- IQVIA, Biostatistics, Frankfurt, Germany
The estimand framework as defined by the ICH E9(R1) addendum aims to clearly define “the treatment effect reflecting the clinical question posed by the trial objective”. It intends to achieve this goal of a clear definition by specifying the 5 estimand attributes: treatment conditions, population, endpoints, handling of intercurrent events (IEs), and population-level summary. However, hybrid clinical/observational research like External Comparators (ECs) leads to new reflections on existing attributes and to considerations for additional ones. Specifically, treatment conditions and exposure may be more difficult to handle in the EC, and especially Standard of Care (SoC) treatment needs detailed attention. The external population typically cannot be based on the classical Intention-to-treat population and constitutes also an approximation only. Endpoints may not be comparable across cohorts, and IEs may be more different than in an RCT setting, such that the hypothetical treatment policy according to the ICH E9(R1) addendum may become of greater interest especially for long-term endpoints. Finally, the necessary assumptions for some population-level summaries (e.g., the proportional hazards assumption) can become more fragile when joining data from different sources due to induced heterogeneity. Finally, it is shown that the baseline definition and the marginal estimator are candidates for additional estimand attributes in case the estimand framework is revised to account for observational study needs.
1 Introduction
Though there is global agreement that the Randomized Controlled Trial (RCT) is the gold standard design for drug approval studies, there are cases where other study designs are needed. External Comparators (ECs) assess clinical trial data like Single-Arm Trials (SATs) against an external cohort (U.S. Food and Drug Administration, 2023; Ghadessi et al., 2020; Burger et al., 2021; Seeger et al., 2020; Skovlund et al., 2018; Thorlund et al., 2020). This mixed clinical/observational research set-up leads to new considerations about the estimand framework as defined by the International Conference of Harmonisation (ICH) E9(R1) addendum (ICH E9(R1) Expert Working Group, 2021). The addendum states that an estimand is the clear definition of “the treatment effect reflecting the clinical question posed by the trial objective” (ICH E9(R1) Expert Working Group, 2021). It intends to achieve this goal of a clear definition by specifying the 5 estimand attributes: treatment conditions, population, endpoints, handling of intercurrent events (IEs) and population-level summary. However, hybrid clinical/observational research like External Comparators (ECs) leads to new reflections on existing attributes and to considerations for additional ones. These have been sketched before (Rippin et al., 2022), and the paper builds up on this previous work to further clarify the application of the estimand framework to ECs.
More general introductions to the estimand framework are available (e.g., Gogtay et al., 2021), and some specific considerations about observational studies have been derived as well (Li et al., 2022; Chen et al., 2023; Wu et al., 2023). Although they are relevant for EC studies, they do not discuss estimands in the EC setting specifically, such that a concrete discussion in the context of EC studies provides added value.
2 Estimand attributes
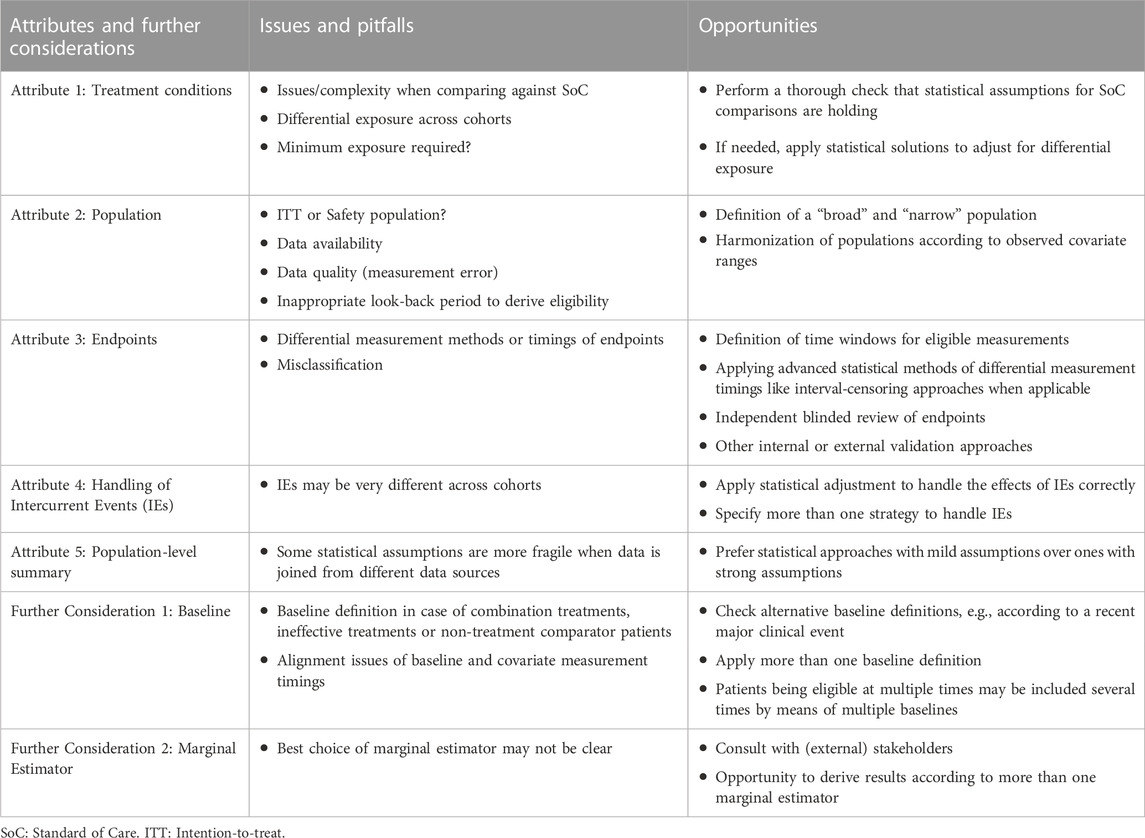
For a summary of issues/pitfalls and opportunities regarding estimand attributes and further considerations see Table 1.
2.1 Estimand attribute 1: treatment conditions
The eligible treatment conditions like doses, route of administration, etc., need to be specified and clearly defined. If different doses or other treatment conditions have different expected treatment effects for the analyzed endpoints, separate analyses must be performed. This is due to the required consistency assumption of causal inference methodology, which involves homogeneity of treatment effects (Faries et al., 2020; Hernán and Robins, 2023).
While comparing the SAT against another single treatment is less complex, comparing against Standard of Care (SoC) can lead to additional difficulties. First, the actual treatments belonging under the umbrella term of SoC should be characterized thoroughly by means of descriptive statistics, both for best medical understanding and transparency. The definition of SoC can vary by country/region (so stratification of descriptive statistics may be helpful), and comparisons against multiple SoC definitions (for example, for multiple national Health Technology Assessment stakeholders) are possible.
SoC can also be not as consistent in the RW as it is in a controlled setting. Hence, a critical review of the RW SoC may be necessary to decide whether treatments should be excluded because they are considered to be inappropriate. If the eligible SoC treatments are narrowed down in such a way, some patient groups may no longer be included in the analysis population. Thus, this estimand attribute of treatment conditions may be related to the population attribute. The remaining eligible treatments must also have the same expected treatment effects (again due to the consistency assumption of causal inference). A check for homogeneity of SoC treatment effects is possible and should be performed, at least for the most common treatments with adequate sample size.
Challenges also arise in situations where the treatment exposure (compliance/adherence) is very different between the SAT and the EC. It is possible that the exposure in the SAT’s controlled setting is high, while the RW setting may lead to lower treatment exposure. This is due to a strict SAT protocol and strong monitoring of sites and patients to follow the protocol, while there is no such situation in the RW. In such circumstances, it is particularly important to describe treatment exposure in detail by statistical tables. Researchers also need to think about the requirement for any needed minimum exposure or whether applying a hypothetical estimand strategy (see Section 2.4) is a reasonable approach to overcome potentially substantial exposure differences. Requesting a minimal exposure would introduce immortal time bias, which needs to be handled statistically in a correct manner. Special challenges may arise if the trial has (or needs to be compared against) a dynamic treatment strategy. For example, when the trial’s design (or the comparator) includes dose escalation, the statistical handling of post-baseline dosing events may involve again hypothetical estimands (see Section 2.4).
Although some of the considerations above are inherited from the broader class of observational research designs, it needs to be pointed out that differential exposure is a challenge which is more likely to be seen in ECs, and that due to this or due to dynamic treatment plans (or other IEs) the hypothetical estimand strategy (Section 2.4) may become more relevant in the EC setting.
2.2 Estimand attribute 2: population
2.2.1 Intention-to-treat (ITT) population versus Safety population
As a general principle, there is a preference to compare populations which share a common definition to compare like-for-like (Pocock, 1976; Gray et al., 2020). However, the EC is typically compiled from real word (RW) data sources, where no Intention-to-treat (ITT) population exists, which denotes the population which was intended to be treated (for example, at enrollment or randomization). This may lead to utilizing for both cohorts rather the population which received the treatment (the Safety population), which has the advantage of straightforward results interpretation but the disadvantage of applying an approach which is outside of common RCT standards.
As an alternative, it is possible to argue that taking the trial’s ITT population and the external Safety population leads to a conservative analysis, because the treatment effect should be lower in the population which is just intended to be treated compared to the population which actually has been treated. The advantage is that such an approach is in line with the trial analysis, which typically relies on the ITT population. The disadvantage is that the comparison is only unbiased if there is no difference for the EC in terms of the ITT and Safety population. Also, by taking the trial’s ITT population and the external Safety population, the intentionally induced bias (conservativeness) is potentially beyond a reasonable margin.
A practical example for considering either approach could be the chimeric antigen receptor (CAR)–T cell treatment where there are usually several weeks after baseline (index date, time zero) until the treatment is received, which could mean that there is a meaningful drop-out after baseline but before the treatment is taken. Considering this drop-out or not by means of choosing the ITT or Safety analysis population leads to a likely difference in the estimated treatment effect. Specifically, the treatment effect based on the ITT population is expected to be lower than the one based on the Safety population. Specifying more than one estimand (one as the primary estimand and the other as a supplementary analysis) could be helpful to generate multiple perspectives.
2.2.2 Approximation of the target population
Although the trial’s eligibility criteria are mimicked in the EC as much as possible, some baseline information is likely to not be available in the RW. For example, an HIV test result or an ECOG value is not always measured or documented in RW datasets. Hence, the RW analysis population is in most cases (if not in all) an approximation of the population of interest. However, if the SAT’s baseline covariates’ values are already available, there is an additional possibility to refine populations. Instead of just applying the trial’s eligibility criteria to the EC as much as possible, it can also be considered to restrict the covariate value ranges according to what has been observed in the trial. If, for example, the eligibility criteria require patients to be at least 18 years old, there is the possibility to look up the actual trial’s age range, which hypothetically may be between 43 and 82 years. Then, the EC could be restricted to the same age range and this approach could be applied for all other important covariates as well. Although this seems to make sense statistically and is in line with the concept of exchangeability (Pocock, 1976; Gray et al., 2020), there is no guidance yet whether such an approach would be considered preferable from a regulatory or payers’ perspective.
Another approximation regarding the included population occurs due to eligibility values being typically taken from a time window (look-back period) before the EC baseline (e.g., 3, 6 or 12 months before true baseline). This is often needed because the RW measurements for important eligibility criteria are not necessarily available at the exact time of baseline. However, the longer the eligibility measurements are retrieved from the past, the more likely the occurrence of measurement error, which can either be pure random error or a systematic shift; for example, for an indication where the patient’s condition is deteriorating fast. Both types of errors would lead to issues, but a systematic shift could directly lead to the analysis becoming anti-conservative.
Often, there is a trade-off between the quality of the target population approximation in the EC and sample size, for example, in case of baseline data being substantially missing, leading to the question whether some of the inclusion criteria should not be applied because of a substantial reduction in sample size. One approach is to derive not a single but two populations: one being a narrow data subset approximating the target population to a high degree, and one being a broad subset of the data with a less accurate population approximation but higher sample size. Presenting results based on more than one population may be helpful to check for the robustness of the generated evidence.
The comparator population can be derived either by a retrospective approach or by a new prospective cohort study. The retrospective approach has the advantage to generate results quicker, which can be an important aspect regarding the drug submission timelines, while the prospective approach comes with the advantage of fully customized data collection, which leads most probably to a better target population approximation.
2.3 Estimand attribute 3: endpoints
While endpoints like Overall Survival (OS) are typically comparable across cohorts (Rippin et al., 2022), others like progression-free survival (PFS) or overall response rate (ORR) may be less comparable. This is due to progression being typically not measured in the RW by internationally standardized classification rules like Response Evaluation Criteria in Solid Tumours (RECIST) (Eisenhauer et al., 2009) or Lugano classification (Cheson et al., 2014). Thus, comparisons of response-based endpoints in the context of ECs require extra caution to handle potential issues of endpoint misclassification, for example, by statistical approaches involving internal or external validation (e.g., central and blinded independent review of endpoint data).
One other source of non-comparability may occur for composite time-to-event (TTE) endpoints, such as PFS, Time to Next Treatment or Death, or Duration of Response until Progression or Death. These endpoints are not automatically comparable across different data sources due to potentially different censoring proportions for the earlier part of the composite endpoint. As an example, consider PFS with high missingness for progression but low missingness for survival. In such a case, PFS is shifted towards the endpoint survival. Because there is typically more missingness in RW data sources, using composite TTE endpoints may lead to biased analysis results.
The endpoint specification needs also a clear definition of what constitutes an acceptable measurement timepoint, since bias may occur when endpoints are measured at different times across cohorts. One option is to define time windows around a specific target time (e.g., 3 months after baseline +/−2 weeks). For repeated measurements analyses (e.g., for patient reported outcomes) there are further options by applying additional statistical assumptions modelling the endpoint over time. For TTE endpoints, different measurement times can be handled appropriately by interval-censoring methods (Bogaerts et al., 2017), which would also handle the case when more frequent follow-up visits occur for more severe cases (intensity bias).
Safety outcomes are typically not as exhaustively documented in the RW as in trials, therefore these endpoints are typically not comparable. However, there may be a complete list of major/life-threatening events or hospitalizations/doctor’s visits in some RW data sources, which may enable comparisons of key safety events.
2.4 Estimand attribute 4: handling of intercurrent events
The ICH E9(R1) guideline defines IEs as “Events occurring after treatment initiation that affect either the interpretation or the existence of the measurements associated with the clinical question of interest.” (ICH E9(R1) Expert Working Group, 2021). It outlines 5 strategies on how to handle IEs. Although a full discussion of all IE handling strategies is out of scope of this paper, the treatment policy strategy and the hypothetical estimand strategy will be highlighted briefly. For the former strategy “the intercurrent event is considered irrelevant in defining the treatment effect of interest” (ICH E9(R1) Expert Working Group, 2021), while the latter is setting up a hypothetical scenario where the IE would not have occurred.
The question of which IE handling strategy is best suited for ECs is especially important to clarify because IEs can differ more in EC studies compared to RCTs due to different settings and data temporality across cohorts. For example, the kind and timing of subsequent treatments may be completely different in the EC. In such cases, the differential effect of follow-up therapies may bias comparisons considerably, especially for long-term endpoints like OS where the effects of subsequent treatments may be substantial. In this case, alternatives to the treatment policy estimand may be important to consider, for example, by applying the hypothetical estimand strategy. These strategies are connected to missing data handling and include methods like Marginal Structural Models using IPTW, g-estimation of Structural Nested Models (SNMs) and the g-formula (Daniel et al., 2013; Clare et al., 2019; Faries et al., 2020; Hernán and Robins, 2023).
For short- or medium-term endpoints like progression-based endpoints IEs may occur less often, leading to a stronger rationale for the treatment policy estimand being the primary estimand. It may also be helpful to define more than one estimand for a robust description of study results.
2.5 Estimand attribute 5: population-level summary
Some population-level summaries may also be more appropriate than others for ECs. This is due to the fact that some summaries need assumptions which can become fragile in ECs. As an example, it was seen before that the proportional hazards assumption is easily lost when comparing data across different data sources (Hoogendoorn et al., 2022). This leads to issues when using the Cox model and selecting the hazard ratio as the population-level summary. Note that the Cox model was criticized since the last decade also for other reasons (Hernán, 2010; Aalen et al., 2015; Mao et al., 2018; Rufibach et al., 2019; Stensrud et al., 2019; Martinussen et al., 2020). Accelerated Failure Time (AFT) models (Collett, 2023) may also not be sufficiently flexible to model the data successfully due to the selected parametric approach. Thus, alternative TTE population-level summaries are recommended, e.g., restricted mean survival time (RMST) differences (Collett, 2023).
Other issues with population-level summaries in an EC setting may generally occur due to the induced heterogeneity across cohorts (which may be a consequence of unmeasured confounding). For example, the variance across cohorts is more likely to be heteroscedastic compared to an RCT setting, for example, for a repeated measurements model analyzing quality-of-life questionnaires. Hence, allowing for unequal variances across cohorts is recommended and statistical models without strong assumptions should be preferred generally.
2.6 Further considerations
The ICH E9(R1) addendum focusses primarily on clinical research, such that for ECs further considerations are needed: one is the definition of baseline and another one is the specification of the marginal estimator, which are discussed below.
2.6.1 The definition of baseline
It is not always clear how baseline should be defined for ECs (see also Section 2.2). One example is when the treatment is a combination of two or more drugs. One possibility to define baseline is to take the latest start date of the involved treatments. However, a decision should only be reached after having received input from medical team members. If one element of the combination treatment is the major driver for efficacy/effectiveness or one part of the combination treatment relates to controlling adverse events only, there may be rationale to choose baseline to be the treatment start date which relates to efficacy/effectiveness. Another possibility may be to restrict the population which starts taking all treatment elements either at the same time or within a certain time window (e.g., ±1 week), especially if there are treatment guidelines stating that all components of the treatment should be taken at the same time.
Further, there may be situations where a comparison needs to take place against comparator treatments which are unknown to be effective. In such cases, it is questionable whether any treatment start date in the comparator cohort would constitute the best baseline definition, since the treatment start may be an irrelevant event for disease progression. Also, its timing may happen a long time after an important clinical event like disease progression or diagnosis, because it was not considered critical that the treatment was prescribed or taken.
All of these considerations do show that the comparator’s effectiveness can matter when deciding about the baseline definition. If the comparator drug is effective, it is natural to take the treatment start date as baseline across both cohorts, but if not effective it remains to be checked for the specific study at hand whether such an event date is meaningful to define baseline. Of note, post-baseline events are not allowed to select study populations, but the proven or unknown effectiveness of a comparator treatment (which is associated with post-baseline outcome likelihood) is proper information for baseline considerations.
Moreover, there are cases where no treatment is available at all (Wakabayashi et al., 2023), such that it is simply not possible to define baseline by means of any treatment start date.
As an alternative baseline, it may be possible for some indications to use the last disease progression or another important clinical event (myocardial infarct, stroke, etc.). However, survivor bias is introduced for the trial participants because the time from the clinical event to the treatment start date has been survived in the trial population, and methods to handle survivor bias (Wang et al., 2022) need to be applied for the statistical analysis of the data. Note that if the treatment start date is chosen to constitute baseline, time from an important clinical event to treatment start may be used as a covariate in causal inference approaches.
The longer the index date in the past (relative to the treatment start date), the more pronounced the disadvantages in the general case. Firstly, the immortal time bias may become critically large, and secondly, potentially unnecessary variability in terms of “white noise” is introduced to the analysis.
There are also cases where patients are eligible multiple times, for example, at the start date of the third and higher lines of treatment (Backenroth, 2021; Hatswell et al., 2022). In such cases, using all of the available data by allowing patients to enter the study at multiple timepoints (multiple baselines) may be an efficient solution, though more complex statistical methodology is needed to handle correlated data correctly.
Of note, when discussing best baseline definitions, issues may also arise regarding the timing of covariate measurements. Firstly, covariates measured at the original SAT baseline are becoming post-baseline covariates if the index date is moved to an earlier time point like disease progression. This is problematic from a theoretical statistical perspective, as only baseline variables can be used in standard statistical approaches for covariate adjustment. Secondly, the EC typically applies a look-back period to derive eligible baseline measurements (see Section 2.2.2). This may lead to actual covariate values at true baseline being potentially different from previously recorded values, which is a threat to the validity of causal inference methods.
Of course, the definition of baseline is also related to the population and treatment attributes, but handling this topic separately is considered to be useful, for example, by helping to identify survivor bias and to clarify whether or not patients will be allowed to enter the study at multiple baseline times.
2.6.2 The marginal estimator
While “RCTs are designed to provide estimates of the average treatment effect” (van Amsterdam and Ranganath, 2023), ECs may estimate the Average Treatment Effect (ATE), the Average Treatment Effect on the Treated (ATT), the Average Treatment Effect on the Untreated (ATU) or the Average Treatment Effect in the Overlap Population (ATO) (Rippin et al., 2022). As an example, the ATE standardizes the estimated treatment effect according to the overall population (of both cohorts), while the ATT does do this standardization according to the baseline covariates of the clinical trial. In other words, for the ATE the overall sample is assumed to be representative or of interest, while for the ATT the trial population is.
While each of these marginal estimators come with their own advantages and disadvantages (Rippin et al., 2022), none of them are yet directly included in the ICH estimand framework. Although the framework speaks about estimators, the estimators are supposed to affect the estimate only in terms of how well model assumptions are met. A conceptual change in the estimand cannot be attributed to the estimator level. Hence, the natural idea to classify the marginal estimator to the estimator level does fail.
One idea to integrate marginal estimators into the framework is to use a sub-attribute to the population-level summary attribute, for example, by saying that the “ATE hazard ratio” or the “ATT relative risk” is estimated. However, the population-level summary (e.g., a relative risk) is specified independently from the marginal estimator, such that this would constitute an artificial mix of two independent concepts.
3 Discussion
The ICH E9(R1) focuses on clinical trials but states that its principles are relevant for observational studies as well (ICH E9(R1) Expert Working Group, 2021). However, setting up an EC estimand is less straightforward than in clinical trials, which is in line with Wu et al., 2023 stating that “constructing estimands for real-world evidence (RWE) studies might require additional considerations”. These additional considerations have been presented for EC studies in this paper. It was seen that due to specific challenges which are typically not seen in clinical trials, nuanced reflections are needed for the 5 existing estimand attributes. Moreover, there are further important aspects which need to be addressed in the EC setting: The first is the definition of baseline, and the second is the specification of the marginal estimator. The Li paper (Li et al., 2022) suggested that there is a relationship of the marginal estimator with the population attribute, but this seems to be against the spirit of the ICH E9(R1) addendum, which treats the population attribute in the sense of defining eligibility (population selection). Hence, rather a separate attribute is suggested or a specification as per the population-level summary.
In case the estimand framework is revised to include RW study needs, the baseline definition and the marginal estimator are candidates for additional estimand attributes. Further new attributes may be the quality and completeness of data and covariates because valid causal inference depends on data being fit for purpose, as mentioned by Chen et al., 2023, who have been discussing estimands in the broader context of RW studies.
We hope that this paper not only fosters discussions around the set-up of EC estimands but also more generally regarding the real world application of the framework.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GR: Writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This article was not funded.
Acknowledgments
The author would like to thank Tom Kwon (IQVIA) for additional review of the manuscript.
Conflict of interest
GR is full time employee and shareholder of IQVIA.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fdsfr.2023.1332040/full#supplementary-material
References
Aalen, O. O., Cook, R. J., and Roysland, K. (2015). Does Cox analysis of a randomized survival study yield a causal treatment effect? Lifetime Data Anal. 21 (4), 579–593. doi:10.1007/s10985-015-9335-y
Backenroth, D. (2021). How to choose a time zero for patients in external control arms. Pharm. Stat. 20, 783–792. doi:10.1002/pst.2107
Bogaerts, K., Komarek, A., and Lesaffre, E. (2017). Survival analysis with interval-censored data. A practical approach with examples in R, SAS and BUGS. New York, NY, USA: Chapman and Hall/CRC. doi:10.1201/9781315116945
Burger, H. U., Gerlinger, C., Harbron, C., Koch, A., Posch, M., Rochon, J., et al. (2021). The use of external controls: to what extent can it currently be recommended? Pharm. Stat. 20 (6), 1002–1016. doi:10.1002/pst.2120
Chen, J., Scharfstein, D., Wang, H., Yu, B., Song, Y., He, W., et al. (2023). Estimands in real-world evidence studies. Stat Biopharm Res, 1–13. doi:10.1080/19466315.2023.2259829
Cheson, B. D., Fisher, R. I., Barrington, S. F., Cavalli, F., Schwartz, L. H., Zucca, E., et al. (2014). Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Onc 32 (27), 3059–3068. doi:10.1200/JCO.2013.54.8800
Clare, P. J., Dobbins, T. A., and Mattick, R. P. (2019). Causal models adjusting for time-varying confounding – a systematic review of the literature. Int. J. Epi 48 (1), 254–265. doi:10.1093/ije/dyy218
Collett, D. (2023). Modelling survival data in medical research. New York, NY, USA: Chapman and Hall/CRC. doi:10.1201/9781003282525
Daniel, R. M., Cousens, S. N., De Stavola, B. L., Kenward, M. G., and Sterne, J. A. (2013). Methods for dealing with time-dependent confounding. Stat. Med. 32 (9), 1584–1618. doi:10.1002/sim.5686
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247. doi:10.1016/j.ejca.2008.10.026
Faries, D. E., Zhang, X., Zbigniew, K., et al. (2020). Real world health care data analysis using SAS: causal methods and implementation using SAS. Cary, NC, USA: SAS Institute Inc.
Ghadessi, M., Tang, R., Zhou, J., Liu, R., Wang, C., Toyoizumi, K., et al. (2020). A roadmap to using historical controls in clinical trials – by Drug Information Association Adaptive Design Scientific Working Group (ADSWG). Orphanet J. Rare Dis. 15 (69), 1–19. doi:10.1186/s13023-020-1332-x
Gogtay, N. J., Ranganathan, P., and Aggarwal, R. (2021). Understanding estimands. Perspect. Clin. Res. 12 (2), 106–112. doi:10.4103/picr.picr_384_20
Gray, C. M., Grimson, F., Layton, D., Pocock, S., and Kim, J. (2020). A framework for methodological choice and evidence assessment for studies using external comparators from real-world data. Drug Saf. 43, 623–633. doi:10.1007/s40264-020-00944-1
Hatswell, A. J., Deighton, K., Snider, J. T., Brookhart, M. A., Faghmous, I., and Patel, A. R. (2022). Approaches to selecting ‘‘time zero’’ in external control arms with multiple potential entry points: a simulation study of 8 approaches. Medic Dec Mak. 42 (7), 893–905. doi:10.1177/0272989X221096070
Hernán, M. A. (2010). The hazards of hazard ratios. Epidem 21 (1), 13–15. doi:10.1097/EDE.0b013e3181c1ea43
Hernán, M. A., and Robins, J. M. (2023). Causal inference: what if. Boca Raton, FL, USA: Chapman and Hall/CRC.
Hoogendoorn, W. E., Sanz, H., Quinten, C., et al. (2022). A methodology study to evaluate ECA study results versus RCT results: multiple Myeloma case study. https://pharmacoepi.org/pub/?id=A15839AD-B6E4-D28E-1C20-FA5A5B1D2B50 (Accessed October 8, 2023).
ICH E9(R1) Expert Working Group (2021). ICH E9(R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials. https://www.fda.gov/media/148473/download (Accessed October 8, 2023).
Li, H., Wang, C., Chen, W.-C., Lu, N., Song, C., Tiwari, R., et al. (2022). Estimands in observational studies: some considerations beyond ICH E9 (R1). Pharm. Stat. 21 (5), 835–844. doi:10.1002/pst.2196
Mao, H., Li, L., Yang, W., and Shen, Y. (2018). On the propensity score weighting analysis with survival outcome: estimands, estimation, and inference. Stat. Med. 37 (26), 3745–3763. doi:10.1002/sim.7839
Martinussen, T., Vansteelandt, S., and Andersen, P. K. (2020). Subtleties in the interpretation of hazard contrasts. Lifetime Data Anal. 26 (4), 833–855. doi:10.1007/s10985-020-09501-5
Pocock, S. (1976). The combination of randomized and historical controls in clinical trials. J. Chronic Dis. 29, 175–188. doi:10.1016/0021-9681(76)90044-8
Rippin, G., Ballarini, N., Sanz, H., Largent, J., Quinten, C., and Pignatti, F. (2022). A review of causal inference for external comparator arm studies. Drug Saf. 45 (8), 815–837. doi:10.1007/s40264-022-01206-y
Rufibach, K. (2019). Treatment effect quantification for time-to-event endpoints – estimands, analysis strategies, and beyond. Pharm. Stat. 18 (2), 145–165. doi:10.1002/pst.1917
Seeger, J. D., Davis, K. J., Iannacone, M. R., Zhou, W., Dreyer, N., Winterstein, A. G., et al. (2020). Methods for external control groups for single arm trials or long-term uncontrolled extensions to randomized clinical trials. Pharmacoepidemiol Drug Saf. 29, 1382–1392. doi:10.1002/pds.5141
Skovlund, E., Leufkens, H. G. M., and Smyth, J. F. (2018). The use of real-world data in cancer drug development. Eur. J. Cancer 101, 69–76. doi:10.1016/j.ejca.2018.06.036
Stensrud, M. J., Aalen, J. M., Aalen, O. O., and Valberg, M. (2019). Limitations of hazard ratios in clinical trials. Eur. Heart J. 40, 1378–1383. doi:10.1093/eurheartj/ehy770
Thorlund, K., Dron, L., Park, J. J. H., and Mills, E. J. (2020). Synthetic and external controls in clinical trials – a primer for researchers. Clin. Epi 12, 457–467. doi:10.2147/CLEP.S242097
U.S. Food and Drug Administration (2023). Considerations for the design and conduct of externally controlled trials for drug and biological products. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-design-and-conduct-externally-controlled-trials-drug-and-biological-products (Accessed October 8, 2023).
van Amsterdam, W. A. C., and Ranganath, R. (2023). Conditional average treatment effect estimation with marginally constrained models. J. Caus Inf. 11 (1), 1–26. doi:10.1515/jci-2022-0027
Wakabayashi, R., Hirano, T., Laurent, T., Kuwatsuru, Y., and Kuwatsuru, R. (2023). Impact of ‘time zero’ of follow-up settings in a comparative effectiveness study using real-world data with a non-user comparator: comparison of 6 different settings. Drugs - Real World Outc 10, 107–117. doi:10.1007/s40801-022-00343-1
Wang, J., Peduzzi, P., Wininger, M., et al. (2022). Statistical methods for accommodating immortal time: a selective review and comparison. https://arxiv.org/abs/2202.02369 (Accessed October 8, 2023).
Keywords: external comparator cohort studies, external comparator studies, external comparator, externally controlled Trials, historical control studies, estimands
Citation: Rippin G (2024) External comparators and estimands. Front. Drug Saf. Regul. 3:1332040. doi: 10.3389/fdsfr.2023.1332040
Received: 02 November 2023; Accepted: 12 December 2023;
Published: 02 February 2024.
Edited by:
Deborah Layton, Independent Researcher, United KingdomReviewed by:
Simone Pinheiro, AbbVie, United StatesCopyright © 2024 Rippin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Rippin, Z2VyZC5yaXBwaW5AaXF2aWEuY29t
 Gerd Rippin
Gerd Rippin