
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Earth Sci., 20 January 2022
Sec. Paleontology
Volume 9 - 2021 | https://doi.org/10.3389/feart.2021.812910
This article is part of the Research TopicHuman-Animal Interactions in Prehistoric ChinaView all 11 articles
Zooarchaeology studies the human-animal interactions over long periods, and can be used to evaluate the sustainable exploitation of animal resources. Sika deer (Cervus nippon), a National Class Ⅰ protected wild animal species of China, used to be commonly found at Neolithic sites across China. In the Yangtze River region, although the Neolithic faunal assemblages show diversity in deer species, sika deer has always been one of the most important components. This research aims at discussing the exploitation of the environmental resources via the hunting strategy of sika deer at Tianluoshan, a Neolithic site in the lower Yangtze River region. The cull pattern and sex ratio of sika deer are reconstructed to display the pattern of prey selection. The results show a specific pattern targeting larger individuals including adults and juveniles, and targeting male over female. This pattern is able to maximize the yield, and keep the deer population sustainable. The sustainable hunting of sika deer probably is why the Tianluoshan site lasted for nearly a thousand years, during which sika deer had been a major prey for meat.
Zooarchaeology investigates the human-animal interactions over centuries and millennia, and thus provides irreplaceable tools for evaluating long-term sustainable use of natural resources (Butler and Delacorte, 2004; Frazier, 2007; Lyman, 1996; Wolverton and Lyman, 2012). In the long history of hunting, the unsustainable exploitation, together with other natural and anthropogenic factors, has caused the decline and extinction of many animal species, and this situation is getting more and more serious (Davis, 1987; Boivin et al., 2016; Frazier, 2007). Meanwhile in many cases, although capable of modifying the ecosystem, the exploitation was managed to a sustainable level, represented by the settlements which were inhabited for hundreds or thousands of years. There is a saying in Chinese: history is a mirror for us to see the faults. Zooarchaeology is not only to study the past, but also for the mankind to have a better future. However not realized, the fact is zooarchaeology has helped to reintroduce the extirpated populations of Père David’s deer (Elaphurus davidianus) in China, and relocate the conservation parks to the Huai River and Yangtze River region which were their original habitat (Cao, 2005).
Sika deer (Cervus nippon) is a medium sized cervid native to East Asia, inhabiting temperate and subtropical forests and shrubs with dense understory (Sheng, 1992; McCullough et al., 2009b). The IUCN Red List of Threatened Species now label sika deer as “least concern”, indicating that they are not under the threat of extinction (Harris, 2015). However, a glance at the distribution map shall realize that most sika deer are concentrated in Japan; their distribution area in China is limited into a few isolated patches, which is harmful for population continuity (Guo and Zheng, 1992; Sheng, 1992; Guo and Zheng, 2000; McCullough et al., 2009a). As a matter of fact, the subspecies C. n. mandarinus, and C. n. Grassianus are already extinct (Guo and Zheng, 1992; Guo and Zheng, 2000). The wild populations of sika deer have been listed as National Class Ⅰ Protected Wild Animal Species of China (National Forestry and Grassland Administration, 2021).
Archaeological records draw an entirely different distribution map of sika deer in the Neolithic period (approx. 10000–2000 BC). Sika deer remains have been found in sites across China (Figure 1) (Institute of Archaeology Chinese Academy of Social Sciences, 1991; Institute of Archaeology Chinese Academy of Social Sciences, 1994; Institute of Archaeology Chinese Academy of Social Sciences, 1995; Henan Provincial Institute of Relics and Archaeology, 1999; Zhang et al., 1999; Zhejiang Provincial Institute of Cultural Relics and Archaeology, 2004; Zhang and Hung, 2008; Innes et al., 2009; Ren and Wu, 2010; Institute of Archaeology Chinese Academy of Social Sciences, Institute of Earth Environment Chinese Academy of Sciences, 2011; Wu et al., 2012), and usually take up a rather high proportion in the faunal assemblage (e.g., Henan Provincial Institute of Relics and Archaeology, 1999; Zhejiang Provincial Institute of Cultural Relics and Archaeology, 2003; Yuan and Yang, 2004; Wang, 2011; Song, 2017; Song, 2019; Li et al., 2021). The hunting strategies of sika deer have been discussed in the past few years, featuring the middle Yellow River region which was an important prehistoric cultural center in China. The study of the Wayaogou site (3400–2700 BC) suggested that deer hunting was organized according to seasonality and the conditions of the deer population (Wang, 2011; Wang et al., 2014). A recent research on the Zaoshugounao (1250–1050 BC) sika deer remains reveals a sustainable hunting strategy that took place in the Bronze Age (Li et al., 2021).
This research intends to investigate whether sika deer were hunted sustainably in the Neolithic Yangtze River region, by interpreting the deer hunting strategy at the Tianluoshan site 7,000 to 6,000 years ago. The culling profile, sex ratio, and death seasons are used to discuss the choice of prey and the hunting seasons, which are key issues in the hunting programme.
1. Hemudu (5000–4000 BC); 2. Songze (3500–3000 BC); 3. Majiabang (5000–4000 BC) 4. Kuahuqiao (6200–4200 BC); 5. Longqiuzhuang (3000–2300 BC); 6. Xianrendong (22550–10000 BC); 7. Jiahu (6100–4800 BC); 8.Xipo (2800–2300 BC); 9. Wayaogou (3400–2700 BC); 10. Zaoshugounao (1250–1050 BC).
The site of Tianluoshan is located in a small valley in Yuyao County, Zhejiang province, in the lower Yangtze River plain (Figure 1). It has been under the spotlight since its first excavation in 2004, due to the extremely rich material culture which resembles the famous Hemudu Culture which is known worldwide for rice cultivation 7,000 to 6,000 years ago (Bellwood, 2005; Chang, 1987; Fuller et al., 2009; Scarre, 2018). Radiocarbon dating shows that the site was occupied approximately 5000–4000 BC. As excavations and research went on, it was revealed that Tianluoshan was another representative site of the Hemudu culture, with the typical black pottery, worked bone manufacture, and most importantly rice domestication (Fuller et al., 2011; Fuller et al., 2009; Sun, 2011; Zhejiang Provincial Institute of Cultural Relics and Archaeology, 2003). The researchers divided the culture layers into three phases based on stratigraphy, and this division was also supported by radiocarbon data: phase 1 included layer 8 and 7 and lasted approximately from 5000 to 4700 BC; phase 2 included layer 6 and 5, at 4700–4300 BC; phase 3 consisted of layer 4 and 3, at about 4300–4000 BC (Figure 2) (Sun, 2011; Wu et al., 2011; Nakamura et al., 2016). Therefore, the Tianluoshan Neolithic village was inhabited from 5,000 to 4000 BC. The radiocarbon dates are summarized in Supplementary Table S1.
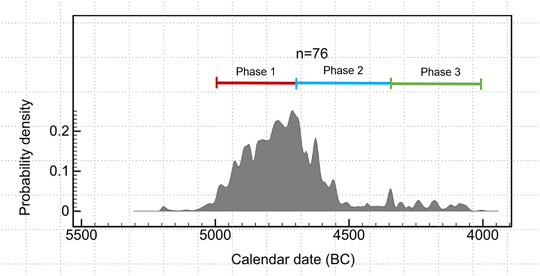
FIGURE 2. The calibrated radiocarbon dates of Tianluoshan. The dates from 76 samples form a time sequence of Tianluoshan.
The well-preserved materials by the waterlogged environment provided great information to reconstruct the village life six to seven thousand years ago. Plant and animal remains indicated a subsistence economy greatly relied on hunting, gathering, and fishing wild resources such as acorns (Quercus), water chestnuts (Trapa), fox-nuts (Euryale), sika deer (Cervus nippon), muntjac (Muntiacus reevesi), snakehead (Channa argus), crucian carp (Carassius auratus), etc. Among the mammalian remains, several species of deer were major prey; sika deer outnumbered the other species e.g. muntjac, sambar (Cervus unicolor), and Père David’s deer (Elaphurus davidianus), indicating sika deer hunting should be an important part in the entire subsistence strategy.
All sika deer remains were collected during the excavations from 2004 to 2010. Medium to large mammal remains, sika deer included, were mainly retrieved by hand collecting. Wet sieving was crucial for retrieving small animal remains, such as fish, small deer, etc., but only contributed a small number of deer, mostly carpals, tarsals, and phalanx. Animal remains were stored by context (trench and layer).
The mammal remains from each context were first separated into “identifiable” and “unidentifiable” categories. The “unidentified” was sorted into large, medium, and small mammals based on the bone size and shaft thickness. The “identifiable” was further sorted by element, and then identified to family, genus, and species if possible, using the reference collection in School of Archaeology and Museology, Peking University; published atlases were also used in identification (Schmid, 1972; Hillson, 1992, 2005; Matsui, 2001-2005). The results of species/genus, element, ageing, sexing, worked traces, gnawing traces, and measurements were recorded in a database. Number of Identified Specimens (NISP) and Minimum Number of Individuals (MNI) were used for quantitative analysis (Grayson, 1984; Lyman, 2008; Reitz and Wing, 2008).
Sika deer remains from the faunal assemblage were taken for further observation, and the sika deer records were selected from the database for quantification. The mammalian assemblage contained 13718 pieces of remains, and 6,378 pieces could be identified to order, family and beyond. The total number of sika deer remains was 2486, was the largest in the identifiable specimens (Figure 3). 341 sika deer specimens were identified from phase 1, taking up 36.7% of the identifiable mammalian specimens; 1618 were from phase 2, taking up 38.3% of the mammalian NISP, and 527 were from phase 3, taking up 43.4% of the NISP. For the concerning of sample size, 141 mandibles from both sides were used for dental ageing, including 30 mandibles from phase 1, 87 from phase 2, and 24 from phase 3. The mandibles were checked not to be paired morphologically.
Dental records and the epiphyseal fusion of postcranial bones have been widely used to estimate the age at death of a mammal in zooarchaeology (Grant, 1982; Silver, 1969). The ageing and sexing methods for different deer species have been developed based on the research of modern specimens. Tooth eruption, development, and replacement show precise age of fawns, juveniles, and sub-adults (Brown and Chapman, 1991a; Koike and Ohtaishi, 1985; Sheng, 1992). For adults, attritional wear on the occlusive surface is commonly used, by reading the wear pattern or using a scoring scheme (Brown and Chapman, 1991b; Brown and Chapman, 1990; Chapman et al., 2005; Ohtaishi, 1980). Crown height measurement and count of annuli in cementum are also used (Klein et al., 1981; Koike and Ohtaishi, 1985). The epiphyseal fusion on postcranial bones of fallow deer (Dama dama) has been researched (Carden et al., 2006).
As sika deer has been the one and only native deer species in Japan, Japanese scholars contributed plenty work in relative research (Ohtaishi, 1975, 1976, 1978, 1980; Koike and Ohtaishi, 1985, 1987; Ohtaishi and Gao, 1990; Sheng et al., 1998; Uchiyama, 1999; McCullough et al., 2009b). Ohtaishi and colleagues established the sequence of teeth eruption, replacement, and wear by studying modern sika deer in Japan, and applied to reconstruct the death age and cull pattern of the sika deer remains from Jomon sites (Ohtaishi, 1980; Koike and Ohtaishi, 1985, Koike and Ohtaishi, 1987). There was also an attempt to apply Brown and Chapman’s scoring scheme on red deer to sika deer (Wang et al., 2014). As Ohtaishi’s approach is easy to use, here we employ Ohtaishi’s methods to estimate the death age of each sika deer individual, to generate the cull pattern of the captured sika population, and to discuss the hunting strategy at Tianluoshan.
Female and male sika deer are different morphologically, and sexing skeletal parts are usually undertaken by two means. First, the presence and absence of antlers on cranium. Male deer (stags) start to grow antlers in the second summer after birth, and bear full antlers at 3–4 years old; females do not grow antlers throughout their lives (Hayden et al., 1994; Yang et al., 1990). Second, sexual dimorphism exists between two sexes: body measurements of adult males averages 8.7% greater than those of females (Feldhamer, 1980). The measurements of the postcranial bones may show the difference, but the existence of juvenile and sub-adult individuals can blur the boundary. Research showed that the measurements of mandibles and dental sequence show the indication between females and males (Uchiyama, 1999). In this research, we attempt to use cranium and mandibles to analyze whether there was a preference of sika deer sex in hunting.
A-C represent 3 life-stages of sika deer: A-fawn, B-juvenile, and C-adult. The span of each block represents the time length of the life-stage: fawn–1 year, juvenile— years, and adult—10 years. The “abundant” life-stages are shown by the larger letters. The histograms are recreated using data from Koike and Ohtaishi (1987).
The lifespan of captive sika deer is 15–18 years, but wild individuals rarely live over 10 years (Ohtaishi, 1978; Koike and Ohtaishi, 1985; Landesman, 1999). The life history of sika deer can be classified into three life-stages: fawn (under 1 year old), juvenile (1–4 years old), and adult (5 years and older). Sika deer of different life-stages show different biological features and act differently, so the following age profiles shall be named after these terms.
Koike and Ohtaishi (Koike and Ohtaishi, 1987) established three models for age structure based on their research on the archaeological remains from 14 sites: adult-abundant pattern, which contained over 60% adults, represented by Ishiyama shell midden site (Early Jomon period, about 5000–3520 BC); Juvenile-dominant pattern, in which the proportion of juveniles is around 60%, represented by Fuyuki site of Late Jomon period (2470–500 BC); and fawn-abundant pattern represented by Onnemoto site of Okhotsk culture. These three patterns are modified using original data, and displayed by life-stages (Figure 4).
Cull pattern can reflect the hunting techniques. A cull with age composition similar to the live population is to be expected from random-capture harvesting by intensive trapping techniques such as the drive-in traps; and the proportion of young animals may be slightly higher as some older ones are likely to escape the traps based on their experience. By contrast, hunting techniques that targeting single animals, such as with a bow and arrow or gun, should produce stronger selection among the game animal. The indicator for distinguishing two hunting techniques is the frequency of fawns and yearlings: a high frequency of fawns and yearlings refers to catastrophic hunting such as trapping, while a low frequency indicates individual hunting. Therefore, the three models indicate different hunting techniques that were used at the sites. Individual hunting was practiced at Jomon sites such as Ishiyama and Fuyuki, and trapping was used at Onnemoto, an Okhotsk Culture site.
The hunting seasons are important for us to learn about the exploitation of the animal resources, and labour and time management of year. Sika deer have regular life cycles that breeding and birth occur in fixed time of year. According to the research on the modern sika deer population in the lower Yangtze River region, breeding occurs from September to December, and birth occurs around mid-May (Yu, 2008). Antler shedding is also seasonal, which occurs in april and May, and thereafter stags grow a new pair during summer to get ready for the rutting season in autumn (Yang et al., 1990).
The hunting season of sika deer is estimated by means of two approaches. First, the death season of an individual can be calculated by adding the age at death which is already estimated for cull pattern reconstruction, to birth month. In order to get a precise result, we only choose the individuals younger than 2 years old, the age of which are estimated from teeth eruption and replacement. Second, the presence, absence, and the structure of antlers represent the death season of the stag. The stag bears antler from June to March the next year, and hunting during these months shall get deer with antlers growing stiffly on the heads (Yang et al., 1990). During the early time of the antler-growing cycle, also known as the velvet antler period, the structure of the antler is spongy, thus it shall indicate a very specific time of hunting. If hunting occurs when the stags shed antlers, the skull shall only have antler base with a flat natural shedding surface on top.
The cull pattern distributions of three phases share similarities. First, the age span in each phase is wide, containing individuals from under 1 year old to over 8 years old. The teeth of sika deer older than 8 years old are heavily worn, thus the death age cannot be estimated accurately. The percentages of adults is 43.3% in phase 1, 41.4% in phase 2, and 50% in phase 3, and juveniles is 40% in phase 1, 34.5% in phase 2, and 41.7% in phase 3, respectively. The proportions of fawns are quite low, 16.7% in phase 1, 10.3% in phase 2, 8.3% and in phase 3, respectively, decreasing slightly through time (Figure 5).
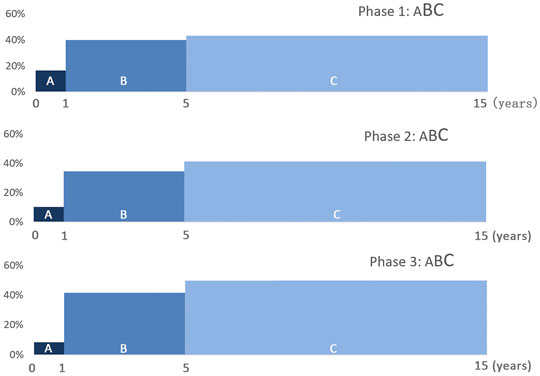
FIGURE 5. Cull patterns of Tianluoshan sika deer. (A): fawn; (B): juvenile; (C): adult. The span of each block represents the time length of a life-stage: fawn–1 year, juvenile—4 years, and adult—10 years. Large letter indicates a high proportion of the life-stage.
The Tianluoshan cull patterns can be classified as the adult-abundant pattern, as the adult individuals have the highest proportions (40–50%), although do not reach the proportion of the model pattern (60%). The proportions of the juveniles and fawns are also higher than the adult-abundant pattern. Therefore, the Tianluoshan pattern can be seen as the adult-abundant pattern with a few juvenile and fawn factors.
28 fragments with frontlets are recorded in this study, and 27 of them have antler bases attached, indicating male predominance. As cranial fragments are difficult to identify to species, the proportion of male sika can be exaggerated.
Therefore, we also examine the measurements on the mandibles following Uchiyama’s (1999) procedure. The height of anterior mandible (a) and the length of premolar sequence (PL) were measured, both of which were related to age and sex due to bone development. As both measurements were taken on the mandible rather than on teeth, they were not influenced by tooth attrition. The mandibles which show information of age are marked separately.
Three clusters of plots can be recognized in Figure 6. Group A is the smallest in measurement, and mainly consist of fawns and yearlings. The developing mandibles bring variety to this group. The mandible size of group B is larger than group A, and possibly consist of females; sub-adult males may also fall in this group. Group C is the largest in measurement, possibly representing the males. Group C also has the largest number of individuals (n = 31), comparing to group A (n = 11) and group B (n = 16), indicating that adult male were hunted more frequently than the females. All measurements for this analysis can be found in Supplementary Table S2.

FIGURE 6. Mandible measurements showing sexual dimorphism. The plots cluster into three groups: (A) fawns and yearlings; (B) females and young males; (C) males.
In brief, both female and male sika deer were captured, and male deer were hunted more frequently.
24 mandibles representing 24 individuals under the age of 2 years old were carefully examined, their age were identified to month. Research shows that the rutting season of sika deer in the Yangtze River region is from August to November, and the birth of fawns occurs from May to July, mostly in June. give birth to the fawns in mid-May (Yang et al., 1990). Here we use June as the birth month for the calculation of death month.
Over half (15 individuals) were killed in winter from December to February the next year (Figure 7). Sika deer hunting also occurred in the other three seasons, but less frequently. The antler growth and shed cycle agreed with the dental ageing. Sika stags start to grow a new pair of antlers in April-May, and wear them throughout the year until april the next year (Yang et al., 1990). 26 out of the total 27 male frontlets have antlers attached, indicating that hunting mostly took place between summer and the next spring.
Therefore, winter hunting could avoid the birth season, and diminish the harmful effect to the population, indicating that deer hunting was conducted based on the knowledge of sika deer’s life history.
From the cull pattern, sex ratio, and seasonality analysis above, we can now draw an image of the sika deer hunting strategy practiced at Tianluoshan 7,000–6,000 years ago.
First, adults and juveniles were preferred, possibly to maximize the hunting profit. According to the body growth model of sika deer, body weight increases rapidly until 3 years, stabilizes until about 10 years, and gradually declines thereafter (Miura and Tokida, 2009). The adults and many juveniles fall into this range, indicating that the hunting prey were chosen, probably by their body size.
Second, the sika deer cull patterns indicate that single animals, rather than the deer population, were targeted in each hunting. Hunting strategy which targets the entire population, such as the drive-in traps, can result in a cull pattern that similar to the live population, with large proportions of fawns and yearlings. By contrast, hunting techniques that targeting single animals, such as guns, bows and arrows, should produce stronger selection among the game animals, e.g., the larger individuals or animals bearing larger antlers, to get a better return. Therefore, the fawn-abundant pattern can be interpreted as population hunting. The adult- and juvenile-abundant patterns, on the other hand, can be seen as the results of individual hunting. We may propose that the Tianluoshan people were targeting one or a few sika deer in each hunt.
Third, both male and female sika deer were hunted, but males were probably hunted more frequently than the females. Due to sexual dimorphism, male sika deer are larger than the females. Therefore, hunting males can get a better return, including meat and antlers for making tools.
Fourth, sika deer was an important stable meat resource for the Tianluoshan residents throughout the year, and was even more important for them to overcome the food shortage in winter. Seasonality analysis shows that deer hunting was practiced in different months of year, and more frequently in winter time. Similar hunting calendar was used by the Neolithic Jomon people living on the Japanese archipelago, that sika deer were mostly hunted in winter time (Habu, 2004; Kobayashi, 2004). The body weight of animals usually varies between different seasons. Research on the modern sika deer revealed that sika deer was heaviest in autumn, with a mean body weight of 62.6kg, and would lose a few kilos in winter (mean body weight 57.4 kg) (Masuko and Souma, 2009).
The culling profiles, sex ratio, and hunting seasons indicate that a sustainable hunting strategy was practiced at Tianluoshan. In the sika deer population, adult male deer were mostly targeted; conversely, the proportions of females and fawns were rather low. This selection of prey left out females and fawns which were crucial for population reproduction. Most hunting took place in winter, avoiding the rutting season in autumn and the birth seasons from May to July.
In the study of modern animals, life table and survivorship curves are commonly used to examine the life history of a population. The survivorship curve is displayed by the logarithm of the frequency of surviving individuals against age. There are three theoretical survivorship curves showing different life history patterns according to Deevey (1947) (Figure 8). The type Ⅰ curve indicates that the animals have a high survivorship rate until old age, represented by large animals e.g. elephants. The type Ⅲ indicates a high level of mortality at a very young age, but the survivors have a good chance of reaching maturity. The type Ⅱ curve lies in between, indicating that the probability of death remains constant throughout life. Most animals’ survivorship curves fall into type Ⅰ and type Ⅱ or intermediate. Reef fish and oysters fall into the type Ⅲ range.
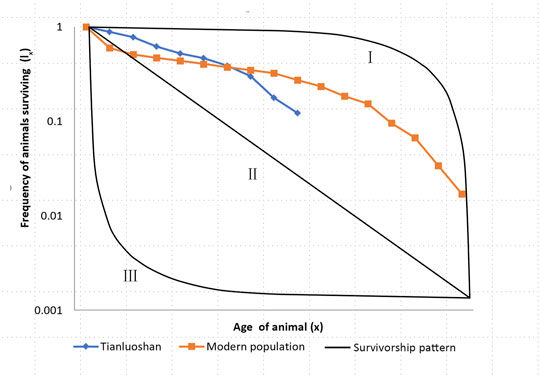
FIGURE 8. The survivorship curves of Tianluoshan and modern sika deer. The frequency of individuals surviving is plotted on a logarithmic scale on the Y axis against the age of animal on the X axis. The survivorship curves Ⅰ, Ⅱ and Ⅲ are theoretical curves following Deevey (1947).
The survivorship curve of Tianluoshan sika deer is generated from the cull structure. Due to the similarity between the cull structures of three phases, the individuals are added together as a large sample. The survivorship curve of modern sika deer is generated following Koike and Ohtaishi (1987), a stationary population protected from hunting. The survivorship curve of Tianluoshan sika deer fall into the area between type Ⅰ and type Ⅱ. It has lower mortality rate for fawns and individuals younger than 6 years old than the protected population, but the mortality rate for adult individuals older than 6 years old increases significantly (Figure 8). Clearly hunting has influenced the survivorship of the sika deer population near Tianluoshan, especially comparing to the Japanese population which only face natural death and predators; but the hunting strategy that deliberately avoid young individuals did not threaten the population. This probably is why the Tianluoshan site lasted for nearly a thousand years, during which sika deer had been a major prey for meat.
This research interpreted the sika deer hunting strategy at the site of Tianluoshan, from the perspectives of culling profile, sex ratio, and death seasons. The results show that sika deer were hunted individually, and adult male deer were preferred over females and fawn, possibly for the consideration of maximize the harvest; although sika deer could be hunted throughout the year, the hunting mostly took place in winters, avoiding the breeding seasons in autumn and spring. All the factors indicate that deer hunting was conducted orderly, following the rules of population sustainability. This hunting strategy supplied the Tianluoshan people with stable and consistent food resources, so that the Hemudu culture could develop prosperously.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YZ, GS, and XY contributed to conception and design of the study, and funding acquisition. GS and YW contributed to the investigation of the research materials. YZ organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. YH and HK contributed sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the National Social Science Fund of China (grant number 16CKG015) and the National Natural Science Foundation of China (grant number 41771231).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2021.812910/full#supplementary-material
Boivin, N. L., Zeder, M. A., Fuller, D. Q., Crowther, A., Larson, G., Erlandson, J. M., et al. (2016). Ecological Consequences of Human Niche Construction: Examining Long-Term Anthropogenic Shaping of Global Species Distributions. Proc. Natl. Acad. Sci. USA 113, 6388–6396. doi:10.1073/pnas.1525200113
Brown, W. A. B., and Chapman, N. G. (1991a). Age Assessment of Red Deer ( Cervus elaphus ): from a Scoring Scheme Based on Radiographs of Developing Permanent Molariform Teeth. J. Zoolog. 225, 85–97. doi:10.1111/j.1469-7998.1991.tb03803.x
Brown, W. A. B., and Chapman, N. G. (1990). The Dentition of Fallow Deer (Dama Dama) : a Scoring Scheme to Assess Age from Wear of the Permanent Molariform Teeth. J. Zoolog. 221, 659–682. doi:10.1111/j.1469-7998.1990.tb04023.x
Brown, W. A. B., and Chapman, N. G. (1991b). The Dentition of Red Deer ( Cervus elaphus ): a Scoring Scheme to Assess Age from Wear of the Permanent Molariform Teeth. J. Zoolog. 224, 519–536. doi:10.1111/j.1469-7998.1991.tb03783.x
Butler, V. L., and Delacorte, M. G. (2004). “Doing Zooarchaeology as if it Mattered: Use of Faunal Data to Address Current Issues in Fish Conservation Biology in Owens Valley, California,” in Zooarchaeology and Conservation Biology. Editors L. Lyman, and K. P. Cannon (Salt Lake City: University of Utah Press), 25–44.
Carden, R. F., Hayden, T. J., and Ruscillo, D. (2006). “Epiphyseal Fusion in the Postcranial Skeleton as an Indicator of Age at Death of European Fallow Deer (Dama Dama Dama, Linnaeus, 1758),” in Recent advances in ageing and sexing animal bones. Editor D. Ruscillo (Oxford: Oxbow Books), 227–236.
Chang, K. C. (1987). The Archaeology of Ancient China. 4th ed. New Haven and London: Yale University Press.
Chapman, N. G., Brown, W. A. B., and Rothery, P. (2005). Assessing the Age of Reeves' Muntjac ( Muntiacus reevesi ) by Scoring Wear of the Mandibular Molars. J. Zoolog. 267, 233–247. doi:10.1017/s0952836905007405
Deevey, E. S. (1947). Life Tables for Natural Populations of Animals. Q. Rev. Biol. 22, 283–314. doi:10.1086/395888
Frazier, J. (2007). Sustainable Use of Wildlife: The View from Archaeozoology. J. Nat. Conservation 15, 163–173. doi:10.1016/j.jnc.2007.08.001
Fuller, D. Q., Qin, L., Zhao, Z., Zheng, Y., Hosoya, A., Chen, X., et al. (2011). “Archaeobotanical Analysis at Tianluoshan: Evidence for Wild-Food Gathering, rice Cultivation and the Process of the Evolution of Morphologically Domesticated rice,” in Centre for the Study of Chinese Archaeology in Peking University, Zhejiang Province Institute of Archaeology and CulturalIntegrated Studies on the Natural Remains from Tianluoshan (Beijing: Cultural Relics Press), 47–96.
Fuller, D. Q., Qin, L., Zheng, Y., Zhao, Z., Chen, X., Hosoya, L. A., et al. (2009). The Domestication Process and Domestication Rate in Rice: Spikelet Bases from the Lower Yangtze. Science 323, 1607–1610. doi:10.1126/science.1166605
Grant, A. (1982). “The Use of Tooth Wear as a Guide to the Age of Domestic Ungulates,” in Ageing and Sexing Animal Bones from Archaeological Sites. Editors B. Wilson, C. Grigson, and S. Payne (Oxford: BAR British Series).
Grayson, D. K. (1984). Quantitative Zooarchaeology: Topics in the Analysis of Archaeological Faunas. London: Academic Press.
Guo, Y., and Zheng, H. (1992). Geographic History of Sika Deer in China. J. China West Normal Univ. (Natural Sciences) 13, 1–9.
Guo, Y., and Zheng, H. (2000). On the Geological Distribution, Taxonomic Status of Species and Evolutionary History of Sika Deer in China. Acta Theriologica Sinica 20, 168–179.
Harris, R. B. (2015). Cervus Nippon. The IUCN Red List of Threatened Species 2015, e.T41788A22155877. doi:10.2305/IUCN.UK.2015-2.RLTS.T41788A22155877.en
Hayden, T. J., Lynch, J. M., and O'Corry‐Crowe, G. (1994). Antler Growth and Morphology in a Feral Sika Deer (Cervus Nippon) Population in Killarney, Ireland. J. Zoolog. 232, 21–35. doi:10.1111/j.1469-7998.1994.tb01557.x
Hillson, S. (1992). Mammal Bones and Teeth : An Introductory Guide to Methods of Identification London: University College London Institute of Archaeology.
Innes, J. B., Zong, Y., Chen, Z., Chen, C., Wang, Z., and Wang, H. (2009). Environmental History, Palaeoecology and Human Activity at the Early Neolithic Forager/cultivator Site at Kuahuqiao, Hangzhou, Eastern China. Quat. Sci. Rev. 28, 2277–2294. doi:10.1016/j.quascirev.2009.04.010
Institute of Archaeology Chinese Academy of Social Sciences (1991). Radiocarbon Dates in Chinese Archaeology (1965-1991). Beijing: Cultural Relics Press.
Institute of Archaeology Chinese Academy of Social Sciences (1994). Report of 14C Dates (XXI). Kaogu (Archaeology), 662–664. https://t.cnki.net/kcms/detail?v=P7vMvggqYY18yHXDGTwC1Yja15bcFHA8PAp9MaSsbdplEJbFuzrrHKJTYdavzEXlOhoyXI84P0zXPMTvri32R7gCj7gaSTALSXBcvU3LpvUsGXFIhNFBGg==;uniplatform=NZKPT
Institute of Archaeology Chinese Academy of Social Sciences (1995). Report of 14C Dates(XXII). Kaogu (Archaeology), 655–659. https://t.cnki.net/kcms/detail?v=P7vMvggqYY2mDIU7jIzWuKUinOBjxz6dFrEbqstVipTUzjH9MiC-1jbRTlD0Nc67qaPIBGmZsrAlohA9g8AJQel9veiWAJsc4mHHAw6vkYw77ro8sEpJFQ==;uniplatform=NZKPT
Institute of Archaeology Chinese Academy of Social Sciences, Institute of Earth Environment Chinese Academy of Sciences (2011). Report of 14C Dates(XXXVII). Kaogu (Archaeology), 65–67. https://t.cnki.net/kcms/detail?v=P7vMvggqYY2KNVaZM1FB2LkQx206dRsDpwTq7ChLFOAq4CnBd4FGFVviZsdyPeaIbBxJa7pvMyLJD5f8q9wcYOsh8U5FE8Xw_ztI9bx8OIlaNxehukkP5w==;uniplatform=NZKPT
Klein, R. G., Wolf, C., Freeman, L. G., and Allwarden, K. (1981). The Use of Dental crown Heights for Constructing Age Profiles of Red Deer and Similar Species in Archaeological Samples. J. Archaeological Sci. 8, 1–31. doi:10.1016/0305-4403(81)90010-8
Kobayashi, T. (2004). Jomon Reflections: Forager Life and Culture in the Prehistoric Japanese Archipelago. Oxford: Oxbow Books.
Koike, H., and Ohtaishi, N. (1987). Estimation of Prehistoric Hunting Rates Based on the Age Composition of Sika Deer (Cervus Nippon). J. Archaeological Sci. 14, 251–269. doi:10.1016/0305-4403(87)90014-8
Koike, H., and Ohtaishi, N. (1985). Prehistoric Hunting Pressure Estimated by the Age Composition of Excavated Sika Deer (Cervus Nippon) Using the Annual Layer of Tooth Cement. J. Archaeological Sci. 12, 443–456. doi:10.1016/0305-4403(85)90004-4
Landesman, N. (1999). Cervus Nippon, Animal Diversity Web. University of Michigan. Available at: https://animaldiversity.org/accounts/Cervus_nippon/ (Accessed October 6, 2021).
Li, Y., Zhang, C., Chen, H., Wang, Z., and Qian, Y. (2021). Sika Deer in Bronze Age Guanzhong: Sustainable Wildlife Exploitation in Ancient China? Antiquity 95, 940–954. doi:10.15184/aqy.2021.75
Lyman, R. L. (1996). Applied Zooarchaeology: The Relevance of Faunai Analysis to Wildlife Management. World Archaeology 28, 110–125. doi:10.1080/00438243.1996.9980334
Masuko, T., and Souma, K. (2009). “Nutritional Physiology of Wild and Domesticated Japanese Sika Deer,” in Sika Deer: Biology and Management of Native and Introduced Populations. Editors D. R. McCullough, S. Takatsuki, and K. Kaji (Springer), 61–82.
Matsui, A. (2008). Fundamentals of Zooarchaeology in Japan and East Asian Kyoto: Kyoto University Press.
McCullough, D. R., Jiang, Z.-G., and Li, C.-W. (2009a). “Sika Deer in mainland China,” in Sika Deer: Biology and Management of Native and Introduced Populations. Editors D. R. McCullough, S. Takatsuki, and K. Kaji (Springer), 521–539.
McCullough, D. R., Takatsuki, S., and Kaji, K. (2009b). Sika Deer: Biology and Management of Native and Introduced Populations. Tokyo: Springer.
Miura, S., and Tokida, K. (2009). “Management Strategy of Sika Deer Based on Sensitivity Analysis,” in Sika Deer: Biology and Management of Native and Introduced Populations. Editors D. R. McCullough, S. Takatsuki, and K. Kaji (Springer), 453–474.
Nakamura, T., Kikuchi, H., Maruyama, M., Sun, G., Matsui, A., and Nakamura, S. (2016). “Radiocarbon Dating of Wooden Artifacts Excavated from the Tianluoshan Site,” in The Origin and Diffusion of LIvestock and Poultry in Neolithic East Asia: New Zooarchaeological Evidence from China. Editors A. Matsui, and H. Kikuchi (Nara: Nara National Research Institute for Cultural Properties).
National Forestry and Grassland Administration (2021). National Protected Wild Animal Species of China.
Ohtaishi, N. (1980). Determination of Sex, Age, and Death Season of Recovered Remains of Sika Seer (Cervus Nippon) by Jaw and Tooth-Cement. Archaeology Nat. Sci., 51–74.
Ohtaishi, N. (1978). Ecological and Physiological Longevity in Mammals. J. Mamm. Soc. Jpn. 7, 130–134.
Ohtaishi, N., and Gao, Y. (1990). A Review of the Distribution of All Species of Deer (Tragulidae, Moschidae and Cervidae) in China. Mammal Rev. 20, 125–144. doi:10.1111/j.1365-2907.1990.tb00108.x
Ohtaishi, N. (1976). Life Table for Japanese Deer at Nara Park and its Characteristic. Annu. Rep. Nara Deer Res. Assoc., 83–95.
Ohtaishi, N. (1975). Life Table for the Japanese Deer at Nara Park (Preliminary). Annu. Rep. Nara Deer Res. Assoc., 25–35.
Scarre, C. (2018). The Human Past: World Prehistory and the Development of Human Societies. London: Thames & Hudson Ltd.
Schmid, E. (1972). Atlas of Animal Bones for Prehistorians, Archaeologists and Quaternary Geologists. Amsterdam: Elsevier Publishing Company.
Sheng, H., Ohtaishi, N., and Lu, H. (1998). The Mammalian of China. Beijing: Chinese Forestry Press.
Silver, I. A. (1969). The Ageing of Domestic Animals, Science in Archaeology. London: Thames and Hudson, 283–302.
Song, Y. (2017). THe Subsistence Stratery of Early Majiabang Culture: A Zooarchaeological Perspective, Southeast Cult., 72–77. http://dnwh.njmuseum.com/pdf/2017/201705/20170508.pdf
Song, Y. (2019). The Subsistence Research of the Middle and Late Majiabang Culture: A Zooarchaeology Perspective. Southeast Cult., 47–55. http://dnwh.njmuseum.com/pdf/2019/201905/20190505.pdf
Sun, G. (2011). “Report on the 2004 -2008 Excavation at Tianluoshan,” in Centre for the Study of Chinese Archaeology in Peking University, Zhejiang Province Institute of Archaeology and Cultural Heritage Integrated Studies on the Natural Remains from Tianluoshan (Beijing: Cultural Relics Press).
Uchiyama, J. (1999). Seasonality and Age Structure in an Archaeological Assemblage of Sika Deer (Cervus Nippon). Int. J. Osteoarchaeol. 9, 209–218. doi:10.1002/(sici)1099-1212(199907/08)9:4<209:aid-oa457>3.0.co;2-u
Wang, H. (2011). Animal Subsistence of the Yangshao Period in the Wei River Valley: A Case-Study from the Site of Wayaogou in Shaanxi Province, China. London: Institute of Archaeology. University College.
Wang, H., Wang, W., and Hu, S. (2014). Human Strategies of Hunting Sika Deers in the Yangshao Period: A Case Study of the Wayaogou Site, Shaanxi Province. Acta Anthropologica Sinica 33, 90–100. doi:10.16359/j.cnki.cn11-1963/q.2014.01.002
Wolverton, S., and Lyman, R. L. (2012). Conservation Biology and Applied Zooarchaeology. Tucson: University of Arizona Press.
Wu, X., Qin, L., and Sun, G. (2011). “Radiocarbon Dates of Tianluoshan,” in Centre for the Study of Chinese Archaeology in Peking University, Zhejiang Province Institute of Archaeology and Cultural Integrated Studies on the Natural Remains from Tianluoshan. (Beijing: Cultural Relics Press).
Wu, X., Zhang, C., Goldberg, P., Cohen, D., Pan, Y., Arpin, T., et al. (2012). Early Pottery at 20,000 Years Ago in Xianrendong Cave, China. Science 336, 1696–1700. doi:10.1126/science.1218643
Yang, J., Ding, T., and Hu, P. (1990). Research on the Ecology of Southern Sika Deer (Cervus Nippon Kopschi). Wildlife, 17–19.
Yu, J.-a. (2008). Study on the Population Size and Distribution of South China Sika Deer in Qingliangfeng Nature Reserve. MS dissertation. Zhejiang Forestry University.
Yuan, J., and Yang, M. (2004). “Research on Animal Remains,” in Zhejiang Province Institute of Archaeology and Cultural Heritage, Xiaoshan Museum Kuahuqiao (Beijing: Cultural Relic Press), 241–270.
Zhang, C., and Hung, H.-C. (2008). The Neolithic of Southern China—Origin, Development, and Dispersal. Asian Perspect. 47, 299–329.http://www.jstor.org/stable/42928744
Zhang, J., Harbottle, G., Wang, C., and Kong, Z. (1999). Oldest Playable Musical Instruments Found at Jiahu Early Neolithic Site in China. Nature 401, 366–368. doi:10.1038/43865
Zhejiang Provincial Institute of Cultural Relics and Archaeology (2003). Hemudu: Excavation Report of a Neolithic Site. Beijing: Cultural Relics Press.
Keywords: zooarchaeology, subsistence economy, culling profile, sex ratio, seasonality, sustainability
Citation: Zhang Y, Sun G, Wang Y, Huang Y, Kikuchi H and Yang X (2022) Sustainable Hunting Strategy of Sika Deer (Cervus nippon) in the Neolithic Lower Yangtze River Region, China. Front. Earth Sci. 9:812910. doi: 10.3389/feart.2021.812910
Received: 10 November 2021; Accepted: 20 December 2021;
Published: 20 January 2022.
Edited by:
Yue Zhang, Institute of Vertebrate Paleontology and Paleoanthropology (CAS), ChinaReviewed by:
Yue Li, Northwest University, ChinaCopyright © 2022 Zhang, Sun, Wang, Huang, Kikuchi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Yang, xyang@lzu.edu.cn, xyang@itpcas.ac.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.