- 1Department of Materials and Environment, Federal Institute for Materials Research and Testing, Berlin, Germany
- 2Department of Earth Sciences, Freie Universität Berlin, Berlin, Germany
Sub-aerial biofilms (SAB) are ubiquitous, self-sufficient microbial ecosystems found on mineral surfaces at all altitudes and latitudes. SABs, which are the principal causes of weathering on exposed terrestrial surfaces, are characterized by patchy growth dominated by associations of algae, cyanobacteria, fungi and heterotrophic bacteria. A recently developed in vitro system to study colonization of rocks exposed to air included two key SAB participants - the rock-inhabiting ascomycete Knufia petricola (CBS 123872) and the phototrophic cyanobacterium Nostoc punctiforme ATCC29133. Both partners are genetically tractable and we used them here to study weathering of granite, K-feldspar and plagioclase. Small fragments of the various rocks or minerals (1–6 mm) were packed into flow-through columns and incubated with 0.1% glucose and 10 μM thiamine-hydrochloride (90 μL min−1) to compare weathering with and without biofilms. Dissolution of the minerals was followed by: (i) analysing the degradation products in the effluent from the columns via Inductively Coupled Plasma Spectroscopy and (ii) by studying polished sections of the incubated mineral fragments/grains using scanning electron microscopy, transmission electron microscopy and energy dispersive X-ray analyses. K. petricola/N. punctiforme stimulated release of Ca, Na, Mg and Mn. Analyses of the polished sections confirmed depletion of Ca, Na and K near the surface of the fragments. The abrupt decrease in Ca concentration observed in peripheral areas of plagioclase fragments favored a dissolution-reprecipitation mechanism. Percolation columns in combination with a model biofilm can thus be used to study weathering in closed systems. Columns can easily be filled with different minerals and biofilms, the effluent as well as grains can be collected after long-term exposure under axenic conditions and easily analyzed.
Introduction
As life began to spread onto land at least 1.2 Ga ago, the first settlers were oxygenic cyanobacteria along with various organotrophic microorganisms. Present day sub-aerial (bare) rock surfaces are inhabited by similar microbial communities. Sub-aerial biofilms (SAB) are ubiquitous, self-sufficient, miniature microbial ecosystems that are found on mineral surfaces at all altitudes and latitudes. Patchy growth dominated by associations of algae, cyanobacteria, fungi and heterotrophic bacteria are amongst the principal characteristics of SABs. In addition to being one of the principal causes of weathering, SABs also accelerate the decay of cultural heritages (Dornieden et al., 2000a,b; Warscheid and Braams, 2000; de los Ríos and Ascaso, 2005). Microbial colonization of architectural monuments and works of art also causes discoloring as the SAB inhabitants often produce highly colored pigments including carotenoids, chlorophylls and melanins (Gorbushina et al., 1993; Diakumaku et al., 1995; Urzì and Realini, 1998; Gorbushina and Broughton, 2009). Furthermore some organisms induce acidolytic and oxido-reductive corrosion processes, excrete geochemically active substances as siderophores and low molecular weight organic acids, form detrimental crusts and therefore destroy solid materials (Warscheid and Braams, 2000). Limiting or preventing biodeterioration is therefore critical to conserving objects of cultural value.
Various microbes enhance the release of different elements from rocks (Davis et al., 2007; Abdulla, 2009; Bonneville et al., 2011; Brunner et al., 2011; Lapanje et al., 2012; Olsson-Francis et al., 2012). Excreted organic acids and respiration-derived CO2 are often active in these processes (Silverman and Munoz, 1970; Delatorre et al., 1993; Gómez-Alarcón et al., 1994; Machill et al., 1997; Landeweert et al., 2001; Abdulla, 2009; Weber et al., 2011), although fungal hyphae can directly penetrate and widen fissures in rocks (Sterflinger, 2000; Gorbushina et al., 2003; Chertov et al., 2004). The extreme microbial diversity of soil organisms (Jongmans et al., 1997; Landeweert et al., 2001; Schöll et al., 2008; Abdulla, 2009; Bonneville et al., 2009; Rosling et al., 2009; Taylor et al., 2009) and accompanying macroscopic vegetation results in high rates of weathering. As weathering of rocks is the first step in soil formation (Chadwick et al., 1990), surface weathering is an essential component of biogeomorphological processes. Nevertheless, the morphologically simpler microbial ecosystems that prevail on bare rocks also have multiple effects on element cycles (Gorbushina, 2007). The relative simplicity of SAB communities as compared to microbes in soils means that they are ideal in the study of biotic impact of microbes on the dissolution of minerals. Due to the extreme conditions on bare rock surfaces, only certain stress tolerant microorganisms that can withstand extreme dryness, wide temperature fluctuations, intense solar irradiation and low nutrient availability are able to survive. Often phototrophic cyanobacteria and oligotrophic microcolonial fungi (MCF) initially dominate this ecological niche (Staley et al., 1982; Sterflinger, 1998; Tamaru et al., 2005; Gorbushina, 2007; Knowles and Castenholz, 2008; Ozturk and Aslim, 2010). Both types of organisms are extremely tolerant of many kinds of stress conditions and are therefore involved in biodeterioration of monuments (Gorbushina et al., 1993, 2003; Diakumaku et al., 1995; Ortega-Calvo et al., 1995; Büdel et al., 2004; Olsson-Francis et al., 2012).
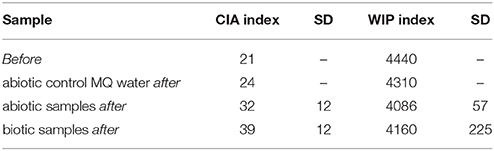
Various systems have been proposed to study the dissolution of minerals under different environmental conditions but flow-through systems result in high aeration and dissolution rates, which are important for cultivation of aerobic microorganisms and ideal for modeling SAB growth on rocks and minerals. Flow-through percolation columns allow for long-term microbiologically and geochemically stable experiments. Furthermore, quantification of dissolution can be performed on the liquid phase by measuring released elements via spectrometric methods like ICP-MS and ICP-OES (inductively coupled plasma mass spectrometry/optical emission spectrometry) (Olesik, 1991). Changes in the mineral phase can be analyzed via spectroscopic methods including EDX (energy dispersive X-ray spectroscopy), SEM (scanning electron microscope) or TEM (transmission electron microscope) (Eggert, 2005). Additionally, the solid mineral phase can be chemically digested and the contents measured by ICP-MS/OES. Degradation of solid phases is often described by “weathering indices” which are the ratios of mobile and immobile elements (Price and Velbel, 2003; Takahashi and Shimaoka, 2012). To be useful, weathering indices must take into account a certain spectrum of mobile elements in the particular mineral and be applicable to as broader range of different rock-types as possible. Two widely accepted indices are the Weathering Index of Parker (WIP) and the Chemical Index of Alteration (CIA), calculated according to Equations (1) and (2). WIP values decrease and CIA values increase with rising degrees of weathering.
Equally, the types of rocks used in model weathering studies should be of global relevance and contain components that weather rapidly. Granite, being among the most abundant rock types within the Earth crust and crucially important in the construction of buildings and monuments was an obvious choice. Mica and feldspar are typical components of granite, which have relatively high dissolution rates and are sensitive to microbial degradation (Lasaga et al., 1994; Bonneville et al., 2011).
Finally, reproducible studies of microbial effects on the weathering of rocks are only possible if the number of variables is limited (i.e., simplified laboratory models are a prerequisite) and under well-controlled, laboratory conditions. In a previous study, a laboratory rock-inhabiting biofilm consisting of the heterotrophic MCF Knufia petricola and the nitrogen-fixing cyanobacterium Nostoc punctiforme was established (Gorbushina and Broughton, 2009). Here we used this biofilm to study the biological impact on weathering of granite and related minerals in a new setting of a geomicrobiologically-modified percolation column.
Materials and Methods
Model Biofilm—Starting Cultures and Inoculum Preparation
Nostoc punctiforme ATCC29133 was kindly supplied by J.M. Meeks (University of California, Davis, CA, USA) (Ekman et al., 2013). Cultures used for the experiments were grown in BG11 medium (Stanier et al., 1971) at pH 7.5 25°C and 90 μM photons of photosynthetically active light m2 s−1 for 24 h d−1 and shaking (160 rpm). Knufia petricola (CBS 123872) was isolated from a weathered marble monument in Athens (Greece) (Nai et al., 2013). Cultures used for the experiments were grown in 2% malt extract broth [MEB: 2% (w/v) malt extract; 0.1% (w/v) casein-digested peptone; 2% (w/v) D-(+)-glucose] under shaking (100 rpm) at 25°C. Both organisms were transferred weekly by diluting 1:100 into fresh media. Seven to 14 d old cultures were used for the preparation of inocula for rock/mineral dissolution experiments.
To prepare inocula, biomass of both organisms was dispersed in a ball-mill, centrifuged (6603 RCF for 15 min), the pellets were washed three times and finally re-suspended in 5 mM glucose and 10 μM thiamine-hydrochloride for the rock/mineral dissolution experiments. Dispersion of clumped cells was performed in stainless steel beakers containing eight stainless steel beads (ca. 5 mm diameter) for 10 min at 30 Hz (for K. petricola) or with four glass beads for 5 min at 25 Hz (for N. punctiforme) in a mixer mill (MM400, Retsch GmbH, Haan, Germany).
Experimental Setting
Percolation experiments were run for 180 d in columns modified from those used in the investigation of the environmental compatibility of materials (DIN 19528, 2009-01). The glass columns were filled with 750 g of rock/mineral (mineralogical details in 2.3) with a grain size between 1 and 6 mm, homogenized using a gyro wheel mixer and subsequently subdivided into representative portions using a sample splitter. Rock/mineral-filled columns and solutions were sterilized by autoclaving for 15 min at 121°C. Inocula were pipetted into the columns under aseptic conditions. Closed, circulation systems were imposed and a single glass bottle served both as the reservoir for the nutrient solution and as the eluate collector for each column (Figure 1). One L glucose/thiamine solution was supplied from the top by a peristaltic pump (ICP12, IDEX, Oak Harbor, USA) in a parallel mode. The flow-rate was 5.4 mL h−1 which meant that it took 185 h for 1 L nutrient solution to flow through a column. In experiments involving granite, non-inoculated columns (“abiotic experiments”) and columns initially inoculated with 105 cells of both organisms per g rock (“biotic experiments”) were run as triplicates. An additional column was used as abiotic control using MilliQ (MQ) water in place of nutrient solution. Plagioclase and K-feldspar dissolution experiments were performed in the same way using single biotic and abiotic samples. Sterile filters were placed at the inlet and outlet positions of the columns to ensure that microorganisms in biotic experiments remained within the columns.

Figure 1. Flow-through experiments in percolation columns. A glucose/thiamine nutrient solution was cycled through the column via silicon tubing attached to a peristaltic pump (1) from glass bottles (2) to the top (3) of the columns (4). The columns were filled with chips of granite or K-feldspar or plagioclase. The eluate was diverted from the bottom of the columns into glass bottles (2). Sterile filters were placed at the input (3) and output (5) positions of the columns to ensure that the microorganisms in biotic experiments remained within the columns.
All rock/mineral dissolution experiments were incubated in Percival climate chambers (Percival Scientific Inc., 505 Research Drive, Perry, IA 50220, USA) at 25°C and 90 μM photons of photosynthetically active light m2 s−1for 24 h d−1.
Rocks/Minerals
Samples used in the weathering experiments were granite blocks (Bauhaus AG, Zug, Switzerland). A chemical and mineralogical characterization of the rock samples was done before use. The overall chemical composition of the granite is given in Table 1 illustrating more than 2-fold higher amounts of K as compared to Na.
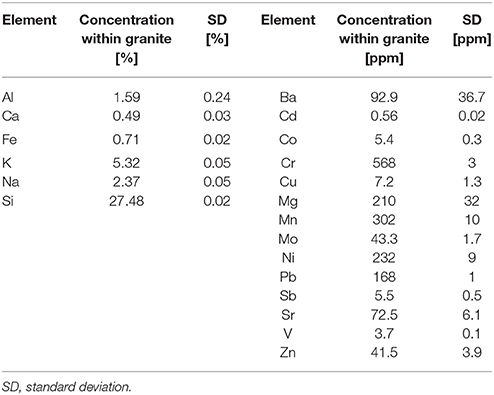
Table 1. Elemental composition of the granite based on ICP-OES or ICP-MS measurements after digestion of the rock material (n = 2).
Polished granite sections contained a plagioclase with an elemental composition toward the albite end member (NaAlSi3O8) (Table 2), a K-rich feldspar (KAlSi3O8), quartz (SiO2) and biotite (K(Mg,Fe)3AlSi3O10(F,OH)2) as mica group. Sporadically, magnetite (Fe3O4), titanite (CaTiSiO5), ilmenite (FeTiO3), zircon (ZrSiO4), calcite (CaCO3) and apatite (Ca10(PO4)6(OH,F,Cl)2) were also found. The K-rich feldspar was microporous with an elemental composition toward microcline (KAlSi3O8) but containing low amounts of Na. Chloritization, the transformation of biotite into chlorite ((Mg,Fe)3(Si,Al)4O10(OH)2(Mg,Fe)3(OH)6), was observed in the peripheral zones of the grains. Plagioclase showed a central sericite formation (KAl2(OH,F)2AlSi3O10) through K accumulation (sericitization).
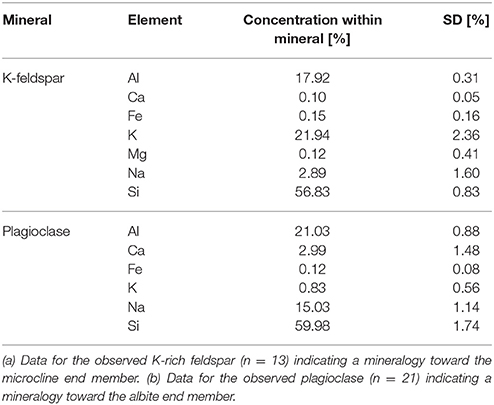
Table 2. Elemental composition of feldspars within the granite used before exposition to the experimental conditions, based on EDX measurements.
As the presumably best weatherable minerals within the granite were plagioclase and the K-rich feldspar, these minerals (obtained from Rheinisches Mineralien-Kontor GmbH, Bonn, Germany) were used in additional experiments within the percolation columns. The plagioclase used contained small inclusions with a more microcline-like chemistry, the K-feldspar used contained albite-like inclusions with an estimated fraction of 20–30%.
The original rock material was crushed to small grains using a jaw crusher (BB 300, Retsch GmbH, Haan, Germany), rinsed with MQ water to remove fine dust, dried and sieved to obtain grain sizes between 1 and 6 mm. To ensure a homogeneous distribution of the varying grain sizes within different samples the mineral grains were homogenized and sub-divided by a rotary sample divider (PT 100, Retsch GmbH) and collected in glass vessels. Then, the homogenized materials were transferred to the incubation columns.
Biological Analyses
Cell numbers were quantified using both qPCR (quantitative polymerase chain reaction) with specific primer pairs and the corresponding PCR protocols (Sherwood and Presting, 2007; Bates and Garcia-Pichel, 2009) as well as by viable counts for K. petricola and by measuring chlorophyll a (Chl a) for N. punctiforme [Meeks and Cohen (Meeks and Castenholz, 1971; Cohen et al., 1994)]. Unknown amounts of DNA were estimated by calibrating DNA extracts with known numbers of each organism.
Chemical Analysis
Analyses of the mineralogical and elemental composition of polished sections of rock/mineral grains were performed by microprobe recording and EDX measurements using a JXA-8230 SuperProbe Electron Probe Microanalyzer (JEOL Ltd, Shin-Suzuharu BLDG. 3F 2-8-3 Akebono-cho, Tachikawa, Tokyo 190-0012, Japan) (n between 13 and 21) on clearly differentiated mineral fractions of the polished sections. Polished mineral sections were compared by scanning electron microscopy-energy-dispersive X-ray spectroscopy SEM-EDX (XL30, 5350 NE Dawson Creek Drive, Hillsboro, Oregon 97124 USA) and transmission electron microscopy- energy-dispersive X-ray (TEM-EDX)(CM12, Philips, Amsterdam, Netherlands) in central and peripheral positions of the polished sections before and after imposition of the treatments. Stimulation of the emission of characteristic X-rays was performed using a 5 μm thick electron beam at 15 kV for 10 s, while TEM-EDX stimulation was at 120 kV for 300 s. With some samples, microprobe-EDX measurements were additionally performed (15 kV, five times for 2 s at the same position with subsequent averaging). Analyses of the total chemical composition were done by ICP-OES (ICAP 7400 Duo, Thermo Fisher Scientific Inc., Waltham, MA 02451, USA) or depending on the concentration by ICP-MS (ICAP-Q, Thermo Fisher Scientific) after acid digestion of powdered sample materials both before and after dissolution experiments. To do this, 0.2 g of ground rock material was digested with an acid mixture (4 mL 65% HNO3; 2. mL 32% HCl and 1 mL 48% HF) in a microwave oven (1 h, maximum temperature 210°C). To minimize the danger of HF and complex formation of barely soluble fluorides, 10 mL cold saturated boric acid was added. Weathering indices WIP and CIA were calculated according to Fiantis et al. (2010) using the data obtained by ICP-OES or ICP-MS analysis of solid mineral phases. Before and after pH measurements of the liquid phase were taken with a pH meter. Elemental concentrations in the eluted materials were determined after 45 and 180 d incubation by ICP-OES or ICP-MS measurement after acidification of the eluate with concentrated HNO3. External calibration with matrix matching was used for all eluted and digested samples.
Results
Experiments with Granite
Abiotic experiments remained aseptic while the number of cells of both organisms increased during 180 d experimental period in the inoculated columns (Figure 2). Biofilm growth was distributed over mineral fragments in patches, both partners were present in the layers near to the glass as well as in the deeper regions of columns. Permanent circulation/percolation of the medium has supported even distribution of cells and especially of their metabolic products in the percolation columns. qPCR-based estimations and Chla measurements indicated similar numbers of N. punctiforme, but with K. petricola the DNA-based methods gave clearly higher numbers than viable counts (Figure 2), perhaps indicating lack of viability.
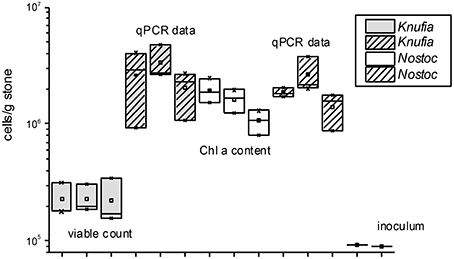
Figure 2. Estimated numbers (n = 3) of K. petricola and N. punctiforme based on qPCR data (both organisms), viable counts (K. petricola) and Chla contents (N. punctiforme). Box plots show triplicate values as cross lines and mean values as squares, the inoculum cell number was the same for all biotic samples and is shown as a cross line.
It is clear that even with short incubation times (45 d), seven elements were released from the granite. In decreasing quantity these were Ca > Na > K > Mg > Mn > Zn > Ba. Longer incubation times resulted in greater accumulation of the elements in the eluate (Figure 3). Perhaps surprisingly though only Mn, Mg and Ca concentrations were higher for the biotic experiments than the abiotic ones (Figures 3A,B). In other words Ba, K, and Zn were released from the minerals by MQ water or sterile medium alone (Figures 3B,C).
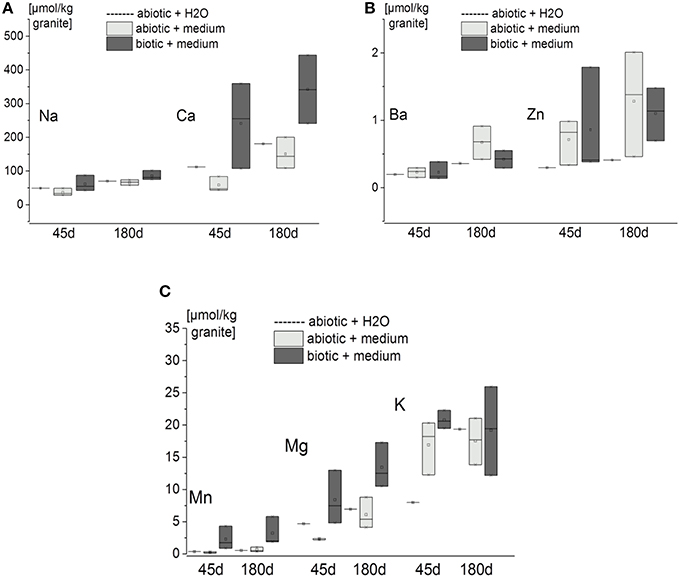
Figure 3. Quantity of elements within the eluates from the flow-through columns containing granite [expressed as amount of element per kg of granite after 45 and 180 d incubation (n = 3 for biotic and abiotic samples with nutrient solution, n = 1 for abiotic controls with MQ water)]. Measurements were done by ICP-OES or ICP-MS. Box-plots show the triplicate values as cross lines and mean values as squares (for MQ water controls only single cross lines are shown). (A) Released amount of Na and Ca. (B) released amount of Ba and Zn. (C) released amount of Mn, Mg, and K.
SEM-EDX analyses of polished sections showed Ca depletion near the granite surface (Figure 4). CIA weathering indices were generally higher after the experiments as well in biotic as opposed to abiotic conditions (Table 3). Unfortunately, the variance in weathering indices was too high to permit definite conclusions. Similarly, the pH of the eluates ranged from 5.6 initially to 5.5 after 180 d and differences between biotic and abiotic conditions were not observed.
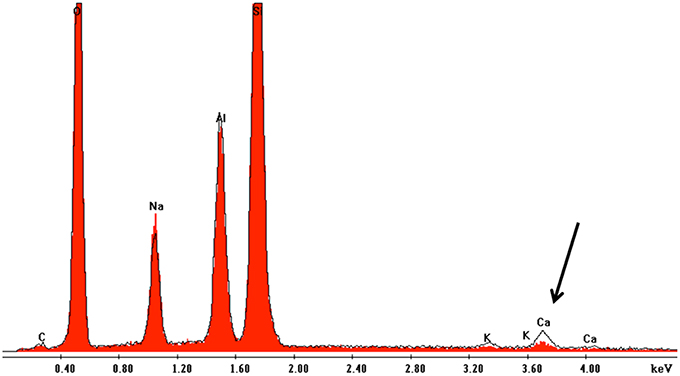
Figure 4. Overlay of EDX spectra near the surface and in the center of granite grains after 180 d incubation in flow-through columns containing K. petricola and N. punctiforme. The spectrum marked in red shows the situation near the mineral surface while the black edging shows the spectrum for the central part of mineral. The black arrow indicates Ca depletion near to the mineral surface.
Experiments with Plagioclase and K-Feldspar
Again, the abiotic treatments remained aseptic while both the cyanobacterium and the fungus grew in the inoculated columns. Ca, K, and Na were released from both minerals over the 180 d experimental period. Ca and Na were especially rapidly released from plagioclase (for Ca ≈ 2 orders of magnitude higher than in K-feldspar) although K release was slightly higher in K-feldspar (Figure 5). Ca release was enhanced in the biotic samples containing plagioclase. With K-feldspar, the release also included Na. Ca depletion was confirmed in polished sections of samples via SEM-EDX analyses. A darker area near the surface of the mineral grains within the polished sections occurred down to ≥ 5 μm (Figure 6A). A line-scan for Ca concentrations starting from the darker to the lighter area within the mineral grains revealed an abrupt rise in Ca concentration at the border between the two areas (Figure 6B). Such an abrupt change in concentration was not observed in line-scans of Na and only slightly for Al. These findings were supported by microprobe analyses showing almost complete depletion of Ca and a slight depletion of Al in the darker area with Na being constant compared to the lighter area. Depletion of Na within plagioclase and K within K-feldspar was shown via TEM-EDX analyses to occur in the direct vicinity of the surface at ≦ 2 μm depth (Figure 7).
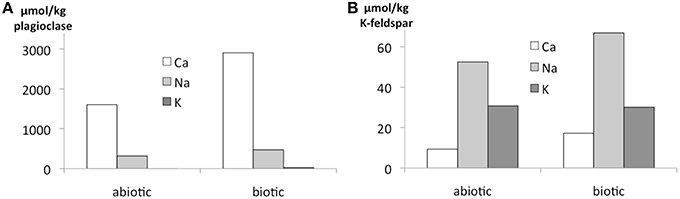
Figure 5. Release of elements in the flow-through experiments expressed as amount per kg of biofilm-incubated plagioclase (A) and K-feldspar (B) after 180 d incubation. Measurements were done by ICP-OES or ICP-MS for one biotic and one abiotic sample with nutrient solution.
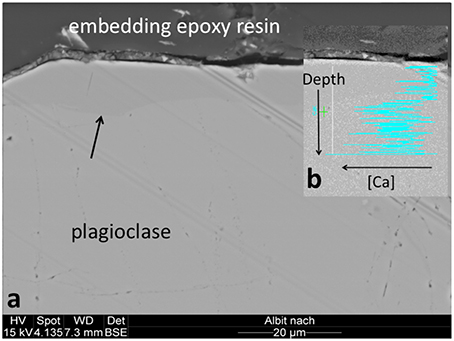
Figure 6. Ca depletion near the surface of plagioclase grains. (A) Microprobe recording of a polished plagioclase grain section with a darker area near the surface is indicated by a black arrow. (B) Line-scan of the Ca concentration starting from the surface and crossing the border to the lighter area positioned more centrally (Ca concentration increases from right to left).
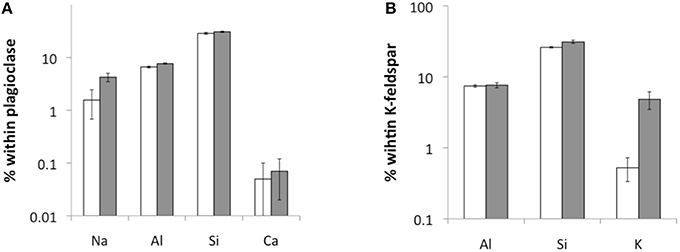
Figure 7. TEM-EDX measurements of element concentrations within plagioclase (A) and K-feldspar (B) near the surface (white) and 1–2 μm below the surface (gray) (n = 10).
Discussion
Although the absolute amount of K within the granite was 2-fold higher than Na and 10-fold higher than Ca (Table 1), release of Ca and Na exceeded the one of K explicitly during incubation in the flow-through columns with Ca being more released than Na (Figure 3). Considering the mainly found minerals within the granite used and known mineral dissolution rates from other studies (Lasaga et al., 1994), plagioclase is expected to be dissolved first, followed by other feldspars, micas and finally quartz (White, 2008). This is in agreement with the found amounts of elements in eluates, as Ca and Na are most probably released from the plagioclase obeying faster dissolution kinetics and resulting in higher final concentrations compared to presumably K-feldspar- and biotite-derived K, Mg and Mn. This is additionally supported by experiments using only plagioclase or K-feldspar: for plagioclase Ca concentrations within eluates were 10-fold and Na concentrations 5-fold higher than the respective values for granite, indicating that within the latter most of the released Ca and Na derived from its plagioclase. In granite other minerals with much lower dissolution rates like quartz occupy a relevant part of the surface area and reduce therefore the overall release of Ca and Na. If only plagioclase is provided as weathering substrate, the relative area with faster dissolving minerals is increased resulting in comparatively higher Ca concentrations in the eluate. For K-feldspar experiments K concentrations in the eluates were within the same range as for granite experiments despite the fact, that only K-feldspar was offered as weathering substrate, indicating that a relevant part of the released K in the granite experiments derived from other minerals than K-feldspar. Biotite is the most likely alternative K-containing mineral within granite that may have contributed to the amount of released K. The concomitant release of Mg and Mn (Figure 3) supports this hypothesis.
Within plagioclases the dissolution rates increase with increasing anorthite fractions implying a faster release of Ca (Huang and Kiang, 1972; Oxburgh et al., 1994). Faster release of Ca might be reflected also in larger depletion distances from the surface to the center of mineral grains. This would explain why Ca is depleted up to more than 5 μm into the mineral grains, whereas Na and K depletion are detected only in less than 2 μm distance from the mineral surface in examined polished mineral sections.
In granite the release of Ca, Mg, and Mn was biotically enhanced (Figures 3A,C), for K there was no difference compared to abiotic samples with regard to the concentrations in the eluate (Figure 3C). Ca2+, Na+, K+, and Mg2+ are the most abundant metal ions within biology (Cowan, 1991) and can be both absorbed in the biofilm/biofilm matrix as well as incorporated into the cells. Mg dependent enzymes participate in general metabolic and essential nucleotide processing reactions in all organisms (Cowan, 2002). Ca is of crucial importance for calmodulin and calcineurin in eukaryotes (Kraus and Heitman, 2003) as well as for various processes like chemotaxis, DNA replication, phospholipid synthesis and protein phosphorylation in prokaryotes (Norris et al., 1991). Na and K are important electrolytes maintaining electrochemical gradients within cells and contributing to charge neutralization (Black et al., 1994). As these elements are essential for various cell processes, they are expected to occur at least in low amounts within all organisms and are found there also (Li, 1984). So, dissolution of rocks like granite can not only be enhanced in the presence of microorganisms, but the concentration of released elements in solution can be changed also by its accumulation in organisms. If released K would be accumulated within microbial cells in significant amounts, its detection within the eluate would be decreased and a biotic enhancement of K release would be masked. Such an effect could have been caused in the presented experiments by K accumulation of 5–10 μm. Data for measured cell numbers lead to an estimated weight of 0.5 g for the final biomass. K accumulations of 5–10 μm would correspond to 0.1–0.2 mg K per g cells. K accumulations of this range and even higher have been shown for mycorrhizal fungi (Wallander et al., 2002, 2003). The estimated weight of the inoculum is 15 mg. The content of the elements Ca, Na, Mg, Mn, and K within microorganisms related to K. petricola and N. punctiforme (Gao, 1998; Tashpulatov et al., 2000) makes a relevant contribution of the starting biomass to the final element concentrations within the eluate implausible. The measured enhancement of mineral dissolution in biotic samples is therefore assumed to be caused by growing microorganisms. As no direct physical attacks by fungal hyphae or cyanobacterial filaments were observable via microscopical methods (SEM, TEM, light microscopy) within mineral samples, an indirect process resulting from microbial metabolism is suggested. For example, production of organic acids or respiration-derived CO2 would lower the pH, which is a possible factor in biodeterioration of minerals (Delatorre et al., 1993; Gómez-Alarcón et al., 1994; Machill et al., 1997; Weber et al., 2011). The fact that pH decreased only slightly and in the same way in all experiments despite varying extent of dissolution (Figure 3) is not contradictory with this explanation as pH values in the direct micro-environment of cells between a biofilm and its substrate can differ significantly from those in the macro-environment (Bonneville et al., 2011). The lower cell numbers for K. petricola in viable counts as compared to qPCR data (Figure 2) could imply that a significant part of the fungal cells died during the experiments or was finally in an uncultivable state. This would not exclude a biotic influence of these cells, as a release of acidifying metabolites would be still possible and could have occurred also in an earlier stage during the experiment. The mere presence of various organic ligands is known to increase element release from granites (Neaman et al., 2006; Hausrath et al., 2009), and the growth of the model biofilm that has steadily increased its biomass during the experiment is unequivocally connected to the production of various accompanying metabolites.
The abrupt decrease of Ca concentration in peripheral mineral areas observed in polished plagioclase sections favors an interface-coupled dissolution-reprecipitation mechanism on the interface between mineral and environment as described recently in silicates (Putnis, 2009; Hellmann et al., 2012; Ruiz-Agudo et al., 2014). A leaching mechanism would have resulted in the formation of a Ca concentration gradient, which was not observed. This implies dissolution of Ca-containing plagioclase and reprecipitation of a plagioclase with much less Ca and a composition more toward the albite end member. This process, known as albitisation, occurs widespread in natural rock types and is especially related to hydrothermal alteration (Hövelmann et al., 2010) as well as low-temperature water-rock exchange during burial diagenesis of feldspar-bearing sandstones (Perez and Boles, 2005).
The calculated WIP and CIA weathering indices indicate increasing weathering after 180 d incubation within the flow-through columns and for percolation with the used nutrient solution as compared to MQ water. A significant difference between biotic and abiotic samples was not observed, probably due to the short incubation time.
Concluding Remarks
The bipartite model laboratory biofilm including Knufia petricola A95 and N. punctiforme (Gorbushina and Broughton, 2009; Seiffert et al., 2014) is capable of growing symbiotically and interacting with mineral substrates. The present work demonstrates that dissolution of granite, plagioclase and K-feldspar is enhanced in the presence of model biofilms under flow-through conditions. This effect is most likely indirectly caused by released metabolites of the microorganisms. Interestingly, the major nutrient Ca is preferentially released from Na-rich feldspar grains. The dissolution mechanisms could be explained by dissolution-reprecipitation. The mechanism of the biodeteriorating process still has to be elucidated—and a combination of a genetically amenable model fungal/phototroph biofilm (Gorbushina and Broughton, 2009; Seiffert et al., 2014) grown in percolation columns offers a new instrument of biologically as well as geochemically precise quantification-based studies. A statistically-relevant number of grains that are exposed to biofilm-induced weathering allows for a quantifying geochemical analysis of the process, while microscopic/analytic analysis of single grains can be used to demonstrate local activity of single cells and their excreted matrices. Acidification in the microenvironment between biofilm and mineral surface is a hypothesis that can be tested via high-spatial resolution pH measurements. Genetic amenability of both model biofilm partners (Noack-Schönmann et al., 2014) will be used to study different combinations of genes possibly involved in mineral weathering.
Author Contributions
FS performed all the experiments, actively designed the study, and intensively worked on the manuscript. NB and UK provided the know-how on the percolation columns and conducted and interpreted chemical analysis by ICP-OES and ICP-MS at the BAM. RM supported the choice of mineral substrates, performed mineralogical characterization of the rock samples and interpreted the data. AAG conceived the study, participated in its design, and helped to interpret the data and write the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the Federal Institute for Materials Research and Testing. We thank Ines Feldmann for her help with SEM-EDX measurements, Peter Schubert-Bischoff for TEM-EDX measurements and polished sections preparation and Katja Nordhauß for ICP-OES and ICP-MS measurements. Furthermore we thank Kerstin Erdmann, Andreas Krüger and Renate Helm for their help with the preparation and homogenization of the rock material. William J. Broughton is thanked for his valued help in English editing of the manuscript.
References
Abdulla, H. (2009). Bioweathering and biotransformation of granitic rock minerals by actinomycetes. Microb. Ecol. 58, 753–761. doi: 10.1007/s00248-009-9549-1
Bates, S. T., and Garcia-Pichel, F. (2009). A culture-independent study of free-living fungi in biological soil crusts of the Colorado Plateau: their diversity and relative contribution to microbial biomass. Environ. Microbiol. 11, 56–67. doi: 10.1111/j.1462-2920.2008.01738.x
Black, C. B., Huang, H. W., and Cowan, J. A. (1994). Biological coordination chemistry of magnesium, sodium, and potassium-ions - protein and nucleotide-binding sites. Coord. Chem. Rev. 135, 165–202. doi: 10.1016/0010-8545(94)80068-5
Bonneville, S., Morgan, D. J., Schmalenberger, A., Bray, A., Brown, A., Banwart, S. A., et al. (2011). Tree-mycorrhiza symbiosis accelerate mineral weathering: evidences from nanometer-scale elemental fluxes at the hypha-mineral interface. Geochim. Cosmochim. Acta 75, 6988–7005. doi: 10.1016/j.gca.2011.08.041
Bonneville, S., Smits, M. M., Brown, A., Harrington, J., Leake, J. R., Brydson, R., et al. (2009). Plant-driven fungal weathering: early stages of mineral alteration at the nanometer scale. Geology 37, 615–618. doi: 10.1130/G25699A.1
Brunner, I., Plötze, M., Rieder, S., Zumsteg, A., Furrer, G., and Frey, B. (2011). Pioneering fungi from the Damma glacier forefield in the Swiss Alps can promote granite weathering. Geobiology 9, 266–279. doi: 10.1111/j.1472-4669.2011.00274.x
Büdel, B., Weber, B., Kühl, M., Pfanz, H., Sültemeyer, D., and Wessels, D. (2004). Reshaping of sandstone surfaces by cryptoendolithic cyanobacteria: bioalkalization causes chemical weathering in arid landscapes. Geobiology 2, 261–268. doi: 10.1111/j.1472-4677.2004.00040.x
Chadwick, O. A., Brimhall, G. H., and Hendricks, D. M. (1990). From a black to a gray box - a mass balance interpretation of pedogenesis. Geomorphology 3, 369–390. doi: 10.1016/0169-555X(90)90012-F
Chertov, O., Gorbushina, A., and Deventer, B. (2004). A model for microcolonial fungi growth on rock surfaces. Ecol. Model. 177, 415–426. doi: 10.1016/j.ecolmodel.2004.02.011
Cohen, M. F., Wallis, J. G., Campbell, E. L., and Meeks, J. C. (1994). Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology 140(Pt 12), 3233–3240. doi: 10.1099/13500872-140-12-3233
Cowan, J. A. (1991). Metallobiochemistry of magnesium - coordination-complexes with biological substrates - site specificity, kinetics and thermodynamics of binding, and implications for activity. Inorg. Chem. 30, 2740–2747. doi: 10.1021/ic00013a008
Cowan, J. A. (2002). Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals 15, 225–235. doi: 10.1023/A:1016022730880
Davis, K. J., Nealson, K. H., and Luttge, A. (2007). Calcite and dolomite dissolution rates in the context of microbe-mineral surface interactions. Geobiology 5, 191–205. doi: 10.1111/j.1472-4669.2007.00112.x
Delatorre, M. A., Gómez-Alarcón, G., Vizcaino, C., and Garcia, M. T. (1993). Biochemical-mechanisms of stone alteration carried out by filamentous fungi living in monuments. Biogeochemistry 19, 129–147. doi: 10.1007/BF00000875
de los Ríos, A., and Ascaso, C. (2005). Contributions of in situ microscopy to the current understanding of stone biodeterioration. Int. Microbiol. 8, 181–188. Available online at: http://scielo.isciii.es/scielo.php?pid=S1139-67092005000300005&script=sci_arttext&tlng=pt
DIN 19528 (2009-01). Leaching of Solid Materials - Percolation Method for the Joint Examination of the Leaching Behaviour of Organic Inorganic Substances for Materials with a Particle Size upto 32 mm - Basic Characterization Using a Comprehensive Column Test Compliance Test Using a Quick Column Test. Deutsches Institut für Normung German Standardization Organization, Berlin.
Diakumaku, E., Gorbushina, A. A., Krumbein, W. E., Panina, L., and Soukharjevski, S. (1995). Black fungi in marble and limestones - an aesthetical, chemical and physical problem for the conservation of monuments. Sci. Total Environ. 167, 295–304. doi: 10.1016/0048-9697(95)04590-W
Dornieden, T., Gorbushina, A. A., and Krumbein, W. E. (2000a). Biodecay of cultural heritage as a space/time-related ecological situation - an evaluation of a series of studies. Int. Biodeter. Biodegrad. 46, 261–270. doi: 10.1016/S0964-8305(00)00107-4
Dornieden, T., Gorbushina, A. A., and Krumbein, W. E. (2000b). “Patina – physical and chemical interactions of sub-aerial biofilms with objects of art,” in Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage, eds O. Ciferri, P. Tiano, and G. Mastromei (New York, NY; Boston, MA; Dordrecht; London; Moscow: Kluwer), 105–119.
Ekman, M., Picossi, S., Campbell, E. L., Meeks, J. C., and Flores, E. (2013). A Nostoc punctiforme sugar transporter necessary to establish a Cyanobacterium-plant symbiosis. Plant Physiol. 161, 1984–1992. doi: 10.1104/pp.112.213116
Fiantis, D., Nelson, M., Shamshuddin, J., Goh, T. B., and Van Ranst, E. (2010). Determination of the geochemical weathering indices and trace elements content of New Volcanic ash deposits from Mt. Talang (West Sumatra) Indonesia. Eur. Soil Sci. 43, 1477–1485. doi: 10.1134/S1064229310130077
Gao, K. (1998). Chinese studies on the edible blue-green alga, Nostoc flagelliforme: a review. J. Appl. Phycol. 10, 37–49. doi: 10.1023/A:1008014424247
Gómez-Alarcón, G., Munoz, M. L., and Flores, M. (1994). Excretion of organic-acids by fungal strains isolated from decayed sandstone. Int. Biodeter. Biodegr. 34, 169–180. doi: 10.1016/0964-8305(94)90006-X
Gorbushina, A. A. (2007). Life on the rocks. Environ. Microbiol. 9, 1613–1631. doi: 10.1111/j.1462-2920.2007.01301.x
Gorbushina, A. A., and Broughton, W. J. (2009). Microbiology of the atmosphere-rock interface: how biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 63, 431–450. doi: 10.1146/annurev.micro.091208.073349
Gorbushina, A. A., Krumbein, W. E., Hamman, C. H., Panina, L., Soukharjevski, S., and Wollenzien, U. (1993). Role of black fungi in colour change and biodeterioration of antique marbles. Geomicrobiol. J. 11, 205–221. doi: 10.1080/01490459309377952
Gorbushina, A. A., Krumbein, W. E., Rullkötter, J., and Volkmann, M. (2003). How and why do rocks turn black - A history of surface biogeochemistry. Geochim. Cosmochim. Acta 67:A123.
Hausrath, E. M., Neaman, A., and Brantley, S. L. (2009). Elemental release rates from dissolving basalts and granite with and without organic ligands. Am. J. Sci. 309, 633–660. doi: 10.2475/08.2009.01
Hellmann, R., Wirth, R., Daval, D., Barnes, J. P., Penisson, J. M., Tisserand, D., et al. (2012). Unifying natural and laboratory chemical weathering with interfacial dissolution–reprecipitation: a study based on the nanometer-scale chemistry of fluid–silicate interfaces. Chem. Geol. 294–295, 203–216. doi: 10.1016/j.chemgeo.2011.12.002
Hövelmann, J., Putnis, A., Geisler, T., Schmidt, B. C., and Golla-Schindler, U. (2010). The replacement of plagioclase feldspars by albite: observations from hydrothermal experiments. Contribut. Mineral. Petrol. 159, 43–59. doi: 10.1007/s00410-009-0415-4
Huang, W. H., and Kiang, W. C. (1972). Laboratory dissolution of plagioclase feldspars in water and organic-acids at room-temperature. Am. Mineral. 57, 1849–1859.
Jongmans, A. G., van Breemen, N., Lundström, U. P., van Hees, A. W., Finlay, R. D., and Olsson, M. (1997). Rock-eating fungi. Nature 389, 682–683. doi: 10.1038/39493
Knowles, E. J., and Castenholz, R. W. (2008). Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms. Fems Microbiol. Ecol. 66, 261–270. doi: 10.1111/j.1574-6941.2008.00568.x
Kraus, P. R., and Heitman, J. (2003). Coping with stress: calmodulin and calcineurin in model and pathogenic fungi. Biochem. Biophys. Res. Commun. 311, 1151–1157. doi: 10.1016/S0006-291X(03)01528-6
Landeweert, R., Hoffland, E., Finlay, R. D., Kuyper, T. W., and van Breemen, N. (2001). Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 16, 248–254. doi: 10.1016/S0169-5347(01)02122-X
Lapanje, A., Wimmersberger, C., Furrer, G., Brunner, I., and Frey, B. (2012). Pattern of elemental release during the granite dissolution can be changed by aerobic heterotrophic bacterial strains isolated from damma glacier (central alps) deglaciated granite sand. Microb. Ecol. 63, 865–882. doi: 10.1007/s00248-011-9976-7
Lasaga, A. C., Soler, J. M., Ganor, J., Burch, T. E., and Nagy, K. L. (1994). Chemical-weathering rate laws and global geochemical cycles. Geochim. Cosmochim. Acta 58, 2361–2386. doi: 10.1016/0016-7037(94)90016-7
Li, Y.-H. (1984). Why are the chemical compositions of living organisms so similar? Schweizerische Zeitschrift Hydrologie 46, 177–184. doi: 10.1007/bf02538059
Machill, S., Althaus, K., Krumbein, W. E., and Steger, W. E. (1997). Identification of organic compounds extracted from black weathered surfaces of Saxonean sandstones, correlation with atmospheric input and rock inhabiting microflora. Organ. Geochem. 27, 79–97. doi: 10.1016/S0146-6380(97)00041-7
Meeks, J. C., and Castenholz, R. W. (1971). Growth and photosynthesis in an extreme thermophile, Synechoccoccus lividus (Cyanophyta). Arch. Mikrobiol. 78, 25. doi: 10.1007/BF00409086
Nai, C., Wong, H. Y., Pannenbecker, A., Broughton, W. J., Benoit, I., de Vries, R. P., et al. (2013). Nutritional physiology of a rock-inhabiting, model micro-colonial fungus from an ancestral lineage of the Chaetothyriales (Ascomycetes). Fungal Genet. Biol. 56, 54–66. doi: 10.1016/j.fgb.2013.04.001
Neaman, A., Chorover, J., and Brantley, S. L. (2006). Effects of organic ligands on granite dissolution in batch experiments at pH 6. Am. J. Sci. 306, 451–473. doi: 10.2475/06.2006.03
Noack-Schönmann, S., Bus, T., Banasiak, R., Knabe, N., Broughton, W. J., Den Dulk-Ras, H., et al. (2014). Genetic transformation of Knufia petricola A95 - a model organism for biofilm-material interactions. AMB Express 4:80. doi: 10.1186/s13568-014-0080-5
Norris, V., Chen, M., Goldberg, M., Voskuil, J., McGurk, G., and Holland, I. B. (1991). Calcium in bacteria: a solution to which problem? Mol. Microbiol. 5, 775–778. doi: 10.1111/j.1365-2958.1991.tb00748.x
Olesik, J. W. (1991). Elemental analysis using ICP-OES and ICP/MS. Anal. Chem. 63, 12A–21A. doi: 10.1021/ac00001a711
Olsson-Francis, K., Simpson, A. E., Wolff-Boenisch, D., and Cockell, C. S. (2012). The effect of rock composition on cyanobacterial weathering of crystalline basalt and rhyolite. Geobiology 10, 434–444. doi: 10.1111/j.1472-4669.2012.00333.x
Ortega-Calvo, J. J., Arino, X., Hernandez-Marine, M., and Saiz-Jimenez, C. (1995). Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci. Total Environ. 167, 329–341. doi: 10.1016/0048-9697(95)04593-P
Oxburgh, R., Drever, J. I., and Sun, T. (1994). Mechanism of plagioclase dissolution in acid solution at 25°C. Geochim. Cosmochim. Acta 58, 661–669. doi: 10.1016/0016-7037(94)90496-0
Ozturk, S., and Aslim, B. (2010). Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Poll. Res. 17, 595–602. doi: 10.1007/s11356-009-0233-2
Perez, R., and Boles, A. R. (2005). An empirically derived kinetic model for albitization of detritic plagioclase. Am. J. Sci. 305, 312–343. doi: 10.2475/ajs.305.4.312
Price, J. R., and Velbel, M. A. (2003). Chemical weathering indices applied to weathering profiles developed on heterogeneous felsic metamorphic parent rocks. Chem. Geol. 202, 397–416. doi: 10.1016/j.chemgeo.2002.11.001
Putnis, A. (2009). Mineral replacement reactions. Rev. Mineral. Geochem. 70, 87–124. doi: 10.2138/rmg.2009.70.3
Rosling, A., Roose, T., Hermann, A. M., Davidson, F. A., Finlay, R. D., and Gadd, G. M. (2009). Approaches to modelling mineral weathering by fungi. Fungal Biol. Rev. 23, 138–144. doi: 10.1016/j.fbr.2009.09.003
Ruiz-Agudo, E., Putnis, C., and Putnis, A. (2014). Coupled dissolution and precipitation at mineral–fluid interfaces. Chem. Geol. 383, 132–146. doi: 10.1016/j.chemgeo.2014.06.007
Schöll, L., Kuyper, T. W., Smits, M. M., Landeweert, R., Hoffland, E., and van Breemen, N. (2008). Rock-eating mycorrhizas: their role in plant nutrition and biogeochemical cycles. Plant Soil 303, 35–47. doi: 10.1007/s11104-007-9513-0
Seiffert, F., Bouchez, J., von Blanckenburg, F., and Gorbushina, A. A. (2014). Microbial colonization of bare rocks: laboratory biofilm enhances mineral weathering. Proc. Earth Plan. Sci. 12, 123–129. doi: 10.1016/j.proeps.2014.08.042
Sherwood, A. R., and Presting, G. G. (2007). Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 43, 605–608. doi: 10.1111/j.1529-8817.2007.00341.x
Silverman, M. P., and Munoz, E. F. (1970). Fungal attack on rock - solubilization and altered infrared spectra. Science 169, 985. doi: 10.1126/science.169.3949.985
Staley, J. T., Palmer, F., and Adams, J. B. (1982). Microcolonial fungi-common inhabitants on desert rocks. Science 215, 1093–1095. doi: 10.1126/science.215.4536.1093
Stanier, R. Y., Kunisawa, R., Mandel, M., and Cohen-Bazire, G. (1971). Purification and properties of unicellular blue-green algae (order Cchroococcales). Bacteriol. Rev. 35, 171.
Sterflinger, K. (1998). Temperature and NaCl- tolerance of rock-inhabiting meristematic fungi. Anton. Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 74, 271–281. doi: 10.1023/A:1001753131034
Sterflinger, K. (2000). Fungi as geologic agents. Geomicrobiol. J. 17, 97–124. doi: 10.1080/01490450050023791
Takahashi, F., and Shimaoka, T. (2012). The weathering of municipal solid waste incineration bottom ash evaluated by some weathering indices for natural rock. Waste Manag. 32, 2294–2305. doi: 10.1016/j.wasman.2012.06.009
Tamaru, Y., Takani, Y., Yoshida, T., and Sakamoto, T. (2005). Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 71, 7327–7333. doi: 10.1128/AEM.71.11.7327-7333.2005
Tashpulatov, Z., Baibaev, B. G., and Shul'man, T. S. (2000). Chemical composition of mycelium of the thermotolerant fungus penicillium atrovenetum. Chem. Nat. Comp. 36, 518–520. doi: 10.1023/A:1002851826381
Taylor, L. L., Leake, J. R., Quirk, J., Hardy, K., Banwart, S. A., and Beerling, D. J. (2009). Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7, 171–191. doi: 10.1111/j.1472-4669.2009.00194.x
Urzì, C., and Realini, M. (1998). Colour changes of Noto's calcareous sandstone as related to its colonisation by microorganisms. Int. Biodeter. Biodegr. 42, 45–54. doi: 10.1016/S0964-8305(98)00045-6
Wallander, H., Johansson, L., and Pallon, J. (2002). PIXE analysis to estimate the elemental composition of ectomycorrhizal rhizomorphs grown in contact with different minerals in forest soil. FEMS Microbiol. Ecol. 39, 147–156. doi: 10.1111/j.1574-6941.2002.tb00916.x
Wallander, H., Mahmood, S., Hagerberg, D., Johansson, L., and Pallon, J. (2003). Elemental composition of ectomycorrhizal mycelia identified by PCR–RFLP analysis and grown in contact with apatite or wood ash in forest soil. FEMS Microbiol. Ecol. 44, 57–65. doi: 10.1016/s0168-6496(02)00456-7
Warscheid, T., and Braams, J. (2000). Biodeterioration of stone: a review. Int. Biodeter. Biodegr. 46, 343–368. doi: 10.1016/S0964-8305(00)00109-8
Keywords: biotic weathering, flow-through columns, plagioclase, K-feldspar, granite
Citation: Seiffert F, Bandow N, Kalbe U, Milke R and Gorbushina AA (2016) Laboratory Tools to Quantify Biogenic Dissolution of Rocks and Minerals: A Model Rock Biofilm Growing in Percolation Columns. Front. Earth Sci. 4:31. doi: 10.3389/feart.2016.00031
Received: 03 August 2015; Accepted: 15 March 2016;
Published: 21 April 2016.
Edited by:
Dina M. Bower, NASA Goddard Space Flight Center/University of Maryland, USAReviewed by:
Eric D. Van Hullebusch, University Paris-Est, FranceAlan W. Decho, University of South Carolina, USA
Copyright © 2016 Seiffert, Bandow, Kalbe, Milke and Gorbushina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna A. Gorbushina, YW5uYS5nb3JidXNoaW5hQGJhbS5kZQ==
 Franz Seiffert
Franz Seiffert Nicole Bandow
Nicole Bandow Ute Kalbe
Ute Kalbe Ralf Milke
Ralf Milke Anna A. Gorbushina
Anna A. Gorbushina