- 1Department of Biology, University of Toronto Mississauga, Mississauga, ON, Canada
- 2Australian Centre for Neutron Scattering, Australian Nuclear Science and Technology Organisation, Lucas Heights, NSW, Australia
- 3International Centre of Future Science, Dinosaur Evolution Research Center, Jilin University, Changchun, China
Trematopids are a clade of terrestrial dissorophoid temnospondyls documented primarily from terrestrial Permo-Carboniferous environments in North America and Europe. Here we describe the complete skull and articulated mandibles of a diminutive trematopid specimen (OMNH 79318) from the Early Permian karst deposits near Richards Spur, Oklahoma. Based on aspects of the neurocranium (e.g., unossified sphenethmoid, prootics, epipterygoids), the specimen represents one of the best examples of a markedly immature trematopid, an important data point for understanding the early ontogeny of trematopids. Specifically, it provides evidence that variation in otic notch structure can be ontogenetically influenced, not only among eucacopine dissorophids but also among trematopids. We provisionally refer the specimen to cf. Acheloma based on the presence of a denticulate vomerine ridge and other qualitative features. However, we emphasize that the taxonomic referral is complicated by several factors that more broadly confound trematopid taxonomy. This includes a low sample size (n = 1) of many taxa and marked size, and presumed ontogenetic, disparity between the known size range of different taxa. Complementary reexamination of both Acheloma cumminsi and Acheloma dunni as part of this study also reveals that the former possesses lateral exposures of palatal bones, the presence/absence of which was the only formal character that previously differentiated the two species, although other qualitative features (e.g., size of the internarial fontanelle) may differentiate these two species. With respect to OMNH 79318, the taxonomic referral is tentative because the specimen also shares many qualitative attributes with Phonerpeton pricei, a trematopid represented only by small-bodied, probably immature individuals. However, many of these shared features are likely to be influenced by ontogeny or size. The subsequent challenges that we encountered in our taxonomic referral suggest that ontogeny may be confounding taxonomy in both diagnoses and phylogenetic analyses of trematopids and emphasize the need for careful study of how this affects our understanding of trematopid intrarelationships.
Introduction
Trematopids are a Permo-Carboniferous clade of dissorophoid temnospondyls that are well-documented from North American and European regions of the supercontinent Pangea. Together with the armored dissorophids, the trematopids form Olsoniformes, a clade of medium-large, terrestrial dissorophoids (e.g., Anderson et al., 2008; Schoch, 2018). Trematopids are most readily identified by the presence of an elongate, typically subdivided (“keyhole-shaped”) naris, the function of which remains unresolved (e.g., Dilkes, 1993). Although trematopids are well-represented in many terrestrial environments, the majority of taxa are represented only by a single individual (Actiobates peabodyi, Anconastes vesperus, Fedexia striegeli, Mordex calliprepes, Rotaryus gothae, and Tambachia trogallas) (Eaton, 1973; Berman et al., 1987, 2010, 2011; Sumida et al., 1998; Milner, 2018). Subsequently, the ontogeny of trematopids in general remains poorly resolved (but see Maddin et al., 2010 for an example). This is particularly salient because of the large size range of trematopids in conjunction with a notable size disparity between taxa. Some taxa are represented primarily by large individuals (e.g., Acheloma cumminsi, Acheloma dunni), while others are represented only by small individuals (e.g., Phonerpeton pricei). As such, both taxonomic diagnoses and phylogenetic analyses of the clade may be biased due to inadvertent capture of ontogenetic variation in addition to taxonomic differentiation. Thus, the study of small-bodied trematopid specimens and additional work to discern ontogenetic patterns within the clade is important for more accurately resolving the phylogenetic relationships of the group and for a more complete understanding of the evolution of the clade. Here we describe the complete skull and mandibles of a small-bodied trematopid from the Early Permian karst deposits near Richards Spur, Oklahoma that we utilize to inform a discussion of trematopid ontogeny and taxonomy.
Materials and Methods
Specimens
OMNH 79318, cf. Acheloma sp.—small, complete skull with articulated mandibles. As part of this study, the holotype of Acheloma cumminsi (AMNH FARB 4205), a nearly complete skull with associated postcrania, and the holotype of “Trematops willistoni” (= A. cumminsi) (FMNH UC 1584), a complete skull with associated postcrania, were also examined.
Locality
OMNH 79318 comes from the fossiliferous karst deposits near Richards Spur, Oklahoma, which have been dated to 289 ± 0.68 Ma (Artinskian) via radioisotopic dating of speleothems by Woodhead et al. (2010). These deposits were formed by infillings of fissures in the uplifted and tilted Ordovician Arbuckle limestone (e.g., Gregory et al., 1956; Olson, 1967, 1991; MacDougall et al., 2017) As noted by previous workers (e.g., MacDougall et al., 2017), the relatively unique nature of the locality and collection at the locality (from waste heaps produced during quarrying) precludes any stratigraphic control within the quarry. OMNH 79318 was collected from a rich pocket that produced most of the specimens of Acheloma dunni (Polley and Reisz, 2011). Both AMNH FARB 4205 and FMNH UC 1584 are from the Arroyo Formation (Clear Fork Group) of Texas. This unit (and much of the classic Texas red beds) has traditionally been interpreted to be Kungurian in age but has never been dated absolutely. The Richards Spur locality was also thought to be of a similar age based on vertebrate biostratigraphy (e.g., Fox and Bowman, 1966; Olson, 1967; see Sullivan and Reisz, 1999 for a full discussion) until the work by Woodhead et al. (2010) produced an older age.
Methods
This study utilized the DINGO neutron radiography/tomography/imaging station (Garbe et al., 2011, 2015), located on the thermal HB 2 beam, tangentially facing the 20 MW Open-Pool Australian Lightwater (OPAL) reactor housed at the Australian Nuclear Science and Technology Organisation (ANSTO), Lucas Heights, New South Wales, Australia to non-invasively image this specimen.
The DINGO facility utilizes a quasi-parallel collimated beam of thermal neutrons from OPAL with a maximum spectrum intensity at 1.08 Å (70 meV) and full-width-at-half-maximum (FWHM) of 0.9 Å (100 meV). For this study, a collimation ratio (L/D) of 1000 (Garbe et al., 2011, 2015) was used to ensure highest available spatial resolution, where L is the neutron aperture-to-sample length and D is the neutron aperture diameter. The field of view was set to 100 × 100 mm2 and scan time was 14 h with a voxel size of 45.5 × 45.5 × 45.5 μm. Neutrons were converted to photons with a 30 μm thick terbium-doped Gadox scintillator screen (Gd2O2S:Tb, RC Tritec AG); photons were then detected by an Andor IKON-L CCD camera (liquid-cooled, 16-bit, 2048 × 2048 pixels) coupled with a Makro Planar 100 mm Carl Zeiss lens. A total of 1,000 equally-spaced angle shadow-radiographs were obtained every 0.18° as the sample was rotated 180° about its vertical axis. Both dark (closed shutter) and beam profile (open shutter) images were obtained for calibration before initiating shadow-radiograph acquisition. A cosmic ray filter was applied to all images to reduce data noise associated with non-neutron background radiation detection events. To further reduce anomalous noise, a total of three individual radiographs with an exposure length of 10 s were acquired at each angle (Mays et al., 2017). These individual radiographs were summed in post-acquisition processing using the “Grouped ZProjector” plugin in ImageJ v.1.51h (National Institutes of Health); this plugin was developed by Holly (2004).
Tomographic reconstruction of the 16-bit raw data was performed using Octopus Reconstruction v.8.8 (Inside Matters NV), yielding virtual slices perpendicular to the rotation axis. When these slices are stacked in a sequence, they form a three-dimensional volume image of the sample. Visualization of the data was performed using ImageJ and Avizo 9.3.0. The unprocessed 16-bit TIFF files used in this study are publicly available at Morphobank (project #3339): http://morphobank.org/permalink/?P3339 and upon reasonable request made to either the authors or to the Sam Noble Oklahoma Museum of Natural History (OMNH).
Description
OMNH 79318 is a small specimen measuring 7.65 cm in length from the tip of the premaxilla to the posteriormost extent of the tabulars when measured along the midline and 5.93 cm in width when measured across the posterior skull table between the quadratojugals (Figures 1–3). The skull roof slopes gently anteroventrally without any inflection along the dorsal margin (Figure 2). The following description focuses primarily on a comparison with Acheloma dunni (Polley and Reisz, 2011), Acheloma cumminsi (Dilkes and Reisz, 1987), and Phonerpeton pricei (Dilkes, 1990), to which OMNH 79318 is most similar. We note that Schoch and Milner (2014) placed another species within Phonerpeton, P. whitei (formerly Acheloma whitei), but the material has not been figured or re-described since the study of Olson (1941), which does not contain sufficient detail to be informative in this comparative study.
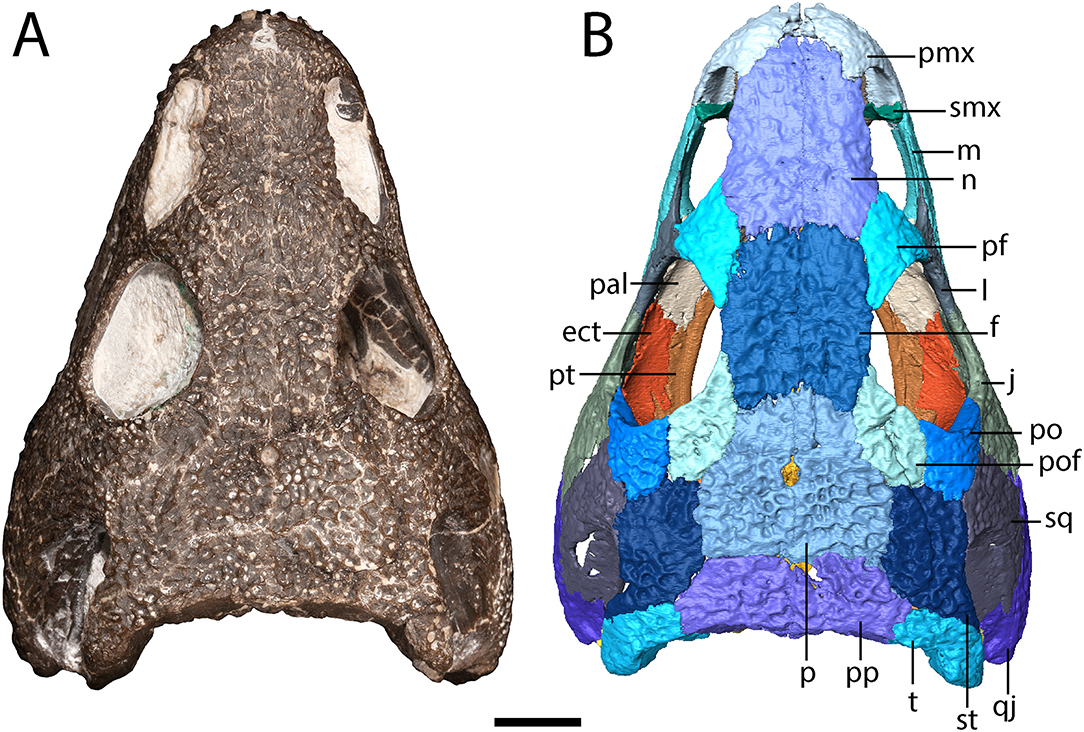
Figure 1. OMNH 79318, cf. Acheloma, entire specimen in dorsal profile. (A) photograph; (B) digitally segmented rendering of the skull roof and palate. Scale bar equal to 1 cm.
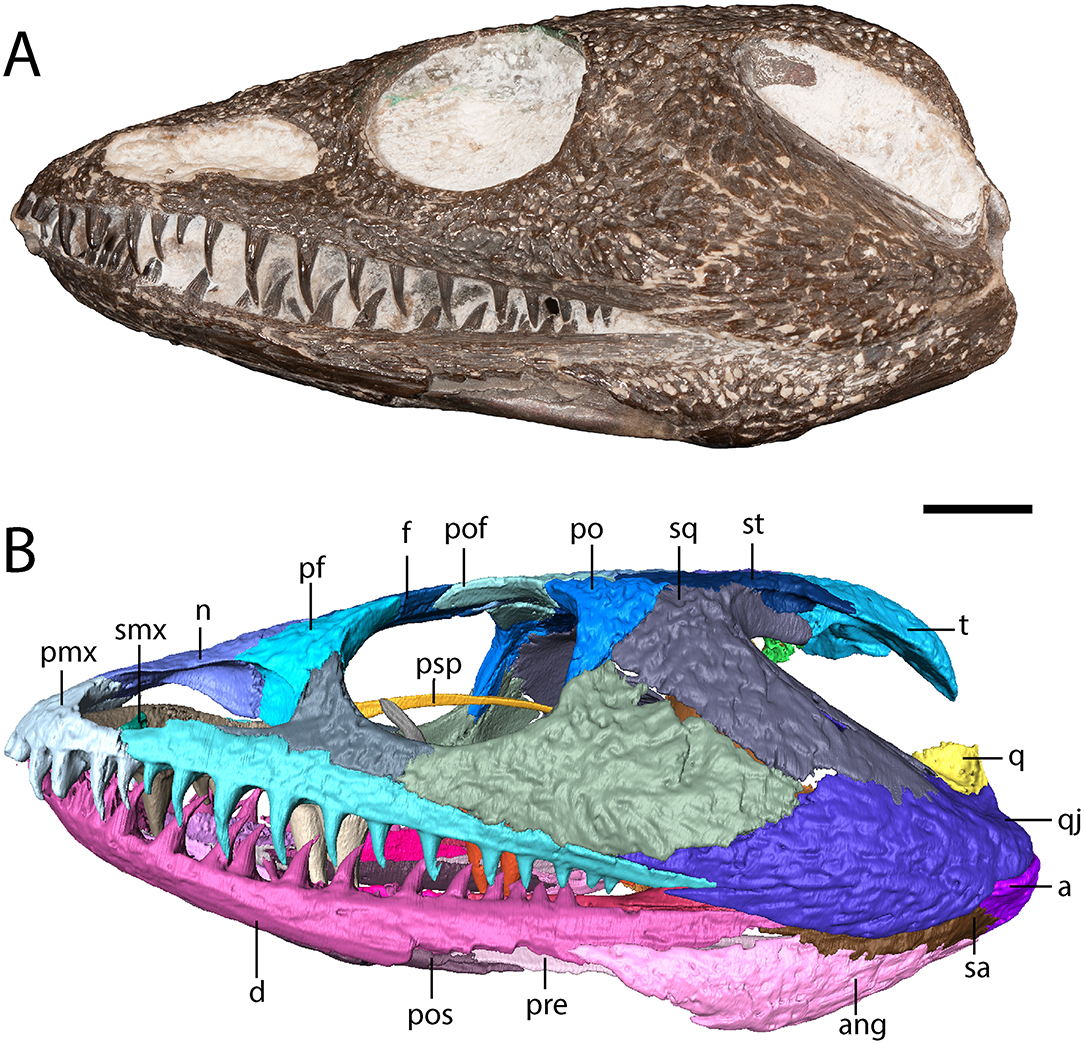
Figure 2. OMNH 79318, cf. Acheloma, entire specimen in left lateral profile. (A) photograph; (B) digitally segmented rendering. Scale bar equal to 1 cm.
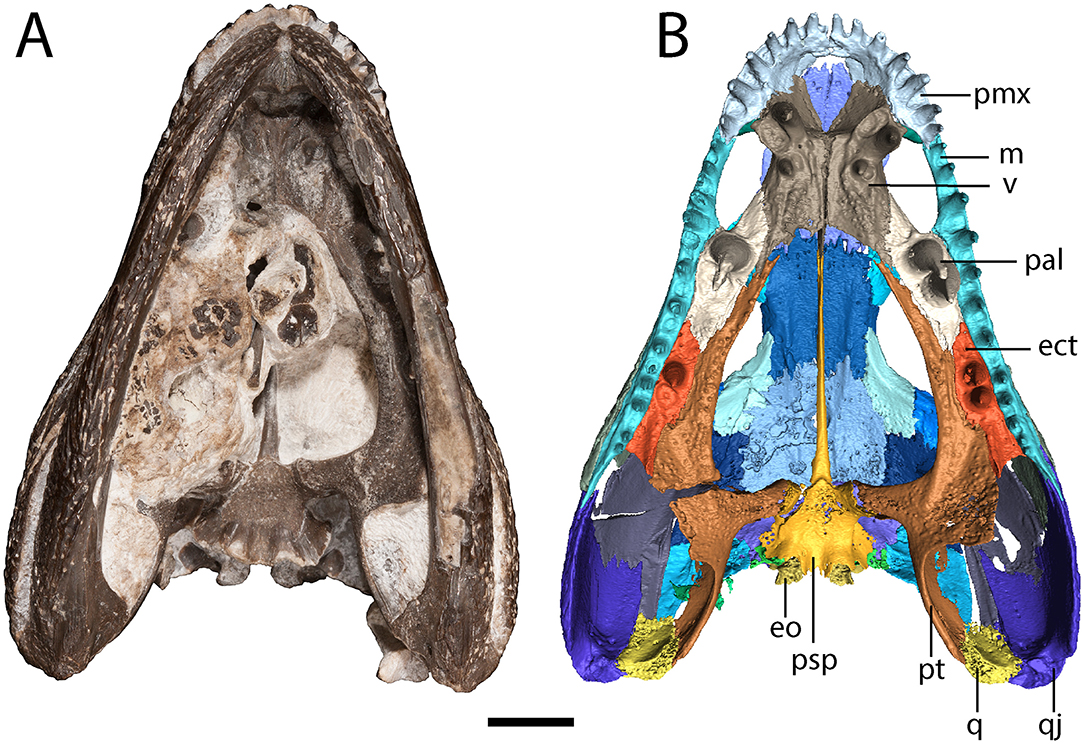
Figure 3. OMNH 79318, cf. Acheloma, entire specimen in ventral profile. (A) photograph; (B) digitally segmented rendering of the skull roof and palate; Scale bar equal to 1 cm.
Skull Roof
The premaxilla is a triangular element that sutures to the maxilla ventrolaterally and to the nasal posteriorly (Figure 1). A relatively blunt, posteriorly directed alary process separates the anterior region of the nasal and the anteromedial narial margin (Figure 1). A circular internarial fontanelle is framed anteromedially by the premaxillae; it is relatively large in OMNH 79318 (Figure 1), Acheloma cumminsi, and Phonerpeton pricei, especially when compared to Acheloma dunni. A total of twelve tooth positions are identified on each premaxilla, with six teeth preserved on the right side and seven on the left side (Figure 3). Polley and Reisz (2011) noted thirteen positions in A. dunni whereas Dilkes (1990) noted only eight to nine positions in P. pricei. Many of the crowns are broken, but based on the size of the exposed bases, the premaxillary teeth exhibit the predicted trematopid caniniform pattern (Figure 2).
The maxilla is a slender element that sutures to the premaxilla anteriorly, to the lacrimal and to the jugal dorsally (which exclude it from the orbit), and to the quadratojugal posteriorly (Figures 1, 2). It is tallest anteriorly and then tapers to a narrow point at the end of the tooth row. Twenty-two tooth positions (14 teeth present) are identified on the right side, and 24 positions are identified on the left side (12 teeth present) (Figure 3). Dilkes (1990) counted 26 positions in Phonerpeton pricei, and Polley and Reisz (2011) estimated at least 28 positions in Acheloma dunni. The tooth row of OMNH 79318 extends posterior to the posterior orbital margin but does not appear to reach the point at which the element overlaps the quadratojugal (Figure 2). The anterior teeth display the typical caniniform pattern seen in other trematopids, being largest at the fifth to sixth positions (Figure 2). Both the maxillary and the premaxillary teeth are notably recurved and gracile, more so than those found in P. pricei and both species of Acheloma.
The septomaxilla is a thin element that extends medially into the naris dorsal to the premaxilla-maxilla suture (Figure 1). It is anteriorly concave and slightly angled ventromedially.
The lacrimal is a triangular element that sutures to the maxilla ventrally, to the prefrontal dorsally, and to the jugal posterodorsally (Figure 1). It contributes to the posterior narial margin and to the anterior orbital margin (Figure 2). The lacrimal contributes to the narial flange, framing the naris posteriorly (Figure 2). A small perforation in this region is interpreted as the nasolacrimal duct.
The nasal is a rectangular element that sutures to the premaxilla anteriorly, to the prefrontal posterolaterally, and to the frontal posteriorly (Figure 1). It contributes to the anterior portion of the narial flange that descends from the skull roof (Figure 2). The nasal's contribution forms a triangle in lateral profile that tapers anteriorly.
The frontal is a rectangular element that sutures to the nasal anteriorly, to the prefrontal anterolaterally, to the postfrontal posterolaterally, and to the parietal posteriorly (Figure 1). It divides the pre- and post-frontal to enter the orbit.
The parietal is a quadrangular element that sutures to the frontal anteriorly, to the postfrontal anterolaterally, to the supratemporal laterally, and to the postparietal posteriorly (Figure 1). The parietals frame the pineal foramen in their anterior half, as in Phonerpeton pricei (Dilkes, 1990) but in contrast to Acheloma dunni in which the foramen is just posterior to the mid-length of the parietals (Figure 1). The foramen is proportionately larger in OMNH 79318 and P. pricei. The parietals of OMNH 79318 and A. dunni are more equant in proportions than the longer and narrower parietals of P. pricei.
The postparietal is a rectangular element that sutures to the parietal anteriorly, to the supratemporal anterolaterally, to the tabular laterally, and to the exoccipital ventrally (Figure 1). It contributes to the occipital flange and frames the foramen magnum dorsally (Figure 4).
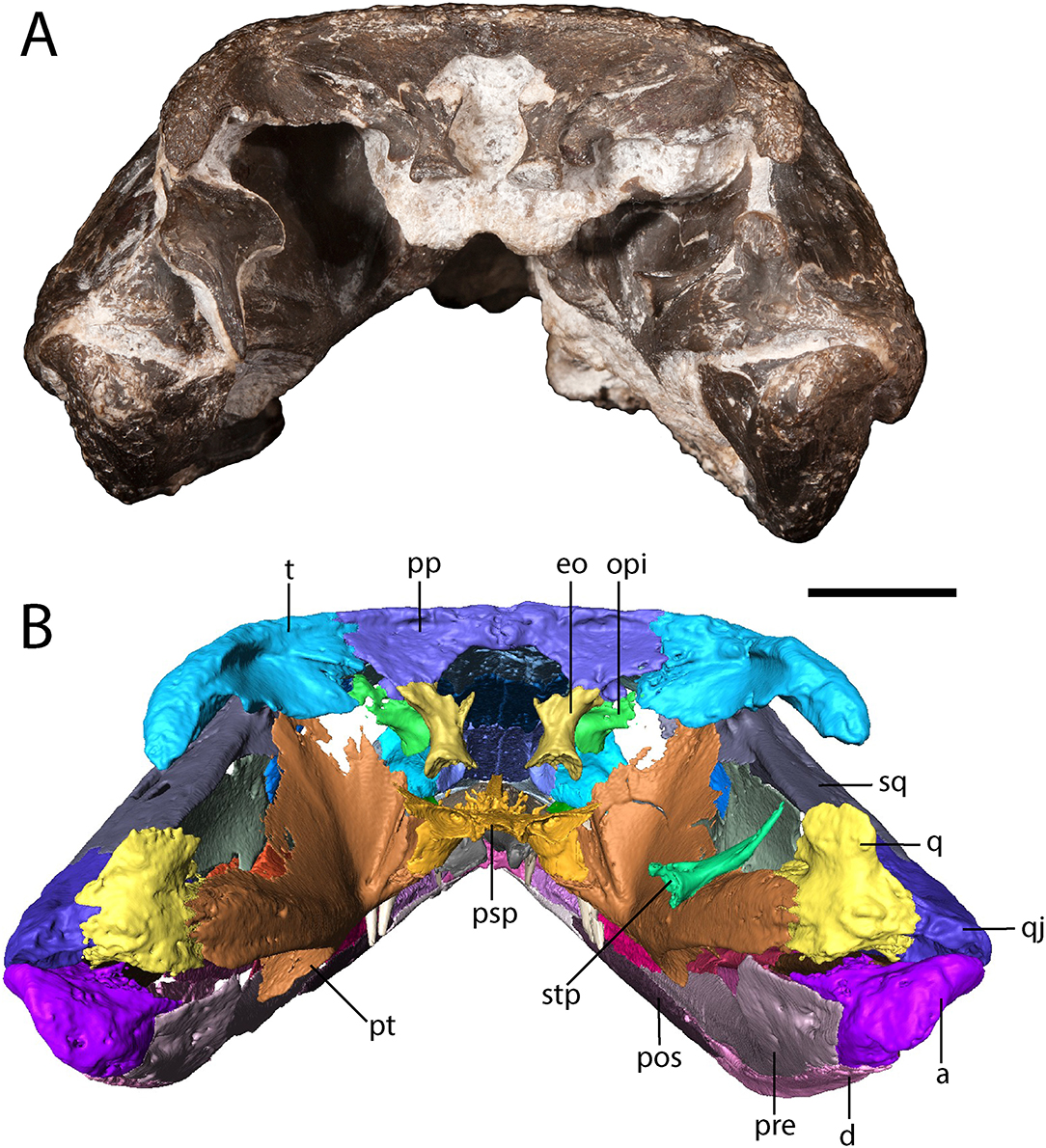
Figure 4. OMNH 79318, cf. Acheloma, entire specimen in occipital profile. (A) photograph; (B) digitally segmented rendering. Scale bar equal to 1 cm.
The postfrontal is a crescentic element that sutures to the frontal medially, to the parietal posteromedially, to the supratemporal posteriorly, and to the postorbital laterally (Figure 1). The postfrontal is more similar in proportions to the slender postfrontal of Phonerpeton pricei (Dilkes, 1990), although its anterior extent (mid-length of the orbit) and posterior extent (terminating just anterior to the level of the otic notch) are the same as in Acheloma dunni (Polley and Reisz, 2011).
The postorbital is a crescentic element that sutures to the postfrontal medially, to the supratemporal and to the squamosal posteriorly, and to the jugal ventrolaterally (Figure 1).
The jugal is a triangular element that sutures to the lacrimal anteriorly, to the maxilla ventrally, to the quadratojugal posteriorly, to the squamosal posterodorsally, and to the postorbital dorsally (Figures 1, 2). It tapers more pronouncedly posteroventrally, similar to Phonerpeton pricei (Dilkes, 1990) and in contrast to Acheloma dunni and Acheloma cumminsi (Dilkes and Reisz, 1987; Polley and Reisz, 2011).
The squamosal is a crescentic element that forms most of the otic notch, suturing to the quadrate posteriorly, to the quadratojugal posteroventrally, to the jugal anteroventrally, to the postorbital anteriorly, to the supratemporal dorsomedially, and to the tabular posterodorsally (Figures 1, 2). An unornamented part of the squamosal forms the otic notch, which is framed by two flanks of the supratympanic flange. The dorsal flank is formed by the squamosal, the supratemporal, and the tabular and extends horizontally and straight back (Figure 2). The descending flank extends posteroventrally and is formed only by the squamosal, terminating at an overlap with the quadratojugal (Figure 2). The angle enclosed by the flanks is ~30 degrees, being closer to some dissorophids [e.g., Cacops (Reisz et al., 2009)] and some trematopids [e.g., Phonerpeton pricei (Dilkes, 1990)]than in the holotype specimens of Acheloma dunni and A. cumminsi in which the flanks are nearly parallel (Dilkes and Reisz, 1987; Polley and Reisz, 2011).
The supratemporal is a rectangular element that sutures to the postfrontal anteromedially, to the parietal medially, to the postparietal posteromedially, to the tabular posterolaterally, to the squamosal ventrally, and to the postorbital anterolaterally (Figure 1). It contributes to the dorsal flank of the supratympanic flange (Figure 2) but is otherwise mostly restricted to a dorsal exposure.
The tabular is a crescentic element that sutures to the supratemporal anteriorly and to the postparietal medially (Figure 1). It extends posteroventrolaterally toward the quadrate (Figures 2, 4), although it remains cleanly separated from the latter, similar to the ontogenetically influenced condition seen in immature dissorophids [e.g., Cacops (Reisz et al., 2009)]. The tabular of Phonerpeton is not reconstructed as curving downward (Dilkes, 1990), but it is not well-preserved in any specimen. The tabular contributes to the occipital flange, with its contribution contacting the opisthotic ventromedially (Figure 4). It also contributes a minute portion to the posteriormost end of the dorsal flank of the supratympanic flange (Figure 2).
Notably, the specimen lacks both a lateral exposure of the palatine (LEP) and a similar exposure of the ectopterygoid (LEE). These features are seen across the variably sized specimens assigned to Acheloma dunni by Polley and Reisz (2011) and commonly occur in other trematopids [e.g., Phonerpeton pricei (Dilkes, 1990)]. Sometimes only the LEP is present [e.g., Fedexia (Berman et al., 2010)]; this condition is commonly found in dissorophids as well (Schoch, 2012). Among olsoniforms, the exposures usually enter the orbital margin, dividing the lacrimal and the jugal (e.g., P. pricei, Fedexia striegeli, Ecolsonia cutlerensis, Cacops morrisi). Alternatively, they partially divide the maxilla from the lacrimal and the jugal without entering the orbit (both species of Acheloma). The purported absence of both an LEP and an LEE in Acheloma cumminsi (Dilkes and Reisz, 1987; Polley and Reisz, 2011) is a significant feature that distinguishes the two species of Acheloma. However, careful restudy and additional preparation of the large-bodied holotype (AMNH FARB 4205) of A. cumminsi and the small-bodied holotype of “Trematops willistoni” (now synonymized with A. cumminsi) (FMNH UC 1584) revealed the presence of these lateral exposures (Figure 5). The configuration and placement in AMNH FARB 4205 are the same as in A. dunni, being situated between the maxilla and the jugal and excluded from the orbit (Figures 5A,B). We can only identify one LEE (there are two in A. dunni), but it is as long as the pair of exposures in A. dunni. The exposures of FMNH UC 1584 are much smaller and less well-defined (Figures 5C,D). They are widely separated from each other; due to their size, they do not substantially separate the lacrimal and the jugal from the maxilla. In OMNH 79318, the lacrimal, jugal, and maxilla are in complete contact with each other (Figure 2). However, the tomographic data show that the ectopterygoid mostly divides the jugal and the maxilla internally, and that it may actually have a minute lateral exposure that simply cannot be recognized by the naked eye (Figure 6). The palatine buttresses the maxilla and the lacrimal internally but does not substantially intercede between them.
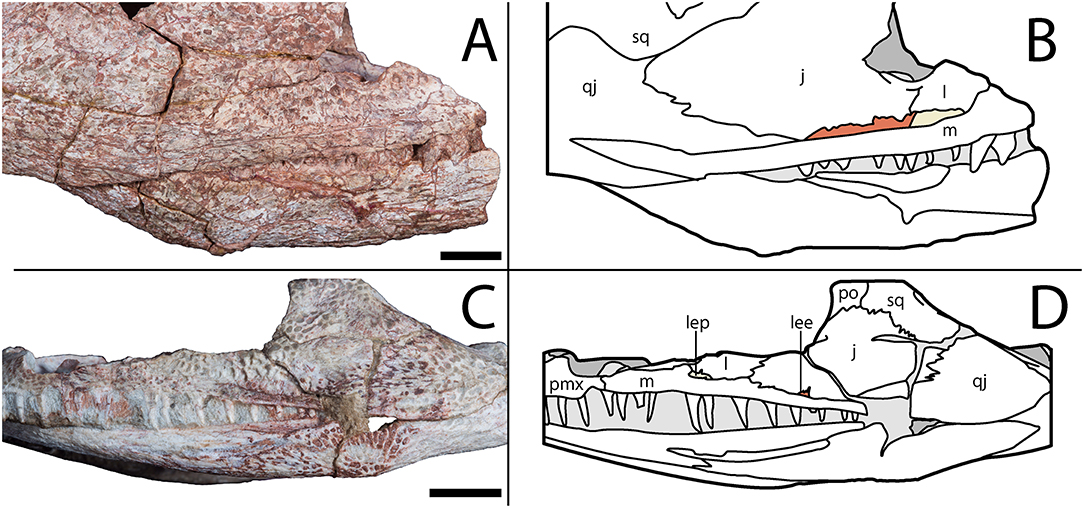
Figure 5. AMNH FARB 4205, holotype of Acheloma cumminsi, in right lateral profile and FMNH UC 1584, holotype of “Trematops willistoni,” in left lateral profile. (A) Photograph showing lateral exposures of the palate in AMNH FARB 4205; (B) line drawing of the same; (C) photograph showing lateral exposures of the palate in FMNH UC 1584; (D) line drawing of the same. Lateral exposure of the palatine (beige) and ectopterygoid (red) are shaded following their colors in the digital segmentation of OMNH 79318 (see Figure 7 for isolated palate). Scale bars equal to 1 cm.
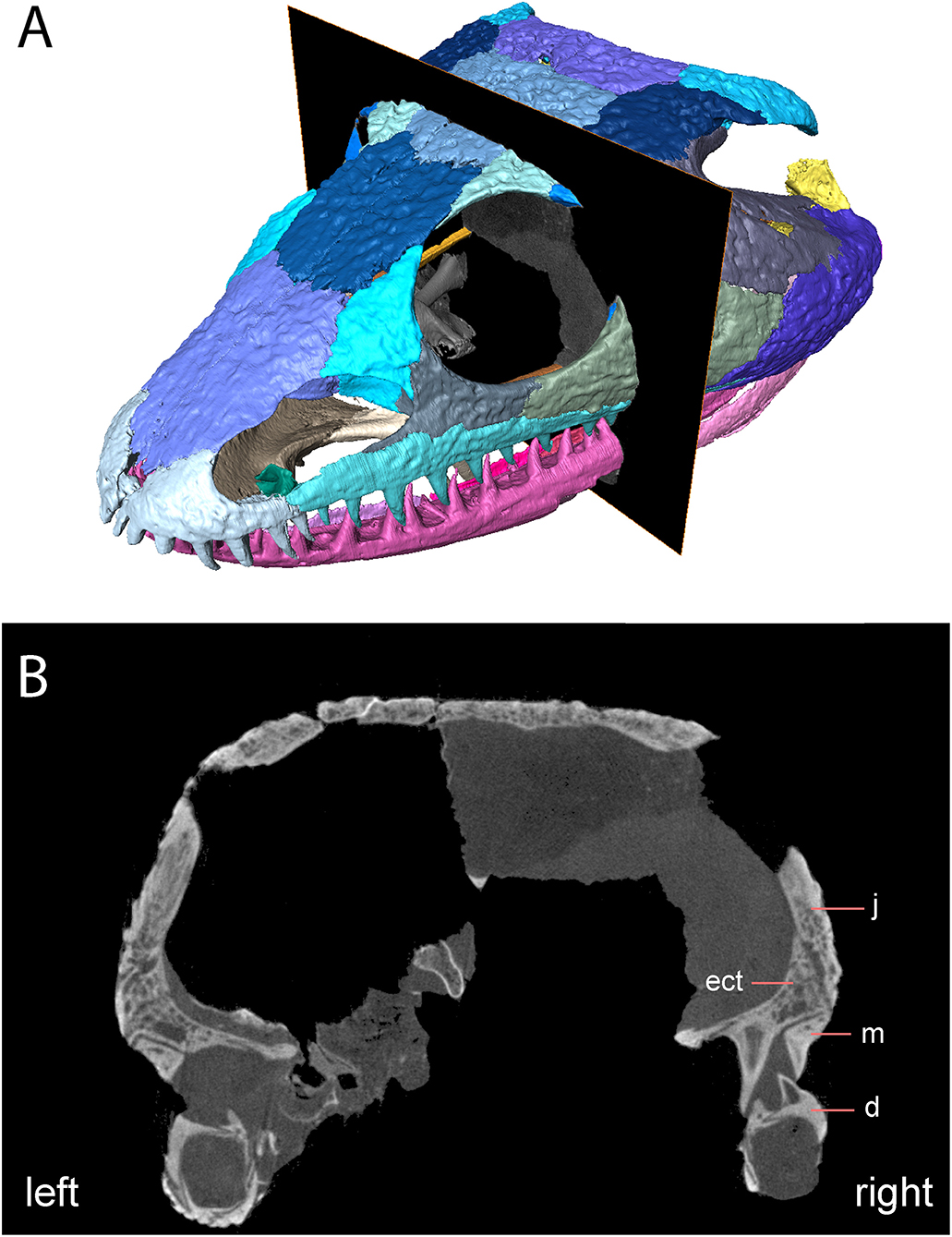
Figure 6. OMNH 79318, cf. Acheloma, skull roof in anterolateral profile with digital slice showing trace of lateral exposure of the ectopterygoid. (A) Rendering of skull roof with transverse slice; (B) transverse digital slice showing sutural relationships of the ectopterygoid, the jugal, and the maxilla.
Cranial ornamentation is similar to that seen in other trematopids, comprising irregularly sized circular to polygonal pitting throughout the skull roof. More elongate grooves are found in the temporal region, particularly extending down the ornamented portion of the squamosal and for much of the length of the quadratojugal. Less developed grooves are also found on the jugal and on the maxilla. The ornamentation is less evenly developed across the skull, similar to Phonerpeton pricei (Dilkes, 1990) and in contrast to Acheloma dunni (Polley and Reisz, 2011). However, the specimen may have been partially prepared prior to study, as some artificial alteration appears to have occurred in regions of minimal ornamentation.
A portion of the articulated scleral ring is present in the ventral orbit, being visible when the skull is viewed from above (Figure 1A). The thin, rectangular plates are not well-resolved in the tomographic data and are not segmented here. They are differentiated from the much smaller denticulate palatal plates by their large size (see description of palate).
Occiput
The exoccipital is constricted at the mid-height with expanded ends that bear unfinished bone surfaces for articulation with other elements (Figure 4). In OMNH 79318, the exoccipitals only contact the occipital flange of the post-parietals, being widely separated ventrally from the parasphenoid.
The opisthotic is a small element positioned slightly anterolateral to the exoccipital such that is partially obscured when viewed in occipital profile (Figure 4). There is no evidence for an ossification of the synotic tectum or of the prootic, let alone for co-ossification of the opisthotic with the prootic [seen in large specimens of Acheloma dunni (Maddin et al., 2010)].
The right stapes is dislodged to lie on top of the quadrate ramus of the pterygoid (Figure 4). It comprises a slightly bifurcated footplate that is perforated by a circular stapedial foramen and a narrow shaft that would have been anteriorly convex, with the distal end curving posterolaterally.
Palate
The parasphenoid comprises the square basal plate and the long and slender cultriform process (Figures 3, 7). The anterior half of the basal plate is covered in denticles, although this is not resolvable in the tomographic data. The basal tubera are not developed. Anterolaterally, the parasphenoid has two stout processes that meet the pterygoids in a distinct and unfused suture. The cultriform process is transversely compressed, forming a blade-like structure that arches dorsally along its mid-length before descending toward the ascending vomerine flanges (Figure 7C).
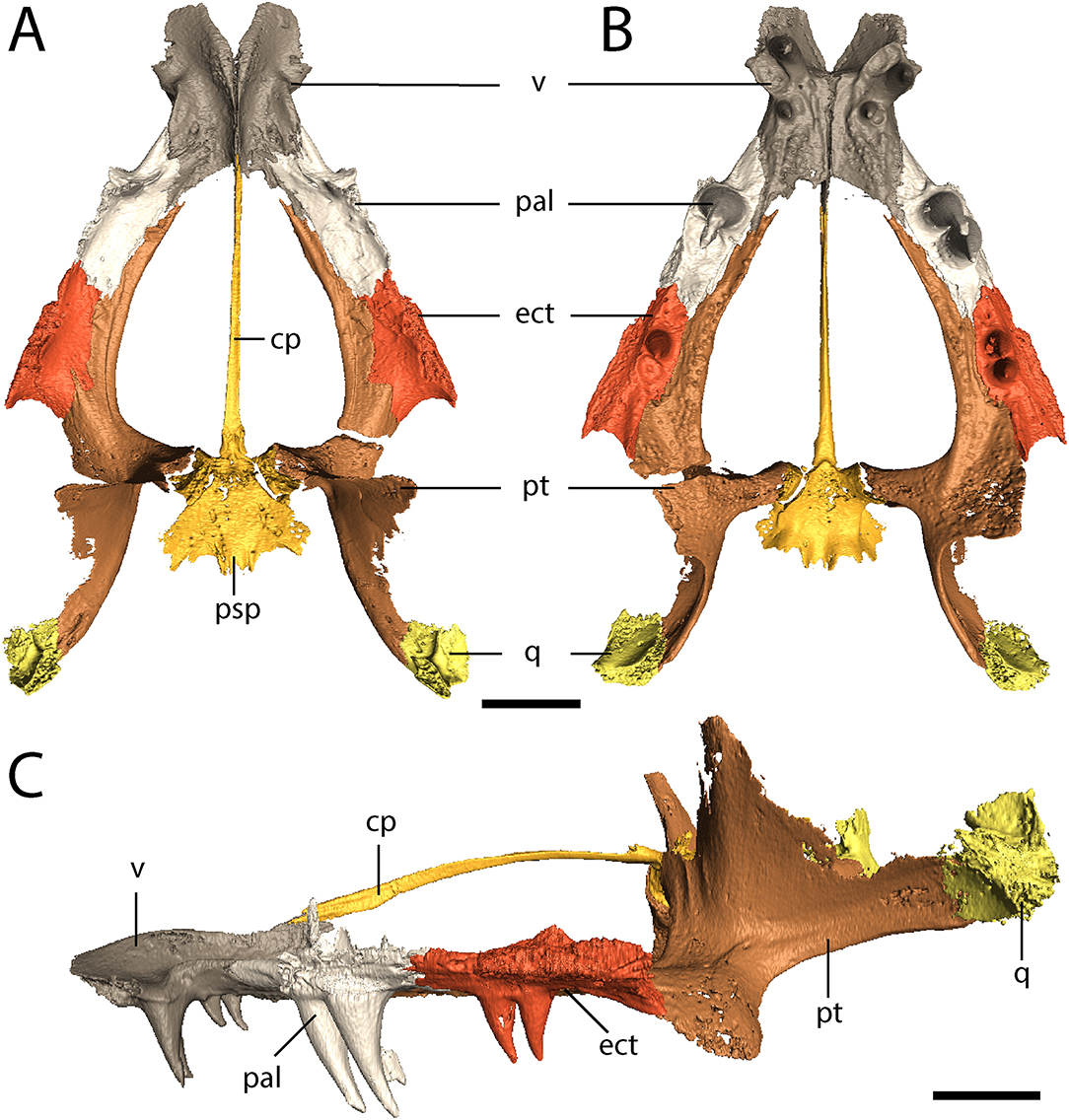
Figure 7. OMNH 79318, cf. Acheloma, isolated renderings of the palate. (A) dorsal profile; (B) ventral profile; (C) left lateral profile. Scale bar equal to 1 cm.
The vomer is a rectangular element that sutures to the premaxilla anterolaterally, to the palatine laterally, to the pterygoid posterolaterally, and to the parasphenoid posteriorly (Figures 3, 7A,B). The vomers frame the posterior extent of a deep fossa, the intervomerine fossa (Figures 3, 7A,B). Two pairs of enlarged teeth (“fangs”) are present. The larger pair is positioned on the anterolateral process of the vomer that meets the premaxilla, separating the choana and the intervomerine fossa, with virtually no room on either side of the tooth sockets. The smaller pair is slightly posterior and medial to the first pair. Only one pair is documented in Phonerpeton pricei (Dilkes, 1990). A denticulate ridge originating medial to the smaller fang pair and extending posterolaterally toward the pterygoid is present; this is an autapomorphy of Acheloma sensu Polley and Reisz (2011). Dorsally, each vomer has an ascending process along its midline contact that meets to form the median vomerine septum and that extends posterodorsally to meet the cultriform process (Figure 7C). The septum contacts the skull roof in Acheloma cumminsi (Dilkes and Reisz, 1987), is of unclear relationship in Acheloma dunni (Polley and Reisz, 2011), and is distinctly separated in Phonerpeton pricei (Dilkes, 1990); the condition of OMNH 79318 is the same as that of P. pricei.
The palatine is a rectangular element that frames the choana posteriorly with the vomer and the maxilla (Figures 3, 7A,B). Its extent along the medial margin of the choana is comparable to that of Acheloma dunni (Polley and Reisz, 2011), being greater than in Phonerpeton pricei in which it is mostly formed by the vomer (Dilkes, 1990). A large pair of fangs occupies most of the ventral surface. The element is rather thin and mostly flat, but it extends dorsolaterally along the internal surface of the ventral orbital rim, mostly buttressing the lacrimal from within (Figure 1). It also extends for a short distance anterodorsally to contribute to the wider antorbital process that is mostly formed by the prefrontal and to a lesser degree by the lacrimal. A few scattered denticles are preserved on the posterior half of the palatine, but they are not well-resolved in the tomographic data.
The ectopterygoid is of similar morphology to the palatine but with a smaller pair of fangs (Figures 3, 7A,B). Like the palatine, it is mostly flat but extends dorsolaterally to buttress the ventral orbital rim, in this case mostly the jugal (Figure 1). As with the palatine, a few scattered denticles are preserved on the posterior half of the ectopterygoid, but they are not well-resolved.
The pterygoid is a large, triradiate element, with processes directed anteriorly along the lateral margin of the interpterygoid vacuity (palatine ramus), medially to meet the parasphenoid in the basicranial articulation, and posterolaterally to meet the quadrate (Figures 3, 4, 7). The anteromedial process narrows to a thin point, similar to Phonerpeton pricei (Dilkes, 1990) and in contrast to Acheloma dunni in which it tapers only slightly to abut the vomer (Polley and Reisz, 2011). The medial margin is curved, forming more oval vacuities, as with P. pricei and in contrast to A. dunni in which it is straight, forming a more trapezoidal vacuity. The transverse flange forms a broad, denticulate lobe that is angled posteroventrally to descend below the ventral margin of the skull (Figure 4). A thin flange ascends from the basipterygoid ramus and extends posteriorly along the quadrate ramus (Figure 4). This partially sheaths the squamosal posteromedially from within (Figure 4), but the flange appears incompletely ossified and does not completely enclose the cheek region. The entire pterygoid is denticulate, save for the posteriormost extent of the quadrate ramus.
A number of small, denticulate palatal plates are located medial to the ventral margin of the right mandible (Figure 3A). They are positioned just anterior to the level of the pararticular foramen and resemble those that are recognized in other olsoniforms and widely found among temnospondyls (Gee et al., 2017). A few other plate-like fragments of uncertain skeletal or taxonomic affinity are exposed in a more anteromedial position within the palate.
Also located within the palate is the left femur and partial pelvis of an amphibamiform temnospondyl (Figure 3B; Supplemental Figure 1). The taxonomic identification is made on the basis of the relatively advanced development of ontogenetically variable features in the femur (e.g., adductor crest) in combination with the small size of these elements. The iliac crest is long, narrow, and extends posterodorsally. This is in contrast to the crest seen in olsoniforms in which the crest is more rectangular and oriented parallel (horizontal) to the rest of the pelvis.
Mandible
The dentary is the longest element of the mandible, comprising the entirety of the symphysial region and extending far posteriorly to partially divide the anterior half of the surangular via a thin process (Figure 8). There are between 30 (right) and 31 (left) tooth positions in the mandibles, compared to at least 28 in Acheloma dunni (Polley and Reisz, 2011) and 26 in Phonerpeton pricei (Dilkes, 1990). This does not include two slightly enlarged symphysial teeth that are positioned internal to the marginal row (Figure 8).
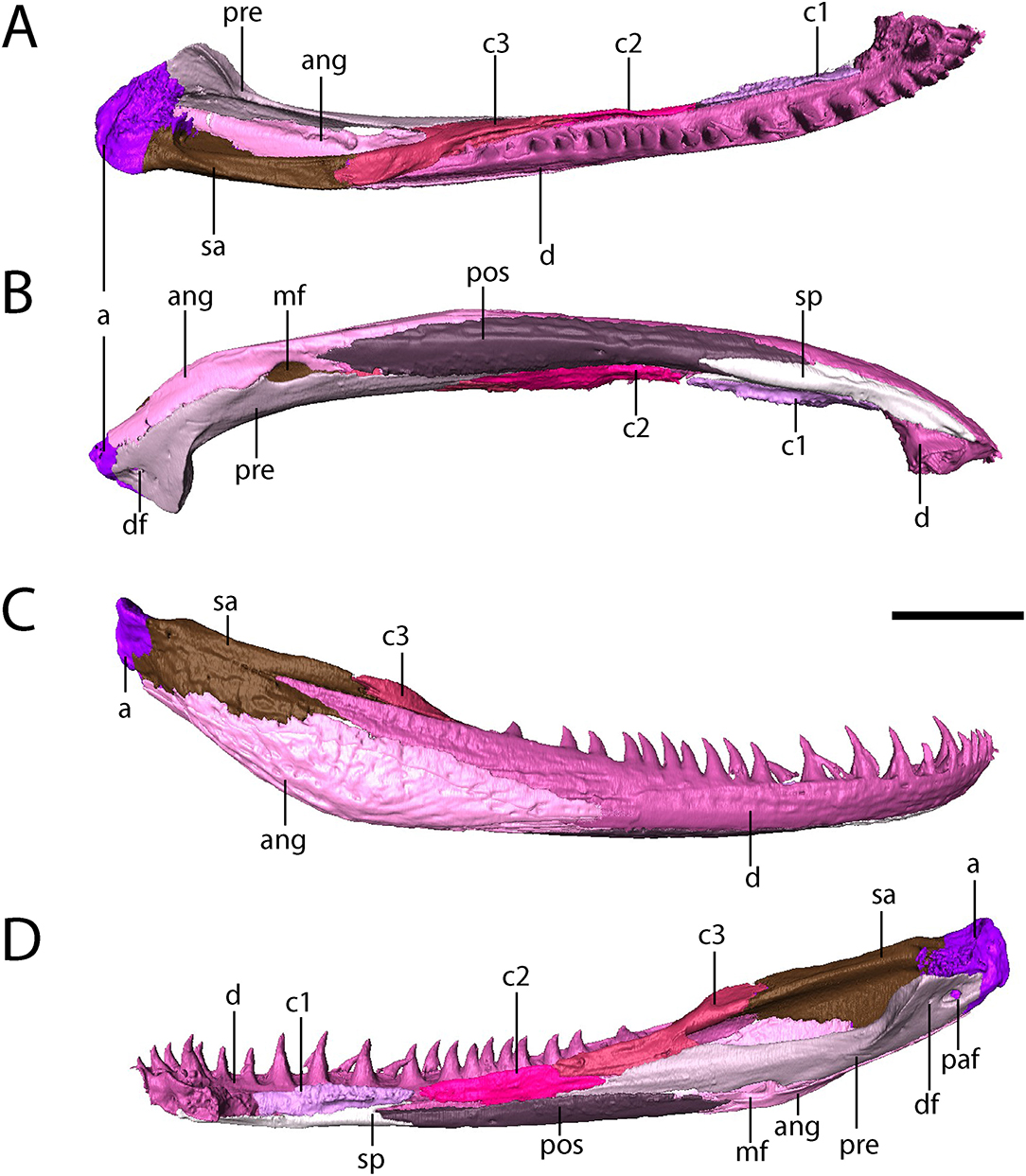
Figure 8. OMNH 79318, cf. Acheloma, isolated renderings of the right mandible. (A) dorsal profile; (B) ventral profile; (C) lateral profile; (D) medial profile. Scale bar equal to 1 cm.
The splenial is a flat, rectangular element that extends posteriorly from beneath the symphysis and along the anteroventral margin of the mandible, where it contacts the first coronoid dorsally and the postsplenial posteriorly (Figure 8).
The postsplenial is a flat, rectangular element that extends from the splenial to meet the angular, lying ventral to the second and third coronoids and the prearticular and ventrolingual to the dentary. It thus forms the ventral margin of the mandible along the mid-length region. Two small perforations in the anterior half represent the mandibular foramina (sensu Dilkes, 1990).
Three coronoids are present (Figure 8). A clearly-defined denticulate texture is present only on the first and second coronoids, which are flat, rectangular elements that lie adjacent to the marginal tooth row of the dentary. Limitations on scan resolution may have obscured denticles on the third coronoid; they are found on all three in Phonerpeton pricei, for example (Dilkes, 1990). The third coronoid is taller and wider, with a ventral flange partially overlapped by the prearticular and a thickened posterodorsal process that contributes to a low coronoid process.
The angular is a long, convex element that forms the posteroventral margin of the adductor chamber (Figure 8). It sutures to the dentary anterodorsally, to the surangular posterodorsally, to the articular posteriorly, and to the prearticular dorsolingually.
The surangular is a tall yet relatively slender element that contributes to the labial wall of the adductor chamber, where it meets the third coronoid anteriorly and the articular posteriorly (Figure 8). Anteriorly, along the labial surface, it is partially divided by a process of the dentary.
The prearticular is a long, slender element that contributes to the lingual surface (Figure 8). It contacts the second and third coronoids anteriorly and dorsally to frame the adductor chamber and contacts the postsplenial and the angular ventrally, framing the Meckelian foramen with the latter. Posteriorly, the prearticular curves lingually with the expanded jaw articulation. A smaller foramen that extends into the articular (the pararticular foramen of Dilkes, 1990) is present; this is inferred to be for the chorda tympani branch of cranial nerve VII. A third, much smaller foramen is slightly anterodorsal to the pararticular foramen (the dental foramen of Dilkes, 1990).
The articular forms the jaw articulation, with a slightly convex dorsal surface that tightly conforms to the ventrally convex margins of the quadrate and the quadratojugal (Figures 4, 6). It is longitudinally short, extending only for a short distance anterior to the contact with the prearticular and the surangular.
The mandible is ornamented in a fashion similar to other temnospondyls. The most intensive ornamentation is along the ventral mid-length of the angular with a few large, oval pits that radiate outward into elongate grooves and ridges (Figure 8A). The ornamentation also extends onto the surangular, the postsplenial, and the splenial but is less developed on these elements (Figure 8A). Small pits and faint ridges are present along the labial surface of the dentary as well.
Discussion
Ontogenetic Interpretation
Studies relating to trematopid ontogeny are relatively rare, as most taxa are known from only the holotype. Most studies are based on a single taxon or on certain skeletal regions. Olson (1985) described what he interpreted as a larval trematopid, although this was refuted by Dilkes (1991) this specimen was most recently erected as the holotype of a new amphibamid genus, Nanobamus (Schoch and Milner, 2014). Dilkes (1993) also discussed ontogeny of the rostrum in a few trematopids as part of a larger study of the unusual trematopid naris. Maddin et al. (2010) presented a discussion of ontogeny in the neurocranium and the occiput of specimens that are now referred to Acheloma dunni. Milner (2018) is the most recent work to address this topic (focusing on Mattauschia), with an emphasis on closure of the otic embayment and the posterior elongation of the naris.
Despite the paucity of work on trematopid ontogeny, numerous features indicate that OMNH 79318 is a relatively immature individual. The specimen conforms to the patterns identified in Acheloma dunni by Maddin et al. (2010), although it precedes even the earliest ontogenetic stage described in that study. For example, the basioccipital and the basal tubera are absent, the paroccipitals are short, and the otic capsule is unfused (with a point of emphasis that the prootic is not even ossified) (Figures 3, 4, 7). There is no evidence for an ossification of the synotic tectum (the “supraoccipital” of Olson, 1941:162). Furthermore, the specimen lacks ossification of the sphenethmoid and the epipterygoid, features that are seen in larger trematopid specimens, including the type of A. dunni. Given the immaculate quality of preservation and articulation of the skull, it seems implausible that all of these elements would have been lost during preservation, but this cannot be unequivocally determined. Regardless, poor ossification (e.g., unfused otic capsule) and distinct sutures support hypothesized immaturity. Lastly, the tabular and the dorsal process of the quadrate remain well-separated, in contrast to large specimens of Acheloma in which they contact each other to close the otic notch posteriorly (e.g., Dilkes and Reisz, 1987). Dilkes (1993) identified a few features of the rostrum that he interpreted as evidence of relative maturity in trematopids: development of the caniniform dentition and posterior elongation of the naris toward the antorbital bar. However, a large component of this study of the narial region in trematopids was the sample of Phonerpeton pricei. As we expand upon further below, if P. pricei is represented mostly by juvenile individuals, rather than small-bodied adults, this would indicate that some of these features actually appear relatively early in ontogeny. Both of these features, as well as the narial flange (stated by Dilkes to appear early in ontogeny), are already developed in OMNH 79318 (more so than in P. pricei), which further corroborates a hypothesized early appearance regardless of the interpretation of P. pricei. Without good constraints on timing of ontogenetic transformations, it cannot be assumed that features typical of large-bodied individuals only appear late in ontogeny. OMNH 79318 thus, irrespective of its somewhat uncertain taxonomy (see below for details), represents an important data point as a relatively immature trematopid.
Taxonomic Affinities
Our ontogenetic interpretation unfortunately complicates a resolution of the taxonomic affinities of OMNH 79318. The specimen is readily identified as a trematopid based on the presence of six of the seven diagnostic features listed by Schoch and Milner (2014), such as a greatly enlarged naris with a reduced lacrimal and a narial flange joining the antorbital bar. The seventh feature (supinator process of the humerus) cannot be evaluated in this specimen. However, a phylogenetic analysis cannot be used to place OMNH 79318 within the Trematopidae because of the ontogenetic immaturity of the specimen and the associated problems that this can produce in analyses (e.g., Tykoski, 2005; Wiens et al., 2005; Lamsdell and Selden, 2013; Tsai and Fordyce, 2014; Woodruff et al., 2018). The potential pitfalls of ontogenetic disparity among currently recognized trematopid genera have been discussed by Milner (2018), and it is beyond the scope of this paper to more fully evaluate the phylogeny of trematopids with this caveat in mind. It may be reasonably predicted that the skeletal immaturity of OMNH 79318 could result in a non-taphonomic variation of “stemward slippage” (Sansom et al., 2010), in which the absence or reduced degree of ossification results in the recovery of a more basal position. However, this would only be possible to test given a well-resolved phylogeny that incorporates mostly skeletally mature individuals. More than half of all trematopid taxa (Actiobates peabodyi, Anconastes vesperus, Fedexia striegeli, Mordex calliprepes, Phonerpeton pricei, Rotaryus gothae, and Tambachia trogallas), including both short- and long-snouted forms, may be represented by type specimens that are immature (Milner, 2018). Accordingly, separating ontogeny from taxonomy (and also taphonomy) becomes difficult. Even when major considerations of ontogenetic disparity are not explicitly considered, there is a lack of consensus in trematopid intrarelationships based on the most recent studies (Berman et al., 2011; Polley and Reisz, 2011). As suggested by Milner's (2018) conceptual phylogeny, the long-snouted forms (Acheloma, Phonerpeton, Rotaryus) are probably the most derived (in line with Berman et al., 2011). We emphasize that further quantitative work is necessary to assess whether ontogeny is exerting a major influence on the tree topology for this clade and to recover a better consensus on intrarelationships of the Trematopidae.
At first glance, OMNH 79318 closely resembles Phonerpeton pricei, differing from Acheloma cumminsi and Acheloma dunni by a large number of qualitative observations (Table 1). For example, the posterior skull is narrower, the lateral margin of the entire skull is without a prominent medial inflection, the otic notch remains open posteriorly, with the flanks of the embayment aligned at an angle to each other rather than being horizontal (the latter being a autapomorphy of Acheloma sensu Polley and Reisz, 2011), and the pineal foramen is situated in the anterior half of the parietals, not at their mid-length. However, all of these features are likely to be ontogenetically influenced, as there is a strong correlation between the observed disparities and the relative size of trematopid specimens (see Milner, 2018). Furthermore, there is good reason to suspect that P. pricei is actually represented only by relatively immature individuals based on our current understanding of ontogenetic changes in trematopids (see below). Subsequently, the apparent similarities between OMNH 79318 and P. pricei likely reflect patterns of trematopid ontogeny, as they are represented by much smaller individuals than the larger specimens of A. cumminsi and A. dunni from which they differ and that serve as the foundation for the taxonomic diagnoses.
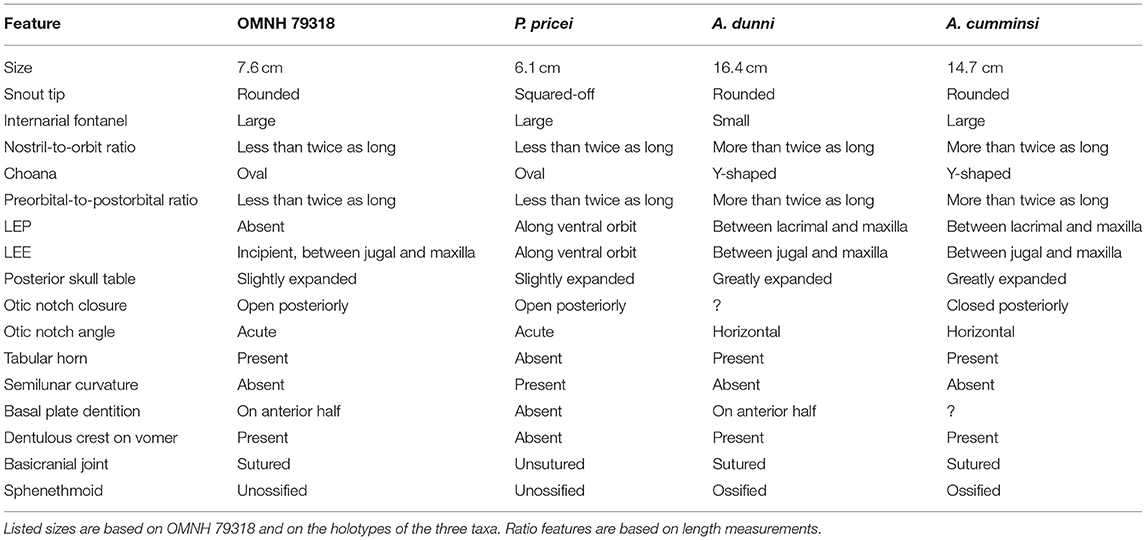
Table 1. Summary table of differences between OMNH 79318, Phonerpeton pricei, Acheloma dunni, and Acheloma cumminsi.
Schoch and Milner (2014) recently placed a second species of trematopid within Phonerpeton, P. whitei (Acheloma whitei of Olson, 1941). As with Phonerpeton pricei, P. whitei is only represented by small skulls (6 cm in length) (Schoch and Milner, 2014). The only apparent difference between P. whitei and P. pricei is a shorter and rounder external naris in the former. The naris of OMNH 79318 is more or less identical to that of P. pricei (and Acheloma dunni and Acheloma cumminsi), being both long and strongly keyhole-shaped in lateral profile. Phonerpeton whitei is based on fewer, more poorly preserved specimens. It has not been redescribed or figured since Olson (1941), and even that description was relatively brief, containing only one largely generic figure of the skull. Beyond this, there are few published details, and it is worth noting that ontogenetic changes to the snout (Dilkes, 1993) could possibly account for the slight distinction in the shape of the external naris between these two species of Phonerpeton. However, we have not examined these specimens ourselves, and in this study, we consider the difference in the shape of the naris to be sufficient to exclude P. whitei from consideration. The remainder of the discussion focuses on P. pricei.
The stratigraphic (and inferred temporal) occurrences of the various long-snouted trematopids does not provide much additional insight. Phonerpeton pricei comes from exposures of the Archer City Formation (Bowie Group) that are interpreted to be Asselian-Sakmarian (Wolfcampian in North American stages) in age (e.g., Dilkes, 1990; Schoch and Milner, 2014). Phonerpeton whitei is from the Petrolia Formation (Wichita Group; Wolfcampian). These inferred ages compare favorably with the Richards Spur locality (earliest Artinskian; Wolfcampian). They contrast with the inferred age of Acheloma cumminsi, which comes from the Arroyo Formation (Clear Fork Group) and equivalents that are traditionally interpreted as latest Artinskian to Kungurian (Leonardian in North American stages) (e.g., Nelson et al., 2013). However, these correlations should be treated with some caution. As noted in the Materials and Methods, the Richards Spur locality was previously correlated with the Arroyo Formation based on tetrapod occurrences and is now considered to be widely temporally disparate following speleothem sampling by Woodhead et al. (2010). Additional work will be needed to assess the current stratigraphic models and inferred ages of the Early Permian deposits of south-central North America before robust conclusions can be drawn from the historical models.
It is beyond the scope of this paper to reappraise Phonerpeton. However, details from Dilkes' work inform the interpretation of Phonerpeton pricei as it relates to the taxonomy of OMNH 79318 and are discussed here. OMNH 79318 preserves virtually all of the formally diagnostic features of P. pricei as prescribed by Dilkes (1990), such as a large and posteriorly open otic notch, an unossified sphenethmoid, and a posterodorsal process of the quadrate formed by two slender sheets that are separated by a weakly ossified region. The potential conundrum is that some of these features are not unique to P. pricei (e.g., open otic notch), and all of them are probably ontogenetically influenced, as with the qualitative observations that are noted above. For example, the sphenethmoid is unossified in all specimens of P. pricei and in OMNH 79318. It should be noted that Dilkes (1990) noted a weakly ossified sphenethmoid in one specimen (MCZ 1767) that he referred to P. pricei but that was more recently restored as the holotype of Phonerpeton whitei by Schoch and Milner (2014). The sphenethmoid ossifies in other trematopids that are represented by larger specimens (e.g., Olson, 1941; Maddin et al., 2010; Polley and Reisz, 2011). The otic notch is known to become closed posteriorly in dissorophids (e.g., Reisz et al., 2009) via posteroventral growth of the tabular and ossification of the dorsal process of the quadrate to a lesser degree throughout ontogeny, and the same transition likely occurred in trematopids (Milner, 2018). Most trematopids other than Acheloma (all of a smaller size) possess similar otic notches to those of dissorophids and OMNH 79318, with a more laterally open notch (e.g., Berman et al., 1985, 2010; Milner, 2018). All of this suggests that the similarities between P. pricei and OMNH 79318 may reflect general patterns of trematopid ontogeny. Other features shared by both that are noted to vary ontogenetically in Acheloma dunni (closely related to P. pricei) by Maddin et al. (2010) include poorly ossified otic capsules, partial gaps between the occipital flanges of the postparietals and the exoccipitals, and the absence of dorsomedial contact between the exoccipitals. One feature formally separates P. pricei from OMNH 79318: the absence of a semilunar curvature of the supratympanic flange. However, this feature appears to be ontogenetically variable in the dissorophid Cacops morrisi (Gee and Reisz, 2018), so caution should be exercised in the interpretation of this feature for taxa in which ontogeny is poorly constrained. The revised diagnosis of Phonerpeton by Schoch and Milner (2014) also supports differentiation of OMNH 79318 from this genus. Although both share an open otic notch, in OMNH 79318, the snout is not squared off, there are denticles on the anterior half of the basal plate of the parasphenoid, and the tabular horn is well-developed (Table 1). The other features shared between OMNH 79318 and Phonerpeton (presence of an internarial fenestra and frontals extending beyond the anterior level of the orbit) are also shared with Acheloma.
Because taxonomic diagnoses are typically based on mature individuals, the genus-level diagnosis for Acheloma is also challenging in the identification of OMNH 79318. Two autapomorphies were previously identified for Acheloma: (1) a dentulous, anteroposteriorly oriented ridge on the vomer; and (2) an otic notch with a nearly horizontal ventral margin (Polley and Reisz, 2011). OMNH 79318 meets the first criterion (Figure 3) but fails to meet the second (Figure 2). However, this disparity in the otic notch morphology may be plausibly explained by ontogeny. The temporal region of large specimens of Acheloma is very tall, especially ventral to the otic notch (jugal, quadratojugal) and more so than in any other olsoniform (Dilkes and Reisz, 1987; Polley and Reisz, 2011). If allometric growth of this region resulted in dorsal expansion in a fashion that constricted the otic notch, it could lead to the horizontal ventral margin considered to be diagnostic for Acheloma by Polley and Reisz (2011) but only in large individuals. An examination of a small referred specimen of Acheloma dunni (BMRP 2007.3.4) and of the holotype of “Trematops willistoni” (FMNH UC 1584) corroborates a hypothesis that the otic notch may change throughout ontogeny. In these specimens, the ventral margin is not as angled as in most other olsoniforms (greater angling in FMNH UC 1584), but neither is it horizontal as in the larger holotype of the taxon. The same is true of a small trematopid specimen of uncertain taxonomy (FMNH UR 2400) from Richards Spur that was described by Bolt (1974a). Also noteworthy is the fact that the holotypes of A. dunni and A. cumminsi are among the largest trematopid specimens to have ever been described and simultaneously have the most constricted notches.
A further seven characters were listed for Acheloma by Schoch and Milner (2014). OMNH 79318 possesses several of these features in addition to the dentulous vomerine ridge noted above: the skull margin widens at the level at and behind the orbit (character 6); the palatine and ectopterygoid bear tall fangs (character 7); and the intervomerine fenestra is large (character 8). However, the specimen differs in several regards: the pre-orbital region is not even close to being approximately twice as long as the skull table (character 3); the naris is similarly not close to being twice as long as the orbit (character 4); the posterior skull table is not substantially widened and posterolaterally expanded (character 5); and the choana is not curved in a fashion that produces a Y-shape (character 9). Of these, characters 3, 4, and 9 relate to the snout region, which is known to extend anteroposteriorly throughout ontogeny based on the work of Dilkes (1993). Thus, it may be reasonably proposed that such features are ontogenetically variable; OMNH 79318 is similar in all of these aspects to Phonerpeton pricei, suggesting a size correlation. The absence of posterolateral expansion of the skull table in OMNH 79318 is a feature that occurs in other small-bodied trematopids (e.g., those referred to P. pricei and FMNH UC 1584), suggestive of ontogeny.
One feature which has been previously suggested to be informative is the presence or absence of lateral exposures of the palatine (LEP) and the ectopterygoid (LEE). Such exposures are found in variably sized specimens of Acheloma dunni, ventral to the jugal and the lacrimal (thus being excluded from the orbit) (Polley and Reisz, 2011). They are also found in almost all specimens of Phonerpeton pricei, but the relationship is different, as they intercede between the jugal and the lacrimal along the ventral orbital margin (Dilkes, 1990). The position of the exposures in P. pricei is similar to that of both other trematopids and dissorophids, whereas the exclusion of these exposures from the orbit in Acheloma is unique. As noted in the description, these palatal exposures were thought not to occur in Acheloma cumminsi (Dilkes and Reisz, 1987), but they were identified in our restudy of the holotype and are of a similar nature to those of Acheloma dunni (i.e. excluded from the orbital margin) (Figure 5). Previous characterizations of the absence of these exposures may reflect the historic nature of most specimens of A. cumminsi, discovered and described in the first half of the twentieth century that could have led to an overlooking of this feature (not identified until the work of Bolt, 1974b). Poor preservation and classical preparation methods may also have obscured these features.
Dilkes suggested that the presence or absence of these exposures may be uninformative because one referred specimen of Phonerpeton pricei (MCZ 1414) lacks these exposures. However, there is only one other documentation of intraspecific variation in this regard among olsoniforms at present. Both an LEP and an LEE were identified in the holotype (MCZ 1419) and in a referred specimen of “Acheloma” (Phonerpeton) pricei (MCZ 1485) by Bolt. These specimens are about the same size as OMNH 79318, but the exposures are separated from each other by a dorsal expansion of the maxilla in MCZ 1485, whereas they contact each other in MCZ 1419 (Bolt, 1974b). In both specimens, the exposures form part of the ventral orbital margin, in contrast to Acheloma and OMNH 79318. Especially because the ontogeny of Acheloma cumminsi remains poorly known, the referral of MCZ 1414 to Phonerpeton pricei should be treated with some skepticism. MCZ 1414 lacks the skeletal regions with the features that would distinguish it from Acheloma, and it further appears to possess a quadratojugal that extends far anteriorly to nearly meet the lacrimal, an unusual condition for an olsoniform (and for tetrapods in general). The suborbital bar of this specimen is also much taller than in other specimens of P. pricei, being closer in this regard to OMNH 79318 and to specimens of both species of Acheloma. The LEE is known to become indistinguishably fused either with the jugal or the LEP during ontogeny in the dissorophid Cacops (e.g., Reisz et al., 2009), but this is not the case in Acheloma dunni in which even the largest individuals maintain their exposures.
Thus far, an appearance of such exposures late in ontogeny is unknown in olsoniforms. However, the presence or absence of lateral exposures in trematopids may correlate with the relative height of the skull (taller in adults). With respect to Acheloma, the only olsoniform in which the exposures do not enter the orbit, the same dorsoventral growth of the skull that we propose could have constricted the otic notch could also lead to the prominent height of the suborbital bar. It is thus possible that such allometric growth could result in exposures of the palate appearing in larger individuals through a lateral incision to separate the maxilla from the lacrimal and the jugal. As noted in OMNH 79318, the ectopterygoid more or less produces a very small, slender lateral exposure (Figure 6) that is not detectable externally, even given the immaculate state of preservation. This is in contrast to the condition of other olsoniforms in which the palatal elements are expressed laterally at the ventral orbital margin. Formation of these exposures may result from dorsal, rather than lateral, extension to override the adjacent circumorbital bones. The relatively small exposures found in FMNH UC 1584 also lend support to our hypothesis for the exposures of Acheloma (Figures 5C,D). However, the discrete presence of the palatal exposures in the similarly small BMRP 2007.3.4 (Acheloma dunni) offers some evidence contrary to an appearance during ontogeny. Collectively, this suggests that caution should be exercised in interpretations of this feature in small specimens until more refined constraints on the ontogenetic development of palatal exposures are produced.
Based on the above lines of reasoning, we tentatively refer OMNH 79318 to cf. Acheloma. OMNH 79318 is very similar in many aspects to Phonerpeton pricei, but we ascribe this to the probable ontogenetic immaturity of that taxon. Various features not found in the formal diagnosis but reflected in phylogenetic codings also separate OMNH 79318 from P. pricei: examples include the presence of a posterolateral process of the vomer that extends along the palatine (as in Acheloma) and a postorbital that tapers to a discrete posterior point. OMNH 79318 is otherwise most similar in morphology to Acheloma, and it shares the longitudinal dentulous ridge on the vomer that also diagnoses the genus sensu Polley and Reisz (2011). The nascent lateral exposure of the ectopterygoid is also in the same fashion as that of Acheloma in interceding between the jugal and the maxilla and thus being excluded from the orbit. Simply because a smaller and presumably much more immature specimen does not align with a taxonomic diagnosis based primarily on much larger individuals (and possibly with ontogenetically influenced characters) should not preclude it from consideration as being taxonomically aligned with that taxon. A more specific referral, however tentative, is not possible because our identification of lateral exposures of the palatal elements in the holotype of Acheloma cumminsi now complicates the taxonomy of both species of Acheloma. As noted above, the presence of these exposures in Acheloma dunni is the only autapomorphy of the taxon. Various qualitative differences were noted by Polley and Reisz (2011), such as the relative size of the internarial fontanelle, but the formal distinction between A. cumminsi and A. dunni is now non-existent. Resolution of the taxonomy of these two species is beyond the scope of this paper and will be addressed in future work.
A final consideration in this convoluted discussion of the taxonomy of OMNH 79318 is based on the presence of clear and distinct lateral exposures in comparably sized (and presumably comparably immature) specimens of Acheloma (e.g., BMRP 2007.3.4 [Acheloma dunni]). This could be interpreted as evidence that OMNH 79318 represents a distinct and novel taxon, regardless of the possibly synonymy of Acheloma cumminsi and Acheloma dunni. However, FMNH UC 1584 (identically sized to OMNH 79318) has much smaller exposures that are separated from the orbit but also separated from each other and that do not substantially separate the lacrimal and the jugal from the maxilla. Without strong evidence indicating that the presence of lateral exposures is both taxonomically informative and not ontogenetically influenced, we refrain from erecting a new taxon, as this is the only feature separating them. There are a few other subtle distinctions (e.g., more triangular postorbital, similar to A. cumminsi; greater recurvature of marginal dentition and palatal fangs than in other trematopids), but these are of uncertain utility at this time. In many extant lissamphibians (e.g., Halliday and Verrell, 1988), size is often a weak predictor of maturity such that comparably sized individuals may not represent comparably mature individuals. Similar developmental plasticity is thus to be expected in temnospondyls and indeed is documented within the clade (e.g., Sanchez and Schoch, 2013; Schoch, 2014; Canoville and Chinsamy, 2015). We believe that this conservative approach is appropriate, given the relatively poor knowledge of early stages of ontogeny in trematopids. Aside from the nature of the otic notch, the morphology of the skull roof, the palate, and the mandible strongly agrees with that seen in both species of Acheloma.
Philosophical Considerations
The complicated pathway to the proposed taxonomic identity of OMNH 79318 emphasizes two key points related to taxonomic practices. The first is that caution should be exercised in diagnosing and/or erecting taxa based only on diminutive and probably immature specimens. Dilkes (1990) diagnosed Phonerpeton pricei as a small-bodied taxon but did not provide any rationale for the interpretation of assigned specimens as being of comparable maturity to much larger-bodied trematopids. Phonerpeton whitei is also noted to be represented only by small individuals (Schoch and Milner, 2014). With more recent work that informs on ontogenetic changes in olsoniforms (e.g., posterior closure of the otic notch, increased ossification of neurocranium), it now seems more plausible to interpret Phonerpeton as a taxon represented only by immature individuals. Thus, as we noted above, purported autapomorphies or differential features may be confounded by ontogenetic variation, among other sources of intraspecific variation.
This leads to the second problem highlighted by this study, which is the challenge of properly referring specimens. There are no formal guidelines for referrals, but more conservative methods such as apomorphy-based identification (e.g., Nesbitt and Stocker, 2008; Bell et al., 2010) may experience major challenges in the case of trematopids. Based on what we now know of trematopid development, especially of the neurocranium (Maddin et al., 2010), it is reasonable to infer that the sphenethmoid and otic capsule (absent in most specimens of Phonerpeton pricei) should ossify at some point in ontogeny in all taxa. There is no evidence suggesting that P. pricei differs greatly from other trematopids in any way that would suggest a heterochronic pathway to small adult size. Thus, if P. pricei is only represented by juveniles, it would be impossible to identify a more mature individual of P. pricei (if it is truly distinct from a known taxon) because it would likely possess an ossified sphenethmoid (among other “adult” features), which is in contrast to the taxonomic diagnosis. By the same token, characters in the genus-level diagnosis of Acheloma by Polley and Reisz (2011), such as the character related to the otic notch, and the diagnosis of Acheloma by (Schoch and Milner, 2014), such as the relative length of the external naris, are based on very large specimens. This excludes the possibility of ever properly identifying a juvenile if our hypothesis about dorsoventral growth in the temporal region is true, for example. It cannot be excluded that the marked constriction of the notch truly separates Acheloma from all other trematopids. However, the general correlation of intraspecific variation with size (i.e. less constriction with smaller individuals) and the aforementioned observation that Acheloma is the largest known trematopid suggest that a proposed ontogenetic change is plausible. The same is true of the naris, which is known to expand anteroposteriorly in the long-snouted trematopids (Dilkes, 1993). It is notable that small specimens of long-snouted trematopids (e.g., FMNH UC 1584 [Acheloma dunni], FMNH UC 1584 [Acheloma cumminsi], all specimens of Phonerpeton pricei) are characterized by a naris less than twice the length of the orbit. As a result, the relative length of the orbit compared to the naris may preclude juveniles from being properly referable.
Caution should thus be exercised in diagnosing taxa based on features that may be reasonably inferred to change during ontogeny, especially when such changes are known in related taxa or clades. We emphasize that we are not proposing a departure from the traditional practice of diagnosing taxa based on mature specimens. Rather, we are emphasizing that the inclusion of clearly ontogenetically influenced characters in diagnoses will inhibit or prevent proper identification and taxonomic referral of immature specimens. This is particularly important for the use of apomorphy-based identification, a method that eliminates the subjectivity of resemblance-based identification but that inherently relies on current and reliable taxonomic diagnoses. Questionable taxonomy (either diagnoses or referrals) can inhibit the study of ontogeny, and existing knowledge about ontogeny should subsequently inform some aspects of taxonomic practices (e.g., Steyer, 2000; Gee and Parker, 2018). The same considerations should be extended to the construction and coding of character matrices (e.g., Tykoski, 2005; Griffin and Nesbitt, 2016; Griffin, 2018 and references therein) for both temnospondyls and for Paleozoic tetrapods more broadly.
Conclusion
The various challenges that we identified in this study for the identification of OMNH 79318 underscore the need for further work to better understand temnospondyl ontogeny. Particularly in the context of this study, current interpretations of trematopid intrarelationships may be biased by disparities and gaps in the fossil record, such as limited sample size for some taxa and a wide size range among holotypes. Although OMNH 79318 is an immaculately preserved skull that contributes important information regarding early stages of trematopid ontogeny, these data have resulted in greater taxonomic uncertainty. Reappraisal of taxonomic diagnoses, referred specimens, and phylogenetic characters in order to identify potential biases and confounding factors will be essential for a more complete and thorough understanding of this clade of terrestrial temnospondyls.
Data Availability
The unprocessed 16-bit TIFF files used in this study are publicly available at Morphobank (project #3339): http://morphobank.org/permalink/?P3339 and upon reasonable request made to either the authors or to the Sam Noble Oklahoma Museum of Natural History (OMNH).
Author Contributions
RR contributed specimens and conceptualized the study. JB performed the experiment. BG analyzed the data. BG drafted the manuscript and prepared figures. BG, JB, and RR edited the manuscript and approved it for submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Richard Cifelli, Jennifer Larson, and their colleagues at the OMNH for their continued support of our work on the Richards Spur fauna. Thanks to Diane Scott for preparation of the specimen and photographs and to Andrew Milner for sharing his manuscript on the Nýrany trematopids ahead of publication. Thanks to Graciela Piñeiro, Liz Freedman Fowler, and David Marjanović and to the editor, Michel Laurin, for constructive feedback that improved this work. This research was funded by an NSERC Discovery Grant to RR, an Ontario Graduate Scholarship (OGS) grant to BG, and the University of Toronto.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/feart.2019.00038/full#supplementary-material
Abbreviations
Institutional abbreviations: AMNH FARB, American Museum of Natural History: Fossil Amphibians, Reptiles, and Birds, New York, NY, USA; BMRP, Burpee Museum of Natural History, Rockford, IL, USA; FMNH, Field Museum, Chicago, IL, USA; MCZ, Museum of Comparative Zoology, Cambridge, MA, USA; OMNH, Sam Noble Oklahoma Museum of Natural History, Norman, OK, USA.
Anatomical abbreviations: a, articular; ang, angular; c, coronoid; cp, cultriform process; d, dentary; df, dental foramen; eo, exoccpital; f, frontal; j, jugal; l, lacrimal; n, nasal; m, maxilla; mf, Meckelian foramen; maf, mandibular foramen; op, opisthotic; p, parietal; pa, prearticular; paf, pararticular foramen; pal, palatine; para, parasphenoid; pf, prefrontal; pmx, premaxilla; po, postorbital; pof, postfrontal; pos, postsplenial; pp, postparietal; pt, pterygoid; qj, quadratojugal; sa, surangular; smx, septomaxilla; sp, splenial; sq, squamosal; st, supratemporal; stp, stapes; t, tabular; v, vomer.
References
Anderson, J. S., Henrici, A. C., Sumida, S. S., Martens, T., and Berman, D. S. (2008). Georgenthalia clavinasica, a new genus and species of dissorophoid temnospondyl from the early Permian of Germany, and the relationships of the family Amphibamidae. J. Vert. Paleontol. 28, 61–75. doi: 10.1671/0272-4634(2008)28[61:GCANGA]2.0.CO;2
Bell, C. J., Gauthier, J. A., and Bever, G. S. (2010). Covert biases, circularity, and apomorphies: a critical look at the North American quaternary herpetofaunal stability hypothesis. Quat. Int. 217, 30–36. doi: 10.1016/j.quaint.2009.08.009
Berman, D. S., Henrici, A. C., Brezinski, D. K., and Kollar, A. D. (2010). A new trematopid amphibian (Temnospondyli: Dissorophoidea) from the Upper Pennsylvanian of western Pennsylvania: earliest record of terrestrial vertebrates responding to a warmer, drier climate. Ann. Carnegie Mus. 78, 289–318. doi: 10.2992/007.078.0401
Berman, D. S., Henrici, A. C., Martens, T., Sumida, S. S., and Anderson, J. S. (2011). Rotaryus gothae, a new trematopid (Temnospondyli: Dissorophoidea) from the Lower Permian of Central Germany. Ann. Carnegie Mus. 80, 49–65. doi: 10.2992/007.080.0106
Berman, D. S., Reisz, R. R., and Eberth, D. A. (1985). Ecolsonia cutlerensis, an early Permian dissorophid amphibian from the cutler formation of north-central New Mexico. N. M. Bur. Mines Miner. Resour. 191, 5–31.
Berman, D. S., Reisz, R. R., and Eberth, D. A. (1987). A new genus and species of trematopid amphibian from the late Pennsylvanian of north-central New Mexico. J. Vert. Paleontol. 7, 252–269. doi: 10.1080/02724634.1987.10011659
Bolt, J. R. (1974a). A trematopsid skull from the lower Permian, and analysis of some characters of the dissorophoid (Amphibia, Labyrinthodontia) otic notch. Fieldiana Geol. 30, 67–79. doi: 10.5962/bhl.title.3432
Bolt, J. R. (1974b). Evolution and functional interpretation of some suture patterns in Paleozoic labyrinthodont amphibians and other lower tetrapods. J. Paleontol. 48, 434–458.
Canoville, A., and Chinsamy, A. (2015). Bone microstructure of the stereospondyl Lydekkerina huxleyi reveals adaptive strategies to the harsh post Permian-extinction environment. Anat. Rec. 298, 1237–1254. doi: 10.1002/ar.23160
Dilkes, D. W. (1990). A new trematopsid amphibian (Temnospondyli: Dissorophoidea) from the lower Permian of Texas. J. Vert. Paleontol. 10, 222–243. doi: 10.1080/02724634.1990.10011809
Dilkes, D. W. (1991). Reinterpretation of a larval dissorophoid amphibian from the lower Permian of Texas. Can. J. Earth Sci. 28, 1488–1492. doi: 10.1139/e91-130
Dilkes, D. W. (1993). Biology and evolution of the nasal region in trematopid amphibians. Palaeontol. 36, 839–839.
Dilkes, D. W., and Reisz, R. (1987). Trematops milleri Williston, 1909, identified as a junior synonym of Acheloma cumminsi Cope, 1882: with a revision of the genus. Am. Mus. Novit. 2902, 1–12.
Eaton, T. H. (1973). A Pennsylvanian Dissorophid Amphibian From Kansas. Occasional Papers of the Museum of Natural History, The University of Kansas.
Fox, R. C., and Bowman, M. C. (1966). Osteology and relationships of Captorhinus aguti (Cope) (Reptilia: Captorhinomorpha). U. Kansas Paleontol. Contrib. Vertebr. 11, 1–79.
Garbe, U., Randall, T., and Hughes, C. (2011). The new neutron radiography/tomography/imaging station DINGO at OPAL. Nucl. Instrum. Methods Phys. Res. A 651, 42–46. doi: 10.1016/j.nima.2011.02.017
Garbe, U., Randall, T., Hughes, C., Davidson, G., Pangelis, S., and Kennedy, S. J. (2015). A new neutron radiography / tomography / imaging station DINGO at OPAL. Phys. Procedia 69, 27–32. doi: 10.1016/j.phpro.2015.07.003
Gee, B. M., Haridy, Y., and Reisz, R. R. (2017). Histological characterization of denticulate palatal plates in an Early Permian dissorophoid. PeerJ 5:e3727. doi: 10.7717/peerj.3727
Gee, B. M., and Parker, W. G. (2018). Morphological and histological description of small metoposaurids from Petrified Forest National Park, AZ, USA and the taxonomy of Apachesaurus. Hist. Biol. doi: 10.1080/08912963.2018.1480616. [Epub ahead of print].
Gee, B. M., and Reisz, R. R. (2018). Cranial and postcranial anatomy of Cacops morrisi, a eucacopine dissorophid from the early Permian of Oklahoma. J. Vert. Paleontol. 38:e1433186. doi: 10.1080/02724634.2018.1433186
Gregory, J. T., Peabody, F. E., and Price, L. I. (1956). Revision of the Gymnarthridae, American Permian microsaurs. Peabody Mus. Nat. Hist. Yale U 10, 1–77.
Griffin, C. T. (2018). Developmental patterns and variation among early theropods. J. Anat. 232, 604–640. doi: 10.1111/joa.12775
Griffin, C. T., and Nesbitt, S. J. (2016). The femoral ontogeny and long bone histology of the middle Triassic (? late Anisian) dinosauriform Asilisaurus kongwe and implications for the growth of early dinosaurs. J. Vert. Paleontol. 36:e1111224. doi: 10.1080/02724634.2016.1111224
Halliday, T. R., and Verrell, P. A. (1988). Body size and age in amphibians and reptiles. J. Herpetol. 22, 253–265. doi: 10.2307/1564148
Holly, C. (2004). Grouped ZProjector (for ImageJ). Available online at: https://imagej.nih.gov/ij/plugins/group.html (Waterville, ME: Holly Mountain Software) (Accessed Jan 21, 2017).
Lamsdell, J. C., and Selden, P. A. (2013). Babes in the wood–a unique window into sea scorpion ontogeny. BMC Evol. Biol. 13:98. doi: 10.1186/1471-2148-13-98
MacDougall, M. J., Tabor, N. J., Woodhead, J., Daoust, A. R., and Reisz, R. R. (2017). The unique preservational environment of the early Permian (Cisuralian) fossiliferous cave deposits of the Richards Spur locality, Oklahoma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 475, 1–11. doi: 10.1016/j.palaeo.2017.02.019
Maddin, H. C., Reisz, R. R., and Anderson, J. S. (2010). Evolutionary development of the neurocranium in Dissorophoidea (Tetrapoda: Temnospondyli), an integrative approach. Evol. Dev. 12, 393–403. doi: 10.1111/j.1525-142X.2010.00426.x
Mays, C., Bevitt, J., and Stilwell, J. (2017). Pushing the limits of neutron tomography in palaeontology: three-dimensional modelling of in situ resin within fossil plants. Palaeontol. Electron. 20:3.57A. doi: 10.26879/808
Milner, A. R. (2018). Two primitive trematopid amphibians (Temnospondyli: Dissorophoidea) from the upper Carboniferous of the Czech Republic. Earth Environ. Sci. Trans. R. Soc. Edinb. doi: 10.1017/S1755691018000725. [Epub ahead of print].
Nelson, J. W., Hook, R., and Chaney, D. (2013). Lithostratigraphy of the lower Permian (Leonardian) clear fork formation of north-central Texas. N. M. Mus. Nat. Hist. Sci. Bull. 60, 286–311.
Nesbitt, S. J., and Stocker, M. R. (2008). The vertebrate assemblage of the late triassic canjilon quarry (northern New Mexico, USA), and the importance of apomorphy-based assemblage comparisons. J. Vert. Paleontol. 28, 1063–1072. doi: 10.1671/0272-4634-28.4.1063
Olson, E. C. (1967). Early Permian vertebrates of Oklahoma. Oklahoma Geol. Survey Circular 74, 1–111.
Olson, E. C. (1985). A larval specimen of a trematopsid (Amphibia: Temnospondyli). J. Paleontol. 59, 1173–1180. doi: 10.2307/1305010
Olson, E. C. (1991). An eryopid (Amphibia: Labyrinthodontia) from the Fort Sill fissures, lower Permian, Oklahoma. J. Vert. Paleontol. 11, 130–132. doi: 10.1080/02724634.1991.10011379
Polley, B. P., and Reisz, R. R. (2011). A new lower Permian trematopid (Temnospondyli: Dissorophoidea) from Richards Spur, Oklahoma. Zool. J. Linn. Soc. 161, 789–815. doi: 10.1111/j.1096-3642.2010.00668.x
Reisz, R. R., Schoch, R. R., and Anderson, J. S. (2009). The armoured dissorophid Cacops from the early Permian of Oklahoma and the exploitation of the terrestrial realm by amphibians. Naturwissenschaften 96:789. doi: 10.1007/s00114-009-0533-x
Sanchez, S., and Schoch, R. R. (2013). Bone histology reveals a high environmental and metabolic plasticity as a successful evolutionary strategy in a long-lived homeostatic Triassic temnospondyl. Evol. Bio. 40, 627–647. doi: 10.1007/s11692-013-9238-3
Sansom, R. S., Gabbott, S. E., and Purnell, M. A. (2010). Non-random decay of chordate characters causes bias in fossil interpretation. Nature 463, 797–800. doi: 10.1038/nature08745
Schoch, R. R. (2012). Character distribution and phylogeny of the dissorophid temnospondyls. Foss. Rec. 15, 121–137. doi: 10.1002/mmng.201200010
Schoch, R. R. (2014). Life cycles, plasticity and palaeoecology in temnospondyl amphibians. Palaeontology 57, 517–529. doi: 10.1111/pala.12100
Schoch, R. R. (2018). The putative lissamphibian stem-group: phylogeny and evolution of the dissorophoid temnospondyls. J. Paleontol. 93, 1–20. doi: 10.1017/jpa.2018.67
Schoch, R. R., and Milner, A. R. (2014). Handbook of Paleoherpetology: Temnospondyli I (Part 3A2). München: Verlag Dr. Friedrich Pfeil.
Steyer, J. S. (2000). Ontogeny and phylogeny in temnospondyls: a new method of analysis. Zool. J. Linn. Soc. Lond. 130, 449–467. doi: 10.1111/j.1096-3642.2000.tb01637.x
Sullivan, C., and Reisz, R. R. (1999). First record of Seymouria (Vertebrata: Seymouriamorpha) from early Permian fissure fills at Richards Spur, Oklahoma. Can. J. Earth Sci. 36, 1257–1266. doi: 10.1139/e99-035
Sumida, S. S., Berman, D. S., and Martens, T. (1998). A new trematopid amphibian from the Lower Permian of Central Germany. Palaeontology 4, 605–630.
Tsai, C. H., and Fordyce, R. E. (2014). Juvenile morphology in baleen whale phylogeny. Naturwissenschaften 101, 765–759. doi: 10.1007/s00114-014-1216-9
Tykoski, R. S. (2005). Anatomy, Ontogeny, and Phylogeny of Coelophysoid Theropods. dissertation. Austin, TX: University of Texas Austin.
Wiens, J. J., Bonett, R. M., and Chippindale, P. T. (2005). Ontogeny discombobulates phylogeny: paedomorphosis and higher-level salamander relationships. Sys. Biol. 54, 91–110. doi: 10.1080/10635150590906037
Woodhead, J., Reisz, R., Fox, D., Drysdale, R., Hellstrom, J., Maas, R., et al. (2010). Speleothem climate records from deep time? exploring the potential with an example from the Permian. Geology 38, 455–458. doi: 10.1130/G30354.1
Keywords: trematopid, Acheloma, Permian, dissorophoid, temnospondyl, Richards Spur
Citation: Gee BM, Bevitt JJ and Reisz RR (2019) A Juvenile Specimen of the Trematopid Acheloma From Richards Spur, Oklahoma and Challenges of Trematopid Ontogeny. Front. Earth Sci. 7:38. doi: 10.3389/feart.2019.00038
Received: 17 December 2018; Accepted: 15 February 2019;
Published: 12 March 2019.
Edited by:
Michel Laurin, UMR7207 Centre de Recherche sur la Paléobiodiversité et les Paléoenvironnements (CR2P), FranceReviewed by:
Graciela Helena Piñeiro, Universidad de la República, UruguayElizabeth Anne Freedman Fowler, Dickinson State University, United States
David Marjanović, Museum für Naturkunde, Germany
Copyright © 2019 Gee, Bevitt and Reisz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bryan M. Gee, YnJ5YW4uZ2VlQG1haWwudXRvcm9udG8uY2E=
 Bryan M. Gee
Bryan M. Gee Joseph J. Bevitt
Joseph J. Bevitt Robert R. Reisz
Robert R. Reisz