Mg isotopic composition of human teeth enamel and individual diet is still unclear, which dramatically hinders the application of the Mg isotopic system for tracing ancient human dietary structures. In this study, elemental and Mg isotopic composition of modern human dental enamel from four Chinese regions with different nutritional characteristics were measured to explore the relationship between the δ26Mg in dental enamel and human dietary structure. This first database of the Mg isotopic compositions in human dental enamel provides novel information on ancient human recipes and evolution: the δ26Mg in dental enamel is mainly controlled by plant-based dietary structure and the various intake proportions of rice and wheat indicate that the Mg isotopic system has the potential to trace specific plant species within the C3 group.
Based on the geographical Qinling-Huaihe Line, China can be divided into northern China and southern China (Figure 1A). Northern China has lower precipitation and wheat and maize are the main food crops; while the wetter southern China produces rice as the main food crop. To cover various typical dietary characteristics, 29 modern human permanent teeth from were selected four regions of China (Figure 1). More specifically, four teeth were collected from Weinan (WN), central Shaanxi, which is located in the middle reaches of the Yellow River, eastern Guanzhong Plain. It belongs to the semi-humid and semi-arid monsoon climate zone, and wheat is the main crop in this area. Two teeth were collected from Puyang (PY), northeast of Henan, which seats in the lower reaches of the Yellow River in the central plains. It has a warm temperate sub-humid continental monsoon climate, while wheat and maize are the main crops here. Eight teeth were selected from southern Shaanxi regions (SN), which is located right below the Qinling-Huaihe line and bears a wet climate and mainly lives on rice. The rest 13 teeth were from Hangzhou (HZ), Zhejiang Province, located on the south side of the Yangtze River Delta in the subtropical monsoon region and mainly produces rice. In short, WN and PY are typical northern China cities, while SN and HZ represent southern cities.
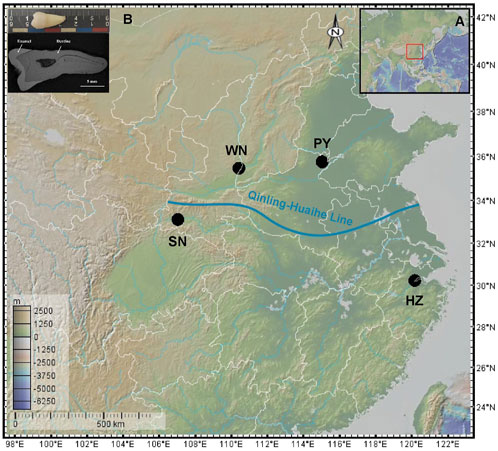
FIGURE 1. (A) Location of the sampling sites. WN-Weinan, PY-Puyang, SN-Southern Shaanxi regions, HZ-Hangzhou. (B) The morphology of a tooth sample and Back-scattered electron image of a tooth sample section.
Except for one fragment without morphological characteristics (WN1, Table 1), samples include 17 molars, eight premolars, two incisors, and one canine. All the samples were collected at local clinics or hospitals. Detailed information about the samples is provided in Table 1.
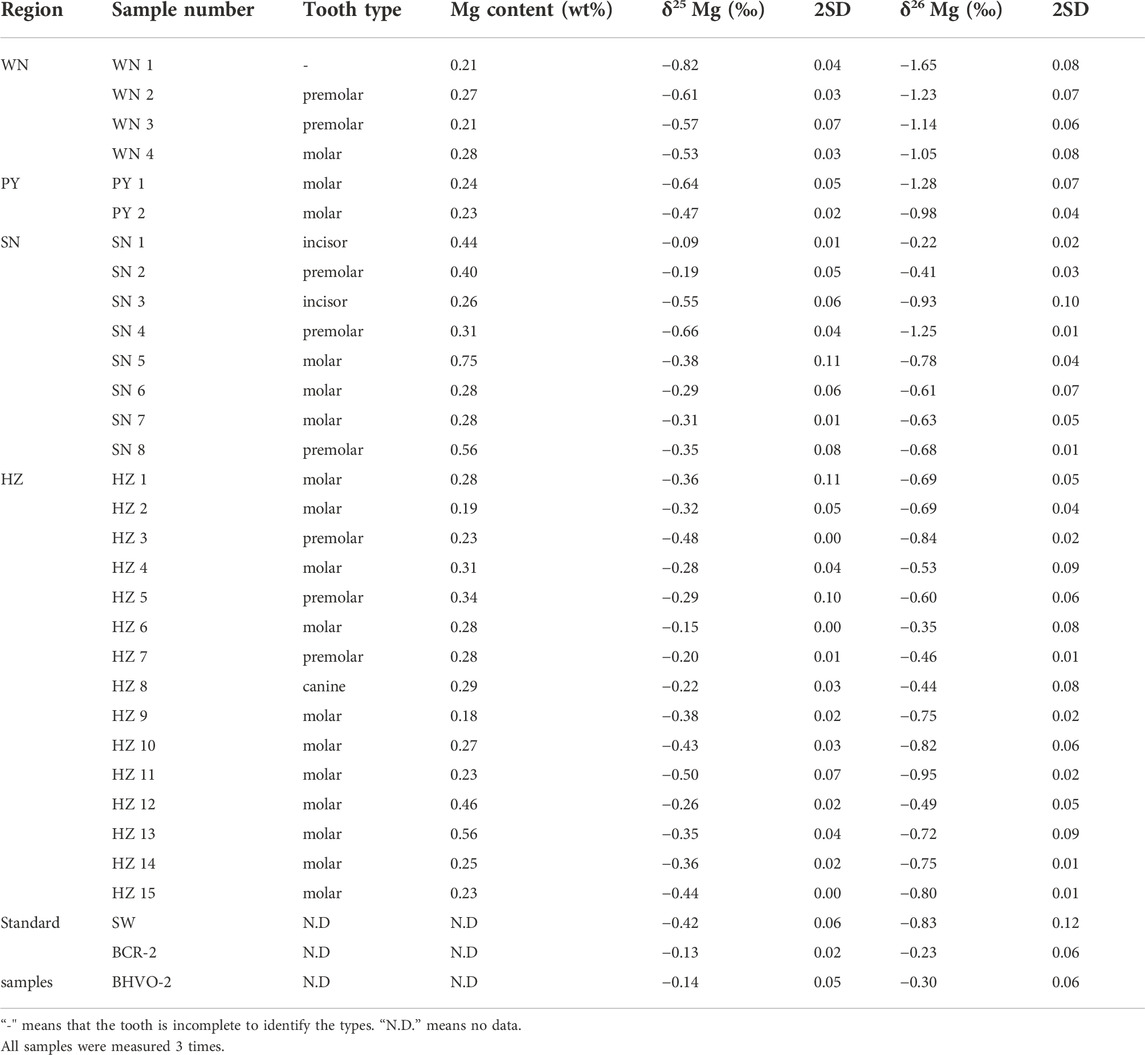
TABLE 1. Magnesium elemental contents and isotopic compositions of modern human teeth enamel from WN (Weinan), PY (Puyang), SN (Southern Shaanxi regions) and HZ (Hangzhou).
The tartar on the teeth surface was removed mechanically by drill firstly and then freeze-dried at -60°C for 7 days. After that, ∼10 mg enamel powder closed to enamel surface of each tooth (Figure 1B) was drilled and dissolved in 1 ml double-distilled 14 N HNO3 on an electric hot plate overnight. After completely dissolved, the sample solution was dried and re-dissolved in 0.5 ml double-distilled 14 N HNO3. Then aliquots of solutions were extracted and diluted for elemental concentration analyses on iCAP PRO ICP-OES at the State Key Laboratory of Continental Dynamics (SKLCD), department of geology, Northwest University, China. Gravitationally prepared standard solutions were used to calibrate the concentration curve of measured elements; multi-element standard solutions were used for monitoring and the uncertainties of measured elements were better than 10%.
Then the solutions containing ∼10–50 μg Mg were dried and dissolved by 12 N distilled HCl to pass through two columns for the purification of Mg. Detailed separation methods have been established in previous studies (Bao et al., 2019). Column #1, filled with 2.0 ml of Bio-Rad AG-50 W-X12 resin (200–400 mesh), was used to separate Mg from Ca. Column #2, filled with 0.5 ml of Bio-Rad AG50 W-X12 resin (200–400 mesh), was designed to separate Mg from all other matrix metals (Al, Na, Fe, etc.). To achieve the complete elution of matrix metals, each sample was passed through both columns twice. At least two standard references (seawater, BCR-2, BHVO-2) were passed through the columns along with samples to test the effect of separation and purification of each batch. The recovery of Mg was as much as 99% in the whole procedure, and the total blank was negligible compared to the mass of Mg in samples. After that, all purified Mg solutions were dried down on the electric hot plate and then re-dissolved in 14 N HNO3 for the following isotopic measurement.
Magnesium isotopic ratios were measured using Nu plasma Ⅱ MC-ICP-MS at the SKLCD. The pure Mg solution was introduced with 2% HNO3 as the acid media and pure argon as the carrier gas through an autosampler, a self-priming nebulizer, and a dual-path quartz fog chamber. During the test, three Mg isotopes (24, 25, 26) were monitored simultaneously in L5, Ax, and H5 Faraday cups under low-resolution mode. The background signal for 24Mg (∼2×10−3 V) was negligible relative to the sample signal (>8 V). The standard-sample bracketing technique (SSB) was used to correct the instrumental mass bias to obtain the Mg isotopic ratios. The analysis results are expressed as the per mil deviation from the isotopic compositions of the DSM3 international standard (Dead Sea Magnesium Ltd., Israel):
where x refers to mass 25 or 26. Uncertainties for δ25Mg and δ26Mg of standards and samples are given as two standard deviations (2SD) based on repeated measurements. During the analytical session, data for seawater (−0.83 ± 0.12‰), BCR-2 (−0.23 ± 0.06‰), and BHVO-2 (−0.30 ± 0.06‰) obtained in this study are all identical within errors to the established values (Huang et al., 2018; Bao et al., 2019; Ma et al., 2019). In addition, the δ26Mg and δ25Mg values of all samples and standards in this study lie on a mass-dependent fractionation line with a slope of 0.519 (Figure 2), which is consistent with the theoretical mass-dependent fractionation law (Young et al., 2002).
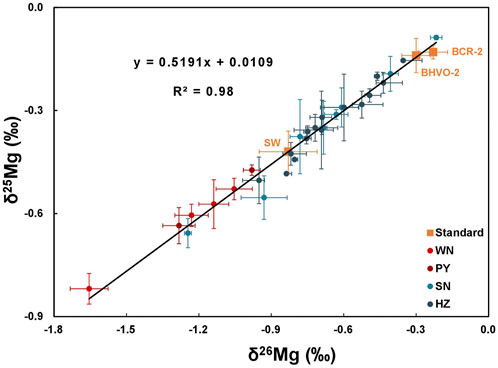
FIGURE 2. Diagram of δ26Mg vs δ25Mg values for human dental enamel. The solid line represents the fractionation line with a slope of 0.519. Error bars indicate the 2SD of the mean value.
The Mg contents of Chinese dental enamel vary by region, ranging from 0.18 to 0.75 wt% (Table 1). Overall, human teeth enamel from southern China has higher Mg contents than those from northern China (Figure 3A). Teeth enamel from WN and PY have almost the same level of Mg content, with a mean value of 0.24 wt%. Teeth enamel from HZ has higher Mg contents (averagely 0.29 wt%). Remarkably, teeth enamel from SN has a mean Mg content of 0.41 wt%, which is the highest value among these four areas.
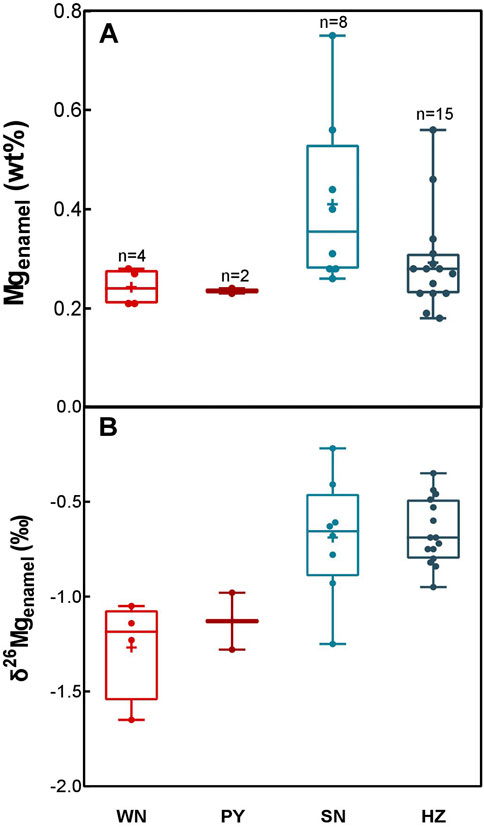
FIGURE 3. Magnesium content (A) and δ26Mg values (B) in modern human teeth enamel from different regions. “+” represent the average value. The boxes range between min to max and show the median value.
The δ26Mg values of human dental enamel from different China regions exhibit a large variation, ranging from -1.65‰ to -0.22‰ (Figure 3B). Dental enamel from southern China has overall heavier Mg isotopic compositions than those from northern China. It is worth noting that WN samples are highly depleted in heavy Mg isotopes with an average δ26Mg value of -1.27 ± 0.27‰ (SD). Samples from PY have intermediate δ26Mg values, with an average value of -1.13 ± 0.21‰ (SD). Teeth enamel in southern China has notable heavier mean δ26Mg values: 0.69‰ ± 0.31‰ (SD) for SN samples and -0.66 ± 0.17‰ (SD) for HZ samples, respectively. Overall, the Mg isotopic composition of dental enamel gradually becomes heavier from northern China (WN and PY) to southern China (SN and HZ).
Our data show distinct geographical differences in both Mg content and Mg isotopic composition of human dental enamel. Teeth enamel from southern China has overall higher Mg contents and heavier Mg isotopic compositions than those from northern China (Figure 3). Magnesium in the human body firstly involves the uptake of Mg from food and drinking water, which Mg and δ26Mg features are controlled by the local context (including soil and water) through cultivating agricultural products. Furthermore, the partition and fractionation during the growth of these products may influence the Mg and δ26Mg features of the food and drinking water intake. Then, the dietary preference and the digestion and migration of Mg in the human body may also produce the variations of Mg contents and δ26Mg values in enamel among individuals and groups of people. Therefore, these systematic differences in Mg contents and δ26Mg values in human enamel may be generated by provenance heterogeneity, physiological metabolism, dietary structure, and the associated Mg isotope signature inheritance and fractionation, which will be discussed accordingly in the followings.
The regional context may inevitably affect the Mg isotopic signatures of human tissues by controlling the isotope ratios of soil and regional water. Briefly, drinking water could involve the human body by direct absorption. Soil and soil water characteristics could be transferred to the human body via producers into the food web. Published Mg isotopic compositions of natural waters and soils span a relatively wide range (Tipper et al., 2006; Wimpenny et al., 2014; Fan et al., 2016; Gao et al., 2018; Zhao et al., 2022), and the Mg isotopic signatures are highly controlled by several factors, such as local lithologies, seasonality, and runoff (Tipper et al., 2006; Nitzsche et al., 2019). Furthermore, the plants and animals also show large Mg isotope variability (Black et al., 2008; Bolou-Bi et al., 2012; Martin et al., 2014; Martin et al., 2015b). Some variations in plants and animals are suggested to involve the provenance (Martin et al., 2014), although the direct evidence is lacking. These contexts indicate that it is imperative to evaluate the provenance effect before further interpretation of the Mg isotopic variability in human enamel.
The precise local isotopic signatures of each region are unavailable, which are also meaningless since the exact isotopic compositions of the context may vary among each product and are impractical to be traced. Therefore, δ26Mg data from the Yellow River and loess here are tentatively used to represent the Mg isotopic signatures of the water and soil in northern China, and the limited δ26Mg data for the Yangtze River and southern red soils are also used for the representative of the substrate of southern China (Tipper et al., 2006; Wimpenny et al., 2014; Fan et al., 2016; Gao et al., 2018; Xu et al., 2022). Compiling these data, we found that the mean δ26Mg value of river water in southern China (−1.46‰) is lighter than that of northern China (−1.14‰). Similarly, the mean δ26Mg value of soil in southern China (−0.72‰) is also lighter than that of northern China (−0.47‰). This compilation dataset indicates that the context δ26Mg in southern China is relatively lighter than that of northern China (Figure 4), which cannot account for the relatively heavier δ26Mg values of human enamel in southern China. Therefore, the provenance likely has limited effects on the distinct Mg isotopic compositions in human teeth enamel between southern and northern regions.
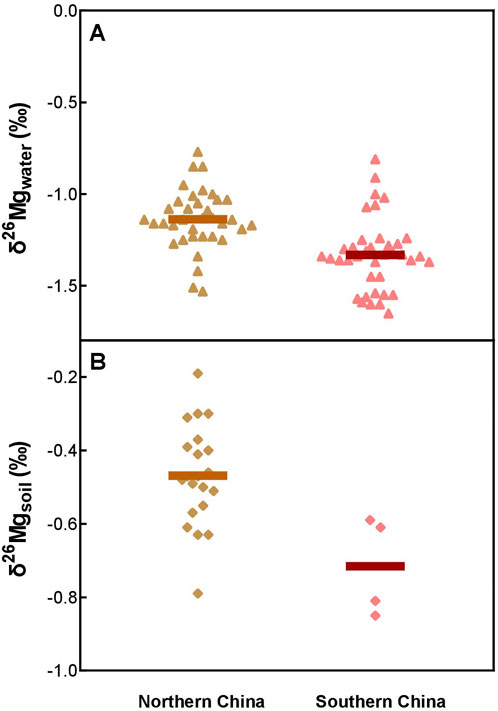
FIGURE 4. Mg isotopic compositions of soils (A) and regional water (B) in northern and southern China. Data are from Fan et al., 2016; Gao et al., 2018; Tipper et al., 2006; Wimpenny et al., 2014; Xu et al., 2022. The horizontal bars are mean values.
Magnesium is indispensable for maintaining structural and biochemical homeostasis in vertebrate animals, including humans (Ryan, 1991; Brown, 1999). It assists in activating more than 600 enzymes in the body and regulating human cells (Mark and Mediawerks, 2007; Faryadi, 2012). The pervasive participation of Mg in the physiological activities of human beings reflects that the potential migration and re-distribution of Mg inside human tissues and organs may generate Mg isotope fractionations. Thus, it should be considered whether the physiology, gender, age or tooth types have an impact on the δ26Mg values in human enamel.
The Han Chinese, the most widely distributed ethnic group in China, belongs to the typical East Asian ethnic group in terms of their origins and formation, with homogeneous and differentiated physical characteristics (Zhao, 2016). There should be no significant physiological differences between modern Chinese if the effects of the disease are not considered. That means the isotopic fractionation of Mg produced by human metabolism can be considered consistent among individuals. Hence, the differences in population metabolism are unlikely to generate such huge a regional discrepancy in enamel δ26Mg.
Unlike iron isotopes, which behave divergently between males and females (Walczyk and Blanckenburg, 2002; Jaouen and Balter, 2014; Van Heghe et al., 2014), Mg isotopes do not show a direct discrepancy between the sexes due to their existing status and physiological activities involved (Elin, 1987; Nadler and Rude, 1995; Faryadi, 2012; De Baaij et al., 2015). Furthermore, although our teeth samples do not have specific age information due to incomplete sample collection, dental enamel normally developed completely in childhood and did not change once formed and thus records the isotopic composition of adolescence (Grupe, 1998). Therefore, it can be inferred that age could not lead to such a vast distinction in the Mg isotopic composition of enamel.
In addition to metabolism, gender, and age effects, the influence of tooth types on enamel δ26Mg also should be considered. The δ26Mg of different types of teeth (canine, incisor, premolar, and molar) display overlapped variations (Table 1), and the Student’s t test for every two tooth types does not show significant differences (p > 0.05). It thus suggests that the influence of tooth types on the Mg isotopic difference in dental enamel between southern Chinese and northern Chinese is negligible. Overall, the metabolic effect is thought to have limited influence on Mg isotopic variation in human teeth enamel.
Food-intaking is the main way humans derive nutrients, including Mg. Individuals have various dietary habits which contain Mg with large variability. Published studies have shown significant variations of δ26Mg among plants and animals (Black et al., 2008; Bolou-Bi et al., 2012; Martin et al., 2014; Martin et al., 2015b). Moreover, animals feeding on different types of plants or different trophic levels of food display distinct Mg isotopic compositions and variations (Martin et al., 2014; Martin et al., 2015a), reflecting that enamel δ26Mg can be significantly affected by food and dietary structure. Thus, the effect of dietary structure on the systematic Mg isotopic differences in human enamel from southern and northern China, which rely on different dietary habits, should be considered and evaluated.
The Chinese Balanced Dietary Pagoda (2016) gives the Chinese a scientifically daily dietary quantity model that an adult should take ∼1600 ml water, ∼350 g grain, ∼400 g vegetable, ∼300 g fruit, ∼50 g egg, etc. The contributions of Mg from different dietary sources can be estimated based on this criterion and the food Mg content from The China Food Composition (Yang et al., 2019). Drinking water contributes less than 1‰ Mg to the human body, while meat and plant foods account for ∼24% and ∼75.7% respectively. It directly demonstrates that plant foods, including grain and vegetables, are primarily responsible for human Mg sources. Thus, the dietary diversities, especially plant-based nutritional structures may be deemed to result in the regional Mg isotopic differences in human enamel. However, it is impractical to directly calculate the Mg content and Mg isotopic compositions of meals for individuals or regional people. Here we tentatively use the output proportion of local crops to quantitively evaluate the average grain-food consumption of local humans. Assuming the people’s dietary preference is in proportion to the local crops’ yield, that means the southern diet is dominated by rice and the northern diet is wheat (Chen et al., 2018; Li et al., 2020). According to these, the uptake of Mg from food of people from four regions can be estimated. Then given that the enrichment coefficient of Mg in enamel is the same for all the people, the entire enamel Mg content acquired by grain food should proportionally equal to the sum of Mg content that all local crops contain. Furthermore, since wheat and rice are two dominant staple food in the regions studied, the leverage of these two crops on Mg content and Mg isotopic compositions of human teeth enamel can also be evaluated. To make a qualitative comparison of dietary structure, we give some calculations as follows:
where Mcrop is the total Mg content provided by all local crops, CS is the crop species, Ycrop is the crop yield, Ccrop is the crop unit Mg content, Mwheat is the Mg content provided by local wheat, Ywheat is the wheat yield, Cwheat is the unit Mg content of wheat, Mrice is the Mg mass fraction provided by local rice, Yrice is the rice yield, and Crice is the unit Mg content of rice. More information is in Table 2.
The calculated Mcrop values are positively correlated to the Mg content in local human enamel (Figure 5). This result convinces that it is plausible to use dietary structure and local crop yield to estimate the uptake of Mg from food for people from different regions. It also qualitatively explains the phenomenon that southerners have higher enamel Mg content and northerners have lower enamel Mg content (Figure 3A). Besides, there is a straightforward linear positive (negative) correlation between δ26Mgenamel and Mwheat/Mcrop (Mrice/Mcrop) (Figure 6). These trends support that staple diet composition likely controls the enamel δ26Mg values differences among regions. From WN to PY to SN to HZ, people become increasingly reliant on rice and less dependent on wheat, corresponding to the sequentially heavier Mg isotopic composition in teeth enamel (Figure 6). Moreover, the opposite tendency of the two crops indicates that wheat provides isotopically lighter Mg while rice has heavier Mg isotopes (Figure 6). Although it is still unclear whether wheat preferentially incorporates lighter Mg isotopes into its seed compared to rice, the limited published data show isotopically lighter wheat seed compared to rice seed (δ26Mg -0.8‰ ∼ -1.83‰ vs -0.67‰) (Black et al., 2008; Bolou-Bi et al., 2009; Bolou-Bi et al., 2010; Bolou-Bi et al., 2012; Martin et al., 2014; Mavromatis et al., 2014; Martin et al., 2015b; Gao et al., 2018; Kimmig et al., 2018; Grigoryan et al., 2020; Wang et al., 2020; Wrobel et al., 2020). However, current research conditions on wheat and rice are not consistent and the specific mechanism is not clear. Hence, it is unavailable to compare δ26Mg values directly. Moreover, the growth conditions of wheat and rice in northern and southern China need to be taken into account. Thus, published data only demonstrate the δ26Mg values indeed differ between rice and wheat, which potentially supports our findings, but more research limitations on rice and wheat are needed. Furthermore, both rice and wheat are typical C3 plants. The difference in the intake proportions of rice and wheat is reflected in the Mg isotopic composition of human enamel (Figure 6), which offers a potentially novel tool to understand human dietary structure ulteriorly. It indicates that the δ26Mg value of human dental enamel is related to a cereal-based food structure. The Mg isotopic system may trace more details and potentially distinguish specific plant species within the C3 plants. Additionally, the enamel δ26Mg of people from all regions display large variations (Figure 3B), which may involve the diversity of dietary structures among individuals and the possible influence of other factors abovementioned.
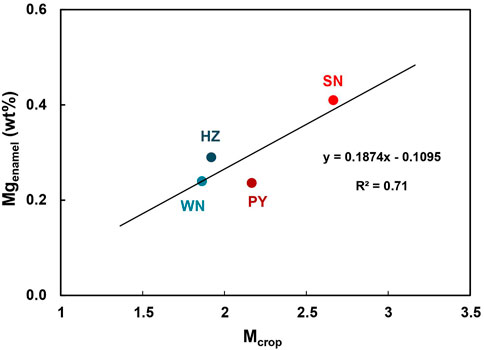
FIGURE 5. Relationship between Mcrop and Mgenamel. Regional Mcrop is measured by the local crop yield structure. Detailed calculation is referred to Table 2.
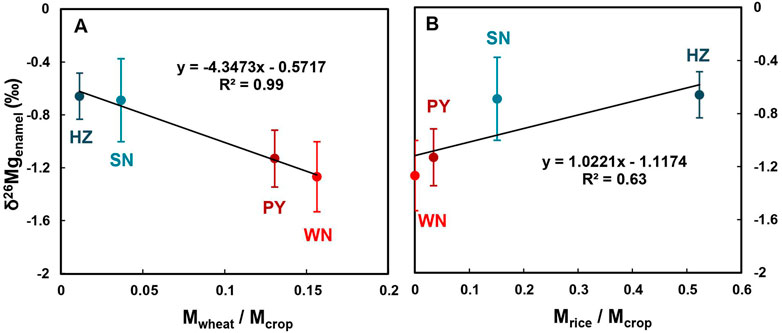
FIGURE 6. The Mwheat/Mcrop (A) and Mrice/Mcrop (B) in different regions and the δ26Mgenamel in local people (mean with 2SD, refer to Table 2 for detailed calculation method). Error bars indicate the 2SD of the mean value.
To date, it is still unclear about the isotope fractionation from diet to enamel. Limited studies brought up with a biological isotopic fractionation model of Mg in mammals that there is no fractionation between bone and diet, while muscle accumulates heavier Mg isotopes and feces is the 26Mg-depleted reservoir (Martin et al., 2015b). We could not elucidate the physiology of Mg isotope fractionation, but the higher Mg content and higher δ26Mg values in human enamel from southern China are consistent with their rice-dependent dietary characteristic, demonstrating the dietary structure dominates enamel Mg isotopic compositions. Also, the distinct δ26Mg values in human enamel of southerners and northerners relying on different C3 staple crops indicate that Mg isotopic system potentially distinguishes crop species among the C3 group.
Diet reconstruction is vital for studying the paleoecology and evolution of early hominins (Ungar, 2012). Direct food remains are often absent or under-represented in the depositional record, but stable isotopes in human apatite remain greatly documented the dietary inputs of ancient humans and are well-established dietary tracers (Makarewicz and Sealy, 2015; Katzenberg and Waters-Rist, 2018). The high abundance and biological isotopic fractionation of Mg make the Mg isotopic system promising for exploring human diet information. Bone Mg concentration has proven to be a poor dietary discriminator due to the unpredictable results (Lambert et al., 1979; Francalacci, 1989; Klepingera, 1990), but the Mg isotopic signatures detected in human enamel could provide another dimensional evidence.
In this study, we found a remarkable geographical distribution pattern that dental enamel of southerners has higher Mg content and heavier Mg isotopic composition than those of northerners. The strong correlation between the enamel δ26Mg and diet structure shows that Mg isotopes in enamel are more responsible for human nutritional structure, especially cereal-based dietary structure. Briefly, the northerners who prefer wheat have lower Mg content and lighter Mg isotopic composition in teeth enamel, while the southerner is opposite. Thereinto, the specific dietary structure means the proportion of wheat and rice, which are both C3 plants. The intake ratio of different C3 plants is reflected in the Mg isotopic composition in enamel, indicating that the Mg isotopic system may potentially distinguish specific plant species within the C3 plants. In addition, human hard tissues inherit all dietary sources’ weighted average Mg isotopic ratio characteristics (Martin et al., 2020), the other food intake, such as meat, may potentially be measured according to Mg isotopes.
Since the limited dataset has shown the large variability of δ26Mg in human enamel and the distinct characteristics among groups with different dietary structures, more work can be done to develop Mg isotopes as a promising tracer in diet and relevant areas. The large δ26Mg variability in one region and δ26Mg differences between food and enamel may involve associated intake preference and isotope fractionation, which cannot be addressed by the current study. Feeding experiments on mammals may reveal the physiological processes associated with Mg isotope fractionation, the dominant control among various foods, and other key factors. The Mg isotope signatures of the source of foods also require systematic investigations.
In this study, we present the first dataset for Mg isotopic compositions of the human apatite tissues to our knowledge. The linear correlation between the Mg isotopic compositions of human enamel and the intake proportion of cereal crops reveals the Mg isotopic composition in human enamel relative to the plant-based diet structure. The regional δ26Mg diversity in human enamel resulting from diet structure helps make the Mg isotopic system promising for identifying specific species among C3 (C4) plants. It suggests that Mg isotopes in human enamel could serve as a promising new tracer for human dietary structure, especially cereal food structure. Furthermore, the dense structure of enamel, which can resist diagenetic contamination and preserve the dietary records during the period of enamel deposition, provides an approach to exploring the ancient human recipe. Considering that the Mg isotopic signature is a relatively novel tool for reestablishing human diet structure and only a small sample dataset is analyzed, the application of this work still needs to be explored more by subsequent research.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of Northwest University. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Northwest University Medical Ethics Committee.
K-JH and XL conceived this study and Huang led the overall scientific questions. FG and PZ performed the test and data analysis. KL contributed to the sample pretreatment and data discussion. FG prepared and drafted the paper with contributions from all authors.
We thank Dr. Mao-Yong He (Institute of Earth Environment, Chinese Academy of Sciences) for the help with providing SN teeth samples. We also thank Zihua Tang (Institute of Geology and Geophysics, Chinese Academy of Sciences) for stimulating discussions. This work is financially supported by the National Natural Science Foundation of China (No. 41973008), and the project of The Key Laboratory of Cultural Heritage Research and Conservation, Ministry of Education, Northwest University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bao, Z., Huang, K.-J., Huang, T., Shen, B., Zong, C., Chen, K.-Y., et al. (2019). Precise magnesium isotope analyses of high-K and low-Mg rocks by MC-ICP-MS. J. Anal. At. Spectrom. 34, 940–953. doi:10.1039/C9JA00002J
Black, J., Epstein, E., Rains, W., Yin, Q.-Z., and Casey, W. (2008). Magnesium-isotope fractionation during plant growth. Environ. Sci. Technol. 42, 7831–7836. doi:10.1021/es8012722
Black, J., Yin, Q.-Z., and Casey, W. (2006). An experimental study of magnesium isotope fractionation in Chlorophyll-a Photosynthesis. Geochim. Cosmochim. Acta 70, 4072–4079. doi:10.1016/j.gca.2006.06.010
Black, J., Yin, Q.-Z., Rustad, J., and Casey, W. (2007). Magnesium isotopic equilibrium in chlorophylls. J. Am. Chem. Soc. 129, 8690–8691. doi:10.1021/ja072573i
Bolou-Bi, E. B., Poszwa, A., Leyval, C., and Vigier, N. (2010). Experimental determination of magnesium isotope fractionation during higher plant growth. Geochim. Cosmochim. Acta 74, 2523–2537. doi:10.1016/j.gca.2010.02.010
Bolou-Bi, E. B., Vigier, N., Brenot, A., and Poszwa, A. (2009). Magnesium isotope compositions of natural reference materials. Geostand. Geoanal. Res. 33, 95–109. doi:10.1111/j.1751-908X.2009.00884.x
Bolou-Bi, E. B., Vigier, N., Poszwa, A., Boudot, J.-P., and Dambrine, E. (2012). Effects of biogeochemical processes on magnesium isotope variations in a forested catchment in the Vosges Mountains (France). Geochim. Cosmochim. Acta 87, 341–355. doi:10.1016/j.gca.2012.04.005
Chen, Y., Zhang, Z., and Tao, F. (2018). Impacts of climate change and climate extremes on major crops productivity in China at a global warming of 1.5 and 2.0°C. Earth Syst. Dyn. 9, 543–562. doi:10.5194/esd-9-543-2018
De Baaij, J., Hoenderop, J., and Bindels, R. (2015). Magnesium in man: Implications for health and disease. Physiol. Rev. 95, 1–46. doi:10.1152/physrev.00012.2014
Elin, R. (1987). Assessment of magnesium status. Clin. Chem. 33, 1965–1970. doi:10.1093/clinchem/33.11.1965
Fan, B.-L., Zhao, Z.-Q., Tao, F., Li, X., Tao, Z., Gao, S., et al. (2016). The geochemical behavior of Mg isotopes in the Huanghe basin, China. Chem. Geol. 426, 19–27. doi:10.1016/j.chemgeo.2016.01.005
Faryadi, Q. (2012). The magnificent effct of magnesium to human health: A critical review. Int. J. Environ. Sci. Technol. 2, 118–126.
Francalacci, P. (1989). Dietary reconstruction at Arene Candide Cave (Liguria, Italy) by means of trace element analysis. J. Archaeol. Sci. 16, 109–124. doi:10.1016/0305-4403(89)90060-5
Gao, T., Ke, S., Wang, S.-J., Li, F., Liu, C., Lei, J., et al. (2018). Contrasting Mg isotopic compositions between Fe-Mn nodules and surrounding soils: Accumulation of light Mg isotopes by Mg-depleted clay minerals and Fe oxides. Geochim. Cosmochim. Acta 237, 205–222. doi:10.1016/j.gca.2018.06.028
Grigoryan, R., Costas-Rodríguez, M., Vandenbroucke, R., and Vanhaecke, F. (2020). High-precision isotopic analysis of Mg and Ca in biological samples using multi-collector ICP-mass spectrometry after their sequential chromatographic isolation – application to the characterization of the body distribution of Mg and Ca isotopes in mice. Anal. Chim. Acta X. 1130, 137–145. doi:10.1016/j.aca.2020.07.045
Huang, K.-J., Teng, F.-Z., Plank, T., Staudigel, H., Hu, Y., and Bao, Z.-Y. (2018). Magnesium isotopic composition of altered oceanic crust and the global Mg cycle. Geochim. Cosmochim. Acta 238, 357–373. doi:10.1016/j.gca.2018.07.011
Jaouen, K., and Balter, V. (2014). Menopause effect on blood Fe and Cu isotope compositions. Am. J. Phys. Anthropol. 153, 280–285. doi:10.1002/ajpa.22430
Katzenberg, M. A., and Waters-Rist, A. L. (2018). Biological anthropology of the human skeleton. New York: Wiley-Liss.
Kimmig, S., Holmden, C., and Bélanger, N. (2018). Biogeochemical cycling of Mg and its isotopes in a sugar maple forest in Québec. Geochim. Cosmochim. Acta 230, 60–82. doi:10.1016/j.gca.2018.03.020
Kiple, K. F., and Ornelas, K. C. (2000). The Cambridge World history and food. Cambridge: Cambridge University Press.
Klepingera, L. (1990). Magnesium ingestion and bone magnesium concentration in paleodietary reconstruction: Cautionary evidence from an animal model. J. Archaeol. Sci. 17, 513–517. doi:10.1016/0305-4403(90)90032-Z
Lambert, J. B., Simpson, S. V., Szpunar, C. B., and Buikstra, J. E. (1984). Ancient human diet from inorganic analysis of bone. Acc. Chem. Res. 17, 298–305. doi:10.1021/ar00105a001
Lambert, J. B., Szpunar, C. B., and Buikstra, J. E. (1979). Chemical analysis of excavated human bone from Middle and Late Woodland sites. Archaeometry 21, 115–129. doi:10.1111/j.1475-4754.1979.tb00248.x
Li, F., Zhou, M., Shao, J., Chen, Z., Wei, X., and Yang, J. (2020). Maize, wheat and rice production potential changes in China under the background of climate change. Agric. Syst. 182, 102853. doi:10.1016/j.agsy.2020.102853
Ling, X. (2010). “Study on dietary of ancient Qin people,”. Dissertation thesis (Xi’an: Northwest University).
Ma, L., Sun, Y., Jin, Z.-D., Bao, Z., Zhang, P., Meng, Z.-K., et al. (2019). Tracing changes in monsoonal precipitation using Mg isotopes in Chinese loess deposits. Geochim. Cosmochim. Acta 259, 1–16. doi:10.1016/j.gca.2019.05.036
Makarewicz, C. A., and Sealy, J. (2015). Dietary reconstruction, mobility, and the analysis of ancient skeletal tissues: Expanding the prospects of stable isotope research in archaeology. J. Archaeol. Sci. 56, 146–158. doi:10.1016/j.jas.2015.02.035
Martin, J. E., Tacail, T., Adnet, S., Girard, C., and Balter, V. (2015a). Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem. Geol. 415, 118–125. doi:10.1016/j.chemgeo.2015.09.011
Martin, J. E., Vance, D., and Balter, V. (2015b). Magnesium stable isotope ecology using mammal tooth enamel. Proc. Natl. Acad. Sci. U. S. A. 112, 430–435. doi:10.1073/pnas.1417792112
Martin, J. E., Vance, D., and Balter, V. (2014). Natural variation of magnesium isotopes in mammal bones and teeth from two South African trophic chains. Geochim. Cosmochim. Acta 130, 12–20. doi:10.1016/j.gca.2013.12.029
Martin, J., Tacail, T., Braga, J., Cerling, T., and Balter, V. (2020). Calcium isotopic ecology of Turkana Basin hominins. Nat. Commun. 11, 3587–7. doi:10.1038/s41467-020-17427-7
Mavromatis, V., Prokushkin, A. S., Pokrovsky, O. S., Viers, J., and Korets, M. A. (2014). Magnesium isotopes in permafrost-dominated Central Siberian larch forest watersheds. Geochim. Cosmochim. Acta 147, 76–89. doi:10.1016/j.gca.2014.10.009
Nadler, J. L., and Rude, R. K. (1995). Disorders of magnesium metabolism. Endocrinol. Metab. Clin. North Am. 24, 623–641. doi:10.1016/S0889-8529(18)30035-5
Nitzsche, K., Kato, Y., Shin, K.-C., and Tayasu, I. (2019). Magnesium isotopes reveal bedrock impacts on stream organisms. Sci. Total Environ. 688, 243–252. doi:10.1016/j.scitotenv.2019.06.209
Ryan, M. F. (1991). The role of magnesium in clinical biochemistry: An overview. Ann. Clin. Biochem. 28, 19–26. doi:10.1177/000456329102800103
Teng, F.-Z. (2017). Magnesium isotope Geochemistry. Rev. Mineral. Geochem. 82, 219–287. doi:10.2138/rmg.2017.82.7
Tipper, E., Galy, A., Gaillardet, J., Bickle, M., Elderfield, H., and Carder, E. A. (2006). The magnesium isotope budget of the modern ocean: Constraints from riverine magnesium isotope ratios. Earth Planet. Sci. Lett. 250, 241–253. doi:10.1016/j.epsl.2006.07.037
Ungar, P., Grine, F., Teaford, M., and El Zaatari, S. (2006). Dental microwear and diets of African early Homo. J. Hum. Evol. 50, 78–95. doi:10.1016/j.jhevol.2005.08.007
Ungar, P. S. (2012). Dental evidence for the reconstruction of diet in african early Homo. Curr. Anthropol. 53, S318–S329. doi:10.1086/666700
Van Heghe, L., Deltombe, O., Delanghe, J., Depypere, H., and Vanhaecke, F. (2014). The influence of menstrual blood loss and age on the isotopic composition of Cu, Fe and Zn in human whole blood. J. Anal. At. Spectrom. 29, 478–482. doi:10.1039/C3JA50269D
Vormann, J. (2003). Magnesium: Nutrition and metabolism. Mol. Asp. Med. 24, 27–37. doi:10.1016/S0098-2997(02)00089-4
Walczyk, T., and Blanckenburg, F. (2002). Natural iron isotope variations in human blood. Science 295, 2065–2066. doi:10.1126/science.1069389
Wang, Y., Wu, B., Berns, A., Xing, Y., Kuhn, A., and Amelung, W. (2020). Magnesium isotope fractionation reflects plant response to magnesium deficiency in magnesium uptake and allocation: A greenhouse study with wheat. Plant Soil 455, 93–105. doi:10.1007/s11104-020-04604-2
Weber, K., Weber, M., Menneken, M., Kral, A. G., Mertz-Kraus, R., Geisler, T., et al. (2021). Diagenetic stability of non-traditional stable isotope systems (Ca, Sr, Mg, Zn) in teeth – an in-vitro alteration experiment of biogenic apatite in isotopically enriched tracer solution. Chem. Geol. 572, 120196. doi:10.1016/j.chemgeo.2021.120196
Wimpenny, J., Yin, Q.-Z., Tollstrup, D., Xie, L., and Sun, J. (2014). Using Mg isotope ratios to trace cenozoic weathering changes: A case study from the Chinese loess plateau. Chem. Geol. 376, 31–43. doi:10.1016/j.chemgeo.2014.03.008
Wrobel, K., Karasiński, J., Tupys, A., Arroyo Negrete, M., Halicz, L., Wrobel, K., et al. (2020). Magnesium-isotope fractionation in chlorophyll-a extracted from two plants with different pathways of carbon fixation (C3, C4). Molecules 25, 1644–1655. doi:10.3390/molecules25071644
Xu, Y., Jin, Z.-D., Gou, L.-F., Galy, A., Jin, C.-Y., Chen, C., et al. (2022). Carbonate weathering dominates magnesium isotopes in large rivers: Clues from the Yangtze River. Chem. Geol. 588, 120677. doi:10.1016/j.chemgeo.2021.120677
Yang, Y.-X., Wang, G.-Y., and Pan, X.-C. (2019). China food composition. Beijing: Peking University Medical Press.
Young, E. D., Galy, A., and Nagahara, H. (2002). Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim. Cosmochim. Acta 66, 1095–1104. doi:10.1016/S0016-7037(01)00832-8
Zhao, D.-Y. (2016). The origin and formation of the Han population —a new perspective from physical anthropology. Dissertation thesis (Changchun: Jilin University).
Keywords: stable isotope, magnesium, dental enamel, plant food, dietary indicator
Citation: Gao F, Zhang P, Liu K, Ling X and Huang K-J (2023) Magnesium isotopic composition of modern human teeth enamel and its implications for dietary reconstructions. Front. Earth Sci. 10:1056881. doi: 10.3389/feart.2022.1056881
Received: 29 September 2022; Accepted: 31 October 2022;
Published: 23 January 2023.
Edited by:
Xiao-Ping Xia, Guangzhou Institute of Geochemistry (CAS), ChinaReviewed by:
Yaowu Hu, Fudan University, ChinaCopyright © 2023 Gao, Zhang, Liu, Ling and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang-Jun Huang, aGtqQG53dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.