- 1Department of Biology, Laurentian University, Sudbury, ON, Canada
- 2Biomolecular Sciences Program, Laurentian University, Sudbury, ON, Canada
- 3Department of Biology, Lakehead University, Thunder Bay, ON, Canada
- 4Medical Sciences Division, Northern Ontario School of Medicine, Thunder Bay, ON, Canada
- 5Medical Sciences Division, Northern Ontario School of Medicine, Sudbury, ON, Canada
- 6Bruce Power, Tiverton, ON, Canada
Biological research conducted in deep-underground environments is limited due to the lack of scientific infrastructure to accommodate the investigations, and only a few studies have utilized complex whole organism models. In this study, lake whitefish (Coregonus clupeaformis) embryogenesis was examined in two different unique laboratory environments; at the Earth’s surface and 2 km deep underground shielded from cosmic radiation. Established developmental endpoints and morphometric analysis were utilized to investigate differences between lake whitefish embryos reared in these two laboratories. No significant differences were observed between the surface and underground laboratories with respect to the timing of hatch or percent survival. However, a significant increase in body length and body weight of up to 10% was observed in embryos reared underground. These findings have been interpreted and discussed in the context of the novel research challenges faced in an inherently difficult to control deep-underground environment. This study represents one of the few investigations with an established whole organism model deep-underground and provides an opportunity to discuss the highly unique technical and logistical challenges of conducting biological experiments in this novel field of scientific research.
Introduction
There is a scarcity of empirical biological research in deep-underground environments, due to the lack of access to infrastructure available to facilitate these endeavors. There are only a small number of underground research laboratories located across the world, which vary in depth as well as type of space, including active mines, caves, tunnels, and a nuclear waste repository (Liu et al., 2018). The majority of these current facilities were established to conduct astroparticle physics experiments, predominantly based in dark matter and neutrino detection (Smith, 2012). These types of explorations necessitate a deep-underground research facility with a significant rock overburden in order to shield out background noise caused by cosmic radiation. Even further limited has been the use of subterranean laboratory facilities to conduct biological experiments, though studies to this end have been gaining traction, with notable work performed at the Waste Isolation Pilot Plant (WIPP) in New Mexico, United States and Gran Sasso National Laboratory (LNGS) in Abruzzo, Italy (Castillo et al., 2015, 2018; Fratini et al., 2015; Castillo and Smith, 2017; Morciano et al., 2018).
The majority of these biological studies have investigated the effects of sub-natural background radiation (NBR) exposures, relying on underground laboratories to provide a sub-background radiation environment. Previous experiments have shown in a variety of model organisms, including paramecium, blue-green algae, and mammalian cells, that protracted incubation in an environment shielded from NBR can induce negative biological effects including attenuation of growth rates (Planel et al., 1976; Conter et al., 1983; Kawanishi et al., 2012). Interestingly, this attenuation could be rescued by the exogenous re-introduction of a radiation source at levels comparable to natural background (Conter et al., 1983; Kawanishi et al., 2012). Additionally, in biological systems cultured underground an increase in background and induced damage, mutation rates, and apoptotic sensitivity were observed as well as greater micronuclei formation and reduced free radical scavenging capacity (Satta et al., 2002; Carbone et al., 2009, 2010; Smith et al., 2011). However, most of this previous research has been conducted using simple cell culture or single-celled organisms.
One of the most important scientific gaps in the current deep-underground biology research is experimentation with complex multicellular whole organisms. However, logistical challenges with working in underground environments make numerous classic research species, for example murine models, non-viable options to fill these needs. It is therefore critical that research models require minimal maintenance in terms of both space and resources. Researching the Effects of the Presence and Absence of Ionizing Radiation (REPAIR) is a recently established research project within SNOLAB. The goal of this project is to study the effects of exposure to a deep underground environment shielded from naturally occurring background radiation on biological systems, including whole organism models (Pirkkanen et al., 2017; Thome et al., 2017c). One of the whole organism models that REPAIR is utilizing is the embryonic development of lake whitefish (Coregonus clupeaformis).
Significant work has been done characterizing developmental staging in lake whitefish as well as their response to a variety of stressors, including chemicals, thermal stress, and ionizing radiation (Sreetharan et al., 2015; Thome et al., 2017a, b). Based on this robust previous characterization, the minimal requirements needed for rearing embryos, and their prolonged embryonic developmental period of several months, lake whitefish represent a unique whole organism for investigating the effects of a deep-underground environment. Lake whitefish embryos can be raised in significantly large numbers in commercially available refrigerator units within basic laboratory petri dishes filled with lake water or dechlorinated municipal tap water (Mitz et al., 2014). Additionally, lake whitefish embryos have a protracted developmental period of up to 200 days depending on temperature (Sreetharan et al., 2015), providing an excellent whole organism model for examining potentially subtle effects over an extended period in a deep-underground environment.
The REPAIR project is located within SNOLAB, a research facility located 2 km underground within an active mine in Sudbury, ON, Canada. The technical details of the research facility and the scientific goals of the REPAIR project have been previously discussed (Boger et al., 2000; Smith, 2012; Thome et al., 2017c). The overall long-term goals of the REPAIR project are to explore the effects of a below natural background radiation environment in a variety of biological models. This article reports on an initial pilot project, where lake whitefish embryos were raised in the REPAIR project’s research space within SNOLAB, as well as at an above ground control laboratory at Laurentian University. These data represent one of the only whole organism model experiments conducted deep-underground, and an excellent opportunity to review the technical, logistical, and scientific challenges of conducting these types of niche investigations.
Materials and Methods
Embryo Collection
Lake whitefish embryos were produced through in vitro fertilization. Milt and eggs were collected from spawning adult fish that were gillnetted in Eastern Lake Huron (44.995, −81.386) on December 1, 2016. Eggs were wet fertilized for 10 min and then disinfected with 5 mL L–1 Ovadine (Syndel Laboratories, BC, Canada) for 30 min. Fertilized eggs were transported on ice to Laurentian University in 1 L plastic bottles filled with water from Lake Huron. Following transportation, and for the remainder of embryonic development, embryos were incubated in water taken from Ramsey Lake adjacent to Laurentian University (46.472, −80.973).
Embryo Rearing
Embryos were raised in two different experimental environments. The “surface” laboratory was located at Laurentian University in the Vale Living with Lakes Centre. The “underground” laboratory was located 2 km underground within SNOLAB in the REPAIR project’s research space (Thome et al., 2017c). The SNOLAB facility is located approximately 27 km from Laurentian University and the Vale Living with Lakes Centre. The SNOLAB surface facility and the Vale Living with Lakes Centre are located at similar altitudes of 305 and 288 m above sea level, respectively.
On the day of fertilization (day 0) embryos were stored overnight in a 3°C refrigeration unit in the surface laboratory. The following morning (day 1) half of the embryos were transported underground to SNOLAB in 1 L plastic bottles on ice. As a transportation sham, the embryos remaining on the surface were also temporarily transferred to 1 L plastic bottles on ice. The total transportation time was approximately 2 h. It is unlikely that there were any significant changes to water quality during transportation since newly fertilization lake whitefish embryos have a very low metabolic activity and minimal oxygen consumption (Mueller et al., 2015).
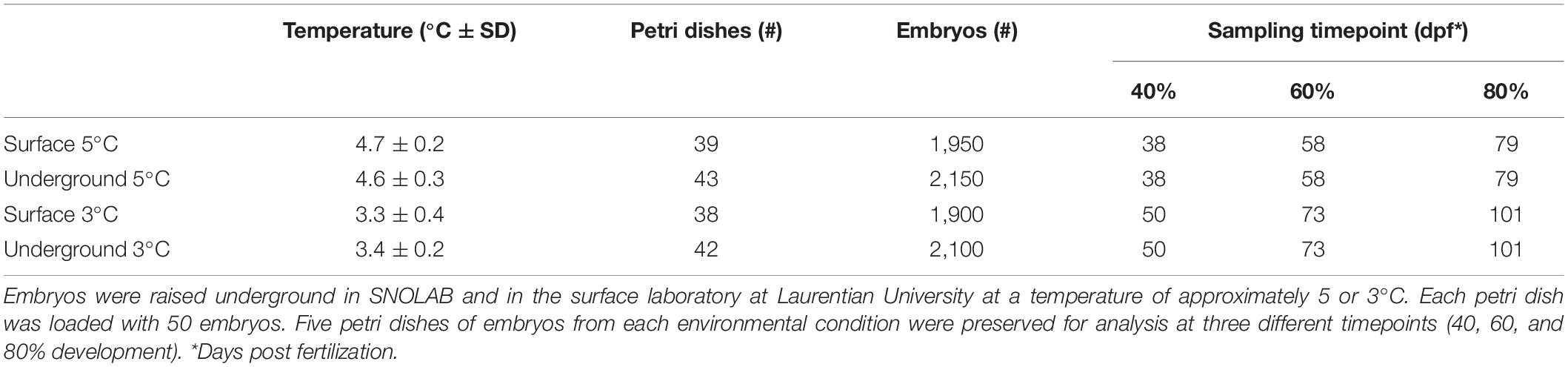
In both the surface and underground laboratories, embryos were reared in refrigeration units (Figure 1A) using methods described previously (Mitz et al., 2014). Culturing conditions within the units were kept as similar as possible, i.e., controlled respective incubation temperature and all ambient room light was blocked from entering the inside of the unit. Embryos were incubated in 10 cm petri dishes (0875711Z, Thermo Fisher Scientific) with 50 embryos per dish (Figure 1B). Petri dishes were filled with chilled water collected from Ramsey Lake. Natural water sources were used for embryonic rearing as they are free from additives such as chlorine found in municipal water sources. Water was transported underground throughout the experiment in 1 L plastic bottles.
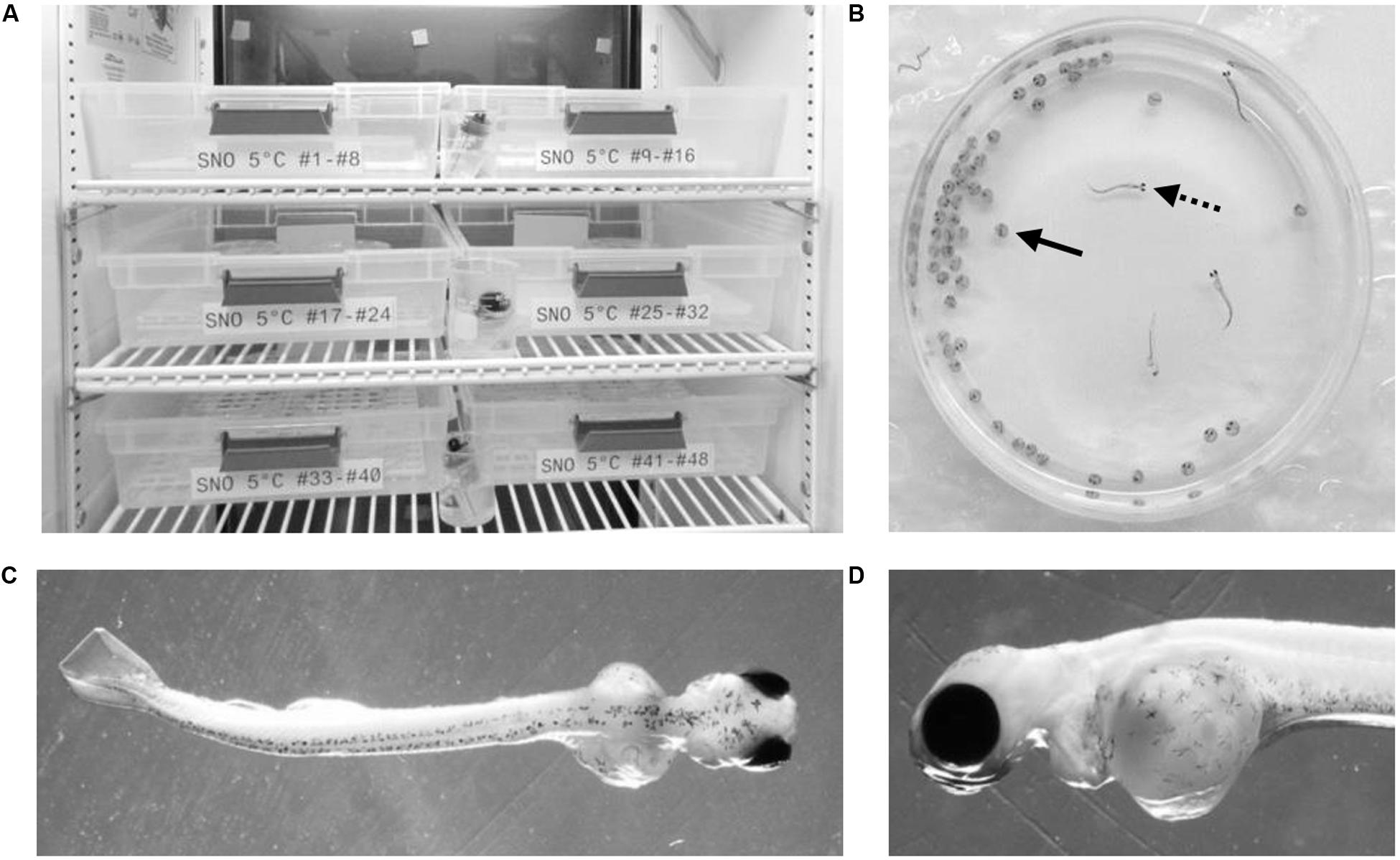
Figure 1. Embryonic development experimental setup. Embryos were raised in temperature controlled refrigeration units at the surface and underground (A). Embryonic development took place within petri dishes (B) where chorionated embryos (solid arrow) were raised until hatch (dotted arrow). A dorsal image of each hatched embryo (C) was used for body length measurements while a lateral image (D) was taken of the yolk sac in order to calculate the yolk area.
Each of the laboratory environments contained two refrigeration units (GDM-10PT, True Manufacturing Company, O’Fallon, MO, United States). The refrigeration units were retrofitted with a digital temperature controller (JC-104, Juchuang Electronic Science and Technology Co., Ltd.) for more precise temperature regulation. The units in each experimental location were set to an approximate temperature of either 3 or 5°C. There were slight variations in temperature depending on the specific location of petri dishes within the units as well as the frequency at which the compressor turned on and off. Actual temperatures were continuously monitored using HOBO pendant data loggers (UA-002, Onset, Bourne, MA, United States). Temperature readings were collected from three different spatial location within each unit and analyzed using HOBOware software (Onset, Bourne, MA, United States). The mean temperature within the 3 and 5°C units in each laboratory were within 0.1°C of each other (Table 1). Between 38 and 43 petri dishes were housed in each refrigeration unit, resulting in between 1,900 and 2,150 embryos being raised in each environmental condition (Table 1). Water was changed on all petri dishes twice per week for the duration of embryonic development, approximately 100 mL per petri dish. At the time of water change, dead or hatched embryos were removed from the petri dish and were numerically recorded.
Morphometric Analysis
Embryos were preserved for analysis at three different time points, corresponding to approximately 40, 60, and 80% development (Table 1). The number of days post fertilization (dpf) to reach each of these development stages was estimated based on the lake whitefish temperature model developed previously (Mitz et al., 2017b). Embryos raised at the warmer 5°C temperature develop faster and were therefore preserved at earlier time points (38, 58, and 79 dpf) compared to 3°C (50, 73, and 101 dpf). Embryos were fixed in 10% neutral buffered formalin (5701, Richard-Allan Scientific) for 7 days and were then transferred to 50% ethanol (A995-4, Thermo Fisher Scientific) for long term storage. Size and mass measurements were taken according to the methods previously established (Sreetharan et al., 2015). Briefly, the preserved embryo and yolk sac were removed from the chorion and separated under an Axio Zoom V16 stereomicroscope (ZEISS) with a Canon EOS Rebel T1i camera mounted (Canon Inc.). A dorsal image was taken of the embryo from which a body length was measured (Figure 1C). A lateral image was taken of the yolk sac from which two perpendicular diameters were measured and used to calculate the yolk area (Figure 1D). Post imaging, the embryo and yolk were dried overnight at 70°C in an oven (1500EM, VWR) and weighed on an analytical balance (XA105DU, ±0.01 mg, Mettler Toledo). All size and mass measurements were taken with the researcher blinded to the rearing location. A subset of 10 embryos were fixed on day 1 post fertilization and weighed. At each of the three development stages, a yolk conversion efficiency (YCE) was calculated according to the equation:
Statistical Analysis
Statistical analysis was conducted using JMP V13.0 (SAS Institute Inc., Cary, NC, United States). Cumulative percent mortality at hatch and the time to 50% hatch were compared between the petri dishes that were not fixed for morphometric analysis (n = 24 for surface 5°C, n = 28 for underground 5°C, n = 23 for surface 3°C, n = 27 for underground 3°C). Mortality was compared using a two-way ANOVA followed by Tukey’s HSD test with location (surface, underground) and temperature (5, 3°C) as the independent variables. Due to a temperature spike in the underground 3°C incubator prior to 50% hatch, a time to 50% hatch could only be calculated for the underground 5°C embryos and it was compared to the surface 5°C embryos using a t-test. Morphometric measurements and YCE were compared between embryos (n = 46–106) using a three-way ANOVA followed by Tukey’s HSD test with location (surface, underground), temperature (5, 3°C) and percent development (40, 60, 80%) as the independent variables.
Results
Embryonic Survival and Hatching
Across all incubation conditions the majority of embryonic mortality occurred during the first month of development (Figure 2A). Mortality then remained minimal from approximately day 30 until hatch. The cumulative percent survival at hatch ranged from 33–37% (Figure 2C) but was not significantly impacted by temperature (p = 0.91) or by the laboratory location (p = 0.30). Similarly, the laboratory location did not impact the timing of hatch. Embryos incubated at 5°C began to hatch earlier compared to those at 3°C (Figure 2B). The hatch window lasted approximately 60 days in embryos incubated at 5°C but the average time to 50% hatch was not significantly different underground (128 ± 11 days) or on the surface (128 ± 10 days, p = 0.98, Figure 2D). Embryos incubated at 3°C in both laboratories started hatching at approximately the same time and the hatching rate followed the same trend for the first 4 weeks (Figure 2B). However, on April 25, 2016 (145 dpf) the compressor failed on the refrigeration unit underground resulting in a temperature spike. At that point, only approximately 30% of the embryos in each petri dish had hatched. The temperature spike resulted in a premature hatching of all the remaining embryos. Therefore, a time to 50% hatch could not be accurately calculated for 3°C embryos.
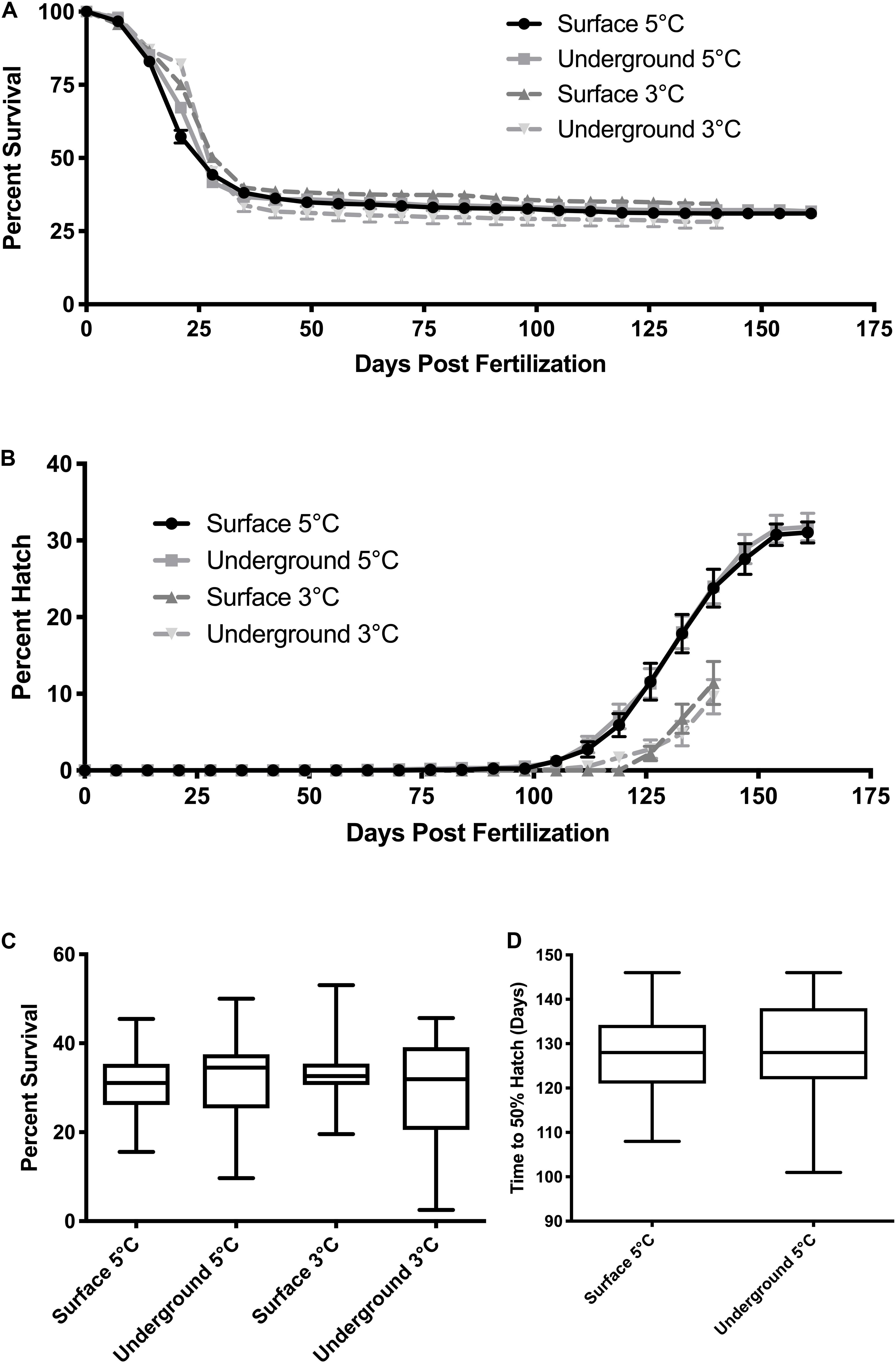
Figure 2. Embryo survival and hatch timing. The cumulative percent survival (A) and percent hatch (B) was recorded each week starting at fertilization. Data points represent the mean ± SE across the replicate petri dishes (n = 38–43). Quartiles and range for the cumulative survival at hatch (C) and the time to 50% hatch (D) were calculated across the replicate petri dishes. Due to equipment failure, data from the 3°C conditions were only collected until day 140 post hatch and a time to 50% hatch could not be calculated. Mortality was compared using a two-way ANOVA followed by Tukey’s HSD test with location (surface, underground) and temperature (5, 3°C) as the independent variables. Time to 50% was compared using a t-test.
Morphometric Analysis
Morphometric measurements were taken at three stages of development from both laboratories at each temperature. The temperature spike in the underground 3°C refrigeration unit did not impact these measurements as it occurred during the hatching stage, after the 80% development time point. As was expected, morphometric measurements correlated with the stage of development (p < 0.05). Body length, body mass and YCE were progressively larger (Figures 3, 5) while yolk mass and yolk size were progressively smaller (Figure 4) as development progressed. The incubation temperature had a significant impact on body length, body mass, and yolk mass (p < 0.05). In general, embryos raised at the colder temperature had a larger body mass and body length (Figure 3) and a smaller yolk mass (Figure 4A). However, temperature did not significantly impact yolk area (p = 0.18) or YCE (p = 0.80). The laboratory location (surface vs underground) significantly impacted body and yolk morphometric measurements (p < 0.05) but not YCE (p = 0.80). Specifically, body size was greater in embryos incubated underground compared to the surface. This was evident in the 3°C embryos at 40 and 60% development, as well as the 5°C embryos at 80% development. The opposite trend was observed in yolk measurements which were smaller in embryos incubated underground compared to the surface. This was observed in the 3°C embryos at 40% development and the 5°C embryos at 80% development.
Figure 3. Embryo size measurements. Body mass (A) and body length (B) were compared between embryos using a three-way ANOVA followed by Tukey’s HSD test with location (surface, underground), temperature (5, 3°C) and percent development (40, 60, 80%) as the independent variables. Asterisk denote statistical differences (p < 0.05). Bars represent means ± 95% confidence intervals (n = 46–106).
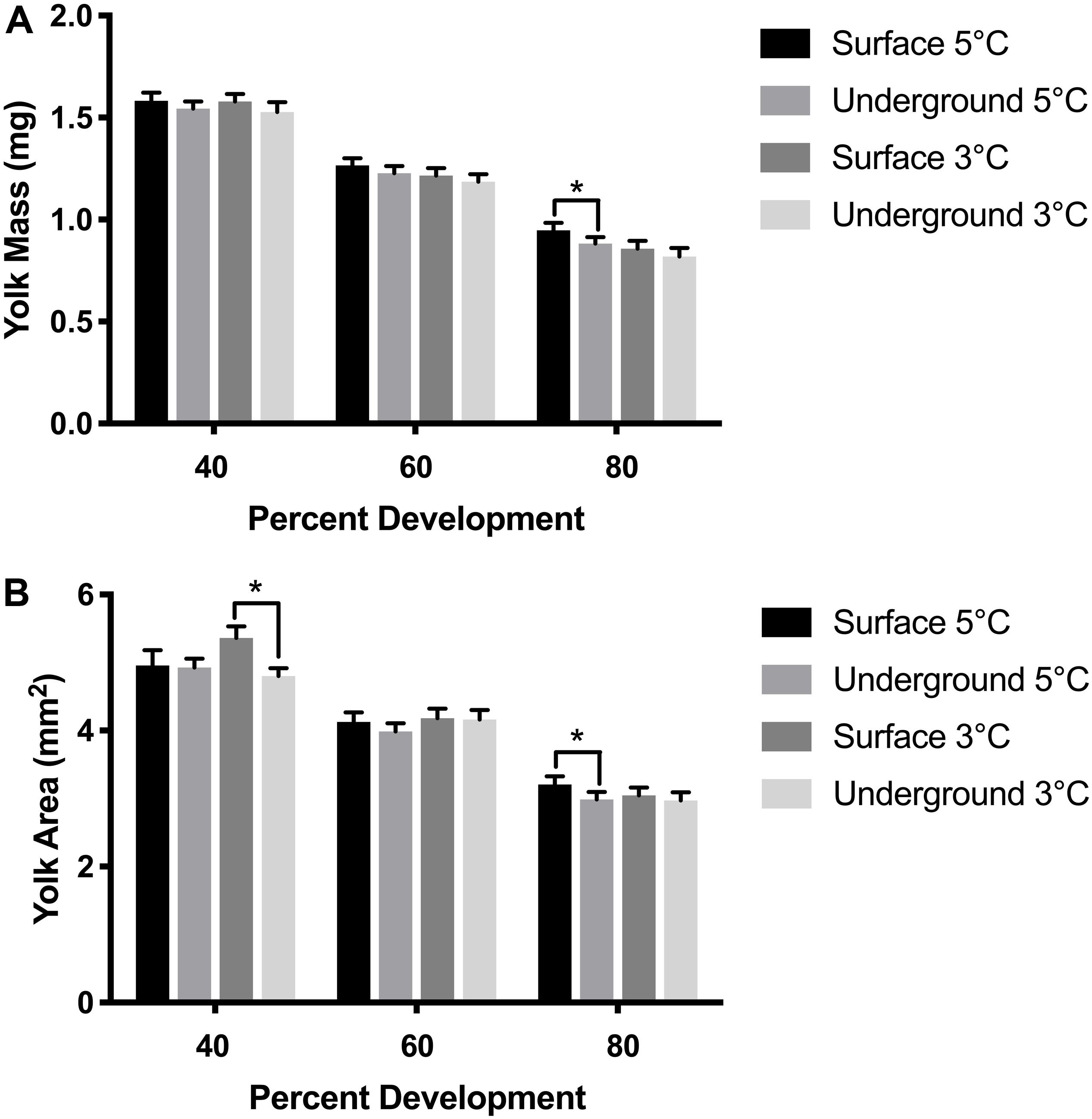
Figure 4. Yolk size measurements. Yolk mass (A) and yolk area (B) were compared between embryos using a three-way ANOVA followed by Tukey’s HSD test with location (surface, underground), temperature (5, 3°C) and percent development (40, 60, 80%) as the independent variables. Asterisk denotes statistical difference (p < 0.05). Bars represent means ± 95% confidence intervals (n = 57–105).
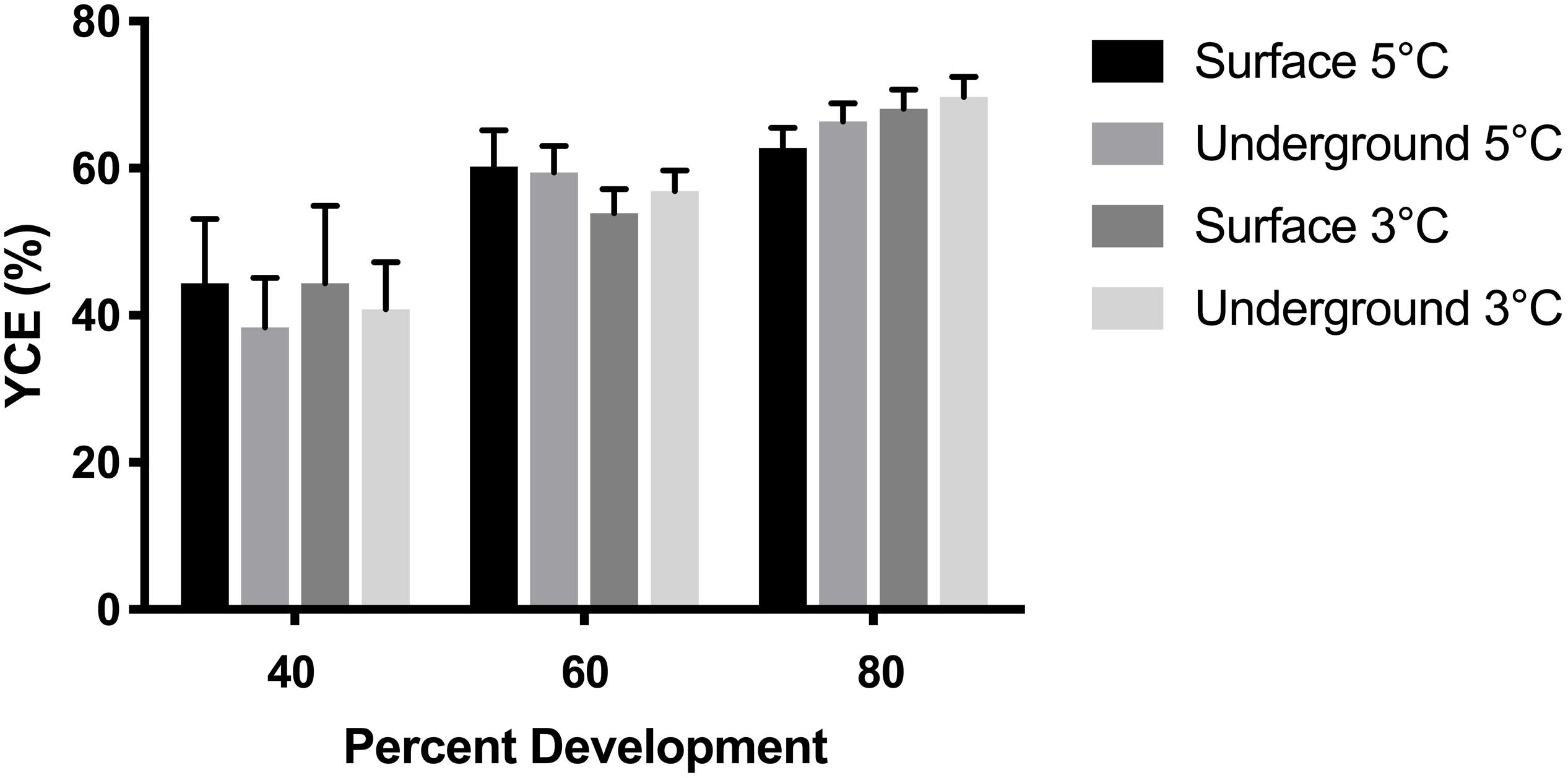
Figure 5. Yolk conversion efficiency (YCE) was compared between embryos using a three-way ANOVA followed by Tukey’s HSD test with location (surface, underground), temperature (5, 3°C) and percent development (40, 60, 80%) as the independent variables. Bars represent means ± 95% confidence intervals (n = 53–105).
Discussion
There is a significant lack of published scientific studies with whole organism biological models in deep-underground environments. The data from this pilot study represent one of the first experiments to successfully raise a complex, multicellular, whole organism model in an underground laboratory. However, this study also highlights some of the unique challenges that are associated with this type of research.
In this study, we investigated growth and development during embryogenesis in lake whitefish (C. clupeaformis) within SNOLAB and in our surface control laboratory. Approximately 4,000 fertilized embryos were reared in each environment (Table 1). Embryonic mortality was highest in the first month post fertilization and then remained minimal for the remainder of development, which is common during lake whitefish laboratory rearing (Brooke, 1975; Mitz et al., 2014). The initial spike in mortality was therefore expected and was observed under all experimental conditions (Figure 2A). Rearing temperature did not significantly impact embryonic survival, but it did have an effect on time of hatch, with embryos incubated at the warmer 5°C temperature hatching earlier than at 3°C. Both of these effects were also expected and have been previously observed in lake whitefish (Brooke, 1975; Mitz et al., 2019). Both 3 and 5°C are within the optimal range for lake whitefish development, so mortality should be similar across the two temperatures (Brooke, 1975). At the same time, however, warmer temperatures are known to increase embryonic development rate, explaining why 5°C embryos hatched earlier (Mitz et al., 2019). The laboratory location (surface vs underground) did not significantly impact the cumulative percent survival, percent hatch or the timing of hatch (Figures 2A,B). This was expected as lake whitefish embryos are more robust to changes in rearing conditions with respect to survival and hatching, and changes in these two parameters would represent a more significant environmental stress.
Morphometric measurements are a sensitive endpoint that can be used to detect more subtle physiological changes. Morphometric analysis was performed in embryos raised in all experimental conditions at three stages of development based on previously established methods (Sreetharan et al., 2015). These morphometric endpoints: body and yolk mass, body length, and YCE, are ideal to evaluate the biological effects of different rearing environments. Body mass, length, and YCE increased with developmental advancement (Figures 3, 5) whereas yolk size and mass decreased (Figure 4) as expected. Interestingly, the laboratory location (surface or underground) was also found to significantly affect body mass and length. The body size at several development points was greater when reared underground (Figure 3), which was paralleled by a reduction in yolk mass and area (Figure 4). Taken together, these data suggest that incubating embryos within SNOLAB can have a subtle yet significant effect on embryonic growth and development.
Changes in embryonic size have previously been shown in lake whitefish following different environmental stressors. In the current study, embryos incubated underground were up to 10% larger in size compared to the surface (Figure 3). A similar magnitude of size change has been shown following a temperature shift, where embryos incubated at 3°C were significantly larger compared to 5°C (Lim et al., 2017). Similarly, chronic chemical exposure to morpholine (1,000 mg/L) resulted in a reduction in embryo size of approximately 10% (Thome et al., 2017b). Of note, low dose radiation has been previously shown to stimulate growth in developing lake whitefish embryos when it was delivered as a chronic or fractionated exposure (Mitz et al., 2017a; Thome et al., 2017a).
The exact cause of the growth changes in the current experiment is not known. Deep-underground research is subject to unique experimental challenges, in terms of controlling variables compared to surface laboratories. In previous studies, challenges have included temperature, humidity, type of rock environment, radiation, and air pressure (Liu et al., 2018). The SNOLAB facility, where the REPAIR project is located is a Class 2000 cleanroom with highly regulated and monitored temperature, air quality, and humidity (Boger et al., 2000). Within SNOLAB, the rock overburden provides significant shielding from cosmic radiation. However radon, a radioactive gas that emanates from the decay of natural uranium found in the surrounding rock, is significantly higher in the air underground (Boger et al., 2000; Smith, 2012). Radon concentrations in SNOLAB vary depending on the time of day and the time of year, ranging from 100–150 Bq/m3 (Hall, 2020). Conversely, ambient surface air radon levels in Sudbury are approximately 10 times lower ranging from 11–14 Bq/m3 (Grasty, 1994). Future experiments planned by REPAIR will incorporate technologies to reduce radon levels in the underground laboratory. However, during this initial pilot project, radon was not controlled for. Radon gas comprises more than half of the natural background radiation dose (NCRP, 2009). Therefore, even though cosmic radiation levels are reduced underground, the elevated radon levels likely resulted in a greater total background dose to embryos. Our previous studies have shown that exposure to low dose gamma radiation stimulated growth in lake whitefish (Thome et al., 2017a). The higher radon levels underground may be one explanation for why embryos reared in SNOLAB were significant larger in body size. Another variable that was not controlled was air pressure, which is up to 25% higher underground (Duncan et al., 2010). This represents a variable that could potentially effect biological results as previously reviewed and discussed (Lampe et al., 2017; Liu et al., 2018; Xie et al., 2018). Future studies conducted by REPAIR will also add in experimental controls for the pressure differences underground.
One of the most challenging technical and logistical considerations with performing deep-underground research in the active mine where SNOLAB and the REPAIR project are located, is access to the facility. To access the underground laboratory facility, researchers assemble dressed in full personal protective equipment (PPE) mining gear and travel underground on an access cage to a depth of 2 km. Travel on foot is approximately 1.5 km through a mine drift to reach the facility. Once at SNOLAB, mine gear is removed, and a decontamination shower is required to eliminate remaining mine dust on the skin or in hair before re-dressing in Class 2000 cleanroom attire and PPE. Travel from the surface to the underground laboratory is limited by cage (elevator) runs, which are not available on every day of the week and can be variable dependent on the status of active mine work. This can be problematic for many biological experiments that require daily maintenance. Low maintenance models, such as lake whitefish, are ideal in this respect since they can survive multiple days between water changes. The restricted access to the laboratory also means that unexpected equipment issues become a challenge. For example, in the current experiment, at 145 dpf the 3°C underground refrigeration unit’s compressor unexpectedly failed, resulting in a considerable temperature spike that led to a premature hatching of all remaining embryos. Although this did not influence morphometric analysis as 80% development had already been reached, this is an example of the types of novel challenges faced with this field of experimentation. Under normal surface laboratory conditions, researchers are usually available to mitigate unexpected situations. Limited access to the underground facility makes these types of events more probable and difficult to remediate. Finally, unexpected or planned power outages are another factor that needs to be considered when planning experiments in an underground facility. All of the REPAIR equipment is connected to uninterrupted power supply units that can provide battery backup power for several hours. Taken together, this emphasizes the highly unique nature of conducting experimental investigations within such a facility, and highlights the atypical logistical and technical challenges that researchers face in these environments.
The long-term research goals of the REPAIR project are focused on understanding the effects of a deep-underground environment in a variety of biological model systems (Pirkkanen et al., 2017; Thome et al., 2017c). Lake whitefish embryogenesis was chosen as a model system in this pilot project in order to evaluate the viability of performing biological experiments deep-underground within SNOLAB, where REPAIR is located. We have successfully demonstrated that a whole organism investigation is feasible. We have gained important insight into the unique technical and scientific challenges of this type of research. Based on this experience, projects currently underway by REPAIR were designed to control these special variables (e.g., underground radiological conditions). Moving forward, this will allow improved more precise investigations of effects of deep-underground environments on biological systems.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study as per Canadian Council on Animal Care guidelines section 4.1.2.2: “Work not requiring protocols or inclusion in animal use inventories: fish eggs, embryos or larvae that have not developed beyond exclusive reliance on their own yolk nutrients.” All embryos in this study were preserved for analysis prior to consumption of yolk nutrients.
Author Contributions
JP, AZ, TL, and CT performed embryonic rearing experiments underground and at the surface. JP performed morphometric analysis. CT performed statistical analysis and prepared figures. DB and CT designed and supervised experiments. JP and CT wrote sections of the first draft of the manuscript. SL and TT were collaborators and hold the NSERC CRD grants supporting this work. All authors contributed to manuscript editing, revision, and approved the submitted version.
Funding
The REPAIR project was supported through a Natural Sciences and Engineering Research Council (NSERC) Collaborative Research and Development (CRD), and MITACS grants in partnership with Bruce Power Inc.
Conflict of Interest
DB was employed by the company Bruce Power.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank SNOLAB and its staff for support through underground space, logistical, and technical services. SNOLAB operations are supported by the Canada Foundation for Innovation and the Province of Ontario Ministry of Research and Innovation, with underground access provided by Vale at the Creighton mine site. The authors would like to additionally thank E. Hulley, S. Sreetharan, and C. West for technical assistance with morphometric analysis.
References
Boger, J., Hahn, R. L., Rowley, J. K., Carter, A. L., Hollebone, B., Kessler, D., et al. (2000). The sudbury neutrino observatory. Nucl. Instrum. Methods Phys. 449, 172–207.
Brooke, L. T. (1975). Effect of different constant incubation temperatures on egg survival and embryonic-development in lake whitefish (Coregonus-clupeaformis). Trans. Am. Fish. Soc. 104, 555–559. doi: 10.1577/1548-86591975104<555:Eodcit<2.0.Co;2
Carbone, M. C., Pinto, M., Antonelli, F., Amicarelli, F., Balata, M., Belli, M., et al. (2009). The cosmic silence experiment: on the putative adaptive role of environmental ionizing radiation. Radiat. Environ. Biophys. 48, 189–196. doi: 10.1007/s00411-008-0208-6
Carbone, M. C., Pinto, M., Antonelli, F., Balata, M., Belli, M., Devirgiliis, L. C., et al. (2010). Effects of deprivation of background environmental radiation on cultured human cells. Nuovo Cim. Della Soc. Ital. Fisica B Basic Top. Phys. 125, 469–477. doi: 10.1393/ncb/i2010-10889-y
Castillo, H., Li, X., Schilkey, F., and Smith, G. B. (2018). Transcriptome analysis reveals a stress response of Shewanella oneidensis deprived of background levels of ionizing radiation. PLoS One 13:e0196472. doi: 10.1371/journal.pone.0196472
Castillo, H., Schoderbek, D., Dulal, S., Escobar, G., Wood, J., Nelson, R., et al. (2015). Stress induction in the bacteria Shewanella oneidensis and Deinococcus radiodurans in response to below-background ionizing radiation. Int. J. Radiat. Biol. 91, 749–756. doi: 10.3109/09553002.2015.1062571
Castillo, H., and Smith, G. B. (2017). Below-background ionizing radiation as an environmental cue for bacteria. Front. Microbiol. 8:177. doi: 10.3389/fmicb.2017.00177
Conter, A., Dupouy, D., and Planel, H. (1983). Demonstration of a biological effect of natural ionizing radiations. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 43, 421–432. doi: 10.1080/09553008314550481
Duncan, F., Noble, A. J., and Sinclair, D. (2010). The construction and anticipated science of SNOLAB. Annu. Rev. Nucl. Part Sci. 60, 163–180. doi: 10.1146/annurev.nucl.012809.104513
Fratini, E., Carbone, C., Capece, D., Esposito, G., Simone, G., Tabocchini, M. A., et al. (2015). Low-radiation environment affects the development of protection mechanisms in V79 cells. Radiat. Environ. Biophys. 54, 183–194. doi: 10.1007/s00411-015-0587-4
Grasty, R. L. (1994). Summer outdoor radon variations in Canada and their relation to soil moisture. Health Phys. 66, 185–193. doi: 10.1097/00004032-199402000-00009
Hall, J. (2020). The SNOLAB underground laboratory. J. Phys. Conf. Ser. 1468:012252. doi: 10.1088/1742-6596/1468/1/012252
Kawanishi, M., Okuyama, K., Shiraishi, K., Matsuda, Y., Taniguchi, R., Shiomi, N., et al. (2012). Growth retardation of Paramecium and mouse cells by shielding them from background radiation. J. Radiat. Res. 53, 404–410. doi: 10.1269/jrr.11145
Lampe, N., Breton, V., Sarramia, D., Sime-Ngando, T., and Biron, D. G. (2017). Understanding low radiation background biology through controlled evolution experiments. Evol. Appl. 10, 658–666. doi: 10.1111/eva.12491
Lim, M. Y., Manzon, R. G., Somers, C. M., Boreham, D. R., and Wilson, J. Y. (2017). The effects of fluctuating temperature regimes on the embryonic development of lake whitefish (Coregonus clupeaformis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 214, 19–29. doi: 10.1016/j.cbpa.2017.08.010
Liu, J., Ma, T., Liu, Y., Zou, J., Gao, M., Zhang, R., et al. (2018). History, advancements, and perspective of biological research in deep-underground laboratories: a brief review. Environ. Int. 120, 207–214. doi: 10.1016/j.envint.2018.07.031
Mitz, C., Thome, C., Cybulski, M. E., Laframboise, L., Somers, C. M., Manzon, R. G., et al. (2014). A self-contained, controlled hatchery system for rearing lake whitefish embryos for experimental aquaculture. North Am. J. Aquac. 76, 179–184. doi: 10.1080/15222055.2014.893472
Mitz, C., Thome, C., Cybulski, M. E., Somers, C. M., Manzon, R. G., Wilson, J. Y., et al. (2017a). Is there a trade-off between radiation-stimulated growth and metabolic efficiency? Radiat. Res. 188, 486–494. doi: 10.1667/RR14665.1
Mitz, C., Thome, C., Thompson, J., Manzon, R. G., Wilson, J. Y., and Boreham, D. R. (2017b). A method to transform a variable thermal regime to a physiologically equivalent effective temperature. J. Therm. Biol. 65, 21–25. doi: 10.1016/j.jtherbio.2016.12.011
Mitz, C., Thome, C., Cybulski, M. E., Somers, C. M., Manzon, R. G., Wilson, J. Y., et al. (2019). Thermal dependence of size-at-hatch in the lake whitefish (Coregonus clupeaformis). Can. J. Fish. Aquat. Sci. 76, 2069–2079. doi: 10.1139/cjfas-2018-0097
Morciano, P., Iorio, R., Iovino, D., Cipressa, F., Esposito, G., Porrazzo, A., et al. (2018). Effects of reduced natural background radiation on Drosophila melanogaster growth and development as revealed by the FLYINGLOW program. J. Cell Physiol. 233, 23–29. doi: 10.1002/jcp.25889
Mueller, C. A., Eme, J., Manzon, R. G., Somers, C. M., Boreham, D. R., and Wilson, J. Y. (2015). Embryonic critical windows: changes in incubation temperature alter survival, hatchling phenotype, and cost of development in lake whitefish (Coregonus clupeaformis). J. Comp. Physiol. B 185, 315–331. doi: 10.1007/s00360-015-0886-8
NCRP (2009). Ionizing Radiation Exposure of the Population of the United States. Report No. 160. Bethesda, MD: NCRP.
Pirkkanen, J. S., Boreham, D. R., and Mendonca, M. S. (2017). The CGL1 (HeLa x Normal Skin Fibroblast) human hybrid cell line: a history of ionizing radiation induced effects on neoplastic transformation and novel future directions in SNOLAB. Radiat. Res. 188, 512–524. doi: 10.1667/RR14911.1
Planel, G., Soleilhavoup, J. P., Tixador, R., Croute, F., and Richoilley, G. (1976). Demonstration of a Stimulating Effect of Natural Ionizing Radiation and of Very Low Radiation Doses on Cell Multiplication. IAEA: International Atomic Energy Agency (IAEA).
Satta, L., Antonelli, F., Belli, M., Sapora, O., Simone, G., Sorrentino, E., et al. (2002). Influence of a low background radiation environment on biochemical and biological responses in V79 cells. Radiat. Environ. Biophys. 41, 217–224. doi: 10.1007/s00411-002-0159-2
Smith, G. B., Grof, Y., Navarrette, A., and Guilmette, R. A. (2011). Exploring biological effects of low level radiation from the other side of background. Health Phys. 100, 263–265. doi: 10.1097/hp.0b013e318208cd44
Sreetharan, S., Thome, C., Mitz, C., Eme, J., Mueller, C. A., Hulley, E. N., et al. (2015). Embryonic development of lake whitefish Coregonus clupeaformis: a staging series, analysis of growth and effects of fixation. J. Fish Biol. 87, 539–558. doi: 10.1111/jfb.12725
Thome, C., Mitz, C., Hulley, E. N., Somers, C. M., Manzon, R. G., Wilson, J. Y., et al. (2017a). Initial characterization of the growth stimulation and heat-shock-induced adaptive response in developing lake whitefish embryos after ionizing radiation exposure. Radiat. Res. 188, 475–485. doi: 10.1667/RR14574.1
Thome, C., Mitz, C., Sreetharan, S., Mitz, C., Somers, C. M., Manzon, R. G., et al. (2017b). Developmental effects of the industrial cooling water additives morpholine and sodium hypochlorite on lake whitefish (Coregonus clupeaformis). Environ. Toxicol. Chem. 36, 1955–1965. doi: 10.1002/etc.3727
Thome, C., Tharmalingam, S., Pirkkanen, J., Zarnke, A., Laframboise, T., and Boreham, D. R. (2017c). The REPAIR project: examining the biological impacts of sub-background radiation exposure within SNOLAB, a deep underground laboratory. Radiat. Res. 188, 470–474. doi: 10.1667/RR14654.1
Keywords: deep-underground environment, Coregonus clupeaformis, lake whitefish, embryonic development, morphometric analysis, SNOLAB
Citation: Pirkkanen J, Zarnke AM, Laframboise T, Lees SJ, Tai TC, Boreham DR and Thome C (2020) A Research Environment 2 km Deep-Underground Impacts Embryonic Development in Lake Whitefish (Coregonus clupeaformis). Front. Earth Sci. 8:327. doi: 10.3389/feart.2020.00327
Received: 01 April 2020; Accepted: 14 July 2020;
Published: 31 July 2020.
Edited by:
Maria Antonella Tabocchini, Istituto Superiore di Sanità (ISS), ItalyReviewed by:
Deborah Oughton, Norwegian University of Life Sciences, NorwayGeoffrey Battle Smith, New Mexico State University, United States
Copyright © 2020 Pirkkanen, Zarnke, Laframboise, Lees, Tai, Boreham and Thome. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Thome, Y3Rob21lQG5vc20uY2E=
 Jake Pirkkanen
Jake Pirkkanen Andrew M. Zarnke
Andrew M. Zarnke Taylor Laframboise1
Taylor Laframboise1 T. C. Tai
T. C. Tai Christopher Thome
Christopher Thome